Abstract
Background
Diabetes mellitus and human immunodeficiency virus (HIV) are independent risk factors for poor outcomes among people with tuberculosis (TB). To date, information on the joint impact of diabetes and HIV on TB outcomes is limited. We aimed to estimate (1) the association between hyperglycemia and mortality and (2) the effect of joint exposure to diabetes and HIV on mortality.
Methods
We conducted a retrospective cohort study among people with TB in the state of Georgia between 2015 and 2020. Eligible participants were 16 or older, did not have a previous TB diagnosis, and were microbiologically confirmed or clinical cases. Participants were followed during TB treatment. Robust Poisson regression was used to estimate risk ratios for all-cause mortality. Interaction between diabetes and HIV was assessed on the additive scale using the attributable proportion and on the multiplicative scale with product terms in regression models.
Results
Of 1109 participants, 318 (28.7%) had diabetes, 92 (8.3%) were HIV positive, and 15 (1.4%) had diabetes and HIV. Overall, 9.8% died during TB treatment. Diabetes was associated with an increased risk of death among people with TB (adjusted risk ratio [aRR] = 2.59; 95% confidence interval [CI], 1.62–4.13). We estimated that 26% (95% CI, −43.4% to 95.0%) of deaths among participants with diabetes mellitus and HIV were due to biologic interaction.
Conclusions
Diabetes alone and co-occurring diabetes and HIV were associated with an increased risk of all-cause mortality during TB treatment. These data suggest a potential synergistic effect between diabetes and HIV.
Keywords: diabetes, hyperglycemia, interaction, mortality, tuberculosis
Among people with tuberculosis in Georgia between 2015–2020, diabetes was associated with increased risk of all-cause mortality (aRR = 2.59; 95% CI, 1.62–4.13). Approximately one quarter of deaths among people with both diabetes and HIV were due to diabetes-HIV synergy.
Tuberculosis (TB) represents the second leading cause of death by an infectious disease worldwide. In 2021, there were 7882 reported cases of TB in the United States, an incidence rate of 2.4 cases per 100 000 persons [1]. In 2020, there were 600 TB-related deaths, a rate of 0.2 deaths per 100 000 persons [2]. Since 1992, TB-related deaths in the United States have declined by 69.1% [3]. However, the risk of TB mortality remains high for patients with comorbidities such as human immunodeficiency virus (HIV) and diabetes [4].
In low TB-burden settings, such as the United States, HIV has been estimated to increase the risk of death 4-fold among people with TB (risk ratio [RR] = 4.30; 95% confidence interval [CI], 2.31–7.99) [5]. Co-occurrence with diabetes also greatly complicates the management of TB disease. Tuberculosis disease may progress faster and present with more systemic symptoms in people with diabetes. In addition, the immunosuppressive properties of diabetes itself and drug interactions can adversely affect TB treatment outcomes in people with diabetes [6]. A meta-analysis conducted in both high and low TB-burden settings suggests that diabetes is associated with a 1.89-fold increased risk of death among patients with TB (95% CI, 1.52–2.36) [7]. The increase in the prevalence of diabetes mellitus (DM) presents a new challenge for TB control and elimination in the country. In 2020, 4.8% of patients with TB had HIV in the United States [1]. That same year, an estimated 22.5% of patients with TB in the United States had diagnosed diabetes [1].
Previously published epidemiologic studies conducted in a variety of settings have reported associations of diabetes and increased mortality among people with TB, but the reported magnitude of associations are widely heterogeneous [8–29]. Studies conducted in low- and middle-income countries with a high burden of TB, such as Indonesia and Ethiopia, have reported strong associations between diabetes and mortality, with risk ratios ranging from 28.47 to 2.28 [8–18]. However, in high-income countries with a low TB burden, such as the United States, the association between diabetes and mortality may be attenuated [19–29]. Inadequate access to healthcare and poor diabetes management in high-burden settings may contribute to a greater risk of mortality in people with TB and diabetes. However, none of these previous studies assessed the joint impact of exposure to HIV and diabetes on mortality and other TB treatment outcomes. Given current gaps in knowledge, we aimed to (1) estimate the association between hyperglycemia (including diabetes and prediabetes) and all-cause mortality before, during, and after TB treatment and (2) estimate the impact of interaction between diabetes and HIV with all-cause mortality.
METHODS
Study Design and Population
We conducted a retrospective cohort study among all tuberculosis cases reported in the state of Georgia, USA, between January 2015 and December 2020. In the state of Georgia, all clinical and laboratory-confirmed cases of tuberculosis are reported to the Georgia Department of Health. Patients were eligible for this cohort study if they were 16 or older at time of TB diagnosis and if they had pulmonary or extrapulmonary TB diagnosed by a positive culture of sputum or tissue, a positive nucleic acid amplification result, or clinical diagnosis based on symptoms and radiological findings. Participants were excluded from the study if they did not have a diabetes status indicated in their medical record, or if they were missing either blood glucose or hemoglobin A1c laboratory results. In subgroup analyses, participants were followed after TB treatment to determine all-cause mortality rates by cross-referencing patient data with the National Death Index [30].
Study Measures and Data Collection
Study data were abstracted from the State Electronic Notifiable Disease Surveillance System (SendSS), a web-based database used to capture notifiable diseases in Georgia. The SendSS records included patient-level demographic and clinical characteristics, medical history, and treatment outcomes, including death during TB treatment.
The primary study outcome was all-cause mortality before or during TB treatment. Death before TB treatment was defined as death from any cause after TB diagnosis prior to TB treatment initiation. Death during TB treatment was defined as death from any cause before treatment completion or within 7 days of the treatment completion date.
