ABSTRACT
The emergence of cell and gene therapies has dramatically changed the treatment paradigm in oncology and other therapeutic areas. Kymriah® (tisagenlecleucel), a CD19-directed genetically modified autologous T-cell immunotherapy, is currently approved in major markets for the treatment of relapsed/refractory (r/r) pediatric and young adult acute lymphoblastic leukemia, r/r diffuse large B-cell lymphoma, and r/r follicular lymphoma. This article presents a high-level overview of the clinical development journey of tisagenlecleucel, including its efficacy outcomes and safety considerations.
KEYWORDS: Cell and gene therapy, CAR-T therapy, clinical development, immunotherapy, leukemia, lymphoma
Introduction
Chimeric antigen receptor (CAR) T-cell therapies have changed the treatment landscape of hematological malignancies in patient populations with a high unmet medical need. This innovative approach involves T lymphocytes isolated from a patient and genetically modified to express CARs on their surface. These modified T cells, upon infusion into the patient, identify and eliminate tumor cells expressing the targeted antigen. CAR-T therapy was developed by the University of Pennsylvania and evaluated in a study in pediatric B-cell acute lymphoblastic leukemia (ALL) in collaboration with the Children’s Hospital of Philadelphia. In 2012, Novartis Pharmaceuticals and the Perelman School of Medicine at the University of Pennsylvania entered into a collaboration to further research, develop, and commercialize CAR-T therapies. Kymriah® (tisagenlecleucel), a CD19-directed CAR-T, was the first cell-based gene therapy approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of relapsed/refractory (r/r) pediatric and young adult ALL, following a unanimous recommendation for approval by the FDA’s Oncology Drug Advisory Committee.
Tisagenlecleucel is currently approved for the treatment of r/r B-cell ALL (pediatric and young adult patients), r/r diffuse large B cell lymphoma (DLBCL), and r/r follicular lymphoma (FL) in major markets, including the United States, the European Union, and Japan. It is currently available for use in at least one of the aforementioned indications in 34 countries and at more than 430 certified treatment centers, and thus far, it has been shipped for more than 7000 patients in clinical trials and the real-world setting. The Prix Galien Award, considered as the Pharmaceutical Industry’s Nobel Prize that recognizes excellence in scientific innovation, was awarded to tisagenlecleucel for Best Biotechnology Product in 2018 and for Best Orphan/Rare Disease Product in 2022 (Golden Jubilee Award) (Source: The Galien Foundation). Table 1 summarizes the pivotal (and supportive) clinical studies of tisagenlecleucel in with patients ALL, DLBCL, or FL along with the response rates. Figure 1 displays the history of key milestones in the clinical development of tisagenlecleucel.
Table 1.
List of pivotal studies to support registration of tisagenlecleucel.
| Study population | Pivotal study for registration | First patient first visit | Protocol-specified dose range for single infusion | Response rate/Efficacy outcomes |
|---|---|---|---|---|
| Pediatric and young adult patients with B-cell ALL who are chemo-refractory, have relapsed after allogeneic SCT, or are otherwise ineligible for allogeneic SCT | CTL019B2202 (ELIANA), pivotal; CTL019B2205J (ENSIGN), supportive | April 8, 2015 |
|
N = 79 infused (ELIANA)
*median not reached Reference: Rives et al.1 |
| Adult patients with r/r DLBCL after failure of two or more lines of therapy who are not eligible for HSCT | CTL019C2201 (JULIET) | July 29, 2015 |
|
N = 115 infused
*median not reached for patients who had CR at 3 months or 6 months Reference: Schuster et al.2 |
| Adult patients with r/r FL grade 1, 2, or 3A after failure of two or more lines of therapy | CTL019E2202 (ELARA) | November 12, 2018 |
|
N = 97 infused
Reference: Dreyling et al.3 |
ALL, acute lymphoblastic leukemia; CI, confidence interval; CR, complete response; CRR, complete response rate; DLBCL, diffuse large B-cell lymphoma; DOR, duration of response; FL, follicular lymphoma; HSCT, hematopoietic stem cell transplantation; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; RFS, relapse-free survival; r/r, relapsed or refractory; SCT, stem cell transplantation.
Figure 1.
