Abstract
Persistent symptomatic coronavirus disease 2019 (COVID-19) is a distinct clinical entity among patients with hematologic cancer and/or profound immunosuppression. The optimal medical management is unknown. We describe 2 patients who had symptomatic COVID-19 for almost 6 months and were successfully treated in the ambulatory setting with extended courses of nirmatrelvir-ritonavir.
Keywords: extended nirmatrelvir-ritonavir, hematologic cancer, persistent COVID-19
Persistent COVID-19 infection is a challenging management dilemma among immunosuppressed individuals, particularly those with B-cell dysfunction. We describe 2 patients with symptomatic COVID-19 for almost 6 months who were successfully treated in the ambulatory setting with extended courses of nirmatrelvir/ritonavir.
Patients with hematologic cancer, particularly those with B-cell dysfunction or receiving B-cell targeted therapies [1–3], are at increased risk of persistent symptomatic coronavirus disease 19 (COVID-19). This is a distinct clinical entity among immunocompromised patients and is characterized by a protracted illness with progressive or waxing and waning respiratory symptoms, radiographic findings, and persistent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA detection well beyond 30 days [4]. Persistent symptomatic COVID-19 can adversely affect cancer-related outcomes by necessitating delays or alterations in treatment. Extremely rapid intrahost viral evolution of SARS-CoV-2 has been described during persistent infection [5–7], and there is growing concern that immunosuppressed individuals may serve as key reservoirs of potentially globally significant viral variants [8].
The optimal management of patients with persistent symptomatic COVID-19 is unknown. Here, we describe our experiences with extended-duration nirmatrelvir-ritonavir obtained as an emergency investigational new drug application (eIND) for 2 ambulatory patients with persistent symptomatic COVID-19 who were ineligible for treatment under existing emergency use authorization.
CASE REPORTS
Case 1
A 34-year-old man with Philadelphia-negative chromosome B-cell acute lymphoblastic leukemia in remission on vincristine and rituximab maintenance chemotherapy, who had previously received 3 doses of Pfizer messenger RNA (mRNA) vaccine, had SARS-CoV-2 infection diagnosed in February 2022. He received intravenous sotrovimab on day 1 after diagnosis but had no improvement in symptoms. On day 19, he presented to the emergency department with shortness of breath, cough, and posttussive emesis. His oxygenation was normal, and chest radiography showed basilar atelectasis, for which he was prescribed azithromycin and discharged to home. His symptoms persisted, along with positive polymerase chain reaction (PCR) results for SARS-CoV-2 (Figure 1A).
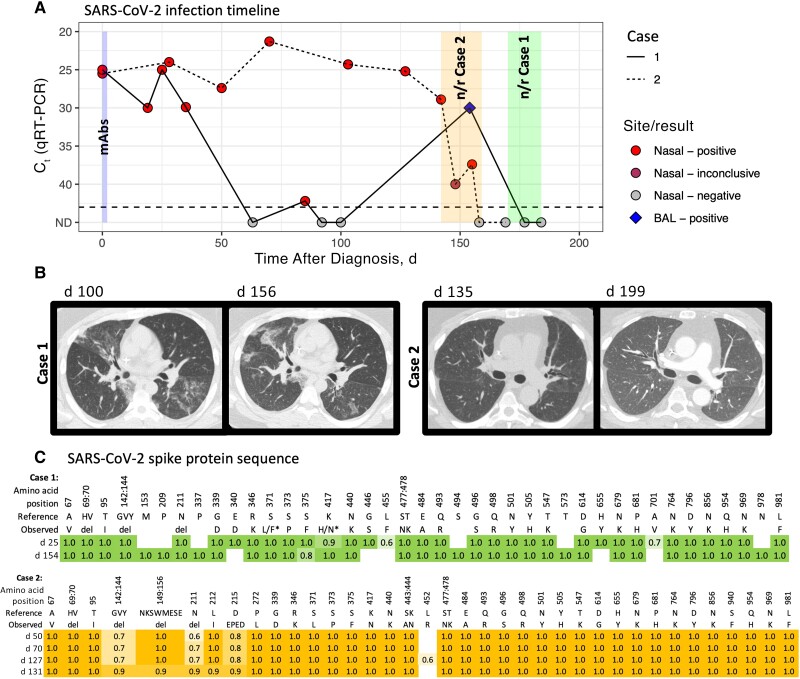
Figure 1.
