Abstract
The suprachiasmatic nucleus (SCN) in the anterior hypothalamus is the major circadian pacemaker in humans. Melatonin is a key hormone secreted by the pineal gland in response to darkness. Light-induced stimuli are transmitted along the retinohypothalamic tract to the SCN. Activation of the SCN inhibits the production of melatonin by the pineal gland through a complex neural pathway passing through the superior cervical ganglion. Accordingly, when light is unavailable, the pineal gland secretes melatonin. The circadian rhythm modulates sleep-wake cycles as well as many physiological functions of the endocrine system, including core body temperature, pulse rate, oxygen consumption, hormone levels, metabolism, and gastrointestinal function. In neurodegenerative disorders, the sleep-wake cycle is disrupted and circadian regulation is altered, which accelerates disease progression, further disrupting circadian regulation and setting up a vicious cycle. Melatonin plays a critical role in the regulation of circadian rhythms and is a multifunctional pleiotropic agent with broad neuroprotective effects in neurodegenerative disorders, viral or autoimmune diseases, and cancer. In this review, I discuss the neuroprotective functions of melatonin in circadian regulation and its roles in promoting anti-inflammatory activity, enhancing immune system functions, and preventing alterations in glucose metabolism and mitochondrial dysfunction in neurodegenerative disorders and autoimmune central nervous system diseases.
Keywords: Melatonin, Circadian rhythm, Neurodegenerative diseases, Autoimmune diseases of the nervous system, Neuroprotection
Introduction
Various life phenomena, such as development, physiology, metabolism, and behavior, are governed by periodic patterns that arise from the body’s internal biological clocks (central and peripheral oscillators), collectively referred to as the circadian rhythm or circadian clock network. The human circadian period generally has a cycle length of approximately 24.2 hours [1]. The circadian rhythm modulates various physiological functions of the endocrine system, including core body temperature, pulse rate, metabolism, sleep-wake cycles, and gastrointestinal function [2,3]. In humans, the suprachiasmatic nucleus (SCN) in the anterior hypothalamus is the major circadian pacemaker. Melatonin controls SCN activity through a feedback mechanism involving two G protein-coupled melatonin receptors, MT1 and MT2, expressed within the SCN. Melatonin activity in the SCN regulates circadian rhythms such as the sleep-wake, neuroendocrine, and body temperature cycles [4]. Circadian rhythms allow humans to adapt their biological functions to cyclical changes occurring in their environment, such as the daylight cycle. Moreover, synchronization of peripheral oscillators by melatonin reflects adaptation to internal and external environmental cues, such as humans waking up in the morning, eating breakfast, and getting energized [5].
Alzheimer disease (AD) and Parkinson disease (PD) are chronic neurodegenerative diseases (ND). The degenerative process in ND often produces sleep disorders, including irregular sleep patterns, such as daytime napping and nighttime awakening. Emerging evidence has linked sleep disorders to manifestation of aggravated symptoms of memory loss and increased risk of developing AD [6,7]. In a recent multicenter study, 66% of patients with PD complained of sleep problems and these disturbances were associated with reduced quality of life and a greater burden of non-motor symptoms [8]. Multiple lines of evidence have suggested that dysregulation of circadian rhythms associated with the occurrence of non-motor symptoms of PD, especially sleep-wake disorders [2]. Isolated or idiopathic rapid eye movement (REM) sleep behavior disorder (iRBD) is an established prodromal biomarker of synucleinopathies, such as dementia with Lewy bodies (DLB) or PD. Several recent studies have shown that altered regulation of circadian rhythms may be an early marker of the conversion of iRBD into clinically evident synucleinopathies [9,10]. As such, it may be important to employ disease-modifying treatments to promote circadian regulation in patients experiencing iRBD.
In addition to acting as a chronobiotic hormone, many preclinical studies have demonstrated that melatonin is also a cytoprotective hormone with anti-inflammatory activity, enhances the immune system, and prevents dysregulation of glucose metabolism. Melatonin has therefore been used as an oncostatic agent in several cancers [11], and the effectiveness of melatonin therapy for viral infection or autoimmune central nervous system (CNS) disease has been evaluated in preclinical and clinical studies [12,13].
In this review, I discuss the neuroprotective functions of melatonin with respect to circadian regulation and evaluate the therapeutic benefits of melatonin-mediated anti-inflammatory activity, immune system enhancement, and prevention of dysregulation of glucose metabolism in neurodegenerative disorders and autoimmune CNS disease.
Melatonin
Endogenous melatonin
Melatonin or 5 methoxy-N-acetyltryptamine was discovered in and extracted from the bovine pineal gland in 1958 by Lerner et al. [14]. Melatonin is mainly secreted by the pineal gland. Extrapineal melatonin synthesis has been detected in the cerebellum, platelets, lymphocytes, bone marrow cells, retina, skin, and especially in the gastrointestinal tract [5]. Serum melatonin concentrations vary with age. Secretion of melatonin is very low in the period from infancy to 3 months of age, and then increases and acquires a normal diurnal rhythm at 6 months of age. After reaching a peak at 1 to 3 years of age, nocturnal concentrations are maximal between the 4th and 7th years of age [4,15], and then are dramatically reduced from ages of 15 to 20 years, probably due to the rapid increase in body size during childhood and puberty without an accompanying increase in the rate of secretion. Melatonin secretion declines progressively in adulthood until 70 to 90 years of age, which is attributable to the age-related degeneration of the pineal gland [16].
