Abstract
The short-chain fatty acid metabolite acetyl-coenzyme A (acetyl-CoA) has emerged as a major signal transducer that can broadly affect cell fate and function, at least partly by influencing acetylation of key proteins. The mechanism by which acetyl-CoA regulates CD4+ T-cell fate determination remains poorly understood. Herein, we report that acetate modulates glyceraldehyde-3-phosphate dehydrogenase (GAPDH) acetylation and CD4+ T helper 1 (Th1) cell differentiation by altering acetyl-CoA levels. Our transcriptome profiling shows that acetate is a robust positive regulator of CD4+ T-cell gene expression typical of glycolysis. We further show that acetate potentiates GAPDH activity, aerobic glycolysis, and Th1 polarization through regulation of GAPDH acetylation levels. This acetate-dependent GAPDH acetylation occurs in a dose- and time-dependent manner, while decreasing acetyl-CoA levels by fatty acid oxidation inhibition results in a decline in acetyl-GAPDH levels. Thus, acetate functions as a potent metabolic regulator in CD4+ T-cells by promoting GAPDH acetylation and Th1 cell fate decision.
INTRODUCTION
Acetyl-coenzyme A (acetyl-CoA) is a central metabolic intermediate and represents a key node connecting cellular catabolic and anabolic processes (Pietrocola et al., 2015; Xiong, 2018a,b). There is increasing evidence that acetyl-CoA operates as a second messenger that signals the program of cell fate determination by functioning as the sole donor of the acetyl groups for acetylation (Wellen et al., 2009; Cai et al., 2011; Lee et al., 2014; Moussaieff et al., 2015). Addition of the short-chain free fatty acid precursor acetate to the culture medium can directly increase cellular acetyl-CoA levels through the action of acyl-CoA synthetase short-chain family member 2 (ACSS2), and this in turn can broadly affect cell fate and function by influencing the patterns of protein acetylation (Balmer et al., 2016; Wong et al., 2017; Xiong et al., 2018; Moffett et al., 2020; Lyu et al., 2022). Although metabolic rewiring is intrinsically linked to T-cell differentiation and function (Geltink et al., 2018), the role of acetate and hence that of acetyl-CoA levels in CD4+ T-cell fate commitment are largely unknown.
T-cells are central to adaptive immunity, and they can develop into either CD8+ or CD4+ subtypes (Bantug et al., 2018). CD4+ T-cells are also named as helper T-cells because they help coordinate immune responses by regulating other immune and nonimmune cells (Dong, 2021). CD4+ T-cells experience dynamic and adaptive metabolic remodeling through their life cycles (Bantug et al., 2018; Jung et al., 2019). Upon activation, naive CD4+ T-cells differentiate into distinct effector cell subsets that produce immune mediators such as cytokines. For example, the T helper type 1 (Th1) is a major subtype of helper T-cells. Th1 cells primarily secrete interferon γ (IFN-γ) (Dong, 2021). A metabolic hallmark of activated T-cells and Th1 differentiation is aerobic glycolysis (the Warburg effect), the conversion of glucose to lactate in the presence of oxygen (Peng et al., 2016; Geltink et al., 2018; Menk et al., 2018). Aerobic glycolysis is made up of a series of biochemical reactions driven by many metabolic enzymes, including glyceraldehyde-3-phosphate dehydrogenase (GAPDH). GAPDH is a rate-limiting enzyme and among the most abundantly expressed glycolytic enzymes (Shestov et al., 2014; Wisniewski et al., 2015).
In this report, we present a series of studies aimed at demonstrating a novel role for acetate in promoting GAPDH acetylation, aerobic glycolysis, and Th1 polarization. To begin, we looked at whether the addition of acetate to the cell culture medium would enhance CD4+ T-cell aerobic glycolysis, a process dependent on the activity of GAPDH. We then examined, through dose- and time-dependent regulation of acetyl-GAPDH levels, the role that changing the levels of acetyl-CoA plays in modulating GAPDH activity. Finally, we performed gene ontology analysis to demonstrate that acetate-mediated GAPDH activity is essential for the regulation of Th1 polarization.
RESULTS AND DISCUSSION
Acetate modulates GAPDH activity and aerobic glycolysis in CD4 + T-cells
Acetyl-CoA is both a key central metabolite and an important signal transducer (Pietrocola et al., 2015). Thus, altering acetyl-CoA levels not only directly affects cellular metabolism but has broad effects on gene expression, protein function, and cell fate (Balmer et al., 2016; Wong et al., 2017; Xiong et al., 2018; Lyu et al., 2022). We therefore reasoned that supplementing the culture media with the acetyl-CoA precursor acetate might modulate the gene expression profile and cell metabolism in CD4+ T-cells. We found that treating isolated primary mouse CD4+ T-cells (activated with anti-CD3/CD28 antibodies) with acetate increased total cellular acetyl-CoA levels (Figure 1, A and B). Moreover, we performed a transcriptome profile in primary mouse CD4+ T-cells cultured in the presence or absence of acetate, and surprisingly, this analysis revealed that acetate is a strong inducer of CD4+ T-cell gene expression, as evidenced by a marked alternation in global gene expression (Figure 1, C and D, and Supplemental Table S1).
