ABSTRACT
The mRNA-based BNT162b2 and inactivated whole-virus CoronaVac are two widely used COVID-19 vaccines that confer immune protection to healthy individuals. However, hesitancy toward COVID-19 vaccination appeared to be common for patients with neuromuscular diseases (NMDs) due to the paucity of data on the safety and efficacy in this high-risk patient population. Therefore, we examined the underlying factors associated with vaccine hesitancy across time for NMDs and assessed the reactogenicity and immunogenicity of these two vaccines. Patients aged 8–18 years with no cognitive delay were invited to complete surveys in January and April 2022. Patients aged 2–21 years were enrolled for COVID-19 vaccination between June 2021 and April 2022, and they recorded adverse reactions (ARs) for 7 days after vaccination. Peripheral blood was obtained before and within 49 days after vaccination to measure serological antibody responses compared to healthy children and adolescents. Forty-one patients completed vaccine hesitancy surveys for both timepoints, while 22 joined the reactogenicity and immunogenicity arm of the study. Two or more family members vaccinated against COVID-19 was positively associated with intention of vaccination (odds ratio 11.7, 95% CI 1.81–75.1, p = .010). Pain at the injection site, fatigue, and myalgia were the commonest ARs. Most ARs were mild (75.5%, n = 71/94). All 19 patients seroconverted against the wildtype SARS-CoV-2 after two doses of either vaccine, similar to 280 healthy counterparts. There was lower neutralization against the Omicron BA.1 variant. BNT162b2 and CoronaVac were safe and immunogenic for patients with NMDs, even in those on low-dose corticosteroids.
KEYWORDS: BNT162b2, COVID-19, CoronaVac, neuromuscular diseases, vaccine hesitancy
Introduction
Since the onset of the COVID-19 pandemic, infection by the SARS-CoV-2 virus has been associated with significant morbidity, mortality, and negative socioeconomic impact throughout the world1–3 Certain patient populations, such as those with neuromuscular diseases (NMDs), have greater risks of severe disease and death from infections due to their muscle weakness of the chest wall or diaphragmatic muscle, cardiac involvement, and immunosuppressed state.4 Vaccination is highly effective against symptomatic infection, hospital admission, and severe COVID-19 in healthy adults and children.5–10 As such, the mRNA-based BNT162b2 and inactivated whole-virus CoronaVac vaccines are amongst the most widely used COVID-19 vaccines globally since authorization for emergency use by World Health Organization.11–13 Based on these findings in healthy individuals, several national neurology associations, and neuromuscular disease networks recommend COVID-19 vaccination for those with NMDs, but data on this important high-risk patient population specifically remain scarce.4
Although COVID-19 vaccination is expected to reduce infectious disease severity in the NMD population, vaccine hesitancy appears to be a major potential barrier.14 As an example, our group found that in mid-2021 when the COVID-19 vaccines initially became available for adolescents, only 39% of healthy adolescents planned for vaccination.15 For NMDs, only 69.0% of the parents would want their children vaccinated during the early pandemic period in December 2020.16 It is also concerning that as little as 42.6% of those with Duchenne muscular dystrophy (DMD) were vaccinated against COVID-19 by November 2021 in Poland.17 Their reasons for not opting for vaccination during this early, pre-Omicron variant period included the potential for reduced efficacy due to their use of immunosuppressive or immunomodulating therapies and uncertainties regarding possible interactions between the vaccines and treatments for NMDs.18–20 Despite the availability of two different COVID-19 vaccine types, BNT162b2 and CoronaVac, in our locality, our NMD patients also appeared reluctant on vaccination. Some of these patients had cited the risks of adverse effects, disease complications, and reduced efficacy as some of their main concerns.
The reason that there has been a paucity of NMD-specific safety and immunogenicity data despite the rollout of the many types of COVID-19 vaccines is because NMD is a group of rare diseases and NMD patients are hesitant to volunteer for receiving novel vaccines, and so large-scale studies had not yet been possible. In fact, the prevalence of NMDs is as low as 1–10 per 100,000 of the total population.21 Therefore, scientific evidence on COVID-19 vaccination in NMD patients have been based on small cohorts only thus far. For 14 adult NMD patients, BNT162b2 and mRNA-1273 vaccines generally seemed safe and immunogenic, and similar outcomes were observed in 53 adult inpatients with muscular dystrophies who received 2 doses of BNT162b2.22,23 The mRNA-1273 vaccine achieved robust humoral and cellular immune responses in 100 adult patients with myasthenia gravis.24 Unfortunately, there are still no available safety and immunogenicity data on COVID-19 vaccines in children with NMDs. Importantly, there has been no previous research on the inactivated COVID-19 vaccines in adult or pediatric patients with NMDs, including immunogenicity against the novel variants, such as Omicron. Data on both vaccines are essential because some individuals experience significant adverse effects on one vaccine type and are only able to tolerate the other type.25
Therefore, this study investigated in-depth the underlying reasons and temporal changes in vaccine hesitancy for pediatric patients with NMDs during the Omicron wave in 2022, using our previously published survey, with an expanded questionnaire tailored specifically for pediatric NMDs.15 Additionally, we assessed the safety and immunogenicity of two types of COVID-19 vaccines, the BNT162b2 and CoronaVac, by recording adverse effects and measuring serum antibody levels and neutralization against the wild type (WT) SARS-CoV-2 virus and Omicron B.1.1.529 variant.
Patients and methods
Study population
All participants were screened from the Hong Kong (HK) NMD registry.26 This registry has been approved by the Institutional Review Board (IRB) and collects patient and clinician-reported demographic and clinical information from those with confirmed diagnoses of NMDs after their consent.26 The diagnosis was determined by clinical pediatric neurologists and supported by genetic testing and/or muscle biopsy results. The neurology clinical study team is based at HK Children’s Hospital, Duchess of Kent Children’s Hospital at Sandy Bay and Queen Mary Hospital that receives referrals from throughout the entire HK territory for clinical care and research in pediatric NMDs.26 Participation in the vaccine hesitancy survey required patients to have reached a neurodevelopmental age that could comprehend and provide credible responses to the detailed questionnaire independently without direct parental influence. As such, the inclusion criteria were 8–18 years old and no cognitive deficit in this survey arm of the study. In another arm of this study, which did not necessitate such neurodevelopmental age limitations, patients aged 2–21 years were invited for COVID-19 vaccination to study the reactogenicity and immunogenicity of the BNT162b2 and CoronaVac vaccines. Both arms of the study required at least one parent to accompany the patient during the study process. Potential participants needed to be in stable condition. A patient could join either or both arms of the study, if eligible (Figure 1).
