Abstract
Background:
African trypanosomiasis is a protozoan disease with huge socio-economic burden to sub-Saharan African exceeding US$4.6 annual loss. To mitigate the incidence of trypanosomal drug resistance, efforts are geared towards discovery of molecules, especially from natural products, with potential to inhibit important molecular target (trypanosome alternative oxidase, TAO) in trypanosomes that are critical to their survival.
Method:
Crude methanol extract of Anogeissus leiocarpa was subjected to in vitro bioassay-guided antitrypanosomal assay to identify the most active extract with trypanocidal activity. The most active extract was run on a column chromatography yielding five fractions, F1-F5. The fractions were assayed for inhibitory effect on TAO. The most promising TAO inhibitor was subjected to antitrypanosomal evaluation by trypanosome count, drug incubation infectivity test (DIIT) and in vivo studies. Gas chromatography-mass spectrometry (GC-MS) was used to identify and quantify phytochemical constituents of the potential TAO-inhibiting fraction.
Results:
Ethyl acetate extract (EtOAc) significantly (p<0.05) produced trypanocidal effect and was the most active extract. Of the five fractions, only F4 significantly (p<0.05) inhibited TAO compared to the control. F4 completely immobilised the trypanosomes up to 0.5 μg/μl, yielding an EC50 of 0.024 μg/μl compared to the 0.502 μg/μl of diminazene aceturate positive control group. The DIIT showed that F4 was significantly (p<0.05) potent up to 0.1 μg/μl. F4 significantly (p<0.05) suppressed parasite multiplication in systemic circulation of the treated rats and significantly (p<0.05) maintained high PCV when compared to the 5% DMSO group. Furthermore, F4 significantly (p<0.05) lowered serum concentrations of malondialdehyde. Phytoconstituents identified by the GC-MS include tetradecene; cetene; 3-(benzylthio) acrylic acid, methyl ester; 1-octadecene; 9-heptadecanone; hexadecanoic acid, methyl ester; dibutyl phthalate; eicosene; octadecenoic acid, methyl ester; oleic acid; 2-methyl-Z,Z-3,13-octadecadienol; 1-docosene; 3-phenylthiane, s-oxide; phenol, 3-methyl; phthalic acid, di(2-propylpentyl) ester and 1,4-benzenedicarboxylic acid, bis (2-ethylhexyl) ester.
Conclusion:
F4 from EtOAc contains six carbohydrates (9.58%), two free fatty acids (6.48%), five fatty acid esters (27.73%), two aromatic compounds (50.63%) and one organosulphide (5.61%). It inhibited TAO and demonstrated antitrypanosomal effects.
Keywords: Anaemia amelioration, Drug discovery, Enzyme inhibition assay, Medicinal plant, Target-based screening, Trypanosoma congolense
1. Introduction
Trypanosomiasis is a tropical disease affecting animals and human caused by extracellular haemoprotozoan parasites of the genus Trypanosoma. The disease is transmitted by the bite of Glossina species. Trypanosomiasis affects over 50 million cattle (FAO, 2003) and 70 million poor people (Kennedy, 2019) in sub-Saharan Africa (SSA). Human African trypanosomiasis (HAT) is caused by T. brucei gambiense and T. b. rhodesiense, while African animal trypanosomiasis (AAT) is caused by Trypanosoma congolense, T. vivax and T. brucei. AAT hinders agricultural productivity and is a threat to sustainable food security in SSA (Holt et al., 2016) with estimated adverse effects on agricultural production and socioeconomic wellbeing to the tune of US$5 billion (Yaro et al., 2016). AAT affects close to 10 million km2 of land in 37 countries of SSA (PATTEC, 2000). Thus, control of the disease will help improve the wellbeing and economic prosperity of the people of SSA.
The current drugs for the treatment and management of trypanosomiasis are far from satisfactory owing to high incidences of drug resistance (Giordani et al., 2016). Additionally, vaccine development against the disease, which is often the most promising approach for the containment of highly infectious diseases, is far from reality because of the efficient immune evasion mechanism of trypanosomes. This mechanism is aided by a highly conserved variable surface glycoprotein of the parasites and it enables the parasites escape the immune system of the host (Black and Mansfield, 2016). For these reasons, attention for newer and more potent antitrypanosomal drugs is being focused on the identification of a hit molecule (especially from natural products) with potential to inhibit validated important molecular targets in trypanosomes necessary for the survival of the trypanosomes. Exploiting successes recorded with the validated drug-target (ornithine decarboxylase), which is the target for eflornithine used in the treatment of HAT, Menzies et al. (2018) identified trypanosome alternative oxidase (TAO) as an important drug-target critical for energy metabolism and survival of trypanosomes.
One important area of attention for the identification and development of potential lead drug candidates is natural products (NPs) (Harvey et al., 2015). NPs are secondary metabolites produced by plants or animals used primarily for defending or adapting purposes (Bernardini et al., 2017). NPs are good sources of bioactive metabolites for drug discovery. Approximately two-thirds of therapeutic compounds approved by the FDA in the US in the last two decades were derived from NP, with close to 70% of pharmaceutical compounds currently in use or in clinical trials emanating from natural products (Harvey et al., 2015). One source of NP for drug discovery is medicinal plants. Anogeissus leiocarpa (DC.) Guill. & Perr. (Combretaceae) is a medicinal plant reputed in ethnomedicine for the treatment of trypanosomiasis (Singh et al., 2016). Our aim is to conduct bioassay-guided antitrypanosomal studies of A. leiocarpa, evaluate inhibitory effect of the most active column fractions of the plant on TAO and conduct antitrypanosomal studies of the potential TAO inhibitor.
