Abstract
Background and aims:
Recent advancements in coronary computed tomography angiography (CCTA) have allowed for the quantitative measurement of high-risk lipid rich plaque. Determination of the optimal threshold for Hounsfield units (HU) by CCTA for identifying lipid rich plaque remains unknown. We aimed to validate reliable cut-points of HU for quantitative assessment of lipid rich plaque.
Methods:
8 post-mortem sudden coronary death hearts were evaluated with CCTA and histologic analysis. Quantitative plaque analysis was performed in histopathology images and lipid rich plaque area was defined as intra-plaque necrotic core area. CCTA images were analyzed for quantitative plaque measurement. Low attenuation plaque (LAP) was defined as any pixel < 30, 45, 60, 75, and 90 HU cut-offs within a coronary plaque. The area of LAP was calculated in each cross-section.
Results:
Among 105 cross-sections, 37 (35.2%) cross-sectional histology images contained lipid rich plaque. Although the highest specificity for identifying lipid rich plaque was shown with <30 HU cut-off (88.2%), sensitivity (e.g. 55.6% for <75 HU, 16.2% for <30 HU) and negative predictive value (e.g. 75.9% for <75 HU, 65.9% for <30 HU) tended to increase with higher HU cut-offs. For quantitative measurement, <75 HU showed the highest correlation coefficient (0.292, p = 0.003) and no significant differences were observed between lipid rich plaque area and LAP area between histology and CT analysis (Histology: 0.34 ± 0.73 mm2, QCT: 0.37 ± 0.71 mm2, p = 0.701).
Conclusions:
LAP area by CCTA using a <75 HU cut-off value demonstrated high sensitivity and quantitative agreement with lipid rich plaque area by histology analysis.
Keywords: Atherosclerosis, Coronary computed tomography, angiography, Histology, Lipid rich plaque, Necrotic core
1. Introduction
The most common mechanism of acute coronary syndrome (ACS) is plaque rupture overlying advanced atheroma [1,2]. Histopathology studies have identified vulnerable plaque characteristics (VPC) indicative of plaque instability and high risk of rupture [3,4]. Increased lipid content in coronary atherosclerotic plaque, such as in intra plaque necrotic core, is a typical pathologic feature of VPC. Previous coronary imaging studies showed that lipid content in plaque is closely associated with a future ACS event [5,6].
Recently, improving spatial and temporal resolution of coronary computed tomography angiography (CCTA) has allowed for detailed plaque assessment, including VPCs [5,7,8]. Quantitative coronary atherosclerotic plaque analysis CT (QCT) software can perform accurate, semi-automated quantification of atherosclerotic plaque volume that is well correlated with intravascular ultrasound [9–11]. By reducing the need for invasive testing and time to analyze, CCTA with QCT may be a promising tool to bring VPC identification into routine clinical practice.
Plaque characterization by CCTA relies upon differences in Hounsfield units (HU) between fibrous and lipid rich plaque, identifying low-attenuation plaque (LAP) as a marker of plaque vulnerability [5]. However, the HU threshold for LAP has been inconsistent in previous studies between 30 and 90 [5,12–15]. In addition, for accurate differentiation of plaque characteristics, a reliable HU cut-off value for LAP needed to be confirmed. We, therefore, aimed to validate the cut-off value of HU by CCTA for quantitative assessment of LAP using semi-automated QCT software against necrotic core area, as lipid rich plaque, by histopathologic plaque analysis.
2. Materials and methods
2.1. Study overview
In this prospective, cross-sectional study, CCTA was compared to histopathology in de-identified ex-vivo human hearts. Necropsy hearts were explanted from decedents at the Office of the Chief Medical Examiner in Baltimore, Maryland, USA, who required consultation for suspected coronary cause of death. Hearts were excluded from analysis if they could not undergo ex-vivo CCTA or histology due to tissue disruption or decomposition, or if the patient had a history of coronary artery bypass graft surgery. Hearts underwent ex-vivo CCTA, then histopathologic examination (CVPath Institute, Gaithersburg, MD). CCTA images were compared and co-registered to serial histology cross-sections, and underwent blinded QCT. All procedures were approved by the institutional review board.
2.2. CCTA image acquisition
CCTA examinations were performed using a 64-detector row CT scanner (Discover CT750 HD; GE Healthcare, Milwaukee, WI). After cleansing the explanted heart with normal saline, a cardiovascular pathology expert (R.K.) dissected epicardial fat away from the surface of the left main and proximal RCA, to expose approximately 1 cm or less of each vessel. A small tunnel was made under each proximal vessel to allow passage of a short length of twine under the vessel. A metal probe with an introducer sheath was inserted into each proximal vessel. The tip of the introducer sheath was lightly heat flared so that it could be secured in place with a twine ligature after vessel insertion. The scan parameters were as follows: prospective ECG-triggering, 64 × 0.625 mm collimation, 120 kVp tube voltage, 440 mA tube current, 350 ms gantry rotation time. The dual-phase contrast protocol was: Iohexol (Omnipaque 350, GE healthcare) 5 cc in 95 ml saline at 3 cc/sec for 8 s with 6 s delay. The contrast protocol was developed by trial and error to achieve complete end-vessel and branch opacification with a typical in vivo lumen contrast density of 250–400 HU without undue myocardial enhancement. Axial images were reconstructed with a slice thickness of 0.6 mm and adaptive statistical iterative reconstruction technique (ASIR; GE Healthcare, Milwaukee, WI) with a blending factor of 40%. CCTA images were interpreted using an offline 3D workstation (Advantage Workstation version 4.3/4.4, General Electric, Milwaukee, WI).
