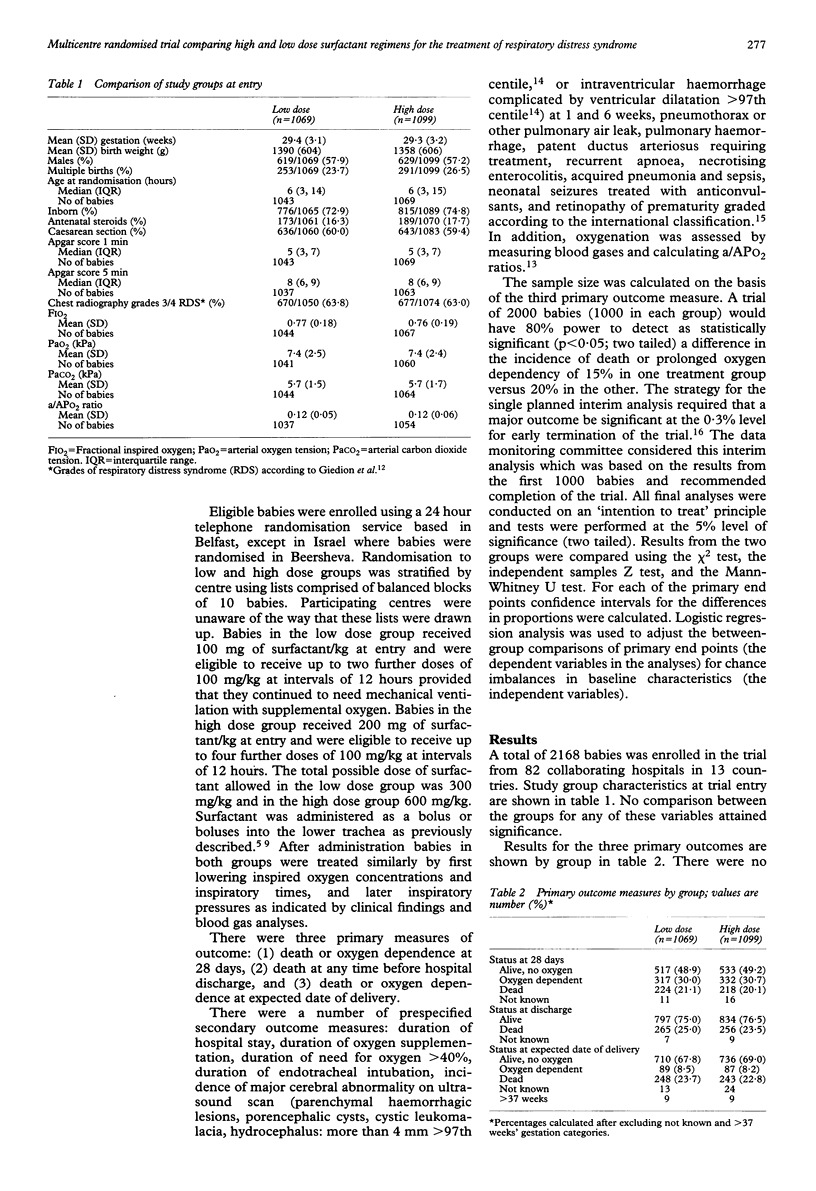
Abstract
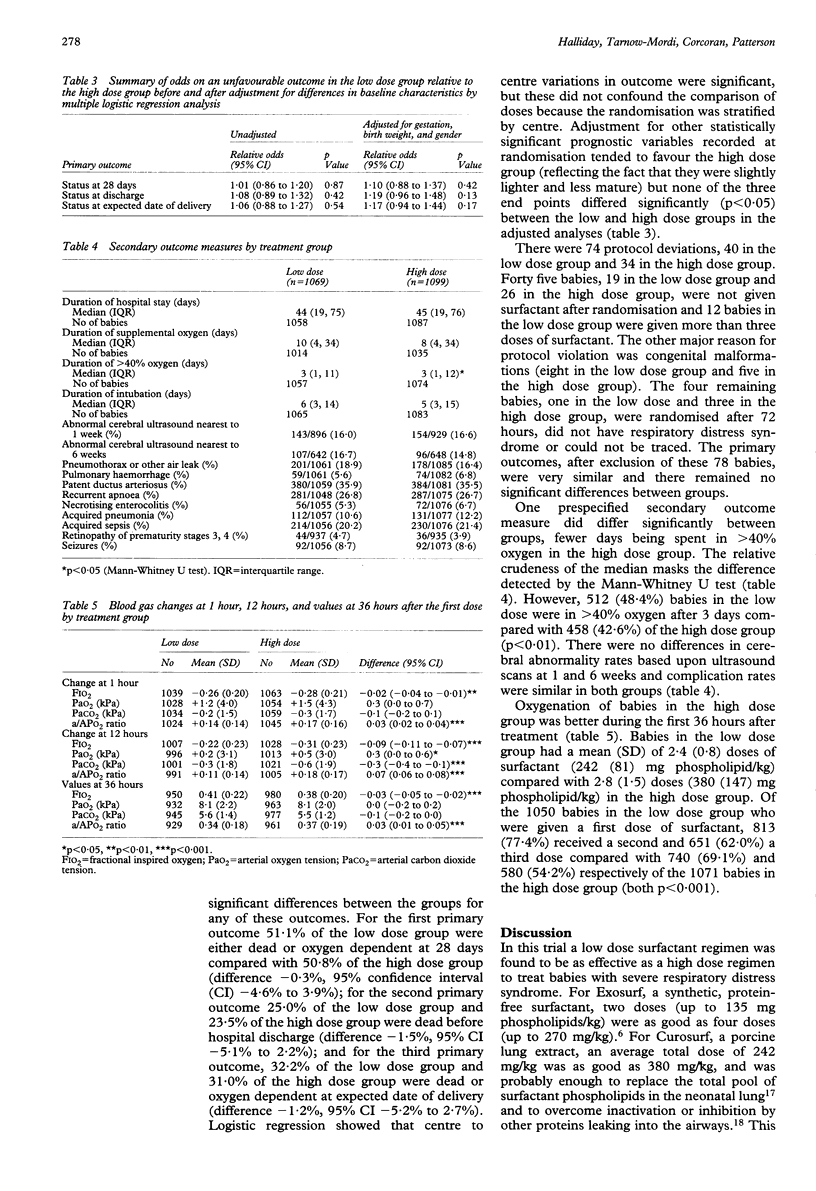
A randomised trial was conducted in 82 centres using the porcine surfactant extract, Curosurf, to compare two regimens of multiple doses to treat infants with respiratory distress syndrome and arterial to alveolar oxygen tension ratio < 0.22. Infants were randomly allocated to a low dosage group (100 mg/kg initially, with two further doses at 12 and 24 hours to a maximum cumulative total of 300 mg/kg; n = 1069) or a high dosage group (200 mg/kg initially with up to four further doses of 100 mg/kg to a maximum cumulative total of 600 mg/kg; n = 1099). There was no difference between those allocated low and high dosage in the rates of death or oxygen dependency at 28 days (51.1% v 50.8%; difference -0.3%, 95% confidence interval (CI) -4.6% to 3.9%), death before discharge (25.0% v 23.5%; difference -1.5%, 95% CI -5.1% to 2.2%), and death or oxygen dependency at the expected date of delivery (32.2% v 31.0%; difference -1.2%, 95% CI -5.2% to 2.7%). For 14 predefined secondary measures of clinical outcome there were no significant differences between the groups but the comparison of duration of supplemental oxygen > 40% did attain significance; 48.4% of babies in the low dose group needed > 40% oxygen after three days compared with 42.6% of those in the high dose group. The total amount of surfactant administered in the low dose regimen (mean 242 mg phospholipid/kg) was probably enough to replace the entire pulmonary surfactant pool. Adopting the low dose regimen would lead to considerable cost savings, with no clinically significant loss in efficacy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cummings J. J., Holm B. A., Hudak M. L., Hudak B. B., Ferguson W. H., Egan E. A. A controlled clinical comparison of four different surfactant preparations in surfactant-deficient preterm lambs. Am Rev Respir Dis. 1992 May;145(5):999–1004. doi: 10.1164/ajrccm/145.5.999. [DOI] [PubMed] [Google Scholar]