As noted above, two types of melatonin receptors are highly expressed in the SCN. When melatonin binds to the MT1 receptor, it activates protein kinase C, resulting in a reduction of the SCN-alerting signal. The MT2 receptor is a guanine cyclase pathway inhibitor, and when melatonin binds to MT2 receptor, it results in a phase shift in the circadian rhythm [1].
Exogenous melatonin
Melatonin is an indoleamine secreted by the mammalian pineal gland as a neurohormone; however, it has also been found in both vertebrate and invertebrate animals, bacteria, fungi, algae, and plants [17,18]. In all melatonin-producing organisms, melatonin is synthesized from the aromatic amino acid tryptophan. In mammalian systems, tryptophan is hydroxylated to 5-hydroxytryptophan, followed by decarboxylation to form serotonin (5-hydroxytryptamine, 5-HT) [19]. In plant systems, the hydroxylation and decarboxylation steps are reversed, and serotonin synthesis proceeds through an intermediate tryptamine metabolite [20]. Melatonin can also be synthesized by enterochromaffin cells and released into the circulation in response to ingestion of food containing tryptophan [21]. Because of the potential health effects of melatonin, the melatonin content of many foods has been tested over the past decades, and melatonin has been identified and quantified in both animal foods and edible plants [16]. Melatonin is a popular supplement and can be easily purchased from drug and health food stores in a variety of formulations. However, the melatonin content often differs significantly from label claims across brands, supplement types, and lots. In addition, serotonin—a precursor of melatonin, is an important neurotransmitter and a controlled substance—is not available in supplement form. Therefore, manufacturers should ensure that melatonin supplements meet label requirements and are free from contaminants like serotonin [22].
In the last decade, numerous melatonin receptor agonists and immediate-release melatonin (IRM) or prolonged-release melatonin (PRM) products have been introduced into the market. At higher doses (3–5 mg) of IRM, a direct chronobiotic and hypnotic effect is observed. However, the hypnotic effects of IRM are limited because of its short half-life and consumption at night, when the concentration of endogenous plasma melatonin is already high [1]. Ramelteon is a selective MT1 and MT2 receptor agonist and exhibits a 10-fold greater affinity for MT1 than MT2. The absolute bioavailability of ramelteon is 1.8% (range, 0.5%–12%), with maximum concentrations achieved within 0.5–1 hour, and the elimination half-life is 1.36 ± 0.49 hours [23]. This drug has been approved for the treatment of sleep-onset insomnia in adults [24]. Agomelatine, which is an MT1 and MT2 agonist and also a serotonin 5-HT2C antagonist, has been approved for the treatment of depression in both Europe and Australia. Tasimelteon was approved in the United States in 2014 for the treatment of non-24-hour sleep-wake disorder [25]. The potency of PRM appeared to increase over time, reaching a plateau after 3 weeks [25]. Based on clinical evidence, PRM was not approved for the treatment of sleep-onset or sleep-maintenance insomnia in the United States; however, it was approved in Europe and Korea and is recommended as a first-line treatment for insomnia characterized by poor sleep quality in patients aged 55 years and older [26,27]. Although it is not covered by insurance, PRM is the only prescribed melatonin product in Korea.
Melatonin and neurodegenerative disorders
Circadian rhythm dysregulation in neurodegenerative disorders
Circadian dysregulation and disease progression are interrelated. Aging is associated with reduced production of melatonin, which is considered a crucial modifying factor of neurodegenerative disorders. In AD, due to degenerative changes in the pineal gland and impairment of the SCN, production of the pineal hormone melatonin is reduced, and the circadian rhythm is altered [28]. Chronobiological disturbances such as sundowning also play a role in accelerating the progression of mental decline, agitation and confusion in patients with AD [29]. Sleep-wake status is an important factor in the production of amyloid beta peptide (Aβ), and Aβ concentrations have been shown to increase during awakening and decrease during sleep [30].
PD is characterized by motor and non-motor symptoms and is the second most common ND. Circadian rhythm dysregulation is considered one of the non-motor features of PD and appears prior to the onset of motor symptoms [31]. Changes in the thermoregulatory circadian rhythm have been recently shown to be associated with REM sleep behavior disorder (RBD) with α-synucleinopathy [32], and while decreased heart rate variability is found in PD patients through-out the day, it is more severe at night [33]. Gastrointestinal dysfunction in PD has also been associated with dysregulated circadian rhythms [34]. A study reported a blunted regulation of secretion of melatonin by the circadian rhythm in PD, which is characterized by the reduced secretion of melatonin for 24 hours and decreased amplitude of the melatonin secretion rhythm [35]. Increasing evidence indicates that the expression of circadian rhythm genes is abnormal in various PD animal models. The Bmal1 and Bmal2 genes were significantly decreased in patients with PD, while relative Bmal1 expression was positively correlated with PD severity [36,37]. Several mechanisms of dysregulation of circadian rhythms in PD have been postulated: overexpression of alpha-synuclein resulted in this SCN protein in a transgenic mouse PD. In the midbrain, MT1 and MT2 receptors modulate the mesocorticolimbic and nigrostriatal dopaminergic pathways, and the substantia nigra of patients with PD exhibited decreased expression of MT1 and MT2 receptors [38].
