Juvenile delta smelt displayed decreased stress and increased energy and condition factor when exposed to higher turbidities (10–11 nephelometric turbidity units [NTU]) compared with clearer waters (1–2 NTU). Increased temperatures (21 vs 17°C) resulted in reduced energy in fish. Predator cues had negligible effects on delta smelt’s stress response.
Keywords: supplementation, San Francisco Estuary, delta smelt, conservation, climate change
Abstract
The San Francisco Estuary (SFE) is one of the most degraded ecosystems in the United States, and organisms that inhabit it are exposed to a suite of environmental stressors. The delta smelt (Hypomesus transpacificus), a small semi-anadromous fish endemic to the SFE and considered an indicator species, is close to extinction in the wild. The goal of this study was to investigate how environmental alterations to the SFE, such as reductions in turbidities, higher temperatures and increased prevalence of invasive predators affect the physiology and stress response of juvenile delta smelt. Juvenile delta smelt were exposed to two temperatures (17 and 21°C) and two turbidities (1–2 and 10–11 NTU) for 2 weeks. After the first week of exposure, delta smelt were exposed to a largemouth bass (Micropterus salmoides) predator cue at the same time every day for 7 days. Fish were measured and sampled on the first (acute) and final (chronic) day of exposures to predator cues and later analyzed for whole-body cortisol, glucose, lactate, and protein. Length and mass measurements were used to calculate condition factor of fish in each treatment. Turbidity had the greatest effect on juvenile delta smelt and resulted in reduced cortisol, increased glucose and lactate, and greater condition factor. Elevated temperatures reduced available energy in delta smelt, indicated by lower glucose and total protein, whereas predator cue exposure had negligible effects on their stress response. This is the first study to show reduced cortisol in juvenile delta smelt held in turbid conditions and adds to the growing data that suggest this species performs best in moderate temperatures and turbidities. Multistressor experiments are necessary to understand the capacity of delta smelt to respond to the multivariate and dynamic changes in their natural environment, and results from this study should be considered for management-based conservation efforts.
Introduction
Coastal estuaries are among the most degraded ecosystems worldwide with rapid human population growth and increased industrialization and urbanization depleting >90% of species (Lotze et al., 2006). This loss of biodiversity has triggered increased dead zones, disease outbreaks, and introduction of invasive species, reducing ecosystem resilience and recovery potential (Worm et al., 2006). Due to the importance of estuaries and the organisms that inhabit them, large-scale restoration and supplementation strategies are being used to promote conservation (Cloern and Jassby, 2012; Moyle et al., 2018). An understanding of the impacts of environmental change and degradation and how they affect the physiology and fitness of native species is critical for these efforts to be successful.
The San Francisco Estuary (SFE) is one of the largest estuaries in the United States and is comprised of the San Francisco Bay, San Pablo Bay, Suisun Bay, and the Sacramento-San Joaquin Delta (hereafter, Delta) (Service, 2007). The SFE supports >750 species of plants and animals and is considered an international hotspot of biodiversity (Myers et al., 2000; Healey et al., 2016). Starting in the 19th century during the California Gold Rush, the SFE underwent associated habitat modifications, including extensive infrastructure to support increasing water demands (Nichols et al., 1986). The Delta currently supplies water to >20 million California residents, and water diversions consume between 35 and 65% of the annual freshwater outflow, depending on the season (Sommer et al., 2011). As a result of continued population growth and development, increased water diversions have caused significant damage to the SFE and the organisms within it.
The delta smelt (Hypomesus transpacificus) is a small, semi-anadromous fish endemic to the upper SFE (Moyle, 2002; Moyle et al., 2018; Yanagitsuru et al., 2022). Due to their exceptionally sensitive nature and unique life history, delta smelt have become an indicator species for ecosystem health (Moyle et al., 2016, 2019). Most delta smelt live a year or less and have low fecundity (1200–2600 eggs per female) (Moyle et al., 1992; LaCava et al., 2015). Consequently, the species is vulnerable to even a single year of poor recruitment. Once a very abundant fish, delta smelt are now rare and the species is on the brink of extinction (Bennett, 2005; Hobbs et al., 2017; Moyle et al., 2018). Rapid declines in delta smelt population began in the 1980s and resulted in its listing as threatened under the State and Federal Endangered Species Acts in 1993 (U.S. Office of the Federal Register, 1993). The delta smelt population has continued to decrease, with all abundance indices pointing downwards since 2002, coinciding with declines in several other pelagic fishes in the upper estuary (Thomson et al., 2010; Hobbs et al., 2017). This phenomenon is known as the ‘Pelagic Organism Decline’ and much like delta smelt’s decline, is not a consequence of one single variable, but rather the cumulation of multiple anthropogenic factors altering the SFE (Sommer et al., 2007; Brooks et al., 2012).
Alterations to the estuary due to water diversions and climate change include, but are not limited to, habitat loss, reduced turbidity, higher salinity and temperature, and increased predation and competition caused by the introduction of invasive species (Moyle et al., 2016, 2018; Hobbs et al., 2017). These changes have negatively impacted delta smelt abundance, as these fish are commonly associated with the low salinity zone (<6 psu), moderate temperatures (7–25°C), and mid-turbid waters (10–50 NTU) (Swanson et al., 2000; Feyrer et al., 2007; Nobriga et al., 2008; Sommer and Mejia, 2013). Understanding how habitat degradation and change affect the physiology and performance of delta smelt is imperative for setting environmental regulations and offering recommendations for rearing and supplementation of the wild population (Yanagitsuru et al., 2022).
Turbidity is an understudied but critical variable affecting delta smelt life history, physiology, and survival. Turbidity is caused by the scattering and absorption of light by suspended particles and is often referred to as the ‘cloudiness’ of water (Kirk, 1985; Henley et al., 2000). The interaction of light intensity, water depth, and the physical properties of suspended material all contribute to the intensity and effect of turbidity (Davies-Colley and Smith, 2001). Turbidity can have significant effects on ecosystems, such as alterations to species compositions and trophic interactions in estuaries (Lunt and Smee, 2014). It can also have both positive and negative effects in fish, depending on its intensity and the species of fish in question (Utne-Palm, 2002; Hasenbein et al., 2016). Low levels of turbidity are often associated with reduced feeding and growth rates, increased predation, and higher levels of stress in fish (Boehlert and Morgan, 1985; Rieger and Summerfelt, 1997). High turbidity, on the other hand, may also lead to reduced feeding, clogged and damaged gills, diminished visual acuity, and increased entrainment (Gardner, 1981; Hecht and Van der Lingen, 1992; Sutherland and Meyer, 2007; Grimaldo et al., 2009; Hess et al., 2017). Turbidity in the SFE has declined significantly over the last couple of decades with a 36% decrease in suspended sediment concentration between years 1991–1998 and 1999–2007, due in part to sediment trapping in reservoirs and dams, riverbank protection, and depletion of erodible sediment from hydraulic mining (Wright and Schoellhamer, 2004; Schoellhamer, 2011). Decreased turbidity is thought to be one of several factors contributing to the Pelagic Organism Decline and the population collapse of delta smelt (Sommer et al., 2007).
High temperatures and the increased occurrence of heat waves present a significant threat to the future of delta smelt (Moyle et al., 2016). Water temperature projections coupled with thermal sensitivity metrics suggest that temperatures in the SFE will result in both lethal and sublethal effects in delta smelt, causing an overall reduction in its population and a substantial compression of their habitat (Brown et al., 2016; Hung et al., 2022b). In addition, critical life history events, such as the spawning and migration of delta smelt, are known to coincide with the most widespread temperature increases in the SFE (Bashevkin et al., 2022). Habitat change due to climate change and water diversions has made the estuary more desirable to competitors and predators, such as the overbite clam (Potamocorbula amurensis), Asian clams (Corbicula fluminea), and warm-water fishes such as the largemouth bass (Micropterus salmoides) and Mississippi silversides (Menidia beryllina) (Kimmerer, 2006; Moyle et al., 2016; Baumsteiger et al., 2017; Komoroske et al., 2020). These non-native and filter-feeding clams are responsible for significant water clearing, thus likely contributing to reductions in turbidity in the SFE (Carlton et al., 1990; Paganini et al., 2010). Introduction of invasive submersed aquatic vegetation (SAV), such as the Brazilian waterweed (Egeria densa), has slowed water movement, further increasing temperature and reducing turbidity (Durand, 2015). In addition, this SAV has reduced the pelagic habitat preferred by delta smelt and increased their risk of predation by introducing vegetated areas that predators such as the largemouth bass can use to ambush open-water prey (Ferrari et al., 2014; Conrad et al., 2016).
Based on climate change projections, industrial competition for water usage, and knowledge of their physiological vulnerabilities and complex life histories, delta smelt are likely to go extinct in the wild in the next 1–5 years without effective and immediate management intervention (Brown et al., 2013, 2016; Komoroske et al., 2014; Jeffries et al., 2016; Moyle et al., 2018, 2019; Hobbs et al., 2019; Bork et al., 2020). Consequently, current research aimed to conserve the delta smelt has shifted to focus on informing hatchery-based supplementation, which was initiated in winter 2021–2022 (Yanagitsuru et al., 2022). The UC Davis Fish Conservation and Culture Laboratory (FCCL) is currently rearing a genetically managed refuge population for the purpose of both research and safeguarding the species against extinction (Baskerville-Bridges et al., 2005; Fisch et al., 2013; Lindberg et al., 2013). As part of genetic management of the cultured delta smelt population, FCCL has been permitted to collect 100 wild adult delta smelt every year. In the last couple of years, this goal has become increasingly challenging to meet. Only two wild delta smelt were captured during the 2020–2021 season, exemplifying the need for rapid and efficient supplementation. The first experimental release of delta smelt occurred between December 2021 and February 2022 and the second-year release started in November 2022 (USFWS, 2019, 2020). To be successful in these efforts, physiological data from controlled laboratory experiments can help us gain a better understanding of delta smelt’s tolerances to key environmental parameters, such as temperature, turbidity, and predation by non-native species. This in turn can provide crucial information when selecting optimal transportation conditions and release environments to minimize stress and maximize survival in this highly sensitive species (Swanson et al., 1996; Lessard et al., 2018).
