Abstract
Simple Summary
The use of probiotic bacteria in the aquaculture of salmonids, which are very sensitive to stress, represents a possibility to increase their resistance to diseases. We have developed a new, low-cost application form ensuring the rapid revitalization of probiotic bacteria. We tested continuous and cyclic feeding regimes with regard to their effect on the intestinal immune response and microbiota of rainbow trout. We found that continuous application stabilizes the intestinal microbiota in favor of beneficial lactic acid bacteria and does not cause unnecessary excessive stimulation of immunity, which can lead to an unwanted increase in energy demand. Cyclic application of probiotic feed has the same positive effect on the intestinal microbiota but provides the opportunity to stimulate the intestinal immune system of trout, for example, in periods of increased stress.
Abstract
Intensive fish farming is associated with a high level of stress, causing immunosuppression. Immunomodulators of natural origin, such as probiotics or phytoadditives, represent a promising alternative for increasing the immune function of fish. In this study, we tested the autochthonous trout probiotic strain L. plantarum R2 in a newly developed, low-cost application form ensuring the rapid revitalization of bacteria. We tested continuous and cyclic feeding regimes with regard to their effect on the intestinal immune response and microbiota of rainbow trout. We found that during the continuous application of probiotic feed, the immune system adapts to the immunomodulator and there is no substantial stimulation of the intestinal immune response. During the cyclic treatment, after a 3-week break in probiotic feeding and the reintroduction of probiotics, there was a significant stimulation of the gene expression of molecules associated with both cellular and humoral immunity (CD8, TGF-β, IL8, TLR9), without affecting the gene expression for IL1 and TNF-α. We can conclude that, in aquaculture, this probiotic feed can be used with a continuous application, which does not cause excessive immunostimulation, or with a cyclic application, which provides the opportunity to stimulate the immunity of trout, for example, in periods of stress.
Keywords: salmonid, probiotic feed, intestinal immune answer, gut microbiota
1. Introduction
Aquaculture is one of the fastest-growing branches of the economy, providing employment and food for millions of people worldwide, with production, consumption, and fish trade expected to increase steadily in the upcoming years [1]. Capacities to produce such quantities of fish are limited. Therefore, the concentration of fish on these farms increases. This leads to a higher level of stress that inevitably causes a risk of diseases and mortality with high economic significance. Such stress also causes worse reproduction and growth in animals [2,3,4]. Farm owners find themselves in a situation where they have to invest significant efforts and funds to prevent and control emerging diseases that can decimate fish monoculture, since the European Union banned the use of antibiotics as growth promoters in 2006 (European Commission in 2003). With stricter legislation to stop the development of antimicrobial resistance being enacted every year, and the focus on healthier foods that do not contain antimicrobial residues, there has been a need for alternative solutions that serve a similar purpose (e.g., probiotics, prebiotics, humic substances, phytoaditives, etc.).
A probiotic in aquaculture are defined as ‘a live, dead or component of a microbial cell that when administered via the feed or to the rearing water benefits the host by improving either disease resistance, health status, growth performance, feed utilization, stress response or general vigor, which is achieved at least in part via improving the host’s microbial balance or the microbial balance of the ambient environment’ [5]. In general, the most frequently used and studied are lactic-acid-producing bacteria (LAB), as reviewed by Chizhayeva et al. [6]. The interest in LAB is based on the fact that these bacteria are part of the natural gastrointestinal tract (GIT) microbiota and have the ability to tolerate the acidic environment and the action of bile acids in the digestive tract. By the fermentation of sugars, LAB produce lactic acid or other organic acids that lower the pH in the GIT and therefore create conditions that naturally protect the digestive tract from colonization by undesirable bacteria [7]. Other mechanisms by which probiotic LAB positively affect the intestinal microbiota include the production of inhibitory substances such as hydrogen peroxide or bacteriocins [8]. Probiotics affect the adherence of pathogens to the intestinal mucosa by blocking binding sites [9] and include other beneficial effects such as nutritional competition and immunomodulation [10]. The increasing economic importance of bacterial infections and stress in aquaculture has enhanced the interest in the study of defense mechanisms against these diseases. Teleosts, as well as warm-blooded animals, including humans, naturally possess components of innate and acquired immunity. Commensal gastrointestinal microbiota (CGIM) is the key to the optimal development of the immune system, and therefore, maintaining the physiological structure of CGIM is necessary to maintain the health of the host [11]. Currently, one of the most used methods for the regulation of the composition of CGIM is the application of probiotics and other bioactive compounds such as short-chain fatty acids [12], various plant extracts [13], or microalgae [14]. The immunomodulatory effect of probiotic bacteria has been confirmed in many studies by an observed increase in phagocytic activity, metabolic activity of phagocytes, complement activity, levels of lysozyme, the number of Ig+ cells in the gut, and the gene expression for the immunologically important molecules (e.g., cytokines), resulting in the improvement of the functions of the intestinal systems of fish as well as the resistance of fish to infections [6,15]. For these reasons, we consider the use of probiotics in aquaculture to be a potentially valuable tool going forward.
In our previous research, we isolated and identified two strains of autochthonous LAB from the intestine of healthy rainbow trout—Lactobacillus plantarum R2 (CCM 8674) and Lactobacillus fermentum R3 (CCM 8675), both with strong inhibitory activity against Aeromonas salmonicida and Yersinia ruckeri, which are currently among the most serious bacterial pathogens impacting salmonid fish. Both strains of LAB showed a high tolerance to the trout gut environment and the best growth abilities at different temperatures. Moreover, the strains complied with antibiotic susceptibility requirements, as regulated by the European Food Safety Authority. Both strains of LAB were sensitive to ampicillin, gentamicin, kanamycin, erythromycin, clindamycin, tetracycline, and chloramphenicol, and in addition, L. fermentum R3 was also sensitive to streptomycin [16,17]. Lactobacillus plantarum R2 also affected the cytokine response in the intestinal cell primary culture, which was challenged with A. salmonicida and Y. ruckeri. Strain R2 stimulated the immune response after Y. ruckeri-induced immunosuppression and the reduced inflammation caused by A. salmonicida [18].
A successful practical application of probiotics is highly dependent on the method of their application and the dosage form, as this is a relatively sensitive biological material. Choosing appropriate and effective dosage forms is necessary to obtain optimum probiotic effects. The ability of probiotic microorganisms to survive and multiply in the host strongly influences their positive effect [19]. Bacteria should be metabolically stable and active in the product and survive during the passage through the intestinal tract in large numbers. Under the directive of the FAO/WHO, the probiotic effects of food or feed may be declared only if they contain at least 106 to 107 live probiotic bacteria per gram. The current dosage forms for the large-scale production systems of animals are prepared for application to powdery and granular mixtures or water. Probiotics are incorporated in compound feed in various ways—during and after the processing of feed, by mixing in, or by spraying on the surface of the feed. The production of animal feed comprises granulation and compression, requiring high temperatures and pressures, which negatively affect the viability of the probiotic bacteria in the feed. Live probiotics stabilized by freeze-drying and mixed into powdery feed mixtures place high demands on storage conditions and demand often practically unreasonable demands for their activation. Fresh inoculants are unstable, and they have often limited, usually short shelf lives, which complicates storage; they are therefore are less useful in practice. For our probiotic strains, we have developed a new, cheap, and stable application form suitable for aquaculture. The mixture of biodegradable compounds together with microencapsulation proved to be effective in protecting the probiotic strain, which was applied directly to the surface of commercial pelleted fish feed. This technology, together with cold storage, ensured high concentrations of live probiotic bacteria for months after the preparation of this feed [20].
This technological procedure was used for the preparation of probiotic feed, the effect of which was subsequently monitored under in vivo conditions on rainbow trout. One of the experiment’s goals was to find a suitable application schedule (continuous versus cyclic application scheme) based on the evaluation of the influence on the immune response and intestinal microbiota. The effect on the immune response was assessed by monitoring the relative gene expression of immunologically important molecules in the trout intestines.
2. Materials and Methods
2.1. The Preparation of Probiotic Feed for Aquaculture
2.1.1. Cultivation of Probiotic Bacteria and Preparation of Bacterial-Starch Hydrogel
The strain Lactobacillus plantarum R2 (CCM 8674) (according to the new taxonomy Lactiplantibacillus plantarum [21]) was used to prepare a probiotic diet. The strain was isolated from the intestinal content of rainbow trout (Oncorhynchus mykiss) reared at the fish farm Rybárstvo Požehy s.r.o. in the Slovak Republic [17]. An 18 h strain culture in 1 L MRS broth (HiMedia, Karnataka, India) was prepared for coating 1 kg of commercial pellets (EFICO Enviro 921 3 mm, BioMar, Brande, Denmark) at 37 °C in a shaker (PSU-20i Orbital Multi-Platform Shaker, Biosan, Riga, Latvia). After incubation, the bacterial culture in the MRS broth was centrifuged at 1000× g for 20 min at 22 °C (ROTINA 420R, Hettich, Tuttlingen, Germany). The cell pellets were washed twice in PBS (MP Biomedicals, Illkirch-Graffenstanden, France). The resulting cell pellet was considered a 100% cell suspension, from which a 25% suspension of L. plantarum R2 probiotic bacterial cells was prepared by resuspension in sterile saline. The dispersion was blended for 60 min on an electromagnetic plate (AccuPlate-Stirrer, Labnet International Inc., Edison, NJ, USA) with Starch 1500® (Colorcon, Dartford, Kent, England) until a probiotic bacterial-starch hydrogel was formed.
2.1.2. Coating of Commercial Aquafeed
The first coating layer on the commercial pellets (EFICO Enviro 921 3 mm, BioMar, Brande, Denmark) was created with colloidal silicon dioxide (Aerosil® 200, Evonik Industries, Hanau, Germany) for 5 min at 40 rpm using a cube mixer (Erweka KB 20, Langen, Germany). Aerosil® 200 adhered to the surface of the pellets formed a dry sorption layer for the next liquid layer. Then, the bacterial-starch hydrogel was evenly poured onto the aerosol-coated pellets and mixed again for 5 min at 40 rpm using a cube mixer (Erweka KB 20, Langen, Germany) (Table 1). The control diet was prepared via the same multiple-coating procedure, except that bacterial cells of L. plantarum R2 were added to the starch hydrogel layer. Finally, the pellets were dried at 35 °C for 4 h in a hot-air oven (Universal Oven UN 55, Memmert, Büchenbach, Germany).
Table 1.
Mass of components for preparation of probiotic aquafeed.
| Components | Weight (g) |
|---|---|
| Pellets (EFICO Enviro 921 3 mm) | 1000 |
| 25% suspension of L. plantarum R2 in sterile saline | 120 |
| Colloidal silicon dioxide (Aerosil® 200) | 10 |
| Pregelatinized maize starch (Starch 1500®) | 10 |
The analytical constituents of both feeds are presented in Table 2. Feed analyses were performed in the laboratory of the Research Institute for Animal Production Nitra, National Agricultural and Food Center, Slovakia. The contents of dry matter, crude protein, fiber, fat, and ash were determined according to Commission Regulation (EC) No. 152/2009 [22]. Briefly, dry matter was determined by weighing and drying at 103 ± 2 °C. Nitrogenous compounds were measured according to the Kjeldahl method using the Kjeltec 8400 (FOSS, Hilleroed, Denmark) machine, fiber was determined via the Hennenberg-Stohman method using a Fibertec 8000 (FOSS, Hilleroed, Denmark), fat content was measured according to the Soxhlett-Henkel method using an SER 148 (Velp Scientifica Srl, Usmate Velate, Italy), and ash content was determined by weighing the sample and heating to 550 °C in a muffle furnace. Amino acids were measured by ion exchange chromatography on an automatic amino acid analyzer AAA 400 (Ingos, Prague, Czechia). Macro- and micronutrients were analyzed using an atomic absorption spectrometer, the iCE 3500Z (FOSS, Hilleroed, Denmark).
