Abstract
Herbivore-Induced Plant Volatiles (HIPVs) are volatile signals emitted by plants to deter herbivores and attract their natural enemies. To date, it is unknown how lychee plants, Litchi chinensis, respond to the induction of leaf galls (erinea) caused by the lychee erinose mite (LEM), Aceria litchii. Aiming to reveal the role of HIPVs in this plant-mite interaction, we investigated changes in the volatile profile of lychee plants infested by LEM and their role on LEM preferences. The volatile profile of uninfested (flower buds, fruit, leaves and new leaf shoots) and infested plant tissue were characterized under different levels of LEM infestation. Volatiles were collected using head-space-solid phase microextraction (HS-SPME) followed by gas chromatography-mass spectrometry (GC-MS) analyses. Fifty-eight volatiles, including terpenoids, alcohols, aldehydes, alkanes, esters, and ketones classes were identified. Using dual-choice bioassays, we investigated the preference of LEM to uninfested plant tissues and to the six most abundant plant volatiles identified. Uninfested new leaf shoots were the most attractive plant tissues to LEM and LEM attraction or repellence to volatiles were mostly influenced by compound concentration. We discuss possible applications of our findings in agricultural settings.
Keywords: herbivore-induced plant volatiles (HIPVs), behavioral bioassays, eriophyoid mites, gas chromatography-mass spectrometry (GC-MS), head-space-solid phase microextraction (HS-SPME)
1. Introduction
Plants have sophisticated defense systems to recognize and counterattack herbivorous mites and insects. Such defensive responses involve changes in plant traits that interfere with herbivores directly or indirectly. Direct defenses include the production of toxicants and volatile organic compounds (VOCs) that repel or deter herbivores, while indirect defenses include the production of VOCs that attract natural enemies of the herbivore (for reviews of VOCs as direct and indirect defenses see Howe et al. [1] and Dudareva et al. [2] and the references therein). VOCs with toxic, repellent, or attractive properties include various groups of terpenoids (monoterpenes, sesquiterpenes and homoterpenes), phenylpropanoids and benzenoids, fatty acid derivatives, and amino acid derivatives [3,4,5]. Upon mechanical damage, mite, or insect attack, the plants’ immune responses trigger the production of novel VOCs, known as Herbivore-Induced Plant Volatiles (HIPVs), locally and systemically within the plant [4].
The most well-documented function of HIPVs is the attraction of natural enemies [4,5,6], which indirectly promotes plant fitness [7]. Several studies have shown that terpenoids and other VOCs, emitted in response to herbivory, allow parasitoids and predators to distinguish infested from non-infested plants, helping them locate their prey. For instance, Lima bean [8], apple [9], and cucumber [10] plants infested with the two-spotted spider mite (Tetranychus urticae, Acari: Tetranychidae) attracted significantly more natural enemies than non-infested plants. The composition of HIPVs can vary significantly within plant species when attacked by the same herbivore. For example, feeding by T. urticae induces different HIPV blends from a range of host plants [11], and the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae) is attracted to different HIPV blends from different T. urticae -damaged plant species [12].
In addition to directly repelling herbivores [13] and indirectly attracting natural enemies, HIPV’s are also involved in within-plant communication [14,15] and plant-plant communication in response to herbivore attack [16,17,18,19]. Herbivory releases distinct volatile blends in damaged plants that are potent elicitors of defenses in systemic uninduced leaves of the same plant or nearby plants [20,21,22]. Since HIPVs can be used to recruit natural enemies and repel herbivores, they can be implemented as a sustainable Integrated Pest Management (IPM) strategy for controlling plant pests. Furthermore, transgenic plants can be engineered to enhance emission of VOCs to increase attraction of natural enemies [23,24,25,26] and repel important pests [27,28].
