Abstract
Lateral pelvic lymph node dissection (LPND) is a technically demanding procedure. This study aimed to compare the short-term outcomes of laparoscopic and robotic LPNDs. This multi-institutional retrospective study included 108 consecutive patients who underwent laparoscopic or robotic total mesorectal excision with LPND for locally advanced rectal cancer. There were 74 patients in the laparoscopic and 34 in the robotic groups. The median operation time was longer in the robotic group than in the laparoscopic group (353 vs. 275 min, p < 0.001). No patients underwent conversion to open surgery in either group. Pathological LPN metastases were observed in 24 and 8 patients in the laparoscopic and robotic groups, respectively (p = 0.347). Although the number of harvested mesorectal lymph nodes was similar (15.5 vs. 15.0, p = 0.968), the number of harvested LPNs was higher in the robotic than in the laparoscopic group (7.0 vs. 5.0, p = 0.004). Postoperative complications and length of hospital stay were similar (robotic vs. laparoscopic, 35.3% and 7 days vs. 37.8% and 7 days, respectively). Both laparoscopic and robotic LPND are safe and feasible for locally advanced rectal cancers, but robotic LPND showed more harvested lateral lymph node than laparoscopic LPND.
Keywords: lateral pelvic lymph node dissection, robotic surgery, laparoscopic surgery, rectal cancer
1. Introduction
Surgical eradication of the lateral pelvic lymph nodes (LPNs) in rectal cancer has attracted much attention recently. In Japan, total mesorectal excision (TME) combined with lateral pelvic lymph node dissection (LPND) without preoperative chemoradiation (CRT) has been routinely performed in locally advanced mid-to-low rectal cancer [1,2]. On the other hand, preoperative CRT, followed by TME, has been widely accepted as a standard treatment in the same situation in Western countries [3,4]. Two disparate treatment strategies have been performed because of the ambivalence of LPND, which reduces local recurrence and improves survival [5,6] while increasing postoperative morbidity such as sexual or urinary dysfunction [7]. A recent collaborative study on LPNs showed that combining LPND and TME decreased local recurrence in patients with suspected LPN metastasis even after preoperative CRT [6]. Therefore, interest in LPN treatment has been moving toward performing LPND in cases with suspected LPN metastases in both Western and Eastern countries [5,6].
Another concern is the technical aspects of LPND. Despite its potential oncological benefits, LPND is still a procedure many colorectal surgeons are reluctant to perform because of its complexity, difficulty, and potential postoperative morbidity. Rectal cancer surgery challenges have inspired innovations because of the high recurrence rates, high morbidity, and the technical difficulties of operating in the deep and narrow pelvis. Robotic surgery has overcome some of these challenges and has shown promising results [8]. Laparoscopic surgery has been widely accepted as the standard modality for colon cancer [9]. However, the laparoscopic approach for rectal cancer has technical limitations due to the use of straight and fixed instruments. Robotic systems have several advantages over laparoscopic platforms. Robotic systems have endo-wrists, which enable free movement, provide high-quality three-dimensional stereoscopic images and stable traction, and prevent natural hand tremors [10]. Thus, the robotic system could be a better surgical option for LPND than the laparoscopic approach.
However, few studies have reported the potential advantages of robotic LPND compared to the laparoscopic approach. This study aimed to identify the potential advantages of robotic LPND over laparoscopic LPND by comparing the two approaches.
2. Materials and Methods
Between January 2015 and December 2021, 108 consecutive patients who underwent laparoscopic or robotic TME with LPND for locally advanced mid-to-low rectal cancer (clinically T3 or N+) and clinically suspected LPN metastasis were included from three study centers: Seoul St. Mary’s Hospital (n = 46), Incheon St. Mary’s Hospital (n = 34), and St. Vincent’s Hospital (n = 28). Inclusion criteria were as follows: 1. Locally advanced rectal cancer (clinically T3 or N+), 2. Mid-to-low rectal cancer (tumor located within 10 cm from anal verge (AV)). 3. Pathologically proven adenocarcinoma, 4. Clinically suspected LPN metastasis, 5. Curative intent surgery (R0 resection) 6. Age < 80 years, 7. Elective surgery. The exclusion criteria were as follows: 1. Early-stage rectal cancer (clinically T1-2 and N-), 2. Upper rectal cancer (tumor located above 10 cm from AV), 3. Unresectable distant metastasis, 4. No magnetic resonance imaging (MRI) before surgery, 5. No suspected LPN metastasis. Data were collected from three hospitals affiliated with the College of Medicine, The Catholic University of Korea. This study was approved by the Institutional Review Board of the Ethics Committee of the College of Medicine, The Catholic University of Korea (XC21RADI0112). The procedures were carried out in accordance with the ethical standards of the committee responsible for human experimentation and with the guidelines of the Helsinki Declaration, 1975, as revised in 1983. All patient records were anonymized and de-identified before the analysis.