Figure 1.
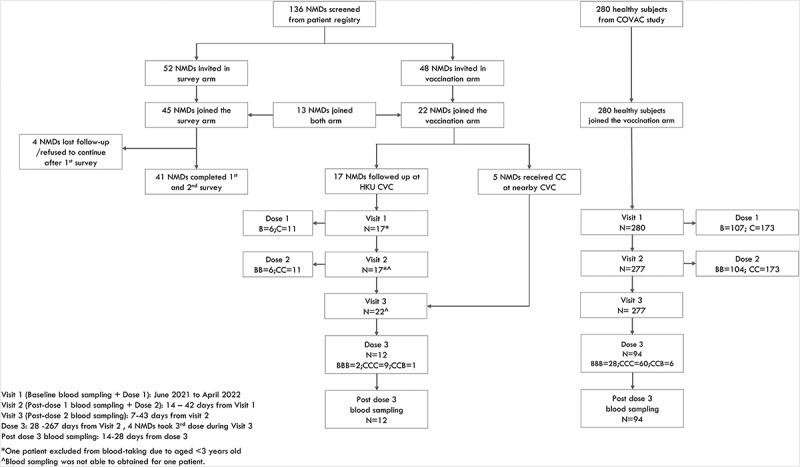
Flow diagram of study participants.
One hundred and thirty-six patients with neuromuscular diseases (NMDs) were screened from the patient registry. Fifty-two patients were invited to complete the hesitancy survey arm, and 48 patients were invited to join the reactogenicity and immunogenicity arm of the study. Forty-five patients completed the first hesitancy survey, and 41 (91.1%) of them completed both first and second surveys. For the reactogenicity and immunogenicity arm of the study, 22 patients joined and 17 were inoculated with BNT162b2 or CoronaVac at our University of Hong Kong (HKU) Community Vaccination Centers (CVCs) research site. These patients recorded adverse reactions in a 7-day diary system after vaccination for reactogenicity/safety analyses and had blood sampling. Additionally, five patients who received two doses of CoronaVac at nearby CVCs and had blood sampling after the second dose. Reactogenicity and immunogenicity data from healthy children and adolescents (n = 280) used for comparisons with patients with NMDs were retrieved from our previous publication.27 CVCs = Community Vaccination Centers research site, NMDs = patients with neuromuscular diseases, COVAC = Coronavirus disease-19 (COVID-19) Vaccination in Adolescents and Children, HKU = University of Hong Kong. B, 1 dose of BNT162b2; BB, 2 doses of BNT162b2; C, 1 dose of CoronaVac; CC, 2 doses of CoronaVac; BBB, 3 dose of BNT162b2; CCC, 3 doses of CoronaVac; CCB, 2 doses of CoronaVac and 1 dose of BNT162b2.
COVID-19 vaccine hesitancy survey
Patients received and completed the first survey through online or phone interviews in January 2022. The survey was based on our previous publication on 2,609 healthy adolescents, supplemented with more tailored queries pertinent to NMD patients and younger age groups that were added into this study.15 In summary, it included 21 yes/no or multiple-choice questions on patient demographics, presence of medical complexity, history of past COVID-19 infection, influenza and COVID-19 vaccination, intention of receiving COVID-19 vaccination and the reasons for their choice (Supplementary Data). Concerns about receiving COVID-19 vaccination included perception of risks, challenges in access to vaccination centers, adverse effects, less efficacy than their healthy counterparts and vaccine–drug interactions that potentially affect their current NMD treatments. The expected time required to complete the survey was 15 min. At least one parent/legally authorized representative/legal guardian accompanied the child participants in completing the survey. A follow-up survey was sent to patients in April 2022 to longitudinally assess changes in attitudes, hesitancy, and associated reasons shortly after the peak of the first major COVID-19 wave due to the SARS-CoV-2 Omicron variant in HK.
Reactogenicity and immunogenicity study of COVID-19 vaccines
The reactogenicity and immunogenicity arm is a sub-study under the registered Coronavirus disease-19 (COVID-19) Vaccination in Adolescents and Children (COVAC) (NCT04800133). The COVID-19 vaccines were administered at the Community Vaccination Centers research sites supported by the University of Hong Kong (HKU) and the HK Government’s COVID-19 Vaccination Program. Patients received two doses of either BNT162b2 or CoronaVac, given 21 or 28 days apart, respectively, followed by an option of either vaccine types as a third dose at least 28 days after their second dose. Dosages of 0.3 mL and 0.1 mL (equivalent to 30 μg and 10 μg of COVID-19 mRNA vaccine embedded in lipid nanoparticles) according to drug regulatory approval by the United States Food and Drug Administration and HK Government were used for aged ≥12 years and 5–11 years, respectively.5,27 The dosage of CoronaVac was 0.5 mL (600 SU, equivalent to 3 μg, of inactivated SARS-CoV-2 virus as antigen) for all ages.5
Vaccine recipients were monitored for 30 min after each injection and reported the types, duration, and severity of adverse reactions (ARs) in a diary using an online or paper format for 7 days after vaccination. Peripheral blood consisting of 15 mL was obtained before the first dose, second dose, 7–43 days after the second dose and 14–49 days after the third dose (if any) for measuring the serological antibody responses. These time intervals as optimal for assessing immunogenicity were based on previous publications and guideline recommendations.28–31 The SARS-CoV-2 S receptor-binding domain (S-RBD) IgG enzyme linked immunosorbent assay (ELISA) (Chondrex Inc, Redmond, USA) and surrogate virus neutralization test (sVNT) (GenScript, New Jersey, USA) performed in our laboratory on the serum isolated from blood samples of patients had been validated and described in our previous publication.32 Levels of S-RBD IgG are expressed as optical density (OD450). The cutoff considered as seroconversion was OD450 ≥0.50, while values below would be inputted as 0.25.33 Neutralizing antibodies against SARS-CoV-2 WT and Omicron BA.1 were evaluated by sVNT with inhibition percentages (%) as the readout.34 The cutoff for positive neutralizing antibody inhibition was ≥30%, and values below 30% would be inputted as 10%. Data from healthy children and adolescents (n = 280) were retrieved from our COVAC study for comparison to this NMD cohort, which are available from our previous publication.28
Social and contact avoidance was common during this study period when the HK Omicron wave occurred, particularly for vulnerable patients as neurological and respiratory complications surged rapidly.35 Therefore, some NMD patients were only able to attend our vaccination research sites and provide blood samples at the time-point of 7–43 days after the second dose.
