Abstract
Bamboo (Phyllostacys edulis J. Houz) has become an emerging forest resource of economic and ecological significance with health benefits. Since the beneficial effects of the non-edible parts of bamboo have not been thoroughly explored, we characterized in this study bamboo leaf (BL) and sheath (BS) extracts. The total phenol and flavonoid content (TPC and TFC), antioxidant activity (ABTS, DPPH, FRAP and β-carotene bleaching test) and anti-inflammatory properties were determined. Leaves exhibited a TPC value of 73.92 mg equivalent (eq) gallic acid/g fresh weight (FW) and a TFC value of 56.75 mg eq quercetin/g FW. Ultra-High-Performance Liquid Chromatography (UHPLC) coupled with photo diode array detector (PDA) analysis revealed evidence for the presence of protocatechuic acid, isoorientin, orientin and isovitexin in BL, whereas BS was rich in phenolic acids. Both samples demonstrated a significant ability to scavenge radicals against ABTS·+, with an inhibitory concentration of 50% of 3.07 μg/mL for BL and 6.78 μg/mL for BS. At a concentration of 0.1 and 0.2 mg/mL, BS decreased reactive oxygen species production without hampering cell viability in HepG2 liver cells, while at the same concentrations, BL exhibited cytotoxicity in HepG2 cells. In addition, 0.1 and 0.2 mg/mL BS and BL reduced Interleukin-6 and Monocyte Chemoattractant Protein-1 production in human lipopolysaccharide-stimulated THP-1 macrophages, without affecting cell viability. These findings highlight the anti-inflammatory and antioxidant properties of BL and BS, corroborating their different potential applications in the nutraceutical, cosmetic and pharmaceutical industries.
Keywords: bamboo, by-products, leaves, sheath, antioxidant, anti-inflammatory, bioactivity, human cell lines
1. Introduction
Bamboo (Phyllostacys edulis J. Houz) is an economically important member of the family Poaceae, subfamily Bambusoideae. Bamboo species can adapt to a wide variety of climatic conditions and ecosystems. A total of 1718 different bamboo species are currently known and described, divided into 91 genera [1]. However, bamboo cannot be found on all continents. Bamboo resources are especially rich in Asia, where China and India represent the largest bamboo reserve [2]. Among its uses, we find food (shoots), a substitute for wood (the cane, used for support sticks for horticultural crops, furniture, toothpicks, etc.), fuel and, finally, an anti-erosion crop [3,4].
The sheath represents 1/3 of the biomass of the raw bamboo shoot and it is often considered a waste in bamboo shoot industrial processing [5]. Few studies have investigated the phytochemical composition and bioactivity of the sheath. Phenols, flavonoids, dietary fibers, xylan and polysaccharides were identified as its main compounds [6,7,8,9,10]. However, up to now, the limited knowledge surrounding bamboo sheath has strongly influenced how it is used.
Industrial waste re-use can help to achieve a higher level of sustainability [11]. Recently, the European Commission has undertaken a series of actions aimed at minimizing waste through recovery and regeneration methods which re-introduce waste, even in a different form, into a new life cycle. In this context, significant attention has been given to the recovery of healthy molecules from various by-products, which may be useful for the development of functional foods or nutraceutical products [12].
In recent decades, research interest in natural plant antioxidant and anti-inflammatory activities has been promising and innovative with respect to potential applications in fields such as food, cosmetics and pharmaceuticals [13,14,15]. When endogenous and exogenous antioxidants fail to counteract free radicals, a condition of oxidative stress is generated, resulting in cellular damage and related diseases [16]. Many natural compounds rich in bioactive molecules, including bamboo, are potential antioxidants, able to protect against reactive oxygen species (ROS) and, ultimately, ameliorate oxidative stress–related diseases, such as cancer, cardiovascular and neurodegenerative diseases and inflammatory disorders [17,18,19].
In addition, the bioactive agents naturally contained in plants are important in defence responses due to their anti-inflammatory properties, proving to be beneficial for human health [20]. Mounting evidence has shown a close link between inflammation and a wide range of chronic health conditions, although the mechanism by which inflammation is involved in pathological alterations is still not fully understood. Nevertheless, the state of chronic inflammation usually involves an imbalance of pro-inflammatory cytokines and anti-inflammatory cytokines. Several studies have proven that extracts and their constituents exhibit anti-inflammatory properties by blocking signaling pathways which play a key role in the production of pro-inflammatory mediators, or by inhibiting pro-inflammatory cytokines [21].
The anti-inflammatory activities of bamboo leaf extracts specifically have been described in different cellular contexts [22,23,24]. Despite the growing identification of chemical compounds in bamboo, most of the pharmacological studies have focused on bamboo leaf flavonoids [19]. Thus, further studies are needed to evaluate the activities of different bamboo by-products and to perform toxicity studies on this plant.
In this context, this work aimed to evaluate the potential bioactivity of two processing by-products of bamboo: leaves and sheaths. For this purpose, the phytochemical profile of bamboo extracts was studied, along with their potential antioxidant and anti-inflammatory activities, using as cellular model systems the human hepatocellular carcinoma HepG2 and THP-1 monocytic cell lines.
2. Materials and Methods
2.1. Chemicals and Reagents
All reagents were obtained from Sigma Aldrich (Milan, Italy), with the following exceptions: solvents, protocatechuic acid, isoorientin, orientin, isovitexin, chlorogenic acid and rutin were purchased from VWR International s.r.l. (Milan, Italy); p-Coumaric acid was purchased from Fluka (Steinheim, Germany); caffeic acid was procured from Extrasynthèse (Lyon, France).
2.2. Extraction Process
The leaves (the size of about 7–10 × 40 cm) and shoots of bamboo (Phyllostacys edulis) were collected in April 2022 in Falco Farm (Corigliano Calabro, Cosenza, Italy) (39°35′45″60 N; 16°31′6″60 E; 210 m above sea level). Leaves were washed and cut into small pieces for subsequent extraction. Shoots were manually peeled to remove the sheath for further analysis. Both leaves and shoots (500 g) were subjected to ultrasound-assisted maceration using a hydroalcoholic solution of ethanol/water (8:2 v/v, 700 mL) as solvent in a Branson model 3800-CPXH water bath (Branson, Milan, Italy) with a frequency of 40 kHz at 25 °C for 45 min. The extraction procedure was repeated 4 times and after each extraction cycle, solutions were filtered and the solvent was removed using a rotary vacuum evaporator.