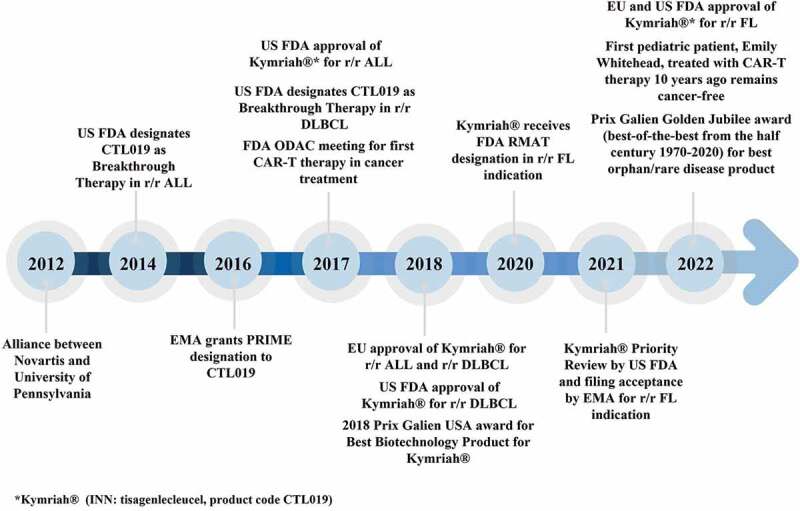
Key events in the clinical development of tisagenlecleu.
ALL, acute lymphoblastic leukemia; CAR-T, chimeric antigen receptor T cell; DLBCL, diffuse large B-cell lymphoma; EMA, European Medicines Agency; EU, European Union; US FDA, United States Food and Drug Administration; FL, follicular lymphoma; ODAC, Oncologic Drugs Advisory Committee; RMAT, regenerative medicine advanced therapy; r/r, relapsed or refractory.
As a general outline of the process, for a certain patient and their specific product, in tisagenlecleucel clinical trials, the non-mobilized peripheral blood mononuclear cells (PBMCs) are collected via leukapheresis. The leukapheresed material is then cryopreserved and shipped to the manufacturing facility. Manufacturing of CAR-T cells is a complex process that encompasses enrichment, selection, and activation of T cells followed by the transduction with lentiviral vectors that integrate anti-CD19 CAR transgene in the T cells. The ex vivo expansion is allowed for a sufficient yield of CAR-T cells, following which the cells are harvested and evaluated for critical quality attributes. The cryopreserved product is shipped to the treatment site where it is thawed prior to infusion into the same patient for whom the leukapheresis was performed.4
While the product was being manufactured, the patient could receive optional bridging chemotherapy as well as lymphodepleting chemotherapy prior to the infusion of the final product. Patients receive bridging chemotherapy to stabilize the disease after leukapheresis and prior to the infusion of tisagenlecleucel, at the discretion of investigators. Hence, the need for bridging chemotherapy is indicative of aggressive disease and high disease burden. Bridging chemotherapy includes combinations of rituximab, gemcitabine, etoposide, dexamethasone, cisplatin, cytarabine, and agents such as ibrutinib and lenalidomide. In the JULIET and ELIANA trials, 90%2 and 87%,5 of the patients received bridging chemotherapy, respectively. Following bridging chemotherapy, patients with more than 103 cells/mm3 of white cell count within 1 week before tisagenlecleucel infusion received lymphodepletion chemotherapy containing fludarabine-cyclophosphamide or bendamustine in DLBCL patients6 or fludarabine-cyclophosphamide or cytarabine-etoposide in ALL patients.5
Efficacy
Although a vast majority of pediatric and young adult patients with ALL have good long-term outcomes after conventional first-line therapy, approximately 15% of patients are refractory or relapse, therefore raising a significant medical need for further therapies. For this group of patients, tisagenlecleucel had a strong impact. For example, the first patient treated with tisagenlecleucel in a clinical trial for pediatric ALL recently celebrated 10 years in remission. In the pivotal ELIANA trial conducted in pediatric and young adult patients with r/r ALL (ClinicalTrials.gov: NCT02435849), the overall response rate (ORR; complete remission [CR] plus complete remission with incomplete count recovery [CRi]) was 82%. The most recent analysis of this study reported that the 5-year relapse-free survival of patients who initially achieved a CR or CRi after tisagenlecleucel administration was 49%.1 The impressive overall efficacy in this patient population was also confirmed in the real-world setting.7
Prior to the availability of CAR-T therapies, the outcomes in patients with r/r DLBCL were dismal.8,9 For patients with r/r FL, the quality of life was limited owing to patients becoming more refractory or having shorter intervals of response with each line of therapy.10 In the pivotal clinical trials of tisagenlecleucel for patients with r/r DLBCL (JULIET; ClinicalTrials.gov: NCT02445248) and for patients with FL (ELARA; ClinicalTrials.gov: NCT03568461), the ORR and CR rates were 53% and 39% (JULIET)2 and 86% and 68% (ELARA),3 respectively. Real-world data from the Center for International Blood and Marrow Transplant Research (CIBMTR) and the CAR-T Consortium show consistent response and survival results in patients treated with DLBCL in the post-approval setting.11 According to the most recent report from the CIBMTR, with 1159 patients receiving tisagenlecleucel for aggressive B-cell non-Hodgkin’s lymphoma (NHL), the ORR was 59.5% and the best overall response rate was 44.5%. The 24-month overall (OS) and progression-free survival (PFS) rates were 43.6% and 28.4%, respectively.11 The CAR-T Consortium consisted of 7 US academic medical centers and reported a 90-day ORR and CR rates of 41% and 35%, respectively. The 12-month OS and PFS rates were 59% and 32%, respectively.12