Statistical analysis
Associations between the categorical variables (presence of medically complexity, history of past COVID-19 infection, influenza, and COVID-19 vaccination) and intention of receiving COVID-19 vaccination (i.e., have received or plan to receive vs do not plan to receive COVID-19 vaccination) were analyzed by the Fisher’s exact test. Intention of receiving COVID-19 vaccination between January and April 2022 was compared by the Cochran’s Q test. Reasons for receiving the COVID-19 vaccines in January and April 2022 were compared by the Fisher’s exact test. The proportions of ARs reported between NMD patients and the healthy population, also between 2–11 and 12–21 year-old NMD patients were compared by the Fisher’s exact test. Age, S-RBD IgG levels, and sVNT% inhibition against WT were compared between NMD patients and the healthy population, also between the two vaccine types, by the Mann-Whitney U-test. S-RBD IgG levels and sVNT% inhibition against WT were compared between different disease subtypes by the Kruskal–Wallis test. Comparisons between the sVNT% inhibition against WT and Omicron BA.1 for each patient were computed using the Wilcoxon matched-pairs signed-rank test. The correlation between ELISA and log10-transformed sVNT% inhibition against WT was evaluated by the Pearson correlation coefficient. p < .05 was considered statistically significant. Data analyses and graphing were performed using GraphPad Prism (version 9.3.1).
Standard protocol approval, registration, consent, and assent
The NMD patient registry and COVAC were approved by the HKU/Hospital Authority HK West Cluster IRB Committee (UW19–356 and UW 21–157, respectively). Written informed consent was obtained from adult participants or parents/legally authorized representatives/legal guardians of the child participants. Pediatric patients who were neurodevelopmentally capable (11–21 years old) also provided written assent in the reactogenicity and immunogenicity arm of the study.
Results
Of the 136 patients in the HK NMD registry, 52 were sent invitations to complete the COVID-19 vaccine hesitancy survey, while the others did not fulfill criteria due to age or cognitive delay. Most patients, which were 41 (78.8%) of the 52, completed both the first and follow-up surveys (Figure 1). Eighteen (43.9%) of 41 who filled the survey had spinal muscular atrophy (SMA), while 26 (63.4%) had complex medical needs, including wheelchair mobility, tube or gastrostomy tube feeding, ventilator use, or brace or spinal surgery for scoliosis (Supplementary Table S1).
Two or more family members vaccinated against COVID-19 was positively associated with a higher intention of vaccination (OR: 11.7, 95% CI: 1.81–75.1, p = .010) (Table 1). Patients who received an influenza vaccine in the last three consecutive years tended to have higher intention of receiving COVID-19 vaccines, albeit not reaching statistical significance (24 of 30 vs 5 of 11, or 80.0% vs 45.5%, p = .052). The major reasons that the NMD patients favored COVID-19 vaccination included their hopes for preventing infection (26 of 40, or 65.0%), protecting their family (16, or 40.0%) and returning to normal life (14, or 35.0%) (Table 2).
Table 1.
Factors associated with intention of receiving COVID-19 vaccination.
| Have received or plan to receive vaccination (n = 30) | Do not plan to receive vaccination (n = 11) | p-values | |
|---|---|---|---|
| Mean age (SD) | 14.0 (3.4) | 13.4 (3.3) | .648 |
| Female | 15 (50.0) | 3 (27.3) | .291 |
| Two or more family members are vaccinated against COVID-19^ |
28 (93.3) | 6 (54.5) | .010* |
| Received the influenza vaccine in the previous year | 22 (73.3) | 5 (45.5) | .140 |
| Received the influenza vaccine in the last three continuous years | 24 (80.0) | 5 (45.5) | .052 |
| Know someone diagnosed with COVID-19 | 0 (0) | 2 (18.2) | - |
| Previously completed compulsory COVID-19 testing | 1 (3.3) | 3 (27.3) | .052 |
| Wheelchair-mobile | 16 (53.3) | 6 (54.5) | 1.000 |
| Nasogastric or PEG tube feeding use | 2 (6.7) | 2 (18.2) | .288 |
| Ventilator support | 10 (33.3) | 4 (36.4) | 1.000 |
| Using spinal brace or had spinal surgery | 7 (23.3) | 1 (9.1) | .412 |
| Presence of one type of disability | 7 (23.3)) | 5 (45.5) | .247 |
| Presence of two or more type of disabilities | 11 (36.7) | 3 (27.3) | .719 |
SD = standard deviation, PEG = percutaneous endoscopic gastrostomy.
^odds ratio is presented for those with significant association according to the Fisher’s exact test.
*p < .05.
Odds ratio = 11.7, 95% confidence interval: 1.81–75.1.
Table 2.
Major reasons for receiving COVID-19 vaccination.
| January 2022 |
April 2022 |
p-values | |
| Planning to vaccinate/already vaccinated |
n = 30 |
n = 40 |
|
| Worried to be infected | 17 (56.7) | 26 (65.0) | .620 |
| To return full-scale face-to-face teaching | 16 (53.3) | 16 (40.0) | .335 |
| To protect their family | 17 (56.7) | 14 (35.0) | .091 |
| To return to normal life | 19 (63.3) | 13 (32.5) | .015* |
| I think I am high risk group of COVID-19 | 17 (23.3) | 12 (30.0) | .596 |
| Because people around me received vaccination | 15 (16.7) | 11 (27.5) | .391 |
| I am tired of the social distancing policies | 19 (30.0) | 10 (25.0) | .787 |
| I want to socialize with large groups of friends | 13 (10.0) | 10 (25.0) | .132 |
| I want to socialize with participate in large-scale events | 112 (6.7) | 19 (22.5) | .100 |
| I want to travel | 14 (46.7) | 18 (20.0) | .022* |
| I want to play sports at school with friends or in a competition without wearing mask | 11 (36.7) | 17 (17.5) | .098 |
| To play music safely E.g.: wind instruments, band, choir |
16 (20.0) | 17 (17.5) | 1.000 |
| I want to join study tours E.g.: day trip, school visits or other extra-curricular activities |
12 (40.0) | 15 (12.5) | .011* |
Data presented as number (%). *p < .05.