2. Materials and methods
2.1. Plant materials
Stem-bark of A. leiocarpa was harvested on the main campus of the Ahmadu Bello University, Zaria (ABUZ) at 11°9ʹ48.21048ʺN 7°38ʹ5.91828ʺE. The specimen of the plant- leaf and flower was taken to the Herbarium Section of the Department of Botany, Faculty of Life Sciences, ABUZ for identification and 1738 voucher specimen number was issued for reference. The stem-bark of the plant was spread out in the laboratory and dried to a constant weight. It was then pulverised to fine-coarse particles with mortar and pestle and stored in a fridge at 4°C.
2.2. Plant extraction
Five hundred gram of the pulverised stem-bark of A. leiocarpa was extracted with methanol for preliminary study and this extract served as crude methanol extract (MeOHE). Thereafter, 2 kg of the pulverised stem-bark of A. leiocarpa was subjected to successive exhaustive extraction; starting with hexane (Hex), ethyl acetate (EtOAc) and methanol (MeOH) using Soxhlet apparatus. Each filtrate was concentrated to dryness with a rotary evaporator and the extracts stored in appropriately labeled bottles at 4°C.
2.3. Parasites
The Trypanosoma congolense used for the study was kindly donated by the Nigerian Institute for Trypanosomiasis Research, Unguwar Rimi, Kaduna, Kaduna State, Nigeria. The parasite was maintained in the laboratory throughout the experimental period with continuous passage. The passage was done two weeks (usually 12–14 days) following the first detection of parasites in the infected rats. Parasitaemic blood was collected from the tip of the mouse’s tail at peak parasitaemia (8.7 × 107 trypanosomes per milliliter of blood, usually in the range of 110–130 trypanosomes per microscopic field), diluted with physiological saline to 2–3 trypanosomes per microscopic field and inoculated into another prospective donor mouse.
2.4. Preliminary bioassay-guided antitrypanosomal screening of the extracts
In vitro drug incubation of 20, 2 and 0.2 μg/μl of MeOHE and Hex, ETOAc and MeOH extracts were carried in triplicates to identify most promising extract against T. congolense for purification and enzyme inhibition assay. In brief, 50 μl each of 20, 2 and 0.2 μg/μl was mixed with 50 μl of blood-containing 8.7 × 107 trypanosomes per milliliter of blood (prasitaemic blood), giving final concentrations of 10, 1 and 0.1 μg/μl, respectively, in a 96-well round bottom microtitre plate and the plate agitated gently. Similarly, wells containing diminazene aceturate, phosphate-buffered saline and parasitemic blood alone served as positive, negative and untreated controls, respectively. The content was incubated at 25°C and the antitrypanosomal efficacy of the assay (trypanosome viability) was evaluated at 1 h interval for 6 h. Trypanosome viability-complete cessation of motility or relative reduction in motility of the parasites compare to the negative control was scored from 0 (no viable/active trypanosomes) to 6 (highest number of active trypanosomes) (Table 1) (Tauheed et al., 2020) and was used as a measure of efficacy.
Table 1.
Score chart for in vitro antitrypanosomal study (Tauheed et al., 2020)
| Trypanosomes density | ||
|---|---|---|
| Score | Remark | |
|
| ||
| 0 | 0 | No motile (active) parasite in ≥ 20 fields |
| 1 – 2 | 1 | Moribund parasites in ≥ 20 fields |
| 3 – 5 | 2 | Motile parasites in ≥ 3 fields |
| 6 – 10 | 3 | Motile parasites in ≥ 3 fields |
| 11 – 20 | 4 | Motile parasites in ≥ 3 fields |
| 21 – 40 | 5 | Motile parasites in ≥ 3 fields |
| > 40 | 6 | Motile parasites in ≥ 3 fields |
2.5. Purification of the most active extract
A 1-L column was packed with silica gel (60–120 mesh) and 9.5 g of the EtOAc (most active) extract of A. leiocarpa was dissolved in 200 ml of hexane (Hex) to form slurry. The slurry was gently poured into the column and allowed to saturate for 3 h before collection commenced. The mobile phase used were Hex 100%; Hex:EtOAc (9:1, 8:2, 7:3, 6:4, 5:5, 4:6); EtOAc 100%; EtOAc:MeOH 9:1 and MeOH 100%. At various time intervals, 30 ml of eluent was collected into 50 ml each. The eluents were monitored on thin-layer chromatography to identify similar eluents (fractions).
2.6. Trypanosome alternative oxidase inhibition assay
The enzyme inhibition assay of the fractions and extracts was done by assessing ubiquinol oxidase activity (Kido et al., 2010; Balogun et al., 2019). The purified recombinant TAO (rTAO) was a kind gift of Dr Emmanuel O. Balogun, Department of Biomedical Chemistry, Graduate School of Medicine, University of Tokyo, Japan. Each fraction/extract (sample) was pre-incubated with 75 ng rTAO and 50 mM Tris-HCl, pH7.4 for 2 min in a 1 ml quartz cuvette and ubiquinol-1 (150 μM) was added to start the reaction. A pre-incubated rTAO, Tris-HCl and ubiquinol-1 only served as control. The inhibitory activity of the samples on the enzyme was measured by recording change in absorbance at 278 nm with UV-Vis spectrophotometer (Cary 300 UV-Vis®: Agilent Technologies).
2.7. Chemical characterisation of bioactive metabolites in the potential TAO inhibitor
Gas chromatography-mass spectrometry, GC-MS, (Agilent Technologies 7890A GC and 5977B MSD, California, United States) was used to identify secondary metabolites present in the most promising TAO inhibitor. Briefly, the oven temperature was set at 110°C (minimum) and 325°C (maximum) with 2 min hold time, 1 min equilibration time, 50°C post run and rate of 5°C/min. It was operated at an ionization voltage of 70 eV. Split sampling was used to inject the sample at 5 ml/min. The identification of compounds from the spectral data was done using the National Institute of Standards and Technology spectral record.