Table 2.
Analytical constituents of control feed (coated with silicon-starch hydrogel) and probiotic feed (containing L. plantarum R2 in the silicon-starch hydrogel).
| Constituents | Control (Coated) | Probiotic |
|---|---|---|
| Dry matter (DM), g/kg | 850.7 | 858.3 |
| Nitrogenous compounds, g/kg | 439.8 | 439.4 |
| Crude fiber, g/kg | 21.9 | 20.3 |
| Fat, g/kg | 190.4 | 192.5 |
| Ash, g/kg | 74.1 | 75.1 |
| Organic matter, g/kg | 776.6 | 793.3 |
| Total calcium, g/kg | 10.4 | 10.4 |
| Total phosphorus, g/kg | 5.9 | 5.6 |
| Magnesium, g/kg | 2.2 | 2.2 |
| Sodium, g/kg | 3.5 | 3.5 |
| Potassium, g/kg | 6.2 | 6.3 |
| Iron, mg/kg | 232.9 | 244.9 |
| Manganese, mg/kg | 17.1 | 20.3 |
| Zinc, mg/kg | 85.8 | 90.7 |
| Copper, mg/kg | 70.0 | 78.4 |
| Aspartic acid, g/kg DM | 51.3 | 50.7 |
| Threonine, g/kg DM | 24.6 | 24.3 |
| Serine, g/kg DM | 25.8 | 25.9 |
| Glutamic acid, g/kg DM | 93.5 | 89.3 |
| Proline, g/kg DM | 26.7 | 25.4 |
| Glycine, g/kg DM | 27.8 | 26.6 |
| Alanine, g/kg DM | 30.9 | 28.6 |
| Valine, g/kg DM | 21.3 | 24.0 |
| Isoleucine, g/kg DM | 14.7 | 16.5 |
| Leucine, g/kg DM | 42.8 | 42.9 |
| Tyrosine, g/kg DM | 14.4 | 15.3 |
| Phenylalanine, g/kg DM | 23.7 | 24.4 |
| Histidine, g/kg DM | 19.8 | 18.7 |
| Lysine, g/kg DM | 35.9 | 36.4 |
| Arginine, g/kg DM | 30.8 | 31.1 |
| Methionine, g/kg DM | 10.5 | 9.5 |
| Cystine, g/kg DM | 5.9 | 5.7 |
After drying the coated pellets, the total number of L. plantarum R2 was ~108 CFU/g, as determined using the spread plating method on MRS agar (Merck, Darmstadt, Germany), anaerobically incubated (GasPak system, Becton Dickinson, San Diego, CA, USA) at 37 °C for 48 h. The probiotic diet was stored at 4 °C until it was fed to the experimental fish.
2.2. Experimental Animals and Sampling
For this experiment, 1000 juvenile rainbow trout (Oncorhynchus mykiss) with an average weight of 77.74 ± 0.38 g were divided into three groups based on their subsequent feeding regime, and each group was further separated and reared in three 1000 L tanks for a total of 9 tanks (3 tanks/group and 111 fish per tank) with continuous aeration and independent recirculation. Fish were maintained in a 12 h photoperiod (12 h light and 12 h dark). After a two-week adaptation period, during which the fish received the commercial feed (EFICO Enviro 921 3 mm, BioMar, Brande, Denmark) without the silicon-starch hydrogel coating, the fish started receiving probiotic feed or commercial feed with the silicon-starch hydrogel coating, respectively. The first group received probiotic feed continually (CON) during the whole duration of the experiment (11 weeks). The second group received the probiotic feed in cycles (CYC)—after 4 weeks of application, there was a 3-week break during which the fish received commercial feed coated with protective components and then were again fed with the probiotic feed for another 4 weeks. The third group served as a control group (CTRL) and received commercial feed coated with protective components. It was observed that the fish in each group consumed feed with a good appetite. The feed ratio was 2.68% of the fish’s body weight. Water temperature, pH, and oxygen saturation were monitored two times every day. The water properties during the experiment were as follows: temperature 17.5 ± 0.3 °C, oxygen saturation 89% ± 3%, and pH 7.1 ± 0.1.
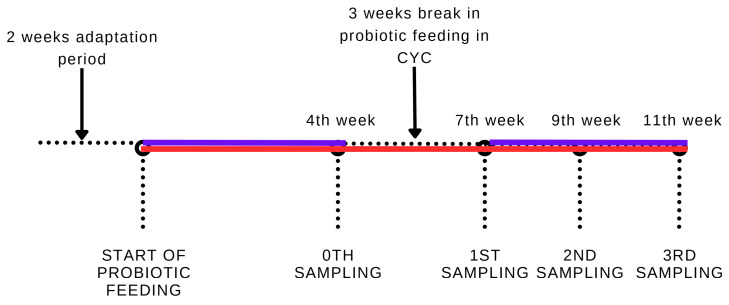
Samples for relative gene expression analysis were taken 7 (1st sampling), 9 (2nd sampling), and 11 (3rd sampling) weeks after the start of the experiment, when 8 fish were randomly selected from each group. The fish were sacrificed by stunning with a blow to the back of the head, followed by a spinal cord transection. Each fish was dissected and samples of the middle intestine (the part immediately behind the pyloric intestine) were taken for further analysis. Samples were stored in RNAlaterTM solution (Invitrogen, Thermo Fisher Scientific Baltics, Vilnius, Lithuania) at −20 °C until processing. For better visualization of the sampling scheme, see Figure 1.
Figure 1.
Scheme of application and sampling of rainbow trout fed probiotic feed. The red line group received probiotic feed continually (CON); the blue line group received probiotic feed cyclically (CYC).
Ethical Statement
The experimental steps complied with national legislation—Act No. 246/1992 Coll., on the Protection of Animals against Cruelty, as amended, and Decree No. 419/2012 Coll., on the Protection, Breeding, and Use of Experimental Animals, as amended.
2.3. Measurement of Relative Gene Expression
Further processing of samples was conducted at the University of Veterinary Medicine and Pharmacy in Kosice. Samples were homogenized by a Precellys 24 Tissues Homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France) using 6000 rpm in 2 × 30 s cycles with a 15 s break between them. RNA was isolated using an Omega E-Z Total RNA Kit (Omega Bio-tek, Norcross, GA, USA). The purity and concentration of RNA was measured using a spectrophotometer Nanodrop 8000 (Thermo Scientific, Waltham, MA, USA) at 260/280 nm. The isolated RNA was then transcribed into cDNA using a Quantitect Reverse Transcription Kit (Qiagen, Hilden, Germany). The PCR reaction was performed in 10 μL reactions, with each reaction consisting of 5 μL iQ™ SYBR® Green Supermix (BioRad, Hercules, CA, USA), 0.5 μL of forward and 0.5 μL of rear primer (c = 10 μM/μL) for selected genes (Table 3), and 4 μL of cDNA with a concentration of 10 ng/μL. β-actin was used as a reference gene. All reactions were performed as triplicates and each assay contained a negative control without a cDNA template. Relative gene expression was analyzed in the samples of cDNA by qPCR using thermocycler iCycler CFX96 (BioRad, Hercules, CA, USA). The protocols for each gene are in Table 3. Relative gene expression was measured as ΔΔCt (±SD) with CFX96 Manager Software (BioRad, Hercules, CA, USA).
Table 3.
Sequences of primers used in qPCR and relevant references containing qPCR conditions.
| Gene | Primer Sequence | Reference |
|---|---|---|
| β-actin F | GGACTTTGAGCAGGAGATGG | [23] |
| β-actin R | ATGATGGAGTTGTAGGTGGTCT | |
| igm F | ACCTTAACCAGCCGAAAG | [24] |
| igm R | TGTCCCATTGCTCCAGTC | |
| cd4 F | CCTGCTCATCCACAGCCT | [24] |
| cd4 R | CTTCTCCTGGCTGTCTGA | |
| cd8 F | AGTCGTGCAAAGTGGGA | [24] |
| cd8 R | GGTTGCAATGGCATACAG | |
| il1-β F | ACATTGCCAACCTCATCATCG | [25] |
| il1-β R | TTGAGCAGGTCCTTGTCCTTG | |
| tnf-α F | GGGGACAAACTGTGGACTGA | [26] |
| tnf-α R | GAAGTTCTTGCCCTGCTCTG | |
| il8 F | CACAGACAGAGAAGGAAGGAAAG | [23] |
| il8 R | TGCTCATCTTGGGGTTACAGA | |
| tgf-β F | TCTGAATGAGTGGCTGCAAG | [27] |
| tgf-β R | GGTTTCCCACAATCACAAGG | |
| tlr9 F | GCAACCAGTCCTTCCACATT | [28] |
| tlr9 R | AAACCCAGGGTAAGGGTTTG |
F—forward, R—reverse.
2.4. Microbiological Screening
For microbiological screening, the determination of the counts of lactic acid bacteria, coliform bacteria, and total aerobic bacteria in the intestinal contents as well as adhered to the intestinal mucosa of trout was used. Samples were collected on the 4th (0th sampling), 7th (1st sampling), and 11th (3rd sampling) weeks. The middle part of the intestine was separated, and the content was gently squeezed into a sterile container. Subsequently, the intestine was rinsed three times with a sterile PBS and then cut longitudinally. The mucosa was scraped with the edge of a sterile glass slide. Feces and mucosal scraping samples were homogenized in isotonic saline solution (Stomacher, Seward, Worthing, UK), diluted by tenfold dilution, and bacterial counts were determined using the plate method. MRS agar plates (Merck, Darmstadt, Germany) incubated for 48 h at 37 °C under anaerobic conditions (GasPak system, Becton Dickinson, San Diego, CA, USA) were used to determine the amounts of LAB. Coliform bacteria were counted on MacConkey agar plates (HiMedia, Karnataka, India), and total aerobic bacteria were counted on blood agar plates composed of Columbia agar (HiMedia, Mumbai, India) and 5% of defibrinated sheep blood. The MacConkey and blood agar plates were incubated aerobically for 24 h at 37 °C. The bacterial counts are expressed in log10 of colony-forming units per gram of content/mucosa (log10 CFU/g) ± standard deviation.
2.5. Histo-Fluorescent In Situ Hybridization Analysis of L. plantarum in the Trout Intestines
The histo-FISH method was used to detect the location and abundance of L. plantarum in the trout’s middle gut. The samples were processed according to the HISTO-FISH protocol, which was standardized in our laboratory according to Madar et al. [29]. In brief, segments of the middle intestine, approximately 2–3 cm long, were ligated and collected without removing the intestinal contents. They were washed in PBS (pH 7.2) and immediately fixed for 4 h at 4 °C in Carnoy’s solution, composed of 60% ethanol, 30% chloroform, 10% glacial acetic acid, and 1 g of ferric chloride. After fixation, the samples were dehydrated through an alcohol series with ethanol concentrations of 70%, 90%, 96%, and 100%, embedded in paraffin, and subsequently, 10 µm thick sections were prepared. The sections were mounted on coated microscopic glass slides (Dako, Agilent Technologies, Glostrup, Denmark). In the next step, the slides were deparaffinized with xylene, rehydrated in a graded alcohol series from 100% to 50%, and washed in PBS. The slides of the highest quality were selected for processing using the above-mentioned standardized FISH procedure. For the detection of Lactobacillus plantarum, probe Lpla 990 5′ATCTCTTAGATTTGCATAGTATG3′, labeled with Texas red on the 5′ end with excitation 587 nm and emission 647–670 nm (red color), was used (Sigma Aldrich, St. Louis, MO, USA). The hybridization was carried out overnight at 52 °C in a dark humid atmosphere. After hybridization, the slides were stained with 4′,6-diamidino-2-phenylindole (DAPI) dye (excitation at 365 nm and emission at 445–450 nm) for 10 min. Finally, the slides were immersed in Vectashield mounting medium and covered by coverslips (Vector Laboratories, Burlingame, CA, USA). An epifluorescence microscope Carl Zeiss Axio Observer Z1 equipped with Software Carl Zeiss Zen 2 (ver. 3.3. blue edition) (Carl Zeiss, Oberkochen, Germany) was used for evaluation.