2.3. Histology preparation and plaque analysis
Histology plaque analysis was conducted by personnel (R.K., K.Y., S.T., R.V.) in a specialized cardiovascular pathology laboratory (CVPath laboratory, Gaithersburg, MD). Following perfusion-fixation with 10% neutral buffered formalin, coronary arteries were sectioned serially at 3 mm intervals and submitted to paraffin embedding. Histologic sections were cut at 6 μm and stained with hematoxylin-eosin and Movat pentachrome. Quantitative plaque measurements were performed in each histology cross-section for vessel area, lumen area, and plaque area. Plaque area was further classified by plaque type: plaque area, calcification area, and necrotic core area. For the purpose of this study, lipid rich plaque area was defined as necrotic core area. Morphometric measurement of coronary sections was performed using image-processing software (IPLabs, Scanalytics, Rockville, MD) on slides stained with Movat pentachrome. Quantitative planimetry included area analysis of the vessel area, lumen area, and plaque area.
2.4. Co-registration and quantitative CT image analysis
CT image data were transferred to an offline workstation for plaque analysis by semi-automated plaque analysis software (QAngioCT Research Edition v2.1.9.1; Medis Medical Imaging Systems, Leiden, the Netherlands). Independent level III–experienced readers performed analyses on the CCTA data blinded to clinical and histology results. After an automatic extraction of centerline, straightened multiplanar reformatted reconstruction was performed with automatic detection of inner lumen and outer vessel wall contours and manual editing if needed. Standard displays (e.g., width 740 Hounsfield Unit (HU), level 220 HU) were adjusted by contrast level of the most proximal site of the coronary lumen (155% and 65% mean luminal intensity). CCTA images were co-registered with corresponding histology slides based on coronary branch points, distance from the cannula tip, and calcium landmarks by an investigator who did not participate in the QCT measurement (D.H.). We included histology slides that were located at proximal or middle coronary segments according to the 18-segment model in the current guidelines [16] with a vessel diameter more than 2 mm. Then, blinded QCT plaque analyses were performed in each co-registered cross-section: vessel area, lumen area, plaque area [10]. LAP was defined as any pixel <30, <45, <60, <75, and <90 HU threshold within a coronary plaque. Lipid-rich plaque on CCTA was defined as the cross-section with presence of LAP. LAP area was measured in each cross-section according to the HU threshold (Fig. 1). Calcified plaque was measured using the threshold HU ≥ 130. Plaque composition was defined as none, non-calcified (>70% non-calcified), partially calcified (30–70% non-calcified or calcified), and calcified (>70% calcified) [16]. The mean HU of lumen was measured in 105 cross-sections by manually placing the region of interest in the center of the vessel lumen.
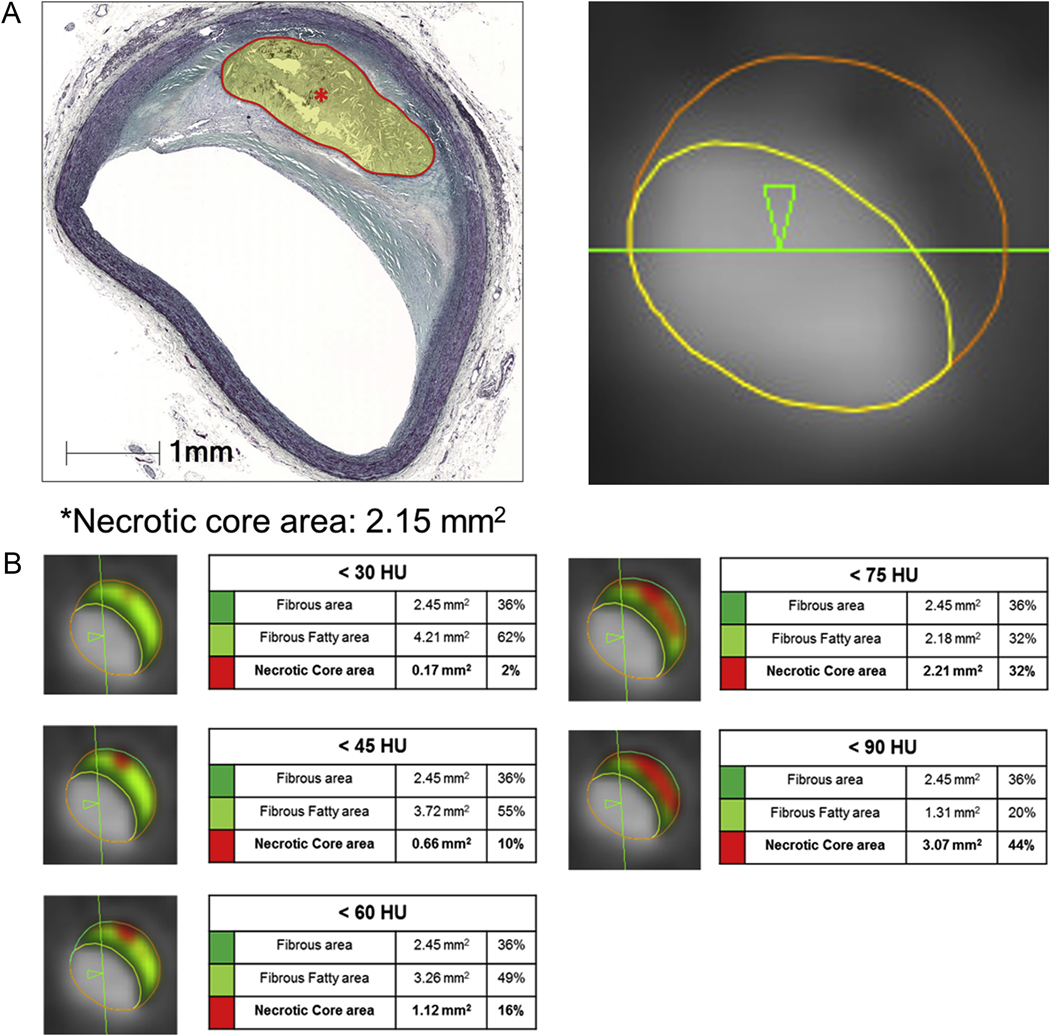
Fig. 1.