FIGURE 1:
Acetate promotes CD4+ T-cell glycolysis by potentiating the activity of GAPDH. (A) Flow cytometric analysis of CD4+ T-cell purity in isolated primary mouse CD4+ T-cells (results are representative of three independent experiments). The x-axis represents CD4 and the y-axis side scatter (SSC). (B) Levels of acetyl-CoA in control or acetate-treated primary mouse CD4+ T-cells (n = 3 independent experiments). (C, D) Volcano plot (C) and tabular form (D) of significantly differentially expressed genes in primary mouse CD4+ T-cells with or without acetate treatment for 24 h following 3-d anti-CD3/CD28 activation. The −log10(adjusted p values) were plotted against the log2(fold change) in gene expression. Up-regulated genes in CD4+ T-cells (n = 6237; log2[fold change] > 0.5; adjusted p value < 0.05) are depicted as red dots, while down-regulated genes (n = 34; log2[fold change] < –0.5; adjusted p value < 0.05) depicted in blue (n = 3 biological replicates). (E) Heat map of RNA-Seq data for the genes in the KEGG pathway “Glycolysis/gluconeogenesis” enriched by highly up-regulated genes (fold change > 3; adjusted p value < 0.05; number = 194). (F) Glycolysis analysis of primary mouse CD4+ T-cells following acetate treatment for 3 d (n = 4 biological replicates). (G, H) Analyses of GAPDH activity (G) and glycolysis (H) in primary mouse CD4+ T-cells with or without treatment of cells with acetate and the GAPDH inhibitor heptelidic acid (HA) as indicated for 3 d (n = 4 biological replicates). Data represent mean ± SEM and significance via one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test (F–H). *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant.
To meet the metabolic needs, both energy and carbon, for gene expression and proliferation, T-cell activation engages aerobic glycolysis, where pyruvate is fermented to lactate in the presence of oxygen rather than being oxidized in mitochondria to support anabolic demands (Lunt and Vander Heiden, 2011; Chang et al., 2013; Menk et al., 2018). Interestingly, a Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the highly up-regulated genes in our RNA-sequencing (RNA-Seq) data pinpointed a significant change for “glycolysis” (Figure 1, C and E, and Supplemental Table S1). In this context, we hypothesized that acetate might reinforce glycolysis in CD4+ T-cells to fulfill the bioenergetic demands of global gene expression. Our metabolic assays confirmed that acetate treatment enhanced lactate production in a dose-dependent manner (Figure 1F).
Because our KEGG analysis showed an increase in glycolysis-associated genes (Figure 1, C and E), we next asked how acetate could augment aerobic glycolysis. Because the abundantly expressed enzyme GAPDH plays a rate-controlling and obligate role in aerobic glycolysis (Shestov et al., 2014; Wisniewski et al., 2015), we reasoned that acetate treatment might help to drive CD4+ T-cell glycolysis by increasing GAPDH enzymatic activity. Our results show that acetate supplementations did increase the activity of GAPDH and hence glycolysis (Figure 1, G and H). In contrast, after treatment with the potent, selective, irreversible GAPDH inhibitor, heptelidic acid (HA) (Balmer et al., 2016), these metabolic effects of acetate were absent (Figure 1, G and H). These results suggest that acetate supplementation can increase the intracellular pool of acetyl-CoA and promote GAPDH-associated aerobic glycolysis.
Acetate modulates GAPDH acetylation in a dose- and time-dependent manner
To examine the mechanisms by which acetate might control GAPDH activity, we took note of the fact that acetylation of GAPDH increases its catalytic activity (Li et al., 2014; Balmer et al., 2016). Thus, we next explored the possibility that acetate mechanistically exerts an effect on acetylation of GAPDH (Figure 2A). As expected, exogenous acetate supplementation in primary mouse CD4+ T-cells resulted in a dose- and time-dependent increase in the levels of GAPDH acetylation (Figure 2, B and C).
FIGURE 2:
Acetate induces a dose- and time-dependent acetylation of GAPDH. (A) Schematic illustration showing that acetate replenishes cellular acetyl-CoA pools through the action of ACSS2 and thus increases the levels of GAPDH acetylation. Ac, acetylation. ACSS2, acyl-CoA synthetase short chain family member 2. (B, C) Western blot analysis of GAPDH acetylation in primary mouse CD4+ T-cells following acetate treatment for 24 h, in a dose-dependent (B) and time-dependent (C) manner (n = 3 independent experiments). (D, E) Western blot (D) and quantification (E) of GAPDH acetylation in human Jurkat T-cells following acetate treatment for 24 h in a dose-dependent manner. (F, G) Western blot (F) and quantification (G) of GAPDH acetylation in human Jurkat T-cells with treatment of acetate at a final concentration of 10 mM in a time-dependent manner. *p < 0.05; ***p < 0.001.
To further pursue the implications of these observations in human T-cells, we first used an immortalized human CD4+ T-cell line, Jurkat. We found an apparent dose- and time-dependent effect of acetate on GAPDH acetylation in Jurkat cells (Figure 2, D–G) that was consistent with the effect on primary mouse CD4+ T-cell acetyl-GAPDH levels. Similar results were obtained in primary human CD4+ T-cells and primary human total activated T-cells from peripheral blood mononuclear cells (PBMCs) (Supplemental Figure S1, A–E). Our findings support a role for acetate in mediating GAPDH acetylation that is dependent on both dose and time.
Acetyl-CoA levels modulate GAPDH acetylation
Our recent work, together with other studies, has demonstrated that mitochondrial fatty acid β-oxidation (FAO) is essential to maintaining cellular acetyl-CoA levels through the tricarboxylic acid (TCA) cycle flux (Wong et al., 2017; Xiong et al., 2018). The rate-limiting step in FAO is the carnitine shuttle that transports fatty acyl-CoA from the cytoplasm into the mitochondrial matrix and requires the enzymatic activity of carnitine palmitoyltransferase 1A (CPT1A) (Houten et al., 2016). Accordingly, inhibition of CPT1A expression causes a fall in cellular acetyl-CoA levels (Wong et al., 2017; Xiong et al., 2018).