Their concerns regarding COVID-19 vaccination included adverse effects that could be potentially worse than the healthy population (9 of 41, 22.0%), safety (9, or 22.0%), suitability (6, or 14.6%), effects on current NMD treatments (4, or 9.8%) and reduced efficacy (4, or 9.8%) (Figure 2). There were also 5 (12.2%) of 41 patients who expressed that their intention of vaccination depended on the progress of the pandemic. Indeed, in April 2022, which was shortly after the peak of the Omicron wave in HK, more respondents had or planned to receive the COVID-19 vaccines than in January 2022 (97.6% vs 73.3%, p = .003) (Supplementary Table S2). Also, 30 (73.2%) patients expressed their intention of receiving future boosters, if necessary, in April 2022 (Supplementary Table S2). Twenty (48.8%) of 41 patients expressed a vaccination history/intention of vaccination for at least 1 dose of BNT162b2 (B) or CoronaVac (C) in April 2022 (Supplementary Table S3).
Figure 2.
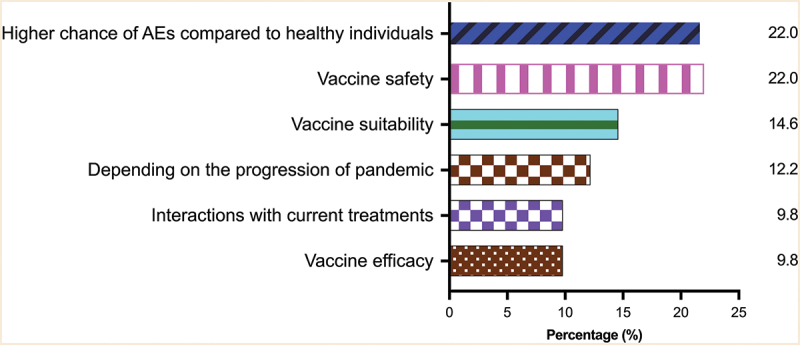
Major concerns about receiving COVID-19 vaccination in patients with neuromuscular diseases.
The three commonest concerns from patients with neuromuscular diseases were having a higher chance of AEs compared to healthy individuals (22.0%), safety (22.0%), and suitability (14.3%). AEs = adverse effects.
Forty-eight patients were invited from the NMD patient registry to complete the reactogenicity and immunogenicity arm from June 2021 to April 2022, while the others were not recruited due to the age exclusion criterion. Twenty-two (45.8%) patients joined the study (Supplementary Table S4). Nine patients had DMD, seven patients had SMA, three patients had congenital myopathy (CM), and one patient had Becker muscular dystrophy (BMD), chronic inflammatory demyelinating polyneuropathy (CIDP) or myotonic disorder (MD). Fourteen (63.6%) were in the late ambulatory or wheelchair mobile stage. All 9 DMD patients were on corticosteroids (ranges of dosage: 10–30 mg/day or 0.30–0.74 mg/kg/day). Seven SMA patients were on nusinersen or risdiplam. One CIDP patient was on intravenous immunoglobulin therapy infused regularly (dosage: 2 g/kg/3 months). There were 6 (27.3%) and 16 (72.7%) of 22 patients who received two doses of BNT162b2 (BB) or CoronaVac (CC), respectively. One case of CM and DMD each had COVID-19 before enrollment into the study, while 1 patient with SMA reported contracting COVID-19 3 weeks after the first dose, and all three patients fully recovered subsequently. 280 healthy children and adolescents were recruited for the immunogenicity study for comparison (BNT162b2: 107, CoronaVac: 173) (Figure 1). Patients with NMDs were younger than the healthy controls (B: 11.3 years vs 13.9 years; C: 13.1 years vs 14.0 years) (Supplementary Table S5).
NMD patients had similar proportions of ARs compared to 280 healthy children and adolescents (Table 3). Pain at the injection site (BB: 3 of 6, 50.0%, 6 of 11, CC: 54.5%), fatigue (BB: 4 of 6, 66.7%, 5 of 11, CC: 45.5%), and myalgia (BB: 1 of 6, 16.7%, 3 of 11, CC: 27.3%) were the commonest ARs in NMD patients. Most ARs were mild (75.5%, n = 71/94). No severe adverse events, such as apparent NMD deterioration, hospitalization, life-threatening complications, disabilities, or deaths occurred) (Supplementary Figure S1). Similar ARs were reported between patients aged 2–11 and 12–21 years (Supplementary Table S6).
Table 3.
Reactogenicity for patients with neuromuscular diseases.
| Dosage | B | C | BB | CC | BBB | CCC | CCB |
|---|---|---|---|---|---|---|---|
| Pain at injection site NMDs | |||||||
| NMDs | 5 (83.3) | 5 (45.5) | 3 (50.0) | 6 (54.5) | 1 (100) | 5 (55.6) | 1 (100) |
| Healthy | 93 (89.4) | 94 (54.3) | 88 (84.6) | 89 (51.5) | 11 (84.6) | 28 (46.7) | 6 (100) |
| p-value | 0.509 | 0.757 | 0.063 | 1.000 | 1.000 | 0.731 | 1.000 |
| Swelling, erythema and induration at injection site | |||||||
| NMDs | 0 (0) | 2 (18.2) | 0 (0) | 1 (9.09) | 0 (0) | 1 (11.1) | 0 (0) |
| Healthy | 10 (9.6) | 30 (17.3) | 11 (10.6) | 37 (21.4) | 4 (30.8) | 1 (1.7) | 0 (0) |
| p-value | 1.000 | 1.000 | 1.000 | 0.465 | 1.000 | 0.224 | N/A |
| Headache | |||||||
| NMDs | 0 (0) | 1 (9.09) | 3 (50.0) | 1 (9.09) | 1 (100) | 2 (22.2) | 0 (0) |
| Healthy | 22 (21.2) | 33 (19.1) | 47 (45.2) | 22 (12.7) | 9 (69.2) | 5 (8.3) | 3 (50.0) |
| p-value | 0.598 | 0.692 | 1.000 | 1.000 | 1.000 | 0.224 | 1.000 |
| Fatigue | |||||||
| NMDs | 3 (50.0) | 4 (36.4) | 4 (66.7) | 5 (45.5) | 0 (0) | 5 (55.6) | 1 (100) |
| Healthy | 50 (48.1) | 74 (42.8) | 65 (62.5) | 59 (34.1) | 9 (69.2) | 17 (28.3) | 4 (66.7) |
| p-value | 1.000 | 0.762 | 1.000 | 0.518 | 0.357 | 0.132 | 1.000 |
| Myalgia | |||||||
| NMDs | 2 (33.3) | 3 (27.3) | 1 (16.7) | 3 (27.3) | 0 (0) | 2 (22.2) | 0 (0) |
| Healthy | 25 (24.0) | 22 (12.7) | 16 (15.4) | 16 (9.25) | 8 (61.5) | 4 (6.7) | 1 (16.7) |
| p-value | 0.634 | 0.174 | 1.000 | 0.091 | 0.429 | 0.172) | 1.000 |
| Fever | |||||||
| NMDs | 0 (0) | 2 (18.2) | 1 (16.7) | 1 (9.1) | 1 (100) | 0 (0) | 0 (0) |
| Healthy | 4 (3.9) | 2 (1.16) | 16 (15.4) | 0 (0) | 5 (38.5) | 0 (0) | 1 (16.7) |
| p-value | 1.000 | 0.018 | 1.000 | 0.060 | 0.429 | 1.000 | 1.000 |
| Abdominal pain | |||||||
| NMDs | 0 (0) | 1 (9.09) | 0 (0) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) |
| Healthy | 9 (8.7) | 17 (9.8) | 9 (8.7) | 9 (5.2) | 1 (7.7) | 3 (5.0) | 1 (16.7) |
| p-value | 1.000 | 1.000 | 1.000 | 0.469 | 1.000 | 1.000 | 1.000 |
| Antipyretic use | |||||||
| NMDs | 1 (16.7) | 0 (0) | 2 (33.3) | 0 (0) | 1 (100) | 0 (0) | 0 (0) |
| Healthy | 7 (6.7) | 2 (1.2) | 25 (24.0) | 2 (1.2) | 3 (23.1) | 0 (0) | 1 (16.7) |
| p-value | 0.371 | 1.000 | 0.634 | 1.000 | 0.286 | 1.000 | 1.000 |
Healthy = healthy children and adolescents, NMDs = patients with neuromuscular diseases. B, one dose of BNT162b2; BB, two doses of BNT162b2; C, one dose of CoronaVac; CC, two doses of CoronaVac; BBB, three doses of BNT162b2; CCC, three doses of CoronaVac; CCB, two doses of CoronaVac, and one dose of BNT162b2.