The primary exposures of interest were diabetes status and HIV status. Diabetes status was ascertained through medical and laboratory records in SendSS. Study participants’ diabetes status was defined as either (1) no diabetes, (2) prediabetes, or (3) diabetes. Participants were classified as no diabetes if they did not have a diagnosis of DM (type 1 or 2) in their medical record and did not have a nonfasting blood glucose result greater than 140 mg/dL or a hemoglobin A1c result greater than 5.7%. If study participants did not have a diagnosis of diabetes mellitus (type 1 or 2) in their medical record but had at least 1 nonfasting blood glucose result between 140 and 199 mg/dL or a hemoglobin A1c result between 5.7% and 6.5%, their diabetes status was prediabetes. Study participants’ diabetes status was defined as diabetes if they had a diagnosis of diabetes mellitus (type 1 or 2) in their medical record or they had at least 1 nonfasting blood glucose result greater than 200 mg/dL or 1 hemoglobin A1c result greater than 6.5%. Human immunodeficiency virus status was ascertained through medical records in SendSS. If a study participant had a missing HIV result, they were classified as HIV unknown.
Other variables of interest in this study included demographic and social information, participant comorbidities, and clinical characteristics. Demographic information, such as age, sex, race, and ethnicity, was abstracted from the Report of Verified Case of Tuberculosis (RVCT) form in SendSS. The RVCT form is completed by interview by healthcare staff when a person is diagnosed with TB. Social information, such as recent homelessness, incarceration, and alcohol and other substance use, was also obtained from the RVCT form in SendSS. Information on participant comorbidities, such as end-stage renal disease (ESRD), was abstracted from medical records in SendSS. Finally, information on TB clinical features, such as baseline culture, site of TB disease, tuberculin skin test (TST) status, and drug-susceptibility profile, was obtained from the RVCT form in SendSS.
Data Analyses
Bivariate analyses were conducted to compare unadjusted relationships between participant characteristics with diabetes status (primary exposure) and mortality (primary outcome). To calculate P values, we used the χ2 test for categorical variables and the Wilcoxon rank-sum test for nonnormally distributed continuous variables. Robust Poisson regression models were used to estimate the RRs and 95% CIs for the relationship between diabetes and death before or during TB treatment [31]. Covariates known to be associated with the outcomes and exposures based on review of literature and directed acyclic graph theory were included in the adjusted robust Poisson regression models [32]. Biological interaction between HIV and diabetes and HIV and hyperglycemia was assessed to determine whether both risk factors worked synergistically to increase all-cause mortality among people with TB. We evaluated biological interaction using 3 measures: (1) relative excess risk due to interaction (RERI), (2) attributable proportion due to interaction (AP), and (3) synergy index (S). If the 95% CI of the RERI and AP included 0.0 and the 95% CI of the S included 1.0, we concluded there was no significant biological interaction [33]. Statistical interaction on the multiplicative scale was evaluated by including cross-product terms within multivariable models and calculating a likelihood ratio test statistic. Statistical analyses were performed using R software, version 1.3.1093 (R Core Team, Vienna, Austria).
Subgroup Analyses
To evaluate the relationship between diabetes and all-cause mortality after TB treatment, a competing risk analysis was conducted on a subgroup of the study population. Eligible participants for the subgroup analysis included those with a verified diagnosis of TB in the state of Georgia between 2015 and 2019, were 18 years or older, did not have a previous diagnosis of TB, and had a known diabetes status (Supplemental FigureA). Study participants were followed during TB treatment until death, loss to follow up, or December 2019, whichever event occurred first. Participants lost to follow up were censored at the time of their last documented clinical visit date. Death after TB treatment was defined as death from any cause 7 or more days after the treatment completion date. Data on date of death for eligible participants were obtained from the National Death Index. Survival analyses and the Cox proportional hazards model, which included subdistribution hazard and cause-specific hazard models, were used to estimate competing risk hazard ratios and 95% confidence intervals for the relationship between diabetes and all-cause mortality separately for (1) death before or during treatment and (2) death after treatment.
Additional subgroup analyses were performed to compare the demographic characteristics of participants without diabetes status information to those with a known diabetes status. Bivariate analyses were conducted to compare unadjusted relationships between characteristics with diabetes status and TB outcomes. To calculate P values comparing values for participants without diabetes to those with an unknown diabetes status, the χ2 test for categorical variables and the Wilcoxon rank-sum test for nonnormally distributed continuous variables were used.
