Abstract
Background
Influenza is known to predispose to secondary bacterial infections including invasive group A streptococcal (iGAS) disease. The universal pediatric live attenuated influenza vaccine (LAIV) program introduced in England from the 2013/2014 influenza season was implemented incrementally, introducing cohorts of children annually to 2–16 years of coverage. Additionally, from the beginning of the program, discrete pilot areas offered LAIV vaccination to all primary school–age children, allowing for a unique comparison of infection rates between pilot and nonpilot areas during the program rollout.
Methods
Cumulative incidence rate ratios (IRRs) of GAS infections (all), scarlet fever (SF), and iGAS infection within each season by age group were compared for pilot and nonpilot areas using Poisson regression. The overall effect of the pilot program in the pre- (2010/2011–2012/2013 seasons) and postintroduction (2013/2014–2016/2017 seasons) periods was assessed using negative binomial regression by comparing changes in incidence between pilot/nonpilot areas (ratio of IRR [rIRR]).
Results
Reductions in IRRs of GAS and SF were observed within most post-LAIV program seasons, among the age groups 2–4 and 5–10 years. Significant reductions were seen among 5–10 years (rIRR, 0.57; 95% CI, 0.45–0.71; P < .001), 2–4 years (rIRR, 0.62; 95% CI, 0.43–0.90; P = .011), and 11–16 years (rIRR, 0.63; 95% CI, 0.43–0.90; P = .018) for GAS infections when assessing the overall effect of the program.
Conclusions
Our findings suggest that vaccination with LAIV may be associated with a reduced risk of GAS infection and support attaining high uptake of childhood influenza vaccination.
Keywords: influenza, LAIV, group A Streptococcus
Group A Streptococcus (GAS) bacterium causes a range of clinical presentations including pharyngitis, scarlet fever, and invasive group A streptococcal (iGAS) infection including pneumonia, with the latter identified as potentially secondary to influenza [1–6].
In England, reports of scarlet fever have been low since the 1960s; however, in the winter of 2013/2014, a resurgence was first observed and continued until coronavirus disease 2019 (COVID-19) restrictions were introduced [7,8]. A number of countries in Asia similarly reported increases in scarlet fever [9, 10]. Both scarlet fever and invasive iGAS infection are notifiable diseases in England. A pronounced increase in scarlet fever and iGAS infection notifications, particularly in children under 10 years of age, was seen during 2022 [11, 12].
The pediatric live attenuated influenza (LAIV) intranasal immunization program in England commenced in the 2013/2014 influenza season with the aim of reducing the annual burden of influenza by vaccinating all children aged 2 to 16 years, offering direct protection to children themselves and indirectly to others [13]. Before 2013/2014, the United Kingdom had a longstanding national selective influenza immunization program offering inactivated influenza vaccines to individuals aged >65 years, pregnant women, health care workers, and individuals aged 6 months to 64 years in a clinical risk group, with uptake averaging 73%, 40%, 45%, and 51%, respectively, in 2011/2012 and 2012/2013 [14]. Since 2013/2014, the program has been incrementally rolled out by preschool and school year groups annually nationwide, reaching moderate uptake levels of ∼50% [15, 16–19]. Simultaneously several geographically discrete pilot areas also began to vaccinate all primary school and some secondary school–aged children, which provided a unique opportunity to measure the direct and indirect impacts of the program on the population. Previous studies of LAIV pilot areas have demonstrated significant differences in the reductions of influenza among the targeted age groups themselves and indirect effects due to reduction of influenza transmission on other age groups in pilot areas compared with nonpilot areas [16–19]. Similar observations of reduced infections among countries vaccinating with LAIV have been noted previously [20, 21].
This study aimed to investigate the potential epidemiological impact of the pediatric LAIV program on the incidence of GAS infections including scarlet fever and iGAS infection by comparing infection rates in the periods pre- and postintroduction of the program in LAIV pilot and nonpilot areas using data from the 2010/2011 to 2016/2017 influenza seasons in England.
METHODS
Data Sources
Data on invasive and noninvasive GAS infections were extracted from a live national laboratory reporting system, Second Generation Surveillance System (SGSS), managed by the UK Health Security Agency (UKHSA) for collation of laboratory notifications of infectious diseases across England, alongside other reportable infections [22]. Data on scarlet fever, based on clinical symptoms, were extracted from the UKHSA statutory notifications of infectious diseases (NOIDs) database, which contains statutory notifications submitted by diagnosing clinicians to local public health officials [23].
Data on the LAIV program pilot areas have been described previously, where an average of 6 pilot areas were selected by the national team to take part in the pilot program year after year [16–19].