2.6. Morphometry of Intestine
During the second sampling, samples from the intestines of 6 fish per group were collected to assess the morphology of the intestine. Samples were washed in a physiological solution and fixed using 4% neutral formaldehyde. After fixation, dehydration was conducted using increasing alcohol concentrations (30%, 50%, 70%, 90%, and absolute ethanol). Samples were then clarified using benzene and embedded in paraffin. Histological sections of 5 µm (10 from each sample) were stained using hematoxylin–eosin. The height, width, and cross-section surface of the intestinal villi were measured using the Image C analytic system (Imtronic GmbH, Berlin, Nemecko) and the IMES program, using a color camera (SONY 3 CCD) and a light microscope (Axiolab, Carl Zeiss Jena, Nemecko) at 40× magnification.
2.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism Version 9.0.0 software (GraphPad Software, Inc., San Diego, CA, USA). Data normality was analyzed using the Shapiro–Wilk test for each individual parameter in each sampling. Since the data showed normality, they were further tested by one-way ANOVA followed by post hoc multi-comparison Tukey’s test to determine differences between the groups in each sampling. Differences between groups were considered significant if the p-value was lower than 0.05. Data are expressed as means ± standard deviations.
3. Results
3.1. Intestinal Immune Response
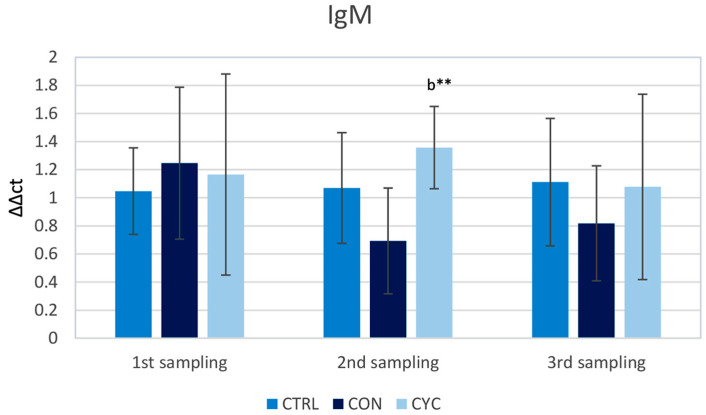
The level of relative gene expression of igm was measured as a marker of antibody response. In the first and the last sampling, no significant difference between groups was observed. In the second sampling, trout showed a significant increase in the intestinal igm gene expression compared to continually fed fish. (Figure 2).
Figure 2.
Influence of continuous and cyclic application of probiotic feed on the relative igm gene expression in the trout gut. b—significantly different from group CON; ** p < 0.01.
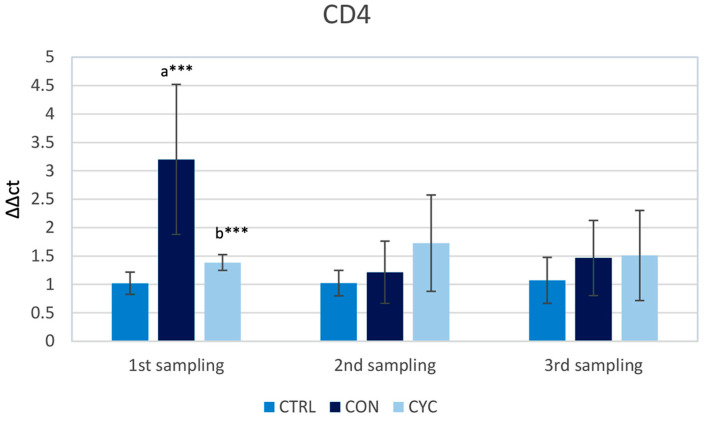
The gene expression of cd4 and cd8 T cell co-receptors was studied to assess the cellular immune response at the gut level. The relative gene expression of cd4 in the gut was significantly higher in the first sampling in fish continuously fed probiotics in comparison with the control as well as the cyclic group. During the second and the third sampling, there was no significant difference in cd4 gene expression between the groups, but there appeared to be a trend of increasing expression in the CON and CYC treatments, respectively (Figure 3).
Figure 3.
Influence of continuous and cyclic application of probiotic feed on the relative gene expression of cd4 gene in the trout gut. a—significantly different from the control group; b—significantly different from group CON; *** p < 0.001.
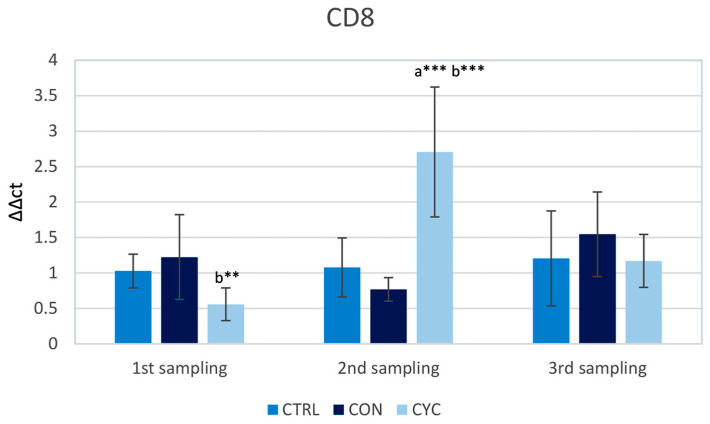
In the first sampling, the highest level of expression of cd8 was recorded in the continuously fed group, with the cyclic group having a significantly lower level of expression in comparison to the continually fed group. On the contrary, in the second sampling, the cyclic group had a significantly higher expression when compared to the control and continual group, and in the third sampling, the values did not differ significantly in individual groups (Figure 4).
Figure 4.
Influence of continuous and cyclic application of probiotic feed on the relative gene expression of cd8 gene in the trout gut. a—significantly different from the control group; b—significantly different from group CON; ** p < 0.01, *** p < 0.001.
The cytokine response was assessed based on the gene expressions of pro-inflammatory cytokines (il1-β and tnf-α) and chemokine (il8). tgf-β was selected as a representative of anti-inflammatory cytokines.
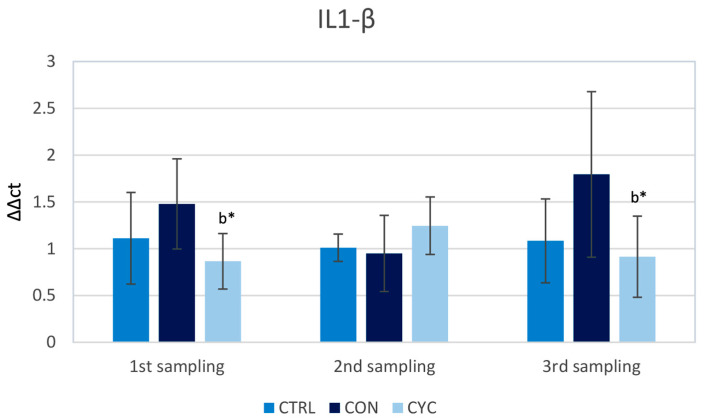
The results show that il1-β relative gene expression was slightly, but not significantly, elevated in the continually fed group during the first and last sampling; however, such an increase was not observed during the second sampling. The cyclic group showed significantly lower levels during the first and third samplings when compared to the continual group, but these were not significantly different from the control (Figure 5).
Figure 5.
Influence of continuous and cyclic application of probiotic feed on the relative gene expression of cytokine il1-β in the trout gut. b—significantly different from group CON; * p < 0.05.
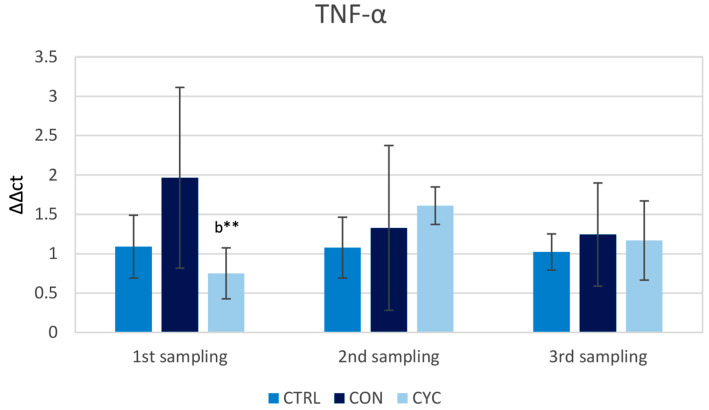
The expression levels of tnf-α had the biggest variance among individual fish in their respective groups, contributing to a relatively high standard deviation. The cyclic group had a significantly lower level of tnf-α gene expression when compared to the continual group during the first sampling, but this was not significantly different from the control. During the second sampling, both experimental groups showed an increasing trend over the control, Lastly, during the third sampling, the expression levels of the tnf-α gene were approximately equal in both experimental groups and not significantly elevated above control levels (Figure 6).
Figure 6.
Influence of continuous and cyclic application of probiotic feed on the relative gene expression of cytokine tnf-α in the trout gut. b—significantly different from group CON; ** p < 0.01.
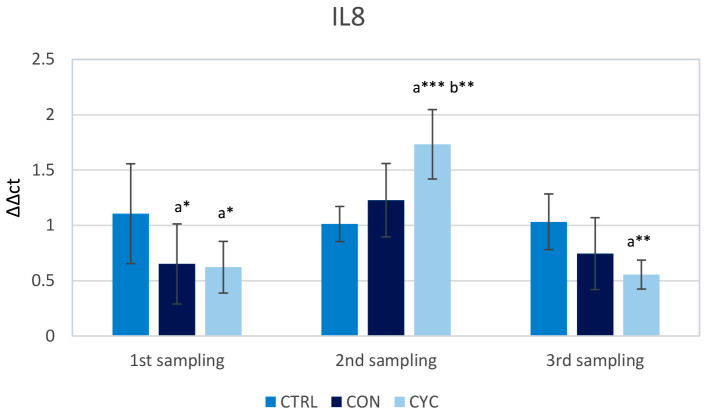
The il8 gene showed a significant decrease in expression in the experimental groups during the first sampling. During the second sampling, the expression levels of il8 were significantly increased in the cyclical group when compared with the control. In the third sampling, both experimental groups had lower relative gene expression for this gene in comparison to the control, with the cyclic group’s expression being significantly lower than the control (Figure 7).
Figure 7.
Influence of continuous and cyclic application of probiotic feed on the relative gene expression of cytokine il8 in the trout gut. a—significantly different from the control group; b—significantly different from group CON; * p < 0.05, ** p < 0.01, *** p < 0.001.
