Abstract
Non-judicious antimicrobial use (AMU) is a major driver of antimicrobial resistance (AMR). In human hospitals, cumulative antibiograms are often used by clinicians to evaluate local susceptibility rates and to select the most appropriate empiric therapy with the aim of minimizing inappropriate AMU. However, the use of cumulative antibiograms to guide empiric antimicrobial therapy in veterinary hospitals in the United States is limited, and there are no specific guidelines or standardized methods available for the construction of antibiograms in veterinary clinical settings. The objective of this methods article is to describe the approaches that were used to construct antibiograms from clinical samples collected from dogs seen at a veterinary teaching hospital. Laboratory data for 563 dogs for the period from 1 January 2015 to 31 December 2015 was utilized. We used the Clinical and Laboratory Standards Institute (CLSI) guidelines for use in the construction of the antibiograms in human healthcare settings as the basis for the veterinary antibiograms. One general antibiogram and antibiograms stratified by hospital section, the anatomic region of sample collection/by sample type, were created and the challenges encountered in preparing these antibiograms were highlighted. The approaches described could be useful in guiding veterinary antibiogram development for empiric therapy.
Keywords: antimicrobial resistance, canine patients, empiric antimicrobial therapy, Gram-negative bacteria, Gram-positive bacteria, veterinary antibiograms
1. Introduction
Antimicrobial resistance (AMR) is a serious healthcare problem that is increasing in incidence worldwide [1], and it is a threat to global health [2]. Any use of antimicrobials, whether judicious or non-judicious, creates selection pressure that can lead to AMR emergence [3]. However, non-judicious use of antimicrobials in human and veterinary medicine is a modifiable driver of AMR, leading to the emergence of bacterial strains resistant to many antimicrobials [4,5,6]. Like in human medicine [7], initial antimicrobial therapy in veterinary medicine is often empiric and is guided by clinical signs of disease. Under ideal clinical practice situations, empirical use of antimicrobials is discouraged and antimicrobial prescription based on a bacterial culture and antimicrobial susceptibility testing is preferred [8]. However, high costs and long turnaround times associated with the existing bacterial culture and antimicrobial susceptibility testing methods influence the decision to use them [9,10].
In human hospitals, summaries of the patterns of antimicrobial susceptibilities (antibiograms), constructed from laboratory samples that have been submitted institutionally are often used by clinicians. The antibiograms report the percentage of isolates susceptible to a set of antimicrobials, may be stratified (by patient population, medical service or specimen type), and are shared with clinicians, hospital pharmacists and infection-control personnel in the form of web postings and pocket guides [11]. These antibiograms can complement rapid diagnostic techniques in clinical microbiology [12], allow clinicians to evaluate the local susceptibility rates to specific antimicrobials and aid them in selection of the most appropriate empiric therapy [13,14,15].
The integration of antibiogram use in veterinary practice to guide empiric antimicrobial therapy is necessary for antimicrobial stewardship [16]. However, in the United States, the use of cumulative antibiograms to guide empiric antimicrobial therapy in veterinary medicine is still limited to just a few tertiary veterinary hospitals. Even the two tertiary veterinary hospitals that in the past publicly provided cumulative antibiograms to their clinicians via their hospital websites do not have up-to-date antibiograms posted on their websites. The limited use of cumulative antibiograms in veterinary medicine may be due in part to the fact that there are no specific guidelines or standardized methods available for the construction of veterinary antibiograms. Here, we examined the practicality of constructing cumulative antibiograms for a veterinary tertiary care hospital. This paper describes and documents the approaches used, and the challenges that were encountered in constructing cumulative antibiograms for a veterinary teaching hospital in Indiana, the United States.
2. Results
2.1. The Isolates
Of the 563 canine antimicrobial susceptibility profiles in the analyzed dataset, 318 (56.5%) isolates were Gram-positive and these included Staphylococcus spp., Enterococcus spp. Streptococcus spp., Corynebacterium spp., Bacillus spp., Aerococcus spp., and Actinomyces spp. Two hundred forty-five isolates (43.5%) were Gram-negative and these included Escherichia coli, Pseudomonas spp., Proteus mirabilis, Pasteurella spp., Enterobacter spp., Klebsiella spp., and other Gram-negative organisms.
2.2. General Antibiograms
One general antibiogram with a section for Gram-negative organisms (Table 1) and another for Gram-positive organisms were prepared (Table 2). The Gram-negative isolates were 100 Escherichia coli, 27 Pseudomonas spp., and 26 Proteus mirabilis (Table 1). For the Gram-positive isolates, there were 170 Staphylococcus spp. isolates, 64 Streptococcus spp., 30 Corynebacterium spp., and 65 Enterococcus spp. isolates (Table 2).
Table 1.
