Abstract
Anti-NMDAR encephalitis has been associated with multiple antigenic triggers (i.e., ovarian teratomas, prodromal viral infections) but whether geographic, climatic, and environmental factors might influence disease risk has not been explored yet. We performed a systematic review and a meta-analysis of all published papers reporting the incidence of anti-NMDAR encephalitis in a definite country or region. We performed several multivariate spatial autocorrelation analyses to analyze the spatial variations in the incidence of anti-NMDA encephalitis depending on its geographical localization and temperature. Finally, we performed seasonal analyses in two original datasets from France and Greece and assessed the impact of temperature using an exposure-lag-response model in the French dataset. The reported incidence of anti-NMDAR encephalitis varied considerably among studies and countries, being higher in Oceania and South America (0.2 and 0.16 per 100,000 persons-year, respectively) compared to Europe and North America (0.06 per 100,000 persons-year) (p < 0.01). Different regression models confirmed a strong negative correlation with latitude (Pearson’s R = −0.88, p < 0.00001), with higher incidence in southern hemisphere countries far from the equator. Seasonal analyses showed a peak of cases during warm months. Exposure-lag-response models confirmed a positive correlation between extreme hot temperatures and the incidence of anti-NMDAR encephalitis in France (p = 0.03). Temperature analyses showed a significant association with higher mean temperatures and positive correlation with higher ultraviolet exposure worldwide. This study provides the first evidence that geographic and climatic factors including latitude, mean annual temperature, and ultraviolet exposure, might modify disease risk.
Keywords: anti-NMDAR encephalitis, geoepidemiology, seasonality
1. Introduction
The description of the N-Methyl-d-Aspartate receptor (NMDAR) encephalitis in 2007 [1] represented a paradigm shift in the field of neuroimmunology. Anti-NMDAR encephalitis is autoimmune encephalitis related to the presence of autoantibodies of the IgG1 subclass targeting the GluN1 subunit of the NMDAR, a glutamate receptor highly expressed on the surface of hippocampal neurons. Anti-NMDAR autoantibodies have demonstrated to be directly pathogenic, causing reversible synaptic dysfunction in neuronal cultures [2] and animal models [3].
Anti-NMDAR encephalitis primarily affects young females [4,5], one of the triggers being the presence of an underlying ovarian teratoma, which is detected in about half of the cases [4,5]. Preceding viral infections [5], not limited to herpes simplex [6] and Japanese encephalitis [7], represent additional triggers of the disease. Genetic factors such as HLA profile (HLA-I B*07:02 in European patients and HLA-II DRB1*16:02 in Chinese populations) have also been suggested to modulate disease risk [8,9], supporting the hypothesis that, similarly to several other autoimmune disorders, both genetic and environmental factors may concur to disease pathogenesis [10].
The possible impact of environmental factors such as latitude, sun exposure, and air pollution has not yet been evaluated in autoimmune encephalitis, although they have shown to heavily influence the risk and disease activity of multiple sclerosis [11,12], another immune-mediated disorder affecting the central nervous system.
Herein, we performed a systematic review and a meta-analysis of the literature, including unpublished datasets from four additional countries, to assess the incidence of anti-NMDAR encephalitis in different countries, searching for elements suggesting an influence from geographic, climatic, and environmental factors. To strengthen our findings, we performed seasonal and climatic analyses on two original datasets from France and Greece.
2. Materials and Methods
The literature review was conducted and reported following PRISMA statements [13]. The PubMed (https://pubmed.ncbi.nlm.nih.gov/, accesed on 15 January 2020) and Google Scholar (https://scholar.google.com/, accessed on 15 January 2020) research was performed between 20 December 2019 and 15 January 2020 using the keywords ‘autoimmune encephalitis’ and ‘NMDA encephalitis’ in combination with each of the 177 country names included in the ISO list of world countries (Supplementary Table S1). No restriction was applied concerning language or year of publication.
Two investigators (AA, GB) independently reviewed the articles retrieved from the research, extracting relevant information using a standardized data extraction sheet, as recommended by quality standards for reporting meta-analyses of observational studies in epidemiology [14]. The assessments performed separately by the two investigators were then cross-checked and, if any disagreement arose, a third reviewer (DP) was consulted to achieve a final decision.
To be included in the meta-analysis, studies needed to provide the number of incident cases or crude and/or age-specific incidence estimates for anti-NMDAR encephalitis, study period, and referring population. Studies reporting incidence estimates inferred from a subset of patients not representative of the whole anti-NMDAR encephalitis population (i.e., concerning intensive care unit or epileptic patients) were excluded. The list of the 68 studies [9,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] included in the meta-analysis is given in the Supplementary Data.
Literature data were complemented by unpublished data on the incidence of NMDAR encephalitis in Colombia, Chile, France, and Greece, collected by coauthors actively working in these countries. This allowed having information on the incidence of anti-NMDAR encephalitis in three countries, with no literature data available (Colombia, Chile, and Greece). This study was approved by the local ethics committee of the Pitié Salpêtrière Hospital and informed consent was waived (reference CPP SUD-EST II).
Two unpublished datasets of patients diagnosed with “definite” anti-NMDAR encephalitis according to the 2016 criteria [83], one from Greece and one from France, were used as exploratory datasets for additional analyses on seasonal and climatic trends. Individual data on Greek patients’ dataset were collected retrospectively during the period 2010–2019 from two diagnostic neuroimmune laboratories considered as nation-wide referral centers. Individual data on French patients were drawn from the database of the National Reference Centre for Paraneoplastic Neurological Syndromes and refer to the period 2008–2018.
Incidence rates were calculated using the number of incident cases per year over the referring population, assuming that reference populations were stable throughout the study period. We calculated an age and sex standardized incidence as directly standardized rates (DSR) using a gamma distribution [84] with the World Health Organization standard population with five-year intervals. We also obtained the female/male ratio with the 95% confidence interval (CI) using the Wald normal approximation and considering counts and person-year [85].
Overall incidence estimates were calculated using both fixed and random-effects models, weighted for inverse variance following DerSimonian’s method [86]. Heterogeneity between studies was assessed using a chi-square test (Cochran’s Q statistic) and quantified using the I² statistic [87].
Publication bias was evaluated with the aid of a funnel plot, the asymmetry of which was assessed with the Egger’s test [88]. The differences between different subgroups within the meta-analysis were assessed using different meta-regression models (Supplementary Methods).
Spatial autocorrelation refers to the correlation of a variable with itself in space. In our case, the variable was the incidence of anti-NMDAR encephalitis: a positive spatial autocorrelation existed if high incidence was associated with high incidence in neighboring countries, while a negative spatial autocorrelation existed if low incidence was associated with high incidence in neighboring countries. Global spatial autocorrelation was assessed using the Moran’s I and the Geary’s C indexes.
We assessed different multivariate spatial regression models (i.e., geographically weighted regression, ordinary least square regression, generalized additional model, and conditional and simultaneously autoregressive models) adjusting with the mean temperature of each country to further characterize in a multivariate model the spatial correlation of anti-NMDAR encephalitis with its spatial distribution. We selected the model with the best performance according to the minimum Akaike Information Criteria (AIC). From these models, we obtained the local R2 that were mapped. In addition, these models provided a prediction of anti-NMDAR encephalitis at a worldwide level.
2.1. Correlating the Incidence of Anti-NMDAR Encephalitis with Different Climatic, Environmental, and Demographic Factors
We performed several linear regressions to correlate the incidence of anti-NMDAR encephalitis with different environmental, climatologic, or demographic features. The degree of correlation was assessed using the coefficient R of Pearson’s correlation. Demographic features included the urban population percentage or the socio-demographic index (SDI) [89]. Climatic and environmental variables included mean annual temperature, particulate matter air pollution (PM2.5) exposure, the median CO2 emissions per country, and the ultraviolet exposure in each included country (Supplementary Methods).