2.3. Phytochemical Content
The determination of total phenol content (TPC) was carried out following the procedure previously reported [25]. For total flavonoid content (TFC) evaluation, the procedure described by Leporini et al. [25] was carried out. Results were reported as mg of chlorogenic acid equivalents (CAE)/g of fresh weight (FW) for TPC and mg quercetin equivalents (QE)/g of FW for TFC.
2.4. UHPLC Quantification of Mainly Phenolic and Flavoinoid Compounds
Ultra-High-Performance Liquid Chromatography (UHPLC) PLATINblue (Knauer, Berlin, Germany) with a PDA-1 (photo diode array detector) was used for quantification of mainly phenolic and flavoinoid compounds occurring in bamboo leaves and sheaths [26]. For chromatographic separation, a C18 column (Knauer, 1.8 µm, 150 × 3 mm) with a mobile phase consisting of H2O (pH 3.10 with acetic acid) (A) and CH3CN (B) was used. Compounds were detected by UV absorption at 245 nm, 280 nm and 303 nm. The gradient elution is reported in Table S1 (Supplementary Materials). External standards were used for the quantification of each individual component.
2.5. In Vitro Antioxidant Activity
To evaluate the ability of bamboo extracts to counteract oxidative stress, various in vitro methods, based on different mechanisms of action, were assessed. For the evaluation of radical scavenging activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic) acid (ABTS) assays were used [26]. For the ABTS test, ABTS radical solution was combined with bamboo samples at different concentrations, and after incubation the absorbance was read at 734 nm [25]. In the DPPH assay, the radical was prepared and mixed with bamboo extracts. After 30 min of incubation, the bleaching of the radical was measured at 517 nm. In both tests, ascorbic acid was used as a positive control.
The ability of extracts to induce the reduction of tripyridyltriazine (TPTZ)-Fe3+ was evaluated spectrophotometrically at 595 nm, using a Ferric reducing ability of plasma (FRAP) assay, whereas a β-carotene bleaching test was used to determine the inhibition of the lipid peroxidization in a liposome model system [26]. Butylhydroxytoluene (BHT) and propyl gallate were used as the positive controls in the FRAP and β-carotene bleaching test, respectively.
2.6. Cell Culture
HepG2 and THP-1 cell lines (American Type Culture Collection, ATCC, Manassas, VA, USA) were authenticated and stored in accordance with the instructions provided by the supplier. HepG2 and THP-1 cells were cultured in Eagle’s Minimum Essential Medium (ATCC) and Roswell Park Memorial Institute (RPMI)-1640 medium (Lonza, Verviers, Belgium), respectively, in the presence of 10% fetal bovine serum (FBS, Life Technologies, Monza, Italy) and 1% penicillin-streptomycin in a humidified 5% CO2 atmosphere at 37 °C. THP-1 monocytes were plated and differentiated into macrophages (M0) with phorbol 12-myristate 12-acetatate (PMA) 100 nM for 24 h and then within a medium without PMA for 1 day, as described by Gionfriddo et al. [27]. M0 macrophages were exposed to 0.1 and 0.2 mg/mL of BL and BS, dissolved in distilled water and dimethylsulfoxide (DMSO), respectively, prior to treatment for 24 h with Lipopolysaccharide (LPS) 10 ng/mL, which was able to polarize M0 into the inflammatory M1 macrophages.
2.7. Cell Viability Assay
HepG2 and THP-1 cells were plated and treated with 0.1 and 0.2 mg/mL of BL and BS for 24 h. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay was used to evaluate cell viability, as previously reported [28]. The absorbance was measured in Multiskan SkyHigh Photometer at a test wavelength of 570 nm (Thermo Fisher Scientific, Milan, Italy). Viable cells were expressed as a percentage with respect to the control (100%).
2.8. Real-Time PCR Assays
Extraction of total RNA was performed as previously described [29]. Real-time PCR was used to analyze cDNA in an iCycler iQ Detection System (Bio-Rad, Hercules, CA, USA). cDNA was determined in duplicates using SYBR Green Universal PCR Master Mix. Each sample was normalized by 18S mRNA content, as previously reported [30]. For gene amplification Interleukin 6 (IL-6, Gene ID: 3569): forward 5′-CCAGGAGCCCAGCTATGAAC-3′ and reverse 5′-CCCAGGGAGAAGGCAACTG-3′; Monocyte Chemoattractant Protein-1 (MCP-1, Gene ID: 6347): forward 5′-CAGCCAGATGCAATCAATGCC-3′ and reverse 5′-TGGAATCCTGAACCCACTTCT-3′; 18s rRNA (18S, Gene ID: 106631781): forward 5′-CCCACTCCTCCACCTTTGAC-3′ and reverse 5′-TGTTGCTGTAGCCAAATTCGTT-3′ primers were used.
2.9. Measurement of Intracellular Reactive Oxygen Species Production
Cells were exposed to BL and BS at concentrations of 0.1 and 0.2 mg/mL for 4 h, prior to treatment with 10 mM of hydrogen peroxide (H2O2) for 30 min. Cells treated with H2O2 were used as positive controls. Then, CellROX® Green Reagent (5 μM), a fluorogenic probe designed to reliably measure the levels of intracellular reactive oxygen species (ROS) in live cells, was added to the medium for 30 min at 37 °C. Upon oxidation, it binds to DNA, localizing mainly in the nucleus and mitochondria. After incubation, cells were washed with PBS and fixed for 15 min with 3.7% formaldehyde. Intracellular ROS levels were evaluated by a Olympus BX51 fluorescence microscope (Olympus, Tokyo, Japan) 10× objective and quantified by ImageJ.
2.10. Statistical Analysis
Data are expressed as means ± standard deviations (S.D.) or standard error mean (SEM). Prism GraphPad Prism Software (GraphPad Software, San Diego, CA, USA) was used to calculate the concentration giving 50% inhibition (IC50). In the total phytochemical content, Tukey’s test was used to determine any significant difference among investigated samples. In the antioxidant assays, differences within and between groups were evaluated by ANOVA followed by the Dunnett’s test. In cell culture-based tests, differences between means were analyzed by the Student’s t-test. Pearson’s correlation coefficient, mean, SD or SEM calculation were completed using Microsoft Excel (Microsoft, Redmond, WA, USA).
