Abstract
Lung transplantation is often the only viable treatment option for a patient with end-stage lung disease. Lung transplant results have improved substantially over time, but ischemia-reperfusion injury, primary graft dysfunction, acute rejection, and chronic lung allograft dysfunction (CLAD) continue to be significant problems. Mesenchymal stromal cells (MSC) are pluripotent cells that have anti-inflammatory and protective paracrine effects and may be beneficial in solid organ transplantation. Here, we review the experimental studies where MSCs have been used to protect the donor lung against ischemia-reperfusion injury and alloimmune responses, as well as the experimental and clinical studies using MSCs to prevent or treat CLAD. In addition, we outline ex vivo lung perfusion (EVLP) as an optimal platform for donor lung MSC delivery, as well as how the therapeutic potential of MSCs could be further leveraged with genetic engineering.
Keywords: mesenchymal stromal cell, lung transplantation, cell therapy, gene therapy, ex vivo lung perfusion
1. Introduction
Lung transplantation is standard therapy for various end-stage lung diseases such as interstitial lung disease, obstructive lung disease, pulmonary hypertension, and cystic fibrosis. Almost 5000 lung transplants are reported annually in the International Society of Heart and Lung Transplantation Registry, and the results have improved over time [1]. However, the median survival of lung transplant recipients is limited to less than 7 years, and the main obstacles are primary graft dysfunction (PGD), acute rejection, chronic lung allograft dysfunction (CLAD), and side-effects of immunosuppressive drugs [2,3,4,5].
Mesenchymal stromal cells (MSC) are pluripotent cells present in most adult tissues [6,7]. MSC therapy in solid organ transplantation has gained attraction as MSCs possess anti-inflammatory, immunomodulatory, and protective features that could theoretically be utilized to counteract non-immunological and immunological injuries related to organ transplantation [8,9]. In lung transplantation, MSCs have been delivered in experimental studies to the donor lung to protect the transplant against ischemia-reperfusion injury (IRI) and PGD and to induce immunomodulatory effects [10]. In addition, small Phase 1 clinical trials have been performed in lung transplant patients with established CLAD [11,12,13], and additional clinical PGD and CLAD trials are ongoing [14].
Here, we review MSC biology, the most important pathological mechanisms involved in lung transplantation, and how these pathways could be targeted with MSC therapy. In addition, we describe how ex vivo lung perfusion (EVLP) could be used to administer MCSs into the donor lung ex vivo before transplantation [10,15], and highlight the potential of MSC genetic engineering to obtain enhanced therapeutic properties [16,17].
2. Mesenchymal Stromal Cells
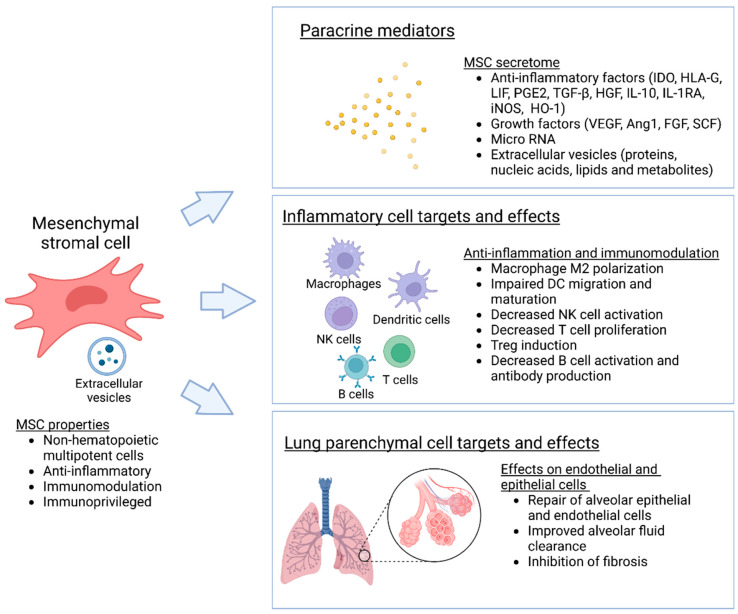
Mesenchymal stromal cells (MSC) and multipotent adult progenitor cells (MAPC) are mesenchymal, non-hematopoietic progenitor cells that are found in most tissues and are capable of self-renewal and differentiation into various types of stromal cells (Figure 1). Resident MSCs are also present in the lung, localized in perivascular spaces, where they act as important regulators of pulmonary homeostasis and the balance between lung injury and repair [18]. For therapeutic purposes, MSCs are commonly isolated from bone marrow, adipose tissue, the umbilical cord, and the placenta [6,7]. As considerable heterogeneity in cell sources, and isolation and expansion methods exist, the International Society for Cell Therapy has recommended minimal standard criteria to identify and characterize MSCs. Accordingly, MSCs should adhere to plastic in standard culture conditions, express typical stromal cell surface markers, lack expression of hematopoietic markers, and differentiate into osteoblasts, adipocytes, and chondroblasts in vitro under appropriate conditions [19]. While MAPCs resemble MCSs, they are biologically more primitive and have greater differentiation potential than classical MSCs [20].
Figure 1.
Biological properties and effects of mesenchymal stromal cells. MSC are non-hematopoietic multipotent cells that have anti-inflammatory, immunomodulatory and protective effects mainly mediated though paracrine mechanisms.
Initially, MSCs and MAPCs were considered to result in cell replacement and renewal of damaged tissues; however, the current view is that the therapeutic effects of these cells are largely mediated by paracrine signaling, through the secretion of numerous soluble mediators, including growth factors, cytokines, and microRNA. This MSC secretome is considered to modulate inflammation and immune responses, and to stimulate innate tissue repair [6,21]. MSC therapies have been investigated in numerous clinical trials of cardiovascular disease, graft-versus host disease, and neurological disease, but the success has so far been variable [7]. Several studies have also examined the use of MSC in lung disease, especially in acute respiratory distress syndrome (ARDS) [22,23]. Here we will concentrate on the use of MSCs in lung transplantation.
2.1. MSC Surface Receptors and Immunomodulatory Effects
According to the minimal standard criteria, MSCs express stromal cell markers CD105, CD73, and CD90 and lack the expression of hematopoietic surface molecules CD45, CD34, CD14 or CD11b, CD79alpha or CD19, and HLA-DR in vitro [19]. MSCs are considered immunoprivileged as they have HLA-I antigens but lack the expression of HLA-II and the costimulatory molecules/receptors CD80 and CD86 that are needed for T cell activation. This has important therapeutic implications for MSCs, as allogenic MSC may be used without eliciting a robust immunoreaction or cytolysis [6,17].
Several studies indicate that MSCs have immunomodulatory effects. They suppress mixed lymphocyte reactions in vitro and alloimmune reactions in vivo through various mechanisms [17]. Similarly, resident MSCs isolated from human lung transplants inhibit T cell activation in vitro, indicating that MSCs may mediate immunological responses in an allogeneic lung environment [24,25]. MSCs interact with numerous innate and adaptive immune cells, both through direct contact with the target cells and also via various paracrine factors [17]. Important for solid organ transplantation, MSCs polarize naïve and memory CD4+ T cells towards regulatory T cells (Treg) and promote tolerance and long-term allograft acceptance. Additionally, MSCs induce the formation of regulatory CD8+ T cells and regulatory B cells. MSCs also have suppressive effects on antigen presenting cells such as dendritic cells and induce polarization of macrophages from the pro-inflammatory M1 to the anti-inflammatory M2 subtype [6,17,26].