Patient data, including demographic and clinicopathological characteristics, were collected from each hospital’s rectal cancer patient registry. All patients included in this study were diagnosed with clinically suspected LPN metastases using pretreatment MRIs. After preoperative CRT, an MRI re-assessment was performed to check the tumor response and change in LPN size. Clinically suspected LPN metastasis was defined as the enlargement of lymph nodes (LNs) greater than 5 mm in short-axis diameter with speculated borders. Mid-to-low rectal cancer was defined as a tumor with an inferior margin within 10 cm from the AV and below the peritoneal reflection, as assessed on MRI (mid, 5.1–10.0 cm; low, 0–5.0 cm) [11]. The numbers of harvested and metastatic LNs in the mesorectum and lateral compartment were pathologically reported. Postoperative complications were recorded within 30 days after surgery using the Clavien–Dindo classification.
Preoperative CRT was recommended as the initial treatment for all patients with suspected metastatic LPNs. However, it was omitted in some patients who refused preoperative CRT, had distant metastases, or were expected to have low compliance owing to their advanced age or comorbidities. The choice of long-course and short-course radiation was made based on the surgeon’s preference and multidisciplinary team discussion. Surgery was performed 7–8 weeks after completion of any CRT [12]. All surgeries were performed by colorectal surgeons certified in the subspecialty of colorectal surgery by the Korean Surgical Society. Five surgeons who participated in the study belonged to the same university and held monthly meetings to standardize the procedure. The surgery details were the same for both laparoscopic and robotic approaches.
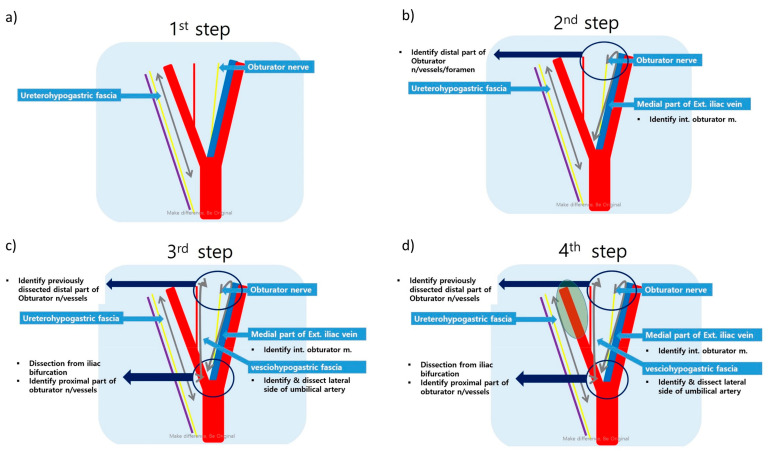
In the robotic approach, the da Vinci Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) was used with a standardized procedure [13]. Step 1 was the dissection of the uretero-hypogastric fascia, which envelopes the ureter and the hypogastric nerve. Step 2 was the dissection of the medial side of the external iliac vein located at the lateral border of the obturator LN group. Step 3 was the dissection of the vesico-hypogastric fascia at the medial border of the obturator LN group. The final step was the dissection of the internal iliac artery to Alcock’s canal (Figure 1 and Figure 2).
Figure 1.
Standardized procedure for right lateral pelvic lymph node dissection: (a) Dissection of the uretero-hypogastric fascia; (b) Dissection of the obturator space; (c) Dissection of the vesico-hypogastric fascia; (d) Dissection of the internal iliac vessels.
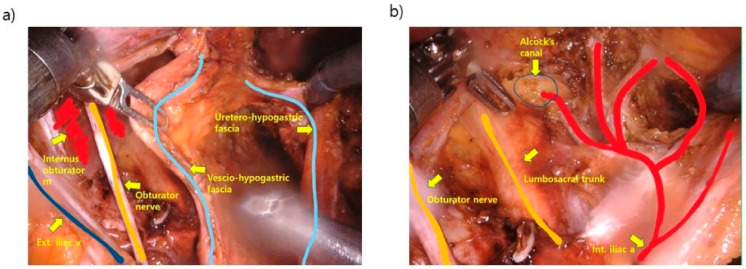
Figure 2.
Surgical view after completion of left lateral pelvic lymph node dissection: (a) External view of the lateral pelvic area; (b) Internal view of the lateral pelvic area.
Surveillance was performed every 3–6 months until 2 years postoperatively and then every 6–12 months until 5 years postoperatively. Tumor markers including carcinoembryonic antigen and carbohydrate antigen 19–9, abdominopelvic computed tomography (CT), and chest CT were performed according to the surveillance schedule. Local recurrence was defined as the disease recurrence in the pelvic cavity, including the lateral pelvic area. Local-recurrence-free survival and recurrence-free survival were defined as the interval from the date of surgery until the date of local recurrence and any recurrence detection by radiological or pathological examination or, in case of no recurrence, until the date of last follow-up. The follow-up was completed in April 2023.