We performed an exposure-lag-response regression between the number of anti-NMDAR encephalitis cases and the temperature in France and in Ile-de-France, as previously described [90] (Supplementary Methods).
2.2. Seasonal and Monthly Trends
After comparing the accuracy of different seasonal and non-seasonal models, we used the X13-Seasonal Extraction in Autoregressive Integrated Moving Average (ARIMA) Time-Series (SEATS)-ARIMA algorithm [91] in the French dataset, and the Seasonal and Trend decomposition using locally weighted running line smoother (LOESS), STL [92] in the Greek dataset, to assess temporal trends (Supplementary Methods).
We compared average monthly counts of anti-NMDAR encephalitis, using a Quasi-Poisson regression, with the number of cases as the outcome, month as the sole predictor (with February as baseline), and the log of the population as an offset (Supplementary Methods).
All statistical analyses were performed using the software “R” (version 4.0.1). The threshold for statistical significance was p < 0.05, all tests were bilateral. Details from the different version of R packages as well as the R scripts, datasets, and the methodological details used to reproduce the vast majority of the results are provided in the Supplementary Methods and can be found at https://osf.io/u5hjf/?view_only=bb4ed5d417b6410c8c3a9ebad81bee09, accessed on 15 October 2022.
3. Results
3.1. Literature Meta-Analysis
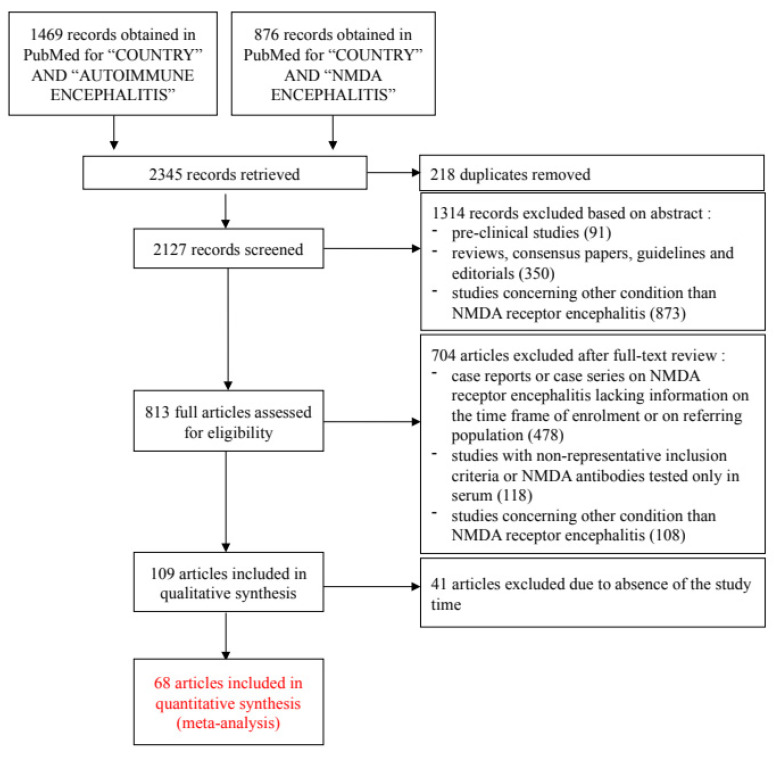
Our research strategy yielded 2127 unique records in PubMed. After a systematic process of exclusion (Figure 1), we were left with 68 articles that, with the addition of four unpublished studies, provided information on the incidence of anti-NMDAR encephalitis in 30 different countries, Supplementary Table S2.
Figure 1.
Flow chart for study selection according to the PRISMA guidelines.
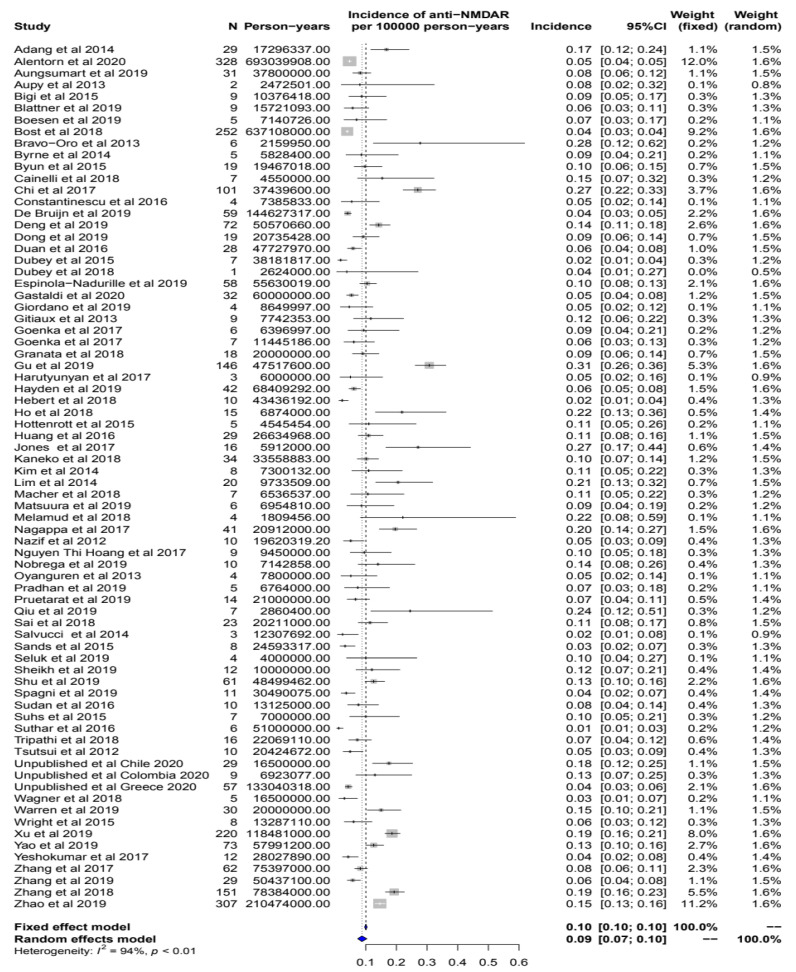
The crude population-based incidences of anti-NMDAR encephalitis within the different studies included in the meta-analysis are summarized in the Forest plot in Figure 2. Pooling together the data from all the studies, the crude overall incidence estimate for anti-NMDAR encephalitis, calculated using a random effect model, was 0.09 per 100,000 inhabitants-year (95% CI: 0.07–0.10). The value of statistical heterogeneity for this analysis was high (I² = 94%), reflecting how the crude incidence of anti-NMDAR encephalitis varied across studies (from 0.01 to 0.31 cases per 100,000 inhabitants-year). The Funnel plot (Supplementary Figure S2) revealed a relatively symmetrical distribution of the studies, with Egger’s bias test p = 0.3, suggesting a non-significant asymmetry of studies.
Figure 2.
Forest plot summarizing the estimates for the population-based incidence of anti-NMDAR encephalitis within the different studies included in the meta-analysis. Summary is expressed as the number of cases of anti-NMDAR encephalitis per 100,000 inhabitants-year. An I² value (statistical heterogeneity) of 94% indicates high variability.
3.2. Unpublished Data
Literature data were complemented by unpublished data on the incidence of NMDAR encephalitis in Colombia, Chile, France, and Greece, collected by coauthors actively working in these countries. This allowed having information on the incidence of anti-NMDAR encephalitis in three countries, with no literature data available (Colombia, Chile, and Greece).