Data Collection
Individual-level data on laboratory-confirmed GAS infections (all sample types) were extracted for the seasonal influenza surveillance period of each year (week 40 [October] to week 20 [May]) between 2010 and 2017. Preprogram seasons (before the introduction of the LAIV program) were defined as 2010/2011, 2011/2012 and 2012/2013; postprogram seasons were defined as 2013/2014, 2014/2015, 2015/2016, and 2016/2017 (Supplementary Figure 1).
Patient-level GAS records were assigned to pilot or nonpilot areas at the Local Authority (LA) level using residential postcodes supplied at the time of reporting [24].
A pilot area was defined as a discrete geographical LA in England, which participated in the full implementation of the rollout of the LAIV program among all primary school–age children (4 to 10 years) in their area in a given season (Supplementary Figure 1) [15–17]. Methods of delivery included via General Practices (GPs), schools, and/or pharmacies (Supplementary Figure 1).
A nonpilot area was defined as any LA not participating as a pilot area in the LAIV pilot program that followed the national incremental rollout of the LAIV program [16–19].
Data were categorized into age groups, with LAIV targeted age groups being 2 to 4 years (all areas) and 5 to 10 years (pilot areas only), to assess the direct effects of the program. Children aged 2 to 4 years were offered the LAIV vaccine in both pilot and nonpilot areas from the beginning of the program (2013/2014). Nontargeted age groups were categorized as <2 years, 11 to 16 years, 17 to 44 years, 45 to 64 years, and 65+ years to assess the indirect impact of the program in these groups.
Additionally, for the LAIV targeted age groups, data on laboratory-confirmed noninvasive GAS infections, scarlet fever, and iGAS (GAS detected from a normally sterile site) notifications were also analyzed.
Population denominators were derived from the Office for National Statistics’ yearly population estimates for each influenza season for each LA to generate denominators for pilot and nonpilot areas and by age group [25].
Statistical Analyses
Cumulative incidence rates were calculated as the number of GAS, scarlet fever, and iGAS infections detected in a defined age group and season divided by the total population within the defined age group and pilot or nonpilot area. Exact Poisson CIs were calculated.
Two analyses were performed. First, incidence rate ratios (IRR) for GAS infection (invasive and noninvasive), scarlet fever, or iGAS infection comparing pilot and nonpilot areas were estimated using Poisson regression within each season by age group.
Second, to assess whether the IRRs changed before and after program implementation, an indicator variable was created (0 for each of 2010/2011 to 2012/2013 and 1 for each of 2013/2014 to 2016/2017), and this was included in a negative binomial model along with pilot area as the interaction term. This gives a ratio of incidence rate ratios of rIRR = (postpilot incidence/prepilot incidence)/(post-nonpilot incidence/pre-nonpilot incidence), where a value <1 would indicate a potential impact of being in a pilot area on reducing rates. Negative binomial regression was used to allow for the fact that the incidence rate ratios varied within the pre- and postimplementation time periods.
Additional sensitivity analyses were carried out on targeted groups to determine the variability in the reporting of infections in each LA between pilot and nonpilot areas and pre- and postprogram periods. This was calculated by selecting all LAs within an area where at least 1 case was reported in every season and where the ratio of the largest total in a season over the lowest was <10—to remove any LAs where reporting variability was high—and where the total number of infections over all seasons was at least 50—to calculate the ratio of post- to preprogram introduction within each LA in a pilot or nonpilot area. The medians of these values were then compared using a Kruskal-Wallis test.
All analyses were computed in STATA, version 15.
RESULTS
LAIV Targeted Age Groups
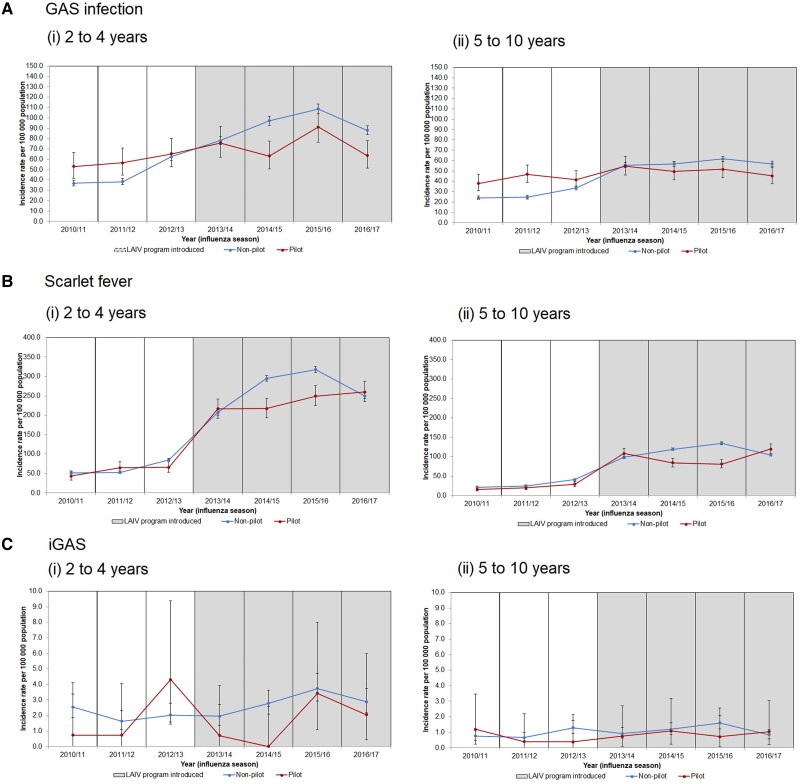
In the preprogram seasons, incidence rates of all laboratory-confirmed GAS infections in both target age groups (2 to 4 and 5 to 10 years) were consistently higher in pilot areas than in nonpilot areas, with IRRs of >1.0 observed within each season (Figure 1A, Table 1A). Incidence rates of scarlet fever and iGAS infection in both targeted age groups in pilot and nonpilot areas in the preprogram seasons varied and showed no significant differences, with the exception of the 2012/2013 season, where rates were significantly lower in pilot areas for both age groups for scarlet fever (Figure 1B and C, Table 1B).