Proportions and medians (interquartile ranges (IQR)) are presented for categorical and continuous variables, respectively. Chi-square or Fisher’s exact tests assessed the association between categorical variables and surgical approaches. Continuous variables were compared using independent t-tests. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS version 25.0 for Windows (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Baseline Characteristics
A total of 108 patients with rectal cancer who underwent TME with LPND were included in this study. The baseline characteristics are summarized in Table 1. Laparoscopic and robotic LPND was performed in 74 (68.5%) and 34 (31.5%) patients, respectively. The median age was 61 years (IQR 54–68 years), and male and female patients were 71 (65.7%) and 37 (34.3%), respectively. Tumors were located in the mid rectum (5.1 cm to 10.0 cm from the AV) and low rectum (0 cm to 5.0 cm from AV) in 67 (62.0%) and 41 (38.0%) patients, respectively. Preoperative CRT was performed in 84 (77.8%) patients. The two groups had no significant differences between the baseline patient, tumor, or LPN characteristics.
Table 1.
Baseline characteristics of patients who underwent laparoscopic and robotic total mesorectal excision with lateral pelvic lymph node dissection.
| Variables | Total Patients (N = 108) |
Laparoscopic LPND (N = 74) |
Robotic LPND (N = 34) |
p-Value |
|---|---|---|---|---|
| Age (years) | 61 (54–68) | 63 (54–71) | 60 (53–66) | 0.129 |
| Sex | 0.555 | |||
| Male | 71 (65.7) | 50 (67.6) | 21 (61.8) | |
| Female | 37 (34.3) | 24 (32.4) | 13 (38.2) | |
| BMI (kg/m2) | 0.111 | |||
| <25 | 71 (65.7) | 45 (60.8) | 26 (46.5) | |
| ≥25 | 37 (34.3) | 29 (39.2) | 8 (23.5) | |
| ASA score | 0.233 | |||
| <3 | 105 (97.2) | 73 (98.6) | 32 (94.1) | |
| ≥3 | 3 (2.8) | 1 (1.4) | 2 (5.9) | |
| Tumor level from AV (cm) | 0.641 | |||
| >5 | 67 (62.0) | 47 (63.5) | 20 (58.8) | |
| ≤5 | 41 (38.0) | 27 (36.5) | 14 (41.2) | |
| cT stage | 0.107 | |||
| 2–3 | 72 (66.7) | 53 (71.6) | 19 (55.9) | |
| 4 | 36 (33.3) | 21 (28.4) | 15 (44.1) | |
| cN stage | 0.132 | |||
| 1 | 56 (51.9) | 42 (56.8) | 14 (41.2) | |
| 2 | 52 (48.1) | 32 (43.2) | 20 (58.8) | |
| cM stage | 0.722 | |||
| 0 | 98 (90.7) | 68 (91.9) | 30 (88.2) | |
| 1 | 10 (9.3) | 6 (8.1) | 4 (11.8) | |
| LPN location | 0.953 | |||
| Unilateral | 95 (88.0) | 65 (87.8) | 30 (88.2) | |
| Bilateral | 13 (12.0) | 9 (12.2) | 4 (11.8) | |
| LPN region | 0.104 | |||
| Internal iliac | 66 (61.1) | 50 (67.6) | 16 (47.1) | |
| Obturator | 32 (29.6) | 17 (23.0) | 15 (44.1) | |
| External iliac | 8 (7.4) | 5 (6.8) | 3 (8.8) | |
| Multiple | 2 (1.9) | 2 (2.7) | 0 (0) | |
| a Initial LPN size (mm) | 10.0 (7.0–13.0) | 10.0 (7.5–13.0) | 8.0 (6.0–11.0) | 0.232 |
| b Initial LPN size (mm) | 10.0 (6.4–12.0) | 10.0 (7.5–13.0) | 7.1 (5.9–11.0) | 0.186 |
| a Initial CEA level (ng/mL) | 4.3 (9.5–2.3) | 4.6 (2.6–11.4) | 3.4 (1.8–8.0) | 0.808 |
| Preoperative CRT | 0.076 | |||
| No | 24 (22.2) | 20 (27.0) | 4 (11.8) | |
| Yes | 84 (77.8) | 54 (73.0) | 30 (88.2) | |
| b Post-CRT LPN size (mm) | 6.0 (4.3–8.0) | 7.0 (5.0–9.0) | 5.0 (3.0–6.2) | 0.261 |
| b Post-CRT CEA level (ng/mL) | 2.2 (1.4–4.3) | 2.3 (1.4–5.0) | 2.0 (1.4–2.9) | 0.745 |
| Radiotherapy | 0.430 | |||
| Short course | 21 (25.0) | 12 (22.2) | 9 (30.0) | |
| Long course | 63 (75.0) | 42 (77.8) | 21 (70.0) | |
| Chemotherapy | 0.101 | |||
| FL | 23 (27.4) | 18 (33.3) | 5 (16.7) | |
| Capecitabine | 61 (72.6) | 36 (66.7) | 25 (83.3) |
ASA = American Society of Anesthesiologists; AV = anal verge; BMI = body mass index; CEA = carcinoembryonic antigen; CRT = chemoradiotherapy; LPN = lateral pelvic lymph node; LPND = lateral pelvic lymph node dissection. Proportions ( ) are presented for categorical data. Medians and interquartile ranges (IQR) are presented for continuous data. a median value (IQR) in whole patients. b median value (IQR) of patients who underwent preoperative CRT.