3.3. The Incidence of Anti-NMDAR Encephalitis Differs between Continents
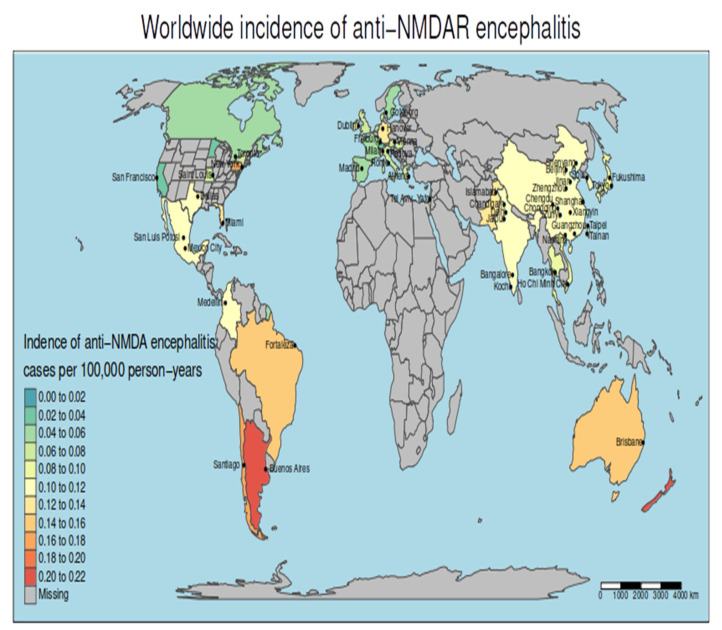
We graphically represented crude incidence rates in the countries included in our meta-analysis in a world map (Figure 3). The crude incidence of anti-NMDAR encephalitis differed based on the continent, varying from 0.06 per 100,000 inhabitants-year (95% CI 0.05–0.07) in Europe (Supplementary Figure S2) and 0.06 (0.04–0.09) in North America (Supplementary Figure S3) to 0.11 per 100,000 inhabitants-year (0.09–0.13) in Asia (Supplementary Figure S4), 0.16 (0.12–0.21) in South America (Supplementary Figure S5), and 0.2 (0.11–0.35) in Oceania (including Australia and New Zealand) (Supplementary Figure S6). According to different meta-regressions, the incidence of anti-NMDAR encephalitis was significantly lower in Europe than in South America, Oceania and Asia (p < 0.0001, p < 0.0001 and p = 0.001, respectively), while it was very similar to North America (p = 0.9). Similarly, South America, Asia, and Oceania countries, had a higher incidence than North America countries, p = 0.01, p < 0.001, and p = 0.02, respectively. Finally, Asia showed an intermediate incidence of 0.11 per 100 000 inhabitants-year (0.09–0.13), with no significant differences compared to South America (p = 0.2) or Oceania (p = 0.5).
Figure 3.
Worldwide distribution of anti-NMDAR encephalitis using the data from the systematic review and meta-analysis. A city name appears when the study considered for that country used data from that city and its referral area while, when no city is indicated, studies were performed at a country-wide level.
3.4. Geographical Clusters of Higher and Lower Incidence
Global spatial autocorrelation analyses showed a moderate but significant correlation between the incidence of anti-NMDAR encephalitis and geography (Moran’s I = 0.23, p < 0.00001; Geary’s C = 0.39, p < 0.00001).
3.5. Multivariate Spatial Analyses: The Impact of Temperature and Latitude
We compared several multivariate spatial regression models (Supplementary Methods) and we selected the GWR model because it had the highest adjusted R² (0.89) and the lowest AIC (−841). The GWR was chosen to assess spatial autocorrelation (Supplementary Figure S7A) in a multivariate model, adjusting by the mean annual temperature of each country (Supplementary Figure S7B). We used the GWR to calculate local R² for each country and obtained high local R2 values (0.75–1), reflecting high goodness of fit of a model, in North America, in Oceania, and most European countries (Supplementary Figure S7C). The GWR was then used to produce a map of the predicted probability of anti-NMDAR encephalitis, which showed a higher risk for southern hemisphere countries (Supplementary Figure S7D).
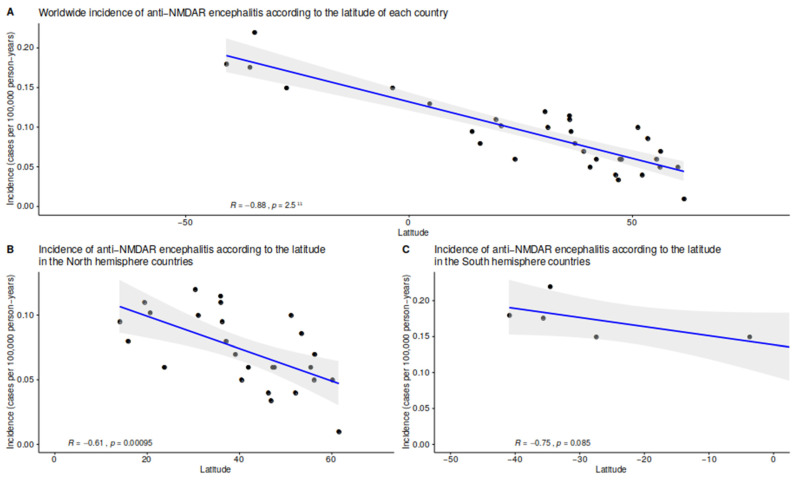
In addition to GWR, multiple linear regression models were used to explore the correlation between the incidence of anti-NMDAR encephalitis and geographic, climatic, environmental, and demographic factors. Interestingly, we observed a strong negative correlation between latitude and the incidence of anti-NMDAR encephalitis (R = −0.88, p < 0.00001) (Figure 4), the incidence of anti-NMDAR encephalitis increasing progressively from the North of Europe to Argentina. The incidence of anti-NMDAR encephalitis showed a positive correlation with mean annual temperature (R = 0.45, p = 0.01) (Supplementary Figure S8) and ultraviolet exposure (R = 0.46, p = 0.02) (Supplementary Figure S9), increasing with higher mean annual temperatures and higher ultraviolet exposure. In northern hemisphere countries, the incidence of anti-NMDAR encephalitis showed an inverse correlation with CO2 emissions (R = −0.5, p = 0.008, Supplementary Figure S10), particulate matter air pollution PM2.5 (R = 0.55, p = 0.005) (Supplementary Figure S11), urban population percentage (R = −0.4, p = 0.03) (Supplementary Figure S12), and SDI (R = −0.4, p = 0.02) (Supplementary Figure S13). These observations did not apply to southern hemisphere countries, where most p-values did not reach statistical significance.
Figure 4.
Linear regressions assessing the association between the latitude of the country and the incidence of anti-NMDAR encephalitis from the meta-analysis, worldwide (A–C). The grey band around the regression line represents the 95% CI. The R is estimated using the Pearson correlation.
3.6. Spatial and Temporal Analyses on the French and Greek Dataset
To better assess the impact of some climatic variables on the incidence of anti-NMDAR encephalitis, we analyzed two unpublished cohorts, one from France (n = 329, 328 with age available) and one from Greece (n = 57). The crude and standardized incidences of anti-NMDAR encephalitis in the French and Greek datasets are provided in Supplementary Tables S3 and S4. We represented the female/male ratio according to a five-year interval in two datasets with a higher proportion of females. Female predominance was stronger in younger patients (Supplementary Figure S14). The frequency of the different tumors associated with anti-NMDAR encephalitis is described in Supplementary Figure S15.
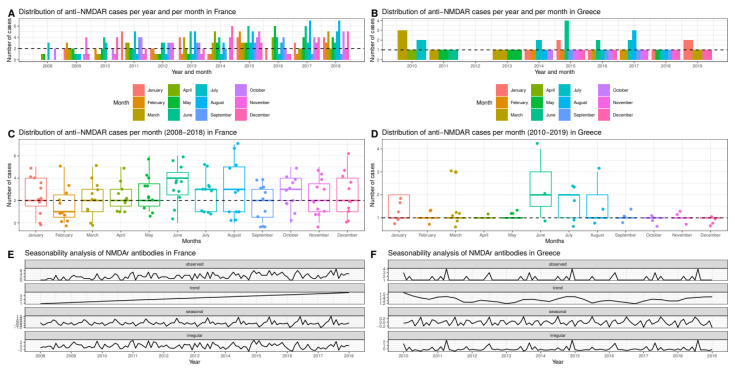
These two datasets were used for temporal distribution analyses. In the French dataset, the median number of cases was 2 per month, with a recurrent lower number of cases in February and a recurrent higher number of cases in June and August (Figure 5, panel C). In the Greek dataset, where the median number of cases was 1 per month, this trend was far less evident (Figure 5, panel D). To better circumstantiate monthly variations, we performed a Quasi-Poisson regression using February as the month of reference. The Quasi-Poisson regression disclosed a significant higher number of cases in June in both countries, with a relative risk (RR) of 2.1 (95% CI 1.2–4) and p = 0.02 in France and a RR of 2.3 (1.2–4.7) and p = 0.02 in the Greek dataset.