3. Results and discussion
3.1. Extraction Yield, Phytochemical Contents and UHPLC Analysis
Extraction yields of 12.45 and 6.43% were obtained with leaves and sheath, respectively. The TPC ranged from 19.36 to 73.92 mg eq CAE/g FW for BL and BS samples, respectively, whereas values from 4.69 to 56.75 mg eq QE/g FW were recorded for TFC.
The impact of the extraction procedure on the TPC and TFC of the leaves of three commonly cultivated bamboo species (Gigantochloa verticillate, G. atter and Dendrocalamus asper) collected in Indonesia were previously investigated. Ethyl acetate extracts were richest in TPC with values in the range of 25.50–26.09 mg GAE/g for G. verticillata and D. asper, respectively, followed by ethanol 70% (v/v) and hot water extracts. A similar situation was observed for TFC with values in the range of 84.03 to 92.67 mg QE/g for G. atter and G. verticillate, respectively [31]. All these values are in agreement with our results.
More recently, Benjamin et al. [32] evaluated the impact of drying methods (freeze-drying, microwave, oven, shade and sun) on the leaves of six different species of bamboo from Malaysia, namely Dinochloa sublaevigata, Bambusa tuldoides, B. vulgaris, B. multiplex, Schizostachyum brachycladum and Gigantochloa levis. Results clearly showed that the drying process considerably affects the retention of phytochemicals in the plant matrix. Leaves subjected to the freeze-drying process are characterized by the highest TPC and TFC, with values from 2.69 to 12.59 mg GAE/g for G. levis and D. sublaevigata, and 0.87–2.12 mg QE/g for G. levis and S. brachycladum, respectively. Values of 14.6 mg GAE/g powder and 6.71 mg quercetin equivalent/g powder were found for Bambusa arundinacea methanol extracts [33]. TPC values in the range of 28.056–29.586 mg GAE/g for Phyllostachys Tao Kiang and P. pubescens leaves ethanol (80%) extract obtained by an ultrasound-assisted maceration process, and TFC values in the range of 17.678–25559 mg rutin eq/g for Phyllostachys aureosuleata and P. heterocycla, respectively, were found [8].
Compared to the leaves, much less investigated is the plant matrix derived from the shoot processing, namely the external sheath. Previously, Jiang et al. [5] investigated the TPC in Phyllostachys pracecox sheath and found a high TPC (85.3 mg GAE/g DW), whereas values in the range of 9.103–16.692 mg GAE/g for Phyllostachys spectabilis and P. heterocycle, respectively, were found by Li et al. [8]. In the same study, TFC levels were quantified in the range of 1.292–6.325 mg rutin eq/g for P. spectabilis and P. heterocycle, respectively.
Selected phytochemicals were identified in bamboo leaf and sheath extracts (Figure S1). According to Ma et al., 2020 [34], a BL sample chemical profile was characterized by a high concentration of protocatechuic acid (911.14 μg/g), isoorientin (1197.64 μg/g), orientin (302.54 μg/g) and isovitexin (575.1 μg/g), whereas BS was mainly characterized by phenolic acids, particularly protocatechuic acid (650.73 μg/g), chlorogenic acid (742.81 μg/g), caffeic acid (155.57 μg/g) and p-cumaric acid (41.20 μg/g). Rutin (119.79 μg/g) was also identified.
Data obtained in our study are in agreement with data found by Wang et al. [35], except with respect to p-cumaric acid (41.20 μg/g) and ferulic acid (64.46 μg/g).
3.2. In Vitro Antioxidant Activity
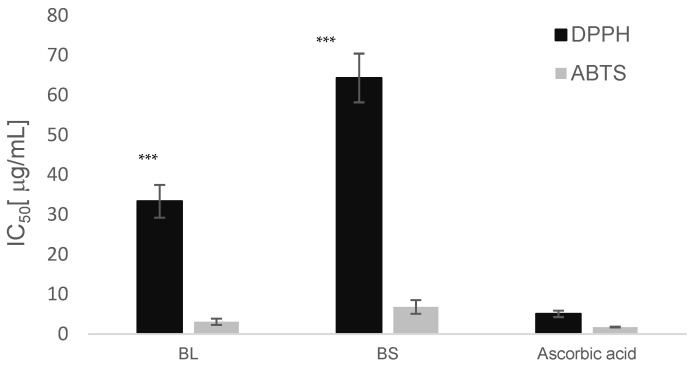
Leaf extract showed the most promising radical scavenging potential with IC50 of 3.07 and 44.32 μg/mL in the ABTS and DPPH tests, respectively (Figure 1). A promising ABTS radical scavenging activity was also observed for the sheath extract (IC50 of 6.78 μg/mL).
Figure 1.
Radical scavenging activity assessed by DPPH and ABTS assays. The values represent the means ± S.D. (n = 3). BL: Bamboo leaves; BS: Bamboo sheath. Differences within and between groups were evaluated by One-way ANOVA followed by a multicomparison Dunnett’s test compared to ascorbic acid (*** p < 0.001).
The protection of β-carotene from the oxidant activity carried out by the thermo-degradation products of linoleic acid was assessed using a β-carotene bleaching test. The obtained data showed that leaves increase the activity after 60 min of incubation (IC50 of 41.04 and 19.61 μg/mL at 30 and 60 min, respectively) (Table 1). A low ferric-reducing ability was found in both samples with FRAP values of 7.71 and 10.25 for BS and BL, respectively (Table 1). A positive correlation was independently found between antioxidant activity and total phytochemical content (TPC and TFC), using the test applied to investigate the bioactivity.
Table 1.
Protection of lipid peroxidation and Ferric Reducing Ability Power of bamboo.
| Samples | β-Carotene Bleaching Test IC50 (µg/mL) |
FRAP μM Fe (II)/g |
|
|---|---|---|---|
| t = 30 min | t = 60 min | ||
| BL | 41.04 ± 4.81 **** | 19.61 ± 2.58 **** | 10.25 ± 2.07 **** |
| BS | 92.48 ± 8.18 **** | 79.13 ± 7.21 **** | 7.71 ± 1.91 **** |
BL: Bamboo leaves; BS: Bamboo sheath. Data are expressed as means ± S.D. (n = 3). Differences within and between groups were evaluated by One-way ANOVA followed by Dunnett’s test compared with the positive controls (propyl gallate in β-Carotene Bleaching Test (IC50 values of 0.09 ± 0.01 µg/mL) at both incubation times) and butylhydroxytoluene in FRAP test (FRAP value 62.27 ± 4.29 μM Fe (II)/g) (**** p < 0.0001).