RESULTS
Study Population and Baseline Characteristics
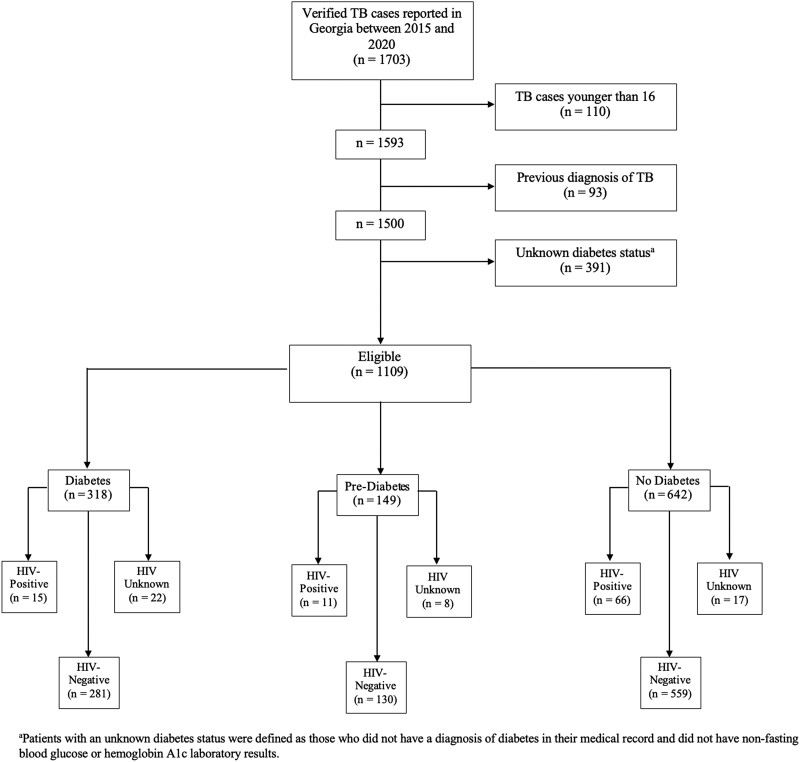
A total of 1703 verified TB cases were reported in SendSS between 2015 and 2020, 1109 (65.1%) of whom were 16 years or older, did not have a previous diagnosis of TB, and had a known diabetes status (Figure 1). Among the 1109 participants included in the analysis, 318 (28.7%) had diabetes, 149 (13.4%) were classified as having prediabetes, and 642 (57.9%) did not have diabetes (Table 1). A total of 391 participants had an unknown diabetes status and were excluded from the analysis (Supplemental Table A). Of the 1109 participants analyzed, 92 (8.3%) were HIV positive, 970 (87.5%) were HIV negative, and 47 (4.2%) had an unknown HIV status. A total of 15 (1.4%) participants had both HIV and diabetes, and 11 (1.0%) had HIV and prediabetes (Table 1). Most people with TB were male (66.6%), non-Hispanic Black (45.3%), and non-US-born (51.6%), with a median age of 48.0 years (interquartile range = 28.0) (Table 1).
Figure 1.
Flow diagram of adult patients with tuberculosis (TB) in the state of Georgia, 2015–2020 included in study analyses.
Table 1.
Patient and Clinical Characteristics of Adults With Tuberculosis in the State of Georgia, 2015–2020 by Diabetes Mellitus Status
| Patient Characteristics | Total (n = 1109) N (%) |
Diabetes (n = 318) N (%) |
Prediabetes (n = 149) N (%) |
No Diabetes (n = 642) N (%) |
P Valuea,c | P Valueb,c |
|---|---|---|---|---|---|---|
| Age (Years) | ||||||
| Median (IQR) | 48.0 (28.0) | 58.0 (21.0) | 50.0 (24.0) | 41.0 (27.0) | <.01 | <.01 |
| Sex | ||||||
| Male | 739 (66.6) | 216 (67.9) | 99 (66.4) | 424 (66.0) | .56 | .75 |
| Race/Ethnicity | ||||||
| NH Asian | 244 (22.0) | 108 (34.0) | 28 (18.8) | 108 (16.8) | <.01 | <.01 |
| NH Black | 502 (45.3) | 106 (33.3) | 63 (42.3) | 333 (51.9) | … | … |
| NH White | 145 (13.1) | 36 (11.3) | 25 (16.8) | 84 (13.1) | … | … |
| Hispanic | 202 (18.2) | 65 (20.4) | 28 (18.8) | 109 (17.0) | … | … |
| Otherd | 16 (1.4) | 3 (1.0) | 5 (3.3) | 8 (1.2) | … | … |
| Occupation | ||||||
| Employed | 463 (44.0) | 110 (36.2) | 70 (49.3) | 283 (46.8) | <.01 | .03 |
| Unemployed | 441 (42.0) | 125 (41.1) | 48 (33.8) | 268 (44.3) | … | … |
| Retired | 147 (14.0) | 69 (22.7) | 24 (16.9) | 54 (8.9) | … | … |
| Foreign Born | ||||||
| Yes | 571 (51.6) | 181 (57.1) | 74 (50.0) | 316 (49.4) | .02 | .13 |
| Recent Homelessness | ||||||
| Yes | 76 (6.9) | 15 (4.8) | 7 (4.8) | 54 (8.5) | .04 | .98 |
| Heavy Alcohol Use | ||||||
| Yes | 162 (15.3) | 43 (14.2) | 30 (20.8) | 89 (14.6) | .88 | .08 |
| ESRD | ||||||
| Yes | 23 (2.1) | 14 (4.4) | 1 (0.7) | 8 (1.3) | <.01 | .03 |
| HIV Status | ||||||
| Positive | 92 (8.7) | 15 (5.1) | 11 (7.8) | 66 (10.6) | <.01 | .26 |
| Clinical characteristics | ||||||
| AFB Smear Status | ||||||
| Positive | 450 (44.6) | 152 (52.8) | 60 (46.2) | 238 (40.2) | <.01 | .21 |
| Culture-Confirmed | ||||||
| Yes | 858 (80.3) | 267 (87.3) | 109 (77.9) | 482 (77.4) | <.01 | .01 |
| Site of TB Disease | ||||||
| PTB only | 790 (71.4) | 243 (76.4) | 113 (76.3) | 434 (67.7) | .02 | .88 |
| PTB and EPTB | 106 (9.5) | 25 (7.9) | 10 (6.8) | 71 (11.1) | … | … |
| EPTB only | 211 (19.1) | 50 (15.7) | 25 (16.9) | 136 (21.2) | … | … |
| TST Status | ||||||
| Positive | 262 (71.2) | 74 (69.2) | 30 (63.8) | 158 (73.8) | .38 | .52 |
| Miliary TB | ||||||
| Yes | 52 (7.3) | 19 (8.4) | 5 (5.1) | 28 (7.2) | .59 | .30 |
Abbreviations: AFB, acid-fast bacilli; EPTB, extrapulmonary TB; ESRD, end-stage renal disease; HIV, human immunodeficiency virus; IQR, interquartile range; NH, non-Hispanic; PTB, pulmonary tuberculosis; TB, tuberculosis; TST, tuberculin skin test.