Figure 1.
Incidence rates per 100 000 population (95% CI) of (A) GAS infection (invasive and noninvasive), (B) scarlet fever, and (C) invasive GAS infections in LAIV pilot and nonpilot areas by influenza seasons (2010/2011 to 2016/2017) for targeted age groups, England. Abbreviations: GAS, group A Streptococcus; LAIV, live attenuated influenza vaccine.
Table 1.
Counts, Crude Incidence Rates per 100 000 Population (95% CI), and Incidence Rate Ratios (95% CI) of (A) All GAS Infections (Invasive and Noninvasive), (B) Scarlet Fever, and (C) Invasive GAS Infections by LAIV Pilot and Nonpilot Areas and Influenza Seasons, 2010/2011 to 2016/2017 for Targeted Age Groups, 2 to 4 Years and 5 to 10 Years
| Season/Pilot Type | 2 to 4 Years | 5 to 10 Years | ||||
|---|---|---|---|---|---|---|
| Count | Crude Rate per 100 000 Population (95% CI) | Incidence Rate Ratio (95% CI) | Count | Crude Rate per 100 000 Population (95% CI) | Incidence Rate Ratio (95% CI) | |
| (A) GAS | ||||||
| 2010/2011 | ||||||
| Pilot | 72 | 53.0 (41.5–66.8) | 1.44 (1.13–1.84) | 96 | 38.0 (30.8–46.4) | 1.59 (1.29–1.96) |
| Nonpilot | 667 | 36.8 (34.1–39.7) | … | 784 | 23.9 (22.3–25.7) | … |
| 2011/2012 | ||||||
| Pilot | 78 | 56.7 (44.8–70.8) | 1.48 (1.17–1.87) | 119 | 46.7 (38.7–55.9) | 1.89 (1.56–2.30) |
| Nonpilot | 707 | 38.4 (35.6–41.3) | … | 819 | 24.7 (23–26.4) | … |
| 2012/2013 | ||||||
| Pilot | 91 | 65.3 (52.6–80.2) | 1.05 (0.85–1.29) | 108 | 41.6 (34.2–50.3) | 1.24 (1.01–1.50) |
| Nonpilot | 1170 | 62.5 (58.9–66.1) | … | 1144 | 33.7 (31.8–35.7) | … |
| 2013/2014 | ||||||
| Pilot | 107 | 75.9 (62.2–91.8) | 0.97 (0.80–1.18) | 146 | 54.7 (46.1–64.3) | 0.98 (0.83–1.16) |
| Nonpilot | 1481 | 78.1 (74.2–82.2) | … | 1952 | 55.7 (53.2–58.2) | … |
| 2014/2015 | ||||||
| Pilot | 91 | 63.1 (50.8–77.5) | 0.65 (0.53–0.80) | 136 | 49.5 (41.5–58.5) | 0.87 (0.73–1.04) |
| Nonpilot | 1885 | 97.2 (92.8–101.7) | … | 2043 | 56.7 (54.3–59.2) | … |
| 2015/2016 | ||||||
| Pilot | 133 | 91.1 (76.3–108.0) | 0.83 (0.70–1.00) | 145 | 51.7 (43.6–60.8) | 0.84 (0.71–0.99) |
| Nonpilot | 2124 | 108.7 (104.1–113.4) | … | 2282 | 61.7 (59.2–64.3) | … |
| (B) Scarlet Fever | ||||||
| 2016/2017 | ||||||
| Pilot | 93 | 63.7 (51.4–78.0) | 0.72 (0.59–0.89) | 130 | 45.2 (37.8–53.7) | 0.79 (0.67–0.95) |
| Nonpilot | 1713 | 88.1 (84.0–92.4) | … | 2158 | 56.9 (54.5–59.4) | … |
| 2010/2011 | ||||||
| Pilot | 59 | 43.5 (33.1–56.1) | 0.85 (0.65–1.10) | 40 | 15.8 (11.3–21.6) | 0.73 (0.53–1.01) |