3.2. Intraoperative Outcomes
Intraoperative outcomes are shown in Table 2. The ratios of surgical procedures (low anterior resection, intersphincteric resection, and abdominoperineal resection) were not different between the two groups. Bilateral LPND was performed in six (8.1%) and four (11.8%) patients in the laparoscopic and robotic LPND groups, respectively (p = 0.722). The median operation time was longer in the robotic group than in the laparoscopic group (353 vs. 275 min, p < 0.001), while the estimated blood loss (EBL) was similar (p = 0.854). None of the patients in either group required conversion to open surgery.
Table 2.
Intraoperative outcomes of patients who underwent laparoscopic and robotic total mesorectal excision with lateral pelvic lymph node dissection.
| Variables | Total Patients (N = 108) |
Laparoscopic LPND (N = 74) |
Robotic LPND (N = 34) |
p-Value |
|---|---|---|---|---|
| Surgical procedure | 0.681 | |||
| Low anterior resection | 66 (61.1) | 47 (63.5) | 19 (55.9) | |
| Intersphincteric resection | 32 (29.6) | 20 (27.0) | 12 (35.3) | |
| Abdominoperineal resection | 10 (9.3) | 7 (9.5) | 3 (8.8) | |
| LPND direction | 0.722 | |||
| Unilateral | 98 (90.7) | 68 (91.9) | 30 (88.2) | |
| Bilateral | 10 (9.3) | 6 (8.1) | 4 (11.8) | |
| Operation time (min) | 300 (241–374) | 275 (230–347) | 353 (285–447) | <0.001 |
| Estimated blood loss (ml) | 100 (50–150) | 100 (50–120) | 100 (50–200) | 0.854 |
| Conversion to open surgery | 0 | 0 | 0 | 1.000 |
LPND = lateral pelvic lymph node dissection. Proportions ( ) are presented for categorical data. Medians and interquartile ranges are presented for continuous data.
3.3. Pathological Outcomes
Pathological outcomes are shown in Table 3. The pathological T and N stages were similar between the two groups. Pathological LPN metastases were observed in 24 (32.4%) and 8 (23.5%) patients in the laparoscopic and robotic groups, respectively (p = 0.347). Although the number of harvested mesorectal LNs was similar, the number of harvested LPNs was higher in the robotic than in the laparoscopic group (7.0 vs. 5.0, p = 0.004). The number of metastatic LPNs was similar between the groups. Other pathological factors such as margin status, differentiation, and lymphatic, vascular, and perineural invasion were not different between the groups.
Table 3.
Pathological outcomes of the patients who underwent laparoscopic and robotic total mesorectal excision combined with lateral pelvic lymph node dissection.
| Variables | Total Patients (N = 108) |
Laparoscopic LPND (N = 74) |
Robotic LPND (N = 34) |
p-Value |
|---|---|---|---|---|
| pT stage | 0.999 | |||
| T0 (CR) | 12 (11.1) | 8 (10.8) | 4 (11.8) | |
| T1 | 7 (6.5) | 5 (6.8) | 2 (5.9) | |
| T2 | 29 (26.9) | 20 (27.0) | 9 (26.5) | |
| T3 | 53 (49.1) | 36 (48.6) | 17 (50.0) | |
| T4 | 7 (6.5) | 5 (6.8) | 2 (5.9) | |
| pN stage | 0.375 | |||
| N0 | 55 (50.9) | 39 (52.7) | 16 (47.1) | |
| N1 | 32 (29.6) | 19 (25.7) | 13 (38.2) | |
| N2 | 21 (19.4) | 16 (21.6) | 5 (14.7) | |
| LPN metastasis | 32 (29.6) | 24 (32.4) | 8 (23.5) | 0.347 |
| No. of harvested LN (mesorectum) | 15.0 (12.0–21.5) | 15.5 (12.0–23.0) | 15.0 (12.0–21.0) | 0.968 |
| No. of metastatic LN (mesorectum) | 0.0 (0.0–1.0) | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) | 0.733 |
| No. of harvested LN (LPN) | 5.5 (3.0–8.0) | 5.0 (2.0–7.0) | 7.0 (4.0–10.0) | 0.004 |
| No. of metastatic LN (LPN) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.320 |
| Tumor size (cm) | 3.0 (1.1–4.5) | 3.1 (1.6–5.0) | 2.9 (0.5–4.0) | 0.131 |
| DRM (cm) | 1.8 (1.0–3.0) | 2.0 (1.0–3.0) | 1.6 (0.5–3.0) | 0.321 |
| CRM | 1.000 | |||
| Free (≥1 mm) | 100 (92.6) | 68 (91.9) | 32 (94.1) | |
| Involvement | 8 (7.4) | 6 (8.1) | 2 (5.9) | |
| Lymphatic invasion, yes | 42 (38.9) | 30 (40.5) | 12 (35.3) | 0.603 |
| Vascular invasion, yes | 18 (16.7) | 12 (16.2) | 6 (17.6) | 0.853 |
| Perineural invasion, yes | 19 (17.6) | 11 (14.9) | 8 (23.5) | 0.272 |
| Poorly differentiated, yes | 10 (9.3) | 5 (6.8) | 5 (14.7) | 0.282 |
CR = complete response; CRM = circumferential radial margin; DRM = distal resection margin; LN = lymph node; LPND = lateral pelvic lymph node dissection. Proportions ( ) are presented for categorical data. Medians and interquartile ranges are presented for continuous data.