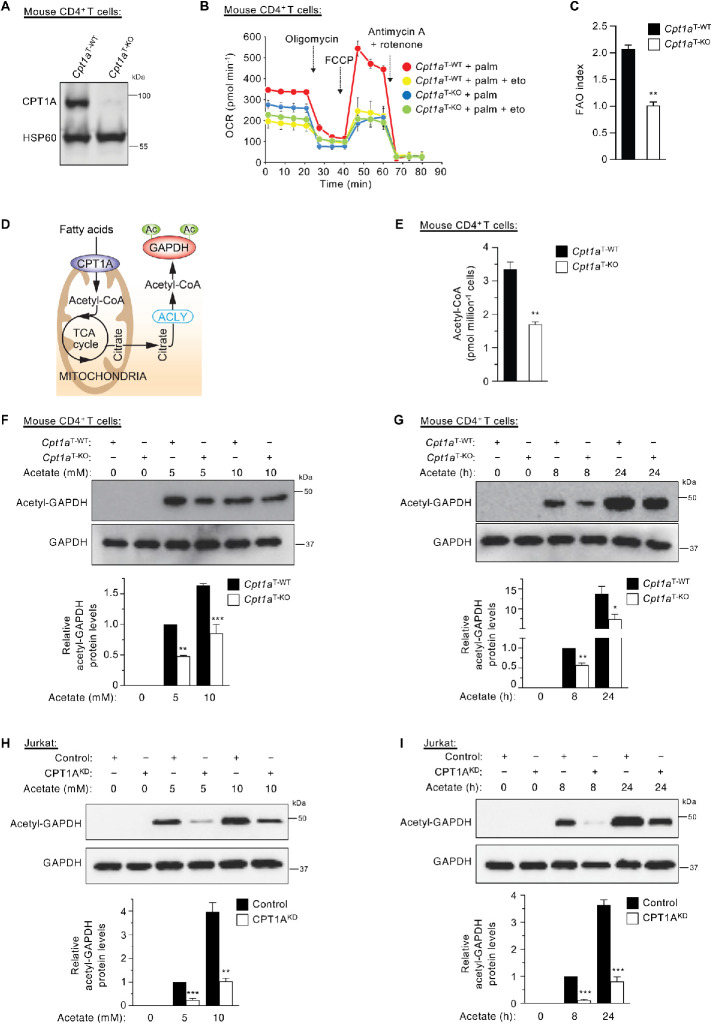
To better understand the importance of acetate and hence acetyl-CoA levels in GAPDH acetylation, we generated floxed Cpt1a (Cpt1aflox/flox) mice that have loxP sites flanking exon 4 of the Cpt1a gene (Supplemental Figure S2A). Then we bred Cpt1aflox/flox mice with CD4-Cre transgenic mice to generate T-cell–specific Cpt1a knockout (KO) (Cpt1aT-KO) mice (Supplemental Figure S2, B and C). Analysis of viable CD4-Cre–positive offspring from CD4 Cre+/–; Cpt1aflox/flox and Cpt1aflox/flox intercrosses revealed the expected percentage of viable mice for each possible genotype, indicating that there is no significant lethality associated with this KO in unchallenged mice (Supplemental Figure S2B). As anticipated, expression of CPT1A was markedly reduced in primary CD4+ T-cells isolated from Cpt1aT-KO animals (Figure 3A and Supplemental Figure S2C). Moreover, whereas wild-type (WT) CD4+ T-cells responded to an exogenous fatty acid (palmitate) challenge with an increase in their oxygen consumption rate (OCR), this metabolic response was not present in primary CD4+ T-cells derived from Cpt1aT-KO mice (Figure 3, B and C). In addition, total cellular acetyl-CoA levels were reduced by approximately 50% in primary CD4+ T-cells isolated from Cpt1aT-KO mice (Figure 3, D and E).
FIGURE 3:
Acetyl-CoA levels regulate GAPDH acetylation. (A) Western blot analysis of CPT1A expression in primary CD4+ T-cells derived from Cpt1aT-WT or Cpt1aT-KO mice. (B) Seahorse analysis of FAO as measured by the ability of palmitate (palm) to stimulate OCR in the presence or absence of the inhibitor etomoxir (eto) in primary CD4+ T-cells derived from Cpt1aT-WT or Cpt1aT-KO mice. (C) Quantifying FAO using the FAO index, calculated as a ratio of the FCCP-stimulated OCR in the presence of palmitate to the FCCP-stimulated OCR in the presence of palmitate plus the inhibitor etomoxir in primary CD4+ T-cells derived from Cpt1aT-WT or Cpt1aT-KO mice. (D) Schematic illustration showing how FAO generates acetyl-CoA through the action of CPT1A and then increases GAPDH acetylation. ACLY, ATP citrate lyase. TCA, tricarboxylic acid. (E) Levels of acetyl-CoA in primary mouse CD4+ T-cells derived from Cpt1aT-WT or Cpt1aT-KO mice (n = 3 independent experiments). (F, G) Western blot analysis of GAPDH acetylation in primary mouse CD4+ T-cells derived from Cpt1aT-WT or Cpt1aT-KO mice following treatment of cells with or without acetate for 24 h in a dose-dependent manner (F) and with or without 10 mM acetate in a time-dependent manner (G). (H, I) Western blot analysis of GAPDH acetylation levels in control or CPT1AKD human Jurkat T-cells following treatment of cells with or without acetate for 24 h in a dose-dependent manner (H) and with or without 10 mM acetate in a time-dependent manner (I). *p < 0.05; **p < 0.01; ***p < 0.001.
