Abstract
Background
The Global Influenza Hospital Surveillance Network (GIHSN) was established in 2012 to conduct coordinated worldwide influenza surveillance. In this study, we describe underlying comorbidities, symptoms, and outcomes in patients hospitalized with influenza.
Methods
Between November 2018 and October 2019, GIHSN included 19 sites in 18 countries using a standardized surveillance protocol. Influenza infection was laboratory-confirmed with reverse-transcription polymerase chain reaction. A multivariate logistic regression model was utilized to analyze the extent to which various risk factors predict severe outcomes.
Results
Of 16 022 enrolled patients, 21.9% had laboratory-confirmed influenza; 49.2% of influenza cases were A/H1N1pdm09. Fever and cough were the most common symptoms, although they decreased with age (P < .001). Shortness of breath was uncommon among those <50 years but increased with age (P < .001). Middle and older age and history of underlying diabetes or chronic obstructive pulmonary disease were associated with increased odds of death and intensive care unit (ICU) admission, and male sex and influenza vaccination were associated with lower odds. The ICU admissions and mortality occurred across the age spectrum.
Conclusions
Both virus and host factors contributed to influenza burden. We identified age differences in comorbidities, presenting symptoms, and adverse clinical outcomes among those hospitalized with influenza and benefit from influenza vaccination in protecting against adverse clinical outcomes. The GIHSN provides an ongoing platform for global understanding of hospitalized influenza illness.
Keywords: age, epidemiology, hospitalization, influenza, mortality
Influenza is an important global cause of morbidity and mortality. Each year, across the globe, there are an estimated 1 billion cases of influenza, 3–5 million of which are severe cases and 290 000–650 000 lead to influenza-related deaths [1, 2]. Nevertheless, the annual impact of influenza may vary depending on the virus, host, and contextual factors, including the virulence of circulating strains, degree of match achieved between vaccine and circulating strains, vaccine coverage, and pre-existing population immunity [3]. Prevention and control of seasonal influenza is a global priority because influenza represents a substantial burden on people's health worldwide.
Although globalization has increased the viral spread between countries and regions, it also offers opportunities for collaborative efforts to combat influenza's far-reaching health and social effects. In addition to the vital work of individual jurisdictions in understanding their influenza epidemiology, it is essential to consider a global perspective. Seasonality differs geographically, with the Northern and Southern Hemispheres generally experiencing a winter influenza season, whereas intertropical zones may experience prolonged or even year-round transmission [4]. It is notable that some countries such as China, Brazil, and India have subregional variations in influenza circulation, mixing areas with northern and southern hemisphere circulation patterns [5].
The Global Influenza Hospital Surveillance Network (GIHSN) was established in 2012 to provide a coordinated influenza surveillance platform. Since its inception, the GIHSN has expanded to provide an evolving picture of the experience and burden of severe influenza leading to acute care hospitalization worldwide and has aimed to become a platform to assess the effectiveness of influenza vaccines. In this study, we present results from the 2018/2019 influenza season, focusing on age differences in underlying comorbidities, presenting symptoms, and adverse clinical outcomes.
METHODS
The GIHSN's coordinated active influenza surveillance has grown steadily since its establishment [3, 6–9]. During the 2018/2019 influenza season, 19 international sites representing 18 countries participated in GIHSN. These included 11 Northern Hemisphere sites in 10 countries (Canada, China, France, Lebanon, Mexico, Romania, Serbia, Spain, Romania, Russia-Moscow, and Russia-St. Petersburg), 4 intertropical sites (Columbia, India, Ivory Coast, and Kenya) and 4 Southern Hemisphere sites (Argentina, Brazil, Peru, and South Africa).
Enrollment
Enrollment was based on an active surveillance protocol with case definitions and data collection standardized across all participating sites. Patients were recruited according to 2 protocols: one for those <5 years of age and one for ages ≥5 years. Inclusion criteria for patients aged ≥5 years were based on the modified European Centre for Diseases Control (ECDC) definition of influenza-like illness (ILI), requiring (1) acute illness (onset within 7 days from hospital admission), (2) at least 1 systemic symptom of fever or feverishness, headache, myalgia, or malaise, and (3) at least 1 of 3 respiratory symptoms: cough, sore throat, or shortness of breath [10]. Patients aged <5 years were included if an illness met the above definition, although specific symptoms were not recorded. Due to differences in local public health surveillance priorities, patient volumes, and capacity (funding and human resource) for enrollment, the protocol was necessarily subject to some local adaptations at each site. For example, sites could enroll all patients or systematically target enrollment to patients presenting on certain days of the week. Local priorities included a focus on the assessment of vaccine effectiveness; enrollment focused on including all positive patients and a matched subset of negative controls. As such, the percentage positivity in each site does not necessarily reflect circulating case positivity in the cities and regions represented. However, coordinating the case definition and clinical data collection protocol across sites allows for robust comparison of clinical characteristics or influenza cases across participating sites.
Laboratory Testing
Laboratory-confirmed influenza (LCI) virus infection was defined by the GIHSN protocol [3]. For influenza virus detection, posterior nasopharyngeal swab samples were collected from patients (and, in some cases, an additional nasal sample for those aged <14 years and a pharyngeal swab for those aged ≥14 years). Influenza virus infection was confirmed using reverse-transcription polymerase chain reaction (RT-PCR) testing and was further subtyped at local laboratories or sent to regional reference laboratories according to local protocols. A subset of sites performed multiplex testing for a panel of other respiratory viruses.
Measures
Demographic and clinical data were retrieved from the GIHSN database, which contains data for laboratory-confirmed cases of influenza from all sites throughout the season. Demographic data collected for this study included sex and age; the latter was further divided into age categories (<5 years, 5–17 years, 18–49 years, 50–64 years, 65–79 years, and ≥80 years) to gain a better understanding of the age structure of the population. Each case's minimum data consisted of symptoms, symptom onset date, laboratory test result date, number and type of comorbidities, smoking status, influenza vaccination status, and disease outcome. Comorbidities were determined from a predefined list, including human immunodeficiency virus (HIV), cardiovascular disease (CVD, chronic obstructive pulmonary disease (COPD), asthma, neuromuscular disease, immunodeficiency/transplant, liver/kidney disease, neoplasm, diabetes, obesity, rheumatologic/autoimmune disease, and active tuberculosis (TB). Mortality was determined at both 30 days and after the entire period of clinical follow-up. Time to death was calculated starting with the time of hospital admission.
Statistical Analysis
We used descriptive statistics focusing on comparisons across age groups. Kruskal-Wallis's test compared continuous variables between groups and Pearson's χ2 test categorical variables. The Mann-Kendall test was used to evaluate trends in the association between age groups and categorical proportions, whereas the Cochran-Mantel-Haenszel test was conducted to calculate mortality proportions per age group within each stratum of vaccination status.