3.4. Postoperative Outcomes
The postoperative outcomes are summarized in Table 4. The median length of the hospital stay after surgery was 7 days (IQR: 6–9 days). There were 40 (37.0%) postoperative complications and 11 (10.2%) major complications, defined as Clavien–Dindo classification ≥3 within 30 days after surgery. There was one case of mortality due to sepsis caused by anastomotic leakage. There were no significant differences between the two groups. The postoperative complications directly related to LPND were lymphocele in four cases and bleeding of the internal iliac artery that required reoperation in the LPND group. The postoperative urinary dysfunction rates in the laparoscopic and robotic LPND groups were 12.2% and 5.9%, respectively. Among them, only one case of lymphocele was observed in the robotic group (p = 0.497).
Table 4.
Postoperative outcomes of the patients who underwent laparoscopic and robotic total mesorectal excision combined with lateral pelvic lymph node dissection.
| Variables | Total Patients (N = 108) |
Laparoscopic LPND (N = 74) |
Robotic LPND (N = 34) |
p-Value |
|---|---|---|---|---|
| Postoperative hospital stay (days) | 7 (6–9) | 7 (6–8) | 7 (5–11) | 0.932 |
| Postoperative complication within 30 days after surgery (CDC) | 40 (37.0) | 28 (37.8) | 12 (35.3) | 0.799 |
| 1 | 3 (2.8) | 3 (4.1) | 0 (0.0) | |
| 2 | 26 (24.1) | 19 (25.7) | 7 (20.6) | |
| 3 | 6 (5.6) | 3 (4.1) | 3 (8.8) | |
| 4 | 4 (3.7) | 3 (4.1) | 1 (2.9) | |
| 5 | 1 (0.9) | 0 (0.0) | 1 (2.9) | |
| Major complication ≥ CDC 3 | 11 (10.2) | 6 (8.1) | 5 (14.7) | 0.317 |
| Postoperative complication details | ||||
| Anastomosis leakage | 13 (12.0) | 8 (10.8) | 5 (14.7) | 0.542 |
| Urinary dysfunction | 11 (10.2) | 9 (12.2) | 2 (5.9) | 0.497 |
| Wound complication | 7 (6.5) | 5 (6.8) | 2 (5.9) | 1.000 |
| Lymphocele | 4 (3.7) | 3 (4.1) | 1 (2.9) | 1.000 |
| Ileus | 5 (4.6) | 2 (2.7) | 3 (8.8) | 0.323 |
| Anastomosis bleeding | 2 (1.9) | 2 (2.7) | 0 | 1.000 |
| Internal iliac a. branch bleeding | 1 (0.9) | 1 (1.4) | 0 | 1.000 |
| Mortality within 30 days after surgery | 1 (0.9) | 0 | 1 (2.9) | 1.000 |
CDC = Clavien–Dindo classification; LPND = lateral pelvic lymph node dissection. Proportions ( ) are presented for categorical data. Medians and interquartile ranges are presented for continuous data.