All 19 patients with NMDs seroconverted against WT after BB or CC (Figure 3a). NMD patients had similar antibody responses compared to 280 healthy children and adolescents (ELISA-CC: 2.03 vs 1.59; ELISA-BB: 2.19 vs 2.86; sVNT-CC: 85.2% vs 83.2%) (Figure 3a, 3b). There was a high correlation between ELISA and surrogate virus neutralization in our samples (n = 690, r = 0.897, p < .0001) (Supplementary Figure S2). Although after receiving CC, DMD patients using corticosteroids had slightly lower S-RBD IgG than those not on corticosteroids (OD450: 1.82 vs 2.37, p = .02) or other disease subtypes (OD450: 1.82 vs 2.54 (SMA) vs 2.03 (CM) vs 1.97 (BMD) vs 2.43 (MD), p = .01), corticosteroids did not affect their seroconversion rates (Supplementary Table S7 and 8). There was lower neutralization against Omicron BA.1. The median sVNT% inhibition against WT and Omicron BA.1 was 96.3% and 10.0% after BB (p = .063) (Figure 3c), respectively, while it was 85.2% and 10.0% after CC (p < .001) (Figure 3d). The other three patients with COVID-19 were excluded from the main immunogenicity analyses (Supplementary Table S9).
Figure 3.
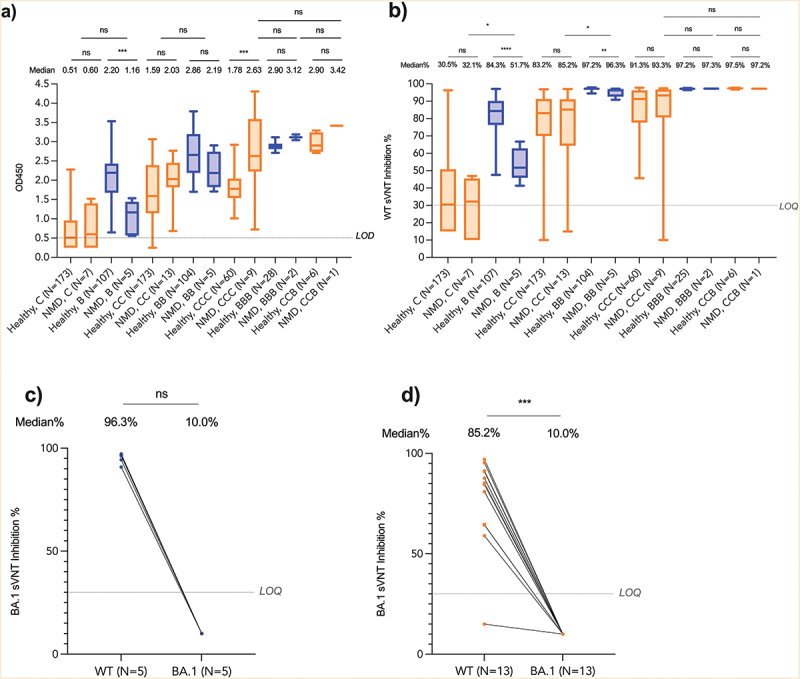
Immunogenicity for patients with neuromuscular diseases.
Antibody responses were determined before the first dose, second dose, 7–43 days after the second dose and 14–49 days after the third dose of BNT162b2 or CoronaVac in patients with neuromuscular diseases (NMDs) (n = 19) and healthy children and adolescents (n = 280). Three NMDs who had COVID-19 infection were excluded from the final analysis. ELISA=enzyme linked immunosorbent assay, LOQ=Limit of quantitation, Healthy=heathy children and adolescents, NMD=patients with neuromuscular diseases, sVNT=surrogate virus neutralization test, WT= wildtype SARS-CoV-2 virus. B, one dose of BNT162b2; BB, two doses of BNT162b2; C, one dose of CoronaVac; CC, two doses of CoronaVac; BBB, three doses of BNT162b2; CCC, three doses of CoronaVac; CCB, two doses of CoronaVac, and one dose of BNT162b2. BNT162b2 was represented as blue while CoronaVac was represented as orange color. Patients with NMDs using corticosteroids (deflazacort or prednisolone) daily were indicated as square dots in (c) & (d). *p < .05, **p < .01, ***p < .001, ****p < .0001 or NS (not significant).
Discussion
This is the first in-depth study to understand the underlying reasons for vaccine hesitancy in NMDs, who have specific concerns based on their particular disease, treatments, and prognosis. The findings revealed COVID-19 vaccination in family members is highly influential on the intention of receiving the vaccines for pediatric patients with NMDs, and those who received either the mRNA-based or inactivated whole-virus vaccines did not encounter severe ARs and had antibody responses similar to their healthy counterparts. This is consistent with the notion that family decision and support are key factors on COVID-19 vaccination for pediatric populations, as several recent studies observed this finding for healthy adolescents and children with neurodevelopmental disorders.15,36,37 Additionally, patients who received the influenza vaccines in the recent consecutive years tended toward having the greater intention of COVID-19 vaccination, and we speculate this was due to higher vaccine confidence and complacency.14,38 This information will be useful for patient counseling as they continued to raise questions in our clinic regarding the need for the third dose and subsequent boosters in the future.