Example of QCT analysis for LAP area corresponding to lipid rich plaque area by histology image according to 5 different HU cut-off points.
(A) Lipid rich plaque area in histology and QCT image. (B) QCT measurement according to HU cut-off points. This figure shows an example of QCT plaque component analysis according to 5 different HU cut-off values. Histology analysis showed that the lipid rich plaque area was 2.15 mm2 (A). Among 5 different HU cut-offs, a similar lipid rich plaque area was shown in <75 HU cut-off (2.15 mm2 in histology vs. 2.21 mm2 in QCT analysis) (B).
2.5. Statistical analysis
Continuous variables were reported as means with standard deviation and categorical variables were reported as counts with proportions. Vessel and plaque parameters were compared between QCT and histology analysis using the Pearson correlation or Spearman correlation coefficient (r) with two sided p-values. The paired t-test was performed for comparison of covariates between histology and QCT analysis. Bland-Altman plots with 95% confidence intervals (CI) were calculated for correlation. The diagnostic performance of QCT identification of lipid rich plaque at varying HU cut-off points was compared to histology analysis to calculate sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) with 95% Cis using McNemar test of proportion. The optimal cut-off value for the likelihood (%) to differentiate lipid rich plaque in each HU threshold was determined using the Youden index [17]. Area under the receiver operating characteristic curve (AUC) analyses were used to examine the discrimination, and AUC values were compared using the method reported by DeLong et al. [18]. To test whether the lumen HU affected the association between LAP area in CT and lipid rich plaque area in histology, a linear regression was performed to test the interaction between lumen HU and LAP area with lipid rich plaque area in histology as the outcome variable. In addition, cross-sections were categorized by tertiles. LAP area in QCT and lipid rich plaque area in histology were compared across lumen HU tertiles. A two sided p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using STATA (version 14; StataCorp, College Station, TX, USA).
3. Results
3.1. Baseline characteristics
Ex-vivo hearts were analyzed from 8 decedents with sudden cardiac death (Table 1.). Their median age was 53 years (range 34–71) and most were male (n = 7, 88%). Of a total of 131 histology cross-sections in 8 patients, 26 could not be analyzed by QCT analysis due to insufficient contrast in the vessel lumen or image artifact (n = 16) and the inability to co-register histology sections and CT cross-section images (n = 10). Out of the 105 cross-sections used for final analysis, 48 cross-sections were of the left anterior descending coronary artery (LAD), 41 of the right coronary artery (RCA), and 16 of the left circumflex coronary artery (LCX). CCTA identified exclusively non-calcified plaque in 52 (49.5%), partially calcified plaque in 44 (41.9%), and exclusively calcified in 9 (8.6%) cross-sections. (Table 1).
Table 1.
Baseline characteristics.
| Per patient characteristics (n = 8) | |
|---|---|
| Age | 53 (34–71) |
| Male | 7 (88) |
| BMI | 27.5 ± 3.9 |
| Hypertension | 4 (50) |
| Diabetes | 0 (0) |
| Dyslipidemia | 2 (25) |
| Smoking | 3 (37.5) |
| Renal failure | 1 (12.5) |
|
| |
| Per cross-section characteristics (n = 105) | |
|
| |
| Plaque type by CCTA | |
| Calcified | 9 (8.6) |
| Partially calcified | 44 (41.9) |
| Non-calcified | 52 (49.5) |
| Vessel distribution | |
| Left anterior descending | 48 (45.7) |
| Left circumflex | 16 (15.2) |
| Right coronary artery | 41 (39.0) |
BMI, body mass index; CCTA, coronary computed tomography angiography.
