Abstract
Objective
Tinnitus is defined as the perception of sound in the absence of an external source. We propose the hypothesis that migraine can cause exacerbation of tinnitus in some patients.
Methods
English literature from PubMed has been reviewed.
Results
Studies have reported a high prevalence of cochlear symptoms in patients with migraine headaches and up to 45% of tinnitus patients have been shown to concomitantly suffer from migraine. Both conditions are thought to stem from central nervous system disturbances, involving disruption of the auditory and trigeminal nerve pathways. One proposed mechanism of this association is the modulation of sound sensitivity by trigeminal nerve activation of the auditory cortex during migraine attacks, resulting in tinnitus fluctuation in some patients. Increased brain and inner ear vascular permeability resulting from trigeminal nerve inflammation, can also cause observed headache and auditory symptoms. Tinnitus and migraine also share a number of symptom triggers including stress, sleep disturbances, and dietary factors. These shared features may help explain promising results of migraine therapies for the treatment of tinnitus.
Conclusion
Given the complex association between tinnitus and migraine, further investigation is needed to identify the underlying mechanisms and determine the optimal treatment strategies for managing migraine‐related tinnitus patients.
Keywords: association, cochleovestibular migraine, migraine, otologic migraine, tinnitus
INTRODUCTION
Tinnitus is defined as a perception of sound “in the absence of a sound source.” 1 Its earliest historic reference is debated but it is believed to have been a known symptom in ancient Egypt, with its first written reference in the Papyrus Ebers, an Egyptian compilation of medical literature dating back to 1550 B.C. 2 In A.D. 980, Avicenna dedicated an entire treatise in his Canon of Medicine to otology, in which he described tinnitus as a ringing sound generated by the movement of air inside the ears of people with either powerful or weak hearing. 3 Although tinnitus is a commonly reported symptom in clinical care, clinicians still lack standard diagnostic objective test for tinnitus, largely relying on patient self‐reporting. Tinnitus falls into one of two categories of classification: objective and subjective. Objective tinnitus, which is generally pulsatile or clicking in nature, is the rarer form, where a sound is heard by both the patient and physician (e.g., arterial bruits, venous hums, and palatal and stapedial myoclonus). Subjective tinnitus is the most common form, in which the noise is only perceived by the patient. For the purposes of this paper, the usage of the word “tinnitus” will henceforth refer exclusively to subjective nonpulsatile tinnitus, and not objective tinnitus.
In the results of a nationwide survey conducted in the United States, 50 million individuals, aged 20 years or older, reported having tinnitus in the past year, and of those individuals, 16 million reported having tinnitus at least once daily. 4 A cross‐sectional analysis in the United States found that 2.5 million individuals aged 12–19 years have experienced one or more episodes of tinnitus, and that 1.6 million individuals in this group suffer from chronic tinnitus. 5 The socioeconomic burden of tinnitus is substantial, as it is the most common disability among veterans. 6 Over 1.5 million veterans receive disability benefits for tinnitus, which amounts to $1.3 billion annually. 6 The psychological and mental toll on tinnitus patients is another concern, as unresolved tinnitus may lead to symptoms of depression and anxiety, though sometimes depressed and anxious patients may somaticize the tinnitus.
Tinnitus is caused by the loss of function in the cochlea, which is caused by damage to the hair cells in the cochlea and the resulting loss of auditory input. 7 Specifically, damage to hair cells in the high‐frequency region of the cochlea is responsible for subclinical or clinically detectable hearing loss, which leads to a rearrangement in the tonotopic organization of the auditory cortex. 8 Following cochlear hair cell damage, the tonotopic organization in the auditory cortex will change such that cortical neurons will respond to frequencies in the less affected cochlear cells. The spontaneous firing of these neurons results in tinnitus. An alternative theory for the etiology of tinnitus is the dorsal cochlear nucleus (DCN) theory. The DCN receives direct auditory input from the vestibulocochlear (8th cranial) nerve as well as indirect somatosensory input from the trigeminal (5th cranial) nerve via granule cells. Animal studies have found that self‐produced sounds are cancelled in the DCN by somatosensory signals from the spinal trigeminal nucleus via mossy fibers. 9 These findings altogether suggest that the spontaneous and self‐generating nature of tinnitus may be due to a disruption of auditory and changes to trigeminal somatosensory input. Both theories for the pathophysiology of tinnitus involve the auditory pathway, which is modulated by the central nervous system (Figure 1). This suggests that there may be a pathophysiological link to migraine headaches, specifically the changes in central hypersensitivity involving the trigeminal nerve which may aggravate the tinnitus. Although not all migraine patients experience tinnitus, many migraine patients both clinically and in our literature review report auditory symptoms in association with migraine, with tinnitus being one of the most common auditory symptoms. In this paper, we propose that increased tinnitus perception in some patients may be associated with migraine, and that treatments for migraine may be used to treat tinnitus in patients with variable or bothersome tinnitus.
Figure 1.