A general antibiogram showing percent antimicrobial susceptibility of all Gram-negative bacteria isolated from a veterinary teaching hospital in Indiana, the United States in 2015 and grouped at genus/species level.
| Escherichia coli | Pseudomonas spp. | Proteus mirabilis | |
|---|---|---|---|
| Number of Isolates | 100 | 27 | 26 |
| Antimicrobial | |||
| Amikacin | 99% | 100% | 92% |
| Amoxicillin/clavulanic acid | 78% | 4% | 100% |
| Ampicillin | 64% | 4% | 84% |
| Cefazolin | 79% | 4% | 88% |
| Cefovecin | 82% | 7% | 96% |
| Cefoxitin | 83% | 4% | 96% |
| Cefpodoxime | 82% | 4% | 96% |
| Ceftiofur | 79% | 7% | 96% |
| Cephalothin ** | 100% | 100% | |
| Chloramphenicol | 84% | 4% | 69% |
| Clindamycin ¥ | 2% | 4% | 0% |
| Doxycycline | 78% | 7% | 0% |
| Enrofloxacin | 85% | 70% | 92% |
| Erythromycin ¥ | 10% | 4% | 0% |
| Gentamycin | 94% | 85% | 85% |
| Imipenem | 100% | 100% | 96% |
| Marbofloxacin | 83% | 92% | |
| Oxacillin + 2%NACL | 0% | 7% | 0% |
| Penicillin | 0% | 4% | 0% |
| Ticarcillin | 64% | 100% | 85% |
| Ticarcillin/clavulanic acid | 75% | 100% | 96% |
| Trimethoprim/sulfamethoxazole | 85% | 11% | 85% |
** Number of isolates tested for cephalothin susceptibility were as follows: E. coli = 67, Proteus mirabilis = 20. ¥ indicates the drug is primarily used for Gram-positive organisms. Grey-shading indicates that susceptibility to a drug was not tested with that organism.
Table 2.
A general antibiogram showing percent antimicrobial susceptibility of Gram-positive bacteria isolated from a veterinary teaching hospital in Indiana, the United States in 2015 and grouped at genus level.
| Staphylococcus spp. | Streptococcus spp. | Corynebacterium spp. | Enterococcus spp. | |
|---|---|---|---|---|
| Number of Isolates | 170 | 64 | 30 | 65 |
| Antimicrobial | ||||
| Amikacin | 89% | 84% | 93% | 0% |
| Amoxicillin/clavulanic acid | 84% | 100% | 59% | 72% |
| Ampicillin | 16% | 95% | 38% | 72% |
| Cefazolin | 85% | 100% | 50% | 0% |
| Cefovecin | 80% | 95% | 48% | 0% |
| Cefoxitin | 84% | 95% | 41% | 0% |
| Cefpodoxime | 80% | 95% | 38% | 0% |
| Ceftiofur | 81% | 97% | 53% | 0% |
| Cephalothin ** | 100% | 100% | ||
| Chloramphenicol | 86% | 92% | 80% | 88% |
| Clindamycin ¥ | 68% | 90% | 69% | 0% |
| Doxycycline | 67% | 78% | 90% | 51% |
| Enrofloxacin | 72% | 34% | 38% | 6% |
| Erythromycin ¥ | 68% | 3% | 63% | 31% |
| Gentamycin | 76% | 92% | 87% | 2% |
| Imipenem | 85% | 100% | 59% | 74% |
| Marbofloxacin | 79% | 60% | ||
| Oxacillin + 2%NACL | 85% | 98% | 21% | 6% |
| Penicillin | 0% | 92% | 0 | 69% |
| Ticarcillin | 75% | 97% | 60% | 15% |
| Ticarcillin/clavulanic acid | 84% | 97% | 52% | 18% |
| Trimethoprim/sulfamethoxazole | 75% | 94% | 87% | 3% |
** Number of isolates tested for cephalothin susceptibility were as follows: Staphylococcus spp. = 105, Streptococcus spp. = 55. ¥ indicates the drug is primarily used for Gram-positive organisms. Grey-shading indicates that susceptibility to a drug was not tested with that organism.
2.3. Antibiograms Stratified by Hospital Section (Medical Service), Sample Type/Anatomic Region of Sample Collection
There were enough isolates collected from different hospital sections, the ears, skin, and urine to create individual antibiograms for the different hospital sections and anatomic regions/sample types. Antibiograms with sections for patients admitted to each hospital department were prepared for Gram-positive and Gram-negative organisms (Table 3, Table 4, and Table 5, respectively). Twenty-one Staphylococcus spp. isolates from the ear and 43 Staphylococcal isolates from the skin were used to create antibiograms for these anatomic regions (Table 6). One antibiogram with two parts, one for Gram-negative and the other for Gram-positive bacteria, was prepared for the isolates from urine (Table 7)
Table 3.
Antibiogram showing percent antimicrobial susceptibility of Gram-positive bacteria isolated from the dermatology, surgery and oncology sections of a veterinary teaching hospital in Indiana, the United States, 2015.