A randomized phase 3 trial for tisagenlecleucel versus standard of care (SoC) was conducted in aggressive B-cell lymphoma patients who were refractory or relapsed within 12 months after first-line therapy (BELINDA; ClinicalTrials.gov number: NCT03570892). Bridging therapy was allowed, and in fact, 83% of the patients in the tisagenlecleucel arm received bridging therapy,13 which may be an indication for the advanced or aggressive nature of the patient population included in this study14,15 While the primary endpoint of event-free survival (EFS) was not met, favorable responses were observed in 46.3% of patients that received tisagenlecleucel and in 42.5% of patients in the SoC arm. Of note, several patients responded to tisagenlecleucel, but due to the design of the study, they were recorded as having an event owing to stable or progressive disease before or soon after infusion.13
A phase 1/2 study in patients with highly refractory primary central nervous system lymphoma, who had been excluded from the pivotal trials, demonstrated response in 7 and CR in 6 out of 12 patients, with 3 patients still in CR at the reported data cutoff.16 Safety appeared manageable, with seven patients experiencing grade 1 cytokine release syndrome (CRS) and five patients experiencing low-grade immune-effector cell-associated neurotoxicity syndrome (ICANS), and only one patient experiencing severe (grade 3) ICANS. No treatment-related deaths were observed. CAR-T cell expansion in peripheral blood was observed in all, except for one patient. The median level of CAR-T cells in cerebrospinal fluid was higher in responding patients (with CR as the best overall response) compared to non-CR patients. The authors concluded that tisagenlecleucel may offer a valuable option in this patient setting.16
Overall, results from the pivotal studies with CAR-T therapy demonstrated noticeable improvement in response and survival rates for patients with r/r B-cell malignancies.
Safety considerations
Cytokine release syndrome
CRS is an on-target and the most common type of toxicity of CAR-T therapy17 and represents a systemic inflammatory response resulting from the overproduction of inflammatory cytokines.17,18 Clinically, patients with CRS present with fever, hypotension, hypoxia, and a range of other symptoms, usually within the first few days after CAR-T infusion. CRS manifests with marked elevation in interleukin levels, with interleukin-6 (IL-6) as the most dominant cytokine. Tocilizumab, an IL-6 receptor monoclonal antibody, ameliorates the symptoms associated with CRS without an untoward impact on the clinical efficacy and in vivo expansion of CAR-T cells; thus, the application of tocilizumab is established to improve the clinical management of CRS.19 Today, tocilizumab is the mainstay of CRS management. With continued clinical experience, a CRS management algorithm has been developed on the basis of CRS severity grade. CRS grading, however, has long been heterogeneous due to the use of several grading scales; for example, the grading scales developed by clinicians at the University of Pennsylvania (Penn)20 or the National Institutes of Health (Lee).21 The emerging need to harmonize grading schemes led to the development of the American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading.22 The key differences between these grading scales were previously reported by Schuster et al.23 The management of CRS with early intervention using tocilizumab for low CRS grades may reduce the probability of worsening of CRS symptoms toward a higher grade.23
Neurological adverse reactions
Neurological toxicity is another common adverse reaction associated with CAR-T therapy and usually includes clinical features such as headache, dizziness, delirium, seizures, dysphasia, hallucinations, and impaired cognitive skills.24 Most cases of neurological events occur within the first 8 weeks of tisagenlecleucel infusion and are often resolved within few days with supportive treatment including corticosteroids. These events can occur at around the time of onset of CRS, following the resolution of CRS, or even in the absence of CRS. The neurological symptoms commonly seen after CAR-T therapy have been summarized using the new term ‘ICANS,’ and grading scales and management algorithms have been developed.25
Other important identified safety risks with CAR-T therapy are infections, tumor lysis syndrome, and prolonged cytopenia, in particular, prolonged depletion of the target cells (i.e., prolonged depletion of normal B-cells for CD19-directed CAR-T therapies). Administration of immunoglobulins is a common practice for the prophylaxis of infections in patients with severe hypogammaglobulinemia resulting from B-cell aplasia.