It is thought that iRBD is a manifestation of the prodromal stages of most, if not all, cases of the synucleinopathies PD and DLB, and less commonly of multiple system atrophy [39]. Clinical synucleinopathies are generally accompanied by substantial dysfunction of the circadian system. Since endogenous melatonin signaling is dampened in synucleinopathies, it can be hypothesized that melatonin may improve RBD by restructuring and resynchronizing circadian rhythmicity. In a clinical study, patients with RBD did not show circadian rhythmicity for the clock genes Per2, Bmal1, and Nr1d1. In addition, the melatonin profile in patients with RBD was delayed by 2 hours compared to that in controls [40]. Two recent prospective studies showed that isolated RBD patients had altered circadian rest-activity patterns as compared with healthy controls [9,10] and that patients who converted to PD took more naps and had lower levels of physical activity during the active period with higher activity fragmentation than those who did not convert to PD at baseline. These results suggest that alterations in rest-activity patterns are temporally related to the conversion of α-synucleinopathies [10].
Disruption of the circadian rhythm leads to changes in neuroinflammation, increased oxidative stress, and reduced metabolic clearance in microglial and neuronal cells within the brain. Activation of the glymphatic system, by which the brain clears neurotoxic waste products such as amyloid plaques produced during wakefulness, is a major function of sleep that is impaired in neurodegenerative disorders [30]. Reduced sleep and fragmented sleep-wake cycles have been suggested to increase the risk of ND onset and exacerbate disease progression. The neurodegeneration caused by neurodegenerative disorders such as AD and PD can negatively affect neural pathways and subsequently desynchronize the clock, which negatively affects sleep. Additionally, these phenomena contribute to a vicious cycle of bidirectional interactions between circadian rhythms dysregulation and neurodegeneration [38].
Neuroprotective role of melatonin in alteration of circadian rhythms
In addition to its functions as a chronobiotic hormone, several preclinical studies have demonstrated that melatonin has also been shown to be a cytoprotective hormone. Melatonin can exert such effects either by acting through specific receptors on the plasma membrane or nucleus or by binding with intracellular proteins. In AD, melatonin inhibits the secretion of beta amyloid precursor protein soluble derivatives, suppresses Aβ β-sheet and amyloid fibril formation, and inhibits Aβ aggregation [41]. Abnormal Aβ deposition induces functional insufficiency of mitochondrial respiratory chain complexes, leading to mitochondrial dysfunction and increased oxidative stress [42]. Melatonin prevents mitochondrial transition pore opening, intramitochondrial lipid peroxidation, mitochondrial DNA oxidation, and cell death [43]. Calcium overload, glutamate excitotoxicity, and reactive oxygen species (ROS) in the brain can cause AD-related neurodegeneration [44]. Melatonin prevents calcium overload by inhibiting voltage-gated calcium channels, suppresses glutamate excitotoxicity by inhibiting N-methyl-ᴅ-aspartate glutamate receptors, and reduces oxidative stress by scavenging ROS. Melatonin also repairs neuronal glucose metabolism, increasing tau N-acetylglucosamine acylation and thereby decreasing tau hyperphosphorylation [45]. In PD, the ability of melatonin to reverse insulin resistance is also important [46]. It has been reported that melatonin has a neuroprotective effect on dopaminergic neurons in animal models of PD [38].
Melatonin therapy and neurodegenerative disorders
Many open-label pilot studies and case series have been published addressing melatonin therapy for neurodegenerative disorders. I reviewed randomized placebo-controlled trials (RCTs) specifically focusing on AD, PD, and RBD for this review. Five RCTs evaluated AD [47-51], four assessed PD [52-55], and two focused on RBD with or without PD [56,57]. The most important limitation of the interpretation of the results was that the melatonin formulations and dosages identified in these RCTs were different. All studies found that melatonin is a safe drug with low toxicity, and several studies suggested that melatonin treatment improves daytime performance and subjective sleep quality in AD, and improved non-motor symptoms and cognition in PD. However, none of these studies found significant improvements in nighttime sleep variables such as sleep latency and total sleep time. With regard to subjective symptoms of RBD as assessed using actigraphy, there were no effective changes attributable to melatonin administration. The results of these studies are summarized in Table 1. The duration of the trial, method of sleep assessment, variables regarding the intake of melatonin (e.g., drug intake time, dosage, etc.), heterogeneity of disease, and expression of melatonin receptors may have been responsible for the negative outcomes. One recent study has shown that the preferred time for melatonin administration is around 10 to 11 ᴘᴍ, and the timing of impulses to circadian system seems decisive and needs to be kept “always-at-the-same-clock-time.” [58]. In AD, the symptoms of sleep problems gradually improved and reached stable plateau levels after 12 weeks of melatonin supplementation, and a significantly reduced rate of decline in cognitive function has been reported after 12 and 24 weeks of treatment with PRM [51]. The effects of long-term melatonin therapy on early AD might be important; however, it is unclear whether the increased efficacy of long-term melatonin therapy in patients with AD represents recovery of MT1/MT2 receptors lost in AD or an enhancement of circadian rhythms, and further clinical studies are needed to identify the mechanisms responsibly. In addition, the required dosage, timing of administration, and duration of melatonin therapy required for treating a given neurodegenerative disorder will likely differ and will require optimization.
Table 1.