3.2. Histology and QCT measurement
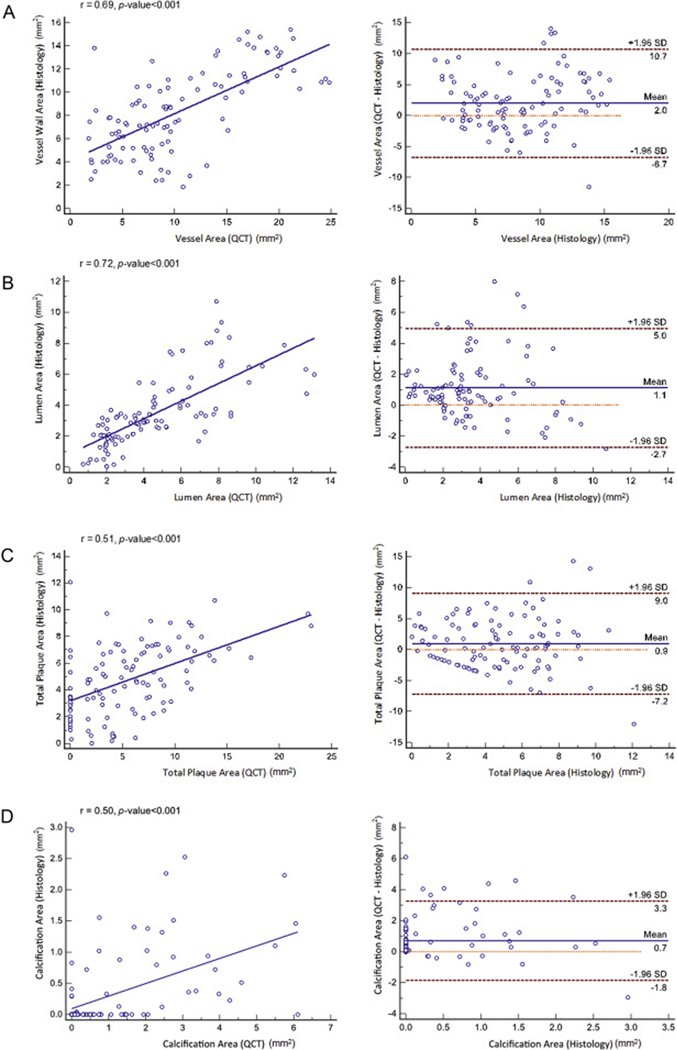
Areas corresponding to the vessel, lumen, total plaque, and calcified plaque area displayed significant correlation between histology and QCT analysis (r = 0.69, 0.72, 0.51, and 0.50, respectively, p < 0.001, for all) (Fig. 2). Bland-Altman analyses showed a mean bias of 2.0 with 95% limits of agreement extending from −6.7 to 10.7 for vessel area, 1.1 with −2.7 to 5.0 for lumen area, 0.9 with −7.2 to 9.0 for total plaque area, and 0.7 with −1.8 to 3.3 for calcified plaque area between histology and QCT analysis. Proportional bias is apparent in total plaque area and calcified plaque area, with lower discrepancies at smaller plaque areas.
Fig. 2.
Correlation and Bland-Altman analyses.
Correlation and Bland-Altman analysis for (A) vessel area, (C) lumen area, (E) total plaque area and (G) calcified plaque area between histology and QCT analysis.
For quantitative measurement, vessel area did not differ between histology and QCT area (histology: 10.2 ± 4.2 mm2, QCT: 10.3 ± 6.0 mm2, mean difference: 0.07 ± 4.4 mm2, p = 0.878). However, lumen area and total plaque area were significantly overestimated by QCT analysis compared to histology analysis (histology: 3.5 ± 2.2 mm2, QCT: 4.6 ± 2.8 mm2, mean difference 1.12 ± 2.0 mm2, p < 0.001; histology: 4.8 ± 2.6 mm2, QCT: 5.7 ± 4.8 mm2, mean difference 0.92 ± 4.14 mm2, p = 0.026). Calcium area was also significantly over-estimated by QCT compared to histology (histology: 0.30 ± 0.60 mm2, QCT: 0.99 ± 1.50 mm2, mean difference: 0.70 ± 1.3 mm2, p = 0.001).
3.3. Diagnostic performance to detect lipid rich plaque by LAP
37 (35.2%) histology sections contained lipid rich plaque. Histology analysis identified a lipid rich plaque area of 0.34 ± 0.73 mm2. Although the highest specificity was shown in <30 HU cut-off, sensitivity and negative predictive value tended to increase with higher HU cut-offs (Table 2). Overall AUCs for detection of lipid rich plaque were somewhat higher in <75 and < 90 HU cut-off than other cut-offs (Table 3). Optimal cut-point of LAP area were derived in each HU cut-offs. A LAP area 0.14 mm2 in <75 HU cut-off showed 57% sensitivity and 74% specificity (Table 3). The AUC did not significantly differ between the overall performance and the AUC from optimal cut-point of LAP area (0.14 mm2) at <75 HU cut-off (0.64 vs. 0.65, Table 3). (See Table 4).
Table 2.
Diagnostic performance to detect lipid rich plaque according to HU cut-off.
| Total detected lipid rich plaque by histology | Total detected lipid rich plaque by CT measure | Accuracy (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| −30–30 HU | 37 (35.2%) | 14 (13.3%) | 62.9, 66/105 (52.3–72.1) | 16.2, 6/37 (6.2–32) | 88.2, 60/68 (78.1–94.8) | 42.9, 6/14 (17.7–55.3) | 65.9, 60/91 (55.3–75.5) |
| −30–45 HU | 37 (35.2%) | 25 (23.8%) | 58.1, 61/105 (48.1–67.7) | 24.3, 9/37 (11.8–41.2) | 76.5, 52/68 (64.6–85.9) | 36, 9/25 (18–57.5) | 65, 52/80 (53.5–75.3) |
| −30–60 HU | 37 (35.2%) | 40 (38.1%) | 59.0, 62/105 (49–68.5) | 45.9, 17/37 (29.5–63.1) | 66.2, 45/68 (53.7–77.2) | 42.5, 17/40 (27–59.1) | 69.2, 45/65 (56.6–80.1) |
| −30–75 HU | 37 (35.2%) | 47 (44.8%) | 63.8, 67/105 (53.9–73) | 62.2, 23/37 (44.8–77.5) | 64.7, 44/68 (52.2–75.9) | 48.9, 23/47 (34.1–63.9) | 75.9, 44/58 (62.8–86.1) |
| −30–90 HU | 37 (35.2%) | 56 (53.3%) | 61, 64/105 (50.9–70.3) | 70.3, 26/37 (53–84.1) | 55.9, 33/68 (43.3–67.9) | 46.4, 26/56 (33–60.3) | 77.6, 38/49 (63.4–88.2) |
HU, hounsfield unit; CT, computed tomography, CI, confidence interval.