This is the first study to investigate the safety of both the novel mRNA-based and inactivated-whole virus COVID-19 vaccines in children with NMDs, which showed BNT162b2 and CoronaVac were well tolerated. There were similar profiles of ARs between pediatric patients with NMDs, our healthy cohort, as well as adolescents and adults with NMDs and multiple sclerosis who received two doses of BNT162b2 or mRNA-1273.22,27,39,40 This is also the first study to demonstrate the CoronaVac is immunogenic in pediatric patients with NMDs. Antibody responses were robust, an observation which was consistent with adolescent and adult patients with NMDs and myasthenia gravis who were able to generate antibody responses against WT.22–24, 40 It is reassuring that even for pediatric patients with NMDs and on corticosteroids, all patients had successful seroconversion after at least two doses of BNT162b2 or CoronaVac, which was also observed in adolescent and adult patients who received BNT162b2.22,40 Additionally, there were no apparent interactions between the COVID-19 vaccines and treatments for NMDs, and our cohort of patients did not encounter NMD-related complications or hospitalization.
This study included immunogenicity against Omicron, which is important as some studies showed reduced vaccine effectiveness (VE) of BNT162b2 and CoronaVac against infection or mild COVID-19 due to this variant.7,8,41–43 Indeed, our findings revealed reduced neutralizing activity against Omicron BA.1 in pediatric patients with NMDs. As neutralization correlates with protective efficacy against symptomatic COVID-19, we expect breakthrough infections to be more common in NMD patients due to Omicron than pre-Omicron variants, as similar to the rest of the population. However, although recent studies indicated that VE of BNT162b2 and CoronaVac against infection due to Omicron were merely~50% after two doses of vaccination in healthy adolescents, both vaccines remained highly protective against hospitalization and moderate-to-severe COVID-19 according to our population studies.5, 44–46 This is likely because T cell responses are preserved against Omicron,47,48 which is correlated with clinical protection against severe diseases.49 Therefore, this study supports the notion that patients with NMDs should become vaccinated with either BNT162b2 or CoronaVac to attain protection against severe COVID-19. Further boosters may enhance neutralization responses against Omicron subvariants for maximal protection.
There were several limitations in this study. First, it was not possible for participants to be enrolled into a study with a blinded and randomized design because these patients are already hesitant to receive novel vaccines and restriction on their choice on the type of vaccine would be an additional deterrent. There can be potential selection bias because more older males with NMDs favored BNT162b2. Also, patients with NMDs were younger than the healthy controls and likely received a higher dosage based on age or size, which could have contributed to the higher antibody responses after 3 injections of CoronaVac. This observation should be investigated in more detail in the future. However, the current findings have more real-life applicability and reflect the reality of outcomes for patients who can choose between vaccines. Reactogenicity and immunogenicity results were not available for all the timepoints, as we encouraged patients to receive the vaccines as soon and conveniently as possible for protection from severe COVID-19. This is because informed choice and prompt preventative treatment for overcoming a surging wave of infection-related deaths in high-risk patients during the peak of our pandemic period is paramount and should be respected. Additionally, this study was able to evaluate common adverse effects, but a different study design, such as large-scale post-market surveillance, will be required for reporting on rare adverse reactions. Finally, the current small sample size limits the generalizability of the study conclusions. Due to the small size of each group, it is difficult to derive strong data. Nevertheless, this is the largest COVID-19 vaccine study in pediatric NMDs to date, with comparison to as many as 280 healthy individuals on immunogenicity after 2 vaccine doses and comprises a total of 690 blood samples. The results from this study greatly contribute to the currently available scientific evidence that serves as the basis for appropriate clinical practice recommendations and policy-making decisions for patients with NMDs.
Taken together, these present findings and overall evidence support the routine schedule of vaccination for patients with NMDs, and the dosages of immunosuppressives used for the treatment of NMDs in relation to the BNT162b2 and CoronaVac vaccines are inconsequential.4 We recommend that counseling for these patients should incorporate these informative points and to include several close relatives, if possible, because their decisions appear to be strongly influential toward vaccine hesitancy. Reassurance by reminding the patients about their tolerance to other vaccines, such as influenza, can also be considered. The in-depth understanding on the reasons for vaccine hesitancy in specific, rare diseases acquired from this study is necessary to devise future follow-up research on interventions. Future studies are required to confirm that these counseling techniques are effective on addressing vaccine hesitancy. Additionally, questions remain in terms of the safety, efficacy, long-lasting T cell immunity, and durability of booster doses against other emerging variants,50 such as Omicron BA2.75, BA.5, XBB, BQ1.1, and BF.7 for these patients.
Supplementary Material
Acknowledgments
We thank Dr. Davy Chun-Wai Lee and Mr. Koon-Wing Chan at the Department of Paediatrics and Adolescent Medicine, LKS Faculty of Medicine, HKU for their laboratory management of the collected blood samples.
Funding Statement
This work was supported by the research grant [COVID19F02, COVID19F10, and COVID19F12] from the Health Bureau, the Government of Hong Kong Special Administrative Region.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, Jaime S Rosa Duque, upon reasonable request.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2206278
References
- 1.Khetan AK, Yusuf S, Lopez-Jaramillo P, Szuba A, Orlandini A, Mat-Nasir N, Oguz A, Gupta R, Avezum A, Rosnah I, et al. Variations in the financial impact of the COVID-19 pandemic across 5 continents: a cross-sectional, individual level analysis. EClin Med. 2022;44:1. doi: 10.1016/j.eclinm.2022.101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Covid-19 dashboard Johns Hopkins coronavirus resource center.
- 3.Who coronavirus (covid-19) dashboard World Health Organisation.
- 4.Covid-19 and people with neuromuscular disorders: world muscle society position and advice April 23rd, 2022, 8th update World Muscle Society.