A, Timeline of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection with quantitative reverse-transcription polymerase chain reaction (qRT-PCR) from respiratory site samples (nasal swab and bronchoalveolar lavage [BAL]) in cases 1 (solid line) and 2 (dotted line). Tests where no virus was detected are indicated in gray (not detected [ND]). qRT-PCR assays were performed in a clinical laboratory, which uses multiple testing platforms, including Cepheid, Hologic, Cobas, Abbott, and Diasorin. Cycle threshold (Ct) values are presented, with lower values indicating higher viral loads, and higher values, lower viral loads. Nirmatrelvir-ritonavir (n/r) treatment was started on day 170 in case 1 (continued for 15 days) and on day 142 in case 2 (continued for 18 days). Nasal swab samples are depicted by a circle, and BAL samples by a diamond. Monoclonal antibodies (mAbs) were administered on day 1 or 2 of symptoms, sotrovimab in case 1 and bebtelovimab in case 2. B, Chest computed tomographic scans in cases 1 (left) and 2 (right) during illness and treatment. C, SARS-CoV-2 spike protein amino acid sequences over the course of illness by day after symptom onset. Consensus sequence (>50% allelic frequency) changes from reference sequence NC_045512.2 (Wuhan Hu 1) are shown. Omicron variant BA.1.1 was detected in both cases. The asterisks in the sequence for case 1 indicate 2 positions where amino acid consensus differed between the 2 samples (L/F and H/N, where the day 25 sequence is indicated first). Abbreviation: del, deletion.
On day 85, the patient presented to the emergency department with cough affecting his ability to work. He had persistent viral detection, and chest radiography showed patchy bibasilar opacities. He was sent home on antibiotics. On days 92 and 100, PCR tests of nasal swab samples had negative results, but chest computed tomography (CT) showed new patchy bilateral ground-glass opacification, for which the patient received another course of antibiotics. His symptoms persisted, and repeated chest CT on day 156 demonstrated some areas of improvement but multiple new ground-glass opacities (Figure 1B).
Bronchoscopy was performed to distinguish between viral lower respiratory tract infection organizing pneumonia, or another contributing condition, and SARS-CoV-2 was detected in a bronchoalveolar lavage fluid sample with PCR, with a cycle threshold of 30, despite negative nasal swab sample. No other pathogens were identified. Sequence analysis from several samples confirmed BA.1.1 lineage with mutations associated with sotrovimab resistance [9] (Figure 1C and Supplementary material) The patient started treatment with nirmatrelvir-ritonavir as an eIND on day 170. On day 6 of therapy, his cough was improving, though still present, and the treatment was continued. After 15 days his cough was nearly resolved, he had returned to full activity, and therapy was discontinued. PCR results with nasal swab samples remained negative. The patient reported no adverse drug effects, and 1 month after completing treatment, he remained asymptomatic.
Case 2
A 55-year-old man with Philadelphia-negative B-cell acute lymphoblastic leukemia after hematopoietic cell transplantation 16 months earlier, with hypogammaglobulinemia but off immunosuppression, had COVID-19 diagnosed in June 2022. After transplantation, the patient had received 2 doses of Moderna mRNA vaccine. He was treated with bebtelovimab on day 1 after COVID-19 diagnosis but reported persistent cough, postnasal drip, and fatigue. Over the ensuing months, he continued to experience a disruptive and worsening cough. A chest radiograph obtained on day 88 was normal, but chest CT on day 135 (Figure 1B) demonstrated subtle scattered ground-glass opacities in the lingula and posterior lower lobes. SARS CoV-2 continued to be detected in nasal swab samples (Figure 1A). Sequencing performed on available isolates from days 50, 70, and 127 revealed Omicron variant BA.1.1 (Figure 1C and Supplementary material) with the K444N mutation, associated with bebtelovimab resistance [10].
Because of the patient’s progressive symptoms with continued PCR detection of SARS-CoV-2, an eIND was obtained to administer nirmatrelvir-ritonavir on day 142. On day 7 of therapy, the patient reported a slight improvement in his cough. By day 14, he reported noticeable improvement, with inconclusive detection of SARS CoV-2 with nasal swab sampling. By day 18, his symptoms were much improved, the SARS CoV-2 PCR result was negative, and nirmatrelvir-ritonavir was discontinued. Results of SARS CoV-2 PCR performed 11 days after cessation of therapy remained negative.
The patient experienced mild transaminitis on day 14 of therapy (aspartate aminotransferase, 50 U/L; alanine aminotransferase, 79 U/L), which resolved after completion of therapy. Owing to a history of hypothyroidism with levothyroxine, a baseline thyroid-stimulating hormone (TSH) level was measured, at 4.7 IU/mL (reference range, 0.4–5 IU/mL). Four days after the end of therapy, the TSH level was still elevated, at 7.0 IU/mL, but 1 week later it had normalized.