| Nonpilot | 931 | 51.4 (48.2–54.8) | … | 710 | 21.7 (20.1–23.3) | … |
| 2011/2012 | ||||||
| Pilot | 89 | 64.7 (52.0–79.7) | 1.22 (0.98–1.52) | 50 | 19.7 (14.6–25.9) | 0.78 (0.59–1.04) |
| Nonpilot | 976 | 53.0 (49.7–56.4) | … | 833 | 25.1 (23.4–26.9) | … |
| 2012/2013 | ||||||
| Pilot | 92 | 66.0 (53.2–81.0) | 0.78 (0.63–0.96) | 76 | 29.3 (23.1–36.7) | 0.72 (0.57–0.90) |
| Nonpilot | 1594 | 85.1 (81.0–89.4) | … | 1390 | 40.9 (38.8–43.2) | … |
| 2013/2014 | ||||||
| Pilot | 305 | 216.5 (192.8–242.2) | 1.05 (0.93–1.18) | 290 | 108.6 (96.4–121.8) | 1.10 (0.98–1.24) |
| Nonpilot | 3922 | 206.92 (200.5–213.5) | … | 3447 | 98.3 (95.1–101.7) | … |
| 2014/2015 | ||||||
| Pilot | 313 | 217.1 (193.7–242.5) | 0.74 (0.66–0.82) | 232 | 84.4 (73.9–96.0) | 0.71 (0.62–0.81) |
| Nonpilot | 5722 | 295.0 (287.4–302.7) | … | 4297 | 119.3 (115.7–122.9) | … |
| 2015/2016 | ||||||
| Pilot | 364 | 249.3 (224.4–276.3) | 0.79 (0.71–0.87) | 227 | 80.9 (70.7–92.1) | 0.60 (0.53–0.69) |
| Nonpilot | 6206 | 317.5 (309.6–325.5) | … | 4980 | 134.7 (131.0–138.5) | … |
| 2016/2017 | ||||||
| Pilot | 380 | 260.2 (234.7–287.7) | 1.04 (0.94–1.16) | 345 | 119.9 (107.6–133.3) | 1.15 (1.03–1.28) |
| Nonpilot | 4859 | 249.9 (243.0–257.1) | … | 3966 | 104.6 (101.4–107.9) | … |
| (C) iGAS | ||||||
| 2010/2011 | ||||||
| Pilot | 1 | 0.7 (0.0–4.1) | 0.29 (0.04–2.10) | 3 | 1.2 (0.2–3.5) | 1.56 (0.47–5.16) |
| Nonpilot | 46 | 2.5 (1.9–3.4) | … | 25 | 0.8 (0.5–1.0) | … |
| 2011/2012 | ||||||
| Pilot | 1 | 0.7 (0.0–4.1) | 0.45 (0.06–3.28) | 1 | 0.4 (0.0–2.2) | 0.59 (0.08–4.40) |
| Nonpilot | 30 | 1.6 (1.1–2.3) | … | 22 | 0.7 (0.4–1.0) | … |
| 2012/2013 | ||||||
| Pilot | 6 | 4.3 (1.6–9.4) | 2.12 (0.90–5.02) | 1 | 0.4 (0–2.2) | 0.30 (0.04–2.16) |
| Nonpilot | 38 | 2.0 (1.4–2.8) | … | 44 | 1.3 (0.9–1.7) | … |
| 2013/2014 | ||||||
| Pilot | 1 | 0.7 (0.0–4.0) | 0.36 (0.05–2.65) | 2 | 0.7 (0.1–2.7) | 0.82 (0.20–3.42) |
| Nonpilot | 37 | 2.0 (1.4–2.7) | … | 32 | 0.9 (0.6–1.3) | … |
| 2014/2015 | ||||||
| Pilot | 0 | 0.0 (0.0–2.6) | — | 3 | 1.1 (0.2–3.2) | 0.91 (0.28–2.95) |
| Nonpilot | 54 | 2.8 (2.1–3.6) | … | 43 | 1.2 (0.9–1.6) | … |
| 2015/2016 | ||||||
| Pilot | 5 | 3.4 (1.1–8.0) | 0.92 (0.37–2.27) | 2 | 0.7 (0.1–2.6) | 0.45 (0.11–1.83) |
| Nonpilot | 73 | 3.7 (2.9–4.7) | … | 59 | 1.6 (1.2–2.1) | … |
| 2016/2017 | ||||||
| Pilot | 3 | 2.1 (0.4–6.0) | 0.71 (0.22–2.28) | 3 | 1.0 (0.2–3.1) | 1.24 (0.38–4.04) |
| Nonpilot | 56 | 2.9 (2.2–3.7) | … | 32 | 0.8 (0.6–1.2) | … |
Significant IRRs with P values <.05 are highlighted in bold.
