Abstract
Melatonin is a fascinating molecule that has captured the imagination of many scientists since its discovery in 1958. In recent times, the focus has changed from investigating its natural role as a transducer of biological time for physiological systems to hypothesized roles in virtually all clinical conditions. This goes along with the appearance of extensive literature claiming the (generally) positive benefits of high doses of melatonin in animal models and various clinical situations that would not be receptor-mediated. Based on the assumption that melatonin is safe, high doses have been administered to patients, including the elderly and children, in clinical trials. In this review, we critically review the corresponding literature, including the hypotheses that melatonin acts as a scavenger molecule, in particular in mitochondria, by trying not only to contextualize these interests but also by attempting to separate the wheat from the chaff (or the wishful thinking from the facts). We conclude that most claims remain hypotheses and that the experimental evidence used to promote them is limited and sometimes flawed. Our review will hopefully encourage clinical researchers to reflect on what melatonin can and cannot do and help move the field forward on a solid basis.
Keywords: melatonin, clinical trials, controversies, preclinical data, mitochondria, bacteria, scavenging hypothesis
1. Foreword
A review is a survey of the existing literature on a given subject and the analysis of it by the author(s). Even if the interpretation of the author(s) about the facts is important, it should be identified as such. The present review presents a critical assessment of the existing literature on melatonin effects, including the conclusions drawn by their authors. Such a critical assessment is, by nature, subjective and has to be further discussed by the community in order to reach a consensus on where the field stands in terms of its hypotheses and facts. The new consensual hypothesis and perspective should then guide future research, which will go through the usual cycle of data acquisition that will then be submitted for peer review.
2. Background, Facts
Melatonin is a natural compound synthesized in the pineal gland during the nighttime, discovered by Lerner et al. [1].
Melatonin is synthesized in the pineal gland under the control of the hypothalamic suprachiasmatic nucleus, such that it is high during the night and low during the day. In humans, circulating melatonin is lowest during the day, at <2 pg/mL (8 pM), and is at 30–70 pg/mL (130–300 pM) during the night [2]. Of course, absolute concentrations can be misleading, as they depend on the age, conditions and health status of the patients; however, these levels are generally accepted by the community and were also validated in post-mortem measures of melatonin in human pineal glands [3,4]. The biosynthesis of melatonin is well established [5,6]. This cycle commands the wake/sleep cycle of almost all animals [7]. As the day length varies during the year, melatonin also indirectly commands the circannual rhythm and thus the reproduction season [8].
The main targets of endogenous melatonin in mammals are its two G-protein coupled receptors, MT1 and MT2, which have high affinities for the hormone 1 nM and lower. The receptors are activated by circulating levels of melatonin at night and are believed to translate most of its physiological actions [9].
3. Background, History
The history of melatonin use can be divided into two periods:
The first period is characterized by the establishment of a wide consensus about the nature of melatonin’s role, its synthesis and its regulation with outstanding discoveries on those subjects, and was followed by progress on the molecular mechanisms by which melatonin exerts its effect on biological rhythms [9] and sleep initiation [10,11]. In the meantime, the pharmaceutical industry described analogs of melatonin that serve as drugs in disordered sleep conditions [11,12], such as delayed sleep-wake phase disorder and jetlag, but also in cases where disrupted melatonin rhythmicity was associated with neurological conditions such as depression and anxiety [13], where they have been promising results based on standard clinical trials [14,15]. The present essay does not address this aspect of melatonin or its analogs (e.g., agomelatine, tasimelteon or ramelteon).
The second period of melatonin research is still ongoing and addresses the possible role of melatonin as an antioxidant and scavenger molecule; however, this is characterized by the absence of a general consensus in the field [16]. Hundreds of publications have proposed that melatonin can act as an agent to treat almost all the main disease conditions, such as cancers, obesity, Alzheimer’s, Parkinson’s and various virological problems, such as AIDS, Ebola and COVID-19 (see Table 1). We have previously reviewed several of these conditions, raising doubts about some claims [17,18], questioning whether a natural molecule could be used to treat so many diseases without actually addressing the mechanism(s) of actions or suggesting mechanisms of action that were independent of the melatonin receptors. Table 1 is mostly restricted to the more recent or significant claims. In short, this table essentially regroups the reviews in which the activity of melatonin on a given pathology is indicated/extrapolated. A common trait of most of these studies is the use of supra-physiological concentrations of melatonin (sometimes by six orders of magnitude higher than physiological concentrations). As pointed out earlier, “In principle, the established role of melatonin in rhythmic function is not necessarily incompatible with the use of high doses for ‘protective’ effects.” [19]; however, researchers need to carefully distinguish between the role of endogenous melatonin and the effects observed at high doses of exogenous melatonin. In a clinical setting, the question of specificity and side effects have to be carefully addressed at these supra-physiological concentration levels (see below).
Table 1.
A non-exhaustive list of the many claimed wonders of melatonin.
| Pathological Conditions | Title | Exp/Rev | Melatonin Concentration |
|
|---|---|---|---|---|
| Aging | Melatonin as an Anti-Aging Therapy | [20] | Rev | |
| Aging | Protective Role of Melatonin and Its Metabolites in Skin Aging | [21] | Rev | |
| Alzheimer’s Disease | Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. | [22] | Rev | |
| Anxiety | Melatonin as a Potential Approach to Anxiety Treatment. | [23] | Rev | |
| Atherosclerosis | Melatonin-based therapeutics for atherosclerotic lesions and beyond: | [24] | Rev | |
| Bacteria infection | Melatonin inhibits Gram-negative pathogens | [25] | Exp | 1 to 4 mM * |
| Cancer | Melatonin and cancer suppression: … | [26] | Rev | |
| Cancer | Melatonin Reverses the Warburg-Type Metabolism | [27] | Exp | 3.2 mM |
| Cancer | Oncostatic activities of melatonin: … | [28] | Rev | |
| Cardiovascular diseases | Evidence for the Benefits of Melatonin in Cardiovascular Disease | [29] | Rev | |
| Coronavirus infection | Melatonin and other indoles show antiviral activities | [30] | Exp | 3 mM ** |
| Diabetes | Coadministration of Melatonin and Insulin Improves Diabetes-Induced… | [31] | Exp | ~40 µM ** |
| Dislipidemia | The Mechanism of Oral Melatonin Ameliorates Intestinal and Adipose Lipid Dysmetabolis | [32] | Exp | 0.4 mg/mL *** |
| Ischemy (cellular) | Melatonin Attenuates Ischemic-like Cell Injury | [33] | Exp | 50 µM |
| Neurodegeneration | …New insights into the role of melatonin in neuronal recovery | [34] | Rev | |
| Neurodegeneration | Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders | [35] | Rev | |
| Parkinson’s Disease | Melatonin and Parkinson Disease: Current Status… | [36] | Rev | |
| SARS infection | Melatonin use for SARS-CoV-2 infection: … | [37] | Rev | |
| SARS infection | Melatonin: highlighting its use as a potential treatment for SARS-CoV-2 infection | [38] | Rev |
* Assuming 100% passage in 30 g mice with 4 mL of blood; ** melatonin is not the best inhibitor among the indoles tested; *** of drinking water.
4. Melatonin Goes to Clinic Why? For What?
In order to be entered into a clinical trial, a compound should have shown activity on the defined pathological model(s), exhibit low or no toxicity and be superior to the existing drugs (if there are any). Dietary “supplements”, as melatonin is defined in the USA, do not have the same legal constraints as pharmaceuticals, and their marketing information must not infer their use to treat diseases.
In general, such clinical trials are directed at a specific pathological condition and are based on a body of relevant preclinical studies. In the case of melatonin, it seems that almost all the pathological conditions one can think of have been reported in the literature to be ameliorated by melatonin! Thus, the conditions for which melatonin has been tested in clinical studies are extensive and are listed in Table 2 and Table 3. It is difficult to find a category that is not listed, and the immense spectrum of conditions reported to be treatable by melatonin is the obvious weakness of those claims.
Table 2.
“Main” categories under which the melatonin trials have been conducted.
| Abnormalities, Multiple | Colitis | Genetic Diseases, Inborn | Musculoskeletal Abnormalities | REM Sleep Parasomnias |
| Acute Graft versus Host Disease | Colitis, Ulcerative | Genetic Diseases, X-Linked | Musculoskeletal Diseases | Renal Insufficiency |
| Acute Kidney Injury | Collagen Diseases | Genital Neoplasms, Female | Musculoskeletal Pain | Renal Insufficiency, Chronic |
| Acute Lung Injury | Colonic Diseases | Glioma | Myalgia | Reperfusion Injury |
| Acute Respiratory Distress Syndrome | Colonic Diseases, Functional | Glucose Intolerance | Myocardial Infarction | Respiration Disorders |
| Adnexal Diseases | Communicable Diseases | Glucose Metabolism Disorders | Myocardial Ischemia | Respiratory Aspiration |
| Alcoholism | Confusion | Gonadal Disorders | Narcolepsy | Respiratory Distress Syndrome |
| Alcohol-Related Disorders | Congenital Abnormalities | Growth Disorders | Necrosis | Respiratory Distress Syndrome, Infant |
| Alzheimer’s Disease | Connective Tissue Diseases | Head and Neck Neoplasms | Necrotizing Enterocolitis | Respiratory Distress Syndrome, Newborn |
| Anaplastic Astrocytoma | Consciousness Disorders | Head Injuries, Closed | Neoplasm Metastasis | Respiratory Hypersensitivity |
| Anaplastic Ependymoma | Constriction, Pathologic | Headache | Neoplasms by Histologic Type | Respiratory Tract Diseases |
| Anaplastic Oligodendroglioma | Coronary Artery Disease | Headache Disorders | Neoplasms, Germ Cell and Embryonal | Respiratory Tract Infections |
| Aneurysm | Coronary Disease | Headache Disorders, Primary | Neoplasms, Glandular and Epithelial | Respiratory Tract Neoplasms |
| Angelman Syndrome | Coronaviridae Infections | Heart Diseases | Neoplasms, Nerve Tissue | Retinal Diseases |
| Anorexia | Coronavirus Infections | Heart Failure | Neoplastic Processes | Rheumatic Diseases |
| Anxiety Disorders | COVID-19 | Hemorrhage | Nervous System Neoplasms | RNA Virus Infections |
| Apnea | Cranial Nerve Diseases | Heredodegenerative Disorders, Nervous System |
Neurobehavioral Manifestations | Schizophrenia |
| Arrhythmias, Cardiac | Craniocerebral Trauma | Hot Flashes | Neurocognitive Disorders | Schizophrenia Spectrum and Other Psychotic Disorders |
| Arterial Occlusive Diseases | Craniofacial Abnormalities | Hyperglycemia | Neurodegenerative Diseases | Sclerosis |
| Arteriosclerosis | Critical Illness | Hyperinsulinism | Neurodevelopmental Disorders | Seasonal Affective Disorder |
| Arthritis | Cysts | Hyperkinesis | Neuroectodermal Tumors | Seizures |
| Asphyxia | Death | Hyperlipidemias | Neuroectodermal Tumors, Primitive | Sensation Disorders |
| Asphyxia Neonatorum | Deglutition Disorders | Hyperlipoproteinemias | Neuroendocrine Tumors | Sepsis |
| Asthma | Delirium | Hyperphagia | Neuroepithelioma | Shock |
| Astrocytoma | Dementia | Hyperplasia | Neurologic Manifestations | Shock, Septic |
| Atrophy | Demyelinating Autoimmune Diseases, CNS | Hypersensitivity | Neuromuscular Diseases | Signs and Symptoms, Digestive |
| Attention Deficit and Disruptive Behavior Disorders |
Demyelinating Diseases | Hypersensitivity, Immediate | Neurotoxicity Syndromes | Signs and Symptoms, Respiratory |
| Attention Deficit Disorder with Hyperactivity |
Depression | Hypertension | Nevi and Melanomas | Skin Diseases |
| Autism Spectrum Disorder | Depression, Postpartum | Hyperthermia | Nevus | Skin Diseases, Eczematous |
| Autistic Disorder | Depressive Disorder | Hypothermia | Nevus, Pigmented | Skin Diseases, Genetic |
| Autoimmune Diseases | Depressive Disorder, Major | Hypoxia | Nidovirales Infections | Skin Manifestations |
| Autoimmune, Diseases of the Nervous System |
Dermatitis | Hypoxia, Brain | Non-24-Hour Sleep-Wake Disorder | Sleep Apnea Syndromes |
| Autonomic Nervous System Diseases | Dermatitis, Atopic | Hypoxia-Ischemia, Brain | Nutrition Disorders | Sleep Apnea, Obstructive |
| Basal Ganglia Diseases | Developmental Disabilities | Immune System Diseases | Obesity | Sleep Deprivation |
| Behavioral Symptoms | Diabetes Complications | Infant, Newborn Diseases | Obstetric Labor Complications | Sleep Disorders, Circadian Rhythm |
| Bipolar and Related Disorders | Diabetes Mellitus | Infant, Premature, Diseases | Obstetric Labor, Premature | Sleep Disorders, Intrinsic |
| Bipolar Disorder | Diabetes Mellitus, Type 1 | Infarction | Occupational Diseases | Sleep Initiation and Maintenance Disorders |
| Blindness | Diabetes Mellitus, Type 2 | Infections | Oculo-cerebral Syndrome With Hypopigmentation |
Sleep-Wake Disorders |
| Body Temperature Changes | Diabetic Angiopathies | Infertility | Oligodendroglioma | Sleepiness |
| Body Weight | Diabetic Retinopathy | Infertility, Female | Orthostatic Intolerance | Smith-Magenis Syndrome |
| Body Weight Changes | Digestive System Diseases | Inflammation | Osteoporosis | Spinal Cord Diseases |
| Bone Diseases | Digestive System Neoplasms | Inflammatory Bowel Diseases | Ovarian Cysts | Spinal Cord Injuries |
| Bone Diseases, Metabolic | Disease Attributes | Insulin Resistance | Ovarian Diseases | ST Elevation |
| Brain Concussion | Disease Progression | Intellectual Disability | Overnutrition | Myocardial Infarction |
| Brain Diseases | Disorders of Excessive Somnolence | Intestinal Diseases | Overweight | Stomatitis |
| Brain Diseases, Metabolic | Dyskinesias | Intracranial Hemorrhages | Pain | Stomatognathic Diseases |
| Brain Infarction | Dyslipidemias | Irritable Bowel Syndrome | Pain, Post-operative | Stomatognathic System |
| Brain Injuries | Dyspepsia | Ischemia | Paralysis | Abnormalities |
| Brain Injuries, Traumatic | Dyssomnias | Ischemic Stroke | Parasomnias | Stress Disorders, Traumatic |
| Brain Ischemia | Eczema | Jet Lag Syndrome | Parkinson’s Disease | Stress, Psychological |
| Brain Neoplasms | Emergence Delirium | Joint Diseases | Parkinsonian Disorders | Stroke |
| Breast Diseases | Emergencies | Kidney Diseases | Pathological Conditions, Anatomical | Substance-Related Disorders |
| Breast Neoplasms | Encephalomyelitis | Kidney Failure, Chronic | Pediatric Obesity | Syndrome |
| Bronchial Diseases | Endocrine System Diseases | Lens Diseases | Periodontal Diseases | Synovial Cyst |
| Burning Mouth Syndrome | Endometriosis | Lipid Metabolism Disorders | Periodontitis | Synucleinopathies |
| Burns | Enterocolitis | Liver Cirrhosis | Personality Disorders | Systemic Inflammatory Response Syndrome |
| Cachexia | Enterocolitis, Necrotizing | Liver Diseases | Photophobia | Tauopathies |
| Calcinosis | Ependymoma | Lower Urinary Tract Symptoms | Pneumonia | Thoracic Neoplasms |
| Calcium Metabolism Disorders | Epilepsy | Lung Diseases | Pneumonia, Viral | Tooth Diseases |
| Carcinoma | Esophageal Diseases | Lung Diseases, Obstructive | Poisoning | Toxemia |
| Carcinoma, Non-Small-Cell Lung | Esophageal Motility Disorders | Lung Injury | Polycystic Ovary Syndrome | Trauma, Nervous System |
| Cardiomyopathies | Esophageal Spasm, Diffuse | Lung Neoplasms | Post-operative Complications | Travel-Related Illness |
| Carotid Artery Diseases | Essential Hypertension | Mania | Precancerous Conditions | Tuberous Sclerosis |
| Carotid Stenosis | Eye Diseases | Maxillofacial Abnormalities | Prediabetic State | Tuberous Sclerosis Complex |
| Cataract | Familial Alzheimer Disease | Melanoma | Pregnancy Complications | Ulcer |
| Central Nervous System Diseases | Fatigue | Menopause | Premature Birth | Urogenital Neoplasms |
| Central Nervous System Infections | Fatigue Syndrome, Chronic | Menstruation Disturbances | Primary Dysautonomias | Urologic Diseases |
| Central Nervous System Neoplasms | Feeding and Eating Disorders | Mental Disorders | Problem Behavior | Urological Manifestations |
| Cerebral Infarction | Fetal Diseases | Metabolic Diseases | Prostatic Diseases | Uterine Cervical Diseases |
| Cerebral Palsy | Fetal Growth Retardation | Metabolic Syndrome | Pruritus | Uterine Cervical Neoplasms |
| Cerebrovascular Disorders | Fever | Migraine Disorders | Psychomotor Agitation | Uterine Diseases |
| Chemically-Induced Disorders | Fibrosis | Mood Disorders | Psychomotor Disorders | Uterine Neoplasms |
| Child Development Disorders, Pervasive | Flaviviridae Infections | Mouth Diseases | Psychotic Disorders | Vascular Diseases |
| Chromosome Disorders | Fractures, Bone | Movement Disorders | Puerperal Disorders | Vascular System Injuries |
| Chronic Graft Versus Host Disease | Frailty | Mucinoses | Quadriplegia | Virus Diseases |
| Chronic Pain | Ganglion Cysts | Mucositis | Quality of Life | Vision Disorders |
| Chronic Periodontitis | Gastroenteritis | Multiple Sclerosis | Radiation Injuries | Wasting Syndrome |
| Chronobiology Disorders | Gastroesophageal Reflux | Multiple Sclerosis, Relapsing-Remitting | Radiodermatitis | Weight Gain |
| Cognition Disorders | Gastrointestinal Diseases | Muscular Atrophy | Recurrence | Weight Loss |
| Cognitive Dysfunction | Gastrointestinal Neoplasms | Muscular Diseases | REM Sleep Behavior Disorder |
Table 3.