2.2. MSC Secretome and Paracrine Effects
Several cytokines and pathways are considered important for the anti-inflammatory and immunomodulatory effects of MSC [21]. One of the most important factors is indoleamine 2,3-dioxygenase (IDO), an enzyme catalyzing the breakdown of tryptophan that induces immunosuppression through the generation of Tregs and tolerogenic dendritic cells (DC) [17,27]. Another key immunomodulatory molecule is HLA-G. MSCs express both the membrane-bound HLA-G1 and soluble HLA-G5 forms, which are considered to polarize T helper cells into Th2 cells [17]. An additional important molecule is leukemia inhibitory factor (LIF), which is expressed at high levels by MSCs and induces direct inhibition of effector T cells and promotes the generation of Tregs [17]. Several other secreted factors also play a role in the immunomodulatory effects of MSCs (Figure 1) [6,21]. It has been suggested that two different immunoregulatory networks may be essential, with the induction of tolerogenic genes IDO, HLA-G, and LIF functioning in a contact-independent manner and direct links with MSCs and target cells leading to modulation of IL-10 and TGF-β expression by the T cells [28].
Although MSCs from different sources are similar, their protein, transcriptomic, and secretory profiles vary to some extent. These differences likely reflect distinctive biological properties and indicate that it is important to consider the MSC source when planning, predicting, or interpreting possible therapeutic effects [17,21].
2.3. MSC-Derived Extracellular Vesicles
Extracellular vesicles (EVs) are lipid bilayer particles released from various cells, such as MSCs that are considered important for cell-to-cell interactions. EVs contain lipids, nucleic acids, and proteins and are categorized as exosomes, microparticles, and apoptotic bodies based on the EV size. Interestingly, several studies have shown that administration of EVs derived from MSCs, rather than the MSCs themselves, protects against lung injury [23,29,30,31]. These results indicate that EVs contain bioactive components that are important for intercellular communication and may in fact mediate many of the beneficial effects of MSCs [23,31]. Consequently, there has been considerable interest in developing cell-free EV-based therapies that could elicit therapeutic effects without some of the potential concerns of MSCs such as oncogenesis or intravascular clustering and embolization [23].
2.4. MSCs in Lung Injury
MSCs protect lung epithelial cells in vitro through paracrine mechanisms by downregulating inflammatory and upregulating anti-apoptotic factors [32,33] and promoting epithelial repair [34]. MSCs also have protective effects on pulmonary endothelial cells by enhancing endothelial barrier function and inhibiting endothelial-to-mesenchymal transition [35,36,37,38]. Additionally, effects on alveolar macrophages, which constitute the first-line defense for the lung, are considered important [26]. When given systemically, MSCs home to the site of damage: this involves an interplay of chemokine signals and adhesion molecules and is considered one of the advantages of using MSCs in lung injury [22]. Interestingly, the lung microenvironment may also affect MSC properties, as the MSC secretome can change, for example, due to inflammation or acidic conditions present in severe lung injury [35,39,40].
MSCs have been shown to be beneficial in various experimental models of lung injury and disease [10,23,31], and these effects likely involve antimicrobial, anti-inflammatory, regenerative, anti-oxidative stress, angiogenic, and antifibrotic signals [41]. Several clinical studies have investigated the safety and efficacy of MSC therapy in ARDS [41]. A recent meta-analysis of 13 clinical randomized controlled trials with a total of 655 patients using intravenous administration of MSCs in ARDS indicated that MSC therapy is safe and may decrease mortality [22]. However, the majority of these studies were performed in patients with COVID-19, and only 2 studies were on non-COVID-19 ARDS. Therefore, more large-scale clinical trials are needed to confirm the efficacy of MSC therapy in ARDS [22].
3. Lung Transplant MSC Therapy
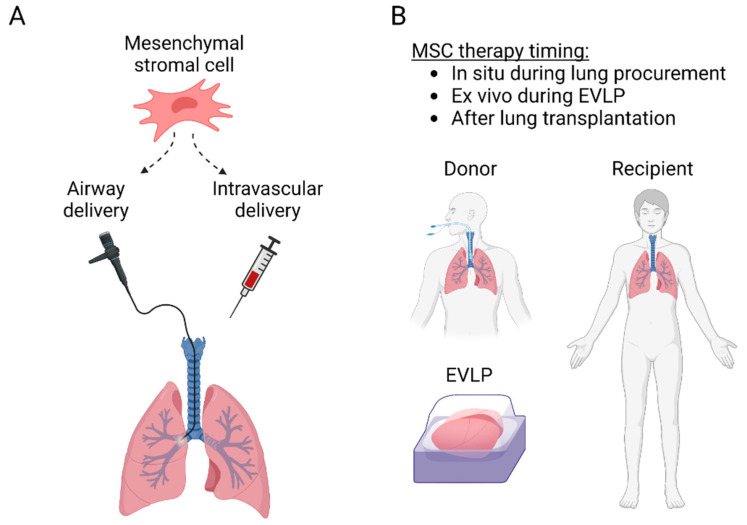
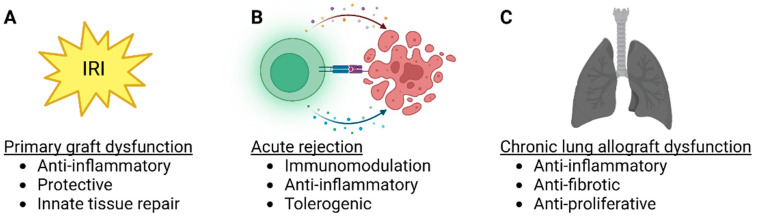
The protective anti-inflammatory and immunomodulatory properties make MSCs attractive therapeutic candidates for lung transplantation. MSCs have been delivered to the lungs through the airways or intravascularly and can be administered into the donor lung either before transplantation or to the recipient before or after transplantation (Figure 2). Most of the preclinical studies have used MSCs to inhibit IRI, while some experiments have targeted acute rejection, and some clinical trials have explored MSC therapy in patients with CLAD (Table 1). Importantly, there is significant interaction between IRI, acute rejection, and CLAD (Figure 3), as lung injury may trigger alloimmune responses, and various non-immunological and immunological factors participate in the development of CLAD [5,42].
Figure 2.
(A) MSC delivery routes to the lung transplant. MSC can be administered into the lungs through the airways with a flexible bronchoscope or intravascularly either intravenously, or directly through the pulmonary artery. (B) MSC administration can be performed before transplantation in situ in the donor or ex vivo during ex vivo lung perfusion, or after transplantation to the recipient.
Table 1.