3.5. Oncological Outcomes
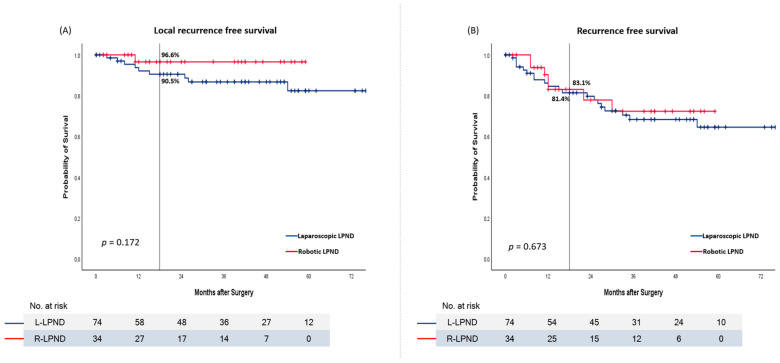
The local recurrence and recurrence rates were compared between the laparoscopic and robotic LPND groups. No significant differences in local recurrence and recurrence-free survival were observed between the two groups (p = 0.172 and 0.673). However, the 18-months local-recurrence-free survival rate tended to be higher in robotic LPND than in laparoscopic LPND. That was 96.6% in robotic group and 90.5% in laparoscopic group. The 18-months recurrence-free-survival rates were 83.1% in robotic LPND and 81.4% in laparoscopic LPND (Figure 3).
Figure 3.
Kaplan–Meier curve in laparoscopic and robotic LPNDs. (A) Local-recurrence-free survival. (B) Recurrence-free survival.
4. Discussion
This study compared the short-term outcomes between laparoscopic and robotic LPND for locally advanced mid-to-low rectal cancer with suspected LPN metastases. Most perioperative outcomes, such as operative details and pathologic and postoperative outcomes, were not different between the two groups. The number of retrieved LPNs was significantly greater in the robotic group than in the laparoscopic group (7.0 vs. 5.0, p = 0.004), while the operating time was significantly longer in the robotic group (353 vs. 275 min, p < 0.001).
With the development of minimally invasive surgery, the scope of surgery using robotic systems has gradually expanded. Rectal cancer surgery is one of the most well-established surgeries in terms of the safety and feasibility of the robotic platform [14]. Robotic surgery enables precise dissection in the narrow pelvic cavity compared to the laparoscopic approach. A meta-analysis of randomized controlled trials demonstrated the safety and feasibility of robotic rectal cancer surgery, along with its lower conversion rate to open surgery and longer operating time than the laparoscopic approach [15]. Two other meta-analyses reported similar results. The first showed that the robotic platform was superior to laparoscopy regarding blood loss, open conversion, hospital stay, and postoperative complications, whereas conventional laparoscopy had an advantage regarding operative time [16]. The other also showed that robotic surgery for rectal cancer was superior to laparoscopy in terms of conversion, and the rest of the perioperative outcomes were similar between the two approaches [17].
Unlike TME, cumulative evidence of the clinical efficacy of robotic LPND is scanty. Several reports have described the surgical techniques or case series for robotic LPND [13,18,19,20]. However, only one retrospective study compared short-term outcomes between laparoscopic and robotic LPNDs in rectal cancer, in 2018 [21]. Kim et al. demonstrated that robotic TME with LPND is safe and feasible, with favorable surgical outcomes compared to the laparoscopic approach [21]. Although a previous study and ours show similar results in an overall context, there are some differences in details. Although they described no differences in the operating time between laparoscopic and robotic LPNDs, the operating time was longer in the robotic group in the present study. This may be due to differences in the surgeons’ experience participating in each study. In Kim’s study, all the procedures were performed by one highly experienced surgeon. In contrast, five surgeons were included in the present study. Furthermore, several studies that compared laparoscopic and robotic TME have reported a significantly longer operation time in robotic TME; we thought that could explain our longer operation time in the robotic group [22,23].
Regarding pathological outcomes, the number of harvested LPNs was significantly higher in the robotic group (7.0 vs. 5.0, p = 0.004). Although there is no consensus about the optimal number of harvested lateral pelvic lymph nodes, this finding could be interpreted to indicate that the completeness of the LPND could be better in the robotic approach than in the laparoscopic approach.
The robotic procedure has the following advantages, in terms of being a surgical technique: it provides fixed third-arm retraction, a magnified three-dimensional view, and endo-wristed instruments. In rectal cancer surgery, both TME and LPND are performed within the narrow pelvic cavity. Therefore, the advantages of robotic surgery in LPND coincide with those of TME in many respects. However, there are some differences between the two technics. TME is dissected along the avascular plane between the mesorectal fascia and the presacral fascia. On the other hand, since LPND only requires dissection of lymphatic tissue, the complex vascular and nerve complexes in the lateral pelvic area should be preserved while skeletalizing. Excessive traction and trembling during lymphatic dissection could cause postoperative urinary dysfunction without direct damage to the major pelvic nerve plexus [7]. During robotic LPND, very stable traction or counter-traction can be performed in the very narrow pelvic side wall, so surgeons can expect less injury to the neural tissue of the lateral pelvic area. Although there was no significant difference in our study, we observed a lower tendency of postoperative urinary dysfunction in the robotic group (12.2% vs. 5.9%). Additionally, fluorescence imaging combined with infrared optics may be helpful to identify metastatic lymph nodes and to reduce the risk of missing hidden pelvic nodes in real time when using the robotic system.