Testing for replication-competent lentivirus
Generation of replication-competent lentivirus (RCL) is a theoretical concern and a potential risk, given the use of replication-deficient lentiviral vectors for the genetic engineering of the CAR-T cells. Safeguards have been incorporated in the design of the vector system to minimize vector recombination and generation of RCL, thus making the generation of RCL highly unlikely.26,27 In addition, the viral vector used to transduce the product undergoes sensitive assays for detecting RCL before it can be released for manufacture. Nevertheless, generation of RCL following infusion of the vector product remains a theoretical possibility. Thus far, there is no evidence of a patient developing RCL after treatment with tisagenlecleucel.
Long-term follow-up
Patients from clinical trials involving CAR-T therapy are offered participation in long-term follow-up studies to monitor the long-term safety profile of CAR-T therapy. There are also registries for the long-term follow-up of patients treated in the real-world setting. A potential risk that is followed in such studies is the development of second primary malignancies. Although such new malignancies can occur independent of CAR-T therapy (e.g., due to prior chemotherapy), there is a theoretical concern and potential risk that the integration of the CAR transgene can lead to malignant transformation.
Overall, the introduction of CAR-T therapy into clinical practice has sparked tremendous efforts to improve the safety of these highly effective therapies including the use of innovative agents such as tocilizumab to manage side effects, efforts of medical societies to develop and harmonize grading scales and recommendations/guidelines, and the collaboration of multiple stakeholders in the setup and monitoring of patients through long-term follow-up studies.
Cellular kinetics and biomarkers
Characterization of the in vivo expansion of CAR-T cells post infusion can provide insights into the relationship between cellular kinetics and safety/efficacy endpoints. Detailed analyses have been performed to understand these relationships in ALL and DLBCL indications28,29 using the cellular kinetics based on the time course of CAR transgene levels measured by quantitative polymerase chain reaction (qPCR). Overall, cellular kinetic data in pediatric ALL demonstrated higher mean expansion in responding patients relative to nonresponding patients, whereas the mean expansion levels were similar between responding and non-responding patients with DLBCL. However, the mean expansion in patients with ALL was nearly sixfold higher than the expansion in patients with DLBCL, potentially because cellular kinetics was measured in the peripheral blood in patients with ALL, which is also the site of disease in ALL (along with bone marrow), whereas in patients with DLBCL, the disease is mainly located outside the peripheral blood, i.e., in extramedullary tissues and lymph nodes. Of note, the expansion levels in both the indications were associated with high inter-individual variability.
Recently, a decade-long persistence of the CAR transgene was demonstrated in a patient with chronic lymphoblastic leukemia who was treated with CAR-T therapy, with the patient having achieved and remaining in CR.30 The robust expansion and long persistence of CAR-T cells following a single infusion is associated with favorable clinical outcomes; however, some patients with ALL and DLBCL have shown durable responses despite early loss of the CAR transgene. This could be due to the complete regression of the tumor with no reappearance, which potentially resulted from the initial expansion and persistence.31
Dose of CAR-T cells
The dose of CAR-T cells is expressed as the number of CAR-positive viable T cells. The dose range for tisagenlecleucel is presented by indication in Table 1. Although manufacturing is aimed at producing doses toward the upper end of the dose range, in some cases, owing to manufacturing challenges and/or patient’s leukapheresis characteristics, higher doses may not be feasible. Across all indications, favorable clinical responses have been observed at doses toward the lower end of the dose range.
Interestingly, no clear relationship was observed between the dose of CAR-T cells and in vivo expansion in ALL and DLBCL, which can be attributed to the multi-log fold in vivo expansion of infused CAR-T cells in patients.
Pasquini et al. demonstrated no significant association between dose and best overall response using logistic regression analyses in patients with ALL and NHL from the CIBMTR database.32 The impact of dose on time-to-event efficacy endpoints was investigated by the Pediatric Real World CAR Consortium (PRWCC), which collected the retrospective data of 185 pediatric and young adult patients with ALL treated with commercial tisagenlecleucel.33 Although higher doses within the approved dose range appeared to be associated with improved OS, PFS and EFS rates with no increased risk of toxicity (CRS, neurotoxicity, or grade 3 or higher adverse events), the authors acknowledge that there could be important confounders such as the lower cytogenetic risk of the patients in the higher dose groups.33
Impact of baseline disease burden
Real-world data from patients with ALL treated with tisagenlecleucel showed an association between higher baseline tumor burden (≥5% bone marrow lymphoblasts, CNS3, or non-CNS extramedullary disease) and inferior outcomes, compared to patients with lower baseline tumor burden.34
In adult patients with aggressive NHL, the probability of response increased with an increase in the dose in patients with stable disease or progressive disease prior to tisagenlecleucel infusion, whereas no apparent impact was observed on dose–response relationship in patients that demonstrated favorable clinical response prior to infusion.13
These findings may underscore the importance of reducing tumor burden prior to tisagenlecleucel infusion, but they may also be associated with differences in the underlying biology of the tumor, which cannot be modified by reducing the tumor burden before CAR-T therapy.