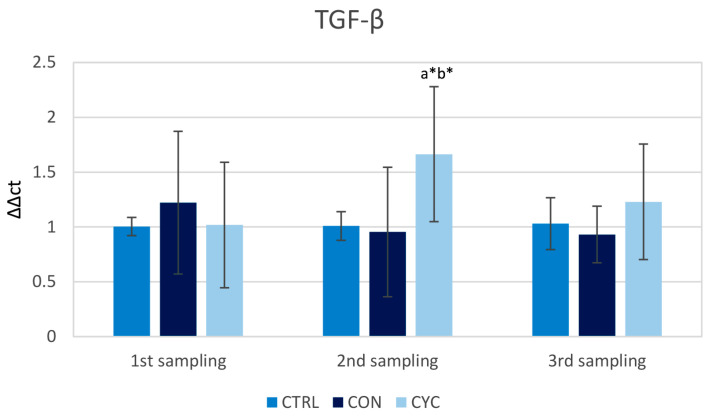
The relative gene expression of tgf-β did not differ between the groups in the first sampling. It was observed that the cyclic group had a higher level of expression in the second sampling after receiving probiotic feeding following the pause in feeding. tgf-β gene expression returned to the level of the control group after 2 weeks of receiving the probiotic feed, i.e., in the last sampling. The continually fed group did not show a significant change in the relative gene expression of tgf-β during the whole experiment (Figure 8).
Figure 8.
Influence of continuous and cyclic application of probiotic feed on the relative gene expression of cytokine tgf-β in the trout gut. a—significantly different from the control group; b—significantly different from group CON; * p < 0.05.
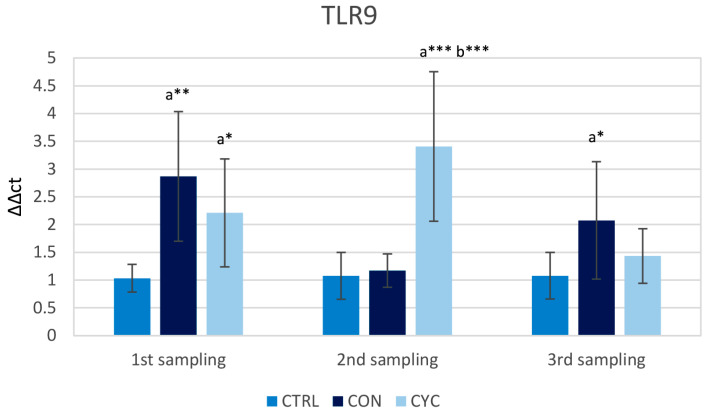
tlr9 belongs to a family of molecules that detect the presence of foreign cells in the intestine. During the first sampling, both experimental groups showed a significantly higher level of tlr9 gene expression than the control group. During the second sampling the relative gene expression of tlr9 was increased in the CYC group when compared to the control and continual group. During the last sampling, a significant increase in expression in the CON group over the control was observed, but the CYC group’s expression was not significantly different from that of the control (Figure 9).
Figure 9.
Influence of continuous and cyclic application of probiotic feed on the relative gene expression of tlr9. a—significantly different from the control group; b—significantly different from group CON; * p < 0.05, ** p < 0.01, *** p < 0.001.
3.2. Microbiological Results
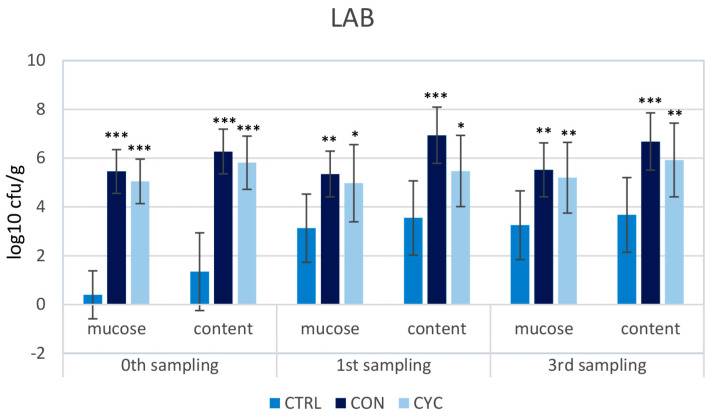
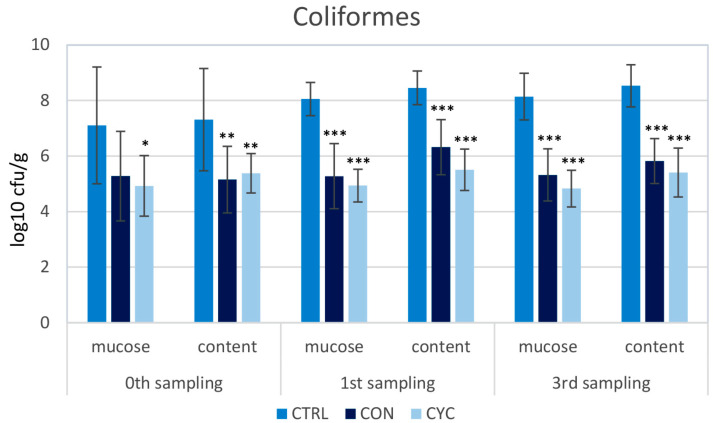
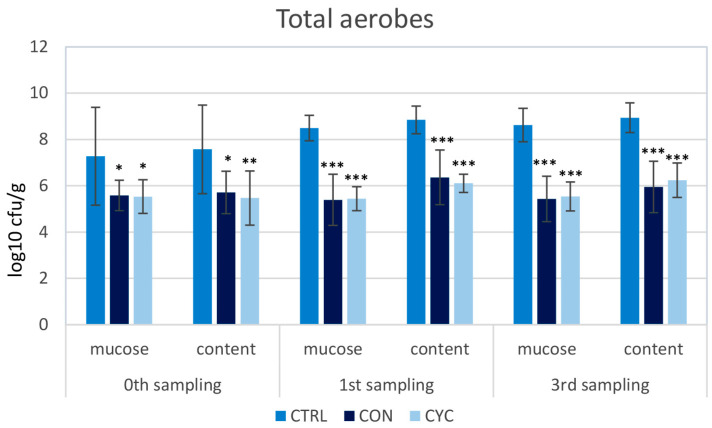
To assess the impact of the new probiotic feed and feeding regime on the intestinal microbiota of trout, a screening of the representation of LAB, coliform bacteria, and total aerobes was carried out. The proportions of the monitored species of bacteria were very similar in the continuously and cyclically fed groups; no significant differences were recorded between these groups in any sampling. LAB counts were significantly higher in all three samplings in both probiotic-fed groups compared to the control (Figure 10). Conversely, the numbers of coliform and total aerobic bacteria were lower in the experimental groups than in the control group (Figure 11 and Figure 12). Before starting the application of probiotic feed, screening of LAB representation was carried out: the numbers were very low and ranged from 0–102 CFU/g of mucosa or intestinal contents (data not shown). For this reason, the difference between the representation of LAB in the control and experimental groups is the most pronounced in the zeroth sampling, i.e., 4 weeks after the start of the administration of the probiotics.
Figure 10.
Influence of continuous and cyclic application of probiotic feed on the amounts of lactic acid bacteria adhered to the gut mucosa and in the gut content of rainbow trout. Significant differences from the control group are marked with stars. Levels of significance: * p < 0.05; ** p < 0.01; *** p < 0.001.
Figure 11.
Influence of continuous and cyclic application of probiotic feed on the amounts of coliform bacteria adhered to the gut mucosa and in the gut content of rainbow trout. Significant differences from the control group are marked with stars. Levels of significance: * p < 0.05; ** p < 0.01; *** p < 0.001.
Figure 12.
Influence of continuous and cyclic application of probiotic feed on the amounts of total aerobic bacteria adhered to the gut mucosa and in the gut content of rainbow trout. Significant differences from the control group are marked with stars. Levels of significance: * p < 0.05; ** p < 0.01; *** p < 0.001.
3.3. Histo-FISH Analysis
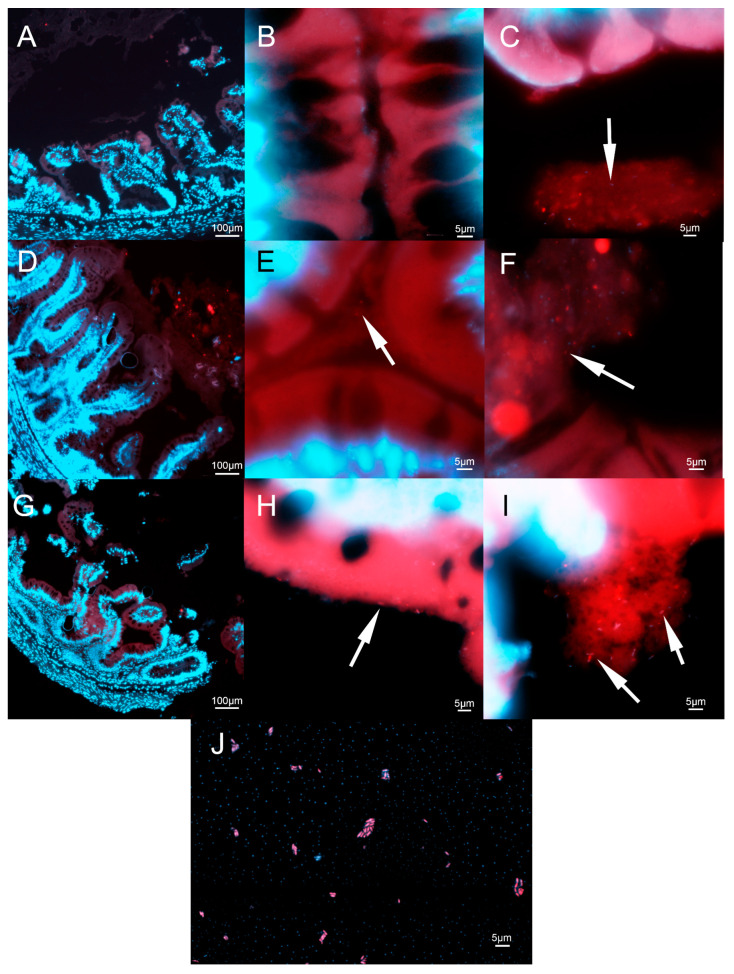
For confirmation of the location and abundance of L. plantarum in the intestines of fish, histological FISH analysis was performed. A control in the form of a bacterial smear that was stained using the same method is presented in Figure 13J. No obvious changes in the morphology of intestines in either experimental group were observed (Figure 13D,G) when compared to the control (Figure 13A). No L. plantarum adhered to the intestinal wall was found in the control group (Figure 13B), and the concentration of bacteria observed in intestinal content was also low (Figure 13C). In the continually fed group, there were some L. plantarum present near the gut wall, although at a low concentration (Figure 13E). A high amount of L. plantarum was observed in the intestinal content of fish from this group (Figure 13F). Lastly, high amounts of L. plantarum and other bacteria were detected in the mucosa of the intestine in the cyclic group (Figure 13H), as well as in the gut content (Figure 13I). Overall, higher concentrations were observed in both experimental groups’ intestinal content compared to the control group.
Figure 13.
The intestine of rainbow trout after staining. The intestinal section of the control (A–C), continually fed (D–F), and cyclically fed groups (G–I). Bacterial smear of L. plantarum for confirmation of the function of staining (J). Arrows indicate L. plantarum.
