Abstract
Cellular metabolism (or energetics) and epigenetics are tightly coupled cellular processes. It is arguable that of all the described cancer hallmarks, dysregulated cellular energetics and epigenetics are the most tightly coregulated. Cellular metabolic states regulate and drive epigenetic changes while also being capable of influencing, if not driving, epigenetic reprogramming. Conversely, epigenetic changes can drive altered and compensatory metabolic states. Cancer cells meticulously modify and control each of these two linked cellular processes in order to maintain their tumorigenic potential and capacity. This review aims to explore the interplay between these two processes and discuss how each affects the other, driving and enhancing tumorigenic states in certain contexts.
Keywords: cancer metabolism, cancer epigenetics, Warburg effect, cancer hallmarks
1. Introduction
All cancer cells, irrespective of originating tissue, have altered cellular metabolism. Despite being first observed in 1927 by Otto Warburg and colleagues [1], it was not until 2011 that altered cellular metabolism was recognized as a cancer hallmark [2,3]. It is now well established that both liquid and solid tumors upregulate metabolic pathways other than oxidative phosphorylation (OXPHOS) even in the presence of oxygen, a phenomenon termed the “Warburg effect” [4,5]. Cancers have been shown to exhibit increased gluconeogenesis [6], increased glutaminolytic activity [7,8], modified amino acid metabolism [9], increased de novo fatty acid synthesis with reduced fatty acid oxidation (FAO) [10,11,12], and increased pentose phosphate pathway activity [13]. Although Warburg’s original observation identified increased glycolytic rates in cancers, decades of research have shown that cancers near universally show decreased dependence on OXPHOS for ATP production and a greater reliance on other metabolic pathways such as glycolysis, amino acid metabolism, and fatty acid oxidation (to various degrees) to drive ATP production and numerous cellular processes. These observations therefore refine Warburg’s original observations, rather than contradict. Evidence has also accumulated establishing metabolic dysfunction as a strong contributor and predisposing factor to tumorigenesis. It has been shown that prolonged metabolic dysfunction can initiate tumorigenesis via retrograde (RTG) responses, reactive oxygen species (ROS) mediation, and apoptotic or hypoxia inducible factor (HIF)-mediated pathways [14,15,16,17,18,19,20].
The zeal to investigate altered metabolism waned with the advent of gene sequencing in the 1970s and 1980s (the beginning of the “genomics era”) and did not receive significant attention until large-scale next-generation sequencing (NGS) studies began to identify mutations in metabolic genes and pathways [21,22,23,24,25,26,27,28,29,30,31,32]. Decades of research have established causal links between metabolic dysfunction and metabolic disease, neurodegenerative disease, and cancer. These findings have since then reinvigorated research efforts studying metabolic dysfunction in cancers.
While cancers are complex multi-factorial diseases, previous research makes a compelling case implicating metabolic dysfunction as a contributing and/or predisposing factor in many cancers. These observations, however, gloss over the intimate coupling between a cell’s metabolic and epigenetic states. Cellular metabolic states regulate and drive epigenetic changes, while also playing key roles in epigenetic rewiring in response to cell states. Conversely, epigenetic changes can drastically alter cellular metabolics and dependencies. We are therefore only now beginning to piece together the puzzle of how cancer cells meticulously modify and control these two linked and coupled cellular processes in order to maintain their tumorigenic potential and capacity.
In this review, we will be discussing the cellular “powerhouse”, the mitochondria, with a specific focus on the mitochondrial respiratory chain (MRC). The MRC is composed of the five mitochondrial complexes I to V (C-I to C-V). Closely tied to MRC function is the tricarboxylic acid (TCA) cycle. Discussions will then outline how cells respond to metabolic dysfunction and what conditions can arise as a consequence, focusing on MRC and TCA dysfunction and cancer. We will then discuss the epigenome, how it is regulated, and how it is maintained. Discussions will then progress to outline how epigenetic regulators are critically dependent on metabolic cofactors. We will discuss how perturbation of cofactor concentrations adversely affects and alters the function and efficiency of said regulators, with consequent adverse effects on the epigenome. We will then close with a discussion on how closely intertwined metabolism and epigenetic processes truly are, and what targeted therapies are currently in development for cancer.
2. The Mitochondrion—Discovery and Structure
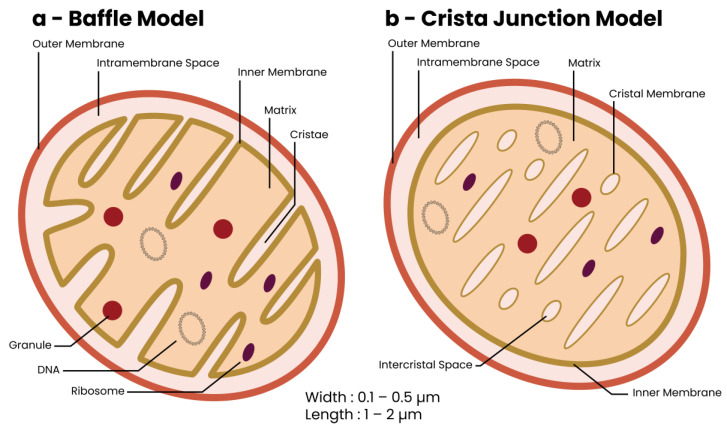
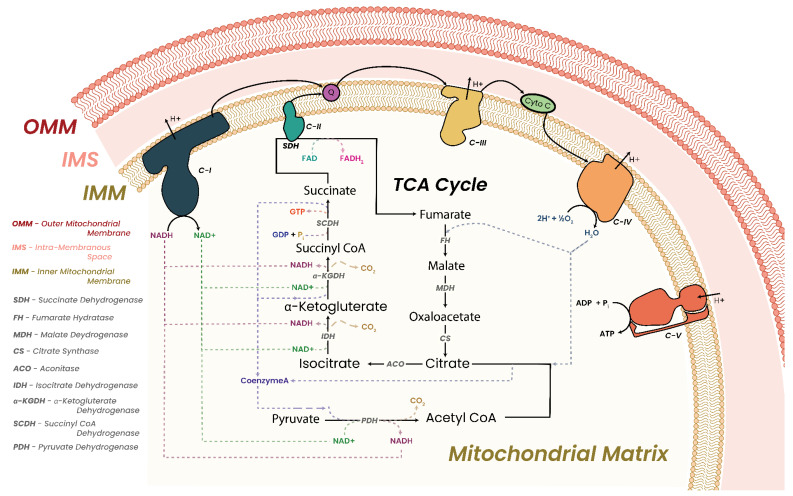
First observed in the late 1800s, mitochondria are abundant organelles in all mammalian eukaryotic cell types except erythrocytes [33]. Mitochondrial function was linked to cellular respiration in the early 1900s to 1950s, when the advent of electron microscopy enabled observation of their morphological structure for the first time [34]. Mitochondria are oval or rod-shaped double-membraned organelles with outer and inner mitochondrial membranes (OMM and IMM, respectively) separated by an inter-mitochondrial space (Figure 1) [35,36,37,38]. EM studies have led to the development of two models describing the internal structure of mitochondria, the baffle model and the crista junction model (Figure 1a,b, respectively) [34,39]. The baffle model sees the cristae as random in-folds of the IMM while the crista junction model sees cristae as stacks of independent membranous lamellae. High-resolution EM has shown the internal structure of a mitochondrion to be a hybrid of both models in that cristae are stacks of independent membranous lamellae with random in-folds [39,40].
Figure 1.
Internal structure of a mitochondrion. The mitochondrion is a double-membrane organelle with an outer membrane (OMM), an inner membrane (IMM), and numerous stacked independent membranous lamellae named cristae. Two models have been proposed describing the internal structure of a mitochondrion, (a) The baffle model sees cristae as random in-folds of the IMM with in-folds extending inwards towards the center of the mitochondrion. (b) The crista junction model sees cristae as independent membranous lamellae. These can be considered as “bubbles” within the inner mitochondria. EM micrographs have shown that the internal structure of a mitochondrion is a hybrid of both proposed models as has been shown in the literature [39,40]. Those interested are urged to view the EM micrographs in the respective publications to appreciate the internal structure of the mitochondria.
The mitochondrial OMM separates the organelle from the cytoplasm. Compositionally, it resembles the cellular membrane albeit with a higher lipid concentration, thus facilitating diffusion of lipophilic molecules into the intramembranous space [41,42]. Hydrophilic and small proteins are transported across the OMM through the voltage-dependent anion channels (VDACs), which are ubiquitously expressed in the OMM [43,44,45]. In contrast, the IMM is structurally and compositionally different from the OMM. It is composed of almost 50% more protein and nearly 50% less lipid [41,42,46]. Almost exclusively found in the IMM is the lipid cardiolipin, which has been shown to be essential for normal mitochondrial function [41,42,46,47,48]. The IMM is impermeable to uncharged proteins > 150 daltons in size and contains numerous translocator proteins that facilitate import of charged molecules required for physiological functions (such as adenosine triphosphate (ATP), glutamate, and α-ketoglutarate (α-KG)) [49,50]. Structurally, the IMM forms folds/invaginations named cristae which drastically increase the IMM’s surface area, maximizing ATP production through the MRC [34,36,40].
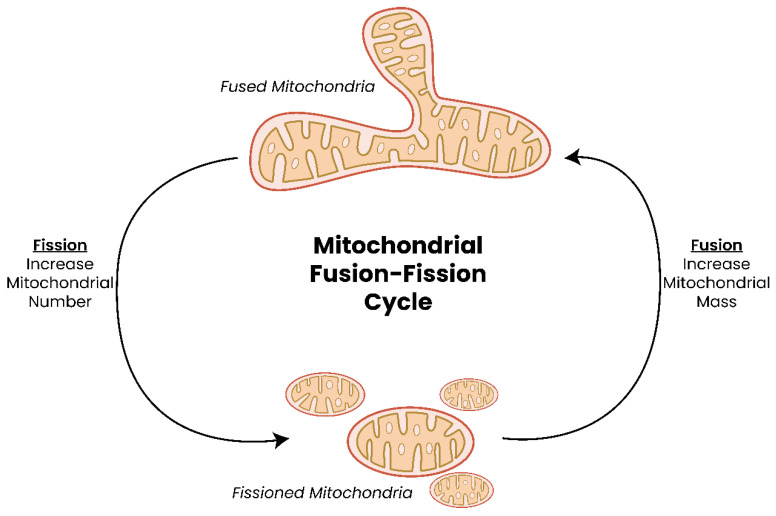
Under conditions of high energy usage, such as during replication or stress, mitochondria fuse to form extended reticular networks which are disassembled when energy requirements stabilize through a process called mitochondrial fission (Figure 2) [34,51,52,53,54,55,56]. Numerous proteins have been implicated in facilitating fusion and fission events and include myeloid cell leukemia (Mcl1, encoded by MCL1), B-cell lymphoma-extra-large (Bcl-xL, encoded by BCL2L1), mitochondrial dynamin-like 120 kDa protein (encoded by OPA1), mitofusion 1 or 2 (encoded by MFN1/2), dynamin-1-like protein (Drp1, encoded by DNM1L), phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (encoded by PINK1), and parkin RBR E3 ubiquitin protein ligase (PARKIN, encoded by PARK2) [55,57,58,59,60].
Figure 2.
Mitochondrial fusion and fission. Mitochondria readily fuse to form large extended networks to produce greater amounts of ATP under conditions of stress or replication. The extended network is then able to break apart through the process of fission, giving rise to individual mitochondria once more. If a mitochondrion is identified to be extensively damaged or experiences sustained depolarization, it undergoes lysosomal degradation; a process known as mitophagy.
Mitochondria are unique in that they are the only eukaryotic organelle to contain their own genetic material, mitochondrial DNA (mtDNA) [61,62,63]. Mammalian mtDNA encodes two ribosomal and twenty-two transfer RNAs along with thirteen MRC subunit proteins (seven proteins for C-I, one protein for C-III, three proteins for C-IV, and two proteins for C-V [53]). Mitochondria are thus heavily dependent on nuclear-encoded proteins for their biological functions. Transcription of mtDNA has been reported to depend on over 100 nuclear-encoded proteins [53,64], and over 1000 nuclear-encoded proteins must be imported into the mitochondria via translocator of outer membrane (TOM) and translocator of inner membrane (TIM) proteins [50,53,64,65]. Such a high interdependence between mitochondrial and nuclear compartments requires regulation and thus necessitates extensive mitochondrial–nuclear crosstalk [66,67,68]. Research has shown that disruption to this bi-directional communication and/or damage to either compartment triggers compensatory mechanisms in an attempt to restore normal cellular functions [20,67,69,70,71].
3. Mitochondrial Cellular Roles
Mitochondria are typically referred to as cellular “powerhouses”, a term first coined in the 1950s [72]. However, mitochondria are also responsible for other cellular functions such as iron–sulfur (Fe-S) cluster formation [73], calcium homeostasis [74], apoptosis regulation [75,76,77], cell signaling, generation of ROS [15,18,50,78,79,80], and generating essential metabolite cofactors required for epigenetic cellular processes (as will be discussed later). Evidence has also implicated mitochondrial involvement in lipid metabolism [81] and autophagosome formation [82].
3.1. Oxidative Phosphorylation and Energy Production
Energy production in the mitochondria is performed by the five MRC complexes via OXPHOS. C-I to C-IV form the electron transport chain (ETC), and together with C-V, they make up the five MRC complexes. In a three-step process, energy is released from the ETC and stored as an electrochemical gradient or transmembrane potential across the IMM. This electrochemical gradient drives the conversion of adenosine diphosphate (ADP) to ATP by ATP synthase (C-V) [83]. Generating ATP via OXPHOS has been shown to be up to 15 times more efficient than glycolysis under anaerobic conditions. It has been established that a critically important contributing factor for this increased efficiency is the spatial arrangement of the MRC complexes in the IMM [53].
To date, two models have been proposed for the arrangement of MRC complexes on the IMM, the random collision model and the solid-state model [84,85]. The random collision model proposed that the MRC complexes randomly distribute across the IMM and electron transfer is a random encounter between individual complexes and electron carriers. Contrary to this, the solid-state model proposed that electron transfer is directed from one enzyme to the next within supercomplexes/respirasomes. EM evidence has accumulated supporting the solid-state model and the organization of the MRC complexes in respirasome/supercomplex structures [86,87,88,89,90,91]. These respirasomes are constructed from the ETC complexes (NADH dehydrogenase (C-I), succinate dehydrogenase (C-II), ubiquinol-cytochrome c oxidase (C-III), cytochrome c oxidase (C-IV)) and are arranged in numerous configurations such as complexes I/III2 (1*C-I, 2*C-III), III2/IV1–2 (2*C-III, 1 or 2*C-IV), I/III2/IV (1*C-I, 2*C-III, 1*C-IV), and II/III/IV (1*C-II, 1*C-III, 1*C-IV) [89,92,93,94,95,96,97]. Interestingly, a large number of individual C-II units are not found incorporated into respirasomes, possibly owing to the involvement of C-II in the TCA cycle as well as the ETC. The most commonly observed respirasome structure is the I/III2/IV configuration, although tissue and species differences have been observed [88,89].
In addition to the ETC supercomplexes, ATP synthase has been shown to form dimer pairs (C-V2) which arrange linearly throughout the IMM in a manner separate to respirasomes [89,95].
3.2. Iron–Sulfur (Fe-S) Cluster Formation
Iron–sulfur clusters are inorganic cofactors found in many proteins. These cofactors are essential for numerous biological processes such as, but not limited to, enzyme catalysis, electron transfer, and gene expression [98,99,100]. Iron–sulfur cluster assembly has been shown to take place within the mitochondrial matrix, cytosol, and nucleus [98,101].
Within the mitochondria, C-I, C-II, and C-III utilize Fe-S clusters as part of their “catalytic cores” owing to their ability to readily donate and accept electrons. MRC C-I contains eight Fe-S clusters, all of which can be found in the “matrix arm” of C-I. C-II contains three Fe-S clusters while C-III contains only one Fe-S cluster. These cofactors are essential components of these MRC complexes.
3.3. Calcium Homeostatic Control
Controlling intracellular calcium concentrations is a critically important task, one in which the mitochondria play a central role [102,103]. Free calcium ions (Ca2+) are involved in numerous cellular processes, including being primary and/or secondary messenger molecules or apoptosis-initiating factors [102,104,105,106].
Intracellular Ca2+ is drawn into the mitochondria through the VDAC in the OMM and through the Ca2+ uniporter in the IMM, as a result of transmembrane potential [107,108,109]. Ca2+ efflux is controlled by the mitochondrial sodium/calcium (Na+/Ca2+) exchanger [110,111]. In doing so, mitochondria control intracellular Ca2+ concentrations in parallel to controlling the efficiency of the TCA cycle. This is because three TCA cycle dehydrogenases (pyruvate dehydrogenase, oxoglutarate dehydrogenase, and nicotinamide adenine dinucleotide (NAD+)-dependent isocitrate dehydrogenase (NAD+-IDH)) are regulated directly and/or indirectly by mitochondrial Ca2+ concentration [104,111,112,113,114].
3.4. Mitochondria and Cell Death
Four forms of cell death have been documented—apoptosis, necrosis, autophagy, and parthanatos—all four of which require mitochondrial involvement.
4. Apoptosis
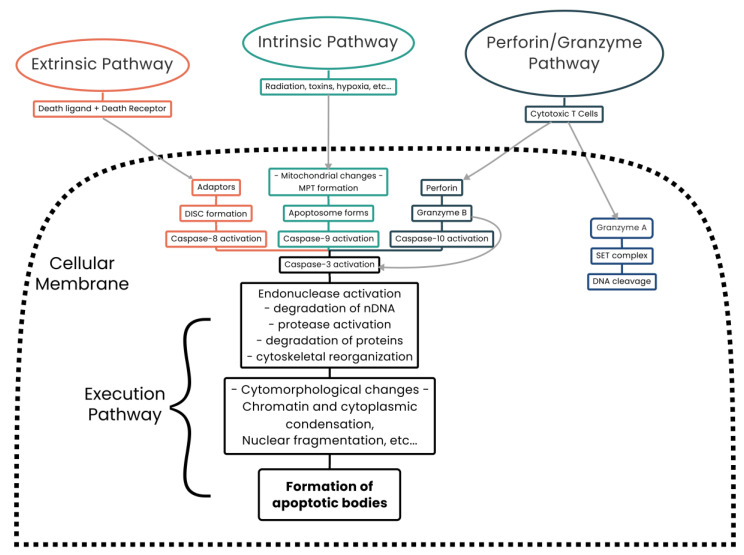
Apoptosis is triggered once a genetically predetermined set of conditions is reached, hence the term “programmed cell death”. In mammalian cells, two apoptotic pathways exist, the intrinsic and extrinsic pathways. A third apoptotic pathway has also been described involving T-cell-mediated cytotoxicity and perforin–granzyme-dependent killing of cells [115,116,117,118,119] (Figure 3).
Figure 3.
The cellular apoptotic pathways. Three apoptotic pathways are defined in the literature—intrinsic, extrinsic and perforin/granzyme-mediated apoptosis, which is unique to T-cell lymphocytes and natural killer cells. Each pathway has unique triggering conditions with all three converging on caspase-3 activation, after which the execution apoptotic pathway is common.
The intrinsic pathway has two initiating conditions. The first is the absence of growth factors, hormones, and cytokines, leading to a failure of suppression of pro-apoptotic proteins (bcl-2-like protein 4 (Bax) and Bcl-2 homologous antagonist/killer (Bak)).
The second is by external stimuli such as radiation, toxins, infection, and free radicals. Intrinsic pathway stimulation results in Bax and Bak localizing to the OMM and forming the mitochondrial permeability transition (MPT) pore, releasing cytochrome c (C-IV), second mitochondria-derived activator of caspases (SMAC, also known as DIABLO), and the mitochondrial high-temperature requirement serine protease HTRA2 (also known as Omi) [120,121,122,123,124,125,126]. This results in activation of the caspase-dependent mitochondrial pathway and culminates in cell death [117].
The extrinsic apoptotic pathway, also known as the death receptor pathway, requires a ligand–receptor interaction wherein a ligand, such as tumor necrosis factor-α (TNF-A) or TNF-related apoptosis-inducing ligand (TRAIL, also known as APO2L), binds to a member of the tumor necrosis factor (TNF) receptor superfamily [127]. On ligand binding, cytoplasmic proteins are recruited to bind to the receptor and a death-inducing signaling complex (DISC) is formed (also known as the Fas-associated death domain protein (FADD)) which activates caspase-8 [128,129]. Activated caspase-8 then cleaves BH3-interacting domain death agonist (Bid), which interacts with Bax–Bak, triggering their localization to the OMM and facilitating the formation of an MPT pore [130]. Opening of this pore causes transmembrane depolarization and cytochrome c (C-IV) release and triggers the remainder of the apoptotic cascade [131].
The third form of apoptosis identified is unique to cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells which are capable of killing target cells via the extrinsic apoptotic pathway or via a separate and unique cascade of events. On target cell binding, CTLs release the pore-forming molecule perforin which facilitates transfer of cytoplasmic granules [115,116,132]. Granzyme B, a serine protease which cleaves Bid, is the most important component of these granules [115,116,132,133]. Cleaved Bid then interacts with Bax and Bak, triggering their localization to the OMM where an MPT pore is formed, causing transmembrane depolarization and cytochrome c (C-IV) release and triggering target cell apoptosis [132,133]. Thus, irrespective of which apoptotic cascade is utilized, mitochondria play a central role in the activation of apoptosis.
5. Necrosis
Traditionally, four types of necrosis are observed microscopically—coagulative, colliquative, fibrinoid, and caseating necrosis [134]. Necrosis has been viewed as an unregulated form of cell death that occurs due to physical injury of the cell [135]. However, studies have shown that necroptosis, a fifth form of necrosis, is a highly controlled form of cell death that is controlled by “death machinery” (receptor-interacting serine/threonine protein kinases (RIPK)) and is triggered by stimuli such as cell death ligands, infection, DNA damage, and oxidative stress [119,134,135]. Signs of necrosis include cellular content leakage, cytoplasmic granulation, and organelle and/or cellular swelling [136].
An initiating factor of necrosis is ATP depletion [137]. This leads to increased intracellular Ca2+ concentrations resulting in mitochondrial calcium overload. Calcium overload then triggers the opening of the mitochondrial permeability transition pore (mPTP), of which the VDAC is a component [65,138,139]. The opening of the mPTP significantly affects the transmembrane gradient, disrupts OXPHOS, leads to mitochondrial morphological changes, and triggers the necrosis cascade [65,139]. Additionally, ROS are also able to trigger necrosis by disrupting lipids, proteins, and DNA, resulting in mitochondrial dysfunction and loss of OMM integrity [139].
6. Parthanatos
Parthanatos is a regulated cell death process dependent on the activity of poly ADP-ribose-polymerase-1 (PARP-1) [140,141]. DNA damage due to nicks, breaks, ROS, or ionizing radiation results in overactivation of PARP-1 which consumes NAD+ and depletes ATP stores which potentially inhibits both OXPHOS and glycolysis [140,141,142]. Overactivation of PARP-1 leads to poly ADP-ribose (PAR) synthesis and accumulation, which binds to apoptosis-inducing factor (AIP) on the OMM, facilitating its release from the mitochondria and its translocation to the nucleus [140,142]. Once in the nucleus, PAR induces DNA fragmentation and chromatin condensation which is believed to trigger the caspase-independent cell death cascade [140,141,142].
7. Autophagy
Autophagy is the process by which non-essential or damaged cellular constituents are broken down and recycled [143,144,145,146]. Three types of autophagy have been described—macroautophagy (commonly referred to as simply “autophagy”), microautophagy, and chaperone-mediated autophagy [147,148].
Macroautophagy involves vesicle (autophagosome) formation around the target cellular component which then fuses with lysosomes to degrade the contents by acidic hydrolases [148]. Microautophagy occurs when lysosomes directly wrap around cellular contents to be degraded and chaperone-mediated autophagy occurs when chaperone proteins translocate target proteins into lysosomes directly [148]. A specialized role of autophagy, known as mitophagy, has also been described which targets mitochondria requiring degradation and recycling [82,149]. Of note, mitophagy is the only known means by which mitochondria are recycled by the cell [150].
In order for cells to carry out autophagic processes, cells require functional mitochondria. This is because mitochondrially produced ROS are essential inducers of autophagy [147,149,151].
7.1. Cell Signaling and ROS
Mitochondria are the main producers of ROS intracellularly as a by-product of OXPHOS generated by C-I, C-II, and C-IV [152,153]. Increased levels of ROS can be generated by the ETC due to subunit damage due to mutation or by ETC inhibition by environmental factors or chemical compounds [154,155,156]. Unsequestered ROS can damage numerous intracellular components such as lipids, proteins, RNA, and DNA [152,157,158,159,160,161]. Increased ROS levels are regularly observed in tumor cells and have been linked to enhancing tumor phenotypes [152,162,163]. It is now accepted also that ROS are intracellular signaling molecules and can modulate cellular functions such as protein function, signaling cascade efficiency, autophagy, or stem cell differentiation, especially in hematopoietic stem cells (HSCs) [147,152,164,165,166].
Modulation of cellular differentiation by ROS is believed to occur via modulation of gene expression of p38 mitogen-associated protein kinases (MAPKs) [167], p53 [168], forkhead box (FOXO) proteins [169], nuclear factor-κB (NF-κB) [170], histone deacetylases (HDACs), and polycomb proteins [171] and through the phosphatidylinositol-4,5-bisphosphate 3-kinase/protein kinase B/mechanistic target of rapamycin (PI3K/AKT/mTOR) signaling pathway [172]. HSCs have been shown to contain low levels of ROS while committed myeloid lineage progenitors contain significantly greater levels [169]. Therefore, controlling ROS production (by controlling OXPHOS) and ROS scavenging mechanisms are critically important for stem and progenitor cells, dysregulation or damage of which could enable intracellular damage or drive unintended premature, or possibly block, differentiation [155,173,174].
7.2. Lipid Metabolism
Lipid metabolism occurs intracellularly on the endoplasmic reticulum (ER) [175,176]. Evidence has also shown that mitochondria contribute to this process by synthesizing phosphatidylethanolamine (PE) and cardiolipin [176,177,178]. In yeast, membrane PE is synthesized primarily in the IMM and then transported out to the cytosol. This process involves the protein ubiquitin-specific peptidase 1 (Usp1) [175,176,179]. Cardiolipin, which is found almost exclusively in the IMM, is essential for mitochondrial function and mitochondrial morphology maintenance [176,177,178].
8. Mitochondria and Disease
Thus far, we have discussed how mitochondria play significant roles in numerous cellular processes. It is therefore unsurprising that mitochondrial dysfunction due to deficiency, damage, or inhibition has been linked to numerous diseases. We will next discuss the cellular consequences of mitochondrial dysfunction and how mitochondrial dysfunction has been implicated in tumorigenesis and progression.
Cellular Consequences of Mitochondrial Dysfunction
Mitochondrial function can be affected by numerous conditions such as age [180,181,182], IMM morphological alterations [183,184], lipid concentration perturbations [48], inflammation [185], viruses [186], carcinogens [187,188], hypoxia [189], radiation [188], nuclear and mitochondrial DNA mutation [190,191], and altered expression of the MRC complexes [192,193]. All of these can lead to mitochondrial dysfunction which, phenotypically, can be mimicked by inhibiting MRC complexes using chemical compounds [156].
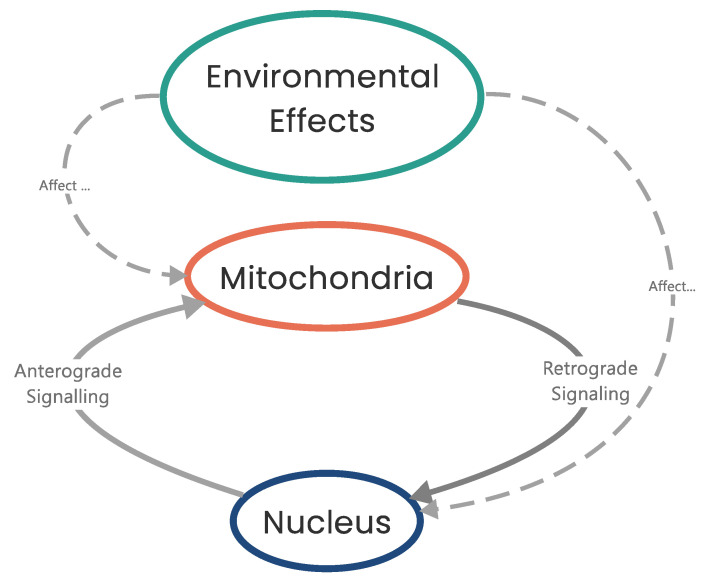
Mitochondrial dysfunction results in impaired respiration/ATP production which triggers a mitochondrial-to-nuclear signaling cascade of events known as an RTG response (Figure 4) [18,67,70,71,194,195]. An RTG response is triggered when changes to respiration and mitochondrial function are detected, resulting in a metabolic switch from OXPHOS to substrate-level phosphorylation (SLP) by modulating gene expression to maintain the intracellular ATP concentrations required for viability [20,28,69,70,71,194]. So, cells effectively trigger SLP whenever OXPHOS becomes compromised. Although RTG responses have been intensively studied in Saccharomyces cerevisiae [195], in mammalian systems, NF-κB/Rel factor responses have been found (through computational homology studies) to most closely resemble an RTG response in yeast [194].
Figure 4.
Nucleus-to-mitochondria communication pathways. Mitochondria communicate with the nucleus via retrograde (RTG) signaling pathways which are triggered on detection of mitochondrial dysfunction. The nucleus responds by anterograde signaling pathways. Environmental effects can trigger either RTG or anterograde signaling cascades.
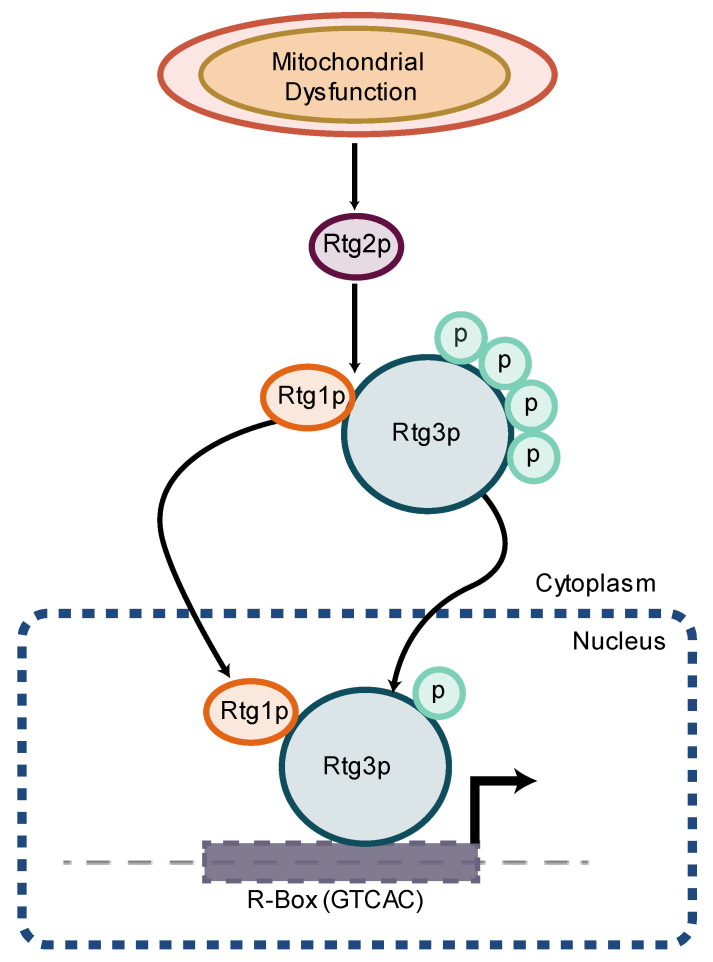
In yeast, activation of an RTG response results in the formation of a heterodimer between the two helix–loop–helix/leucine zipper proteins retrograde regulation proteins 1 and 3 (Rtg1 and Rtg3, respectively) in the cytoplasm which, with the aid of Rtg2, translocate to the nucleus and bind to the GTCAC (R box) sequence in the promoter region of RTG response target genes (Figure 5) [196,197,198]. In mammals, the V-Myc avian myelocytomatosis viral oncogene homolog–MYC-associated factor X (Myc-Max) transcription factors are homologous to the Rtg1–Rtg3 transcription factors [194]. In order to translocate to the nucleus, Rtg3 must be partially dephosphorylated and this is believed to be controlled by Rtg2 [197,199,200].
Figure 5.
RTG response in Saccharomyces cerevisiae. In Saccharomyces cerevisiae, an RTG response results in the formation of a heterodimer between Rtg1 and Rtg3 in the cytoplasm. Rtg2 then partially dephosphorylates Rtg3, facilitating translocation of Rtg1 and Rtg3 into the nucleus. The transcription factors then bind to the “R box” sequence in the promoter region of RTG target proteins. In mammals, the Myc-Max transcription factors are homologous to Rtg1–Rtg3 and parallels have been drawn between an RTG response in Saccharomyces cerevisiae and an NF-κB response in mammals.
Negative regulators of RTG signaling include negative regulator of RAS-cAMP pathway (Mks1) and the Bmh1 and Bmh2 proteins [195,200]. Mks1 forms a complex with Bmh1 and Bmh2 (yeast homologs of the 14-3-3 proteins in mammals) and maintain Rtg3 in a phosphorylated state, thus inhibiting translocation [197,201,202,203]. Additionally, Lst8, a subunit of the target of rapamycin (TOR) complex, negatively regulates RTG signaling in a manner distinct to RTG activation by mitochondrial dysfunction [201,202,204].
In mammalian systems, impaired respiration triggers an NF-κB/Rel factor response which causes upregulation of target genes such as Myc and Ras [17,19,20,205]. In a hypoxic setting, however, mammalian systems will also, in conjunction, upregulate the HIFα cascade [206,207,208]. Irrespective of whichever of these signaling pathways is triggered (NF-κB/Rel or HIF1α), the consequent result for the cell is increased Myc expression which enhances ROS production, modulates p53 function, and modulates the expression of genes required for SLP along with other cellular responses [209,210]. (Increased ROS has numerous cellular consequences and will be discussed later [67,211,212]).
P53, a well-established tumor suppressor, modulates numerous metabolic pathways [213,214]. P53 has been shown to modulate the expression of hexokinase and phosphoglycerate mutase, two key enzymes in the metabolism of glucose [215,216]. P53 mediates expression of tumor protein 53 (TP53)-induced glycolysis and apoptosis regulator (TIGAR) to inhibit glycolysis [217]. Loss-of-function p53 mutations, therefore, potentially lead to a loss of glycolysis control. Furthermore, overexpression of transmembrane glucose transporters GLUT1 and GLUT4, a common finding in many cancers [218,219], is implicated to be due, in part, to loss-of-function p53 mutation. P53 also activates the PI3K/Akt/MAPK/Ras signaling pathway [220], directly interacts with Bax, Bak, Bcl-2, and Bcl-xl, facilitating apoptotic signaling [221,222,223,224,225], and is regulated by liver kinase B1 (LKB1) [226]. LKB1 knockout mice are hyperglycemic and LKB1+/− mice crossed with p53 null mice show increased tumor incidences and significantly shorter lifespans compared to either mutation/deletion alone [227,228].
Apart from the direct modulation of RTG target gene expression, impaired mitochondrial respiration also triggers numerous compensatory mechanisms through mTORC1 and mTORC2, including, but not limited to, altering expression of genes required for SLP [229,230,231]. Together, these findings describe scenarios wherein mitochondrial dysfunction can lead to increased levels of DNA damage, upregulation of compensatory metabolic and regulatory pathways, and pathology [212,232,233]. Indeed, it is well established that metabolic dysfunction and oxidative stress are linked to metabolic and neurodegenerative diseases and, in certain cases, tumorigenesis and tumor persistence [212,234,235,236,237,238,239].