Randomized placebo-controlled study of melatonin and melatonin receptor agonist therapy in neurodegenerative disorders
| Study | Year | No. of subjects | Melatonin/melatonin agonist (medication duration) | Participants | Outcome measurements | Results |
|---|---|---|---|---|---|---|
| Alzheimer disease (AD) | ||||||
| Serfaty et al. [47] | 2002 | 25 | PRM, 6 mg (2 wk) | AD diagnosed with DSM-IV + sleep problems (agitation/shouting or wandering occurring at least two nights per week) | Actigraphy, sleep daily diary, MMSE | Melatonin had no effect on median total sleep time, number of awakenings, or sleep efficiency, nor were there any significant changes in the MMSE. |
| Singer et al. [48] | 2003 | 157 | PRM, 2.5 mg or IRM, 10 mg (8 wk) | Probable AD diagnosed with NINCDS-ADRDA + sleep problems (averaged less than 7 hours of sleep per night as documented by wrist actigraphy, and had 2 or more episodes per week of nighttime awakenings reported by the caregiver) | Actigraphy, MMSE, ADAS-Cog, IADL, Hamilton depression scale, NPI, SDI, sleep quality rating | No statistically significant differences in objective sleep measures were noted between baseline and treatment periods for any of the three groups. |
| Dowling et al. [49] | 2008 | 50 | Melatonin, 5 or 10 mg, with 1 hr of morning light exposure (≥2,500 lux in gaze direction) (10 wk) | Probable AD diagnosed with NINCDS-ADRDA + sleep problems (rest-activity rhythm disruptions included insomnia, frequent nighttime awakenings, wandering at night, unusually early morning awakenings, sundowning, and excessive daytime sleepiness) | Actigraphy | No significant differences in nighttime sleep variables were found between groups. At the end of the intervention, the light-exposure + melatonin group showed significant improvement in daytime somnolence as indicated by a reduction in the duration of daytime sleep, an increase in daytime activity, and an improvement in the day:night sleep ratio. |
| Gehrman et al. [50] | 2009 | 41 | IRM, 8.5 mg + PRM, 1.5 mg (10 days) | Probable AD diagnosed with NINCDS-ADRDA | Actigraphy, Agitated Behavior Rating Scale, Cohen-Mansfield Agitation Inventory | No significant effects of melatonin on sleep, circadian rhythms, or agitation as compared with placebo |
| Wade et al. [51] | 2014 | 60 | PRM, 2 mg (24 wk) | Mild to moderate AD diagnosed with MMSE of ≥15 | ADAS-Cog, IADL, MMSE, PSQI, CGI, NPI, SDI | Melatonin group had a significantly better cognitive performance as measured by the IADL and MMSE. Sleep efficacy was significantly improved in melatonin group. |
| Parkinson disease (PD) | ||||||
| Ortiz et al. [52] | 2017 | 13 | Melatonin, 25 mg every 12 hr (48 wk) | PD diagnosed according to clinical diagnostic criteria of the UKPDSBB | UPDRS, measurement of COX-2, nitric oxide, lipoperoxides, and glutathione peroxidase activity in serum | Melatonin decreased COX-2 activity and improved some antioxidant markers. UPDRS score decreased in the melatonin-treated patients, but not in the placebo group. |
| Daneshvar Kakhaki et al. [53] | 2020 | 60 | Melatonin, 10 mg (12 wk) | PD diagnosed according to clinical diagnostic criteria of the UKPDSBB | UPDRS, PSQI, BDI, BAI, glycemic controls, lipids, biomarkers of oxidative stress | Melatonin supplementation significantly reduced UPDRS part I score, PSQI, BDI and BAI, increased antioxidant capacity, and reduced serum insulin, and total and LDL-cholesterol concentrations. |
| Ahn et al. [54] | 2020 | 34 | PRM, 2 mg (4 wk) | PD diagnosed according to clinical diagnostic criteria of the UKPDSBB + poor sleep quality (PSQI of >5) | PSQI, RBDSQ, ESS, NMSS, PDQ-39, UPDRS-III | Melatonin treatment was associated with improvements in PSQI, NMSS, and PDQ-39. No changes were observed in UPDRS-III. |
| Delgado-Lara et al. [55] | 2020 | 26 | Melatonin, 25 mg (12 wk) | PD | ESS, SCOPA-sleep, UPDRS, Hoehn and Yahr scale, relative expression of the PER1 and BMAL1 genes (RT-qPCR) | Melatonin increased BMAL1 expression but did not improve sleep parameters. |
| REM sleep behavior disorder with or without PD | ||||||
| Gilat et al. [56] | 2020 | 30 | PRM, 4 mg (8 wk) | PD with RBD diagnosed with ICSD-3 and confirmed polysomnography finding | Actigraphy, polysomnography, DEB frequency recorded by patients on daily diary, several RBD questionnaires, CGI-I, SF-36v2 | There was no difference in CGI-1 and related symptoms between the two groups. However, sleep latency and ‘energy fatigue’ component significantly decreased in the melatonin group. |
| Jun et al. [57] | 2019 | 25 | PRM, 2 or 6 mg (4 wk) | Isolated RBD diagnosed with ICSD-3 and confirmed polysomnography finding | CGI-I, RBDQ-KR, ESS, PSQI, DEB frequency recorded by patients on daily diary, SF-36v2 | There were no significant differences in any measured outcomes as compared with the placebo. |
PRM, prolonged released melatonin; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders IV; MMSE, Mini-Mental State Examination; IRM, immediate released melatonin; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke/the Alzheimer Disease and Related Disorders Association; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognition Subscale; IADL, instrumental activities of daily living; NPI, Neuropsychiatric Inventory; SDI, Sleep Disorders Inventory; PSQI, Pittsburgh Sleep Quality Index; CGI, Clinical Global Impression; UKPDSBB, the United Kingdom Parkinson’s Disease Society Brain Bank; UPDRS, Unified Parkin-son’s Disease Rating Scale; COX-2, cyclooxygenase-2; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; LDL, low-density lipoprotein; RBDSQ, Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire; ESS, Epworth Sleepiness Scale; NMSS, Non-Motor Symptom Scale; PDQ-39, Parkinson’s Disease Quality of Life-39; UPDRS-III, UPDRS part III; SCOPA-sleep, Scales for Outcomes in Parkinson’s Disease-Sleep; RT-qPCR, real-time quantitative polymerase chain reaction; REM, rapid eye movement; RBD, REM sleep behavior disorder; ICSD-3, International Classification of Sleep Disorders, 3rd ed; DEB, dream enactment behavior; CGI-I, CGI-Improvement; SF-36v2. Short Form Health Survey version 2; RBDQ-KR, RBD questionnaire-Korean version.