B-cell recovery as a functional marker of CAR persistence
B cell aplasia, an on-target effect of CAR-T therapy, is considered as a pharmacodynamic marker for the functional persistence of CAR-T cells.29 Recovery of B cells is usually defined as >1% of B cells in total white blood cells or >3% of B cells in total lymphocytes in the peripheral blood.29 In ALL, patients with early B-cell recovery (<6 months following infusion) showed rapid loss of transgene and lower expansion relative to patients with late B-cell recovery or sustained B-cell aplasia.29 In DLBCL, however, B-cell recovery was not associated with relapse.28,35
B-cell recovery within the first 6 months appears to be associated with inferior clinical outcomes; however, B-cell aplasia monitoring in itself is not sufficient to predict relapses. Pulsipher et al. demonstrated the importance of next-generation sequencing in predicting relapse in patients with ALL treated with tisagenlecleucel. Monitoring of the minimal residual disease in bone marrow using next-generation sequencing was shown to be the most sensitive biomarker for predicting relapse.36
Evolution and changes in manufacturing over time
Manufacturing of tisagenlecleucel involves leukapheresis (collection of immobilized PBMCs), followed by cryopreservation of the leukapheresed material.4 The cryopreserved material is then thawed at the manufacturing site and washed to remove the cryomedium. Enrichment, selection, and activation of T cells are carried out, followed by transduction of the CAR transgene. The cells are washed and allowed to expand ex vivo to attain the required cell dose. The critical quality attributes, indicative of purity and activity, of the final product are assessed prior to release of the cell product to the site for infusion.
The transition of the tisagenlecleucel manufacturing process from an academic center (University of Pennsylvania) to the industry setting was accompanied by steps aimed at improving the process performance and robustness while maintaining the potency of the product. Refinements were made to various aspects of manufacturing to address increasing demands for the supply of the product to multicenter global trials, to improve robustness of the process, and to overcome rate-limiting steps such as the supply of human AB serum and time required to perform sterility testing. As an example, the modification to T cell enrichment pathway that can accommodate a range of incoming cell population led to improved ex vivo cellular growth with comparable product quality, while the implementation of validated rapid qPCR assay for mycoplasma sterility testing shortened the testing time prior to the release of the final product. In addition, improvements were made in the facility and operational fronts via optimization of training programs and increase in personnel, logistics, etc. The optimization in the manufacturing process for the clinical trials resulted in high manufacturing success rate and low throughput time during commercial manufacturing.4
Product attributes
Key attributes such as the percentage of T cells, cell viability, transduction efficiency and total cell count, which are indicative of the purity and quality of the final CAR-T cell product, are assessed before the release of the final product for infusion into the patient. Analyses have been performed to understand the relationship between these attributes and the in vivo expansion of CAR-T cells, and no apparent correlation was observed in patients with ALL and DLBCL.28,29
Next-generation CAR-T: the T-Charge™ platform
Despite the clinical advances that tisagenlecleucel and other CAR-T therapies have brought to patients, there is room to further improve response rates, durability of remission, and the side effect profile. A next-generation CAR-T platform has been developed with the aim to preserve the T-cell stemness in the final product and to confer higher proliferative potential with reduced exhausted T cells while reducing the manufacturing time to less than 2 days. Preliminary data from the first-in-human study of YTB323 (Rapcabtagene Autoleucel), a next-generation anti-CD19 CAR-T, manufactured using the novel T-Charge™ platform demonstrated efficacy and a favorable safety profile in patients with DLBCL with preservation of CD4 and CD8 naive and stem cell memory cells in the final product.37
Healthcare resource utilization and hospitalization cost associated with tisagenlecleucel treatment
Total cost of CAR-T treatment includes both drug and non-drug costs. Recent real-world studies in US showed that mean total health care expenditures of CAR-T, including tisagenlecleucel, ranged from $380,000 to $526,000. The cost was higher for patients who were experienced severe neurological events and CRS.38,39 Therefore, a CAR-T with a better toxicity profile is associated with reduced non-drug related cost. Real-world studies of tisagenlecleucel showed that the rates of severe neurological events and CRS were lower in the commercial setting than those reported in early clinical trials, likely due to continuous improvement in adverse event management.12,32
The cost-effectiveness analysis (CEA) of CAR-Ts was reported in both literatures and health technology assessment reports. The reported CEA results based on the visible prices in ex-US are less informative since final negotiated prices are unknown. CAR-Ts including tisagenlecleucel were granted reimbursement in over 30 countries globally – including CEA-driven countries such as UK, Canada, and Australia.