We next asked whether the loss of CPT1A might suppress GAPDH acetylation. While levels of endogenous acetyl-GAPDH were almost below the detection limit of our immunoblotting, the levels of acetylated GAPDH in cells treated with exogenous acetate were reduced in CPT1A-deficient CD4+ T-cells. This effect was modulated in a dose- and time-dependent manner (Figure 3, F and G). Furthermore, we examined this effect in human cells. We took advantage of small hairpin RNAs (shRNAs) to stably knock down CPT1A (CPT1AKD) in Jurkat T-cells (Supplemental Figure S2, D and E). After treatment with acetate, Jurkat T-cells with reduced expression of CPT1A showed a marked dose- and time-dependent decrease in acetyl-GAPDH levels (Figure 3, H and I). When CPT1A was knocked down in primary human total activated T-cells, an increase in acetyl-GAPDH followed acetate treatment and the levels of GAPDH catalytic activity were reduced (Supplemental Figure S2, F–J). Taken together, these data indicate that manipulation of cellular acetyl-CoA levels by exogenous acetate administration can regulate GAPDH acetylation, GAPDH activity and aerobic glycolysis.
Acetate modulates Th1 cell differentiation via the activity of GAPDH
To dissect the roles of acetate in regulating CD4+ T-cell fate and function, we used gene ontology analysis to categorize the genes that were regulated by exogenous acetate in our RNA-Seq data (Supplemental Table S1). The most highly associated biological processes in red are all important for T-cell differentiation (Figure 4A). Th1 cell polarization and its associated effector functions are dependent on a shift to aerobic glycolysis (Chang et al., 2013; Peng et al., 2016; Geltink et al., 2018). Moreover, IFN-γ is a signature cytokine produced by Th1 cells and associated with their functions (Ren et al., 2020; Dong, 2021). Thus, we measured the levels of Ifng mRNA encoding IFN-γ and the amount of secreted IFN-γ proteins. Acetate treatment enhanced the stimulation of both Ifng mRNA and secreted IFN-γ protein (Supplemental Figure S3, A and B).
FIGURE 4:
Acetate promotes Th1 cell differentiation. (A) RNA-Seq bubble plot depicts top 10 enriched gene ontology (GO) biological processes for the highly up-regulated genes in acetate-treated primary mouse CD4+ T-cells (fold change > 3; adjusted p value < 0.05; number = 194). (B) Levels of IFN-γ, TNF-α, and IL-2 production from differentiating Th1 cell culture on day 3, where acetate or the GAPDH inhibitor heptelidic acid (HA) was added as indicated on day 0 (n = 6 biological replicates). (C, D) Flow cytometric analysis (D) and quantification (E) of the percentage of CD4+ IFN-γ+ cells under Th1 polarization condition. After 3-d Th1 polarization culture, cells were treated with or without acetate and/or GAPDH inhibitor heptelidic acid (HA) for 2 d under the Th1 polarization condition (n = 6 biological replicates). The x-axis represents CD4 and the y-axis IFN-γ. (E) Model for how acetate, a regulator of acetyl-CoA levels, substantially increases GAPDH acetylation and glycolysis, promoting Th1 cell differentiation. Ac, acetylation. Data represent mean ± SEM and significance via one-way ANOVA with Tukey’s multiple comparison test (B and D). *p < 0.05; **p < 0.01; ***p < 0.001.
To further investigate the role of acetate and GAPDH in Th1 cell fate determination, we showed that increasing or decreasing GAPDH activity by treatment with acetate or the GAPDH inhibitor resulted in a corresponding increase or decrease in the levels of IFN-γ and other Th1 effector cytokines, IL-2 and tumor necrosis factor α (TNF-α), as well as its critical transcription factor, T-bet, in differentiating Th1 cells (Figure 4B and Supplemental Figure S3C). Similar results were obtained in Th1-polarized T-cells (Supplemental Figure S3D). Moreover, acetate treatment had no effect on the survival and proliferation of differentiating Th1 cells (Supplemental Figure S3, E and F). In accordance with these findings, our flow cytometric analysis of single T-cells revealed that acetate supplementation significantly increased the percentage of CD4+IFN-γ+ cells, while treatment with the GAPDH inhibitor reduced the levels of Th1 polarization (Figure 4, C and D).
In contrast, exogenous acetate did not affect Th2 differentiation and the differentiation rate of FoxP3+CD25+ regulatory T-cells (Treg) (Supplemental Figure S3, G–I). These results are consistent with recent observations that different Th cell subsets exhibit distinct metabolic requirements (Geltink et al., 2018; Stark et al., 2019). On the basis of these results, we propose a model in which the addition of acetate results in a rise in acetyl-CoA levels and a subsequent increase in GAPDH acetylation and activity, leading to an elevation in aerobic glycolysis, which results in enhanced Th1 cell differentiation (Figure 4E).
Recent studies of intracellular switching between proinflammatory IFN-γ and immunosuppressive interleukin-10 (IL-10) in Th1 cells provide evidence for a more complex interaction of molecular pathways that control Th1 life cycle and functional T-cell plasticity (Bluestone et al., 2009; Zhu et al., 2010; Cope et al., 2011). In this regard, we observed an increased expression of IL-10 following relatively short-term (24 h) treatment with acetate (Supplemental Table S1). Although our data linked acetate to Th1 cell differentiation through the regulation of GAPDH acetylation, we cannot exclude the possibility that acetate and hence acetyl-CoA levels might have additional epigenetic effects on CD4+ T-cells (e.g., through acetylation of histones or RNAs) (Zhang and Cao, 2019). For example, acetate/acetyl-CoA could promote Ifng transcription through histone acetylation (Peng et al., 2016). Despite this caveat, our results offer key mechanistic clues that influence Th1 differentiation.