The objective of this study was to investigate the individual and combined effects of turbidity, temperature, and predator cues on the physiology and stress response of juvenile delta smelt. We hypothesized that delta smelt would display reduced stress and greater available energy in conditions with higher turbidity, lower temperature, and in the absence of predation cues. We also hypothesized that reduced temperatures and higher turbidities have mitigating effects on stress induced by predator cue exposure. To test these hypotheses, juvenile delta smelt were held at two temperatures (17 and 21°C) and two turbidities (1–2 and 10–11 , NTU) for a week before exposure to a largemouth bass predator cue. Delta smelt were then exposed to a similar largemouth bass predator cue at the same time every day for 7 days to compare the effects of an acute versus a chronic predation stress at different temperatures and turbidities. At the end of both the acute and chronic exposures, fish were measured and sampled for quantification of whole-body cortisol, glucose, lactate, protein, and condition factor. Maintaining homeostasis after stressor exposure is energetically expensive and can result in reduced aerobic scope and reduced energy available to allocate to basic physiological needs, such as metabolism, growth, and reproduction (Pörtner, 2001, 2010; Sokolova et al., 2012). Therefore, the long-term effects of elevated stress on fish can be detrimental to their survival and result in population-level effects. A greater understanding of how environmentally relevant variables affect delta smelt’s stress response and physiology will help identify optimal conditions for rearing, transportation, and supplementation, and in turn, reduce risk of its extinction in the wild.
Methods
Study species and maintenance
Juvenile delta smelt (42.7 ± 0.3 mm fork length, 0.53 ± 0.01 g) were obtained from the University of California Davis FCCL in Byron, CA. Information about the design of the aquaculture facility, captive breeding program, and genetic management plan can be found in Lindberg et al. (2013), Tigan et al. (2020) and Hung et al., (2021). In June 2021, delta smelt (~160 days post-hatch, dph) were transferred from FCCL to the UC Davis Putah Creek Aquaculture Facility (PCF). Fish were immediately introduced into 36, 15-gallon black polyethylene tubs (hereafter, sub tanks), each covered with circular lids made from shade cloth to avoid water contamination, prevent fish escapement, and to manage light intensity. The PCF includes a recirculating aquaculture system with external temperature control units and 12, 400-L tanks (hereafter, holding tanks) that function as experimental water baths. Each holding tank housed three sub tanks, with 30 fish per sub tank (Supplementary Fig. S1).
To optimize holding conditions and maintain turbidities, outdoor reservoir tanks with treatment water provided flow-through water to sub tanks. Excess water in sub tanks overflowed through a 1-inch hole covered in mesh to prevent fish from escaping. In addition, each sub tank contained its own external biofiltration unit, where water in the sub tanks was pushed through the unit filled with k1 biomedia and returned to the sub tanks using an airlift mechanism. The airlift unit also maintained oxygen levels by introducing freshly oxygenated water into sub tanks.
Delta smelt were acclimated to ambient conditions of 17°C and 1.4 NTU for 2 weeks before the start of experimentation (Supplementary Table S1). Fish were fed dry feed [BioVita starter (50% of #0 crumble and 50% #1 crumble)] at 2% of their body weight per day during two separate feedings and exposed to a natural photoperiod (12 L:12 D). Light was provided by indoor fluorescent light bulbs and light intensity was kept at a constant low level (55.74 lx ± 1.0) to match culture protocols (Lindberg et al., 2013). Light intensity was measured using a portable digital light meter (LX1330B; Drmeter) to ensure values were consistent between sub tanks. To monitor water quality, daily temperature (°C), dissolved oxygen (mg/L), and salinity (psu) readings were taken using a handheld YSI 556 MPS meter (YSI Inc., Yellow Springs, OH) and turbidity using a Hach 2100q portable turbidity meter (Hach Company, Loveland, CO). pH was measured every other day using a pinpoint pH monitor (American Marine Inc., Ridgefield, CT). Ammonia, nitrate, and nitrite were measured two to three times a week using a Hach pocket colorimeter (Hach Company, Loveland, CO) for ammonia and a marine care multitest kit (Red Sea, Houston, TX) for ammonia, nitrite, and nitrate. Mortality was quantified daily, and any dead fish were immediately removed from sub tanks to maintain water quality. All handling, care, and experimental procedures used were reviewed and approved by the UC Davis Institutional Animal Care and Use Committee (IACUC Protocol 16 591).
Temperature and turbidity treatments
After the 2-week acclimation period (17°C and 1.4 NTU), delta smelt were exposed to temperatures of 17 or 21°C and turbidities of 1–2 or 10–11 NTU for a week before predator cue introduction. Temperatures and turbidities were gradually increased (1.3°C and 3 NTU per day) from acclimation levels to treatment levels over the course of 3 days. To reach desired turbidities, Nannochloropsis algae (Nanno 3600—High-yield grow-out feed; Reed Mariculture Inc., USA) were spiked into individual sub tanks. This algal suspension is also used by FCCL to increase turbidities when rearing larval delta smelt (Tigan et al., 2020). To maintain treatment turbidity throughout the experimental period, Nannochloropsis algae were added to reservoir tanks connected to individual sub tanks via PFA standard tubing (inner diameter = 0.5 cm) connected to a standpipe located in the middle of each sub tank, allowing for daily introduction of fresh algae-spiked water. Salinity (1.6 ± 0.14 psu) was maintained in a similar manner by adding Instant Ocean (Aquarium Systems, Mentor, OH) to outdoor reservoir tanks so that fresh saline water was added via the same PFA standard tubing. Salinity levels were chosen to reflect conditions delta smelt are commonly associated with in their natural habitat (Bennett, 2005). In addition to daily temperature measurements, 12 HOBO temperature loggers were exchanged between sub tanks every couple of days and recorded temperatures (°C) every 15 min. All acclimation and treatment temperature and turbidity data are presented as mean ± standard error of the mean (SEM) in Supplementary Table S1.
Predator cue
To test the effects of different temperatures and turbidities on both the acute and chronic predator stress response of juvenile delta smelt, a largemouth bass predator cue was introduced to sub tanks every day for 8 days. Predator cues were inserted at the same time each day (~1300), 3 h after the last feeding. Fish were then sampled 15 min after cue insertion on both the first (day 1) and last (day 8) day to assess the generalized stress response to acute and chronic predator cue exposure, respectively. Previous experiments on juvenile delta smelt confirmed that 15 min was sufficient timing for fish to display increased cortisol after a predator cue (Pasparakis et al., 2022). Details on the preparation and insertion of predator cues are outlined in Pasparakis et al., 2022. Briefly, predator cues were prepared the day before trials by housing a largemouth bass in an insulated and aerated cooler for 24 h with 50 mL of water per gram of bass. Cues were injected through PFA standard tubing (inner diameter = 0.5 cm) outside holding tanks with closed lids over sub tanks to avoid disturbance to fish. Tubing was fastened to sub tanks via zip ties so that cues were introduced 1 cm below water level and 3 cm downstream of outflow valves to maximize distribution. To prime the tubing, avoid air bubbles, and ensure all the predator cue was effectively pushed through tubing, 60 mL of tank water was injected before and after the 60 mL of predator cue. Control tanks received three 60-mL syringes of tank water. Fish were netted from individual sub tanks and immediately euthanized with an overdose of tricaine methanesulfonate (MS-222; Finquel) (500 mg/L MS-222; Finquel) buffered to a neutral pH with sodium bicarbonate. Fish were weighed, measured, and snap frozen in liquid nitrogen within <3 min after netting to ensure cortisol levels were not affected by handling stress. Fork length (mm) and wet mass (g) data for juvenile delta smelt measured on sampling days are presented in Supplementary Table S2.
Biochemical Analysis of Stress Response
Whole-body homogenization
A total of 144 delta smelt (4–5 replicates per treatment and 4 fish per replicate) from both the acute and chronic predator stress trials were analyzed for whole-body cortisol, glucose, lactate, and protein concentrations. Due to the complex dynamics of the hypothalamic-pituitary-interrenal axis and resulting variability in cortisol levels, the head of each frozen fish was cut off with a sterile razor blade. The remaining tissue was ground into a fine powder using a mortar and pestle over liquid nitrogen. Whole-body fish powder was weighed and then homogenized in 4 mL ice-cold 1× phosphate-buffered saline [PBS buffer: 137 mM sodium chloride, 2.7 mM potassium chloride, 10 mM disodium phosphate and 1.8 mM monopotassium phosphate (pH = 7.4)] and protease inhibitors (Roche Molecular Systems, Inc.), using a handheld homogenizer. The homogenate was then split into four equal parts for analysis of cortisol, glucose, lactate, and protein. Glucose, lactate, and protein samples were centrifuged for 30 min at 14 500g at 4°C, and supernatant was extracted and stored at −80°C for later analysis.
Cortisol extraction and analysis
Cortisol extraction was performed following methodology outlined in Pasparakis et al., 2022. Briefly, 1 mL of homogenate was transferred to a 9-mL Pyrex glass tube on the same day as tissue homogenization. Tissue homogenate was spiked with 2.5 mL of diethyl ether, vortexed for 1 min and then centrifuged for 7 min at 3200g at 4°C. Without touching the pellet, supernatant was extracted and transferred to a new 9-mL Pyrex glass tube. This process was repeated two more times for maximal cortisol extraction, and extracted supernatant from all three washes was combined. The pellet was then discarded, and the combined supernatant was left in a fume hood overnight to ensure full evaporation of diethyl ether. On the following day, samples were resuspended in 200 μL 1× PBS, vortexed and stored at −80°C until later analysis. Cortisol analysis was performed using an enzyme immunoassay (EIA) kit, following the manufacturer’s instructions (Salivary Cortisol Immunoassay, Salimetrics LLC). Samples were run in duplicate, and cortisol concentrations (μg·dL−1) were calculated using a four-parameter sigmoid standard curve. Cortisol levels were normalized to both mass (ng cortisol per g fish) and total protein (pg cortisol per μg protein) of each sample. Cortisol values corrected by fish mass were used in statistical analysis.