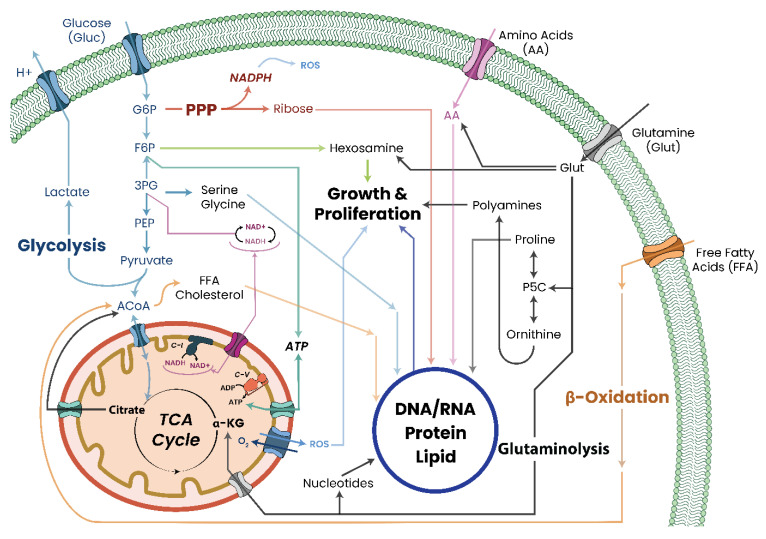
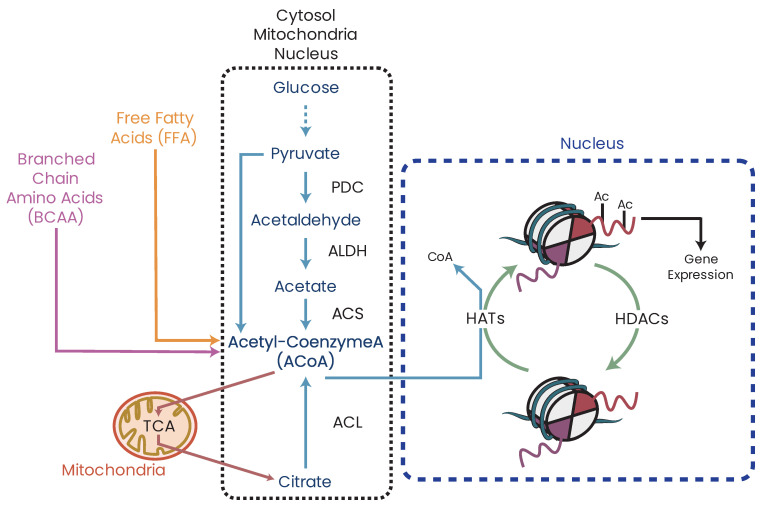
While metabolic deficiency can arise from direct damage/inhibition of MRC or TCA cycle proteins, it is not limited to these two systems only. As the mitochondria are the central metabolic regulators of the cell, damage, disruption, or inhibition of other metabolic pathways, such as glycolysis, FAO, or transamination, will have cascading effects on the mitochondria and their function by disrupting the availability of metabolites needed for respiration, particularly acetyl coenzyme A (ACoA) (Figure 6). This therefore implies that even dietary intakes, such as high-fat Western diets, can potentially induce chronic changes to cellular metabolic states and function which, over time, can trigger pathological states.
Figure 6.
Schematic outlining the relationships between cellular metabolic pathways and the mitochondria. Although separate, each metabolic pathway within the cell feeds back into the mitochondria by producing metabolites required for ATP production. Deficiency/damage/inhibition to any of metabolic pathway can result in metabolic deficiency, potentially triggering an RTG response. Legend: G6P—glucose-6-phosphate; F6P—fructose-6-phosphate; 3PG—glycerate-3-phosphate; PEP—phosphoenolpyruvate; ACoA—acetyl coenzyme A; α-KG—α-ketoglutarate; PPP—pentose phosphate pathway; P5C—1-pyrroline-5-carboxylate; TCA—tricarboxylic acid; ROS—reactive oxygen species and conditions [240]. Mitochondrial dysfunction can therefore be a major contributing factor to a varied range of pathologies with potentially serious debilitating and/or life-threatening consequences.
9. Mitochondrial Dysfunction and Cancer Initiation/Progression
Cancer cells, irrespective of originating tissue, have altered cellular metabolics and mitochondria. Over the years, comparative studies between healthy and tumor cell mitochondria have shown molecular, microscopic, metabolic, biochemical, and genetic differences in cancer contexts, while electron microscopy comparisons have shown tumors typically have fewer, structurally altered, and larger mitochondria [241,242,243,244,245,246]. Differential expression of MRC components, which is indicative of mitochondrial dysfunction, has also been linked to numerous cancers [23,247,248,249,250].
With the increasing prevalence of NGS studies, mitochondrial (mt) and nuclear (n) DNA mutations have been identified in numerous cancers including leukemia, breast, lung, liver, kidney, thyroid, ovarian, colon, and brain cancers [21,23,24,25,26,28,31,32]. Unlike nDNA, mtDNA mutations are identified in most cancers with varying prevalence rates (Table 1) [251,252]. Unfortunately, the functional and clinical consequences of the vast majority of these mutations have yet to be elucidated, although many are predicted to impact protein function [252,253]. Of the mtDNA variants that have been studied, consequences are varied with reports of both increased [254] and decreased survival in AML, decreased survival in renal cell carcinoma [255], and enhanced tumorigenicity [256]. Additionally, a survey of mtDNA copy number variation across 22 tumor types identified significant variation in mtDNA copy numbers across the surveyed tumors [257], with additional evidence reporting suppression of MRC gene expression across many cancers as well [23]. Research has therefore been presented highlighting the prevalence of mtDNA mutations, gene expression changes, and copy number variations in cancers, all of which likely lead to metabolic dysfunction and the altered metabolic phenotypes observed in cancers.
Table 1.
Selected somatic mtDNA mutation frequencies in cancers.
| Cancer | Somatic Mutation Frequency (%) |
|---|---|
| Skin | 16–75 |
| Head and neck | 49–78 |
| Thyroid | 23–100 |
| Breast | 30–93 |
| Lung | 43–79 |
| Esophageal | 5–55 |
| Gastric | 18–81 |
| Colorectal | 16–70 |
| Pancreatic | 16–92 |
| Liver | 40–68 |
| Renal | 27–79 |
| Urinary Bladder | 64–100 |
| Prostate | 19–88 |
| Ovarian | 20–80 |
| Endometrial | 9–63 |
| Cervical | 38–90 |
| Nervous system | <35 |
| Hematological | 30–50 |
| Connective tissue | <70 |
Summary of selected somatic mtDNA mutation frequencies across numerous cancers.
As discussed, mitochondrial insufficiency and/or dysfunction, possibly due to mtDNA mutation or copy number alterations, are sufficient triggers to initiate a cellular RTG response. This results in upregulation of genes required for SLP, genes that are commonly reported to be upregulated in both solid and liquid cancers [4,5]. Furthermore, persistent RTG response due to respiratory insufficiency has been shown to cause genomic instability and aberrant growth, two hallmarks of cancers [2,18,70,71,156,205,212,233]. Beyond metabolism, an RTG response also has implications for the cell epigenome, as it radically alters produced metabolite concentrations, which results in modulated or inhibited epigenetic modifier function and efficiency.
Due to the prevalence of the altered metabolic phenotype across nearly all cancers, an important question to raise is whether altered metabolism is causative or a result of transformation. This is discussed below.
10. Metabolic Dysfunction and Genomic Instability
In Saccharomyces cerevisiae, MRC dysfunction was modeled using small-molecule inhibitors. Sublethal doses of oligomycin (an inhibitor of C-V), antimycin A (an inhibitor of C-III), and potassium cyanide (an inhibitor of C-IV) were applied to yeast so as to ascertain what effects mitochondrial dysfunction has on genomic stability [212]. In doing so, Rasmussen et al. [212] noted an increased prevalence of nDNA mutations in all test groups compared to control cells. Oligomycin-, potassium cyanine-, and antimycin A-treated cells showed a 1.5-, 2-, and 3-fold increase in nDNA mutation frequency, respectively. Furthermore, antimycin A inhibition was found to increase both superoxide (O2−) and hydrogen peroxide (H2O2) levels in treated cells. These results showed that MRC inhibition has a nuclear mutator phenotype that is potentially a consequence of increased oxidative stress [212]. Other studies, though, investigated the matter further.
To further characterize the effects mitochondrial dysfunction had on genomic stability, yeast strains lacking their mitochondrial genome (rho0 strains) or with fragments of their mitochondrial genome deleted (rho− strains) were created [212]. Both yeast strains (rho0 and rho−) showed mitochondrial dysfunction and a 2–3-fold increase in nDNA mutation rates over wild-type strains (rho+ strains). Decreased levels of ROS were also measured in both strains, potentially due to decreased mitochondrial function. Therefore, the nuclear mutator phenotype observed was independent of oxidative stress-related mechanisms [212]. Rasmussen et al. therefore showed that mitochondrial dysfunction can result in increased nDNA mutation rates and is tumorigenic in nature, involving multiple pathways.
11. Metabolic Dysfunction and Aberrant Growth
Apart from gene mutation, mtDNA depletion is a commonly observed feature of many cancers [257,258,259,260]. To investigate the effects of mtDNA depletion on breast cancer tumorigenesis, Kulaweic et al. [233] developed a breast epithelial cell line devoid of mtDNA (ρ0 cells). Their results showed that, in vitro, ρ0 cells showed a tumorigenic phenotype with enhanced proliferative rates and invasive growth, increased rates of double-stranded DNA breaks, and unique chromosomal rearrangements [233]. Furthermore, ρ0 cells in a xenograft SCID mouse model showed a gain of tumorigenicity in normally non-tumorigenic cells and an enhanced tumorigenic phenotype in already transformed cells [233].
To better understand the effects of mtDNA depletion in their ρ0 cells, gene expression and pathway analysis was performed. Kulaweic et al. [233] identified 19 regulatory networks with genes showing > 10-fold change in expression in ρ0 compared to parental cell lines. One of the highest ranked networks identified had the genomic gatekeeper TP53 as a focus gene. Kulaweic et al. [233] found TP53 significantly downregulated in ρ0 cell lines and this was recapitulated in primary breast tumors as well [233]. To conclude, Kulaweic et al. [233] suggested that mtDNA depletion plays a role in breast epithelial cell transformation and involves multiple pathways.
Similar observations of increased tumorigenicity have also been documented in mouse C2C12 monocytes and human pulmonary carcinoma A549 cells [17,18,205]. Partial depletion of mtDNA or treatment with metabolic inhibitors resulted in invasiveness of normally non-invasive cells, phenotypes that were reversible on restoration of normal mitochondrial function [17,18,205]. Additionally, mtDNA-depleted cells showed evidence of cellular RTG responses when gene expression was assessed [17,18,205].
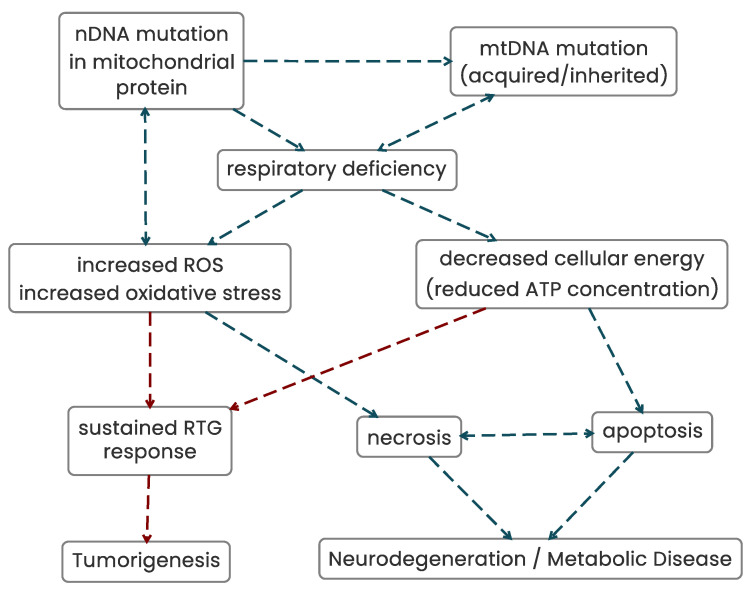
The evidence presented therefore suggests that mitochondrial dysfunction can potentially initiate genomic instability, trigger invasive growth, and initiate metabolic and neuronal diseases (Figure 7). However, the question arises, once genomic instability occurs, which phenotype, the mitochondrial dysfunction or the genomic instability, perpetuates the tumorigenic phenotype? To address this question, nuclear-to-cytoplasmic transfer experiments can be interrogated to shed light on this.
Figure 7.
The interactions between mitochondrial mutation, dysfunction, neurodegenerative disease, and tumorigenesis. Mutations in mtDNA and nDNA result in mitochondrial dysfunction. While metabolic dysfunction typically leads to metabolic disease and/or neurodegeneration, evidence suggests that metabolic dysfunction can trigger genomic instability and trigger tumorigenesis.
12. Mitochondrial Dysfunction and the Tumorigenic Phenotype
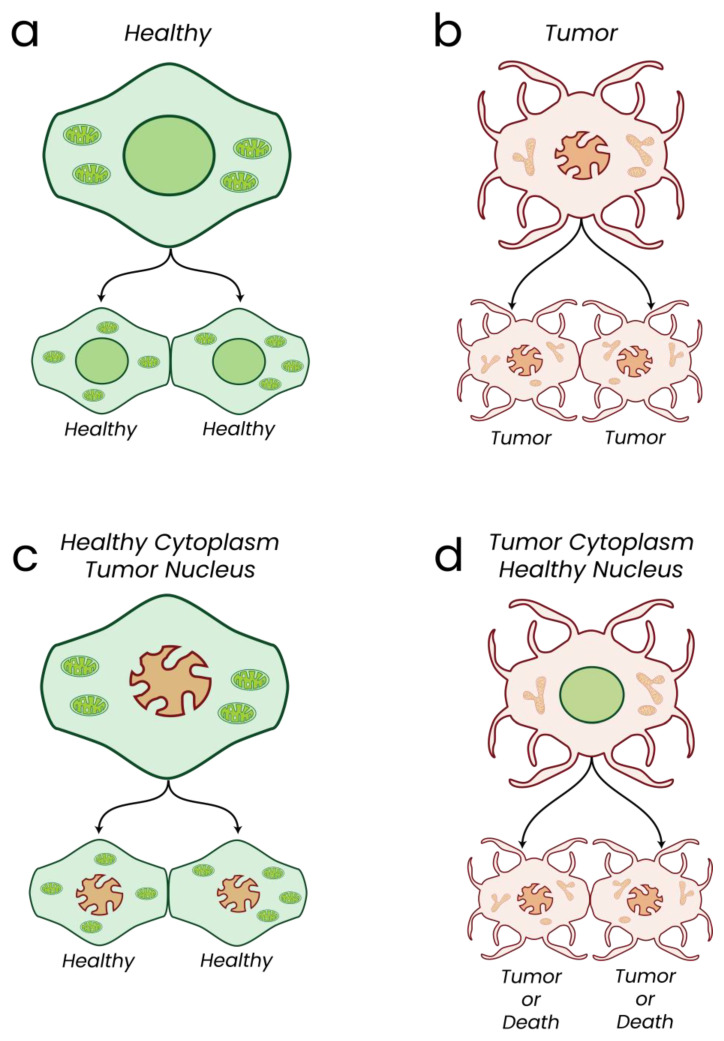
Healthy parent cells replicate, giving rise to healthy daughter cells (Figure 8a). Likewise, tumor parent cells give rise to tumor daughter cells (Figure 8b). Transfer of tumor-derived nuclei into healthy enucleated cells (cybrids) results in a suppression of tumorigenicity in vitro and in vivo (Figure 8c) [261,262,263,264,265,266,267,268,269,270,271,272]. Cytoplasmic elements can therefore drive the tumorigenic phenotype. To support this, healthy embryonic murine tissue derived from tumor nuclei showed normal phenotypic characteristics despite the persistence of melanoma and brain-associated nDNA mutations, while embryos derived from tumor nuclei did not develop tumors despite persistence of tumor-associated aneuploidy and nDNA mutations [269,273]. Genetic mutation or genomic rearrangements are therefore not necessarily sufficient to induce tumorigenesis alone [274]. Investigations transferring normal mitochondria into tumor cell cytoplasm showed a suppression of tumorigenicity in vitro, suggesting the cytoplasmic elements controlling the tumorigenic phenotypes are indeed the mitochondria [275,276]. Finally, introduction of mtDNA mutations in non-tumorigenic cybrids reverses the anti-tumorigenic effects, giving tumorigenic growth [277]. In 2014, Wahlestedt et al. determined that induced pluripotent stem cells with heavy mtDNA mutation burdens displayed extensive differentiation defects, thus highlighting that mtDNA mutation can lead to aberrant differentiation phenotypes of stem cells [278]. In 2011, Sharma and colleagues observed tumorigenic growth in the presence of C-I mtDNA mutation and a reversal of the tumorigenic phenotype on C-I function rescue. The presented findings showed that healthy mitochondria are able to suppress tumorigenesis despite the continued persistence of tumor-associated DNA mutations and rearrangements. In contrast, tumor-derived mitochondria induced an enhanced tumorigenic phenotype on transfer into healthy cell cytoplasm, suggesting mitochondrial function could be the stronger perpetuating force in continuing a tumorigenic phenotype (Figure 8d) [263,277]. Evidence has therefore been presented from both in vitro and in vivo data implicating mitochondria and mitochondrial dysfunction as key drivers of tumorigenesis and persistence. While DNA mutations undoubtedly can result in dysregulated and non-functional proteins with aberrant function, published evidence shows that mitochondrial dysfunction can have a strong influence on the presentation and continued persistence of tumorigenic phenotypes as shown in numerous in vitro and in vivo models. Future investigations to thoroughly characterize the mitochondrial and metabolic state of cancers would therefore likely lead to significant findings for better and longer-lasting patient treatment as they would be targeting a significant driving force of the tumorigenic phenotype. It is important to clarify, however, that once somatic mutations accumulate within the genome, the combined deleterious effects of both metabolic dysfunction and abnormal/aberrant protein function would unquestionably be significantly deleterious to the state of the cell.
Figure 8.
Key findings of nuclear-to-cytoplasmic transfer experiments. Nuclear-to-cytoplasmic transfer experiments highlight the significant role mitochondria play in initiation and persistence of a tumorigenic phenotype. Details of models utilized can be found in text.
13. Mitochondrial Horizontal Transfer Experiments
Over the years, evidence has been presented describing horizontal transfer of mtDNA and/or mitochondria from non-cancerous to cancerous cells which results in restoration of cellular respiration and, in certain scenarios, enhances the tumorigenic potential of recipient cells [279,280,281,282,283]. While such findings initially seem to refute evidence presented that healthy mitochondria can suppress a tumorigenic phenotype, careful examination of these findings only further supports previous evidence and, arguably, partially validates Warburg’s original observations and hypothesis.
Healthy phenotypes are colored green while cancerous phenotypes are colored red.
a—Healthy cells give rise to healthy cells.
b—Tumor cells give rise to tumor cells.
c—Transfer of tumor nucleus into a healthy cytoplasm leads to healthy cells despite persistence of tumor-associated genomic mutations and instability.
d—Transfer of a healthy nucleus into a tumor cytoplasm gives rise to tumor cells or death.
For the following discussions, one should take note that the state of a mitochondrion is dependent on four characteristics, (i) mtDNA integrity (i.e., lack of a significant number of mutations and/or insertions/deletions), (ii) sufficient mtDNA copy numbers (which are tissue specific), (iii) the morphological shape of the mitochondrion (both internally and externally), and (iv) the function of internal metabolic pathways such as the MRC and TCA. Cancers show abnormalities in multiple aspects of the mitochondrial state. MtDNA mutations have been linked to, and are causative of, many diseases, including some cancers. MtDNA copy number alterations (both increased and decreased copy numbers) have been associated with numerous diseases, including Parkinson’s disease [284], heart disease [285], and autism [286], and copy number alterations are observed in most cancers [257]. Finally, cancers universally show abnormal mitochondrial morphology and distinct metabolic phenotypes [25,212,287,288,289,290,291,292,293].
In 2006, Spees and colleagues described mitochondrial transfer from human MSCs to A549 lung adenocarcinoma cells. In their studies, Spees et al. reported that the A549 cells with donor mitochondria and mtDNA showed restoration of mitochondrial respiration [279]. While these claims are valid, on examination of the published results, the restoration of the cancer cells’ respiratory capacity was not complete and the recipient clones showed gene expression and gross mitochondrial number differences compared to donor MSCs. Consequently, the mitochondria did not recover to the state of their healthy donor counterparts and could not be classified as “healthy” in all aspects of their mitochondrial state despite partial restoration of respiration. Tan et al. [281] made similar observations that mtDNA was found to transfer to donor cell lines, restoring cellular respiration. Tan et al. also observed an enhancement of tumorigenic potential of their cell lines on acquisition of mtDNA [281]. Similar to the findings of Spees et al., examination of the published findings identifies that recipient clones differ in numerous mitochondrial characteristics from their donor cells, ranging from gross mitochondrial number deficits, morphological abnormalities, mtDNA copy number alterations, and gene expression differences [281]. The enhanced tumorigenic phenotype observed can therefore be expected and parallels previously published results that abnormal mitochondria can perpetuate a tumorigenic phenotype [263,277]. These experiments therefore do not contradict the evidence presented previously but, rather, support it.
An additional caveat in interpreting the aforementioned studies relates to the circumstances and direction in which such mitochondrial transfers occurred [280,283,294]. Cho et al. [294] were able to deduce that gross mitochondrial transfer (not mtDNA transfer) only occurred between human MSCs and 143B human osteosarcoma cells that were depleted of mtDNA and did not occur between MSCs and 143B human osteosarcoma clones with mutated mtDNA. These results suggest that mtDNA damage (such as is commonly seen in cancers) may not be a sufficient trigger to instigate horizontal mitochondrial transfer and only extreme levels of mitochondrial damage and depletion will trigger such cell–cell transfers. These results also suggest that neighboring cells can “assist” a cell suffering from extreme mitochondrial dysfunction in order to maintain its respiratory capacity and avoid cell death. Wang and Gerdes [283] observed uni-directional transfer of mitochondria from untreated PC12 rat pheochromocytomas to UV-treated PC12 rat pheochromocytoma cells, which prevented the UV-treated cells from undergoing apoptosis. As discussed, ATP insufficiency is sufficient to trigger cellular death and restoration of mitochondrial respiration by mitochondrial transfer would prevent activation of the apoptotic cascade. Finally, Lou et al. [295] reported mitochondrial transfer between mesothelioma cell lines or between primary human mesothelioma cells but not between cancerous and normal cells [295]. These results therefore suggest that mitochondrial horizontal transfer is context dependent and may not necessarily be a universal phenomenon.
In conclusion, results obtained by mitochondrial transfer experiments, while insightful, do not contradict the nuclear transfer experiments previously described as, despite the transfer of mtDNA and/or mitochondria, numerous aspects of the mitochondria’s state remain altered and the resultant hybrids are not identical to “healthy” donor mitochondria. Furthermore, the assistance of “healthy” cells to donate mitochondria to their metabolically compromised neighboring cancer cells is indicative of cancer cells having defective mitochondria and/or mitochondrial respiration. As this process is predominantly uni-directional (i.e., “healthy” to “cancer”), this potentially supports Warburg’s original hypothesis that cancer cells have compromised mitochondria, a phenomenon that neighboring cells can become aware of (potentially through paracrine signaling) and they can assist in restoring partial mitochondrial function of compromised cells.
14. Investigations of Altered Metabolism in Cancers
Many researchers have investigated altered metabolism in cancers [296,297]. Cancers universally show altered metabolic phenotypes and abnormal mitochondria [25,212,288,289,290,291,292,293]. Genomic studies have reported catalogs of mtDNA mutations in numerous cancers [1,21,25,31,252,254,256,257,278,287,296,298,299,300,301,302,303,304,305,306,307,308,309,310]. Despite retaining partial functionality, mitochondria in tumors consistently show mtDNA mutation, structural abnormalities, and diminished functional capacities and tumor cells universally have distinct, reprogrammed metabolic phenotypes which are not observed in non-transformed tissues and cells. To date, no study has yet described a tumor with mitochondria that are functionally, structurally, and genetically indistinguishable from non-transformed cells, to the best of our knowledge. While upregulation of metabolic pathways other than OXPHOS can compensate for any potential defects in OXPHOS function, evidence has yet to be presented establishing MRC dysfunction as resultant to stresses within tumor cells and environments.
To establish that MRC dysfunction is consequent to tumor cell genomic and environmental stresses, doxycycline-inducible models have been investigated. While mutation-inducible models have been described [311,312,313,314], the investigations by Ying and colleagues [311] assessed the metabolic state of their model post doxycycline induction. In their study, Ying et al. [311] described a doxycycline–KRAS-inducible model wherein activation of a KRAS G12D mutant resulted in development of pancreatic ductal adenocarcinoma in nude mice. On activation of the oncogenic KRAS mutant, altered metabolic pathway dependencies were observed, including an increased dependence on glycolysis [311]. However, a caveat in interpreting these studies is that doxycycline has known effects on cellular metabolism and considerably shifts cellular metabolism towards a more glycolytic phenotype at commonly used concentrations of 100 ng/mL–5 μg/mL [315]. Therefore, without appropriate experimental controls, use of doxycycline confounds the effects of the mutation under investigation and the true consequent metabolic effects become ambiguous at best. Furthermore, the work of Chang and colleagues [316] showed that doxycycline directly activates the PI3K-Akt signaling pathway, which has been shown to enhance survival and self-renewal in vitro [156,172,316]. Investigations performed using doxycycline-induced models must therefore be carefully analyzed in light of these findings to accurately ascertain the oncogenic effect of the induced mutation and any consequent metabolic changes that are potentially resultant.
15. MRC Dysfunction and Cancer
15.1. C-I Dysfunction and Cancer
NADH dehydrogenase (C-I) (Figure 9) is the largest complex of the ETC and is a main site of mitochondrial ROS production [317,318]. Of the five MRC complexes, C-I dysfunction is the most common mitochondrial defect observed, leading to disease and cell death [319,320]. Somatic mutations in mitochondrial and nuclear C-I genes have been found in numerous cancers, including leukemia, breast, thyroid, bladder, prostate, colon, pancreatic, and head and neck cancers, and renal carcinomas (select variants listed in Table 2) [31,300,302,303,305,321,322,323,324,325,326,327,328]. Reported C-I variants are predominantly mitochondrially encoded, with fewer data available on somatic nDNA C-I variants. Germline data are more difficult to acquire still, with few publications highlighting their functional significance [329]. Mutations in C-I have also been linked to increased ROS production and increased metastatic potential in mouse tumor cell lines, colorectal cancer cell lines, and HeLa cells [156,304,330]. The degree to which C-I dysfunction plays a role in tumorigenesis and progression therefore varies and depends on both the degree of complex function disruption and tissue type [331,332,333].
Figure 9.
The MRC and TCA cycles. The MRC and TCA are tightly coupled. Metabolites from both pathways feed into each other to maintain function. Mutations in both the MRC and TCA have been identified in numerous cancers with established links of said mutations being tumor inducing.
Table 2.
Select somatic C-I variants identified in cancers.
| Cancer (Reference) | C-I Gene Mutations Identified |
|---|---|
| Acute Myeloid Leukemia [31] | NDUFA12—3′ UTR—somatic NDUFA13—p.S57P—somatic NDUFAF2—intronic e2-16687—somatic NDUFS4—intronic e3-5101—somatic —5′ UTR—somatic NDUFS7—intronic e2+243—somatic NDUFV2—intronic e2-1653—somatic MT-ND1—p.Y43H—somatic —p.E204—somatic —p.E204—somatic MT-ND2—p.V43A—somatic MT-ND4—p.I8T—somatic —p.T350—somatic —p.V234—somatic MT-ND5—p.P242fs—somatic —p.L260P—somatic —p.S345P—somatic —p.S476P—somatic —p.N452S—somatic |
| Acute Lymphoid Leukemia [324] | MT-ND4—p.L-P variant—somatic |
| Chronic Lymphoid Leukemia [324] | MT-ND1—p.F-S variant—somatic |
| Thyroid Cancer [321] | MT-ND2—p.S-F variant—somatic —p.I-V variant—somatic —frameshift—somatic MT-ND4—p.S-F variant—somatic —p.E-K variant—somatic MT-ND5—p.L-K variant—somatic —p.S-F variant—somatic —p.I-V variant—somatic —p.D-G variant—somatic —p.A-G variant—somatic —p.S-M variant—somatic —p.I-V variant—somatic MT-ND6—p.V-A variant—somatic —p.W-R variant—somatic |
| Oncocytomas [303] | MT-ND1—p.G120X—somatic —p.G244X—somatic MT-ND4—p.374X—somatic MT-ND5—p.540X—somatic MT-ND6—p.87X—somatic |
15.2. C-II Dysfunction and Cancer
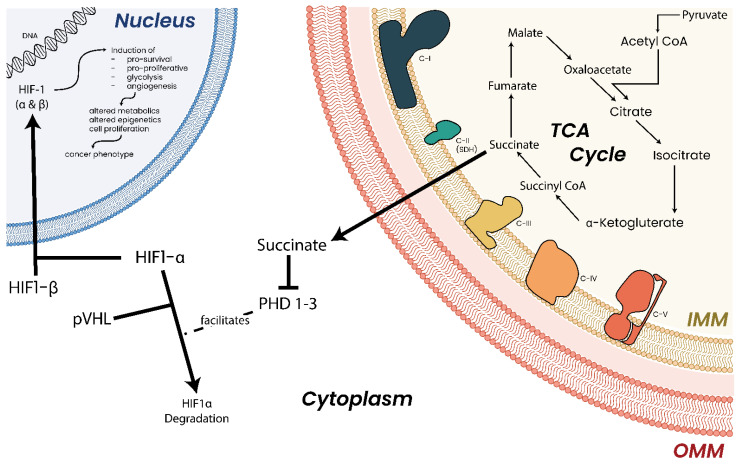
Succinate dehydrogenase (C-II) (Figure 9) is part of both the ETC and the TCA cycle. Of the MRC complexes, C-II has the strongest and most well-established link to cancers with succinate dehydrogenase proteins classified as bona fide tumor suppressor genes [236,334,335]. Germline heterozygous C-II mutations have been shown to predispose individuals to cancer, while homozygous mutations in the same genes lead to neuronal diseases [336]. Mutations affecting C-II function and assembly have been linked to hereditary paragangliomas and pheochromocytomas [191], renal carcinoma [337], gastrointestinal stromal tumor [338], and breast cancer [339]. The tumorigenic effects of C-II mutations appear to be due to the accumulation of succinate, which, in excess, inhibits prolyl hydroxylases (PHDs), HIFs, DNA and histone demethylases, and JmjC domain-containing histone demethylation (JHDM) proteins [340,341] (Figure 10), giving rise to a hypermethylation phenotype which resembles that observed in tumors with (for example) somatic IDH mutations [235,236,335,342,343,344].
Figure 10.
Hypothesized tumorigenic pathway consequent to succinate accumulation. C-II dysfunction leads to accumulation of the succinate. Excess succinate is transported into the cytoplasm where it inhibits prolyl hydroxylases (PHDs), thereby inhibiting HIF degradation. This leads to constitutive activation of HIF, facilitating increased expression of genes commonly associated with tumorigenesis.
15.3. C-III Dysfunction and Cancer
In addition to C-I and C-IV, ubiquinol-cytochrome c oxidase (C-III) (Figure 9) is a major producer of mitochondrial ROS. Mutations affecting C-III function have been identified in numerous cancers including gastric [345], colorectal [346], ovarian [347], thyroid [348], breast [349] bladder, lung, and head and neck tumors [350]. In bladder cancer, expression of a truncated form of mitochondrial cytochrome b (MT-CYTB), the sole mitochondrial encoded subunit of C-III, has been shown to increase cellular growth rates and promote invasion in vitro and in vivo in MB49 bladder cancer cells [351]. This growth phenotype also correlated with increased ROS production and activation of NF-κB2, two characteristics of an RTG response [351]. Additionally, in SV-40-transformed human uroepithelial HUC-1 cells, overexpression of a truncated MT-CYTB protein, a truncation previously reported in bladder cancer, resulted in aberrant and sustained cell growth [352]. C-III dysfunction due to mutation can therefore contribute to sustained aberrant growth of tumor cells.
15.4. C-IV Dysfunction and Cancer
Cytochrome c oxidase (C-IV) (Figure 9) is the final complex in the ETC. C-IV is unique amongst the MRC complexes in that it is the key regulator and the rate-limiting step of OXPHOS [353]. It also has strong links with apoptosis control [354]. C-IV is also the only complex of the MRC that has known tissue-specific isoforms of nDNA-encoded subunits and has been shown to be modulated by p53 at the mRNA level [353,355]. It has been discovered that p53 regulates expression of cytochrome c oxidase 2 (SCO2) [213]. SCO1 and SCO2 are involved in transporting copper to the catalytic core of C-IV during assembly and TP53−/− mice and p53-deficient colon cancer cell lines show decreased oxygen consumption (a key indicator of C-IV function) and ATP generation capacities [213,356].
Of the five MRC complexes, C-IV shows the weakest link between dysfunction and cancer, possibly suggesting a limited tolerance to genetic variation of this complex. Function affecting mutations in C-IV have only been identified in prostate and ovarian cancers [277,357]. In contrast, however, nDNA-encoded C-IV subunits have been found upregulated in leukemia [358] and A549 lung adenocarcinoma cells [359]. In leukemia, increased expression of COX5A and COX5B correlated with increased ROS [358]. The contribution of ROS to tumorigenesis will be discussed in a coming section.
15.5. C-V Dysfunction and Cancer
C-V (Figure 9) is the final complex in the MRC and is the site of ATP generation in the mitochondria. Investigations have observed that C-V forms part of the mitochondrial permeability transition pore (mPTP) [360] and can also be found on the surface of vacuoles [361]. It has therefore been hypothesized that the link between C-V and tumorigenesis could potentially relate to its function as part of the mPTP, with potential links to defective autophagic processes.
C-V subunits have been reported mutated in pancreatic [362], thyroid [348], and prostate cancers [277]. Cybrids containing mutated mitochondrial ATP synthase 6 (MT-ATP6) were found to show faster growth rates in vitro and in vivo in xenografted nude mice; a trait which was reversed on reintroduction of wild-type MT-ATP6 [363]. MT-ATP6 mutations were also associated with increased levels of ROS, a potential consequence of an RTG response due to mitochondrial dysfunction [360].
16. TCA Cycle Dysfunction and Cancer
16.1. Isocitrate Dehydrogenase Dysfunction and Cancer
Five isocitrate dehydrogenase (IDH) enzymes are found in human cells. Three NAD+-dependent IDH enzymes (IDH3A, IDH3B, IDH3G) are localized in the mitochondria along with the NADP+-dependent IDH2 (Figure 9). IDH1 is also NADP+ dependent but is localized in the cytoplasm. The IDH enzymes catalyze the reversible conversion of isocitrate to α-KG. Both IDH1 and IDH2 are somatically mutated in AML predominantly showing hotspot mutations [31,364,365], colon cancer [366], glioma [30], enchondromas [367,368], spindle cell hemangiomas [367], osteosarcoma [369], glioblastoma [27], chondrosarcomas [368], intrahepatic cholangiocarcinoma [370], prostate cancer, and B-acute lymphoblastic leukemia [371].