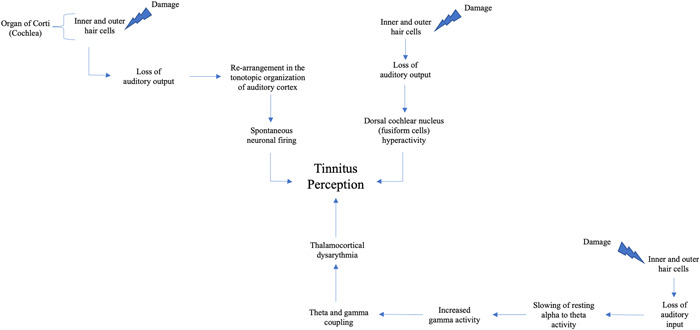
Representation of the possible central nervous system pathways involved in tinnitus
HYPOTHESIS
We hypothesize that fluctuating tinnitus in some patients may be associated with a migraine phenomenon, as both are similarly elusive in etiology but possibly share a pathophysiology linked by the central nervous system, specifically the activation of the trigeminal ganglion, and altered blood flow to the inner ear. This common risk factor can therefore explain why some migraine patients can experience inner ear symptoms such as a heightened awareness of tinnitus during the activation of migraine. This hypothesis will also explain in more detail the epidemiological and mechanistic associations between fluctuating tinnitus and migraine as well as their overlapping trigger factors. Finally, this theory will explain the potential for migraine treatments to be considered in the management and treatment of significant bothersome and fluctuating tinnitus.
EVALUATION OF THE HYPOTHESIS
An epidemiological and mechanistic association between tinnitus and migraine
Tinnitus is caused by central activity secondary to damage to the auditory periphery. It has been found that migraine is more common in patients with tinnitus and subjective hearing loss. In an analysis of the National Health and Nutrition Examination Survey (NHANES) database, we found that among 12,962 patients with tinnitus or subjective hearing loss, from 1999 to 2004, migraine was reported in 36.6% and 24.5% respectively. Multivariate logistic regression showed that patients with tinnitus were more likely to have migraine, and that patients with migraine were more likely to have subjective hearing loss and tinnitus. 10 Additionally, a cohort study in Taiwan found that among 1056 patients with migraine, 61.5% of patients with vestibular migraine experienced auditory symptoms with tinnitus being the most common symptom. 11 Overall, this study found that cochlear symptoms (tinnitus, hearing loss, sudden deafness) were significantly more common in patients with a history of migraine headaches than in those without. Another retrospective analysis of questionnaire data from tinnitus patients found that 44.6% of subjects suffered from migraine. 12 These findings heavily suggest that there may be a mechanistic link between migraine and enhanced attention to the dysregulation of the central auditory pathway in a subpopulation of tinnitus.
Migraine has only recently been explored as a possible cause of inner ear disorders including Meniere's disease (MD), vertigo, sensorineural hearing loss, and potentially tinnitus. 13 , 14 Two forms of migraine—vestibular migraine and cochlear migraine—exhibit associations with inner ear dysfunction. Vestibular migraine is the more commonly understood form of migraine, consisting of migraine and vertigo symptoms. 15 Patients with vestibular migraine have been shown to have significantly higher rates of tinnitus compared to controls. 16 In 2018, the concept of a “cochlear migraine” was introduced. Cochlear migraine consists of migraine and non‐vestibular ear symptoms. 11 Even earlier, in 1966, Atkinson termed MD “migraine in the ear,” characterized by hearing loss secondary to vasospasms in the inner ear. 17 Therefore, MD is theorized to be a manifestation of cochleovestibular migraine as both conditions share a common pathophysiology. In fact, a hydropic inner ear (ELH) is unable to autoregulate its blood flow in response to calcitonin gene‐related peptide and substance P secreted from the trigeminal ganglion during a migraine attack. This appears to make the ELH more vulnerable to triggering factors (detailed below) and the patient will manifest MD. 18 Recently, we found that migraine prophylaxis medications significantly improve MD symptoms, which further strengthens this link. 19 As low‐frequency tinnitus is one of the features of MD, this lends more credence to the association between low‐frequency hearing loss‐associated tinnitus and migraine.