Glucose and lactate analyses
Frozen tissue homogenate samples were thawed on ice, and analyses were conducted using commercial kits according to the manufacturer’s instructions for both glucose (glucose assay kit, Sigma-Aldrich) and lactate (lactate assay kit II, Sigma-Aldrich). Samples were run in duplicate, concentrations (ng·μL−1) were calculated using a linear standard curve and values were normalized to fish mass (μg·g−1).
Protein to mass ratios
Protein concentrations were determined using the bicinchoninic acid method (Pierce, Thermo Fisher Scientific Inc.) following the manufacturer’s instructions. Samples were diluted 5-fold with 1× PBS buffer to match the kit’s serum albumin standards and then run in duplicate. Protein concentration (μg·mL−1) was calculated using a linear standard curve. Protein-to-mass ratios were calculated by dividing total protein (μg·mL−1) by mass of fish (g).
Samples outside the range of standard curve values for all four assays were eliminated from analyses. Standard curves of all assays had r2 values ≥0.99.
Condition factor
Mass and fork length measurements of delta smelt were taken on both sampling days, during the acute and chronic predation stress exposures (Supplementary Table S2). These measurements were used to calculate Fulton condition factor using the equation CF = (WB/FL3) × 100, where WB is the body mass (g) and FL is the fork length (cm).
Statistical analysis
Statistical analyses were conducted using R version 4.1.2 (R Core Team, 2021), with the packages ‘nlme’ (Pinheiro et al., 2019) and ggplot2 (Wickham, 2009). Non-parametric tests were used due to the necessity of including random effects, i.e. to account for effects of sub tanks. Linear mixed-effect models (LMEs) that incorporated sub tank as a random effect were used to analyse whole-body cortisol, glucose, and lactate data, protein-to-mass ratios, and condition factor. Fixed effects included temperature, turbidity, predator cue, and the timing of that cue (acute vs chronic). Multiple LMEs using singular, combined, and interactive effects of biological relevance were run, and Akaike information criterion (AICc) were calculated to determine the model of best fit for the data. Statistical output for these models can be found in Supplementary Table S3, and AICc scores are reported in Supplementary Table S4. The full LME was the most parsimonious model for glucose, lactate, and protein-to-mass ratios. The model exploring the interaction of turbidity and largemouth bass predator cue followed by the full model were the best fit models for cortisol data, whereas the model exploring timing of cue alone followed by the interaction of turbidity and timing were the most parsimonious models for condition factor (Supplementary Tables S3 & S4). Data were tested for normality and homogeneity using the ‘shapiro.test’ function from the stats package and the ‘leveneTest’ function from the ‘car’ package, respectively. The Kruskal-Wallis rank sum test was used to test the effect of water conditions (temperature and turbidity) on delta smelt mortality. Data are presented as means ± SEM, and differences between means were deemed significant at P < 0.05.
Results
Mortality
There was no effect of temperature nor turbidity on delta smelt survival (Kruskal-Wallis: χ2 = 5.93; P = 0.12), suggesting that these experimental treatments only induced sublethal effects to the fish tested.
Cortisol
There was a significant effect of turbidity on whole-body cortisol in juvenile delta smelt such that fish displayed reduced cortisol at higher turbidities (10–11 NTU) compared with lower turbidities (1–2 NTU) (P < 0.01) (Fig. 1). There was no effect of temperature, predator cue, or time point (acute vs chronic predator cue) on cortisol values. There were also no significant interactions of any fixed effects (Supplementary Table S3).
Figure 1.
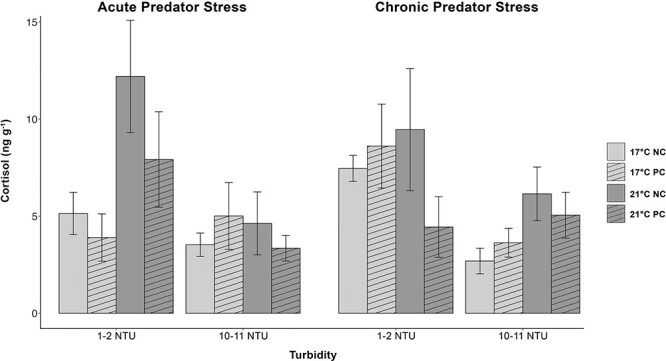
Whole-body cortisol measurements (ng·g−1) in juvenile delta smelt during an acute and chronic predator cue exposure (NC = no cue; PC = predator cue) held at two temperatures (light grey = 17°C; dark grey = 21°C) and two turbidities (1–2 NTU & 10–11 NTU). Fish were sampled 15 min after exposure to a largemouth bass predator cue (vertical angled bars) or tank water (blank bars). Cortisol levels were significantly lower in fish held in turbid compared with low-turbidity conditions. Data (n = 14–20) are presented as mean ± SEM.
Glucose
Both temperature (P < 0.0001) and turbidity (P < 0.001) had a significant effect on whole-body glucose in delta smelt such that fish displayed increased glucose at 17°C compared with 21°C and at higher turbidities (10–11 NTU) compared with lower turbidities (1–2 NTU) (Fig. 2). There was no effect of predator cue, time point, or interaction of fixed effects on glucose levels (Supplementary Table S3).
Figure 2.
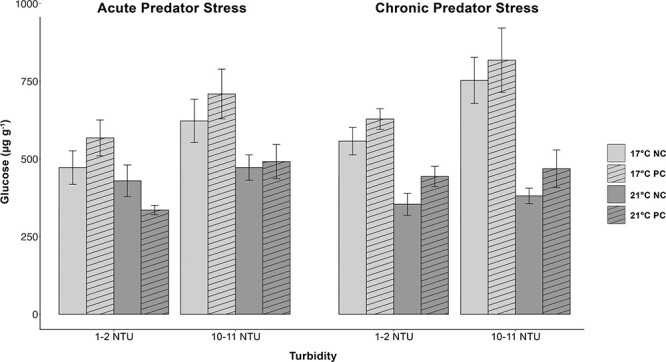
Whole-body glucose measurements (μg·g−1) in juvenile delta smelt during an acute and chronic predator cue exposure (NC = no cue; PC = predator cue) held at two temperatures (light grey = 17°C; dark grey = 21°C) and two turbidities (1–2 NTU & 10–11 NTU). Fish were sampled 15 min after exposure to a largemouth bass predator cue (vertical angled bars) or tank water (blank bars). Glucose levels were significantly greater in fish held at lower temperatures and greater turbidities. Data (n = 12–20) are presented as mean ± SEM.
Lactate
There was a significant effect of both turbidity (P < 0.005) and time point (P < 0.0001) on whole-body lactate in delta smelt such that fish displayed higher lactate levels when held at higher turbidities (10–11 vs 1–2 NTU) and during the chronic predator cue time point compared with the acute predator time point a week earlier (Fig. 3). There was no effect of temperature, predator cue, or interaction of fixed effects on lactate levels (Supplementary Table S3).
Figure 3.
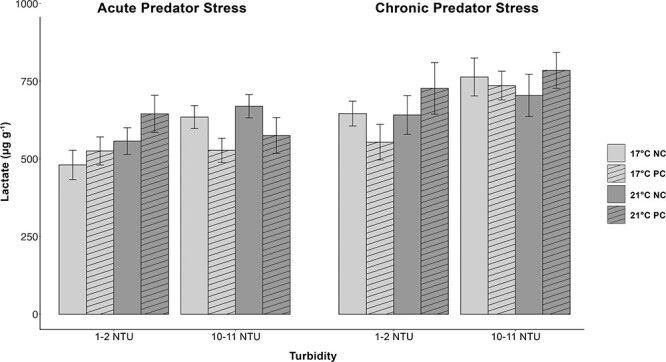
Whole-body lactate measurements (μg·g−1) in juvenile delta smelt during an acute and chronic predator cue exposure (NC = no cue; PC = predator cue) held at two temperatures (light grey = 17°C; dark grey = 21°C) and two turbidities (1–2 NTU & 10–11 NTU). Fish were sampled 15 min after exposure to a largemouth bass predator cue (vertical angled bars) or tank water (blank bars). Lactate levels were significantly greater in fish held in turbid conditions and during the chronic predator stress. Data (n = 14–20) are presented as mean ± SEM.
Protein-to-mass ratio
Delta smelt held at 17°C had significantly higher protein-to-mass ratios compared with fish held at 21°C (P < 0.05) (Fig. 4). There was no effect of turbidity, predator cue, or time point on protein-to-mass ratios. There were also no significant interactions of fixed effects (Supplementary Table S3).
Figure 4.
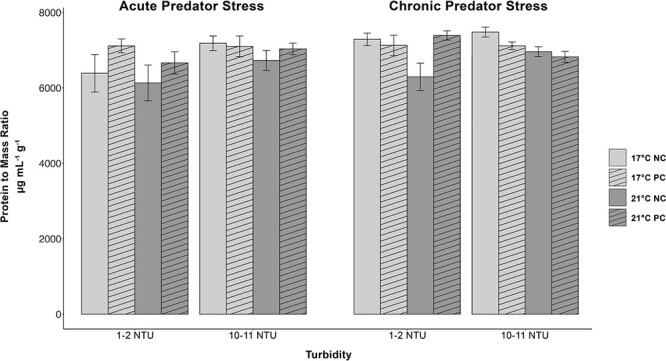
Protein-to-mass ratios (μg·mL−1·g−1) in juvenile delta smelt during an acute and chronic predator cue exposure (NC = no cue; PC = predator cue) held at two temperatures (light grey = 17°C; dark grey = 21°C) and two turbidities (1–2 NTU & 10–11 NTU). Fish were sampled 15 min after exposure to a largemouth bass predator cue (vertical angled bars) or tank water (blank bars). Protein-to-mass ratios were significantly greater in fish held at 17°C compared with those held at 21°C. Data (n = 15–20) are presented as mean ± SEM.