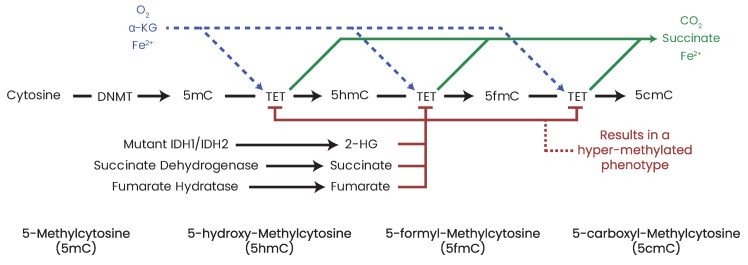
Somatic mutations in IDH1 and IDH2 result in neomorphic enzymatic activity whereby the oncometabolite R-2-hydroxyglutarate (2-HG) is produced [372]. TF-1 human erythroleukemia cell lines incubated with 2-HG became cytokine independent and showed differentiation blockage [373]. 2-HG has also been shown to inhibit α-KG-dependent enzymes such as the PHDs [374], the ten–eleven translocation (TET) family demethylases [375], and the JHDMs [376], which results in DNA and/or histone hypermethylation phenotypes in leukemia and breast cancers [377,378,379]. While 2-HG accumulation in leukemia patients is a direct result of neomorphic IDH mutation, in breast cancer, the accumulation of 2-HG is a result of metabolic dysfunction driven by Myc overexpression [378]. Accumulation of the oncometabolite 2-HG can therefore accumulate in cells independent of IDH mutation due to metabolic dysfunction or hypoxia which has consequent effects on the epigenome [380].
Apart from epigenetic effects, somatic IDH1 mutations have significantly hindered enzymatic function and catalytic abilities [372,381,382]. In glioma, mutant IDH1 is deficient in performing its oxidative reaction by >80% [383] with an approximate 38% reduction in NADPH generation [384], thus highlighting the metabolic consequences of mutation on its enzymatic and metabolic function.
16.2. Fumarate Hydratase and Cancer
Fumarate hydratase (FH) (also known as fumarase) (Figure 9) catalyzes the conversion of fumarate to malate in the TCA cycle. FH has been shown to be an essential regulator of HSC functions, deletion of which results in defective hematopoiesis [385]. Germline mutations in FH have been identified in leiomyomatosis and renal cell cancers [386] as well as paragangliomas and pheochromocytomas [335,387]. Aberrant expression [388,389] and deletion [390] of FH have also been reported in various cancers. Similar to succinate and 2-HG, high concentrations of fumarate have been shown to inhibit α-KG-dependent enzymes such as PHDs and DNA/histone demethylases [235,391,392], leading to hypermethylated DNA phenotypes and aberrant expression profiles.
16.3. Citrate Synthase and Cancer
Citrate synthase (CS) (Figure 9) catalyzes the irreversible conversion of ACoA and oxaloacetate into citrate, which can then be exported to the cytoplasm for FAO or processed further by the TCA cycle in the mitochondria. Aberrant expression of CS has been documented in renal oncocytomas, pancreatic ductal carcinoma, and numerous cervical cancer cell lines [192,393,394]. While it is unclear how CS promotes tumorigenesis, the link between CS and cancer could potentially be indirect, wherein CS dysfunction results in mitochondrial dysfunction, resulting in a compensatory and persistent RTG response, giving rise to aberrant growth. Another possibility could be the depletion of citrate metabolite stores, which would result in insufficient ACoA concentrations required to maintain metabolic and epigenetic homeostasis while simultaneously removing a noted glycolysis inhibitor from the cell [395]. However, further investigations are still required.
16.4. Aconitate Hydratase and Cancer
Aconitate hydratase (AH) (Figure 9) is an Fe-S cluster enzyme that catalyzes the reversible conversion of citrate to isocitrate. In FH-deficient cell lines, the accumulation of fumarate inactivates the Fe-S of AH and abolishes its enzymatic function, likely triggering an RTG response due to metabolic deficiency [396]. Decreased AH expression has been previously reported in gastric cancers and is also a prognostic marker of disease progression [397].
16.5. Malic Enzyme and Cancer
Malic enzyme (ME) (also known as malic dehydrogenase) (Figure 9) catalyzes the conversion of malate into pyruvate and CO2. It has been observed that mitochondria isolated from L-1210 mouse leukemia cells showed increased conversion of malate to pyruvate [398]. More recently it was established that knockdown of ME2 diminished proliferation and increased apoptotic rates of K562 erythroleukemia cells in vitro and completely inhibited growth of K562 xenografts in nude mice in vivo [399]. It has also been determined that p53 represses expression of ME1 and ME2, decreasing lipogenesis and glutamine metabolism [305]. These findings therefore link p53 and active metabolic pathways within leukemic cells through modulation of MEs. As deactivating TP53 mutations can be found in ~10% of AML cases [31], these mutations would enable TP53 mutant clones to decrease their dependence on OXPHOS, upregulation of which is essential for HSC differentiation, allowing cells to remain in an undifferentiated state, enabling disease establishment and progression, while also making them reliant on pyruvate stores [230].
17. ROS and Cancer
ROS produced by the MRC has generally been considered to be deleterious to cells. Recent findings show that ROS are actually important signaling molecules and can modulate numerous cellular functions [147,152,165,166]. Under normal circumstances, ROS production is kept within tight boundaries [400] but tumor cells are often reported as having increased levels of ROS as compared to healthy counterparts [162].
Elevated levels ROS have been shown to affect cell cycle progression and growth factor signaling [401] and promote differentiation in myeloid cells [402] and have been linked to tumorigenesis [403,404]. Excess production of ROS within a cell leads to a state of oxidative stress [405] which is commonly seen in hematopoietic malignancies, including ALL [406], AML [407], and CML [407,408]. While the relationship between ROS, tumorigenesis, and tumor persistence is complex, increased ROS in tumors may support cell survival [409,410], migration [411], metastasis [304], proliferation [412], and genomic instability [413] or even facilitate drug resistance [163] depending on the cancer type by modulating metabolic pathways [408].
18. Cellular Consequences of Metabolic Reprogramming
Independent of discussions on the origins of cancer, the universal prevalence of metabolic reprogramming in tumors is indisputable [2,7,414,415,416,417,418,419]. As mitochondria play critical roles in macromolecular biosynthesis, cellular metabolism, and epigenomic control, metabolic reprogramming has consequent effects on these and other cellular pathways affecting cell state. Literature evidence has also been presented implicating the tumor microenvironment (TME) and local inflammation as key factors in supporting and maintaining metabolic phenotypes [420,421,422,423,424], although these too (TME and inflammation) can be controlled by internal cellular metabolic states modulating their surrounding environments [425,426,427].
It has been elucidated that, once glucose is imported into a cell from the TME, cancers utilize large amounts of glucose in a step-wise manner, converting it to pyruvate even in the presence of oxygen [1,188,418,428,429]. Evidence has emerged that a portion of glucose-derived pyruvate is transported into the mitochondria where it is processed for use by the TCA cycle and utilized in subsequent anabolic reactions [428,430,431,432]. Additionally, in tumors which show evidence of defective mitochondrial respiration and/or TCA function, glutamine dependence is observed [430,433,434,435]. Glutamine is becoming recognized as a critical substrate required by many cancers as it is a carbon source for macromolecular synthesis and can also be utilized in producing ATP in a respiration-independent manner [7,430,431,432,433,436,437,438]. Glutamine metabolism is also utilized by tumors for both amino acid and de novo lipid synthesis where required [428,437]. Ultimately, though, tumor cells have metabolic preferences, whether for glucose, glutamine, amino acids, or lipids, that support their growth and proliferative requirements but unfortunately can become potent dysregulators of the epigenome [439,440,441].
In order for a cell to regulate its genome, numerous post-translational modification (PTM) systems are utilized including, but not limited to, phosphorylation, methylation, and acetylation. However, these same epigenetic mechanisms require metabolites such as citrate, pyruvate, NAD+, ACoA, ATP, NADH, and flavin adenine dinucleotide (FAD), which originate from metabolic pathways, some of which can be inhibitory for epigenomic regulator function at specific concentrations (such as succinate or fumarate [439]). Metabolic reprogramming resulting in altered metabolite concentrations can therefore have significant effects on the epigenome which could potentially further drive tumorigenic phenotypes, as will be discussed.
As mitochondria are key central functional hubs within the cell, reprogramming of metabolic circuits and pathways in tumor cells has far-reaching consequences, including disrupted energetic pathway or dependencies and alterations to the epigenomic landscape. Such varied and far-reaching consequences of cellular metabolism only add complexity when studying altered metabolics of tumor cells.
19. Epigenetics and Epigenome
Apart from DNA nucleotides (A, C, T, G), there exists an additional layer of information encoded in the nucleotide bases [442]: the epigenome. The epigenetic layer enables genomic regulation without necessitating coding sequence changes [443,444]. Epigenetic PTMs can include chemical alterations to DNA, such as the addition of methyl or acetyl groups, or alternations to the histone tails, such as (but not limited to) deposition of methyl, acetyl, or phosphoryl groups [445]. For each of these reactions, a series of protein families are employed capable of “writing”, “reading”, and “erasing” said PTM marks (Table 3). Depending on the chemical nature, PTMs possess different lifetimes. While phosphatases readily reverse phosphorylation [446,447,448], tri-methylated lysine modifications can persist for extended periods of time [448,449,450]. These modifications are utilized by the cell to regulate gene expression and chromatin architecture, which can have significant impacts on the development and function of the cell in response to internal/external stimuli or in diseased states such as cancer. Investigations have shown that many of these PTM deposition and/or excision reactions require metabolites as cofactors [451,452]. This, therefore, intimately couples cellular metabolic and epigenomic states owing to the shared generation and use of cofactors and metabolites. As such, a cell’s metabolic state can drastically shape and alter its epigenetic state and epigenetic alterations can propagate and alter a cell’s metabolic state [451,452,453,454].
Table 3.
Epigenetic Regulator Families.
| Modification | Writers | Readers | Erasers | Genes Mutated in Cancer |
|---|---|---|---|---|
| DNA Methylation | DNA Methyltransferases (DNMTs) |
Methyl-CpG-Binding Domain Proteins (MBDs) |
Ten–Eleven Translocation Proteins (TETs) |
DNMT3A DNMT3B TET1 TET2 MBD4 IDH1/2 |
| Histone Acetylation | Histone Acetyltransferases (HATs) including GNAT, p300/CBP, MYST Protein Families |
Bromodomain and Extra-Terminal Proteins (BETs) |
Histone Deacetylases (HDACs) |
CBP EP300 KAT6A KAT6B BRD1 BRD2 BRD3 BRD4 TRIM33 |
| Histone Methylation | Histone Methyltransferase (HMTs)/ Histone Lysine Methyltransferase (KMTs) |
PHD Finger (PHF); Chromodomain-Containing (CHD); Malignant Brain Tumor (MBT); Tudor Domain; PWWP Domain |
Histone Demethylases (HDMs)/ Histone Lysine Demethylases (KDMs) including LSD1 and Jumonji-C Proteins (JHDM/JmjC) |
EZH2 MLL2 KDM3A KDM4C KDM5A KDM5C KDM6A MSD1/KMT3B SETD2/KMT3A EHMT1 |
| Histone Phosphorylation | Protein Tyrosine Kinases (PTKs) |
14-3-3 Proteins | Protein Tyrosine Phosphatases (PTPs) |
PTPN1 PTPN11 PTPN13 PTPRB PTPRC PTPRD |
20. DNA Packaging and Histones
Within the nucleus, DNA can take on states of euchromatin or heterochromatin [455]. Euchromatin, or an open state of chromatin, enables transcription and gene expression. Conversely, heterochromatin is tightly packed and condensed DNA around histone proteins. For this condensation to occur, a 147 bp stretch of DNA is wrapped around a core of eight histone proteins (2*(H3/H4) dimers linked to 2*(H2A/H2B) dimers), with a stretch of 20–90 bp of DNA linking adjacent histones (linker DNA) [443,456,457,458,459,460]. Histone H3/H4 dimers occupy the core of the nucleosome, while H2A/H2B dimers are more loosely associated. Histone proteins have a globular domain with a characteristic alpha-helical “histone-fold” arrangement [461,462]. The histone octamer has a positively charged surface interacting with the negatively charged DNA backbone [463]. Physically, DNA has a double-helix structure and is wound in a right-handed orientation which is then wound in a left-handed orientation around histone octamers. Owing to the size of the DNA molecule and histone proteins, the DNA molecule only winds approximately 1.65 times around a histone octamer which results in a spatial arrangement where sites 70 bp along the DNA molecule are in proximity to each other on the apical surface of the wound nucleosome [460,464,465].
Nucleosome structure varies due to differences in histone composition, which itself can show tissue-specific differences [466]. Through deposition of chemical modifications onto the N-terminus of histone tails, which are readily accessible for modifications, the function of nucleosomes can be changed, thus affecting the function of the associated DNA. Histone chemical modifications described in the literature include methylation, phosphorylation, and acetylation, with other modifications such as SUMOylation, ubiquitinylation, ribosylation, glycosylation, crotonylation, and serotonylation also being described [459,467,468,469]. More specifically, histone methylation is reported to be deposited at lysine (K) and arginine (R) residues, with deposition of multiple methyl groups possible (mono-, di-, tri-methyl group deposition). Phosphorylation can occur at serine (S) residues, with acetylation and ubiquitination also potentially occurring at lysine (K) residues. The nomenclature describing these changes follows the convention of
< histone protein—H2A/H2A.z/H2B/H3/H3.1/H3.3/H4 >
< single character amino acid code—K (lysine)/R (arginine)/S (serine) >
< position of the amino acid carrying the modification >
< abbreviation of the chemical modification—me1 (mono-methylation) /
me2 (di-methylation)/me3 (tri-methylation)/p (phosphorylation)/ac (acetylation) >
In the following sections, we will introduce DNA methylation, followed by a discussion on histone methylation, acetylation, and phosphorylation. Although other histone modifications have been described in the literature, their exploration is beyond this review.
21. DNA Methylation
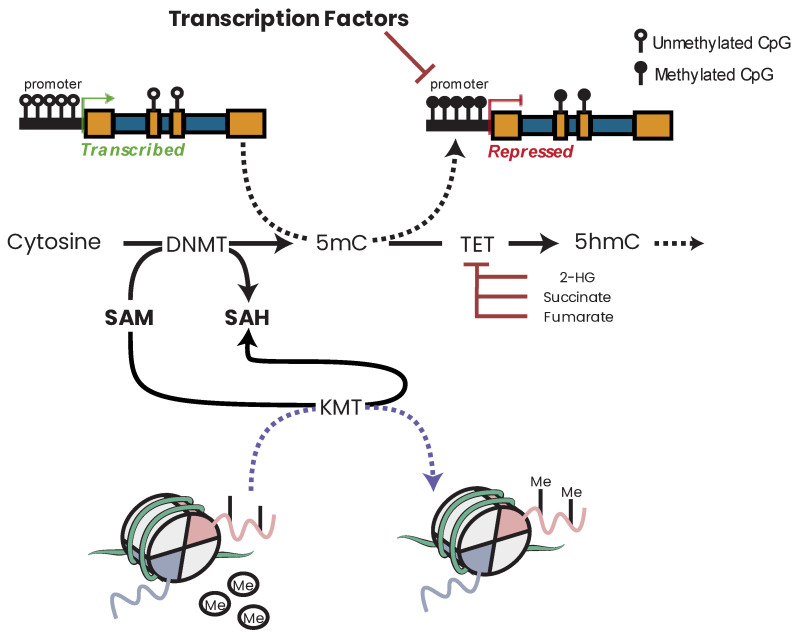
In animal and plant genomes, DNA cytosine bases can be modified with the chemical addition of a methyl group, resulting in 5-methyl-cytosine (5mC) [470,471]. In humans, the 5mC reaction is catalyzed by the activity of a DNA methyltransferases (DNMTs) and utilizes the metabolite cofactor S-adenosyl-methionine (SAM) as a methyl group donor [472,473]. Chemically, this reaction involves a modification of the 5th position of the cytosine ring by the thiol group of a cysteine, leading to the formation of a covalent bond between the cytosine and the DNMT protein. Once the 5mC methyl group is attached, it can be physically located in the major groove of a DNA helix where it can be accessed by DNA methylation readers, the methyl-CpG-binding domain (MBD) protein family (Table 3) [474]. De novo methylation is described to occur by the DNMT3 family of proteins, namely DNMT3A/B, with DNA methylation maintenance being performed by DNMT1 [442,475,476,477,478,479] (a detailed review of the DNMT family can be found in reference [480]). Despite these canonical roles, evidence has emerged that each protein is capable of having a compensatory function for the other DNMTs should the cell require it; i.e., DNMT1 can show de novo methylation function, while DNMT3A/B can show DNA maintenance function [481,482]. Such compensatory behavior is predicted to occur in disease states, such as cancer, where mutations can result in loss of enzymatic function of particular family members [483,484].
In mammalian genomes, 5mC is preferentially found in the context of CG dinucleotides. This CG dinucleotide sequence is palindromic across both sense and anti-sense strands, representing a symmetrical arrangement of nucleotides. During replication, when methylated DNA is copied, the synthesized (daughter) strand does not contain the methylation of the parental strand, resulting in hemi-methylated DNA [478,485,486]. Following synthesis, DNMT1 is recruited to the daughter strand to restore the methylation pattern read on the parental strand [485]. If for any reason DNA methylation maintenance is inhibited at this stage, owing to mutation, molecular inhibition, or metabolite insufficiency, the integrity of the methylation signature can be diluted or lost if this persists for subsequent cellular divisions [473,480,487].
Although DNA methylation is now a ubiquitously described epigenetic control mechanism, the significance and relevance of DNA methylation were questioned when initially observed [488,489]. Over the decades the regulatory role DNA methylation plays for a cell has become indisputable as well as its ubiquity in many organisms [490,491]. Evidence has accumulated describing the essential role DNA CpG methylation plays in regulating the epigenome, providing a stable gene-silencing mechanism in most contexts (in concert with chromatin architectural changes) without necessitating genomic sequence changes [471,481,492,493]. Methylation of CpG-rich “islands” (Figure 11) in the promoter regions of genes has been found to correlate with transcriptional repression, while unmethylated CpG islands correlate with active transcription [494,495,496]. However, there exists ambiguity as to whether CpG islands are the master methylation-sensitive regulatory loci controlling gene expression since studies have documented stronger correlations between CpG “shore” methylation and active transcription (CpG shores are described as the 2 kb regions flanking a CpG island) [493] (Figure 11). Additionally, only 30–70% of gene promoters contain CpG “island” regions (depending on the identification criteria and genome build), prompting the question, what about other genes without CpG islands? They too can show methylation-dependent transcription regulation [497]. These findings blur the lines as to which methylation-sensitive loci are truly and definitively capable of controlling gene expression [493,498,499,500,501,502,503]. Moving beyond gene promoter regions, evidence has been presented correlating unmethylated CpGs with active enhancers similar to gene promoters, however, in opposition to this trend, methylation within gene bodies correlates with active expression, rather than repression [494,495,496].
Figure 11.
Commonly Accepted CpG Island-Centric Annotations. The commonly accepted definition of the CpG island-centric landscape situates a CpG -ich “island” in the center. Flanking the island are CpG shores, which extend for 2 kb on each flank. Thereafter, CpG shelves extend 2 kb beyond that, with any regions beyond that falling in the open sea window. The definition of what constitutes as a CpG island is dependent on the investigators’ question, what thresholds they set, and what genome build is being utilized for the definitions.
Depending on the site of modification, DNA methylation can facilitate or inhibit protein or transcription factor binding [504,505,506,507,508,509,510,511,512]. Examples of how different proteins interact with methylated or unmethylated DNA include the transcription factors CCCTC-binding factor (CTCF) and CCAAT enhancer-binding protein beta (CEBPB). CTCF can recognize methylated DNA, while CEBPB will recognize methylated or unmethylated DNA depending on its binding partners ATF4 and CEBPD, respectively [504,512,513]. Integrative studies such as these showcase the breadth of transcription factor (TF) binding in different contexts and how transcription can be facilitated or inhibited simply depending on the underlying methylation state of the genome even if the canonical binding motif is present.
In contrast to the methylation “writing” DNMT family, the ten–eleven translocation (TET) family is capable of oxidizing 5mC into its other chemical forms, leading to eventual demethylation or “erasing” of DNA methylation [514,515,516,517]. The TET family of proteins consists of TET1–3 which are ferrous ion (Fe2+)- and α-KG-dependent dioxygenases that use oxygen to oxidize the methyl group of 5mC [514,515,516,517,518]. In this reaction, 5mC is oxidized to 5-hydroxy-methyl-cytosine (5hmC), then 5-formyl-cytosine (5fC), and finally 5-carboxyl-cytosine (5caC) [514,515,516,517] (Figure 12). Through these TET-mediated oxidation reactions, a cell is able to actively and/or passively demethylate DNA [485,519]. It is pertinent to note that the intermediates and cofactors of these successive reactions are derived from other cellular processes, primarily metabolism [451,452,453,454]. Intermediates such as α-KG, O2, CO2, succinate, fumarate, and 2-HG are all metabolic factors [451,452,453,454]. As such, perturbations to the availability and concentration of these intermediates either through metabolic regulation, metabolic inhibition, diet, or environmental factors will affect a cell’s ability to regulate methylation adequately.
Figure 12.
Oxidation of 5-Methylcytosine by TET Proteins. The TET enzymes sequentially oxidize 5mC to 5cmC. For these reactions, ferrous iron (Fe2+), molecular oxygen (O2), and α-KG are utilized. In cancer contexts, the production of 2-HG, hypersuccinate, and hyperfumarate contributes to inhibition of TET enzymatic function, resulting in a progression towards a hypermethylated genome.
The last family of proteins associated with DNA methylation is the methylation “readers”; proteins capable of reading the deposited methylated residues and interpreting the signature for the cell. All of these proteins contain a methyl-CpG-binding domain. These proteins are described to mechanistically function by binding to methylated DNA and directly reducing accessibility for transcription factors, resulting in transcriptional repression [520,521].
22. Histone Methylation
Histones (namely H3 and H4) have lysine (K) and arginine (R) residues in their tails which can be methylated [444,522]. In contrast to acetylation and phosphorylation, methylation does not alter the charge of the histone protein [523]. Additionally, while DNA methylation is used as a repressive mark, histone methylation is able to both facilitate or repress transcription depending on the residue and the type of methylation applied. Histone lysine methyltransferase (KMT) enzymes utilize SAM as an intermediate donor to transfer a methyl group onto the lysine residues of histones and contain a conserved SET domain (with the exception of DOT1) [523,524,525,526]. Lysine residues show mono-, di-, or tri-methyl group deposition which is capable of facilitating or repressing transcription depending on position of deposition [463,522,527]. Arginine residues, on the other hand, show mono- or symmetrical or asymmetrical di-methylation [528].
Through numerous studies, general consensus profiles for histone lysine methylation have been observed (Table 4) such as active transcription being marked by tri-methylated H3K4 (H3K4me3), the 5′ end of transcribed genes being marked by di-methylated H3K4 (H3K4me2), active enhancers marked by mono-methylated H3K4 (H3K4me1), actively transcribed gene bodies marked by tri-methylated H3K36 (H3K36me3), and gene repression marked by di- or tri-methylated H3K9 (H3K9me2/3) and tri-methylated H3K27 (H3K27me3) [505,508,529,530,531,532,533,534,535]. While such individual observations are insightful regarding genome dynamics, integrating such observations can be more insightful by identifying potentially novel elements, as was carried out to identify low-abundance transcripts by overlapping H3K4me3 and H3K36me3 marks [536]. Other histone marks such as H3K79 and H4K20 show methylation-specific behavior depending on whether they are mono-, di-, or tri-methylated, which dictates whether they are associated with active or repressed expression [505,529,533,534]. One should keep in mind that not all genomic elements possess a single histone mark in isolation. There are many genomic loci that show bi- and tri-valency where they show deposition of activation and repressive histone marks simultaneously [444,505,527,529,533,534,537].
Table 4.
Epigenetic Modifications and Function.
| Modification | Function | Reference(s) |
|---|---|---|
| DNA Methylation | ||
| CpG Islands, CpG Shores | Inversely associated with transcription | [490,492,521,538,539] |
| Gene Bodies | Positively associated with transcription | [495,529,540] |
| Intergenic | Positively associated with transcription | [541] |
| Enhancers | Inversely associated with transcription | [495,529,540] |
| Histone Modifications | ||
| H3K4me1 | Active enhancers and promoters; Poised enhancers and promoters when in conjunction with H3K4me3 + H3K27me3 |
[529,532,540] |
| H3K4me2 | Active promoters when in conjunction with H3K4me3; Poised marker when alone |
[535,542,543,544] [545,546,547] |
| H3K4me3 | Active promoters and transcription | [535,548,549,550] |
| H3K9ac | Active promoters | [529,532,548] |
| H3K9me1 | Active promoters | [529] |
| H3K9me2 | Silenced promoters and transcription | [551,552] |
| H3K9me3 | Silenced promoters and transcription | [553,554] |
| H3K27ac | Active enhancers and promoters | [506,555] |
| H3K27me1 | Active transcription | [556,557,558] |
| H3K27me2 | Active transcription | [558] |
| H3K27me3 | Silenced promoters and transcription | [559,560] |
| H3K36me3 | Active gene bodies | [529,532] |
| H3K79me2 | Active transcriptional elongation | [561] |
| H3R2me2 | Counter-correlates with H3K4me3 at promoters; Enriched in gene bodies |
[562] |
| H3R8me2 | Silenced promoters | [563] |
| H3R17me2 | Active transcription | [564] |
| H3R42me2 | Active transcription | [565] |
| H4K20me1 | Active enhancers and promoters | [566] |
| Variant Histones | ||
| H2A.X | DNA repair site | [567] |
| H2A.Z | Active promoters and DNA repair sites | [568,569] |
| H3.3 | Active transcription | [570] |
| Non-coding RNA | ||
| miRNA | Repression of gene expression | [571,572,573,574] |
| lncRNA | Gene expression regulation | [575,576,577] |
In contrast to histone methylation deposition, in order to remove histone methylation, cells utilize the histone demethylases (HDMs), also known as the histone lysine demethylases (KDMs), which consist of the LSD1 and Jumonji proteins [530,578,579,580] (Table 3). LSD1 proteins are dependent on the metabolite FAD, while the Jumonji family of proteins require Fe2+ and α-KG to perform their catalytic activity [578,579,580,581]. All demethylases have been shown to have high substrate specificity for their lysine target with some enzymes capable of only demethylating mono- and di-methyl targets, while others can demethylate all methylated lysine states [523].
In contrast to writing and erasing histone methylation, reading histone methylation is performed by a number of protein classes, including Tudor domain proteins, chromodomain proteins, and malignant brain tumor (MBT) proteins to name a few [582,583,584,585,586,587,588] (Table 3).
23. Histone Acetylation
Histone acetylation is regulated through histone acetyl transferases (HATs; acetylation writers), histone deacetyl transferases (HDACs; acetylation erasers) with bromodomain and extra-terminal proteins (BETs) acting as acetylation readers (Table 3). HATs include three protein families, the GNAT, p300/CBP, and MYST proteins [589,590,591]. Most described HATs can be categorized as type A HATs which are (mainly) nuclear enzymes and are responsible for acetylation of histones and non-histone proteins in the nucleus with implied functions in the epigenetic regulation of gene expression [592]. Type B HATs are instead cytoplasmic enzymes, specifically KAT1 and HAT4, and modify free histones in the cytoplasm after synthesis [592]. Histone acetylation utilizes a zinc- and ACoA-dependent process to transfer an acetyl group to histone lysine side chains. This, in turn, reduces lysine residue charge, which weakens the electrochemical connection between the positively charged histone tails and the negatively charged DNA backbone [523,589]. This causes dissociation of the DNA and histones, leaving the DNA exposed and readily accessible for transcription [523,589,593,594]. As such, histone acetylation is typically considered to facilitate gene expression and is critically dependent on ACoA stores and availability for its deposition reaction to occur [595,596,597].
In order to remove deposited histone acetylation, cells utilize the HDAC family of proteins. Four classes of HDACs have been described to date [598,599,600,601,602]. Class I HDACs are Rpd3-like proteins with members HDAC1–3 and HDAC8. Class II HDACs are Hda1-like proteins and are split into two subgroups. Class IIa shuttles between the cytoplasm and nucleus and regulates TF activity. Class IIa consists of members HDAC4, HDAC5, HDAC7, and HDAC9. Class IIb proteins, on the other hand, appear to predominantly have cytoplasmic roles and include the proteins HDAC6 and HDAC10. Class III HDACs are Sir2-like proteins and include members SIRT1–7. SIRT1, 6, and 7 localize to the nucleus where they affect gene expression. SIRT2 localizes to both the nucleus and cytosol and modulates cell cycle control. SIRT3, 4, and 5 localize to the mitochondria and respond to caloric restriction by switching cells to favor mitochondrial OXPHOS [603]. In contrast to other HDAC proteins, class III proteins utilize the metabolic cofactor NAD+ to catalyze their reactions, rather than ACoA and zinc [589,598,599,600]. Finally, class IV consists of only HDAC11. As a side note, in plants, a fifth group of HDACs exist, the HD2 family, which is not found in animals [604].
A number histone lysine residues have been reported to be sites of acetylation deposition, including, but not limited to, H3K4, H3K9, H3K14, H3K18, H3K23, H3K27, H3K36, H3K79, H4K5, H4K12, H4K15, H4K20, and H3K24 [605]. Of these reported marks, the most well studied is H3K27ac which, when observed in conjunction with H3 methylation, marks a spectrum of active/poised transcriptional states [506,509,540,606,607]. As noted, primed enhancers are marked by sole deposition of H3K4me1. When H3K4me1 marks are found in conjunction with H3K27ac, this marks active enhancers. In contrast, deposition of H3K27me3, H3K4me1, and H3K27ac marks poised enhancers. Additionally, co-occurrence of H3K27ac and H3K4me3 is typically associated with promoters and enhancers of actively transcribed genes [506,509]. H3K27ac deposition in intergenic regions is regarded by some to also mark superenhancers [606,608]. While such characterizations are indeed extremely insightful, the broad nature of histone peaks in sequencing studies means that their presence alone is not sufficient to accurately define which exact genomic bases perform the “enhancer” function. For this, more detailed characterizations are required to truly identify where, within the broad region identified by histone peaks, the true regulatory locus is actually located [609].
24. Histone Phosphorylation
Histone phosphorylation has been shown to play roles in regulating numerous cellular processes such as gene expression, DNA damage repair, apoptosis, cell cycle regulation, and cell division [468,610]. Owing to the chemical nature of this PTM, histone phosphorylation alters the overall charge of the histone protein which affects the structure of local chromatin [468,523]. Histone phosphorylation is reported to be “written” by protein kinases and is reported to occur at either serine (S), threonine (T), or tyrosine (Y) residues of histone tails [456,468,523]. Furthermore, the phosphorylation state of S, T, and Y residues has been shown to drastically alter the rate of nucleosome sliding and rearrangement [468,611,612,613,614].
All identified histone kinases transfer a phosphate group from metabolically derived ATP to the target amino acid side chain [523]. It has been shown that histone methylation sites H3K9 and H3K27 have subsequent S (phosphorylation) residues. It is hypothesized that phosphorylation at these “KS” domains may, in part, alter the affinity and/or accessibility of methylation readers and writers at the K residues [615,616]. In order to “erase” histone phosphorylation, cells utilize protein phosphatases [468]. Histone phosphatases have been shown to be essential for regulating DNA repair, mitosis, and apoptosis [468,617,618,619].
25. Deposition of Epigenetic Marks Is Dependent on Mitochondrial Metabolites
Cellular metabolism involves biochemical reactions to maintain the state of a cell. Metabolism impacts every cellular process and can be influenced by a cell’s epigenetic state, genetic state, environment, and dietary intake [472]. Cells use their metabolic networks to sense nutrient availability and then propagate this information to direct cellular compensatory behavior through signaling networks such as RTG responses. Downstream of this, chromatin-modifying enzymes rely on metabolites in order to carry out their PTMs. ACoA, SAM, and ATP are used for the acetylation, methylation, and phosphorylation of histones (and DNA where applicable), respectively. Therefore, concentrations of these metabolites affect gene expression by modulating epigenetic pathways, processes, and associated chromatin-modifying enzymes.
25.1. ACoA and Epigenetics
Acetyl coenzyme A (ACoA) is utilized by many metabolic and cellular reactions. As such, concentrations of ACoA reflect the cell’s energetic state [620]. ACoA is derived from enzymatic reactions involving glucose, fatty acids, and amino acid catabolism [620] (Figure 13). ACoA has two cellular pools, the mitochondrial and nuclear/cytoplasmic [595]. The inner mitochondrial membrane is impermeable to ACoA which is in contrast to the nuclear membrane which is freely permeable to it, thus linking the nuclear and cytoplasmic pools [595].
Figure 13.
Generation of ACoA for HATs. ACoA is processed in either the mitochondria or in the nuclear/cytosolic pool. ACoA is generated primarily from glucose/pyruvate, free fatty acids, and BCAAs. Once produced, ACoA is shuttled to the nucleus where it is used by HATs as a cofactor enabling deposition of acetyl groups onto histone tails, thus enabling gene transcription.
The majority of ACoA within the cell is produced and consumed in the mitochondria [621]. Within the mitochondria, ACoA is produced through the carboxylation of pyruvate to form ACoA, CO2, and NADH by pyruvate dehydrogenase [620]. When the cell experiences high/adequate levels of glucose, ACoA is produced by the oxidation of glucose with any excess glucose being shuttled to the cytoplasm to be stored as fat [597,620]. Conversely, in states of low-glucose concentrations, ACoA is produced as an end-product of β-oxidation of fatty acids that were previously stored in the cytoplasm [620,622,623,624]. Within the cytoplasm, ACoA levels are maintained by ATP-citrate lyase (ACL) and acetyl CoA synthetase (ACS) [625] (Figure 13). ACL converts citrate into ACoA and oxaloacetate [625]. This cytoplasmic pool of ACoA can also originate from reductive carboxylation of glutamine [623,624]. Functionally, cytosolic glutamine is converted into glutamate, which is then transported into the mitochondria and converted into α-KG while also generating citrate in the TCA cycle [623,624]. Citrate can then be exported back to the cytoplasm where it is converted into oxaloacetate and ACoA [623,624].
Once generated, ACoA is a key cofactor that is essential for HAT function and de novo fatty acid synthesis [597]. Cells must carefully balance the availability and usage of ACoA across these two critical cellular processes with dire phenotypic consequences should this balance be chronically perturbed [596,597,626,627]. It has been reported that hypoxic conditions, such as those found in/surrounding tumors, repress cell differentiation through reducing cellular ACoA levels which in turn leads to reduced global histone acetylation and chromatin accessibility [628]. This means that histone acetylation is highly dependent on cellular ACoA stores and availability [596,597,629,630]. When cellular ACoA concentrations are perturbed, rapid histone deacetylation occurs which can result in significant changes in chromatin structure and accessibility that can result in disease [595,597,626,627,630]. The opposing cellular mechanism to histone acetylation is the process of histone deacetylation. Regarding this, high intracellular concentrations of acetate, the precursor of ACoA, block HDAC function, which can lead to aberrant expression profiles and diseased phenotypes [631,632].
25.2. NNMT and Epigenetics
Nicotinamide N-methyl transferase (NNMT) is a cytosolic enzyme that catalyzes the transfer of a methyl group from SAM to nicotinamide, generating SAH and 1-methylnicotinamide (1-MNA) [633,634,635]. NNMT effectively links NAD+ and methionine metabolic pathways (Figure 14). As such, NNMT is capable of regulating the enzymatic reactions controlling both intracellular NAD+ and SAM/SAH. By being situated between these two critical cellular pathways, NNMT is able to exert effects on both metabolism (directly) and the epigenome (indirectly), which has consequent effects in diseased states such as cancer [633,636,637,638].
Figure 14.
NNMT Links NAD Metabolism and the Methionine Cycle. NNMT catalyzes the transfer of a methyl group from SAM to NAM, generating SAH and 1-MNA. NAM is generated by the metabolizing of NAD+ by NAD+-consuming enzymes such as SIRTs and PARPs. Conversely, SAM is generated through the methionine cycle. NNMT therefore links NAD+ metabolism and the methionine cycles, with consequent effects on cellular metabolism and the epigenome.