2.8. Experimental animals
Nine females and three males Wistar rats and mice were obtained from the Animal House of the Department of Veterinary Pharmacology and Toxicology, ABUZ and were used as the parent stock to raise the experimental animals. The animals were kept in clean mouse cages with sawdust as bedding. The bedding was changed every 4 days. Rat chaw and water were provided ad libitum throughout the period of the experiment. Approval for the use of the animals was given by the Ethical Committee on Animal Use and Care, ABUZ (reference number: ABUCAUC/2019/005).
2.9. Antitrypanosomal studies of the potential TAO inhibitors
2.9.1. Drug incubation infectivity test of the potential TAO inhibitors
In vitro drug incubation described above was similarly carried out for the five fractions (potential TAO inhibitors) but advanced to drug incubation infectivity test (ex vivo) (Kaminsky et al., 1990). In this case, however, six concentrations from 20 to 0.1 μg/μl were used to increase the degree of precision. Furthermore, the content of each well was inoculated into one mouse each any time score 0 was attained or at the end of the 6-hour incubation period for the concentrations that did not kill the parasites completely and the mice were observed daily for possible establishment of infection/development of parasitaemia .
2.10. In vivo assay of the potential TAO inhibitor
The median lethal dose (LD50) of the extracts was carried out according to the OECD guideline (OECD, 2008) and the dose optimisation of the extracts was evaluated. Furthermore, MeOH extract (MeOHE) and EtOAc extract (EtOAcE) of A. leiocarpa from where F4 was obtained were equally tested.
2.11. Experimental design
Twenty-five parasitaemic (4.2 × 103 trypanosomes per milliliter of blood) adult Wistar rats were randomly divided into five groups of 5 rats each. Rats in groups I, II and III were treated, intraperitoneally with F4, MeOHE and EtOAcE at 200 mg/kg, respectively, for 5 days. Rats in group IV were similarly treated intraperitoneally once with diminazene aceturate at 7 mg/kg while rats in group V were treated with 5% dimethyl sulfoxide (DMSO) intraperitoneally at 2 ml/kg for 7 days. To assess the efficacy of treatments, a drop of blood was collected from the tip of the tail of each rat onto a clean microscope slide, covered with a coverslip and observed at × 400 magnification; and the number of trypanosomes in ≥ 10 fields was enumerated. At the end of the experiment, the rats were humanely sacrificed by jugular venesection and haematological parameters and malondialdehyde concentration were evaluated.
2.12. Haematology of the experimental rats treated with the potential TAO inhibitor
Blood collected into ethylenediaminetetracetic acid sample bottles was used to determine packed cell volume (PCV), total leukocytes and erythrocytic indices with haematology autoanalyser (Mindray Hematology Analyzer BC3600, Hoevelaken). Mean corpuscular volume, mean corpuscular hemoglobin and mean corpuscular hemoglobin concentrations were also determined.
2.13. Malondialdehyde of the experimental rats treated with the potential TAO inhibitor
Three milliliter of blood from each rat was also collected into plain sample bottles without anticoagulant and was allowed to clot. The bottles were centrifuged at 1000 × g for 15 min and the serum carefully decanted into pre-labeled Eppendorf tubes. Serum malondialdehyde (MDA) was determined as described by Draper and Hadley (1999). Briefly, a centrifuge tube containing a mixture of 0.5 ml of serum sample and 2.5 ml of 100 g/l trichloroacetic acid (TCA) solution was placed in a boiling water bath for 15 min. The tube was brought to room temperature under running tap water and centrifuged at 1000 × g for 10 min. Thereafter, 2 ml of the supernatant was added to 1 ml of 6.7 g/l thiobarbituric acid (TBA) in a test tube and returned to the water bath for another 15 min. It was cooled again by running tap water and its absorbance was taken at 532 nm with a spectrophotometer (Spectrum lab 23A China). A tube containing 1 ml of 10% TCA and 1 ml of 0.67% TBA only served as blank. Lipid peroxidation is measured by the intensity of pink coloration.
2.14. Data analysis
Data were expressed as mean ± standard error of mean and subjected to one-way analysis of variance followed by Tukey post-test. The results of the in vitro studies were subjected to Kruskal-Wallis and Dunns post-tests. GraphPad Prism version 5.0 was used and values of p<0.05 were considered statistically significant. EC50 values were obtained with the linear regression eqation of best fit: y = ax+b using Excel. Where a = intercept (the value of y when x = 0), b = slope, x = explanatory variable, y = dependent variable (EC50).
3. Results
3.1. Chemical characterisation of F4
Figure 1 shows the GC-MS profile of the F4, obtained as the most active fraction from the EtOAc extract of A. leiocarpa. The F4 comprises six carbohydrates (9.58%): 1, 2, 4, 5, 8 and 12; two aromatic compounds (50.63%): 3 and 7; two free fatty acids (6.48%): 10 and 11; five fatty acid esters (27.73%): 6, 9 and 14–16; and one organosulphide (5.61%): 13 (Table 2).
Figure 1.
The GC-MS profile of fraction 4 of from ethyl acetate extract of Anogeissus leiocarpa showing the peaks and their retention time.
Table 2.