Lung transplant and EVLP studies with MSCs.
| Target | Species | Model | MSC | Administration | Effect | Ref |
|---|---|---|---|---|---|---|
| IRI | Rat | Lung hilar clamping | Engineered BM-MSC (MSC-vIL-10) | Intravenous | Improved oxygenation, inflammation and permeability | [43] |
| IRI | Pig | SLTx | BM-MSC | Pulmonary artery vs. endobronchial | Endobronchial MSC delivery improved lung compliance | [44] |
| IRI | Human | EVLP | MAPC | Airways | Decreased edema and inflammation | [45] |
| IRI | Human | EVLP | BM-MSC | Intravascular | Restored alveolar fluid clearance | [46] |
| IRI | Mouse | SLTx | BM-MSC | Recipient intravenous | Decreased IRI, MSC homing preferentially into the lung transplant | [47] |
| IRI | Pig | EVLP | UC-MSC | Airway vs. intravascular, 3 different doses | Intravascular delivery improved MSC lung retention, optimal dose 150 × 106 MSC decreased IL-8 and increased VEGF | [48] |
| IRI | Mouse | SLTx | BM-MSC | Ex vivo pulmonary artery | Decreased IRI | [49] |
| IRI | Pig | SLTx | BM-MSC | Pulmonary artery vs. endobronchial | No short-term differences detected | [50] |
| IRI | Mouse | Lung hilar clamping and EVLP | Human UC-MSC vs. MSC-EVs | Intravascular | MSCs and MSC-EVs attenuate IRI | [51] |
| IRI | Human | EVLP | MAPC | Airways | Decreased BAL neutrophilia, TNF-α, IL-1β and IFN-γ | [52] |
| IRI | Pig | SLTx | BM-MSC | Intravenous or intrabronchial | Heterogenous localization, in alveoli after endobronchial and in blood vessels after intravascular administration | [53] |
| IRI | Rat | SLTx | BM-MSC | Intravenous | Protection against IRI | [54] |
| IRI | Pig | EVLP and SLTx | UC-MSC | Intravascular | Decreased IRI during EVLP and after TX | [55] |
| IRI | Rat | EVLP | UC-MSC | Intravascular | Improved inflammation and IRI | [56] |
| IRI | Rat | EVLP | BM-MSC-EVs | Intravascular | Multiple influences on pulmonary energetics, tissue integrity and gene expression | [30] |
| IRI | Human | EVLP | Engineered UC-MSC (MSCIL−10) | Intravascular | Safe and feasible, results in rapid IL-10 elevation | [40] |
| IRI | Rat | SLTx | Donor vs. recipient adipose tissue MSC | Intravenous | MSCs, regardless of their origin, exert similar immunosuppressive effects | [57] |
| IRI/ ARDS |
Human | EVLP/endotoxin | BM-MSC | Airways | Restored alveolar fluid clearance | [58] |
| IRI/ ARDS |
Human | EVLP/e.coli pneumonia | BM-MSC | Airways | Restored alveolar fluid clearance, reduced inflammation and increased antimicrobial activity | [59] |
| Acute rejection | Rat | SLTx | BM-MSC | 1 vs. 2 recipient intravenous doses | Protection from acute rejection, best result with 2 recipient doses | [60] |
| Acute rejection/ CLAD |
Mouse | Ortotopic tracheal Tx | iPSC-MSC | Intravascular | Induces immune tolerance and supports long-term graft survival | [61] |
| CLAD | Mouse | Heterotopic tracheal Tx | MSC (various sources) | Intravenous | Prevents airway occlusion | [62] |
| CLAD | Mouse | Ortotopic tracheal Tx | BM-MSC | Intravenous | Prevents airway occlusion through macrophage cytokines | [63] |
| CLAD | Mouse | Heterotopic tracheal Tx | BM-MSC | Local vs. systemic vs. combination | Prevents airway occlusion through modulation of immune response, best effect with combination treatment | [64] |
| CLAD | Human | Clinical Tx | BM-MSC | Intravenous twice weekly for 2 weeks | Safe and feasible in patients with advanced CLAD | [13] |
| CLAD | Human | Clinical Tx | BM-MSC | Intravenous | Safe and feasible in patients with moderate CLAD | [12] |
| CLAD | Human | Clinical Tx | BM-MSC | Intravenous | Well tolerated in moderate-to-severe CLAD, low-dose may slow progression of CLAD in some patients | [11] |
Figure 3.
MSC therapy targets in lung transplantation. (A) Lung transplant ischemia-reperfusion injury and primary graft dysfunction, (B) acute rejection and (C) chronic lung allograft dysfunction (CLAD) are potential targets for MSC therapy. In each lung transplant pathology, possible beneficial effects of MSCs are listed.
3.1. MSC Therapy in Lung Transplant IRI and PGD
PGD occurs in almost one-third of lung transplant recipients [65]. Severe PGD is especially problematic as mortality is high and treatment options are mainly supportive [66,67,68]. The clinical importance of PGD is further emphasized by its negative long-term effects [69,70]. Overproduction of reactive oxygen species after ischemia-reoxygenation is considered the main trigger in IRI and PGD [71,72,73,74,75,76]. This results in widespread molecular and cellular dysfunction that involves cytokines and damage-associated molecular patterns (DAMP), innate and adaptive immunity activation, mitochondrial dysfunction, ion imbalance, apoptosis, and the activation of complement, coagulation, and platelet-aggregation cascades. The ensuing microvascular dysfunction and breakdown of the alveolar-capillary barrier result in pulmonary edema and hypoxia—the clinical manifestations of PGD [71,72]. The main cells involved in IRI consist of both parenchymal and inflammatory cells. Endothelial and epithelial cells, and donor alveolar macrophages, are considered essential contributors to the initial IRI [67], and danger signals from these cells augment the infiltration of recipient monocytes, macrophages, neutrophils, natural killer cells [76,77], and lymphocytes.
Several experimental studies have shown that MSC therapy is beneficial for lung IRI (Table 1). The majority of these studies have used bone marrow-derived cells, but also MSCs from the umbilical cord or adipose tissue have also been effective. Airway or intravascular delivery results in alveolar or blood vessel MSC localization, respectively [53], and intravascular delivery has been shown to yield better MSC retention in the lung parenchyma in a pig EVLP model [48]. Multiple studies indicate that MSC therapy protects the alveolar-epithelial barrier during lung injury, as improved permeability, alveolar fluid clearance, and oxygenation have been reported (Table 1). Anti-inflammatory effects are likely also important, as MSC therapy decreases proinflammatory cytokine levels and neutrophilia [10,15,45,46,48,52,55,58,59,78]. Several studies have also reported that administration of extracellular vesicles (EV) derived from MSCs instead of MSCs during EVLP protects against lung injury [29,30]. No clinical lung transplant IRI studies have been reported yet, but one Phase 1–2 trial is investigating the effect of adipose tissue-derived MSCs on PGD (ClinicalTrials.gov identifier NCT04714801).
3.2. MSC Therapy in Acute Rejection
Acute rejection remains a significant problem after lung transplantation, and about 27% of the recipients experience at least one acute rejection episode during the first year [79]. Acute rejection most often responds to anti-rejection treatments, but recurrent rejection episodes are problematic and may increase the risk of the later development of chronic rejection [3,80]. In addition, the augmented immunosuppression predisposes the patients to infections, malignancies, and metabolic problems [81]. Allorecognition after solid organ transplantation occurs in lymph nodes or the spleen, but in the case of the lung, it can also take place locally in the transplanted graft itself [82,83]. Co-stimulatory signals and cytokine-mediated lymphocyte differentiation and expansion are also needed to mount an efficient alloimmune response, and the effector mechanisms involve CD4+ helper T cells, CD8+ cytotoxic T cells, B cells, antibody production, and complement-mediated damage. Importantly, Tregs function to limit the alloimmune response [84].