The interplay of both forms of migraine and tinnitus can be explained by a close examination of the mechanism connecting them. Tinnitus occurs in the setting of peripheral auditory system damage. 20 The organ of Corti is the specialized organ responsible for hearing located within the cochlea. It consists of one row of inner hair cells and three rows of outer cells, as well as three primary sections—apical, middle, and basal turns, which are tuned to respond to low, medium, and high frequencies of sound, respectively. Hearing loss most commonly (e.g., continuous loud noise indued and age‐related) begins in high frequencies, with low frequencies being preserved. This may be due to selective damage of hair cells in the basal turn of the cochlea. 21 The functional loss of hair cells in the basal region results in reduction of lateral inhibition at the central level, which causes a reorganization of the tonotopic map in the auditory cortex. This leads to spontaneous neuronal firings in the cortex which is experienced as tinnitus by the patient. 22
During migraine attacks, sensory sensitivity may increase, which enhances sensitivity to visual, auditory, and olfactory stimuli. Therefore, migraine attacks may modulate migraineurs’ sensitivity to sound and enhance the perception of tinnitus. One magnetic resonance imaging (MRI) study found that in response to cutaneous noxious stimulation to the forehead (or the first branch of the trigeminal nerve), migraineurs exhibit greater activation in areas of the brain such as the parahippocampal gyrus. As the trigeminal nerve directly innervates the inner ear blood supply and cause neurogenic inflammation, its activation in migraine attacks may cause hearing loss or increase central sensitivity or attention to the tinnitus percept in the auditory cortex. 21 Furthermore, the significant association between tinnitus and headache laterality found in several studies supported that trigeminal nerve sensitization might be the common pathophysiological factor. 23 , 24 The role of cochlear dysfunction in migraine mediated by outer hair cell activity has also been studied and observed in otoacoustic emission (OAE) testing in migraine patients. One study found that the amplitude of otoacoustic admissions were significantly lower in migraine patients compared to controls, and there was an absence of suppression of transiently evoked OAEs by contralateral sound stimulation in migraine patients, which may be part of the mechanism of phonophobia in migraine. 25
Alternatively, another proposed mechanism of tinnitus supported by animal models is that it originates in the DCN. This hypothesis suggests that tinnitus can be traced to spontaneous activity in the DCN due to loss of input from the cochlear nerve. In 1970, a study involving nerve fiber recordings in a cat model with sensorineural hearing loss found that auditory nerve fibers had lost all spontaneous activity and became unresponsive to acoustic stimulation. 26 In the years that followed, it was discovered that the DCN develops spontaneous activity in the region that tonotopically corresponds to the damaged region of the cochlea in subjects with cochlear hearing loss. 27 Several studies corroborated this finding in animal models with behavioral evidence of tinnitus, finding that the fusiform cells (the main integrative cells) in the DCN in these species exhibited spontaneous activity. Other studies have found that the same DCN neurons with increased firing rates in hearing loss‐associated tinnitus show increased sensitivity to trigeminal electric stimulation, suggesting that the trigeminal nerve is also at play in its etiology. 28 The DCN hypothesis for tinnitus, therefore, posits that loss of or disruption of input from cochlear nerve leads to inhibition of dorsal cochlear input, which subsequently manifests as spontaneous activity arising from the DCN resulting in tinnitus symptoms. 29
The disruption of input to the auditory nerve can occur as a result of altered vascular permeability in the inner ear. A study by Vass et al. demonstrated that trigeminal ganglion neurons innervate the cochlear arterial supply within the cochlea, which points to the trigeminal ganglion as the source of blood flow change within the cochlea. 30 More specifically, trigeminal stimulation can cause changes in vascular permeability within the cochlea and neurogenic inflammation, which may then contribute to the inhibition of the DCN which leads to tinnitus, in cases of sudden hearing loss. These vascular changes that affect inner ear perfusion are also observed in migraine headaches. Previously, we proposed that the mechanism for migraine‐related cochlear symptoms (i.e., tinnitus and hearing loss) is changes to the blood flow of the inner ear caused by vasospasm or vasodilation of the posterior cerebral arterial circulation and inflammation caused by the trigeminal nerve. 31
The main proposed mechanism for migraine is spreading cortical depression or altered electrical activity propagating across the cortex. This is the underlying cause of aura, a common sensory disturbance characterized by flashes of light and blind spots experienced just before a migraine attack in some patients. Spreading cortical depression is believed to produce a state of inflammation in the intracranial meninges and subsequent activation of trigeminal meningeal nociceptors. 32 Specifically, this state of inflammation comprises the release of neuropeptides such as substance P and calcitonin gene‐related peptide from the trigeminal ganglion, which then causes secondary vasodilation, capillary leakage, and edema which underlies the headache experienced in migraine (Figure 2). 32 Another proposed model of the association of auditory dysfunction and tinnitus is thalamocortical dysrhythmia (TCD), resulting from disruption of auditory input (similar to the DCN hypothesis). 33 In the TCD model, loss of auditory input leads to a slowing of resting‐state alpha to theta activity, which causes an increase in surrounding gamma activity. This is followed by theta and gamma coupling, which may result in tinnitus‐related focal auditory gamma activity becoming consciously perceived (Figure 1). 34 This model is supported by findings by Muhlinickel et al., 35 which showed a strong positive correlation between subjective strength of tinnitus stimulus and the amount of cortical reorganization, which is observed in phantom limb pain. This then led to clinical trials that have tested the use of anticonvulsant drugs for phantom limb pain (e.g., topiramate) for tinnitus. A meta‐analysis of seven clinical trials testing this has shown a slight improvement in tinnitus. 36
Figure 2.