Collectively, our results warrant further studies on the potential role of acetate in Th1 cell differentiation and function in additional physiological and pathophysiological conditions. Given the growing appreciation that altered T-cell metabolism underlies many pathological conditions (Bantug et al., 2018), our results suggest that therapeutic manipulation of acetate production and levels could potentially provide important new approaches for a wide range of T-cell–associated diseases such as cardiovascular disease, autoimmune disease, and cancer. In summary, we demonstrate that the addition of acetate to cells and the corresponding increase in cellular acetyl-CoA levels regulate Th1 cell metabolism and differentiation. Dysregulation of Th1 cell differentiation may result in susceptibility to a variety of immune-related diseases such as autoimmune diabetes (Hung et al., 2005; Ren et al., 2020; Dong, 2021).
MATERIALS AND METHODS
Animals
Cpt1aflox/flox mice (Strain#: 035711; Jackson Laboratory) were crossed with CD4 Cre transgenic mice (Strain #:017336; Jackson Laboratory) to generate CD4 Cre+/-; Cpt1aflox/flox mice (Cpt1aT-KO) containing a T-cell–specific deletion of CPT1A and corresponding controls (Cpt1aflox/flox, designated WT, Cpt1aT-WT). The above-mentioned mice were on a C57BL/6J background. All mice were genotyped by standard PCR-based methods and housed in a pathogen-free animal facility at the Johns Hopkins University School of Medicine. Both female and male mice from 6 to 12 wk old were used in experiments, and all experiments were approved by the Johns Hopkins Animal Care and Use Committee.
Cell culture
Mouse CD4+ and naive CD4+ T-cells were isolated from mouse spleen and lymph nodes using EasySep cell isolation kits (#19852, #19853, and #19765, respectively; STEMCELL Technologies). Mouse T-cells were cultured in RPMI 1640 (R8758; Sigma-Aldrich) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; HyClone), l-glutamine (2 mM), 2-mercaptoethanol (55 μM), 100 U/ml penicillin, 100 μg/ml streptomycin, and recombinant human IL-2 (rhIL-2; 100 U/ml). Mouse CD4+ T-cells were activated with plate-bound anti-CD3ε (2 μg/ml; BioLegend) plus soluble anti-CD28 (1 μg/ml; BioLegend) for 3 d. For Th1 cell polarization, 5 × 105 mouse naive CD4+ T-cells were cultured with plate-bound anti-CD3ε (2 μg/ml; BioLegend), soluble anti-CD28 (1 μg/ml; BioLegend), anti-mouse IL-4 (10 μg/ml), rhIL-2 (100 U/ml), and recombinant mouse IL-12 (10 ng/ml). For Th2 cell polarization, 5 × 105 mouse naive CD4+ T-cells were cultured with plate-bound anti-CD3ε (2 μg/ml), soluble anti-CD28 (1 μg/ml), rhIL-2 (100 U/ml), anti-mouse IFN-γ (10 μg/ml), and mouse IL-4 (20 ng/ml). For Treg cell polarization, 5 × 105 mouse naive T-cells were cultured in the presence of plate-bound anti-CD3ε (2 μg/ml), soluble anti-CD28 (1 μg/ml), rhIL-2 (100 U/ml), and rhTGF-β1 (5 ng/ml). The GAPDH inhibitor heptelidic acid was used at a concentration of 0.5 μM for Th1 cells or 10 μM for mouse CD4+ T-cells. Acetate (S5636; Sigma-Aldrich) was used at a final concentration of 10 mM unless mentioned otherwise. Because glycolysis and T-cell effector function are affected by changes in cellular pH (Wu et al., 2020), an acetate solution (pH 7.4) with the same pH as culture media was used. Unless mentioned otherwise, on day 3 under 5-d polarization conditions, acetate or heptelidic acid was added as indicated for 2 d. For differentiating Th cells under 3-d cell polarization, acetate or heptelidic acid was added as indicated on day 0. Trypan blue solution (15250061; Thermo Fisher Scientific) was used as a cell stain (1:1 dilution of 0.4% solution) to assess cell viability using the dye exclusion test. Metabolic active cells were incubated with alamarBlue (resazurin) dye (10%; DAL1025; Thermo Fisher Scientific) for 2 h to quantitatively analyze cell proliferation.