Several studies have demonstrated the antioxidant potential of the bamboo leaves. The variability of antioxidant activity, in accordance with the analyses in the literature, is related to different factors including bamboo species, place of collection, season of collection, pre-treatment of the plant matrix, solvent used for extraction and extraction procedure.
Ni et al. [36,37] reported the radical scavenging potential and ferric-reducing ability of Indocalamus latifolius and Sasa argenteastriatus, two bamboo species particularly common in East Asia. Results indicated that the higher altitude reduced the secondary metabolite accumulation and consequently the antioxidant potential. Moreover, the bamboo leaf extract’s DPPH radical scavenging activity was influenced by season, since it was higher in autumn and winter compared to other seasons, with maximum values in the range of 284.08–457.42 μg/mL. Values in the range of 234.57 to 422.87 μmol/L were found using a FRAP test. A similar trend was observed for bamboo leaves from Phyllostachys species [38].
Gong et al. [39] investigated the DPPH radical scavenging potential of Phyllostachys nigra var. henonis leaves and found an IC50 value of 1.81 μg/mL, which is significantly lower than those found in our sample. A lower DPPH radical scavenging potential was independently observed for Gigantochloa atter, Dendrocalamus asper and Gigantochloa verticillate, in comparison with our data, using the solvent used for extraction with values in the range of 566.79 to 1825.07 μg/mL for G. verticillata and G. atter, respectively [31]. An IC50 value of 164.11 μg/mL was found with Bambusa vulgaris leaf extract [40].
A lower DPPH radical scavenging potential was found for three different bamboo species collected in China with IC50 values in the range of 2.10–10.17, 1.25–5.07 and 1.59–2.72 mg/mL for Lophatherum gracile, Pleioblastus amarus and Phyllostachys nigra, respectively [34]. A similar situation was also observed when comparing our data with those obtained from the leaves of Bambusa arundinacea with IC50 values in the range of 273–1103 μg/mL [33].
Data found using the ABTS test are in line with those obtained by Benjamin et al. [32], which found IC50 values in the range of 1.89 to 3.47 μg/mL for B. tuldoides leaves subjected to oven drying and S. brachycladum leaves subjected to sun drying, respectively, whereas all extracts exhibited a higher DPPH radical scavenging potential with IC50 values of 2.92–4.73 μg/mL. A comparable radical scavenging potential was observed with data obtained by Kim et al. [41], with extracts obtained using Phyllostachys nigra leaves with IC50 values in the range of 1.79–32.64 and 2.05–47.90 mg/mL for ABTS and DPPH, respectively.
Li et al. [8] evaluated the DPPH radical scavenging potential and ferric-reducing ability of eight different Phyllostachys species and found that the leaves of P. pubescens resulted the most active, whereas among the sheath the strongest antioxidant potential was observed in the golden thread moso bamboo. More recently, Cao et al. [42] investigated the impact of the extraction procedure on the recovery of bioactive compounds using Bambusa chungii culms processing waste, as well as its influence on antioxidant activity. The best extraction conditions were found at a temperature of 160 °C and with a solvent ratio of 1:30 g/mL for 14 min. The obtained extract showed IC50 values of 27.22 and 125.07 mg/L for the ABTS and DPPH tests, respectively.
A certain variability was observed using the FRAP test with values in the range of 6.40–36.65 mg Trolox equivalent/g. An ABTS radical scavenging potential from 1.373 and 1.650 mg eq di Vitamin C/mL was found for Phyllostachys pubescens leaves subjected to different extraction procedures in terms of temperature and time [43].
Regarding sheath, Jiang et al. [5] found values of 33 and 77 μmol ascorbic acid equivalent (AAE)/g DW for FRAP and DPPH, respectively, using Phyllostachys pracecox ultrasound-assisted ethanol extract.
3.3. Antioxidant Power of Bamboo Leaves and Sheaths in Human Liver HepG2 Cells
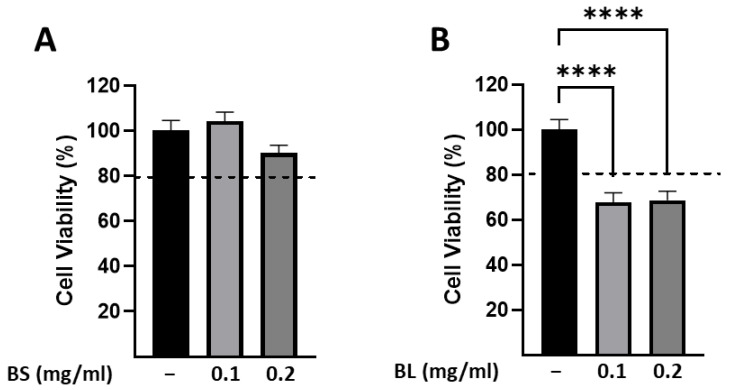
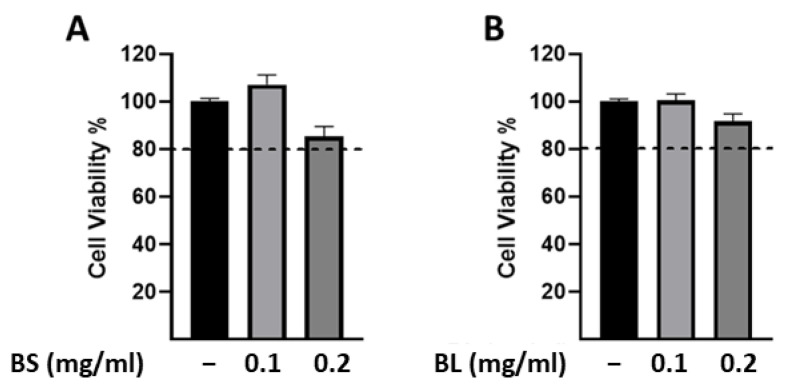
The human hepatoma HepG2 cells are the most commonly used cell model in hepatotoxicity studies since they display an epithelial-like morphology [44]. Based on our results, showing that different in vitro tests revealed the antioxidant activities of extracts derived from bamboo in a dose-dependent manner, we first assessed whether the highest IC50 values of both extracts are cytotoxic in HepG2 cells. As shown in Figure 2A, the exposure of cells to BS for 24 h, at concentrations of 0.1 and 0.2 mg/mL, did not result in cell toxicity, while both concentrations significantly reduced cell viability upon treatment with BL (Figure 2B). We hypothesize that the different qualitative and quantitative compositions of the bamboo extracts may influence viability in a cell context-dependent manner. In particular, by chromatography we identified isoorientin in BL, but not in BS extracts, which is an antiproliferative and apoptotic agent in HepG2 cells [45]. Moreover, both extracts contained the protocatechuic acid able to exhibit antiproliferative effects in various cell lines, including HepG2 cells [46]. However, the protocatechuic acid amount in BL was about 3 times higher than that of BS (1911.14 mg/g and 650.73 mg/g, respectively). Based on these data, it appears that the BL extract may potentially cause liver damage, and further investigations are necessary to determine its possible applications beyond the food industry. BS did not elicit any cytotoxic effects and could serve as a beneficial additive component in food and nutraceuticals.