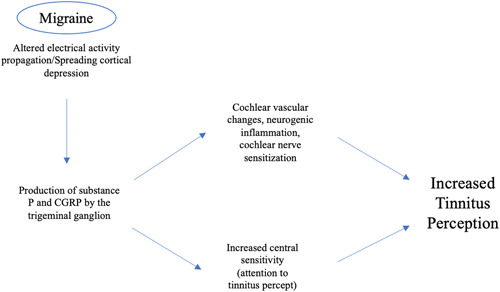
Representation of the migraine mechanisms leading to increased tinnitus perception
Additionally, some studies have identified differences in the pathogenesis of migraine in acute versus chronic tinnitus. Ma et al. 37 suggests that the pathogenesis of migraine in acute tinnitus is due to central sensitization of migraine producing high and distorted auditory signals in central compensation, which is perceived as tinnitus. This theory is supported by case reports of patients with primary migraine disorders who report increase in tinnitus intensity during migraine attacks. 38 The authors also propose the neuropsychological model of tinnitus and the involvement of anxiety and depression in the onset of chronic tinnitus. 37 One study found that gray and white matter volumes of brain were smaller in migraine patients with depression than patients with migraine alone, depression alone, or neither. The authors suggested that there was a common pathophysiological mechanism of depression and migraine involving serotonin and other transmitters. 39
Tinnitus and migraine share similar triggers
In our experience treating thousands of tinnitus patients, we have found that tinnitus and migraine share a number of triggering factors including sleep disturbance, stress, diet, and weather changes. In particular, high sodium foods contain preservatives (most commonly glutamate) or byproducts of protein breakdown including tyramine, which are common migraine triggers. 40 Consumption of sodium‐containing foods combined with dehydration is a trigger of migraine. Tyramine is also a byproduct of fermentation, found in fermented products including alcoholic beverages such as wine and beer, and aged cheese. It is an endogenously produced compound that appears to modulate dopamine release, and is degradation by monoamine oxidase (MAO) produces hydrogen peroxide, which causes oxidative stress that potentially leads to inflammatory symptoms of migraine. Reduced sodium in the diet is commonly recommended as a first‐line treatment for patients with inner ear symptoms, as it is believed that a low‐salt diet helps to increase plasma aldosterone which then affects endolymph regulation in the inner ear. Due to a lack of randomized controlled trials to prove the link between tinnitus and dietary sodium intake; however, this link can only be hypothesized based on clinical observations. 41 In our observation of patients who report improvement with a low sodium diet, we have found that most of the food items they eliminated were glutamate or tyramine‐containing foods. In addition, we have found that patients who link high sodium with increased inner ear symptoms do not experience exacerbation if increased water intake accompanies the higher sodium intake. Therefore, adequate hydration and the unintended reduction in glutamate and tyramine likely caused the benefit rather than the sodium reduction.
Additionally, caffeine overusage or withdrawal has been identified as a trigger of migraine. Caffeine acts by blocking adenosine receptors, and adenosine is important in modulating neuronal activity, particularly neurotransmitter release. Caffeine, therefore, acts to increase wakefulness and arousal by blocking adenosine receptors, which inhibits GABAergic output to the hypothalamus. Evidence has shown that caffeine overuse and withdrawal cause headaches and migraine symptoms, due to upregulated dopaminergic activity. 42 It is unclear if caffeine intake is a trigger for tinnitus; however, one study found that high caffeine intake (>450 mg/day) correlated with lower risk of tinnitus in women. 43 This study found a 15%–21% reduction in incidence in the high caffeine‐consuming groups, but only looked at caffeine as the primary variable and did not examine other dietary, lifestyle issues (stress/sleep), or audiogram. The results of that study should be interpreted with caution.
Stress is another well‐understood trigger of migraine, as perceived stress has been shown to be the most commonly reported trigger of migraine. 38 A randomized clinical trial found that mindfulness‐based stress reduction decreased episodic migraine frequency in 52% of participants compared to 23% in the control group. 44 Although the directionality of the causality is unclear, stress is associated with tinnitus also. A cross‐sectional study performed in Korea investigating the link between stress and tinnitus discovered that the adjusted odds ratio of tinnitus increased with perceived stress. 45
Meteorological changes such as weather, temperature, atmospheric pressure changes have also been proposed as migraine triggers. A retrospective review found that of 130 patients with vestibular migraine, 26% reported weather changes as being migraine trigger. 46 A prospective study also found a correlation between depressed barometric pressure with headache frequency. The study, involving a headache diary over a 1‐year period, found that among 28 migraine patients, 18 reported headaches associated with weather, the majority of whom citing barometric pressure as the cause. 47 Barometric data confirmed that the frequency of headaches increased significantly just before barometric depression, such as in a typhoon. Some studies, however, have not found a significant correlation between meteorological changes and migraine. 48 This may be because only a subpopulation of migraine sufferers is sensitive to weather and atmospheric pressure changes, as we have observed in our clinical practice and in other studies. This may also explain why some studies, which rely on pooled analysis, show inconclusive results. 49 In the clinic, we often see patients who report that their tinnitus can be triggered by weather changes. This is most commonly observed with low atmospheric pressure which has been found to be associated with the exacerbation of the MD symptoms including tinnitus. 50
Treatments for migraine may help treat tinnitus
Traditional treatments for tinnitus are categorized into dietary and lifestyle modifications, medications, cognitive behavioral therapy (CBT), and sound therapy. However, there is no FDA‐approved medication for the treatment of tinnitus. Most medications for tinnitus are prescribed to manage the psychological effects of chronic tinnitus, such as depression and anxiety.
