Abstract
Sarcopenia refers to the loss of muscle strength and mass in older individuals and is a major determinant of fall risk and impaired ability to perform activities of daily living, often leading to disability, loss of independence, and death. Owing to its impact on morbidity, mortality, and healthcare expenditure, sarcopenia in the elderly has become a major focus of research and public policy debates worldwide. Despite its clinical importance, sarcopenia remains under-recognized and poorly managed in routine clinical practice, partly owing to the lack of available diagnostic testing and uniform diagnostic criteria. Since the World Health Organization and the United States assigned a disease code for sarcopenia in 2016, countries worldwide have assigned their own disease codes for sarcopenia. However, there are currently no approved pharmacological agents for the treatment of sarcopenia; therefore, interventions for sarcopenia primarily focus on physical therapy for muscle strengthening and gait training as well as adequate protein intake. In this review, we aimed to examine the latest information on the epidemiology, molecular mechanisms, interventions, and possible treatments with new drugs for sarcopenia.
Keywords: sarcopenia, muscle strength, muscle atrophy, frailty, aging, senescence, skeletal muscle mass loss, intervention, clinical trials, treatment
1. Introduction
The term sarcopenia (Greek, “sarx” meaning “flesh” and “penia” meaning “loss”) was first proposed by Rosenberg in 1997 and refers to the reduction of both muscular mass and function during the natural process of aging [1]. Decreased muscle strength negatively affects steady walking and contributes to a high incidence of falls among elderly individuals. Sarcopenia is strongly linked to self-reported physical disability in both men and women, regardless of ethnicity, age, morbidity, obesity, income, or health behaviors [2]. Reduced muscle strength with age leads to reduced functional ability and is a significant cause of disability, mortality, and other negative health outcomes [3]. As the number of elderly people continues to increase, there is a growing demand for healthcare resources to address sarcopenia-related morbidities.
Initially, the definition of sarcopenia only considered the loss of muscle mass and not muscle strength or physical impairment as part of the disease process [3]. However, in 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) recognized sarcopenia as a syndrome characterized by the progressive and widespread loss of skeletal muscle mass and strength, with an increased risk of negative outcomes such as physical disability, poor quality of life, and mortality [4,5,6]. They acknowledged that loss of muscle mass and strength and reduction in physical performance are important characteristics of sarcopenia. Sarcopenia can occur in sarcopenic obesity in which both muscle mass loss and obesity occur simultaneously, and osteosarcoma visceral obesity can result in bone and muscle loss and accumulation of fat in the abdomen [7]. In 2018, the EWGSOP revised its diagnostic criteria to consider low muscle strength (LMS) as the primary parameter for diagnosing sarcopenia. Muscle strength is currently considered the most reliable measure of muscle function according to the revised guidelines (EWGSOP2) [8]. The updated guidelines also recognize muscle strength as a more effective predictor of adverse outcomes than muscle mass [9,10,11,12].
Timely identification and treatment can help prevent the negative outcomes associated with sarcopenia. Despite being recognized as a significant clinical issue, sarcopenia has only recently been officially categorized as a disease. In 2016, sarcopenia was added to the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) by the United States, a global disease classification system published by the World Health Organization, and given a specific code (M62.84) [13]. The eighth revision of the Korean Classification of Diseases (KCD) in 2021 also included sarcopenia as a disease and assigned it an ICD-10-CM code (M62.5) [14].
2. Epidemiology and Pathophysiology
Sarcopenia is prevalent in older individuals, with rates ranging from 11% to 50% in those over 80 years of age and up to 29% in community healthcare settings [15]. This condition is characterized by an imbalance between anabolic and catabolic processes, leading to a decline in the size and number of type II muscle fibers and intramuscular and intermuscular fat infiltration. Additionally, the number and function of satellite cells, responsible for repairing damaged muscle fibers, decrease [16,17,18]. Several factors, including neuromuscular junction dysfunction, decreased number of motor units [19], inflammation [20], insulin resistance [21], mitochondrial dysfunctions [22,23], and oxidative stress [24], contribute to muscle loss in sarcopenia. Moreover, the denervation of single muscle fibers can cause a substantial reduction in type II fibers, which are replaced by type I fibers and fat tissue. These changes in muscle structure and function can result in adverse outcomes such as physical disability and poor quality of life. Early recognition and intervention can mitigate these negative consequences.
3. Risk Factors for Primary Sarcopenia
Most people believe that sarcopenia is an inevitable aspect of the aging process; however, its severity can vary significantly and is influenced by particular risk factors. Therefore, classification of muscle atrophy as primary or secondary sarcopenia may be useful in clinical practice [8]. The following sections summarize the risk factors for primary sarcopenia:
3.1. Lack of Exercise
Insufficient exercise is considered a primary risk factor for sarcopenia [25]. Around the age of 50 years, a gradual reduction in the number of muscle fibers begins to occur [26]. The reduction in muscle fiber and strength is more evident in sedentary individuals than in those who engage in physical activity. However, even professional athletes, such as weightlifters and motorcycle runners, show a gradual decrease in strength and speed with age, albeit at a slower rate [26].
3.2. Imbalance in Hormones and Cytokines
Age-associated reductions in the levels of hormone, such as growth hormone, testosterone, thyroid hormone, and insulin-like growth factor, can result in the loss of muscle mass and strength. In many cases, severe muscle loss arises due to a combination of reduced hormonal anabolic signals and the promotion of catabolic signals influenced by pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) [27]. Research has revealed that both IL-6 and TNF-α levels are elevated in the skeletal muscles of older individuals [28,29].
3.3. Insufficient Protein Synthesis
The inability of the body to effectively synthesize protein, combined with an insufficient intake of calories and/or protein to maintain muscle mass, is frequently observed in sarcopenia. With aging, oxidized proteins within the skeletal muscle increase, leading to the accumulation of lipofuscin and cross-linked proteins that are not adequately cleared by the proteolysis system. Consequently, non-contractile and dysfunctional proteins accumulate in skeletal muscles, contributing to the significant decline in muscle strength observed in sarcopenia [30].
3.4. Dysfunction of Motor Units
Another age-related issue is the decline in motor neurons, responsible for transmitting signals from the brain to the muscles to initiate movement. Satellite cells are small, single-nucleus cells that line muscle fibers and are typically activated during exercise or injury. These signals prompt the differentiation of satellite cells, which then merge with muscle fibers, thereby aiding in the maintenance of muscle function. It has been proposed that sarcopenia is partially due to an inability to activate satellite cells [27].
3.5. Lifestyle of Individual
Evolutionary theories propose that genes regulating muscle mass and function are responsible for the inability of the body to maintain these traits with age [31]. According to this hypothesis, the genes that supported the high levels of muscle strength required for survival in the Late Paleolithic period are no longer necessary in the modern lifestyle, which is characterized by prolonged periods of sedentary behavior.
3.6. Physical Condition at Birth
Studies on the developmental origins of health and disease have revealed that environmental factors during early growth and development can have long-lasting effects on human health. Low birth weight, indicative of a suboptimal early environment, has been linked to low muscle mass and strength in adulthood [32,33]. Research has shown that a decrease in muscle fiber score is significantly associated with low birth weight, indicating that developmental factors affecting muscle physiology may account for the relationship between low birth weight and sarcopenia [34].
3.7. Nutritional Status
Malnutrition is a significant contributor to the development of sarcopenia, and a multidisciplinary approach should be implemented for its management, including nutritional screening and care plans similar to those used for cachexia [35,36]. Protein–energy malnutrition, along with other factors, is frequently associated with sarcopenia [37]. Maintaining a high protein intake above the Recommended Daily Allowance, in the range of 1.2–1.6 g/kg per day, has been suggested as a preventative measure for age-related sarcopenia [38].
4. Risk Factors for Secondary Sarcopenia
Sarcopenia is frequently associated with other medical conditions, and understanding the mechanisms that lead to muscle loss in secondary sarcopenia can offer valuable information regarding age-related sarcopenia [8]. The approach to managing secondary sarcopenia should prioritize the treatment of the primary underlying condition using the methods mentioned earlier to enhance skeletal muscle mass and strength.
4.1. Cachexia
Severe muscle wasting is often associated with severe systemic diseases such as cancer, cardiomyopathy, and end-stage renal disease, known as cachexia [39]. Cachexia is defined as a metabolic syndrome associated with an underlying illness, characterized by the loss of muscle and fat mass [40], and is frequently linked to inflammation, insulin resistance, anorexia, and increased breakdown of muscle proteins [41,42]. Although sarcopenia is one of the elements of the proposed definition of cachexia, not all sarcopenic individuals are considered cachectic [40]. Based on this information, a consensus definition has been developed by the Special Interest Group on cachexia–anorexia in chronic wasting diseases of the European Society for Clinical Nutrition and Metabolism (ESPEN-SIG) to differentiate between cachexia and other conditions associated with sarcopenia [43].
4.2. Frailty
Frailty is a syndrome that affects older adults and is caused by a gradual decline in multiple physiological systems with age, resulting in a reduced capacity to handle stress and reduced homeostatic reserve. This syndrome is associated with an increased risk of negative health outcomes, such as falls, institutionalization, hospitalization, and mortality [44]. Frailty is defined by the presence of three or more of the following criteria: unintentional weight loss, weakness, exhaustion, slow gait speed, and low physical activity [44,45]. Frailty and sarcopenia are often associated, with most frail older adults experiencing sarcopenia, suggesting a shared underlying mechanism. Frailty extends beyond physical characteristics and may also include psychological and social dimensions, such as cognitive decline, lack of social support, and environmental factors [45].
4.3. Sarcopenic Obesity
Sarcopenic obesity (SO) occurs when a person has low lean body mass due to sarcopenia combined with high fat mass. This medical condition has been linked to a range of problems, including impaired functional capacity, metabolic issues, disability, and increased mortality rates [8,46]. The reported prevalence of SO varies widely, ranging from 2% to 21.7%, and may be due to factors such as a lack of awareness among healthcare providers, genetic differences, nutrition, and lifestyle factors [47]. Under certain conditions, such as malignancy and rheumatoid arthritis, individuals may experience a loss of lean body mass while preserving or even increasing fat mass [46]. Furthermore, low muscle mass combined with high fat mass is a typical aspect of the aging process. However, identifying SO in older people can be challenging because the reduction in muscle mass and strength due to aging may occur independently of body mass index.
For a long time, it was believed that the age-related decline in weight and muscle mass was the main cause of muscle weakness in the elderly [48]. However, recent research has suggested that changes in muscle composition also contribute to this weakness. In particular, marbling or fat infiltration into the muscles reduces muscle quality and function [49]. Studies examining the development of sarcopenic obesity have identified age-associated changes in muscle and fat composition. For example, older men experience an initial increase in the percentage of fat mass, which later stabilizes or decreases [49]. There is also a redistribution of fat in the body, with an increase in intramuscular and visceral fat and a decrease in subcutaneous fat [50,51]. These changes may play a role in the development of SO.
5. Pathogenesis
The activation, differentiation, and proliferation of myoblasts in skeletal muscles are crucial for the regeneration of muscle fibers with mechanical, chemical, or degenerative damage [52,53]. The differentiation of myoblasts into myotubes is vital for the development and regeneration of skeletal muscles because these monocytic myoblasts proliferate, differentiate, and eventually fuse with existing muscle fibers to form multinucleated myotubes and myofibers [54]. The process of muscle cell proliferation and differentiation occurs during birth and postnatal development [55]. Hence, promoting the proliferation and differentiation of myoblasts and inducing myotube hypertrophy could potentially benefit muscle regeneration and the preservation of muscle mass.