Figure 2.
Cell viability assessed by MTT assay in HepG2 cells. HepG2 cells were untreated (−) or treated with 0.1 and 0.2 mg/mL of bamboo sheaths (BS) (A) and bamboo leaves (BL) (B) for 24 h. The histograms represent the means ± SEM of three independent experiments, each performed in triplicate. **** p < 0.0001.
Yu et al. [47] explored the antioxidant properties of bamboo leaf flavonoids extracts in human HepG2 cells in which oxidative-stress was induced by oleic acid. A reduction of high ROS production and a modulation of related antioxidant defense responses carried out by bamboo flavonoids was shown, suggesting the ability of the extracts to alleviate oxidative stress in HepG2 cells. In line with these findings, Zhang et al. [48] provided evidence that bamboo leaf flavonoids suppressed hepatocellular injury and death of apoptotic cells induced by carbon tetrachloride chemical liver injury, indicating that the protective effects exhibited by this biological compound on acute liver damage are related to its strong antioxidant capacity.
In our study, we used HepG2 cells as a model system to investigate the potential antioxidant effects of BS extracts on oxidative stress induced by H2O2. It has been reported that exogenous H2O2 penetrates the cell membrane easily and generates elevated levels of free radicals which attack the mitochondrial membrane, leading to excessive ROS production in the cells [49]. In particular, the abnormal accumulation of ROS can directly induce cell damage, through the oxidation of macromolecules, such as DNA, RNA, carbohydrates, proteins and lipids, or can indirectly alter different intracellular signaling pathways implicated in the development of ageing, as well as of various chronic and degenerative diseases [50]. Therefore, antioxidants that can prevent the production of ROS are a major therapeutic means of reinforcing treatments against a wide spectrum of chronic diseases.
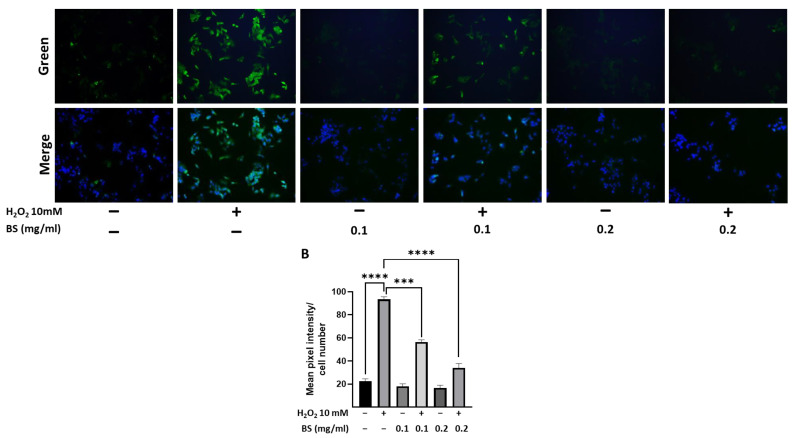
Our data showed that treatment with 10 mM H2O2 increased, as expected, the accumulation of ROS (p < 0.0001), whereas BS significantly inhibited the ROS production induced by H2O2 in a dose-dependent manner (Figure 3), suggesting that BS prevented oxidative stress damage caused by H2O2 in HepG2 cells.
Figure 3.
Reactive oxygen species production in HepG2 cells upon treatment with extracts from bamboo sheaths. (A) Representative green images (CellROX dye) of reactive oxygen species (ROS) captured by fluorescent microscopy in HepG2 cells without treatment (−) or with 0.1 and 0.2 mg/mL of bamboo sheaths (BS) for 4 h and then treated with 10 mM H2O2 for 30 min. Merged images of green and blue (DAPI (40,6-diamidino-2-phenylindole) dye for staining DNA) are shown. (B) ROS production is reported as mean pixel intensity (green dye) normalized to cell number (blue dye) in three independent experiments each performed in triplicate. The histograms represent means ± SEM. *** p < 0.001, **** p < 0.0001.
3.4. Anti-Inflammatory Effects of Bamboo Extracts in LPS-Stimulated Human Macrophages
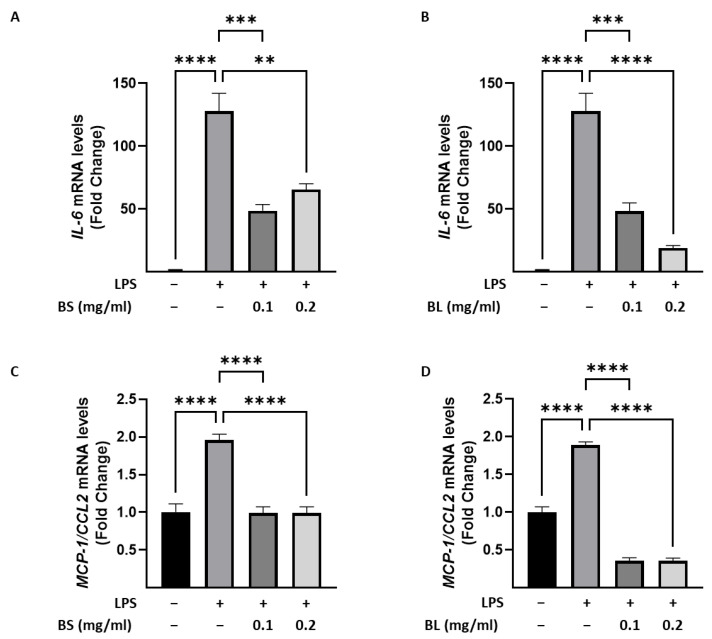
Recently, it has been reported that bamboo extracts from Sasa albomarginata are able to inhibit LPS-induced inflammatory responses at nontoxic concentrations in macrophages [22]. Thus, we aimed to explore the potential anti-inflammatory activities of bamboo extracts from Phyllostacys edulis, using LPS-stimulated humanmacrophages, as an in vitro model of acute inflammation. Specifically, we evaluated by Real Time PCR the gene expression levels of pro-inflammatory cytokines in human M1 macrophages treated with 0.1 and 0.2 mg/mL of BS and BL. As shown in Figure 3, untreated macrophages, named M0 macrophages, produce low levels of Interleukin (IL)-6 (Figure 4A,B) and Monocyte Chemoattractant Protein (MCP)-1/C-C motif chemokine ligand 2 (CCL-2) (Figure 4C,D), while treatment with LPS stimulated macrophages to enhance IL-6 (Figure 4A,B) and MCP-1/CCL-2 levels (Figure 4C,D). Interstingly, BS and BL at both concentrations tested, were able to dampen the inflammation, suggesting that they have an anti-inflammatory effect in macrophages.