3.4. Morphometry of the Intestine
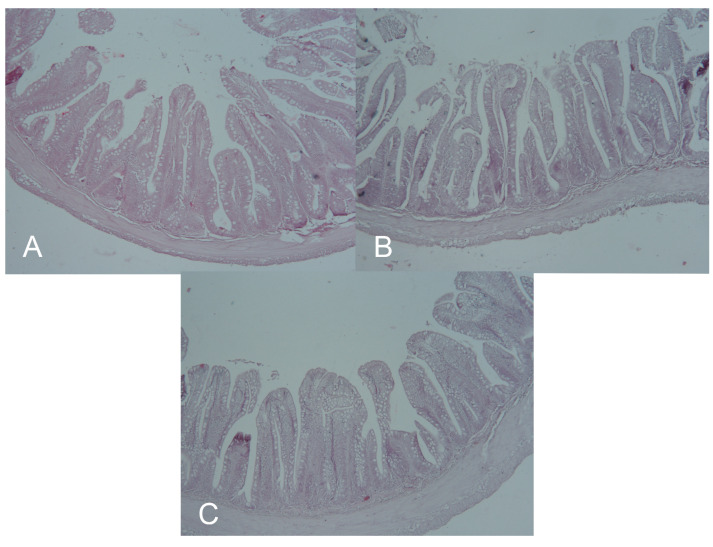
In both probiotic groups, the surface of the villi was increased significantly compared to the control group. In the case of the cyclic group, a significant increase when compared to the continual group was observed. The height of the villi was not significantly different between the experimental groups and the control; however, the cyclic group also had a significantly increased height in comparison to the continual group. Lastly, the width of the intestinal villi was not significantly different between groups (Table 4). A microscopic view of the intestines is presented on Figure 14.
Table 4.
Morphometric parameters of the intestine of rainbow trout fed probiotics continually (CON) and cyclically (CYC) and the control (CTRL).
| CTRL | CON | CYC | |
|---|---|---|---|
| Average cross-section surface (µm2) | 368,505.4 ± 235,495.5 | 533,749.5 ± 218,978.6 a *** | 706,911.7 ± 267,860.5 a *** b * |
| Average villus height (µm) | 1404.6 ± 289.3 | 1374.8 ± 296.4 | 1512.7 ± 396.6 b * |
| Average villus width (µm) | 460.9 ± 92.0 | 432.6307 ± 101.9 | 435.8 ± 93.2 |
a—a significant difference to CTRL, b—a significant difference to CON. Levels of significance: * p < 0.05; *** p < 0.001.
Figure 14.
Histological sections of rainbow trout intestine from the CTRL (A), CYC (B) and CON (C) groups.
4. Discussion
LAB are part of the normal intestinal microbiota of fish [30]. The majority of successfully used probiotic preparations contain LAB, both in human and veterinary practice, including use in aquatic animals. When using autochthonous strains of probiotic microorganisms, there is a higher probability of their good adaptation to the conditions of the organism of the relevant animal species or its environment, as compared to non-autochthonous strains. Several experiments have been carried out in which the impact of the application of probiotics on the health and production parameters of trout was monitored. Some studies have used autochthonous strains [31,32,33], while others have used non-autochthonous ones [34,35,36,37], with varying results. In our experiment, we focused on testing the influence of the L. plantarum R2 strain, isolated from the intestine of healthy rainbow trout, which has demonstrated positive probiotic properties in previous in vitro [17,18] and in vivo experiments in Atlantic salmon [38]. In addition, we applied the strain using a newly developed, inexpensive application form, which is based on placing live probiotic bacteria in the protective starch hydrogel layer. This application form ensures the rapid revitalization of bacteria in the digestive tract and aquatic environment, as well as their long-term survival in storage conditions [20]. This new application form was used in an in vivo experiment for the first time. An important finding was that the trout willingly accepted it, so the food intake was not affected, as confirmed by fish weights in the experimental groups that did not differ or were even higher than in the control group (unpublished data). In addition, we tested two different feeding regimes—the continuous and intermittent application of probiotic feed to monitor the immune response of the fish. Often during the long-term application of immunomodulators, the immune system “adapts” and stops reacting to them. For this reason, we hypothesized that after a break in administration, cyclic application could activate stronger immunomodulation/immunostimulation than continuous application. Meanwhile, continuous application, which would be appropriate in farms with poor water quality, low zoohygienic standards, or higher levels of environmental risks, could cause unnecessary long-term immunostimulation, which is not desirable. Therefore, we focused primarily on monitoring the immune responses of fish, namely in the place of the direct action of the probiotic feed—in the trout intestine. We focused on selected markers of cellular as well as humoral immunity. Based on our previous results, we hypothesized that our probiotic strain could stimulate the expression of genes associated with non-specific immune response (TLR9, IL8) as well as for anti-inflammatory cytokine (TGF-β), but not for pro-inflammatory cytokines (IL1, TNFα). We also assumed there would be an influence on gene expression for some markers of cellular immunity. In order to examine the established hypotheses, in the cyclic group, samples were taken for immunological analyses after a 3-week break in the application of probiotics (first sampling); 2 weeks after reintroducing the probiotic feed (second sampling), where we expected stimulation; and after another 2 weeks (third sampling), where we expected adaptation to the long-term application of probiotics.
IgM is one of three classes of immunoglobulins that we can find in teleost fish, with the other two being IgD and IgT (IgT is sometimes called IgZ in certain teleosts) [39]. IgM is the most abundant Ig in teleost plasma, where it can be found in monomeric and tetrameric forms [40], and its concentration increases following bacterial infection, with a shift of isoform from monomer to tetramer [41]. Our results show that relative gene expression was elevated in fish from the CYC group after the reintroduction of probiotic bacteria into the intestinal tract when compared to continually fed fish. This difference between the CYC and CON groups was evident only 2 weeks after the re-introduction of the probiotic feed (second sampling); after another 2 weeks of probiotic feeding, there were no more differences between the groups (Figure 2). This difference between the groups could be explained by a slight decrease in the expression of the CON group during the second sampling, and a slight increase in that of the CYC group, even though the important takeaway is that none of the experimental groups differed significantly from the control. Gene expression in the continually fed group did not differ from the control throughout the experiment. This is in line with an experiment performed by Vazirzadeh et al. [42], who fed rainbow trout with three strains of probiotic bacteria, L. buchneri, L. fermentum, and Saccharomyces cerevisiae, and compared their influence on immune response after 30 and 130 days of feeding. After 30 days of application, they recorded non-significantly higher levels of total serum globulins in all three strains. After 130 days of application, globulin levels were lower compared to the previous sampling and did not differ from the control. An increase in total plasma immunoglobulins was also observed by Balcázar et al. [43] after the administration of probiotic LAB to brown trout. The highest levels were recorded after 3 weeks of Lactococcus lactis feeding. It was proven that fish have intestinal mechanisms to induce immune tolerance to nonpathogenic microorganisms [44] and this may also be the reason why we did not see a significant change in the continually fed group, as the fish organisms had adapted to the presence of probiotic in the intestine. After several days, the levels of the cyclical group decreased to the same levels as the control. It is important to note that expression in CYC-treated group also did not differ from the control during the whole experiment. One of the reasons for this may be that no immune response on the level of IgM is triggered by our probiotic once the organism receives it for a prolonged period, which can also be seen in the CON group. Another reason may be that although the expression has not changed, the serum level of IgM may be increased, as observed by the studies mentioned above, and therefore, a change in expression is not triggered.
Molecule CD4 is found on the surface of Th lymphocytes, where it functions as a coreceptor in the process of the recognition of antigens. CD4+ T cells are responsible, then, for the production of cytokines and thus are essential in the regulation of immune response [45]. Studies on different fish species with different probiotics showed only insignificant changes in gene expression for cd4 in the intestine of experimental animals [46]. A significant increase in the number of CD4 cells at the intestinal level was recorded after infection with viral hemorrhagic septicemia virus in rainbow trout [47]. Our results show an increase in gene expression for cd4 in the CON group as compared to the control as well as the CYC group, but only during the first sampling. In the following samplings, the level of cd4 gene expression in the groups did not differ, and surprisingly, even in the second sampling in the CYC group, there was no significant increase: only a weak tendency to increase was recorded (Figure 3). This difference from the work of Picchietti et al. [46] could be the result of the use of an autochthonous strain of probiotics. The increase in relative expression could potentially prove beneficial to the host organism since its response to pathogens could be more rapid, while the level of expression of cd4 was not high enough to cause any detrimental effect on the organism as a whole. This hypothesis would need to be proven by further research.
CD8 can be found as a co-receptor on the surface of cytotoxic T-lymphocytes. They are predominantly found in the thymus, gill, and intestine, with low concentrations in the blood, spleen, and pronephros of fish [48]. The function of these cells in teleosts is similar to that of these cells in mammals, which is a defense against virus-infected cells [49] and foreign cells [50]. Apart from cytotoxic T lymphocytes, there is also a population of dendritic-like cells, that have CD8 molecules, and their role is thought to be in the regulation of local mucosal immunity and immune tolerance in teleosts [51]. This function is confirmed in mammals [52]. Since our measurement of relative gene expression is not specific to a certain population of CD8 cells, it identifies all subpopulations of CD8+ cells of fish including dendritic-like cells. As observed in our experiment, the relative gene expression for this molecule was highly elevated after the fish in the cyclic group started receiving the probiotic feed again (Figure 4), indicating the immunomodulatory potential of this feeding regime. As we assumed, this stimulation is only temporary, and in the third sampling, when fish in both the CYC and CON groups received probiotic feed for 4 weeks, there were no significant differences between the groups. The significant downregulation in the CYC group compared to the CON group during the first sampling may be the result of a feeding break, as at this point the fish in the CYC group had not received the probiotic feed for 3 weeks. Importantly, however, there were no significant differences between the experimental groups and the control in this sampling. The levels of the continually fed group, which across the whole experiment did not significantly exceed those of the control group, further suggest some sort of stabilization of the CD8+ cell population in the intestine, although as stated before, it is not clear what proportion of the CD8+ cell population is formed by DC-like cells.
Interleukin 1 is considered to be one of the most important pro-inflammatory cytokines in fish [53]. During the experiment, measured levels of gene expression were not significantly different between the CON or CYC groups and the control group (Figure 5). After a prolonged period of consuming probiotic strains (21 and 28 days), similar results were recorded in grass carps, where levels of il1-β in the experimental group did not exceed the control [54], although in the mentioned work there was significant upregulation of il1-β expression after 7 days of receiving the probiotic feed. We did not observe such a phenomenon in the cyclically fed group after these fish had started receiving probiotics after a pause. Interestingly, the gene expression of il1-β was, in our experiment, significantly lowered in the cyclic group when compared to the continual group (Figure 4). This difference was, however, observed after the pause in probiotic feeding of the CYC group and during the third sampling, when both groups were receiving the same feed. The statistically significant difference between the experimental groups appears to be primarily due to the slight increase in values in the CON group. It is also worth noting that there has been research that observed lowered levels of this cytokine in fish fed probiotics [46], and also that these levels were increased [55] even after several weeks, further proving that interaction between different probiotic bacteria and different species of fish and conditions yields specific results. Overall, the lack of an increase in the expression of il1-β could mean that our probiotic has not triggered an inflammatory response at the intestinal level, which would be considered unwanted stress for the fish organism.