Condition factor
Condition factor in juvenile delta smelt was affected by both timing (acute vs chronic) (P < 0.0005) and turbidity (P < 0.05). Condition factor increased from acute to chronic exposures and decreased at lower turbidities (Table 1). There was no interaction of turbidity and timing (Supplementary Table S3).
Table 1.
Condition factor data for juvenile delta smelt held at different temperatures (17 or 21°C) and turbidities (1–2 or 10–11 NTU) and exposed to an acute or chronic predator cue. Values are presented as mean ± SEM
| Temperature (°C) | Turbidity (NTU) | Timing | Condition Factor |
|---|---|---|---|
| 17 | 1–2 | Acute | 0.61 ± 0.01 |
| 21 | 1–2 | Acute | 0.58 ± 0.01 |
| 17 | 10–11 | Acute | 0.64 ± 0.01 |
| 21 | 10–11 | Acute | 0.61 ± 0.01 |
| 17 | 1–2 | Chronic | 0.63 ± 0.01 |
| 21 | 1–2 | Chronic | 0.64 ± 0.01 |
| 17 | 10–11 | Chronic | 0.66 ± 0.01 |
| 21 | 10–11 | Chronic | 0.65 ± 0.01 |
Discussion
The goal of this study was to explore how environmental changes, such as decreased turbidity, higher temperature, and greater predation affected the stress response and physiology of delta smelt. Juvenile delta smelt were exposed to varying temperatures (17 and 21°C) and turbidities (1–2 and 10–11 NTU) for 2 weeks before the introduction of an acute and chronic largemouth bass predator cue. Turbidity had the greatest effect on delta smelt, and fish held in more turbid conditions displayed reduced stress, greater condition factors, and increased energy in the form of both whole-body glucose and lactate. Lower temperature resulted in a significant increase in both whole-body glucose and protein-to-mass ratios. Finally, a largemouth bass predator cue had negligible effects on delta smelt.
Chronic stress and reduced energy can have significant impacts on populations of fish (Wendelaar Bonga, 1997). During stress, energy necessary for regaining homeostasis and inducing defense mechanisms is diverted away from growth and development (Pankhurst, 2011). Prolonged stress and increased cortisol have also been correlated to reduced immune function and increased susceptibility to disease (Tort, 2011). Reductions in growth and development and suppression of the immune system can ultimately lead to reduced fitness, fecundity, and survival in nature (Barton and Iwama, 1991; Barton, 2002). The results of this study add valuable information that can inform rearing conditions in aquaculture and release environments for supplementation of delta smelt.
Turbidity
The importance of turbidity for the sustainability of delta smelt populations is becoming increasingly apparent. It is likely an important factor mediating the completion of their life cycle because increased pulse turbidity during the ‘first flush’ of the rainy season is thought to act as a cue for their annual spawning migration (Grimaldo et al., 2009; Sommer et al., 2011; Bennett and Burau, 2015). Similar to protocols at FCCL, our turbidity treatments were maintained using Nannochloropsis algae (Tigan et al., 2020). Turbidity in the SFE is made up of a complex and variable mixture of suspended sediment, dissolved organic matter, and algae; therefore, our turbidity treatments are not representative of all combinations of environmental conditions. However, our results correspond well to field observations, where delta smelt are commonly found in mid-turbid waters ranging from 10 to 50 NTU. (Feyrer et al., 2007). In our study, we chose to test two turbidities: a low-turbidity treatment of 1–2 NTU, reflecting reductions in turbidity due to environmental degradation and increased prevalence of invasive species, and a turbid treatment of 10–11 NTU, representative of delta smelt’s preferred habitat (Schoellhamer, 2011). These turbidities were chosen to facilitate comparisons with similar peer-reviewed studies, reflect ecological relevance, and to consider feasibility of maintenance in an aquaculture setting. The maintenance of turbidity in aquaculture is both time-consuming and expensive, and thus careful consideration must be made when assessing its cost–benefit analysis. Because there are limited data on the effects of turbidity on cultured juvenile delta smelt, a main goal of this study was to fill this knowledge gap.
Lower turbidity significantly increased cortisol levels, reduced metabolites glucose and lactate, and decreased condition factor in juvenile delta smelt over a 2-week exposure period (Fig. 1, Fig. 2, Fig. 3 and Table 1). These data suggest that juvenile delta smelt held in clearer waters of 1–2 NTU had increased stress, reduced available energy, and were in poorer condition than fish held at higher turbidities of 10–11 NTU. One possible explanation for these results is that turbidity influenced delta smelt’s feeding. Juvenile delta smelt are visual zooplankton feeders with a diet that consists of pelagic copepods, cladocerans, and mysid shrimp (Hobbs et al., 2006; Moyle et al., 2018). As visual feeders, their feeding success is determined by visual range and prey density (Hecht and Van der Lingen, 1992; Utne-Palm, 2002). In the current experiment, pellet density was kept consistent between sub tanks and visual range was affected by suspended particles or the turbidity in each treatment. Turbidity is thought to increase delta smelt’s visual acuity by enhancing the contrast between prey and its background and thus assist in feeding (Baskerville-Bridges et al., 2004).
Although feeding rates were not quantified in this current study, previous studies show that delta smelt’s feeding success is greatly affected by turbidity (Baskerville-Bridges et al., 2004; Hasenbein et al., 2013, 2016; Tigan et al., 2020). Larval delta smelt were found to have the highest feeding rates at 11 NTU, with a sharp decline in feeding at lower algal concentrations and lower light levels (Baskerville-Bridges et al., 2004). Hasenbein et al. (2016) reported highest feeding rates in late-larval delta smelt between 25 and 80 NTU and reduced feeding at lower (5 and 12 NTU) and higher (120 and 250) turbidities, whereas juvenile delta smelt displayed reduced feeding at >250 NTU, consistent feeding between 12 and 120 NTU and highest feeding at <12 NTU (Hasenbein et al., 2013). In the same studies (Hasenbein et al., 2013, 2016), there were no documented effects of turbidity (0–250 NTU) on whole-body cortisol levels in juvenile delta smelt, but although non-significant, cortisol trends in late-larval delta smelt indicated minimal stress at mid-range turbidities (35–80 NTU) and elevated stress at low turbidities (5, 12 and 25 NTU). In addition, low and high turbidities resulted in reduced survival at the late-larval stage (Hasenbein et al., 2016). Turbidity exposures in these experiments were performed over a 2-h period, likely explaining differences in our findings, which occurred over a 2-week period. Our study is the first, to our knowledge, to show that turbidity reduces stress in juvenile delta smelt (Fig. 1). These combined results suggest that reduced turbidity in the SFE may be having significant effects on delta smelt physiology and growth and likely contribute to population decline.
Whether a certain level of turbidity will provide benefit or harm to a fish is dependent on life stage, feeding style, intensity of turbidity, and other interacting environmental conditions. For example, turbidity resulted in reduced feeding rates of planktivorous bluegills (Lepomis macrochirus) and cape silversides (Atherina breviceps), whereas the effect of turbidity on the spotted grunter (Pomadasys commefsonnii), a macrobenthivore, was not as pronounced (Gardner, 1981; Hecht and Van der Lingen, 1992). Turbidity (up to 160 NTU) had no effect on the feeding rate of juvenile rainbow trout (Oncorhynchus mykiss), which the authors suggest was accomplished by alternating senses of prey detection depending on turbidity conditions (Rowe et al., 2003). Adult European smelt (Osmerus eperlanus) had the highest feeding rates at 20 NTU turbidity, irrespective of light levels (Horppila et al., 2004). Turbidity enhanced the feeding and/or growth rates of larval Pacific herring (Clupea pallasii), Atlantic halibut (Hippoglossus hippoglossus), walleye (Stizostedion vitreum), and rainbow smelt (O. mordax) (Boehlert and Morgan, 1985; Naas and Harboe, 1992; Rieger and Summerfelt, 1997; Sirois and Dodson, 2000). Because there was no evidence of increased prey ingestion in rainbow smelt larvae held in turbid conditions, the authors attributed their greater growth to a reduction in energy expenditure (Sirois and Dodson, 2000).
In the current study, turbidities previously demonstrated as favorable (Feyrer et al., 2007) maintained delta smelt in better condition, and low turbidities decreased energy stores in the form of free glucose and lactate (Table 1, Fig. 2 and Fig. 3). Lactate is a substrate of glucose and is known to increase its concentrations, likely explaining the correlation of these two metabolites (Suarez and Mommsen, 1987; Polakof et al., 2012). Previous studies have attributed high glucose and lactate and lower cortisol to fish in better condition (Chase et al., 2016; Pasparakis et al., 2022). In support of this, delta smelt in the current study displayed lower cortisol, greater glucose and lactate, and increased condition factor in higher turbidities (Table 1). Turbidity may have reduced the energetic cost of locating food. Alternatively, decreased glucose and lactate may be linked to prolonged cortisol induction in fish held in clearer waters. Stress adaptation involves the relocation of metabolic energy toward the maintenance of homeostasis and away from processes such as growth and reproduction (Wendelaar Bonga, 1997). Fish under chronic stress frequently display reduced growth, and parameters such as condition factor and food conversion efficiency are common indicators of stress in fish (Barton and Iwama, 1991; Barton, 2002). Future studies investigating the effects of long-term turbidity exposures on the feeding and growth rates of juvenile delta smelt are needed.