This study had some limitations. Firstly, because this study was retrospective, inherent and unintentional selection bias could not be dismissed. Secondly, we could not compare the postoperative functional outcomes between the two groups. But to our knowledge, this is the second retrospective study comparing perioperative outcomes between the two approaches to LPND. Thirdly, we reported only a short period of oncological outcomes because the follow-up was too short. However, the 18-months local-recurrence-free survival rate tended to be higher with robotic LPND than with laparoscopic LPND. Regarding pathological outcomes, the number of retrieved LPNs was significantly higher in the robotic surgery group. Based on these findings, we plan to report the long-term oncological outcomes of robotic and laparoscopic LPND in the future.
5. Conclusions
In conclusion, the robotic approach is a safe and feasible surgery option for TME with LPND, with more harvested lateral lymph nodes than the laparoscopic approach. A large prospective study would help validate the safety, feasibility, and potential benefits of robotic TME and LPND in locally advanced rectal cancer.
Acknowledgments
We thank our colleagues who helped with data collection in the colorectal division of the Surgery Department at The Catholic University of Korea.
Author Contributions
Conceptualization, J.H.B. and Y.S.L.; methodology, J.H.B. and Y.S.L.; software, J.H.B., B.-H.K. and I.K.L.; validation, J.H.B., I.K.L. and Y.S.L.; formal analysis, J.H.B. and Y.S.L.; investigation, J.S., R.N.Y. and J.H.K.; resources, J.H.B., J.H.K., B.-H.K. and Y.S.L.; data curation, J.H.B., J.S., J.H.K., B.-H.K. and H.-M.C.; writing—original draft preparation, J.H.B.; writing—review and editing, Y.S.L.; visualization, J.H.B., J.S. and R.N.Y.; supervision, J.H.K., B.-H.K., I.K.L. and H.-M.C.; project administration, J.H.K., B.-H.K., I.K.L. and Y.S.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Ethics Committee of the College of Medicine, The Catholic University of Korea (XC21RADI0112). The records were anonymized and de-identified before the analysis.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patients’ privacy concerns.
Conflicts of Interest
The authors have no potential conflict of interest to declare.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Watanabe T., Muro K., Ajioka Y., Hashiguchi Y., Ito Y., Saito Y., Hamaguchi T., Ishida H., Ishiguro M., Ishihara S., et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moriya Y., Sugihara K., Akasu T., Fujita S. Nerve-sparing surgery with lateral node dissection for advanced lower rectal cancer. Eur. J. Cancer. 1995;31:1229–1232. doi: 10.1016/0959-8049(95)00164-E. [DOI] [PubMed] [Google Scholar]
- 3.Bosset J.F., Collette L., Calais G., Mineur L., Maingon P., Radosevic-Jelic L., Daban A., Bardet E., Beny A., Ollier J.C. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 4.Kusters M., Beets G.L., van de Velde C.J., Beets-Tan R.G., Marijnen C.A., Rutten H.J., Putter H., Moriya Y. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann. Surg. 2009;249:229–235. doi: 10.1097/SLA.0b013e318190a664. [DOI] [PubMed] [Google Scholar]
- 5.Kim M.J., Chang G.J., Lim H.K., Song M.K., Park S.C., Sohn D.K., Chang H.J., Kim D.Y., Park J.W., Jeong S.Y., et al. Oncological Impact of Lateral Lymph Node Dissection After Preoperative Chemoradiotherapy in Patients with Rectal Cancer. Ann. Surg. Oncol. 2020;27:3525–3533. doi: 10.1245/s10434-020-08481-y. [DOI] [PubMed] [Google Scholar]
- 6.Ogura A., Konishi T., Cunningham C., Garcia-Aguilar J., Iversen H., Toda S., Lee I.K., Lee H.X., Uehara K., Lee P., et al. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J. Clin. Oncol. 2019;37:33–43. doi: 10.1200/JCO.18.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita S., Akasu T., Mizusawa J., Saito N., Kinugasa Y., Kanemitsu Y., Ohue M., Fujii S., Shiozawa M., Yamaguchi T., et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): Results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol. 2012;13:616–621. doi: 10.1016/S1470-2045(12)70158-4. [DOI] [PubMed] [Google Scholar]
- 8.Tribuzi A., Guagni T., Paolini C., Di Marino M., Coratti A. Robotic intersphincteric resection with total mesorectal excision and coloanal anastomosis—A video vignette. Colorectal. Dis. 2020;22:1777–1778. doi: 10.1111/codi.15179. [DOI] [PubMed] [Google Scholar]