MSC delivery into the lung graft to prevent or treat acute rejection would be attractive based on the immunomodulatory properties of MSCs. However, although MSC therapy has early protective effects in lung transplant IRI (Table 1), it remains largely unknown whether this reflects decreased alloimmunity later after transplantation. In a rat major histocompatibility complex (MHC)-mismatched single lung transplant model, MSC administration on days 0 and 3 decreased histological signs of rejection and resulted in a beneficial change in PD-L1 and IL-17A gene expression at 6 days [60]. Similarly, in a mouse MHC-mismatched orthotopic tracheal transplant model with follow-up until 90 days, human induced pluripotent stem cell (iPSC)-derived MSCs resulted in immunomodulation and an increase of Tregs [61]. These experimental studies indicate that MSCs can inhibit the development of alloimmune responses, but more translational experiments and clinical trials are needed. In addition, it remains to be determined whether MSCs could also be used, for example, as salvage therapy to treat established or recurrent acute rejection episodes.
3.3. MSC Therapy in CLAD
CLAD results in progressive and irreversible deterioration of graft function and remains the leading cause of late deaths after lung transplantation [85,86]. CLAD currently consists of different subtypes. Bronchiolitis obliterans syndrome (BOS) is characterized by obstruction and small airway obliteration [85], whereas restrictive allograft syndrome (RAS) results in restrictive lung function and fibrosis [87]. Additionally, mixed and undefined phenotypes are recognized [88,89]. Therapeutic options for CLAD are limited, and it is known that the prognosis of RAS is poor [85,90]. These factors highlight the need for novel therapeutic options to prevent CLAD development or treat established diseases. It is believed that chronic non-immunological and immunological injury to the lung is central to causing pathological distal airway fibroproliferation, leading to the irreversible lung damage related to CLAD [42,86,91,92]. Encouragingly, in mouse tracheal transplantation models, MSC therapy has successfully prevented airway obliteration, possibly by modulating immune responses [61,62,63,64].
MSCs have also been used in three important Phase 1 clinical trials that have established the safety and feasibility of MSC therapy in lung transplant patients [11,12,13]. All of these studies have used cryopreserved 3rd party allogeneic bone marrow-derived MSCs with intravenous administration to patients with obstructive CLAD, i.e., BOS. Chambers et al. delivered 2 × 106 MSCs/kg to 10 lung transplant recipients with BOS grade ≥ 1, or to patients with grade 1 with risk factors for CLAD progression. The treatment was feasible and safe; no procedure-related serious adverse effects were detected, and there was a trend in the lung function decline measured by forced expiratory flow in 1 s (FEV1) was smaller after MSC infusion compared with the pre-treatment situation [13]. Keller et al. used a single intravenous infusion in nine patients, comparing three doses of 1, 2, or 4 × 106 MSCs/kg. MSC therapy was feasible, safe, and well tolerated and did not change functional or laboratory parameters within 30 days after infusion [12]. Erasmus et al. treated 13 patients with moderate-to-severe BOS using 0.5 or 1 × 106 MSCs/kg. Lung function decline is measured by FEV1 was significant before treatment but not after treatment, indicating possible CLAD stabilization [11]. An ongoing Phase 2 trial investigates whether 4 intravenous doses of 2 × 106 MSCs/kg given over 2 weeks improve CLAD-progression-free survival (ClinicalTrials.gov identifier NCT02709343). The results of this study will hopefully help clarify the therapeutic potential of MSCs in lung transplantation. In addition, more studies are needed to determine the optimal MSC source and dose, CLAD severity, and possible effects in other CLAD subtypes than BOS.
4. Strategies to Improve MSC Delivery and Therapeutic Potential
Although the lung is well accessible for MSC therapy with either airway or intravenous in vivo administration, cell delivery into an isolated donor lung during ex vivo lung perfusion (EVLP) would have several advantages. We review the concept of EVLP and how it could be used as a unique therapeutic opportunity and platform for MSC therapy. In addition, we discuss how MSC genetic engineering could be used to improve cell properties and achieve improved therapeutic efficacy.
4.1. MSC Delivery into the Donor Lung during EVLP
In EVLP, the isolated donor lung is perfused and ventilated in normothermia ex vivo (outside the body), enabling confirmation of donor lung quality before transplantation. This has revolutionized lung transplantation, as marginal donor lungs that would otherwise have been rejected from transplantation can be tested and transplanted if graft function is adequate [93]. A landmark clinical study evaluated high-risk donor lungs during EVLP and established that transplantation of lungs that were physiologically stable during 4 h of EVLP leads to post-transplant results similar to those obtained with conventionally selected lungs [94]. EVLP has safely and significantly increased the available pool of donor organs for transplantation, and later studies have shown that medium- and long-term lung transplant results remain excellent after EVLP evaluation [95,96].
EVLP has been used as a platform for cell therapy in several experimental studies using airway or intravascular delivery (Table 1). Mordant et al. compared different MSC doses and administration routes during pig lung EVLP. Intravascular administration resulted in better MSC retention in the lung, and most of the MSCs engraftment occurred within the first minutes after pulmonary artery injection [48]. Moreover, comparison of three different doses revealed that intravascular administration of 150 × 106 MSCs, the middle dose of the experiment, improved lung oxygenation and compliance. In contrast, a higher 300 × 106 MSCs dose temporarily increased pulmonary vascular resistance [48], possibly related to clustering of the cells into the lung microvasculature. These results highlight the efficacy of intravascular MSC delivery but also indicate that high cell doses may result in adverse embolic effects [48]. Interestingly, the optimal cell dose (150 × 106 MSCs; 5 × 106 per kg of animal weight) is close to the doses used in the clinical MSC CLAD studies [11,13,70]. In the studies administering MSCs during EVLP, decreased proinflammatory cytokine levels and protection of the alveolar-epithelial barrier function during ischemia- or endotoxin-induced lung injury have been observed [45,46,48,52,55,58,59]. Several studies have also reported that administration of MSC-derived EVs protects against lung injury [29,30].
4.2. Genetically Engineered MSCs
Genetic engineering of cell-based therapeutics with viral and non-viral vectors has been used to achieve modified cells with enhanced therapeutic properties. Genetic engineering modulates the MSC secretome and aims, for example, to enhance MSC migration to target sites, facilitate intrinsic repair properties, improve cell survival, or change MSCs into gene delivery vehicles [16].
Several different genetically modified MSCs have been studied in lung injury models, and for example, MSC engineered to produce anti-inflammatory IL-10 have been beneficial in lung IRI, endotoxemia, and ARDS [43,97,98]. In our recent study, we transduced human umbilical cord-derived MSC with adenoviral vectors encoding IL-10 and administered these pre-modified and cryopreserved MSCIL−10 cells during EVLP to human lungs rejected from clinical transplantation. This rapidly elevated EVLP perfusate IL-10 levels in minutes, MSCIL−10 cells were retained in the lung, and IL-10 was increased also in lung tissue and the airway compartment [40]. While possible therapeutic or immunomodulatory effects of MSCIL−10 therapy are not known yet, these results indicate that therapy with genetically modified cells during standard clinical EVLP is feasible.