Each category corresponds to a single trial for melatonin effect(s).
| Acanthosis Nigricans | Chromosome Deletion | Hepatitis | Marijuana Abuse | Prognathism |
| Acid-Base Imbalance | Chronic Disease | Hepatitis A | Melanosis | Prostatic Hyperplasia |
| Acidosis | Cluster Headache | Hepatitis C | MELAS Syndrome | Prostatic Neoplasms |
| Acquired Immunodeficiency Syndrome | Colic | Hepatitis C, Chronic | Memory Disorders | Psychophysiologic Disorders |
| ACTH-Secreting Pituitary Adenoma | Colonic Neoplasms | Hepatitis, Chronic | Mental Retardation, X-Linked | Rare Diseases |
| Acute Coronary Syndrome | Colorectal Neoplasms | Hepatitis, Viral, Human | Metabolism, Inborn Errors | Retinal Degeneration |
| Acute Mountain Sickness | Communication Disorders | Herpes Genitalis | Microvascular Angina | Retrognathia |
| Adamantinoma | Constipation | Herpes Simplex | Migraine with Aura | Rupture |
| Adrenal Gland Diseases | Contusions | Herpesviridae Infections | Migraine without Aura | Salivary Gland Diseases |
| Adrenal Insufficiency | Craniopharyngioma | Hip Fractures | Monosomy | Sarcopenia |
| Adrenocortical Hyperfunction | Crohn Disease | Hip Injuries | Mouth Neoplasms | Scoliosis |
| Aggression | Cushing Syndrome | HIV Infections | Mouth, Edentulous | Seizures, Febrile |
| Alcohol Drinking | Delayed Emergence from Anesthesia | Hodgkin Disease | Multiple Myeloma | Self-Injurious Behavior |
| Alternating Hemiplegia of Childhood | Dengue | Hodgkin Lymphoma | Multiple System Atrophy | Septo-Optic Dysplasia |
| Altitude Sickness | Dengue Fever | Huntington Disease | Myocardial Reperfusion Injury | Septo-optic Dysplasia Spectrum |
| Alveolar Bone Loss | Dentofacial Deformities | Hyperadrenalism | Myofascial Pain Syndromes | Severe Acute Respiratory Syndrome |
| Ameloblastoma | Depressive Disorder, Treatment-Resistant |
Hyperandrogenism | Narcotic-Related Disorders | Sex Chromosome Disorders |
| Amino Acid Metabolism, Inborn Errors | Diabetes Insipidus | Hyperpigmentation | Neonatal Sepsis | Sexually Transmitted Diseases |
| Anaplastic Oligoastrocytoma | Diabetes Insipidus, Neurogenic | Hypertension, Portal | Neoplasms, Neuroepithelial | Sexually Transmitted Diseases, Viral |
| Aneuploidy | Diarrhea | Hypertension, Pregnancy-Induced |
Neoplasms, Plasma Cell | Shy-Drager Syndrome |
| Anodontia | Diffuse Large B-Cell Lymphoma | Hypo-hidrotic Ectodermal Dysplasia |
Neoplasms, Squamous Cell | Skin Abnormalities |
| Anorexia Nervosa | DNA Virus Infections | Hypokinesia | Nervous System Malformations | Skin Diseases, Infectious |
| Aortic Aneurysm | Drug-Resistant Epilepsy | Hypopituitarism | Neuromuscular Manifestations | Skin Diseases, Viral |
| Aortic Aneurysm, Abdominal | Drug-Related Side Effects and Adverse Reactions |
Hypotension | Night Eating Syndrome | Somatoform Disorders |
| Aortic Diseases | Dysbiosis | Hypotension, Orthostatic | Nocturia | Speech Disorders |
| Aphasia | Dyskinesia, Drug-Induced | Hypothalamic Diseases | Nocturnal Enuresis | Spinal Curvatures |
| Arbovirus Infections | Dysmenorrhea | Hypothalamic Obesity | Obesity, Morbid | Spinal Diseases |
| Arthritis, Juvenile | Ear Diseases | Idiopathic Hypersomnia | Oligoastrocytoma | Squamous Cell Carcinoma of Head and Neck |
| Arthritis, Rheumatoid | Ectodermal Dysplasia | Immunoproliferative Disorders | Opioid-Related Disorders | Stillbirth |
| Atrial Fibrillation | Ectodermal Dysplasia 1, Anhidrotic | Inborn Amino Acid Metabolism Disorder |
Optic Nerve Diseases | Stress Disorders, Traumatic, Acute |
| B-cell Lymphoma | Emaciation | Influenza, Human | Optic Nerve Hypoplasia | Substance Withdrawal Syndrome |
| Back Pain | Endotoxemia | Intestinal Neoplasms | Oral Cancer | Sunburn |
| Bacteremia | Enterovirus Infections | Intracranial Aneurysm | Oral Leukoplakia | Tachycardia |
| Barrett Esophagus | Enuresis | Intracranial Arterial Diseases | Oral Squamous Cell Carcinoma | Tachycardia, Sinus |
| Binge-Eating Disorder | Epilepsy, Generalized | Intracranial Hemorrhage, Traumatic |
Osteoarthritis | Tardive Dyskinesia |
| Birth Weight | Epileptic Syndromes | Intraocular Melanoma | Osteoarthritis, Knee | Thoracic Injuries |
| Blood-Borne Infections | Erythema | Jaw Abnormalities | Osteoporosis, Postmenopausal | Tic Disorders |
| Bone Neoplasms | Eye Diseases, Hereditary | Jaw Diseases | Otorhinolaryngologic Diseases | Tics |
| Bone Resorption | Eye Neoplasms | Lacerations | Pancreatic Cancer | Tinnitus |
| Borderline Personality Disorder | Facies | Language Disorders | Pancreatic Neoplasms | Tobacco Use Disorder |
| Brain Damage, Chronic | Femoral Fractures | Leg Injuries | Pelvic Pain | Tooth Abnormalities |
| Brain Diseases, Metabolic, Inborn | Fetal Alcohol Spectrum Disorders | Lennox Gastaut Syndrome | Periodontal Atrophy | Tooth Loss |
| Bronchial Neoplasms | Fetal Death | Leukoencephalopathies | Phenylketonurias | Tooth, Impacted |
| Bronchitis | Fetal Membranes, Premature Rupture |
Leukoplakia | Photosensitivity Disorders | Tourette Syndrome |
| Bulimia | Fibromyalgia | Leukoplakia, Oral | Picornaviridae Infections | Trauma and Stressor Related Disorders |
| Bulimia Nervosa | Flavivirus Infections | Lewy Body Disease | Pigmentation Disorders | Trigeminal Autonomic Cephalalgias |
| Burnout, Psychological | Flushing | Lightning Injuries | Pinealoma | Unconsciousness |
| Carcinogenesis | Fragile X Syndrome | Liver Failure | Pineocytoma | Urinary Incontinence |
| Carcinoma, Bronchogenic | Genital Neoplasms, Male | Low Back Pain | Pituitary ACTH Hypersecretion | Urination Disorders |
| Carcinoma, Squamous Cell | Headache Disorders, Secondary | Lymphatic Diseases | Pituitary Diseases | Uterine Hemorrhage |
| Cardiac Conduction System Disease | Hearing Disorders | Lymphoma | Post-Concussion Syndrome | Uveal Diseases |
| Caregiver Burden | Heat Stress Disorders | Lymphoma, B-Cell | Post-operative Cognitive Complications |
Uveal Neoplasms |
| Central Diabetes Insipidus | Hematoma | Lymphoma, Large B-Cell, Diffuse | Postpartum Hemorrhage | Vector Borne Diseases |
| Cerebral Hemorrhage | Hematoma, Subdural | Lymphoma, Non-Hodgkin | Postural Orthostatic Tachycardia Syndrome |
Viral Hemorrhagic Fever |
| Childhood Acute Lymphoblastic Leukemia | Hematoma, Subdural, Chronic | Lymphoproliferative Disorders | Prader-Willi Syndrome | Vision, Low |
| Chorea | Hemiplegia | Lymphosarcoma | Prehypertension | X-linked Hypo-hidrotic Ectodermal Dysplasia |
| Chorioamnionitis | Hemorrhagic Fevers, Viral | Macular Degeneration | Premenstrual Dysphoric Disorder |
Xerostomia |
| Chromosome 17p Deletion | Hepatic Encephalopathy | Malocclusion, Angle Class III | Premenstrual Syndrome | |
| Chromosome Aberrations | Hepatic Insufficiency | Mandibular Diseases | Primary Orthostatic Hypotension |
In brief, whenever a clinical trial is launched, if declared on the official site (www.ClinicalTrials.gov, accessed on 23 January 2023, see below), the declaration comprises a series of keywords/categories, according to which the compound tested would have an effect—presumably—on the disease discussed (See Table 2 and Table 3 for an exhaustive list of such keywords linked to actual clinical studies, past, present and future).
One should recall (i) all the studies on a compound are not necessarily declared on this site, and (ii), more surprisingly, the data obtained are not necessarily published or made public. They belong to the initiator of the study and remain its property, translating into an absence of published results in most cases.
One should also point out that some of these studies are purely observational, aimed at measuring the levels of melatonin in patients with particular pathological conditions.
5. Clinical Studies
Melatonin is sold over the counter in several Western countries, such as Western Europa and the USA, in doses ranging from 1 to 10 mg. In clinical trials, doses of up to 100 mg have been reported (see Table 4). Several studies on humans showed that 100 mg of melatonin would result in a plasma Cmax of 1,252,500 pg/mL [i.v., bolus [39]] or 101 163 pg/mL [oral [40]]. Incidentally, 100 mg can be considered a huge dosage—the maximal level recorded in the complete review of Harpsøe et al. [41]. This raises the question of the safety of such doses. Because melatonin is considered a natural agent and not a drug, it is accessible without prescription in the USA, Canada and some other Western countries. There have been no large, long-term, high-quality randomized clinical trials specifically addressing melatonin safety in adults or children, probably because no Phase I information is required prior to commencing a clinical trial. It is unlikely that manufacturers and suppliers of melatonin as a dietary supplement would ever sponsor such an expensive trial. Nevertheless, melatonin is said to have a benign safety profile [42], yet this flies in the face of the multitude of physiological systems that melatonin has been associated with that are unrelated to any role in sleep. In a recent review, interactions with the cardiovascular, reproductive, endocrine and metabolic systems in humans were discussed, along with prescription drug interactions [43]. These interactions were discovered in controlled experiments in healthy subjects; we do not know what the effects of melatonin might be for people with cardiovascular diseases, diabetes, cancer, etc. A drug can only be termed safe in relation to what has been investigated. Nevertheless, even in 2023, researchers are calling for studies on the long-term safety of melatonin [44,45,46]. In the meantime, we are left with a somewhat patchy data collection via poison centers receiving information on adverse events (www.poison.org/ accessed on 4 February 2023). Indeed, the review by Vines et al. [47] indicated that no formal safety trials had been performed. All the available information in the public domain comes from poison and other centers, as briefly summarized here: “Health Information” given by the NIH on the side effects of melatonin mentions headaches, dizziness, nausea and sleepiness, with the possible long-term side effects remaining unclear [48]. Furthermore, a recent report by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) reached a similar conclusion based on 90 responses collected in a nutri-vigilance program between 2009 and 2017 [49], which was complemented by similar data available from other European countries and Canada [50]. Of note, the alert put forward a possible link between melatonin and infant sudden death syndrome [51]. While this study could not directly attribute these infant deaths to the use of melatonin, they concluded that “Melatonin is not known to be acutely toxic; however, it causes a multitude of systemic effects by way of mechanisms of action that are not entirely understood, especially in developing infants.” More guidance is critical for parents and pediatricians who use melatonin as a routine sleep aid for young children. This caution is also shared by another group in their recent communication [52] (see also Section 6).