Melatonin and autoimmune central nervous system disease
Maladaptive responses to environmental stress weaken the body’s resistance to other environmental stimuli, such as pathogenic organisms. Environmental stimuli to the nervous system affect the immune, inflammatory, and endocrine systems. On this conceptual basis, the day/light photoperiod is a basic environmental cue for all organisms and can also influence the immune and inflammatory systems [59]. Melatonin is a major mediator of these effects. Additionally, melatonin has anti-inflammatory, immunomodulatory, and antiapoptotic functions, as well as neuroprotective effects. Figure 1 depicts the signaling cascades that give rise to the protective effect of melatonin against neurodegenerative disorders and autoimmune CNS diseases. Owing to the multiple locations of formation and expression of melatonin receptors, this hormone is gaining interest as a possible therapeutic agent for autoimmune and inflammatory processes. The therapeutic effects of melatonin include providing potential relief in some systemic autoimmune diseases; however, the evidence is still weak.
Figure 1. Mechanisms on therapeutic targets of melatonin for neurodegenerative disorders and autoimmune CNS.
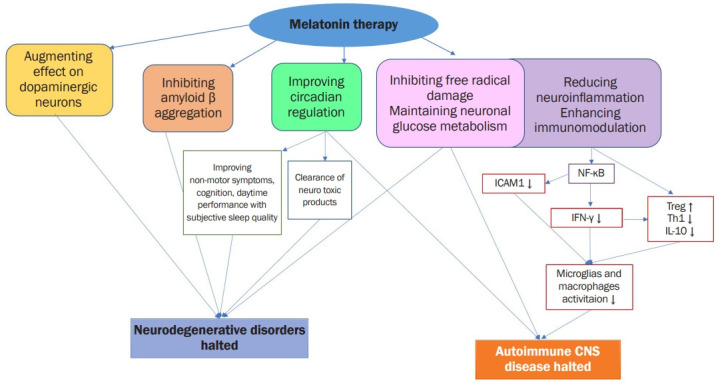
ICAM, intracellular adhesion molecule; NF-κB, nuclear factor kappa B; IFN, interferon; Treg, T regulatory; Th, T helper; IL, interleukin; CNS, central nervous system disease.
Anti-inflammatory and immunomodulatory effects of melatonin
Several animal and clinical studies have evaluated the role of melatonin in multiple sclerosis (MS), which is one of the chronic neuroinflammatory demyelinating diseases and occurs mainly in young- to middle-aged women. Clinically, MS is characterized by recurrent attacks of neuroinflammation in the CNS, causing demyelinating neurological injury and physical disability [60].
The exact etiology of MS is unknown; however, epidemiological data indicate both environmental and genetic factors are involved [61]. In MS, T cells are activated outside the CNS and play critical roles in disrupting the blood-brain barrier, activating macrophages, and attacking myelin [62]. T cells are reactivated by surface antigen-presenting cells by releasing associated antigens to CD4+ T helper cells in the periphery and by generating autoreactive proinflammatory cytokines. These immune cells trigger a cascade of inflammatory events, in the CNS, including increased expression of proinflammatory cytokines such as interleukin-12 (IL-12), IL-23, interferon-γ (IFN-γ), tumor necrosis factor-α, which induce additional inflammatory cells along with astrocyte and microglial cells [63]. Therefore, MS disease-modifying therapies aim to improve the regeneration of CNS by modulating the inflammatory/immune state [64]. In vivo studies have demonstrated that high doses of melatonin stimulated the immune system by increasing T-cell activity and lymphocytes, producing several humoral responses. Melatonin has been shown to decrease the peripheral and central Th1/Th17 ratios, and to increase regulatory responses such as IL-10 synthesis and T regulatory (Treg) cell frequency. The expressions of IFN-γ, IL-17, IL-6, and CCL20 were also suppressed following melatonin treatment [65].
Experimental autoimmune encephalitis (EAE) is an animal model of MS that shares many of the clinical and histopathological features of human MS and is used to understand the pathophysiological characteristics of MS. When melatonin was administered to mice daily beginning the day of EAE induction, the severity of clinical symptoms was reduced without altering the time of onset EAE [66]. In another study, the duration and severity of the disease were significantly reduced in the melatonin group although paralysis was evident in both melatonin-treated and vehicle-treated groups [67]. There have been few case-control or cohort studies regarding melatonin therapy in patients with MS. Most of the clinical studies used melatonin as a supplementary therapy in addition to INF-β-1b [68]. There are limitations to the interpretation of melatonin therapy in these clinical studies.