5. Conclusions
MSCs have several anti-inflammatory, immunomodulatory, and repair capabilities that make them attractive therapeutic options in lung transplantation. Experimental studies show that perioperative MSC administration protects the donor lung against IRI, acute rejection, and the development of CLAD mainly through paracrine mechanisms. As these conditions remain major obstacles to the long-term survival of lung transplant patients, novel therapeutic options are indeed warranted. However, although small clinical trials indicate that MSC therapy is safe and feasible in patients with established CLAD, randomized studies are needed to confirm therapeutic efficacy. In addition, the optimal MSC source, dose, and preparation need to be determined. It will also be important to define the potential role of cell-free MSC-derived EVs. In the future, it may be possible to use EVLP to deliver genetically modified MSCs with improved properties to achieve advanced lung transplant cellular engineering for enhanced protection and immunomodulation.
Acknowledgments
M.L. is James and Mary Davie Chair in Lung Injury, Repair and Regeneration at the University Health Network and University of Toronto. Figures created with BioRender.com (Accessed on 17 January 2023).
Author Contributions
Conceptualization, A.I.N., M.L. and S.K.; writing—original draft preparation, A.I.N.; writing—review and editing, M.L. and S.K.; visualization, A.I.N. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
A.I.N. declares no conflict of interest. S.K. serves as Chief Medical Officer of Traferox Technologies; S.K. receives personal fees from Lung Bioengineering and Traferox Technologies. S.K. is an inventor of IP licensed to SQI Diagnostics and Traferox Technologies. M.L. serves as a consultant to Traferox Technologies.
Funding Statement
The study was Supported by Canadian Institutes of Health Research (CIHR) Open Operating Grant #312227. A.I.N. was supported by Sigrid Juselius Foundation Fellowship Grant.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chambers D.C., Perch M., Zuckermann A., Cherikh W.S., Harhay M.O., HayesJr D., Hsich E., Khush K.K., Potena L., Sadavarte A., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report—2021; Focus on recipient characteristics. J. Heart Lung Transpl. 2021;40:1060–1072. doi: 10.1016/j.healun.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chacon-Alberty L., Fernandez R., Jindra P., King M., Rosas I., Hochman-Mendez C., Loor G. Primary Graft Dysfunction in Lung Transplantation: A Review of Mechanisms and Future Applications. Transplantation. 2023 doi: 10.1097/TP.0000000000004503. [DOI] [PubMed] [Google Scholar]
- 3.Parulekar A.D., Kao C.C. Detection, classification, and management of rejection after lung transplantation. J. Thorac. Dis. 2019;11:S1732–S1739. doi: 10.21037/jtd.2019.03.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohm B., Jungraithmayr W. B Cell Immunity in Lung Transplant Rejection—Effector Mechanisms and Therapeutic Implications. Front. Immunol. 2022;13:845867. doi: 10.3389/fimmu.2022.845867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venado A., Kukreja J., Greenland J.R. Chronic Lung Allograft Dysfunction. Thorac. Surg. Clin. 2022;32:231–242. doi: 10.1016/j.thorsurg.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger M.F., Discher D.E., Péault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. npj Regen. Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galderisi U., Peluso G., Di Bernardo G. Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years? Stem Cell Rev. Rep. 2022;18:23–36. doi: 10.1007/s12015-021-10231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansourabadi A.H., Mohamed Khosroshahi L., Noorbakhsh F., Amirzargar A. Cell therapy in transplantation: A comprehensive review of the current applications of cell therapy in transplant patients with the focus on Tregs, CAR Tregs, and Mesenchymal stem cells. Int. Immunopharmacol. 2021;97:107669. doi: 10.1016/j.intimp.2021.107669. [DOI] [PubMed] [Google Scholar]
- 9.Vandermeulen M., Erpicum P., Weekers L., Briquet A., Lechanteur C., Detry O., Beguin Y., Jouret F. Mesenchymal Stromal Cells in Solid Organ Transplantation. Transplantation. 2020;104:5. doi: 10.1097/TP.0000000000003077. [DOI] [PubMed] [Google Scholar]
- 10.Miceli V., Bertani A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells. 2022;11:826. doi: 10.3390/cells11050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erasmus D.B., Durand N., Alvarez F.A., Narula T., Hodge D.O., Zubair A.C. Feasibility and Safety of Low-Dose Mesenchymal Stem Cell Infusion in Lung Transplant Recipients. Stem Cells Transl. Med. 2022;11:891–899. doi: 10.1093/stcltm/szac051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keller C.A., Gonwa T.A., Hodge D.O., Hei D.J., Centanni J.M., Zubair A.C. Feasibility, Safety, and Tolerance of Mesenchymal Stem Cell Therapy for Obstructive Chronic Lung Allograft Dysfunction. Stem Cells Transl. Med. 2018;7:161–167. doi: 10.1002/sctm.17-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambers D.C., Enever D., Lawrence S., Sturm M.J., Herrmann R., Yerkovich S., Musk M., Hopkins P.M. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl. Med. 2017;6:1152–1157. doi: 10.1002/sctm.16-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller C.L., Jane M.O., Allan J.S., Madsen J.C. Novel approaches for long-term lung transplant survival. Front. Immunol. 2022;13:931251. doi: 10.3389/fimmu.2022.931251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niroomand A., Hirdman G., Olm F., Lindstedt S. Current Status and Future Perspectives on Machine Perfusion: A Treatment Platform to Restore and Regenerate Injured Lungs Using Cell and Cytokine Adsorption Therapy. Cells. 2022;11:91. doi: 10.3390/cells11010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damasceno P.K.F., de Santana T.A., Santos G.C., Orge I.D., Silva D.N., Albuquerque J.F., Golinelli G., Grisendi G., Pinelli M., Ribeiro dos Santos R., et al. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front. Cell Dev. Biol. 2020;8:737. doi: 10.3389/fcell.2020.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida-Porada G., Atala A.J., Porada C.D. Therapeutic Mesenchymal Stromal Cells for Immunotherapy and for Gene and Drug Delivery. Mol. Methods Clin. Dev. 2020;16:204–224. doi: 10.1016/j.omtm.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty D.F., Roets L., Krasnodembskaya A.D. The Role of Lung Resident Mesenchymal Stromal Cells in the Pathogenesis and Repair of Chronic Lung Disease. Stem Cells. 2023;41:431–443. doi: 10.1093/stmcls/sxad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 20.Khan R.S., Newsome P.N. A Comparison of Phenotypic and Functional Properties of Mesenchymal Stromal Cells and Multipotent Adult Progenitor Cells. Front. Immunol. 