TNF-α is a pro-inflammatory cytokine that has many immunological functions, which are aimed at the regulation of cellular response as well as the modulation of other cytokines. The tnf-α gene is commonly expressed in many tissues of rainbow trout after stimulations with pathogens, their components, prebiotics [56], and also vaccines [57]. Similar to the results of the il1 gene, the gene expression for TNF-α in the experimental groups did not differ from the control (Figure 6), and we also noted a significantly lower expression of the tnf-α gene in fish that did not receive probiotics for 3 weeks (CYC group) compared to the continuously fed group. This is in contrast to the work of Wu et al. [53], who administered probiotics based on autochthonous strains of Shewanella xiamenensis to grass carp and recorded a long-term significant increase in the relative expression of the tnf-α gene compared to the control, lasting from the seventh to the twenty-eighth day after the start of the application. Increased levels of gene expression for tnf-α in fish post probiotic feeding have also been demonstrated by other works [33,58]. However, it is important to note that the levels of tnf-α in the mentioned works were highest in the days immediately after feeding experimental animals with probiotics. As with il1-β, the lack of a significant increase in the levels of the experimental groups in comparison to CTRL suggests no negative stimulation of the immune system towards inflammation in healthy fish.
Interleukin 8 is a typical pro-inflammatory cytokine with chemotactic properties that responds very quickly to antigenic stimulation. The observed increase in relative expression in the CYC group after those fish started receiving probiotics again (Figure 7) correlates with the results of Wu et al. [54], where a sharp increase in the expression of il8 over that of the control was recorded within a week of feeding fish probiotics. In the same research, it was shown that after 14 days, the levels of il8 relative expression remained just slightly increased in the experimental groups when compared to the control. An increase in il8 mRNA abundance in the intestine of fish fed probiotics after 21 days was also previously recorded [33]. On the contrary, when the CYC group did not receive the probiotic feed for 3 weeks, the expression level of the il8 gene was reduced below the control level. This confirms the rapid stimulation of IL8 production and also the rapid stabilization in the absence of antigenic stimulation. We observed a decrease in gene expression for IL8 below the levels of the control group in the CON group after 7 weeks of consuming our probiotic feed, but no differences in the following two samplings. We suppose that in the fish fed continuously, the immune system adapted to the presence of beneficial bacteria, and the expression of this gene was stabilized. Finally, it was also confirmed in an in vitro experiment on trout cells that the effect of probiotics on the expression of the il8 gene is strain-dependent. While some strains of probiotic bacteria increase expression, others decrease it. However, the most significant changes were induced by pathogenic bacteria [23].
TGF-β is a cytokine that possesses many regulatory functions. It is responsible for the suppression of the immune system to protect the organism against autoimmune diseases and maintains immune tolerance [59]. Its regulatory function, which is parallel to that in the mammalian immune system, was also found in teleosts [60]. It was observed that the relative gene expression of tgf-β increases after infection of the internal organs of fish, including the intestine [61]. As with the relative expression of previously mentioned molecules, an increase in the expression of tgf-β could be seen in the cyclically fed group after the reintroduction of probiotic feed, with a decrease over time (Figure 8). Probiotic-bacteria-fed Nile tilapia also showed an increase in the relative gene expression of tgf-β [62]. It is also important to note that the expression of tgf-β did not decrease significantly below the levels of the control group after the fish received our feed; in the CON group, the level of expression was the same as in the control group throughout the experiment. A decrease was observed in Atlantic salmon following a soybean meal diet that promoted proinflammatory reactions in the intestine [63]. This result in the CON group would suggest that our diet is safe even after prolonged consumption and does not weaken the immune system. We hypothesize that since no increase in the gene expression for TGF-β was observed in the CON group, continuous treatment with probiotics may not be as effective in treating intestinal inflammation as cycle feeding. However, this hypothesis will have to be confirmed in further experiments. Furthermore, an increase in TGF-β can have a therapeutic effect during intestinal inflammation [64]; therefore, the use of our probiotic feed could also prove effective in the treatment of such inflammation based on stimulating effect observed in the CYC group. Moreover, the anti-inflammatory potential of our R2 strain was confirmed in Atlantic salmon with enteritis induced by a pro-inflammatory soybean meal-based feed [37].
The innate part of the immune system recognizes the molecular structures of microorganisms. These pathogen-associated molecular patterns (PAMPs) are highly conserved and specific to certain microorganisms. The host organisms have developed a set of receptors to recognize PAMPs, one of them being TLR9 [65]. TLR9 in teleosts recognizes and binds to procaryotic (or viral) DNA after it is internalized in endosomes of mononuclear phagocytes [66] but can be also located on the surface of intestinal epithelial cells [67]. It has been suggested that TLR9 is stimulated by probiotics or their DNA [68], and our work shows similar results, with a higher increase in the gene expression of tlr9 in the CYC group after the reintroduction of probiotics in the feed (Figure 9). Elevation of tlr9 expression was also observed during the first sampling in both experimental groups, and in the CON group during the last sampling, when the level of expression in the CYC group was equal to the control. These increased levels of tlr9 may have activated the innate immune system of the host, providing better preparedness for potential infection. As for the reason behind the overall increase in tlr9 expression in both experimental groups across the whole experiment, there is a possibility that no tolerance is established since TLRs are a part of an innate immune response that triggers rapidly and has no memory function. Therefore, continuous exposure to bacteria stimulates the continual expression of this gene. However, this does not explain the low levels of expression in the CON group in the second sampling and in the CYC group in the third sampling, and further study is needed for a complete understanding of TLR–probiotic interaction.
One of the most important effects of probiotic preparations lies in their positive influence on the intestinal microbiome. In our previous experiment on Atlantic salmon, to which we administered probiotic strains L. plantarum R2 or L. fermentum R3 sprayed onto the surface of feed granules using a high-pressure coulter, intestinal bacterial communities were analyzed via high-throughput 16S rRNA gene amplicon sequencing. We found that the diversity of microbiota adhered to the intestinal mucosa was significantly increased. Both of the used strains significantly dominated in the intestinal contents as well as on the surface of the mucosa [69]. Similarly, in the present study, after the administration of L. plantarum R2 to trout, high numbers of LAB were recorded in the intestinal contents as well as on the mucosa of the middle intestine (Figure 10). On the contrary, a significantly lower representation of enterobacteria (Figure 11) and total aerobic bacteria (Figure 12) was observed compared to the control. As shown by the results of the HISTO-FISH analysis (Figure 13), L. plantarum dominated both in the digesta and on the mucosal surface. No significant differences in the representation of the observed bacterial species were found between the continuously and cyclically fed groups. Interestingly, even after a 3-week break in the feeding of probiotic feed, there were no differences in the amounts of LAB in the digesta or on the intestinal mucosa between the cyclic and continuous groups. In the salmon experiment, after the end of the application, the amounts of L. plantarum R2 and L. fermentum R3 slowly decreased, and after 14–20 days, they reached only 101–102 CFU/g (unpublished data). This means that the R2strain better colonized the gut of trout as compared to salmon, which confirms the better adaptation of the strain to the conditions of the digestive tract of the trout, from which it was originally isolated, as well as to fresh-water conditions. Although many enterobacteria are symbiotic, many are pathogenic, or potentially pathogenic, bacteria. Therefore, the ratio between LAB and enterobacteria is often considered as an indicator of the health of the intestinal microbiota. The shift in the favor of LAB indicates a positive effect of L. plantarum R2 on the intestinal microbiota.
In both probiotic groups, the surface of the villi was increased significantly compared to the control group (Table 4), which was caused by the density of the villi. Similar results where the application of commercial probiotics increased the density of villi in the intestine have been observed in poultry [70]. Such an increase allows for better absorption of nutrients, thus providing a better growth rate and conversion rate. The height of the villi was not significantly different between the experimental groups and the control. In a different study, it was observed that probiotic bacteria Lactobacillus plantarum and Lactobacillus rhamnosus caused an increase in the height of the villi in common carp [71], suggesting that not all probiotics have an equal effect on the target organism. Lastly, the width of the intestinal villi was not significantly different between groups, and the same result was recorded in a different study using multiple species probiotic preparation in pigs [72].
5. Conclusions
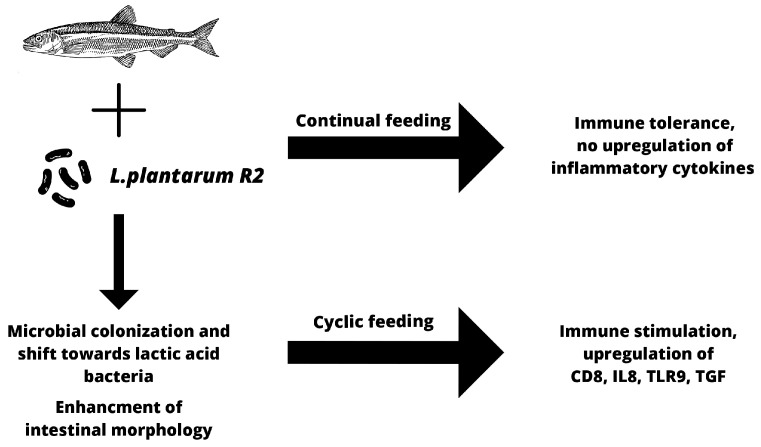
In conclusion, autochthonous trout probiotic strain L. plantarum R2 showed very good colonization abilities with a positive effect on the intestinal microbiota. LAB dominated in the intestinal contents as well as on the mucosa of the middle intestine of trout, even 3 weeks after the end of probiotic feed application. By comparing the two feeding regimens, we found that during the continuous application of probiotic feed, the immune system adapts to the immunomodulator, and most of the tested cytokines, including pro-inflammatory cytokines, did not significantly stimulate the intestinal immune response. The exception was the increased expression of the tlr9 and cd4 genes. On the other hand, after a 3-week break in probiotic feeding and subsequent reintroduction of probiotics, there was a significant temporary stimulation of the gene expression of molecules associated with both cellular and humoral immunity (cd8, tgf-β, il8, tlr9), without affecting the relative expression of the igm gene or the pro-inflammatory il1 and tnf-α genes. Moreover, the probiotic feed significantly increased the absorption area of the intestine (Figure 15). Based on the obtained results, we can conclude that continuous application can be used for stabilization of gut microbiota in favor of LAB without overstimulation of intestinal immunity. However, we prefer the cyclical application, which provides the opportunity to modulate the immune response in critical periods associated with stress, such as transport, change in feed, bad weather conditions, etc. In addition, the newly developed application form proved to be suitable, as it ensures the long-term survival of the probiotic strain, is cheap with a simple preparation technology, and is accepted by the fish.
Figure 15.
Summarization of effects of probiotic feed using autochthonous bacteria L. plantarum R2.
Acknowledgments
A microscope Carl Zeiss Axio Observer Z1 equipped with Software Carl Zeiss Zen 2 (ver. 3.3. blue edition) was purchased thanks to the support of the operational program: “Open scientific community for modern interdisciplinary research in medicine (OPENMED)”, ITMS2014+: 313011V455, supported by the Operational Programme Integrated Infrastructure, funded by the ERDF. We would like to thank you for the opportunity to use this device.