In a study involving 75 patients with rheumatoid arthritis, a daily dose of 10-mg melatonin shows a slowly developing antioxidant profile in patients, but no improvement in clinical symptoms was evident [69]. Furthermore, in a study using the pristane-induced mouse model of systemic lupus erythematosus, melatonin had a beneficial effect by decreasing IL-6 and IL-13 production [70]. Several experimental studies reported beneficial effects of melatonin against ulcerative colitis due to modulation of the inflammatory pathway and oxidative stress [71]. In a clinical study, 60 patients with ulcerative colitis have prescribed either mesalazine with melatonin (5 mg) or a placebo daily at bedtime. In the melatonin group, serum C-reactive protein concentrations remained within normal range and remission states were sustained during the study period (12 months) [72]. Although many experimental studies have found beneficial effects of melatonin in animal models of systemic autoimmune diseases, several clinical studies had small sample sizes, and some measured only indices of quality of life with clinical symptoms and did not quantify biomarkers of immune and inflammatory modulation.
These results highlight the need for more systematic and detailed clinical studies regarding the immunomodulatory and anti-inflammatory effects of melatonin as a neuroprotective agent.
Conclusions
Circadian regulation is a key factor contributing to the progression of neurodegenerative disorders. Neurodegenerative disorders may increase the vulnerability of the body’s internal clock network to the disruptive effect of external conditions, such as a weak light/dark zeitgeber. Maladaptive responses to environmental stress could affect the immune, inflammatory, and endocrine systems, and, it can either be a risk or trigger for autoimmune disease. Melatonin is an important mediator of circadian regulation and stabilization of environmental stress. In addition, many in vivo and in vitro studies have demonstrated the neuroprotective effects of melatonin therapy. Given the ability of exogenously administered melatonin to mitigate the loss of endogenous night signals, improve circadian rhythm, and enhance other anti-inflammatory effects with immunomodulation, melatonin therapy might be a promising early intervention for many disease states. However, it should be noted that high-quality clinical studies are lacking, and additional studies are needed to assess the potential roles of supplemental melatonin in disease management.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Berry RB. In: Fundamentals of sleep medicine. Berry RB, editor. Elsevier/Saunders; 2012. Circaidian rhythm sleep disorders; pp. 515–544. [Google Scholar]
- 2.Li S, Wang Y, Wang F, Hu LF, Liu CF. A new perspective for Parkinson’s disease: circadian rhythm. Neurosci Bull. 2017;33:62–72. doi: 10.1007/s12264-016-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karasek M, Winczyk K. Melatonin in humans. J Physiol Pharmacol. 2006;57 Suppl 5:19–39. [PubMed] [Google Scholar]
- 5.Tordjman S, Chokron S, Delorme R, et al. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017;15:434–443. doi: 10.2174/1570159X14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyata S, Noda A, Iwamoto K, Kawano N, Okuda M, Ozaki N. Poor sleep quality impairs cognitive performance in older adults. J Sleep Res. 2013;22:535–541. doi: 10.1111/jsr.12054. [DOI] [PubMed] [Google Scholar]
- 7.Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70:1544–1551. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos-García D, Castro ES, de Deus Fonticoba T, et al. Sleep problems are related to a worse quality of life and a greater non-motor symptoms burden in Parkinson’s disease. J Geriatr Psychiatry Neurol. 2021;34:642–658. doi: 10.1177/0891988720964250. [DOI] [PubMed] [Google Scholar]
- 9.Liguori C, Zuccarelli V, Spanetta M, Izzi F, Biagio Mercuri N, Placidi F. Sleep-wake cycle dysregulation in idiopathic REM sleep behaviour disorder. J Sleep Res. 2021;30:e13234. doi: 10.1111/jsr.13234. [DOI] [PubMed] [Google Scholar]
- 10.Feng H, Chen L, Liu Y, et al. Rest-activity pattern alterations in idiopathic REM sleep behavior disorder. Ann Neurol. 2020;88:817–829. doi: 10.1002/ana.25853. [DOI] [PubMed] [Google Scholar]
- 11.Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179–188. doi: 10.1385/ENDO:27:2:179. [DOI] [PubMed] [Google Scholar]
- 12.Bahrampour Juybari K, Pourhanifeh MH, Hosseinzadeh A, Hemati K, Mehrzadi S. Melatonin potentials against viral infections including COVID-19: current evidence and new findings. Virus Res. 2020;287:198108. doi: 10.1016/j.virusres.2020.198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bondy SC, Campbell A. Melatonin and regulation of immune function: impact on numerous diseases. Curr Aging Sci. 2020;13:92–101. doi: 10.2174/1874609813666200711153223. [DOI] [PubMed] [Google Scholar]
- 14.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587. [Google Scholar]
- 15.Sadeh A. Sleep and melatonin in infants: a preliminary study. Sleep. 1997;20:185–191. [PubMed] [Google Scholar]
- 16.Meng X, Li Y, Li S, et al. Dietary sources and bioactivities of melatonin. Nutrients. 2017;9:367. doi: 10.3390/nu9040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erland LA, Murch SJ, Reiter RJ, Saxena PK. A new balancing act: the many roles of melatonin and serotonin in plant growth and development. Plant Signal Behav. 2015;10:e1096469. doi: 10.1080/15592324.2015.1096469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnao MB. Phytomelatonin: discovery, content, and role in plants. Adv Bot. 2014;2014:815769. [Google Scholar]