| Dermatology | Surgery | Oncology | ||||||
|---|---|---|---|---|---|---|---|---|
| Staphylococcus spp. | Streptococcus spp. | Staphylococcus spp. | Staphylococcus spp. | Streptococcus spp. | Enterococcus spp. | |||
| Number of Isolates | 41 | 12 | Number of Isolates | 20 | Number of Isolates | 33 | 20 | 17 |
| Antimicrobial | ||||||||
| Amikacin | 93% | 75% | Amikacin | 85% | Amikacin | 91% | 85% | 0% |
| Amoxicillin/clavulanic acid | 85% | 100% | Amoxicillin/clavulanic acid | 65% | Amoxicillin/clavulanic acid | 85% | 100% | 65% |
| Ampicillin | ⱡ 10% | 100% | Ampicillin * | 13% | Ampicillin | 24% | 95% | 65% |
| Cefazolin | 85% | 100% | Cefazolin | 65% | Cefazolin | 85% | 100% | 0% |
| Cefovecin | 78% | 100% | Cefovecin | 60% | Cefovecin | 85% | 100% | 0% |
| Cefoxitin | 85% | 100% | Cefoxitin | 65% | Cefoxitin | 85% | 95% | 0% |
| Cefpodoxime | 78% | 100% | Cefpodoxime | 60% | Cefpodoxime | 85% | 100% | 0% |
| Ceftiofur | 85% | 100% | Ceftiofur | 60% | Ceftiofur | 79% | 100% | 0% |
| Cephalothin ** | 100% | 100% | Cephalothin ** | 100% | Cephalothin ** | 100% | 100% | |
| Chloramphenicol | 88% | 100% | Chloramphenicol | 85% | Chloramphenicol | 76% | 95% | 88% |
| Clindamycin | 68% | 92% | Clindamycin | 55% | Clindamycin | 67% | 95% | 0% |
| Doxycycline | 71% | 92% | Doxycycline | 90% | Doxycycline | 61% | 75% | 47% |
| Enrofloxacin | 68% | 42% | Enrofloxacin | 60% | Enrofloxacin | 76% | 25% | 0% |
| Erythromycin | 71% | 0% | Erythromycin | 55% | Erythromycin | 67% | 5% | 35% |
| Gentamycin | 66% | 92% | Gentamycin | 70% | Gentamycin | 82% | 95% | 0% |
| Imipenem | 85% | 100% | Imipenem | 70% | Imipenem | 85% | 100% | 71% |
| Marbofloxacin | 71% | 67% | Marbofloxacin | 75% | Marbofloxacin | 85% | 50% | |
| Oxacillin + 2%NACL | 85% | 100% | Oxacillin + 2%NACL | 70% | Oxacillin + 2%NACL | 85% | 100% | 6% |
| Penicillin * | 0% | 100% | Penicillin * | 0% | Penicillin * | 0% | 95% | 65% |
| Ticarcillin * | 79% | 100% | Ticarcillin * | 44% | Ticarcillin * | 80% | 100% | 24% |
| Ticarcillin/clavulanic acid | 85% | 100% | Ticarcillin/clavulanic acid | 65% | Ticarcillin/clavulanic acid | 85% | 100% | 24% |
| Trimethoprim/sulfamethoxazole | 68% | 100% | Trimethoprim/sulfamethoxazole | 80% | Trimethoprim/sulfamethoxazole | 79% | 95% | 0% |
Dermatology section: ⱡ Number of isolates tested: Staphylococcus spp. = 29. ** Number of isolates tested for cephalothin susceptibility were as follows: Staphylococcus spp. = 26, Streptococcus spp. = 11. * Number of Staphylococcus spp. isolates tested for penicillin and ticarcillin susceptibility was 29. Surgery section: * Number of Staphylococcus spp. isolates tested for ampicillin; penicillin and ticarcillin susceptibility was 16. ** Number of isolates tested for cephalothin susceptibility was 10. Oncology section: ** Numbers of isolates tested for cephalothin susceptibility were as follows: Staphylococcus spp. = 20, Streptococcus spp. = 19. * Number of Staphylococcus spp. isolates for penicillin and ticarcillin susceptibility was 25. Grey-shading indicates that susceptibility to a drug was not tested with that organism.
Table 4.
Antibiogram showing percent antimicrobial susceptibility of Gram-positive bacteria isolated from the medicine and emergency sections of a veterinary teaching hospital in Indiana, the United States, 2015.
| Medicine | Emergency | ||||
|---|---|---|---|---|---|
| Staphylococcus spp. | Enterococcus spp. | Staphylococcus spp. | Enterococcus spp. | ||
| Number of Isolates | 28 | 15 | Number of Isolates | 16 | 13 |
| Antimicrobial | |||||
| Amikacin | 86% | 0% | Amikacin | 88% | 0% |
| Amoxicillin/clavulanic acid | 82% | 80% | Amoxicillin/clavulanic acid | 94% | 69% |
| Ampicillin | * 6% | 80% | Ampicillin * | 13% | 69% |
| Cefazolin | 82% | 0% | Cefazolin | 94% | 0% |
| Cefovecin | 79% | 0% | Cefovecin | 88% | 0% |
| Cefoxitin | 82% | 0% | Cefoxitin | 94% | 0% |
| Cefpodoxime | 79% | 0% | Cefpodoxime | 88% | 0% |
| Ceftiofur | 79% | 0% | Ceftiofur | 88% | 0% |
| Cephalothin ** | 100% | Cephalothin ** | 100% | ||
| Chloramphenicol | 86% | 100% | Chloramphenicol | 94% | 62% |
| Clindamycin | 61% | 0% | Clindamycin | 69% | 0% |
| Doxycycline | 54% | 60% | Doxycycline | 75% | 31% |
| Enrofloxacin | 71% | 7% | Enrofloxacin | 69% | 8% |
| Erythromycin | 61% | 27% | Erythromycin | 69% | 8% |
| Gentamycin | 75% | 7% | Gentamycin | 81% | 0% |
| Imipenem | 82% | 80% | Imipenem | 94% | 695 |
| Marbofloxacin | 71% | Marbofloxacin | 88% | ||
| Oxacillin + 2%NACL | 82% | 7% | Oxacillin + 2%NACL | 94% | 0% |
| Penicillin | * 0% | 73% | Penicillin | * 0% | 69% |
| Ticarcillin | * 72% | 13% | Ticarcillin | * 88% | 0% |
| Ticarcillin/clavulanic acid | 82% | 20% | Ticarcillin/clavulanic acid | 94% | 0% |
| Trimethoprim/sulfamethoxazole | 61% | 7% | Trimethoprim/sulfamethoxazole | 81% | 0% |
Medicine section: * Number of Staphylococcus spp. isolates for ampicillin, penicillin and ticarcillin susceptibility was 18. ** Number of Staphylococcus spp. isolates for cephalothin susceptibility was 16. Emergency section: * Number of Staphylococcus spp. isolates for ampicillin, penicillin and ticarcillin susceptibility was 8. ** Number of Staphylococcus spp. isolates for cephalothin susceptibility was 13. Grey-shading indicates that susceptibility to a drug was not tested with that organism.