Abbreviations: GAS, group A Streptococcus; iGAS, invasive group A Streptococcus; IRR, incidence rate ratio.
In the postprogram seasons, incidence rates of GAS infection, scarlet fever, and iGAS infection in the 2 to 4 years and 5 to 10 years groups were lower in pilot areas than in nonpilot areas, with the exception of the 2013/2014 and 2016/2017 seasons for scarlet fever, which saw slightly greater IRRs of 1.05 and 1.10 in the 2 to 4 years group and 1.04 and 1.15 in the 5 to 10 years group, respectively, and in the 2016/2017 season for iGAS with an IRR of 1.24 in the 5 to 10 years group (Figure 1A(i)–C(i), Table 1A–C).
Significant reductions in incidence rates of GAS infection were noted for 2- to 4-year-olds in 2 of 4 postprogram seasons, 2014/2015 and 2016/2017 (IRR, 0.65; 95% CI, 0.53–0.80; and IRR, 0.72; 95% CI, 0.52–0.89), as well as in the 2015/2016 and 2016/2017 seasons for the 5 to 10 years group (IRR, 0.84; 95% CI, 0.71–0.99; and IRR, 0.79; 95% CI, 0.67–0.95) (Table 1A). Significant reductions in incidence rates of scarlet fever in pilot areas were also noted among the targeted age groups, 2 to 4 years and 5 to 10, in postprogram seasons, 2014/2015 and 2015/2016 (2 to 4 years: IRR, 0.74; 95% CI, 0.66–0.82; and IRR, 0.79; 95% CI, 0.71–0.87; 5 to 10 years: IRR, 0.71; 95% CI, 0.62–0.81; and IRR, 0.60; 95% CI, 0.53–0.69) (Table 1C).
Comparing the changes pre- with postprogram in pilot and nonpilot areas for GAS using negative binomial regression showed that for 2- to 4-year-olds the 1.26-fold increase in pilot areas was lower than the 2.03-fold increase in nonpilot areas (rIRR, 0.62; 95% CI, 0.43–0.90; P = .011). In 5- to 10-year-olds, the 1.19-fold increase in pilot areas was lower than the 2.10-fold increase in nonpilot areas (rIRR, 0.57; 95% CI, 0.45–0.71; P < .001). For iGAS infection, the fold change was also lower in pilot areas for 2- to 4-year-olds, but not reaching statistical significance (rIRR, 0.58; 95% CI, 0.21–1.65; P = .31). For iGAS infection in 5- to 10-year-olds and for scarlet fever in both age groups, changes were similar in pilot and nonpilot areas (rIRR, 1.1; 95% CI, 0.34–3.60; rIRR, 0.96; 95% CI, 0.66–1.39; rIRR, 1.16; 95% CI, 0.75–1.81; for iGAS age 5 to 10, scarlet fever age 2 to 4, and scarlet fever age 5 to 10, respectively).
The sensitivity analyses showed the median change to be a 1.26-fold increase in pilot areas where 6 pilot areas could be included compared with a median change of 1.53 increase in nonpilot areas where 34 nonpilot areas could be included.
The ratio of these median increases between the pilot and nonpilot areas (similar to an rIRR) was 0.83, representing a 17% lower increase in the pilot areas. Although this is a smaller effect than seen when using all pilot and nonpilot areas (as described in the previous paragraph), it is still significant (Kruskal-Wallis test P = .049).
Nontargeted Age Groups
Differences in incidence rates of GAS between pilot and nonpilot areas in the preprogram seasons among the nontargeted groups were minimal and varied (Figure 2; Supplementary Table 1).