P value comparing values for patients with diabetes versus patients with no diabetes.
P value comparing values for patients with prediabetes versus patients with no diabetes.
Calculated using the χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
Other race/ethnicity includes American Indian/Alaskan Native (NH), multiracial (NH), and unknown.
Compared to participants without diabetes, participants with diabetes were more likely to be non-US-born (57.1% vs 49.4%, P = .02) and have ESRD (4.4% vs 1.3%, P < .01); they were less likely to experience recent homelessness (4.8% vs 8.5%, P = .04) and be HIV positive (5.1% vs 10.6%, P < .01). Participants with diabetes were more likely to have a positive acid-fast bacilli (AFB) smear (52.8% vs 40.2%, P < .01), culture-confirmed TB (87.3% vs 77.4%, P < .01), and have pulmonary tuberculosis only (76.4% vs 67.7%, P = .02) compared to those without diabetes. Compared to participants without diabetes, participants with prediabetes were less likely to have ESRD (0.7% vs 1.3%, P = .03). Participants with prediabetes were more likely to have culture-confirmed TB (77.9% vs 77.4%, P = .01) compared to participants without diabetes (Table 1).
All-Cause Mortality Before or During Tuberculosis Treatment
Of the 1109 participants with TB, 109 (9.8%) died before or during TB treatment, including 56 (17.6%) of those with diabetes, 17 (11.4%) with prediabetes, and 36 (5.6%) with no diabetes (P = .04) (Table 2). In unadjusted analyses, diabetes (crude risk ratio [cRR] = 3.14; 95% CI, 2.11–4.67) and prediabetes (cRR = 2.03; 95% CI, 1.18–3.52) were associated with an increased risk of death among people with TB (Table 2). After adjusting for covariates, diabetes status was associated with an increased risk of death among people with TB (aRR = 2.60; 95% CI, 1.61–4.18); prediabetes was not significantly associated with an increased risk of death (aRR = 1.89; 95% CI, .99–3.61) (Table 2). Other factors associated with all-cause mortality included older age, occupation, heavy alcohol use, ESRD, positive AFB smear status, culture-confirmed TB, and miliary TB. Being born outside of the United States and having a positive TST at baseline were associated with a decreased risk of death (Supplemental TableB).
Table 2.
Associations Between Diabetes Mellitus and Mortality Before or During Tuberculosis Treatment, Stratified by HIV Status
| HIV Status | Diabetes Status | Mortality Risk N/T (%) |
Risk Ratio (95% CI) |
Adjusted Risk Ratioa,b (95% CI) |
|---|---|---|---|---|
| Total cohort (n = 1109) |
Diabetes | 56/318 (17.6) | 3.14 (2.11–4.67) | 2.60 (1.61–4.18) |
| Prediabetes | 17/149 (11.4) | 2.03 (1.18–3.52) | 1.89 (.99–3.61) | |
| No diabetes | 36/642 (5.6) | REF | REF | |
| HIV positive (n = 92) |
Diabetes | 4/15 (26.7) | 2.93 (.94–9.12) | 3.03 (.86–10.63) |
| Prediabetes | 1/11 (9.1) | 1.00 (.13–7.53) | 0.63 (.07–5.42) | |
| No diabetes | 6/66 (9.1) | REF | REF | |
| HIV negative (n = 970) |
Diabetes | 45/281 (16.0) | 3.44 (2.17–5.46) | 2.81 (1.60–4.96) |
| Prediabetes | 12/130 (9.2) | 1.98 (1.03–3.83) | 1.97 (.91–4.24) | |
| No diabetes | 26/559 (4.7) | REF | REF | |
| HIV unknown (n = 47) |
Diabetes | 7/22 (31.8) | 1.35 (.47–3.88) | 1.25 (.58–2.71) |
| Prediabetes | 4/8 (50.0) | 2.13 (.71–6.40) | 1.69 (.62–4.62) | |
| No diabetes | 4/17 (23.5) | REF | REF |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; N/T, number experiencing mortality/total; REF, reference.
Bold indicates statistically significant.
Adjusted for age, sex, alcohol use, foreign born, race/ethnicity, occupation, and baseline culture.
Multiplicative effect of diabetes status on all-cause mortality risk is nonsignificantly different across HIV status (P = .54).
Biologic and Statistical Interaction Between Diabetes and Human Immunodeficiency Virus With Mortality
In analyses to assess biologic interaction between diabetes and HIV among participants with TB, we found the risk of all-cause mortality was highest among participants with HIV and diabetes (4 of 15, 26.7%) and participants without HIV but with diabetes (52 of 303, 17.2%). Participants with HIV only (7 of 77, 9.1%) and participants with neither diabetes nor HIV (46 of 714, 6.4%) had the lowest risk of all-cause mortality (Table 3). We observed a trend toward synergism between HIV and diabetes (RERI = 1.06, 95% CI = −2.62 to 4.74; AP = 25.70%, 95% CI = −43.40% to 94.90%; S = 1.51, 95% CI = .43–5.35), yet results were not statistically significant (Table 3). In evaluating biologic interaction between hyperglycemia and HIV among people with TB, the risk of all-cause mortality was highest among participants with HIV and hyperglycemia (5 of 26, 19.2%) and participants without HIV but with hyperglycemia (68 of 441, 15.4%). Participants with HIV only (6 of 66, 9.1%) and neither hyperglycemia nor HIV (30 of 576, 5.2%) had the lowest risk of mortality (Table 3). We did not find evidence of biologic interaction between HIV and hyperglycemia in people with TB (RERI = −0.01, 95% CI = −3.30 to 3.28; AP = −0.40%, 95% CI = −89.70% to 88.90%; S = 0.99, 95% CI = .29–3.37) (Table 3).