Table 4.
Details of the currently recruiting trials on melatonin.
| Conditions | Number Patients | Treatment | Duration (Days) (Years) | Observational | |
|---|---|---|---|---|---|
| Smith-Magenis syndrome | 8 | 1 | Mel measure | NCT02180451 | |
| Autism | 105 | 3 | Mel measure | NCT02878499 | |
| Cushing disease | 15 | 90 | Mel measure | NCT03343470 | |
| Breast Neoplasms | 27 | 270 | Mel measure | NCT04364347 | |
| Alzheimer’s Disease | 60 | * | Mel measure | NCT04522960 | |
| Sleep deprivation | 20 | 32 | Mel measure | NCT04868539 | |
| Frail Elderly | 300 | 3 | Mel measure | NCT05107947 | |
| Hypo-hidrotic Ectodermal Dysplasia | 80 | 10 | Mel measure | NCT05378932 | |
| Bipolar disorder | 80 | 2 | Mel measure | NCT05413486 | |
| Alzheimer’s Disease | 164 | 42 | Mel measure | NCT05543681 | |
| Sleep Disturbance | 60 | 3 | Mel measure | NCT05647148 | |
| Hypoxic-ischemic Encephalopathy | 70 | 0.5–5 mg | 14 | NCT02621944 | |
| Delirium | 190 | 4 mg | 28 | NCT03438526 | |
| Multiple Progressive Primary Sclerosis | 50 | 100 mg daily | 2 | NCT03540485 | |
| Alzheimer’s Disease | 230 | 5 mg daily | 231 | NCT03954899 | |
| Osteopenia | 40 | 5 mg daily | 1 | NCT04233112 | |
| Attention Deficit Hyperactivity Disorder | 80 | 3 mg daily | 0.5 | NCT04318067 | |
| Postoperative delirium | 790 | 4 mg daily | 10 | NCT04335968 | |
| Huntington’s Disease | 20 | 5 mg daily | 63 | NCT04421339 | |
| COVID-19 infection | 30 | 3 × 10 mg daily | 28 | NCT04474483 | |
| Diabetic Eye Problems | 36 | 3 mg daily | 14 | NCT04547439 | |
| Post-operative pain | 60 | 10 mg 3 days | 21 | NCT04791943 | |
| Osteoarthritis | 252 | 5 mg daily | 60 | NCT04795336 | |
| Traumatic Brain Injury | 110 | 3–5 mg daily | 30 | NCT04932096 | |
| Necrotizing Enterocolitis | 100 | 6 mg daily | 150 | NCT05033639 | |
| Emergence Agitation | 117 | 5 mg pre-op *** | 1 | NCT05223010 | |
| Ischemic Stroke | 80 | 3 mg daily | 14 | NCT05247125 | |
| Hypertension | 23 | 1 mg daily | 1 | NCT05257291 | |
| PD | 50 | 5 mg daily | 28 | NCT05307770 | |
| Epilepsy | 120 | 5 mg daily | 28 | NCT05439876 | |
| Post-menopause insomnia | 14 | 2 mg daily | 15 | NCT05440734 | |
| Chronic Fatigue Syndrome | 106 | 1 mg daily | 28 | NCT05454683 | |
| Uveal Melanoma | 100 | 20 mg daily | 5 | NCT05502900 | |
| Severe Preterm Fetal Growth Restriction | 336 | 3 × 10 mg daily ** | 126 | NCT05651347 | |
* From 2 days to 2 years. ** Treatment to the mother, not clearly stating for how many days.*** Pre-op means pre-operation.
Interrogating the www.ClinicalTrials.gov site (accessed on 23 January 2023), we found an impressive number (742) of clinical studies on melatonin on the conditions listed in Table 2 and Table 3. The condition list can be divided into two parts: the “generic” ones; these 399 conditions regroup several trials (Table 2), for example, “Mental disorders” containing 279 studies, such as “psychosis & sleep”, “Rapid Eye Movement, Sleep Behavior Disorder & Parkinson Disease” etc., and the “unique” ones (296), corresponding to a single study, such as “Digestive system diseases or Necrotizing Enterocolitis” or “Bacteremia or Human Endotoxaemia”, etc. (Table 3). By tapping the conditions into the search query on the site, one can have access to the trials and their details.
This body of trials comprises all the declared trials in the past, present and future. As a basis for comparison, we did the same query for “resveratrol”, a natural product also reported having many beneficial actions in traditional medicine, and found 147 studies, or “vitamin C”, and found 453 trials (400 of which are completed). Melatonin has attracted a lot of attention, as the diversity of conditions (and thus of pathological conditions) covers most, if not all, human diseases.
From the trial list, we have removed 118 conditions where the status is “unknown”. Another 40 studies were reportedly withdrawn, without obvious reasons, but one might speculate that this was due to difficulties in recruiting patients or to the lack of results, particularly on delirium prophylaxis, Parkinson’s disease, coronary artery calcification, intensive care elderly population or “melatonin inhibition of NLRP3 inflammasome in COVID19 patients”, to name only but a few.
Looking at the 56 trials currently recruiting, one can separate them into two main categories: observational and interventional. The first section, “observational”, comprises all the studies in which the patient’s melatonin levels are measured as a function of (i) their pathological conditions and (ii) their daily circadian rhythms. Those studies are basically clinical biochemistry research trials and are extremely useful for our understanding of the dependence of circadian rhythms on pathological conditions. Among the pathological conditions that have been studied are Cushing’s syndrome, autism, hypo-hidrotic ectodermal dysplasia, bipolar disorder, frail elderly, etc. The data arising from those studies may be expected to have a major impact on our understanding of how melatonin synthesis and release are influenced by pathological conditions. In our view, those studies are quite important, hopefully leading to scientific paper(s) explaining the results, as those in the Harpsoe et al. review [41], including the negative results in which the melatonin rhythm remains completely “normal”.
The intervention group comprises all the studies in which melatonin is tested as a therapeutic agent. Table 4 lists 35 recruiting studies, which are both interventional and observational. It is clear that the conditions studied are numerous and diverse, including cognitive dysfunction, bone diseases, attention deficit and disruptive behavior disorders, bone fractures, chorea, coronavirus infections, diabetes complications, post-operative pain, arthritis and traumatic brain injuries.
Because cancer is one of the most documented and searched domains in pathology, we have selected the trials in which cancer patients were treated with melatonin. Sixty-five studies tested its effectiveness in various cancer-related parameters, such as a loss of appetite, sleep quality, etc. Those studies also comprised trials in which the effects of melatonin as an anti-cancer adjuvant or drug, with direct effects on the disease itself, were reported. Half of the trials are reported as completed (32 studies), yet only the following four had published their results. We have briefly summarized their results and their conclusions:
Trial NCT00513357 (USA): Conclusion: “In cachectic patients with advanced cancer, oral melatonin 20 mg at night did not improve appetite, weight, or quality of life compared with placebo” [53].
Trial NCT00668707 (Canada): Conclusion: “Melatonin may benefit cancer patients who are also receiving chemotherapy, radiotherapy, supportive therapy or palliative therapy by improving survival and ameliorating the side effects of chemotherapy” [54].
Trial NCT00925899 (Denmark): Conclusion: “In the current study, oral melatonin at a dose of 20 mg was not found to improve fatigue or other symptoms in patients with advanced cancer” [55].
Trial NCT04137627 (Indonesia): Conclusion: “In patients with squamous cell carcinoma of the oral cavity, the addition of 20-mg melatonin to neoadjuvant chemotherapy reduced the expression of miR-210 and CD44 and decreased the percentage of tumor residue; however, no statistically significant result was observed”.
The survey of those trials currently recruiting is a good image of the other ongoing trials: from those using small to large dosages of melatonin, 50 to 100 patients, spanning a month to more than a year. We would argue that the spectrum of diseases and/or conditions cannot seriously be attributed to a single melatonin-related cause.
It is very often under-regarded that melatonin has poor bioavailability. Indeed, the vast majority (>80%) of melatonin is hydroxylated by cytochrome P450 [56], conjugated with sulphate or glucuronic acid and excreted as conjugates [57], which is a classical scheme in drug metabolism. Melatonin is also a substrate of the indoleamine 2,3-dioxygenase, leading to the opening of the indole cycle, leading to AFMK (N1-acetyl-N2-formyl-5-methoxykynurenamine), which is then rapidly metabolized further to AMK (N1-acetyl-5-methoxykynurenamine) [58].
The wide range of dosages for the patients undergoing these recruiting trials varies from 0.5 to 100 mg, with most of them in the low milligram range. Once again, one should recall that at low ‘physiological’ doses—those in the 1 to 3 mg range, the resulting Cmax is low (~10 nM), even if it is two orders of magnitude higher than the nocturnal produced level. If one is given 100 mg, the circulating concentration of the compound is in the 0.5 µM range [40], as reviewed by Harpsoe et al. [41], although both concentrations vary widely between the various studies. Furthermore, the first pass principle of drug metabolism [59] clearly states that roughly half of the dose in the blood is eliminated by the liver minutes after ingestion and even shorter if injected intravenously [60]. This principle translates into the fact that melatonin remains in the µM range concentrations in the blood for a few minutes.
In the preclinical studies upon which trials are usually based, melatonin was generally used at concentrations higher than 1 µM—and even 1 mM. Thus, those concentrations are not expected to be naturally reached in living animals or in humans, and certainly not for prolonged periods. Thus, the transposition to clinical trials of those data might be considered more an act of faith than an experimental reality.
The main issue to be considered here is that nearly 4000 patients have entered these trials and have been treated with 0.5 mg to 100 mg melatonin daily. Furthermore, some of those studies are designed to run for very long periods (for several years, in some cases). In one clinical trial on melatonin and autoimmune diseases (ClinicalTrials.gov Identifier: NCT03540485, accessed on 23 January 2023), it is planned that 50 patients recruited because of their neurological conditions will receive a daily administration of 100 mg of melatonin, or a placebo, orally between 10 pm and 11 pm for 24 months. Finally, several studies are using newborn children (from a few hours to a few days old) with quite desperate health conditions—a feature that is commented on below.
In a survey (meta-analysis) of melatonin used for cardio-protection, the authors stated that the data was difficult to generalize due to the numerous possible biases, the low number of patients and their high heterogeneity [61]. Note that the combined number of patients was 396, half of which were tested with a placebo across six studies. Three of these studies were labeled as ‘no or poor effect’ (of melatonin as cardio-protectors). The doses were from 12 to 50 mg orally plus 1 or 2 mg intracoronary. Finally, the only study where the myocardial IR injury site was assessed using resonance imaging was classified as ‘no effect’ [62,63]. Incidentally, one of those articles stated, “…treatment with melatonin was associated with a larger infarct size in the group of patients” [63].
In conclusion, many clinical studies appear to have been based on scientifically poor experimental (preclinical) studies or overenthusiastic interpretations of the preclinical experimental data, thus explaining the overall poor outcome of these studies. The use of small populations of patients—elderly people as well as infants and toddlers in desperate health conditions—may have ethical considerations.
6. Melatonin in Kids
The first reports of the administration of melatonin to children were conducted by Jan and colleagues more than 28 years ago [64,65,66] in attempts to modify their disordered sleep resulting from neurodevelopmental disorders or blindness. Since that time, there have been many studies published on the pediatric use of melatonin, generally in children with comorbidities, such as autism spectrum disorder or attention deficit/hyperactivity disorder [44], as opposed to typically developing children with sleep problems. The majority of studies conducted in both adults and children have been small and short-term, although there are reports of treatments continuing for many years outside of the trials. The melatonin used in trials has usually been an immediate release preparation from non-pharmaceutical companies (dietary supplement manufacturers or chemical suppliers), with the first pharmaceutical preparation, Circadin, being approved in Europe in 2007. Circadin, a prolonged-release formulation, is authorized for the short-term treatment of primary insomnia in adults aged 55 years and older. It was not until 2018 that a formulation, Slenyto, was approved in Europe for the treatment of insomnia in children from 2 years of age with autism spectrum disorder and Smith–Magenis syndrome. With respect to the efficacy of melatonin in improving sleep in children presenting with sleep disorders, there is evidence that melatonin can have a role in those with neurodevelopmental disorders [42,67,68]; however, the effects are modest [43].
Since melatonin in the USA is not considered a drug, there is no requirement to report adverse events. The latest such report, covering 2012–21, documented more than 250,000 pediatric ingestions, 45,000 symptomatic effects, 3211 serious outcomes and 2 deaths [69]. Further, unintentional melatonin ingestion and its related hospitalizations and serious outcomes are increasing in the USA [69]. A separate recent study reported seven undetermined deaths of infants and toddlers with high exogenous melatonin as a result of deliberate or incidental ingestion of melatonin [51]. Based on these observations, careful evaluation and caution might be wise before administering melatonin to young children.
It is true that one cannot be sure that melatonin played a significant role in the deaths of infants. It is, however, an indication that melatonin is being recklessly administered to children in the absence of any formal safety information. Even the pharmaceutical companies that sell melatonin say not to take melatonin during pregnancy or when breastfeeding.
The uncertainty about the use of (high dosages of) melatonin by mothers during pregnancy and lactation remains. Indeed, in their thorough review of the subject, Vine et al. [47] concluded, “clinical studies to date suggest that melatonin use during pregnancy and breastfeeding is probably safe in humans and emphasizes the need for clinical studies…, including exogenous melatonin, during pregnancy and lactation”. The key word, for us, is certainly “probably safe”. To the best of our knowledge, no actual trial results whose primary outcomes were the safety or efficacy of melatonin for insomnia or other sleep disorders during pregnancy and lactation have been issued. Furthermore, no trial comprising “lactation” has been declared on the trial site.
Overall, our feeling is that, while the study could not directly attribute the deaths to the use of melatonin, they concluded that “Melatonin is not known to be acutely toxic; however, it causes a multitude of systemic effects by way of mechanisms of action that are not entirely understood, especially in developing infants. More guidance is critical for parents and pediatricians who use melatonin as a routine sleep aid for young children” [51].
7. Some Proposed Mechanisms behind the Claimed Beneficial Effects of Melatonin
7.1. Melatonin as an Antioxidant
7.1.1. Melatonin as a Scavenger
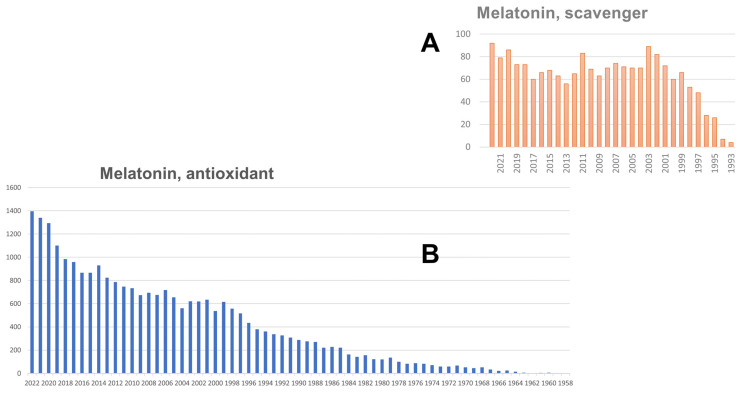
In an initial influential paper, melatonin, as well as other indole-based molecules, was shown to be a good scavenger in an a-cellular system. Then, to render the experiment transposable to a “living” system, the tissue homogenate was added to the medium, and it was found that the trapping operation still occurred. Thus, melatonin was called a scavenger. As a direct consequence, more than 1727 (23 February 2023) articles have been published using the words “melatonin” and “scavenger”. The initial publications can be pinpointed to Poeggeler et al. [70] and. The influence of this statement can be seen through the evolution of the number of papers appearing in the literature over the years after this publication (Figure 1A). The possibility that melatonin could be an antioxidant molecule appeared early in 1958. Ever since this date, publications formulating hypotheses or reporting experiments started to appear in the literature. On 25 March 2023, a query of “melatonin” + “antioxidant” returned 24,271 results (see Figure 1B).