One month after viral clearance, the patient presented with new fever, nausea, vomiting, and diarrhea. SARS CoV-2 virus was detected by PCR testing of a nasal swab sample, with a cycle threshold of 40.7. He received a 5-day course of nirmatrelvir-ritonavir, with swift resolution of symptoms. The chest CT appearance was similar to earlier findings (Figure 1B). It was not clear whether this detection reflected recurrence, a false-positive result, as suggested by the low-level viral detection, or a new COVID-19 exposure.
For both cases, written patient consent was obtained for publication, and all information presented was anonymized as much as possible. Data collection was approved by the Fred Hutchinson Cancer Center Institutional Review Board.
DISCUSSION
We present 2 patients who had symptomatic COVID-19 for almost 6 months despite treatment at diagnosis with monoclonal antibodies recommended for viral strains circulating at the time and in whom symptom resolution and viral clearance was ultimately achieved with extended-duration nirmatrelvir-ritonavir alone. These cases illustrate the unique management challenges of persistent symptomatic COVID-19 among immunocompromised patients and the need for further clinical trials. Our group and others have previously described patients with persistent, severe lower respiratory tract COVID-19 who received multiple different therapies over the course of illness but ultimately had a successful response when treated with an extended course of nirmatrelvir-ritonavir with remdesivir [11, 12]. The cases reported here illustrate that persistent COVID-19 may also present more indolently, without precipitous escalation in disease severity necessitating hospitalization and in the absence of detectable virus in nasal swab samples, with possible discordant results between upper and lower respiratory tract specimens [13]. Despite the absence of life-threatening illness, both patients nonetheless required intervention owing to prolonged symptoms severely affecting their quality of life.
Profoundly immunosuppressed patients, particularly those with humoral dysfunction and B-cell dyscrasias, as noted in both of our patients, are at risk of persistent symptomatic COVID-19 [1]. In a study of 382 patients with hematologic cancer, chronic lymphopenia (absolute lymphocyte count, <500/μL), receipt of anti-CD20 therapy, and hematopoietic cell transplantation or cellular therapy within 1 year were independent predictors of prolonged SARS-CoV-2 detection [3]. In another study, receipt of anti-CD20 therapy within 1 year, age >70 years, and relapsed or refractory lymphoma status were identified as predictors of persistent COVID-19 requiring prolonged hospitalization (for >30 days) [2].
Treatment guidelines currently recommend a 5-day course of nirmatrelvir-ritonavir as first-line therapy for acute COVID-19 and previously recommended a single infusion of monoclonal antibody when active against circulating variants [14, 15]. Our 2 patients were initially treated with a single dose of monoclonal antibody; neither had previously received tixagevimab-cilgavimab prophylaxis. It is unknown whether “upfront” treatment with a direct-acting antiviral at the time of diagnosis or combination therapy would have reduced the likelihood of persistent symptomatic COVID-19. Furthermore, it is unknown whether a standard 5-day treatment course of nirmatrelvir-ritonavir is sufficient to promote viral clearance in the context of profound immunosuppression. A trial to explore duration of initial treatment with nirmatrelvir-ritonavir among immunocompromised individuals is currently in progress [16].
Data are lacking to guide the optimal management of patients with persistent symptomatic COVID-19. There is a single report of a successful outcome in a patient treated with an extended 30-day course of remdesivir [17], but others have used repeated infusions of remdesivir with relapse of symptoms and SARS-CoV-2 detection following cessation of therapy and unfavorable outcomes [18, 19]. One group has reported successful clinical and virologic response to 5 days of nirmatrelvir-ritonavir administered several months after initial diagnosis among patients with persistent or relapsed COVID-19 [20], but our experience with these 2 patients and reports by others [21] suggest that durations of 14–20 days may be needed to halt viral replication and promote symptom resolution. Although a clinical trial is needed to directly address this question, we hypothesize that sustained viral clearance may be achieved in patients with an inadequate host immune response only through an extended course of antiviral therapy.
Extended-duration nirmatrelvir-ritonavir was well tolerated in both patients, who had laboratory monitoring and telehealth visits on a weekly basis over a 1-month period. One of our patients had transient transaminitis and elevated TSH levels, which resolved with cessation of therapy, but did not observe any serious adverse effects. Close follow-up is necessary when considering the use of extended-durations nirmatrelvir-ritonavir treatment, as its safety profile of has not been systematically evaluated.
Persistent symptomatic COVID-19 has thus far not been considered in clinical treatment trials. Nirmatrelvir-ritonavir beyond the acute treatment window for an extended duration, along with appropriate clinical and laboratory monitoring, should be considered a therapeutic option for immunocompromised individuals with persistent symptomatic COVID-19. Defining the safety and efficacy of this approach in management of persistent symptomatic COVID-19 should be a priority, given the significant impact on immunocompromised patients as well as potential public health implications.