Smelt are known to have light sensitivities and actively avoid light in their natural environments (Appenzeller and Leggett, 1995; Horppila et al., 2004). Preference for low-light conditions is found to ultimately influence smelt distribution (Dembiński, 1971; Heist and Swenson, 1983). Delta smelt in the current study did not have the option of evading greater light intensities in their sub tanks, providing another possible explanation for elevated cortisol at low turbidities. To reduce stress to fish at the FCCL, early-stage delta smelt larvae are reared at a turbidity of 5.5 NTU. Because delta smelt are believed to have life stage-dependent sensitivities to light, late-larval, juveniles, and adults are reared in clear waters. Reduced lighting provided by shade cloth was found to increase juvenile survival during their 3-day transition to outdoor tanks (Lindberg et al., 2013). Results from the current study suggest that the maintenance of higher turbidity may provide benefit to juvenile delta smelt as well as larval and late-larval stages and thus should be considered for rearing. In addition, environments with moderate turbidities should be prioritized during delta smelt supplementation to reduce stress, preserve energy, and maximize survival.
Temperature
Temperature is a critical determinant of a species’ fitness, performance, and distribution, and warming waters due to climate change have resulted in the range shifts and niche compressions of many aquatic organisms (Pörtner, 2002; Schulte et al., 2011; Schulte, 2015). Laboratory experiments are a tool we can use to investigate the sublethal effects of warming temperatures on the physiological performance of a species of interest to understand how future climate projections will affect their survival and sustainability (Helmuth, 2009). In the current experiment, juvenile delta smelt held at an increased temperature of 21°C displayed significantly reduced energy in the form of both available glucose and protein compared with fish held at 17°C (Fig. 2 and Fig. 4). Optimal physiological performance typically occurs over a narrow range of temperatures, and temperatures above or below this range may impose a significant energetic cost on a species (Pörtner, 2002, 2010; Schulte et al., 2011). Reductions in glucose and protein are likely a consequence of increased energy demand associated with maintenance of homeostasis and induction of thermal stress defense mechanisms in juvenile delta smelt.
Delta smelt are especially sensitive to high temperatures and have lower thermal tolerances than many other native and non-native fish in the SFE including green sturgeon (Acipenser medirostris), Sacramento splittail (Pogonichthys macrolepidotus), Mississippi silversides (M. beryllina), largemouth bass (M. salmoides), and some populations of Chinook salmon (Oncorhynchus tshawytscha) (Young and Cech Jr, 1996; Sardella et al., 2008; Davis et al., 2019a; Zillig et al., 2020). Depending on life stage and acclimation temperature, the critical thermal maximum (CTMax) temperature in delta smelt has been found to range from 24°C to just under 30°C (Swanson et al., 2000; Komoroske et al., 2014; Jeffries et al., 2016; Davis et al., 2019a). Most juvenile and pre-adult delta smelt are caught at temperatures <24°C, and a mean daily temperature of 25°C has been identified as a threshold for high mortality (Swanson et al., 2000; Nobriga et al., 2008; Cloern et al., 2011). Although these temperatures are important to consider for management regulations, sublethal critical thresholds have been found to occur much below (4–6°C) upper tolerance limits and likely have significant impacts on delta smelt’s population and future survival (Komoroske et al., 2015; Jeffries et al., 2016; Davis et al., 2019b).
In line with results from the current study, juvenile delta smelt acutely exposed to 20°C displayed increased metabolic rate as well as increased expression of genes relating to metabolic processes, ion regulation, and protein synthesis, indicating that this temperature imposed a substantial energetic demand (Jefferies et al., 2016). Gene expression data suggests that delta smelt experience physiological stress 4–6°C below their upper tolerance limits at all life stages (Komoroske et al., 2015). In addition, delta smelt displayed limited thermal acclimation capacity, suggesting they are unable to modify cellular mechanisms and physiology to cope with higher temperatures (Komoroske et al., 2014, 2015). Upregulation of genes and protein products is energetically expensive, and continued upregulation despite sufficient acclimation periods implies that delta smelt are living close to their thermal maximum (Feder and Hofmann, 1999; Komoroske et al., 2015). After a 7-day exposure at 21°C, juvenile delta smelt displayed elevated swimming velocities, further supporting the notion that temperatures several degrees below thermal thresholds incur an energetic cost and provide a possible explanation for reduced energy in delta smelt observed in the current study (Davis et al., 2019b). These combined results suggest that to sustain delta smelt populations in the wild, management thresholds need to incorporate a thermal buffer several degrees below upper tolerance limits. In addition, supplementation release efforts should continue to occur in winter months when temperature stress is reduced.
Predation
Turbidity is thought to act as a cover for and decrease predation of open water prey species such as delta smelt. Laboratory studies showed that largemouth bass predation on delta smelt was reduced in turbid conditions (Ferrari et al., 2014). Field studies also found that turbidity was an important predictor of predation because delta smelt DNA was more frequently found in the stomachs of Mississippi silversides caught in non-turbid waters (Schreier et al., 2016). Therefore, we predicted that a largemouth bass predator cue would induce a stress response in juvenile delta smelt, which would be mitigated by turbid conditions. Predator cue exposures had no effect on delta smelt’s primary or secondary stress response as indicated by negligible differences in whole-body cortisol, glucose, or lactate levels (Fig. 1, 2 and 3). However, we did find that there was a significant increase in whole-body lactate and condition factor after 7 days of chronic predator cue exposure compared with fish that were only acutely exposed for 1 day (Fig. 3, Table 1). Due to the lack of direct effects from acute predator cues, it is possible that increases in lactate and condition factor were a result of a week of extra growth in captivity versus a week of chronic predator cues.
Many species of fish respond to predation cues, whether visual, acoustic, or olfactory in nature, by inducing their primary stress response and increasing cortisol levels (Remage-Healey et al., 2006; Barcellos et al., 2007; Barreto et al., 2014; Barkhymer et al., 2019). However, in some cases, fish are found to display signs of increased stress in the absence of significant increases in cortisol (Hasenbein et al., 2016; Miyai et al., 2016). Late-larval delta smelt exposed to high turbidities of 250 NTU exhibited elevated mortality and gene expression suggestive of oxidative and osmotic stress but displayed surprisingly low levels of cortisol (Hasenbein et al., 2016). Nile tilapia (Oreochromis niloticus) exposed to a predator odor displayed decreased activity and increased ventilation rate, indicative of increased stress, with no corresponding changes in plasma levels of cortisol or glucose (Miyai et al., 2016). Although previous studies found a similar largemouth bass predator cue elicited a stress response in juvenile delta smelt, cortisol results from the current study suggest that this cue was not strong enough to increase stress in delta smelt (Pasparakis et al., 2022). Alternatively, due to delta smelt’s limited tolerance to stress and known variability of cortisol results, it is possible that increased stress was not detected through whole-body cortisol or metabolite results in this study (Hasenbein et al., 2013; Moyle et al., 2016). Plasma cortisol levels may have revealed increased stress but this analysis was not possible due to the size of juvenile delta smelt. Future studies should investigate how predator and competitor cues from other species of fish affect delta smelt’s stress response.
The significant increase in lactate without corresponding increases in cortisol or glucose between acute and chronic predator stress exposures was an unexpected finding (Fig. 3). Increased lactate may indicate that chronic exposure to a predator cue affected delta smelt’s swimming behaviors, such that increased swim velocity resulted in a shift to anaerobic respiration and thus higher lactate production (Pankhurst, 2011). In support of this, juvenile delta smelt exposed to a largemouth bass predator cue displayed increased swimming speeds and other swim behaviors suggestive of increased stress (Davis et al., 2019b).
An alternative explanation for the lack of results is that delta smelt used in this study were raised in captivity and did not recognize the largemouth bass odor as a predator cue. Because largemouth bass are an invasive species, it is possible that delta smelt born and raised in the SFE would perceive this odor as a threat due to learned associations, whereas delta smelt in the current study, without a reference, perceived this cue as innocuous. Because supplementation is now necessitated for the maintenance of the delta smelt population in the SFE, fish raised in captivity will become the new wild delta smelt. Therefore, understanding how hatchery-raised fish respond to environmental signals, such as a predation cue, is important and environmentally relevant for this endangered species.
Concluding Remarks
The SFE is a complex ecosystem with many interacting variables that have contributed to the long-term population declines of delta smelt. Laboratory experiments incorporating a multistressor design can be used to better understand how individual stressors and their interactions will affect the physiology of a species in question. Temperature and turbidity were both important variables affecting the physiological stress response of juvenile delta smelt. Increased temperatures caused a reduction in delta smelt’s energy in the form of available glucose and protein, whereas lower turbidities resulted in reduced available glucose and lactate, increased cortisol, and decreased condition factor. As the frequency and severity of events such as heat waves and droughts increase due to climate change, the challenges associated with delta smelt conservation will continue to intensify. Increased water demands and habitat alterations, such as continued declines in turbidity and increases in temperature in the SFE, will further reduce the delta smelt population and compress their limited distribution (Nobriga et al., 2008; Moyle et al., 2016). Findings from the current study should be used to inform regulations and initiatives to prevent delta smelt extinction. Specifically, mid-range turbidities and temperatures should be prioritized in the rearing, transportation, and release of delta smelt. This will facilitate the supplementation of delta smelt with greater condition factor, increased energy, and reduced stress, favoring their chances of survival in nature.
Funding
This research was made possible by funding from the U.S. Fish and Wildlife Service no. F19AC00943 to A.E.T., R.E.C., T-C.H. and N.A.F., and the University of California, Davis Agricultural Experiment Station (CA-D-ASC-2252-H to A.E.T. and CA-D-ASC-2098-H to N.A.F.). USBOR, R20AC00027 funding to T-C.H. was used for delta smelt rearing and maintenance. Funding for F.B. was provided by the Bayerische Forschungsstiftung Scholarship (DOK-181-19, Geist). The findings and conclusions in this article are those of the authors and do not necessarily represent the view of the U.S. Fish and Wildlife Service.