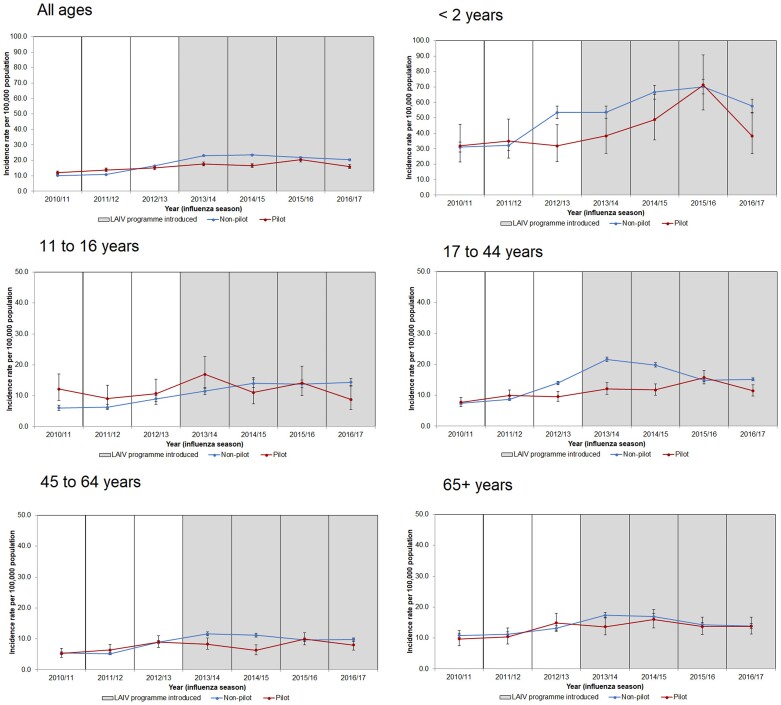
Figure 2.
Incidence rates per 100 000 population (95% CI) of GAS infections (invasive and noninvasive) by LAIV pilot and nonpilot areas and influenza seasons (2010/2011 to 2016/2017) for nontargeted age groups, England. Abbreviations: GAS, group A Streptococcus; LAIV, live attenuated influenza vaccine.
All nontargeted age groups, with the exception of all ages and 65+ years, saw greater incidence rates of GAS infection among pilot areas than in at least 1 of the postprogram seasons and IRRs >1.0 (Figure 2; Supplementary Table 1). The all-age and 65+ years age groups observed lower albeit nonsignificant IRRs for GAS in pilot areas compared with nonpilot areas within all postprogram seasons (Supplementary Table 1).
Comparing the changes pre- with postprogram in pilot and nonpilot areas for GAS using negative binomial regression showed no significant differences except for age 11 to 16, where the 1.20-fold increase in pilot areas was lower than the 1.89-fold increase in nonpilot areas (rIRR, 0.63; 95% CI, 0.43–0.92; P = .018). In the other ages, increases were also lower in the pilot areas but were not significant (range of rIRRs, 0.73–0.94).
DISCUSSION
Our findings suggest that there were relative reductions in the incidence rates of GAS infection for 2- to 4-, 5- to 10-, and 11- to 16-year-olds in areas where the LAIV program was piloted for these age groups compared with areas that introduced the program gradually. Reductions were also observed in incidence rates of GAS infection between pilot and nonpilot areas within seasons in at least 2 of the postprogram influenza seasons in all age groups, with significant reductions noted in the 2 to 4 years and <2 years groups. Reductions were also noted when assessing scarlet fever and iGAS infection rates within seasons in the targeted age groups (2 to 10 years), but significant reductions were noted only for scarlet fever in both 2 to 4 and 5 to 10 years. These findings are consistent with a positive impact of the LAIV program on reducing GAS infections.
Many prior studies have identified influenza as a predisposing secondary bacterial infection, particularly when influenza activity is high [2–6]. A review study summarized that increases in noninvasive GAS and iGAS infections were noted in or after the 2009 influenza A(H1N1) pdm09 pandemic, with a further study in England finding that high influenza activity in the 2010/2011 season contributed to an increased risk of concurrent invasive bacterial infections [2, 6]. Prior findings from LAIV impact studies in England have shown a reduction in influenza infections among LAIV targeted age groups when comparing rates in pilot and nonpilot areas in England [16–19]. Our findings suggest that the reductions in influenza among children also contribute to a reduction in secondary bacterial infections.
The reduction in GAS infections was most apparent in the 5 to 10 years age group; this is also the age group where the highest burden of GAS infections is mainly observed. This is expected given that it is these age groups where vaccine coverage differed most between the pilot and nonpilot areas (pilot areas having offered vaccination to all these age cohorts, nonpilot areas having offered vaccination sequentially each year to an additional age group) [16, 18, 27]. While both pilot and nonpilot areas offered vaccination to 2- to 4-year olds, the finding of significant reductions in the rates of GAS in the 2 to 4 years group post–introduction of the LAIV program may be due to the higher and accelerated LAIV vaccine uptake in these age groups in pilot areas in comparison with the uptake in nonpilot areas, as well as the indirect effect of vaccinating 5 to 10 year olds [27]. Both findings provide encouraging evidence that an increase in the LAIV vaccine uptake, particularly through an accelerated pilot program, may reduce the incidence of GAS infections.