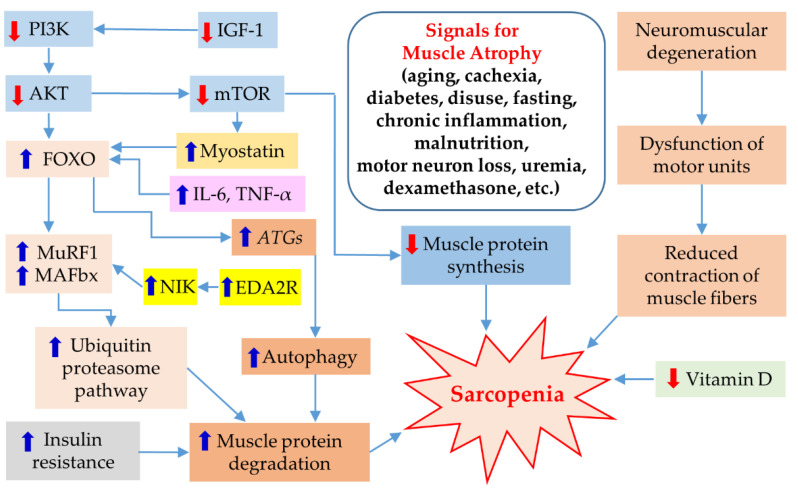
The main mechanism for muscle regeneration is to activate serine/threonine kinase, which amplifies mammalian target of rapamycin (mTOR), ultimately leading to an increase in muscle protein synthesis [56]. This process is stimulated by anabolic factors such as insulin-like growth factor-1 (IGF-1), testosterone, and exercise. Testosterone also plays a role in repairing muscle cells by promoting myoblasts and inhibiting myostatin. However, with aging, the levels of testosterone, IGF-1, and exercise decrease, resulting in a decrease in muscle protein synthesis and an increase in muscle breakdown. There are several signals that contribute to muscle atrophy, including aging, cachexia, diabetes, disuse, fasting, chronic inflammation, malnutrition, motor neuron loss, uremia, and dexamethasone. These factors lead to the development of sarcopenia through various signaling pathways. However, this paper provides a brief summary without mentioning the detailed molecular mechanisms of each factor (Figure 1). Muscle wasting occurs due to increased protein breakdown, which is caused by activation of the ubiquitin–proteasome pathway [57]. The muscle-specific ligases muscle atrophy F-box (MAFbx; also known as atrogin-1) and muscle RING Finger-1 (MuRF-1) are expressed early in the muscle atrophy process and play crucial roles in muscle protein degradation such as myogenic differentiation 1 (MyoD) and myogenin [56,57,58,59,60,61]. Insulin resistance, elevated proinflammatory cytokines, genetic factors, and poor nutritional status (including insufficient energy and protein intake leading to weight loss and decreased levels of vitamin D) can further accelerate muscle breakdown [56,58].
Figure 1.
Schematic of the molecular mechanism of sarcopenia induced by several signals. AKT, protein kinase B; ATGs, autophagy genes; EDA2R, ectodysplasin A2 receptor; FOXO, forkhead box O; IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; MAFbx, muscle atrophy F-box; mTOR, mammalian target of rapamycin; MuRF1, muscle RING Finger-1; NIK, NF-κB-inducing kinase; PI3K, phosphoinositol-3-kinase; TNF-α, tumor necrosis factor-α.
6. Diagnosis of Sarcopenia
An accurate definition is crucial for the diagnosis of sarcopenia. In 2010, the EWGSOP proposed three criteria based on muscle mass, strength, and physical performance [6]. Low muscle mass was defined as a skeletal muscle mass (SMM) index of less than 8.90 kg/m2, handgrip strength, indicative of muscle strength, below 30 kg in men and 20 kg in women, and gait speed, indicative of physical performance, less than 0.8 m/s. To confirm the diagnosis of sarcopenia, the presence of low muscle mass and either LMS or low physical performance (LPP) is required. Sarcopenia is classified into pre-sarcopenia, sarcopenia, and severe sarcopenia, based on the presence of low muscle mass and functional impairment. However, in 2018, the EWGSOP2 revised its guidelines and regarded LMS as the primary parameter for sarcopenia diagnosis because it is considered the most reliable measure of muscle function [62]. The revised guidelines recognize that muscle strength is more effective than muscle mass in predicting adverse outcomes [9,10,11,12,63,64,65,66]. Sarcopenia is probable when LMS is detected and the diagnosis is confirmed by the presence of low muscle quantity or quality. However, using muscle quality, which involves micro- and macroscopic aspects of muscle architecture and composition, as the primary parameter for defining sarcopenia is challenging owing to technological limitations [67,68,69].
Severe sarcopenia is diagnosed when there is LMS, low muscle quantity or quality, or LPP. Patients who exhibit indications or symptoms of sarcopenia, such as muscle wasting, weight loss, falls, weakness, difficulty in rising from a chair, or slow gait speed, should undergo case-finding during clinical practice. To confirm the diagnosis, the EWGSOP2 recommends the use of either the SARC-F questionnaire, which consists of five items related to strength, assistance in walking, rising from a chair, climbing stairs, and falls [70,71,72], or the Ishii screening tool, which includes age, grip strength, and calf circumference as variables [73]. To evaluate skeletal muscle power, grip strength can be measured using Jamar or Smedley instruments for the arms [74] and the chair stand test, which involves performing five sit-to-stand repetitions in 30 s intervals, for the legs [75,76].
There are various methods for estimating muscle quantity or mass, such as dual energy X-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA); the results can be adjusted based on height or body mass index (BMI) [77,78]. Muscle quantity can be expressed in different ways, including total body SMM, appendicular skeletal muscle mass (ASM), or the muscle cross-sectional area of specific muscle groups using computed tomography (CT) or magnetic resonance imaging (MRI) [79,80,81,82]. Muscle mass and body size are correlated; therefore, it is important to adjust for body size. Although calf circumference is not a good measure of muscle mass, it predicts performance and survival in older people [83,84,85]. Physical performance, which involves both muscle and nerve function, can be measured using various tests such as gait speed, short physical performance battery (SPPB), timed up and go test (TUG), and 400 m walk test [6,86,87,88,89,90,91].
However, using Western criteria for diagnosing sarcopenia may not be appropriate for individuals residing in other continents, including Asia, who generally have a smaller body size, more adipose tissue, and a more physically active life style than Western people. Therefore, both the International Working Group on Sarcopenia (IWGS) in 2011 [92,93] and the Asian Working Group for Sarcopenia (AWGS) in 2014 worked on developing a consensus for sarcopenia diagnosis based on evidence from research studies conducted within certain regions. In 2014, the AWGS proposed an algorithm for sarcopenia diagnosis in Asians based on the EWGSOP guidelines but with clearly defined cutoff values for each diagnostic component [94]. These cutoff values included muscle mass measurements using DXA or BIA, handgrip strength, and usual gait speed. The AWGS revised its algorithm for sarcopenia diagnosis at a consensus meeting held in Hong Kong in May 2019 [95].
The AWGS 2019 maintained the previous definition of sarcopenia but revised the diagnostic algorithm and some criteria. For handgrip strength, the cutoffs are <28.0 kg for men and <18.0 kg for women. Physical performance can be assessed using the 6 m walk test (with a cutoff speed of ≤1.0 m/s), SPPB (with a cutoff score of ≤9), and the five-time chair stand test (with a cutoff time of ≤12 s) [6,94,96]. Additionally, calf circumference (men: <34 cm; women: <33 cm), SARC-F (with a score of ≥4 indicating sarcopenia), and SARC-Calf (with a score of ≥11 indicating sarcopenia) were used. The AWGS 2019 also introduced “possible sarcopenia” as a diagnosis when LMS is present with or without reduced physical performance. Low ASM plus LMS or LPP are considered to indicate sarcopenia, whereas low ASM, LMS, and LPP are considered to indicate severe sarcopenia. Finally, the original cutoffs for LMM in sarcopenia diagnosis were retained: <7.0 kg/m2 for men and <5.4 kg/m2 for women using DXA and <7.0 kg/m2 for men and <5.7 kg/m2 for women using BIA.
7. Histopathology
In its early stages, sarcopenia is marked by a decline in muscle size, followed by a deterioration in muscle quality over time. The decline in muscle tissue quality is caused by the replacement of muscle fibers with fat, increased fibrosis, metabolic changes, oxidative stress, and degeneration of neuromuscular junctions. As a result of these changes, there is a gradual loss of muscle function leading to frailty [27].
Studies have shown that sarcopenia mainly affects type II (fast twitch) muscle fibers, whereas type I (slow twitch) fibers are less affected [97]. The reduction in the size of type II fibers in sarcopenia can be up to 50%, which is moderate compared with the overall muscle mass reduction, suggesting that sarcopenia involves a decrease in the number and size of muscle fibers. Studies comparing muscle cross-sections of elderly and younger individuals have shown a significant reduction in both type I and type II fibers in the elderly [98]. Anatomical and electrophysiological studies also indicate a loss of motor neurons with age, suggesting that a chronic neuropathic process may contribute to a reduction in muscle mass [99,100]. Histological changes observed in sarcopenia may also be influenced by lifestyle, hormones, inflammatory cytokines, and genetic factors.
8. Intervention
Early detection and timely intervention are crucial for improving outcomes in patients with sarcopenia. Screening elderly patients for any physical function or impairment of daily activities during routine healthcare visits is recommended. Patients who have difficulties with activities of daily living should undergo specific testing for sarcopenia using the methods described earlier. It is also important to assess the living environment for fall hazards and take appropriate safety measures as part of the treatment plan.
8.1. Non-Pharmacologic Treatment
An inactive lifestyle is associated with a loss of muscle mass and strength. Therefore, exercise is considered a crucial component in the management of sarcopenia. Short-term resistance exercise enhances the capacity of the skeletal muscles to produce proteins [101]. Resistance training (RT) and strength training (ST) are effective interventions for sarcopenia prevention and treatment. RT has a positive effect on the neuromuscular system, hormone levels, and protein synthesis rates [102]. There is evidence that RT, aerobic exercise, balance training, and even walking are effective in preventing primary and secondary sarcopenia but also secondary sarcopenia [103]. A recent meta-analysis showed that a combination of dietary supplements and exercise may have some benefits in treating sarcopenia; however, the results varied across populations [104,105].
8.2. Pharmacological Therapies
Currently, no United States Food and Drug Administration (FDA)-approved drugs are available for the treatment of sarcopenia. The use of steroid hormones, such as dehydroepiandrosterone (DHEA), testosterone, and anabolic steroids, has been studied as a potential treatment for sarcopenia. While these agents have shown some positive effects on muscle strength and mass, their use is limited owing to adverse effects, such as an increased risk of prostate cancer in men, virilization in women, and an overall high risk of cardiovascular events [106,107].
Novel sarcopenia treatments are currently being developed and tested in clinical trials. One potential therapy is selective androgen receptor modulators (SARMs), which can selectively target androgen receptors and promote muscle growth. The mechanism of action of SARMs involves their ability to selectively activate androgen receptors in muscle and bone tissues while minimizing the activation of androgen receptors in other tissues, such as the prostate gland [108]. As of April 2023, several SARMs had been tested in clinical trials, including GTx-024 (also known as enobosarm, ostarine, or MK-2866), GSK2881078, RAD140, and S-23. These trials primarily focused on evaluating the safety and efficacy of SARMs in treating muscle-wasting conditions and improving physical function in older adults. We have summarized the clinical trial status of the representative SARMs GTx-024 and GSK2881078 (Table 1).
Table 1.
Recent clinical status of SARMs.
| Title | Intervention | Conditions | Primary Outcome | Phase | Status | Clinical Trials Identifier | Refs. |
|---|---|---|---|---|---|---|---|
| GTx-024 as a treatment for stress urinary incontinence in women |
GTx-024 | Stress urinary incontinence |
The mean percent change in number of stress incontinence episodes/day as assessed by patient completion of the 3-day voiding diary |
Phase 2 | Completed | NCT02658448 | [109] |
| Study to assess enobosarm (GTx-024) in postmenopausal women with stress urinary incontinence (ASTRID) | GTx-024, placebo |
Stress urinary incontinence |
Number of participants with a ≥50% reduction from baseline in the mean number of stress incontinence episodes per day at week 12 | Phase 2 | Completed | NCT03241342 | [110] |
| Durability extension study to assess clinical activity and safety of enobosarm (GTx-024) in stress urinary incontinence | GTx-024, matching placebo |
Stress urinary incontinence |
Durability of response, stress incontinence | Phase 2 | Terminated | NCT03508648 | [111] |
| Study of GTx-024 on muscle wasting (cachexia) cancer. |
GTx-024, placebo |
Cachexia | The efficacy of GTx-024 on total body lean mass |
Phase 2 | Completed | NCT00467844 | [112,113] |
| Add-on study for protocol G200802 (NCT02463032): effect of GTx-024 on maximal neuromuscular function and lean body mass | GTx-024, 9 or 18 mg |
ER+ and AR+ breast cancer | Maximal power production (assessed by inertial-load cycle ergometry) |
Phase 2 | Completed | NCT02746328 | [114] |
| Effect of GTx-024 on muscle wasting in patients with NSCLC on first line platinum |
GTx-024, placebo |
Muscle wasting, NSCLC |
Physical function (measure is the percentage of subjects at day 84 with stair climb power change), lean body mass (measure is the percentage of subjects at day 84 with lean body mass change) |
Phase 3 | Completed | NCT01355497 | [115,116] |
| Phase III study on the effect of GTx-024 on muscle wasting in patients with NSCLC |
GTx-024, placebo |
Muscle wasting, NSCLC |
Physical function (measure is the percentage of subjects at day 84 with stair climb power change), lean body mass (measure is the percentage of subjects at day 84 with lean body mass change) |
Phase 3 | Completed | NCT01355484 | [116,117] |
| Enobosarm and anastrozole in pre-menopausal women with high mammographic breast density |
Enobosarm (GTx-024) |
Mammographic density | Mammographic breast density, breast tissue elasticity |
Phase 1 | Completed | NCT03264651 | [118] |
| Study to evaluate the safety and efficacy of 13 weeks of the SARM GSK2881078 in chronic obstructive pulmonary disease (COPD) |
GSK2881078, matching placebo | Cachexia | Change from baseline in SBP, DBP, heart rate and urinalysis parameter, number of participants with SAEs, percentage change from baseline in maximum leg press strength following 1 RM |
Phase 2 | Completed | NCT03359473 | [119,120] |
AR, androgen receptor; DBP, diastolic blood pressure; ER, estrogen receptor; NSCLC, non-small-cell lung cancer; SAEs, serious adverse events; SARM, selective androgen receptor modulator; SBP, systolic blood pressure; 1 RM, 1 repetition maximum.