Supplemental Data Section
Supplementary material is available at Conservation Physiology online. Data in this section include an experimental schematic figure, a table with temperature and turbidity (mean ± SEM) in different treatments and during the acclimation period, a table including length and mass data for juvenile delta smelt on sampling days, a table with the statistical output for linear mixed-effects models and a table with the AICc scores for determination of best fit LMEs.
Author Contributions
This manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. C.P., D.E.C., R.E.C., N.A.F. and A.E.T. designed the experiment. C.P., T.L. and F.B. collected the data. E.W.C. contributed biological expertise and expertise in the management of delta smelt. T-C.H. provided experimental fish. C.P. wrote the manuscript. R.E.C., N.A.F. and A.E.T. secured the funding that supported the work.
Data Availability
The data underlying this article are available in the online supplementary material.
Supplementary Material
Acknowledgements
We thank the hatchery staff and volunteers at UC Davis Fish Conservation and Culture Lab for maintaining the captive population of delta smelt used in this research. We also thank Jordan Colby, Sarah Baird, Alexis Lundquist, Hali Schwasnick and Susan Landa for their help with fish care, experimental setup and sampling. And a special thanks to Richelle Tanner for her advice on statistics.
Contributor Information
Christina Pasparakis, Department of Environmental Toxicology, University of California Davis, 1 Shields Ave., Davis, CA, USA; Bodega Marine Laboratory, University of California Davis, 2099 Westshore Rd., Bodega Bay, CA, USA.
Toni Lohroff, Department of Wildlife, Fish and Conservation Biology, University of California Davis, 1 Shields Ave., Davis, CA, USA; Department of Animal Science, University of California Davis, 1 Shields Ave., Davis, CA, USA.
Felix Biefel, School of Veterinary Medicine, Department of Anatomy, Physiology and Cell Biology, University of California Davis, 1 Shields Ave., Davis, CA, USA.
Dennis E Cocherell, Department of Wildlife, Fish and Conservation Biology, University of California Davis, 1 Shields Ave., Davis, CA, USA.
Evan W Carson, San Francisco Bay-Delta Fish and Wildlife Office, U.S. Fish and Wildlife Service, 650 Capitol Mall, Sacramento, CA, USA.
Tien-Chieh Hung, Fish Conservation and Culture Laboratory, Department of Biological and Agricultural Engineering, University of California Davis, 1 Shields Ave., Davis, CA, USA.
Richard E Connon, School of Veterinary Medicine, Department of Anatomy, Physiology and Cell Biology, University of California Davis, 1 Shields Ave., Davis, CA, USA.
Nann A Fangue, Department of Wildlife, Fish and Conservation Biology, University of California Davis, 1 Shields Ave., Davis, CA, USA.
Anne E Todgham, Department of Animal Science, University of California Davis, 1 Shields Ave., Davis, CA, USA.
References
- Appenzeller AR, Leggett WC (1995) An evaluation of light-mediated vertical migration of fish based on hydroacoustic analysis of the diel vertical movements of rainbow smelt (Osmerus mordax). Can J Fish Aquat Sci 52: 504–511. 10.1139/f95-051. [DOI] [Google Scholar]
- Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, Silva LB, Bedin AC, Finco J, Cericato L (2007) Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture 272: 774–778. 10.1016/j.aquaculture.2007.09.002. [DOI] [Google Scholar]
- Barkhymer AJ, Garrett SG, Wisenden BD (2019) Olfactorily-mediated cortisol response to chemical alarm cues in zebrafish Danio rerio. J Exp Biol 95: 287–292. 10.1111/jfb.13860. [DOI] [PubMed] [Google Scholar]
- Barreto RE, Barbosa-Júnior A, Urbinati EC, Hoffmann A (2014) Cortisol influences the antipredator behavior induced by chemical alarm cues in the Frillfin goby. Horm Behav 65: 394–400. 10.1016/j.yhbeh.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42: 517–525. 10.1093/icb/42.3.517. [DOI] [PubMed] [Google Scholar]
- Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1: 3–26. [Google Scholar]
- Bashevkin SM, Mahardja B, Brown LR (2022) Warming in the Upper San Francisco Estuary: Patterns of Water Temperature Change From Five Decades of Data. Limnol, Oceanogr [Google Scholar]
- Baskerville-Bridges B, Lindberg JC, Doroshov SI (2004) The effect of light intensity, alga concentration, and prey density on the feeding behavior of delta smelt larvae. In American Fisheries Society Symposium 39: 219–227. [Google Scholar]
- Baskerville-Bridges B, Lindberg JC, Doroshov SI (2005) Manual for the Intensive Culture of Delta Smelt (Hypomesus transpacificus). University of California-Davis, Sacramento (CA), Report to CALFED Bay-Delta Program. ERP-02-P31. [Google Scholar]
- Baumsteiger J, Schroeter RE, O'Rear T, Cook JD, Moyle PB (2017) Long-term surveys show invasive overbite clams (Potamocorbula amurensis) are spatially limited in Suisun Marsh, California. San. Franc. Estuary Watershed Sci 15: 6. 10.15447/sfews.2017v15iss2art6. [DOI] [Google Scholar]
- Bennett WA (2005) Critical assessment of the delta smelt population in the San Francisco Estuary. California San Franc Estuary Watershed Sci 3: 1–71. 10.15447/sfews.2005v3iss2art1. [DOI] [Google Scholar]
- Bennett WA, Burau JR (2015) Riders on the storm: selective tidal movements facilitate the spawning migration of threatened delta smelt in the San Francisco Estuary. Estuar Coast 38: 826–835. 10.1007/s12237-014-9877-3. [DOI] [Google Scholar]
- Boehlert GW, Morgan JB (1985) Turbidity enhances feeding abilities of larval Pacific herring, Clupea harengus pallasi. Hydrobiologia 123: 161–170. 10.1007/BF00018978. [DOI] [Google Scholar]
- Bork K, Moyle P, Durand J, Hung TC, Rypel AL (2020) Small populations in jeopardy: a Delta smelt case study. Envtl L Rep 50: 10714. [Google Scholar]
- Brooks ML, Fleishman E, Brown LR, Lehman PW, Werner I, Scholz N, Mitchelmore C, Lovvorn JR, Johnson ML, Schlenk Det al. (2012) Life histories, salinity zones, and sublethal contributions of contaminants to pelagic fish declines illustrated with a case study of San Francisco Estuary, California, USA. Estuar Coast 35: 603–621. 10.1007/s12237-011-9459-6. [DOI] [Google Scholar]
- Brown LR, Bennett WA, Wagner RW, Morgan-King T, Knowles N, Feyrer F, Schoellhamer DH, Stacey MT, Dettinger M (2013) Implications for future survival of Delta smelt from four climate change scenarios for the Sacramento–San Joaquin Delta, California. Estuaries Coast 36: 754–774. 10.1007/s12237-013-9585-4. [DOI] [Google Scholar]
- Brown LR, Komoroske LM, Wagner RW, Morgan-King T, May JT, Connon RE, Fangue NA (2016) Coupled downscaled climate models and ecophysiological metrics forecast habitat compression for an endangered estuarine fish. PloS One 11: e0146724. 10.1371/journal.pone.0146724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JT, Thompson JK, Schemel LE, Nichols FH (1990) Remarkable invasion of San Francisco Bay (California, USA) by the Asian clam Potamocorbula amurensis. I. Introduction and dispersal. Mar Ecol Prog Ser 66: 81–94. 10.3354/meps066081. [DOI] [Google Scholar]
- Chase DA, Flynn EE, Todgham AE (2016) Survival, growth and stress response of juvenile tidewater goby, Eucyclogobius newberryi, to interspecific competition for food. Conserv Physiol 4: cow013. 10.1093/conphys/cow013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloern JE, Jassby AD (2012) Drivers of change in estuarine-coastal ecosystems: discoveries from four decades of study in San Francisco Bay. Rev Geophys 50: RG4001. 10.1029/2012RG000397. [DOI] [Google Scholar]
- Cloern JE, Knowles N, Brown LR, Cayan D, Dettinger MD, Morgan TL, Schoellhamer DH, Stacey MT, Van der Wegen M, Wagner RWet al. (2011) Projected evolution of California's San Francisco Bay-Delta-River system in a century of climate change. PloS One 6: 24465. 10.1371/journal.pone.0024465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad JL, Bibian AJ, Weinersmith KL, De Carion D, Young MJ, Crain P, Hestir EL, Santos MJ, Sih A (2016) Novel species interactions in a highly modified estuary: association of largemouth Bass with Brazilian waterweed Egeria densa. Trans Am Fish Soc 145: 249–263. 10.1080/00028487.2015.1114521. [DOI] [Google Scholar]
- Davies-Colley RJ, Smith DG (2001) Turbidity suspended sediment, and water clarity: a review. Water resources bulletin 37: 1085–1101. 10.1111/j.1752-1688.2001.tb03624.x. [DOI] [Google Scholar]
- Davis BE, Cocherell DE, Sommer T, Baxter RD, Hung TC, Todgham AE, Fangue NA (2019a) Sensitivities of an endemic, endangered California smelt and two non-native fishes to serial increases in temperature and salinity: implications for shifting community structure with climate change. Conserv Physiol 7: coy076. 10.1093/conphys/coy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BE, Hansen MJ, Cocherell DE, Nguyen TX, Sommer T, Baxter RD, Fangue NA, Todgham AE (2019b) Consequences of temperature and temperature variability on swimming activity, group structure, and predation of endangered delta smelt. Freshw biol 64: 2156–2175. 10.1111/fwb.13403. [DOI] [Google Scholar]