Indirect impacts were also noted among nontargeted age groups, albeit not reaching statistical significance (other than in the age group most proximal to the pilot intervention ages), similar to previous impact studies [16–18]. The observation of higher infections in the 2015/2016 season coincides with an increase in iGAS notifications in England that year likely caused by the dominance of a new emm1 S pyogenes lineage as well as the predominant circulation of influenza A(H1N1)pdm09, which is known to mainly affect children [7, 8, 28]. However, moderate influenza vaccine effectiveness of almost 60% was observed for LAIV that season among the 2- to 17-year-olds, and therefore the increase in iGAS notifications that year may not only be attributable to influenza-related factors [26, 27].
There are key strengths to our study, including the unique opportunity to compare the impact of the LAIV program rollout due to its pilot program and the use of national infection data. There are, however, a number of limitations to this study. As a population-level ecological study, our results should be interpreted with caution as they cannot be used to infer a causal association to the LAIV vaccine only as there may be other contributing factors such as changes in laboratory testing and surveillance reporting over time. We have mitigated this limitation by using both historical (pre– and post–LAIV program data) and geographical controls (pilot and nonpilot areas). Our findings of significant relative incidence reductions in the LAIV targeted age groups when comparing pilot with nonpilot areas were seen in both the main analysis and in the sensitivity analysis, where there was restriction to areas where reporting was assessed to be most complete, which further mitigates these limitations. Second, it is important to note that most GAS infections are not laboratory confirmed, and there may be regional differences in the acquisition of swabs and reporting over time; this and other “surveillance artefacts” could vary between pilot and nonpilot areas over time and could contribute to apparent differences in rates of GAS over time. Third, it is important to note that some LAIV pilot areas targeted vaccination among secondary school (children aged 11 to 16 years) children in the 2014/2015 influenza season, and this may have been reflected in our results. Lastly, both the national rollout and the pilot program targeted 2- to 4-year-olds from the commencement of the program, with pilot areas achieving higher LAIV vaccine uptake rates [27].
Our study suggests that vaccinating children with LAIV may reduce the incidence of GAS infections, including potentially life-threatening iGAS infections. In the context of the increasing incidence of GAS observed in England during the current 2022/2023 season and the potential for similar increases elsewhere following the COVID-19 pandemic and easing of associated social distancing measures, our findings support maximizing childhood influenza vaccine uptake.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Richard G. Pebody for the original concept of this study and initiating this work.
Author contributions. M.S., F.W., and N.A. conceptualized the study and its design. Data collection and analysis were prepared by M.S., F.W., and N.A. The first draft of the manuscript was written by M.S. All authors commented, read, and approved the final manuscript.
Data availability . All data produced in the study are contained in the manuscript. Data cannot be made publicly available for ethical and legal reasons; that is, public availability would compromise patient confidentiality as data tables list single counts of individuals rather than aggregated data.
Patient consent. This study was a public health evaluation with data processed for the monitoring of the LAIV immunization program. UKHSA has legal permission under Regulation 3 of the Health Service (Control of Patient Information) Regulations 2002 to process confidential patient information.
Contributor Information
Mary A Sinnathamby, UK Health Security Agency, London, UK.
Fiona Warburton, UK Health Security Agency, London, UK.
Rebecca Guy, UK Health Security Agency, London, UK.
Nick Andrews, UK Health Security Agency, London, UK.
Theresa Lamagni, UK Health Security Agency, London, UK.
Conall Watson, UK Health Security Agency, London, UK.
Jamie Lopez Bernal, UK Health Security Agency, London, UK.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. UK Health Security Agency . Group A streptococcal infections: guidance and data. Available at: https://www.gov.uk/government/collections/group-a-streptococcal-infections-guidance-and-data. Accessed December 10, 2022.
- 2. Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol 2017; 8:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph C, Togawa Y, Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respir Viruses 2013; 7(Suppl 2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheykhsaran E, Hemmat N, Baghi HB. Influenza A virus and related secondary bacterial infections. Rev Med Microbiol 2019; 30:205–11. [Google Scholar]
- 5. Herrera A, Huber V, Chaussee M. The association between invasive group A streptococcal diseases and viral respiratory tract infections. Front Microbiol 2016; 7:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zakikhany K, Degail MA, Lamagni T, et al. . Increase in invasive Streptococcus pyogenes and Streptococcus pneumoniae infections in England, December 2010 to January 2011. Euro Surveill 2011; 16:19785. [PubMed] [Google Scholar]
- 7. Lamagni T, Guy R, Chand M, et al. . Resurgence of scarlet fever in England, 2014-16: a population-based surveillance study. Lancet Infect Dis 2018; 18:180–7. [DOI] [PubMed] [Google Scholar]
- 8. Public Health England . Group A streptococcal infections: activity during the 2015 to 2016 season. Available at: https://www.gov.uk/government/publications/group-a-streptococcal-infections-activity-during-the-2015-to-2016-season. Accessed December 10, 2022.