2019;10:1952. doi: 10.3389/fimmu.2019.01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Y., Yang J., Fang J., Zhou Y., Candi E., Wang J., Hua D., Shao C., Shi Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022;7:92. doi: 10.1038/s41392-022-00932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F., Li Y., Wang B., Li J., Peng Z. The safety and efficacy of mesenchymal stromal cells in ARDS: A meta-analysis of randomized controlled trials. Crit. Care. 2023;27:31. doi: 10.1186/s13054-022-04287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu A., Zhang X., He H., Zhou L., Naito Y., Sugita S., Lee J.W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020;20:125–140. doi: 10.1080/14712598.2020.1689954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvinen L., Badri L., Wettlaufer S., Ohtsuka T., Standiford T.J., Toews G.B., Pinsky D.J., Peters-Golden M., Lama V.N. Lung Resident Mesenchymal Stem Cells Isolated from Human Lung Allografts Inhibit T Cell Proliferation via a Soluble Mediator1. J. Immunol. 2008;181:4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolandsson S., Andersson Sjöland A., Brune J.C., Li H., Kassem M., Mertens F., Westergren A., Eriksson L., Hansson L., Skog I., et al. Primary mesenchymal stem cells in human transplanted lungs are CD90/CD105 perivascularly located tissue-resident cells. BMJ Open Respir. Res. 2014;1:e000027. doi: 10.1136/bmjresp-2014-000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerkic M., Szaszi K., Laffey J.G., Rotstein O., Zhang H. Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury. Int. J. Mol. Sci. 2023;24:3376. doi: 10.3390/ijms24043376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge W., Jiang J., Arp J., Liu W., Garcia B., Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 28.Nasef A., Chapel A., Mazurier C., Bouchet S., Lopez M., Mathieu N., Sensebé L., Zhang Y., Gorin N.C., Thierry D., et al. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13:217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gennai S., Monsel A., Hao Q., Park J., Matthay M.A., Lee J.W. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am. J. Transpl. 2015;15:2404–2412. doi: 10.1111/ajt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lonati C., Bassani G.A., Brambilla D., Leonardi P., Carlin A., Maggioni M., Zanella A., Dondossola D., Fonsato V., Grange C., et al. Mesenchymal stem cell-derived extracellular vesicles improve the molecular phenotype of isolated rat lungs during ischemia/reperfusion injury. J. Heart Lung Transpl. 2019;38:1306–1316. doi: 10.1016/j.healun.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Worthington E.N., Hagood J.S. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int. J. Mol. Sci. 2020;21:2318. doi: 10.3390/ijms21072318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmelzer E., Miceli V., Chinnici C.M., Bertani A., Gerlach J.C. Effects of Mesenchymal Stem Cell Coculture on Human Lung Small Airway Epithelial Cells. BioMed Res. Int. 2020;2020:9847579. doi: 10.1155/2020/9847579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miceli V., Bertani A., Chinnici C.M., Bulati M., Pampalone M., Amico G., Carcione C., Schmelzer E., Gerlach J.C., Conaldi P.G. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2021;22:510. doi: 10.3390/ijms22020510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruk D.M.L.W., Wisman M., Noordhoek J.A., Nizamoglu M., Jonker M.R., de Bruin H.G., Arevalo Gomez K., ten Hacken N.H.T., Pouwels S.D., Heijink I.H. Paracrine Regulation of Alveolar Epithelial Damage and Repair Responses by Human Lung-Resident Mesenchymal Stromal Cells. Cells. 2021;10:2860. doi: 10.3390/cells10112860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Zheng R., Chen Q., Shao J., Yu J., Hu S. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF) Stem Cell Res. Ther. 2017;8:211. doi: 10.1186/s13287-017-0662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Z., Zhang Y., Yan F. Potential of Mesenchymal Stem Cell-Based Therapies for Pulmonary Fibrosis. DNA Cell Biol. 2022;41:951–965. doi: 10.1089/dna.2022.0327. [DOI] [PubMed] [Google Scholar]
- 37.Huang J., Lu W., Ouyang H., Chen Y., Zhang C., Luo X., Li M., Shu J., Zheng Q., Chen H., et al. Transplantation of Mesenchymal Stem Cells Attenuates Pulmonary Hypertension by Normalizing the Endothelial-to-Mesenchymal Transition. Am. J. Respir. Cell Mol. Biol. 2020;62:49–60. doi: 10.1165/rcmb.2018-0165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu S., Park J., Liu A., Lee J., Zhang X., Hao Q., Lee J.W. Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem Cells Transl. Med. 2018;7:615–624. doi: 10.1002/sctm.17-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss D.J., Rolandsson Enes S. MSCs interaction with the host lung microenvironment: An overlooked aspect? Front. Immunol. 2022;13:1072257. doi: 10.3389/fimmu.2022.1072257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nykänen A.I., Mariscal A., Duong A., Estrada C., Ali A., Hough O., Sage A., Chao B.T., Chen M., Gokhale H., et al. Engineered mesenchymal stromal cell therapy during human lung ex vivo lung perfusion is compromised by acidic lung microenvironment. Mol. Methods Clin. Dev. 2021;23:184–197. doi: 10.1016/j.omtm.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernández-Francos S., Eiro N., González-Galiano N., Vizoso F.J. Mesenchymal Stem Cell-Based Therapy as an Alternative to the Treatment of Acute Respiratory Distress Syndrome: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2021;22:7850. doi: 10.3390/ijms22157850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawashima M., Juvet S.C. The role of innate immunity in the long-term outcome of lung transplantation. Ann. Transl. Med. 2020;8:412. doi: 10.21037/atm.2020.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manning E., Pham S., Li S., Vazquez-Padron R.I., Mathew J., Ruiz P., Salgar S.K. Interleukin-10 delivery via mesenchymal stem cells: A novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum. Gene. 2010;21:713–727. doi: 10.1089/hum.2009.147. [DOI] [PubMed] [Google Scholar]
- 44.Wittwer T., Rahmanian P., Choi Y.H., Zeriouh M., Karavidic S., Neef K., Christmann A., Piatkowski T., Schnapper A., Ochs M., et al. Mesenchymal stem cell pretreatment of non-heart-beating-donors in experimental lung transplantation. J. Cardiothorac. Surg. 2014;9:151. doi: 10.1186/s13019-014-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.La Francesca S., Ting A.E., Sakamoto J., Rhudy J., Bonenfant N.R., Borg Z.D., Cruz F.F., Goodwin M., Lehman N.A., Taggart J.M., et al. Multipotent adult progenitor cells decrease cold ischemic injury in ex vivo perfused human lungs: An initial pilot and feasibility study. Transpl. Res. 2014;3:19. doi: 10.1186/2047-1440-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAuley D.F., Curley G.F., Hamid U.I., Laffey J.G., Abbott J., McKenna D.H., Fang X., Matthay M.A., Lee J.W. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:L809–L815. doi: 10.1152/ajplung.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian W., Liu Y., Zhang B., Dai X., Li G., Li X., Zhang Z., Du C., Wang H. Infusion of mesenchymal stem cells protects lung transplants from cold ischemia-reperfusion injury in mice. Lung. 2015;193:85–95. doi: 10.1007/s00408-014-9654-x. [DOI] [PubMed] [Google Scholar]