In cancers, NNMT has been observed to be overexpressed in breast [441,639], colon [639,640], bladder [641], neuroblastoma [642,643], lung [644], liver [639], and renal cancers [645]. The overexpression of NNMT in tumors has also been associated with the aggressiveness of certain tumors [633]. This is believed to be in part due to NNMT decreasing cellular SAM/SAH ratios which has consequent effects on reducing H3K4, H3K9, H3K27, and H4K20 methylation with downstream consequences on cell cycle- and cancer-related pathways [633,637,646,647]. As previous authors have noted, overexpression of NNMT serves as a “sink” for cellular SAM [633] thereby drastically reducing the cellular SAM pool and depriving HMTs of this essential cofactor, the deprivation of which is capable of stalling or inhibiting enzymatic function [648,649,650]. Additionally, in other cellular contexts, overexpression of NNMT has been shown to result in decreased cellular NAD+ concentrations while also decreasing expression of fatty acid oxidation pathway genes by inhibiting NAD+-dependent sirtuin function [634]. Therefore, although indirect, NNMT is capable of exerting enough influence to perturb and alter metabolite concentrations and epigenomic states of a cell.
25.3. NAD+ and Epigenetics
NAD+ is an essential metabolite utilized in numerous cellular processes. NAD+ levels have been shown to decrease under high-fat diets and increase with exercise and caloric restriction [651,652,653,654]. There has also been a link established between NAD+ levels and aging which has driven investigation into manipulating NAD+ concentrations in therapies aimed at disease prevention and lifespan extension therapies [651,652,654,655,656,657].
Within the cell, NAD+ can be synthesized from a number of dietary sources, including nicotinic acid (vitamin B3) and nicotinamide and tryptophan [653,654,658]. The NAD+ salvage pathway is also a key pathway for maintaining cellular NAD+ levels since it recycles nicotinamide generated as a by-product of the enzymatic activities of NAD+-consuming enzymes, the sirtuins (SIRTs), adenosine diphosphate (ADP)-ribose transferases (ARTs) and poly (ADP-ribose) polymerases (PARPs), and the cyclic ADP-ribose (cADPR) synthases [603,653,654,658]. Nicotinamide, a potential precursor of NAD+, also serves as an inhibitory factor of SIRTs, ARTs, and PARPs [653,654,658].
NAD+ serves both as a cofactor for enzymes that catalyze redox reactions and as a cosubstrate for the SIRTs, ARTs, PARPs, and cADPR synthases [651,652,657,659] (Figure 15). SIRTs, as discussed, are HDACs that remove acyl groups from lysine residues (e.g., H3K9ac, H3K14ac, and H4K16ac) on proteins in a NAD+-dependent manner [589,598,599,600]. Sirtuin’s function is therefore linked to cellular metabolism and is also sensitive to caloric restriction that alters NAD+ concentrations. PARP proteins, on the other hand, have established links with methylation rather than acetylation [660,661,662,663,664]. PARP proteins are enzymes that mechanistically catalyze the addition of ADP-ribose units from NAD+ donor molecules to target substrates [661]. Investigations have detailed how NAD+ regulates PARP function, as it is a required cofactor for the catalytic reaction to occur, with PARPs thereafter able to regulate DNA methylation and gene expression [653,658,660,661,662,663,664,665,666,667,668,669].
Figure 15.
NAD’s Regulation of Histone Acetylation and Methylation. NAD+ is primarily generated by NADH dehydrogenase (C-I) in the mitochondria and is used as a cofactor for a number of cellular processes such as the processing of glucose into ACoA. In the nucleus, NAD+ is a cosubstate for histone deacetylases (SIRT family). ARTs and PARPs have established roles in regulating DNA methylation by blocking DNMT function. As such, NAD+ is therefore indirectly capable of regulating not only histone acetylation but DNA methylation as well. Through both of these mechanisms, NAD+ concentrations are capable of regulating gene expression.
There are 17 PARP-related enzymes described in the literature [670]. PARP1 and PARP2 catalyze the polymerization of ADP-ribose units from NAD+, resulting in the attachment of either linear or branched poly-(ADP-ribose) (PAR) polymers to itself or other target proteins.
PARP1, in particular, has been linked to epigenetic and chromatin regulation [660,661,662,663,664,666,667,668,669]. PARP1 can modulate chromatin structure and can act as a transcriptional coregulator. There therefore exists an indirect link between NAD+ and cellular methylation control. It has been recently shown that hyper-NAD+ treatment of the leukemic cell line HL-60 resulted in impairment of DNMT1 methylation maintenance function for set gene loci, which resulted in hypomethylation and subsequent activation of the CCAAT enhancer-binding protein alpha (CEBPA) locus [665]. Although these findings have only been reported for a single genomic locus, when taken in conjunction with the additional literature evidence, these results strongly implicate a potentially undescribed, indirect epigenetic regulatory mechanism in which NAD+ is able to control DNA methylation through modulation of PARP proteins. Owing to the ubiquity of NAD+ within the cell, it therefore is plausible that cells utilize NAD+ for both HDAC and DNMT regulation through differing control mechanisms.
25.4. SAM and Epigenetics
SAM is a cofactor of DNA and histone methyltransferases and is the second most common enzymatic cofactor after ATP [648]. The methyl group of SAM serves as a donor for methyltransferase reactions as it is converted into SAH. SAH is a potent inhibitor of all methyltransferases [648,649,650]. The SAM/SAH intracellular ratio, and its control over DNA methylation and histones, is an example of how a metabolite, which can be controlled by diet and nutrition, is capable of affecting the epigenome by controlling epigenetic modifier function. SAM is produced by one-carbon metabolism through a combination of the folate cycle and the methionine cycles [648,649,650].
Enzymes that use SAM as a cofactor are DNMTs, KMTs, and PRMTs [472,473,523,524,525,526] (Figure 16). Crosstalk between metabolism and chromatin is therefore regulated by each enzyme and the physiological concentrations of its required metabolites. This is of particular importance for methyltransferases which respond to low physiological concentrations of metabolic substrates with limited and/or reduced enzymatic activity [648,649,650]. Competition for available SAM may regulate the contrasting methylation events of histone H3K4, that is associated with transcription activity, and methylation of DNA and histone H3K9, that is associated with transcriptional silencing [671,672]. Thus, changes in one-carbon metabolism, and hence alterations in the levels of SAM, can have an impact on gene expression [672,673].
Figure 16.
SAM as a Methyltransferase Regulator. SAM is a regulator of DNA and lysine histone methyltransferases. Cytosine is converted to 5mC by the DNMT family of proteins on DNA and utilizes the cofactor SAM as a methyl group donor. Methylated DNA results in transcriptional repression through blocking access to transcription actors. SAM is also used as a methyl group donor for the KMT family of proteins. Deposition of methylation on histone tails can have activation or repressive functions depending on the lysine residue and its position.
25.5. FAD and Epigenetics
FAD is a cofactor required in many cellular oxidative reactions such as mitochondrial fatty acid β-oxidation and respiratory metabolism and is most notably required as a cofactor for lysine demethylase 1 (KDM1 or LSD1) enzymatic function [674,675,676,677,678] (Figure 17). Intracellularly, riboflavin (vitamin B2) is converted into flavin mononucleotide (FMN) followed by FAD in the cytosol [679,680]. Cytoplasmic FAD is them transported into the mitochondria where it is reduced to FADH2 in the TCA cycle by SDH as it requires FAD to catalyze the oxidation of succinate to fumarate [681,682]. In this reaction, FAD is used as a proton accepter instead of NAD+. The converse oxidizing reaction of FADH2 is also carried out can by reoxidized oxygen, resulting in production of FAD and peroxide to replenish cellular FAD concentrations [681].
Figure 17.
FAD and Histone Demethylation. Flavin adenine dinucleotide (FAD) is a cofactor required for lysine demethylase 1 (KDM1 or LSD1) enzymatic function. Lysine-specific demethylate 1 (LSD1), in complex with CoREST, utilizes FAD to demethylate mono- and di-methylated histone H3K4 residues, with evidence presented also supporting its role in demethylating H3K9 residues.
Lysine-specific demethylate 1 (LSD1), in complex with CoREST, utilizes FAD to demethylate mono- and di-methylated histone H3K4 residues [578,678,683,684,685]. LSD1 has also been shown to be involved in the demethylation of H3K9 [685] as well as other non-histone proteins such as p53 [686].
25.6. α-KG, 2-HG, and Epigenetics
α-KG-dependent dioxygenases catalyze hydroxylation reactions on diverse substrates, including proteins and nucleic acids [687]. TETs and Jumonji C family (JmjC) histone demethylases are dependent on ferrous ion (Fe2+) and α-KG for their enzymatic activity to facilitate the removal of methyl groups from cytosine bases and histone residues, respectively [530,578,579,580]. TET proteins have been described as DNA demethylation enzymes (Table 3), although there still exists some ambiguity regarding the exact mechanism of DNA demethylation and the degree to which active versus passive demethylation is employed within a cell. Conversely, JmJC demethylases are a group within the KDMs [688]. There are several subfamilies of JmjC KDMs that have been identified [530,578,579,580].
TETs and JmjC KDMs both require ferrous ion (Fe2+) as a cofactor in addition to α-KG as cosubstrate to catalyze reactions in which a singular oxygen from molecular oxygen (O2) is attached to form a hydroxyl group in the substrate, while the second oxygen is taken up by α-KG, leading to the decarboxylation of α-KG and subsequent release of carbon dioxide (CO2) and succinate [578,579,580,581,687]. While KDM and TET proteins require α-KG for their required enzymatic activity, they are also sensitive to other TCA cycle intermediates, such as succinate and fumarate, which have been shown to block and inhibit their function [235,236,689]. Cells must therefore balance metabolite concentrations in order to drive/inhibit particular enzymatic activities and maintain particular cellular states and enzymatic functions. Naive embryonic stem cells (ESCs), for example, utilize both glucose and glutamine catabolism to maintain a high level of α-KG [690]. The elevated α-KG/succinate ratio in naive ESCs promotes histone and DNA demethylation and maintains pluripotency [690]. Direct manipulation of the intracellular α-KG/succinate ratio is sufficient to regulate multiple chromatin modifications, including H3K27me3 and TET-dependent DNA demethylation, which contribute to the regulation of pluripotency-associated gene expression [690].
Enzymatic reactions that utilize 2-HG have an additional inhibitory mechanism to contend with and potentially overcome: the presence of α-KG. α-KG is an oncometabolite that is formed as a consequence of somatic mutation in IDH enzymes. Although numerous somatic mutations have been identified in IDH1/2 in tumors [691,692], there appears to be a predilection for specific binding site “hotspot” mutations (Arg 132 for IDH1, Arg 172 for IDH2) in certain diseases such as leukemia and glioma. Instead of producing α-KG, these mutations instead result in the production of 2-HG, an oncometabolite that competitively inhibits αKG-dependent enzymes such as JmjC KDMs and TETs (Figure 18) [372,379,381,691,692,693,694,695]. Structurally, 2-HG and α-KG are similar with the exception of the 2-ketone group in α-KG being replaced by a hydroxyl group in 2-HG. Structural analysis showed that 2-HG occupies the same space and binding orientation as α-KG in the active site of histone demethylases, thereby preventing the binding of α-KG to the active site of the enzymes, thus it is a competitive inhibitor of 2-HG [696,697]. 2-HG also strongly inhibits histone demethylases [379]. The strongest inhibition was observed with KDM4A, which demethylates H3K9 and H3K36 [698], followed by the H3K9/H3K36 demethylase KDM4C [379] and the H3K36 demethylase KDM2A [699]. Human tumors expressing IDH1 and IDH2 mutations had increased histone methylation at H3K4, H3K9, H3K27, and H3K79 [379]. 2-HG inhibits TET activity and this inhibition could be overcome by the addition of α-KG [375]. Mutations affecting TET2 and IDH1 are mutually exclusive in AML, suggesting that their biological effect is similar and that they have overlapping roles in AML pathogenesis [377,700].
Figure 18.
α-KG, 2-HG, and Epigenetic Control. Under healthy conditions (upper panel), the cytosol and the mitochondria IDH enzymes produce the metabolite α-KG which is utilized as a cofactor in DNA demethylation and histone deacetylation reactions. In cancer conditions (lower panel), the IDH enzymes instead produce the oncometabolite 2-HG, an α-KG analog which competitively inhibits DNA demethylases and histone deacetylases. Relationship between altered metabolism and an altered epigenome.
The evidence presented thus far has discussed the mitochondria and the plethora of cellular processes which they can control and direct independent of nucleus input. Evidence has also been presented discussing how perturbation to mitochondrial function, be it due to environmental factors, nutrition deprivation, molecular inhibition, or mutation (somatic or germline), can trigger numerous cellular cascades and compensatory mechanisms in order to preserve cellular survival, the most important of which is an RTG response. An RTG response triggers a cascade of changes that upregulate numerous cellular pathways and changes (such as substrate-level phosphorylation and HIF) which, if left uncorrected, drive the cell to a persistent tumor phenotype that displays aberrant growth profiles and has a resistance to apoptotic signals in conjunction with aberrant execution of other cellular processes from which it may not be able to recover should these triggering signals dissipate or subside.
An (un)intended consequence of disrupted mitochondrial function, however, is the effects it has on the cell’s epigenome. Perturbations to metabolite production, concentrations, or even the production of oncometabolites owing to somatic mutation all have inhibitory effects on the function of epigenome maintainers (Figure 19). This affects the cell’s ability to regulate its epigenome to maintain normal states and results in dysregulation of DNA methylation as well as histone methylation, acetylation, and phosphorylation profiles (Figure 19). If the epigenome is held captive in an aberrant state for an extended period of time, the cell may lose its ability to recover to a pre-perturbed state even if triggering signals are removed. How deleterious the consequent dysregulation is depends on the epigenetic mark in question and the duration of aberrance as each mark has its own duration of persistence. It therefore becomes evident that repeat incidences of mitochondrial and epigenetic dysregulatory episodes are required before cells are pushed into a chronic and persistent tumorigenic state, which can require numerous perturbation episodes over a number of years. This therefore might be why the presentation of many cancers is in older members of the population.
Figure 19.
Summary of the Metabolic Regulators of Epigenetics.
Taken together, the evidence clearly describes how intimately linked cellular metabolism and the epigenome truly are. The triggers that perturb and alter one will undoubtedly alter and perturb the other. Tumors are therefore critically dependent on these two cellular systems and their coupling in order to maintain tumorigenic phenotypes to support and drive essential metabolite generation, reprogrammed transcriptional circuits, reconfigured epigenetic profiles, apoptotic resistance, and essential cellular growth circuits that are typically tightly controlled and regulated by said cellular systems.
26. Therapies Targeting Dysregulated Metabolics and Epigenetics in Cancers
Through decades of research, evidence has been accumulated as to how essential dysregulated metabolic and epigenomic profiles contribute to continued tumor cell persistence and survival. This naturally has led to development of small-molecule inhibitors aiming to target these tumor cell dependencies for various therapies. What follows is a brief summary of compounds in development along with their intended cellular targets (Table 5).
Table 5.
Select Metabolic and Epigenetic Inhibitors Being Tested or Used in Cancer Therapies.
| Drug | Target | Mode of Action | Epigenetic Consequence | Treats | References |
|---|---|---|---|---|---|
| 5-Aza-2′-deoxycytidine (Dacogen/Inqovi) |
DNMTs | Integration into DNA to block DNMTs | Induces hypomethylation | AML and MDS | [701,702,703,704] |
| 5-azacytidine (Onureg/Vidaza) |
DNMTs | Integration into DNA to block DNMTs | Induces hypomethylation | AML, MDS, CMML | [703,704,705] |
| 3-Bromopyruvate (BrPA) |
Glyceraldehyde 3-phosphate dehydrogenase | Suppress production of ACoA resulting in depletion | Reduced histone acetylation | Prostate, breast, hepatic cancers | [706] |
| Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide (BPTES) |
Glutaminase | Suppress production of ACoA resulting in depletion Suppress 2-HG levels |
Reduced histone acetylation | Breast cancer | [707] |
| CB-839 (Telaglenastat) |
Glutaminase | Suppress production of ACoA resulting in depletion Suppress 2-HG levels |
Reduced histone acetylation | Multiple cancers | [708,709,710] |
| Compound 968 (Glutaminase C-IN-1) |
Glutaminase | Suppress production of ACoA resulting in depletion Suppress 2-HG levels |
Reduced histone acetylation Reduced H3K4me3 methylation |
Breast cancer | [711,712] |
| Zaprinast | Glutaminase | Suppress production of ACoA resulting in depletion Suppress 2-HG levels Reduces H3K27me2/me3 |
IDH1 mutant cancers | AML, glioblastoma | [709,713] |
| Butyrate | HDACs | Reduces glucose update | Restores histone acetylation balance | T-cell lymphoma, colorectal cancer | [714,715,716,717,718] |
| Vorinostat (Zolinza) |
HDACs | Reduces glucose update | Restores histone acetylation balance | T-cell lymphoma | [719,720] |
| Romidepsin | HDACs | Reduces glucose update | Restores histone acetylation balance | T-cell lymphoma | [721,722] |
| 2-Deoxyglucose (2-DG) |
Hexokinases | Depletes ACoA stores | Reduced histone acetylation | Multiple cancers | [723,724] |
| FT-2102 (Olutasidenib) |
Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | AML, glioma, MDS | [725] |
| LY3410738 | Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | AML and MDS | [726] |
| DS-1001b | Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | Glioma | [727] |
| AG-881 (Vorasidenib) |
Mutant IDH1 and IDH2 | Suppress 2-HG production | Enables proper TET function | AML, glioma, MDS, chondrosarcoma | [728] |
| AG-120 (Ivosidenib) |
Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | AML, glioma, MDS, chondrosarcoma | [729] |
| BAY1436032 | Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | AML | [730] |
| IDH305 | Mutant IDH1 | Suppress 2-HG production Reduces H3K9me3 and H3K27me3 methylation |
Enables proper TET function | Glioma, AML, MDS | [731] |
| AGI-5198 | Mutant IDH1 | Suppress 2-HG production Reduces H3K9me3 and H3K27me3 methylation |
Enables proper TET function | AML, glioma, MDS, chondrosarcoma | [732] |
| GSK321 | Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | AML | [372,733] |
| GSK864 (Derivative of CSK321) |
Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | AML | [733] |
| ML309 | Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | AML, glioblastoma | [734] |
| 2-(3-Trifluoromethylphenyl) isothiazol-3(2H)-one | Mutant IDH1 | Suppress 2-HG production | Enables proper TET function | Glioma | [735] |
| AG-221 (Enasidenib) |
Mutant IDH2 | Suppress 2-HG production | Enables proper TET function | AML, glioma, T-cell lymphomas, chondrosarcoma, cholangiocarcinoma | [736,737] |
| AGI-6780 | Mutant IDH2 | Suppress 2-HG production | Enables proper TET function | AML | [738] |
| CPI-613 (Devimistat) |
TCA Cycle Intermediates | Pyruvate dehydrogenase alpha-ketoglutarate dehydrogenase |
Blocks enzymatic activity | Burkitt’s lymphoma, MDS, T-cell lymphoma | Company press releases |
| IACS-010759 | NADH dehydrogenase (C-I) |
Quinone-site inhibitor | Blocks C-I function and electron transfer | Multiple cancers | [739,740] |
| DZNep (3-deazaneeplanocin A) |
SAH hydrolase | Increases SAH:SAM ratio Degrades EZH2 |
Inhibits H3K27me3 and H4K20me3 | Multiple cancers | [741] |
| Adenosine dialdehyde | SAH hydrolase | Increases SAH:SAM ratio | Inhibits DNA and histone methylation Downregulates MMP-9 and inhibits Ras/Raf-1/ERK/AP-1 |
Multiple cancers | [742] |
| N-methylnicotinamide | NNMTT | Increases SAM levels | Impairs methylation | AML, multiple cancers |
NCT02746081, NCT03127735 |
| TVB-2640 | Fatty acid synthase | Blocks fatty acid processing | Solid tumors, lung, ovarian, breast | [743,744] |
DNA methylation regulators, as well as histone methylation and acetylation regulators, can be controlled by mitochondrially derived metabolites. By carefully orchestrating and controlling metabolism, tumor cells can drive whichever genetic program they require to drive their growth and survival, promoting hyper- or hypophenotypes depending on the required state and/or phenotype.
The data presented show numerous critical pathways and enzymes can be targeted, especially those upregulated or mutated in various cancers. Many of the compounds listed still require years of development and possibly refinement before they make their way to the clinic, but preliminary data, at least for some compounds, show clear efficacy for targeting the metabolic and epigenetic abnormalities frequently observed in tumors.
27. Concluding Remarks
This review aimed to summarize the body of knowledge gained investigating cellular metabolism and the epigenome. We firstly discussed the mitochondria’s essential roles and how they regulate cellular function. Next, we discussed how mitochondrial dysfunction relates to and drives key tumor hallmarks and included a detailed discussion of the altered metabolic profiles seen in cancers. These discussions outlined how numerous tumor cell characteristics are driven by dysregulation of metabolic circuits.
Next, we discussed the cellular epigenome. Owing to the breadth of PTMs possible, we focused only on methylation (DNA and histone), acetylation (histone), and phosphorylation (histone). In this review, we aimed to outline how cellular metabolism and epigenetics intersect by connecting which pathways and metabolites are linked, how they are linked, and the consequence of how metabolic dysregulation affects epigenomic regulation. We aimed to discuss how coupled and intertwined these two cellular circuits are. This coupling is fortunate for researchers since one need only identify a targetable pathway step for therapy development. As research continues and better therapies are developed, it is foreseeable that effective combinatorial and personalized treatment regimens will be developed for cancer patients which include a component targeting tumor metabolic–epigenetic dependencies and dysregulation.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The author declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Warburg O., Wind F., Negelein E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Schulz T.J., Thierbach R., Voigt A., Drewes G., Mietzner B., Steinberg P., Pfeiffer A.F., Ristow M. Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J. Biol. Chem. 2006;281:977–981. doi: 10.1074/jbc.M511064200. [DOI] [PubMed] [Google Scholar]
- 5.Seyfried T.N., Shelton L.M. Cancer as a metabolic disease. Nutr. Metab. 2010;7:7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altenberg B., Greulich K.O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 7.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise D.R., DeBerardinis R.J., Mancuso A., Sayed N., Zhang X.Y., Pfeiffer H.K., Nissim I., Daikhin E., Yudkoff M., McMahon S.B., et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananieva E. Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J. Biol. Chem. 2015;6:281–289. doi: 10.4331/wjbc.v6.i4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohrig F., Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer. 2016;16:732–749. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 11.Brusselmans K., Swinnen J.V. The Lipogenic Switch in Cancers. In: Singh K.K., Costello L.C., editors. Mitochondria and Cancer. 1st ed. Springer; New York, NY. USA: 2009. pp. 39–60. [DOI] [Google Scholar]
- 12.Samudio I., Harmancey R., Fiegl M., Kantarjian H., Konopleva M., Korchin B., Kaluarachchi K., Bornmann W., Duvvuri S., Taegtmeyer H., et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J. Clin. Investig. 2010;120:142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baysal B.E. On the association of succinate dehydrogenase mutations with hereditary paraganglioma. Trends Endocrinol. Metab. 2003;14:453–459. doi: 10.1016/j.tem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Bonello S., Zahringer C., BelAiba R.S., Djordjevic T., Hess J., Michiels C., Kietzmann T., Gorlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 16.Linnartz B., Anglmayer R., Zanssen S. Comprehensive scanning of somatic mitochondrial DNA alterations in acute leukemia developing from myelodysplastic syndromes. Cancer Res. 2004;64:1966–1971. doi: 10.1158/0008-5472.CAN-03-2956. [DOI] [PubMed] [Google Scholar]
- 17.Biswas G., Adebanjo O.A., Freedman B.D., Anandatheerthavarada H.K., Vijayasarathy C., Zaidi M., Kotlikoff M., Avadhani N.G. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: A novel mode of inter-organelle crosstalk. EMBO J. 1999;18:522–533. doi: 10.1093/emboj/18.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amuthan G., Biswas G., Zhang S.Y., Klein-Szanto A., Vijayasarathy C., Avadhani N.G. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 2001;20:1910–1920. doi: 10.1093/emboj/20.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erol A. Retrograde regulation due to mitochondrial dysfunction may be an important mechanism for carcinogenesis. Med. Hypotheses. 2005;65:525–529. doi: 10.1016/j.mehy.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Miceli M.V., Jazwinski S.M. Common and cell type-specific responses of human cells to mitochondrial dysfunction. Exp. Cell Res. 2005;302:270–280. doi: 10.1016/j.yexcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Tuppen H.A., Blakely E.L., Turnbull D.M., Taylor R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 2010;1797:113–128. doi: 10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Ye F.D.C.S., Yan G. Detection of Mitochondrial Mutations in Cancer by Next Generation Sequencing. J. Next Gener. Seq. Appl. 2014;1:1. doi: 10.4172/2469-9853.1000102. [DOI] [Google Scholar]
- 23.Reznik E., Wang Q., La K., Schultz N., Sander C. Mitochondrial respiratory gene expression is suppressed in many cancers. Elife. 2017;6:e21592. doi: 10.7554/eLife.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ley T.J., Mardis E.R., Ding L., Fulton B., McLellan M.D., Chen K., Dooling D., Dunford-Shore B.H., McGrath S., Hickenbotham M., et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456:66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace D.C. Mitochondria and cancer. Nat. Rev. Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace D.C. Genetics: Mitochondrial DNA in evolution and disease. Nature. 2016;535:498–500. doi: 10.1038/nature18902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons D.W., Jones S., Zhang X., Lin J.C., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.M., Gallia G.L., et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seyfried T.N., Flores R.E., Poff A.M., D’Agostino D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis. 2014;35:515–527. doi: 10.1093/carcin/bgt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dang C.V. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan H., Parsons D.W., Jin G., McLendon R., Rasheed B.A., Yuan W., Kos I., Batinic-Haberle I., Jones S., Riggins G.J., et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grzybowska-Szatkowska L., Slaska B. Mitochondrial NADH dehydrogenase polymorphisms are associated with breast cancer in Poland. J. Appl. Genet. 2014;55:173–181. doi: 10.1007/s13353-013-0190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder G.K., Sheafor B.A. Red Blood Cells: Centerpiece in the Evolution of the Vertebrate Circulatory System. Am. Zool. 1999;39:189–198. doi: 10.1093/icb/39.2.189. [DOI] [Google Scholar]
- 34.Palade G.E. The fine structure of mitochondria. Anat. Rec. 1952;114:427–451. doi: 10.1002/ar.1091140304. [DOI] [PubMed] [Google Scholar]
- 35.Sottocasa G.L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. a biochemical and morphological study. J. Cell Biol. 1967;32:415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons D.F., Williams G.R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann. N. Y. Acad. Sci. 1966;137:643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- 37.Schnaitman C., Greenawalt J.W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J. Cell Biol. 1968;38:158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnaitman C., Erwin V.G., Greenawalt J.W. The submitochondrial localization of monoamine oxidase. an enzymatic marker for the outer membrane of rat liver mitochondria. J. Cell Biol. 1967;32:719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins G.A., Frey T.G. Recent structural insight into mitochondria gained by microscopy. Micron. 2000;31:97–111. doi: 10.1016/S0968-4328(99)00065-7. [DOI] [PubMed] [Google Scholar]
- 40.Logan D.C. The mitochondrial compartment. J. Exp. Bot. 2006;57:1225–1243. doi: 10.1093/jxb/erj151. [DOI] [PubMed] [Google Scholar]
- 41.Comte J., Maisterrena B., Gautheron D.C. Lipid composition and protein profiles of outer and inner membranes from pig heart mitochondria. Comparison with microsomes. Biochim. Biophys. Acta. 1976;419:271–284. doi: 10.1016/0005-2736(76)90353-9. [DOI] [PubMed] [Google Scholar]
- 42.Hallermayer G., Neupert W. Lipid composition of mitochondrial outer and inner membranes of Neurospora crassa. Biol. Chem. 1974;355:279–288. doi: 10.1515/bchm2.1974.355.1.279. [DOI] [PubMed] [Google Scholar]
- 43.Colombini M. The VDAC channel: Molecular basis for selectivity. Biochim. Biophys. Acta. 2016;1863:2498–2502. doi: 10.1016/j.bbamcr.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Parsons D.F., Bonner W.D., Jr., Verboon J.G. Electron microscopy of isolated plant mitochondria and plastids using both the thin-section and negative-staining techniques. Can. J. Bot. 1965;43:647–655. doi: 10.1139/b65-072. [DOI] [Google Scholar]
- 45.Werkheiser W.C., Bartley W. The study of steady-state concentrations of internal solutes of mitochondria by rapid centrifugal transfer to a fixation medium. Biochem. J. 1957;66:79–91. doi: 10.1042/bj0660079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Getz G.S., Bartley W., Lurie D., Notton B.M. The phospholipids of various sheep organs, rat liver and of their subcellular fractions. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1968;152:325–339. doi: 10.1016/0005-2760(68)90040-4. [DOI] [PubMed] [Google Scholar]
- 47.Chicco A.J., Sparagna G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 48.Fry M., Green D.E. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J. Biol. Chem. 1981;256:1874–1880. doi: 10.1016/S0021-9258(19)69888-1. [DOI] [PubMed] [Google Scholar]
- 49.Pfaff E., Klingenberg M., Heldt H.W. Unspecific permeation and specific exchange of adenine nucleotides in liver mitochondria. Biochim. Biophys. Acta. 1965;104:312–315. doi: 10.1016/0304-4165(65)90258-8. [DOI] [PubMed] [Google Scholar]
- 50.Ernster L., Schatz G. Mitochondria: A historical review. J. Cell Biol. 1981;91:227s–255s. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Formosa L.E., Ryan M.T. Mitochondrial fusion: Reaching the end of mitofusin’s tether. J. Cell Biol. 2016;215:597–598. doi: 10.1083/jcb.201611048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter V., Singh A.P., Kvansakul M., Ryan M.T., Osellame L.D. Splitting up the powerhouse: Structural insights into the mechanism of mitochondrial fission. Cell. Mol. Life Sci. 2015;72:3695–3707. doi: 10.1007/s00018-015-1950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadenbach B. Introduction to Mitochondrial Oxidative Phosphorylation. In: Cohen I.R., Lajtha A., Lambris J.D., Paoletti R., editors. Advances in Experimental Medicine and Biology. Volume 748. Springer; Berlin/Heidelberg, Germany: 2012. pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 54.Chan D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 55.Ferree A., Shirihai O. Mitochondrial Dynamics: The Intersection of Form and Function. In: Kabenbach B., editor. Mitochondrial Oxidative Phosphorylation. Springer; New York, NY, USA: 2012. pp. 13–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat. Reviews. Mol. Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 57.Opferman J.T., Iwasaki H., Ong C.C., Suh H., Mizuno S., Akashi K., Korsmeyer S.J. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y.B., Aon M.A., Hsu Y.T., Soane L., Teng X., McCaffery J.M., Cheng W.C., Qi B., Li H., Alavian K.N., et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J. Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alavian K.N., Li H., Collis L., Bonanni L., Zeng L., Sacchetti S., Lazrove E., Nabili P., Flaherty B., Graham M., et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat. Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perciavalle R.M., Stewart D.P., Koss B., Lynch J., Milasta S., Bathina M., Temirov J., Cleland M.M., Pelletier S., Schuetz J.D., et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat. Cell Biol. 2012;14:575–583. doi: 10.1038/ncb2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nass M.M.K., Nass S. Intramitochondrial fibers with DNA characteristics. I. Fixation and Electron Staining Reactions. J. Cell Biol. 1963;19:593–611. doi: 10.1083/jcb.19.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schatz G., Haslbrunner E., Tuppy H. Deoxyribonucleic acid associated with yeast mitochondria. Biochem. Biophys. Res. Commun. 1964;15:127–132. doi: 10.1016/0006-291X(64)90311-0. [DOI] [PubMed] [Google Scholar]
- 63.Luck D.J.L., Reich E. DNA in mitochondria of neurospora crassa. Proc. Natl. Acad. Sci. USA. 1964;52:931–938. doi: 10.1073/pnas.52.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt O., Pfanner N., Meisinger C. Mitochondrial protein import: From proteomics to functional mechanisms. Nat. Rev. Mol. Cell Biol. 2010;11:655–667. doi: 10.1038/nrm2959. [DOI] [PubMed] [Google Scholar]
- 65.Simcox E., Reeve A. An Introduction to Mitochondria, Their Structure and Functions. In: Reeve A., Simcox E., Duchen M., Turnbull D., editors. Mitochondrial Dysfunction in Neurodegenerative Disorders. Springer International Publishing; Cham, Switzerland: 2016. pp. 3–31. [DOI] [Google Scholar]
- 66.Kelly D.P., Scarpulla R.C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 67.Woodson J.D., Chory J. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 2008;9:383–395. doi: 10.1038/nrg2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerrero-Castillo S., Baertling F., Kownatzki D., Wessels H.J., Arnold S., Brandt U., Nijtmans L. The Assembly Pathway of Mitochondrial Respiratory Chain Complex I. Cell Metab. 2016;25:128–139. doi: 10.1016/j.cmet.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Schroeder P., Pohl C., Calles C., Marks C., Wild S., Krutmann J. Cellular response to infrared radiation involves retrograde mitochondrial signaling. Free Radic. Biol. Med. 2007;43:128–135. doi: 10.1016/j.freeradbiomed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Singh K.K., Kulawiec M., Still I., Desouki M.M., Geradts J., Matsui S. Inter-genomic cross talk between mitochondria and the nucleus plays an important role in tumorigenesis. Gene. 2005;354:140–146. doi: 10.1016/j.gene.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 71.Butow R.A., Avadhani N.G. Mitochondrial signaling: The retrograde response. Mol. Cell. 2004;14:1–15. doi: 10.1016/S1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- 72.Siekevitz P. Powerhouse of the Cell. Sci. Am. 1957;197:131–144. doi: 10.1038/scientificamerican0757-131. [DOI] [Google Scholar]