We used Kaplan-Meier analysis to estimate the probability of survival and log-rank testing for between-group comparison. We also used a multivariate logistic regression to model the composite outcome of admission to intensive care unit (ICU) or death as a function of age groups, adjusted for comorbidities and vaccination status for the current influenza season. The regression results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). A sensitivity analysis was conducted using mixed effects in the regression models to account for variability between sites, and this is presented in the Supplementary Materials.
Statistical significance was set at a 2-sided P < .05. We used R version 4.2.3 (The R Foundation for Statistical Computing, Vienna, Austria) in R-Studio 2023.03.0 + 386 “Cherry Blossom” Release (RStudio Inc., Boston, MA, USA) to conduct all analyses.
Ethics and Patient Consent
The GIHSN protocol was reviewed and approved by the respective Research Ethics Boards of all participating sites, and patient consent was acquired in adherence to local regulations after the approval.
RESULTS
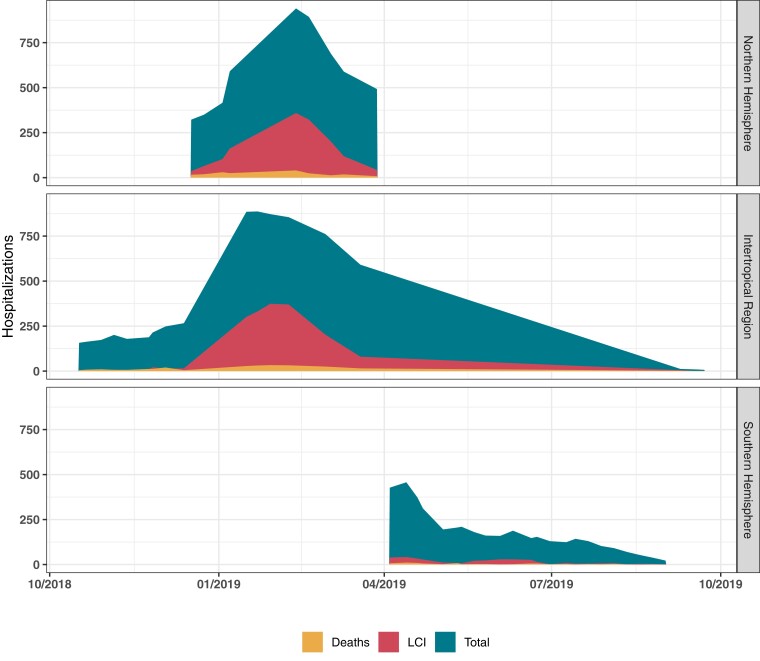
We enrolled 16 022 patients, 3512 (21.9%) of whom had LCI. Table 1 presents the enrollment by recruitment site, including the breakdown of patients by influenza test outcome and age distribution. Figure 1 shows the overall number of hospital admissions, LCI, and deaths according to the epidemiological week from October 2018 to October 2019. Influenza vaccine uptake by site is illustrated graphically in Supplementary Figure 1.
Table 1.
Characteristics of Patients Enrolled in the GIHSN Study by Site in the 2018/2019 Influenza Season
| Recruiting Site | Laboratory Confirmed Influenza N (%Within Site) | Influenza Negative N (%Within Site) |
Age (Years) Median [IQR] |
Influenza Vaccine Uptake N (%Within Site)a |
|---|---|---|---|---|
| Overall | 3512 (21.9) | 12 510 (78.1) | 9.0 [1.1–61.0] | 2094 (13.1) |
| Northern Hemisphere | ||||
| France—Lyon | 43 (40.2) | 64 (59.8) | 71.0 [57.5–84.0] | 48 (44.9) |
| Spain—Valencia | 297 (9.8) | 2748 (90.2) | 73.0 [49.0–84.0] | 1220 (40.1) |
| Romania | 416 (44.5) | 518 (55.5) | 13.0 [2.5–53.0] | 100 (16.8) |
| Serbia | 336 (44.7) | 415 (55.3) | 60.0 [39.0–69.0] | 21 (2.8) |
| Russia-Moscow | 174 (21.2) | 648 (78.8) | 24.0 [2.3–52.0] | 29 (3.5) |
| Russia-St. Petersburg | 1096 (36.0) | 1945 (64.0) | 4.3 [1.6–22.0] | 127 (4.1) |
| Canada | 252 (53.8) | 216 (46.2) | 67.0 [52.0–79.0] | 203 (43.4) |
| Mexico | 163 (27.4) | 432 (72.6) | 4.5 [1.2–49.0] | 100 (16.8) |
| China-Shanghai | 164 (14.2) | 992 (85.8) | 0.8 [0.2–2.7] | 6 (0.5) |
| Lebanon | 124 (24.7) | 379 (75.3) | 37.0 [6.0–66.0] | 46 (9.1) |
| Intertropical Region | ||||
| India | 230 (23.5) | 748 (76.5) | 58.0 [38.0–70.0] | 15 (1.5) |
| Ivory Coast | 32 (7.8) | 377 (92.2) | 2.0 [0.7–28.0] | 17 (4.1) |
| Kenya | 22 (4.3) | 484 (95.7) | 1.2 [0.5–3.0] | 0 (0) |
| Colombia | 15 (12.2) | 108 (87.8) | 1.3 [0.4–28.0] | 27 (22) |
| Southern Hemisphere | ||||
| South Africa | 90 (5.6) | 1504 (94.4) | 0.7 [0.1–2.9] | 0 (0) |
| Brazil | 23 (6.2) | 346 (93.8) | 1.5 [0.4–5.0] | 149 (40.4) |
| Argentina | 18 (4.3) | 404 (95.7) | 1.0 [0.4–2.0] | 11 (2.6) |
| Peru | 17 (8.5) | 182 (91.5) | 3.2 [0.7–15.5] | 27 (13.6) |
Abbreviations: GIHSN, Global Influenza Hospital Surveillance Network; IQR, interquartile range.
NOTE: Influenza vaccine uptake by site is illustrated graphically in Supplementary Figure 1.
To calculate the influenza vaccine uptake, the entire enrolled hospitalized sample within the site was used as the denominator.
Figure 1.
Hospital admissions, laboratory-confirmed influenza virus infection, and deaths grouped by epidemiological weeks from October 2018 to October 2019.
Characteristics of Patients With Laboratory-Confirmed Influenza Virus Infection
Table 2 shows the characteristics of LCI cases stratified by age. Among patients with LCI, presenting symptoms were not collected for children <5 years, whereas the symptoms varied by age for those 5 years and older. Fever and cough were the most common presenting symptoms, although the prevalence decreased with age; for example, 98.9% of those aged 5–17 years reported fever and 95.0% reported cough, falling to 80.7% with fever and 85.5% with cough in those aged ≥80 years (P for temporal trend = .027). A majority of adults aged ≥50 years presented with shortness of breath: 58.1% aged 50–64 years, 61.7% aged 65–79 years, and 75.3% aged ≥80 years (P for temporal trend = .027). Shortness of breath was uncommon among those aged <50 years but increased with age.