- Dembiński W (1971) Vertical distribution of vendace Coregonus albula L. and other pelagic fish species in some Polish lakes. J Fish Biol 3: 341–357. 10.1111/j.1095-8649.1971.tb03689.x. [DOI] [Google Scholar]
- Durand JR (2015) A conceptual model of the aquatic food web of the upper San Francisco Estuary. San. Franc. Estuary Watershed Sci 13. 10.15447/sfews.2015v13iss3art5. [DOI] [Google Scholar]
- Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61: 243–282. 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Ferrari MC, Ranåker L, Weinersmith KL, Young MJ, Sih A, Conrad JL (2014) Effects of turbidity and an invasive waterweed on predation by introduced largemouth bass. Environ Biol Fishes 97: 79–90. 10.1007/s10641-013-0125-7. [DOI] [Google Scholar]
- Feyrer F, Nobriga ML, Sommer TR (2007) Multidecadal trends for three declining fish species: habitat patterns and mechanisms in the San Francisco Estuary, California, USA. Can J Fish Aquat Sci 64: 723–734. 10.1139/f07-048. [DOI] [Google Scholar]
- Fisch KM, Ivy JA, Burton RS, May B (2013) Evaluating the performance of captive breeding techniques for conservation hatcheries: a case study of the delta smelt captive breeding program. J Hered 104: 92–104. 10.1093/jhered/ess084. [DOI] [PubMed] [Google Scholar]
- Gardner MB (1981) Effects of turbidity on feeding rates and selectivity of bluegills. T Am Fish Soc 110: 446–450. . [DOI] [Google Scholar]
- Grimaldo LF, Sommer T, Van Ark N, Jones G, Holland E, Moyle PB, Herbold B, Smith P (2009) Factors affecting fish entrainment into massive water diversions in a tidal freshwater estuary: can fish losses be managed? N Am J Fish Manag 29: 1253–1270. 10.1577/M08-062.1. [DOI] [Google Scholar]
- Hasenbein M, Fangue NA, Geist J, Komoroske LM, Truong J, McPherson R, Connon RE (2016) Assessments at multiple levels of biological organization allow for an integrative determination of physiological tolerances to turbidity in an endangered fish species. Conserv Physiol 4: cow004. 10.1093/conphys/cow004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenbein M, Komoroske LM, Connon RE, Geist J, Fangue NA (2013) Turbidity and salinity affect feeding performance and physiological stress in the endangered delta smelt. Integr Comp Biol 53: 620–634. 10.1093/icb/ict082. [DOI] [PubMed] [Google Scholar]
- Healey M, Goodwin P, Dettinger M, Norgaard R (2016) The state of Bay–Delta science 2016: an introduction. San. Franc. Estuary Watershed Sci 14. 10.15447/sfews.2016v14iss2art5. [DOI] [Google Scholar]
- Hecht T, Van der Lingen CD (1992) Turbidity-induced changes in feeding strategies of fish in estuaries. Afr Zool 27: 95–107. 10.1080/02541858.1992.11448269. [DOI] [Google Scholar]
- Heist BG, Swenson WA (1983) Distribution and abundance of rainbow smelt in western Lake Superior as determined from acoustic sampling. J Great Lakes Res 9: 343–353. 10.1016/S0380-1330(83)71905-2. [DOI] [Google Scholar]
- Helmuth B (2009) From cells to coastlines: how can we use physiology to forecast the impacts of climate change? J Exp Biol 212: 753–760. 10.1242/jeb.023861. [DOI] [PubMed] [Google Scholar]
- Henley WF, Patterson MA, Neves RJ, Lemly AD (2000) Effects of sedimentation and turbidity on lotic food webs: a concise review for natural resource managers. Rev Fish Sci 8: 125–139. 10.1080/10641260091129198. [DOI] [Google Scholar]
- Hess S, Prescott LJ, Hoey AS, McMahon SA, Wenger AS, Rummer JL (2017) Species-specific impacts of suspended sediments on gill structure and function in coral reef fishes. Proc R Soc B 284: 20171279. 10.1098/rspb.2017.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J, Moyle PB, Fangue N, Connon RE (2017) Is extinction inevitable for Delta smelt and longfin smelt? An opinion and recommendations for recovery. San. Franc. Estuary Watershed Sci 15. 10.15447/sfews.2017v15iss2art2. [DOI] [Google Scholar]
- Hobbs JA, Bennett WA, Burton JE (2006) Assessing nursery habitat quality for native smelts (Osmeridae) in the low-salinity zone of the San Francisco estuary. J Fish Biol 69: 907–922. 10.1111/j.1095-8649.2006.01176.x. [DOI] [Google Scholar]
- Hobbs JA, Lewis LS, Willmes M, Denney C, Bush E (2019) Complex life histories discovered in a critically endangered fish. Sci Reps 9: 16772–16712. 10.1038/s41598-019-52273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horppila J, Liljendahl-Nurminen A, Malinen T (2004) Effects of clay turbidity and light on the predator prey interaction between smelts and chaoborids. Can J Fish Aquat Sci 61: 1862–1870. [Google Scholar]
- Hung TC, Ellison L, Stevenson T, Sandford M, Schultz AA, Eads AR (2022a) Early weaning in endangered Delta smelt: effect of weaning time on growth and survival. N Am J Aquac 84: 249–260. 10.1002/naaq.10230. [DOI] [Google Scholar]
- Hung TC, Hammock BG, Sandford M, Stillway M, Park M, Lindberg JC, Teh SJ (2022b) Temperature and salinity preferences of endangered Delta smelt (Hypomesus transpacificus, Actinopterygii, Osmeridae). Sci Rep 12: 16558–16511. 10.1038/s41598-022-20934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries KM, Connon RE, Davis BE, Komoroske LM, Britton MT, Sommer T, Todgham AE, Fangue NA (2016) Effects of high temperatures on threatened estuarine fishes during periods of extreme drought. J Exp Biol 219: 1705–1716. 10.1242/jeb.134528. [DOI] [PubMed] [Google Scholar]
- Kimmerer WJ (2006) Response of anchovies dampens effects of the invasive bivalve Corbula amurensis on the San Francisco Estuary foodweb. Mar Ecol Prog Ser 324: 207–218. 10.3354/meps324207. [DOI] [Google Scholar]
- Kirk JT (1985) Effects of suspensoids (turbidity) on penetration of solar radiation in aquatic ecosystems. Hydrobiologia 125: 195–208. 10.1007/BF00045935. [DOI] [Google Scholar]
- Komoroske LM, Connon RE, Jeffries KM, Fangue NA (2015) Linking transcriptional responses to organismal tolerance reveals mechanisms of thermal sensitivity in a mesothermal endangered fish. Mol Ecol 24: 4960–4981. 10.1111/mec.13373. [DOI] [PubMed] [Google Scholar]
- Komoroske LM, Connon RE, Lindberg J, Cheng BS, Castillo G, Hasenbein M, Fangue NA (2014) Ontogeny influences sensitivity to climate change stressors in an endangered fish. Conserv Physiol 2: cou008. 10.1093/conphys/cou008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoroske LM, Jeffries KM, Whitehead A, Roach JL, Britton M, Connon RE, Verhille C, Brander SM, Fangue NA (2021) Transcriptional flexibility during thermal challenge corresponds with expanded thermal tolerance in an invasive compared to native fish. Evol Appl 14: 931–949. 10.1111/eva.13172. [DOI] [Google Scholar]
- LaCava M, Fisch K, Nagel M, Lindberg JC, May B, Finger AJ (2015) Spawning behavior of cultured Delta smelt in a conservation hatchery. N Am J Aquacul 77: 255–266. 10.1080/15222055.2015.1007192. [DOI] [Google Scholar]
- Lessard J, Cavallo B, Anders P, Sommer T, Schreier B, Gille D, Schreier A, Finger A, Hung TC, Hobbs Jet al. (2018) Considerations for the use of captive-reared delta smelt for species recovery and research. San Fran Estuar Watershed Sci 16. 10.15447/sfews.2018v16iss3art3. [DOI] [Google Scholar]
- Lindberg JC, Tigan G, Ellison L, Rettinghouse T, Nagel MM, Fisch KM (2013) Aquaculture methods for a genetically managed population of endangered Delta smelt. N Am J Aquacult 75: 186–196. 10.1080/15222055.2012.751942. [DOI] [Google Scholar]
- Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson JB (2006) Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312: 1806–1809. 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- Lunt J, Smee DL (2014) Turbidity influences trophic interactions in estuaries. Limnol Oceanogr 59: 2002–2012. 10.4319/lo.2014.59.6.2002. [DOI] [Google Scholar]
- Miyai CA, Sanches FHC, Pinho-Neto CF, Barreto RE (2016) Effects of predator odour on antipredator responses of Nile tilapia. Physiol Behav 165: 22–27. 10.1016/j.physbeh.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Moyle PB (2002) Inland Fishes of California. University of California Press, Berkeley [Google Scholar]
- Moyle PB, Bork K, Durand J, Hung TC, Rypel A (2019) Futures for Delta Smelt. Center for Watershed Sciences White Paper. University of California—Davis, Davis, CA, p. 15 [Google Scholar]
- Moyle PB, Brown LR, Durand JR, Hobbs JA (2016) Delta smelt: life history and decline of a once-abundant species in the San Francisco Estuary. San Franc Estuary Watershed Sci 14. 10.15447/sfews.2016v14iss2art6. [DOI] [Google Scholar]
- Moyle PB, Herbold B, Stevens DE, Miller LW (1992) Life history and status of Delta smelt in the Sacramento-San Joaquin Estuary, California. Trans Am Fish Soc 121: 67–77. . [DOI] [Google Scholar]
- Moyle PB, Hobbs JA, Durand JR (2018) Delta smelt and water politics in California. Fisheries 43: 42–50. 10.1002/fsh.10014. [DOI] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853–858. 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Naas KE, Harboe T (1992) Enhanced first feeding of halibut larvae (Hippoglossus hippoglossus L.) in green water. Aquaculture 105: 143–156. 10.1016/0044-8486(92)90126-6. [DOI] [Google Scholar]