Treatments that are traditionally used for migraine are promising alternatives for treating tinnitus and other inner ear disorders. Adjuvant migraine medications such as nortriptyline—a tricyclic antidepressant that inhibits the reuptake of serotonin and norepinephrine—and topiramate—a second‐generation antiepileptic that acts as a GABA agonist, in conjunction with oral steroids and intratympanic dexamethasone injections produced greater recovery in sudden sensorineural hearing loss patients than the steroids alone. 51 This suggests that traditional treatments for migraine may also be used to treat closely related inner ear pathologies such as tinnitus. Antidepressants such as nortriptyline have previously been prescribed for treatment of tinnitus, with serotonergic and antimuscarinic mechanisms. 52 They appear to be more effective in patients who are already anxious or depressed with a severe phenotype. In particular, selective serotonin reuptake inhibitors (SSRIs) appear to be more tolerable than tricyclic antidepressants. 53 While some may suggest that antidepressants only treat the emotional and psychological effects of unresolved tinnitus, a clinical trial by Sullivan et al. showed that nortriptyline's effect on tinnitus may be independent of depression and anxiety symptoms. Specifically, pretreatment depression scores did not change following a course of nortriptyline, but tinnitus was found to be improved. 52 This finding suggests that the efficacy of nortriptyline may be due to its migraine prophylaxis effect, which helps to lessen the severity of tinnitus.
Neuroimaging studies have shown that limbic system structures are activated in tinnitus. These areas (fronto‐subcortical loop circuits) are also affected in psychiatric disorders, suggesting that there is a joint mechanism at play, which supports the use of antidepressants for tinnitus. 54 The Cochrane Library reported the findings of six clinical trials that examined the usefulness of antidepressants for tinnitus. 55 However, due to large drop‐out rates and inadequate outcome measures, they were unable to draw any conclusions about the efficacy of antidepressants.
Nonpharmacological interventions including CBT to reduce stress and sound therapy typically used for migraine may also help to alleviate tinnitus. A meta‐analysis found that the pooled odds ratios of clinically significant improvement post‐CBT treatment and 3‐month follow‐up in children and adolescents showed significant improvement with CBT compared to placebo only. 56 Similarly, one randomized controlled trial of internet‐based CBT for distress associated with tinnitus found that participants in the treatment group improved at least 50% on the Tinnitus Reaction Questionnaire that assesses the distress associated with tinnitus (Supporting Information: Table S1). 57 One randomized cross‐over trial of customized sound therapy compared to generic white noise found that customized sound therapy was more effective in reducing the tinnitus loudness and score on the Tinnitus Handicap Inventory (a questionnaire that measures the severity of tinnitus; Supporting Information: Table S2). 58 In a proof‐of‐concept study, we showed that undergoing our rigorous 8‐week course of CBT modules (comprised of behavioral coaching, stress and sleep management, meditation and breathing exercises, dietary recommendations, etc.) and personalized sound therapy (pitch and frequency‐matched sounds with the ability to be mixed with music) resulted in improved tinnitus‐specific quality of life measures. 59 A summary of proposed algorithm for step‐wise tinnitus treatment regimen with migraine medications is shown in Figure 3.
Figure 3.
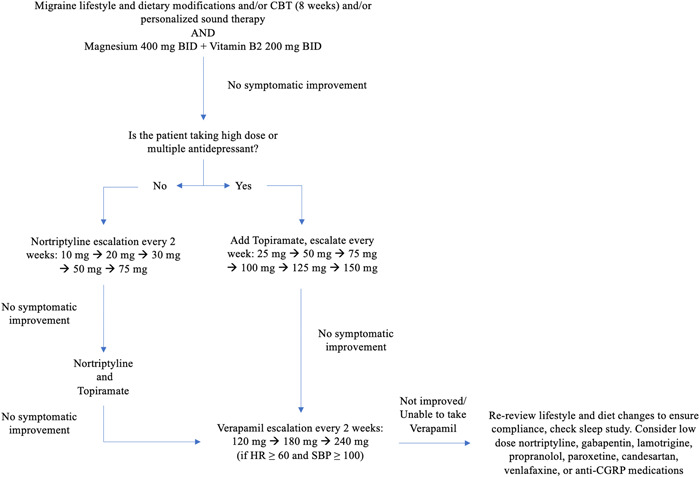
Proposed algorithm for step‐wise tinnitus treatment regimen with migraine medications. BID, twice daily; CBT, cognitive behavioral therapy; CGRP, calcitonin‐gene related peptide.