- Dunn M. S., Shennan A. T., Possmayer F. Single- versus multiple-dose surfactant replacement therapy in neonates of 30 to 36 weeks' gestation with respiratory distress syndrome. Pediatrics. 1990 Oct;86(4):564–571. [PubMed] [Google Scholar]
- Early versus delayed neonatal administration of a synthetic surfactant--the judgment of OSIRIS. The OSIRIS Collaborative Group (open study of infants at high risk of or with respiratory insufficiency--the role of surfactant. Lancet. 1992 Dec 5;340(8832):1363–1369. [PubMed] [Google Scholar]
- Fuchimukai T., Fujiwara T., Takahashi A., Enhorning G. Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol (1985) 1987 Feb;62(2):429–437. doi: 10.1152/jappl.1987.62.2.429. [DOI] [PubMed] [Google Scholar]
- Giedion A., Haefliger H., Dangel P. Acute pulmonary X-ray changes in hyaline membrane disease treated with artificial ventilation and positive end-expiratory pressure (PEP). Pediatr Radiol. 1973 Oct;1(3):145–152. doi: 10.1007/BF00974058. [DOI] [PubMed] [Google Scholar]
- Gilbert R., Keighley J. F. The arterial-alveolar oxygen tension ratio. An index of gas exchange applicable to varying inspired oxygen concentrations. Am Rev Respir Dis. 1974 Jan;109(1):142–145. doi: 10.1164/arrd.1974.109.1.142. [DOI] [PubMed] [Google Scholar]
- Hjalmarson O. Epidemiology and classification of acute, neonatal respiratory disorders. A prospective study. Acta Paediatr Scand. 1981 Nov;70(6):773–783. doi: 10.1111/j.1651-2227.1981.tb06228.x. [DOI] [PubMed] [Google Scholar]