Author Contributions
M.R. and I.C.M.: investigation, formal analysis, writing; P.P.: conceptualization, investigation; A.F., J.K. and N.C.: investigation; J.M.: conceptualization, supervision, resources; O.M.: investigation, formal analysis; R.Ž.: investigation; M.F.: conceptualization, funding acquisition; D.M.: conceptualization, formal analysis, writing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the project ERDF/ESF “Profish” [No. CZ.02.1.01/0.0/0.0/16_019/0000869, Czech Republic] and by the Slovak Research and Development Agency under the contract no. [APVV-19-0234].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.FAO . The State of World Fisheries and Aquaculture 2020. Sustainability in Action. FAO; Rome, Italy: 2020. [Google Scholar]
- 2.Billard R., Bry C., Gillet C. Gillet Stress, Environment and Reproduction in Teleost Fish. Stress Fish. 1981;4:185–201. [Google Scholar]
- 3.Pickering A.D., Pottinger T. Pottinger Stress Responses and Disease Resistance in Salmonid Fish: Effects of Chronic Elevation of Plasma Cortisol. Fish Physiol. Biochem. 1989;7:253–258. doi: 10.1007/BF00004714. [DOI] [PubMed] [Google Scholar]
- 4.Conde-Sieira M., Chivite M., Míguez J.M., Soengas J.L. Soengas Stress Effects on the Mechanisms Regulating Appetite in Teleost Fish. Front. Endocrinol. 2018;9:631. doi: 10.3389/fendo.2018.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrifield D.L., Dimitroglou A., Foey A., Davies S.J., Baker R.T., Bøgwald J., Castex M., Ringø E. The Current Status and Future Focus of Probiotic and Prebiotic Applications for Salmonids. Aquaculture. 2010;302:1–18. doi: 10.1016/j.aquaculture.2010.02.007. [DOI] [Google Scholar]
- 6.Chizhayeva A., Amangeldi A., Oleinikova Y., Alybaeva A., Sadanov A. Lactic Acid Bacteria as Probiotics in Sustainable Development of Aquaculture. Aquat. Living Resour. 2022;35:10. doi: 10.1051/alr/2022011. [DOI] [Google Scholar]
- 7.Van Doan H., Soltani M., Ringø E. In Vitro Antagonistic Effect and in Vivo Protective Efficacy of Gram-Positive Probiotics versus Gram-Negative Bacterial Pathogens in Finfish and Shellfish. Aquaculture. 2021;540:736581. doi: 10.1016/j.aquaculture.2021.736581. [DOI] [Google Scholar]
- 8.Vijayabaskar P., Somasundaram S. Somasundaram Isolation of Bacteriocin Producing Lactic Acid Bacteria from Fish Gut and Probiotic Activity against Common Fresh Water Fish Pathogen Aeromonas Hydrophila. Biotechnology. 2008;7:124–128. doi: 10.3923/biotech.2008.124.128. [DOI] [Google Scholar]
- 9.Chabrillon M., Ouwehand A.C., Diaz-Rosales P., Arijo S., Martinez-Manzanares E., Balebona M.C. Adhesion of Lactic Acid Bacteria to Mucus of Farmed Gilthead Seabream, and Interactions with Fish Pathogenic Microorganisms. Bull. Eur. Assoc. Fish Pathol. 2006;26:202–210. [Google Scholar]
- 10.SNikoskelainen S., Ouwehand A., Bylund G., Salminen S., Lilius E.-M. Immune Enhancement in Rainbow Trout (Oncorhynchus mykiss) by Potential Probiotic Bacteria (Lactobacillus rhamnosus) Fish Shellfish. Immunol. 2003;15:443–452. doi: 10.1016/S1050-4648(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 11.Kosiewicz M.M., Zirnheld A.L., Alard P. Gut Microbiota, Immunity, and Disease: A Complex Relationship. Front. Microbiol. 2011;2:180. doi: 10.3389/fmicb.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhu N., Wang J., Yu L., Zhang Q., Chen K., Liu B. Modulation of Growth Performance and Intestinal Microbiota in Chickens Fed Plant Extracts or Virginiamycin. Front. Microbiol. 2019;10:1333. doi: 10.3389/fmicb.2019.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagaram U.S., Gaikwad M.S., Nandru R., Dasgupta S. Dasgupta Microalgae as Feed Ingredients: Recent Developments on Their Role in Immunomodulation and Gut Microbiota of Aquaculture Species. FEMS Microbiol. Lett. 2021;368:71. doi: 10.1093/femsle/fnab071. [DOI] [PubMed] [Google Scholar]
- 15.Nayak S.K. Probiotics and Immunity: A Fish Perspective. Fish Shellfish. Immunol. 2010;29:2–14. doi: 10.1016/j.fsi.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 16.EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Guidance on the Assessment of Bacterial Susceptibility to Antimicrobials of Human and Veterinary Importance. EFSA J. 2012;10:2740. doi: 10.2903/J.EFSA.2012.2740. [DOI] [Google Scholar]
- 17.Fečkaninová A., Koščová J., Mudroňová D., Schusterová P., Maruščáková I.C., Popelka P. Characterization of Two Novel Lactic Acid Bacteria Isolated from the Intestine of Rainbow Trout (Oncorhynchus mykiss, Walbaum) in Slovakia. Aquaculture. 2019;506:294–301. doi: 10.1016/j.aquaculture.2019.03.026. [DOI] [Google Scholar]
- 18.Maruščáková I.C., Schusterová P., Popelka P., Gancarčíková S., Csank T., Fečkaninová A., Ratvaj M., Mudroňová D. Effect of Autochthonous Lactobacilli on Immunologically Important Molecules of Rainbow Trout after Bacterial Infection Studied on Intestinal Primoculture. Fish Shellfish. Immunol. 2021;119:379–383. doi: 10.1016/j.fsi.2021.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Nemcová R. Criteria for selection of lactobacilli for probiotic use. Vet. Med. 1997;42:19–27. [PubMed] [Google Scholar]
- 20.Fečkaninová A., Koščová J., Franc A., Mudroňová D., Popelka P. Surviving of Production Probiotic Strains in a Selected Application Form. Ceska Slov. Farm. 2022;71:27–33. doi: 10.5817/CSF2022-1-27. [DOI] [PubMed] [Google Scholar]
- 21.Zheng J., Wittouck S., Salvetti E., Franz C.M.A.P., Harris H.M.B., Mattarelli P., O’Toole P.W., Pot B., Vandamme P., Walter J., et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 22.European Commission COMMISSION REGULATION (EC) No 152/2009 of 27 January 2009 Laying down the Methods of Sampling and Analysis for the Official Control of Feed. 2009. [(accessed on 1 June 2023)]. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32009R0152.
- 23.Kim D.-H., Austin B. Cytokine Expression in Leucocytes and Gut Cells of Rainbow Trout, Oncorhynchus Mykiss Walbaum, Induced by Probiotics. Vet. Immunol. Immunopathol. 2006;114:297–304. doi: 10.1016/j.vetimm.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Li S., Xie H., Yan Z., Li B., Wu P., Qian X., Zhang X., Wu J., Liu J., Zhao X. Development of a Live Vector Vaccine against Infectious Hematopoietic Necrosis Virus in Rainbow Trout. Fish Shellfish. Immunol. 2019;89:516–524. doi: 10.1016/j.fsi.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Yarahmadi P., Miandare H.K., Fayaz S., Caipang C.M.A. Increased Stocking Density Causes Changes in Expression of Selected Stress- and Immune-Related Genes, Humoral Innate Immune Parameters and Stress Responses of Rainbow Trout (Oncorhynchus mykiss) Fish Shellfish. Immunol. 2016;48:43–53. doi: 10.1016/j.fsi.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Jørgensen T.R., Raida M.K., Kania P.W., Buchmann K. Response of Rainbow Trout (Oncorhynchus mykiss) in Skin and Fin Tissue during Infection with a Variant of Gyrodactylus salaris (Monogenea: Gyrodactylidae) Folia Parasitol. 2013;56:251–258. doi: 10.14411/fp.2009.029. [DOI] [PubMed] [Google Scholar]
- 27.Chettri J.K., Kuhn J., Jaafar R.M., Kania P.W., Møller O.S., Buchmann K. Epidermal Response of Rainbow Trout to Ichthyobodo Necator: Immunohistochemical and Gene Expression Studies Indicate a Th1-/Th2-like Switch. J. Fish Dis. 2014;37:771–783. doi: 10.1111/jfd.12169. [DOI] [PubMed] [Google Scholar]
- 28.Fierro-Castro C., Barrioluengo L., López-Fierro P., Razquin B., Villena A. Fish Cell Cultures as in Vitro Models of Inflammatory Responses Elicited by Immunostimulants. Expression of Regulatory Genes of the Innate Immune Response. Fish Shellfish. Immunol. 2013;35:979–987. doi: 10.1016/j.fsi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Madar M., Slizova M., Czerwinski J., Hrckova G., Mudronova D., Gancarcikova S., Popper M., Pistl J., Soltys J., Nemcova R. Histo-FISH Protocol to Detect Bacterial Compositions and Biofilms Formation in Vivo. Benef. Microbes. 2015;6:899–907. doi: 10.3920/BM2015.0016. [DOI] [PubMed] [Google Scholar]
- 30.Ringø E., Gatesoupe F.-J. Lactic Acid Bacteria in Fish: A Review. Aquaculture. 1998;160:177–203. doi: 10.1016/S0044-8486(97)00299-8. [DOI] [Google Scholar]
- 31.Balcã¡Zar J.L., de Blas I., Ruiz-Zarzuela I., Vendrell D., Gironã©S O., Muzquiz J.L. Enhancement of the Immune Response and Protection Induced by Probiotic Lactic Acid Bacteria against Furunculosis in Rainbow Trout (Oncorhynchus mykiss) FEMS Immunol. Med. Microbiol. 2007;51:185–193. doi: 10.1111/j.1574-695X.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- 32.Vendrell D., Balcázar J.L., de Blas I., Ruiz-Zarzuela I., Gironés O., Múzquiz J.L. Protection of Rainbow Trout (Oncorhynchus mykiss) from Lactococcosis by Probiotic Bacteria. Comp. Immunol. Microbiol. Infect. Dis. 2008;31:337–345. doi: 10.1016/j.cimid.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Sánchez T., Balcázar J.L., Merrifield D.L., Carnevali O., Gioacchini G., de Blas I., Ruiz-Zarzuela I. Expression of Immune-Related Genes in Rainbow Trout (Oncorhynchus mykiss) Induced by Probiotic Bacteria during Lactococcus garvieae Infection. Fish Shellfish. Immunol. 2011;31:196–201. doi: 10.1016/j.fsi.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Merrifield D., Dimitroglou A., Bradley G., Baker R., Davies S. Probiotic Applications for Rainbow Trout (Oncorhynchus mykiss Walbaum) I. Effects on Growth Performance, Feed Utilization, Intestinal Microbiota and Related Health Criteria. Aquac. Nutr. 2010;16:504–510. doi: 10.1111/j.1365-2095.2009.00689.x. [DOI] [Google Scholar]
- 35.Merrifield D., Bradley G., Harper G., Baker R., Munn C., Davies S. Assessment of the Effects of Vegetative and Lyophilized Pediococcus Acidilactici on Growth, Feed Utilization, Intestinal Colonization and Health Parameters of Rainbow Trout (Oncorhynchus mykiss Walbaum) Aquac. Nutr. 2011;17:73–79. doi: 10.1111/j.1365-2095.2009.00712.x. [DOI] [Google Scholar]
- 36.Gümüş E., Kubilay A., Guney Z., Guzel-Seydim T., Kok-Tas S., Ulukoy M.G. Effect of Dietary Kefir on the Growth Performance, Feed Utilization and Fatty Acid Profile of Juvenile Rainbow Trout, Oncorhynchus Mykiss. Aquac. Nutr. 2017;23:964–972. doi: 10.1111/anu.12464. [DOI] [Google Scholar]