Figure 1.
PubMed extraction of queries “melatonin + scavenger” (A); and “melatonin + antioxidant” (B).
The statement that melatonin and many other purported antioxidants are scavengers is common in the introduction of hundreds of publications. We consider the term “scavengers” to be misleading because, while these compounds can react with oxidants, they are generally no more reactive than the thousands of other compounds in cells [71]. Thus, to be 50% effective, they would need to be at a concentration approximately equal to all the other compounds in the cell that could react with the oxidant. In cell culture experiments, scavenging can seem effective; however, it is likely to be because the ratio of the compound to its putative targets is likely thousands of times higher than would occur in vivo [72].
Scavengers do not exist in complex media, living systems, etc. A true antioxidant defense is provided by enzymes that remove superoxide and hydrogen peroxide with rate constants that are hundreds of thousands to more than a million times greater than that of melatonin. For hydroxyl radical, no compound is an effective scavenger, and the antioxidant defense involves the prevention of its formation rather than its removal (see discussions in [73,74,75]).
Many of the compounds purported to have antioxidant activity can react with Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (Keap1), sparing Nrf2 from degradation and thereby activating transcription of the genes for antioxidant enzymes. Several compounds have been reported as such protectors for Keap1 degradation: TBE-31 [76,77], CDDO, a triterpenoid [78], or curcumin and caffeic acid [79,80], to name only but a few. However, many of those compounds also affect other signaling pathways that result in the protection of cells from injury [73].
7.1.2. If Not a Scavenger, Then What?
The toxicity of ROS and oxidative stress is controlled by several enzymes, the activity of which detoxifies ROS, such as the three superoxide dismutases (see discussion in Keller et al. [81]. These enzymes are under the control of oxidative response elements that, once activated, induce their expression. This process is dependent on a nuclear receptor, such as nrf2, and melatonin has been suggested to be able to induce the expression of cellular defenses against oxidative stress (see also discussion in Amoroso et al. [82]). Many years later, this nuclear receptor has still not been found. Nrf2 might be a candidate [83,84], as might be Hem oxidase 1 [85], or both [86]; however, their role is yet to be experimentally demonstrated (see also Section 7.2).
7.2. Melatonin Nuclear Receptor
The proposed potential role of the nuclear receptors, promoted by many authors, remains an example of the enthusiastic support of a study that has not been reproduced (and thus confirmed) anywhere. In 1994, it was reported that melatonin binds to the receptor RZRα and activates it [87]. Another paper from the same group was published at the same time, reporting the binding affinities of iodo-melatonin in nuclear homogenates from cells in which RZR was cloned and expressed [88], which has been cited ever since 314 times. After several years of unsuccessful attempts to confirm the results in several laboratories, the 1994 paper was withdrawn in 1997 [89]. It has been cited and was continued to be cited 546 times, including 271 times since it was retracted. One possible explanation for that might be the seductiveness of the idea of a nuclear melatonin receptor, knowing that melatonin easily penetrates cells and nuclei, and the number of reported transcriptional effects of melatonin. The goal of the community is to discover the nuclear factor that is activated by melatonin leading to the neo-synthesis of antioxidant proteins, possibly explaining the melatonin antioxidant properties. The possibility that melatonin could be an antioxidant molecule appeared early in the 1990s [90]. Ever since this date, experiments and hypotheses publications started to appear in the literature; as a query, “melatonin” + “antioxidant” returned 24,271 results on 25 March 2023 (see Figure 1B).
7.3. Melatonin in Bacteria and Mitochondria
The idea that bacteria produce melatonin has been formulated in statements such as the following one: “Evolution’s best idea” is that melatonin is supposedly produced in bacteria. From there, because bacteria colonized cells several million years ago, eukaryotic cells became mitochondria; mitochondria are thus full of melatonin. Additionally, melatonin is there to protect mitochondria, cells and living organisms from oxidative stress [91].
7.3.1. Melatonin in Bacteria
This statement can be challenged as follows:
Melatonin synthesis in microorganisms has been reviewed by Que et al. [92]. Indeed, numerous yeast, protozoa and algae are reported as capable of synthesizing the hormone, as optimistically reportedly reviewed by Hardeland et al. [93,94]. Algae, protozoa and fungi are eukaryotic organisms. On the contrary, bacteria are prokaryotic organisms, and they might have the capacity to synthesize melatonin, as reported in just six publications, listed in Table 5.
Table 5.
Bacteria that produce melatonin, as reported by Que et al. [92].
| Bacteria Type | Reference | Comments (See Text) |
|---|---|---|
| Cyanobacteria, | [95] | Comment A |
| Rhodospirillum rubrum | [95,96] | Comments A & B |
| Bacillus cereus CS-17 | [97] | Comment C |
| Ensifer sp. VA11, | [97] | Comment C |
| Pseudomonas sp. | [97] | Comment C |
| Variovorax sp. | [97] | Comment C |
| Agrobacterium tumefaciens | [97] | Comment C |
| Bacillus amyloliquefaciens | [97] | Comment C |
| Bacillus thuringiensis | [97] | Comment C |
| Sphingomonas sp. | [97] | Comment C |
| Bifidobacterium breve, | [98] | Comment D |
| Lactobacillus brevis, | [98] | Comment D |
| Lactobacillus casei, | [98] | Comment D |
| Bifidobacterium longum, | [98] | Comment D |
| Enterococcus faecalis TH10, | [98] | Comment D |
| Lactobacillus acidophilus, | [98] | Comment D |
| Lactobacillus bulgaricus, | [98] | Comment D |
| Lactobacillus fermentum, | [98] | Comment D |
| Lactobacillus helveticus | [98] | Comment D |
| Lactobacillus plantarum , | [98] | Comment D |
| Streptococcus thermophilus, | [98] | Comment D |
| Erythrobacter longus , | [99] | Comment E |
Color code: green: melatonin synthesis proven; orange: melatonin synthesis claimed (by patents); red: absence of melatonin synthesis reported.
It is interesting to look closely at those articles cited to provide proof of bacterial melatonin synthesis.
In an article by Byeon and Back on the cloning of E. coli using the necessary enzymatic machinery for this bacterium to produce melatonin, the only mention of two bacterial melatonin productions is in the following sentence: “Melatonin is predicted to have evolved from the precursor bacteria of mitochondria and chloroplasts, such as Rhodospirillum rubrum and Cyanobacteria, respectively, via an endosymbiotic event with their ancestral eukaryotic host” [95]. This is not evidence of these bacteria producing melatonin.
Manchester et al. reported a melatonin immunoreactivity in this Rhodospirillum rubrum [96]; however, this observation has never been reported independently, nor has melatonin been directly measured in the bacterium. Given the capital importance of Rhodospirillum rubrum and other purple non-sulfur bacteria, predicted to be at the origin of mitochondria according to the endosymbiotic theory of mitochondria [100], it is surprising that no more effort has been spent to clarify this point.
Jiao et al. [97] reported a thorough study on eight strains of bacteria and found melatonin in only four: Agrobacterium tumefaciens, Bacillus amyloliquefaciens, Bacillus thuringiensis and barely in Pseudomonas sp.
A review by Tan et al., while not providing any experimental data on bacteria melatonin production [98], stated that a series of Lactobacillus had been patented for their production of melatonin. Furthermore, they wrote: “If these speculations are valid, the beneficial effects in consumption of these products may, at least partially, be explained by the presence of melatonin and its isomers” [98]. This statement was later used as evidence that several Lactobacillus produce melatonin. For example, in a trial on abdominal pain in children, Lactobacillus and exogenous melatonin were used for the health of the patient [101], as if melatonin produced by Lactobacillus was not enough to obtain the desired effect. In another study, melatonin was shown to increase the amount of Lactobacillus previously decreased by sleep deprivation as if the production of melatonin by Lactobacillus was not enough to counterbalance the decrease in circulating melatonin [102]. Finally, in an earlier study on the possible relationship between several “antioxidant” molecules and their capacity to inhibit bacterial growth in the presence of mycotoxins, melatonin did not show any significant effect on the growth of Lactobacillus [103]; however, if this bacterium produces melatonin, those authors should have seen an effect of the addition of melatonin to the bacteria. In conclusion, the situation of Lactobacillus as a source of melatonin production remains, at the very least, unclear.
In Erythrobacter longus, Tilden et al. [99] reported the presence of melatonin by using a radioimmunoassay, although there is reason to be cautious when using immunoassay melatonin by radioimmunoassay in complex matrices [2,104].
Very recently, Chen et al. reported the discovery of the gene encoding for a serotonin N-acetyltransferase gene, xoSNAT3, in the bacteria Xanthomonas oryzae [105]. The synthetic melatonin capacity of this bacterium was not reported in this paper, nor was the presence of melatonin. This very large family of enzymes has been reported from many organisms [106], including by us [107,108]; however, its expression does not grant the capacity to synthesize melatonin.
We conclude that whether bacteria widely produce melatonin remains to be proven.
7.3.2. Melatonin in Mitochondria
Melatonin is claimed to be present/enriched in mitochondria [91,109].
However, robust and, in particular, quantitative data are not available. To our knowledge, massive amounts of melatonin have not been reported in mitochondria. Furthermore, if melatonin is a ROS scavenger, it would be expected to be transformed into hydroxy-melatonin or possibly into a kynurenamine, as shown by mass spectrometry analyses [110,111]. To the best of our knowledge, the generation of such metabolites was never directly correlated with the trapping of ROS by melatonin in mitochondria—or in other systems. However, 2-hydroxymelatonin, 4-hydroxymelatonin and 6-hydroxymelatonin have been reported in various other situations. Further examples are 2-hydroxymelatonin in the signaling pathways in Arabidopsis [112], the generation of 6- and 2-hydroxymelatonin upon the action of cytochrome P450 on melatonin [113] or the a-cellular system scavenging property of 4-hydroxymelatonin, which was described as superior to that of 2-hydroxymelatonin [114].
It is assumed that the mitochondria occupy a tenth to a twentieth of the total cellular volume [115]. Therefore, any concentration of melatonin in the mitochondria would translate as its tenth or twentieth volume in the whole cells. Assuming a high concentration of melatonin in mitochondria of 1 mM for its role in neutralizing ROS production, it would lead to cell homogenates in a melatonin concentration of ca. 50 to 100 µM (~12 to 23 µg/mL). A feature never measured in the literature is where, in blood, the melatonin concentration during the night is about 60 to 70 pg/mL [116], up to 300 pM. Similarly, in sheep brain tissue, melatonin concentrations of ~1 nM (i.e., 232 pg/mL) and 10 nM have been measured during the day and night, respectively [117]. This would translate into a concentration 10 times higher in mitochondria: 10 to 100 nM, which is far from any capacity to protect those organelles from ROS injuries. One can argue that melatonin concentrations are not in equilibrium between mitochondria and the rest of the cell because mitochondria have the capacity to either enrich melatonin or retain the melatonin synthesized in mitochondria. However, for the moment, there is no experimental evidence supporting these hypotheses. The well-characterized physical properties of melatonin show that exogenous melatonin equilibrates within seconds with the cytoplasm, confirming that melatonin crosses biological membranes rapidly [118].
The question of intra-mitochondrial melatonin synthesis has been recently reexamined by Suofo et al. [119]. The authors identified, using a Western blot, the two enzymes of the biosynthesis pathway, AANAT and ASMT, in preparations of purified mouse non-synaptosomal brain mitochondria. These bands were fully resistant to proteinase K digestion and digitonin treatment, indicating their localization in the mitochondrial matrix. In contrast to the pineal AANAT levels, these mitochondrial AANAT levels did not change along the circadian cycle. The authors demonstrated further that, in mouse neuroblastoma (N2a) cell knockout for AANAT, mitochondrial-generated superoxide production was increased. No alteration of the mitochondrial membrane potential was seen in these cells. In an attempt to address melatonin synthesis in mitochondria more directly, purified mitochondria were treated with deuterated (d4)-serotonin, the AANAT substrate. The generation of d4-n-acetylserotonin and d4-melatonin was observed by mass spectrometry, indicating that mitochondria can indeed synthesize melatonin if the serotonin precursor is available. These data represent, by far, the most convincing dataset in support of melatonin synthesis in mitochondria. However, several important questions still have to be solved. Furthermore, as whole-brain mitochondria were used, the question of whether melatonin synthesis can occur in the mitochondria of all brain cells or of a subset of cells is unknown. Whether this capacity of melatonin synthesis also exists in mitochondria from other tissues remains to be studied, as the quantity of mitochondria varies widely from cell type to cell type, and the repertoire of proteins imported into mitochondria largely depends on the cell type. Importantly, whether the generated quantities of melatonin are significant or anecdotal remains an open question, as no quantitative conclusion can be drawn from the data presented by Suofo et al. [119]. To show that melatonin is really synthesized by mitochondria in vivo, in other words, whether the precursor concentrations and enzyme levels are sufficient, remains an important goal for future studies.
7.3.3. Conclusions
In summary, evidence on melatonin synthesis in bacteria remains weak and warrants independent replications. Other bacterial strains should be investigated to determine how generalizable they are. Studies should focus on Rhodospirillum rubrum and related species to provide evidence for the assumption that mitochondria are producing melatonin because the bacteria engulfed millions of years ago already produced this molecule. Collectively, the experimental evidence for melatonin synthesis in mitochondria and, in particular, its quantitative aspects and generalization to all mitochondria-containing cells is still weak. This includes the presence of its main precursors, such as serotonin, as well as its main metabolites that could be expected, assuming the highly oxidative environment of the mitochondrial matrix.
7.4. Melatonin as a Co-Substrate of NQO2
Although not really related to the previous points but providing an example of how ideas may emerge, a third melatonin receptor was reported [120] and systematized later on, particularly with a specific ligand, MCA-NAT [121,122]. The identity between this third melatonin binding site and the enzyme NQO2 has been reported [123,124]. In the following years, a mini-review was published hypothesizing that melatonin is, in fact, a co-substrate of NQO2 [125]. That would explain how melatonin regulates the antioxidant capacity of the enzyme. This hypothesis was then experimentally tested by another group and proven to be wrong [126]. Despite the disapproval of the initial hypothesis, it still continued to be mentioned in the literature by citing the mini-review with some citations, even transforming the hypothesis into a fact. Unfortunately, this persistence of the wrong hypothesis may have retarded the field by redirecting future research in the wrong direction.