Figure 4.
Anti-inflammatory effects of bamboo extracts in M1 macrophages. Evaluation of interleukin-6 (IL-6), Monocyte Chemoattractant Protein (MCP)-1/C-C motif chemokine ligand 2 (CCL-2) mRNA expression, by Real Time PCR assay, in macrophages untreated (−) or treated for 1 h with bamboo sheaths (BS) (A,C) and bamboo leaves (BL) (B,D) at concentrations of 0.1 and 0.2 mg/mL and then stimulated with Lipopolysaccharide (LPS) 10 ng/mL for 24 h. Data represent the means ± SEM of three different experiments each performed in duplicate. ** p < 0.005, *** p < 0.001, **** p < 0.0001.
Importantly, to ascertain whether bamboo extracts elicit any cytotoxic effects, an MTT assay was performed in human THP-1 macrophages. The exposure of cells for 24 h to BS and BL, at concentrations of 0.1 and 0.2 mg/mL, did not affect cell viability (Figure 5), supporting the potential use of these extracts by the healthcare industry.
Figure 5.
Cell viability assessed by MTT assay in human THP-1 derived macrophages. Human THP-1 derived macrophages were untreated (−) or treated with 0.1 and 0.2 mg/mL of bamboo sheaths (BS) (A) and bamboo leaves (BL) (B) for 24 h. Cell viability is expressed as percentage of control (−). The histograms represent the means ± SEMs of three different experiments, each performed in triplicate.
Besides their application in the pharmacological industries, they can be incorporated into skincare products to help reduce skin inflammation and redness, which are often associated with conditions such as acne, rosacea and eczema. Moreover, in the food industry, BS extracts can be used as natural food additives to reduce inflammation and oxidative stress, which are known to contribute to the development of chronic diseases.
4. Conclusions
Our results provide insight into the antioxidant and anti-inflammatory properties of bamboo leaf and sheath processing by-products, emphasizing the potential application of this plant in food, nutraceutical, cosmetic and pharmaceutical industries. Of particular interest is that the leaf extract, characterized by a higher content of total phenols and flavonoids than the sheath processing by-products, was shown to be most active in all antioxidant assays. Moreover, both extracts impair cytokine production in a model of cellular inflammation, indicating their potential exploitation in therapeutic strategies to prevent and/or treat inflammatory diseases. Further studies are needed to investigate the mechanism of action and toxicological properties of different bamboo extracts aimed at the valorisation of plant biomass to be exploited for human health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12061239/s1, Table S1: UHPLC gradient elution. Figure S1: Chromatographic profile of bamboo sheath. Figure S2: Chromatographic profile of bamboo leaves.
Author Contributions
Methodology: D.B. and R.T.; software validation: V.S.; formal analysis: A.V., G.A. and R.T.; data curation: G.A., R.R. and R.T.; writing-original draft preparation: M.R.L. and R.T.; writing-review and editing, D.B.; project administration. M.R.L. and D.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Funding Statement
This work was partially financially supported by the PSR Calabria 2014–2020 (Calabrian Rural Development Programme) within Project Action 16.2: “Messa in coltura di Phyllostachis edulis, studio di shelf-life su germogli di bambù da impiegare nell’industria food e non-food (BAMBOO) (CUP J26G20000320005)”.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ohrnberger D. The Bamboos of the World: Annotated Nomenclature and Literature of the Species and the Higher and Lower Taxa. Elsevier; Amsterdam, The Netherlands: 1999. [Google Scholar]
- 2.Canavan S., Richardson D.M., Visser V., Roux J.J., Vorontsova M.S., Wilson J.R. The global distribution of bamboos: Assessing correlates of introduction and invasion. AoB Plants. 2016;9:plw078. doi: 10.1093/aobpla/plw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nirmala C., Bisht M.S., Laishram M. Bioactive compounds in bamboo shoots: Health benefits and prospects for developing functional foods. Int. J. Food Sci. Technol. 2014;49:1425–1431. doi: 10.1111/ijfs.12470. [DOI] [Google Scholar]
- 4.van Dam J.E.G., Elbersen H.W., Montaño C.M.D. Perennial Grasses for Bioenergy and Bioproducts. Academic Press; Cambridge, MA, USA: 2018. 6—Bamboo Production for Industrial Utilization; pp. 175–216. [DOI] [Google Scholar]
- 5.Jiang L., Belwal T., Huang H., Ge Z.-w., Limwachiranon J., Zhao Y., Li L., Ren G., Luo Z. Extraction and Characterization of Phenolic Compounds from Bamboo Shoot Shell under Optimized Ultrasonic-Assisted Conditions: A Potential Source of Nutraceutical Compounds. Food Bioprocess Technol. 2019;12:1741–1755. doi: 10.1007/s11947-019-02321-y. [DOI] [Google Scholar]
- 6.Jiang L., Jiang L.-K., Chen K.W. Ultrasonic extraction conditions of bamboo shell flavones and its antioxidative activity on oil. Nat. Prod. Res. Devel. 2009;21:146–151. [Google Scholar]
- 7.Jin Y., Yuan K. Studies on the Functional Components and Bioactivity and the Relativity of Bamboo Shoots and Shells. Appl. Mech. Mater. 2011;108:314–319. doi: 10.4028/www.scientific.net/AMM.108.314. [DOI] [Google Scholar]
- 8.Li Y.-X., Cheng F.-R., Jin Y.-C., Yuan K. Studies on the Active Components and Antioxidant Activity of the Extracts from Different Parts of Bamboo. Asian J. Chem. 2013;25:6354–6360. doi: 10.14233/ajchem.2013.14585. [DOI] [Google Scholar]