In a Phase 2A clinical trial (NCT03359473, as of 10 April 2023), GSK2881078 was studied in conjunction with exercise in patients with chronic obstructive pulmonary disease (COPD) and impaired physical function [120]. The results showed that GSK2881078 increased leg strength in men but not in women. Lean body mass also increased; however, there were no improvements in the patient-reported outcomes. Some safety concerns were noted, including a reversible reduction in high-density lipoprotein cholesterol and transient elevation in hepatic transaminase levels. Nonetheless, GSK2881078 was well tolerated, and short-term treatment increased leg strength to a greater extent than physical training alone in men with COPD. SARMs hold promise for achieving gains in skeletal muscle mass and strength without the adverse effects associated with other anabolic steroids [120]. Although SARMs have shown promise in clinical trials, they have not yet been approved for use by the US FDA or other regulatory agencies. As with any new drug, more research is needed to fully understand the potential benefits and risks of SARMs and determine the appropriate dosages and treatment regimens for different patient populations.
Myostatin inhibitors are a class of drugs that target the protein myostatin, which is a negative regulator of muscle growth [121,122,123,124]. Myostatin inhibitors block the action of myostatin, thereby allowing for increased muscle growth and preventing muscle loss, especially in conditions such as sarcopenia. The mechanism of action of myostatin inhibitors against sarcopenia involves their ability to inhibit myostatin activity in muscle tissue. Myostatin normally binds to and activates a receptor on the surface of muscle cells, activin receptor type IIB (ActRIIB), which suppresses muscle growth and protein synthesis [125]. When myostatin inhibitors are administered, they bind to and neutralize myostatin, thereby preventing its binding to the ActRIIB receptor and suppressing muscle growth. This leads to increased protein synthesis and muscle growth, resulting in improved muscle mass and function. Furthermore, myostatin inhibitors have other beneficial effects on muscles, including increased satellite cell activation and differentiation, improved mitochondrial function, and decreased inflammation [121,122,123,124,125]. As of April 2023, several myostatin inhibitors have been tested in clinical trials, including apitegromab (SRK-015), bimagrumab (BYM338), domagrozumab (PF-06252616), landogrozumab (LY2495655), taldefgrobep alfa (BMS 986089), trevogrumab (REGN1033), and rAAV1.CMV.huFollistatin344. These trials primarily focused on evaluating the safety and efficacy of myostatin inhibitors in treating muscle-wasting conditions and improving physical function in older adults. We have summarized the clinical trial status of representative myostatin inhibitors (Table 2). However, myostatin inhibitors are still in the experimental stage of development and have not yet been approved for clinical use. Further research is needed to determine their long-term safety and efficacy and the optimal dosage and duration of treatment for sarcopenia.
Table 2.
Recent clinical status of myostatin inhibitor drugs.
| Title | Intervention | Conditions | Primary Outcome | Phase | Status | Clinical Trials Identifier | Refs. |
|---|---|---|---|---|---|---|---|
| A phase 2 study to evaluate the safety, efficacy, pharmacokinetics, and pharmacodynamics of PF-06252616 in Duchenne muscular dystrophy |
PF-06252616, placebo | Duchenne muscular dystrophy |
Number of participants with TEAEs by week 49, change from baseline on the 4SC as compared to placebo at weeks 17, 33, and 49 |
Phase 2 | Terminated | NCT02310763 | [126,127,128,129,130,131,132,133] |
| An open-label extension study to evaluate safety of PF-06252616 in boys with Duchenne muscular dystrophy |
PF-06252616 | Duchenne muscular dystrophy |
Number of participants with dose reduced or temporary discontinuation due to AEs, number of participants with severe TEAEs |
Phase 2 | Terminated | NCT02907619 | [127,134] |
| Efficacy and safety of apitegromab in patients with later-onset spinal muscular atrophy treated with nusinersen or risdiplam (SAPPHIRE) |
Apitegromab, placebo |
SMA, muscular atrophy |
Main efficacy population: change from baseline in HFMSE total score | Phase 3 | Recruiting | NCT05156320 | [135] |
| Study evaluating MYO-029 in adult muscular dystrophy |
MYO-029 | Becker muscular dystrophy, facioscapulohumeral muscular dystrophy, limb–girdle muscular dystrophy |
Safety assessment | Phase 1, Phase 2 | Completed | NCT00104078 | [136] |
| An active treatment study of SRK-015 in patients with type 2 or type 3 spinal muscular atrophy (TOPAZ) |
SRK-015 | SMA | Change from baseline in the RHS total score at day 364, change from baseline in HFMSE total score at day 364 |
Phase 2 | Active, not recruiting | NCT03921528 | [137] |
| Long-term safety and efficacy of apitegromab in patients with SMA who completed previous trials of apitegromab-ONYX (ONYX) | Apitegromab | SMA | Evaluate the long-term safety and tolerability of apitegromab in patients with type 2 and type 3 SMA | Phase 3 | Not yet recruiting | NCT05626855 | [138] |
| Study of efficacy and safety of bimagrumab in patients after hip fracture surgery |
Bimagrumab | Muscle wasting (Atrophy) after hip fracture surgery |
Change from baseline in total lean body mass measured by DXA at weeks 12 and 24 | Phase 2 | Completed | NCT02152761 | [139,140] |
| Safety, pharmacokinetics, and efficacy of bimagrumab in overweight and obese patients with type 2 diabetes |
BYM338 10 mg/kg, placebo |
Diabetes mellitus type 2 |
Change from baseline in total body fat mass by DXA at week 48 | Phase 2 | Completed | NCT03005288 | [141,142] |
| An extension study of the efficacy, safety, and tolerability of BYM338 (Bimagrumab) in patients with sporadic inclusion body myositis who previously participated in the core study CBYM338B2203 |
Bimagrumab, placebo |
sIBM | Number of participants with AEs, SAEs, and deaths, change from core study baseline in 6MWD |
Phase 3 | Completed | NCT02573467 | [143,144] |
| Dose range finding study of bimagrumab in sarcopenia | Bimagrumab, placebo |
Sarcopenia | Change from baseline in Total SPPB score to week 25 | Phase 2 | Completed | NCT02333331 | [145,146] |
| Study of long-term safety, efficacy tolerability of BYM338 in patients with sIBM (BYM338) | BYM338 (Bimagrumab) |
sIBM | Number of participants with AE a measure of safety and tolerability | Phase 2 Phase 3 |
Completed | NCT02250443 | [147,148] |
| Efficacy and safety of bimagrumab/BYM338 at 52 weeks on physical function, muscle strength, mobility in sIBM patients (RESILIENT) | BYM338/ Bimagrumab, placebo |
sIBM | Change from baseline in 6MWD test at week 52 | Phase 2 Phase 3 |
Completed | NCT01925209 | [149,150] |
| A multi-center study to assess the effects of BYM338 on skeletal muscle in sarcopenic adults |
BYM338, placebo |
Skeletal muscle | Muscle volume of the thigh (measurement gathered using MRI, magnetic resonance imaging) | Phase 2 | Completed | NCT01601600 | [151] |
| Efficacy, safety, and tolerability of BYM338 in patients with sIBM | BYM338, placebo |
sIBM | Effect of BYM338 on thigh muscle volume by MRI | Phase 2 | Completed | NCT01423110 | [152,153] |
| A 24-week off-drug extension study in sarcopenic elderly who completed treatment in the 6-month core study | Bimagrumab, placebo |
Sarcopenia | SPPB total score at week 49 | Phase 2 | Completed | NCT02468674 | [154] |
| BYM338 in COPD patients with cachexia | BYM338, placebo |
COPD with cachexia | Percentage change from baseline of TMV by MRI Scan at week 4, 8, 16, and 24 | Phase 2 | Completed | NCT01669174 | [155,156] |
| Clinical study of BYM338 for the treatment of unintentional weight loss in patients with cancer of the lung or the pancreas | BYM338 active drug, placebo |
Cachexia | Percentage change from baseline of TMV by MRI Scan at week 8 | Phase 2 | Completed | NCT01433263 | [157] |
| A study of LY2495655 in older participants undergoing elective total hip replacement | LY2495655, placebo |
Muscular atrophy | Change from baseline in aLBM at week 12 | Phase 2 | Completed | NCT01369511 | [158,159] |
| A study in older participants who have fallen and have muscle weakness | LY2495655, placebo |
Muscle weakness | Change from baseline to 24 weeks endpoint in aLBM | Phase 2 | Completed | NCT01604408 | [160,161] |
| Study of the safety and efficacy of REGN1033 (SAR391786) in patients with sarcopenia | REGN1033 (SAR391786), placebo | Sarcopenia | Percent change in total lean body mass | Phase 2 | Completed | NCT01963598 | [162] |
| A study to evaluate the efficacy and safety of taldefgrobep Alfa in participants with spinal muscular atrophy (RESILIENT) | Taldefgrobep alfa, placebo |
SMA, neuromuscular diseases |
Efficacy of taldefgrobep alfa compared to placebo in change in the MFM-32 total score | Phase 3 | Recruiting | NCT05337553 | [163] |
| Clinical intramuscular gene transfer of rAAV1.CMV.huFollistatin344 trial to patients with Duchenne muscular dystrophy | rAAV1.CMV.huFollistin344 | Duchenne muscular dystrophy |
Number of DLT adverse events as assessed by 21 CFR 312.32. | Phase 1 Phase 2 | Completed | NCT02354781 | [164] |
| Follistatin gene transfer to patients with Becker muscular dystrophy and sporadic inclusion body myositis | rAAV1.CMV.huFollistatin344 | Becker muscular dystrophy, sIBM |
Safety | Phase 1 | Completed | NCT01519349 | [165,166] |
AE, adverse event; aLBM, appendicular lean body mass; COPD, chronic obstructive pulmonary disease; DLT, dose limiting toxicity; DXA, dual energy X-ray absorptiometry; HFMSE, hammersmith functional motor scale expanded; MRI, magnetic resonance imaging; MFM-32, 32 item motor function measure; RHS, revised hammersmith scale; SAEs, serious adverse events; sIBM, sporadic inclusion body myositis; SMA, spinal muscular atrophy; SPPB, short physical performance battery; TEAEs, treatment-emergent adverse events; TMV, thigh muscle volume; 4SC, 4 stair climb; 6MWD, 6 min walking distance test.