- Nichols FH, Cloern JE, Luoma SN, Peterson DH (1986) The modification of an estuary. Science 231: 567–573. 10.1126/science.231.4738.567. [DOI] [PubMed] [Google Scholar]
- Nobriga ML, Sommer TR, Feyrer F, Fleming K (2008) Long-term trends in summertime habitat suitability for delta smelt, Hypomesus transpacificus. San Franc Estuary Watershed Sci 6: 1. 10.15447/sfews.2008v6iss1art1. [DOI] [Google Scholar]
- Paganini A, Kimmerer WJ, Stillman JH (2010) Metabolic responses to environmental salinity in the invasive clam Corbula amurensis. Aquat Biol 11: 139–147. 10.3354/ab00304. [DOI] [Google Scholar]
- Pankhurst NW (2011) The endocrinology of stress in fish: an environmental perspective. Gen Comp Endocr 170: 265–275. 10.1016/j.ygcen.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Pasparakis C, Wampler AN, Lohroff T, DeCastro F, Cocherell DE, Carson EW, Hung TC, Connon RE, Fangue NA, Todgham AE (2022) Characterizing the stress response in juvenile Delta smelt exposed to multiple stressors. Comp Biochem Phys A 274: 111303. 10.1016/j.cbpa.2022.111303. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2019) Nlme: linear and nonlinear mixed effects models. R package version 3.1–140 https:// CRAN. R-project. org/package=nlme. [Google Scholar]
- Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Phys B 182: 1015–1045. 10.1007/s00360-012-0658-7. [DOI] [PubMed] [Google Scholar]
- Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88: 137–146. 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Phys A 132: 739–761. 10.1016/S1095-6433(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Pörtner HO (2010) Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213: 881–893. 10.1242/jeb.037523. [DOI] [PubMed] [Google Scholar]
- R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Remage-Healey L, Nowacek DP, Bass AH (2006) Dolphin foraging sounds suppress calling and elevate stress hormone levels in a prey species, the Gulf toadfish. J Exp Biol 209: 4444–4451. 10.1242/jeb.02525. [DOI] [PubMed] [Google Scholar]
- Rieger PW, Summerfelt RC (1997) The influence of turbidity on larval walleye, Stizostedion vitreum, behavior and development in tank culture. Aquaculture 159: 19–32. 10.1016/S0044-8486(97)00187-7. [DOI] [Google Scholar]
- Rowe DK, Dean TL, Williams E, Smith JP (2003) Effects of turbidity on the ability of juvenile rainbow trout, Oncorhynchus mykiss, to feed on limnetic and benthic prey in laboratory tanks. New Zeal J Mar Fresh 37: 45–52. 10.1080/00288330.2003.9517145. [DOI] [Google Scholar]
- Sardella BA, Sanmarti E, Kültz D (2008) The acute temperature tolerance of green sturgeon (Acipenser medirostris) and the effect of environmental salinity. J Exp Zool Part A 309A: 477–483. 10.1002/jez.477. [DOI] [PubMed] [Google Scholar]
- Schoellhamer DH (2011) Sudden clearing of estuarine waters upon crossing the threshold from transport to supply regulation of sediment transport as an erodible sediment pool is depleted: San Francisco Bay, 1999. Estuar Coast 34: 885–899. 10.1007/s12237-011-9382-x. [DOI] [Google Scholar]
- Schreier BM, Baerwald MR, Conrad JL, Schumer G, May B (2016) Examination of predation on early life stage Delta smelt in the San Francisco estuary using DNA diet analysis. Trans Am Fish Soc 145: 723–733. 10.1080/00028487.2016.1152299. [DOI] [Google Scholar]
- Schulte PM (2015) The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218: 1856–1866. 10.1242/jeb.118851. [DOI] [PubMed] [Google Scholar]
- Schulte PM, Healy TM, Fangue NA (2011) Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51: 691–702. 10.1093/icb/icr097. [DOI] [PubMed] [Google Scholar]
- Service, R.F (2007) Delta blues, California style. Science 317: 442–445. 10.1126/science.317.5837.442. [DOI] [PubMed] [Google Scholar]
- Sirois P, Dodson JJ (2000) Influence of turbidity, food density and parasites on the ingestion and growth of larval rainbow smelt Osmerus mordax in an estuarine turbidity maximum. Mar Ecol Prog Ser 193: 167–179. 10.3354/meps193167. [DOI] [Google Scholar]
- Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA (2012) Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res 79: 1–15. 10.1016/j.marenvres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Sommer T, Armor C, Baxter R, Breuer R, Brown L, Chotkowski M, Culberson S, Feyrer F, Gingras M, Herbold Bet al. (2007) The collapse of pelagic fishes in the upper San Francisco Estuary. Fisheries 32: 270–277. 10.1577/1548-8446(2007)32[270:TCOPFI]2.0.CO;2. [DOI] [Google Scholar]
- Sommer T, Mejia F (2013) A place to call home: a synthesis of Delta smelt habitat in the upper San Francisco Estuary. San Franc Estuary Watershed Sci 11. 10.15447/sfews.2013v11iss2art4. [DOI] [Google Scholar]
- Sommer T, Mejia FH, Nobriga ML, Feyrer F, Grimaldo L (2011) The spawning migration of Delta smelt in the Upper San Francisco Estuary. San Franc Estuary Watershed Sci 9: 2. 10.15447/sfews.2011v9iss2art2. [DOI] [Google Scholar]
- Suarez RK, Mommsen TP (1987) Gluconeogenesis in teleost fishes. Can J Zool 65: 1869–1882. 10.1139/z87-287. [DOI] [Google Scholar]
- Sutherland AB, Meyer JL (2007) Effects of increased suspended sediment on growth rate and gill condition of two southern Appalachian minnows. Environ Biol Fishes 80: 389–403. 10.1007/s10641-006-9139-8. [DOI] [Google Scholar]
- Swanson C, Mager RC, Doroshov SI, Cech JJ Jr (1996) Use of salts, anesthetics, and polymers to minimize handling and transport mortality in delta smelt. T Am Fish Soc 125: 326–329. . [DOI] [Google Scholar]
- Swanson C, Reid T, Young PS, Cech JJ Jr (2000) Comparative environmental tolerances of threatened delta smelt (Hypomesus transpacificus) and introduced wakasagi (H. nipponensis) in an altered California estuary. Oecologia 123: 384–390. 10.1007/s004420051025. [DOI] [PubMed] [Google Scholar]
- Thomson JR, Kimmerer WJ, Brown LR, Newman KB, Nally RM, Bennett WA, Feyrer F, Fleishman E (2010) Bayesian change point analysis of abundance trends for pelagic fishes in the Upper San Francisco Estuary. Ecol Appl 20: 1431–1448. 10.1890/09-0998.1. [DOI] [PubMed] [Google Scholar]
- Tigan G, Mulvaney W, Ellison L, Schultz A, Hung TC (2020) Effects of light and turbidity on feeding, growth, and survival of larval Delta smelt (Hypomesus transpacificus, Actinopterygii, Osmeridae). Hydrobiologia 847: 2883–2894. 10.1007/s10750-020-04280-4. [DOI] [Google Scholar]
- Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35: 1366–1375. 10.1016/j.dci.2011.07.002. [DOI] [PubMed] [Google Scholar]
- U.S. Fisheries and Wildlife Service (USFWS) (2019) Biological Opinion For the Reinitiation of Consultation on the Coordinated Operations of the Central Valley Project and State Water Project
- U.S. Fisheries and Wildlife Service (USFWS) (2020) California Department of Water Resources, United States Bureau of Reclamation, California Department of Fish and Wildlife, and University of California Davis. Delta Smelt Supplementation Strategy [Google Scholar]
- U.S. Office of the Federal Register (1993) Endangered and threatened wildlife and plants: determination of threatened status for the Delta smelt. Federal Register 58 425 March 1993: 12854–11286. [Google Scholar]
- Utne-Palm AC (2002) Visual feeding of fish in a turbid environment: physical and behavioural aspects. Mar Freshw Behav Phy 35: 111–128. 10.1080/10236240290025644. [DOI] [Google Scholar]
- Wendelaar Bonga SW (1997) The stress response in fish. Physiol Rev 77: 591–625. 10.1152/physrev.1997.77.3.591. [DOI] [PubMed] [Google Scholar]
- Wickham H (2009) Ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York, NY [Google Scholar]
- Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JB, Lotze HK, Micheli F, Palumbi SRet al. (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314: 787–790. 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- Wright SA, Schoellhamer DH (2004) Trends in the sediment yield of the Sacramento River, California, 1957–2001. San. Franc. Estuary Watershed Sci. 2: 1–14. 10.15447/sfews.2004v2iss2art2. [DOI] [Google Scholar]
- Yanagitsuru YR, Davis BE, Baerwald MR, Sommer TR, Fangue NA (2022) Using Physiology to Recover Imperiled Smelt Species. Academic Press, Cambridge, MA, USA [Google Scholar]
- Young PS, Cech JJ Jr (1996) Environmental tolerances and requirements of splittail. Trans Am Fish Soc 125: 664–678. . [DOI] [Google Scholar]
- Zillig, K.W., Cocherell, D.E., Fangue, N.A.. 2020. Interpopulation variation among juvenile Chinook salmon from California and Oregon. The United States Environmental Protection Agency Region 9 – Pacific Southwest Region, San Francisco, CA. https://www.epa.gov/sfbay-delta/2020-chinook-salmon-interpopulation-thermal-tolerance-investigation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the online supplementary material.