Table 2.
Characteristics of Laboratory-Confirmed Influenza Cases, Stratified by Age Group
| Feature, N (%) | <5 Years | 5–17 Years | 18–49 Years | 50–64 Years | 65–79 Years | 80+ Years | P for Temporal Trend |
|---|---|---|---|---|---|---|---|
| N | 1149 (32.7) | 359 (10.2) | 769 (21.9) | 456 (13.0) | 504 (14.4) | 275 (7.8) | … |
| Male sex | 516 (44.9) | 153 (42.6) | 477 (62.0) | 233 (51.1) | 236 (46.8) | 156 (56.7) | .452 |
| Smoking Habit | |||||||
| Current smoker (parent smoking status for children) | 348 (30.3) | 101 (28.1) | 186 (24.2) | 149 (32.7) | 105 (20.8) | 20 (7.3) | .132 |
| Never smoker | 635 (55.3) | 212 (59.1) | 499 (64.9) | 180 (39.5) | 201 (39.9) | 160 (58.2) | 1.000 |
| Past smoker | 121 (10.5) | 40 (11.1) | 84 (10.9) | 127 (27.9) | 198 (39.3) | 95 (34.5) | 1.000 |
| Not available | 45 (3.9) | 6 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Influenza Vaccination in the Current Season | 39 (3.4) | 18 (5.0) | 33 (4.3) | 37 (8.1) | 99 (19.6) | 97 (35.3) | .024 |
| Not available | 31 (2.7) | 5 (1.4) | 23 (3.0) | 31 (6.8) | 37 (7.3) | 26 (9.5) | … |
| Presenting Symptoms | |||||||
| Fever | 0 (0.0) | 355 (98.9) | 743 (96.6) | 431 (94.5) | 455 (90.3) | 222 (80.7) | .027 |
| Not available | 1149 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Cough | 0 (0.0) | 341 (95.0) | 695 (90.4) | 425 (93.2) | 441 (87.5) | 235 (85.5) | 0.086 |
| Not available | 1149 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Shortness of breath | 0 (0.0) | 75 (20.9) | 267 (34.7) | 265 (58.1) | 311 (61.7) | 207 (75.3) | 0.027 |
| Not available | 1149 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Malaise | 0 (0.0) | 282 (78.6) | 574 (74.6) | 327 (71.7) | 323 (64.1) | 186 (67.6) | 0.086 |
| Not available | 1149 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Headache | 0 (0.0) | 158 (44.0) | 470 (61.1) | 222 (48.7) | 213 (42.3) | 75 (27.3) | .220 |
| Not available | 1149 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Myalgias | 0 (0.0) | 120 (33.4) | 384 (49.9) | 236 (51.8) | 230 (45.6) | 111 (40.4) | 1.000 |
| Not available | 1149 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Sore throat | 0 (0.0) | 175 (48.7) | 372 (48.4) | 184 (40.4) | 167 (33.1) | 65 (23.6) | .027 |
| Not available | 1149 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | … |
| Chronic Comorbidities | |||||||
| Any chronic comorbidities | 124 (10.8) | 58 (16.2) | 283 (36.8) | 352 (77.2) | 460 (91.3) | 261 (94.9) | .008 |
| Not available | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) | 1 (0.2) | 0 (0) | … |
| Cardiovascular disease/high blood pressure | 17 (1.5) | 6 (1.7) | 55 (7.2) | 185 (40.6) | 318 (63.1) | 190 (69.1) | .024 |
| Not Available | 183 (15.9) | 15 (4.2) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Chronic obstructive pulmonary disease | 13 (1.1) | 5 (1.4) | 21 (2.7) | 81 (17.8) | 149 (29.6) | 78 (28.4) | 0.024 |
| Not available | 35 (3.0) | 4 (1.1) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Asthma | 9 (0.8) | 17 (4.7) | 42 (5.5) | 28 (6.1) | 35 (6.9) | 26 (9.5) | .008 |
| Not available | 183 (15.9) | 15 (4.2) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Diabetes mellitus | 1 (0.1) | 0 (0.0) | 44 (5.7) | 103 (22.6) | 153 (30.4) | 74 (26.9) | .060 |
| Not available | 35 (3.0) | 4 (1.1) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Immunodeficiency (except HIV)/organ transplant | 7 (0.6) | 2 (0.6) | 27 (3.5) | 17 (3.7) | 19 (3.8) | 6 (2.2) | .259 |
| Not available | 35 (3.0) | 4 (1.1) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Renal impairment | 1 (0.1) | 0 (0.0) | 19 (2.5) | 37 (8.1) | 53 (10.5) | 44 (16.0) | .024 |
| Not available | 35 (3.0) | 4 (1.1) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Rheumatologic disease/autoimmune disease | 0 (0.0) | 1 (0.3) | 18 (2.3) | 18 (3.9) | 27 (5.4) | 24 (8.7) | .008 |
| Not available | 35 (3.0) | 4 (1.1) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Neurological or neuromuscular disease | 9 (0.8) | 8 (2.2) | 14 (1.8) | 20 (4.4) | 37 (7.3) | 39 (14.2) | .024 |
| Not available | 35 (3.0) | 4 (1.1) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Cirrhosis/liver disease | 2 (0.2) | 2 (0.6) | 14 (1.8) | 20 (4.4) | 19 (3.8) | 6 (2.2) | .132 |
| Not available | 35 (3.0) | 4 (1.1) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Neoplasm | 1 (0.1) | 8 (2.2) | 24 (3.1) | 42 (9.2) | 66 (13.1) | 29 (10.5) | .024 |
| Not available | 35 (3.0) | 4 (1.1) | 4 (0.5) | 6 (1.3) | 9 (1.8) | 4 (1.5) | … |
| Obesity | 0 (0.0) | 0 (0.0) | 52 (6.8) | 48 (10.5) | 66 (13.1) | 49 (17.8) | .012 |
| Not available | 35 (3.0) | 4 (1.1) | 33 (4.3) | 29 (6.4) | 30 (6.0) | 25 (9.1) | … |
| Active tuberculosis | 0 (0.0) | 0 (0.0) | 2 (0.3) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1.000 |
| Not available | 36 (3.1) | 6 (1.7) | 18 (2.3) | 44 (9.6) | 104 (20.6) | 101 (36.7) | … |
| HIV infection | 19 (1.7) | 0 (0.0) | 13 (1.7) | 3 (0.7) | 2 (0.4) | 0 (0.0) | .338 |
| Not available | 36 (3.1) | 6 (1.7) | 18 (2.3) | 44 (9.6) | 104 (20.6) | 101 (36.7) | … |
| Antivirals | 339 (29.5) | 92 (25.6) | 210 (27.3) | 127 (27.9) | 157 (31.2) | 94 (34.2) | .132 |
Abbreviations: HIV, human immunodeficiency virus.