Figure 5.
Temporal distribution of anti-NMDAR encephalitis cases in France (2008–2018) (panel A,C,E) and Greece (2010–2019) (panel B,D,F). (Panel A,B) show the aggregated number of cases per year and month; (panel C,D) show the number of cases per month (dotted lines in both panels indicate median values); (panel E,F) show long-term trends and seasonality.
These findings prompted us to perform more in-depth seasonal analyses using the ARIMA model (French dataset) and the STL model (Greek dataset), details are provided in the Supplementary Methods. These models confirmed the existence of a seasonal trend in both countries, with a recurrent peak of cases during summer (Figure 5, panel E and F). The same models disclosed a progressive increase in the number of cases of anti-NMDAR encephalitis diagnosed in France over the study interval (2008–2018) (Figure 5, panel E) that was not observed in Greece, where the yearly number of cases remained relatively constant from 2010 to 2019 (Figure 5, panel F).
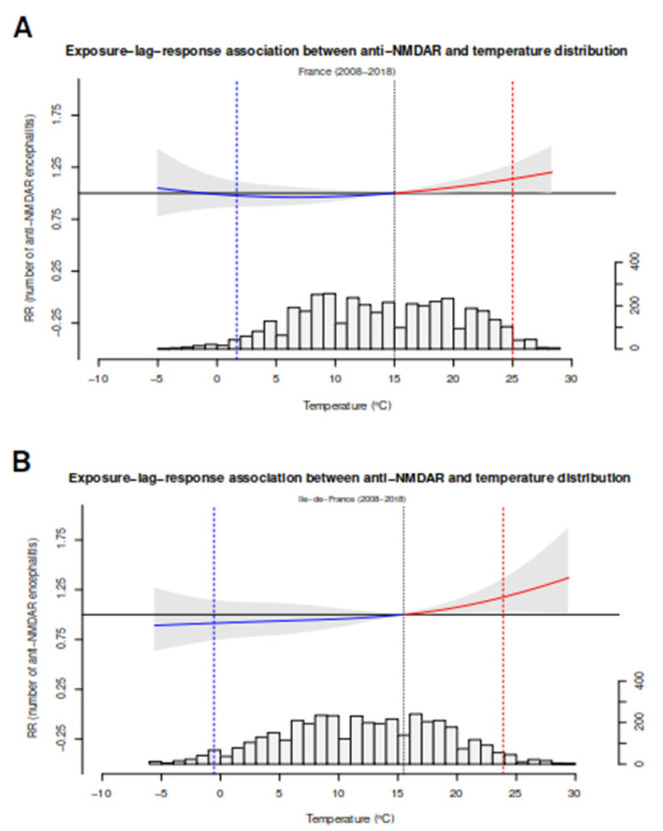
Based on the observation that the incidence of anti-NMDAR encephalitis seemed to increase during warm months, we used the French dataset to perform temperature analyses. Tlag-he impact of temperature on the incidence of anti-NMDAR encephalitis was assessed using a lag-response association model. Both in the overall dataset (n = 328) and the subset of patients from the Île-de-France region (n = 115), which was the region with more cases, we observed a significant association between hot temperatures and the crude incidence of anti-NMDAR encephalitis in the overall dataset, RR 1.2 [1.02–1.4, p = 0.03, and also in Île-de-France, RR 1.15 [1.02–1.55] p = 0.04 (Figure 6, panel A and B), providing additional evidence in support of this association. This model was used to estimate the quantitative impact of temperature on the incidence of anti-NMDAR encephalitis. We found that high temperatures accounted for approximately 15% (CI 0.7–27%) of cases of anti-NMDAR encephalitis. The Greek dataset was not analyzed using this approach due to the limited number of patients.
Figure 6.
Exposure-lag-association model between anti-NMDAR encephalitis and the temperature in the Ile-de-France region (panel A) and all France (panel B). Exposure–response associations as best linear unbiased prediction (with 95% CI, shaded grey), with related temperature distributions. Solid grey lines are minimum incidence temperatures and dotted lines are the 2·5th and 97·5th percentiles. Note that in both models, the grey area is slightly above the horizontal line (RR = 1), showing a significant association. RR = relative risk.
4. Discussion
In this systematic review and meta-analysis, we explored the association between the incidence of anti-NMDAR encephalitis and several geographic, climatic, and environmental factors. We found that the incidence of anti-NMDAR encephalitis strongly correlated with latitude, mean annual temperature, and ultraviolet exposure. We identified a seasonal distribution, with a peak of cases during warm months and a correlation with extreme hot temperatures. The results were not unanticipated, as several autoimmune diseases show a strong geographical distribution [93,94]. The best paradigm in neurology is multiple sclerosis, which has a higher incidence in countries far from the equator and lower incidence in countries near the equator [95,96]. This phenomenon has been attributed to mean vitamin D levels, which are dependent on sun exposure, and decrease as the distance from the equator increases, exposing to a higher disease risk [93]. Conversely, our meta-analysis suggests that ultraviolet exposure might influence the risk of anti-NMDAR encephalitis, the geographical distribution observed for anti-NMDAR encephalitis differs from the one depicted for multiple sclerosis, suggesting that additional climatic and environmental factors might be implicated.
Similarly to sun exposure, mean annual temperature has shown to modulate the incidence of multiple sclerosis [97] and other autoimmune disorders [98] and might represent one of the factors responsible for the geographical gradient observed in our meta-analysis. Intriguingly, the exposure-lag-associated study pinpointed a non-linear association between hot temperatures and the incidence of anti-NMDAR encephalitis (Figure 6). Interestingly, this type of non-linear association with temperature and other health conditions has been previously described [90].
Consistently with temperature analysis, we observed that anti-NMDAR encephalitis displayed a seasonal pattern, with a higher number of cases during warm months. It should be noted that in the Greek dataset, with a limited number of cases, there was a great number of cases during June, July and August (Figure 5D). A similar observation was previously reported in a small study conducted in the United States on pediatric patients [15]; however, multivariate analyses or seasonal modelling were not included. Although, a higher peak of cases during summer could simply reflect higher seasonal temperatures, other factors that have not been taken into account in the present study, such as recurrent viral epidemics, might also be implicated. Some studies on infectious encephalitis, including herpes simplex virus encephalitis, conducted in western countries have pinpointed a higher incidence of hospital admissions during summer [99,100], while others have failed to disclose any significant seasonal pattern [101]. Besides virus commonly responsible for encephalitis [6,7], other non-neurotropic viruses display a seasonal pattern and might be responsible for the higher number of cases of anti-NMDAR encephalitis.
This study has several limitations related to the limited data available in the literature on this rare disorder. Most of the studies included in the meta-analysis were retrospective and, as such, potentially affected by referral, selection, and misclassification biases. In addition, we did not have patient individual data for most of the studies limiting some of analysis, for example, the possibility to analyze the seasonality at worldwide level or the potential impact of UV on the incidence of anti-NMDAR encephalitis and we could not include age and gender adjustment, due to this limitation. The differences in age composition rendered crude incidence estimates not directly comparable between populations. Data on the incidence of anti-NMDAR encephalitis were unavailable for many world countries, mainly in the southern hemisphere, and completely missing for Africa. Therefore, our results should be interpreted with caution when considering the data of southern hemisphere, including the association between NMDAR encephalitis incidence and ultraviolet radiation exposure and the mean annual temperature.