- 9.Han X.F., Jin J.C. Study on the properties and extraction of insoluble dietary fiber from bamboo shells. Guangzhou Chem. Ind. 2015;43:56–58. [Google Scholar]
- 10.Yu N., Wu N., Wang Y., Tu Y. Study on Extraction of Xylan from Bamboo Shoot Shell; Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012); Tianjin, China. 18–19 October 2012; Berlin/Heidelberg, Germany: Springer; 2014. pp. 923–929. Lecture Notes in Electrical Engineering. [DOI] [Google Scholar]
- 11.Kumar N., Amritphale S.S., Matthews J.C., Lynam J.G., Alam S., Abdulkareem O.A. Synergistic utilization of diverse industrial wastes for reutilization in steel production and their geopolymerization potential. Waste Manag. 2021;126:728–736. doi: 10.1016/j.wasman.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Sharma Y.K., Mangla S.K., Patil P.P., Liu S. When challenges impede the process. Manag. Decis. 2019;57:995–1017. doi: 10.1108/MD-09-2018-1056. [DOI] [Google Scholar]
- 13.Hahn D., Shin S.H., Bae J.S. Natural Antioxidant and Anti-Inflammatory Compounds in Foodstuff or Medicinal Herbs Inducing Heme Oxygenase-1 Expression. Antioxidants. 2020;9:1191. doi: 10.3390/antiox9121191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girgih A.T., He R., Hasan F.M., Udenigwe C.C., Gill T.A., Aluko R.E. Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions. Food Chem. 2015;173:652–659. doi: 10.1016/j.foodchem.2014.10.079. [DOI] [PubMed] [Google Scholar]
- 15.Carpentieri S., Augimeri G., Ceramella J., Vivacqua A., Sinicropi M.S., Pataro G., Bonofiglio D., Ferrari G. Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages. Foods. 2022;11:2250. doi: 10.3390/foods11152250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rani V., Deep G., Singh R.K., Palle K., Yadav U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Chen W., Jia Z., Pan M.H., Anandh Babu P.V. Natural Products for the Prevention of Oxidative Stress-Related Diseases: Mechanisms and Strategies. Oxid. Med. Cell. Longev. 2016;2016:4628502. doi: 10.1155/2016/4628502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonofiglio D., Giordano C., De Amicis F., Lanzino M., Ando S. Natural Products as Promising Antitumoral Agents in Breast Cancer: Mechanisms of Action and Molecular Targets. Mini Rev. Med. Chem. 2016;16:596–604. doi: 10.2174/1389557515666150709110959. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y., Wan S., Yao L., Lin D., Wu T., Chen Y., Zhang A., Lu C. Bamboo leaf: A review of traditional medicinal property, phytochemistry, pharmacology, and purification technology. J. Ethnopharmacol. 2023;306:116166. doi: 10.1016/j.jep.2023.116166. [DOI] [PubMed] [Google Scholar]
- 20.Sun W., Shahrajabian M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants-Natural Health Products for Human Health. Molecules. 2023;28:1845. doi: 10.3390/molecules28041845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016;2016:5276130. doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima S., Hakamata M., Asanuma T., Suzuki R., Tsuruda J.I., Nonoyama T., Lin Y., Fukatsu H., Koide N., Umezawa K. Cellular Anti-Inflammatory and Antioxidant Activities of Bamboo Sasa albomarginata Leaf Extract and Its Constituent Coumaric Acid Methyl Ester. Sci. World J. 2022;2022:8454865. doi: 10.1155/2022/8454865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wedler J., Daubitz T., Schlotterbeck G., Butterweck V. In vitro anti-inflammatory and wound-healing potential of a Phyllostachys edulis leaf extract--identification of isoorientin as an active compound. Planta Med. 2014;80:1678–1684. doi: 10.1055/s-0034-1383195. [DOI] [PubMed] [Google Scholar]
- 24.Ono M., Kantoh K., Ueki J., Shimada A., Wakabayashi H., Matsuta T., Sakagami H., Kumada H., Hamada N., Kitajima M., et al. Quest for anti-inflammatory substances using IL-1beta-stimulated gingival fibroblasts. In Vivo. 2011;25:763–768. [PubMed] [Google Scholar]
- 25.Leporini M., Loizzo M.R., Sicari V., Pellicano T.M., Reitano A., Dugay A., Deguin B., Tundis R. Citrus × Clementina Hort. Juice Enriched with Its By-Products (Peels and Leaves): Chemical Composition, In Vitro Bioactivity, and Impact of Processing. Antioxidants. 2020;9:298. doi: 10.3390/antiox9040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sottile F., Napolitano A., Badalamenti N., Bruno M., Tundis R., Loizzo M.R., Piacente S. A New Bloody Pulp Selection of Myrobalan (Prunus cerasifera L.): Pomological Traits, Chemical Composition, and Nutraceutical Properties. Foods. 2023;12:1107. doi: 10.3390/foods12051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gionfriddo G., Plastina P., Augimeri G., Catalano S., Giordano C., Barone I., Morelli C., Giordano F., Gelsomino L., Sisci D., et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARgamma Ligands as a Potential Target in Breast Cancer. Cells. 2020;9:174. doi: 10.3390/cells9010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rovito D., Gionfriddo G., Barone I., Giordano C., Grande F., De Amicis F., Lanzino M., Catalano S., Ando S., Bonofiglio D. Ligand-activated PPARgamma downregulates CXCR4 gene expression through a novel identified PPAR response element and inhibits breast cancer progression. Oncotarget. 2016;7:65109–65124. doi: 10.18632/oncotarget.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonofiglio D., Qi H., Gabriele S., Catalano S., Aquila S., Belmonte M., Ando S. Peroxisome proliferator-activated receptor gamma inhibits follicular and anaplastic thyroid carcinoma cells growth by upregulating p21Cip1/WAF1 gene in a Sp1-dependent manner. Endocr. Relat. Cancer. 2008;15:545–557. doi: 10.1677/ERC-07-0272. [DOI] [PubMed] [Google Scholar]