8. Melatonin Has Many Effects and Targets
The suspected beneficial effect of melatonin in such a large spectrum of diseases is often justified by the involvement of melatonin in a large spectrum of biological processes, as reported in preclinical (cellular and animal) studies. To illustrate this diversity in terms of the melatonin effects, the following 30 randomly selected examples were extracted from a PubMed interrogation with the words “melatonin” and “inhibit”, which retrieved 400 items in total between 2018 and the end of 2022.
In also the following publications, the effect of melatonin is associated with a molecular target (IGF1, CYP, Akt, etc.) in a way that might suggest to the reader that melatonin is indeed binding to those targets. This is not the case; in most of the publications, the authors suggest that melatonin does “something” that leads to a pathway acting through one of these proteins without convincingly demonstrating a molecular interaction between melatonin and a protein. Melatonin protects from experimental models of newborn hypoxic-ischemic brain injury via the MT1 receptor [127]. Melatonin inhibits LH + insulin-like growth factor 1 (IGF1)-induced androstenedione and progesterone production as well as the expression of steroidogenic acute regulatory protein (StAR) mRNA (via real-time polymerase chain reaction) in Theca cells. Melatonin has no effect on cytochrome P450 11A1 (CYP11A1) and cytochrome P450 17A1 (CYP17A1) mRNA abundance [128]. Melatonin increases apoptosis, induced by cisplatin, by inhibiting the JNK/Parkin/mitophagy axis [129]. Melatonin elevates α-ketoglutarate and diverts adipose-derived exosomes to macrophages in mice [130]. Melatonin downregulates the expression of the dynamin-related protein 1 [131]. Melatonin restores mitochondrial normalcy after MPTP treatment in zebrafish [132]. Melatonin (3 mM), combined with 20 nM of rapamycin, suppresses the AKT/mTOR pathway activation, mitophagy and apoptosis via the regulation of mitochondrial function(s) in cultured cells [133]. Melatonin inhibits ERK phosphorylation [134]. Melatonin attenuates lung ischemia-reperfusion injury by inhibiting oxidative stress and inflammation [135]. Melatonin inhibits the HIF-1α-VEGF pathway in oxygen-induced retinopathy mice [136]. Melatonin activates Src and PKA in parallel and, thus, regulates CRE-dependent gene transcription [137]. Melatonin preserves insulin secretion and hepatic glycogen synthesis in rats [138]. Melatonin inhibits Cav3.2 T-type Ca2+ channels (about 40% at 10 µM) [139]. Melatonin activates the ERK1/2 signaling pathway [140]. Melatonin preserves the YAP expression during doxorubicin-induced cardiotoxicity [141]. Melatonin induces apoptosis in VCR-resistant oral cancer cells [142]. Melatonin induces apoptosis in 3T3-L1 preadipocytes as well [143]. However, melatonin inhibits apoptosis through the upregulation of sestrin2 in vascular smooth muscle cells [144]. Melatonin attenuates Atg5-dependent autophagy and activates the Akt/mTOR pathway [145]. Melatonin inhibits excessive mitophagy through the MT2/SIRT3/FoxO3a signaling pathway in cells [146]. Melatonin blocks the ROS-mediated HIF-1α/miR210/ISCU axis activation [147]. Melatonin inhibits the mitochondrial permeability transition pore opening [148]. Melatonin partially inhibits the NE/AKT/β-catenin/SLUG axis [149]. Melatonin inhibits TRPV4 activity (about 80 % at 1 mM) [150]. Melatonin promotes endocytosis and the subsequent degradation of HER2 [151]. Melatonin (0.2 mM) suppresses O-GlcNAcylation of cyclin-dependent-like kinase 5 [152]. Melatonin activates the ATF6 and PERK signaling pathways [153]. Melatonin activates the Nrf2/HO-1 signaling pathway [154]. Of note, the interaction between melatonin and NF-κB has been reported in numerous pathological cases, such as osteoarthritis [155], breast cancer [156] or, more generally, in inflammatory pathways [157]; however, the nature of the interaction has not been convincingly demonstrated.
This snapshot of articles is not meant to be exhaustive, as the search using keywords will always return only a partial picture. Nevertheless, we found the exercise quite informative on two counts:
-
(i)
The concentration of melatonin needed for most of the abovementioned effects is high, e.g., in the range of 1µM and up to several mMs. This leads to two remarks: (1) whether these effects are specific for melatonin or would they also be observed in structurally related molecules; (2) related to this first point is the question of the molecular target(s) or mechanism(s) behind it. Most studies lack the appropriate experimental conditions to—or at least start to—address these questions appropriately. For the first point, the most obvious structurally related candidate class of molecules is indoles (see below, Section 9); however, other classes of chemical compounds should also be considered, such as primary amines, etc. The second remark on molecular targets and mechanisms is a crucial step toward a better understanding of the observed effects [158]. The most obvious experiments, in this respect, are the establishment of concentration (dose)–response curves to determine an EC50 value. Low EC50 values generally hint at specific molecular targets, whereas a high EC50 value hints at targets with lower specificity. The absence of EC50 values (no saturation) may hint at a general property, such as membrane fluidity or intactness. Unfortunately, many of the articles describing the effects of melatonin use only a single (often high) melatonin concentration/dose.
-
(ii)
The reported effects of melatonin tend to be over-interpreted. The effects are not only system-dependent but, on their own, do not necessarily allow for conclusions on the precise mechanism or targets involved. These ‘descriptive’ data should therefore be interpreted with caution, not only in terms of the molecular mechanism and specificity but also in terms of translatability into another cell type, tissue and its relevance for pathologies. Unfortunately, the often-used perspective phrase “… these findings open new avenues in therapeutics” should be used with more precaution, considering the high melatonin concentrations used and the fact that almost all experimental protocols use melatonin in a preventive paradigm instead of a treatment paradigm.
Another misleading habit is the transformation of the working hypotheses and ideas formulated in an article into a fact in the following article and then propagated from review to review. This is nicely illustrated by the literature associating melatonin with the recent COVID-19 pandemic. A PubMed search using the terms “melatonin” and “COVID” retrieved 217 publications (25 February 2023) in just a three-year period. Incidentally, most of the papers claim that melatonin is active because it is an antioxidant and scavenger and has anti-inflammatory properties [159], and thus it is unsurprising to reach the conclusion that melatonin would be the ideal anti-COVID-19 therapeutic agent, as proclaimed in many reviews. However, a closer look at the more than 200 publications on COVID-19 and melatonin shows that most of them are reviews or comments and that experimental pieces of evidence are actually only reported in a small number of original articles. Interestingly, the anticipated anti-inflammatory effect of melatonin was not observed in K18-hACE2 mice infected with SARS-CoV-2 [160]. Whether this lack of effect is specific to this mouse model and that of a rapid manifestation of severe COVID-19 symptoms occurred remains to be determined in further studies. The totally unexpected effect of melatonin in this mouse model was the protection of the brain from SARS-CoV-2 infection, as compared to the lungs at high doses of melatonin [161]. Even more unexpected, the mechanistic studies suggest that this effect is mediated by the binding of melatonin to an allosteric binding site at the human angiotensin-converting enzyme 2 (ACE2), thus interfering with the ACE2 function as an entry receptor for SARS-CoV-2.
Altogether, we conclude that the use of high melatonin doses can be problematic in terms of specificity and engagement of molecular targets, two aspects that are rarely addressed but need to be clarified based on experimental evidence (see also the discussion and recommendations in Cecon et al. [9]).
9. The Indole Hypothesis
As we detailed previously, particularly in the recommendations on melatonin-related publications [162], the necessity must be emphasized to use the control substances at concentrations similar to melatonin concentrations to evaluate the specificity of an observed effect. This includes the use of other indole-based compounds, including those that may not be oxidized in the same way as melatonin, to address and demonstrate the possible mechanism of action of melatonin for the particular outcome.
Concerning indole-based compounds, it is interesting to evaluate some of the examples that addressed the question of specificity: (a) serotonin was found to be the most effective compound for inhibiting amyloid-β peptide aggregation. Almost all the indole compounds tested in this study prevented amyloid-β peptide fibril formation and increased cell viability between 9 and 25%. Melatonin and serotonin were found to be the most active. Moreover, serotonin increased the expression of SIRT-1 and 2, heat shock protein 70, and heme oxygenase activity—as melatonin is reported to do as well—this being a possible mechanism underlying the observed neuroprotective effect [163]. This paragraph, adapted from the abstract of the paper, is a rare break from the melatonin-does-everything system, suggesting that either high concentrations of natural compounds may have beneficial effects on the disease models or that indole-based compounds have the propensity to interfere with proteins due to their chemical natures. (b) Zhai et al. demonstrated that a series of indole-based compounds (including tryptamine and tryptophane but not the unrelated histidine) were able, at the same concentrations (3 mM), to block the virus infectivity, probably via a virus/receptor interaction [30]; see also [161]. (c) Wölfler et al. showed that N-acetylserotonin is a better compound for antioxidant activity than melatonin, especially at 10 µM, at which the concentration of melatonin is almost inactive in this cellular model [164]. (d) When the neuroblastoma SK-N-MC cells were treated either by hydrogen peroxide (H2O2) or following glutamate-induced cell death, N-acetyltryptamine, as well as melatonin, were reported to protect the cells against those injuries, although at concentrations from 10 to 500 µM. The authors suggested that the protection occurred via the induction of NF-κB [165]. The systematic inclusion of melatonin-related compounds and the determination of the EC50 values are highly desirable in future studies to better understand the effects associated with melatonin, in particular, at high concentrations. From a therapeutic point of view, the putative interchangeability of indole-based compounds, such as melatonin, N-acetyltryptamine, etc., would increase the number of therapeutic choices to minimize the harmful effects, depending on the disease context.
10. What to do?
For several years now, a growing number of claims can be said to have distracted melatonin research and trivialized the role that endogenously produced melatonin has in maintaining circadian sleep patterns, metabolism and mental health.
Indeed, the disruption of light rhythms in our cities and the constant use of electronic devices with artificial light have led to questions about the way public health is affected by such changes. In the meantime, significant resources—intellectual, financial and societal—are potentially wasted by moving forward the working repetitive claims/hypotheses that are not, or only insufficiently, supported by experimental evidence with all the required scientific rigor. Clearly, only the clarification of the basics and not the extension of non-consensual theories will lead to a general consensus in the scientific community to eventually move on in a coordinated manner and on a solid scientific basis to address the most relevant questions and challenges.
Another way to reduce the impact of misleading claims is to discourage reviews of the literature that simply and uncritically repeat statements or discuss experiments.
Another point should be stressed: the use of melatonin at very high dosages in patients and infants should be restricted as a precautionary principle. Indeed, as pointed out on several occasions, melatonin toxicity, in vivo, remains unexplored territory at these dosages, even at moderate dosages. There have been no rigorous toxicity studies reported in humans, and the repeated claims that a compound being natural cannot be toxic, are potentially harmful.
Acknowledgments
The authors are indebted to H.J. Forman (University of Southern California at Los Angeles, CA, USA) for his help in Section 7.1.1’s writing. The work of R.J. was supported by the Agence Nationale de la Recherche (ANR-19-CE16-0025-01 « mitoGPCR », ANR-21-CE18-00XX « alloGLP1R », ANR-20-COV4-0001 « MELATOVID »), the Fondation de la Recherche Médicale (Equipe FRM DEQ20130326503), La Ligue Contre le Cancer N/Ref: RS19/75-127, Plan de Relance 2021 and the Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS).