Patient comorbidities varied by site and by age group (Table 2). Among adults, CVDs (22%), diabetes (10.7%), COPD (9.9%), and obesity (6.1%) were the most common comorbidities. Most children <5 years had no underlying conditions, whereas more than half of older adults had cardiovascular illnesses. Some sites in lower-income settings had a relatively high burden of underlying infectious diseases (eg, HIV infection and active TB), whereas others in higher-income settings had more chronic noninfectious diseases.
2018/2019 Influenza Season Circulating Strains
Influenza A/H1N1pdm09 was the most frequently detected strain, at 49.2% of all influenza-positive tests (presumably more, including the un-subtyped Influenza A strains). It is notable that all 4 strains (A/H1N1pdm09, A/H3N2, B/Yamagata-like, and B/Victoria/like) cocirculated from a global perspective. Influenza A strain distribution varied by age (Table 3), with more A/H1N1pdm09 in younger age groups (P for trend = .024) and more A/H3N2 in older age groups. Influenza B was detected predominantly in the youngest (both B lineages with Victoria slightly predominating compared with Yamagata) and oldest (B Yamagata only) age groups. It is notable that enrollment profiles should be kept in mind in interpreting these trends, because some countries enrolled only children and others enrolled only adults, such that age differences in influenza strain/type reflect both differential susceptibility of some age groups to specific strains and also differential geographical circulation patterns. Among patients enrolled at sites where RT-PCR testing was done to detect additional viruses, respiratory syncytial virus was the most tested and detected noninfluenza virus, followed by rhinovirus, metapneumovirus, and seasonal coronavirus. These patterns of viral infection underlying illness differed by age group, with the younger patients being generally more likely to have noninfluenza viruses (Table 3). However, it is worth noting that age distribution and viral types were likely influenced by the characteristics of recruitment sites and their laboratory testing facilities.
Table 3.
Influenza (Strain and Subtype) and Multiplex Results by age Group
| Virus Strain and Subtypes | <5 Years | 5–17 Years | 18–49 Years | 50–64 Years | 65–79 Years | 80+ Years | P for Temporal Trend |
|---|---|---|---|---|---|---|---|
| N (%) | 1149 (32.7) | 359 (10.2) | 769 (21.9) | 456 (13.0) | 504 (14.4) | 275 (7.8) | … |
| Influenza A/H1N1pdm09 | 663 (57.7) | 198 (55.2) | 337 (43.8) | 242 (53.1) | 216 (42.9) | 73 (26.5) | .024 |
| Influenza A/H3N2 | 407 (35.4) | 121 (33.7) | 327 (42.5) | 138 (30.3) | 189 (37.5) | 153 (55.6) | .452 |
| Influenza A unsubtyped | 85 (7.4) | 17 (4.7) | 78 (10.1) | 73 (16.0) | 86 (17.1) | 38 (13.8) | .132 |
| Influenza B Yamagata | 14 (1.2) | 8 (2.2) | 17 (2.2) | 11 (2.4) | 11 (2.2) | 13 (4.7) | .259 |
| Influenza B Victoria | 21 (1.8) | 6 (1.7) | 11 (1.4) | 0 (0.0) | 4 (0.8) | 0 (0.0) | .035 |
| Influenza B unsubtyped | 28 (2.4) | 12 (3.3) | 6 (0.8) | 0 (0.0) | 4 (0.8) | 0 (0.0) | .180 |
| Multiplex virus-virus codetectionsa | |||||||
| Human seasonal coronavirus type not specified | 8 (0.7) | 2 (0.6) | 3 (0.4) | 1 (0.2) | 4 (0.8) | 0 (0.0) | .259 |
| Not available | 119 (10.4) | 7 (1.9) | 67 (8.7) | 34 (7.5) | 28 (5.6) | 11 (4.0) | … |
| Metapneumovirus | 13 (1.1) | 1 (0.3) | 3 (0.4) | 3 (0.7) | 0 (0.0) | 0 (0.0) | .180 |
| Not available | 110 (9.6) | 14 (3.9) | 62 (8.1) | 28 (6.1) | 22 (4.4) | 11 (4.0) | … |
| RSV | 45 (3.9) | 2 (0.6) | 2 (0.3) | 3 (0.7) | 5 (1.0) | 3 (1.1) | .707 |
| Not available | 94 (8.2) | 12 (3.3) | 61 (7.9) | 28 (6.1) | 22 (4.4) | 11 (4.0) | … |
| Adenovirus | 6 (0.5) | 2 (0.6) | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0 (0.0) | .085 |
| Not available | 174 (15.1) | 12 (3.3) | 67 (8.7) | 34 (7.5) | 28 (5.6) | 11 (4.0) | … |
| Bocavirus | 3 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .241 |
| Not available | 174 (15.1) | 12 (3.3) | 67 (8.7) | 34 (7.5) | 28 (5.6) | 11 (4.0) | … |
| Parainfluenza type not specified | 5 (0.4) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) | .314 |
| Not available | 175 (15.2) | 12 (3.3) | 67 (8.7) | 34 (7.5) | 28 (5.6) | 11 (4.0) | … |
| Rhinovirus | 6 (0.5) | 5 (1.4) | 4 (0.5) | 4 (0.9) | 5 (1.0) | 1 (0.4) | .707 |
| Not available | 190 (16.5) | 14 (3.9) | 67 (8.7) | 34 (7.5) | 28 (5.6) | 11 (4.0) | … |
Abbreviations: RSV, respiratory syncytial virus.
Not all recruiting sites have run multiplex tests. For standardization purposes, the totality of laboratory-confirmed influenza cases was considered the denominator to estimate the proportions per age group.
Clinical Adverse Outcomes
Clinical adverse outcomes were generally worst for the youngest children (<5 years) and in adults at older ages. Hospital lengths of hospital stay tended to increase with age. Rates of mechanical ventilation and ICU admission were relatively high in children <5, and for adults these were associated with increasing age in all but the oldest age group: ICU admission peaked in adults aged 50–64 and mechanical ventilation was highest in those age 65–79, and both decreased in adults aged 80+. Mortality was 3.6% for children <5 and was highest in adults aged 50–64 (8.1%), with a slight decrease for the oldest age groups to 7.7% for those 65%–79% and 6.5% for those 80+ (Table 4). The global mortality rate and 30-day mortality rate were similar, with only a negligible difference. Specifically, 3 deaths occurred after 30 days of the initial assessment, and 4 deaths occurred for those under 17. To account for the follow-up period and its effect on the denominator of those at risk of the outcome, we used a time-to-event analysis strategy that censored the deaths after the 30-day mark. The overall survival rate of patients assessed within 30 days was 71.5%, with a 95% CI of 64.5%–79.2%. We found that the median time-to-death was 7 days, with the first quartile set at 4 days and the third set at 12 days, with no significant difference between age groups. Our results revealed a statistically significant difference in survival probability between males and females, with males having higher survival rates (log-rank test, P = .007).