Phytochemical constituents detected in the GC-MS chromatogram of EtOAc fraction 4 of Anogeissus leiocarpa
| Peak No. | Compound name | Ret. Time, min | Area (%) | Quality |
|---|---|---|---|---|
|
| ||||
| 1 | 7-tetradecene and 4-tetradecene | 6.361 | 0.96 | 94, 94 |
| 2 | Cetene | 8.966 | 2.05 | 99 |
| 3 | 3-benzyl-5-chloro-1,2,3-triazole 1-oxide | 10.721 | 1.01 | 38 |
| 4-[(tetracyclo[3.2.0.0(2,7).0(4,6)] hept-3-yl)oxy]benzonitrile | 38 | |||
| 3-(benzylthio)acrylic acid, methyl ester | 35 | |||
| 4 | 1-octadecene and E-15-heptadecenal | 11.362 | 2.22 | 99, 98 |
| 5 | 9-heptadecanone | 12.382 | 0.73 | 91 |
| 6 | hexadecanoic acid, methyl ester | 13.001 | 2.10 | 97 |
| Dodecanoic acid, 10-methyl-, methy ester | 94 | |||
| 7 | 1,2-benzenedicarboxylic acid, butyl 2-methylpropyl ester and dibutyl phthalate | 13.650 | 49.62 | 97, 96 |
| 8 | 3-eicosene; 5-eicosene and 9-eicosene | 13.860 | 2.14 | 98, 96, 96 |
| 9 | 11-octadecenoic acid, methyl ester and | 15.350 | 1.99 | 99 |
| cis-13-octadecenoic acid, methyl ester | 99 | |||
| 10 | trans-13-octadecenoic acid; oleic acid and 2-methyl-Z,Z-3,13-octadecadienol | 16.049 | 3.53 | 95, 95, 90 |
| 11 | Oleic acid; octadec-9-enoic acid and 9-octadecenoic acid, (E) | 16.086 | 2.94 | 99, 96, 86 |
| 12 | 1-docosene; 1-eicosene and 9-eicosene | 16.632 | 1.48 | 99, 98, 96 |
| 13 | 3-phenylthiane, s-oxide; benzonitrile, m-phenethyl and N-benzyl-1H-benzimidazole | 20.601 | 5.61 | 27, 20, 18 |
| 14 | (Z)-3-(pentadec-8-en-1-yl) phenol and phenol, 3-methyl | 21.120 | 4.95 | 64, 52 |
| 15 | Bis(2-ethylhexyl) phthalate and phthalic acid, di(2-propylpentyl) ester | 21.968 | 16.73 | 98, 78 |
| 16 | 1,4-benzenedicarboxylic acid, bis (2-ethylhexyl) ester | 24.866 | 1.96 | 87 |
3.2. Preliminary bioassay-guided antitrypanosomal screening of the extracts
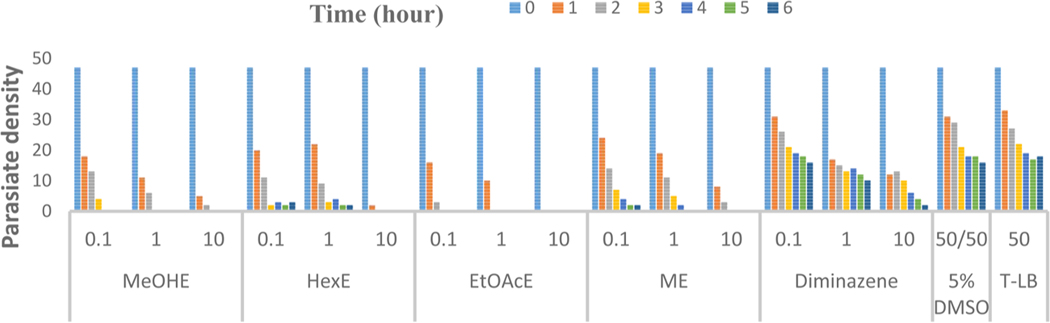
The four extracts significantly (p<0.05) exhibited in vitro antitrypanosomal effect compared to the negative and untreated controls. EtOAcE rapidly immobilized the trypanosomes completely within 1 h at the highest concentration 20 μg/μl. With the exception of the HexE, EtOAcE at the lowest concentration of 0.1 μg/μl showed a comparable antitrypanosomal effect of the highest concentrations of the MeOHE. Overall, EtOAcE exhibited the most promising antitrypanosomal effect. Although the four extracts showed significant antitrypanosomal effects, the activity of the EtOAcE was higher (p<0.05) than the HexE and MeOHE (Figure 2).
Figure 2.
Preliminary in vitro bioassay-guided antitrypanosomal screening of crude methanol and three successive extracts of Anogeissus leiocarpa. MeOHE = Crude methanol extract; EtOAc = Ethyl acetate extract; HexE= Hexane extract; Diminazene = Diminazene aceturate (positive control); 5% DMSO= Dimethyl sulphoxide (negative control); ME= Methanol extract (Successive); T-LB= Trypanosome-laden blood
3.3. Column chromatography
Collecting 30 ml of eluent each resulted in the collection of 128 eluents (fractions) and monitoring of these fractions on thin-layered chromatography yielded 5 pulled fractions (F1-F5). The yields of the fractions are F1: 0.0801 g, F2: 0.7421 g, F3: 0.1289 g, F4: 1.4141 g and F5: 5.0337 g.
3.4. TAO inhibition assay
Only F4 significantly (p<0.05) inhibited TAO compared to the control. EtOAcE inhibited the enzyme though not statistically significant (p<0.05). Other fractions and MeOHE of A. leiocarpa did not show inhibitory effect against the enzyme (Figure 3).
Figure 3.
Trypanosome alternative oxidase inhibition assay of the ethyl acetate fractions (F1-F5) and extracts of Anogeissus leiocarpa. Bars with different letters were exhibited significant effect (P<0.05).