- 73.Sands R.H., Beinert H. Studies on mitochondria and submitochondrial particles by paramagnetic resonance (EPR) spectroscopy. Biochem. Biophys. Res. Commun. 1960;3:47–52. doi: 10.1016/0006-291X(60)90101-7. [DOI] [Google Scholar]
- 74.Hajnóczky G., Csordás G., Das S., Garcia-Perez C., Saotome M., Sinha Roy S., Yi M. Mitochondrial calcium signalling and cell death: Approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green D.R. Apoptotic Pathways. Cell. 1998;94:695–698. doi: 10.1016/S0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 76.Marzo I., Brenner C., Kroemer G. The central role of the mitochondrial megachannel in apoptosis: Evidence obtained with intact cells, isolated mitochondria, and purified protein complexes. Biomed. Pharmacother. 1998;52:248–251. doi: 10.1016/S0753-3322(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 77.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 78.Chandel N.S., McClintock D.S., Feliciano C.E., Wood T.M., Melendez J.A., Rodriguez A.M., Schumacker P.T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: A mechanism of O2 sensing. J. Biol. Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 79.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li X., Fang P., Mai J., Choi E.T., Wang H., Yang X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borkenhagen L.F., Kennedy E.P., Fielding L. Enzymatic Formation and Decarboxylation of Phosphatidylserine. J. Biol. Chem. 1961;236:PC28–PC30. doi: 10.1016/S0021-9258(19)63319-3. [DOI] [Google Scholar]
- 82.Hailey D.W., Rambold A.S., Satpute-Krishnan P., Mitra K., Sougrat R., Kim P.K., Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rees D.M., Leslie A.G., Walker J.E. The structure of the membrane extrinsic region of bovine ATP synthase. Proc. Natl. Acad. Sci. USA. 2009;106:21597–21601. doi: 10.1073/pnas.0910365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chance B., Williams G.R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J. Biol. Chem. 1955;217:409–427. doi: 10.1016/S0021-9258(19)57191-5. [DOI] [PubMed] [Google Scholar]
- 85.Hackenbrock C.R., Chazotte B., Gupte S.S. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J. Bioenerg. Biomembr. 1986;18:331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- 86.Acin-Perez R., Enriquez J.A. The function of the respiratory supercomplexes: The plasticity model. Biochim. Biophys. Acta. 2014;1837:444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Cruciat C.M., Brunner S., Baumann F., Neupert W., Stuart R.A. The cytochrome bc1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J. Biol. Chem. 2000;275:18093–18098. doi: 10.1074/jbc.M001901200. [DOI] [PubMed] [Google Scholar]
- 88.Schagger H., Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dudkina N.V., Kouril R., Peters K., Braun H.P., Boekema E.J. Structure and function of mitochondrial supercomplexes. Biochim. Biophys. Acta. 2010;1797:664–670. doi: 10.1016/j.bbabio.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 90.Vartak R., Porras C.A., Bai Y. Respiratory supercomplexes: Structure, function and assembly. Protein Cell. 2013;4:582–590. doi: 10.1007/s13238-013-3032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Genova M.L., Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys. Acta. 2014;1837:427–443. doi: 10.1016/j.bbabio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 92.Acin-Perez R., Fernandez-Silva P., Peleato M.L., Perez-Martos A., Enriquez J.A. Respiratory active mitochondrial supercomplexes. Mol. Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 93.Shinzawa-Itoh K., Shimomura H., Yanagisawa S., Shimada S., Takahashi R., Oosaki M., Ogura T., Tsukihara T. Purification of Active Respiratory Supercomplex from Bovine Heart Mitochondria Enables Functional Studies. J. Biol. Chem. 2016;291:4178–4184. doi: 10.1074/jbc.M115.680553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Letts J.A., Fiedorczuk K., Sazanov L.A. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 95.Stuart R.A. Supercomplex organization of the oxidative phosphorylation enzymes in yeast mitochondria. J. Bioenerg. Biomembr. 2008;40:411–417. doi: 10.1007/s10863-008-9168-4. [DOI] [PubMed] [Google Scholar]
- 96.Gu J., Wu M., Guo R., Yan K., Lei J., Gao N., Yang M. The architecture of the mammalian respirasome. Nature. 2016;537:639–643. doi: 10.1038/nature19359. [DOI] [PubMed] [Google Scholar]
- 97.Schafer E., Seelert H., Reifschneider N.H., Krause F., Dencher N.A., Vonck J. Architecture of active mammalian respiratory chain supercomplexes. J. Biol. Chem. 2006;281:15370–15375. doi: 10.1074/jbc.M513525200. [DOI] [PubMed] [Google Scholar]
- 98.Johnson D.C., Dean D.R., Smith A.D., Johnson M.K. Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 99.Tong W.-H., Jameson G.N.L., Huynh B.H., Rouault T.A. Subcellular compartmentalization of human Nfu, an iron–sulfur cluster scaffold protein, and its ability to assemble a [4Fe–4S] cluster. Proc. Natl. Acad. Sci. USA. 2003;100:9762–9767. doi: 10.1073/pnas.1732541100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lange H., Kaut A., Kispal G., Lill R. A mitochondrial ferredoxin is essential for biogenesis of cellular iron-sulfur proteins. Proc. Natl. Acad. Sci. USA. 2000;97:1050–1055. doi: 10.1073/pnas.97.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lill R., Muhlenhoff U. Iron-sulfur-protein biogenesis in eukaryotes. Trends Biochem. Sci. 2005;30:133–141. doi: 10.1016/j.tibs.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 102.Brini M., Cali T., Ottolini D., Carafoli E. Intracellular calcium homeostasis and signaling. Met. Ions Life Sci. 2013;12:119–168. doi: 10.1007/978-94-007-5561-1_5. [DOI] [PubMed] [Google Scholar]
- 103.Carafoli E., Crompton M. The regulation of intracellular calcium by mitochondria. Ann. N. Y. Acad. Sci. 1978;307:269–284. doi: 10.1111/j.1749-6632.1978.tb41957.x. [DOI] [PubMed] [Google Scholar]
- 104.McCormack J.G., Halestrap A.P., Denton R.M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 105.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 106.Rasmussen H., Barrett P.Q. Calcium messenger system: An integrated view. Physiol. Rev. 1984;64:938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- 107.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keinan N., Pahima H., Ben-Hail D., Shoshan-Barmatz V. The role of calcium in VDAC1 oligomerization and mitochondria-mediated apoptosis. Biochim. Biophys. Acta. 2013;1833:1745–1754. doi: 10.1016/j.bbamcr.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 109.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A Forty-Kilodalton Protein of the Inner Membrane is the Mitochondrial Calcium Uniporter. [(accessed on 24 April 2023)];Nature. 2011 476:336–340. doi: 10.1038/nature10230. Available online: http://www.nature.com/nature/journal/v476/n7360/abs/nature10230.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palty R., Hershfinkel M., Sekler I. Molecular Identity and Functional Properties of the Mitochondrial Na+/Ca2+ Exchanger. J. Biol. Chem. 2012;287:31650–31657. doi: 10.1074/jbc.R112.355867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palty R., Sekler I. The mitochondrial Na+/Ca2+ exchanger. Cell Calcium. 2012;52:9–15. doi: 10.1016/j.ceca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 112.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta (BBA)-Bioenerg. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 113.Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Szabadkai G., Duchen M.R. Mitochondria: The hub of cellular Ca2+ signaling. Physiology. 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 115.Trapani J.A., Smyth M.J. Killing by cytotoxic T cells and natural killer cells: Multiple granule serine proteases as initiators of DNA fragmentation. Immunol. Cell Biol. 1993;71:201–208. doi: 10.1038/icb.1993.22. [DOI] [PubMed] [Google Scholar]
- 116.Shi L., Kraut R.P., Aebersold R., Greenberg A.H. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J. Exp. Med. 1992;175:553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Estaquier J., Vallette F., Vayssiere J.L., Mignotte B. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012;942:157–183. doi: 10.1007/978-94-007-2869-1_7. [DOI] [PubMed] [Google Scholar]
- 119.Ouyang L., Shi Z., Zhao S., Wang F.T., Zhou T.T., Liu B., Bao J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45:487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Westphal D., Dewson G., Czabotar P.E., Kluck R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011;1813:521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 121.Ma S., Hockings C., Anwari K., Kratina T., Fennell S., Lazarou M., Ryan M.T., Kluck R.M., Dewson G. Assembly of the Bak apoptotic pore: A critical role for the Bak protein alpha6 helix in the multimerization of homodimers during apoptosis. J. Biol. Chem. 2013;288:26027–26038. doi: 10.1074/jbc.M113.490094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cai J., Yang J., Jones D.P. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta. 1998;1366:139–149. doi: 10.1016/S0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 123.Du C., Fang M., Li Y., Li L., Wang X. Smac, a Mitochondrial Protein that Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell. 2000;102:33–42. doi: 10.1016/S0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 124.van Loo G., Saelens X., van Gurp M., MacFarlane M., Martin S.J., Vandenabeele P. The role of mitochondrial factors in apoptosis: A Russian roulette with more than one bullet. Cell Death Differ. 2002;9:1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 125.Garrido C., Galluzzi L., Brunet M., Puig P.E., Didelot C., Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 126.Budihardjo I., Oliver H., Lutter M., Luo X., Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 127.Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/S0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 128.Kischkel F.C., Hellbardt S., Behrmann I., Germer M., Pawlita M., Krammer P.H., Peter M.E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Young M.M., Takahashi Y., Khan O., Park S., Hori T., Yun J., Sharma A.K., Amin S., Hu C.D., Zhang J., et al. Autophagosomal membrane serves as platform for intracellular death-inducing signaling complex (iDISC)-mediated caspase-8 activation and apoptosis. J. Biol. Chem. 2012;287:12455–12468. doi: 10.1074/jbc.M111.309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 131.Pradelli L.A., Beneteau M., Ricci J.E. Mitochondrial control of caspase-dependent and -independent cell death. Cell. Mol. Life Sci. 2010;67:1589–1597. doi: 10.1007/s00018-010-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Russell J.H., Ley T.J. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 133.Barry M., Bleackley R.C. Cytotoxic T lymphocytes: All roads lead to death. Nat. Rev. Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 134.Linkermann A., Vanden Berghe T., Takahashi N., Kunzendorf U., Krautwald S., Vandenabeele P. The Potential Role of Necroptosis in Diseases. In: Shen H., Vandenabeele P., editors. Necrotic Cell Death. Volume 2. Springer Science; New York, NY, USA: 2014. pp. 1–21. [Google Scholar]
- 135.Kroemer G., Dallaporta B., Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 136.Vanden Berghe T., Linkermann A., Jouan-Lanhouet S., Walczak H., Vandenabeele P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 137.Eguchi Y., Shimizu S., Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 138.Crompton M., Barksby E., Johnson N., Capano M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie. 2002;84:143–152. doi: 10.1016/S0300-9084(02)01368-8. [DOI] [PubMed] [Google Scholar]
- 139.Vanlangenakker N., Vanden Berghe T., Krysko D.V., Festjens N., Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 140.Fatokun A.A., Dawson V.L., Dawson T.M. Parthanatos: Mitochondrial-linked mechanisms and therapeutic opportunities. Br. J. Pharmacol. 2014;171:2000–2016. doi: 10.1111/bph.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Andrabi S.A., Dawson T.M., Dawson V.L. Mitochondrial and nuclear cross talk in cell death: Parthanatos. Ann. N. Y. Acad. Sci. 2008;1147:233–241. doi: 10.1196/annals.1427.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Virag L., Robaszkiewicz A., Rodriguez-Vargas J.M., Oliver F.J. Poly(ADP-ribose) signaling in cell death. Mol. Asp. Med. 2013;34:1153–1167. doi: 10.1016/j.mam.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 143.De Duve C. The lysosome. Sci. Am. 1963;208:64–72. doi: 10.1038/scientificamerican0563-64. [DOI] [PubMed] [Google Scholar]
- 144.Katheder N.S., Khezri R., O’Farrell F., Schultz S.W., Jain A., Rahman M.M., Schink K.O., Theodossiou T.A., Johansen T., Juhasz G., et al. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541:417–420. doi: 10.1038/nature20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Duve C., Wattiaux R. Functions of lysosomes. Annu. Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 146.De Duve C. Lysosomes revisited. Eur. J. Biochem. 1983;137:391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]
- 147.Scherz-Shouval R., Elazar Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 148.Lee J., Giordano S., Zhang J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okamoto K., Kondo-Okamoto N. Mitochondria and autophagy: Critical interplay between the two homeostats. Biochim. Biophys. Acta. 2012;1820:595–600. doi: 10.1016/j.bbagen.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 150.Vyas S., Zaganjor E., Haigis M.C. Mitochondria and Cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tafani M., Sansone L., Limana F., Arcangeli T., De Santis E., Polese M., Fini M., Russo M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell. Longev. 2016;2016:3907147. doi: 10.1155/2016/3907147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sun Q., Zhong W., Zhang W., Zhou Z. Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: Role of zinc deficiency. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;310:G205–G214. doi: 10.1152/ajpgi.00270.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kumari U., Ya Jun W., Huat Bay B., Lyakhovich A. Evidence of mitochondrial dysfunction and impaired ROS detoxifying machinery in Fanconi anemia cells. Oncogene. 2014;33:165–172. doi: 10.1038/onc.2012.583. [DOI] [PubMed] [Google Scholar]
- 156.Sharma L.K., Fang H., Liu J., Vartak R., Deng J., Bai Y. Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Hum. Mol. Genet. 2011;20:4605–4616. doi: 10.1093/hmg/ddr395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kwiecien S., Jasnos K., Magierowski M., Sliwowski Z., Pajdo R., Brzozowski B., Mach T., Wojcik D., Brzozowski T. Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress—Induced gastric injury. J. Physiol. Pharmacol. 2014;65:613–622. [PubMed] [Google Scholar]
- 158.Mylonas C., Kouretas D. Lipid peroxidation and tissue damage. In Vivo. 1999;13:295–309. [PubMed] [Google Scholar]
- 159.Kwon J., Lee S.R., Yang K.S., Ahn Y., Kim Y.J., Stadtman E.R., Rhee S.G. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee S.R., Yang K.S., Kwon J., Lee C., Jeong W., Rhee S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 161.Samper E., Nicholls D.G., Melov S. Mitochondrial oxidative stress causes chromosomal instability of mouse embryonic fibroblasts. Aging Cell. 2003;2:277–285. doi: 10.1046/j.1474-9728.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 162.Pelicano H., Carney D., Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 163.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 164.Owusu-Ansah E., Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shadel G.S., Horvath T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell. 2015;163:560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Waypa G.B., Smith K.A., Schumacker P.T. O2 sensing, mitochondria and ROS signaling: The fog is lifting. Mol. Asp. Med. 2016;47:76–89. doi: 10.1016/j.mam.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K., Ohmura M., Naka K., Hosokawa K., Ikeda Y., et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 168.Asai T., Liu Y., Bae N., Nimer S.D. The p53 tumor suppressor protein regulates hematopoietic stem cell fate. J. Cell. Physiol. 2011;226:2215–2221. doi: 10.1002/jcp.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tothova Z., Kollipara R., Huntly B.J., Lee B.H., Castrillon D.H., Cullen D.E., McDowell E.P., Lazo-Kallanian S., Williams I.R., Sears C., et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 170.Hoesel B., Schmid J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Doyle K., Fitzpatrick F.A. Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function. J. Biol. Chem. 2010;285:17417–17424. doi: 10.1074/jbc.M109.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Le Belle J.E., Orozco N.M., Paucar A.A., Saxe J.P., Mottahedeh J., Pyle A.D., Wu H., Kornblum H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oberley L.W. Inhibition of tumor cell growth by overexpression of manganese-containing superoxide dismutase. Age. 1998;21:95–97. doi: 10.1007/s11357-998-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sun W.G., Weydert C.J., Zhang Y., Yu L., Liu J., Spitz D.R., Cullen J.J., Oberley L.W. Superoxide Enhances the Antitumor Combination of AdMnSOD Plus BCNU in Breast Cancer. Cancers. 2010;2:68–87. doi: 10.3390/cancers2010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Daum G., Lees N.D., Bard M., Dickson R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast. 1998;14:1471–1510. doi: 10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 176.Tatsuta T., Scharwey M., Langer T. Mitochondrial lipid trafficking. Trends Cell Biol. 2013;24:44–52. doi: 10.1016/j.tcb.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 177.Tamura Y., Harada Y., Nishikawa S., Yamano K., Kamiya M., Shiota T., Kuroda T., Kuge O., Sesaki H., Imai K., et al. Tam41 is a CDP-diacylglycerol synthase required for cardiolipin biosynthesis in mitochondria. Cell Metab. 2013;17:709–718. doi: 10.1016/j.cmet.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Osman C., Voelker D.R., Langer T. Making heads or tails of phospholipids in mitochondria. J. Cell Biol. 2011;192:7–16. doi: 10.1083/jcb.201006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Connerth M., Tatsuta T., Haag M., Klecker T., Westermann B., Langer T. Intramitochondrial transport of phosphatidic acid in yeast by a lipid transfer protein. Science. 2012;338:815–818. doi: 10.1126/science.1225625. [DOI] [PubMed] [Google Scholar]
- 180.Lee H.C., Wei Y.H. Mitochondria and aging. Adv. Exp. Med. Biol. 2012;942:311–327. doi: 10.1007/978-94-007-2869-1_14. [DOI] [PubMed] [Google Scholar]
- 181.Miquel J., Economos A.C., Fleming J., Johnson J.E., Jr. Mitochondrial role in cell aging. Exp. Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. [DOI] [PubMed] [Google Scholar]
- 182.Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. Am. J. Pathol. 1989;134:1167–1173. [PMC free article] [PubMed] [Google Scholar]
- 183.Detmer S.A., Chan D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 184.Pedersen P.L. Tumor mitochondria and the bioenergetics of cancer cells. Prog. Exp. Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- 185.Hunter R.L., Dragicevic N., Seifert K., Choi D.Y., Liu M., Kim H.C., Cass W.A., Sullivan P.G., Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- 186.Rous P. Surmise and fact on the nature of cancer. Nature. 1959;183:1357–1361. doi: 10.1038/1831357a0. [DOI] [PubMed] [Google Scholar]
- 187.Smith A.E., Kenyon D.H. A unifying concept of carcinogenesis and its therapeutic implications. Oncology. 1973;27:459–479. doi: 10.1159/000224754. [DOI] [PubMed] [Google Scholar]
- 188.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 189.Weinberg J.M., Venkatachalam M.A., Roeser N.F., Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc. Natl. Acad. Sci. USA. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shoubridge E.A. Nuclear genetic defects of oxidative phosphorylation. Hum. Mol. Genet. 2001;10:2277–2284. doi: 10.1093/hmg/10.20.2277. [DOI] [PubMed] [Google Scholar]
- 191.Baysal B.E., Ferrell R.E., Willett-Brozick J.E., Lawrence E.C., Myssiorek D., Bosch A., van der Mey A., Taschner P.E., Rubinstein W.S., Myers E.N., et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 192.Simonnet H., Alazard N., Pfeiffer K., Gallou C., Beroud C., Demont J., Bouvier R., Schagger H., Godinot C. Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma. Carcinogenesis. 2002;23:759–768. doi: 10.1093/carcin/23.5.759. [DOI] [PubMed] [Google Scholar]
- 193.Simonnet H., Demont J., Pfeiffer K., Guenaneche L., Bouvier R., Brandt U., Schägger H., Godinot C. Mitochondrial complex I is deficient in renal oncocytomas. Carcinogenesis. 2003;24:1461–1466. doi: 10.1093/carcin/bgg109. [DOI] [PubMed] [Google Scholar]
- 194.Srinivasan V., Kriete A., Sacan A., Jazwinski S.M. Comparing the yeast retrograde response and NF-kappaB stress responses: Implications for aging. Aging Cell. 2010;9:933–941. doi: 10.1111/j.1474-9726.2010.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu Z., Butow R.A. Mitochondrial retrograde signaling. Annu. Rev. Genet. 2006;40:159–185. doi: 10.1146/annurev.genet.40.110405.090613. [DOI] [PubMed] [Google Scholar]
- 196.Rothermel B.A., Shyjan A.W., Etheredge J.L., Butow R.A. Transactivation by Rtg1p, a basic helix-loop-helix protein that functions in communication between mitochondria and the nucleus in yeast. J. Biol. Chem. 1995;270:29476–29482. doi: 10.1074/jbc.270.49.29476. [DOI] [PubMed] [Google Scholar]
- 197.Sekito T., Thornton J., Butow R.A. Mitochondria-to-nuclear signaling is regulated by the subcellular localization of the transcription factors Rtg1p and Rtg3p. Mol. Biol. Cell. 2000;11:2103–2115. doi: 10.1091/mbc.11.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rothermel B.A., Thornton J.L., Butow R.A. Rtg3p, a basic helix-loop-helix/leucine zipper protein that functions in mitochondrial-induced changes in gene expression, contains independent activation domains. J. Biol. Chem. 1997;272:19801–19807. doi: 10.1074/jbc.272.32.19801. [DOI] [PubMed] [Google Scholar]
- 199.Borghouts C., Benguria A., Wawryn J., Jazwinski S.M. Rtg2 protein links metabolism and genome stability in yeast longevity. Genetics. 2004;166:765–777. doi: 10.1093/genetics/166.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jazwinski S.M. The retrograde response: A conserved compensatory reaction to damage from within and from without. Prog. Mol. Biol. Transl. Sci. 2014;127:133–154. doi: 10.1016/B978-0-12-394625-6.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dilova I., Aronova S., Chen J.C., Powers T. Tor signaling and nutrient-based signals converge on Mks1p phosphorylation to regulate expression of Rtg1.Rtg3p-dependent target genes. J. Biol. Chem. 2004;279:46527–46535. doi: 10.1074/jbc.M409012200. [DOI] [PubMed] [Google Scholar]
- 202.Dilova I., Chen C.Y., Powers T. Mks1 in concert with TOR signaling negatively regulates RTG target gene expression in S. cerevisiae. Curr. Biol. 2002;12:389–395. doi: 10.1016/S0960-9822(02)00677-2. [DOI] [PubMed] [Google Scholar]
- 203.Liu Z., Sekito T., Spirek M., Thornton J., Butow R.A. Retrograde signaling is regulated by the dynamic interaction between Rtg2p and Mks1p. Mol. Cell. 2003;12:401–411. doi: 10.1016/S1097-2765(03)00285-5. [DOI] [PubMed] [Google Scholar]
- 204.Giannattasio S., Liu Z., Thornton J., Butow R.A. Retrograde response to mitochondrial dysfunction is separable from TOR1/2 regulation of retrograde gene expression. J. Biol. Chem. 2005;280:42528–42535. doi: 10.1074/jbc.M509187200. [DOI] [PubMed] [Google Scholar]
- 205.Amuthan G., Biswas G., Ananadatheerthavarada H.K., Vijayasarathy C., Shephard H.M., Avadhani N.G. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene. 2002;21:7839–7849. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- 206.Lv X., Li J., Zhang C., Hu T., Li S., He S., Yan H., Tan Y., Lei M., Wen M., et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2017;4:19–24. doi: 10.1016/j.gendis.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jun J.C., Rathore A., Younas H., Gilkes D., Polotsky V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017;3:1–10. doi: 10.1007/s40675-017-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yeung S.J., Pan J., Lee M.H. Roles of p53, MYC and HIF-1 in regulating glycolysis—The seventh hallmark of cancer. Cell. Mol. Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chung Y.M., Kim J.S., Yoo Y.D. A novel protein, Romo1, induces ROS production in the mitochondria. Biochem. Biophys. Res. Commun. 2006;347:649–655. doi: 10.1016/j.bbrc.2006.06.140. [DOI] [PubMed] [Google Scholar]
- 210.Vafa O., Wade M., Kern S., Beeche M., Pandita T.K., Hampton G.M., Wahl G.M. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: A mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031–1044. doi: 10.1016/S1097-2765(02)00520-8. [DOI] [PubMed] [Google Scholar]
- 211.Seoane M., Mosquera-Miguel A., Gonzalez T., Fraga M., Salas A., Costoya J.A. The mitochondrial genome is a “genetic sanctuary” during the oncogenic process. PLoS ONE. 2011;6:e23327. doi: 10.1371/journal.pone.0023327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rasmussen A.K., Chatterjee A., Rasmussen L.J., Singh K.K. Mitochondria-mediated nuclear mutator phenotype in Saccharomyces cerevisiae. Nucleic Acids Res. 2003;31:3909–3917. doi: 10.1093/nar/gkg446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Matoba S., Kang J.-G., Patino W.D., Wragg A., Boehm M., Gavrilova O., Hurley P.J., Bunz F., Hwang P.M. p53 Regulates Mitochondrial Respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 214.Corcoran C.A., Huang Y., Sheikh M.S. Mitochondria and Cancer. Springer; New York, NY, USA: 2009. Energy Generating Pathways and the Tumor Suppressor p53; pp. 131–150. [DOI] [Google Scholar]
- 215.Kondoh H., Lleonart M.E., Gil J., Wang J., Degan P., Peters G., Martinez D., Carnero A., Beach D. Glycolytic Enzymes Can Modulate Cellular Life Span. Cancer Res. 2005;65:177–185. doi: 10.1158/0008-5472.177.65.1. [DOI] [PubMed] [Google Scholar]
- 216.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bensaad K., Tsuruta A., Selak M.A., Vidal M.N.C., Nakano K., Bartrons R., Gottlieb E., Vousden K.H. TIGAR, a p53-Inducible Regulator of Glycolysis and Apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 218.Lim Y.P., Lim T.T., Chan Y.L., Song A.C.M., Yeo B.H., Vojtesek B., Coomber D., Rajagopal G., Lane D. The p53 knowledgebase: An integrated information resource for p53 research. Oncogene. 2006;26:1517–1521. doi: 10.1038/sj.onc.1209952. [DOI] [PubMed] [Google Scholar]
- 219.Petitjean A., Achatz M.I.W., Borresen-Dale A.L., Hainaut P., Olivier M. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 220.Fang L., Li G., Liu G., Lee S.W., Aaronson S.A. p53 induction of heparin-binding EGF-like growth factor counteracts p53 growth suppression through activation of MAPK and PI3K/Akt signaling cascades. EMBO J. 2001;20:1931–1939. doi: 10.1093/emboj/20.8.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chipuk J.E., Kuwana T., Bouchier-Hayes L., Droin N.M., Newmeyer D.D., Schuler M., Green D.R. Direct Activation of Bax by p53 Mediates MitochondrialMembrane Permeabilization and Apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 222.Erster S., Moll U.M. Stress-induced p53 runs a transcription-independent death program. Biochem. Biophys. Res. Commun. 2005;331:843–850. doi: 10.1016/j.bbrc.2005.03.187. [DOI] [PubMed] [Google Scholar]
- 223.Leu J.I.J., Dumont P., Hafey M., Murphy M.E., George D.L. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 224.Mihara M., Erster S., Zaika A., Petrenko O., Chittenden T., Pancoska P., Moll U.M. p53 Has a Direct Apoptogenic Role at the Mitochondria. Mol. Cell. 2003;11:577–590. doi: 10.1016/S1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 225.Perfettini J.-L., Kroemer R.T., Kroemer G. Fatal liaisons of p53 with Bax and Bak. Nat. Cell Biol. 2004;6:386–388. doi: 10.1038/ncb0504-386. [DOI] [PubMed] [Google Scholar]
- 226.Karuman P., Gozani O., Odze R.D., Zhou X.C., Zhu H., Shaw R., Brien T.P., Bozzuto C.D., Ooi D., Cantley L.C., et al. The Peutz-Jegher Gene Product LKB1 Is a Mediator of p53-Dependent Cell Death. Mol. Cell. 2001;7:1307–1319. doi: 10.1016/S1097-2765(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 227.Shaw R.J., Lamia K.A., Vasquez D., Koo S.-H., Bardeesy N., DePinho R.A., Montminy M., Cantley L.C. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wei C., Amos C.I., Stephens L.C., Campos I., Deng J.M., Behringer R.R., Rashid A., Frazier M.L. Mutation of Lkb1 and p53 Genes Exert a Cooperative Effect on Tumorigenesis. Cancer Res. 2005;65:11297–11303. doi: 10.1158/0008-5472.CAN-05-0716. [DOI] [PubMed] [Google Scholar]
- 229.Betz C., Hall M.N. Where is mTOR and what is it doing there? J. Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kohli L., Passegue E. Surviving change: The metabolic journey of hematopoietic stem cells. Trends Cell Biol. 2014;24:479–487. doi: 10.1016/j.tcb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Laplante M., Sabatini D.M. mTOR signaling at a glance. J. Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gasche C., Chang C.L., Rhees J., Goel A., Boland C.R. Oxidative stress increases frameshift mutations in human colorectal cancer cells. Cancer Res. 2001;61:7444–7448. [PubMed] [Google Scholar]
- 233.Kulawiec M., Safina A.F., Desouki M.M., Still I., Matsui S.-I., Bakin A., Singh K.K. Tumorigenic transformation of human breast epithelial cells induced by mitochondrial DNA depletion. Cancer Biol. Ther. 2008;7:1732–1743. doi: 10.4161/cbt.7.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Di Donato S. Disorders related to mitochondrial membranes: Pathology of the respiratory chain and neurodegeneration. J. Inherit. Metab. Dis. 2000;23:247–263. doi: 10.1023/A:1005684029429. [DOI] [PubMed] [Google Scholar]
- 235.Xiao M., Yang H., Xu W., Ma S., Lin H., Zhu H., Liu L., Liu Y., Yang C., Xu Y., et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Selak M.A., Armour S.M., MacKenzie E.D., Boulahbel H., Watson D.G., Mansfield K.D., Pan Y., Simon M.C., Thompson C.B., Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 237.Cha M.Y., Kim D.K., Mook-Jung I. The role of mitochondrial DNA mutation on neurodegenerative diseases. Exp. Mol. Med. 2015;47:e150. doi: 10.1038/emm.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hroudov J., Singh N., Fi Z. Mitochondrial Dysfunctions in Neurodegenerative Diseases: Relevance to Alzheimer’s Disease. BioMed Res. Int. 2014;2014:9. doi: 10.1155/2014/175062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Christodoulou J. Genetic defects causing mitochondrial respiratory chain disorders and disease. Hum. Reprod. 2000;15((Suppl. S2)):28–43. doi: 10.1093/humrep/15.suppl_2.28. [DOI] [PubMed] [Google Scholar]
- 240.Goncalves M.D., Hopkins B.D., Cantley L.C. Dietary Fat and Sugar in Promoting Cancer Development and Progression. Annu. Rev. Cancer Biol. 2019;3:255–273. doi: 10.1146/annurev-cancerbio-030518-055855. [DOI] [Google Scholar]
- 241.Modica-Napolitano J.S., Singh K. Mitochondria as targets for detection and treatment of cancer. Expert Rev. Mol. Med. 2002;4:1–19. doi: 10.1017/S1462399402004453. [DOI] [PubMed] [Google Scholar]
- 242.Tallini G. Oncocytic tumours. Virchows Arch. 1998;433:5–12. doi: 10.1007/s004280050209. [DOI] [PubMed] [Google Scholar]
- 243.Hruban Z., Mochizuki Y., Morris H.P., Slesers A. Ultrastructure of Morris renal tumors. J. Natl. Cancer Inst. 1973;50:1487–1495. doi: 10.1093/jnci/50.6.1487. [DOI] [PubMed] [Google Scholar]
- 244.White M.T., Arya D.V., Tewari K.K. Biochemical properties of neoplastic cell mitochondria. J. Natl. Cancer Inst. 1974;53:553–559. doi: 10.1093/jnci/53.2.553. [DOI] [PubMed] [Google Scholar]
- 245.Sriskanthadevan S., Jeyaraju D.V., Chung T.E., Prabha S., Xu W., Skrtic M., Jhas B., Hurren R., Gronda M., Wang X., et al. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood. 2015;125:2120–2130. doi: 10.1182/blood-2014-08-594408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Arismendi-Morillo G. Electron microscopy morphology of the mitochondrial network in gliomas and their vascular microenvironment. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011;1807:602–608. doi: 10.1016/j.bbabio.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 247.Strunck E., Frank K., Vollmer G. Regulation of Gene Expression in Endometrial Cancer Cells: Role of Extracellular Matrix in Mitochondrial Gene Expression. In: Kuramoto H., Nishida M., editors. Cell and Molecular Biology of Endometrial Carcinoma. Springer; Tokyo, Japan: 2003. pp. 221–231. [DOI] [Google Scholar]
- 248.Zhang Q., Liang Z., Gao Y., Teng M., Niu L. Differentially expressed mitochondrial genes in breast cancer cells: Potential new targets for anti-cancer therapies. Gene. 2016;596:45–52. doi: 10.1016/j.gene.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 249.Chen X., Sun K., Jiao S., Cai N., Zhao X., Zou H., Xie Y., Wang Z., Zhong M., Wei L. High levels of SIRT1 expression enhance tumorigenesis and associate with a poor prognosis of colorectal carcinoma patients. Sci. Rep. 2014;4:7481. doi: 10.1038/srep07481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sharp M.G., Adams S.M., Walker R.A., Brammar W.J., Varley J.M. Differential expression of the mitochondrial gene cytochrome oxidase II in benign and malignant breast tissue. J. Pathol. 1992;168:163–168. doi: 10.1002/path.1711680203. [DOI] [PubMed] [Google Scholar]
- 251.Dakubo G.D. Mitochondrial Genetic Alterations in Cancer I. In: Dakubo G.D., editor. Mitochondrial Genetics and Cancer. Springer; Berlin/Heidelberg, Germany: 2010. pp. 135–165. [DOI] [Google Scholar]
- 252.Larman T.C., DePalma S.R., Hadjipanayis A.G., Cancer Genome Atlas Research N., Protopopov A., Zhang J., Gabriel S.B., Chin L., Seidman C.E., Kucherlapati R., et al. Spectrum of somatic mitochondrial mutations in five cancers. Proc. Natl. Acad. Sci. USA. 2012;109:14087–14091. doi: 10.1073/pnas.1211502109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Czarnecka A., Bartnik E. Mitochondrial DNA Mutations in Tumors. In: Sarangarajan R., Apte S., editors. Cellular Respiration and Carcinogenesis. Humana Press; Totowa, NJ, USA: 2009. pp. 119–130. [DOI] [Google Scholar]
- 254.Damm F., Bunke T., Thol F., Markus B., Wagner K., Gohring G., Schlegelberger B., Heil G., Reuter C.W., Pullmann K., et al. Prognostic implications and molecular associations of NADH dehydrogenase subunit 4 (ND4) mutations in acute myeloid leukemia. Leukemia. 2012;26:289–295. doi: 10.1038/leu.2011.200. [DOI] [PubMed] [Google Scholar]
- 255.Kim H., Komiyama T., Inomoto C., Kamiguchi H., Kajiwara H., Kobayashi H., Nakamura N., Terachi T. Mutations in the Mitochondrial ND1 Gene Are Associated with Postoperative Prognosis of Localized Renal Cell Carcinoma. Int. J. Mol. Sci. 2016;17:2049. doi: 10.3390/ijms17122049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cruz-Bermudez A., Vallejo C.G., Vicente-Blanco R.J., Gallardo M.E., Fernandez-Moreno M.A., Quintanilla M., Garesse R. Enhanced tumorigenicity by mitochondrial DNA mild mutations. Oncotarget. 2015;6:13628–13643. doi: 10.18632/oncotarget.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Reznik E., Miller M.L., Senbabaoglu Y., Riaz N., Sarungbam J., Tickoo S.K., Al-Ahmadie H.A., Lee W., Seshan V.E., Hakimi A.A., et al. Mitochondrial DNA copy number variation across human cancers. Elife. 2016;5:e10769. doi: 10.7554/eLife.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lee H.C., Yin P.H., Lin J.C., Wu C.C., Chen C.Y., Wu C.W., Chi C.W., Tam T.N., Wei Y.H. Mitochondrial genome instability and mtDNA depletion in human cancers. Ann. N. Y. Acad. Sci. 2005;1042:109–122. doi: 10.1196/annals.1338.011. [DOI] [PubMed] [Google Scholar]
- 259.Selvanayagam P., Rajaraman S. Detection of mitochondrial genome depletion by a novel cDNA in renal cell carcinoma. Lab. Investig. 1996;74:592–599. [PubMed] [Google Scholar]