DISCUSSION AND CONCLUSION
There appears to be an association between fluctuation of tinnitus and migraine, but the mechanism that links them has yet to be fully proven. As more studies continue to investigate this association, particularly the vasculopathies, spreading cortical depression, and neuronal disturbances that factor into both migraine and tinnitus, this connection will be further elucidated. These studies will serve to better inform clinicians to not treat tinnitus and migraine in isolation but consider them as potentially interlinked phenomena in some patients with fluctuating or bothersome tinnitus.
As of today, there are limited treatment options for individuals suffering from significant bothersome and fluctuating tinnitus. Current treatment methods focus on alleviating the tinnitus‐related distress, rather than treating the underlying pathophysiology, such as counseling and sound therapy. 51 As the association between tinnitus and migraine becomes more understood, traditional migraine treatments should be considered in the management of tinnitus. For example, adjuvant migraine medications such as nortriptyline and topiramate may be effective in reducing the severity of tinnitus. Additionally, nonpharmacological approaches to migraine treatment such as CBT may also be applied to severe tinnitus patients to reduce the subjective emotional distress caused by significant bothersome tinnitus.
On the other hand, future studies may examine the common dietary triggers of both tinnitus and migraine. This will better inform the dietary limitations that can also be considered in the management of migraine and tinnitus. Furthermore, studies should continue to explore the neuroanatomy that links migraine, tinnitus fluctuation, and psychiatric disorders. Hopefully, these connections will serve as a stepping stone for advances in tinnitus management and improve the quality of life for tinnitus and migraine sufferers.
AUTHOR CONTRIBUTIONS
Ariel Lee: Conceptualization, Methodology, Writing – original draft, Writing – review and editing; Mehdi Abouzari: Conceptualization, Methodology, Supervision, Writing – review and editing; Meleeka Akbarpour: Methodology, Writing – review and editing; Adwight Risbud: Methodology, Writing – review and editing; Harrison W. Lin: Conceptualization, Supervision, Writing — review and editing; Hamid R. Djalilian: Conceptualization, Supervision, Writing — review and editing.
CONFLICTS OF INTEREST
Hamid R. Djalilian holds equity in MindSet Technologies, Elinava Technologies, and Cactus Medical LLC. He is a consultant to NXT Biomedical.
ETHICS STATEMENT
Given the deidentified and publicly available nature of this database, this study was exempted from Institutional Review Board approval.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Mehdi Abouzari, MD, PhD; is supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant TL1TR001415. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors also wish to acknowledge Dr. Karen Tawk for her help in revising the manuscript and designing the figures.
Lee A, Abouzari M, Akbarpour M, Risbud A, Lin HW, Djalilian HR. A proposed association between subjective nonpulsatile tinnitus and migraine. World J Otorhinolaryngol Head Neck Surg. 2023;9:107‐114. 10.1002/wjo2.81
Ariel Lee and Mehdi Abouzari contributed equally to this study.
Contributor Information
Mehdi Abouzari, Email: mabouzar@hs.uci.edu.
Hamid R. Djalilian, Email: hdjalili@hs.uci.edu.
DATA AVAILABILITY STATEMENT
N/A.
REFERENCES
- 1. Chemali Z, Nehmé R, Fricchione G. Sensory neurologic disorders: tinnitus. Handb Clin Neurol. 2019;165:365‐381. [DOI] [PubMed] [Google Scholar]
- 2. Dietrich S. Earliest historic reference of ‘tinnitus’ is controversial. J Laryngol Otol. 2004;118:487‐488. [DOI] [PubMed] [Google Scholar]
- 3. Hamidi S, Sajjadi H, Boroujerdi A, Golshahi B, Djalilian HR. Avicenna's treatise on otology in Medieval Persia. Otol Neurotol. 2008;29:1198‐1203. [DOI] [PubMed] [Google Scholar]
- 4. Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711‐718. [DOI] [PubMed] [Google Scholar]
- 5. Mahboubi H, Oliaei S, Kiumehr S, Dwabe S, Djalilian HR. The prevalence and characteristics of tinnitus in the youth population of the United States. Laryngoscope. 2013;123:2001‐2008. [DOI] [PubMed] [Google Scholar]
- 6. Lewis MS, Reavis KM, Griest S, Carlson KF, Gordon J, Henry JA. The influence of tinnitus and hearing loss on the functional status of military Service members and veterans. Int J Audiol . 2022;1‐9. [DOI] [PubMed]
- 7. Chen GD, Fechter LD. The relationship between noise‐induced hearing loss and hair cell loss in rats. Hear Res. 2003;177:81‐90. [DOI] [PubMed] [Google Scholar]