Table 4.
Outcomes of Laboratory-Confirmed Influenza Cases by Age Group
| Outcomes Within Each Category | Overall | <5 Years | 5–17 Years | 18–49 Years | 50–64 Years | 65–79 Years | 80+ Years | P |
|---|---|---|---|---|---|---|---|---|
| N | 3512 (100%) | 1149 (32.7) | 359 (10.2) | 769 (21.9) | 456 (13.0) | 504 (14.4) | 275 (7.8) | - |
| Length of stay (days), median [IQR] | 6 [4–8] | 5 [3–7] | 6 [4–7] | 5 [3–8] | 7 [4–11] | 7 [4–11] | 7 [4–11] | <.001 |
| ICU admission, n (%) | 267 (7.6) | 48 (4.2) | 18 (5.0) | 52 (6.8) | 63 (13.8) | 65 (12.9) | 21 (7.6) | <.001 |
| Mechanical ventilation, n (%) | 151 (4.3) | 8 (0.7) | 2 (0.6) | 29 (3.8) | 39 (8.6) | 55 (10.9) | 18 (6.5) | <.001 |
| 30-day mortality, n (%) | 125 (3.5) | 4 (0.3) | 0 (0.0) | 29 (3.8) | 37 (8.1) | 38 (7.5) | 17 (6.1) | <.001 |
| Mortality, n (%) | 128 (3.6) | 4 (0.3) | 0 (0.0) | 30 (3.9) | 37 (8.1) | 39 (7.7) | 18 (6.5) | <.001 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
NOTES: Differences in the length of stay (days) distribution across age groups were tested using the Kruskal-Wallis rank-sum test. We used Pearson's χ2 test for the remaining categorical variables. Mortality was reported at both 30 days and for the entire period of clinical follow up, which ranged from 1 to 274 days.
Patients not admitted to an ICU had higher survival rates than those admitted to an ICU (log-rank test, P < .001). These results, along with survival curves stratified by age, sex and presence of chronic conditions, are illustrated in Supplementary Figure 2. Mortality peaked at age 50–64 (8.1%) and then decreased slightly at older ages. The Cochran-Mantel-Haenszel test, adjusted for strata (vaccination status), was used to compare the percentage of death in the age range stratum and showed what appears to be the effect of the vaccine in attenuating mortality, as higher vaccine uptake was noted at older ages (data not shown, P < .001). The distribution of vaccination status among surviving versus deceased patients is shown in Supplementary Figure 3.
Analysis of influenza strain data from our study revealed statistically significant associations with the probability of survival (log-rank test, P = .009) (Supplementary Figure 4A). Influenza A infection had a higher estimated 30-day survival probability than influenza B infection (log-rank test, P = .034) (Supplementary Figure 4B). Further stratification of influenza A revealed that those infected with influenza A/H1N1pdm09 had a higher estimated 30-day survival probability than those infected with influenza A/H3N2 (log-rank test, P = .006) (Supplementary Figure 4C).
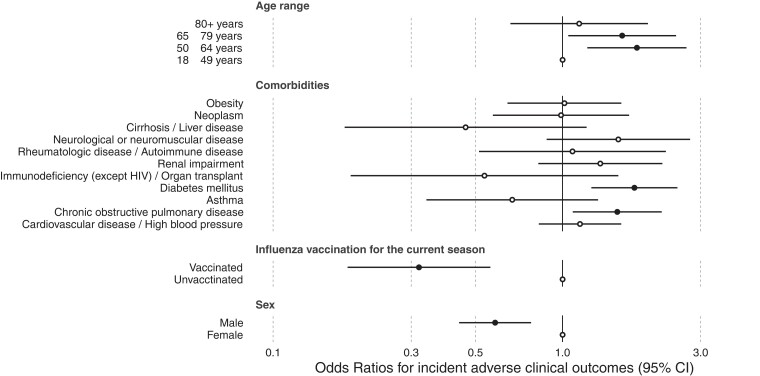
After filtering a sample to include only those with LCI, we evaluated the variables independently associated with ICU admission or death outcomes. Our modeling results revealed that middle-aged adults (50–64 years) had higher mortality, with a 1.80 OR (95% CI, 1.21–2.68), whereas the elderly (65–79 years) had 1.60 OR (95% CI, 1.04–2.46), of ICU admission or death. Patients with COPD and diabetes had 1.54 (95% CI, 1.07–2.19) and 1.77 (95% CI, 1.25–2.48) OR of the worst outcomes, respectively. It is notable that male gender and vaccination for the current influenza season were associated with lower OR of ICU admission or death, with the OR of 0.58 (95% CI, .43–.77) and 0.31 (95% CI, .17–.54), respectively (Figure 2). The Supplementary Materials include a sensitivity analysis using mixed effects in the regression models to account for variability between sites (Supplementary Figure 5), illustrating that the results remained consistent except that age and COPD were no longer statistically significant.
Figure 2.
Forest plot illustrating the odds ratios (circles) and associated 95% confidence intervals ([CI] horizontal bars) from the logistic model for the composite severe outcome of death or intensive care unit admission of laboratory-confirmed influenza cases in the 2018/2019 season. Hollow circles indicate where the confidence interval crosses the null, representing the point estimates for which the covariables have no significant effect. HIV, human immunodeficiency virus.
DISCUSSION
During the 2018/2019 influenza season, 3512 (21.9%) patients of all ages who were admitted to the hospital and enrolled at GIHSN sites had laboratory-confirmed influenza. There was cocirculation of influenza A/H1N1pdm09, A/H3N2, B Yamagata-like, and B Victoria-like, with variability noted between countries and regions. Symptoms varied significantly with age, with younger patients more likely to present with fever and cough and older adults presenting with shortness of breath. Severe outcomes were experienced across the age continuum. Middle and older age (particularly age 50–64 and 65–79) and underlying diabetes or COPD were independently associated with the composite outcome of death or ICU admission, whereas male sex and influenza vaccination were associated with lower odds of these severe outcomes. Very few children and young adults had comorbidities, which were much more frequent in older age groups.