In general, published CEAs in US have demonstrated the cost-effectiveness of CARTs, including tisagenlecleucel.40–44 Research has showed that CAR-T therapies improved health outcomes (measured in incremental quality-adjusted life-years) to a greater extent than recent innovative oncology treatments.45 The magnitude of benefit due to CAR-T therapies depends upon the ability of patients to access these treatments promptly. With treatment delay, the loss of benefit is significant.46 A major limitation of these CEAs is that they were based on results from early clinical trials which lacked long-term outcome data and real-world cost information. More recent long-term follow-up data from CART trials suggest that these early CEAs may underestimate the long-term benefit of CAR-T therapies.1,47 Further CEA research based on emerging longer-term follow-up data, real-world cost information, and real-world practice patterns could help us to better quantify the value of CAR-T therapies.
Coverage and reimbursement decisions for one-time curative therapy are challenging. These decisions require balancing the needs from different stakeholders – urgent patients’ need for life-saving medicine, payers’ need for robust evidence to quantify the value, and manufacturers’ need to preserve the value and sustainable innovation. Irrespective of the selected reimbursement approaches, it is important for policymakers to promptly establish interim reimbursement policies to ensure that patients have timely access to new treatments while allowing longer-term data to be collected and reviewed later. With these new data, reimbursement decisions can be revised accordingly to better reflect the value of CAR-T therapies. By experimenting with novel approaches, we can start to close the gap between regulatory approval and patients’ receipt of treatments. Faster approval of curative therapies is helpful only if patients can access them in time.
Conclusion
Cell and gene therapies have transformed the treatment paradigm of hematological malignancies for patients who otherwise had limited therapeutic options. Extensive research and data exploration have improved the understanding of the various facets of CAR-T therapy, including manufacturing, biomarkers, management of toxicities, and identification of risk factors for poor prognosis. The innovation toward reducing the throughput time, possibility of treating in an outpatient and community setting, and potential for better and durable responses and reduced toxicity risks are aimed to provide improved long-term outcomes in patients with high unmet medical need.
Acknowledgement
The authors would like to thank the patients and their families, the investigators, and the study site personnel who participated in Novartis sponsored tisagenlecleucel studies, and Novartis team members for their contributions to the program.
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure statement
R.A. is an employee of Novartis Institutes for BioMedical Research and owns stock/options. H.J.M. is an employee of Novartis Pharma AG and owns stock/options. JZ is an employee of Novartis Services Inc. and owns stock/options. S.L. is an employee of Novartis Pharmaceuticals Corporation and owns stock/options.
References
- 1.Rives S, Maude SL, Hiramatsu H, Baruchel A, Bader P, Bittencourt H, Buechner J, Laetsch T, De Moerloose B, Qayed M, et al. Tisagenlecleucel in pediatric and young adult patients (Pts) with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-All): final analyses from the eliana study. HemaSphere. 2022;6:1–8. doi: 10.1097/01.HS9.0000843344.19780.98. [DOI] [Google Scholar]
- 2.Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, Waller EK, Jaglowski S, Bishop MR, Damon LE, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:1403–15. doi: 10.1016/S1470-2045(21)00375-2. [DOI] [PubMed] [Google Scholar]
- 3.Dreyling M, Dickinson M, Lopez JM, Kolstad A, Butler JP, Ghosh M, Popplewell LL, Chavez J, Bachy E, Kato K, et al. Long-term clinical outcomes and correlative efficacy analyses in patients (Pts) with relapsed/refractory follicular lymphoma (r/r FL) treated with tisagenlecleucel in the Elara trial. American Society of Hematology (ASH) Annual Meeting and Exposition New Orleans, Louisiana; 2022. [Google Scholar]
- 4.Tyagarajan S, Spencer T, Smith J.. Optimizing CAR-T cell manufacturing processes during pivotal clinical trials. Mol Ther Methods Clin Dev. 2020;16:136–44. doi: 10.1016/j.omtm.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 7.John S, Pulsipher MA, Moskop A, Z-H H, Phillips CL, Hall EM, Margossian SP, Nikiforow S, Martin PL, Oshrine B, et al. Real-world outcomes for pediatric and young adult patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (ALL) treated with tisagenlecleucel: update from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. Blood. 2021;138:428. [Google Scholar]