Table 5.
Antibiogram showing percent antimicrobial susceptibility of Gram-negative bacteria isolated from different sections of a veterinary teaching hospital in Indiana, the United States, 2015.
| Oncology | Medicine | Emergency | |||
|---|---|---|---|---|---|
| Escherichia coli | Escherichia coli | Escherichia coli | |||
| Number of Isolates | 28 | Number of Isolates | 26 | Number of Isolates | 15 |
| Antimicrobial | |||||
| Amikacin | 100% | Amikacin | 100% | Amikacin | 100% |
| Amoxicillin/clavulanic acid | 71% | Amoxicillin/clavulanic acid | 81% | Amoxicillin/clavulanic acid | 67% |
| Ampicillin | 57% | Ampicillin | 69% | Ampicillin | 73% |
| Cefazolin | 79% | Cefazolin | 85% | Cefazolin | 73% |
| Cefovecin | 82% | Cefovecin | 88% | Cefovecin | 73% |
| Cefoxitin | 86% | Cefoxitin | 85% | Cefoxitin | 73% |
| Cefpodoxime | 82% | Cefpodoxime | 88% | Cefpodoxime | 73% |
| Ceftiofur | 82% | Ceftiofur | 81% | Ceftiofur | 67% |
| Cephalothin ** | 100% | Cephalothin ** | 100% | Cephalothin ** | 9% |
| Chloramphenicol | 86% | Chloramphenicol | 85% | Chloramphenicol | 93% |
| Clindamycin ¥ | 0% | Clindamycin | 4% | Clindamycin | 7% |
| Doxycycline | 75% | Doxycycline | 81% | Doxycycline | 87% |
| Enrofloxacin | 82% | Enrofloxacin | 85% | Enrofloxacin | 93% |
| Erythromycin ¥ | 11% | Erythromycin | 12% | Erythromycin | 7% |
| Gentamycin | 93% | Gentamycin | 96% | Gentamycin | 100% |
| Imipenem | 100% | Imipenem | 100% | Imipenem | 100% |
| Marbofloxacin | 82% | Marbofloxacin | 85% | Marbofloxacin | 93% |
| Oxacillin + 2%NACL | 0% | Oxacillin + 2%NACL | 0% | Oxacillin + 2%NACL | 0% |
| Penicillin | 0% | Penicillin | 0% | Penicillin | 0% |
| Ticarcillin | 57% | Ticarcillin | 69% | Ticarcillin | 73% |
| Ticarcillin/clavulanic acid | 71% | Ticarcillin/clavulanic acid | 80% | Ticarcillin/clavulanic acid | 73% |
| Trimethoprim/sulfamethoxazole | 79% | Trimethoprim/sulfamethoxazole | 88% | Trimethoprim/sulfamethoxazole | 100% |
Oncology section: ** Number of isolates for cephalothin susceptibility was 19. Medicine section: ** Number of isolates for cephalothin susceptibility was 18. Emergency section: ** Number of isolates for cephalothin susceptibility was 9. ¥ indicates the drug is primarily used for Gram-positive organisms. Grey-shading indicates that susceptibility to a drug was not tested with that organism.
Table 6.
Antibiogram showing percent antimicrobial susceptibility of Staphylococcus spp. isolated from samples obtained from the ear and skin of canine patients at a veterinary teaching hospital in Indiana, the United States, 2015.
| Samples from the Ear | Samples from the Skin | ||
|---|---|---|---|
| Staphylococcus spp. | Staphylococcus spp. | ||
| Number of Isolates | 21 | Number of Isolates | 43 |
| Antimicrobial | |||
| Amikacin | 90% | Amikacin | 93% |
| Amoxicillin/clavulanic acid | 95% | Amoxicillin/clavulanic acid | 88% |
| Ampicillin * | 29% | Ampicillin * | 3% |
| Cefazolin | 95% | Cefazolin | 88% |
| Cefovecin | 90% | Cefovecin | 81% |
| Cefoxitin | 95% | Cefoxitin | 88% |
| Cefpodoxime | 90% | Cefpodoxime | 81% |
| Ceftiofur | 95% | Ceftiofur | 88% |
| Cephalothin ** | 100% | Cephalothin ** | 100% |
| Chloramphenicol | 95% | Chloramphenicol | 84% |
| Clindamycin | 81% | Clindamycin | 63% |
| Doxycycline | 71% | Doxycycline | 65% |
| Enrofloxacin | 76% | Enrofloxacin | 70% |
| Erythromycin | 81% | Erythromycin | 65% |
| Gentamycin | 86% | Gentamycin | 67% |
| Imipenem | 95% | Imipenem | 88% |
| Marbofloxacin | 81% | Marbofloxacin | 72% |
| Oxacillin + 2%NACL | 95% | Oxacillin + 2%NACL | 88% |
| Penicillin * | 0% | Penicillin * | 0% |
| Ticarcillin * | 93% | Ticarcillin * | 81% |
| Ticarcillin/clavulanic acid | 95% | Ticarcillin/clavulanic acid | 88% |
| Trimethoprim/sulfamethoxazole | 71% | Trimethoprim/sulfamethoxazole | 63% |
Ear samples: * Number of Staphylococcus spp. isolates for ampicillin, penicillin and ticarcillin susceptibility was 14. ** Number of Staphylococcus spp. isolates for cephalothin susceptibility was 17. Skin samples: * Number of Staphylococcus spp. isolates for ampicillin, penicillin and ticarcillin susceptibility was 31. ** Number of Staphylococcus spp. isolates for cephalothin susceptibility was 26. Grey-shading indicates that susceptibility to a drug was not tested with that organism.