Table 3.
Biologic Interaction Between Diabetes and HIV With Mortality Risk Among Adults With Tuberculosis in the State of Georgia, 2015–2020
| Diabetes/Hyperglycemia and HIV Status | Mortality Risk N/T (%) |
Risk Ratio (95% CI) |
|---|---|---|
| Diabetesa | ||
| Diabetes/HIV positive | 4/15 (26.7) | 4.14 (1.71–10.02) |
| Diabetes/HIV negativeb | 52/303 (17.2) | 2.66 (1.83–3.87) |
| No diabetes/HIV positive | 7/77 (9.1) | 1.41 (.66–3.02) |
| No diabetes/HIV negativeb | 46/714 (6.4) | REF |
| RERI 1.06 (95% CI, −2.62 to 4.74); AP 25.70% (95% CI, −43.40% to 94.90%); S 1.51 (95% CI, .43–5.35) | ||
| Hyperglycemiac | ||
| Hyperglycemia/HIV positive | 5/26 (19.2) | 3.69 (1.56–8.74) |
| Hyperglycemia/HIV negativeb | 68/441 (15.4) | 2.96 (1.96–4.47) |
| No hyperglycemia/HIV positive | 6/66 (9.1) | 1.75 (.75–4.04) |
| No hyperglycemia/HIV negativeb | 30/576 (5.2) | REF |
| RERI −.01 (95% CI, −3.30 to 3.28); AP −.40% (95% CI, −89.70% to 88.90%); S .99 (95% CI, .29 to 3.37) | ||
Abbreviations: AP, attributable proportion due to interaction; CI, confidence interval; HIV, human immunodeficiency virus; N/T, number experiencing mortality/total; REF, reference; RERI, relative excess risk due to interaction; S, synergy index.
Bold indicates statistically significant.
Patients for this analysis were classified as having diabetes or not having diabetes (prediabetes and no diabetes).
Patients with an unknown HIV status were classified as HIV negative for this analysis.
Patients for this analysis were classified as having hyperglycemia (diabetes and prediabetes) or not having hyperglycemia (no diabetes).
We also assessed interaction between diabetes and HIV with mortality on the multiplicative scale. Among participants with TB who did not have HIV, diabetes (cRR = 3.44; 95% CI, 2.17– 5.46) and prediabetes (cRR = 1.98; 95% CI, 1.03–3.83) were associated with an increased risk of death before or during TB treatment (Table 2). After adjusting for age, sex, alcohol use, non-US-born status, race/ethnicity, occupation, and culture-confirmed TB, the risk of death among participants without HIV but with diabetes (aRR = 2.81; 95% CI, 1.60–4.96) and prediabetes (aRR = 1.97; 95% CI, .91–4.24) decreased slightly (Table 2). In unadjusted analyses among participants with HIV, diabetes and prediabetes were not significantly associated with an increased risk of mortality (cRR = 2.93, 95% CI = .94–9.12; cRR = 1.00, 95% CI = .13–7.53) (Table 2). The multiplicative effect of diabetes status on all-cause mortality risk was nonsignificantly different across HIV status (P = .54). After adjusting for confounders, we found that the risk ratio of all-cause mortality comparing those with diabetes to those without diabetes was 3.03 (95% CI, .86–10.63) among participants with HIV and 2.81 (95% CI, 1.60–4.96) among participants without HIV (Table 2).
Subgroup Analyses: All-Cause Morality After Tuberculosis Treatment
We conducted a subgroup analysis to determine the association between diabetes and all-cause mortality after TB treatment completion. Of the 927 participants included in the subgroup analysis, 267 (28.8%) had diabetes, 113 (12.2%) had prediabetes, and 547 (59.0%) did not have diabetes (Supplemental FigureA). A total of 78 (8.4%) participants died before or during TB treatment, and 19 (2.0%) died after treatment. Of the 267 participants with diabetes in this cohort, 42 (15.7%) died before or during treatment, and 12 (4.5%) died after treatment. Among the 113 participants with prediabetes, 7 (6.2%) died before or during treatment, and 1 (0.9%) died after treatment. Of the remaining 547 participants without diabetes, 29 (5.3%) died before or during TB treatment, and 6 (1.1%) died after treatment.
In competing risk models, diabetes was not significantly associated with increased all-cause mortality after TB treatment (Table 4). In a multivariable subdistribution model, the hazard rate of death after TB treatment among those with diabetes was 1.85 (95% CI, .70–4.86) times the rate in participants without diabetes (analogous cause-specific model adjusted hazard ratio [aHR] = 2.69; 95% CI, .71–10.16). Diabetes remained significantly associated with all-cause mortality before or during TB treatment in competing risk models. After adjusting for covariates, the relative incidence of morality before and during treatment among participants with diabetes was more than 2 times the mortality rate in those without diabetes (subdistribution aHR = 2.33, 95% CI = 1.34–4.04; cause-specific aHR = 2.42, 95% CI = 1.43–4.07) (Table 4).
Table 4.