Vitamin D is a fat-soluble vitamin that plays a vital role in bone metabolism, calcium absorption, and muscle function. Increasing evidence suggests that vitamin D deficiency is associated with an increased risk of sarcopenia, and vitamin D supplementation has been investigated as a potential treatment option for this condition [167,168]. The mechanism of action of vitamin D in sarcopenia involves its ability to modulate muscle function and protein synthesis. Vitamin D receptors are present in skeletal muscle tissue, and vitamin D stimulates protein synthesis and increases muscle mass and strength by promoting the activity of the mTOR signaling pathway, a key regulator of muscle growth [168]. Moreover, vitamin D improves muscle function by enhancing neuromuscular junction functions and reducing inflammation in muscle tissue. Vitamin D deficiency is associated with reduced muscle strength and an increased risk of falls and fractures in older adults. In addition to its effects on muscle function, vitamin D is also important for maintaining bone health. Vitamin D deficiency can lead to osteoporosis, which is another age-related condition associated with an increased risk of fractures. Overall, the mechanism of action of vitamin D in sarcopenia involves its ability to promote muscle function and protein synthesis, reduce inflammation, and prevent osteoporosis [169]. Additionally, previous studies have reported that in older adults with sarcopenia, consuming a whey protein-based nutritional diet rich in leucine and vitamin D improves muscle mass as well as physical function and function and reduces treatment intensity and cost [170]. As of April 2023, 23 clinical trials related to the treatment of sarcopenia using vitamin D have been registered, most of which have been completed, whereas some are currently recruiting participants. However, the optimal dose and duration of vitamin D supplementation for the treatment of sarcopenia remain under investigation, and further research is required to determine the long-term safety and efficacy of this approach [171,172].
Growth hormone (GH), produced by the pituitary gland, is involved in the regulation of growth and metabolism. GH has anabolic effects on muscle tissues, and its use has been investigated as a potential treatment for sarcopenia. The mechanism of action of GH against sarcopenia involves its ability to stimulate the production of IGF-1, which is a hormone that promotes muscle growth and protein synthesis [173]. GH stimulates the liver and other tissues to produce IGF-1, which acts on muscle cells to stimulate protein synthesis and increase muscle mass. In addition to its anabolic effects, GH improves muscle strength and endurance, possibly by increasing the number of motor units in the muscle tissue and enhancing neuromuscular function [174]. However, the use of GH as a treatment for sarcopenia is controversial and its long-term safety and efficacy are still being studied [175]. GH supplementation is associated with several side effects including fluid retention, joint pain, and an increased risk of diabetes and cardiovascular disease. As of April 2023, there have been two clinical trials related to the treatment of sarcopenia using GH, one of which has been completed, whereas the other is currently recruiting participants. The completed clinical trial [“Effects of an Oral GH Secretagogue (MK-677) on Body Composition and Functional Ability of Older Adults (MOT089; NCT00474279)”] evaluated the effects of MK-677, an orally active GH secretagogue, on body composition and functional ability of older adults. The study involved 65 healthy older adults aged 60–81 years who were randomized to receive either MK-677 or placebo for 12 months. The results of the study showed that the MK-677 treatment resulted in a significant increase in lean body mass and a decrease in fat mass compared to the placebo. Additionally, participants in the MK-677 group showed improvements in physical function and mobility, as measured by a battery of functional tests. However, the study also reported some adverse effects associated with MK-677 treatment, including transient increases in fasting glucose and insulin levels and mild edema and muscle pain [176]. Further research is required to fully understand the potential benefits and risks of using MK-677 to treat age-related changes in body composition and functional abilities. Therefore, GH therapy should only be considered in carefully selected patients under the supervision of healthcare professionals.
Several other compounds, including angiotensin-converting enzyme inhibitors and eicosapentaenoic acid, are being investigated [103,104]. To prevent sarcopenia via the treatment of cachexia, 11 compounds, including thalidomide, OHR/AVR118, celecoxib, VT-122, omega-3 supplements, and anabolic agents such as ghrelin and its analogs, MT-102, and ruxolitinib, have been studied [177]. Among them, MT-102, the first anabolic catabolic transforming agent, has been tested in a phase-II clinical study for treating cachexia in patients with late-stage cancer, and the results indicated a significant increase in body weight compared to placebo treatment [178]. In aged animal models, MT-102 has been shown to reverse sarcopenia, and further studies are currently underway to investigate its potential as a treatment for sarcopenia [179,180].
9. Exercise Mimetics
The most effective treatment for sarcopenia involves combining a consistent exercise program with essential amino acid supplementation. However, certain individuals, such as those with severe sarcopenia, severe frailty, hip fracture, congenital neuromuscular disorders, or in intensive care, are unable to engage in regular exercise and thus cannot benefit from its advantages. In such cases, pharmaceuticals known as “exercise mimetics” or “exercise in a pill” are the only available therapeutic strategies to partially replicate some of the benefits associated with exercise in these specific populations [125]. While no single pharmaceutical compound can fully replicate all the benefits of exercise, research has focused on exploring the benefits of activating specific signaling pathways associated with exercise. One of these molecular targets is the peroxisome proliferator-activated receptor delta (PPARδ or PPARβ), which plays a role in mediating the effects of exercise.
Overexpressing PPARδ and administering the PPARδ agonist GW501516, the expression of genes related to mitochondrial content such as uncoupling protein 3 (Ucp3), carnitine palmitoyltransferase 1b (Cpt1b), and pyruvate dehydrogenase kinase 4 (Pdk4) increased [181]. When combined with exercise, GW501516 also enhanced oxidative myofibers and running endurance in adult mice. In mouse models with muscular dystrophy, GW501516 improved skeletal muscle mass [182], although it did not have an effect on muscle mass in rats [183]. However, clinical trials in humans were discontinued due to a significant increase in cancer occurrence in multiple organs observed in preclinical studies using rats and mice treated with GW501516 [184].
Another example of exercise mimetics is the activator of adenosine monophosphate-activated protein kinase (AMPK) [125]. AMPK is an enzyme that plays a crucial role in regulating energy metabolism and maintaining cellular homeostasis. Activation of AMPK has positive effects on muscle metabolism, including increased glucose uptake, mitochondrial biogenesis, and muscle protein synthesis. Some compounds, such as 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), have been shown to activate AMPK and improve muscle function [185]. In addition to PPARδ agonist and AICAR, there have been numerous studies on exercise mimetics utilizing peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) modulators, nuclear factor erythroid 2–related factor 2 (Nrf2) modulators, irisin, and others [186,187,188]. However, currently, there are no exercise mimetics that are used in clinical practice, and further research is needed to clarify the molecular mechanisms and other aspects. Additionally, clear clinical studies are required to investigate the preventive efficacy of exercise mimetics in sarcopenia.
10. Herbal Supplements and Nutrition
Herbal supplements are of significant interest owing to their potential to promote muscular mass and health in patients with sarcopenia. Recent reviews identified numerous herbal compounds with effects on skeletal muscles [189,190]. Several of these compounds have shown mild effects on the skeletal muscles in human studies. For instance, curcumin from Curcuma longa, alkaloids and steroidal lactones from Withania somnifera (Solanaceae), catechins from Camellia sinensis, proanthocyanidin of grape seeds, and gingerols and shogaols from Zingiber officinale were found to have positive effects on skeletal muscle in human studies [189].
Various phytochemicals in rosemary have been studied for their effects on skeletal muscle cells, and several results have been reported. For instance, rosmarinic acid increases glucose uptake in skeletal muscle cells and activates 5′-adenosine monophosphate-activated protein kinase (AMPK) [191]. Carnosol attenuates C2C12 myotube atrophy and reduces insulin resistance in muscle cells and adipocytes [192,193]. Ursolic acid ameliorated indoxyl sulfate-induced mitochondrial biogenesis disorders in C2C12 cells [194], and together with leucine, it potentiated the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway [195]. An ethanol extract of loquat (Eriobotrya japonica), which contains ursolic acid, prevented dexamethasone-induced muscle atrophy by inhibiting the muscle degradation pathway in Sprague–Dawley rats [196], whereas loquat leaf extract enhanced myogenic differentiation and muscle function in aged Sprague–Dawley rats [197] and healthy human adults [198].
A recent small clinical study revealed that an extract from Schisandra chinensis (SC) not only improves the thigh muscle strength of elderly women but also has a fatigue-improving effect [199]. SC fruit is a well-known traditional herb used for pharmacological purposes in Asian countries, such as Korea, China, and Japan. Studies in animals have suggested that SC extract has beneficial effects, such as decreasing protein degradation, increasing protein synthesis, and exhibiting antioxidant and anti-inflammatory effects on skeletal muscle fibers [200,201]. SC also improves mass, strength, and endurance in mice. Schisandrin A and schisandrin B are the major active ingredients in SC extract, which not only inhibit muscle atrophy in various animal experiments [200,201,202] but also promote muscle cell differentiation [203,204,205]. In addition to these, curcumin, resveratrol, catechin, soy protein, ginseng, and other substances known to have antioxidant, anti-aging, or anti-cancer properties are also being introduced as helpful in preventing sarcopenia without specific side effects on the human body [206]. However, the use of herbal supplements for the treatment and prevention of sarcopenia is not widely recommended until further research establishes their safety and efficacy in humans.
11. Conclusions and Future Direction
Sarcopenia is an increasingly important health issue worldwide, with a prevalence of 5–13% among those aged 60–70 years and up to 50% among those over 80 years old [207,208]. As of 2017, the global population older than 60 years was estimated to be 962 million, which is projected to increase to 1.4 billion by 2030, with one in six people being aged 60 years or over. By 2050, this population is expected to double to 2.1 billion [209], and the number of individuals aged 80 years is predicted to triple to 426 million. Considering these statistics, sarcopenia, which currently affects over 80 million people, is conservatively expected to affect more than 320 million people over the next 30 years.
However, confirming the diagnosis of sarcopenia is challenging. Although comprehensive measurements used in research provide accurate results, they are often not feasible in clinical settings and may not affect patient care planning. Exercise remains the preferred method for managing sarcopenia; however, implementing an exercise program can be difficult for various reasons. The role of nutrition in the prevention and treatment of sarcopenia is not yet fully understood, and there is an ongoing debate regarding the optimal level of protein intake. However, it is generally recommended to ensure adequate protein intake and replace deficient nutrients and vitamins [210].
Future research should investigate the biological mechanisms that contribute to the development of sarcopenia and identify more accurate diagnostic biomarkers. Since 2016, sarcopenia has been recognized as a disease and assigned disease codes in numerous advanced countries worldwide, including the United States, Japan, and South Korea [72,124,211]. Multifaceted efforts are being made for the prevention and treatment of sarcopenia. Particularly, with the ongoing release of recent research findings on gene expression changes and single nucleotide polymorphisms associated with sarcopenia/frailty, as well as their associations with other diseases through genome-wide association studies and more [212,213], it is anticipated that in the near future, not only the approval of primary therapeutic agents but also the discovery of preventive measures will become possible.