- 260.Wu C.-W., Yin P.-H., Hung W.-Y., Li A.F.-Y., Li S.-H., Chi C.-W., Wei Y.-H., Lee H.-C. Mitochondrial DNA mutations and mitochondrial DNA depletion in gastric cancer. Genes Chromosom. Cancer. 2005;44:19–28. doi: 10.1002/gcc.20213. [DOI] [PubMed] [Google Scholar]
- 261.Koura M., Isaka H., Yoshida M.C., Tosu M., Sekiguchi T. Suppression of tumorigenicity in interspecific reconstituted cells and cybrids. Gan. 1982;73:574–580. [PubMed] [Google Scholar]
- 262.Israel B.A., Schaeffer W.I. Cytoplasmic suppression of malignancy. Vitr. Cell. Dev. Biol. 1987;23:627–632. doi: 10.1007/BF02621071. [DOI] [PubMed] [Google Scholar]
- 263.Israel B.A., Schaeffer W.I. Cytoplasmic mediation of malignancy. Vitr. Cell. Dev. Biol. 1988;24:487–490. doi: 10.1007/BF02628504. [DOI] [PubMed] [Google Scholar]
- 264.Shay J.W., Liu Y.N., Werbin H. Cytoplasmic suppression of tumor progression in reconstituted cells. Somat. Cell Mol. Genet. 1988;14:345–350. doi: 10.1007/BF01534642. [DOI] [PubMed] [Google Scholar]
- 265.McKinnell R.G., Deggins B.A., Labat D.D. Transplantation of pluripotential nuclei from triploid frog tumors. Science. 1969;165:394–396. doi: 10.1126/science.165.3891.394. [DOI] [PubMed] [Google Scholar]
- 266.Shay J.W., Lorkowski G., Clark M.A. Suppression of tumorigenicity in cybrids. J. Supramol. Struct. Cell. Biochem. 1981;16:75–82. doi: 10.1002/jsscb.1981.380160107. [DOI] [PubMed] [Google Scholar]
- 267.Shay J.W., Werbin H. Cytoplasmic suppression of tumorigenicity in reconstructed mouse cells. Cancer Res. 1988;48:830–833. [PubMed] [Google Scholar]
- 268.Howell A.N., Sager R. Tumorigenicity and its suppression in cybrids of mouse and Chinese hamster cell lines. Proc. Natl. Acad. Sci. USA. 1978;75:2358–2362. doi: 10.1073/pnas.75.5.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hochedlinger K., Blelloch R., Brennan C., Yamada Y., Kim M., Chin L., Jaenisch R. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004;18:1875–1885. doi: 10.1101/gad.1213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li L., Connelly M.C., Wetmore C., Curran T., Morgan J.I. Mouse embryos cloned from brain tumors. Cancer Res. 2003;63:2733–2736. [PubMed] [Google Scholar]
- 271.Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Jonasson J., Harris H. The analysis of malignancy by cell fusion. VIII. Evidence for the intervention of an extra-chromosomal element. J. Cell Sci. 1977;24:255–263. doi: 10.1242/jcs.24.1.255. [DOI] [PubMed] [Google Scholar]
- 273.Seyfried T.N. Cancer as a Metabolic Disease. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2012. Mitochondria: The Ultimate Tumor Suppressor; pp. 195–205. [DOI] [Google Scholar]
- 274.Bose S., Deininger M., Gora-Tybor J., Goldman J.M., Melo J.V. The presence of typical and atypical BCR-ABL fusion genes in leukocytes of normal individuals: Biologic significance and implications for the assessment of minimal residual disease. Blood. 1998;92:3362–3367. doi: 10.1182/blood.V92.9.3362. [DOI] [PubMed] [Google Scholar]
- 275.Kaipparettu B.A., Ma Y., Park J.H., Lee T.-L., Zhang Y., Yotnda P., Creighton C.J., Chan W.-Y., Wong L.-J.C. Crosstalk from Non-Cancerous Mitochondria Can Inhibit Tumor Properties of Metastatic Cells by Suppressing Oncogenic Pathways. PLoS ONE. 2013;8:e61747. doi: 10.1371/journal.pone.0061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Elliott R.L., Jiang X.P., Head J.F. Mitochondria organelle transplantation: Introduction of normal epithelial mitochondria into human cancer cells inhibits proliferation and increases drug sensitivity. Breast Cancer Res. Treat. 2012;136:347–354. doi: 10.1007/s10549-012-2283-2. [DOI] [PubMed] [Google Scholar]
- 277.Petros J.A., Baumann A.K., Ruiz-Pesini E., Amin M.B., Sun C.Q., Hall J., Lim S., Issa M.M., Flanders W.D., Hosseini S.H., et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl. Acad. Sci. USA. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wahlestedt M., Ameur A., Moraghebi R., Norddahl G.L., Sten G., Woods N.B., Bryder D. Somatic cells with a heavy mitochondrial DNA mutational load render induced pluripotent stem cells with distinct differentiation defects. Stem Cells. 2014;32:1173–1182. doi: 10.1002/stem.1630. [DOI] [PubMed] [Google Scholar]
- 279.Spees J.L., Olson S.D., Whitney M.J., Prockop D.J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. USA. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Pasquier J., Guerrouahen B.S., Al Thawadi H., Ghiabi P., Maleki M., Abu-Kaoud N., Jacob A., Mirshahi M., Galas L., Rafii S., et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J. Transl. Med. 2013;11:94. doi: 10.1186/1479-5876-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tan A.S., Baty J.W., Dong L.-F., Bezawork-Geleta A., Endaya B., Goodwin J., Bajzikova M., Kovarova J., Peterka M., Yan B., et al. Mitochondrial Genome Acquisition Restores Respiratory Function and Tumorigenic Potential of Cancer Cells without Mitochondrial DNA. Cell Metab. 2015;21:81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 282.Berridge M.V., Dong L., Neuzil J. Mitochondrial DNA in Tumor Initiation, Progression, and Metastasis: Role of Horizontal mtDNA Transfer. Cancer Res. 2015;75:3203–3208. doi: 10.1158/0008-5472.CAN-15-0859. [DOI] [PubMed] [Google Scholar]
- 283.Wang X., Gerdes H.H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22:1181–1191. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pyle A., Anugrha H., Kurzawa-Akanbi M., Yarnall A., Burn D., Hudson G. Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurobiol. Aging. 2016;38:216.e7–216.e10. doi: 10.1016/j.neurobiolaging.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Huang J., Tan L., Shen R., Zhang L., Zuo H., Wang D.W. Decreased Peripheral Mitochondrial DNA Copy Number is Associated with the Risk of Heart Failure and Long-term Outcomes. Medicine. 2016;95:e3323. doi: 10.1097/MD.0000000000003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Chen S., Li Z., He Y., Zhang F., Li H., Liao Y., Wei Z., Wan G., Xiang X., Hu M., et al. Elevated mitochondrial DNA copy number in peripheral blood cells is associated with childhood autism. BMC Psychiatry. 2015;15:50. doi: 10.1186/s12888-015-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. doi: 10.1126/science.124.3215.269. [DOI] [PubMed] [Google Scholar]
- 288.Seyfried T.N., Mukherjee P. Targeting energy metabolism in brain cancer: Review and hypothesis. Nutr. Metab. 2005;2:30. doi: 10.1186/1743-7075-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chen Y., Cairns R., Papandreou I., Koong A., Denko N.C. Oxygen Consumption Can Regulate the Growth of Tumors, a New Perspective on the Warburg Effect. PLoS ONE. 2009;4:e7033. doi: 10.1371/journal.pone.0007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ramanathan A., Wang C., Schreiber S.L. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc. Natl. Acad. Sci. USA. 2005;102:5992–5997. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.John A.P. Dysfunctional mitochondria, not oxygen insufficiency, cause cancer cells to produce inordinate amounts of lactic acid: The impact of this on the treatment of cancer. Med. Hypotheses. 2001;57:429–431. doi: 10.1054/mehy.2001.1335. [DOI] [PubMed] [Google Scholar]
- 292.Galluzzi L., Morselli E., Kepp O., Vitale I., Rigoni A., Vacchelli E., Michaud M., Zischka H., Castedo M., Kroemer G. Mitochondrial gateways to cancer. Mol. Asp. Med. 2010;31:1–20. doi: 10.1016/j.mam.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 293.Cuezva J.M., Krajewska M., de Heredia M.L., Krajewski S., Santamaria G., Kim H., Zapata J.M., Marusawa H., Chamorro M., Reed J.C. The bioenergetic signature of cancer: A marker of tumor progression. Cancer Res. 2002;62:6674–6681. [PubMed] [Google Scholar]
- 294.Cho Y.M., Kim J.H., Kim M., Park S.J., Koh S.H., Ahn H.S., Kang G.H., Lee J.-B., Park K.S., Lee H.K. Mesenchymal Stem Cells Transfer Mitochondria to the Cells with Virtually No Mitochondrial Function but Not with Pathogenic mtDNA Mutations. PLoS ONE. 2012;7:e32778. doi: 10.1371/journal.pone.0032778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lou E., Fujisawa S., Morozov A., Barlas A., Romin Y., Dogan Y., Gholami S., Moreira A.L., Manova-Todorova K., Moore M.A.S. Tunneling Nanotubes Provide a Unique Conduit for Intercellular Transfer of Cellular Contents in Human Malignant Pleural Mesothelioma. PLoS ONE. 2012;7:e33093. doi: 10.1371/journal.pone.0033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Carew J.S., Huang P. Mitochondrial defects in cancer. Mol. Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Singh K.K., Costello L.C., editors. Mitochondria and Cancer. 1st ed. Springer; New York, NY, USA: 2009. p. 294. [DOI] [Google Scholar]
- 298.Samuels D.C., Li C., Li B., Song Z., Torstenson E., Boyd Clay H., Rokas A., Thornton-Wells T.A., Moore J.H., Hughes T.M., et al. Recurrent tissue-specific mtDNA mutations are common in humans. PLoS Genet. 2013;9:e1003929. doi: 10.1371/journal.pgen.1003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yeung K.Y., Dickinson A., John J.C.S. The Role of Mitochondrial DNA in Tumorigenesis. In: St. John J.C., editor. Mitochondrial DNA, Mitochondria, Disease and Stem Cells. Humana Press; Totowa, NJ, USA: 2013. pp. 119–155. [DOI] [Google Scholar]
- 300.Chatterjee A., Mambo E., Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- 301.Cline S.D. Mitochondrial DNA damage and its consequences for mitochondrial gene expression. Biochim. Biophys. Acta. 2012;1819:979–991. doi: 10.1016/j.bbagrm.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gasparre G., Porcelli A.M., Bonora E., Pennisi L.F., Toller M., Iommarini L., Ghelli A., Moretti M., Betts C.M., Martinelli G.N., et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc. Natl. Acad. Sci. USA. 2007;104:9001–9006. doi: 10.1073/pnas.0703056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Gasparre G., Hervouet E., de Laplanche E., Demont J., Pennisi L.F., Colombel M., Mege-Lechevallier F., Scoazec J.Y., Bonora E., Smeets R., et al. Clonal expansion of mutated mitochondrial DNA is associated with tumor formation and complex I deficiency in the benign renal oncocytoma. Hum. Mol. Genet. 2008;17:986–995. doi: 10.1093/hmg/ddm371. [DOI] [PubMed] [Google Scholar]
- 304.Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J.-I. ROS-Generating Mitochondrial DNA Mutations Can Regulate Tumor Cell Metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 305.Jin X., Zhang J., Gao Y., Ding K., Wang N., Zhou D., Jen J., Cheng S. Relationship between mitochondrial DNA mutations and clinical characteristics in human lung cancer. Mitochondrion. 2007;7:347–353. doi: 10.1016/j.mito.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 306.Kretzschmar C., Roolf C., Timmer K., Sekora A., Knubel G., Escobar H.M., Fuellen G., Ibrahim S.M., Tiedge M., Baltrusch S., et al. Polymorphisms of the murine mitochondrial ND4, CYTB and COX3 genes impact hematopoiesis during aging. Oncotarget. 2016;7:74460–74472. doi: 10.18632/oncotarget.11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zong W.X., Rabinowitz J.D., White E. Mitochondria and Cancer. Mol. Cell. 2016;61:667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zu X.L., Guppy M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 309.Fan J., Kamphorst J.J., Mathew R., Chung M.K., White E., Shlomi T., Rabinowitz J.D. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 2013;9:712. doi: 10.1038/msb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Gaglio D., Metallo C.M., Gameiro P.A., Hiller K., Danna L.S., Balestrieri C., Alberghina L., Stephanopoulos G., Chiaradonna F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 2011;7:523. doi: 10.1038/msb.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ying H., Kimmelman A.C., Lyssiotis C.A., Hua S., Chu G.C., Fletcher-Sananikone E., Locasale J.W., Son J., Zhang H., Coloff J.L., et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chi T.Y., Chen G.G., Ho L.K., Lai P.B. Establishment of a doxycycline-regulated cell line with inducible, doubly-stable expression of the wild-type p53 gene from p53-deleted hepatocellular carcinoma cells. Cancer Cell Int. 2005;5:27. doi: 10.1186/1475-2867-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Politi K., Zakowski M.F., Fan P.D., Schonfeld E.A., Pao W., Varmus H.E. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Dow L.E., Fisher J., O’Rourke K.P., Muley A., Kastenhuber E.R., Livshits G., Tschaharganeh D.F., Socci N.D., Lowe S.W. Inducible in vivo genome editing with CRISPR-Cas9. Nat. Biotech. 2015;33:390–394. doi: 10.1038/nbt.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ahler E., Sullivan W.J., Cass A., Braas D., York A.G., Bensinger S.J., Graeber T.G., Christofk H.R. Doxycycline alters metabolism and proliferation of human cell lines. PLoS ONE. 2013;8:e64561. doi: 10.1371/journal.pone.0064561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chang M.Y., Rhee Y.H., Yi S.H., Lee S.J., Kim R.K., Kim H., Park C.H., Lee S.H. Doxycycline enhances survival and self-renewal of human pluripotent stem cells. Stem Cell Rep. 2014;3:353–364. doi: 10.1016/j.stemcr.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Fato R., Bergamini C., Bortolus M., Maniero A.L., Leoni S., Ohnishi T., Lenaz G. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim. Biophys. Acta. 2009;1787:384–392. doi: 10.1016/j.bbabio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Hirst J., King M.S., Pryde K.R. The production of reactive oxygen species by complex I. Biochem. Soc. Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 319.Gonzalez-Halphen D., Ghelli A., Iommarini L., Carelli V., Esposti M.D. Mitochondrial Complex I and Cell Death: A Semi-automatic Shotgun Model. [(accessed on 24 April 2023)];Cell Death Dis. 2011 2:e222. doi: 10.1038/cddis.2011.107. Available online: http://www.nature.com/cddis/journal/v/2/n10/suppinfo/cddis2011107s1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Saada A. Complex Subunits and Assembly Genes: Complex I. In: Wong L., editor. Mitochondrial Disorders Caused by Nuclear Genes. Springer; New York, NY, USA: 2013. pp. 186–202. [DOI] [Google Scholar]
- 321.Abu-Amero K.K., Alzahrani A.S., Zou M., Shi Y. High frequency of somatic mitochondrial DNA mutations in human thyroid carcinomas and complex I respiratory defect in thyroid cancer cell lines. Oncogene. 2005;24:1455–1460. doi: 10.1038/sj.onc.1208292. [DOI] [PubMed] [Google Scholar]
- 322.Zimmermann F.A., Mayr J.A., Neureiter D., Feichtinger R., Alinger B., Jones N.D., Eder W., Sperl W., Kofler B. Lack of complex I is associated with oncocytic thyroid tumours. Br. J. Cancer. 2009;100:1434–1437. doi: 10.1038/sj.bjc.6605028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mayr J.A., Meierhofer D., Zimmermann F., Feichtinger R., Kogler C., Ratschek M., Schmeller N., Sperl W., Kofler B. Loss of complex I due to mitochondrial DNA mutations in renal oncocytoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008;14:2270–2275. doi: 10.1158/1078-0432.CCR-07-4131. [DOI] [PubMed] [Google Scholar]
- 324.He L., Luo L., Proctor S.J., Middleton P.G., Blakely E.L., Taylor R.W., Turnbull D.M. Somatic mitochondrial DNA mutations in adult-onset leukaemia. Leukemia. 2003;17:2487–2491. doi: 10.1038/sj.leu.2403146. [DOI] [PubMed] [Google Scholar]
- 325.Chen G.F., Chan F.L., Hong B.F., Chan L.W., Chan P.S. Mitochondrial DNA mutations in chemical carcinogen-induced rat bladder and human bladder cancer. Oncol. Rep. 2004;12:463–472. doi: 10.3892/or.12.2.463. [DOI] [PubMed] [Google Scholar]
- 326.Imanishi H., Hattori K., Wada R., Ishikawa K., Fukuda S., Takenaga K., Nakada K., Hayashi J. Mitochondrial DNA mutations regulate metastasis of human breast cancer cells. PLoS ONE. 2011;6:e23401. doi: 10.1371/journal.pone.0023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ju Y.S., Alexandrov L.B., Gerstung M., Martincorena I., Nik-Zainal S., Ramakrishna M., Davies H.R., Papaemmanuil E., Gundem G., Shlien A., et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. Elife. 2014;3:e02935. doi: 10.7554/eLife.02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zhu W., Qin W., Sauter E.R. Large-scale mitochondrial DNA deletion mutations and nuclear genome instability in human breast cancer. Cancer Detect. Prev. 2004;28:119–126. doi: 10.1016/j.cdp.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 329.Bassal M.A., Samaraweera S.E., Lim K., Benard B.A., Bailey S., Kaur S., Leo P., Toubia J., Thompson-Peach C., Nguyen T., et al. Germline mutations in mitochondrial complex I reveal genetic and targetable vulnerability in IDH1-mutant acute myeloid leukaemia. Nat. Commun. 2022;13:2614. doi: 10.1038/s41467-022-30223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.He X., Zhou A., Lu H., Chen Y., Huang G., Yue X., Zhao P., Wu Y. Suppression of Mitochondrial Complex I Influences Cell Metastatic Properties. PLoS ONE. 2013;8:e61677. doi: 10.1371/journal.pone.0061677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Iommarini L., Kurelac I., Capristo M., Calvaruso M.A., Giorgio V., Bergamini C., Ghelli A., Nanni P., De Giovanni C., Carelli V., et al. Different mtDNA mutations modify tumor progression in dependence of the degree of respiratory complex I impairment. Hum. Mol. Genet. 2013;23:1453–1466. doi: 10.1093/hmg/ddt533. [DOI] [PubMed] [Google Scholar]
- 332.Birsoy K., Possemato R., Lorbeer F.K., Bayraktar E.C., Thiru P., Yucel B., Wang T., Chen W.W., Clish C.B., Sabatini D.M. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Calabrese C., Iommarini L., Kurelac I., Calvaruso M.A., Capristo M., Lollini P., Nanni P., Bergamini C., Nicoletti G., De Giovanni C., et al. Respiratory complex I is essential to induce a Warburg profile in mitochondria-defective tumor cells. Cancer Metab. 2013;1:11. doi: 10.1186/2049-3002-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Evenepoel L., Papathomas T.G., Krol N., Korpershoek E., de Krijger R.R., Persu A., Dinjens W.N.M. Toward an improved definition of the genetic and tumor spectrum associated with SDH germ-line mutations. Genet. Med. 2014;17:610–620. doi: 10.1038/gim.2014.162. [DOI] [PubMed] [Google Scholar]
- 335.Letouzé E., Martinelli C., Loriot C., Burnichon N., Abermil N., Ottolenghi C., Janin M., Menara M., Nguyen A.T., Benit P., et al. SDH Mutations Establish a Hypermethylator Phenotype in Paraganglioma. Cancer Cell. 2013;23:739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 336.Eng C., Kiuru M., Fernandez M.J., Aaltonen L.A. A role for mitochondrial enzymes in inherited neoplasia and beyond. Nat. Rev. Cancer. 2003;3:193–202. doi: 10.1038/nrc1013. [DOI] [PubMed] [Google Scholar]
- 337.Ricketts C., Woodward E.R., Killick P., Morris M.R., Astuti D., Latif F., Maher E.R. Germline SDHB Mutations and Familial Renal Cell Carcinoma. JNCI J. Natl. Cancer Inst. 2008;100:1260–1262. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 338.Janeway K.A., Kim S.Y., Lodish M., Nosé V., Rustin P., Gaal J., Dahia P.L.M., Liegl B., Ball E.R., Raygada M., et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc. Natl. Acad. Sci. USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kim S., Kim D.H., Jung W.-H., Koo J.S. Succinate dehydrogenase expression in breast cancer. SpringerPlus. 2013;2:299. doi: 10.1186/2193-1801-2-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lu C., Thompson C.B. Metabolic regulation of epigenetics. Cell Metab. 2012;16:9–17. doi: 10.1016/j.cmet.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rose N.R., Ng S.S., Mecinovic J., Lienard B.M., Bello S.H., Sun Z., McDonough M.A., Oppermann U., Schofield C.J. Inhibitor scaffolds for 2-oxoglutarate-dependent histone lysine demethylases. J. Med. Chem. 2008;51:7053–7056. doi: 10.1021/jm800936s. [DOI] [PubMed] [Google Scholar]
- 342.Killian J.K., Kim S.Y., Miettinen M., Smith C., Merino M., Tsokos M., Quezado M., Smith W.I., Jahromi M.S., Xekouki P., et al. Succinate Dehydrogenase Mutation Underlies Global Epigenomic Divergence in Gastrointestinal Stromal Tumor. Cancer Discov. 2013;3:648–657. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Cervera A.M., Bayley J.-P., Devilee P., McCreath K.J. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Mol. Cancer. 2009;8:89. doi: 10.1186/1476-4598-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Yang J., Qian J., Yao D.M., Qian S.X., Qian W., Lin J., Xiao G.F., Wang C.Z., Deng Z.Q., Ma J.C., et al. SF3B1 mutation is a rare event in Chinese patients with acute and chronic myeloid leukemia. Clin. Biochem. 2013;46:701–703. doi: 10.1016/j.clinbiochem.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 345.Hung W.-Y., Wu C.-W., Yin P.-H., Chang C.-J., Li A.F.-Y., Chi C.-W., Wei Y.-H., Lee H.-C. Somatic mutations in mitochondrial genome and their potential roles in the progression of human gastric cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010;1800:264–270. doi: 10.1016/j.bbagen.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 346.Polyak K., Li Y., Zhu H., Lengauer C., Willson J.K.V., Markowitz S.D., Trush M.A., Kinzler K.W., Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat. Genet. 1998;20:291–293. doi: 10.1038/3108. [DOI] [PubMed] [Google Scholar]
- 347.Liu V.W.S., Shi H.H., Cheung A.N.Y., Chiu P.M., Leung T.W., Nagley P., Wong L.C., Ngan H.Y.S. High Incidence of Somatic Mitochondrial DNA Mutations in Human Ovarian Carcinomas. Cancer Res. 2001;61:5998–6001. [PubMed] [Google Scholar]
- 348.Máximo V., Soares P., Lima J., Cameselle-Teijeiro J., Sobrinho-Simões M. Mitochondrial DNA Somatic Mutations (Point Mutations and Large Deletions) and Mitochondrial DNA Variants in Human Thyroid Pathology: A Study with Emphasis on Hürthle Cell Tumors. Am. J. Pathol. 2002;160:1857–1865. doi: 10.1016/S0002-9440(10)61132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Owens K.M., Kulawiec M., Desouki M.M., Vanniarajan A., Singh K.K. Impaired OXPHOS Complex III in Breast Cancer. PLoS ONE. 2011;6:e23846. doi: 10.1371/journal.pone.0023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Fliss M.S., Usadel H., Caballero O.L., Wu L., Buta M.R., Eleff S.M., Jen J., Sidransky D. Facile Detection of Mitochondrial DNA Mutations in Tumors and Bodily Fluids. Science. 2000;287:2017. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 351.Dasgupta S., Hoque M.O., Upadhyay S., Sidransky D. Mitochondrial Cytochrome B Gene Mutation Promotes Tumor Growth in Bladder Cancer. Cancer Res. 2008;68:700–706. doi: 10.1158/0008-5472.CAN-07-5532. [DOI] [PubMed] [Google Scholar]
- 352.Dasgupta S., Hoque M.O., Upadhyay S., Sidransky D. Forced cytochrome B gene mutation expression induces mitochondrial proliferation and prevents apoptosis in human uroepithelial SV-HUC-1 cells. Int. J. Cancer. 2009;125:2829–2835. doi: 10.1002/ijc.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Pierron D., Wildman D.E., Huttemann M., Markondapatnaikuni G.C., Aras S., Grossman L.I. Cytochrome c oxidase: Evolution of control via nuclear subunit addition. Biochim. Biophys. Acta. 2012;1817:590–597. doi: 10.1016/j.bbabio.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Huttemann M., Helling S., Sanderson T.H., Sinkler C., Samavati L., Mahapatra G., Varughese A., Lu G., Liu J., Ramzan R., et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim. Biophys. Acta. 2012;1817:598–609. doi: 10.1016/j.bbabio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Okamura S., Ng C.C., Koyama K., Takei Y., Arakawa H., Monden M., Nakamura Y. Identification of seven genes regulated by wild-type p53 in a colon cancer cell line carrying a well-controlled wild-type p53 expression system. Oncol. Res. 1999;11:281–285. [PubMed] [Google Scholar]
- 356.Horng Y.-C., Leary S.C., Cobine P.A., Young F.B.J., George G.N., Shoubridge E.A., Winge D.R. Human Sco1 and Sco2 Function as Copper-binding Proteins. J. Biol. Chem. 2005;280:34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 357.Permuth-Wey J., Chen Y.A., Tsai Y.-Y., Chen Z., Qu X., Lancaster J.M., Stockwell H., Dagne G., Iversen E., Risch H., et al. Inherited Variants in Mitochondrial Biogenesis Genes May Influence Epithelial Ovarian Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2011;20:1131–1145. doi: 10.1158/1055-9965.EPI-10-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Chen Z.X., Pervaiz S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010;17:408–420. doi: 10.1038/cdd.2009.132. [DOI] [PubMed] [Google Scholar]
- 359.Telang S., Nelson K.K., Siow D.L., Yalcin A., Thornburg J.M., Imbert-Fernandez Y., Klarer A.C., Farghaly H., Clem B.F., Eaton J.W., et al. Cytochrome c oxidase is activated by the oncoprotein Ras and is required for A549 lung adenocarcinoma growth. Mol. Cancer. 2012;11:60. doi: 10.1186/1476-4598-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G.D., Petronilli V., Zoratti M., Szabó I., et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Nishi T., Forgac M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 362.Jones J.B., Song J.J., Hempen P.M., Parmigiani G., Hruban R.H., Kern S.E. Detection of Mitochondrial DNA Mutations in Pancreatic Cancer Offers a “Mass”-ive Advantage over Detection of Nuclear DNA Mutations. Cancer Res. 2001;61:1299–1304. [PubMed] [Google Scholar]
- 363.Shidara Y., Yamagata K., Kanamori T., Nakano K., Kwong J.Q., Manfredi G., Oda H., Ohta S. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 2005;65:1655–1663. doi: 10.1158/0008-5472.CAN-04-2012. [DOI] [PubMed] [Google Scholar]
- 364.Mardis E.R., Ding L., Dooling D.J., Larson D.E., McLellan M.D., Chen K., Koboldt D.C., Fulton R.S., Delehaunty K.D., McGrath S.D., et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Gross S., Cairns R.A., Minden M.D., Driggers E.M., Bittinger M.A., Jang H.G., Sasaki M., Jin S., Schenkein D.P., Su S.M., et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J. Exp. Med. 2010;207:339–344. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Sjoblom T., Jones S., Wood L.D., Parsons D.W., Lin J., Barber T.D., Mandelker D., Leary R.J., Ptak J., Silliman N., et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 367.Pansuriya T.C., van Eijk R., d’Adamo P., van Ruler M.A., Kuijjer M.L., Oosting J., Cleton-Jansen A.M., van Oosterwijk J.G., Verbeke S.L., Meijer D., et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat. Genet. 2011;43:1256–1261. doi: 10.1038/ng.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Amary M.F., Bacsi K., Maggiani F., Damato S., Halai D., Berisha F., Pollock R., O’Donnell P., Grigoriadis A., Diss T., et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011;224:334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 369.Liu X., Kato Y., Kaneko M.K., Sugawara M., Ogasawara S., Tsujimoto Y., Naganuma Y., Yamakawa M., Tsuchiya T., Takagi M. Isocitrate dehydrogenase 2 mutation is a frequent event in osteosarcoma detected by a multi-specific monoclonal antibody MsMab-1. Cancer Med. 2013;2:803–814. doi: 10.1002/cam4.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Borger D.R., Goyal L., Yau T., Poon R.T., Ancukiewicz M., Deshpande V., Christiani D.C., Liebman H.M., Yang H., Kim H., et al. Circulating Oncometabolite 2-Hydroxyglutarate Is a Potential Surrogate Biomarker in Patients with Isocitrate Dehydrogenase-Mutant Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. 2014;20:1884–1890. doi: 10.1158/1078-0432.CCR-13-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Kang M.R., Kim M.S., Oh J.E., Kim Y.R., Song S.Y., Seo S.I., Lee J.Y., Yoo N.J., Lee S.H. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int. J. Cancer. 2009;125:353–355. doi: 10.1002/ijc.24379. [DOI] [PubMed] [Google Scholar]
- 372.Dang L., White D.W., Gross S., Bennett B.D., Bittinger M.A., Driggers E.M., Fantin V.R., Jang H.G., Jin S., Keenan M.C., et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Losman J.-A., Looper R.E., Koivunen P., Lee S., Schneider R.K., McMahon C., Cowley G.S., Root D.E., Ebert B.L., Kaelin W.G. (R)-2-Hydroxyglutarate Is Sufficient to Promote Leukemogenesis and Its Effects Are Reversible. Science. 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Koivunen P., Lee S., Duncan C.G., Lopez G., Lu G., Ramkissoon S., Losman J.A., Joensuu P., Bergmann U., Gross S., et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Xu W., Yang H., Liu Y., Yang Y., Wang P., Kim S.-H., Ito S., Yang C., Wang P., Xiao M.-T., et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Chowdhury R., Yeoh K.K., Tian Y.M., Hillringhaus L., Bagg E.A., Rose N.R., Leung I.K., Li X.S., Woon E.C., Yang M., et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Figueroa M.E., Abdel-Wahab O., Lu C., Ward P.S., Patel J., Shih A., Li Y., Bhagwat N., Vasanthakumar A., Fernandez H.F., et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Terunuma A., Putluri N., Mishra P., Mathé E.A., Dorsey T.H., Yi M., Wallace T.A., Issaq H.J., Zhou M., Killian J.K., et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J. Clin. Investig. 2014;124:398–412. doi: 10.1172/JCI71180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Lu C., Ward P.S., Kapoor G.S., Rohle D., Turcan S., Abdel-Wahab O., Edwards C.R., Khanin R., Figueroa M.E., Melnick A., et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Wise D.R., Ward P.S., Shay J.E.S., Cross J.R., Gruber J.J., Sachdeva U.M., Platt J.M., DeMatteo R.G., Simon M.C., Thompson C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Rendina A.R., Pietrak B., Smallwood A., Zhao H., Qi H., Quinn C., Adams N.D., Concha N., Duraiswami C., Thrall S.H., et al. Mutant IDH1 enhances the production of 2-hydroxyglutarate due to its kinetic mechanism. Biochemistry. 2013;52:4563–4577. doi: 10.1021/bi400514k. [DOI] [PubMed] [Google Scholar]
- 382.Ward P.S., Lu C., Cross J.R., Abdel-Wahab O., Levine R.L., Schwartz G.K., Thompson C.B. The potential for isocitrate dehydrogenase mutations to produce 2-hydroxyglutarate depends on allele specificity and subcellular compartmentalization. J. Biol. Chem. 2013;288:3804–3815. doi: 10.1074/jbc.M112.435495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Zhao S., Lin Y., Xu W., Jiang W., Zha Z., Wang P., Yu W., Li Z., Gong L., Peng Y., et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Bleeker F.E., Atai N.A., Lamba S., Jonker A., Rijkeboer D., Bosch K.S., Tigchelaar W., Troost D., Vandertop W.P., Bardelli A., et al. The prognostic IDH1 (R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119:487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Guitart A.V., Panagopoulou T.I., Villacreces A., Vukovic M., Sepulveda C., Allen L., Carter R.N., van de Lagemaat L.N., Morgan M., Giles P., et al. Fumarate hydratase is a critical metabolic regulator of hematopoietic stem cell functions. J. Exp. Med. 2017;214:719–735. doi: 10.1084/jem.20161087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Tomlinson I., Alam A., Rowan A., Barclay E., Jaeger E., Kelsell D., Leigh I., Gorman P., Lamlum H., Rahman S., et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 387.Castro-Vega L.J., Buffet A., De Cubas A.A., Cascón A., Menara M., Khalifa E., Amar L., Azriel S., Bourdeau I., Chabre O., et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum. Mol. Genet. 2013;23:2440–2446. doi: 10.1093/hmg/ddt639. [DOI] [PubMed] [Google Scholar]
- 388.Khalil A.A. Biomarker discovery: A proteomic approach for brain cancer profiling. Cancer Sci. 2007;98:201–213. doi: 10.1111/j.1349-7006.2007.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Sudarshan S., Shanmugasundaram K., Naylor S.L., Lin S., Livi C.B., O’Neill C.F., Parekh D.J., Yeh I.T., Sun L.-Z., Block K. Reduced Expression of Fumarate Hydratase in Clear Cell Renal Cancer Mediates HIF-2α Accumulation and Promotes Migration and Invasion. PLoS ONE. 2011;6:e21037. doi: 10.1371/journal.pone.0021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Fieuw A., Kumps C., Schramm A., Pattyn F., Menten B., Antonacci F., Sudmant P., Schulte J.H., Van Roy N., Vergult S., et al. Identification of a novel recurrent 1q42.2-1qter deletion in high risk MYCN single copy 11q deleted neuroblastomas. Int. J. Cancer. 2012;130:2599–2606. doi: 10.1002/ijc.26317. [DOI] [PubMed] [Google Scholar]
- 391.Isaacs J.S., Jung Y.J., Mole D.R., Lee S., Torres-Cabala C., Chung Y.-L., Merino M., Trepel J., Zbar B., Toro J., et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 392.Zheng L., MacKenzie E.D., Karim S.A., Hedley A., Blyth K., Kalna G., Watson D.G., Szlosarek P., Frezza C., Gottlieb E. Reversed argininosuccinate lyase activity in fumarate hydratase-deficient cancer cells. Cancer Metab. 2013;1:12. doi: 10.1186/2049-3002-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Lin C.-C., Cheng T.-L., Tsai W.-H., Tsai H.-J., Hu K.-H., Chang H.-C., Yeh C.-W., Chen Y.-C., Liao C.-C., Chang W.-T. Loss of the respiratory enzyme citrate synthase directly links the Warburg effect to tumor malignancy. Sci. Rep. 2012;2:785. doi: 10.1038/srep00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Schlichtholz B., Turyn J., Goyke E., Biernacki M., Jaskiewicz K., Sledzinski Z., Swierczynski J. Enhanced citrate synthase activity in human pancreatic cancer. Pancreas. 2005;30:99–104. doi: 10.1097/01.mpa.0000153326.69816.7d. [DOI] [PubMed] [Google Scholar]
- 395.Icard P., Coquerel A., Wu Z., Gligorov J., Fuks D., Fournel L., Lincet H., Simula L. Understanding the Central Role of Citrate in the Metabolism of Cancer Cells and Tumors: An Update. Int. J. Mol. Sci. 2021;22:6587. doi: 10.3390/ijms22126587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ternette N., Yang M., Laroyia M., Kitagawa M., O’Flaherty L., Wolhulter K., Igarashi K., Saito K., Kato K., Fischer R., et al. Inhibition of Mitochondrial Aconitase by Succination in Fumarate Hydratase Deficiency. Cell Rep. 2013;3:689–700. doi: 10.1016/j.celrep.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Wang P., Mai C., Wei Y.-L., Zhao J.-J., Hu Y.-M., Zeng Z.-L., Yang J., Lu W.-H., Xu R.-H., Huang P. Decreased expression of the mitochondrial metabolic enzyme aconitase (ACO2) is associated with poor prognosis in gastric cancer. Med. Oncol. 2013;30:552. doi: 10.1007/s12032-013-0552-5. [DOI] [PubMed] [Google Scholar]
- 398.Hansford R.G., Lehninger A.L. Active oxidative decarboxylation of malate by mitochondria isolated from L-1210 ascites tumor cells. Biochem. Biophys. Res. Commun. 1973;51:480–486. doi: 10.1016/0006-291X(73)91282-5. [DOI] [PubMed] [Google Scholar]
- 399.Ren J.-G., Seth P., Everett P., Clish C.B., Sukhatme V.P. Induction of Erythroid Differentiation in Human Erythroleukemia Cells by Depletion of Malic Enzyme 2. PLoS ONE. 2010;5:e12520. doi: 10.1371/journal.pone.0012520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Linnane A.W., Eastwood H. Cellular redox regulation and prooxidant signaling systems: A new perspective on the free radical theory of aging. Ann. N. Y. Acad. Sci. 2006;1067:47–55. doi: 10.1196/annals.1354.008. [DOI] [PubMed] [Google Scholar]
- 401.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 402.Kusmartsev S., Gabrilovich D.I. Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J. Leukoc. Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 403.Poli G., Leonarduzzi G., Biasi F., Chiarpotto E. Oxidative stress and cell signalling. Curr. Med. Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 404.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:44–84. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 405.Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv. Exp. Med. Biol. 2012;942:93–136. doi: 10.1007/978-94-007-2869-1_5. [DOI] [PubMed] [Google Scholar]
- 406.Battisti V., Maders L.D., Bagatini M.D., Santos K.F., Spanevello R.M., Maldonado P.A., Brule A.O., Araujo Mdo C., Schetinger M.R., Morsch V.M. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin. Biochem. 2008;41:511–518. doi: 10.1016/j.clinbiochem.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 407.Sallmyr A., Fan J., Rassool F.V. Genomic instability in myeloid malignancies: Increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 2008;270:1–9. doi: 10.1016/j.canlet.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 408.Hole P.S., Darley R.L., Tonks A. Do reactive oxygen species play a role in myeloid leukemias? Blood. 2011;117:5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- 409.Clerkin J.S., Naughton R., Quiney C., Cotter T.G. Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Lett. 2008;266:30–36. doi: 10.1016/j.canlet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 410.Nieborowska-Skorska M., Flis S., Skorski T. AKT-induced reactive oxygen species generate imatinib-resistant clones emerging from chronic myeloid leukemia progenitor cells. Leukemia. 2014;28:2416–2418. doi: 10.1038/leu.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53–59. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 412.de Carvalho D.D., Sadok A., Bourgarel-Rey V., Gattacceca F., Penel C., Lehmann M., Kovacic H. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. Int. J. Cancer. 2008;122:1757–1764. doi: 10.1002/ijc.23300. [DOI] [PubMed] [Google Scholar]