Table 3.
Diagnostic performance to detect lipid rich plaque at best cut point LAP area according to HU cut-off.
| Overall |
Values at cut point of plaque area |
|||||||
|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | LAP area (mm2) | AUC | Accuracy (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | |
|
| ||||||||
| −30–30 HU | 0.52 (0.45–0.59) | 0.05 | 0.52 (0.46–0.59) | 0.64 (0.54–0.73) | 0.14 (0.05–0.29) | 0.91 (0.82–0.97) | 0.46 (0.17–0.77) | 0.66 (0.56–0.75) |
| −30–45 HU | 0.52 (0.43–0.61) | 0.17 | 0.54 (0.47–0.62) | 0.65 (0.55–0.74) | 0.19 (0.08–0.35) | 0.90 (0.80–0.96) | 0.50 (0.23–0.77) | 0.67 (0.56–0.77) |
| −30–60 HU | 0.57 (0.46–0.67) | 0.12 | 0.57 (0.48–0.67) | 0.64 (0.54–0.73) | 0.35 (0.20–0.53) | 0.79 (0.68–0.88) | 0.48 (0.29–0.68) | 0.69 (0.58–0.79) |
| −30–75 HU | 0.64 (0.53–0.74) | 0.14 | 0.65 (0.56–0.75) | 0.68 (0.58–0.76) | 0.57 (0.40–0.73) | 0.74 (0.61–0.84) | 0.54 (0.37–0.70) | 0.76 (0.64–0.86) |
| −30–90 HU | 0.64 (0.54–0.75) | 0.41 | 0.64 (0.55–0.74) | 0.67 (0.57–0.76) | 0.57 (0.40–0.73) | 0.72 (0.60–0.82) | 0.53 (0.36–0.69) | 0.75 (0.63–0.85) |
LAP, low attenuation plaque; HU, hounsfield unit; AUC, the area under the curve; CI, confidence interval.
Table 4.
Quantitative measurement of lipid rich plaques using different HU cut-off.
| Histology area (mm2) | LAP area (mm2) | Mean difference (mm2) | p-value for difference | Correlation coefficient | p-value for correlation | |
|---|---|---|---|---|---|---|
|
| ||||||
| −30–30 HU | 0.34 ± 0.73 | 0.02 ± 0.06 | 0.32 ± 0.73 | <0.001 | 0.138 | 0.162 |
| −30–45 HU | 0.34 ± 0.73 | 0.07 ± 0.2 | 0.27 ± 0.71 | <0.001 | 0.228 | 0.019 |
| −30–60 HU | 0.34 ± 0.73 | 0.16 ± 0.36 | 0.17 ± 0.36 | 0.014 | 0.266 | 0.006 |
| −30–75 HU | 0.34 ± 0.73 | 0.37 ± 0.71 | −0.03 ± 0.86 | 0.701 | 0.292 | 0.003 |
| −30–90 HU | 0.34 ± 0.73 | 0.71 ± 1.23 | −0.37 ± 1.24 | 0.029 | 0.280 | 0.004 |
HU, hounsfield unit; LAP, low attenuation plaque.
3.4. QCT measurement for lipid rich plaque area
Cross-section level quantitative measurements of lipid rich plaque area according to different HU cut-offs are shown in Table 4. LAP area identified by QCT analysis tended to increase with higher HU cut-off values. Except for the <30 HU cut-off, all cut-off points showed significant correlation with the corresponding histologic analysis. Notably, the <75 HU cut-off had the highest correlation coefficient (0.292, p = 0.003) and no significant differences were observed in lipid rich plaque area between histology and QCT analysis (histology: 0.34 ± 0.73 mm2, QCT: 0.37 ± 0.71 mm2, p = 0.701). Bland-Altman analyses for <30, <45, <60, <75, and <90 showed a mean bias of −0.4 with 95% limits of agreement extending from −1.8 to 1.0, −0.3 with −1.7 to 1.1, −0.2 with −1.6 to 1.1, 0 with −1.6 to 1.6, and 0.3 with −2.0 to 2.6, respectively (Supplementary Fig. 1). The trend of underestimation of lipid rich plaque is noted among all HU cut-offs in QCT and this trend is progressively decreased with higher HU cut-offs. On the other hand, variances which represent wide 95% CIs tend to increase in higher HU cut-offs. The <75 cut-off showed the lowest bias. The average HU within the coronary lumen in CT was 444 and ranged from 201 to 725 HU. The results of the interaction test between lumen HU and LAP area for lipid rich plaque area in histology was not statistically significant at <75 HU cut-off (p = 0.132). When comparing lipid rich plaque area and LAP area across lumen HU density tertiles (Supplementary Table 1), LAP area was overestimated in cross-sections with lower lumen HU density (1st tertile) and underestimated in cross-sections with higher lumen HU density (3rd tertile).
3.5. Subgroup analysis by age group
There was no significant difference in comorbidities between the age groups (Supplementary Table 2). 49 cross-sections and 56 cross-sections were included in young (<52) and old age (52≥) group, respectively. The old age group demonstrated higher prevalence of calcified plaque (14% vs 2%) and lower prevalence of non-calcified plaque (39% vs. 61%) than the young age group (p = 0.019). A similar trend was noted at higher specificity at lower HU cut-off and increasing sensitivity in higher HU cut-off (Supplementary Table 3). Overall diagnostic performance is slightly higher in the young age group but not statistically significant (all p-value>0.05 comparing accuracy between young and old age group in each HU cut-offs).