- 5.Rosa Duque JS, Leung D, Yip KM, Lee DHL, So H-K, Wong WHS, Lau YL.. Covid-19 vaccines versus pediatric hospitalization. Cell Rep Med. 2023;4(2):100936. doi: 10.1016/j.xcrm.2023.100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming-Dutra KE, Britton A, Shang N, Derado G, Link-Gelles R, Accorsi EK, Smith ZR, Miller J, Verani JR, Schrag SJ. Association of prior bnt162b2 COVID-19 vaccination with symptomatic sars-cov-2 infection in children and adolescents during omicron predominance. JAMA. 2022;327(22):2210–10. doi: 10.1001/jama.2022.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florentino PTV, Alves FJO, Cerqueira-Silva T, Oliveira VA, Junior JBS, Jantsch AG, Penna GO, Boaventura V, Werneck GL, Rodrigues LC, et al. Vaccine effectiveness of coronavac against COVID-19 among children in Brazil during the omicron period. Nat Commun. 2022;13(1):4756. doi: 10.1038/s41467-022-32524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jara A, Undurraga EA, Zubizarreta JR, Gonzalez C, Acevedo J, Pizarro A, Vergara V, Soto-Marchant M, Gilabert R, Flores JC, et al. Effectiveness of CoronaVac in children 3–5 years of age during the SARS-CoV-2 omicron outbreak in Chile. Nat Med. 2022;28(7):1377–80. doi: 10.1038/s41591-022-01874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu P, Leung GM, Cowling BJ. Vaccine effectiveness of one, two, and three doses of bnt162b2 and coronavac against COVID-19 in hong kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–43. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ionescu IG, Skowronski DM, Sauvageau C, Chuang E, Ouakki M, Kim S, De Serres G. Bnt162b2 effectiveness against delta and omicron variants of sars-cov-2 in adolescents aged 12-17 years, by dosing interval and duration. J Infect Dis. 2023. doi: 10.1093/infdis/jiad006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallapaty S, Callaway E, Kozlov M, Ledford H, Pickrell J, Van Noorden R. How covid vaccines shaped 2021 in eight powerful charts. Nature. 2021;600(7890):600–583 580–583. doi: 10.1038/d41586-021-03686-x. [DOI] [PubMed] [Google Scholar]
- 12.Interim recommendations for use of the inactivated COVID-19 vaccine, coronavac, developed by sinovac . Interim recommendations for use of the inactivated COVID-19 vaccine, coronavac, developed by sinovac. World Health Orgnization. 2021.
- 13.Interim recommendations for use of the pfizer–biontech COVID-19 vaccine, bnt162b2, under emergency use listing: Interim guidance . Interim recommendations for use of the pfizer–biontech COVID-19 vaccine, bnt162b2, under emergency use listing: interim guidance. 2021.
- 14.Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. Nat Med. 2021;27(8):1338–9. doi: 10.1038/s41591-021-01459-7. [DOI] [PubMed] [Google Scholar]
- 15.Wong WHS, Leung D, Chua GT, Duque JSR, Peare S, So HK, Chan SM, Kwan MYW, Ip P, Lau YL. Adolescents’ attitudes to the COVID-19 vaccination. Vaccine. 2022;40(7):967–9. doi: 10.1016/j.vaccine.2022.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handberg C, Werlauff U, Hojberg AL, Knudsen LF, Picone GA. Impact of the COVID-19 pandemic on biopsychosocial health and quality of life among danish children and adults with neuromuscular diseases (Nmd)—patient reported outcomes from a national survey. PLoS One. 2021;16(6):e0253715. doi: 10.1371/journal.pone.0253715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasilewska E, Sobierajska-Rek A, Sledzinska K, Malgorzewicz S, Jassem E, Wierzba J. Morbidity, clinical course and vaccination against sars-cov-2 virus in patients with duchenne muscular dystrophy: a patient reported survey. Int J Environ Res Public Health. 2021;19(1):406. doi: 10.3390/ijerph19010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertran Recasens B, Rubio MA. Neuromuscular diseases care in the era of covid-19. Front Neurol. 2020;11(588929). doi: 10.3389/fneur.2020.588929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zivkovic SA, Gruener G, Narayanaswami P, Quality A, Patient Safety C. Doctor-should i get the COVID-19 vaccine? Infection and immunization in individuals with neuromuscular disorders. Muscle Nerve. 2021;63(3):294–303. doi: 10.1002/mus.27179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covid-19 and people with neuromuscular disorders: world muscle society advice - vaccines and therapeutics (updated 23rd April 2022). World Muscle Society. [Google Scholar]
- 21.Deenen JC, Horlings CG, Verschuuren JJ, Verbeek AL, van Engelen BG. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis. 2015;2(1):73–85. doi: 10.3233/JND-140045. [DOI] [PubMed] [Google Scholar]
- 22.Demonbreun AR, Velez MP, Saber R, Ryan DT, Sancilio A, McDade TW, McNally EM. Mrna intramuscular vaccination produces a robust igg antibody response in advanced neuromuscular disease. Neuromuscul Disord. 2021;32(1):33–5. doi: 10.1016/j.nmd.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito T, Saito T, Hashimoto H, Ogata K, Kobayashi M, Takada H, Kuru S, Kimura T, Nakamura A, Matsumura T. Safety and immunogenicity of mrna COVID-19 vaccine in inpatients with muscular dystrophy. Muscle Nerve. 2022;67(2):117–23. doi: 10.1002/mus.27761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes-Leiva D, Lopez-Contreras J, Moga E, Pla-Junca F, Lynton-Pons E, Rojas-Garcia R, Turon-Sans J, Querol L, Olive M, Alvarez-Velasco R, et al. Immune response and safety of sars-cov-2 mrna-1273 vaccine in patients with myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. 2022;9(4):e200002. doi: 10.1212/NXI.0000000000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang V, Leung ASY, Au EYL, Ho MHK, Lee TH, Wu AYY, Wong GWK, Li PH. Updated consensus statements on COVID-19 vaccine allergy safety in Hong Kong. Asia Pac Allergy. 2022;12(1):e8. doi: 10.5415/apallergy.2022.12.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu MHS, Gao Y, Rosa Duque JS, Chan GCF, Chan SHS. Establishment of hong kong neuromuscular disorder patient registry. Hong Kong J Paediatr. 2022;27:80. [Google Scholar]