- 413.Bolton-Gillespie E., Schemionek M., Klein H.U., Flis S., Hoser G., Lange T., Nieborowska-Skorska M., Maier J., Kerstiens L., Koptyra M., et al. Genomic instability may originate from imatinib-refractory chronic myeloid leukemia stem cells. Blood. 2013;121:4175–4183. doi: 10.1182/blood-2012-11-466938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Ward P.S., Thompson C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Anso E., Mullen A.R., Felsher D.W., Matés J.M., DeBerardinis R.J., Chandel N.S. Metabolic changes in cancer cells upon suppression of MYC. Cancer Metab. 2013;1:7. doi: 10.1186/2049-3002-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Boroughs L.K., DeBerardinis R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.DeBerardinis R.J., Chandel N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 419.Erez A., DeBerardinis R.J. Metabolic dysregulation in monogenic disorders and cancer—Finding method in madness. Nat. Rev. Cancer. 2015;15:440–448. doi: 10.1038/nrc3949. [DOI] [PubMed] [Google Scholar]
- 420.Lyssiotis C.A., Kimmelman A.C. Metabolic Interactions in the Tumor Microenvironment. Trends Cell Biol. 2017;27:863–875. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Liberti M.V., Locasale J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Anderson N.M., Simon M.C. The tumor microenvironment. Curr. Biol. 2020;30:R921–R925. doi: 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Singh N., Baby D., Rajguru J.P., Patil P.B., Thakkannavar S.S., Pujari V.B. Inflammation and cancer. Ann. Afr. Med. 2019;18:121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Mishra D., Banerjee D. Metabolic Interactions Between Tumor and Stromal Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2021;1350:101–121. doi: 10.1007/978-3-030-83282-7_5. [DOI] [PubMed] [Google Scholar]
- 425.Scharping N.E., Delgoffe G.M. Tumor Microenvironment Metabolism: A New Checkpoint for Anti-Tumor Immunity. Vaccines. 2016;4:46. doi: 10.3390/vaccines4040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Cassim S., Pouyssegur J. Tumor Microenvironment: A Metabolic Player that Shapes the Immune Response. Int. J. Mol. Sci. 2019;21:157. doi: 10.3390/ijms21010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Wu S., Kuang H., Ke J., Pi M., Yang D.H. Metabolic Reprogramming Induces Immune Cell Dysfunction in the Tumor Microenvironment of Multiple Myeloma. Front. Oncol. 2020;10:591342. doi: 10.3389/fonc.2020.591342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Ahn C.S., Metallo C.M. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3:4. doi: 10.1186/s40170-015-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Hay N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer. 2016;16:635–649. doi: 10.1038/nrc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Kim A. Mitochondria in Cancer Energy Metabolism: Culprits or Bystanders? Toxicol. Res. 2015;31:323–330. doi: 10.5487/TR.2015.31.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Pavlova N.N., Thompson C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Pavlova N.N., Zhu J., Thompson C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34:355–377. doi: 10.1016/j.cmet.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Mullen A.R., Wheaton W.W., Jin E.S., Chen P.H., Sullivan L.B., Cheng T., Yang Y., Linehan W.M., Chandel N.S., DeBerardinis R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Metallo C.M., Gameiro P.A., Bell E.L., Mattaini K.R., Yang J., Hiller K., Jewell C.M., Johnson Z.R., Irvine D.J., Guarente L., et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Emadi A. Exploiting AML vulnerability: Glutamine dependency. Blood. 2015;126:1269–1270. doi: 10.1182/blood-2015-07-659508. [DOI] [PubMed] [Google Scholar]
- 436.Mullen P.J., Yu R., Longo J., Archer M.C., Penn L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer. 2016;16:718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 437.Altman B.J., Stine Z.E., Dang C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer. 2016;16:619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Reitzer L.J., Wice B.M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979;254:2669–2676. doi: 10.1016/S0021-9258(17)30124-2. [DOI] [PubMed] [Google Scholar]
- 439.Kinnaird A., Zhao S., Wellen K.E., Michelakis E.D. Metabolic control of epigenetics in cancer. Nat. Rev. Cancer. 2016;16:694–707. doi: 10.1038/nrc.2016.82. [DOI] [PubMed] [Google Scholar]
- 440.Kim J., DeBerardinis R.J. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019;30:434–446. doi: 10.1016/j.cmet.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Wong C.C., Qian Y., Yu J. Interplay between epigenetics and metabolism in oncogenesis: Mechanisms and therapeutic approaches. Oncogene. 2017;36:3359–3374. doi: 10.1038/onc.2016.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Jeltsch A. Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochem. 2002;3:274–293. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 443.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 444.Lan F., Shi Y. Epigenetic regulation: Methylation of histone and non-histone proteins. Sci. China C Life Sci. 2009;52:311–322. doi: 10.1007/s11427-009-0054-z. [DOI] [PubMed] [Google Scholar]
- 445.Strahl B.D., Allis C.D. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 446.Gurley L.R., D’Anna J.A., Barham S.S., Deaven L.L., Tobey R.A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur. J. Biochem. 1978;84:1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- 447.Zee B.M., Levin R.S., Xu B., LeRoy G., Wingreen N.S., Garcia B.A. In vivo residue-specific histone methylation dynamics. J. Biol. Chem. 2010;285:3341–3350. doi: 10.1074/jbc.M109.063784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Huang C., Xu M., Zhu B. Epigenetic inheritance mediated by histone lysine methylation: Maintaining transcriptional states without the precise restoration of marks? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20110332. doi: 10.1098/rstb.2011.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Chestier A., Yaniv M. Rapid turnover of acetyl groups in the four core histones of simian virus 40 minichromosomes. Proc. Natl. Acad. Sci. USA. 1979;76:46–50. doi: 10.1073/pnas.76.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Jackson V., Shires A., Chalkley R., Granner D.K. Studies on highly metabolically active acetylation and phosphorylation of histones. J. Biol. Chem. 1975;250:4856–4863. doi: 10.1016/S0021-9258(19)41247-7. [DOI] [PubMed] [Google Scholar]
- 451.Mishra P., Beura S., Ghosh R., Modak R. Nutritional Epigenetics: How Metabolism Epigenetically Controls Cellular Physiology, Gene Expression and Disease. Subcell. Biochem. 2022;100:239–267. doi: 10.1007/978-3-031-07634-3_8. [DOI] [PubMed] [Google Scholar]
- 452.Choubey P., Kaur H., Bansal K. Modulation of DNA/RNA Methylation Signaling Mediating Metabolic Homeostasis in Cancer. Subcell. Biochem. 2022;100:201–237. doi: 10.1007/978-3-031-07634-3_7. [DOI] [PubMed] [Google Scholar]
- 453.Mondal P., Tiwary N., Sengupta A., Dhang S., Roy S., Das C. Epigenetic Reprogramming of the Glucose Metabolic Pathways by the Chromatin Effectors During Cancer. Subcell. Biochem. 2022;100:269–336. doi: 10.1007/978-3-031-07634-3_9. [DOI] [PubMed] [Google Scholar]
- 454.Adhikari S., Guha D., Mohan C., Mukherjee S., Tyler J.K., Das C. Reprogramming Carbohydrate Metabolism in Cancer and Its Role in Regulating the Tumor Microenvironment. Subcell. Biochem. 2022;100:3–65. doi: 10.1007/978-3-031-07634-3_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Back F. The variable condition of euchromatin and heterochromatin. Int. Rev. Cytol. 1976;45:25–64. doi: 10.1016/s0074-7696(08)60077-7. [DOI] [PubMed] [Google Scholar]
- 456.North J.A., Simon M., Ferdinand M.B., Shoffner M.A., Picking J.W., Howard C.J., Mooney A.M., van Noort J., Poirier M.G., Ottesen J.J. Histone H3 phosphorylation near the nucleosome dyad alters chromatin structure. Nucleic Acids Res. 2014;42:4922–4933. doi: 10.1093/nar/gku150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Szerlong H.J., Hansen J.C. Nucleosome distribution and linker DNA: Connecting nuclear function to dynamic chromatin structure. Biochem. Cell Biol. 2011;89:24–34. doi: 10.1139/O10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Kornberg R.D. Chromatin structure: A repeating unit of histones and DNA. Science. 1974;184:868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- 459.Ray-Gallet D., Almouzni G. The Histone H3 Family and Its Deposition Pathways. Adv. Exp. Med. Biol. 2021;1283:17–42. doi: 10.1007/978-981-15-8104-5_2. [DOI] [PubMed] [Google Scholar]
- 460.Luger K., Mader A.W., Richmond R.K., Sargent D.F., Richmond T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 461.Arents G., Burlingame R.W., Wang B.C., Love W.E., Moudrianakis E.N. The nucleosomal core histone octamer at 3.1 A resolution: A tripartite protein assembly and a left-handed superhelix. Proc. Natl. Acad. Sci. USA. 1991;88:10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Ramaswamy A., Ioshikhes I. Chapter Four—Dynamics of Modeled Oligonucleosomes and the Role of Histone Variant Proteins in Nucleosome Organization. In: Donev R., editor. Advances in Protein Chemistry and Structural Biology. Volume 90. Academic Press; Cambridge, MA, USA: 2013. pp. 119–149. [DOI] [PubMed] [Google Scholar]
- 463.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 464.Hayes J.J., Clark D.J., Wolffe A.P. Histone contributions to the structure of DNA in the nucleosome. Proc. Natl. Acad. Sci. USA. 1991;88:6829–6833. doi: 10.1073/pnas.88.15.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Tomschik M., Karymov M.A., Zlatanova J., Leuba S.H. The archaeal histone-fold protein HMf organizes DNA into bona fide chromatin fibers. Structure. 2001;9:1201–1211. doi: 10.1016/S0969-2126(01)00682-7. [DOI] [PubMed] [Google Scholar]
- 466.Doenecke D., Albig W., Bode C., Drabent B., Franke K., Gavenis K., Witt O. Histones: Genetic diversity and tissue-specific gene expression. Histochem. Cell Biol. 1997;107:1–10. doi: 10.1007/s004180050083. [DOI] [PubMed] [Google Scholar]
- 467.Farrelly L.A., Thompson R.E., Zhao S., Lepack A.E., Lyu Y., Bhanu N.V., Zhang B., Loh Y.-H.E., Ramakrishnan A., Vadodaria K.C., et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567:535–539. doi: 10.1038/s41586-019-1024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Xhemalce B., Dawson M.A., Bannister A.J. Reviews in Cell Biology and Molecular Medicine. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2011. Histone Modifications. [DOI] [Google Scholar]
- 469.Zentner G.E., Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat. Struct. Mol. Biol. 2013;20:259–266. doi: 10.1038/nsmb.2470. [DOI] [PubMed] [Google Scholar]
- 470.Ehrlich M., Wang R.Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981;212:1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- 471.Razin A., Riggs A.D. DNA methylation and gene function. Science. 1980;210:604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- 472.Ducker G.S., Rabinowitz J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017;25:27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Ouyang Y., Wu Q., Li J., Sun S., Sun S. S-adenosylmethionine: A metabolite critical to the regulation of autophagy. Cell Prolif. 2020;53:e12891. doi: 10.1111/cpr.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Jurkowska R.Z., Jeltsch A. Mechanisms and Biological Roles of DNA Methyltransferases and DNA Methylation: From Past Achievements to Future Challenges. Adv. Exp. Med. Biol. 2022;1389:1–19. doi: 10.1007/978-3-031-11454-0_1. [DOI] [PubMed] [Google Scholar]
- 475.Li E., Bestor T.H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-F. [DOI] [PubMed] [Google Scholar]
- 476.Okano M., Xie S., Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 477.Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 478.Bestor T., Laudano A., Mattaliano R., Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J. Mol. Biol. 1988;203:971–983. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- 479.Hermann A., Goyal R., Jeltsch A. The Dnmt1 DNA-(cytosine-C5)-methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J. Biol. Chem. 2004;279:48350–48359. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- 480.Dan J., Chen T. Genetic Studies on Mammalian DNA Methyltransferases. Adv. Exp. Med. Biol. 2016;945:123–150. doi: 10.1007/978-3-319-43624-1_6. [DOI] [PubMed] [Google Scholar]
- 481.Rhee I., Bachman K.E., Park B.H., Jair K.W., Yen R.W., Schuebel K.E., Cui H., Feinberg A.P., Lengauer C., Kinzler K.W., et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 482.Chen T., Ueda Y., Dodge J.E., Wang Z., Li E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol. 2003;23:5594–5605. doi: 10.1128/MCB.23.16.5594-5605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Lee C.J., Ahn H., Jeong D., Pak M., Moon J.H., Kim S. Impact of mutations in DNA methylation modification genes on genome-wide methylation landscapes and downstream gene activations in pan-cancer. BMC Med. Genom. 2020;13:27. doi: 10.1186/s12920-020-0659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Rasmussen K.D., Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30:733–750. doi: 10.1101/gad.276568.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Shi M., Shen L. The Molecular Basis of DNA Demethylation. In: Kaneda A., Tsukada Y.-I., editors. DNA and Histone Methylation as Cancer Targets. Springer International Publishing; Cham, Switzerland: 2017. pp. 53–73. [DOI] [Google Scholar]
- 486.Bolli N., Manes N., McKerrel T., Chi J., Park N., Gundem G., Quail M.A., Sathiaseelan V., Herman B., Crawley C., et al. Characterization of gene mutations and copy number changes in acute myeloid leukemia using a rapid target enrichment protocol. Haematologica. 2014;100:214–222. doi: 10.3324/haematol.2014.113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Heby O. DNA methylation and polyamines in embryonic development and cancer. Int. J. Dev. Biol. 1995;39:737–757. [PubMed] [Google Scholar]
- 488.Holliday R., Pugh J.E. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. doi: 10.1126/science.187.4173.226. [DOI] [PubMed] [Google Scholar]
- 489.Riggs A.D. X inactivation, differentiation, and DNA methylation. Cytogenet. Cell Genet. 1975;14:9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- 490.Tazi J., Bird A. Alternative chromatin structure at CpG islands. Cell. 1990;60:909–920. doi: 10.1016/0092-8674(90)90339-G. [DOI] [PubMed] [Google Scholar]
- 491.Jeltsch A. Molecular biology. Phylogeny of methylomes. Science. 2010;328:837–838. doi: 10.1126/science.1190738. [DOI] [PubMed] [Google Scholar]
- 492.Lim W.J., Kim K.H., Kim J.Y., Jeong S., Kim N. Identification of DNA-Methylated CpG Islands Associated With Gene Silencing in the Adult Body Tissues of the Ogye Chicken Using RNA-Seq and Reduced Representation Bisulfite Sequencing. Front. Genet. 2019;10:346. doi: 10.3389/fgene.2019.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Irizarry R.A., Ladd-Acosta C., Wen B., Wu Z., Montano C., Onyango P., Cui H., Gabo K., Rongione M., Webster M., et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat. Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.De Smet C., Lurquin C., Lethe B., Martelange V., Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol. Cell. Biol. 1999;19:7327–7335. doi: 10.1128/MCB.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Lister R., Pelizzola M., Dowen R.H., Hawkins R.D., Hon G., Tonti-Filippini J., Nery J.R., Lee L., Ye Z., Ngo Q.M., et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Yang X., Han H., De Carvalho D.D., Lay F.D., Jones P.A., Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577–590. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Nunez J.K., Chen J., Pommier G.C., Cogan J.Z., Replogle J.M., Adriaens C., Ramadoss G.N., Shi Q., Hung K.L., Samelson A.J., et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell. 2021;184:2503–2519.e17. doi: 10.1016/j.cell.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Sakamoto A., Akiyama Y., Shimada S., Zhu W.G., Yuasa Y., Tanaka S. DNA Methylation in the Exon 1 Region and Complex Regulation of Twist1 Expression in Gastric Cancer Cells. PLoS ONE. 2015;10:e0145630. doi: 10.1371/journal.pone.0145630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Hodges E., Molaro A., Dos Santos C.O., Thekkat P., Song Q., Uren P.J., Park J., Butler J., Rafii S., McCombie W.R., et al. Directional DNA methylation changes and complex intermediate states accompany lineage specificity in the adult hematopoietic compartment. Mol. Cell. 2011;44:17–28. doi: 10.1016/j.molcel.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.van Vlodrop I.J., Niessen H.E., Derks S., Baldewijns M.M., van Criekinge W., Herman J.G., van Engeland M. Analysis of promoter CpG island hypermethylation in cancer: Location, location, location! Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011;17:4225–4231. doi: 10.1158/1078-0432.CCR-10-3394. [DOI] [PubMed] [Google Scholar]
- 501.Bibikova M., Barnes B., Tsan C., Ho V., Klotzle B., Le J.M., Delano D., Zhang L., Schroth G.P., Gunderson K.L., et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98:288–295. doi: 10.1016/j.ygeno.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 502.Jeong M., Sun D., Luo M., Huang Y., Challen G.A., Rodriguez B., Zhang X., Chavez L., Wang H., Hannah R., et al. Large conserved domains of low DNA methylation maintained by Dnmt3a. Nat. Genet. 2014;46:17–23. doi: 10.1038/ng.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Li Y., Zheng H., Wang Q., Zhou C., Wei L., Liu X., Zhang W., Zhang Y., Du Z., Wang X., et al. Genome-wide analyses reveal a role of Polycomb in promoting hypomethylation of DNA methylation valleys. Genome Biol. 2018;19:18. doi: 10.1186/s13059-018-1390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Xuan Lin Q.X., Sian S., An O., Thieffry D., Jha S., Benoukraf T. MethMotif: An integrative cell specific database of transcription factor binding motifs coupled with DNA methylation profiles. Nucleic Acids Res. 2019;47:D145–D154. doi: 10.1093/nar/gky1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Wiles E.T., Selker E.U. H3K27 methylation: A promiscuous repressive chromatin mark. Curr. Opin. Genet. Dev. 2017;43:31–37. doi: 10.1016/j.gde.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Creyghton M.P., Cheng A.W., Welstead G.G., Kooistra T., Carey B.W., Steine E.J., Hanna J., Lodato M.A., Frampton G.M., Sharp P.A., et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Karmodiya K., Krebs A.R., Oulad-Abdelghani M., Kimura H., Tora L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genom. 2012;13:424. doi: 10.1186/1471-2164-13-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Mikkelsen T.S., Ku M., Jaffe D.B., Issac B., Lieberman E., Giannoukos G., Alvarez P., Brockman W., Kim T.K., Koche R.P., et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S.A., Flynn R.A., Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Kharchenko P.V., Alekseyenko A.A., Schwartz Y.B., Minoda A., Riddle N.C., Ernst J., Sabo P.J., Larschan E., Gorchakov A.A., Gu T., et al. Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature. 2011;471:480–485. doi: 10.1038/nature09725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Zentner G.E., Tesar P.J., Scacheri P.C. Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Res. 2011;21:1273–1283. doi: 10.1101/gr.122382.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Lin Q.X.X., Thieffry D., Jha S., Benoukraf T. TFregulomeR reveals transcription factors’ context-specific features and functions. Nucleic Acids Res. 2020;48:e10. doi: 10.1093/nar/gkz1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Hashimoto H., Wang D., Horton J.R., Zhang X., Corces V.G., Cheng X. Structural Basis for the Versatile and Methylation-Dependent Binding of CTCF to DNA. Mol. Cell. 2017;66:711–720.e713. doi: 10.1016/j.molcel.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y., Agarwal S., Iyer L.M., Liu D.R., Aravind L., et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Ito S., Shen L., Dai Q., Wu S.C., Collins L.B., Swenberg J.A., He C., Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.He Y.F., Li B.Z., Li Z., Liu P., Wang Y., Tang Q., Ding J., Jia Y., Chen Z., Li L., et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Maiti A., Drohat A.C. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: Potential implications for active demethylation of CpG sites. J. Biol. Chem. 2011;286:35334–35338. doi: 10.1074/jbc.C111.284620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Iyer L.M., Zhang D., Burroughs A.M., Aravind L. Computational identification of novel biochemical systems involved in oxidation, glycosylation and other complex modifications of bases in DNA. Nucleic Acids Res. 2013;41:7635–7655. doi: 10.1093/nar/gkt573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Yin X., Xu Y. Structure and Function of TET Enzymes. Adv. Exp. Med. Biol. 2016;945:275–302. doi: 10.1007/978-3-319-43624-1_12. [DOI] [PubMed] [Google Scholar]
- 520.Jones P.L., Veenstra G.J.C., Wade P.A., Vermaak D., Kass S.U., Landsberger N., Strouboulis J., Wolffe A.P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 521.Vinson C., Chatterjee R. CG methylation. Epigenomics. 2012;4:655–663. doi: 10.2217/epi.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Greer E.L., Shi Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Rea S., Eisenhaber F., O’Carroll D., Strahl B.D., Sun Z.W., Schmid M., Opravil S., Mechtler K., Ponting C.P., Allis C.D., et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 525.Herz H.M., Garruss A., Shilatifard A. SET for life: Biochemical activities and biological functions of SET domain-containing proteins. Trends Biochem. Sci. 2013;38:621–639. doi: 10.1016/j.tibs.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Qian C., Zhou M.M. SET domain protein lysine methyltransferases: Structure, specificity and catalysis. Cell. Mol. Life Sci. 2006;63:2755–2763. doi: 10.1007/s00018-006-6274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Black J.C., Van Rechem C., Whetstine J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell. 2012;48:491–507. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Bedford M.T., Clarke S.G. Protein arginine methylation in mammals: Who, what, and why. Mol. Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Barski A., Cuddapah S., Cui K., Roh T.Y., Schones D.E., Wang Z., Wei G., Chepelev I., Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 530.Bannister A.J., Zegerman P., Partridge J.F., Miska E.A., Thomas J.O., Allshire R.C., Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 531.Kim T., Buratowski S. Dimethylation of H3K4 by Set1 recruits the Set3 histone deacetylase complex to 5’ transcribed regions. Cell. 2009;137:259–272. doi: 10.1016/j.cell.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Heintzman N.D., Stuart R.K., Hon G., Fu Y., Ching C.W., Hawkins R.D., Barrera L.O., Van Calcar S., Qu C., Ching K.A., et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 533.Kimura H. Histone modifications for human epigenome analysis. J. Hum. Genet. 2013;58:439–445. doi: 10.1038/jhg.2013.66. [DOI] [PubMed] [Google Scholar]
- 534.Zhao Y., Garcia B.A. Comprehensive Catalog of Currently Documented Histone Modifications. Cold Spring Harb. Perspect. Biol. 2015;7:a025064. doi: 10.1101/cshperspect.a025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Santos-Rosa H., Schneider R., Bannister A.J., Sherriff J., Bernstein B.E., Emre N.C., Schreiber S.L., Mellor J., Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 536.Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P., et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Steger D.J., Lefterova M.I., Ying L., Stonestrom A.J., Schupp M., Zhuo D., Vakoc A.L., Kim J.E., Chen J., Lazar M.A., et al. DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol. Cell. Biol. 2008;28:2825–2839. doi: 10.1128/MCB.02076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Ziller M.J., Gu H., Muller F., Donaghey J., Tsai L.T., Kohlbacher O., De Jager P.L., Rosen E.D., Bennett D.A., Bernstein B.E., et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500:477–481. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Fenouil R., Cauchy P., Koch F., Descostes N., Cabeza J.Z., Innocenti C., Ferrier P., Spicuglia S., Gut M., Gut I., et al. CpG islands and GC content dictate nucleosome depletion in a transcription-independent manner at mammalian promoters. Genome Res. 2012;22:2399–2408. doi: 10.1101/gr.138776.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Heintzman N.D., Hon G.C., Hawkins R.D., Kheradpour P., Stark A., Harp L.F., Ye Z., Lee L.K., Stuart R.K., Ching C.W., et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Almamun M., Kholod O., Stuckel A.J., Levinson B.T., Johnson N.T., Arthur G.L., Davis J.W., Taylor K.H. Inferring a role for methylation of intergenic DNA in the regulation of genes aberrantly expressed in precursor B-cell acute lymphoblastic leukemia. Leuk. Lymphoma. 2017;58:2156–2164. doi: 10.1080/10428194.2016.1272683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.van Nuland R., Schram A.W., van Schaik F.M., Jansen P.W., Vermeulen M., Marc Timmers H.T. Multivalent engagement of TFIID to nucleosomes. PLoS ONE. 2013;8:e73495. doi: 10.1371/journal.pone.0073495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Vermeulen M., Mulder K.W., Denissov S., Pijnappel W.W., van Schaik F.M., Varier R.A., Baltissen M.P., Stunnenberg H.G., Mann M., Timmers H.T. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell. 2007;131:58–69. doi: 10.1016/j.cell.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 544.Wozniak G.G., Strahl B.D. Hitting the ‘mark’: Interpreting lysine methylation in the context of active transcription. Biochim. Biophys. Acta. 2014;1839:1353–1361. doi: 10.1016/j.bbagrm.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 545.Orford K., Kharchenko P., Lai W., Dao M.C., Worhunsky D.J., Ferro A., Janzen V., Park P.J., Scadden D.T. Differential H3K4 methylation identifies developmentally poised hematopoietic genes. Dev. Cell. 2008;14:798–809. doi: 10.1016/j.devcel.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Abdel-Wahab O., Adli M., LaFave L.M., Gao J., Hricik T., Shih A.H., Pandey S., Patel J.P., Chung Y.R., Koche R., et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22:180–193. doi: 10.1016/j.ccr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Zhou V.W., Goren A., Bernstein B.E. Charting histone modifications and the functional organization of mammalian genomes. Nat. Rev. Genet. 2011;12:7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]
- 548.Bernstein B.E., Kamal M., Lindblad-Toh K., Bekiranov S., Bailey D.K., Huebert D.J., McMahon S., Karlsson E.K., Kulbokas E.J., 3rd, Gingeras T.R., et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 549.Pokholok D.K., Harbison C.T., Levine S., Cole M., Hannett N.M., Lee T.I., Bell G.W., Walker K., Rolfe P.A., Herbolsheimer E., et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 550.Schneider R., Bannister A.J., Myers F.A., Thorne A.W., Crane-Robinson C., Kouzarides T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat. Cell Biol. 2004;6:73–77. doi: 10.1038/ncb1076. [DOI] [PubMed] [Google Scholar]
- 551.Rougeulle C., Chaumeil J., Sarma K., Allis C.D., Reinberg D., Avner P., Heard E. Differential histone H3 Lys-9 and Lys-27 methylation profiles on the X chromosome. Mol. Cell. Biol. 2004;24:5475–5484. doi: 10.1128/MCB.24.12.5475-5484.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Wang Z., Zang C., Rosenfeld J.A., Schones D.E., Barski A., Cuddapah S., Cui K., Roh T.Y., Peng W., Zhang M.Q., et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Ninova M., Fejes Toth K., Aravin A.A. The control of gene expression and cell identity by H3K9 trimethylation. Development. 2019;146:dev181180. doi: 10.1242/dev.181180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Peters A.H., Mermoud J.E., O’Carroll D., Pagani M., Schweizer D., Brockdorff N., Jenuwein T. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat. Genet. 2002;30:77–80. doi: 10.1038/ng789. [DOI] [PubMed] [Google Scholar]
- 555.Consortium E.P. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Cui K., Zang C., Roh T.Y., Schones D.E., Childs R.W., Peng W., Zhao K. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4:80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Vakoc C.R., Sachdeva M.M., Wang H., Blobel G.A. Profile of histone lysine methylation across transcribed mammalian chromatin. Mol. Cell. Biol. 2006;26:9185–9195. doi: 10.1128/MCB.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Ferrari K.J., Scelfo A., Jammula S., Cuomo A., Barozzi I., Stutzer A., Fischle W., Bonaldi T., Pasini D. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol. Cell. 2014;53:49–62. doi: 10.1016/j.molcel.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 559.Schwartz Y.B., Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat. Rev. Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 560.Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R.S., Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 561.van Leeuwen F., Gafken P.R., Gottschling D.E. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell. 2002;109:745–756. doi: 10.1016/S0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- 562.Guccione E., Bassi C., Casadio F., Martinato F., Cesaroni M., Schuchlautz H., Luscher B., Amati B. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature. 2007;449:933–937. doi: 10.1038/nature06166. [DOI] [PubMed] [Google Scholar]
- 563.Pal S., Vishwanath S.N., Erdjument-Bromage H., Tempst P., Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol. Cell. Biol. 2004;24:9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Wu J., Xu W. Histone H3R17me2a mark recruits human RNA polymerase-associated factor 1 complex to activate transcription. Proc. Natl. Acad. Sci. USA. 2012;109:5675–5680. doi: 10.1073/pnas.1114905109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Casadio F., Lu X., Pollock S.B., LeRoy G., Garcia B.A., Muir T.W., Roeder R.G., Allis C.D. H3R42me2a is a histone modification with positive transcriptional effects. Proc. Natl. Acad. Sci. USA. 2013;110:14894–14899. doi: 10.1073/pnas.1312925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Shoaib M., Chen Q., Shi X., Nair N., Prasanna C., Yang R., Walter D., Frederiksen K.S., Einarsson H., Svensson J.P., et al. Histone H4 lysine 20 mono-methylation directly facilitates chromatin openness and promotes transcription of housekeeping genes. Nat. Commun. 2021;12:4800. doi: 10.1038/s41467-021-25051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Bassing C.H., Suh H., Ferguson D.O., Chua K.F., Manis J., Eckersdorff M., Gleason M., Bronson R., Lee C., Alt F.W. Histone H2AX: A dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114:359–370. doi: 10.1016/S0092-8674(03)00566-X. [DOI] [PubMed] [Google Scholar]
- 568.Subramanian V., Mazumder A., Surface L.E., Butty V.L., Fields P.A., Alwan A., Torrey L., Thai K.K., Levine S.S., Bathe M., et al. H2A.Z acidic patch couples chromatin dynamics to regulation of gene expression programs during ESC differentiation. PLoS Genet. 2013;9:e1003725. doi: 10.1371/journal.pgen.1003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Raisner R.M., Hartley P.D., Meneghini M.D., Bao M.Z., Liu C.L., Schreiber S.L., Rando O.J., Madhani H.D. Histone variant H2A.Z marks the 5’ ends of both active and inactive genes in euchromatin. Cell. 2005;123:233–248. doi: 10.1016/j.cell.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Loppin B., Bonnefoy E., Anselme C., Laurencon A., Karr T.L., Couble P. The histone H3.3 chaperone HIRA is essential for chromatin assembly in the male pronucleus. Nature. 2005;437:1386–1390. doi: 10.1038/nature04059. [DOI] [PubMed] [Google Scholar]
- 571.Erson A.E., Petty E.M. MicroRNAs in development and disease. Clin. Genet. 2008;74:296–306. doi: 10.1111/j.1399-0004.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 572.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 573.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Shukla G.C., Singh J., Barik S. MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol. Cell. Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 575.Fernandes J.C.R., Acuna S.M., Aoki J.I., Floeter-Winter L.M., Muxel S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. NonCoding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Cheetham S.W., Gruhl F., Mattick J.S., Dinger M.E. Long noncoding RNAs and the genetics of cancer. Br. J. Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A., Casero R.A., Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 579.Yamane K., Toumazou C., Tsukada Y., Erdjument-Bromage H., Tempst P., Wong J., Zhang Y. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 580.Anand R., Marmorstein R. Structure and mechanism of lysine-specific demethylase enzymes. J. Biol. Chem. 2007;282:35425–35429. doi: 10.1074/jbc.R700027200. [DOI] [PubMed] [Google Scholar]
- 581.Tsai C.L., Shi Y., Tainer J.A. How substrate specificity is imposed on a histone demethylase—Lessons from KDM2A. Genes Dev. 2014;28:1735–1738. doi: 10.1101/gad.249755.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Guo Y., Nady N., Qi C., Allali-Hassani A., Zhu H., Pan P., Adams-Cioaba M.A., Amaya M.F., Dong A., Vedadi M., et al. Methylation-state-specific recognition of histones by the MBT repeat protein L3MBTL2. Nucleic Acids Res. 2009;37:2204–2210. doi: 10.1093/nar/gkp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Jacobs S.A., Khorasanizadeh S. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science. 2002;295:2080–2083. doi: 10.1126/science.1069473. [DOI] [PubMed] [Google Scholar]
- 584.Huang Y., Fang J., Bedford M.T., Zhang Y., Xu R.M. Recognition of histone H3 lysine-4 methylation by the double tudor domain of JMJD2A. Science. 2006;312:748–751. doi: 10.1126/science.1125162. [DOI] [PubMed] [Google Scholar]
- 585.Musselman C.A., Lalonde M.E., Cote J., Kutateladze T.G. Perceiving the epigenetic landscape through histone readers. Nat. Struct. Mol. Biol. 2012;19:1218–1227. doi: 10.1038/nsmb.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Musselman C.A., Avvakumov N., Watanabe R., Abraham C.G., Lalonde M.E., Hong Z., Allen C., Roy S., Nunez J.K., Nickoloff J., et al. Molecular basis for H3K36me3 recognition by the Tudor domain of PHF1. Nat. Struct. Mol. Biol. 2012;19:1266–1272. doi: 10.1038/nsmb.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Wysocka J., Swigut T., Xiao H., Milne T.A., Kwon S.Y., Landry J., Kauer M., Tackett A.J., Chait B.T., Badenhorst P., et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- 588.Shi X., Hong T., Walter K.L., Ewalt M., Michishita E., Hung T., Carney D., Pena P., Lan F., Kaadige M.R., et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature. 2006;442:96–99. doi: 10.1038/nature04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Yang X.J., Seto E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 590.Dawson M.A., Kouzarides T. Cancer Epigenetics: From Mechanism to Therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 591.Yang X.J. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32:959–976. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Wapenaar H., Dekker F.J. Histone acetyltransferases: Challenges in targeting bi-substrate enzymes. Clin. Epigenetics. 2016;8:59. doi: 10.1186/s13148-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Pogo B.G., Allfrey V.G., Mirsky A.E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc. Natl. Acad. Sci. USA. 1966;55:805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Clayton A.L., Hebbes T.R., Thorne A.W., Crane-Robinson C. Histone acetylation and gene induction in human cells. FEBS Lett. 1993;336:23–26. doi: 10.1016/0014-5793(93)81601-U. [DOI] [PubMed] [Google Scholar]