- 37.Hines I.S., Santiago-Morales K.D., Ferguson C.S., Clarington J., Thompson M., Rauschenbach M., Levine U., Drahos D., Aylward F.O., Smith S.A., et al. Steelhead Trout (Oncorhynchus mykiss) Fed Probiotic during the Earliest Developmental Stages Have Enhanced Growth Rates and Intestinal Microbiome Bacterial Diversity. Front. Mar. Sci. 2022;9:2291. doi: 10.3389/fmars.2022.1021647. [DOI] [Google Scholar]
- 38.Nimalan N., Sørensen S.L., Fečkaninová A., Koščová J., Mudroňová D., Gancarčíková S., Vatsos I.N., Bisa S., Kiron V., Sørensen M. Mucosal Barrier Status in Atlantic Salmon Fed Marine or Plant-Based Diets Supplemented with Probiotics. Aquaculture. 2022;547:737516. doi: 10.1016/j.aquaculture.2021.737516. [DOI] [Google Scholar]
- 39.Mashoof S., Criscitiello M.F. Criscitiello Fish Immunoglobulins. Biology. 2016;5:45. doi: 10.3390/biology5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solem S.T., Stenvik J. Antibody Repertoire Development in Teleosts—A Review with Emphasis on Salmonids and Gadus morhua L. Dev. Comp. Immunol. 2006;30:57–76. doi: 10.1016/j.dci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Costa G., Danz H., Kataria P., Bromage E. A Holistic View of the Dynamisms of Teleost IgM: A Case Study of Streptococcus Iniae Vaccinated Rainbow Trout (Oncorhynchus mykiss) Dev. Comp. Immunol. 2012;36:298–305. doi: 10.1016/j.dci.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Vazirzadeh A., Roosta H., Masoumi H., Farhadi A., Jeffs A. Long-Term Effects of Three Probiotics, Singular or Combined, on Serum Innate Immune Parameters and Expressions of Cytokine Genes in Rainbow Trout during Grow-Out. Fish Shellfish. Immunol. 2020;98:748–757. doi: 10.1016/j.fsi.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Balcázar J.L., de Blas I., Ruiz-Zarzuela I., Vendrell D., Calvo A.C., Márquez I., Gironés O., Muzquiz J.L. Changes in Intestinal Microbiota and Humoral Immune Response Following Probiotic Administration in Brown Trout (Salmo trutta) Br. J. Nutr. 2007;97:522–527. doi: 10.1017/S0007114507432986. [DOI] [PubMed] [Google Scholar]
- 44.IAas I.B., Austbø L., Falk K., Hordvik I., Koppang E.O. The Interbranchial Lymphoid Tissue Likely Contributes to Immune Tolerance and Defense in the Gills of Atlantic Salmon. Dev. Comp. Immunol. 2017;76:247–254. doi: 10.1016/j.dci.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Ashfaq H., Soliman H., Saleh M., El-Matbouli M. CD4: A Vital Player in the Teleost Fish Immune System. Vet. Res. 2019;50:1–11. doi: 10.1186/s13567-018-0620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picchietti S., Fausto A.M., Randelli E., Carnevali O., Taddei A.R., Buonocore F., Scapigliati G., Abelli L. Early Treatment with Lactobacillus Delbrueckii Strain Induces an Increase in Intestinal T-Cells and Granulocytes and Modulates Immune-Related Genes of Larval dicentrarchus Labrax (L.) Fish Shellfish. Immunol. 2009;26:368–376. doi: 10.1016/j.fsi.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Ashfaq H., Soliman H., Fajmann S., Sexl V., El-Matbouli M., Saleh M. Kinetics of CD4-1+ Lymphocytes in Brown Trout after Exposure to Viral Haemorrhagic septicaemia Virus. J. Fish Dis. 2021;44:1553–1562. doi: 10.1111/jfd.13476. [DOI] [PubMed] [Google Scholar]
- 48.Takizawa F., Dijkstra J.M., Kotterba P., Korytář T., Kock H., Köllner B., Jaureguiberry B., Nakanishi T., Fischer U. The Expression of CD8α Discriminates Distinct T Cell Subsets in Teleost Fish. Dev. Comp. Immunol. 2011;35:752–763. doi: 10.1016/j.dci.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Utke K., Bergmann S., Lorenzen N., Köllner B., Ototake M., Fischer U. Cell-Mediated Cytotoxicity in Rainbow Trout, Oncorhynchus mykiss, Infected with Viral Haemorrhagic septicaemia Virus. Fish Shellfish. Immunol. 2007;22:182–196. doi: 10.1016/j.fsi.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Toda H., Shibasaki Y., Koike T., Ohtani M., Takizawa F., Ototake M., Moritomo T., Nakanishi T. Alloantigen-Specific Killing Is Mediated by CD8-Positive T Cells in Fish. Dev. Comp. Immunol. 2009;33:646–652. doi: 10.1016/j.dci.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Granja A.G., Leal E., Pignatelli J., Castro R., Abós B., Kato G., Fischer U., Tafalla C. Identification of Teleost Skin CD8α+ Dendritic-like Cells, Representing a Potential Common Ancestor for Mammalian Cross-Presenting Dendritic Cells. J. Immunol. 2015;195:1825–1837. doi: 10.4049/jimmunol.1500322. [DOI] [PubMed] [Google Scholar]
- 52.Skaggs B.J., Singh R.P., Hahn B.H. Induction of Immune Tolerance by Activation of CD8+ T Suppressor/Regulatory Cells in Lupus-Prone Mice. Hum. Immunol. 2008;69:790–796. doi: 10.1016/j.humimm.2008.08.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogryzko N.V., Renshaw S.A., Wilson H.L. The IL-1 Family in Fish: Swimming through the Muddy Waters of Inflammasome Evolution. Dev. Comp. Immunol. 2014;46:53–62. doi: 10.1016/j.dci.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Wu Z.-Q., Jiang C., Ling F., Wang G.-X. Effects of Dietary Supplementation of Intestinal Autochthonous Bacteria on the Innate Immunity and Disease Resistance of Grass Carp (Ctenopharyngodon idellus) Aquaculture. 2015;438:105–114. doi: 10.1016/j.aquaculture.2014.12.041. [DOI] [Google Scholar]
- 55.He S., Zhang Y., Xu L., Yang Y., Marubashi T., Zhou Z., Yao B. Effects of Dietary Bacillus Subtilis C-3102 on the Production, Intestinal Cytokine Expression and Autochthonous Bacteria of Hybrid Tilapia Oreochromis Niloticus ♀ × Oreochromis Aureus ♂. Aquaculture. 2013;412–413:125–130. doi: 10.1016/j.aquaculture.2013.06.028. [DOI] [Google Scholar]
- 56.Li Y., Xiao T., Zou J. Fish TNF and TNF Receptors. Sci. China Life Sci. 2020;64:196–220. doi: 10.1007/s11427-020-1712-4. [DOI] [PubMed] [Google Scholar]
- 57.Wangkahart E., Secombes C.J., Wang T. Dissecting the Immune Pathways Stimulated Following Injection Vaccination of Rainbow Trout (Oncorhynchus mykiss) against Enteric Redmouth Disease (ERM) Fish Shellfish. Immunol. 2019;85:18–30. doi: 10.1016/j.fsi.2017.07.056. [DOI] [PubMed] [Google Scholar]
- 58.Muñoz-Atienza E., Araújo C., Magadán S., Hernández P.E., Herranz C., Santos Y., Cintas L.M. In Vitro and in Vivo Evaluation of Lactic Acid Bacteria of Aquatic Origin as Probiotics for Turbot (Scophthalmus maximus L.) Farming. Fish Shellfish. Immunol. 2014;41:570–580. doi: 10.1016/j.fsi.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Saxena V., Lienesch D.W., Zhou M., Bommireddy R., Azhar M., Doetschman T., Singh R.R. Dual Roles of Immunoregulatory Cytokine TGF-β in the Pathogenesis of Autoimmunity-Mediated Organ Damage. J. Immunol. 2008;180:1903–1912. doi: 10.4049/jimmunol.180.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang M., Wang X., Chen D., Wang Y., Zhang A., Zhou H. Tgf-Β1 Exerts Opposing Effects on Grass Carp Leukocytes: Implication in Teleost Immunity, Receptor Signaling and Potential Self-Regulatory Mechanisms. PLoS ONE. 2012;7:e35011. doi: 10.1371/journal.pone.0035011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X., Mu P., Teng Y., Wu Y., Chen X. Identification of a TGF-Β1 Homologue in the Large Yellow Croaker (Larimichthys crocea) Revealed Its Role in Regulation of Immune Response. Water Biol. Secur. 2022;1:100006. doi: 10.1016/j.watbs.2022.100006. [DOI] [Google Scholar]
- 62.Tan H.Y., Chen S.-W., Hu S.-Y. Improvements in the Growth Performance, Immunity, Disease Resistance, and Gut Microbiota by the Probiotic Rummeliibacillus Stabekisii in Nile Tilapia (Oreochromis niloticus) Fish Shellfish. Immunol. 2019;92:265–275. doi: 10.1016/j.fsi.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 63.Lilleeng E., Penn M.H., Haugland O., Xu C., Bakke A.M., Krogdahl A., Landsverk T., Frøystad-Saugen M.K. Decreased Expression of TGF-β, GILT and T-Cell Markers in the Early Stages of Soybean Enteropathy in Atlantic Salmon (Salmo salar L.) Fish Shellfish Immunol. 2009;27:65–72. doi: 10.1016/j.fsi.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Di Giacinto C., Marinaro M., Sanchez M., Strober W., Boirivant M. Probiotics Ameliorate Recurrent Th1-Mediated Murine Colitis by Inducing IL-10 and IL-10-Dependent TGF-β-Bearing Regulatory Cells. J. Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 65.Medzhitov R., Janeway C.A., Jr. Decoding the Patterns of Self and Nonself by the Innate Immune System. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 66.Iliev D.B., Skjæveland I., Jørgensen J.B. CpG Oligonucleotides Bind TLR9 and RRM-Containing Proteins in Atlantic Salmon (Salmo salar) BMC Immunol. 2013;14:12. doi: 10.1186/1471-2172-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mielcarska M.B., Bossowska-Nowicka M., Toka F.N. Cell Surface Expression of Endosomal Toll-Like Receptors—A Necessity or a Superfluous Duplication? Front. Immunol. 2021;11:3652. doi: 10.3389/fimmu.2020.620972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhong Y., Huang J., Tang W., Chen B., Cai W. Effects of Probiotics, Probiotic DNA and the CpG Oligodeoxynucleotides on Ovalbumin-Sensitized Brown-Norway Rats via TLR9/NF-ΚB Pathway. FEMS Immunol. Med. Microbiol. 2012;66:71–82. doi: 10.1111/j.1574-695X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 69.Gupta S., Fečkaninová A., Lokesh J., Koščová J., Sørensen M., Fernandes J., Kiron V. Erratum: Lactobacillus Dominate in the Intestine of Atlantic Salmon Fed Dietary Probiotics. Front. Microbiol. 2019;10:3247. doi: 10.3389/fmicb.2018.03247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rahimi S., Grimes J.L., Fletcher O., Oviedo E., Sheldon B.W. Effect of a Direct-Fed Microbial (Primalac) on Structure and Ultrastructure of Small Intestine in Turkey Poults. Poult. Sci. 2009;88:491–503. doi: 10.3382/ps.2008-00272. [DOI] [PubMed] [Google Scholar]
- 71.Mohammadian T., Monjezi N., Peyghan R., Mohammadian B. Effects of Dietary Probiotic Supplements on Growth, Digestive Enzymes Activity, Intestinal Histomorphology and Innate Immunity of Common Carp (Cyprinus carpio): A Field Study. Aquaculture. 2022;549:737787. doi: 10.1016/j.aquaculture.2021.737787. [DOI] [Google Scholar]
- 72.Sun Y., Duarte M.E., Kim S.W. Dietary Inclusion of Multispecies Probiotics to Reduce the Severity of Post-Weaning Diarrhea Caused by Escherichia Coli F18+ in Pigs. Anim. Nutr. 2021;7:326–333. doi: 10.1016/j.aninu.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.