Supplementary Material
Acknowledgments
We thank Jared Castor from UW Virology for his assistance with providing SARS-CoV-2 laboratory result and sequencing data.
Financial support. This work was supported by the National Institutes of Health/National Cancer Institute (NIH/NCI) Cancer Center Support Grant P30 CA15704.
Contributor Information
Catherine Liu, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA; Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Leah H Yoke, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA.
Pooja Bhattacharyya, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA.
Ryan D Cassaday, Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Hematology, University of Washington, Seattle, Washington, USA.
Guang-Shing Cheng, Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Pulmonary, Critical Care and Sleep Medicine, University of Washington, Seattle, Washington, USA.
Zahra Kassamali Escobar, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; School of Pharmacy, University of Washington, Seattle, Washington, USA.
Cristina Ghiuzeli, Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Hematology, University of Washington, Seattle, Washington, USA.
Denise J McCulloch, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA; Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Steven A Pergam, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA; Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Pavitra Roychoudhury, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Laboratory Medicine, University of Washington, Seattle, Washington, USA.
Frank Tverdek, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; School of Pharmacy, University of Washington, Seattle, Washington, USA.
Joshua T Schiffer, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA; Clinical Research Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Emily S Ford, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Laracy JC, Kamboj M, Vardhana SA. Long and persistent COVID-19 in patients with hematologic malignancies: from bench to bedside. Curr Opin Infect Dis 2022; 35:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duléry R, Lamure S, Delord M, et al. Prolonged in-hospital stay and higher mortality after COVID-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol 2021; 96:934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee CY, Shah MK, Hoyos D, et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. Cancer Discov 2022; 12:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaguza C, Hahn AM, Petrone ME, et al. Accelerated SARS-CoV-2 intrahost evolution leading to distinct genotypes during chronic infection. Cell Rep Med 2023; 4:100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lynch M, Macori G, Fanning S, et al. Genomic evolution of SARS-CoV-2 virus in immunocompromised patient, Ireland. Emerg Infect Dis 2021; 27:2499–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kemp SA, Collier DA, Datir RP, et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 2021; 592:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nussenblatt V, Roder AE, Das S, et al. Yearlong COVID-19 infection reveals within-host evolution of SARS-CoV-2 in a patient with B-cell depletion. J Infect Dis 2022; 225:1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 2021; 385:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. [. Accessed 19 May 2023.]. https://www.fda.gov/media/149534/download Fact sheet for healthcare providers: emergency use authorization for sotrovimab. 2021. Available at:
- 10.Food and Drug Administration. [. Accessed 19 May 2023.]. https://www.fda.gov/media/156152/download Fact sheet for healthcare providers: emergency use authorization for bebtelovimab. 2022. Available at:
- 11. Ford ES, Simmons W, Karmarkar EN, et al. Successful treatment of prolonged, severe coronavirus disease 2019 lower respiratory tract disease in a B cell acute lymphoblastic leukemia patient with an extended course of remdesivir and nirmatrelvir/ritonavir. Clin Infect Dis 2023; 76:926–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trottier CA, Wong B, Kohli R, et al. Dual antiviral therapy for persistent coronavirus disease 2019 and associated organizing pneumonia in an immunocompromised host. Clin Infect Dis 2023; 76:923–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao CA, Cuttica MJ, Malsin ES, et al. Comparing nasopharyngeal and BAL SARS-CoV-2 assays in respiratory failure. Am J Respir Crit Care Med 2021; 203:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Infectious Diseases Society of America . IDSA guidelines on the treatment and management of patients with COVID-19. 2021. Available at:https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 7 March 2023.
- 15.National Institutes of Health. [. Accessed 7 March 2023.]. https://www.covid19treatmentguidelines.nih.gov/ Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: [PubMed]
- 16. A study to learn about the study medicines (nirmatrelvir plus ritonavir) in people aged 12 years or older with COVID-19 and a compromised immune system. Available at: https://clinicaltrials.gov/ct2/show/NCT05438602. Accessed 7 March 2023.
- 17. Martinez MA, Chen TY, Choi H, et al. Extended remdesivir infusion for persistent coronavirus disease 2019 infection. Open Forum Infect Dis 2022; 9:ofac382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020; 383:2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helleberg M, Niemann CU, Moestrup KS, et al. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis 2020; 222:1103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graziani L, Gori L, Manciulli T, et al. Successful use of nirmatrelvir/ritonavir in immunocompromised patients with persistent and/or relapsing COVID-19. J Antimicrob Chemother 2023; 78:555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breeden M, Aitken SL, Baang JH, et al. Successful treatment of prolonged severe acute respiratory syndrome coronavirus 2 infection in patients with immunodeficiency with extended nirmatrelvir/ritonavir: case series. Open Forum Infect Dis 2023; 10:ofad189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.