- 8.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Den Neste E V, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, Milpied N, Radford J, Ketterer N, Shpilberg O, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51:51–7. doi: 10.1038/bmt.2015.213. [DOI] [PubMed] [Google Scholar]
- 10.Link BK, Day BM, Zhou X, Zelenetz AD, Dawson KL, Cerhan JR, Flowers CR, Friedberg JW.. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational national lymphoCare study. Br J Haematol. 2019;184:660–3. doi: 10.1111/bjh.15149. [DOI] [PubMed] [Google Scholar]
- 11.Landsburg DJ, Frigault M, Heim M, Foley SR, Hill BT, Ho CM, Jacobson CA, Jaglowski S, Locke FL, Ram R, et al. Real-world outcomes for patients with relapsed or refractory (R/R) aggressive B-cell non-hodgkin’s lymphoma (aBNHL) treated with commercial tisagenlecleucel: subgroup analyses from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. Blood. 2022;140:1584–7. [Google Scholar]
- 12.Riedell PA, Hwang WT, Nastoupil LJ, Pennisi M, McGuirk JP, Maziarz RT, Bachanova V, Oluwole OO, Brower J, Flores OA, et al. Patterns of use, outcomes, and resource utilization among recipients of commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B cell lymphomas. Transplant Cell Ther. 2022;28:669–76. doi: 10.1016/j.jtct.2022.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, Kato K, Sureda A, Greil R, Thieblemont C, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386:629–39. doi: 10.1056/NEJMoa2116596. [DOI] [PubMed] [Google Scholar]
- 14.Pinnix CC, Gunther JR, Dabaja BS, Strati P, Fang P, Hawkins MC, Adkins S, Westin J, Ahmed S, Fayad L, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv. 2020;4:2871–83. doi: 10.1182/bloodadvances.2020001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roschewski M, Longo DL, Wilson WH. CAR T-cell therapy for large B-cell lymphoma - who, when, and how? N Engl J Med. 2022;386:692–6. doi: 10.1056/NEJMe2118899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frigault MJ, Dietrich J, Gallagher K, Roschewski M, Jordan JT, Forst D, Plotkin SR, Cook D, Casey KS, Lindell KA, et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood. 2022;139:2306–15. doi: 10.1182/blood.2021014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor–Modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11:35. doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster SJ, Maziarz RT, Rusch ES, Li J, Signorovitch JE, Romanov VV, Locke FL, Maloney DG. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv. 2020;4:1432–9. doi: 10.1182/bloodadvances.2019001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maziarz RT, Schuster SJ, Romanov VV, Rusch ES, Li J, Signorovitch JE, Maloney DG, Locke FL. Grading of neurological toxicity in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv. 2020;4:1440–7. doi: 10.1182/bloodadvances.2019001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, Atkins MB, Brassil KJ, Caterino JM, Chau I, et al. management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. 2021;39:3978–92. doi: 10.1200/JCO.21.01992. [DOI] [PubMed] [Google Scholar]
- 26.Bear AS, Morgan RA, Cornetta K, June CH, Binder-Scholl G, Dudley ME, Feldman SA, Rosenberg SA, Shurtleff SA, Rooney CM, et al. Replication-competent retroviruses in gene-modified T cells used in clinical trials: is it time to revise the testing requirements? Mol Ther. 2012;20:246–9. doi: 10.1038/mt.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awasthi R, Pacaud L, Waldron E, Tam CS, Jager U, Borchmann P, Jaglowski S, Foley SR, van Besien K, Wagner-Johnston ND, et al. Tisagenlecleucel cellular kinetics, dose, and immunogenicity in relation to clinical factors in relapsed/refractory DLBCL. Blood Adv. 2020;4:560–72. doi: 10.1182/bloodadvances.2019000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller KT, Waldron E, Grupp SA, Levine JE, Laetsch TW, Pulsipher MA, Boyer MW, August KJ, Hamilton J, Awasthi R, et al. Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2018;24:6175–84. doi: 10.1158/1078-0432.CCR-18-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, Gao P, Bandyopadhyay S, Sun H, Zhao Z, et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature. 2022;602:503–9. doi: 10.1038/s41586-021-04390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awasthi R, Mueller KT, Yanik GA, Tam CS, Rives S, McGuirk JP, Pulsipher MA, Boyer MW, Jaeger U, Baruchel A, et al. Evaluation of in vivo CAR transgene levels in relapsed/refractory pediatric and young adult ALL and adult DLBCL tisagenlecleucel-treated patients. Blood. 2018;132:899. doi: 10.1182/blood-2018-99-116385. [DOI] [Google Scholar]