The impact of factors such as nutrition and lifestyle could not be assessed in our meta-analysis, although these likely represent important elements, as demonstrated for other autoimmune disorders [102]. However, we used the Socio-Demographic Index (SDI) similar factors. This is a summary measure of a geography’s socio-demographic development. It is based on average income per person, educational attainment, and total fertility rate (TFR) (Supplementary Methods and Supplementary Figure S13). Studies assessing the changes in disease risk as a consequence of changes in the place of living, as performed in patients with multiple sclerosis [103], might help to assess the weight of environmental factors and living habits on disease risk.
Another factor that could not be assessed in our meta-analysis is the genetic risk, which could indeed contribute to the variations in the incidence of anti-NMDAR encephalitis among countries and populations. Studies on genetic risk factors are to date limited to European and Chinese patients [8,9], and only further studies in low- and high-risk populations will elucidate the relative weight of genetics.
To sum up, this study provides the first evidence that geographic and climatic factors might modulate the risk of anti-NMDAR encephalitis, paving the way to a broader range of explorations on the impact of environmental factors in the pathogenesis of this rare disease. The approach described in this study could be applied to other types of autoimmune encephalitis, clarifying if other entities within this spectrum share similar risk factors.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biomedicines11061525/s1.
Author Contributions
Conceptualization, A.A.; methodology, A.A. and P.-Y.B.; formal analysis, A.A. and G.B.; resources, A.A., G.B., D.P., H.A., J.T., S.M.-C., A.V., B.J., G.R.B., F.A.G.J., D.C., J.V.T., A.M.M., C.D., P.S., M.T., L.G., M.G., G.P., V.R., M.B., I.H.V., M.D., N.W., M.C.D. and J.H.; data curation, D.P.; writing—original draft preparation, A.A. and G.B.; writing—review and editing, A.A., G.B., J.-Y.D., P.-Y.B. and D.P.; visualization, A.A.; supervision, A.A., G.B. and D.P.; project administration, A.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board.
Informed Consent Statement
Patient consent was waived due to the retrospective and observational nature of this study.
Data Availability Statement
The data and R scripts as well as the methodological details used to reproduce the vast majority of the results are provided in the Supplementary Methods.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work has been developed within the BETPSY project, supported by a public grant overseen by the French National Research Agency (ANR) (reference ANR-18-RHUS-0012). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dalmau J., Tüzün E., Wu H.-Y., Masjuan J., Ba J.E.R., Voloschin A., Baehring J.M., Shimazaki H., Koide R., King D., et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes E.G., Peng X., Gleichman A.J., Lai M., Zhou L., Tsou R., Parsons T.D., Lynch D.R., Dalmau J., Balice-Gordon R.J. Cellular and Synaptic Mechanisms of Anti-NMDA Receptor Encephalitis. J. Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planagumà J., Leypoldt F., Mannara F., Gutiérrez-Cuesta J., Martín-García E., Aguilar E., Titulaer M., Petit-Pedrol M., Jain A., Balice-Gordon R., et al. Human N-methyl d-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J., Gleichman A.J., Hughes E.G., Rossi J.E., Peng X., Lai M., Dessain S.K., Rosenfeld M.R., Balice-Gordon R., Lynch D.R. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J., Lancaster E., Martinez-Hernandez E., Rosenfeld M.R., Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armangue T., Spatola M., Vlagea A., Mattozzi S., Cárceles-Cordon M., Martinez-Heras E., Llufriu S., Muchart J., Erro M.E., Abraira L., et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma J., Han W., Jiang L. Japanese encephalitis-induced anti-N-methyl-d-aspartate receptor encephalitis: A hospital-based prospective study. Brain Dev. 2020;42:179–184. doi: 10.1016/j.braindev.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Mueller S.H., Färber A., Prüss H., Melzer N., Golombeck K.S., Kümpfel T., Thaler F., Elisak M., Lewerenz J., Kaufmann M., et al. Genetic predisposition in anti-LGI1 and anti-NMDA receptor encephalitis. Ann. Neurol. 2018;83:863–869. doi: 10.1002/ana.25216. [DOI] [PubMed] [Google Scholar]
- 9.Shu Y., Qiu W., Zheng J., Sun X., Yin J., Yang X., Yue X., Chen C., Deng Z., Li S., et al. HLA class II allele DRB1*16:02 is associated with anti-NMDAR encephalitis. J. Neurol. Neurosurg. Psychiatry. 2019;90:652–658. doi: 10.1136/jnnp-2018-319714. [DOI] [PubMed] [Google Scholar]
- 10.Wu C.-Y., Wu J.-D., Chen C.-C. The Association of Ovarian Teratoma and Anti-N-Methyl-D-Aspartate Receptor Encephalitis: An Updated Integrative Review. Int. J. Mol. Sci. 2021;22:10911. doi: 10.3390/ijms222010911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson J.S., Wang W., Otahal P., Blizzard L., Mei I.A.F.V.D., Taylor B.V. Latitude continues to be significantly associated with the prevalence of multiple sclerosis: An updated meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2019;90:1193–1200. doi: 10.1136/jnnp-2018-320189. [DOI] [PubMed] [Google Scholar]
- 12.Bergamaschi R., Cortese A., Pichiecchio A., Berzolari F.G., Borrelli P., Mallucci G., Bollati V., Romani A., Nosari G., Villa S., et al. Air pollution is associated to the multiple sclerosis inflammatory activity as measured by brain MRI. Mult. Scler. J. 2017;24:1578–1584. doi: 10.1177/1352458517726866. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Adang L.A., Lynch D.R., Panzer J.A. Pediatric anti-NMDA receptor encephalitis is seasonal. Ann. Clin. Transl. Neurol. 2014;1:921–925. doi: 10.1002/acn3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aungsumart S., Ha A., Apiwattanakul M. Abnormal level of consciousness predicts outcomes of patients with anti-NMDA encephalitis. J. Clin. Neurosci. 2018;62:184–187. doi: 10.1016/j.jocn.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Aupy J., Collongues N., Blanc F., Tranchant C., Hirsch E., de Seze J. Encéphalites dysimmunitaires, données cliniques, radiologiques et immunologiques. Rev. Neurol. 2013;169:142–153. doi: 10.1016/j.neurol.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Bigi S., Hladio M., Twilt M., Dalmau J., Benseler S.M. The growing spectrum of antibody-associated inflammatory brain diseases in children. Neurol. Neuroimmunol. Neuroinflamm. 2015;2:e92. doi: 10.1212/NXI.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blattner M.S., de Bruin G.S., Bucelli R.C., Day G.S. Sleep disturbances are common in patients with autoimmune encephalitis. J. Neurol. 2019;266:1007–1015. doi: 10.1007/s00415-019-09230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boesen M.S., Born A.P., Lydolph M.C., Blaabjerg M., Børresen M.L. Pediatric autoimmune encephalitis in Denmark during 2011–17: A nationwide multicenter population-based cohort study. Eur. J. Paediatr. Neurol. 2019;23:639–652. doi: 10.1016/j.ejpn.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Bost C., Chanson E., Picard G., Meyronet D., Mayeur M.-E., Ducray F., Rogemond V., Psimaras D., Antoine J.-C., Delattre J.-Y., et al. Malignant tumors in autoimmune encephalitis with anti-NMDA receptor antibodies. J. Neurol. 2018;265:2190–2200. doi: 10.1007/s00415-018-8970-0. [DOI] [PubMed] [Google Scholar]