Cardiovascular illness, diabetes, respiratory conditions, neoplasm, and kidney disease were the most common comorbidities, although comorbidity profiles differed across age groups and sites. It is notable that only a minority of younger patients had any underlying illnesses at all (<1% for ages 0–5 years, < 1.5% for those aged 5–17 years, and <5% for those aged 18–49 years). These findings underscore that influenza virus infection can cause severe illness requiring hospitalization, even among children, adolescents, and previously healthy individuals. The low rate of comorbidities in this international sample contrasts with a study from Canada, which reported that approximately one third of children aged 0–59 months admitted to hospital with influenza had complex underlying comorbidities [11], suggesting that contextual factors such as socioeconomic status and clinical care pathways may play an essential role in profiles of underlying illness. A meta-analysis has identified underlying comorbidity as a critical risk factor for influenza hospitalization among children, listing neurological conditions, prematurity, sickle cell, immunosuppression, diabetes, and age <2 years as higher risk conditions [12]. Children with more than 1 of these factors were at the highest risk.
It is notable that, using an international surveillance network, our study is unique in providing an international comparison of these comorbidities underlying influenza hospital admissions. Our finding that comorbidities increased with age, and was exceptionally high among older adults, is consistent with findings in the literature [13–16]. Not surprisingly, the profile of underlying comorbidities also varied widely between sites, according to local epidemiology of infectious and noninfectious illnesses. This is consistent with other international reports [17]. Among comorbidities, COPD and diabetes were independently associated with increased odds of the most severe outcomes. Similar results were also reported regarding the positive association between these comorbidities and adverse clinical outcomes in patients hospitalized with influenza. Researchers recently showed that older adults with diabetes have higher rates of severe influenza-associated outcomes than those without [18].
On the other hand, others found no association between diabetes and adverse clinical outcomes in hospitalized patients with influenza during Catalonia's 2017–2018 influenza season [19]. However, the authors showed that COPD was associated with mortality in the age group 15 to 49 years, similar to the results found in New Zealand [20]. Although the effect of influenza vaccination among diabetic patients remains inconclusive, a recent study found that influenza vaccination in diabetic patients reduced the risk of hospitalization for influenza by approximately half [21]. Future studies should analyze the effectiveness of influenza vaccination on adverse clinical outcomes in diabetic patients, considering other factors mentioned here.
Fever and cough were the most common presenting symptoms, at over 90%. This finding is not surprising given that the enrollment protocol was based on the ECDC case definition of ILI, in which fever and cough are prominent [10]. We observed age differences in presenting symptoms, with older adults less likely to present with typical ILI symptoms and more likely to experience shortness of breath. This is consistent with prior reports in the literature that the sensitivity of ILI case definitions varies with age and is lower among older adults [22, 23] and has led to suggestions that criteria be modified in older adults and residents of long-term care facilities [22, 24, 25].
Influenza A/H1N1pdm09 (49.2%) was the dominant influenza strain detected in the GIHSN during the 2018/2019 influenza season. However, it is notable that there were substantial differences between sites in the strain distribution pattern, and, from a global perspective, there was cocirculation of A/H1N1pdm09, A/H3N2, B Victoria, and B Yamagata, creating a mixed season overall. This finding is consistent with other reports from the same season, in which both A/H1N1pdm09 and A/H3N2 and, to a minor degree, influenza B were reported [26–28]. Overall, the seasonal influenza vaccine was reported to be well matched to circulating A/H1N1 strains, but the majority of circulating A/H3N2 strains were mismatched [29]. This variability also emphasizes the need for more research to understand why influenza epidemiology can vary widely, even between neighboring countries. The differences in strain circulation are consistent with previous experience in the GIHSN [3]. There were also age differences in the strain prevalence; for example, consistent with prior reports, B Yamagata was more often detected in older adults, whereas B Victoria and B Yamagata were more frequently detected in hospitalized children. This is consistent with prior reports that B Victoria may preponderantly affect younger children, whereas older adults may be more affected by B Yamagata [30–32]. We also found that influenza A > influenza B in their strength of association with mortality (Supplementary Figure 4). This finding is consistent with other reports showing that clinical outcomes of patients with influenza A may be more severe than those with influenza B, regardless of gender, age, and underlying health conditions [33, 34].
Although vaccination rates varied widely between sites, likely reflecting international differences in influenza vaccination policy, programs, and availability [35], we found that influenza vaccination in the current season was associated with statistically significantly lower odds of experiencing the most severe outcomes (ICU admission and mortality). Our analyses also showed that vaccination was associated with the plateau (and indeed slight attenuation) of mortality in the oldest age groups, in whom vaccination uptake was highest. The magnitude of this association is consistent with prior reports that influenza vaccine effectiveness tends to be highest against the most severe outcomes [13]. Care-seeking and access patterns, which vary across sites and resource settings, represent other factors that may have contributed to our finding of differential mortality between age groups; the importance of this variability between sites is highlighted by our sensitivity analyses in which these were accounted for using mixed-effects models.
The RT-PCR testing for additional respiratory viruses was performed in some sites and demonstrates that many other viral illnesses cocirculate during influenza season and can present similarly to influenza with illness severe enough to require acute care hospitalization. This is consistent with prior literature reports [36, 37], although awareness in the public and medical communities remains better for pediatric than older age groups. Future analyses may indicate the role of coinfection between influenza and other respiratory viruses as a determining disease severity factor.
We found that women had higher odds of death or ICU admission. The causes of this association are unclear, although some prior studies have reported differences in influenza mortality between sexes. Although we adjusted our analyses for comorbidity and age, it is also possible that this difference may relate to discrepancies in frailty, which tends to be higher for women than for men [38]. We also found that the likelihood of severe outcomes (particularly in-hospital death and ICU admission) tended to increase with age among adults, although with some reduction at the oldest age groups as discussed above. This increase in adverse outcomes with age is consistent with findings in the existing literature [16, 39]. However, age alone may not explain this finding. A growing body of evidence suggests that individuals vary widely in their health status and vulnerability to adverse outcomes related to influenza, even within defined age groups; this variability can be measured using the concept of frailty [40, 41], particularly at older ages. Starting from the 2019/2020 influenza season, the Clinical Frailty Scale is included in the GIHSN data collection and will be a feature of future season reports and analyses [42].
Our study is not without limitations. Symptoms were not documented for children under age 5, and functional status was documented only for the age group ≥65. Enrollment was based on ILI criteria, which likely resulted in disproportionate underenrollment of older adults with influenza, who tend to present atypically, even without fever. We could not grade the severity of individual comorbidities, and although we relied on hospital medical records rather than patient self-report, the possibility of undiagnosed comorbidity remains. Unmeasured confounding may remain, including in the estimate of vaccine effectiveness. Despite the standardized protocol, there are differences between-country data collection approaches, because individual sites coordinate with internal regional and national surveillance structures. Because individual sites are based at specific hospitals or institutions, some countries over- or underrepresented specific patient groups. For example, some sites enrolled only adults, whereas others in pediatric institutions enrolled only children. Some sites are in specialized infectious disease, respiratory illness, or maternity hospitals, whereas others are in general facilities. Nevertheless, these differences also contribute to the richness of the network in providing a snapshot of the experience of hospitalized influenza illness and care around the world.