Human PBMCs were obtained from healthy donors under the Johns Hopkins Institutional Review Board approved protocol (JHMI-IRB00175372). All protocols followed local ethics recommendations, and informed consent was obtained. PBMCs were isolated using Lymphocyte Separation Medium (25-072-CV; Corning). Human CD4+ T-cells were isolated from PBMCs using the MojoSort Human CD4 T Cell Isolation Kit (#480010; BioLegend). Human T-cells were activated using Dynabeads Human T-Activator CD3/CD28 (11131D; Thermo Fisher Scientific) for 3 d and then expanded in RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated FBS, l-glutamine (2 mM), rhIL-2 (100 U/ml), 100 U/ml penicillin, and 100 μg/ml streptomycin. Human 293T cells (Clontech) and Jurkat cells (American Type Culture Collection) and were cultured in DMEM (D5796; Sigma-Aldrich) and RPMI 1640 medium (R8758; Sigma), respectively, supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Western blotting
For analysis of acetyl-GAPDH and GAPDH protein levels, T-cells were collected in RIPA Lysis Buffer (BP-115; Boston Bioproducts) supplemented with Protease Inhibitor Cocktail (Roche) and Phosphatase Inhibitor Cocktail (Roche). For analysis of CPT1A and HSP60 protein levels, mitochondrial proteins were isolated as previously described (Liao et al., 2020). Briefly, cells were washed with cold phosphate-buffered saline (PBS) and homogenized with a Teflon-glass homogenizer in extraction buffer (0.25 M sucrose, 20 mM HEPES-KOH, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride [PMSF]), and then centrifuged at 750 × g for 10 min. The supernatants were collected and then centrifuged at 10,000 × g for 15 min, after which crude mitochondria were recovered in the pellet using 20–30 μl of extraction buffer. All protein lysates were separated by SDS–PAGE and then transferred to a 0.2 μm nitrocellulose membrane using the Trans-Blot Turbo Transfer System (Bio-Rad). Following blocking using Blotting-Grade Blocker (#1706404; Bio-Rad), the membranes were incubated with the indicated primary antibodies: rabbit polyclonal anti–acetyl-GAPDH (Lys-160) (#07-2184; Sigma-Aldrich; affinity purified; validated for use in Western blotting detection of acetyl-GAPDH [Lys-160]; species reactivity includes human and mouse), rabbit monoclonal anti-GAPDH (#5174; Cell Signaling Technology; produced by immunizing animals with a synthetic peptide corresponding to residues near the carboxy terminus of human GAPDH; validated for use in Western blotting detection of endogenous levels of total GAPDH protein; species reactivity includes human and mouse), goat polyclonal anti-HSP60 (sc-1052; Santa Cruz Biotechnology; raised against recombinant HSP 60 of human origin, with epitope mapping to amino acids 383–447; validated for use in Western blotting detection of HSP60; species reactivity includes human and mouse), mouse monoclonal anti–α-tubulin (T5168; Sigma-Aldrich; validated for use in Western blotting detection of α-tubulin; species reactivity includes human and mouse), mouse monoclonal anti–T-bet (50-5825-80; Thermo Fisher Scientific; validated for use in Western blotting detection of T-bet; species reactivity includes human and mouse), and mouse monoclonal anti-CPT1A (ab128568; Abcam; immunogen is recombinant fragment, corresponding to amino acids 489–773 of human CPT1A; this monoclonal antibody to CPT1A has been KO validated in Western blot; species reactivity includes human and mouse).
Real-time qRT-PCR
Total RNAs from T-cells were extracted using Direct-zol RNA Miniprep Plus (R2072; ZYMO). cDNA was prepared using the iScript cDNA Synthesis Kit (1708891 Bio-Rad). Real-time quantitative PCR (qPCR) was performed on a CFX Connect Real-Time System (Bio-Rad) using THUNDERBIRD SYBR qPCR mix (QPS-201 Toyobo). Data were analyzed using the comparative threshold cycle (Ct)method. The following primers were synthesized by Integrated DNA Technologies (Coralville, IA) to detect gene expression: 18S rRNA, forward primer, 5′-GTAACCCGTTGAACCCCATT-3′, and reverse primer 5′-CCATCCAATCGGTAGTAGCG-3′; human CPT1A, forward primer, 5′-TCATCAAGAAATGTCGCACG-3′, and reverse primer, 5′-GCCTCGTATGTGAGGCAAAA-3′; mouse Cpt1a, forward primer, 5′-AGATCAATCGGACCCTAGACACCAC-3′, and reverse primer, 5′- AGAAGACCTTGACCATAGCCATCCA-3′; mouse Ifng, forward primer, 5′-ATGAACGCTACACACTGCATC-3′, and reverse primer, 5′-CCATCCTTTTGCCAGTTCCTC-3′.
Flow cytometry
Monoclonal antibodies specific to the following mouse antigens were purchased from BioLegend or eBioscience: FITC-anti-CD4 (GK1.5), PE-anti-IFN-γ (XMG1.2), eFluor450-anti-FOXP3(FJK-16s), and PE/Cyanine5-anti-CD25 (PC61). For staining of cell surface markers, 106 cells were stained in 50 μl of staining buffer (PBS containing 1% bovine serum albumin [BSA] and 0.09% azide). For intracellular cytokine staining, cells were stimulated with phorbol 12-myristate 13-acetate (PMA; 500 ng/ml; Sigma-Aldrich; P1839) and ionomycin (1 μg/ml; Sigma-Aldrich) for 2 h, followed by protein transport inhibitor (BD GolgiStop; 554715; BD Biosciences) for 4 h, and then fixed and permeabilized with the BD Cytofix/Cytoperm Kit (554714; BD Biosciences) before antibody staining. Data were acquired and analyzed using a CytoFLEX Flow Cytometer (Beckman Coulter, Brea, CA) and FlowJo software (Version 10.8.1; BD Biosciences).
Plasmids and lentiviral vectors
The lentiviral vector shCPT1A and a scrambled shRNA control (plasmid #1864; Addgene) were used for stable shRNA-mediated knockdown (KD) of human CPT1A gene expression as previously described (Xiong et al., 2015, 2018). Plasmids were purified using the ZymoPURE Plasmid Miniprep Kit (Zymo Research; D4212) according to the manufacturer’s instructions. Lentiviral supernatants were collected 48 h after transfection of 293T cells. For CPT1A KD, cells were spin infected with lentivirus at 600 × g, 32°C, for 2 h in the presence of polybrene (8 μg/ml; sc-134220; Santa Cruz Biotechnology), followed by incubation and selection with puromycin (2 μg/ml). CPT1A gene expression were measured at 24–48 h post–lentiviral infection.