3.5. In vitro and drug incubation infectivity test of the fractions
All the fractions produced significant (p<0.05), dose-dependent inhibition of trypanosome’s motility compared to the 5% DMSO treated group. F1, F3 and F4 significantly inhibited the viability of the parasites up to 0.1 μg/μl with EC50 of 0.314, 0.088 and 0.025 μg/μl, respectively, when compared with the 977741 μg/μl of the 5% DMSO group (Table 3). Similarly, F5 and F2 significantly inhibited trypanosomes viability up to 0.5 and 1 μg/μl, with EC50 of 0.243 and 0.246 μg/μl, respectively. Interestingly, only F4 completely immobilised motility of the trypanosomes within 1 h of incubation at the concentration of 10 μg/μl tested. Whereas it took F2 2 h to completely immobilise the parasites at 10 μg/μl, F1 and F3 took 3 h while F5 took 4 h to exhibit a similar effect at the same concentration.
Table 3.
Minimum effective concentration (EC50) of the fractions
| Group | F1 | F2 | F3 | F4 | F5 | Diminazene | 5% DMSO |
|---|---|---|---|---|---|---|---|
|
| |||||||
| EC50 (μg/μL) | 0.3137 | 0.2457 | 0.0876 | 0.0248 | 0.2431 | 0.5023 | 977741 |
| R2 | 0.986 | 0.996 | 0.973 | 0.982 | 0.983 | 0.943 | 0.9169 |
EC50= Minimum effective concentration; F= Ethyl acetate fraction of Anogeissus leiocarpa; Diminazene= Diminazene aceturate (positive control); DMSO= Dimethyl sulphoxide (negative control)
All the mice inoculated with concentrations at score 0 (no motile trypanosome) did not develop infection (parasitaemia) throughout the four weeks of observation (Table 4). With the exceptions of F4 and F5, the concentrations containing 1–2 trypanosomes (4.3 × 106 trypanosome/ml of blood, score 1) inoculated into mice did not develop infection throughout the period of observation. Whereas one mouse each from F4 and F5 at 0.5 and 5 μg/μl, respectively, inoculated with concentrations containing similar number of trypanosomes at score 1 developed parasitaemia (infection) and succumbed to the infection with 66.7% survival rate; all the inoculated mice in F4 at 0.1 μg/μl developed infection and succumbed to infection with 100% mortality (zero survival rate). All other concentrations inoculated into mice at the end of the 6-h in vitro enumeration of trypanosome developed infection and the mice succumbed to the infection with zero survival rates.
Table 4.
Percentage survival rate of mice under drug incubation infectivity test
| Concentration (μg/μL) | F1 | F2 | F3 | F4 | F5 | Diminazene | 5% DMSO |
|---|---|---|---|---|---|---|---|
| Survival rate (%) | |||||||
|
| |||||||
| 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.1 | 0 | 0 | 0 | 0‡ | 0 | 0 | 0 |
| 0.5 | 0 | 0 | 0 | 66.7‡ | 0 | 0 | 0 |
| 1 | 0 | 100‡ | 0 | 100 | 0 | 0 | 0 |
| 5 | 100‡ | 100 | 100‡ | 100 | 66.7‡ | 0 | 0 |
| 10 | 100 | 100 | 100 | 100 | 100 | 0 | 0 |
Score 1 at the point of inoculation into the mice. F= Ethyl acetate fraction of Anogeissus leiocarpa; Diminazene= Diminazene aceturate (positive control); DMSO= Dimethyl sulphoxide (negative control)
3.6. In vivo antitrypanosomal effect of F4
The results of the LD50 showed that the extracts are acutely safe at 5,000 mg/kg. None of the rats exhibited signs of toxicity throughout 14-day observation period.
3.7. Levels of parasitaemia in the experimental rats
The experimental and positive control groups significantly (p<0.05) suppressed the levels of parasitaemia in the systemic circulation of the treated rats when compared to the 5% DMSO group. Within the experimental groups, F4- and MeOHE-treated groups significantly (p<0.05) lowered the extent of infection compared to the EtOAcE group (Figure 4). Furthermore, F4 exhibited greater, non-significant (p>0.05) suppression of parasite multiplication than the MeOHE group. Diminazene aceturate completely eliminated the parasites from the systemic circulation of the treated rats within 72 h and showed no relapse infection.
Figure 4.
Effect of treatment with F4, and ethyl acetate and crude methanol extracts of Anogeissus leiocarpa on the levels of parasitaemia in the experimental rats. MeOHE = Crude methanol extract; EtOAcE = Ethyl acetate extract; F4 = Ethyl acetate fraction 4 of Anogeissus leiocarpa; Diminazene = Diminazene aceturate (positive control); DMSO = Dimethyl sulphoxide (negative control). Bars with different letters were exhibited significant effect (P<0.05).
3.8. Measure of the haematological responses of rats to F4 administration
PCV was significantly (p<0.05) higher in the MeOHE-, EtOAcE- and F4- of A. leiocarpa and diminazene-treated groups compared to the 5% DMSO control group (Figure 5). Comparing the extracts and fractions of A. leiocarpa on PCV of the rats, MeOHE yielded highest (p>0.05) PCV than the EtOAcE and F4. However, F4 non-significantly (p>0.05) produced higher PCV than the EtOAcE. Other haematological paramentres such as mean corpuscular volume, mean corpuscular haemoglobin and mean corpuscular haemoglobin concentration were non-significantly (p>0.05) higher than the 5% DMSO group.
Figure 5.
Haematological parameters of rats treated with fraction 4 and extracts of A. leiocarpa. MeOHE = Crude methanol extract; EtOAcE = Ethyl acetate extract; F4 = Ethyl acetate fraction 4 of Anogeissus leiocarpa; Diminazene= Diminazene aceturate (positive control); DMSO = Dimethyl sulphoxide (negative control). Bars with different letters were exhibited significant effect (P<0.05).