- 19.Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- 20.Murch SJ, KrishnaRaj S, Saxena PK. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000;19:698–704. doi: 10.1007/s002990000206. [DOI] [PubMed] [Google Scholar]
- 21.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 22.Erland LA, Saxena PK. Melatonin natural health products and supplements: presence of serotonin and significant variability of melatonin content. J Clin Sleep Med. 2017;13:275–281. doi: 10.5664/jcsm.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams WP, 3rd, McLin DE, 3rd, Dressman MA, Neubauer DN. Comparative review of approved melatonin agonists for the treatment of circadian rhythm sleep-wake disorders. Pharmacotherapy. 2016;36:1028–1041. doi: 10.1002/phar.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:307–349. doi: 10.5664/jcsm.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riha RL. The use and misuse of exogenous melatonin in the treatment of sleep disorders. Curr Opin Pulm Med. 2018;24:543–548. doi: 10.1097/MCP.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 26.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675–700. doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 27.Choi H, Youn S, Um YH, et al. Korean clinical practice guideline for the diagnosis and treatment of insomnia in adults. Psychiatry Investig. 2020;17:1048–1059. doi: 10.30773/pi.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla M, Govitrapong P, Boontem P, Reiter RJ, Satayavivad J. Mechanisms of melatonin in alleviating Alzheimer’s disease. Curr Neuropharmacol. 2017;15:1010–1031. doi: 10.2174/1570159X15666170313123454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moe KE, Vitiello MV, Larsen LH, Prinz PN. Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J Sleep Res. 1995;4:15–20. doi: 10.1111/j.1365-2869.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 30.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology: a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Niu L, Liu X, Cheng C, Le W. Recent progress in non-motor features of Parkinson’s disease with a focus on circadian rhythm dysregulation. Neurosci Bull. 2021;37:1010–1024. doi: 10.1007/s12264-021-00711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raupach AK, Ehgoetz Martens KA, Memarian N, et al. Assessing the role of nocturnal core body temperature dysregulation as a biomarker of neurodegeneration. J Sleep Res. 2020;29:e12939. doi: 10.1111/jsr.12939. [DOI] [PubMed] [Google Scholar]
- 33.Pursiainen V, Haapaniemi TH, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllylä VV. Circadian heart rate variability in Parkinson’s disease. J Neurol. 2002;249:1535–1540. doi: 10.1007/s00415-002-0884-0. [DOI] [PubMed] [Google Scholar]
- 34.Bubenik GA, Konturek SJ. Melatonin and aging: prospects for human treatment. J Physiol Pharmacol. 2011;62:13–19. [PubMed] [Google Scholar]
- 35.Videnovic A, Noble C, Reid KJ, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71:463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding H, Liu S, Yuan Y, Lin Q, Chan P, Cai Y. Decreased expression of Bmal2 in patients with Parkinson’s disease. Neurosci Lett. 2011;499:186–188. doi: 10.1016/j.neulet.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 37.Lin Q, Ding H, Zheng Z, et al. Promoter methylation analysis of seven clock genes in Parkinson’s disease. Neurosci Lett. 2012;507:147–150. doi: 10.1016/j.neulet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Hunt J, Coulson EJ, Rajnarayanan R, Oster H, Videnovic A, Rawashdeh O. Sleep and circadian rhythms in Parkinson’s disease and preclinical models. Mol Neurodegener. 2022;17:2. doi: 10.1186/s13024-021-00504-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iranzo A, Ramos LA, Novo S. The isolated form of rapid eye movement sleep behavior disorder: the upcoming challenges. Sleep Med Clin. 2021;16:335–348. doi: 10.1016/j.jsmc.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Weissová K, Škrabalová J, Skálová K, et al. Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic REM sleep behavior disorder. Sleep Med. 2018;52:1–6. doi: 10.1016/j.sleep.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Prodhan AH, Cavestro C, Kamal MA, Islam MA. Melatonin and sleep disturbances in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2021;20:736–754. doi: 10.2174/1871527320666210804155617. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan V, Spence DW, Pandi-Perumal SR, Brown GM, Cardinali DP. Melatonin in mitochondrial dysfunction and related disorders. Int J Alzheimers Dis. 2011;2011:326320. doi: 10.4061/2011/326320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinivasan V, Kaur C, Pandi-Perumal S, Brown GM, Cardinali DP. Melatonin and its agonist ramelteon in Alzheimer’s disease: possible therapeutic value. Int J Alzheimers Dis. 2010;2011:741974. doi: 10.4061/2011/741974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Havekes R, Heckman PR, Wams EJ, Stasiukonyte N, Meerlo P, Eisel UL. Alzheimer’s disease pathogenesis: the role of disturbed sleep in attenuated brain plasticity and neurodegenerative processes. Cell Signal. 2019;64:109420. doi: 10.1016/j.cellsig.2019.109420. [DOI] [PubMed] [Google Scholar]