Author Contributions
Conceptualization, J.Y.J., D.K., and N.D.K.; Investigation, J.Y.J. and D.K.; Writing—Original Draft Preparation, J.Y.J.; Writing—Review and Editing, D.K. and N.D.K.; Supervision, N.D.K.; Project Administration, J.Y.J. and N.D.K.; Funding Acquisition, N.D.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by a two-year research grant by Pusan National University (N.D.K.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rosenberg I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997;127:990s–991s. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner R.N., Koehler K.M., Gallagher D., Romero L., Heymsfield S.B., Ross R.R., Garry P.J., Lindeman R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R. Origins and clinical relevance of sarcopenia. Can. J. Appl. Physiol. 2001;26:78–89. doi: 10.1139/h01-006. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V., Simonsick E.M., Tylavsky F.A., Visser M., Newman A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 5.Delmonico M.J., Harris T.B., Lee J.S., Visser M., Nevitt M., Kritchevsky S.B., Tylavsky F.A., Newman A.B. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., Martin F.C., Michel J.P., Rolland Y., Schneider S.M., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perna S., Spadaccini D., Nichetti M., Avanzato I., Faliva M.A., Rondanelli M. Osteosarcopenic Visceral Obesity and Osteosarcopenic Subcutaneous Obesity, Two New Phenotypes of Sarcopenia: Prevalence, Metabolic Profile, and Risk Factors. J. Aging Res. 2018;2018:6147426. doi: 10.1155/2018/6147426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaap L.A., van Schoor N.M., Lips P., Visser M. Associations of Sarcopenia Definitions, and Their Components, With the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:1199–1204. doi: 10.1093/gerona/glx245. [DOI] [PubMed] [Google Scholar]
- 10.Van Ancum J.M., Alcazar J., Meskers C.G.M., Nielsen B.R., Suetta C., Maier A.B. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: A clinical perspective. Arch. Gerontol. Geriatr. 2020;90:104125. doi: 10.1016/j.archger.2020.104125. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez E., Salas R., Bouzas C., Pastor R., Tur J.A. Comparison between Original and Reviewed Consensus of European Working Group on Sarcopenia in Older People: A Probabilistic Cross-Sectional Survey among Community-Dwelling Older People. Gerontology. 2022;68:869–876. doi: 10.1159/000519304. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes L.V., Paiva A.E.G., Silva A.C.B., de Castro I.C., Santiago A.F., de Oliveira E.P., Porto L.C.J. Prevalence of sarcopenia according to EWGSOP1 and EWGSOP2 in older adults and their associations with unfavorable health outcomes: A systematic review. Aging Clin. Exp. Res. 2022;34:505–514. doi: 10.1007/s40520-021-01951-7. [DOI] [PubMed] [Google Scholar]
- 13.Anker S.D., Morley J.E., von Haehling S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle. 2016;7:512–514. doi: 10.1002/jcsm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo J.I., Kim J.T., Park C.H., Cha Y. Diagnosis and Management of Sarcopenia after Hip Fracture Surgery: Current Concept Review. Hip Pelvis. 2022;34:1–9. doi: 10.5371/hp.2022.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz-Jentoft A.J., Landi F., Schneider S.M., Zúñiga C., Arai H., Boirie Y., Chen L.K., Fielding R.A., Martin F.C., Michel J.P., et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciciliot S., Rossi A.C., Dyar K.A., Blaauw B., Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013;45:2191–2199. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Verdijk L.B., Snijders T., Drost M., Delhaas T., Kadi F., van Loon L.J. Satellite cells in human skeletal muscle; from birth to old age. Age. 2014;36:545–547. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McPhee J.S., Cameron J., Maden-Wilkinson T., Piasecki M., Yap M.H., Jones D.A., Degens H. The Contributions of Fiber Atrophy, Fiber Loss, In Situ Specific Force, and Voluntary Activation to Weakness in Sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:1287–1294. doi: 10.1093/gerona/gly040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edström E., Altun M., Bergman E., Johnson H., Kullberg S., Ramírez-León V., Ulfhake B. Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol. Behav. 2007;92:129–135. doi: 10.1016/j.physbeh.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Fulop T., Larbi A., Dupuis G., Le Page A., Frost E.H., Cohen A.A., Witkowski J.M., Franceschi C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walrand S., Zangarelli A., Guillet C., Salles J., Soulier K., Giraudet C., Patrac V., Boirie Y. Effect of fast dietary proteins on muscle protein synthesis rate and muscle strength in ad libitum-fed and energy-restricted old rats. Br. J. Nutr. 2011;106:1683–1690. doi: 10.1017/S0007114511002182. [DOI] [PubMed] [Google Scholar]
- 22.Huang J.H., Hood D.A. Age-associated mitochondrial dysfunction in skeletal muscle: Contributing factors and suggestions for long-term interventions. IUBMB Life. 2009;61:201–214. doi: 10.1002/iub.164. [DOI] [PubMed] [Google Scholar]
- 23.Ferri E., Marzetti E., Calvani R., Picca A., Cesari M., Arosio B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020;21:5236. doi: 10.3390/ijms21155236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji L.L. Exercise at old age: Does it increase or alleviate oxidative stress? Ann. N. Y. Acad. Sci. 2001;928:236–247. doi: 10.1111/j.1749-6632.2001.tb05653.x. [DOI] [PubMed] [Google Scholar]
- 25.Abate M., Di Iorio A., Di Renzo D., Paganelli R., Saggini R., Abate G. Frailty in the elderly: The physical dimension. Eur. Medicophys. 2007;43:407–415. [PubMed] [Google Scholar]
- 26.Faulkner J.A., Larkin L.M., Claflin D.R., Brooks S.V. Age-related changes in the structure and function of skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 27.Ryall J.G., Schertzer J.D., Lynch G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 28.Ying L., Zhang Q., Yang Y.M., Zhou J.Y. A Combination of Serum Biomarkers in Elderly Patients with Sarcopenia: A Cross-Sectional Observational Study. Int. J. Endocrinol. 2022;2022:4026940. doi: 10.1155/2022/4026940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visser M., Pahor M., Taaffe D.R., Goodpaster B.H., Simonsick E.M., Newman A.B., Nevitt M., Harris T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.M326. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa H., Fukunishi S., Asai A., Yokohama K., Nishiguchi S., Higuchi K. Pathophysiology and mechanisms of primary sarcopenia (Review) Int. J. Mol. Med. 2021;48:156. doi: 10.3892/ijmm.2021.4989. [DOI] [PubMed] [Google Scholar]
- 31.Booth F.W., Chakravarthy M.V., Spangenburg E.E. Exercise and gene expression: Physiological regulation of the human genome through physical activity. J. Physiol. 2002;543:399–411. doi: 10.1113/jphysiol.2002.019265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayer A.A., Syddall H.E., Gilbody H.J., Dennison E.M., Cooper C. Does sarcopenia originate in early life? Findings from the Hertfordshire cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:M930–M934. doi: 10.1093/gerona/59.9.M930. [DOI] [PubMed] [Google Scholar]
- 33.Sayer A.A., Dennison E.M., Syddall H.E., Jameson K., Martin H.J., Cooper C. The developmental origins of sarcopenia: Using peripheral quantitative computed tomography to assess muscle size in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:835–840. doi: 10.1093/gerona/63.8.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel H.P., Jameson K.A., Syddall H.E., Martin H.J., Stewart C.E., Cooper C., Sayer A.A. Developmental influences, muscle morphology, and sarcopenia in community-dwelling older men. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:82–87. doi: 10.1093/gerona/glr020. [DOI] [PubMed] [Google Scholar]
- 35.Konishi M., Ishida J., von Haehling S., Anker S.D., Springer J. Nutrition in cachexia: From bench to bedside. J. Cachexia Sarcopenia Muscle. 2016;7:107–109. doi: 10.1002/jcsm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Relph W.L. Addressing the nutritional needs of older patients. Nurs. Older People. 2016;28:16–19. doi: 10.7748/nop.28.3.16.s22. [DOI] [PubMed] [Google Scholar]
- 37.Sieber C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019;31:793–798. doi: 10.1007/s40520-019-01170-1. [DOI] [PubMed] [Google Scholar]
- 38.Phillips S.M., Chevalier S., Leidy H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016;41:565–572. doi: 10.1139/apnm-2015-0550. [DOI] [PubMed] [Google Scholar]
- 39.Thomas D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Evans W.J., Morley J.E., Argilés J., Bales C., Baracos V., Guttridge D., Jatoi A., Kalantar-Zadeh K., Lochs H., Mantovani G., et al. Cachexia: A new definition. Clin. Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Morley J.E., Anker S.D., Evans W.J. Cachexia and aging: An update based on the Fourth International Cachexia Meeting. J. Nutr. Health Aging. 2009;13:47–55. doi: 10.1007/s12603-009-0009-x. [DOI] [PubMed] [Google Scholar]
- 42.Durham W.J., Dillon E.L., Sheffield-Moore M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:72–77. doi: 10.1097/MCO.0b013e32831cef61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muscaritoli M., Anker S.D., Argilés J., Aversa Z., Bauer J.M., Biolo G., Boirie Y., Bosaeus I., Cederholm T., Costelli P., et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Bauer J.M., Kaiser M.J., Sieber C.C. Sarcopenia in nursing home residents. J. Am. Med. Dir. Assoc. 2008;9:545–551. doi: 10.1016/j.jamda.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 46.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., Baracos V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 47.Waters D.L., Baumgartner R.N. Sarcopenia and obesity. Clin. Geriatr. Med. 2011;27:401–421. doi: 10.1016/j.cger.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Stenholm S., Harris T.B., Rantanen T., Visser M., Kritchevsky S.B., Ferrucci L. Sarcopenic obesity: Definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding J., Kritchevsky S.B., Newman A.B., Taaffe D.R., Nicklas B.J., Visser M., Lee J.S., Nevitt M., Tylavsky F.A., Rubin S.M., et al. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am. J. Clin. Nutr. 2007;85:405–410. doi: 10.1093/ajcn/85.2.405. [DOI] [PubMed] [Google Scholar]
- 50.Song M.Y., Ruts E., Kim J., Janumala I., Heymsfield S., Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am. J. Clin. Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 51.Hughes V.A., Roubenoff R., Wood M., Frontera W.R., Evans W.J., Fiatarone Singh M.A. Anthropometric assessment of 10-y changes in body composition in the elderly. Am. J. Clin. Nutr. 2004;80:475–482. doi: 10.1093/ajcn/80.2.475. [DOI] [PubMed] [Google Scholar]
- 52.Karalaki M., Fili S., Philippou A., Koutsilieris M. Muscle regeneration: Cellular and molecular events. In Vivo. 2009;23:779–796. [PubMed] [Google Scholar]
- 53.Ceafalan L.C., Popescu B.O., Hinescu M.E. Cellular players in skeletal muscle regeneration. BioMed Res. Int. 2014;2014:957014. doi: 10.1155/2014/957014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grefte S., Kuijpers-Jagtman A.M., Torensma R., Von den Hoff J.W. Skeletal muscle development and regeneration. Stem Cells Dev. 2007;16:857–868. doi: 10.1089/scd.2007.0058. [DOI] [PubMed] [Google Scholar]
- 55.Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 56.Hollingworth T.W., Oke S.M., Patel H., Smith T.R. Getting to grips with sarcopenia: Recent advances and practical management for the gastroenterologist. Frontline Gastroenterol. 2021;12:53–61. doi: 10.1136/flgastro-2019-101348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodine S.C., Baehr L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014;307:E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riuzzi F., Sorci G., Arcuri C., Giambanco I., Bellezza I., Minelli A., Donato R. Cellular and molecular mechanisms of sarcopenia: The S100B perspective. J. Cachexia Sarcopenia Muscle. 2018;9:1255–1268. doi: 10.1002/jcsm.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bilgic S.N., Domaniku A., Toledo B., Agca S., Weber B.Z.C., Arabaci D.H., Ozornek Z., Lause P., Thissen J.P., Loumaye A., et al. EDA2R-NIK signalling promotes muscle atrophy linked to cancer cachexia. Nature. 2023;617:827–834. doi: 10.1038/s41586-023-06047-y. [DOI] [PubMed] [Google Scholar]
- 60.Tintignac L.A., Lagirand J., Batonnet S., Sirri V., Leibovitch M.P., Leibovitch S.A. Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J. Biol. Chem. 2005;280:2847–2856. doi: 10.1074/jbc.M411346200. [DOI] [PubMed] [Google Scholar]