3.9. Effect of F4 on the serum malondialdehyde concentration of the treated rats
All the treatment groups significantly (p<0.05) lowered serum concentration of malondialdehyde (MDA) when compared to the negative control groups. Interestingly, F4 non-significantly (p>0.05) demonstrated greater lowering effect on the serum MDA concentration than the diminazene aceturate. Surprisingly, however, rats in the MeOHE-treated group exhibited lowest (p>0.05) level of serum MDA concentration when compared to EtOAcE-, F4- and diminazene aceturate-treated groups (Figure 6).
Figure 6.
Mean malondialdehyde of rats treated with the F4, ethyl acetate and crude methanol extracts of Anogeissu leiocarpa. MeOHE = Crude methanol extract; EtOAcE = Ethyl acetate extract; F4 = Ethyl acetate fraction 4 of Anogeissus leiocarpa; Diminazene= Diminazene aceturate (positive control); DMSO = Dimethyl sulphoxide (negative control). Bars with different letters were exhibited significant effect (P<0.05).
4. Discussion
The identification and design of unique small molecules with potential to disrupt specific targets necessary for the survival of parasite but with such targets either absent from or structurally dissimilar to those occurring in the host will revolutionise drug discovery against an age-long disease (trypanosomiasis) ravaging sub-Saharan Africa. The ability of F4 of A. leiocarpa to potently inhibit TAO shows that the plant contains bioactive metabolites capable of inhibiting energy metabolism of the bloodstream-form (BSF) of trypanosomes via inhibition of TAO. TAO-catalysed reaction in the mitochondrion of the BSF of trypanosome is the sole pathway through which trypanosomes derive their adequate energy for survival and inhibition of this pathway often leads to the death of the parasites (Menzies et al., 2018). Interestingly, TAO is completely absent in primates (Menzies et al., 2018). Thus, F4 if optimized could be a 21st-century magic wand to roll back trypanosomiasis and will reassert the importance of natural products in drug discovery.
The results of our in vitro, DIIT (ex vivo) and in vivo showed that F4 demonstrated significant antitrypanosomal effects. Only F4 demonstrated antitrypanosomal activity up to 0.1 μg/μl in the enumeration of cultured trypanosomes and 0.5 μg/μl in the DIIT yielding the lowest EC50 of 0.0248 μg/μl. Enumeration of cultured trypanosomes and DIIT are cheap, rapid and sensitive techniques for screening compounds against trypanosomes (Kaminsky et al., 1990; Gillingwater et al., 2017). Surprisingly, we observed that in vitro antitrypanosomal activity of diminazene aceturate was far lower than what we recorded for F4, and extracts of EtOAc and MeOH of A. leiocarpa. However, the reverse was the case with in vivo antitrypanosomal effects as diminazene aceturate exceedingly lowered parasitaemia and ultimately eliminated the parasites from the systemic circulation within 72 h after single-dose administration. Recently, diminazene and isometamidium have been reported to take 48- to 72-hour incubation to exert their full in vitro antitrypanosomal effects (Gillingwater et al., 2017). Furthermore, high concentrations of Berenil®, a brand of diminazene aceturate, are needed to prevent infectivity of trypanosomes at 24-hour incubation (Kaminsky et al., 1990). Hitherto, in vitro trypanocidal mechanism of action of drugs has not been elucidated, in vivo antitrypanosomal action of diminazene is postulated to be mediated via immunomodulatory effects. Diminazene has been reported to sufficiently stimulate humoral and cell-mediated immune system which mediates clearance of T. congolense from systemic circulation in murine experiments (Kuriakose et al., 2012). According to Tabel et al. (2013), the elimination of trypanosomes from the host is a factor of its ability to mount an adequate immune response to the successive waves of invariant variable surface glycoprotein with resultant phagocytosis of the fixed trypanosomes by liver and spleen macrophages. The exceedingly superior prevention of trypanosome infectivity by natural products under in vitro studies but poor in vivo antitrypanosomal activity manifested by their inability to completely clear the parasites from systemic circulation may warrant studies into pharmacokinetics, eg, absorption, distribution, bioavailability, plasma half-life, excretion, etc, in addition to pharmacodynamics of ethnomedicinal plant extracts.
Anaemia is a consistent and detrimental clinical feature of trypanosomiasis in livestock. Naessens (2006) observed that anaemia in livestock is more related to the productivity of animals than parasitaemia and is, therefore, used as the primary indicator of when treatment should be instituted. Compound with the potential to maintain high PCV in trypanosome-infected animals could be of help in maintaining the productivity of trypanosome-infected animals in endemic areas (Naessens, 2006; Tauheed et al., 2020). F4 in addition to its inhibitory effect on trypanosome metabolism and keeping parasitaemia at bay also maintained significantly high PCV. It could, therefore, be of immense value in trypanosome-endemic areas to maintain livestock in good productivity. Although anaemia occurs in all the stages of trypanosomiasis, consumptive anaemia (acute or early stage) is transient and generally associated with a significant decrease in PCV and usually coincides with peak parasitaemia clearance. Thereafter, a chronic (late--stage) progressive form of anaemia ensues (Stijlemans et al., 2018). Furthermore, anaemia in T. congolense-infected mice has been described as erythropoietin-insensitive, a phenomenon common in chronic infections (Noyes et al., 2009).