- Jobe A., Ikegami M. Surfactant for the treatment of respiratory distress syndrome. Am Rev Respir Dis. 1987 Nov;136(5):1256–1275. doi: 10.1164/ajrccm/136.5.1256. [DOI] [PubMed] [Google Scholar]
- Levene M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child. 1981 Dec;56(12):900–904. doi: 10.1136/adc.56.12.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks L. B., Notter R. H., Oberdorster G., McBride J. T. Ultrasonic and jet aerosolization of phospholipids and the effects on surface activity. Pediatr Res. 1983 Sep;17(9):742–747. doi: 10.1203/00006450-198309000-00012. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettenazzo A., Jobe A. H., Ikegami M., Rider E., Seidner S. R., Yamada T. Cumulative effects of repeated surfactant treatments in the rabbit. Exp Lung Res. 1990 Mar-Apr;16(2):131–143. doi: 10.3109/01902149009087878. [DOI] [PubMed] [Google Scholar]
- Speer C. P., Robertson B., Curstedt T., Halliday H. L., Compagnone D., Gefeller O., Harms K., Herting E., McClure G., Reid M. Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: single versus multiple doses of Curosurf. Pediatrics. 1992 Jan;89(1):13–20. [PubMed] [Google Scholar]
- Tubman T. R., Halliday H. L., Normand C. Cost of surfactant replacement treatment for severe neonatal respiratory distress syndrome: a randomised controlled trial. BMJ. 1990 Oct 13;301(6756):842–845. doi: 10.1136/bmj.301.6756.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh T. F., Torre J. A., Rastogi A., Anyebuno M. A., Pildes R. S. Early postnatal dexamethasone therapy in premature infants with severe respiratory distress syndrome: a double-blind, controlled study. J Pediatr. 1990 Aug;117(2 Pt 1):273–282. doi: 10.1016/s0022-3476(05)80547-5. [DOI] [PubMed] [Google Scholar]