- 45.Cardinali DP, Vigo DE, Olivar N, Vidal MF, Brusco LI. Melatonin therapy in patients with Alzheimer’s disease. Antioxidants (Basel) 2014;3:245–277. doi: 10.3390/antiox3020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amaral FG, Andrade-Silva J, Kuwabara WM, Cipolla-Neto J. New insights into the function of melatonin and its role in metabolic disturbances. Expert Rev Endocrinol Metab. 2019;14:293–300. doi: 10.1080/17446651.2019.1631158. [DOI] [PubMed] [Google Scholar]
- 47.Serfaty M, Kennell-Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. 2002;17:1120–1127. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 48.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dowling GA, Burr RL, Van Someren EJ, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:239–246. doi: 10.1111/j.1532-5415.2007.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. 2009;17:166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wade AG, Farmer M, Harari G, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. 2014;9:947–961. doi: 10.2147/CIA.S65625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ortiz GG, Moráles-Sánchez EW, Pacheco-Moisés FP, et al. Effect of melatonin administration on cyclooxy-genase-2 activity, serum levels of nitric oxide metabolites, lipoperoxides and glutathione peroxidase activity in patients with Parkinson’s disease. Gac Med Mex. 2017;153(Suppl 2):S72–S81. doi: 10.24875/GMM.M000008. [DOI] [PubMed] [Google Scholar]
- 53.Daneshvar Kakhaki R, Ostadmohammadi V, Kouchaki E, et al. Melatonin supplementation and the effects on clinical and metabolic status in Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Clin Neurol Neurosurg. 2020;195:105878. doi: 10.1016/j.clineuro.2020.105878. [DOI] [PubMed] [Google Scholar]
- 54.Ahn JH, Kim M, Park S, et al. Prolonged-release melatonin in Parkinson’s disease patients with a poor sleep quality: a randomized trial. Parkinsonism Relat Disord. 2020;75:50–54. doi: 10.1016/j.parkreldis.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 55.Delgado-Lara DL, González-Enríquez GV, Torres-Mendoza BM, et al. Effect of melatonin administration on the PER1 and BMAL1 clock genes in patients with Parkinson’s disease. Biomed Pharmacother. 2020;129:110485. doi: 10.1016/j.biopha.2020.110485. [DOI] [PubMed] [Google Scholar]
- 56.Gilat M, Coeytaux Jackson A, Marshall NS, et al. Melatonin for rapid eye movement sleep behavior disorder in Parkinson’s disease: a randomised controlled trial. Mov Disord. 2020;35:344–349. doi: 10.1002/mds.27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jun JS, Kim R, Byun JI, et al. Prolonged-release melatonin in patients with idiopathic REM sleep behavior disorder. Ann Clin Transl Neurol. 2019;6:716–722. doi: 10.1002/acn3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kunz D, Stotz S, Bes F. Treatment of isolated REM sleep behavior disorder using melatonin as a chronobiotic. J Pineal Res. 2021;71:e12759. doi: 10.1111/jpi.12759. [DOI] [PubMed] [Google Scholar]
- 59.Maestroni GJ. The immunotherapeutic potential of melatonin. Expert Opin Investig Drugs. 2001;10:467–476. doi: 10.1517/13543784.10.3.467. [DOI] [PubMed] [Google Scholar]
- 60.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 61.Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: current knowledge and future outlook. Eur Neurol. 2014;72:132–141. doi: 10.1159/000360528. [DOI] [PubMed] [Google Scholar]
- 62.Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 2010;9:727–739. doi: 10.1016/S1474-4422(10)70094-6. [DOI] [PubMed] [Google Scholar]
- 63.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 64.Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89:225–240. doi: 10.1016/j.mayocp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Chen SJ, Huang SH, Chen JW, et al. Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2016;31:169–177. doi: 10.1016/j.intimp.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 66.Álvarez-Sánchez N, Cruz-Chamorro I, López-González A, et al. Melatonin controls experimental autoimmune encephalomyelitis by altering the T effector/regulatory balance. Brain Behav Immun. 2015;50:101–114. doi: 10.1016/j.bbi.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 67.Kang JC, Ahn M, Kim YS, et al. Melatonin ameliorates autoimmune encephalomyelitis through suppression of intercellular adhesion molecule-1. J Vet Sci. 2001;2:85–89. [PubMed] [Google Scholar]
- 68.Yeganeh Salehpour M, Mollica A, Momtaz S, Sanadgol N, Farzaei MH. Melatonin and multiple sclerosis: from plausible neuropharmacological mechanisms of action to experimental and clinical evidence. Clin Drug Investig. 2019;39:607–624. doi: 10.1007/s40261-019-00793-6. [DOI] [PubMed] [Google Scholar]
- 69.Forrest CM, Mackay GM, Stoy N, Stone TW, Darlington LG. Inflammatory status and kynurenine metabolism in rheumatoid arthritis treated with melatonin. Br J Clin Pharmacol. 2007;64:517–526. doi: 10.1111/j.1365-2125.2007.02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou LL, Wei W, Si JF, Yuan DP. Regulatory effect of melatonin on cytokine disturbances in the pristane-induced lupus mice. Mediators Inflamm. 2010;2010:951210. doi: 10.1155/2010/951210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jena G, Trivedi PP. A review of the use of melatonin in ulcerative colitis: experimental evidence and new approaches. Inflamm Bowel Dis. 2014;20:553–563. doi: 10.1097/01.MIB.0000436962.32164.6e. [DOI] [PubMed] [Google Scholar]
- 72.Chojnacki C, Wisniewska-Jarosinska M, Walecka-Kapica E, Klupinska G, Jaworek J, Chojnacki J. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J Physiol Pharmacol. 2011;62:327–334. [PubMed] [Google Scholar]