- 22.Bravo-Oro A., Abud-Mendoza C., Quezada-Corona A., Dalmau J., Campos-Guevara V. Anti-N-methyl-d-aspartate (NMDA) receptor encephalitis: Experience with six pediatric patients. Potential efficacy of methotrexate. Rev. Neurol. 2013;57:405–410. [PMC free article] [PubMed] [Google Scholar]
- 23.Byrne S., McCoy B., Lynch B., Webb D., King M.D. Does early treatment improve outcomes in N-methyl-d-aspartate receptor encephalitis? Dev. Med. Child Neurol. 2014;56:794–796. doi: 10.1111/dmcn.12411. [DOI] [PubMed] [Google Scholar]
- 24.Byun J.-I., Lee S.-T., Moon J., Jung K.-H., Shin J.-W., Sunwoo J.-S., Lim J.-A., Shin Y.-W., Kim T.-J., Lee K.-J., et al. Cardiac sympathetic dysfunction in anti-NMDA receptor encephalitis. Auton. Neurosci. 2015;193:142–146. doi: 10.1016/j.autneu.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Cainelli E., Nosadini M., Sartori S., Suppiej A. Neuropsychological and Psychopathological Profile Of Anti-Nmdar Encephalitis: A Possible Pathophysiological Model For Pediatric Neuropsychiatric Disorders. Arch. Clin. Neuropsychol. 2018;34:1309–1319. doi: 10.1093/arclin/acy088. [DOI] [PubMed] [Google Scholar]
- 26.Chi X., Wang W., Huang C., Wu M., Zhang L., Li J., Zhou D. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol. Scand. 2016;136:298–304. doi: 10.1111/ane.12723. [DOI] [PubMed] [Google Scholar]
- 27.Constantinescu R., Krýsl D., Bergquist F., Andrén K., Malmeström C., Asztély F., Axelsson M., Menachem E.B., Blennow K., Rosengren L., et al. Cerebrospinal fluid markers of neuronal and glial cell damage to monitor disease activity and predict long-term outcome in patients with autoimmune encephalitis. Eur. J. Neurol. 2016;23:796–806. doi: 10.1111/ene.12942. [DOI] [PubMed] [Google Scholar]
- 28.De Bruijn M.A., van Sonderen A., van Coevorden-Hameete M.H., Bastiaansen A.E., Schreurs M.W., Rouhl R.P., van Donselaar C.A., Majoie M.H., Neuteboom R.F., Smitt P.A.S., et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABABR encephalitis. Neurology. 2019;92:e2185–e2196. doi: 10.1212/WNL.0000000000007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng S., Qiu K., Liu H., Wu X., Lei Q., Lu W. Clinical Characteristics and Short-Term Prognosis of Autoimmune Encephalitis: A Single-Center Cohort Study in Changsha, China. Front. Neurol. 2019;10:539. doi: 10.3389/fneur.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong X., Zheng D., Nao J. Clinical characteristics and factors associated with short-term prognosis in adult patients with autoimmune encephalitis of non-neoplastic etiology. Neurol. Sci. 2019;40:1567–1575. doi: 10.1007/s10072-019-03883-7. [DOI] [PubMed] [Google Scholar]
- 31.Duan B.-C., Weng W.-C., Lin K.-L., Wong L.C., Li S.-T., Hsu M.-H., Lin J.-J., Fan P.-C., Lin M.-I., Chiu N.-C., et al. Variations of movement disorders in anti-N-methyl-d-aspartate receptor encephalitis. Medicine. 2016;95:e4365. doi: 10.1097/MD.0000000000004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubey D., Pittock S.J., Kelly C.R., McKeon A., Lopez-Chiriboga A.S., Lennon V.A., Gadoth A., Smith C.Y., Bryant S.C., Klein C.J., et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann. Neurol. 2018;83:166–177. doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubey D., Samudra N., Gupta P., Agostini M., Ding K., Van Ness P.C., Vernino S., Hays R. Retrospective case series of the clinical features, management and outcomes of patients with autoimmune epilepsy. Seizure. 2015;29:143–147. doi: 10.1016/j.seizure.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Nadurille M.E., Flores-Rivera J., Rivas-Alonso V., Vargas-Cañas S., Fricchione G.L., Bayliss L., Martínez-Juárez I.E., Hernandez-Vanegas L.E., Martinez-Hernandez R., Bautista-Gomez P., et al. Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin. Neurosci. 2019;73:574–580. doi: 10.1111/pcn.12867. [DOI] [PubMed] [Google Scholar]
- 35.Fominykh V., Brylev L., Gaskin V., Luzin R., Yakovlev A., Komoltsev I., Belousova I., Rosliakova A., Guekht A., Gulyaeva N. Neuronal damage and neuroinflammation markers in patients with autoimmune encephalitis and multiple sclerosis. Metab. Brain Dis. 2019;34:1473–1485. doi: 10.1007/s11011-019-00452-x. [DOI] [PubMed] [Google Scholar]
- 36.Gastaldi M., Mariotto S., Giannoccaro M.P., Iorio R., Zoccarato M., Nosadini M., Benedetti L., Casagrande S., Di Filippo M., Valeriani M., et al. Subgroup comparison according to clinical phenotype and serostatus in autoimmune encephalitis: A multicenter retrospective study. Eur. J. Neurol. 2019;27:633–643. doi: 10.1111/ene.14139. [DOI] [PubMed] [Google Scholar]
- 37.Giordano A., Fazio R., Gelibter S., Minicucci F., Vabanesi M., Anzalone N., Magnani G., Filippi M., Martinelli V. Diagnosing autoimmune encephalitis in a real-world single-centre setting. J. Neurol. 2019;267:449–460. doi: 10.1007/s00415-019-09607-3. [DOI] [PubMed] [Google Scholar]
- 38.Gitiaux C., Simonnet H., Eisermann M., Leunen D., Dulac O., Nabbout R., Chevignard M., Honnorat J., Gataullina S., Musset L., et al. Early electro-clinical features may contribute to diagnosis of the anti-NMDA receptor encephalitis in children. Clin. Neurophysiol. 2013;124:2354–2361. doi: 10.1016/j.clinph.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Goenka A., Jain V., Nariai H., Spiro A., Steinschneider M. Extended Clinical Spectrum of Anti-N-Methyl-d-Aspartate Receptor Encephalitis in Children: A Case Series. Pediatr. Neurol. 2017;72:51–55. doi: 10.1016/j.pediatrneurol.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Granata T., Matricardi S., Ragona F., Freri E., Zibordi F., Andreetta F., Binelli S., Nardocci N. Pediatric NMDAR encephalitis: A single center observation study with a closer look at movement disorders. Eur. J. Paediatr. Neurol. 2018;22:301–307. doi: 10.1016/j.ejpn.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Gu Y., Zhong M., He L., Li W., Huang Y., Liu J., Chen Y., Xiao Z. Epidemiology of Antibody-Positive Autoimmune Encephalitis in Southwest China: A Multicenter Study. Front. Immunol. 2019;10:2611. doi: 10.3389/fimmu.2019.02611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harutyunyan G., Hauer L., Dünser M.W., Moser T., Pikija S., Leitinger M., Novak H.F., Aichhorn W., Trinka E., Sellner J. Risk Factors for Intensive Care Unit Admission in Patients with Autoimmune Encephalitis. Front. Immunol. 2017;8:835. doi: 10.3389/fimmu.2017.00835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayden Z., Böröcz K., Csizmadia Z., Balogh P., Kellermayer Z., Bodó K., Najbauer J., Berki T. Single-center study of autoimmune encephalitis-related autoantibody testing in Hungary. Brain Behav. 2019;9:e01454. doi: 10.1002/brb3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hébert J., Day G.S., Steriade C., Wennberg R.A., Tang-Wai D.F. Long-Term Cognitive Outcomes in Patients with Autoimmune Encephalitis. Can. J. Neurol. Sci. 2018;45:540–544. doi: 10.1017/cjn.2018.33. [DOI] [PubMed] [Google Scholar]