- 48.Mordant P., Nakajima D., Kalaf R., Iskender I., Maahs L., Behrens P., Coutinho R., Iyer R.K., Davies J.E., Cypel M., et al. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J. Heart Lung Transpl. 2016;35:1245–1254. doi: 10.1016/j.healun.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe T., Hoshikawa Y., Ishibashi N., Suzuki H., Notsuda H., Watanabe Y., Noda M., Kanehira M., Ohkouchi S., Kondo T., et al. Mesenchymal stem cells attenuate ischemia-reperfusion injury after prolonged cold ischemia in a mouse model of lung transplantation: A preliminary study. Surg. Today. 2017;47:425–431. doi: 10.1007/s00595-016-1391-8. [DOI] [PubMed] [Google Scholar]
- 50.Schnapper A., Christmann A., Knudsen L., Rahmanian P., Choi Y.H., Zeriouh M., Karavidic S., Neef K., Sterner-Kock A., Guschlbauer M., et al. Stereological assessment of the blood-air barrier and the surfactant system after mesenchymal stem cell pretreatment in a porcine non-heart-beating donor model for lung transplantation. J. Anat. 2018;232:283–295. doi: 10.1111/joa.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stone M.L., Zhao Y., Robert Smith J., Weiss M.L., Kron I.L., Laubach V.E., Sharma A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017;18:212. doi: 10.1186/s12931-017-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martens A., Ordies S., Vanaudenaerde B.M., Verleden S.E., Vos R., Van Raemdonck D.E., Verleden G.M., Roobrouck V.D., Claes S., Schols D., et al. Immunoregulatory effects of multipotent adult progenitor cells in a porcine ex vivo lung perfusion model. Stem Cell Res. 2017;8:159. doi: 10.1186/s13287-017-0603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piatkowski T., Brandenberger C., Rahmanian P., Choi Y.H., Zeriouh M., Sabashnikov A., Wittwer T., Wahlers T.C.W., Ochs M., Mühlfeld C. Localization of Exogenous Mesenchymal Stem Cells in a Pig Model of Lung Transplantation. Thorac. Cardiovasc. Surg. 2018;66:63–70. doi: 10.1055/s-0037-1607051. [DOI] [PubMed] [Google Scholar]
- 54.Wei L., Han Z.J., Xu L., Li J.W. Effect of mesenchymal stem cells on expression of high mobility group box 1 protein in rats with ischemia reperfusion injury after lung transplantation. Zhonghua Yi Xue Za Zhi. 2018;98:2019–2023. doi: 10.3760/cma.j.issn.0376-2491.2018.25.011. [DOI] [PubMed] [Google Scholar]
- 55.Nakajima D., Watanabe Y., Ohsumi A., Pipkin M., Chen M., Mordant P., Kanou T., Saito T., Lam R., Coutinho R., et al. Mesenchymal stromal cell therapy during ex vivo lung perfusion ameliorates ischemia-reperfusion injury in lung transplantation. J. Heart Lung Transpl. 2019;38:1214–1223. doi: 10.1016/j.healun.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Pacienza N., Santa-Cruz D., Malvicini R., Robledo O., Lemus-Larralde G., Bertolotti A., Marcos M., Yannarelli G. Mesenchymal Stem Cell Therapy Facilitates Donor Lung Preservation by Reducing Oxidative Damage during Ischemia. Stem Cells Int. 2019;2019:8089215. doi: 10.1155/2019/8089215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimoyama K., Tsuchiya T., Watanabe H., Ergalad A., Iwatake M., Miyazaki T., Hashimoto Y., Hsu Y.I., Hatachi G., Matsumoto K., et al. Donor and Recipient Adipose-Derived Mesenchymal Stem Cell Therapy for Rat Lung Transplantation. Transpl. Proc. 2022;54:1998–2007. doi: 10.1016/j.transproceed.2022.05.038. [DOI] [PubMed] [Google Scholar]
- 58.Lee J.W., Fang X., Gupta N., Serikov V., Matthay M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J.W., Krasnodembskaya A., McKenna D.H., Song Y., Abbott J., Matthay M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishibashi N., Watanabe T., Kanehira M., Watanabe Y., Hoshikawa Y., Notsuda H., Noda M., Sakurada A., Ohkouchi S., Kondo T., et al. Bone marrow mesenchymal stromal cells protect allograft lung transplants from acute rejection via the PD-L1/IL-17A axis. Surg. Today. 2018;48:726–734. doi: 10.1007/s00595-018-1643-x. [DOI] [PubMed] [Google Scholar]
- 61.Khan M.A., Alanazi F., Ahmed H.A., Shamma T., Kelly K., Hammad M.A., Alawad A.O., Assiri A.M., Broering D.C. iPSC-derived MSC therapy induces immune tolerance and supports long-term graft survival in mouse orthotopic tracheal transplants. Stem Cell Res. 2019;10:290. doi: 10.1186/s13287-019-1397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grove D.A., Xu J., Joodi R., Torres-Gonzales E., Neujahr D., Mora A.L., Rojas M. Attenuation of early airway obstruction by mesenchymal stem cells in a murine model of heterotopic tracheal transplantation. J. Heart Lung Transpl. 2011;30:341–350. doi: 10.1016/j.healun.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Z., Zhou X., Li J., Meng Q., Cao H., Kang L., Ni Y., Fan H., Liu Z. Mesenchymal stem cells reprogram host macrophages to attenuate obliterative bronchiolitis in murine orthotopic tracheal transplantation. Int. Immunopharmacol. 2013;15:726–734. doi: 10.1016/j.intimp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Casey A., Dirks F., Liang O.D., Harrach H., Schuette-Nuetgen K., Leeman K., Kim C.F., Gerard C., Subramaniam M. Bone marrow-derived multipotent stromal cells attenuate inflammation in obliterative airway disease in mouse tracheal allografts. Stem Cells Int. 2014;2014:468927. doi: 10.1155/2014/468927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diamond J.M., Arcasoy S., Kennedy C.C., Eberlein M., Singer J.P., Patterson G.M., Edelman J.D., Dhillon G., Pena T., Kawut S.M., et al. Report of the International Society for Heart and Lung Transplantation Working Group on Primary Lung Graft Dysfunction, part II: Epidemiology, risk factors, and outcomes-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transpl. 2017;36:1104–1113. doi: 10.1016/j.healun.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 66.Van Raemdonck D., Hartwig M.G., Hertz M.I., Davis R.D., Cypel M., Hayes D., Jr., Ivulich S., Kukreja J., Lease E.D., Loor G., et al. Report of the ISHLT Working Group on primary lung graft dysfunction Part IV: Prevention and treatment: A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transpl. 2017;36:1121–1136. doi: 10.1016/j.healun.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Rosenheck J., Pietras C., Cantu E. Early Graft Dysfunction after Lung Transplantation. Curr. Pulmonol. Rep. 2018;7:176–187. doi: 10.1007/s13665-018-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diamond J.M., Lee J.C., Kawut S.M., Shah R.J., Localio A.R., Bellamy S.L., Lederer D.J., Cantu E., Kohl B.A., Lama V.N., et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daud S.A., Yusen R.D., Meyers B.F., Chakinala M.M., Walter M.J., Aloush A.A., Patterson G.A., Trulock E.P., Hachem R.R. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am. J. Respir. Crit. Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 70.Keller M., Bush E., Diamond J.M., Shah P., Matthew J., Brown A.W., Sun J., Timofte I., Kong H., Tunc I., et al. Use of donor-derived-cell-free DNA as a marker of early allograft injury in primary graft dysfunction (PGD) to predict the risk of chronic lung allograft dysfunction (CLAD) J. Heart Lung Transpl. 2021;40:488–493. doi: 10.1016/j.healun.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forgie K.A., Fialka N., Freed D.H., Nagendran J. Lung Transplantation, Pulmonary Endothelial Inflammation, and Ex-Situ Lung Perfusion: A Review. Cells. 