- 8. Saunders JC. The role of central nervous system plasticity in tinnitus. J Commun Disord. 2007;40:313‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singla S, Dempsey C, Warren R, Enikolopov AG, Sawtell NB. A cerebellum‐like circuit in the auditory system cancels responses to self‐generated sounds. Nat Neurosci. 2017;20:943‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goshtasbi K, Abouzari M, Risbud A, et al. Tinnitus and subjective hearing loss are more common in migraine: a cross‐sectional NHANES analysis. Otol Neurotol. 2021;42:1329‐1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hwang JH, Tsai SJ, Liu TC, Chen YC, Lai JT. Association of tinnitus and other cochlear disorders with a history of migraines. JAMA Otolaryngol Head Neck Surg. 2018;144:712‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langguth B, Hund V, Landgrebe M, Schecklmann M. Tinnitus patients with comorbid headaches: the influence of headache type and laterality on tinnitus characteristics. Front Neurol. 2017;8:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dash AK, Panda N, Khandelwal G, Lal V, Mann SS. Migraine and audiovestibular dysfunction: is there a correlation. Am J Otolaryngol. 2008;29:295‐299. [DOI] [PubMed] [Google Scholar]
- 14. Bayazit Y, Yilmaz M, Mumbuç S, Kanlikama M. Assessment of migraine‐related cochleovestibular symptoms. Rev Laryngol Otol Rhinol (Bord). 2001;122:85‐88. [PubMed] [Google Scholar]
- 15. Luzeiro I, Luís L, Gonçalves F, Pavão Martins I. Vestibular migraine: clinical challenges and opportunities for multidisciplinarity. Behav Neurol. 2016;2016:6179805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kırkım G, Mutlu B, Olgun Y, et al. Comparison of audiological findings in patients with vestibular migraine and migraine. Turk Arch Otorhinolaryngol. 2017;55:158‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moshtaghi O, Sahyouni R, Lin HW, Ghavami Y, Djalilian HR. A historical recount: discovering Menière's disease and its association with migraine headaches. Otol Neurotol. 2016;37:1199‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarna B, Abouzari M, Lin HW, Djalilian HR. A hypothetical proposal for association between migraine and Meniere's disease. Med Hypotheses. 2020;134:109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abouzari M, Tan D, Sarna B, et al. Efficacy of multi‐modal migraine prophylaxis therapy on hyperacusis patients. Ann Otol Rhinol Laryngol. 2020;129:421‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rüttiger L, Singer W, Panford‐Walsh R, et al. The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PLoS One. 2013;8:e57247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demarquay G, Mauguière F. Central nervous system underpinnings of sensory hypersensitivity in migraine: insights from neuroimaging and electrophysiological studies. Headache. 2016;56:1418‐1438. [DOI] [PubMed] [Google Scholar]
- 22. Eggermont JJ. Central tinnitus. Auris Nasus Larynx. 2003;30 Suppl:S7‐S12. [DOI] [PubMed] [Google Scholar]
- 23. Langguth B, Hund V, Busch V, et al. Tinnitus and headache. BioMed Res Int. 2015;2015:797416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nowaczewska M, Wiciński M, Straburzyński M, Kaźmierczak W. The prevalence of different types of headache in patients with subjective tinnitus and its influence on tinnitus parameters: a prospective clinical study. Brain Sci. 2020;10:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bolay H, Bayazit Y, Gündüz B, et al. Subclinical dysfunction of cochlea and cochlear efferents in migraine: an otoacoustic emission study. Cephalalgia. 2008;28:309‐317. [DOI] [PubMed] [Google Scholar]
- 26. Kiang NY, Moxon EC, Levine RA. Auditory‐nerve activity in cats with normal and abnormal cochleas. In: Sensorineural hearing loss. Ciba Found Symp . 1970:241‐273. [DOI] [PubMed]
- 27. Kaltenbach JA, McCaslin DL. Increases in spontaneous activity in the dorsal cochlear nucleus following exposure to high‐intensity sound: a possible neural correlate of tinnitus. Audit Neurosci. 3, 1996:57‐78. [PMC free article] [PubMed] [Google Scholar]
- 28. Levine RA, Oron Y. Tinnitus. Handb Clin Neurol. 2015;129:409‐431. [DOI] [PubMed] [Google Scholar]
- 29. Atik A. Pathophysiology and treatment of tinnitus: an elusive disease. Indian J Otolaryngol Head Neck Surg. 2014;66:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vass Z, Shore SE, Nuttall AL, Miller JM. Direct evidence of trigeminal innervation of the cochlear blood vessels. Neuroscience. 1998;84:559‐567. [DOI] [PubMed] [Google Scholar]
- 31. Lin HW, Djalilian HR. The role of migraine in hearing and balance symptoms. JAMA Otolaryngol Head Neck Surg. 2018;144:717‐718. [DOI] [PubMed] [Google Scholar]
- 32. Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40:301‐314. [DOI] [PubMed] [Google Scholar]