Table 7.
Antibiogram showing percent antimicrobial susceptibility of bacteria isolated from urine samples of canine patients at a veterinary teaching hospital in Indiana, the United States, 2015.
| Gram-Negative | Gram-Positive | ||||||
|---|---|---|---|---|---|---|---|
| Escherichia coli | Pseudomonas spp. | Proteus mirabilis | Staphylococcus spp. | Streptococcus spp. | Enterococcus spp. | ||
| Number of Isolates | 71 | 13 | 14 | Number of Isolates | 49 | 27 | 45 |
| Antimicrobial | |||||||
| Amikacin | 99% | 100% | 93% | Amikacin | 90% | 89% | 0% |
| Amoxicillin/clavulanic acid | 80% | 8% | 100% | Amoxicillin/clavulanic acid | 86% | 100% | 73% |
| Ampicillin | 69% | 8% | 86% | Ampicillin | 23% | 93% | 73% |
| Cefazolin | 82% | 8% | 93% | Cefazolin | 86% | 100% | 0% |
| Cefovecin | 86% | 15% | 93% | Cefovecin | 82% | 96% | 0% |
| Cefoxitin | 84% | 8% | 93% | Cefoxitin | 86% | 93% | 0% |
| Cefpodoxime | 84% | 8% | 93% | Cefpodoxime | 82% | 93% | 0% |
| Ceftiofur | 80% | 15% | 93% | Ceftiofur | 76% | 96% | 0% |
| Cephalothin ** | 100% | 100% | Cephalothin ** | 100% | 100% | ||
| Chloramphenicol | 83% | 8% | 71% | Chloramphenicol | 78% | 89% | 89% |
| Clindamycin ¥ | 1% | 8% | 0% | Clindamycin | 69% | 96% | 0% |
| Doxycycline | 82% | 15% | 0% | Doxycycline | 59% | 78% | 49% |
| Enrofloxacin | 87% | 77% | 93% | Enrofloxacin | 76% | 26% | 4% |
| Erythromycin ¥ | 11% | 8% | 0% | Erythromycin | 69% | 7% | 31% |
| Gentamycin | 94% | 100% | 86% | Gentamycin | 80% | 92% | 2% |
| Imipenem | 100% | 100% | 100% | Imipenem | 86% | 100% | 76% |
| Marbofloxacin | 86% | 93% | Marbofloxacin | 82% | 48% | ||
| Oxacillin + 2%NACL | 0% | 15% | 0% | Oxacillin + 2%NACL | 86% | 100% | 7% |
| Penicillin | 0% | 8% | 0% | Penicillin * | 0% | 89% | 69% |
| Ticarcillin | 69% | 100% | 86% | Ticarcillin * | 80% | 96% | 13% |
| Ticarcillin/clavulanic acid | 79% | 100% | 93% | Ticarcillin/clavulanic acid | 86% | 96% | 16% |
| Trimethoprim/sulfamethoxazole | 86% | 23% | 93% | Trimethoprim/sulfamethoxazole | 80% | 89% | 4% |
Gram-negatives: ** Numbers of isolates tested for cephalothin susceptibility were as follows: E. coli = 50, Proteus mirabilis = 12. ¥ indicates the drug is primarily used for Gram-positive organisms. Gram-positives: ** Numbers of isolates tested for cephalothin susceptibility were as follows: Staphylococcus spp. = 29, Streptococcus spp. = 24. * Number of Staphylococcus spp. isolates for penicillin and ticarcillin susceptibility was 35. Grey-shading indicates that susceptibility to a drug was not tested with that organism.
3. Discussion
This article adds to the literature a description of practical approaches used in making antibiograms for a veterinary teaching hospital. This work shows that creating cumulative antibiograms for dogs seen at a veterinary teaching hospital stratified by area of hospital admission and sample type is feasible and practical. Similar to ours, another study conducted in the US demonstrated the feasibility of adapting the existing guidelines for developing antibiograms in human medicine to the veterinary companion animal private practice setting [17]. The results of these antibiograms show the tremendous variability in the antimicrobial susceptibilities of the isolates to specific antimicrobials at a veterinary teaching hospital in Indiana, e.g., in the general antibiograms, 90% of the staphylococcal isolates were susceptible to amoxicillin–clavulanic acid, only 70% were susceptible to enrofloxacin, 73% of E. coli were susceptible to amoxicillin–clavulanic acid and 85% were susceptible to enrofloxacin. These susceptibility differences suggest that antibiograms could be very useful in guiding empiric antimicrobial therapy and could guide the framing of antimicrobial stewardship policies at this hospital.