4. Discussion
In this study, we sought to identify a reliable HU cut-off to assess lipid rich plaque by semi-automated QCT analysis, using histopathology analysis as the reference standard. Our results demonstrated that quantitative measurement of LAP area by QCT was significantly correlated with histologic measurement of lipid rich plaque area. Notably, out of the 5 different HU cut-offs (<30, <45, <60, <75, and <90 HU), the <75 HU cut-off most accurately measured LAP area with a high correlation coefficient, with histologic measurement of lipid rich plaque area.
Lipid rich coronary atherosclerotic plaques such as necrotic core are high risk markers of future ACS events [4]. In CT images, necrotic core is identified as LAP using “below than” HU cut-off points. Early attempts to validate the clinical utility of LAP by CCTA were assessed by the qualitative approach [5,19]. For instance, Motoyama et al. reported that coronary atherosclerotic plaque with presence of low plaque density was associated with ACS [5]. Park et al. also demonstrated that lesions with LAP displayed a 5-fold increased likelihood of being a lesion that caused significant ischemia (i.e. fractional flow reserve< 0.8) [20]. In addition, recent studies suggested that not only the presence or absence of LAP was an independent predictor of adverse clinical outcomes, but also increased LAP area/volume [21,22]. In light of this, a reliable HU cut-off for LAP is needed for accurate detection and quantitative measurement of lipid rich plaque.
Prior studies have examined the optimal HU cut-off for differentiation of lipid rich plaque from fibrous plaque by CCTA, however the HU cut-off for LAP showed inconsistent results (Supplementary Table 4). Marwan and Schlett demonstrated high diagnostic accuracy using a histogram-based approach to count the pixel area of lipid-core plaque. Marwan et al. demonstrated that lipid rich plaque was comprised of a significantly higher percentage of pixels with low CT attenuation as compared to fibrous plaque, using Intravascular ultrasound (IVUS) images as the gold standard [15]. The receiver operating characteristics curve analysis showed that a cut-off with <30 HU has a high sensitivity and specificity (95% and 80%, respectively). Conversely, Schlett and colleagues evaluated 446 cross-sections from 5 ex-vivo hearts to determine the HU cut-off for lipid rich plaque among 3 different cut-offs (i.e. 30, 60, 90 HU) using the quantitative histogram analysis [14]. In that study, the plaque area of less than 60 HU indicated reasonable accuracy (71% sensitivity and 73% specificity) to detect lipid rich plaque confirmed by histology analysis. However, the current investigation found that <75 HU cut-off allows for the most accurate measurement of LAP area, corresponding to histologic lipid rich plaque area. Several possible explanations for this discrepancy can be considered. The latter study reported by Marwan et al. used IVUS image as the reference standard, however prior studies indicated that IVUS shows limited value in identifying lipid rich plaque when compared to histology analysis [23]. In addition, the two prior studies reported that the mean plaque attenuation between LAP and fibrous plaque is 67 HU and 96 HU by Marwan et al., and 11–99 and 77–121 by Schlett and colleagues [14,15]. These mean plaque attenuation differences also indicated that higher HU cut-offs (above 30) may be more reliable. Comparably, Schlett et al. suggested that a 60 HU cut-off is well correlated with histology measurements; this cut-off is much closer in value to the finding of the current investigation (<75 HU). Finally, our study focused on accurate estimation of LAP area, and not only on binary detection of LAP; as such, it is more clinically applicable for vulnerable plaque evaluation inclusive of lipid-rich plaque size.
Previous studies that have assessed the amount of total plaque by QCT analysis reported progressively greater discrepancies with larger sizes of plaque, which represented a funnel shaped plot in the Bland-Altman analysis [10,24]. A similar trend of the quantification error for total plaque area is noted in the current analysis. In contrast, quantitative estimation of lipid rich plaque area indicates lipid rich plaques are progressively underestimated by QCT analysis at every HU cut-offs, especially in lower HU cut-offs. The important finding is that lipid rich plaques, which are closely associated with future adverse outcomes, might be underestimated by QCT analysis. In addition, although lower HU cut-off showed higher specificity to detect lipid rich plaque, low HU cut-offs revealed lower sensitivity as well as a potential risk for underestimation of lipid rich plaque area. To the best of our knowledge, the current study is the first to explore the potential risk of QCT analysis in assessment of the quantification of lipid rich plaque among different HU cut-offs. Previous studies could not demonstrate this measurement error in QCT analysis because histogram method based analyses were used for the estimation of lipid rich plaque [14,15].
The luminal HU density affected the HU values in plaque [25]. Previous studies reported that intra-plaque HU density tended to increase with higher luminal HU density and higher luminal HU density, which possibly reclassified the characterization of non-calcified plaque from lipid rich to fibrous [25,26]. Our study also demonstrated consistent results with previous findings that LAP area by QCT was underestimated in cross-sections with higher luminal HU density (3rd tertile) when compared to lipid rich plaque area in histology. In contrast, the LAP area in the cross-sections with lower luminal HU density (1st tertile) tended to be overestimated. On the other hand, despite our efforts to develop the contrast protocol to achieve the typical in vivo lumen contrast density 250–400 HU, the wide range of luminal HU density in current study (201–725) might affect the detection of the presence of lipid rich plaque since the diagnostic accuracy to detect lipid rich plaque was only 60%. Furthermore, the wide range of luminal HU also influenced precise measurement of lipid rich plaque area. Consequently, although average plaque measurement was comparable between QCT and histology measurement in the current study, the limits of agreement area were too wide, more than 200% of the actual plaque size, in every HU cut-offs (Supplementary Fig. 1). Thus, a more reproducible and standardized contrast protocol to control the lumen HU density is required for accurate and precise evaluation of lipid rich plaque by QCT analysis.