- 45.Ho A.C.-C., Chan S.H.-S., Chan E., Wong S.S.-N., Fung S.T.-H., Cherk S.W.-W., Fung E.L.-W., Ma K.-H., Tsui K.-W., Yau E.K.-C., et al. Anti-N-methyl-d-aspartate receptor encephalitis in children: Incidence and experience in Hong Kong. Brain Dev. 2018;40:473–479. doi: 10.1016/j.braindev.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Hottenrott T., Dersch R., Berger B., Rauer S., Eckenweiler M., Huzly D., Stich O. The intrathecal, polyspecific antiviral immune response in neurosarcoidosis, acute disseminated encephalomyelitis and autoimmune encephalitis compared to multiple sclerosis in a tertiary hospital cohort. Fluids Barriers CNS. 2015;12:27. doi: 10.1186/s12987-015-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Q., Wu Y., Qin R., Wei X., Ma M. Clinical characteristics and outcomes between children and adults with anti-N-Methyl-d-Aspartate receptor encephalitis. J. Neurol. 2016;263:2446–2455. doi: 10.1007/s00415-016-8282-1. [DOI] [PubMed] [Google Scholar]
- 48.Jones H.F., Mohammad S.S., Reed P.W., Dunn P.P.J., Steele R.H., Dale R.C., Sharpe C. Anti-N-methyl-d-aspartate receptor encephalitis in Māori and Pacific Island children in New Zealand. Dev. Med. Child Neurol. 2017;59:719–724. doi: 10.1111/dmcn.13420. [DOI] [PubMed] [Google Scholar]
- 49.Kaneko A., Kaneko J., Tominaga N., Kanazawa N., Hattori K., Ugawa Y., Moriya A., Kuzume D., Ishima D., Kitamura E., et al. Pitfalls in clinical diagnosis of anti-NMDA receptor encephalitis. J. Neurol. 2018;265:586–596. doi: 10.1007/s00415-018-8749-3. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.Y., Choi S.A., Ryu H.W., Kim H., Lim B.C., Hwang H., Chae J.-H., Choi J., Kim K.J., Hwang Y.S., et al. Screening Autoimmune Anti-neuronal Antibodies in Pediatric Patients with Suspected Autoimmune Encephalitis. J. Epilepsy Res. 2014;4:55–61. doi: 10.14581/jer.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim J.-A., Lee S.-T., Jung K.-H., Kim S., Shin J.-W., Moon J., Byun J.-I., Kim T.-J., Shin Y.-W., Lee K.-J., et al. Anti-N-Methyl-D-Aspartate Receptor Encephalitis in Korea: Clinical Features, Treatment, and Outcome. J. Clin. Neurol. 2014;10:157–161. doi: 10.3988/jcn.2014.10.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macher S., Zimprich F., De Simoni D., Höftberger R., Rommer P.S. Management of Autoimmune Encephalitis: An Observational Monocentric Study of 38 Patients. Front. Immunol. 2018;9:2708. doi: 10.3389/fimmu.2018.02708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuura R., Hamano S.-I., Daida A., Nonoyama H., Kubota J., Ikemoto S., Hirata Y., Koichihara R., Kikuchi K., Yamaguchi A., et al. Serum matrix metallopeptidase-9 and tissue inhibitor of metalloproteinase-1 levels in autoimmune encephalitis. Brain Dev. 2020;42:264–269. doi: 10.1016/j.braindev.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Melamud L.I., Fernández V.C., Manin A., Villa A.M. Autoimmune encephalitis and immune therapy: Lessons from Argentina. Acta Neurol. Belg. 2018;120:565–572. doi: 10.1007/s13760-018-1013-x. [DOI] [PubMed] [Google Scholar]
- 55.Nagappa M., Parayil S.B., Mahadevan A., Sinha S., Mathuranath P.S., Taly A.B. Management of Anti- N-Methyl-d-Aspartate (NMDA) Receptor Encephalitis in Children. J. Child Neurol. 2017;32:513–514. doi: 10.1177/0883073816689518. [DOI] [PubMed] [Google Scholar]
- 56.Nazif T.M., Vázquez J., Honig L.S., Dizon J.M. Anti-N-methyl-D-aspartate receptor encephalitis: An emerging cause of centrally mediated sinus node dysfunction. Europace. 2012;14:1188–1194. doi: 10.1093/europace/eus014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoang M.N.T., Hoan P.N., Le Van T., McBride A., Trung N.H.D., Tan T.T., Thu H.N.T., Heemskerk D., Day J., Vincent A., et al. First reported cases of anti-NMDA receptor encephalitis in Vietnamese adolescents and adults. J. Neurol. Sci. 2017;373:250–253. doi: 10.1016/j.jns.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nóbrega P.R., Pitombeira M.S., Mendes L.S., Krueger M.B., Santos C.F., Morais N.M.D.M., Simabukuro M.M., Maia F.M., Braga-Neto P. Clinical Features and Inflammatory Markers in Autoimmune Encephalitis Associated with Antibodies Against Neuronal Surface in Brazilian Patients. Front. Neurol. 2019;10:472. doi: 10.3389/fneur.2019.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oyanguren B., Sánchez V., González F.J., De Felipe A., Esteban L., López-Sendón J.L., Garcia-Barragán N., Millán J.M.-S., Masjuán J., Corral I. Limbic encephalitis: A clinical-radiological comparison between herpetic and autoimmune etiologies. Eur. J. Neurol. 2013;20:1566–1570. doi: 10.1111/ene.12249. [DOI] [PubMed] [Google Scholar]
- 60.Pradhan S., Das A., Das A., Mulmuley M. Antibody negative autoimmune encephalitis—Does it differ from definite one? Ann. Indian Acad. Neurol. 2019;22:401–408. doi: 10.4103/aian.AIAN_206_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pruetarat N., Netbaramee W., Pattharathitikul S., Veeravigrom M. Clinical manifestations, treatment outcomes, and prognostic factors of pediatric anti-NMDAR encephalitis in tertiary care hospitals: A multicenter retrospective/prospective cohort study. Brain Dev. 2019;41:436–442. doi: 10.1016/j.braindev.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Qiu X., Zhang H., Li D., Wang J., Jiang Z., Zhou Y., Xu P., Zhang J., Feng Z., Yu C., et al. Analysis of Clinical Characteristics and Poor Prognostic Predictors in Patients with an Initial Diagnosis of Autoimmune Encephalitis. Front. Immunol. 2019;10:1286. doi: 10.3389/fimmu.2019.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sai Y., Zhang X., Feng M., Tang J., Liao H., Tan L. Clinical diagnosis and treatment of pediatric anti-N-methyl-d-aspartate receptor encephalitis: A single center retrospective study. Exp. Ther. Med. 2018;16:1442–1448. doi: 10.3892/etm.2018.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salvucci A., Devine I.M., Hammond D., Sheth R.D. Pediatric Anti-NMDA (N-methyl d-Aspartate) Receptor Encephalitis. Pediatr. Neurol. 2014;50:507–510. doi: 10.1016/j.pediatrneurol.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Sands T.T., Nash K., Tong S., Sullivan J. Focal seizures in children with anti-NMDA receptor antibody encephalitis. Epilepsy Res. 2015;112:31–36. doi: 10.1016/j.eplepsyres.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Seluk L., Taliansky A., Yonath H., Gilburd B., Amital H., Shoenfeld Y., Kivity S. A large screen for paraneoplastic neurological autoantibodies; diagnosis and predictive values. Clin. Immunol. 2018;199:29–36. doi: 10.1016/j.clim.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Sheikh S., Ahmad A., Ahmed T.A. Anti NMDA receptor antibody encephalitis in Pakistan: Clinicopathological features and treatment outcomes. J. Pak. Med. Assoc. 2019;69:1910–1914. doi: 10.5455/jpma.300185. [DOI] [PubMed] [Google Scholar]
- 68.Spagni G., Iorio R. Grading the severity of autoimmune encephalitis: Advances and pitfalls. Ann. Neurol. 2019;86:150. doi: 10.1002/ana.25496. [DOI] [PubMed] [Google Scholar]
- 69.Sudan Y.S., Vinayan K.P., Roy A.G., Wagh A., Kannoth S., Patil S. Clinical Characteristics and Follow-Up of South Indian Children with Autoimmune Encephalopathy. Indian J. Pediatr. 2016;83:1367–1373. doi: 10.1007/s12098-016-2092-4. [DOI] [PubMed] [Google Scholar]