- 61.Morel S., Hugon G., Vitou M., Védère M., Fons F., Rapior S., Saint N., Carnac G. A Bioassay-Guided Fractionation of Rosemary Leaf Extract Identifies Carnosol as a Major Hypertrophy Inducer in Human Skeletal Muscle Cells. Nutrients. 2021;13:4190. doi: 10.3390/nu13124190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang T., Streeper T., Cawthon P., Baldwin K., Taaffe D.R., Harris T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010;21:543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Auyeung T.W., Lee J.S., Leung J., Kwok T., Woo J. Adiposity to muscle ratio predicts incident physical limitation in a cohort of 3,153 older adults--an alternative measurement of sarcopenia and sarcopenic obesity. Age. 2013;35:1377–1385. doi: 10.1007/s11357-012-9423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim J.H., Choi S.H., Lim S., Yoon J.W., Kang S.M., Kim K.W., Lim J.Y., Cho N.H., Jang H.C. Sarcopenia and obesity: Gender-different relationship with functional limitation in older persons. J. Korean Med. Sci. 2013;28:1041–1047. doi: 10.3346/jkms.2013.28.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ibrahim K., May C.R., Patel H.P., Baxter M., Sayer A.A., Roberts H.C. Implementation of grip strength measurement in medicine for older people wards as part of routine admission assessment: Identifying facilitators and barriers using a theory-led intervention. BMC Geriatr. 2018;18:79. doi: 10.1186/s12877-018-0768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Celis-Morales C.A., Welsh P., Lyall D.M., Steell L., Petermann F., Anderson J., Iliodromiti S., Sillars A., Graham N., Mackay D.F., et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651. doi: 10.1136/bmj.k1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buckinx F., Landi F., Cesari M., Fielding R.A., Visser M., Engelke K., Maggi S., Dennison E., Al-Daghri N.M., Allepaerts S., et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle. 2018;9:269–278. doi: 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masanés F., Rojano I.L.X., Salvà A., Serra-Rexach J.A., Artaza I., Formiga F., Cuesta F., López Soto A., Ruiz D., Cruz-Jentoft A.J. Cut-off Points for Muscle Mass—Not Grip Strength or Gait Speed—Determine Variations in Sarcopenia Prevalence. J. Nutr. Health Aging. 2017;21:825–829. doi: 10.1007/s12603-016-0844-5. [DOI] [PubMed] [Google Scholar]
- 69.Treviño-Aguirre E., López-Teros T., Gutiérrez-Robledo L., Vandewoude M., Pérez-Zepeda M. Availability and use of dual energy X-ray absorptiometry (DXA) and bio-impedance analysis (BIA) for the evaluation of sarcopenia by Belgian and Latin American geriatricians. J. Cachexia Sarcopenia Muscle. 2014;5:79–81. doi: 10.1007/s13539-013-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bahat G., Yilmaz O., Kılıç C., Oren M.M., Karan M.A. Performance of SARC-F in Regard to Sarcopenia Definitions, Muscle Mass and Functional Measures. J. Nutr. Health Aging. 2018;22:898–903. doi: 10.1007/s12603-018-1067-8. [DOI] [PubMed] [Google Scholar]
- 71.Nishikawa H., Asai A., Fukunishi S., Takeuchi T., Goto M., Ogura T., Nakamura S., Kakimoto K., Miyazaki T., Nishiguchi S., et al. Screening Tools for Sarcopenia. In Vivo. 2021;35:3001–3009. doi: 10.21873/invivo.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piotrowicz K., Gryglewska B., Gąsowski J. The usefulness of SARC-F. Aging Clin. Exp. Res. 2021;33:2307. doi: 10.1007/s40520-021-01839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishii S., Tanaka T., Shibasaki K., Ouchi Y., Kikutani T., Higashiguchi T., Obuchi S.P., Ishikawa-Takata K., Hirano H., Kawai H., et al. Development of a simple screening test for sarcopenia in older adults. Geriatr. Gerontol. Int. 2014;14((Suppl. S1)):93–101. doi: 10.1111/ggi.12197. [DOI] [PubMed] [Google Scholar]
- 74.Roberts H.C., Denison H.J., Martin H.J., Patel H.P., Syddall H., Cooper C., Sayer A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 75.Beaudart C., McCloskey E., Bruyère O., Cesari M., Rolland Y., Rizzoli R., Araujo de Carvalho I., Amuthavalli Thiyagarajan J., Bautmans I., Bertière M.C., et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016;16:170. doi: 10.1186/s12877-016-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones C.J., Rikli R.E., Beam W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport. 1999;70:113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 77.Cooper C., Fielding R., Visser M., van Loon L.J., Rolland Y., Orwoll E., Reid K., Boonen S., Dere W., Epstein S., et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 2013;93:201–210. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cawthon P.M., Peters K.W., Shardell M.D., McLean R.R., Dam T.T., Kenny A.M., Fragala M.S., Harris T.B., Kiel D.P., Guralnik J.M., et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014;69:567–575. doi: 10.1093/gerona/glu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mourtzakis M., Prado C.M., Lieffers J.R., Reiman T., McCargar L.J., Baracos V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 80.Kim E.Y., Kim Y.S., Park I., Ahn H.K., Cho E.K., Jeong Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015;10:1795–1799. doi: 10.1097/JTO.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 81.Lee S.J., Janssen I., Heymsfield S.B., Ross R. Relation between whole-body and regional measures of human skeletal muscle. Am. J. Clin. Nutr. 2004;80:1215–1221. doi: 10.1093/ajcn/80.5.1215. [DOI] [PubMed] [Google Scholar]
- 82.Baracos V.E., Reiman T., Mourtzakis M., Gioulbasanis I., Antoun S. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am. J. Clin. Nutr. 2010;91:1133s–1137s. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 83.Kim K.M., Jang H.C., Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016;31:643–650. doi: 10.3904/kjim.2016.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tosato M., Marzetti E., Cesari M., Savera G., Miller R.R., Bernabei R., Landi F., Calvani R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017;29:19–27. doi: 10.1007/s40520-016-0717-0. [DOI] [PubMed] [Google Scholar]
- 85.Landi F., Onder G., Russo A., Liperoti R., Tosato M., Martone A.M., Capoluongo E., Bernabei R. Calf circumference, frailty and physical performance among older adults living in the community. Clin. Nutr. 2014;33:539–544. doi: 10.1016/j.clnu.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 86.Abellan van Kan G., Rolland Y., Andrieu S., Bauer J., Beauchet O., Bonnefoy M., Cesari M., Donini L.M., Gillette Guyonnet S., Inzitari M., et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 87.Peel N.M., Kuys S.S., Klein K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:39–46. doi: 10.1093/gerona/gls174. [DOI] [PubMed] [Google Scholar]
- 88.Studenski S., Perera S., Patel K., Rosano C., Faulkner K., Inzitari M., Brach J., Chandler J., Cawthon P., Connor E.B., et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maggio M., Ceda G.P., Ticinesi A., De Vita F., Gelmini G., Costantino C., Meschi T., Kressig R.W., Cesari M., Fabi M., et al. Instrumental and Non-Instrumental Evaluation of 4-Meter Walking Speed in Older Individuals. PLoS ONE. 2016;11:e0153583. doi: 10.1371/journal.pone.0153583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rydwik E., Bergland A., Forsén L., Frändin K. Investigation into the reliability and validity of the measurement of elderly people’s clinical walking speed: A systematic review. Physiother. Theory Pract. 2012;28:238–256. doi: 10.3109/09593985.2011.601804. [DOI] [PubMed] [Google Scholar]
- 91.Podsiadlo D., Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 92.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., Abellan van Kan G., Andrieu S., Bauer J., Breuille D., et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cesari M., Fielding R.A., Pahor M., Goodpaster B., Hellerstein M., van Kan G.A., Anker S.D., Rutkove S., Vrijbloed J.W., Isaac M., et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J. Cachexia Sarcopenia Muscle. 2012;3:181–190. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S., Chou M.Y., Chen L.Y., Hsu P.S., Krairit O., et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 95.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020;21:300–307.e302. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 96.Chen L.K., Lee W.J., Peng L.N., Liu L.K., Arai H., Akishita M. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2016;17:767.e1–767.e7. doi: 10.1016/j.jamda.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 97.Doherty T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 98.Lexell J., Taylor C.C., Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988;84:275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 99.Doherty T.J., Brown W.F. Age-related changes in the twitch contractile properties of human thenar motor units. J. Appl. Physiol. 1997;82:93–101. doi: 10.1152/jappl.1997.82.1.93. [DOI] [PubMed] [Google Scholar]
- 100.Tomlinson B.E., Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J. Neurol. Sci. 1977;34:213–219. doi: 10.1016/0022-510X(77)90069-7. [DOI] [PubMed] [Google Scholar]
- 101.Yarasheski K.E. Exercise, aging, and muscle protein metabolism. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:M918–M922. doi: 10.1093/gerona/58.10.M918. [DOI] [PubMed] [Google Scholar]
- 102.Roth S.M., Ferrell R.F., Hurley B.F. Strength training for the prevention and treatment of sarcopenia. J. Nutr. Health Aging. 2000;4:143–155. [PubMed] [Google Scholar]
- 103.Supriya R., Singh K.P., Gao Y., Gu Y., Baker J.S. Effect of Exercise on Secondary Sarcopenia: A Comprehensive Literature Review. Biology. 2021;11:51. doi: 10.3390/biology11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Denison H.J., Cooper C., Sayer A.A., Robinson S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging. 2015;10:859–869. doi: 10.2147/cia.S55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McKendry J., Currier B.S., Lim C., McLeod J.C., Thomas A.C.Q., Phillips S.M. Nutritional Supplements to Support Resistance Exercise in Countering the Sarcopenia of Aging. Nutrients. 2020;12:2057. doi: 10.3390/nu12072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sakuma K., Yamaguchi A. Sarcopenia and age-related endocrine function. Int. J. Endocrinol. 2012;2012:127362. doi: 10.1155/2012/127362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wakabayashi H., Sakuma K. Comprehensive approach to sarcopenia treatment. Curr. Clin. Pharmacol. 2014;9:171–180. doi: 10.2174/1574884708666131111192845. [DOI] [PubMed] [Google Scholar]
- 108.Christiansen A.R., Lipshultz L.I., Hotaling J.M., Pastuszak A.W. Selective androgen receptor modulators: The future of androgen therapy? Transl. Androl. Urol. 2020;9:S135–S148. doi: 10.21037/tau.2019.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.GTx-024 as a Treatment for Stress Urinary Incontinence in Women. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT02658448.
- 110.Study to Assess Enobosarm (GTx-024) in Postmenopausal Women With Stress Urinary Incontinence. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT03241342.
- 111.Durability Extension Study to Assess Clinical Activity and Safety of Enobosarm (GTx-024) in Stress Urinary Incontinence. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT03508648.
- 112.Study of GTx-024 on Muscle Wasting (Cachexia) Cancer. [(accessed on 31 May 2023)];2008 Available online: https://ClinicalTrials.gov/show/NCT00467844.
- 113.Dobs A.S., Boccia R.V., Croot C.C., Gabrail N.Y., Dalton J.T., Hancock M.L., Johnston M.A., Steiner M.S. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335–345. doi: 10.1016/S1470-2045(13)70055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Add-on Study for Protocol G200802 ( NCT02463032): Effect of GTx-024 on Maximal Neuromuscular Function and Lean Body Mass. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT02746328.
- 115.Effect of GTx-024 on Muscle Wasting in Patients With Non-Small Cell Lung Cancer (NSCLC) on First Line Platinum. [(accessed on 31 May 2023)];2013 Available online: https://ClinicalTrials.gov/show/NCT01355497.
- 116.Crawford J., Prado C.M., Johnston M.A., Gralla R.J., Taylor R.P., Hancock M.L., Dalton J.T. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials) Curr. Oncol. Rep. 2016;18:37. doi: 10.1007/s11912-016-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Phase III Study of the Effect of GTx-024 on Muscle Wasting in Patients With Non-Small Cell Lung Cancer (NSCLC) [(accessed on 31 May 2023)];2013 Available online: https://ClinicalTrials.gov/show/NCT01355484.
- 118.Enobosarm and Anastrozole in Pre-Menopausal Women with High Mammographic Breast Density. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT03264651.
- 119.Study to Evaluate the Safety and Efficacy of 13 Weeks of the Selective Androgen Receptor Modulator (SARM) GSK2881078 in Chronic Obstructive Pulmonary Disease (COPD) [(accessed on 31 May 2023)];2019 Available online: https://ClinicalTrials.gov/show/NCT03359473.
- 120.Mohan D., Rossiter H., Watz H., Fogarty C., Evans R.A., Man W., Tabberer M., Beerahee M., Kumar S., Millns H., et al. Selective androgen receptor modulation for muscle weakness in chronic obstructive pulmonary disease: A randomised control trial. Thorax. 2023;78:258–266. doi: 10.1136/thorax-2021-218360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J.W., Kim R., Choi H., Lee S.J., Bae G.U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharmacal Res. 2021;44:876–889. doi: 10.1007/s12272-021-01349-z. [DOI] [PubMed] [Google Scholar]
- 122.Cho M.R., Lee S., Song S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022;37:e146. doi: 10.3346/jkms.2022.37.e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee S.J. Myostatin: A Skeletal Muscle Chalone. Annu. Rev. Physiol. 2023;85:269–291. doi: 10.1146/annurev-physiol-012422-112116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baig M.H., Ahmad K., Moon J.S., Park S.Y., Ho Lim J., Chun H.J., Qadri A.F., Hwang Y.C., Jan A.T., Ahmad S.S., et al. Myostatin and its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022;13:876078. doi: 10.3389/fphys.2022.876078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dao T., Green A.E., Kim Y.A., Bae S.J., Ha K.T., Gariani K., Lee M.R., Menzies K.J., Ryu D. Sarcopenia and Muscle Aging: A Brief Overview. Endocrinol. Metab. 2020;35:716–732. doi: 10.3803/EnM.2020.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.A Phase 2 Study to Evaluate the Safety, Efficacy, Pharmacokinetics and Pharmacodynamics of PF-06252616 in Duchenne Muscular Dystrophy. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT02310763.