- 9.Lezoche E., Feliciotti F., Paganini A.M., Guerrieri M., De Sanctis A., Campagnacci R. Laparoscopic colonic resection. J. Laparoendosc. Adv. Surg. Tech. A. 2001;11:401–408. doi: 10.1089/10926420152761932. [DOI] [PubMed] [Google Scholar]
- 10.Kang J., Yoon K.J., Min B.S., Hur H., Baik S.H., Kim N.K., Lee K.Y. The impact of robotic surgery for mid and low rectal cancer: A case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann. Surg. 2013;257:95–101. doi: 10.1097/SLA.0b013e3182686bbd. [DOI] [PubMed] [Google Scholar]
- 11.Cheng L.J., Chen J.H., Chen S.Y., Wei Z.W., Yu L., Han S.P., He Y.L., Wu Z.H., Chen C.Q. Distinct Prognosis of High Versus Mid/Low Rectal Cancer: A Propensity Score-Matched Cohort Study. J. Gastrointest. Surg. 2019;23:1474–1484. doi: 10.1007/s11605-018-04072-1. [DOI] [PubMed] [Google Scholar]
- 12.Lee S.W., Lee J.H., Lee I.K., Oh S.T., Kim D.Y., Kim T.H., Oh J.H., Baek J.Y., Chang H.J., Park H.C., et al. The Impact of Surgical Timing on Pathologic Tumor Response after Short Course and Long Course Preoperative Chemoradiation for Locally Advanced Rectal Adenocarcinoma. Cancer Res. Treat. 2018;50:1039–1050. doi: 10.4143/crt.2017.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae J.H., Koh W., Kim H.H., Lee Y.S. Standardized Step-by-step Technique Using Surgical Landmarks in Robotic Lateral Pelvic Lymph Node Dissection. Ann. Coloproctol. 2021;37:58–60. doi: 10.3393/ac.2020.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leal Ghezzi T., Campos Corleta O. 30 Years of Robotic Surgery. World J. Surg. 2016;40:2550–2557. doi: 10.1007/s00268-016-3543-9. [DOI] [PubMed] [Google Scholar]
- 15.Prete F.P., Pezzolla A., Prete F., Testini M., Marzaioli R., Patriti A., Jimenez-Rodriguez R.M., Gurrado A., Strippoli G.F.M. Robotic Versus Laparoscopic Minimally Invasive Surgery for Rectal Cancer: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2018;267:1034–1046. doi: 10.1097/SLA.0000000000002523. [DOI] [PubMed] [Google Scholar]
- 16.Cui Y., Li C., Xu Z., Wang Y., Sun Y., Xu H., Li Z., Sun Y. Robot-assisted versus conventional laparoscopic operation in anus-preserving rectal cancer: A meta-analysis. Ther. Clin. Risk Manag. 2017;13:1247–1257. doi: 10.2147/TCRM.S142758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S., Jiang H.G., Chen Z.H., Zhou S.Y., Liu X.S., Yu J.R. Meta-analysis of robotic and laparoscopic surgery for treatment of rectal cancer. World J. Gastroenterol. 2011;17:5214–5220. doi: 10.3748/wjg.v17.i47.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alturkistani S.A., Alghanem A.M., Lee I.K. Robotic Lateral Pelvic Lymph Node Dissection: Description of A Technique. J. Minim. Invasive Surg. 2020;23:103–105. doi: 10.7602/jmis.2020.23.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae S.U., Saklani A.P., Hur H., Min B.S., Baik S.H., Lee K.Y., Kim N.K. Robotic and laparoscopic pelvic lymph node dissection for rectal cancer: Short-term outcomes of 21 consecutive series. Ann. Surg. Treat. Res. 2014;86:76–82. doi: 10.4174/astr.2014.86.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong K.Y., Tan A.M. Short term outcomes of minimally invasive selective lateral pelvic lymph node dissection for low rectal cancer. World J. Gastrointest. Surg. 2020;12:178–189. doi: 10.4240/wjgs.v12.i4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.J., Choi G.S., Park J.S., Park S.Y., Lee H.J., Woo I.T., Park I.K. Selective lateral pelvic lymph node dissection: A comparative study of the robotic versus laparoscopic approach. Surg. Endosc. 2018;32:2466–2473. doi: 10.1007/s00464-017-5948-4. [DOI] [PubMed] [Google Scholar]
- 22.Pan J., Wang B., Feng Z., Sun Z., Xia C., Zhang Q., Ren S. Robotic versus laparoscopic total mesorectal excision for mid-low rectal cancer with difficult anatomical conditions. Asian J. Surg. 2022;45:2725–2732. doi: 10.1016/j.asjsur.2022.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Asoglu O., Tokmak H., Bakir B., Aliyev V., Saglam S., Iscan Y., Bademler S., Meric S. Robotic versus laparoscopic sphincter-saving total mesorectal excision for mid or low rectal cancer in male patients after neoadjuvant chemoradiation therapy: Comparison of long-term outcomes. J. Robot. Surg. 2020;14:393–399. doi: 10.1007/s11701-019-01001-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patients’ privacy concerns.