- 9. Park DW, Kim SH, Park JW, et al. . Incidence and characteristics of scarlet fever, South Korea, 2008-2015. Emerg Infec Dis 2017; 23:658–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Chan TC, Yap LW, et al. . Resurgence of scarlet fever in China: a 13-year population-based surveillance study. Lancet Infect Dis 2018; 18:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. UK Health Security Agency . Group A streptococcal infections: first update on seasonal activity in England, 2022 to 2023. Available at: https://www.gov.uk/government/publications/group-a-streptococcal-infections-activity-during-the-2022-to-2023-season/group-a-streptococcal-infections-first-update-on-seasonal-activity-in-england-2022-to-2023#:∼:text=A%20total%20of%206%2C601%20notifications,in%20the%20previous%205%20years. Accessed December 10, 2022.
- 12. Guy R, Henderson KL, Coelho J, et al. . Increase in invasive group A streptococcal infection notifications, England, 2022. Euro Surveill 2023; 28:2200942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baguelin M, Flasche S, Camacho A, Demiris N, Miller E, Edmunds WJ. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med 2013; 10:e1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Public Health England . Surveillance of influenza and other respiratory viruses, including novel respiratory viruses, in the United Kingdom: winter 2012/13. Available at: https://www.gov.uk/government/statistics/annual-flu-reports. Accessed December 10, 2022.
- 15. Public Health England . Seasonal influenza vaccine uptake for children of primary school age: winter 2016/17. Available at: https://www.gov.uk/government/statistics/seasonal-flu-vaccine-uptake-in-children-of-primary-school-age-winter-season-2016-to-2017. Accessed December 10, 2022.
- 16. Pebody RG, Green HK, Andrews N, et al. . Uptake and impact of a new live attenuated influenza vaccine programme in England: early results of a pilot in primary school-age children, 2013/14 influenza season. Euro Surveill 2014; 19:20823. [DOI] [PubMed] [Google Scholar]
- 17. Pebody RG, Green HK, Andrews N, et al. . Uptake and impact of vaccinating school age children against influenza during a season with circulation of drifted influenza A and B strains, England, 2014/15. Euro Surveill 2015; 20. [DOI] [PubMed] [Google Scholar]
- 18. Pebody RG, Sinnathamby MA, Warbuton F, et al. . Uptake and impact of vaccinating primary school-age children against influenza: experiences of a live attenuated influenza vaccine programme, England, 2015/16. Euro Surveill 2018; 23:1700496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sinnathamby MA, Warburton F, Andrews, N, et al. . Uptake and impact of vaccinating primary school children against influenza: experiences in the fourth season of the live attenuated influenza vaccination programme, England, 2016/2017. Influenza Other Respi Viruses 2022; 16:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gallagher N, Jessop L, Sartaj M, Johnston J. Impact of live attenuated influenza vaccination programme for health children in Northern Ireland: a comparison of seven influenza seasons, 2010/11-2016/17. Vaccine 2017; 36:521–6. [DOI] [PubMed] [Google Scholar]
- 21. Sinnathamby MA, Warburton F, Reynolds AJ, et al. . An intercountry comparison of the impact of the paediatric live attenuated influenza vaccine (LAIV) programme across the UK and the republic of Ireland (ROI), 2010 to 2017. Influenza Other Respir Viruses 2023; 17:e13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. UK Health Security Agency . Laboratory reporting to UKHSA: a guide for diagnostic laboratories. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1108438/UKHSA_Laboratory_reporting_guidelines__1_.pdf. Accessed December 10, 2022.
- 23. Public Health England . Guidelines for the public health management of scarlet fever outbreaks in schools, nurseries and other childcare settings. Available at: https://www.gov.uk/government/publications/scarlet-fever-managing-outbreaks-in-schools-and-nurseries. Accessed December 10, 2022.
- 24. Office for National Statistics (ONS) . Population profiles for local authorities in England. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/populationprofilesforlocalauthoritiesinengland/2020-12-14. Accessed December 10, 2022.
- 25. Office for National Statistics (ONS) . Population estimates. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates. Accessed December 10, 2022.
- 26. Pebody RG, Warburton F, Ellis J, et al. . Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Euro Surveill 2016; 21:30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Public Health England (PHE) . Surveillance of influenza and other respiratory viruses in the United Kingdom: winter 2015 to 2016. London. Available at: https://www.gov.uk/government/statistics/annual-flu-reports. Accessed December 10, 2022.
- 28. Lynskey NN, Jauneikaite E, Li Kwong H, et al. . Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet 2019; 19:1209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.