Chronic infections often cause lipid peroxidation and result in a high concentration of MDA in the serum (Ayala et al., 2014). Trypanosomiasis, a chronic debilitating disease of livestock and man, has been reported to cause lipid peroxidation of erythrocyte membrane with resultant high MDA (Kobo et al., 2014). High levels of MDA recorded in the 5% DMSO-treated group but significantly lowered in the experimental treated-groups could translate into perturbation of cellular function with peroxidation of the lipid membrane. Free radicals cause lipoperoxidation in the body and a linear relationship has been established between increased radical generation and MDA production (Ayala et al., 2014). Among the aldehydes formed as secondary products of lipid peroxidation, MDA is the most widely used biomarker of omega-3 and omega-6 fatty acids lipid peroxidation (Pryor, 1989). It is the most reliable and commonly used biomarker for determining oxidative stress in a biological sample (Giera et al., 2012). Polyunsaturated fatty acids of the cell membrane are the primary target of free radical-mediated lipoperoxidation which was mitigated by the presence of antioxidants, polyphenols (Moine et al., 2018). A. leiocarpa is a rich source of polyphenolic compounds (Nduji and Okwute, 1988; Singh et al., 2016). Indeed, crude methanol extract, ethyl acetate extract, and F4 of A. leiocarpa exhibited an equipotent effect in ameliorating MDA production in the present study.
Although F4 demonstrated significant in vitro and ex vivo antitrypanosomal effects, its inability to completely eliminate trypanosomes from the system circulation may not be unconnected with its promising TAO inhibitory activity. Menzies et al. (2018) observed that trypanosomes temporarily utilize the glycerol kinase (GK) pathway to generate ATP when TAO is inhibited. The inhibition of TAO results in the accumulation of glycerol-3-phosphate in the glycosome which is converted to glycerol to generate one molecule of ATP in a reverse reaction catalyzed by GK (Menzies et al., 2018).
Although NP drug discovery has contributed to the identification of promising drug candidates and subsequent design of ligands with specificity, cocktails of compounds in NPs often work synergistically to modulate multiple proteins or ligands in the body rather than single receptor (Hopkins, 2008; Effert and Koch, 2011). This led to renewed calls in polypharmacology to combat poor efficacy often seen with the purification of some compounds from natural products. The poor efficacy of some pure compounds is responsible for high attrition rates of potential drug candidates and a major cause of skyrocketing drug development costs (DiMasi et al., 2016; Waring et al., 2015). Our results showed that though MeOH and EtOAc extracts of A. leiocarpa demonstrated equipotent effects in ameliorating anaemia and MDA production, F4 exhibited significant effects in enzyme inhibition assay and parasitaemia suppression than the two extracts. Thus, isolation of a pure compound from natural products with specificity is worthwhile. Furthermore, the dearth of methods required to validate target combinations and optimization of multiple structure-activity relationships while maintaining drug-like property is another setback of polypharmacology (Hopkins, 2008).
5. Conclusion
Fraction 4 (F4) from EtOAc of A. leiocarpa contains six carbohydrates (9.58%), two free fatty acids (6.48%), five fatty acid esters (27.73%), two aromatic compounds (50.63%) and one organosulphide (5.61%). Furthermore, it potently inhibited TAO and demonstrated antitrypanosomal effects. F4 could be a good source of a potential lead compound capable of inhibiting TAO and its optimization will help revolutionise drug discovery against African trypanosomiasis. Furthermore, F4, of A. leiocarpa ameliorated trypanosome-induced anaemia and MDA and could be useful in the management of trypanosomiasis in livestock to improve productivity.
Supplementary Material
Acknowledgments
We thank E.O. Balogun for donating TAO. The assistance of Mr. Bashir Ibrahim, Department of Biochemistry, ABU, Zaria and Dr Na’imatu A. Sani, Department of Veterinary Physiology and Biochemistry, Bayero University, Kano in the enzyme inhibition assay; and column fractionation and in vitro antitrypanosomal study, respectively, is appreciated. We thank Messrs. Dennis Otie and AbdulWahab Hashim for technical assistance, and Sanusi Musa for the care of experimental animals. EOB is supported by the Fogarty International Center of the National Institutes of Health, USA under Award Number K43TW012015. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This research did not receive any specific grant from funding agency in public, commercial or not for profit sector.
Footnotes
CRediT
AMT: Conceptualisation, methodology, resources, formal analysis, investigation, writing – original draft; MM: Methodology, validation, supervision, writing – review and editing; AA: Methodology, validation, supervision; MMS: Methodology, writing – review and editing; EOB: Conceptualisation, methodology, resources, validation, supervision, writing – review and editing
Declaration of Competing Interest
We declare no conflicting interest.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phyplu.2022.100223.