CONCLUSIONS
The GIHSN contributes to the global understanding of hospitalized influenza illness in diverse international settings and all age groups. Future seasons will include more nuanced consideration of the impact of frailty and add data on whole-genome sequencing in the context of ongoing data collection on clinical presentation and outcomes.
Supplementary Material
Acknowledgments
Author contributions. MKA wrote the initial draft. HP and LS conducted the analyses and contributed to the initial drafts. All authors (MKA, HP, LS, JP, SSC, JRO, JM, JB, BL, MCN, EB, SMR, HIGG, SAM, DG, TZ, PV, PAK, DC, NAO, GD, MLGA, VAL-T, ACD, EB, AS, DD, SM, and JD-D) contributed to data interpretation and manuscript revisions. MKA, MCN, EB, SMR, HIGG, SAM, DG, TZ, PV, PAK, DC, NAO, GD, MLGA, VAL-T, ACD, EB, AS, DD, SM, and JD-D are lead investigators for Global Influenza Hospital Surveillance Network (GIHSN) country sites. All authors have approved the final manuscript.
Disclaimer. None of the donors have access to the data, and they do not participate in the analysis, interpretation, or decision to publish the results. More details about the Foundation and its governance can be found at https://www.gihsn.org/.
Financial support. The GIHSN is a public-private partnership partially supported by unrestricted Foundation for Influenza Epidemiology grants, under the auspices of the Fondation de France. The Foundation receives support from Sanofi, CSL Seqirus, Illumina, Abbott, and International Federation of Pharmaceutical Manufacturers and Associations.
Contributor Information
Melissa K Andrew, Dalhousie University and Canadian Center for Vaccinology, Halifax, Canada.
Henrique Pott, Dalhousie University and Canadian Center for Vaccinology, Halifax, Canada; Department of Medicine, Universidade Federal de São Carlos, São Carlos, Brazil.
Lisa Staadegaard, Netherlands Institute for Health Care Research (Nivel), Utrecht, Netherlands.
John Paget, Netherlands Institute for Health Care Research (Nivel), Utrecht, Netherlands.
Sandra S Chaves, Foundation for Influenza Epidemiology, Fondation de France, Paris, France.
Justin R Ortiz, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
John McCauley, WHO Collaborating Centre for Reference and Research on Influenza, Crick Institute, London, United Kingdom.
Joseph Bresee, Centre for Vaccine Equity, Task Force for Global Health, Atlanta, Georgia, USA.
Marta C Nunes, South African Medical Research Council, Vaccines & Infectious Diseases Analytics (VIDA) Research Unit, and Department of Science and Technology/National Research Foundation, South African Research Chair Initiative in Vaccine Preventable Diseases, Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa.
Elsa Baumeister, National Reference Laboratory for Viral Respiratory Diseases, Virology Department, INEI-ANLIS, Buenos Aires, Argentina.
Sonia Mara Raboni, Molecular Biology/Microbiology Research Laboratory, Universidade Federal do Paraná, Curitiba, Brazil.
Heloisa I G Giamberardino, Epidemiology, Immunization and Infection Control Department—Hospital Pequeno Principe, Curitiba, Paraná, Brazil.
Shelly A McNeil, Dalhousie University and Canadian Center for Vaccinology, Halifax, Canada.
Doris Gomez, Grupo de Investigación UNIMOL, Facultad de Medicina, Universidad de Cartagena, Cartagena de Indias, Colombia.
Tao Zhang, School of Public Health, Fudan University, Shanghai, China.
Philippe Vanhems, Hôpital Edouard Herriot, Lyon, France.
Parvaiz A Koul, Sher-i-Kashmir Institute, Srinagar, India.
Daouda Coulibaly, Institut National d'Hygiène Publique (INHP), Abidjan, Côte d’Ivoire.
Nancy A Otieno, Kenya Medical Research Institute (KEMRI), Nairobi, Kenya.
Ghassan Dbaibo, Center for Infectious Diseases Research, American University of Beirut, Beirut, Lebanon.
Maria Lourdes Guerrero Almeida, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico.
Victor Alberto Laguna-Torres, Clínica Internacional, Instituto de Medicina Tropical Universidad Nacional Mayor de San Marcos, Lima, Peru.
Anca Cristina Drăgănescu, National Institute for Infectious Diseases “Prof. Dr. Matei Bals”, Bucharest, Romania.
Elena Burtseva, FSBI “N.F. Gamaleya NRCEM” Ministry of Health of the Russian Federation (Federal Research Budgetary Institute “National Research Center of Epidemiology and Microbiology named after honorary academician N.F. Gamaleya), Moscow, Russia.
Anna Sominina, Smorodintsev Research Institute of Influenza, St. Petersburg, Russia.
Daria Danilenko, Smorodintsev Research Institute of Influenza, St. Petersburg, Russia.
Snežana Medić, Institute of Public Health of Vojvodina, Novi Sad, Serbia; Faculty of Medicine, University of Novi Sad, Novi Sad, Serbia.
Javier Diez-Domingo, FISABIO, Valencia, Spain.
Bruno Lina, Université de Lyon, Lyon, France.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO launches new global influenza strategy. Available at: https://www.who.int/news/item/11-03-2019-who-launches-new-global-influenza-strategy. Accessed 7 June 2023.