Analysis of GAPDH activity and lactate and cytokine production
GAPDH activity was measured using a GAPDH activity assay kit (ab204732; Abcam) according to manual instructions. Briefly, cells were lysed in ice-cold assay buffer for 10 min and then the supernatants were collected by centrifugation at 10,000 × g for 5 min at 4°C, followed by incubation with GAPDH developer and substrate mix. The output was measured at OD450nm on a Cytation 1 microplate reader (BioTek). Lactate production was measured using a l-lactate assay kit (#1200012002; Eton Bio). Briefly, 50 μl of cell supernatants and l-lactate assay solution were mixed and then incubated for 30 min at 37°C. The absorbance was measured at 490 nm on a microplate reader. The production of IFN-γ, IL-2, IL-4, and TNF-α was measured using a solid-phase sandwich enzyme-linked immunosorbent assay (Sandwich ELISA) kit (KE10001, KE10004, KE10010, and KE10002, respectively; Proteintech) according to the manufacturer’s instructions.
Metabolic assays
FAO of activated T-cells was assessed using the XF Palmitate-BSA FAO substrates and a Seahorse XFe96 Analyzer (Agilent). FAO was measured in FAO assay medium (111 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 2.0 mM MgSO4, 1.2 mM Na2HPO4, 2.5 mM glucose, 0.5 mM carnitine, and 5 mM HEPES, pH 7.4). Cells were plated on XF96 cell culture microplates at a density of 2 × 105 cells per well and during the assay were exposed to 5 μM oligomycin, 1.5 μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), and 1 μM rotenone and antimycin, as indicated. FAO index was calculated as previously described (De Rosa et al., 2015). Acetyl-CoA levels were measured using a PicoProbe acetyl-CoA fluorometric assay kit (K317-100; BioVision).
RNA-Seq analysis
Activated mouse CD4+ T-cells were treated with or without acetate for 24 h, and then total RNA was extracted from cells using the Direct-zol RNA Miniprep Plus kit (R2073; Zymo). The sequencing libraries were constructed from 500 ng of total RNA using the Zymo-Seq RiboFree Total RNA Library Kit (R3000; Zymo), and the concentrations of RNA-Seq libraries were determined using a Qubit 4 instrument (Q33239; Thermo Fisher Scientific). RNA-Seq libraries were sequenced on an Illumina NovaSeq to a sequencing depth of at least 30 million read pairs (150 base pair paired-end sequencing) per sample. The Zymo Research RNA-Seq pipeline was originally adapted from the nf-core/rnaseq pipeline v1.4.2. The pipeline was built using Nextflow. Briefly, quality control of raw reads was carried out using FastQC v0.11.9. Adapter and low-quality sequences were trimmed from raw reads using Trim Galore! v0.6.6. Trimmed reads were aligned to the reference genome using STAR v2.6.1d. BAM file filtering, and indexing was carried out using SAMtools v1.9. RNAseq library quality control was implemented using RSeQC v4.0.0 and QualiMap v2.2.2-dev. Duplicate reads were marked using Picard tools v2.23.9. Library complexity was estimated using Preseq v2.0.3. Duplication rate quality control was performed using dupRadar v1.18.0. Reads overlapping with exons were assigned to genes using featureCounts v2.0.1. Classification of rRNA genes/exons and their reads were based on annotations and RepeatMasker rRNA tracks from the University of California Santa Cruz (UCSC) genome browser when applicable. Differential gene expression analysis was completed using DESeq2 v1.28.0. Functional enrichment analysis was achieved using g:Profiler Python API v1.0.0 (https://biit.cs.ut.ee/gprofiler/gost). Quality control and analysis results plots were visualized using MultiQC v1.9. The RNA-Seq data were analyzed using an R programming environment and deposited in the Gene Expression Omnibus repository with the accession number GSE226252.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 9.4.1; GraphPad Software, La Jolla, CA), and statistical significance was determined using an unpaired Student’s t test unless mentioned otherwise. Results are represented as the mean ± standard error of the mean (SEM), and p values < 0.05 are considered significant. Significant p values are labeled with asterisks, denoting *p < 0.05, **p < 0.01, and ***p < 0.001.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health grant (K22HL146793 to J. X.). We thank Chengyu Liu at the National Heart, Lung, and Blood Institute Transgenic Core, Toren Finkel, and Warren J. Leonard for their help generating mouse models, Krisztian Csomos and Jolan E. Walter for help with PBMC culture, and Timothy F. Osborne for critical reading of the manuscript.
Abbreviations used:
- acetyl-CoA
acetyl-coenzyme A
- CPT1A
carnitine palmitoyltransferase 1A
- FAO
fatty acid oxidation
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- Ifng
mouse gene encoding interferon γ
- IFN-γ
interferon γ
- IL
interleukin
- KD
knockdown
- KO
knockout
- OCR
oxygen consumption rate
- PBMC
peripheral blood mononuclear cell
- RNA-Seq
RNA-sequencing
- Th
T helper
- TNF-α
tumor necrosis factor α
- Treg
regulatory T cells
- WT
wild type.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E23-02-0070) on May 3, 2023.