References
- Ayala A, Munoz MF, Arguelles S, 2014. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Long 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini S, Tiezzi A, Laghezza Masci V, Ovidi E, 2017. Natural products for human health: an historical overview of the drug discovery approaches. Nat. Prod. Res 32, 1926–1950. [DOI] [PubMed] [Google Scholar]
- Balogun EO, Inaoka DK, Shiba T, Tsuge C, May B, Sato T, Kido Y, Nara T, Aoki T, Honma T, Tanaka A, Inoue M, Matsuoka S, Michels PAM, Watanabe Y-I, Moore AL, Harada S, Kita K, 2019. Discovery of trypanocidal coumarins with dual inhibition of both the glycerol kinase and alternative oxidase of Trypanosoma brucei brucei. FASEB J 33. 10.1096/fj.201901342R. [DOI] [PubMed] [Google Scholar]
- Black SJ, Mansfield JM, 2016. Prospects for vaccination against pathogenic African trypanosomes. Parasite Immunol 38, 735–743. [DOI] [PubMed] [Google Scholar]
- DiMasi JA, Grabowski HG, Hansen RW, 2016. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47, 20–33. [DOI] [PubMed] [Google Scholar]
- Draper HH, Hadley M, 1999. Malondialdehyde determination as index of lipid peroxidation. Met. Enzymol 186, 421–431. [DOI] [PubMed] [Google Scholar]
- Effert T, Koch E, 2011. Complex interactions between phytochemicals: the multi-target therapeutic concept of phytotherapy. Curr. Drug Target 12, 122–132. [DOI] [PubMed] [Google Scholar]
- FAO, 2003. Strategic reviews of traps and targets for tsetse and African trypanosomiasis, 9–12. [Google Scholar]
- Giera M, Lingeman H, Niessen WMA, 2012. Recent advancements in the LC- and GC-based analysis of malondialdehyde (MDA): a brief overview. Chromatographia 75, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater K, Kunz C, Braghiroli C, Boykin DW, Tidwell RR, Brun R, 2017. In vitro, ex vivo, and in vivo activities of diamidines against Trypanosoma vivax. Antimicrob. Agents Chemother. 61 e02356–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani F, Morrison LJ, Rowan TG, de Koning H, Barrett MP, 2016. The animaltrypanosomiases and their chemotherapy: a review. Parasitology 143, 1862–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AL, Edrada-Ebel R, Quinn RJ, 2015. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 14, 111–129. [DOI] [PubMed] [Google Scholar]
- Holt HR, Selby R, Mumba C, Napier GB, Guitian J, 2016. Assessment of animal African trypanosomiasis (AAT) vulnerability in cattle-owning communities of sub-Saharan Africa. Par. Vect 9, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL, 2008. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol 4, 682–690. [DOI] [PubMed] [Google Scholar]
- Kaminsky R, Gumm ID, Zweygarth E, Chuma F, 1990. A drug incubation infectivity test (DIIT) for assessing resistance in trypanosomes. Vet. Parasitol 34, 335–343. [DOI] [PubMed] [Google Scholar]
- Kennedy PGE, 2019. Update on human African trypanosomiasis (sleeping sickness). J. Neurol 266, 2334–2337. [DOI] [PubMed] [Google Scholar]
- Kido Y, Sakamoto K, Nakamora K, Hadara M, Suzuki T, Yabu Y, 2010. Purification and kinetic characterization of recombinant alternative oxidase from Trypanosoma brucei brucei. Biochim. Biophys. Acta 1797, 443–450. [DOI] [PubMed] [Google Scholar]
- Kobo PI, Ayo JO, Aluwong T, Zezi AU, Maikai V, Ambali SF., 2014. Flavonoid mixture ameliorates increase in erythrocyte osmotic fragility and malondialdehyde concentration induced by Trypanosoma brucei-infection in Wistar rats. Res. Vet. Sci 96, 139–142. [DOI] [PubMed] [Google Scholar]
- Kuriakose S, Muleme HM, Onyilagha C, Singh R, Jia P, Uzonna JE, 2012. Diminazene aceturate (Berenil) modulates the host cellular and inflammatory responses to Trypanosoma congolense infection. PLoS One 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies SK, Tulloch LB, Florence GJ, Smith TK, 2018. The trypanosome alternative oxidase: a potential drug target? Parasitology 145, 175–183. [DOI] [PubMed] [Google Scholar]
- Moine E, Brabet P, Guillou L, Durand T, Vercauteren J, Crauste C, 2018. New lipophenol antioxidants reduce oxidative damage in retina pigment epithelial cells. Antioxidants 7, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naessens J, 2006. Bovine trypanotolerance: a natural ability to prevent severe anemia and hemophagocytic syndrome? Int. J. Parasitol 36, 521–528. [DOI] [PubMed] [Google Scholar]
- Nduji AA, Okwute SK, 1988. Co-occurrence of 3,3′,•’-tri-O-methylflavellagic acid and3,3′-di-O-methylellagic acid in the bark of Anogeissus schimperii. Phytochemistry 27, 1548–1550. [Google Scholar]
- Noyes HA, Alimohammadian MH, Agaba M, Brass A, Fuchs H, Gailus-Durner V, Hulme H, Iraqi F, Kemp S, Rathkolb B, Wolf E, de Angelis MH, Roshandel D, Naessens J, 2009. Mechanisms controlling anemia in Trypanosoma congolense infected mice. PLoS ONE 4, e5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD- Organization for Economic Co-operation and Development,, 2008. OECD guidelines for the testing of chemicals. OECD/OCDE 425, 1–27. [Google Scholar]
- PATTEC- Pan African Tsetse and Trypanosomosis Eradication Campaign, 2000. A continental plan of action for the eradication of tsetse and trypanosomosis. The Organization of African Unity pathway for the PATTEC initiative 7–16 December. OAU/STRC Publication, Nairobi. [Google Scholar]
- Pryor WA, 1989. On the detection of lipid hydroperoxides in biological samples. Free Rad. Biol. Med 7, 177–178. [DOI] [PubMed] [Google Scholar]
- Singh D, Baghel US, Gautam A, Baghel DS, Yadav D, Malik J, Yadav R, 2016. The genus Anogeissus: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol 194, 30–56. [DOI] [PubMed] [Google Scholar]
- Stijlemans B, De Baetselier P, Magez S, Van Ginderachter JA, De Trez C, 2018. African trypanosomiasis-associated anemia: the contribution of the interplay between parasites and the mononuclear phagocyte system. Front. Immunol 9, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabel H, Wei G, Bull HJ, 2013. Immunosuppression: cause for failures of vaccines against African trypanosomiases. PLoS Neg. Trop. Dis 7, e2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauheed AM, Mamman M, Ahmed A, Suleiman MM, Balogun EO, 2020. In vitro and in vivo antitrypanosomal efficacy of combination therapy of Anogeissus leiocarpa, Khaya senegalensis and potash. J. Ethnopharmacol 258, 112805. [DOI] [PubMed] [Google Scholar]
- Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, Pairaudeau G, Pennie WD, Pickett SD, Wang J, Wallace O, Weir A, 2015. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov 14, 475–486. [DOI] [PubMed] [Google Scholar]
- Yaro M, Munyard KA, Stear MJ, Groth DM, 2016. Combatting African Animal Trypanosomiasis (AAT) in livestock: The potential role of trypanotolerance. Vet. Parasitol 225, 43–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.