Competing Risk Hazard Rates of Mortality Before/During and After Treatment Among Adults With Tuberculosis in the State of Georgia, 2015–2019
| Subdistribution Hazard Model | Cause-Specific Hazard Model | ||||
|---|---|---|---|---|---|
| Before/During Treatment | After Treatment | Before/During Treatment | After Treatment | ||
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | ||
| Total cohorta (n = 927) |
Diabetes | 3.06 (1.91–4.91) | 1.59 (.58–4.37) | 3.09 (1.92–4.95) | 1.44 (.48–4.28) |
| Prediabetes | 1.17 (.52–2.67) | 1.07 (.16–7.28) | 1.18 (.52–2.68) | 1.25 (.14–10.93) | |
| No diabetes | REF | REF | REF | REF | |
| Total cohortb (n = 927) |
Diabetes | 2.33 (1.34–4.04) | 1.85 (.70–4.86) | 2.42 (1.43–4.07) | 2.69 (.71–10.16) |
| Prediabetes | 1.13 (.47–2.76) | 1.18 (.22–6.33) | 1.16 (.50–2.70) | 1.38 (.14–13.91) | |
| No diabetes | REF | REF | REF | REF | |
Abbreviations: CI, confidence interval; REF, reference.
Bold indicates statistically significant.
Unadjusted.
Adjusted for age, sex, alcohol use, foreign born, race/ethnicity, occupation, and baseline culture.
Subgroup Analyses: Patients With Unknown Diabetes Status
In comparing the distribution of participants without diabetes status information to those with a known diabetes status, we found that patient and clinical characteristics of participants without diabetes status information were similar to participants known to be without diabetes. There were no statistical differences in age (P = .17), sex (P = .23), occupation (P = .28), HIV status (P = .26), AFB smear status (P = .50), and culture-confirmed TB (P = .62) among participants without diabetes status information and those without diabetes (Supplemental Table A).
DISCUSSION
Overall, we found that more than one quarter of all people with TB in the state of Georgia during 2015–2020 had co-occurring diabetes. The co-occurrence of diabetes among people with TB (28.7%) was higher than that of coinfection with HIV (8.3%). Few participants in this cohort were both HIV positive and had diabetes (1.4%). Importantly, we reported that approximately 1 in 5 people with TB and diabetes died during TB treatment. For example, diabetes was associated with over a 3-fold relative increased risk of death before or during TB treatment. Among participants without HIV, both diabetes and prediabetes (hyperglycemia) were associated with a 3-fold and 2-fold relative risk of increased death before or during TB treatment, respectively. Although not statistically significant, we also estimated that approximately one quarter of the observed mortality in participants with both diabetes and HIV was due to synergism between the 2 risk factors. Finally, we found that when accounting for competing risks, diabetes increased both the relative incidence and cause-specific hazard of mortality before or during treatment for people with TB but not after TB treatment. Diabetes co-occurrence is common among people with TB in Georgia, and mortality among these individuals is high. These results highlight the need for regular screening of people with TB for diabetes and HIV and careful monitoring of individuals with comorbidities.
Several mechanisms have been proposed to explain why people with diabetes and TB comorbidity are at a higher risk of death compared to people with TB without diabetes. Diabetes can impair host defense against pathogens by suppressing cytokine production, inhibiting phagocytosis, and causing the dysfunction of immune cells, such as neutrophils [34]. Once infected with Mycobacterium tuberculosis, a lower proportion of activated macrophages and a lower proportion of proinflammatory cytokines may influence the frequency of adverse outcomes among people with diabetes and TB [35]. Our findings are consistent with those of other studies examining the association between diabetes and mortality among people with TB. For example, a systematic review of observational studies conducted in both high- and low-burden settings found that diabetes is associated with an approximately 2-fold increased risk of death among people with TB (RR = 1.89; 95% CI, 1.52–2.36) [7]. Three other retrospective studies conducted in the United States found that diabetes was associated with an increased risk of death. A retrospective cohort study from 1993 to 1998 by Fielder et al [17] conducted among 174 participants with TB from Baltimore found that after adjusting for age, the odds of death among people with diabetes were 3.8 times those of people without diabetes (odds ratio [OR] = 3.8; 95% CI, 1.4–10.3). A retrospective cohort study of 139 Baltimore adults with TB diagnosed between 1994 and 1996 by Oursler et al [15] in 2002 found that after adjusting for confounders, the hazard of death among people with TB and DM was 6.7 times that of people with TB and without DM (95% CI, 1.6–29.3). Finally, a retrospective cohort study conducted by Dooley et al [36] among 297 people with TB in Maryland diagnosed between 2004 and 2005 found that the odds of death were 6.5 times greater in people with DM than people without, after adjusting for confounders such as HIV status, age, and weight (95% CI, 1.1–38.0).
We did find evidence of a trend toward synergism between HIV and diabetes in participants with TB in our cohort. Among participants with diabetes and HIV, we estimated 25% of the deaths that occurred during TB treatment resulted from HIV-diabetes interaction. This finding suggests that HIV and diabetes interact together biologically, resulting in an exacerbation of mortality risk during TB treatment. The finding from the current study is not consistent with the results of 2 other studies conducted in African countries. Faurholt-Jepsen et al [37] found that diabetes was associated with TB in people without HIV (adjusted OR [aOR] = 4.2; 95% CI, 1.5–11.6), and not people with HIV (aOR = 0.1; 95% CI, .01–1.8), after adjusting for important confounders. In addition, a 2014 study from Botswana found that there was no difference in severe TB disease among people with HIV and diabetes compared to people without HIV but with diabetes [38]. However, neither of these studies assessed the interaction between HIV and diabetes with the outcome of mortality.