The E. coli isolated from samples obtained from the Medicine service showed susceptibility profiles similar to those observed in the general Gram-negative antibiogram. Of the 43 Staphylococcus spp. isolates from the skin, only 63% showed susceptibility to clindamycin, a drug of choice for treating drug-resistant skin infections (pyoderma) in dogs, and 95% Staphylococcus spp. isolates from the ear were susceptible to amoxicillin–clavulanic acid. In comparison to our findings, a study conducted in Japan found 82% of all the Staphylococcus pseudintermedius isolates from dogs with otitis externa were susceptible to amoxicillin–clavulanic acid [18]. In our antibiograms, no Staphylococcus spp. isolate from the ear was susceptible to penicillin. A study conducted in France reported a 68.5% resistance to penicillin among Staphylococcus pseudintermedius isolated from dogs with otitis [19]. Sixty-nine percent of the 71 E. coli isolated from urine were susceptible to ticarcillin and 73% of the 45 Enterococcus spp. isolated from urine were susceptible to amoxicillin–clavulanic acid and ampicillin. Our urinary antibiogram showed lower susceptibility of Enterobacter spp. to amoxicillin–clavulanic acid when compared to the findings of an Australian study [20] that reported a 95% susceptibility of Enterobacter faecalis to amoxicillin–clavulanic acid. Compared to ours, the Australian study had a larger sample size collected over a five-year period. We observed from our antibiograms that a 100% of the canine E. coli isolated from a veterinary teaching hospital in Indiana in 2015 were susceptible to imipenem. This is similar to a previous report from Marshfield labs, where a 100% susceptibility of canine E. coli isolates tested in the years 2017 through 2018 were susceptible to imipenem [21]. Additionally, a previous study reported a 99.9% cumulative susceptibility of E. coli isolates from human samples to imipenem in Pennsylvania [15]. Resistance to carbapenems (such as imipenem) among veterinary isolates is rare, and imipenem is highly stable against most β-lactamases [22]. Possibly, the 100% susceptibility observed in our antibiograms is due to the pharmacodynamic properties of imipenem or could be because imipenem is not frequently used and is not a first-line drug in the treatment of dogs with bacterial infections. However, it is important to note that the reported proportions of susceptible isolates could be subject to selection bias because selection bias can be an issue in clinical samples submitted to a diagnostic laboratory.
Having an insufficient number of isolates for analysis is a concern when creating antibiograms in smaller human hospitals and for infrequently isolated bacteria [11]. In our analysis, the number of isolates for other animal species (e.g., cattle) and for some individual bacterial species was very low (fewer than 30 isolates), and therefore antibiograms were not constructed. Depending upon the patient population and the frequency of clinical sample submission, we observe that construction of veterinary antibiograms for the various veterinary animal species (except for dogs) and for some bacterial species will be challenging due to the relatively small number of samples submitted for bacterial culture and antimicrobial susceptibility testing. This challenge could be overcome with the use of data collected over a longer period of time.
Periodic preparation and distribution of cumulative antibiograms to clinicians, the hospital pharmacist, and the infection-control personnel at this hospital in the form of pocket guides and website postings will be useful. The impact of the antibiograms on veterinary prescription practices through periodic assessment of hospital pharmacy records and via surveys periodically administered to clinicians should be routinely assessed.
4. Materials and Methods
We used laboratory data for the period from 1 January 2015 to 31 December 2015 provided by the Animal Disease Diagnostic Lab (ADDL) at a veterinary teaching hospital in Indiana, the United States. The data provided did not contain personally identifiable information about the clinician or client and was provided in a Microsoft Excel file. These data consisted of 661 (complete and partial) antimicrobial susceptibility profiles for isolates obtained from dogs (n = 578), cats (n = 68), rats (n = 4), wolves (n = 2), guinea pigs (n = 2), chinchillas (n = 2), fox (n = 1), conure (n = 1), and snake (n = 1). No animal species was recorded for two isolates. The data were verified and examined for completeness. The data captured in the dataset included the hospital section where the patient was admitted, the location from which the sample was collected, the bacterial isolate identified, and the antimicrobial susceptibility profiles for various antimicrobials. Given the low numbers reported for the various animal species, only the dog data were utilized in the construction of the antibiograms. In total, 563 canine antimicrobial susceptibility profiles were analyzed.
We used the Clinical and Laboratory Standards Institute (CLSI) guidelines for the use in the construction of the antibiograms in human healthcare settings as the basis for the veterinary antibiograms [23]. In brief, the CLSI recommends that the isolates included in antibiogram construction are identified during diagnostic sampling and include only the results from the first isolate recovered from each patient. Information from individual bacterial species should only be presented if there are at least 30 isolates tested. In addition, data should be presented as the total percent of the isolates susceptible to drugs that are routinely tested and should not include the percentage of isolates with intermediate susceptibility results. Data included in hospital antibiograms should be updated annually [11].
In our analysis, we utilized only the first isolate of a given bacterial species recovered from each canine patient during 2015 in the calculation of percent susceptibility for each organism/antimicrobial combination. Antibiograms for drugs that are routinely tested were prepared and presented in tabular form. These drugs include amikacin, amoxicillin/clavulanic acid, ampicillin, cefazolin, cefovecin, cefoxitin, cefpodoxime, ceftiofur, cephalothin, chloramphenicol, clindamycin, doxycycline, enrofloxacin, erythromycin, gentamycin, imipenem, marbofloxacin, oxacillin + 2%NACL, penicillin, ticarcillin, ticarcillin/clavulanic acid, trimethoprim/sulfamethoxazole.