- 27.Hause AM, Baggs J, Marquez P, Myers TR, Gee J, Su JR, Zhang B, Thompson D, Shimabukuro TT, Shay DK. Covid-19 vaccine safety in children aged 5-11 years - United States, November 3-December 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1755–60. doi: 10.15585/mmwr.mm705152a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosa Duque JS, Wang X, Leung D, Cheng SMS, Cohen CA, Mu X, Hachim A, Zhang Y, Chan SM, Chaothai S, et al. Immunogenicity and reactogenicity of sars-cov-2 vaccines bnt162b2 and coronavac in healthy adolescents. Nat Commun. 2022;13(1):3700. doi: 10.1038/s41467-022-31485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung D, Mu X, Duque JSR, Cheng SMS, Wang M, Zhang W, Zhang Y, Tam IYS, Lee TSS, Lam JHY, et al. Safety and immunogenicity of 3 doses of bnt162b2 and coronavac in children and adults with inborn errors of immunity. Front Immunol. 2022;13(982155). doi: 10.3389/fimmu.2022.982155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mu X, Cohen CA, Leung D, Rosa Duque JS, Cheng SMS, Chung Y, Wong HHW, Lee AMT, Li WY, Tam IYS, et al. Antibody and t cell responses against wild-type and omicron sars-cov-2 after third-dose bnt162b2 in adolescents. Signal Transduct Target Ther. 2022;7(1):397. doi: 10.1038/s41392-022-01282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, De La Morena M, Kumararatne D, Harville TO, Hesterberg P, Koleilat M, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the basic and clinical immunology interest section of the American Academy of allergy, asthma & immunology. J Allergy Clin Immunol. 2012;130(3):S1–24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Perera RA, Mok CK, Tsang OT, Lv H, Ko RL, Wu NC, Yuan M, Leung WS, Chan JM, Chik TS, et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (sars-cov-2. Euro Surveill. 2020. Mar;25(16). doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau EHY, Tsang OTY, Hui DSC, Kwan MYW, Chan WH, Chiu SS, RLW K, Chan KH, Cheng SMS, Perera R, et al. Neutralizing antibody titres in sars-cov-2 infections. Nat Commun. 2021;12(1):63. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perera R, Ko R, Tsang OTY, Hui DSC, Kwan MYM, Brackman CJ, EMW T, Yen HL, Leung K, Cheng SMS, et al. Evaluation of a sars-cov-2 surrogate virus neutralization test for detection of antibody in human, canine, cat, and hamster sera. J Clin Microbiol. 2021;59(2). doi: 10.1128/JCM.02504-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tso WWY, Kwan MYW, Wang YL, Leung LK, Leung D, Chua GT, Ip P, Fong DYT, Wong WHS, Chan SHS, et al. Severity of sars-cov-2 omicron ba.2 infection in unvaccinated hospitalized children: comparison to influenza and parainfluenza infections. Emerg Microbes Infect. 2022;11(1):1742–50. doi: 10.1080/22221751.2022.2093135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali M, Proma TS, Tasnim Z, Islam MA, Urmi TA, Ahmed S, Sarkar AS, Bonna AS, Khan US. Parental COVID-19 vaccine hesitancy for children with neurodevelopmental disorders: a cross-sectional survey. Trop Med Health. 2022;50(1):24. doi: 10.1186/s41182-022-00415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarus JV, Wyka K, White TM, Picchio CA, Rabin K, Ratzan SC, Parsons Leigh J, Hu J, El-Mohandes A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat Commun. 2022;13(1):3801. doi: 10.1038/s41467-022-31441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oduwole EO, Pienaar ED, Mahomed H, Wiysonge CS. Current tools available for investigating vaccine hesitancy: a scoping review protocol. BMJ Open. 2019;9(12):e033245. doi: 10.1136/bmjopen-2019-033245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briggs FBS, Mateen FJ, Schmidt H, Currie KM, Siefers HM, Crouthamel S, Bebo BF, Fiol J, Racke MK, O’Connor KC, et al. Covid-19 vaccination reactogenicity in persons with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1104. doi: 10.1212/NXI.0000000000001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwayama H, Ishihara N, Kawahara K, Madokoro Y, Togawa Y, Muramatsu K, Murakami A, Kuru S, Kumagai T, Ohashi W, et al. Early immunological responses to the mrna sars-cov-2 vaccine in patients with neuromuscular disorders. Front Immunol. 2022;13(996134). doi: 10.3389/fimmu.2022.996134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higdon MM, Baidya A, Walter KK, Patel MK, Issa H, Espie E, Feikin DR, Knoll MD. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect Dis. 2022;22(8):1114–16. doi: 10.1016/S1473-3099(22)00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price AM, Olson SM, Newhams MM, Halasa NB, Boom JA, Sahni LC, Pannaraj PS, Irby K, Bline KE, Maddux AB, et al. Bnt162b2 protection against the omicron variant in children and adolescents. N Engl J Med. 2022;386(20):1899–909. doi: 10.1056/NEJMoa2202826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, Anyaneji UJ, Bester PA, Boni MF, Chand M, et al. Rapid epidemic expansion of the sars-cov-2 omicron variant in southern africa. Nature. 2022;603(7902):679–86. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung D, Rosa Duque JS, Yip KM, So HK, Wong WHS, Lau YL. Effectiveness of bnt162b2 and coronavac in children and adolescents against sars-cov-2 infection during omicron ba.2 wave in hong kong. Comm Med. 2023;3(1). doi: 10.1038/s43856-022-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng SMS, Mok CKP, Leung YWY, Ng SS, Chan KCK, Ko FW, Chen C, Yiu K, Lam BHS, Lau EHY, et al. Neutralizing antibodies against the sars-cov-2 omicron variant ba.1 following homologous and heterologous coronavac or bnt162b2 vaccination. Nat Med. 2022;28(3):486–9. doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoako DG, et al. Omicron extensively but incompletely escapes pfizer bnt162b2 neutralization. Nature. 2022;602(7898):654–6. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao Y, Cai C, Grifoni A, Muller TR, Niessl J, Olofsson A, Humbert M, Hansson L, Osterborg A, Bergman P, et al. Ancestral sars-cov-2-specific t cells cross-recognize the omicron variant. Nat Med. 2022;28(3):472–6. doi: 10.1038/s41591-022-01700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naranbhai V, Nathan A, Kaseke C, Berrios C, Khatri A, Choi S, Getz MA, Tano-Menka R, Ofoman O, Gayton A, et al. T cell reactivity to the sars-cov-2 omicron variant is preserved in most but not all individuals. Cell. 2022;185(6):1041–51 e1046. doi: 10.1016/j.cell.2022.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moss P. The t cell immune response against sars-cov-2. Nat Immunol. 2022;23(2):186–93. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 50.Canetti M, Barda N, Gilboa M, Indenbaum V, Asraf K, Gonen T, Weiss-Ottolenghi Y, Amit S, Doolman R, Mendelson E, et al. Six-month follow-up after a fourth bnt162b2 vaccine dose. N Engl J Med. 2022;387(22):2092–4. doi: 10.1056/NEJMc2211283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Jaime S Rosa Duque, upon reasonable request.