Our study is subject to several limitations that are important to note. First, we only considered a limited number of laboratory values to classify participants by diabetes status; therefore, our primary exposure was subject to misclassification. However, unlike many previous studies estimating the association between diabetes and TB outcome, we had at least 1 laboratory result (glucose or hemoglobin A1c) for most study participants. Laboratory results of glucose or hemoglobin A1c are typically a more reliable measure of diabetes status than self-report or medical record abstraction alone. Second, because data on our exposure was limited to reported laboratory values, we were unable to distinguish between stress hyperglycemia and type I or type II diabetes. We also did not have access to data on the duration of diabetes disease or use of diabetes medications, and, therefore, we were unable to estimate the effect of chronic controlled versus uncontrolled hyperglycemia. Third, whereas the information on our secondary exposure of interest, HIV infection, was partially complete within the state surveillance system, we did not incorporate viral load and HIV treatment adherence into our analyses. As a result, outcomes among participants with HIV in our cohort may be vastly different depending on CD4 count, viral load, and whether or not they are adhering to treatment for HIV infection. A study conducted by Schechter et al [39] found that among patients diagnosed with TB at a hospital in Atlanta, Georgia, USA between 2008 and 2015, 81% of patients with HIV were receiving antiretroviral therapy during TB treatment and 52% had suppressed viral load at end of treatment. Fourth, regarding our primary outcome, mortality, we were unable to determine whether a participant's cause of death was due to TB disease or an unrelated cause. Last, it is important to note that our study was conducted in a low TB-burden setting. As a result, findings from this study may not be generalizable to high-burden settings. Nonetheless, our study population was diverse, and the data are widely generalizable to the entire state of Georgia and similar settings in the United States.
CONCLUSIONS
We found that diabetes co-occurrence is common among people with TB in Georgia, with prevalence estimates exceeding those of TB/HIV coinfection. This study found that diabetes was associated with an increased risk of all-cause mortality among people with TB, supporting the findings from previous research. We also found that there was a trend toward synergism between HIV and diabetes in people with TB. Finally, when accounting for competing risks, diabetes increased both the relative incidence and cause-specific hazard of mortality before or during treatment but not after TB treatment. Additional research is needed to understand the interactions between hyperglycemia and HIV and evaluate the cause of death in people with these co-occurring conditions. Effective clinical interventions should be identified to reduce mortality within this high-risk group. Overall, our results highlight the need for regular screening of patients with TB for DM and careful management of coprevalent individuals.
Supplementary Material
Acknowledgments
We thank Angie Campbell (Emory University), Dr. Susan Ray (Grady Memorial Hospital), the Emory-Einstein TB Research Group, and the Georgia Department of Public Health Tuberculosis Program.
Author contributions. KH, JAC, MCS, and MJM participated in the study design. KH, TC, and SG collected the data and performed the data analyses. All authors assisted with data interpretation, provided critical revision of the article, and reviewed and approved the final manuscript.
Disclaimer. The sponsors had no role in (1) the design and conduct of the study, (2) collection, management, analysis, and interpretation of the data, and (3) preparation, review, or approval of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (Grant Numbers R01AI153152 [to MJM] and R21AI156161 [to MJM and JAC]) and the Preventive Treatment of Latent Tuberculosis Infection in People with Diabetes Mellitus (PROTID) study, which is part of the EDCTP2 program supported by the European Union (Grant Number RIA2018CO-2514-PROTID; to JAC). This study was also supported in part by the National Institutes of Health grant P30AI168386.
Contributor Information
Kennedy Houck, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Tsira Chakhaia, Department of Population Health Sciences, Georgia State University, Atlanta, Georgia, USA.
Sarah Gorvetzian, Division of Infectious Diseases, Department of Medicine, Emory School of Medicine, Emory University, Atlanta, Georgia, USA.
Julia A Critchley, Population Health Research Institute, St. George's, University of London, London, United Kingdom.
Marcos C Schechter, Division of Infectious Diseases, Department of Medicine, Emory School of Medicine, Emory University, Atlanta, Georgia, USA; Grady Memorial Hospital, Atlanta, Georgia, USA; Georgia Department of Public Health Tuberculosis Program, Atlanta, Georgia, USA.
Matthew J Magee, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA; Division of Infectious Diseases, Department of Medicine, Emory School of Medicine, Emory University, Atlanta, Georgia, USA; Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Centers for Disease Control and Prevention . TB in the United States, 2021. Available at: https://www.cdc.gov/nchhstp/newsroom/fact-sheets/tb/TB-in-the-US.html. Accessed 8 January 2023.
- 2. Centers for Disease Control and Prevention . Reported TB in the US, 2021 Surveillance Report. Available at: https://www.cdc.gov/tb/statistics/reports/2021/default.htm. Accessed 8 January 2023.
- 3. Centers for Disease Control and Prevention . Reported Tuberculosis in the United States, 2019. Available at: https://www.cdc.gov/tb/statistics/reports/2019/default.htm. Accessed 24 February 2022.
- 4. World Health Organization . Addressing TB comorbidities and health-related risk factors. Available at: https://www.who.int/activities/addressing-tb-comorbidities-and-health-related-risk-factors. Accessed 30 January 2023.