- 127.Wagner K.R., Abdel-Hamid H.Z., Mah J.K., Campbell C., Guglieri M., Muntoni F., Takeshima Y., McDonald C.M., Kostera-Pruszczyk A., Karachunski P., et al. Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy. Neuromuscul. Disord. 2020;30:492–502. doi: 10.1016/j.nmd.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 128.Sherlock S.P., Zhang Y., Binks M., Marraffino S. Quantitative muscle MRI biomarkers in Duchenne muscular dystrophy: Cross-sectional correlations with age and functional tests. Biomark. Med. 2021;15:761–773. doi: 10.2217/bmm-2020-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wagner K.R., Guglieri M., Ramaiah S.K., Charnas L., Marraffino S., Binks M., Vaidya V.S., Palmer J., Goldstein R., Muntoni F. Safety and disease monitoring biomarkers in Duchenne muscular dystrophy: Results from a Phase II trial. Biomark. Med. 2021;15:1389–1396. doi: 10.2217/bmm-2021-0222. [DOI] [PubMed] [Google Scholar]
- 130.Sherlock S.P., Palmer J., Wagner K.R., Abdel-Hamid H.Z., Bertini E., Tian C., Mah J.K., Kostera-Pruszczyk A., Muntoni F., Guglieri M., et al. Quantitative magnetic resonance imaging measures as biomarkers of disease progression in boys with Duchenne muscular dystrophy: A phase 2 trial of domagrozumab. J. Neurol. 2022;269:4421–4435. doi: 10.1007/s00415-022-11084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muntoni F., Guglieri M., Mah J.K., Wagner K.R., Brandsema J.F., Butterfield R.J., McDonald C.M., Mayhew A.G., Palmer J.P., Marraffino S., et al. Novel approaches to analysis of the North Star Ambulatory Assessment (NSAA) in Duchenne muscular dystrophy (DMD): Observations from a phase 2 trial. PLoS ONE. 2022;17:e0272858. doi: 10.1371/journal.pone.0272858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wojciechowski J., Purohit V.S., Harnisch L.O., Dua P., Tan B., Nicholas T. Population PK and PD Analysis of Domagrozumab in Pediatric Patients with Duchenne Muscular Dystrophy. Clin. Pharmacol. Ther. 2022;112:1291–1302. doi: 10.1002/cpt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sherlock S.P., Palmer J., Wagner K.R., Abdel-Hamid H.Z., Tian C., Mah J.K., Muntoni F., Guglieri M., Butterfield R.J., Charnas L., et al. Dual-energy X-ray absorptiometry measures of lean body mass as a biomarker for progression in boys with Duchenne muscular dystrophy. Sci. Rep. 2022;12:18762. doi: 10.1038/s41598-022-23072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.An Open-Label Extension Study to Evaluate Safety of PF-06252616 in Boys with Duchenne Muscular Dystrophy. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT02907619.
- 135.Efficacy and Safety of Apitegromab in Patients with Later-Onset Spinal Muscular Atrophy Treated with Nusinersen or Risdiplam. [(accessed on 31 May 2023)];2024 Available online: https://ClinicalTrials.gov/show/NCT05156320.
- 136.Study Evaluating MYO-029 in Adult Muscular Dystrophy. [(accessed on 31 May 2023)]; Available online: https://ClinicalTrials.gov/show/NCT00104078.
- 137.An Active Treatment Study of SRK-015 in Patients with Type 2 or Type 3 Spinal Muscular Atrophy. [(accessed on 31 May 2023)];2021 Available online: https://ClinicalTrials.gov/show/NCT03921528.
- 138.Long-Term Safety & Efficacy of Apitegromab in Patients with SMA Who Completed Previous Trials of Apitegromab-ONYX. [(accessed on 31 May 2023)];2026 Available online: https://ClinicalTrials.gov/show/NCT05626855.
- 139.Study of Efficacy and Safety of Bimagrumab in Patients After Hip Fracture Surgery. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT02152761.
- 140.Hofbauer L.C., Witvrouw R., Varga Z., Shiota N., Cremer M., Tanko L.B., Rooks D., Auberson L.Z., Arkuszewski M., Fretault N., et al. Bimagrumab to improve recovery after hip fracture in older adults: A multicentre, double-blind, randomised, parallel-group, placebo-controlled, phase 2a/b trial. Lancet Healthy Longev. 2021;2:e263–e274. doi: 10.1016/S2666-7568(21)00084-2. [DOI] [PubMed] [Google Scholar]
- 141.Safety, Pharmacokinetics and Efficacy of Bimagrumab in Overweight and Obese Patients with Type 2 Diabetes. [(accessed on 31 May 2023)];2019 Available online: https://ClinicalTrials.gov/show/NCT03005288.
- 142.Heymsfield S.B., Coleman L.A., Miller R., Rooks D.S., Laurent D., Petricoul O., Praestgaard J., Swan T., Wade T., Perry R.G., et al. Effect of Bimagrumab vs Placebo on Body Fat Mass Among Adults With Type 2 Diabetes and Obesity: A Phase 2 Randomized Clinical Trial. JAMA Netw. Open. 2021;4:e2033457. doi: 10.1001/jamanetworkopen.2020.33457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.An Extension Study of the Efficacy, Safety and Tolerability of BYM338 (Bimagrumab) in Patients with Sporadic Inclusion Body Myositis Who Previously Participated in the Core Study CBYM338B2203. [(accessed on 31 May 2023)];2016 Available online: https://ClinicalTrials.gov/show/NCT02573467.
- 144.Amato A.A., Hanna M.G., Machado P.M., Badrising U.A., Chinoy H., Benveniste O., Karanam A.K., Wu M., Tankó L.B., Schubert-Tennigkeit A.A., et al. Efficacy and Safety of Bimagrumab in Sporadic Inclusion Body Myositis: Long-term Extension of RESILIENT. Neurology. 2021;96:e1595–e1607. doi: 10.1212/WNL.0000000000011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dose Range Finding Study of Bimagrumab in Sarcopenia. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT02333331.
- 146.Rooks D., Swan T., Goswami B., Filosa L.A., Bunte O., Panchaud N., Coleman L.A., Miller R.R., Garcia Garayoa E., Praestgaard J., et al. Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults: A Randomized Clinical Trial. JAMA Netw. Open. 2020;3:e2020836. doi: 10.1001/jamanetworkopen.2020.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Study of Long-Term Safety, Efficacy Tolerability of BYM338 in Patients with Sporadic Inclusion Body Myositis. [(accessed on 31 May 2023)];2016 Available online: https://ClinicalTrials.gov/show/NCT02250443.
- 148.Sivakumar K., Cochrane T.I., Sloth B., Ashar H., Laurent D., Tankó L.B., Amato A.A. Long-term safety and tolerability of bimagrumab (BYM338) in sporadic inclusion body myositis. Neurology. 2020;95:e1971–e1978. doi: 10.1212/WNL.0000000000010417. [DOI] [PubMed] [Google Scholar]
- 149.Efficacy and Safety of Bimagrumab/BYM338 at 52 Weeks on Physical Function, Muscle Strength, Mobility in sIBM Patients. [(accessed on 31 May 2023)];2016 Available online: https://ClinicalTrials.gov/show/NCT01925209.
- 150.Hanna M.G., Badrising U.A., Benveniste O., Lloyd T.E., Needham M., Chinoy H., Aoki M., Machado P.M., Liang C., Reardon K.A., et al. Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): A randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019;18:834–844. doi: 10.1016/S1474-4422(19)30200-5. [DOI] [PubMed] [Google Scholar]
- 151.A Multi-Center Study to Assess the Effects of BYM338 on Skeletal Muscle in Sarcopenic Adults. [(accessed on 31 May 2023)];2013 Available online: https://ClinicalTrials.gov/show/NCT01601600.
- 152.Efficacy, Safety and Tolerability of BYM338 in Patients with Sporadic Inclusion Body Myositis. [(accessed on 31 May 2023)];2012 Available online: https://ClinicalTrials.gov/show/NCT01423110.
- 153.Amato A.A., Sivakumar K., Goyal N., David W.S., Salajegheh M., Praestgaard J., Lach-Trifilieff E., Trendelenburg A.U., Laurent D., Glass D.J., et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83:2239–2246. doi: 10.1212/WNL.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.A 24-Week Off-Drug Extension Study in Sarcopenic Elderly Who Completed Treatment in the 6-Month Core Study. [(accessed on 31 May 2023)];2018 Available online: https://ClinicalTrials.gov/show/NCT02468674.
- 155.BYM338 in Chronic Obstructive Pulmonary Disease (COPD) Patients with Cachexia. [(accessed on 31 May 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT01669174.
- 156.Polkey M.I., Praestgaard J., Berwick A., Franssen F.M.E., Singh D., Steiner M.C., Casaburi R., Tillmann H.C., Lach-Trifilieff E., Roubenoff R., et al. Activin Type II Receptor Blockade for Treatment of Muscle Depletion in Chronic Obstructive Pulmonary Disease. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2019;199:313–320. doi: 10.1164/rccm.201802-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Clinical Study of BYM338 for the Treatment of Unintentional Weight Loss in Patients with Cancer of the Lung or the Pancreas. [(accessed on 31 May 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT01433263.
- 158.A Study of LY2495655 in Older Participants Undergoing Elective Total Hip Replacement. [(accessed on 31 May 2023)];2014 Available online: https://ClinicalTrials.gov/show/NCT01369511.
- 159.Woodhouse L., Gandhi R., Warden S.J., Poiraudeau S., Myers S.L., Benson C.T., Hu L., Ahmad Q.I., Linnemeier P., Gomez E.V., et al. A Phase 2 Randomized Study Investigating the Efficacy and Safety of Myostatin Antibody LY2495655 versus Placebo in Patients Undergoing Elective Total Hip Arthroplasty. J. Frailty Aging. 2016;5:62–70. doi: 10.14283/jfa.2016.81. [DOI] [PubMed] [Google Scholar]
- 160.A Study in Older Participants Who Have Fallen and Have Muscle Weakness. [(accessed on 31 May 2023)];2013 Available online: https://ClinicalTrials.gov/show/NCT01604408.
- 161.Becker C., Lord S.R., Studenski S.A., Warden S.J., Fielding R.A., Recknor C.P., Hochberg M.C., Ferrari S.L., Blain H., Binder E.F., et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3:948–957. doi: 10.1016/S2213-8587(15)00298-3. [DOI] [PubMed] [Google Scholar]
- 162.Study of the Safety and Efficacy of REGN1033 (SAR391786) in Patients with Sarcopenia. [(accessed on 31 May 2023)];2015 Available online: https://ClinicalTrials.gov/show/NCT01963598.
- 163.A Study to Evaluate the Efficacy and Safety of Taldefgrobep Alfa in Participants with Spinal Muscular Atrophy. [(accessed on 31 May 2023)];2025 Available online: https://ClinicalTrials.gov/show/NCT05337553.
- 164.Jerry R.M., Duchenne A., Milo T., Nationwide Children’s H. Clinical Intramuscular Gene Transfer of rAAV1.CMV.huFollistatin344 Trial to Patients with Duchenne Muscular Dystrophy. [(accessed on 31 May 2023)];2017 Available online: https://ClinicalTrials.gov/show/NCT02354781.
- 165.Follistatin Gene Transfer to Patients with Becker Muscular Dystrophy and Sporadic Inclusion Body Myositis. [(accessed on 31 May 2023)];2017 Available online: https://ClinicalTrials.gov/show/NCT01519349.