- 70.Sühs K.-W., Wegner F., Skripuletz T., Trebst C., Ben Tayeb S., Raab P., Stangel M. Heterogeneity of clinical features and corresponding antibodies in seven patients with anti-NMDA receptor encephalitis. Exp. Ther. Med. 2015;10:1283–1292. doi: 10.3892/etm.2015.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suthar R., Saini A.G., Sankhyan N., Sahu J.K., Singhi P. Childhood Anti-NMDA Receptor Encephalitis. Indian J. Pediatr. 2016;83:628–633. doi: 10.1007/s12098-015-1988-8. [DOI] [PubMed] [Google Scholar]
- 72.Tripathi M., Tripathi M., Roy S.G., Parida G.K., Ihtisham K., Dash D., Damle N., Shamim S.A., Bal C. Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology. 2017;60:189–198. doi: 10.1007/s00234-017-1956-2. [DOI] [PubMed] [Google Scholar]
- 73.Tsutsui K., Kanbayashi T., Tanaka K., Boku S., Ito W., Tokunaga J., Mori A., Hishikawa Y., Shimizu T., Nishino S. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry. 2012;12:37. doi: 10.1186/1471-244X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner J.N., Kalev O., Sonnberger M., Krehan I., Von Oertzen T.J. Evaluation of Clinical and Paraclinical Findings for the Differential Diagnosis of Autoimmune and Infectious Encephalitis. Front. Neurol. 2018;9:434. doi: 10.3389/fneur.2018.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warren N., O’Gorman C., McKeon G., Swayne A., Blum S., Siskind D. Psychiatric management of anti-NMDAR encephalitis: A cohort analysis. Psychol. Med. 2019;51:435–440. doi: 10.1017/S0033291719003283. [DOI] [PubMed] [Google Scholar]
- 76.Wright S., Hacohen Y., Jacobson L., Agrawal S., Gupta R., Philip S., Smith M., Lim M., Wassmer E., Vincent A. N-methyl-d-aspartate receptor antibody-mediated neurological disease: Results of a UK-based surveillance study in children. Arch. Dis. Child. 2015;100:521–526. doi: 10.1136/archdischild-2014-306795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu X., Lu Q., Huang Y., Fan S., Zhou L., Yuan J., Yang X., Ren H., Sun D., Dai Y., et al. Anti-NMDAR encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:e633. doi: 10.1212/NXI.0000000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao L., Yue W., Xunyi W., Jianhong W., Guoxing Z., Zhen H. Clinical features and long-term outcomes of seizures associated with autoimmune encephalitis: A follow-up study in East China. J. Clin. Neurosci. 2019;68:73–79. doi: 10.1016/j.jocn.2019.07.049. [DOI] [PubMed] [Google Scholar]
- 79.Yeshokumar A.K., Gordon-Lipkin E., Arenivas A., Cohen J., Venkatesan A., Saylor D., Probasco J.C. Neurobehavioral outcomes in autoimmune encephalitis. J. Neuroimmunol. 2017;312:8–14. doi: 10.1016/j.jneuroim.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 80.Zhang X., Wang C., Zhu W., Wang B., Liang H., Guo S. Factors Affecting the Response to First-Line Treatments in Patients with Anti-N-Methyl-d-Aspartate Receptor Encephalitis. J. Clin. Neurol. 2019;15:369–375. doi: 10.3988/jcn.2019.15.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Liu G., Di Jiang M., Li L.P., Su Y.Y. Analysis of electroencephalogram characteristics of anti-NMDA receptor encephalitis patients in China. Clin. Neurophysiol. 2017;128:1227–1233. doi: 10.1016/j.clinph.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 82.Zhao J., Wang C., Xu X., Zhang Y., Ren H., Ren Z., Li G., Zhang J., Guan H. Coexistence of Autoimmune Encephalitis and Other Systemic Autoimmune Diseases. Front. Neurol. 2019;10:1142. doi: 10.3389/fneur.2019.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Cellucci T., Cortese I., Dale R.C., Gelfand J.M., Geschwind M., et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fay M.P., Feuer E.J. Confidence intervals for directly standardized rates: A method based on the gamma distribution. Stat. Med. 1997;16:791–801. doi: 10.1002/(SICI)1097-0258(19970415)16:7<791::AID-SIM500>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 85.Rothman K.J. Epidemiology: An Introduction. Oxford University Press; Oxford, UK: 2012. [Google Scholar]
- 86.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harbord R.M., Egger M., Sterne J. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2005;25:3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 89.GBD 2016 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gasparrini A., Guo Y., Hashizume M., Lavigne E., Zanobetti A., Schwartz J., Tobías A., Tong S., Rocklöv J., Forsberg B., et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet. 2015;386:369–375. doi: 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Findley D.F., Monsell B.C., Bell W.R., Otto M.C., Chen B.-C. New Capabilities and Methods of the X-12-ARIMA Seasonal-Adjustment Program. J. Bus. Econ. Stat. 1998;16:127. [Google Scholar]
- 92.Cleveland R.B., Cleveland W.S., McRae J.E., Terpenning I. STL: A seasonal-trend decomposition. J. Off. Stat. 1990;6:3–73. [Google Scholar]
- 93.Shapira Y., Agmon-Levin N., Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J. Autoimmun. 2010;34:J168–J177. doi: 10.1016/j.jaut.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 94.Shapira Y., Agmon-Levin N., Shoenfeld Y. Geoepidemiology of autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2010;6:468–476. doi: 10.1038/nrrheum.2010.86. [DOI] [PubMed] [Google Scholar]
- 95.Kurtzke J.F. A reassessment of the distribution of multiple sclerosis. Acta Neurol. Scand. 1975;51:137–157. doi: 10.1111/j.1600-0404.1975.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 96.Koch-Henriksen N., Sørensen P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 97.Jin Y.-P., De Pedro-Cuesta J., Söderström M., Link H. Incidence of Optic Neuritis in Stockholm, Sweden, 1990–1995: II. Time and Space Patterns. Arch. Neurol. 1999;56:975–980. doi: 10.1001/archneur.56.8.975. [DOI] [PubMed] [Google Scholar]
- 98.Waernbaum I., Dahlquist G. Low mean temperature rather than few sunshine hours are associated with an increased incidence of type 1 diabetes in children. Eur. J. Epidemiol. 2015;31:61–65. doi: 10.1007/s10654-015-0023-8. [DOI] [PubMed] [Google Scholar]
- 99.George B.P., Schneider E.B., Venkatesan A. Encephalitis Hospitalization Rates and Inpatient Mortality in the United States, 2000–2010. PLoS ONE. 2014;9:e104169. doi: 10.1371/journal.pone.0104169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barbadoro P., Marigliano A., Ricciardi A., D’Errico M.M., Prospero E. Trend of hospital utilization for encephalitis. Epidemiol. Infect. 2011;140:753–764. doi: 10.1017/S0950268811001002. [DOI] [PubMed] [Google Scholar]
- 101.Vora N.M., Holman R.C., Mehal J.M., Steiner C.A., Blanton J., Sejvar J. Burden of encephalitis-associated hospitalizations in the United States, 1998-2010. Neurology. 2014;82:443–451. doi: 10.1212/WNL.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 102.Dehner C., Fine R., Kriegel M. The microbiome in systemic autoimmune disease: Mechanistic insights from recent studies. Curr. Opin. Rheumatol. 2019;31:201–207. doi: 10.1097/BOR.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hammond S.R., English D.R., McLeod J.G. The age-range of risk of developing multiple sclerosis: Evidence from a migrant population in Australia. Brain. 2000;123:968–974. doi: 10.1093/brain/123.5.968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and R scripts as well as the methodological details used to reproduce the vast majority of the results are provided in the Supplementary Methods.