Author Contributions
All the authors equally contributed to the writing and editing of the review. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The work of R.J. was supported by the Agence Nationale de la Recherche (ANR-19-CE16-0025-01 « mitoGPCR », ANR-21-CE18-00XX « alloGLP1R », ANR-20-COV4-0001 « MELATOVID »), the Fondation de la Recherche Médicale (Equipe FRM DEQ20130326503), La Ligue Contre le Cancer N/Ref: RS19/75-127, Plan de Relance 2021 and the Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lerner A.B., Case J.D., Takahashi Y., Lee T.H., Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958;80:2587. doi: 10.1021/ja01543a060. [DOI] [Google Scholar]
- 2.Kennaway D.J. Measuring melatonin by immunoassay. J. Pineal Res. 2020;69:e12657. doi: 10.1111/jpi.12657. [DOI] [PubMed] [Google Scholar]
- 3.Luboshitzky R., Yanai D., Shen-Orr Z., Israeli E., Herer P., Lavie P. Daily and seasonal variations in the concentration of melatonin in the human pineal gland. Brain Res. Bull. 1998;47:271–276. doi: 10.1016/S0361-9230(98)00105-1. [DOI] [PubMed] [Google Scholar]
- 4.Kennaway D.J. The Dim Light Melatonin Onset (DLMO) across ages, methodologies and sex and its relationship with Morningness/Eveningness. Sleep. 2023;46:zsad033. doi: 10.1093/sleep/zsad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein D.C. Arylalkylamine N-acetyltransferase: “The Timezyme”. J. Biol. Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- 6.Falcón J., Coon S.L., Besseau L., Cazaméa-Catalan D., Fuentès M., Magnanou E., Paulin C.-H., Boeuf G., Sauzet S., Jørgensen E.H., et al. Drastic neofunctionalization associated with evolution of the timezyme AANAT 500 Mya. Proc. Natl. Acad. Sci. USA. 2014;111:314–319. doi: 10.1073/pnas.1312634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassone V.M. Effects of melatonin on vertebrate circadian systems. Trends Neurosci. 1990;13:457–464. doi: 10.1016/0166-2236(90)90099-V. [DOI] [PubMed] [Google Scholar]
- 8.Hastings M.H., Herbert J., Martensz N.D., Roberts A.C. Annual reproductive rhythms in mammals: Mechanisms of light synchronization. Ann. N. Y. Acad. Sci. 1985;453:182–204. doi: 10.1111/j.1749-6632.1985.tb11810.x. [DOI] [PubMed] [Google Scholar]
- 9.Cecon E., Boutin J.A., Jockers R. Molecular Characterization and Pharmacology of Melatonin receptors in Animals. Receptors. 2023;2:127–147. doi: 10.3390/receptors2020008. [DOI] [Google Scholar]
- 10.Dijk D.J., Cajochen C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J. Biol. Rhythms. 1997;12:627–635. doi: 10.1177/074873049701200618. [DOI] [PubMed] [Google Scholar]
- 11.Pevet P., Challet E., Felder-Schmittbuhl M.-P. Melatonin and the circadian system: Keys for health with a focus on sleep. Handb. Clin. Neurol. 2021;179:331–343. doi: 10.1016/B978-0-12-819975-6.00021-2. [DOI] [PubMed] [Google Scholar]
- 12.Yue J.-L., Chang X.-W., Zheng J.-W., Shi L., Xiang Y.-J., Que J.-Y., Yuan K., Deng J.-H., Teng T., Li Y.-Y., et al. Efficacy and tolerability of pharmacological treatments for insomnia in adults: A systematic review and network meta-analysis. Sleep Med. Rev. 2023;68:101746. doi: 10.1016/j.smrv.2023.101746. [DOI] [PubMed] [Google Scholar]
- 13.Hickie I.B., Rogers N.L. Novel melatonin-based therapies: Potential advances in the treatment of major depression. Lancet. 2011;378:621–631. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- 14.Kasper S., Corruble E., Hale A., Lemoine P., Montgomery S.A., Quera-Salva M.-A. Antidepressant efficacy of agomelatine versus SSRI/SNRI: Results from a pooled analysis of head-to-head studies without a placebo control. Int. Clin. Psychopharmacol. 2013;28:12–19. doi: 10.1097/YIC.0b013e328359768e. [DOI] [PubMed] [Google Scholar]
- 15.Cipriani A., Furukawa T.A., Salanti G., Chaimani A., Atkinson L.Z., Ogawa Y., Leucht S., Ruhe H.G., Turner E.H., Higgins J.P.T., et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutin J.A., Jockers R. Melatonin controversies, an update. J. Pineal Res. 2021;70:e12702. doi: 10.1111/jpi.12702. [DOI] [PubMed] [Google Scholar]
- 17.Boutin J.A. Quinone reductase 2 as a promising target of melatonin therapeutic actions. Expert Opin. Ther. Targets. 2016;20:303–317. doi: 10.1517/14728222.2016.1091882. [DOI] [PubMed] [Google Scholar]
- 18.Boutin J.A. How Can Molecular Pharmacology Help Understand the Multiple Actions of Melatonin: 20 Years of Research and Trends. In: Manuela Drăgoi C., Crenguţa Nicolae A., editors. Melatonin-Molecular Biology, Clinical and Pharmaceutical Approaches. IntechOpen; London, UK: 2018. [Google Scholar]
- 19.Arendt J. Melatonin: Countering Chaotic Time Cues. Front. Endocrinol. 2019;10:391. doi: 10.3389/fendo.2019.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martín Giménez V.M., de Las Heras N., Lahera V., Tresguerres J.A.F., Reiter R.J., Manucha W. Melatonin as an Anti-Aging Therapy for Age-Related Cardiovascular and Neurodegenerative Diseases. Front. Aging Neurosci. 2022;14:888292. doi: 10.3389/fnagi.2022.888292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bocheva G., Slominski R.M., Janjetovic Z., Kim T.-K., Böhm M., Steinbrink K., Reiter R.J., Kleszczyński K., Slominski A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022;23:1238. doi: 10.3390/ijms23031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla M., Govitrapong P., Boontem P., Reiter R.J., Satayavivad J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017;15:1010–1031. doi: 10.2174/1570159X15666170313123454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Repova K., Baka T., Krajcirovicova K., Stanko P., Aziriova S., Reiter R.J., Simko F. Melatonin as a Potential Approach to Anxiety Treatment. Int. J. Mol. Sci. 2022;23:6187. doi: 10.3390/ijms232416187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajoolabady A., Bi Y., McClements D.J., Lip G.Y.H., Des Richardson R., Reiter R.J., Klionsky D.J., Ren J. Melatonin-based therapeutics for atherosclerotic lesions and beyond: Focusing on macrophage mitophagy. Pharmacol. Res. 2022;176:106072. doi: 10.1016/j.phrs.2022.106072. [DOI] [PubMed] [Google Scholar]
- 25.He F., Liu Y., Li P., Wu X., Xia Y., Zhang D., Li N., Peng Y., Zhu G., Hardeland R., et al. Melatonin inhibits Gram-negative pathogens by targeting citrate synthase. Sci. China Life Sci. 2022;65:1430–1444. doi: 10.1007/s11427-021-2032-9. [DOI] [PubMed] [Google Scholar]
- 26.Davoodvandi A., Nikfar B., Reiter R.J., Asemi Z. Melatonin and cancer suppression: Insights into its effects on DNA methylation. Cell. Mol. Biol. Lett. 2022;27:73. doi: 10.1186/s11658-022-00375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cucielo M.S., Cesário R.C., Silveira H.S., Gaiotte L.B., Dos Santos S.A.A., de Campos Zuccari D.A.P., Seiva F.R.F., Reiter R.J., de Almeida Chuffa L.G. Melatonin Reverses the Warburg-Type Metabolism and Reduces Mitochondrial Membrane Potential of Ovarian Cancer Cells Independent of MT1 Receptor Activation. Molecules. 2022;27:4350. doi: 10.3390/molecules27144350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Targhazeh N., Reiter R.J., Rahimi M., Qujeq D., Yousefi T., Shahavi M.H., Mir S.M. Oncostatic activities of melatonin: Roles in cell cycle, apoptosis, and autophagy. Biochimie. 2022;202:34–48. doi: 10.1016/j.biochi.2022.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Tobeiha M., Jafari A., Fadaei S., Mirazimi S.M.A., Dashti F., Amiri A., Khan H., Asemi Z., Reiter R.J., Hamblin M.R., et al. Evidence for the Benefits of Melatonin in Cardiovascular Disease. Front. Cardiovasc. Med. 2022;9:888319. doi: 10.3389/fcvm.2022.888319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhai X., Wang N., Jiao H., Zhang J., Li C., Ren W., Reiter R.J., Su S. Melatonin and other indoles show antiviral activities against swine coronaviruses in vitro at pharmacological concentrations. J. Pineal Res. 2021;71:e12754. doi: 10.1111/jpi.12754. [DOI] [PubMed] [Google Scholar]
- 31.Hajam Y.A., Rai S., Pandi-Perumal S.R., Brown G.M., Reiter R.J., Cardinali D.P. Coadministration of Melatonin and Insulin Improves Diabetes-Induced Impairment of Rat Kidney Function. Neuroendocrinology. 2022;112:807–822. doi: 10.1159/000520280. [DOI] [PubMed] [Google Scholar]
- 32.Rong B., Wu Q., Reiter R.J., Sun C. The Mechanism of Oral Melatonin Ameliorates Intestinal and Adipose Lipid Dysmetabolism Through Reducing Escherichia Coli-Derived Lipopolysaccharide. Cell. Mol. Gastroenterol. Hepatol. 2021;12:1643–1667. doi: 10.1016/j.jcmgh.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luchetti F., Nasoni M.G., Burattini S., Mohammadi A., Pagliarini M., Canonico B., Ambrogini P., Balduini W., Reiter R.J., Carloni S. Melatonin Attenuates Ischemic-like Cell Injury by Promoting Autophagosome Maturation via the Sirt1/FoxO1/Rab7 Axis in Hippocampal HT22 Cells and in Organotypic Cultures. Cells. 2022;11:3701. doi: 10.3390/cells11223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luchetti F., Carloni S., Nasoni M.G., Reiter R.J., Balduini W. Tunneling nanotubes and mesenchymal stem cells: New insights into the role of melatonin in neuronal recovery. J. Pineal Res. 2022;73:e12800. doi: 10.1111/jpi.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loh D., Reiter R.J. Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders. Antioxidants. 2021;10:1483. doi: 10.3390/antiox10091483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamtaji O.R., Reiter R.J., Alipoor R., Dadgostar E., Kouchaki E., Asemi Z. Melatonin and Parkinson Disease: Current Status and Future Perspectives for Molecular Mechanisms. Cell. Mol. Neurobiol. 2020;40:15–23. doi: 10.1007/s10571-019-00720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiter R.J., Sharma R., Tan D.-X., Neel R.L., Simko F., Manucha W., Rosales-Corral S., Cardinali D.P. Melatonin use for SARS-CoV-2 infection: Time to diversify the treatment portfolio. J. Med. Virol. 2022;94:2928–2930. doi: 10.1002/jmv.27740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiter R.J., Sharma R., Simko F., Dominguez-Rodriguez A., Tesarik J., Neel R.L., Slominski A.T., Kleszczynski K., Martin-Gimenez V.M., Manucha W., et al. Melatonin: Highlighting its use as a potential treatment for SARS-CoV-2 infection. Cell. Mol. Life Sci. 2022;79:143. doi: 10.1007/s00018-021-04102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersen L.P.H., Gögenur I., Rosenberg J., Reiter R.J. Pharmacokinetics of Melatonin: The Missing Link in Clinical Efficacy? Clin. Pharmacokinet. 2016;55:1027–1030. doi: 10.1007/s40262-016-0386-3. [DOI] [PubMed] [Google Scholar]
- 40.Vakkuri O., Leppäluoto J., Kauppila A. Oral administration and distribution of melatonin in human serum, saliva and urine. Life Sci. 1985;37:489–495. doi: 10.1016/0024-3205(85)90412-6. [DOI] [PubMed] [Google Scholar]
- 41.Harpsøe N.G., Andersen L.P.H., Gögenur I., Rosenberg J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015;71:901–909. doi: 10.1007/s00228-015-1873-4. [DOI] [PubMed] [Google Scholar]
- 42.Rolling J., Rabot J., Schroder C.M. Melatonin Treatment for Pediatric Patients with Insomnia: Is There a Place for It? Nat. Sci. Sleep. 2022;14:1927–1944. doi: 10.2147/NSS.S340944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennaway D.J. What do we really know about the safety and efficacy of melatonin for sleep disorders? Curr. Med. Res. Opin. 2022;38:211–227. doi: 10.1080/03007995.2021.2000714. [DOI] [PubMed] [Google Scholar]
- 44.Skrzelowski M., Brookhaus A., Shea L.A., Berlau D.J. Melatonin Use in Pediatrics: Evaluating the Discrepancy in Evidence Based on Country and Regulations Regarding Production. J. Pediatr. Pharmacol. Ther. 2021;26:4–20. doi: 10.5863/1551-6776-26.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zisapel N. Assessing the potential for drug interactions and long term safety of melatonin for the treatment of insomnia in children with autism spectrum disorder. Expert Rev. Clin. Pharmacol. 2022;15:175–185. doi: 10.1080/17512433.2022.2053520. [DOI] [PubMed] [Google Scholar]
- 46.Kuehn B.M. Climbing Melatonin Use for Insomnia Raises Safety Concerns. JAMA. 2022;328:605–607. doi: 10.1001/jama.2022.11506. [DOI] [PubMed] [Google Scholar]
- 47.Vine T., Brown G.M., Frey B.N. Melatonin use during pregnancy and lactation: A scoping review of human studies. Braz. J. Psychiatry. 2022;44:342–348. doi: 10.1590/1516-4446-2021-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seabra M.L., Bignotto M., Pinto L.R., Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000;29:193–200. doi: 10.1034/j.1600-0633.2002.290401.x. [DOI] [PubMed] [Google Scholar]
- 49.ANSE. [(accessed on 22 February 2023)]. Available online: https://www.anses.fr/fr/system/files/NUT2016SA0209.pdf.
- 50.NCI. [(accessed on 21 March 2023)]; Available online: www.nccih.nih.gov/health/melatonin-what-you-need-to-know.