- 32.Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R, Pulsipher MA, Phillips CL, Keating A, Frigault MJ, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4:5414–24. doi: 10.1182/bloodadvances.2020003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanski H, Eaton A, Baggott C, Rossoff J, Verneris MR, Prabhu S, Pacenta HL, Phillips CL, Talano JA, Moskop A, et al. Higher doses of tisagenlecleucel associate with improved outcomes: a report from the pediatric real-world CAR consortium. Blood Adv. 2023;7:541–548. doi: 10.1182/bloodadvances.2022007246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz LM, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, Stefanski HE, Talano J-A, Moskop A, Margossian SP, et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol. 2022;40:945–55. doi: 10.1200/JCO.20.03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulsipher MA, Han X, Maude SL, Laetsch TW, Qayed M, Rives S, Boyer MW, Hiramatsu H, Yanik GA, Driscoll T, et al. Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov. 2022;3:66–81. doi: 10.1158/2643-3230.BCD-21-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flinn IW, Jaeger U, Shah NN, Blaise D, Briones J, Shune L, Boissel N, Bondanza A, Lu D, Zhu X, et al. A first-in-human study of YTB323, a novel, autologous CD19-directed CAR-T cell therapy manufactured using the novel T-Charge TM platform, for the treatment of patients (Pts) with relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL). Blood. 2021;138:740. doi: 10.1182/blood-2021-146268. [DOI] [Google Scholar]
- 38.Keating SJ, Gu T, Jun MP, McBride A. Health care resource utilization and total costs of care among patients with diffuse large B cell lymphoma treated with chimeric antigen receptor T cell therapy in the United States. Transplant Cell Ther. 2022;28:404 e1–e6 . doi: 10.1016/j.jtct.2022.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Maziarz RT, Yang H, Liu Q, Wang T, Zhao J, Lim S, Lee S, Dalal A, Bollu V. Real-world healthcare resource utilization and costs associated with tisagenlecleucel and axicabtagene ciloleucel among patients with diffuse large B-cell lymphoma: an analysis of hospital data in the United States. Leuk Lymphoma. 2022;63:2052–62. doi: 10.1080/10428194.2022.2060503. [DOI] [PubMed] [Google Scholar]
- 40.Chimeric antigen receptor T-cell therapy for B-cell cancers: effectiveness and value. Institute for Clinical and Economic Review; 2018 Mar 23. [Google Scholar]
- 41.Hao Y, Eldjerou LK, Yang H, Qi C, Globe D. Cost-effectiveness analysis of CTL019 for the treatment of pediatric and young adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia in the United States. Blood. 2017;130:609. [Google Scholar]
- 42.Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, Davis KL, Owens DK, Goldhaber-Fiebert JD. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36:3192–202. doi: 10.1200/JCO.2018.79.0642. [DOI] [PubMed] [Google Scholar]
- 43.Roth JA, Sullivan SD, Lin VW, Bansal A, Purdum AG, Navale L, Cheng P, Ramsey SD. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21:1238–45. doi: 10.1080/13696998.2018.1529674. [DOI] [PubMed] [Google Scholar]
- 44.Whittington MD, McQueen RB, Ollendorf DA, Kumar VM, Chapman RH, Tice JA, Pearson SD, Campbell JD. Long-term survival and value of chimeric antigen receptor T-cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018;172:1161–8. doi: 10.1001/jamapediatrics.2018.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumgardner J, Everson K, Brauer M, Zhang J, Hao Y, Liu J, Lakdawalla DN. CAR-T therapy displays favorable gains in health outcomes and competitive cost-effectiveness when compared with past innovative cancer treatments. Blood. 2018;132:322. doi: 10.1182/blood-2018-99-116763. [DOI] [Google Scholar]
- 46.Snider JT, Brauer M, Batt K, Karaca-Mandic P, Zhang J, Goldman DP. The social value of tisagenlecleucel, a CAR-T cell therapy, for the treatment of relapsed or refractory pediatric acute lymphoblastic leukemia in the United States: what are consequences of treatment delays? J Clin Oncol. 2018;36:10529. doi: 10.1200/JCO.2018.36.15_suppl.10529. [DOI] [Google Scholar]
- 47.Neelapu SS, Jacobson CA, Ghobadi A, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, et al. 5-year follow-up supports curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1). Blood. 2023. doi: 10.1182/blood.2022018893. [DOI] [PubMed] [Google Scholar]