REFERENCES
- Balmer ML, Ma EH, Bantug GR, Grahlert J, Pfister S, Glatter T, Jauch A, Dimeloe S, Slack E, Dehio P, et al. (2016). Memory CD8(+) T cells require increased concentrations of acetate induced by stress for optimal function. Immunity 44, 1312–1324. [DOI] [PubMed] [Google Scholar]
- Bantug GR, Galluzzi L, Kroemer G, Hess C (2018). The spectrum of T cell metabolism in health and disease. Nat Rev Immunol 18, 19–34. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B (2009). The functional plasticity of T cell subsets. Nat Rev Immunol 9, 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Sutter BM, Li B, Tu BP (2011). Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell 42, 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. (2013). Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope A, Le Friec G, Cardone J, Kemper C (2011). The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol 32, 278–286. [DOI] [PubMed] [Google Scholar]
- De Rosa V, Galgani M, Porcellini A, Colamatteo A, Santopaolo M, Zuchegna C, Romano A, De Simone S, Procaccini C, La Rocca C, et al. (2015). Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol 16, 1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C (2021). Cytokine regulation and function in T cells. Annu Rev Immunol 39, 51–76. [DOI] [PubMed] [Google Scholar]
- Geltink RIK, Kyle RL, Pearce EL (2018). Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol 36, 461–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houten SM, Violante S, Ventura FV, Wanders RJ (2016). The biochemistry and physiology of mitochondrial fatty acid beta-oxidation and its genetic disorders. Annu Rev Physiol 78, 23–44. [DOI] [PubMed] [Google Scholar]
- Hung JT, Liao JH, Lin YC, Chang HY, Wu SF, Chang TH, Kung JT, Hsieh SL, McDevitt H, Sytwu HK (2005). Immunopathogenic role of TH1 cells in autoimmune diabetes: evidence from a T1 and T2 doubly transgenic non-obese diabetic mouse model. J Autoimmun 25, 181–192. [DOI] [PubMed] [Google Scholar]
- Jung J, Zeng H, Horng T (2019). Metabolism as a guiding force for immunity. Nat Cell Biol 21, 85–93. [DOI] [PubMed] [Google Scholar]
- Lee JV, Carrer A, Shah S, Snyder NW, Wei S, Venneti S, Worth AJ, Yuan ZF, Lim HW, Liu S, et al. (2014). Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab 20, 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao PC, Bergamini C, Fato R, Pon LA, Pallotti F (2020). Isolation of mitochondria from cells and tissues. Methods Cell Biol 155, 3–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Liu M, Feng X, Wang Z, Das I, Xu Y, Zhou X, Sun Y, Guan KL, Xiong Y, et al. (2014). Glyceraldehyde-3-phosphate dehydrogenase is activated by lysine 254 acetylation in response to glucose signal. J Biol Chem 289, 3775–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG (2011). Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27, 441–464. [DOI] [PubMed] [Google Scholar]
- Lyu J, Pirooznia M, Li Y, Xiong J (2022). The short-chain fatty acid acetate modulates epithelial-to-mesenchymal transition. Mol Biol Cell 33, br13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menk AV, Scharping NE, Moreci RS, Zeng X, Guy C, Salvatore S, Bae H, Xie J, Young HA, Wendell SG, et al. (2018). Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep 22, 1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Puthillathu N, Vengilote R, Jaworski DM, Namboodiri AM (2020). Acetate revisited: a key biomolecule at the nexus of metabolism, epigenetics, and oncogenesis—part 2: acetate and ACSS2 in health and disease. Front Physiol 11, 580171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M, et al. (2015). Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21, 392–402. [DOI] [PubMed] [Google Scholar]
- Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO (2016). Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, Kroemer G (2015). Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab 21, 805–821. [DOI] [PubMed] [Google Scholar]
- Ren M, Kazemian M, Zheng M, He J, Li P, Oh J, Liao W, Li J, Rajaseelan J, Kelsall BL, et al. (2020). Transcription factor p73 regulates Th1 differentiation. Nat Commun 11, 1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestov AA, Liu X, Ser Z, Cluntun AA, Hung YP, Huang L, Kim D, Le A, Yellen G, Albeck JG, et al. (2014). Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. eLife 3, e03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JM, Tibbitt CA, Coquet JM (2019). The metabolic requirements of Th2 cell differentiation. Front Immunol 10, 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski JR, Gizak A, Rakus D (2015). Integrating proteomics and enzyme kinetics reveals tissue-specific types of the glycolytic and gluconeogenic pathways. J Proteome Res 14, 3263–3273. [DOI] [PubMed] [Google Scholar]
- Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, Garcia-Caballero M, Missiaen R, Huang H, Bruning U, et al. (2017). The role of fatty acid beta-oxidation in lymphangiogenesis. Nature 542, 49–54. [DOI] [PubMed] [Google Scholar]
- Wu H, Estrella V, Beatty M, Abrahams D, El-Kenawi A, Russell S, Ibrahim-Hashim A, Longo DL, Reshetnyak YK, Moshnikova A, et al. (2020). T-cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat Commun 11, 4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J (2018a). Fatty acid oxidation in cell fate determination. Trends Biochem Sci 43, 854–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J (2018b). A “Nobel” look at metabolism. Trends Endocrinol Metab 29, 809–813. [DOI] [PubMed] [Google Scholar]
- Xiong J, Kawagishi H, Yan Y, Liu J, Wells QS, Edmunds LR, Fergusson MM, Yu ZX, Rovira II, Brittain EL, et al. (2018). A metabolic basis for endothelial-to-mesenchymal transition. Mol Cell 69, 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Todorova D, Su NY, Kim J, Lee PJ, Shen Z, Briggs SP, Xu Y (2015). Stemness factor Sall4 is required for DNA damage response in embryonic stem cells. J Cell Biol 208, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Cao X (2019). Epigenetic regulation of the innate immune response to infection. Nat Rev Immunol 19, 417–432. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE (2010). Differentiation of effector CD4 T cell populations. Annu Rev Immunol 28, 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.