- 51.Bishop-Freeman S.C., Young K.A., Labay L.M., Beuhler M.C., Hudson J.S. Melatonin Supplementation in Undetermined Pediatric Deaths. J. Anal. Toxicol. 2022;46:808–816. doi: 10.1093/jat/bkac033. [DOI] [PubMed] [Google Scholar]
- 52.Rishi M.A., Khosla S., Sullivan S.S. Health advisory: Melatonin use in children. J. Clin. Sleep Med. 2023;19:415. doi: 10.5664/jcsm.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Fabbro E., Dev R., Hui D., Palmer L., Bruera E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: A double-blind placebo-controlled trial. J. Clin. Oncol. 2013;31:1271–1276. doi: 10.1200/JCO.2012.43.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seely D., Wu P., Fritz H., Kennedy D.A., Tsui T., Seely A.J.E., Mills E. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integr. Cancer Ther. 2012;11:293–303. doi: 10.1177/1534735411425484. [DOI] [PubMed] [Google Scholar]
- 55.Lund Rasmussen C., Klee Olsen M., Thit Johnsen A., Petersen M.A., Lindholm H., Andersen L., Villadsen B., Groenvold M., Pedersen L. Effects of melatonin on physical fatigue and other symptoms in patients with advanced cancer receiving palliative care: A double-blind placebo-controlled crossover trial. Cancer. 2015;121:3727–3736. doi: 10.1002/cncr.29563. [DOI] [PubMed] [Google Scholar]
- 56.Skene D.J., Papagiannidou E., Hashemi E., Snelling J., Lewis D.F., Fernandez M., Ioannides C. Contribution of CYP1A2 in the hepatic metabolism of melatonin: Studies with isolated microsomal preparations and liver slices. J. Pineal Res. 2001;31:333–342. doi: 10.1034/j.1600-079X.2001.310408.x. [DOI] [PubMed] [Google Scholar]
- 57.Ma X., Chen C., Krausz K.W., Idle J.R., Gonzalez F.J. A metabolomic perspective of melatonin metabolism in the mouse. Endocrinology. 2008;149:1869–1879. doi: 10.1210/en.2007-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferry G., Ubeaud C., Lambert P.-H., Bertin S., Cogé F., Chomarat P., Delagrange P., Serkiz B., Bouchet J.-P., Truscott R.J.W., et al. Molecular evidence that melatonin is enzymatically oxidized in a different manner than tryptophan: Investigations with both indoleamine 2,3-dioxygenase and myeloperoxidase. Biochem. J. 2005;388:205–215. doi: 10.1042/BJ20042075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pond S.M., Tozer T.N. First-pass elimination. Basic concepts and clinical consequences. Clin. Pharmacokinet. 1984;9:1–25. doi: 10.2165/00003088-198409010-00001. [DOI] [PubMed] [Google Scholar]
- 60.Rowland M. Influence of route of administration on drug availability. J. Pharm. Sci. 1972;61:70–74. doi: 10.1002/jps.2600610111. [DOI] [PubMed] [Google Scholar]
- 61.Domínguez-Rodríguez A., Abreu-González P., Báez-Ferrer N., Reiter R.J., Avanzas P., Hernández-Vaquero D. Melatonin and Cardioprotection in Humans: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021;8:635083. doi: 10.3389/fcvm.2021.635083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ekeloef S., Halladin N., Fonnes S., Jensen S.E., Zaremba T., Rosenberg J., Jonsson G., Aarøe J., Gasbjerg L.S., Rosenkilde M.M., et al. Effect of Intracoronary and Intravenous Melatonin on Myocardial Salvage Index in Patients with ST-Elevation Myocardial Infarction: A Randomized Placebo Controlled Trial. J. Cardiovasc. Transl. Res. 2017;10:470–479. doi: 10.1007/s12265-017-9768-7. [DOI] [PubMed] [Google Scholar]
- 63.Dominguez-Rodriguez A., Abreu-Gonzalez P., de La Torre-Hernandez J.M., Consuegra-Sanchez L., Piccolo R., Gonzalez-Gonzalez J., Garcia-Camarero T., Del Mar Garcia-Saiz M., Aldea-Perona A., Reiter R.J. Usefulness of Early Treatment with Melatonin to Reduce Infarct Size in Patients With ST-Segment Elevation Myocardial Infarction Receiving Percutaneous Coronary Intervention (From the Melatonin Adjunct in the Acute Myocardial Infarction Treated with Angioplasty Trial) Am. J. Cardiol. 2017;120:522–526. doi: 10.1016/j.amjcard.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 64.Espezel H., Jan J.E., O’Donnell M.E., Milner R. The Use of Melatonin to Treat Sleep-Wake-Rhythm Disorders in Children who are Visually Impaired. J. Vis. Impair. Blind. 1996;90:43–50. doi: 10.1177/0145482X9609000109. [DOI] [Google Scholar]
- 65.Jan J.E., Espezel H., Appleton R.E. The treatment of sleep disorders with melatonin. Dev. Med. Child Neurol. 1994;36:97–107. doi: 10.1111/j.1469-8749.1994.tb11818.x. [DOI] [PubMed] [Google Scholar]
- 66.Jan J.E., O’Donnell M.E. Use of melatonin in the treatment of paediatric sleep disorders. J. Pineal Res. 1996;21:193–199. doi: 10.1111/j.1600-079X.1996.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 67.Goldman R.D., Bongiorno P.B., Olcese J.M., Witt-Enderby P.A., Shatkin J.P. Myths and Evidence Regarding Melatonin Supplementation for Occasional Sleeplessness in the Pediatric Population. Pediatr. Ann. 2021;50:e391–e395. doi: 10.3928/19382359-20210823-01. [DOI] [PubMed] [Google Scholar]
- 68.Williams Buckley A., Hirtz D., Oskoui M., Armstrong M.J., Batra A., Bridgemohan C., Coury D., Dawson G., Donley D., Findling R.L., et al. Practice guideline: Treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2020;94:392–404. doi: 10.1212/WNL.0000000000009033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lelak K., Vohra V., Neuman M.I., Toce M.S., Sethuraman U. Pediatric Melatonin Ingestions—United States, 2012–2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71:725–729. doi: 10.15585/mmwr.mm7122a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poeggeler B., Reiter R.J., Tan D.X., Chen L.D., Manchester L.C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J. Pineal Res. 1993;14:151–168. doi: 10.1111/j.1600-079X.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 71.Forman H.J., Davies K.J.A., Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forman H.J., Augusto O., Brigelius-Flohe R., Dennery P.A., Kalyanaraman B., Ischiropoulos H., Mann G.E., Radi R., Roberts L.J., Vina J., et al. Even free radicals should follow some rules: A guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 73.Forman H.J., Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021;20:689–709. doi: 10.1038/s41573-021-00233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- 75.Treml J., Šmejkal K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016;15:720–738. doi: 10.1111/1541-4337.12204. [DOI] [PubMed] [Google Scholar]
- 76.Kostov R.V., Knatko E.V., McLaughlin L.A., Henderson C.J., Zheng S., Huang J.T.-J., Honda T., Dinkova-Kostova A.T. Pharmacokinetics and pharmacodynamics of orally administered acetylenic tricyclic bis(cyanoenone), a highly potent Nrf2 activator with a reversible covalent mode of action. Biochem. Biophys. Res. Commun. 2015;465:402–407. doi: 10.1016/j.bbrc.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinkova-Kostova A.T., Talalay P., Sharkey J., Zhang Y., Holtzclaw W.D., Wang X.J., David E., Schiavoni K.H., Finlayson S., Mierke D.F., et al. An exceptionally potent inducer of cytoprotective enzymes: Elucidation of the structural features that determine inducer potency and reactivity with Keap1. J. Biol. Chem. 2010;285:33747–33755. doi: 10.1074/jbc.M110.163485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Couch R.D., Browning R.G., Honda T., Gribble G.W., Wright D.L., Sporn M.B., Anderson A.C. Studies on the reactivity of CDDO, a promising new chemopreventive and chemotherapeutic agent: Implications for a molecular mechanism of action. Bioorg. Med. Chem. Lett. 2005;15:2215–2219. doi: 10.1016/j.bmcl.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 79.Balogun E., Hoque M., Gong P., Killeen E., Green C.J., Foresti R., Alam J., Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shahcheraghi S.H., Salemi F., Peirovi N., Ayatollahi J., Alam W., Khan H., Saso L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules. 2021;27:167. doi: 10.3390/molecules27010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Auf Dem Keller U., Kümin A., Braun S., Werner S. Reactive oxygen species and their detoxification in healing skin wounds. J. Investig. Dermatol. Symp. Proc. 2006;11:106–111. doi: 10.1038/sj.jidsymp.5650001. [DOI] [PubMed] [Google Scholar]
- 82.Amoroso R., Maccallini C., Bellezza I. Activators of Nrf2 to Counteract Neurodegenerative Diseases. Antioxidants. 2023;12:778. doi: 10.3390/antiox12030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z., Ma C., Meng C.-J., Zhu G.-Q., Sun X.-B., Huo L., Zhang J., Liu H.-X., He W.-C., Shen X.-M., et al. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J. Pineal Res. 2012;53:129–137. doi: 10.1111/j.1600-079X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 84.Kryl’skii E.D., Popova T.N., Safonova O.A., Stolyarova A.O., Razuvaev G.A., de Carvalho M.A.P. Transcriptional Regulation of Antioxidant Enzymes Activity and Modulation of Oxidative Stress by Melatonin in Rats Under Cerebral Ischemia/Reperfusion Conditions. Neuroscience. 2019;406:653–666. doi: 10.1016/j.neuroscience.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 85.Kang J.-W., Lee S.-M. Melatonin inhibits type 1 interferon signaling of toll-like receptor 4 via heme oxygenase-1 induction in hepatic ischemia/reperfusion. J. Pineal Res. 2012;53:67–76. doi: 10.1111/j.1600-079X.2012.00972.x. [DOI] [PubMed] [Google Scholar]
- 86.Zhou X., Zhang Y., Hou M., Liu H., Yang H., Chen X., Liu T., He F., Zhu X. Melatonin Prevents Cartilage Degradation in Early-Stage Osteoarthritis Through Activation of miR-146a/NRF2/HO-1 Axis. J. Bone Miner. Res. 2022;37:1056–1072. doi: 10.1002/jbmr.4527. [DOI] [PubMed] [Google Scholar]
- 87.Becker-André M., Wiesenberg I., Schaeren-Wiemers N., André E., Missbach M., Saurat J.H., Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994;269:28531–28534. doi: 10.1016/S0021-9258(19)61934-4. [DOI] [PubMed] [Google Scholar]
- 88.Wiesenberg I., Missbach M., Kahlen J.P., Schräder M., Carlberg C. Transcriptional activation of the nuclear receptor RZR alpha by the pineal gland hormone melatonin and identification of CGP 52608 as a synthetic ligand. Nucleic Acids Res. 1995;23:327–333. doi: 10.1093/nar/23.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Becker-André M., Wiesenberg I., Schaeren-Wiemers N., André E., Missbach M., Saurat J.H., Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1997;272:16707. doi: 10.1074/jbc.272.26.16707. [DOI] [PubMed] [Google Scholar]
- 90.Pierrefiche G., Topall G., Courboin G., Henriet I., Laborit H. Antioxidant activity of melatonin in mice. Res. Commun. Chem. Pathol. Pharmacol. 1993;80:211–223. [PubMed] [Google Scholar]
- 91.Reiter R.J., Rosales-Corral S., Tan D.X., Jou M.J., Galano A., Xu B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Que Z., Ma T., Shang Y., Ge Q., Zhang Q., Xu P., Zhang J., Francoise U., Liu X., Sun X. Microorganisms: Producers of Melatonin in Fermented Foods and Beverages. J. Agric. Food Chem. 2020;68:4799–4811. doi: 10.1021/acs.jafc.0c01082. [DOI] [PubMed] [Google Scholar]
- 93.Hardeland R., Balzer I., Fuhrberg B., Behrmann G. Melatonin in Unicellular Organisms and Plants1. In: Pang S.F., Reiter R.J., Tang P.L., editors. Melatonin: A Universal Photoperiodic Signal with Diverse Actions. S. Karger AG; Basel, Switzerland: 1996. pp. 1–6. [Google Scholar]
- 94.Hardeland R., Poeggeler B. Non-vertebrate melatonin. J. Pineal Res. 2003;34:233–241. doi: 10.1034/j.1600-079X.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 95.Byeon Y., Back K. Melatonin production in Escherichia coli by dual expression of serotonin N-acetyltransferase and caffeic acid O-methyltransferase. Appl. Microbiol. Biotechnol. 2016;100:6683–6691. doi: 10.1007/s00253-016-7458-z. [DOI] [PubMed] [Google Scholar]
- 96.Manchester L.C., Poeggeler B., Alvares F.L., Ogden G.B., Reiter R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 1995;41:391–395. [PubMed] [Google Scholar]
- 97.Jiao J., Ma Y., Chen S., Liu C., Song Y., Qin Y., Yuan C., Liu Y. Melatonin-Producing Endophytic Bacteria from Grapevine Roots Promote the Abiotic Stress-Induced Production of Endogenous Melatonin in Their Hosts. Front. Plant Sci. 2016;7:1387. doi: 10.3389/fpls.2016.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan D.-X., Hardeland R., Manchester L.C., Rosales-Corral S., Coto-Montes A., Boga J.A., Reiter R.J. Emergence of naturally occurring melatonin isomers and their proposed nomenclature. J. Pineal Res. 2012;53:113–121. doi: 10.1111/j.1600-079X.2012.00979.x. [DOI] [PubMed] [Google Scholar]
- 99.Tilden A.R., Becker M.A., Amma L.L., Arciniega J., McGaw A.K. Melatonin production in an aerobic photosynthetic bacterium: An evolutionarily early association with darkness. J. Pineal Res. 1997;22:102–106. doi: 10.1111/j.1600-079X.1997.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 100.Martin W.F., Garg S., Zimorski V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015;370:20140330. doi: 10.1098/rstb.2014.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dipasquale V., Palermo L., Barbalace A., Tumminello G., Romano C. Randomised controlled trial of melatonin for paediatric functional abdominal pain disorders. J. Paediatr. Child Health. 2023;59:458–463. doi: 10.1111/jpc.16323. [DOI] [PubMed] [Google Scholar]
- 102.Park Y.S., Kim S.H., Park J.W., Kho Y., Seok P.R., Shin J.-H., Choi Y.J., Jun J.-H., Jung H.C., Kim E.K. Melatonin in the colon modulates intestinal microbiota in response to stress and sleep deprivation. Intest. Res. 2020;18:325–336. doi: 10.5217/ir.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Atroshi F., Rizzo A., Westermarck T., Ali-vehmas T. Effects of tamoxifen, melatonin, coenzyme Q10, and L-carnitine supplementation on bacterial growth in the presence of mycotoxins. Pharmacol. Res. 1998;38:289–295. doi: 10.1006/phrs.1998.0363. [DOI] [PubMed] [Google Scholar]
- 104.Kennaway D.J. Can we believe results obtained from plasma melatonin ELISA kits? Chronobiol. Int. 2021;38:616–619. doi: 10.1080/07420528.2021.1886112. [DOI] [PubMed] [Google Scholar]
- 105.Chen X., Zhao Y., Laborda P., Yang Y., Liu F. Molecular Cloning and Characterization of a Serotonin N-Acetyltransferase Gene, xoSNAT3, from Xanthomonas oryzae pv. oryzae. Int. J. Environ. Res. Public Health. 2023;20:1865. doi: 10.3390/ijerph20031865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coon S.L., Klein D.C. Evolution of arylalkylamine N-acetyltransferase: Emergence and divergence. Mol. Cell. Endocrinol. 2006;252:2–10. doi: 10.1016/j.mce.2006.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferry G., Loynel A., Kucharczyk N., Bertin S., Rodriguez M., Delagrange P., Galizzi J.P., Jacoby E., Volland J.P., Lesieur D., et al. Substrate specificity and inhibition studies of human serotonin N-acetyltransferase. J. Biol. Chem. 2000;275:8794–8805. doi: 10.1074/jbc.275.12.8794. [DOI] [PubMed] [Google Scholar]
- 108.Ferry G., Ubeaud C., Dauly C., Mozo J., Guillard S., Berger S., Jimenez S., Scoul C., Leclerc G., Yous S., et al. Purification of the recombinant human serotonin N-acetyltransferase (EC 2.3.1.87): Further characterization of and comparison with AANAT from other species. Protein Expr. Purif. 2004;38:84–98. doi: 10.1016/j.pep.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 109.Venegas C., García J.A., Escames G., Ortiz F., López A., Doerrier C., García-Corzo L., López L.C., Reiter R.J., Acuña-Castroviejo D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012;52:217–227. doi: 10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 110.Xing D., Meng Y., Yuan X., Jin S., Song X., Zare R.N., Zhang X. Capture of Hydroxyl Radicals by Hydronium Cations in Water Microdroplets. Angew. Chem. Int. Ed. Engl. 2022;61:e202207587. doi: 10.1002/anie.202207587. [DOI] [PubMed] [Google Scholar]