- 5. Karo B, Krause G, Hollo V, et al. Impact of HIV infection on treatment outcome of tuberculosis in Europe. AIDS 2016; 30:1089–98. [DOI] [PubMed] [Google Scholar]
- 6. Lin Y, Harries AD, Kumar AMV, et al. Tackling diabetes mellitus and tuberculosis: a new union guide on the management of diabetes-tuberculosis. Int Tuberc Lung Dis 2019; 23:771–2. [DOI] [PubMed] [Google Scholar]
- 7. Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alisjahbana B, Sahiratmadja E, Nelwan EJ, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 2007; 45:428–35. [DOI] [PubMed] [Google Scholar]
- 9. Maalej S, Belhaoui N, Bourguiba M, et al. Pulmonary tuberculosis and diabetes. A retrospective study of 60 patients in Tunisia. Presse Med 2009; 38:20–24. [DOI] [PubMed] [Google Scholar]
- 10. Dooley KE, Chaisson RE: Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009; 9:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faurholt-Jepsen D, Range N, PrayGod G, et al. Diabetes is a strong predictor of mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop Med Int Health 2013; 18:822–9. [DOI] [PubMed] [Google Scholar]
- 12. Tatar D, Senol G, Alptekin S, Karakurum C, Aydin M, Coskunol I. Tuberculosis in diabetics: features in an endemic area. Jpn J Infect Dis 2009; 62:423–7. [PubMed] [Google Scholar]
- 13. Workneh MH, Bjune GA, Yimer SA. Diabetes mellitus is associated with increased mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients in south-eastern Amhara Region, Ethiopia. Infect Dis Pov 2016; 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hasibi M, Rasoulinejad M, Hosseini SM, Davari P, Sahebian A, Khashayar P. Epidemiological, clinical, laboratory findings, and outcomes of disseminated tuberculosis in Tehran, Iran. South Med J 2008; 101:910–3. [DOI] [PubMed] [Google Scholar]
- 15. Oursler KK, Moore RD, Bishai WR, Harrington SM, Pope DS, Chaisson RE. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis 2002; 34:752–9. [DOI] [PubMed] [Google Scholar]
- 16. Mboussa J, Monabeka H, Kombo M, Yokolo D, Yoka-Mbio A, Yala F: Course of pulmonary tuberculosis in diabetics. Rev Pneumol Clin 2003; 59:39–44. [PubMed] [Google Scholar]
- 17. Fielder JF, Chaulk CP, Dalvi M, Gachuhi R, Comstock GW, Sterling TR: A high tuberculosis case fatality rate in a setting of effective tuberculosis control: implications for acceptable treatment success rates. Int J Tuberc Lung Dis 2002; 6:1114–7. [PubMed] [Google Scholar]
- 18. Wang CS, Yang CJ, Chen HC, et al. Impact of type 2 diabetes on manifestations and treatment outcomes of pulmonary tuberculosis. Epidemiol Infect 2009; 137:203–10. [DOI] [PubMed] [Google Scholar]
- 19. Centis R, Migliori GB; Tuberculosis Study Group. National A.I.P.O. (Italian Association of Hospital Pneumologists); SMIRA Group (Multicentre Italian Study on Drug Resistance); National Tuberculosis Project, Istituto Superiore di Sanita : Evaluation of tuberculosis treatment results in Italy, report 1999. Monaldi Arch Chest Dis 2002; 57:297–305. [PubMed] [Google Scholar]
- 20. Pina JM, Dominguez A, Alcaide J, et al. Excess mortality due to tuberculosis and factors associated to death in and annual cohort of patients diagnosed of tuberculosis. Rev Clin Esp 2006; 206:560–5. [DOI] [PubMed] [Google Scholar]
- 21. Vasankari T, Holmstrom P, Ollgren J, Liippo K, Kokki M, Ruutu P: Risk factors for poor tuberculosis treatment outcome in Finland: a cohort study. BMC Public Health 2007; 7:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiang CY, Lee JJ, Yu MC, Enarson DA, Lin TP, Luh KT: Tuberculosis outcomes in Taipei: factors associated with treatment interruption for 2 months and death. Int J Tuberc Lung Dis 2009; 13:105–11. [PubMed] [Google Scholar]
- 23. Kitahara Y, Ikeda A, Kajiki A, et al. An investigation on risk factors relating to the treatment difficulty in originally treated pulmonary tuberculosis cases. Kekkaku 1994; 69:503–11. [PubMed] [Google Scholar]
- 24. Ponce-De-Leon A, Garcia-Garcia L, Garcia-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care 2004; 27:1584–90. [DOI] [PubMed] [Google Scholar]
- 25. Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MK Jr. Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis 2006; 10:1224–30. [PubMed] [Google Scholar]
- 26. Magee MJ, Foote M, Maggio DM, et al. Diabetes mellitus and risk of all-cause mortality among patients with tuberculosis in the state of Georgia, 2009–2012. Ann Epidemiol 2014; 24:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis 2006; 10:74–9. [PubMed] [Google Scholar]
- 28. Baltas I, Sturdy A, Kavallieros K, et al. Diabetes mellitus is not a predictor of poor TB treatment outcomes. Int J Tuberc Lung Dis 2023; 27:140–5. [DOI] [PubMed] [Google Scholar]
- 29. Ambrosetti M, Besozzi G, Codecasa LR, et al. The Italian AIPO study on tuberculosis treatment results, report 1997. National AIPO “Tuberculosis” study group. Monaldi Arch Chest Dis 1999; 54:407–12. [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention . National Death Index. Available at: https://www.cdc.gov/nchs/ndi/index.htm. Accessed 30 January 2023.
- 31. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005; 163:199–200. [DOI] [PubMed] [Google Scholar]
- 32. Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008; 8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005; 20:575–9. [DOI] [PubMed] [Google Scholar]
- 34. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R: Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev 2020; 16:442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Restrepo BI. Diabetes and tuberculosis. Microbiol Spectr 2016; 4(6):10.1128/microbiolspec.TNMI7-0023-2016. doi: 10.1128/microbiolspec.TNMI7-0023-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W: Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 2009; 80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 37. Faurholt-Jepsen D, Range N, Praygod G, et al. Diabetes is a risk factor for pulmonary tuberculosis: a case-control study from Mwanza, Tanzania. PLoS One 2011; 6:e24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reid MJA, Oyewko A, Molosiwa B, McFadden N, Tsima B, Ho-Foster A. Screening for tuberculosis in a diabetes clinic in Gaborone, Botswana. Int J Tuberc Lung Dis 2014; 18:1004. [DOI] [PubMed] [Google Scholar]
- 39. Schechter MC, Bizune D, Kagei M, et al. Challenges across the HIV care continuum for patients with HIV/TB co-infection in Atlanta, GA. Open Forum Infect Dis 2018; 5:ofy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.