- 3. Lina B, Georges A, Burtseva E, et al. Complicated hospitalization due to influenza: results from the global hospital influenza network for the 2017–2018 season. BMC Infect Dis 2020; 20:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019; 7:e1031–e45. [DOI] [PubMed] [Google Scholar]
- 5. Koul PA, Koul HP. Redefining the influenza equator. Lancet Glob Health 2022; 10:e1388. [DOI] [PubMed] [Google Scholar]
- 6. Puig-Barbera J, Tormos A, Trushakova S, et al. The global influenza hospital surveillance network (GIHSN): a new platform to describe the epidemiology of severe influenza. Influenza Other Respir Viruses 2015; 9:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puig-Barbera J, Mira-Iglesias A, Burtseva E, et al. Influenza epidemiology and influenza vaccine effectiveness during the 2015–2016 season: results from the global influenza hospital surveillance network. BMC Infect Dis 2019; 19:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baselga-Moreno V, Trushakova S, McNeil S, et al. Influenza epidemiology and influenza vaccine effectiveness during the 2016–2017 season in the global influenza hospital surveillance network (GIHSN). BMC Public Health 2019; 19:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Puig-Barbera J, Burtseva E, Yu H, et al. Influenza epidemiology and influenza vaccine effectiveness during the 2014–2015 season: annual report from the global influenza hospital surveillance network. BMC Public Health 2016; 16(Suppl 1):757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casalegno JS, Eibach D, Valette M, et al. Performance of influenza case definitions for influenza community surveillance: based on the French influenza surveillance network GROG, 2009–2014. Euro Surveill 2017; 22:30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buchan SA, Chung H, Karnauchow T, et al. Characteristics and outcomes of young children hospitalized with laboratory-confirmed influenza or respiratory syncytial virus in Ontario, Canada, 2009–2014. Pediatr Infect Dis J 2019; 38:362–9. [DOI] [PubMed] [Google Scholar]
- 12. Gill PJ, Ashdown HF, Wang K, et al. Identification of children at risk of influenza-related complications in primary and ambulatory care: a systematic review and meta-analysis. Lancet Respir Med 2015; 3:139–49. [DOI] [PubMed] [Google Scholar]
- 13. Nichols MK, Andrew MK, Hatchette TF, et al. Influenza vaccine effectiveness to prevent influenza-related hospitalizations and serious outcomes in Canadian adults over the 2011/12 through 2013/14 influenza seasons: a pooled analysis from the Canadian immunization research network (CIRN) serious outcomes surveillance (SOS network). Vaccine 2018; 36:2166–75. [DOI] [PubMed] [Google Scholar]
- 14. Ferdinands JM, Gaglani M, Martin ET, et al. Prevention of influenza hospitalization among adults in the United States, 2015–2016: results from the US hospitalized adult influenza vaccine effectiveness network (HAIVEN). J Infect Dis 2019; 220:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pitigoi D, Streinu-Cercel A, Ivanciuc AE, et al. Surveillance of medically-attended influenza in elderly patients from Romania-data from three consecutive influenza seasons (2015/16, 2016/17, and 2017/18). Influenza Other Respir Viruses 2020; 14:530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coleman BL, Fadel SA, Fitzpatrick T, Thomas SM. Risk factors for serious outcomes associated with influenza illness in high- versus low- and middle-income countries: systematic literature review and meta-analysis. Influenza Other Respir Viruses 2018; 12:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hajat C, Stein E. The global burden of multiple chronic conditions: a narrative review. Prev Med Rep 2018; 12:284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Owusu D, Rolfes MA, Arriola CS, et al. Rates of severe influenza-associated outcomes among older adults living with diabetes-influenza hospitalization surveillance network (FluSurv-NET), 2012–2017. Open Forum Infect Dis 2022; 9:ofac131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soldevila N, Acosta L, Martinez A, et al. Behavior of hospitalized severe influenza cases according to the outcome variable in Catalonia, Spain, during the 2017–2018 season. Sci Rep 2021; 11:13587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker TA, Waite B, Thompson MG, et al. Risk of severe influenza among adults with chronic medical conditions. J Infect Dis 2020; 221:183–90. [DOI] [PubMed] [Google Scholar]
- 21. Martinez-Baz I, Navascues A, Portillo ME, et al. Effect of influenza vaccination in preventing laboratory-confirmed influenza hospitalization in patients with diabetes mellitus. Clin Infect Dis 2021; 73:107–14. [DOI] [PubMed] [Google Scholar]
- 22. Andrew MK, McElhaney JE, McGeer AA, et al. Influenza surveillance case definitions miss a substantial proportion of older adults hospitalized with laboratory-confirmed influenza: a report from the Canadian immunization research network (CIRN) serious outcomes surveillance (SOS) network. Infect Control Hosp Epidemiol 2020; 41:499–504. [DOI] [PubMed] [Google Scholar]
- 23. Hartman L, Zhu Y, Edwards KM, Griffin MR, Talbot HK. Underdiagnosis of influenza virus infection in hospitalized older adults. J Am Geriatr Soc 2018; 66:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Falsey AR, Baran A, Walsh EE. Should clinical case definitions of influenza in hospitalized older adults include fever? Influenza Other Respir Viruses 2015; 9(Suppl 1):23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. High KP, Bradley SF, Gravenstein S, et al. Clinical practice guideline for the evaluation of fever and infection in older adult residents of long-term care facilities: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:149–71. [DOI] [PubMed] [Google Scholar]
- 26. Flannery B, Kondor RJG, Chung JR, et al. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis 2020; 221:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. PHAC . FluWatch annual report: 2018–19 influenza season. Ottawa: Public Health Agency of Canada, 2019. [Google Scholar]
- 28. World Health Organization . 2018–2019 influenza season: what we know so far. Available at: https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/news/news/2019/01/20182019-influenza-season-what-we-know-so-far. Accessed 19 April 2023.
- 29. Chung JR, Rolfes MA, Flannery B, et al. Effects of influenza vaccination in the United States during the 2018–2019 influenza season. Clin Infect Dis 2020; 71:e368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Socan M, Prosenc K, Ucakar V, Berginc N. A comparison of the demographic and clinical characteristics of laboratory-confirmed influenza B Yamagata and Victoria lineage infection. J Clin Virol 2014; 61:156–60. [DOI] [PubMed] [Google Scholar]
- 31. Seleka M, Treurnicht FK, Tempia S, et al. Epidemiology of influenza B/Yamagata and B/Victoria lineages in South Africa, 2005–2014. PLoS One 2017; 12:e0177655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emukule GO, Otiato F, Nyawanda BO, et al. The epidemiology and burden of influenza B/Victoria and B/Yamagata lineages in Kenya, 2012–2016. Open Forum Infect Dis 2019; 6:ofz421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murillo-Zamora E, Trujillo X, Huerta M, et al. Survival in influenza virus-related pneumonia by viral subtype: 2016–2020. Int J Infect Dis 2021; 112:288–93. [DOI] [PubMed] [Google Scholar]
- 34. Chen L, Han XD, Li YL, Zhang CX, Xing XQ. Severity and outcomes of influenza-related pneumonia in type A and B strains in China, 2013–2019. Infect Dis Poverty 2020; 9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palache A, Rockman S, Taylor B, et al. Vaccine complacency and dose distribution inequities limit the benefits of seasonal influenza vaccination, despite a positive trend in use. Vaccine 2021; 39:6081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tai CC, Tsai CH, Huang YH, Lee CL, Chen HP, Chan YJ. Detection of respiratory viruses in adults with respiratory tract infection using a multiplex PCR assay at a tertiary center. J Microbiol Immunol Infect 2021; 54:858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weigl JA, Puppe W, Meyer CU, et al. Ten years’ experience with year-round active surveillance of up to 19 respiratory pathogens in children. Eur J Pediatr 2007; 166:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol 2017; 89:30–40. [DOI] [PubMed] [Google Scholar]
- 39. Ersoy E O, Er B, Ciftci F, et al. Outcome of patients admitted to intensive care units due to influenza-related severe acute respiratory illness in 2017–2018 flu season: a multicenter study from Turkey. Respiration 2020; 99:954–60. [DOI] [PubMed] [Google Scholar]
- 40. Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.