- 111.Zhang D., Gong C., Wang J., Xing D., Zhao L., Li D., Zhang X. Unravelling Melatonin’s Varied Antioxidizing Protection of Membrane Lipids Determined by its Spatial Distribution. J. Phys. Chem. Lett. 2021;12:7387–7393. doi: 10.1021/acs.jpclett.1c01965. [DOI] [PubMed] [Google Scholar]
- 112.Lee H.Y., Back K. 2-Hydroxymelatonin Promotes Seed Germination by Increasing Reactive Oxygen Species Production and Gibberellin Synthesis in Arabidopsis thaliana. Antioxidants. 2022;11:737. doi: 10.3390/antiox11040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Semak I., Korik E., Antonova M., Wortsman J., Slominski A. Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes. J. Pineal Res. 2008;45:515–523. doi: 10.1111/j.1600-079X.2008.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pérez-González A., Galano A., Alvarez-Idaboy J.R., Tan D.X., Reiter R.J. Radical-trapping and preventive antioxidant effects of 2-hydroxymelatonin and 4-hydroxymelatonin: Contributions to the melatonin protection against oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2017;1861:2206–2217. doi: 10.1016/j.bbagen.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 115.Szabò I., Zoratti M. Encyclopedia of Biological Chemistry III. Elsevier; Amsterdam, The Netherlands: 2013. Membrane Transport|Potassium Channels in the Inner Membrane of Mitochondria in Various Organisms: From Unicellular Eukaryotes to Higher Plants and Mammals; pp. 986–989. [Google Scholar]
- 116.Arendt J., Aulinas A. Endotext: Physiology of the Pineal Gland and Melatonin. Endotext; South Dartmouth, MA, USA: 2000. [Google Scholar]
- 117.Legros C., Chesneau D., Boutin J.A., Barc C., Malpaux B. Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J. Neuroendocrinol. 2014;26:151–163. doi: 10.1111/jne.12134. [DOI] [PubMed] [Google Scholar]
- 118.Yu H., Dickson E.J., Jung S.-R., Koh D.-S., Hille B. High membrane permeability for melatonin. J. Gen. Physiol. 2016;147:63–76. doi: 10.1085/jgp.201511526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suofu Y., Li W., Jean-Alphonse F.G., Jia J., Khattar N.K., Li J., Baranov S.V., Leronni D., Mihalik A.C., He Y., et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA. 2017;114:E7997–E8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duncan M.J., Takahashi J.S., Dubocovich M.L. 2-125Iiodomelatonin binding sites in hamster brain membranes: Pharmacological characteristics and regional distribution. Endocrinology. 1988;122:1825–1833. doi: 10.1210/endo-122-5-1825. [DOI] [PubMed] [Google Scholar]
- 121.Dubocovich M.L. Melatonin receptors: Are there multiple subtypes? Trends Pharmacol. Sci. 1995;16:50–56. doi: 10.1016/S0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- 122.Molinari E.J., North P.C., Dubocovich M.L. 2-125Iiodo-5-methoxycarbonylamino-N-acetyltryptamine: A selective radioligand for the characterization of melatonin ML2 binding sites. Eur. J. Pharmacol. 1996;301:159–168. doi: 10.1016/0014-2999(95)00870-5. [DOI] [PubMed] [Google Scholar]
- 123.Nosjean O., Ferro M., Coge F., Beauverger P., Henlin J.M., Lefoulon F., Fauchere J.L., Delagrange P., Canet E., Boutin J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000;275:31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 124.Boutin J.A., Ferry G. Is There Sufficient Evidence that the Melatonin Binding Site MT3 Is Quinone Reductase 2? J. Pharmacol. Exp. Ther. 2019;368:59–65. doi: 10.1124/jpet.118.253260. [DOI] [PubMed] [Google Scholar]
- 125.Tan D.-X., Manchester L.C., Terron M.P., Flores L.J., Tamura H., Reiter R.J. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: Hypothesis and significance. J. Pineal Res. 2007;43:317–320. doi: 10.1111/j.1600-079X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 126.Boutin J.A., Marcheteau E., Hennig P., Moulharat N., Berger S., Delagrange P., Bouchet J.-P., Ferry G. MT3/QR2 melatonin binding site does not use melatonin as a substrate or a co-substrate. J. Pineal Res. 2008;45:524–531. doi: 10.1111/j.1600-079X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 127.Sinha B., Wu Q., Li W., Tu Y., Sirianni A.C., Chen Y., Jiang J., Zhang X., Chen W., Zhou S., et al. Protection of melatonin in experimental models of newborn hypoxic-ischemic brain injury through MT1 receptor. J. Pineal Res. 2018;64:e12443. doi: 10.1111/jpi.12443. [DOI] [PubMed] [Google Scholar]
- 128.Feng T., Schutz L.F., Morrell B.C., Perego M.C., Spicer L.J. Effect of melatonin on bovine theca cells in vitro. Reprod. Fertil. Dev. 2018;30:643–650. doi: 10.1071/RD17203. [DOI] [PubMed] [Google Scholar]
- 129.Chen L., Liu L., Li Y., Gao J. Melatonin increases human cervical cancer HeLa cells apoptosis induced by cisplatin via inhibition of JNK/Parkin/mitophagy axis. In Vitro Cell. Dev. Biol. Anim. 2018;54:1–10. doi: 10.1007/s11626-017-0200-z. [DOI] [PubMed] [Google Scholar]
- 130.Liu Z., Gan L., Zhang T., Ren Q., Sun C. Melatonin alleviates adipose inflammation through elevating α-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018;64:e12455. doi: 10.1111/jpi.12455. [DOI] [PubMed] [Google Scholar]
- 131.Zhou H., Cheang T., Su F., Zheng Y., Chen S., Feng J., Pei Z., Chen L. Melatonin inhibits rotenone-induced SH-SY5Y cell death via the downregulation of Dynamin-Related Protein 1 expression. Eur. J. Pharmacol. 2018;819:58–67. doi: 10.1016/j.ejphar.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 132.Díaz-Casado M.E., Rusanova I., Aranda P., Fernández-Ortiz M., Sayed R.K.A., Fernández-Gil B.I., Hidalgo-Gutiérrez A., Escames G., López L.C., Acuña-Castroviejo D. In Vivo Determination of Mitochondrial Respiration in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Zebrafish Reveals the Efficacy of Melatonin in Restoring Mitochondrial Normalcy. Zebrafish. 2018;15:15–26. doi: 10.1089/zeb.2017.1479. [DOI] [PubMed] [Google Scholar]
- 133.Shen Y.-Q., Guerra-Librero A., Fernandez-Gil B.I., Florido J., García-López S., Martinez-Ruiz L., Mendivil-Perez M., Soto-Mercado V., Acuña-Castroviejo D., Ortega-Arellano H., et al. Combination of melatonin and rapamycin for head and neck cancer therapy: Suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J. Pineal Res. 2018;64:e12461. doi: 10.1111/jpi.12461. [DOI] [PubMed] [Google Scholar]
- 134.Proietti S., Catizone A., Masiello M.G., Dinicola S., Fabrizi G., Minini M., Ricci G., Verna R., Reiter R.J., Cucina A., et al. Increase in motility and invasiveness of MCF7 cancer cells induced by nicotine is abolished by melatonin through inhibition of ERK phosphorylation. J. Pineal Res. 2018;64:e12467. doi: 10.1111/jpi.12467. [DOI] [PubMed] [Google Scholar]
- 135.Wang M.-L., Wei C.-H., Wang W.-D., Wang J.-S., Zhang J., Wang J.-J. Melatonin attenuates lung ischaemia-reperfusion injury via inhibition of oxidative stress and inflammation. Interact. Cardiovasc. Thorac. Surg. 2018;26:761–767. doi: 10.1093/icvts/ivx440. [DOI] [PubMed] [Google Scholar]
- 136.Xu Y., Lu X., Hu Y., Yang B., Tsui C.-K., Yu S., Lu L., Liang X. Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1α-VEGF pathway in oxygen-induced retinopathy mice. J. Pineal Res. 2018;64:e12473. doi: 10.1111/jpi.12473. [DOI] [PubMed] [Google Scholar]
- 137.Tao L., Zhu Y. Melatonin regulates CRE-dependent gene transcription underlying osteoblast proliferation by activating Src and PKA in parallel. Am. J. Transl. Res. 2018;10:86–100. [PMC free article] [PubMed] [Google Scholar]
- 138.Li T., Ni L., Zhao Z., Liu X., Lai Z., Di X., Xie Z., Song X., Wang X., Zhang R., et al. Melatonin attenuates smoking-induced hyperglycemia via preserving insulin secretion and hepatic glycogen synthesis in rats. J. Pineal Res. 2018;64:e12475. doi: 10.1111/jpi.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y., Ji H., Wang J., Sun Y., Qian Z., Jiang X., Snutch T.P., Sun Y., Tao J. Melatonin-mediated inhibition of Cav3.2 T-type Ca2+ channels induces sensory neuronal hypoexcitability through the novel protein kinase C-eta isoform. J. Pineal Res. 2018;64:e12476. doi: 10.1111/jpi.12476. [DOI] [PubMed] [Google Scholar]
- 140.Ge J., Zhou Q., Niu J., Wang Y., Yan Q., Wu C., Qian J., Yang H., Zou J. Melatonin Protects Intervertebral Disc from Degeneration by Improving Cell Survival and Function via Activation of the ERK1/2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019;2019:5120275. doi: 10.1155/2019/5120275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li H.-R., Wang C., Sun P., Liu D.-D., Du G.-Q., Tian J.-W. Melatonin attenuates doxorubicin-induced cardiotoxicity through preservation of YAP expression. J. Cell. Mol. Med. 2020;24:3634–3646. doi: 10.1111/jcmm.15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hsieh M.-J., Lin C.-W., Su S.-C., Reiter R.J., Chen A.W.-G., Chen M.-K., Yang S.-F. Effects of miR-34b/miR-892a Upregulation and Inhibition of ABCB1/ABCB4 on Melatonin-Induced Apoptosis in VCR-Resistant Oral Cancer Cells. Mol. Ther. Nucleic Acids. 2020;19:877–889. doi: 10.1016/j.omtn.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee J., Yoo Y.-M., Lee Y.H., Kim C.H. Melatonin Induces Apoptotic Cell Death in 3T3-L1 Preadipocytes. Mol. Biol. 2020;54:233–243. doi: 10.1134/S0026893320020120. [DOI] [PubMed] [Google Scholar]
- 144.Lee S., Byun J.-K., Park M., Woo Kim S., Lee S., Kim J.-G., Lee I.-K., Choi Y.-K., Park K.-G. Melatonin inhibits vascular smooth muscle cell proliferation and apoptosis through upregulation of Sestrin2. Exp. Ther. Med. 2020;19:3454–3460. doi: 10.3892/etm.2020.8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu C.-N., Kong L.-H., Ding P., Liu Y., Fan Z.-G., Gao E.-H., Yang J., Yang L.-F. Melatonin ameliorates pressure overload-induced cardiac hypertrophy by attenuating Atg5-dependent autophagy and activating the Akt/mTOR pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165848. doi: 10.1016/j.bbadis.2020.165848. [DOI] [PubMed] [Google Scholar]
- 146.Wu J., Yang Y., Gao Y., Wang Z., Ma J. Melatonin Attenuates Anoxia/Reoxygenation Injury by Inhibiting Excessive Mitophagy through the MT2/SIRT3/FoxO3a Signaling Pathway in H9c2 Cells. Drug Des. Devel. Ther. 2020;14:2047–2060. doi: 10.2147/DDDT.S248628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He M., Zhou C., Lu Y., Mao L., Xi Y., Mei X., Wang X., Zhang L., Yu Z., Zhou Z. Melatonin Antagonizes Nickel-Induced Aerobic Glycolysis by Blocking ROS-Mediated HIF-1α/miR210/ISCU Axis Activation. Oxid. Med. Cell. Longev. 2020;2020:5406284. doi: 10.1155/2020/5406284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fang Y., Zhao C., Xiang H., Jia G., Zhong R. Melatonin improves cryopreservation of ram sperm by inhibiting mitochondrial permeability transition pore opening. Reprod. Domest. Anim. 2020;55:1240–1249. doi: 10.1111/rda.13771. [DOI] [PubMed] [Google Scholar]
- 149.Bu S., Wang Q., Sun J., Li X., Gu T., Lai D. Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis. Cell Death Dis. 2020;11:644. doi: 10.1038/s41419-020-02906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Özşimşek A., Nazıroğlu M. The involvement of TRPV4 on the hypoxia-induced oxidative neurotoxicity and apoptosis in a neuronal cell line: Protective role of melatonin. Neurotoxicology. 2021;87:136–148. doi: 10.1016/j.neuro.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 151.Liu Z., Sang X., Wang M., Liu Y., Liu J., Wang X., Liu P., Cheng H. Melatonin potentiates the cytotoxic effect of Neratinib in HER2+ breast cancer through promoting endocytosis and lysosomal degradation of HER2. Oncogene. 2021;40:6273–6283. doi: 10.1038/s41388-021-02015-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu J., Tan Z., Li H., Lin M., Jiang Y., Liang L., Ma Q., Gou J., Ning L., Li X., et al. Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O-GlcNAcylation of cyclin-dependent-like kinase 5. J. Pineal Res. 2021;71:e12765. doi: 10.1111/jpi.12765. [DOI] [PubMed] [Google Scholar]
- 153.Qin D.-Z., Cai H., He C., Yang D.-H., Sun J., He W.-L., Li B.-L., Hua J.-L., Peng S. Melatonin relieves heat-induced spermatocyte apoptosis in mouse testes by inhibition of ATF6 and PERK signaling pathways. Zool. Res. 2021;42:514–524. doi: 10.24272/j.issn.2095-8137.2021.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Y., Cong P., Tong C., Jin H., Liu Y., Hou M. Melatonin pretreatment alleviates blast-induced oxidative stress in the hypothalamic-pituitary-gonadal axis by activating the Nrf2/HO-1 signaling pathway. Life Sci. 2021;280:119722. doi: 10.1016/j.lfs.2021.119722. [DOI] [PubMed] [Google Scholar]
- 155.Wang L., He C. Nrf2-mediated anti-inflammatory polarization of macrophages as therapeutic targets for osteoarthritis. Front. Immunol. 2022;13:967193. doi: 10.3389/fimmu.2022.967193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sadoughi F., Dana P.M., Asemi Z., Shafabakhash R., Mohammadi S., Heidar Z., Mirzamoradi M., Targhazeh N., Mirzaei H. Molecular and cellular mechanisms of melatonin in breast cancer. Biochimie. 2022;202:26–33. doi: 10.1016/j.biochi.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 157.Zhao C.-N., Wang P., Mao Y.-M., Dan Y.-L., Wu Q., Li X.-M., Wang D.-G., Davis C., Hu W., Pan H.-F. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019;48:1–10. doi: 10.1016/j.cytogfr.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 158.Liu L., Labani N., Cecon E., Jockers R. Melatonin Target Proteins: Too Many or Not Enough? Front. Endocrinol. 2019;10:791. doi: 10.3389/fendo.2019.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Srinivasan V., Spence D.W., Trakht I., Pandi-Perumal S.R., Cardinali D.P., Maestroni G.J. Immunomodulation by melatonin: Its significance for seasonally occurring diseases. Neuroimmunomodulation. 2008;15:93–101. doi: 10.1159/000148191. [DOI] [PubMed] [Google Scholar]
- 160.Cecon E., Izabelle C., Le Poder S., Real F., Zhu A., Tu L., Ghigna M.R., Klonjkowski B., Bomsel M., Jockers R., et al. Therapeutic potential of melatonin and melatonergic drugs on K18-hACE2 mice infected with SARS-CoV-2. J. Pineal Res. 2022;72:e12772. doi: 10.1111/jpi.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cecon E., Fernandois D., Renault N., Coelho C.F.F., Wenzel J., Bedart C., Izabelle C., Gallet S., Le Poder S., Klonjkowski B., et al. Melatonin drugs inhibit SARS-CoV-2 entry into the brain and virus-induced damage of cerebral small vessels. Cell. Mol. Life Sci. 2022;79:361. doi: 10.1007/s00018-022-04390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cecon E., Legros C., Boutin J.A., Jockers R. Journal of pineal research guideline for authors: Defining and characterizing melatonin targets. J. Pineal Res. 2021;70:e12712. doi: 10.1111/jpi.12712. [DOI] [PubMed] [Google Scholar]
- 163.Hornedo-Ortega R., Da Costa G., Cerezo A.B., Troncoso A.M., Richard T., Garcia-Parrilla M.C. In Vitro Effects of Serotonin, Melatonin, and Other Related Indole Compounds on Amyloid-β Kinetics and Neuroprotection. Mol. Nutr. Food Res. 2018;62:1700383. doi: 10.1002/mnfr.201700383. [DOI] [PubMed] [Google Scholar]
- 164.Wölfler A., Abuja P.M., Schauenstein K., Liebmann P.M. N-acetylserotonin is a better extra- and intracellular antioxidant than melatonin. FEBS Lett. 1999;449:206–210. doi: 10.1016/S0014-5793(99)00435-4. [DOI] [PubMed] [Google Scholar]
- 165.Lezoualc’h F., Sparapani M., Behl C. N-acetyl-serotonin (normelatonin) and melatonin protect neurons against oxidative challenges and suppress the activity of the transcription factor NF-kappaB. J. Pineal Res. 1998;24:168–178. doi: 10.1111/j.1600-079X.1998.tb00530.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.