- 30.Augimeri G., Plastina P., Gionfriddo G., Rovito D., Giordano C., Fazio A., Barone I., Catalano S., Ando S., Bonofiglio D., et al. N-Eicosapentaenoyl Dopamine, A Conjugate of Dopamine and Eicosapentaenoic Acid (EPA), Exerts Anti-inflammatory Properties in Mouse and Human Macrophages. Nutrients. 2019;11:2247. doi: 10.3390/nu11092247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pujiarti R., Suryani S., Sunarta S., Lukmandaru G., Purba B. Extractive Contents and DPPH-Scavenging Activities of Bamboo Leaf Extracts from Gigantochloa atter, Dendrocalamus asper, and Gigantochloa verticillata. Taiwan J. For. Sci. 2020;35:1–12. [Google Scholar]
- 32.Benjamin M.A.Z., Ng S.Y., Saikim F.H., Rusdi N.A. The Effects of Drying Techniques on Phytochemical Contents and Biological Activities on Selected Bamboo Leaves. Molecules. 2022;27:6458. doi: 10.3390/molecules27196458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macwan C., Patel H.V., Kalia K. A comparative evaluation of in vitro antioxidant properties of bamboo Bambusa arundinacea leaves extracts. J. Cell Tissue Res. 2010;10:2413–2418. [Google Scholar]
- 34.Ma N.H., Guo J., Xu Chen S.H., Yuan X.R., Zhang T., Ding Y. Antioxidant and Compositional HPLC Analysis of Three Common Bamboo Leaves. Molecules. 2020;25:409. doi: 10.3390/molecules25020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Chen J., Wang D., Ye F., He Y., Hu Z., Zhao G. A systematic review on the composition, storage, processing of bamboo shoots: Focusing the nutritional and functional benefits. J. Funct. Foods. 2020;71:104015. doi: 10.1016/j.jff.2020.104015. [DOI] [Google Scholar]
- 36.Ni Q., Xu G., Wang Z., Gao Q., Wang S., Zhang Y. Seasonal variations of the antioxidant composition in ground bamboo Sasa argenteastriatus leaves. Int. J. Mol. Sci. 2012;13:2249–2262. doi: 10.3390/ijms13022249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni Q., Wang Z., Xu G., Gao Q., Yang D., Morimatsu F., Zhang Y. Altitudinal variation of antioxidant components and capability in Indocalamus latifolius (Keng) McClure leaf. J. Nutr. Sci. Vitaminol. 2013;59:336–342. doi: 10.3177/jnsv.59.336. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Wu X.Q., Yu Z.Y. Studies on seasonal variation of flavonoids and lactones in bamboo leaves. Chem. Ind. For. Prod. 2002;22:65–69. [Google Scholar]
- 39.Gong J., Xia D., Huang J., Ge Q., Mao J., Liu S., Zhang Y. Functional components of bamboo shavings and bamboo leaf extracts and their antioxidant activities in vitro. J. Med. Food. 2015;18:453–459. doi: 10.1089/jmf.2014.3189. [DOI] [PubMed] [Google Scholar]
- 40.Htwe H.M. Determination of antioxidant activity of bamboo leaves (Bambusa vulgaris Schrad. ex J.C. Wendl.) Yadanabon Univ. Res. J. 2017;8:1–9. [Google Scholar]
- 41.Kim C.Y., Lee H.J., Jung S.H., Lee E.H., Cha K.H., Kang S.W., Pan C.-H., Um B.-H. Rapid identification of radical scavenging phenolic compounds from black bamboo leaves using high-performance liquid chromatography coupled to an online ABTS+-based assay. J. Korean Soc. Appl. Biol. Chem. 2009;52:613–619. doi: 10.3839/jksabc.2009.102. [DOI] [Google Scholar]
- 42.Cao X., Zhang Y., Xun H., Wang J., Tang F. High-Yield Recovery of Antioxidant Compounds from Bambusa chungii Culms Using Pressurized Hot Water Extraction. Antioxidants. 2022;11:2231. doi: 10.3390/antiox11112231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Z., Zhang Q., Dai J., Wang X., Yang Q., Cai C., Mao J., Ge Q. Structural characterization, antioxidant and antimicrobial activity of water-soluble polysaccharides from bamboo (Phyllostachys pubescens Mazel) leaves. Int. J. Biol. Macromol. 2020;142:432–442. doi: 10.1016/j.ijbiomac.2019.09.115. [DOI] [PubMed] [Google Scholar]
- 44.Donato M.T., Tolosa L., Gomez-Lechon M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015;1250:77–93. doi: 10.1007/978-1-4939-2074-7_5. [DOI] [PubMed] [Google Scholar]
- 45.Lin X., Wei J., Chen Y., He P., Lin J., Tan S., Nie J., Lu S., He M., Lu Z., et al. Isoorientin from Gypsophila elegans induces apoptosis in liver cancer cells via mitochondrial-mediated pathway. J Ethnopharmacol. 2016;187:187–194. doi: 10.1016/j.jep.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 46.Xu Q., Wang H., Li T., Chen L., Zheng B., Liu R.H. Comparison of phenolics, antioxidant, and antiproliferative activities of two Hypsizygus marmoreus varieties. J. Food Sci. 2020;85:2227–2235. doi: 10.1111/1750-3841.15173. [DOI] [PubMed] [Google Scholar]
- 47.Yu Y., Li Z., Cao G., Huang S., Yang H. Bamboo Leaf Flavonoids Extracts Alleviate Oxidative Stress in HepG2 Cells via Naturally Modulating Reactive Oxygen Species Production and Nrf2-Mediated Antioxidant Defense Responses. J. Food Sci. 2019;84:1609–1620. doi: 10.1111/1750-3841.14609. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S., Chen J., Sun A., Zhao L. Protective effects and antioxidant mechanism of bamboo leaf flavonoids on hepatocytes injured by CCl4. Food Agric. Immunol. 2014;25:386–396. doi: 10.1080/09540105.2013.810709. [DOI] [Google Scholar]
- 49.Brand M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Checa J., Aran J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020;13:1057–1073. doi: 10.2147/JIR.S275595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.