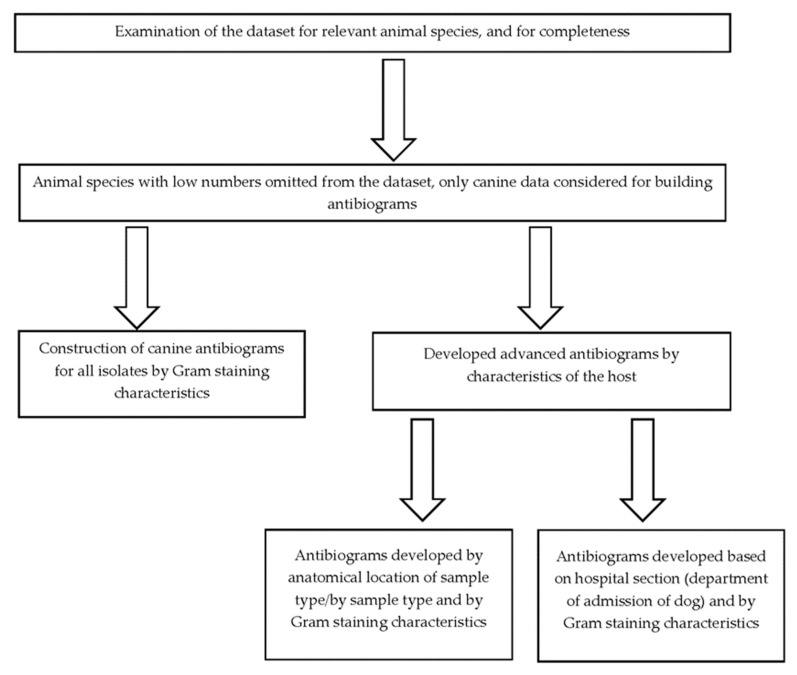
Only the percentage of susceptible isolates and not those which are of intermediate susceptibility were presented. Organisms with fewer than 30 isolates were only included if their inclusion was deemed essential based on existing knowledge showing that resistance against common antimicrobials is possible [22]. In some instances, species within a genus with fewer than 30 individual isolates were grouped together. In cases where the number of isolates used to calculate the percent susceptibility to a given drug were fewer compared to other drugs, a note was appended to indicate that the calculation of percent susceptibility was conducted using fewer isolates. The completed antibiograms were further validated to ensure that only percent susceptibilities for antimicrobial agents that are appropriate for clinical use in the species were reported. However, percent susceptibilities for known intrinsically resistant pathogens such as Pseudomonas aeruginosa and Enterococcus spp. were reported in the antibiograms to guide the antimicrobial selection. Additionally, percentage susceptibilities for drugs primarily used to treat Gram-positive infections (lincosamides, e.g., clindamycin and macrolides, e.g., erythromycin) were reported for Gram-negative pathogens with a footnote added stating that these two drug classes are primarily used against Gram-positive organisms. The percentage susceptibilities were calculated using commercial statistical software (SAS, version 9.4, SAS Institute Inc, Cary, NC, USA) and the steps taken in constructing the antibiograms are given in Figure 1.
Figure 1.
The algorithm used to construct veterinary antibiograms for dogs presented to a veterinary teaching hospital in Indiana, the United States, 1 January 2015 to 31 December 2015.
5. Conclusions
Creating cumulative antibiograms for dogs seen at a veterinary teaching hospital is feasible and practical. Susceptibility differences to specific antimicrobials were identified among bacteria isolated at a veterinary teaching hospital in Indiana from January 2015 through December 2015. Cumulative antibiograms could be useful in guiding empiric antimicrobial therapy and for detecting and monitoring the AMR trends at this teaching hospital.
Acknowledgments
This project was funded by the Integrative Data Science Initiative at Purdue University. We extend our special thanks to Sharon Erdman of Eskenazi Health for guidance in the antibiograms construction and Amanda Martin of Purdue University for assistance in data preparation. We thank the Indiana Animal Disease Diagnostic Laboratory for providing the data used to construct the antibiograms.
Author Contributions
Conceptualization, A.R., L.G. and K.H.; methodology, J.E.E., L.G., M.A., K.H.; software, J.E.E.; validation, J.E.E., L.G., K.H.; formal analysis, K.H.; investigation, J.E.E., L.G., M.A., K.H.; resources, A.R., L.G. and K.H.; data curation, J.E.E. and M.A.; writing—original draft preparation, J.E.E.; writing—review and editing, J.E.E., L.G., M.A., K.H., A.R.; visualization, J.E.E.; supervision, A.R.; project administration, A.R.; funding acquisition, A.R., L.G. and K.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study since no humans or vertebrate animals were involved in this research.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project received funding from the Integrative Data Science Initiative at Purdue University.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Partridge S.R., Kwong S.M., Firth N., Jensen S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018;31:e00088-17. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baekkeskov E., Rubin O., Munkholm L., Zaman W. Antimicrobial Resistance as a Global Health Crisis. [(accessed on 21 April 2023)]. Available online: https://oxfordre.com/politics/display/10.1093/acrefore/9780190228637.001.0001/acrefore-9780190228637-e-1626.