Previous investigations also reported low accuracy for differentiation of lipid rich plaque due to substantial overlap of HU value between vulnerable and stable plaques based on CT attenuation. Indeed, Pohle et al. reported that the mean CT attenuation within plaque of hyper-echogenic and hypo echogenic appearance in IVUS was 121 ± 34 and 58 ± 43, respectively [27]. The latter study indicated that there is significant overlap between the CT densities in hyper-echogenic (range: 60–201 HU) and hypo-echogenic (range: −39 to 167 HU) appearances mainly due to a partial volume effect. In a meta-analysis study, the CT-derived mean HU values in lipid rich plaque and fibrous plaque ranges were also substantially overlapped, 11–79 and 63.8–148.6, respectively [28]. To overcome these limitation, Dual energy CT (DECT), a novel CT technique that employs differences in energy dependent absorption properties of materials, may be useful for differentiation of coronary plaque characteristics [29]. When compared with conventional single energy CT, the use of DECT resulted in reduction of artifact as well as improvement of signal to noise ratio and contrast to noise ratio for coronary artery assessment [30]. In addition, Obaid et al. reported that DECT improves the differentiation of lipid rich plaque and fibrous plaque (sensitivity: 64%, specificity 98% in dual energy CT; sensitivity: 50%, specificity 94% in single energy CT) [31]. Still, further studies are needed to evaluate the utility of DECT for identifying lipid rich coronary atherosclerotic plaque.
4.1. Limitations
In this study, CCTA images were obtained from ex-vivo hearts. Although histology plaque area measurement for necrotic core is known as the gold standard, CCTA images and QCT analysis are potentially different in the ex-vivo condition, with an absence of cardiac motion, and are different in kVp setting as well as noise from adjacent organs. Hence, our result should be interpreted with caution when applied to in-vivo heart imaging. The study sample comprised of only 8 ex-vivo hearts, and therefore, we cannot discount potential selection bias, and generalizability might be limited. For instance, despite the fact that diabetes is well known CVD risk factor, the study sample comprised of only non-diabetics. Therefore, a future study is needed with a larger cohort including both diabetics and non-diabetics. Furthermore, our study sample shows a wide range of age (range 34–71). However, the subgroup analysis showed that there are no significant differences in trends of results between age groups. CCTA images are currently unable to differentiate among the 3-vessel wall layers including the adventitia. Therefore, plaque area directly measured by QCT analysis can potentially be overestimated. In addition, due to the variation of the enhancement of lumen which was not controlled when performing ex-vivo CT scan, the wide range of lumen HU (201–725) in the current study potentially relates to low accuracy and high variance in the evaluation of lipid rich plaque. Previous studies used a histogram based analysis for estimation of HU cut-off for LAP [13,14]. However, histogram-based analysis is beyond the means of most automated QCT programs currently. Finally, current QCT analysis software enables 3D volume quantification of plaque characteristics; however, the present study assessed only cross-sectional LAP area, which co-registered with the histology slide. Thus, our result was not validated to apply the 3D volumetric measurement of LAP by QCT analysis.
4.2. Conclusion
LAP area by CCTA using a <75 HU cut-off value demonstrated high sensitivity and quantitative agreement with lipid rich plaque area by histology analysis. However, diagnostic accuracy for identifying presence of lipid rich plaque was somewhat limited by semi-automated QCT analysis.
Supplementary Material
Abbreviations:
- ACS
acute coronary syndrome
- VPC
vulnerable plaque characteristics
- CCTA
coronary computed tomography angiography
- QCT
quantitative coronary atherosclerotic plaque analysis CT
- HU
Hounsfield units
- LAP
low-attenuation plaque
- NPV
negative predictive value
- PPV
positive predictive value
- IVUS
intravascular ultrasound
- DECT
dual energy CT
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.atherosclerosis.2018.05.024.