2021;10:1417. doi: 10.3390/cells10061417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Talaie T., DiChiacchio L., Prasad N.K., Pasrija C., Julliard W., Kaczorowski D.J., Zhao Y., Lau C.L. Ischemia-reperfusion Injury in the Transplanted Lung: A Literature Review. Transplant. Direct. 2021;7:e652. doi: 10.1097/TXD.0000000000001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Todd J.L., Palmer S.M. Danger signals in regulating the immune response to solid organ transplantation. J. Clin. Investig. 2017;127:2464–2472. doi: 10.1172/JCI90594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iske J., Hinze C.A., Salman J., Haverich A., Tullius S.G., Ius F. The potential of ex vivo lung perfusion on improving organ quality and ameliorating ischemia reperfusion injury. Am. J. Transpl. 2021;21:3831–3839. doi: 10.1111/ajt.16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen-Yoshikawa T.F. Ischemia-Reperfusion Injury in Lung Transplantation. Cells. 2021;10:1333. doi: 10.3390/cells10061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shepherd H.M., Gauthier J.M., Terada Y., Li W., Krupnick A.S., Gelman A.E., Kreisel D. Updated Views on Neutrophil Responses in Ischemia-Reperfusion Injury. Transplantation. 2022;106:2314–2324. doi: 10.1097/TP.0000000000004221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calabrese D.R., Aminian E., Mallavia B., Liu F., Cleary S.J., Aguilar O.A., Wang P., Singer J.P., Hays S.R., Golden J.A., et al. Natural killer cells activated through NKG2D mediate lung ischemia-reperfusion injury. J. Clin. Investig. 2021;131:e137047. doi: 10.1172/JCI137047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson E.R., Connelly C., Ali S., Sheerin N.S., Wilson C.H. Cell therapy during machine perfusion. Transpl. Int. 2021;34:49–58. doi: 10.1111/tri.13780. [DOI] [PubMed] [Google Scholar]
- 79.Chambers D.C., Cherikh W.S., Harhay M.O., Hayes D., Jr., Hsich E., Khush K.K., Meiser B., Potena L., Rossano J.W., Toll A.E., et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: Donor and recipient size match. J. Heart Lung Transpl. 2019;38:1042–1055. doi: 10.1016/j.healun.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinu T., Pavlisko E.N., Chen D.F., Palmer S.M. Acute allograft rejection: Cellular and humoral processes. Clin. Chest Med. 2011;32:295–310. doi: 10.1016/j.ccm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kotecha S., Ivulich S., Snell G. Review: Immunosuppression for the lung transplant patient. J. Thorac. Dis. 2021;13:6628–6644. doi: 10.21037/jtd-2021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gauthier J.M., Li W., Hsiao H.M., Takahashi T., Arefanian S., Krupnick A.S., Gelman A.E., Kreisel D. Mechanisms of Graft Rejection and Immune Regulation after Lung Transplant. Ann. Am. Thorac. Soc. 2017;14:S216–S219. doi: 10.1513/AnnalsATS.201607-576MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gelman A.E., Li W., Richardson S.B., Zinselmeyer B.H., Lai J., Okazaki M., Kornfeld C.G., Kreisel F.H., Sugimoto S., Tietjens J.R., et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J. Immunol. 2009;182:3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi T., Roskin K., Baker B.M., Woodle E.S., Hildeman D. Advanced Genomics-Based Approaches for Defining Allograft Rejection With Single Cell Resolution. Front. Immunol. 2021;12:750754. doi: 10.3389/fimmu.2021.750754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verleden S.E., Vos R., Verleden G.M. Chronic lung allograft dysfunction: Light at the end of the tunnel? Curr. Opin. Organ Transplant. 2019;24:318–323. doi: 10.1097/MOT.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 86.Verleden G.M., Vos R., Vanaudenaerde B., Dupont L., Yserbyt J., Van Raemdonck D., Verleden S. Current views on chronic rejection after lung transplantation. Transpl. Int. 2015;28:1131–1139. doi: 10.1111/tri.12579. [DOI] [PubMed] [Google Scholar]
- 87.Sato M., Waddell T.K., Wagnetz U., Roberts H.C., Hwang D.M., Haroon A., Wagnetz D., Chaparro C., Singer L.G., Hutcheon M.A., et al. Restrictive allograft syndrome (RAS): A novel form of chronic lung allograft dysfunction. J. Heart Lung Transpl. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 88.Verleden G.M., Glanville A.R., Lease E.D., Fisher A.J., Calabrese F., Corris P.A., Ensor C.R., Gottlieb J., Hachem R.R., Lama V., et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transpl. 2019;38:493–503. doi: 10.1016/j.healun.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 89.Glanville A.R., Verleden G.M., Todd J.L., Benden C., Calabrese F., Gottlieb J., Hachem R.R., Levine D., Meloni F., Palmer S.M., et al. Chronic lung allograft dysfunction: Definition and update of restrictive allograft syndrome-A consensus report from the Pulmonary Council of the ISHLT. J. Heart Lung Transpl. 2019;38:483–492. doi: 10.1016/j.healun.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 90.Bedair B., Hachem R.R. Management of chronic rejection after lung transplantation. J. Thorac. Dis. 2021;13:6645–6653. doi: 10.21037/jtd-2021-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bos S., Filby A.J., Vos R., Fisher A.J. Effector immune cells in chronic lung allograft dysfunction: A systematic review. Immunology. 2022;166:17–37. doi: 10.1111/imm.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Royer P.J., Olivera-Botello G., Koutsokera A., Aubert J.D., Bernasconi E., Tissot A., Pison C., Nicod L., Boissel J.P., Magnan A. Chronic Lung Allograft Dysfunction: A Systematic Review of Mechanisms. Transplantation. 2016;100:1803–1814. doi: 10.1097/TP.0000000000001215. [DOI] [PubMed] [Google Scholar]
- 93.Watanabe T., Cypel M., Keshavjee S. Ex vivo lung perfusion. J. Thorac. Dis. 2021;13:6602–6617. doi: 10.21037/jtd-2021-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cypel M., Yeung J.C., Liu M., Anraku M., Chen F., Karolak W., Sato M., Laratta J., Azad S., Madonik M., et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 2011;364:1431–1440. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 95.Tikkanen J.M., Cypel M., Machuca T.N., Azad S., Binnie M., Chow C.W., Chaparro C., Hutcheon M., Yasufuku K., de Perrot M., et al. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J. Heart Lung Transpl. 2015;34:547–556. doi: 10.1016/j.healun.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 96.Divithotawela C., Cypel M., Martinu T., Singer L.G., Binnie M., Chow C.W., Chaparro C., Waddell T.K., de Perrot M., Pierre A., et al. Long-term Outcomes of Lung Transplant With Ex Vivo Lung Perfusion. JAMA Surg. 2019;154:1143–1150. doi: 10.1001/jamasurg.2019.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Islam D., Huang Y., Fanelli V., Delsedime L., Wu S., Khang J., Han B., Grassi A., Li M., Xu Y., et al. Identification and Modulation of Microenvironment is Crucial for Effective MSC Therapy in Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2019;199:1214–1224. doi: 10.1164/rccm.201802-0356OC. [DOI] [PubMed] [Google Scholar]
- 98.Wang C., Lv D., Zhang X., Ni Z.A., Sun X., Zhu C. Interleukin-10-Overexpressing Mesenchymal Stromal Cells Induce a Series of Regulatory Effects in the Inflammatory System and Promote the Survival of Endotoxin-Induced Acute Lung Injury in Mice Model. DNA Cell Biol. 2018;37:53–61. doi: 10.1089/dna.2017.3735. [DOI] [PubMed] [Google Scholar]