- 33. Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222‐15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Ridder D, Vanneste S, Langguth B, Llinas R. Thalamocortical dysrhythmia: a theoretical update in tinnitus. Front Neurol. 2015;6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A. 1998;95:10340‐10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoekstra CE, Rynja SP, van Zanten GA, Rovers MM. Anticonvulsants for tinnitus. Cochrane Database Syst Rev. 2011;2011:CD007960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma X, Ke YJ, Jing YY, Diao TX, Yu LS. Migraine and cochlear symptoms. Curr Med Sci. 2021;41:649‐653. [DOI] [PubMed] [Google Scholar]
- 38. Volcy M, Sheftell FD, Tepper SJ, Rapoport AM, Bigal ME. Tinnitus in migraine: an allodynic symptom secondary to abnormal cortical functioning. Headache. 2005;45:1083‐1087. [DOI] [PubMed] [Google Scholar]
- 39. Gudmundsson LS, Scher AI, Sigurdsson S, et al. Migraine, depression, and brain volume: the AGES‐Reykjavik study. Neurology. 2013;80:2138‐2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Borkum JM. Migraine triggers and oxidative stress: a narrative review and synthesis. Headache. 2016;56:12‐35. [DOI] [PubMed] [Google Scholar]
- 41. Hofmeister M. Do dietary factors significantly influence tinnitus. Aust J Gen Pract. 2019;48:153‐157. [DOI] [PubMed] [Google Scholar]
- 42. Alstadhaug KB, Andreou AP. Caffeine and primary (migraine) headaches‐friend or foe. Front Neurol. 2019;10:1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Glicksman JT, Curhan SG, Curhan GC. A prospective study of caffeine intake and risk of incident tinnitus. Am J Med. 2014;127:739‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seminowicz DA, Burrowes S, Kearson A, et al. Enhanced mindfulness‐based stress reduction in episodic migraine: a randomized clinical trial with magnetic resonance imaging outcomes. Pain. 2020;161:1837‐1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim HJ, Lee HJ, An SY, et al. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS One. 2015;10:e0127578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beh SC, Masrour S, Smith SV, Friedman DI. The spectrum of vestibular migraine: clinical features, triggers, and examination findings. Headache. 2019;59:727‐740. [DOI] [PubMed] [Google Scholar]
- 47. Kimoto K, Aiba S, Takashima R, et al. Influence of barometric pressure in patients with migraine headache. Intern Med. 2011;50:1923‐1928. [DOI] [PubMed] [Google Scholar]
- 48. Elcik C, Fuhrmann CM, Mercer AE, Davis RE. Relationship between air mass type and emergency department visits for migraine headache across the Triangle region of North Carolina. Int J Biometeorol. 2017;61:2245‐2254. [DOI] [PubMed] [Google Scholar]
- 49. Hoffmann J, Schirra T, Lo H, Neeb L, Reuter U, Martus P. The influence of weather on migraine—are migraine attacks predictable. Ann Clin Transl Neurol. 2015;2:22‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmidt W, Sarran C, Ronan N, et al. The weather and Ménière's disease: a longitudinal analysis in the UK. Otol Neurotol. 2017;38:225‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abouzari M, Goshtasbi K, Chua JT, et al. Adjuvant migraine medications in the treatment of sudden sensorineural hearing loss. Laryngoscope. 2021;131:E283‐E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sullivan MD, Dobie RA, Sakai CS, Katon WJ. Treatment of depressed tinnitus patients with nortriptyline. Ann Otol Rhinol Laryngol. 1989;98:867‐872. [DOI] [PubMed] [Google Scholar]
- 53. Robinson S. Antidepressants for treatment of tinnitus. Prog Brain Res. 2007;166:263‐271. [DOI] [PubMed] [Google Scholar]
- 54. Minen MT, Camprodon J, Nehme R, Chemali Z. The neuropsychiatry of tinnitus: a circuit‐based approach to the causes and treatments available. J Neurol Neurosurg Psychiatry. 2014;85:1138‐1144. [DOI] [PubMed] [Google Scholar]
- 55. Baldo P, Doree C, Molin P, McFerran D, Cecco S. Antidepressants for patients with tinnitus. Cochrane Database Syst Rev. 2012;2012:CD003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hesser H, Weise C, Westin VZ, Andersson G. A systematic review and meta‐analysis of randomized controlled trials of cognitive‐behavioral therapy for tinnitus distress. Clin Psychol Rev. 2011;31:545‐553. [DOI] [PubMed] [Google Scholar]
- 57. Andersson G, Strömgren T, Ström L, Lyttkens L. Randomized controlled trial of internet‐based cognitive behavior therapy for distress associated with tinnitus. Psychosom Med. 2002;64:810‐816. [DOI] [PubMed] [Google Scholar]
- 58. Mahboubi H, Haidar YM, Kiumehr S, Ziai K, Djalilian HR. Customized versus noncustomized sound therapy for treatment of tinnitus: a randomized crossover clinical trial. Ann Otol Rhinol Laryngol. 2017;126:681‐687. [DOI] [PubMed] [Google Scholar]
- 59. Abouzari M, Goshtasbi K, Sarna B, et al. Adapting personal therapies using a mobile application for tinnitus rehabilitation: a preliminary study. Ann Otol Rhinol Laryngol. 2021;130:571‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
N/A.