Conflicts of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- [1].Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, et al. , From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I, Circulation 108 (2003) 1664–1672. [DOI] [PubMed] [Google Scholar]
- [2].Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, et al. , Coronary risk factors and plaque morphology in men with coronary disease who died suddenly, N. Engl. J. Med 336 (1997) 1276–1282. [DOI] [PubMed] [Google Scholar]
- [3].Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM, Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions, Arterioscler. Thromb. Vasc. Biol 20 (2000) 1262–1275. [DOI] [PubMed] [Google Scholar]
- [4].Virmani R, Burke AP, Kolodgie FD, Farb A, Vulnerable plaque: the pathology of unstable coronary lesions, J. Intervent. Cardiol 15 (2002) 439–446. [DOI] [PubMed] [Google Scholar]
- [5].Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, et al. , Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes, J. Am. Coll. Cardiol 50 (2007) 319–326. [DOI] [PubMed] [Google Scholar]
- [6].Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, et al. , A prospective natural-history study of coronary atherosclerosis, N. Engl. J. Med 364 (2011) 226–235. [DOI] [PubMed] [Google Scholar]
- [7].Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, et al. , Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography, J. Am. Coll. Cardiol 47 (2006) 1655–1662. [DOI] [PubMed] [Google Scholar]
- [8].Leber AW, Knez A, Becker A, Becker C, von Ziegler F, et al. , Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound, J. Am. Coll. Cardiol 43 (2004) 1241–1247. [DOI] [PubMed] [Google Scholar]
- [9].Versteylen MO, Kietselaer BL, Dagnelie PC, Joosen IA, Dedic A, et al. , Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome, J. Am. Coll. Cardiol 61 (2013) 2296–2305. [DOI] [PubMed] [Google Scholar]
- [10].Park HB, Lee BK, Shin S, Heo R, Arsanjani R, et al. , Clinical feasibility of 3D automated coronary atherosclerotic plaque quantification algorithm on coronary computed tomography angiography: comparison with intravascular ultrasound, Eur. Radiol 25 (2015) 3073–3083. [DOI] [PubMed] [Google Scholar]
- [11].Boogers MJ, Broersen A, van Velzen JE, de Graaf FR, El-Naggar HM, et al. , Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification, Eur. Heart J 33 (2012) 1007–1016. [DOI] [PubMed] [Google Scholar]
- [12].Choi BJ, Kang DK, Tahk SJ, Choi SY, Yoon MH, et al. , Comparison of 64-slice multidetector computed tomography with spectral analysis of intravascular ultrasound backscatter signals for characterizations of noncalcified coronary arterial plaques, Am. J. Cardiol 102 (2008) 988–993. [DOI] [PubMed] [Google Scholar]
- [13].Uetani T, Amano T, Kunimura A, Kumagai S, Ando H, et al. , The association between plaque characterization by CT angiography and post-procedural myocardial infarction in patients with elective stent implantation, JACC Cardiovascular imaging 3 (2010) 19–28. [DOI] [PubMed] [Google Scholar]
- [14].Schlett CL, Maurovich-Horvat P, Ferencik M, Alkadhi H, Stolzmann P, et al. , Histogram analysis of lipid-core plaques in coronary computed tomographic angiography: ex vivo validation against histology, Invest. Radiol 48 (2013) 646–653. [DOI] [PubMed] [Google Scholar]
- [15].Marwan M, Taher MA, El Meniawy K, Awadallah H, Pflederer T, et al. , In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS, Atherosclerosis 215 (2011) 110–115. [DOI] [PubMed] [Google Scholar]
- [16].Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, et al. , SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee, Journal of cardiovascular computed tomography 8 (2014) 342–358. [DOI] [PubMed] [Google Scholar]
- [17].Youden WJ, Index for rating diagnostic tests, Cancer 3 (1950) 32–35. [DOI] [PubMed] [Google Scholar]
- [18].DeLong ER, DeLong DM, Clarke-Pearson DL, Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach, Biometrics 44 (1988) 837–845. [PubMed] [Google Scholar]
- [19].Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, et al. , High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial, J. Am. Coll. Cardiol 64 (2014) 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park HB, Heo R, o Hartaigh B, Cho I, Gransar H, et al. , Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve, JACC Cardiovascular imaging 8 (2015) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gaur S, Ovrehus KA, Dey D, Leipsic J, Botker HE, et al. , Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions, Eur. Heart J 37 (2016) 1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, et al. , Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome, J. Am. Coll. Cardiol 54 (2009) 49–57. [DOI] [PubMed] [Google Scholar]
- [23].Maurovich-Horvat P, Schlett CL, Alkadhi H, Nakano M, Stolzmann P, et al. , Differentiation of early from advanced coronary atherosclerotic lesions: systematic comparison of CT, intravascular US, and optical frequency domain imaging with histopathologic examination in ex vivo human hearts, Radiology 265 (2012) 393–401. [DOI] [PubMed] [Google Scholar]
- [24].de Graaf MA, Broersen A, Kitslaar PH, Roos CJ, Dijkstra J, et al. , Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology, Int. J. Cardiovasc. Imag 29 (2013) 1177–1190. [DOI] [PubMed] [Google Scholar]
- [25].Cademartiri F, Mollet NR, Runza G, Bruining N, Hamers R, et al. , Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography, Eur. Radiol 15 (2005) 1426–1431. [DOI] [PubMed] [Google Scholar]
- [26].Dalager MG, Bottcher M, Andersen G, Thygesen J, Pedersen EM, et al. , Impact of luminal density on plaque classification by CT coronary angiography, Int. J. Cardiovasc. Imag 27 (2011) 593–600. [DOI] [PubMed] [Google Scholar]
- [27].Pohle K, Achenbach S, Macneill B, Ropers D, Ferencik M, et al. , Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: comparison to IVUS, Atherosclerosis 190 (2007) 174–180. [DOI] [PubMed] [Google Scholar]
- [28].Voros S, Rinehart S, Qian Z, Joshi P, Vazquez G, et al. , Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis, JACC Cardiovascular imaging 4 (2011) 537–548. [DOI] [PubMed] [Google Scholar]
- [29].Danad I, Fayad ZA, Willemink MJ, Min JK, New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging, JACC Cardiovascular imaging 8 (2015) 710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Scheske JA, O’Brien JM, Earls JP, Min JK, LaBounty TM, et al. , Coronary artery imaging with single-source rapid kilovolt peak-switching dual-energy CT, Radiology 268 (2013) 702–709. [DOI] [PubMed] [Google Scholar]
- [31].Obaid DR, Calvert PA, Gopalan D, Parker RA, West NE, et al. , Dual-energy computed tomography imaging to determine atherosclerotic plaque composition: a prospective study with tissue validation, Journal of cardiovascular computed tomography 8 (2014) 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.