- 166.Mendell J.R., Sahenk Z., Malik V., Gomez A.M., Flanigan K.M., Lowes L.P., Alfano L.N., Berry K., Meadows E., Lewis S., et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol. Ther. 2015;23:192–201. doi: 10.1038/mt.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Remelli F., Vitali A., Zurlo A., Volpato S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients. 2019;11:2861. doi: 10.3390/nu11122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Uchitomi R., Oyabu M., Kamei Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients. 2020;12:3189. doi: 10.3390/nu12103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cereda E., Pisati R., Rondanelli M., Caccialanza R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients. 2022;14:1524. doi: 10.3390/nu14071524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rondanelli M., Cereda E., Klersy C., Faliva M.A., Peroni G., Nichetti M., Gasparri C., Iannello G., Spadaccini D., Infantino V., et al. Improving rehabilitation in sarcopenia: A randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J. Cachexia Sarcopenia Muscle. 2020;11:1535–1547. doi: 10.1002/jcsm.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bauer J.M., Verlaan S., Bautmans I., Brandt K., Donini L.M., Maggio M., McMurdo M.E., Mets T., Seal C., Wijers S.L., et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015;16:740–747. doi: 10.1016/j.jamda.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 172.Waterhouse M., Sanguineti E., Baxter C., Duarte Romero B., McLeod D.S.A., English D.R., Armstrong B.K., Ebeling P.R., Hartel G., Kimlin M.G., et al. Vitamin D supplementation and risk of falling: Outcomes from the randomized, placebo-controlled D-Health Trial. J. Cachexia Sarcopenia Muscle. 2021;12:1428–1439. doi: 10.1002/jcsm.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Caputo M., Pigni S., Agosti E., Daffara T., Ferrero A., Filigheddu N., Prodam F. Regulation of GH and GH Signaling by Nutrients. Cells. 2021;10:1376. doi: 10.3390/cells10061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Junnila R.K., List E.O., Berryman D.E., Murrey J.W., Kopchick J.J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 2013;9:366–376. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mellen R.H., Girotto O.S., Marques E.B., Laurindo L.F., Grippa P.C., Mendes C.G., Garcia L.N.H., Bechara M.D., Barbalho S.M., Sinatora R.V., et al. Insights into Pathogenesis, Nutritional and Drug Approach in Sarcopenia: A Systematic Review. Biomedicines. 2023;11:136. doi: 10.3390/biomedicines11010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hersch E.C., Merriam G.R. Growth hormone (GH)-releasing hormone and GH secretagogues in normal aging: Fountain of Youth or Pool of Tantalus? Clin. Interv. Aging. 2008;3:121–129. [PMC free article] [PubMed] [Google Scholar]
- 177.Dingemans A.M., de Vos-Geelen J., Langen R., Schols A.M. Phase II drugs that are currently in development for the treatment of cachexia. Expert Opin. Investig. Drugs. 2014;23:1655–1669. doi: 10.1517/13543784.2014.942729. [DOI] [PubMed] [Google Scholar]
- 178.Stewart Coats A.J., Srinivasan V., Surendran J., Chiramana H., Vangipuram S.R., Bhatt N.N., Jain M., Shah S., Ali I.A., Fuang H.G., et al. The ACT-ONE trial, a multicentre, randomised, double-blind, placebo-controlled, dose-finding study of the anabolic/catabolic transforming agent, MT-102 in subjects with cachexia related to stage III and IV non-small cell lung cancer and colorectal cancer: Study design. J. Cachexia Sarcopenia Muscle. 2011;2:201–207. doi: 10.1007/s13539-011-0046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pötsch M.S., Tschirner A., Palus S., von Haehling S., Doehner W., Beadle J., Coats A.J., Anker S.D., Springer J. The anabolic catabolic transforming agent (ACTA) espindolol increases muscle mass and decreases fat mass in old rats. J. Cachexia Sarcopenia Muscle. 2014;5:149–158. doi: 10.1007/s13539-013-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pötsch M.S., Ishida J., Palus S., Tschirner A., von Haehling S., Doehner W., Anker S.D., Springer J. MT-102 prevents tissue wasting and improves survival in a rat model of severe cancer cachexia. J. Cachexia Sarcopenia Muscle. 2020;11:594–605. doi: 10.1002/jcsm.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Narkar V.A., Downes M., Yu R.T., Embler E., Wang Y.X., Banayo E., Mihaylova M.M., Nelson M.C., Zou Y., Juguilon H., et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mosti M.P., Stunes A.K., Ericsson M., Pullisaar H., Reseland J.E., Shabestari M., Eriksen E.F., Syversen U. Effects of the peroxisome proliferator-activated receptor (PPAR)-δ agonist GW501516 on bone and muscle in ovariectomized rats. Endocrinology. 2014;155:2178–2189. doi: 10.1210/en.2013-1166. [DOI] [PubMed] [Google Scholar]
- 183.Sahebkar A., Chew G.T., Watts G.F. New peroxisome proliferator-activated receptor agonists: Potential treatments for atherogenic dyslipidemia and non-alcoholic fatty liver disease. Expert Opin. Pharmacother. 2014;15:493–503. doi: 10.1517/14656566.2014.876992. [DOI] [PubMed] [Google Scholar]
- 184.Kadayat T.M., Shrestha A., Jeon Y.H., An H., Kim J., Cho S.J., Chin J. Targeting Peroxisome Proliferator-Activated Receptor Delta (PPARδ): A Medicinal Chemistry Perspective. J. Med. Chem. 2020;63:10109–10134. doi: 10.1021/acs.jmedchem.9b01882. [DOI] [PubMed] [Google Scholar]
- 185.Višnjić D., Lalić H., Dembitz V., Tomić B., Smoljo T. AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review. Cells. 2021;10:1095. doi: 10.3390/cells10051095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guerrieri D., Moon H.Y., van Praag H. Exercise in a Pill: The Latest on Exercise-Mimetics. Brain Plast. 2017;2:153–169. doi: 10.3233/BPL-160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jang Y.J., Byun S. Molecular targets of exercise mimetics and their natural activators. BMB Rep. 2021;54:581–591. doi: 10.5483/BMBRep.2021.54.12.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cento A.S., Leigheb M., Caretti G., Penna F. Exercise and Exercise Mimetics for the Treatment of Musculoskeletal Disorders. Curr. Osteoporos. Rep. 2022;20:249–259. doi: 10.1007/s11914-022-00739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rondanelli M., Miccono A., Peroni G., Guerriero F., Morazzoni P., Riva A., Guido D., Perna S. A Systematic Review on the Effects of Botanicals on Skeletal Muscle Health in Order to Prevent Sarcopenia. Evid. Based Complement. Altern. Med. 2016;2016:5970367. doi: 10.1155/2016/5970367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Granic A., Sayer A.A., Robinson S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients. 2019;11:745. doi: 10.3390/nu11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vlavcheski F., Naimi M., Murphy B., Hudlicky T., Tsiani E. Rosmarinic Acid, a Rosemary Extract Polyphenol, Increases Skeletal Muscle Cell Glucose Uptake and Activates AMPK. Molecules. 2017;22:1669. doi: 10.3390/molecules22101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kuang J.X., Shen Q., Zhang R.Q., Fang Q.Y., Deng X., Fan M., Cheng C.R., Zhang X.W., Liu X. Carnosol attenuated atrophy of C2C12 myotubes induced by tumour-derived exosomal miR-183-5p through inhibiting Smad3 pathway activation and keeping mitochondrial respiration. Basic Clin. Pharmacol. Toxicol. 2022;131:500–513. doi: 10.1111/bcpt.13795. [DOI] [PubMed] [Google Scholar]
- 193.Den Hartogh D.J., Vlavcheski F., Giacca A., MacPherson R.E.K., Tsiani E. Carnosic Acid Attenuates the Free Fatty Acid-Induced Insulin Resistance in Muscle Cells and Adipocytes. Cells. 2022;11:167. doi: 10.3390/cells11010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sasaki Y., Kojima-Yuasa A., Tadano H., Mizuno A., Kon A., Norikura T. Ursolic acid improves the indoxyl sulfate-induced impairment of mitochondrial biogenesis in C2C12 cells. Nutr. Res. Pract. 2022;16:147–160. doi: 10.4162/nrp.2022.16.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim M., Sung B., Kang Y.J., Kim D.H., Lee Y., Hwang S.Y., Yoon J.H., Yoo M.A., Kim C.M., Chung H.Y., et al. The combination of ursolic acid and leucine potentiates the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway. Int. J. Mol. Med. 2015;35:755–762. doi: 10.3892/ijmm.2014.2046. [DOI] [PubMed] [Google Scholar]
- 196.Noh K.K., Chung K.W., Sung B., Kim M.J., Park C.H., Yoon C., Choi J.S., Kim M.K., Kim C.M., Kim N.D., et al. Loquat (Eriobotrya japonica) extract prevents dexamethasone-induced muscle atrophy by inhibiting the muscle degradation pathway in Sprague Dawley rats. Mol. Med. Rep. 2015;12:3607–3614. doi: 10.3892/mmr.2015.3821. [DOI] [PubMed] [Google Scholar]
- 197.Sung B., Hwang S.Y., Kim M.J., Kim M., Jeong J.W., Kim C.M., Chung H.Y., Kim N.D. Loquat leaf extract enhances myogenic differentiation, improves muscle function and attenuates muscle loss in aged rats. Int. J. Mol. Med. 2015;36:792–800. doi: 10.3892/ijmm.2015.2286. [DOI] [PubMed] [Google Scholar]
- 198.Cho Y.H., Lee S.Y., Kim C.M., Kim N.D., Choe S., Lee C.H., Shin J.H. Effect of Loquat Leaf Extract on Muscle Strength, Muscle Mass, and Muscle Function in Healthy Adults: A Randomized, Double-Blinded, and Placebo-Controlled Trial. Evid. Based Complement. Altern. Med. 2016;2016:4301621. doi: 10.1155/2016/4301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Park J., Han S., Park H. Effect of Schisandra Chinensis Extract Supplementation on Quadriceps Muscle Strength and Fatigue in Adult Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Environ. Res. Public Health. 2020;17:2475. doi: 10.3390/ijerph17072475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim K.Y., Ku S.K., Lee K.W., Song C.H., An W.G. Muscle-protective effects of Schisandrae Fructus extracts in old mice after chronic forced exercise. J. Ethnopharmacol. 2018;212:175–187. doi: 10.1016/j.jep.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 201.Kim J.W., Ku S.K., Han M.H., Kim K.Y., Kim S.G., Kim G.Y., Hwang H.J., Kim B.W., Kim C.M., Choi Y.H. The administration of Fructus Schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015;36:29–42. doi: 10.3892/ijmm.2015.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cao S., Shang H., Wu W., Du J., Putheti R. Evaluation of anti-athletic fatigue activity of Schizandra chinensis aqueous extracts in mice. Afr. J. Pharm. Pharmacol. 2009;3:593–597. doi: 10.5897/AJPP.9000143. [DOI] [Google Scholar]
- 203.Kim J.W., Ku S.K., Kim K.Y., Kim S.G., Han M.H., Kim G.Y., Hwang H.J., Kim B.W., Kim C.M., Choi Y.H. Schisandrae Fructus Supplementation Ameliorates Sciatic Neurectomy-Induced Muscle Atrophy in Mice. Oxid. Med. Cell. Longev. 2015;2015:872428. doi: 10.1155/2015/872428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yeon M., Choi H., Jun H.S. Preventive Effects of Schisandrin A, A Bioactive Component of Schisandra chinensis, on Dexamethasone-Induced Muscle Atrophy. Nutrients. 2020;12:1255. doi: 10.3390/nu12051255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yoo A., Ahn J., Kim M.J., Seo H.D., Hahm J.H., Jung C.H., Ha T.Y. Fruit of Schisandra chinensis and its bioactive component schizandrin B ameliorate obesity-induced skeletal muscle atrophy. Food Res. Int. 2022;157:111439. doi: 10.1016/j.foodres.2022.111439. [DOI] [PubMed] [Google Scholar]
- 206.Bagherniya M., Mahdavi A., Shokri-Mashhadi N., Banach M., Von Haehling S., Johnston T.P., Sahebkar A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle. 2022;13:2772–2790. doi: 10.1002/jcsm.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Morley J.E. Sarcopenia: Diagnosis and treatment. J. Nutr. Health Aging. 2008;12:452–456. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 208.Sayer A.A., Cruz-Jentoft A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing. 2022;51:afac220. doi: 10.1093/ageing/afac220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.World Health Organization Ageing and Health. [(accessed on 31 May 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 210.Papadopoulou S.K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients. 2020;12:1293. doi: 10.3390/nu12051293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kwak J.Y., Kwon K.S. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann. Geriatr. Med. Res. 2019;23:98–104. doi: 10.4235/agmr.19.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Semenova E.A., Pranckevičienė E., Bondareva E.A., Gabdrakhmanova L.J., Ahmetov I.I. Identification and Characterization of Genomic Predictors of Sarcopenia and Sarcopenic Obesity Using UK Biobank Data. Nutrients. 2023;15:758. doi: 10.3390/nu15030758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ye Y., Noche R.B., Szejko N., Both C.P., Acosta J.N., Leasure A.C., Brown S.C., Sheth K.N., Gill T.M., Zhao H., et al. A genome-wide association study of frailty identifies significant genetic correlation with neuropsychiatric, cardiovascular, and inflammation pathways. Geroscience. 2023. online ahead of print . [DOI] [PMC free article] [PubMed]