- 595.Takahashi H., McCaffery J.M., Irizarry R.A., Boeke J.D. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol. Cell. 2006;23:207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 596.Cai L., Sutter B.M., Li B., Tu B.P. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell. 2011;42:426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Galdieri L., Vancura A. Acetyl-CoA carboxylase regulates global histone acetylation. J. Biol. Chem. 2012;287:23865–23876. doi: 10.1074/jbc.M112.380519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Koeneke E., Witt O., Oehme I. HDAC Family Members Intertwined in the Regulation of Autophagy: A Druggable Vulnerability in Aggressive Tumor Entities. Cells. 2015;4:135–168. doi: 10.3390/cells4020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Vaquero A., Sternglanz R., Reinberg D. NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs. Oncogene. 2007;26:5505–5520. doi: 10.1038/sj.onc.1210617. [DOI] [PubMed] [Google Scholar]
- 600.Sauve A.A., Youn D.Y. Sirtuins: NAD+-dependent deacetylase mechanism and regulation. Curr. Opin. Chem. Biol. 2012;16:535–543. doi: 10.1016/j.cbpa.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 601.Seto E., Yoshida M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Xu Y., Zhang S., Lin S., Guo Y., Deng W., Zhang Y., Xue Y. WERAM: A database of writers, erasers and readers of histone acetylation and methylation in eukaryotes. Nucleic Acids Res. 2017;45:D264–D270. doi: 10.1093/nar/gkw1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Chang H.C., Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Pandey R., Muller A., Napoli C.A., Selinger D.A., Pikaard C.S., Richards E.J., Bender J., Mount D.W., Jorgensen R.A. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002;30:5036–5055. doi: 10.1093/nar/gkf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Rodríguez-Paredes M., Esteller M. Cancer epigenetics reaches mainstream oncology. Nat. Med. 2011;17:330–339. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]
- 606.Khan A., Zhang X. dbSUPER: A database of super-enhancers in mouse and human genome. Nucleic Acids Res. 2016;44:D164–D171. doi: 10.1093/nar/gkv1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Ghisletti S., Barozzi I., Mietton F., Polletti S., De Santa F., Venturini E., Gregory L., Lonie L., Chew A., Wei C.L., et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317–328. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 608.Hnisz D., Abraham B.J., Lee T.I., Lau A., Saint-Andre V., Sigova A.A., Hoke H.A., Young R.A. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Liu Y.V., Bassal M.A., Lin Q.X.X., Wu C.-S., Kwon J., Zhou Q., Tan H.K., Ebralidze A.K., Chai L., Benoukraf T., et al. Targeted intragenic demethylation initiates chromatin rewiring for gene activation. BioRxiv. 2020 doi: 10.1101/2020.07.16.205922. [DOI] [Google Scholar]
- 610.Cohen P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 611.Beard P. Mobility of histones on the chromosome of simian virus 40. Cell. 1978;15:955–967. doi: 10.1016/0092-8674(78)90279-9. [DOI] [PubMed] [Google Scholar]
- 612.Meersseman G., Pennings S., Bradbury E.M. Mobile nucleosomes—A general behavior. EMBO J. 1992;11:2951–2959. doi: 10.1002/j.1460-2075.1992.tb05365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Flaus A., Owen-Hughes T. Dynamic properties of nucleosomes during thermal and ATP-driven mobilization. Mol. Cell. Biol. 2003;23:7767–7779. doi: 10.1128/MCB.23.21.7767-7779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Ferreira H., Somers J., Webster R., Flaus A., Owen-Hughes T. Histone tails and the H3 alphaN helix regulate nucleosome mobility and stability. Mol. Cell. Biol. 2007;27:4037–4048. doi: 10.1128/MCB.02229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Sawicka A., Seiser C. Sensing core histone phosphorylation—A matter of perfect timing. Biochim. Biophys. Acta. 2014;1839:711–718. doi: 10.1016/j.bbagrm.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Fischle W., Tseng B.S., Dormann H.L., Ueberheide B.M., Garcia B.A., Shabanowitz J., Hunt D.F., Funabiki H., Allis C.D. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- 617.Cook P.J., Ju B.G., Telese F., Wang X., Glass C.K., Rosenfeld M.G. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458:591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Chowdhury D., Keogh M.C., Ishii H., Peterson C.L., Buratowski S., Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol. Cell. 2005;20:801–809. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 619.Keogh M.C., Kim J.A., Downey M., Fillingham J., Chowdhury D., Harrison J.C., Onishi M., Datta N., Galicia S., Emili A., et al. A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature. 2006;439:497–501. doi: 10.1038/nature04384. [DOI] [PubMed] [Google Scholar]
- 620.Shi L., Tu B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015;33:125–131. doi: 10.1016/j.ceb.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Moussaieff A., Rouleau M., Kitsberg D., Cohen M., Levy G., Barasch D., Nemirovski A., Shen-Orr S., Laevsky I., Amit M., et al. Glycolysis-Mediated Changes in Acetyl-CoA and Histone Acetylation Control the Early Differentiation of Embryonic Stem Cells. Cell Metab. 2015;21:392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 622.McGarry J.D., Foster D.W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 623.Robinson M.B., Lee M.L., DaSilva S. Glutamate Transporters and Mitochondria: Signaling, Co-compartmentalization, Functional Coupling, and Future Directions. Neurochem. Res. 2020;45:526–540. doi: 10.1007/s11064-020-02974-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Counihan J.L., Grossman E.A., Nomura D.K. Cancer Metabolism: Current Understanding and Therapies. Chem. Rev. 2018;118:6893–6923. doi: 10.1021/acs.chemrev.7b00775. [DOI] [PubMed] [Google Scholar]
- 625.Hynes M.J., Murray S.L. ATP-citrate lyase is required for production of cytosolic acetyl coenzyme A and development in Aspergillus nidulans. Eukaryot. Cell. 2010;9:1039–1048. doi: 10.1128/EC.00080-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Friis R.M., Wu B.P., Reinke S.N., Hockman D.J., Sykes B.D., Schultz M.C. A glycolytic burst drives glucose induction of global histone acetylation by picNuA4 and SAGA. Nucleic Acids Res. 2009;37:3969–3980. doi: 10.1093/nar/gkp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Ramaswamy V., Williams J.S., Robinson K.M., Sopko R.L., Schultz M.C. Global control of histone modification by the anaphase-promoting complex. Mol. Cell. Biol. 2003;23:9136–9149. doi: 10.1128/MCB.23.24.9136-9149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Li Y., Gruber J.J., Litzenburger U.M., Zhou Y., Miao Y.R., LaGory E.L., Li A.M., Hu Z., Yip M., Hart L.S., et al. Acetate supplementation restores chromatin accessibility and promotes tumor cell differentiation under hypoxia. Cell Death Dis. 2020;11:102. doi: 10.1038/s41419-020-2303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Chow J.D., Lawrence R.T., Healy M.E., Dominy J.E., Liao J.A., Breen D.S., Byrne F.L., Kenwood B.M., Lackner C., Okutsu S., et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol. Metab. 2014;3:419–431. doi: 10.1016/j.molmet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Rios Garcia M., Steinbauer B., Srivastava K., Singhal M., Mattijssen F., Maida A., Christian S., Hess-Stumpp H., Augustin H.G., Muller-Decker K., et al. Acetyl-CoA Carboxylase 1-Dependent Protein Acetylation Controls Breast Cancer Metastasis and Recurrence. Cell Metab. 2017;26:842–855.e845. doi: 10.1016/j.cmet.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 631.Soliman M.L., Rosenberger T.A. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol. Cell. Biochem. 2011;352:173–180. doi: 10.1007/s11010-011-0751-3. [DOI] [PubMed] [Google Scholar]
- 632.Ropero S., Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007;1:19–25. doi: 10.1016/j.molonc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Ulanovskaya O.A., Zuhl A.M., Cravatt B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 2013;9:300–306. doi: 10.1038/nchembio.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Komatsu M., Kanda T., Urai H., Kurokochi A., Kitahama R., Shigaki S., Ono T., Yukioka H., Hasegawa K., Tokuyama H., et al. NNMT activation can contribute to the development of fatty liver disease by modulating the NAD+ metabolism. Sci. Rep. 2018;8:8637. doi: 10.1038/s41598-018-26882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Gao Y., van Haren M.J., Buijs N., Innocenti P., Zhang Y., Sartini D., Campagna R., Emanuelli M., Parsons R.B., Jespers W., et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021;64:12938–12963. doi: 10.1021/acs.jmedchem.1c01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Campagna R., Pozzi V., Sartini D., Salvolini E., Brisigotti V., Molinelli E., Campanati A., Offidani A., Emanuelli M. Beyond Nicotinamide Metabolism: Potential Role of Nicotinamide N-Methyltransferase as a Biomarker in Skin Cancers. Cancers. 2021;13:4943. doi: 10.3390/cancers13194943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Wang W., Yang C., Wang T., Deng H. Complex roles of nicotinamide N-methyltransferase in cancer progression. Cell Death Dis. 2022;13:267. doi: 10.1038/s41419-022-04713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Nguyen T.T.M., Nguyen T.H., Kim H.S., Dao T.T.P., Moon Y., Seo M., Kang S., Mai V.H., An Y.J., Jung C.R., et al. GPX8 regulates clear cell renal cell carcinoma tumorigenesis through promoting lipogenesis by NNMT. J. Exp. Clin. Cancer Res. 2023;42:42. doi: 10.1186/s13046-023-02607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Tomida M., Ohtake H., Yokota T., Kobayashi Y., Kurosumi M. Stat3 up-regulates expression of nicotinamide N-methyltransferase in human cancer cells. J. Cancer Res. Clin. Oncol. 2008;134:551–559. doi: 10.1007/s00432-007-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Roessler M., Rollinger W., Palme S., Hagmann M.L., Berndt P., Engel A.M., Schneidinger B., Pfeffer M., Andres H., Karl J., et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005;11:6550–6557. doi: 10.1158/1078-0432.CCR-05-0983. [DOI] [PubMed] [Google Scholar]
- 641.Kassem H., Sangar V., Cowan R., Clarke N., Margison G.P. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder cancer. Int. J. Cancer. 2002;101:454–460. doi: 10.1002/ijc.10631. [DOI] [PubMed] [Google Scholar]
- 642.Thomas M.G., Saldanha M., Mistry R.J., Dexter D.T., Ramsden D.B., Parsons R.B. Nicotinamide N-methyltransferase expression in SH-SY5Y neuroblastoma and N27 mesencephalic neurones induces changes in cell morphology via ephrin-B2 and Akt signalling. Cell Death Dis. 2013;4:e669. doi: 10.1038/cddis.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Mistry R.J., Klamt F., Ramsden D.B., Parsons R.B. Nicotinamide N-methyltransferase expression in SH-SY5Y human neuroblastoma cells decreases oxidative stress. J. Biochem. Mol. Toxicol. 2020;34:e22439. doi: 10.1002/jbt.22439. [DOI] [PubMed] [Google Scholar]
- 644.Sartini D., Morganti S., Guidi E., Rubini C., Zizzi A., Giuliante R., Pozzi V., Emanuelli M. Nicotinamide N-methyltransferase in non-small cell lung cancer: Promising results for targeted anti-cancer therapy. Cell Biochem. Biophys. 2013;67:865–873. doi: 10.1007/s12013-013-9574-z. [DOI] [PubMed] [Google Scholar]
- 645.Zhang J., Xie X.Y., Yang S.W., Wang J., He C. Nicotinamide N-methyltransferase protein expression in renal cell cancer. J. Zhejiang Univ. Sci. B. 2010;11:136–143. doi: 10.1631/jzus.B0900249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Dai Z., Mentch S.J., Gao X., Nichenametla S.N., Locasale J.W. Methionine metabolism influences genomic architecture and gene expression through H3K4me3 peak width. Nat. Commun. 2018;9:1955. doi: 10.1038/s41467-018-04426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Sperber H., Mathieu J., Wang Y., Ferreccio A., Hesson J., Xu Z., Fischer K.A., Devi A., Detraux D., Gu H., et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat. Cell Biol. 2015;17:1523–1535. doi: 10.1038/ncb3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Cantoni G.L. Biological methylation: Selected aspects. Annu. Rev. Biochem. 1975;44:435–451. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- 649.Caudill M.A., Wang J.C., Melnyk S., Pogribny I.P., Jernigan S., Collins M.D., Santos-Guzman J., Swendseid M.E., Cogger E.A., James S.J. Intracellular S-Adenosylhomocysteine Concentrations Predict Global DNA Hypomethylation in Tissues of Methyl-Deficient Cystathionine β-Synthase Heterozygous Mice. J. Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- 650.Sibani S., Melnyk S., Pogribny I.P., Wang W., Hiou-Tim F., Deng L., Trasler J., James S.J., Rozen R. Studies of methionine cycle intermediates (SAM, SAH), DNA methylation and the impact of folate deficiency on tumor numbers in Min mice. Carcinogenesis. 2002;23:61–65. doi: 10.1093/carcin/23.1.61. [DOI] [PubMed] [Google Scholar]
- 651.Imai S.-i., Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Radak Z., Koltai E., Taylor A.W., Higuchi M., Kumagai S., Ohno H., Goto S., Boldogh I. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic. Biol. Med. 2013;58:87–97. doi: 10.1016/j.freeradbiomed.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 653.Houtkooper R.H., Canto C., Wanders R.J., Auwerx J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Bogan K.L., Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 655.Guarente L. Sirtuins and ageing—New findings. EMBO Rep. 2013;14:750. doi: 10.1038/embor.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Pirinen E., Lo Sasso G., Auwerx J. Mitochondrial sirtuins and metabolic homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26:759–770. doi: 10.1016/j.beem.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Sebastian C., Satterstrom F.K., Haigis M.C., Mostoslavsky R. From sirtuin biology to human diseases: An update. J. Biol. Chem. 2012;287:42444–42452. doi: 10.1074/jbc.R112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Rongvaux A., Andris F., Van Gool F., Leo O. Reconstructing eukaryotic NAD metabolism. Bioessays. 2003;25:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- 659.German N.J., Haigis M.C. Sirtuins and the Metabolic Hurdles in Cancer. Curr. Biol. 2015;25:R569–R583. doi: 10.1016/j.cub.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Nalabothula N., Al-jumaily T., Eteleeb A.M., Flight R.M., Xiaorong S., Moseley H., Rouchka E.C., Fondufe-Mittendorf Y.N. Genome-Wide Profiling of PARP1 Reveals an Interplay with Gene Regulatory Regions and DNA Methylation. PLoS ONE. 2015;10:e0135410. doi: 10.1371/journal.pone.0135410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Ummarino S., Hausman C., Di Ruscio A. The PARP Way to Epigenetic Changes. Genes. 2021;12:446. doi: 10.3390/genes12030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Caiafa P., Guastafierro T., Zampieri M. Epigenetics: Poly(ADP-ribosyl)ation of PARP-1 regulates genomic methylation patterns. FASEB J. 2009;23:672–678. doi: 10.1096/fj.08-123265. [DOI] [PubMed] [Google Scholar]
- 663.Lobo J., Constâncio V., Guimarães-Teixeira C., Leite-Silva P., Miranda-Gonçalves V., Sequeira J.P., Pistoni L., Guimarães R., Cantante M., Braga I., et al. Promoter methylation of DNA homologous recombination genes is predictive of the responsiveness to PARP inhibitor treatment in testicular germ cell tumors. Mol. Oncol. 2021;15:846–865. doi: 10.1002/1878-0261.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Guastafierro T., Cecchinelli B., Zampieri M., Reale A., Riggio G., Sthandier O., Zupi G., Calabrese L., Caiafa P. CCCTC-binding Factor Activates PARP-1 Affecting DNA Methylation Machinery. J. Biol. Chem. 2008;283:21873–21880. doi: 10.1074/jbc.M801170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Ummarino S., Hausman C., Gaggi G., Rinaldi L., Bassal M.A., Zhang Y., Seelam A.J., Kobayashi I.S., Borchiellini M., Ebralidze A.K., et al. NAD Modulates DNA Methylation and Cell Differentiation. Cells. 2021;10:2986. doi: 10.3390/cells10112986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Kim M.Y., Zhang T., Kraus W.L. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 667.Kraus W.L. Transcriptional control by PARP-1: Chromatin modulation, enhancer-binding, coregulation, and insulation. Curr. Opin. Cell Biol. 2008;20:294–302. doi: 10.1016/j.ceb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Kraus W.L., Lis J.T. PARP goes transcription. Cell. 2003;113:677–683. doi: 10.1016/S0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- 669.Krishnakumar R., Gamble M.J., Frizzell K.M., Berrocal J.G., Kininis M., Kraus W.L. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science. 2008;319:819–821. doi: 10.1126/science.1149250. [DOI] [PubMed] [Google Scholar]
- 670.Ame J.C., Spenlehauer C., de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 671.Mentch S.J., Mehrmohamadi M., Huang L., Liu X., Gupta D., Mattocks D., Gomez Padilla P., Ables G., Bamman M.M., Thalacker-Mercer A.E., et al. Histone Methylation Dynamics and Gene Regulation Occur through the Sensing of One-Carbon Metabolism. Cell Metab. 2015;22:861–873. doi: 10.1016/j.cmet.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Mentch S.J., Locasale J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016;1363:91–98. doi: 10.1111/nyas.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Nishiyama A., Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37:1012–1027. doi: 10.1016/j.tig.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 674.Moore K.E., Gozani O. An unexpected journey: Lysine methylation across the proteome. Biochim. Biophys. Acta. 2014;1839:1395–1403. doi: 10.1016/j.bbagrm.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Upadhyay A.K., Cheng X. Dynamics of histone lysine methylation: Structures of methyl writers and erasers. Prog. Drug Res. 2011;67:107–124. doi: 10.1007/978-3-7643-8989-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Hyun K., Jeon J., Park K., Kim J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017;49:e324. doi: 10.1038/emm.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.Hino S., Sakamoto A., Nagaoka K., Anan K., Wang Y., Mimasu S., Umehara T., Yokoyama S., Kosai K., Nakao M. FAD-dependent lysine-specific demethylase-1 regulates cellular energy expenditure. Nat. Commun. 2012;3:758. doi: 10.1038/ncomms1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Martignago D., Bernardini B., Polticelli F., Salvi D., Cona A., Angelini R., Tavladoraki P. The Four FAD-Dependent Histone Demethylases of Arabidopsis Are Differently Involved in the Control of Flowering Time. Front. Plant Sci. 2019;10:669. doi: 10.3389/fpls.2019.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.De Colibus L., Mattevi A. New frontiers in structural flavoenzymology. Curr. Opin. Struct. Biol. 2006;16:722–728. doi: 10.1016/j.sbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 680.van Berkel W.J., Kamerbeek N.M., Fraaije M.W. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 2006;124:670–689. doi: 10.1016/j.jbiotec.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 681.Rutter J., Winge D.R., Schiffman J.D. Succinate dehydrogenase—Assembly, regulation and role in human disease. Mitochondrion. 2010;10:393–401. doi: 10.1016/j.mito.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Giancaspero T.A., Wait R., Boles E., Barile M. Succinate dehydrogenase flavoprotein subunit expression in Saccharomyces cerevisiae--involvement of the mitochondrial FAD transporter, Flx1p. FEBS J. 2008;275:1103–1117. doi: 10.1111/j.1742-4658.2008.06270.x. [DOI] [PubMed] [Google Scholar]
- 683.Joosten V., van Berkel W.J. Flavoenzymes. Curr. Opin. Chem. Biol. 2007;11:195–202. doi: 10.1016/j.cbpa.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 684.Forneris F., Binda C., Vanoni M.A., Mattevi A., Battaglioli E. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005;579:2203–2207. doi: 10.1016/j.febslet.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 685.Opel M., Lando D., Bonilla C., Trewick S.C., Boukaba A., Walfridsson J., Cauwood J., Werler P.J., Carr A.M., Kouzarides T., et al. Genome-wide studies of histone demethylation catalysed by the fission yeast homologues of mammalian LSD1. PLoS ONE. 2007;2:e386. doi: 10.1371/journal.pone.0000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Huang J., Sengupta R., Espejo A.B., Lee M.G., Dorsey J.A., Richter M., Opravil S., Shiekhattar R., Bedford M.T., Jenuwein T., et al. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 687.Hausinger R.P. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit. Rev. Biochem. Mol. Biol. 2004;39:21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- 688.Tsukada Y., Fang J., Erdjument-Bromage H., Warren M.E., Borchers C.H., Tempst P., Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 689.Alam M.T., Manjeri G.R., Rodenburg R.J., Smeitink J.A., Notebaart R.A., Huynen M., Willems P.H., Koopman W.J. Skeletal Muscle Mitochondria of Ndufs4 Mice Display Normal Maximal Pyruvate Oxidation and Atp Production. Biochim. Biophys. Acta. 2015;1847:526–533. doi: 10.1016/j.bbabio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 690.Carey B.W., Finley L.W., Cross J.R., Allis C.D., Thompson C.B. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Murugan A.K., Alzahrani A.S. Isocitrate Dehydrogenase IDH1 and IDH2 Mutations in Human Cancer: Prognostic Implications for Gliomas. Br. J. Biomed. Sci. 2022;79:10208. doi: 10.3389/bjbs.2021.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692.Yang H., Ye D., Guan K.L., Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: Mechanistic insights and clinical perspectives. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:5562–5571. doi: 10.1158/1078-0432.CCR-12-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Oizel K., Gratas C., Nadaradjane A., Oliver L., Vallette F.M., Pecqueur C. D-2-Hydroxyglutarate does not mimic all the IDH mutation effects, in particular the reduced etoposide-triggered apoptosis mediated by an alteration in mitochondrial NADH. Cell Death Dis. 2015;6:e1704. doi: 10.1038/cddis.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Parker S.J., Metallo C.M. Metabolic consequences of oncogenic IDH mutations. Pharmacol. Ther. 2015;152:54–62. doi: 10.1016/j.pharmthera.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Reitman Z.J., Duncan C.G., Poteet E., Winters A., Yan L.-J., Gooden D.M., Spasojevic I., Boros L.G., Yang S.-H., Yan H. Cancer-associated Isocitrate Dehydrogenase 1 (IDH1) R132H Mutation and d-2-Hydroxyglutarate Stimulate Glutamine Metabolism under Hypoxia. J. Biol. Chem. 2014;289:23318–23328. doi: 10.1074/jbc.M114.575183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Matsunaga H., Futakuchi-Tsuchida A., Takahashi M., Ishikawa T., Tsuji M., Ando O. IDH1 and IDH2 have critical roles in 2-hydroxyglutarate production in D-2-hydroxyglutarate dehydrogenase depleted cells. Biochem. Biophys. Res. Commun. 2012;423:553–556. doi: 10.1016/j.bbrc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 697.Du X., Hu H. The Roles of 2-Hydroxyglutarate. Front. Cell Dev. Biol. 2021;9:651317. doi: 10.3389/fcell.2021.651317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Carbonneau M., Gagné L.M., Lalonde M.E., Germain M.A., Motorina A., Guiot M.C., Secco B., Vincent E.E., Tumber A., Hulea L., et al. The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat. Commun. 2016;7:12700. doi: 10.1038/ncomms12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Chang S., Yim S., Park H. The cancer driver genes IDH1/2, JARID1C/KDM5C, and UTX/KDM6A: Crosstalk between histone demethylation and hypoxic reprogramming in cancer metabolism. Exp. Mol. Med. 2019;51:1–17. doi: 10.1038/s12276-019-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Inoue S., Lemonnier F., Mak T.W. Roles of IDH1/2 and TET2 mutations in myeloid disorders. Int. J. Hematol. 2016;103:627–633. doi: 10.1007/s12185-016-1973-7. [DOI] [PubMed] [Google Scholar]
- 701.de Vos D., van Overveld W. Decitabine: A historical review of the development of an epigenetic drug. Ann. Hematol. 2005;84((Suppl. S1)):3–8. doi: 10.1007/s00277-005-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Momparler R.L. Pharmacology of 5-Aza-2’-deoxycytidine (decitabine) Semin. Hematol. 2005;42:S9–S16. doi: 10.1053/j.seminhematol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 703.Stresemann C., Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer. 2008;123:8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]
- 704.Derissen E.J., Beijnen J.H., Schellens J.H. Concise drug review: Azacitidine and decitabine. Oncologist. 2013;18:619–624. doi: 10.1634/theoncologist.2012-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Sorm F., Piskala A., Cihak A., Vesely J. 5-Azacytidine, a new, highly effective cancerostatic. Experientia. 1964;20:202–203. doi: 10.1007/BF02135399. [DOI] [PubMed] [Google Scholar]
- 706.Ganapathy-Kanniappan S., Vali M., Kunjithapatham R., Buijs M., Syed L.H., Rao P.P., Ota S., Kwak B.K., Loffroy R., Geschwind J.F. 3-bromopyruvate: A new targeted antiglycolytic agent and a promise for cancer therapy. Curr. Pharm. Biotechnol. 2010;11:510–517. doi: 10.2174/138920110791591427. [DOI] [PubMed] [Google Scholar]
- 707.Robinson M.M., McBryant S.J., Tsukamoto T., Rojas C., Ferraris D.V., Hamilton S.K., Hansen J.C., Curthoys N.P. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) Biochem. J. 2007;406:407–414. doi: 10.1042/BJ20070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Varghese S., Pramanik S., Williams L.J., Hodges H.R., Hudgens C.W., Fischer G.M., Luo C.K., Knighton B., Tan L., Lorenzi P.L., et al. The Glutaminase Inhibitor CB-839 (Telaglenastat) Enhances the Antimelanoma Activity of T-Cell-Mediated Immunotherapies. Mol. Cancer. Ther. 2021;20:500–511. doi: 10.1158/1535-7163.MCT-20-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Elhammali A., Ippolito J.E., Collins L., Crowley J., Marasa J., Piwnica-Worms D. A high-throughput fluorimetric assay for 2-hydroxyglutarate identifies Zaprinast as a glutaminase inhibitor. Cancer Discov. 2014;4:828–839. doi: 10.1158/2159-8290.CD-13-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Simpson N.E., Tryndyak V.P., Pogribna M., Beland F.A., Pogribny I.P. Modifying metabolically sensitive histone marks by inhibiting glutamine metabolism affects gene expression and alters cancer cell phenotype. Epigenetics. 2012;7:1413–1420. doi: 10.4161/epi.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Wang D., Meng G., Zheng M., Zhang Y., Chen A., Wu J., Wei J. The Glutaminase-1 Inhibitor 968 Enhances Dihydroartemisinin-Mediated Antitumor Efficacy in Hepatocellular Carcinoma Cells. PLoS ONE. 2016;11:e0166423. doi: 10.1371/journal.pone.0166423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Yuan L., Sheng X., Clark L.H., Zhang L., Guo H., Jones H.M., Willson A.K., Gehrig P.A., Zhou C., Bae-Jump V.L. Glutaminase inhibitor compound 968 inhibits cell proliferation and sensitizes paclitaxel in ovarian cancer. Am. J. Transl. Res. 2016;8:4265–4277. [PMC free article] [PubMed] [Google Scholar]
- 713.Emberley E., Pan A., Chen J., Dang R., Gross M., Huang T., Li W., MacKinnon A., Singh D., Sotirovska N., et al. The glutaminase inhibitor telaglenastat enhances the antitumor activity of signal transduction inhibitors everolimus and cabozantinib in models of renal cell carcinoma. PLoS ONE. 2021;16:e0259241. doi: 10.1371/journal.pone.0259241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Flint H.J., Scott K.P., Louis P., Duncan S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 715.Turner N.D., Lupton J.R. Dietary fiber. Adv. Nutr. 2011;2:151–152. doi: 10.3945/an.110.000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Liu H., Wang J., He T., Becker S., Zhang G., Li D., Ma X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018;9:21–29. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Geng H.W., Yin F.Y., Zhang Z.F., Gong X., Yang Y. Butyrate Suppresses Glucose Metabolism of Colorectal Cancer Cells via GPR109a-AKT Signaling Pathway and Enhances Chemotherapy. Front. Mol. Biosci. 2021;8:634874. doi: 10.3389/fmolb.2021.634874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Luu M., Riester Z., Baldrich A., Reichardt N., Yuille S., Busetti A., Klein M., Wempe A., Leister H., Raifer H., et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021;12:4077. doi: 10.1038/s41467-021-24331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Bubna A.K. Vorinostat-An Overview. Indian J. Dermatol. 2015;60:419. doi: 10.4103/0019-5154.160511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Richon V.M. Cancer biology: Mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor. Br. J. Cancer. 2006;95:S2–S6. doi: 10.1038/sj.bjc.6603463. [DOI] [Google Scholar]
- 721.Valdez B.C., Brammer J.E., Li Y., Murray D., Liu Y., Hosing C., Nieto Y., Champlin R.E., Andersson B.S. Romidepsin targets multiple survival signaling pathways in malignant T cells. Blood Cancer J. 2015;5:e357. doi: 10.1038/bcj.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 722.Grant C., Rahman F., Piekarz R., Peer C., Frye R., Robey R.W., Gardner E.R., Figg W.D., Bates S.E. Romidepsin: A new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev. Anticancer Ther. 2010;10:997–1008. doi: 10.1586/era.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Pajak B., Siwiak E., Soltyka M., Priebe A., Zielinski R., Fokt I., Ziemniak M., Jaskiewicz A., Borowski R., Domoradzki T., et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019;21:234. doi: 10.3390/ijms21010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 724.Chen W., Gueron M. The inhibition of bovine heart hexokinase by 2-deoxy-D-glucose-6-phosphate: Characterization by 31P NMR and metabolic implications. Biochimie. 1992;74:867–873. doi: 10.1016/0300-9084(92)90070-U. [DOI] [PubMed] [Google Scholar]
- 725.Caravella J.A., Lin J., Diebold R.B., Campbell A.M., Ericsson A., Gustafson G., Wang Z., Castro J., Clarke A., Gotur D., et al. Structure-Based Design and Identification of FT-2102 (Olutasidenib), a Potent Mutant-Selective IDH1 Inhibitor. J. Med. Chem. 2020;63:1612–1623. doi: 10.1021/acs.jmedchem.9b01423. [DOI] [PubMed] [Google Scholar]
- 726.Stein E.M., Konopleva M., Gilmour R., Szpurka A.M., Hill E., Ward R., Kantarjian H.M., Dinardo C.D. A Phase 1 Study of LY3410738, a First-in-Class Covalent Inhibitor of Mutant IDH in Advanced Myeloid Malignancies (Trial in Progress) Blood. 2020;136:26. doi: 10.1182/blood-2020-134307. [DOI] [Google Scholar]
- 727.Natsume A., Arakawa Y., Narita Y., Sugiyama K., Hata N., Muragaki Y., Shinojima N., Kumabe T., Saito R., Motomura K., et al. The first-in-human phase I study of a brain-penetrant mutant IDH1 inhibitor DS-1001 in patients with recurrent or progressive IDH1-mutant gliomas. Neuro. Oncol. 2023;25:326–336. doi: 10.1093/neuonc/noac155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 728.Mellinghoff I.K., Penas-Prado M., Peters K.B., Burris H.A., 3rd, Maher E.A., Janku F., Cote G.M., de la Fuente M.I., Clarke J.L., Ellingson B.M., et al. Vorasidenib, a Dual Inhibitor of Mutant IDH1/2, in Recurrent or Progressive Glioma; Results of a First-in-Human Phase I Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021;27:4491–4499. doi: 10.1158/1078-0432.CCR-21-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Popovici-Muller J., Lemieux R.M., Artin E., Saunders J.O., Salituro F.G., Travins J., Cianchetta G., Cai Z., Zhou D., Cui D., et al. Discovery of AG-120 (Ivosidenib): A First-in-Class Mutant IDH1 Inhibitor for the Treatment of IDH1 Mutant Cancers. ACS Med. Chem. Lett. 2018;9:300–305. doi: 10.1021/acsmedchemlett.7b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Heuser M., Palmisiano N., Mantzaris I., Mims A., DiNardo C., Silverman L.R., Wang E.S., Fiedler W., Baldus C., Schwind S., et al. Safety and efficacy of BAY1436032 in IDH1-mutant AML: Phase I study results. Leukemia. 2020;34:2903–2913. doi: 10.1038/s41375-020-0996-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Cho Y.S., Levell J.R., Liu G., Caferro T., Sutton J., Shafer C.M., Costales A., Manning J.R., Zhao Q., Sendzik M., et al. Discovery and Evaluation of Clinical Candidate IDH305, a Brain Penetrant Mutant IDH1 Inhibitor. ACS Med. Chem. Lett. 2017;8:1116–1121. doi: 10.1021/acsmedchemlett.7b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Rohle D., Popovici-Muller J., Palaskas N., Turcan S., Grommes C., Campos C., Tsoi J., Clark O., Oldrini B., Komisopoulou E., et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733.Okoye-Okafor U.C., Bartholdy B., Cartier J., Gao E.N., Pietrak B., Rendina A.R., Rominger C., Quinn C., Smallwood A., Wiggall K.J., et al. New IDH1 mutant inhibitors for treatment of acute myeloid leukemia. Nat. Chem. Biol. 2015;11:878–886. doi: 10.1038/nchembio.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Davis M., Pragani R., Popovici-Muller J., Gross S., Thorne N., Salituro F., Fantin V., Straley K., Su M., Dang L., et al. Probe Reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information (US); Bethesda, MD, USA: 2010. ML309: A potent inhibitor of R132H mutant IDH1 capable of reducing 2-hydroxyglutarate production in U87 MG glioblastoma cells. [PubMed] [Google Scholar]
- 735.Kim H.J., Choi B.Y., Keum Y.S. Identification of a new selective chemical inhibitor of mutant isocitrate dehydrogenase-1. J. Cancer Prev. 2015;20:78–83. doi: 10.15430/JCP.2015.20.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Yen K., Travins J., Wang F., David M.D., Artin E., Straley K., Padyana A., Gross S., DeLaBarre B., Tobin E., et al. AG-221, a First-in-Class Therapy Targeting Acute Myeloid Leukemia Harboring Oncogenic IDH2 Mutations. Cancer Discov. 2017;7:478–493. doi: 10.1158/2159-8290.CD-16-1034. [DOI] [PubMed] [Google Scholar]
- 737.Thomas D., Majeti R. Optimizing Next-Generation AML Therapy: Activity of Mutant IDH2 Inhibitor AG-221 in Preclinical Models. Cancer Discov. 2017;7:459–461. doi: 10.1158/2159-8290.CD-17-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Chen J., Yang J., Sun X., Wang Z., Cheng X., Lu W., Cai X., Hu C., Shen X., Cao P. Allosteric inhibitor remotely modulates the conformation of the orthestric pockets in mutant IDH2/R140Q. Sci. Rep. 2017;7:16458. doi: 10.1038/s41598-017-16427-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 739.Yap T.A., Ahnert J.R., Piha-Paul S.A., Fu S., Janku F., Karp D.D., Naing A., Dumbrava E.E.I., Pant S., Subbiah V., et al. Phase I trial of IACS-010759 (IACS), a potent, selective inhibitor of complex I of the mitochondrial electron transport chain, in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2019;37:3014. doi: 10.1200/JCO.2019.37.15_suppl.3014. [DOI] [Google Scholar]
- 740.Tsuji A., Akao T., Masuya T., Murai M., Miyoshi H. IACS-010759, a potent inhibitor of glycolysis-deficient hypoxic tumor cells, inhibits mitochondrial respiratory complex I through a unique mechanism. J. Biol. Chem. 2020;295:7481–7491. doi: 10.1074/jbc.RA120.013366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Fujiwara T., Saitoh H., Inoue A., Kobayashi M., Okitsu Y., Katsuoka Y., Fukuhara N., Onishi Y., Ishizawa K., Ichinohasama R., et al. 3-Deazaneplanocin A (DZNep), an inhibitor of S-adenosylmethionine-dependent methyltransferase, promotes erythroid differentiation. J. Biol. Chem. 2014;289:8121–8134. doi: 10.1074/jbc.M114.548651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Kim J.H., Kim J.H., Kim S.C., Yi Y.S., Yang W.S., Yang Y., Kim H.G., Lee J.Y., Kim K.H., Yoo B.C., et al. Adenosine dialdehyde suppresses MMP-9-mediated invasion of cancer cells by blocking the Ras/Raf-1/ERK/AP-1 signaling pathway. Biochem. Pharmacol. 2013;86:1285–1300. doi: 10.1016/j.bcp.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 743.Syed-Abdul M.M., Parks E.J., Gaballah A.H., Bingham K., Hammoud G.M., Kemble G., Buckley D., McCulloch W., Manrique-Acevedo C. Fatty Acid Synthase Inhibitor TVB-2640 Reduces Hepatic de Novo Lipogenesis in Males With Metabolic Abnormalities. Hepatology. 2020;72:103–118. doi: 10.1002/hep.31000. [DOI] [PubMed] [Google Scholar]
- 744.Falchook G., Infante J., Arkenau H.T., Patel M.R., Dean E., Borazanci E., Brenner A., Cook N., Lopez J., Pant S., et al. First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors. EClinicalMedicine. 2021;34:100797. doi: 10.1016/j.eclinm.2021.100797. [DOI] [PMC free article] [PubMed] [Google Scholar]