- 3.Weese J.S., Giguère S., Guardabassi L., Morley P.S., Papich M., Ricciuto D.R., Sykes J.E. ACVIM Consensus Statement on Therapeutic Antimicrobial Use in Animals and Antimicrobial Resistance. J. Vet. Intern. Med. 2015;29:487–498. doi: 10.1111/jvim.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes A.H., Moore L.S.P., Sundsfjord A., Steinbakk M., Regmi S., Karkey A., Guerin P.J., Piddock L.J.V. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 5.McEwen S.A., Collignon P.J. Antimicrobial Resistance: A One Health Perspective. Trans. R. Soc. Trop. Med. Hyg. 2017;111:255–260. doi: 10.1128/microbiolspec.ARBA-0009-2017. [DOI] [PubMed] [Google Scholar]
- 6.Hildreth C.J., Burke A.E., Glass R.M. Inappropriate Use of Antibiotics. JAMA. 2009;302:816. doi: 10.1001/jama.302.7.816. [DOI] [PubMed] [Google Scholar]
- 7.Leekha S., Terrell C.L., Edson R.S. General Principles of Antimicrobial Therapy. Mayo Clin. Proc. 2011;86:156–167. doi: 10.4065/mcp.2010.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guardabassi L., Lars Bøgø J., Hilde K. Guide to Antimicrobial Use in Animals. 1st ed. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2008. [Google Scholar]
- 9.De Briyne N., Atkinson J., Pokludová L., Borriello S.P., Price S. Factors Influencing Antibiotic Prescribing Habits and Use of Sensitivity Testing amongst Veterinarians in Europe. Vet. Rec. 2013;173:475. doi: 10.1136/vr.101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts J.L., Sweeney M.T., Lubbers B.V. Antimicrobial Susceptibility Testing of Bacteria of Veterinary Origin. Microbiol. Spectr. 2018;6:6.2.08. doi: 10.1128/microbiolspec.ARBA-0001-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hindler J.F., Stelling J. Analysis and Presentation of Cumulative Antibiograms: A New Consensus Guideline from the Clinical and Laboratory Standards Institute. Clin. Infect. Dis. 2007;44:867–873. doi: 10.1086/511864. [DOI] [PubMed] [Google Scholar]
- 12.Fernández J., Vazquez F. The Importance of Cumulative Antibiograms in Diagnostic Stewardship. Clin. Infect. Dis. 2019;69:1086–1087. doi: 10.1093/cid/ciz082. [DOI] [PubMed] [Google Scholar]
- 13.Lautenbach E., Nachamkin I. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Data (Antibiograms): Substantial Variability across Medical Centers in the United States. Infect. Control. Hosp. Epidemiol. 2006;27:409–412. doi: 10.1086/503342. [DOI] [PubMed] [Google Scholar]
- 14.Joshi S. Hospital Antibiogram: A Necessity. Indian J. Med. Microbiol. 2010;28:277–280. doi: 10.4103/0255-0857.71802. [DOI] [PubMed] [Google Scholar]
- 15.Guarascio A.J., Brickett L.M., Porter T.J., Lee N.D., Gorse E.E., Covvey J.R. Development of a Statewide Antibiogram to Assess Regional Trends in Antibiotic-Resistant ESKAPE Organisms. J. Pharm. Pract. 2019;32:19–27. doi: 10.1177/0897190017735425. [DOI] [PubMed] [Google Scholar]
- 16.Frey E., Jacob M. Commentary: Using Antibiograms to Promote Antimicrobial Stewardship during Treatment of Bacterial Cystitis and Superficial Bacterial Folliculitis in Companion Animal Practice. J. Am. Vet. Med. Assoc. 2020;257:900–903. doi: 10.2460/javma.257.9.900. [DOI] [PubMed] [Google Scholar]
- 17.Frey E., Jacob M. Development of a Method for Creating Antibiograms for Use in Companion Animal Private Practices. J. Am. Vet. Med. Assoc. 2020;257:950–960. doi: 10.2460/javma.257.9.950. [DOI] [PubMed] [Google Scholar]
- 18.Iyori K., Shishikura T., Shimoike K., Minoshima K., Imanishi I., Toyoda Y. Influence of Hospital Size on Antimicrobial Resistance and Advantages of Restricting Antimicrobial Use Based on Cumulative Antibiograms in Dogs with Staphylococcus Pseudintermedius Infections in Japan. Vet. Dermatol. 2021;32:668-e178. doi: 10.1111/vde.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourély C., Cazeau G., Jarrige N., Leblond A., Madec J.Y., Haenni M., Gay E. Antimicrobial Resistance Patterns of Bacteria Isolated from Dogs with Otitis. Epidemiol. Infect. 2019;147:e121. doi: 10.1017/S0950268818003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarborough R., Bailey K., Galgut B., Williamson A., Hardefeldt L., Gilkerson J., Browning G. Use of Local Antibiogram Data and Antimicrobial Importance Ratings to Select Optimal Empirical Therapies for Urinary Tract Infections in Dogs and Cats. Antibiotics. 2020;9:924. doi: 10.3390/antibiotics9120924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshfieldslabs. Canine Prevalent Pathogens & Antimicrobial Susceptibility Patterns: January 1, 2017 to December 31, 2018. [(accessed on 27 January 2023)]. Available online: https://wwwmarshfieldlabsorg/Sites/Ltrm/Vet/Documents/Annual_Cumulative_Antibiogram_Caninepdf.
- 22.Papich M.G. Antibiotic Treatment of Resistant Infections in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2013;43:1091–1107. doi: 10.1016/j.cvsm.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 23.CLSI . Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2014. Approved Guideline—Fourth Edition. CLSI document M39-A4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are available from the corresponding author upon reasonable request.