Lychee (Litchi chinensis Sonn., Sapindaceae) is a commercially important fruit native to Asia that has been cultivated by several countries in the tropical and subtropical regions of the world [29]. A tiny eriophyoid mite called the lychee erinose mite (LEM), Aceria litchii (Keifer) (Acari: Eriophyidae), threatens lychee production worldwide as it infests every plant tissue. LEM is highly host-specific and is only known to attack lychee [30,31]. Upon feeding, LEM induces the formation of a gall called “erineum” which consists of proliferations of hypertrophic trichomes [32] that undergo several stages of development. In early erineum stages, when mite infestations are low, the affected tissue becomes distorted, and white hairs develop as feltlike masses. As mite populations grow, a denser white erineum becomes apparent, and when populations peak, the erineum turns into an amber color. At its final stage, the erineum turns dark in color followed by tissue necrosis [30]. Erinea can be induced in several plant tissues that have not hardened yet, such as new leaf shoots, petioles, flower buds, and fruitlets [32]. LEM management has focused on removing LEM infested plant parts by pruning [33,34] followed by repeated pesticide (sulfur) applications to protect the new shoots [32,35]. However, these strategies are insufficient to mitigate LEM damage, which can reduce yields by up to 80% [36,37].
To date, it is unknown how and why LEM induces the formation of erinea. Recently, a growing body of literature shows that the erinea are a perfect environment for LEM to hide and acquire protection from biotic and abiotic stressors. For instance, pesticides eliminated nearly all LEM when applied directly [38], but not when mites were sheltered inside the erinea [39]. Moreover, although several predatory mites have been found associated with LEM erinea [40,41,42,43] evidence is lacking on their ability to prey on mites inside the erinea. Hence, control methods focused on forcing the mites to exit the erinea to target them with pesticides or natural enemies can contribute to managing this mite pest. In addition, it is unknown what drives the mites to disperse to new young tissues within and among plants. One possibility is that volatile compounds emitted by immature lychee tissues may trigger their initial dispersal.
Previous studies have explored the odor and flavor of lychee fruits, and described them as honey, rose-floral and citrus-fruity [44,45]. Volatiles emitted by fresh fruit [44,46,47], leaves [48], juices [44,49], wines [50] and canned fruit [51] of several lychee cultivars have been characterized. Although the volatile extraction methods varied among different studies, all reported terpenoids and alcohols as major volatile components of lychee fruit [44,45,47,52,53] and young and old lychee leaves [48]. Recently, Gunpal and Patni [54] compared methanolic extracts from non-infested and LEM-infested lychee leaflets to determine which metabolic processes of the leaf were altered by LEM. Leaf extracts were rich in sugars, terpenoids, linoleic acid esters, vitamins, antioxidants, glycerol, and steroids. However, it remains to be investigated which VOCs are emitted by lychee tissues distinctly targeted by LEM, i.e., new leaf shoots, leaves, flower buds, and fruitlets, and which HIPVs are synthesized by the plant after LEM infestation. In addition, LEM preferences to lychee VOCs or HIPVs are unknown and their preferences for specific plant tissues have not been studied.
In line with this goal, laboratory bioassays were performed to assess the importance of volatile compounds in the LEM-lychee interaction. First, the role of VOCs was investigated by characterizing volatile profiles of uninfested lychee plant tissues (floral buds, open flowers, leaves, and new leaf shoots) and LEM’s preference for them. Second, potential HIPVs were assessed by identifying volatiles of plants with different levels of LEM infestation (initial, intermediate, heavy, and overexploited). Third, dual-choice bioassays were carried out to investigate LEM’s attraction or repellence to the most abundant lychee volatiles. The end goal of this study was to address the role of the key VOCs and HIPVs in LEM behavior. This information may help to develop management strategies that force the mites to leave the erinea and/or lure natural enemies to LEM infested plants.
2. Materials and Methods
2.1. Plant Material and LEM Infestation
Lychee plants (var. ‘Mauritius’) were grown from seed in pots 3.7 L filled with ProMix (ProMix BX Mycorhizae, Denver, CO, USA) and kept in a climate room (25 ± 2 °C, 50% RH, and a 12:12 h (L:D) photoperiod) in the biocontainment facility of the Tropical Research and Education Center (TREC), University of Florida, Homestead, FL (25.50° N, 80.49° W) under the Florida Department of Agriculture and Consumer Services, Division of Plant Industry permit 2018-029. The plants were watered twice per week and fertilized every 15 d with 24-8-16 (N-P-K) (Miracle-Gro®, The Scotts Company, Marysville, OH, USA) and 138 chelated EDDHA iron (Sequestrene®, Syngenta, Wilmington, DE, USA). LEM-infested leaflets were detached from the infested lychee plants collected from the FruitScapes Nursery (Bokeelia, FL, USA; 26.38° N 82.07° W) and placed on 4-month-old pest-free lychee seedlings showing new leaf shoots. Mite establishment was confirmed by appearance of erineum which varied per plant from 6 to 30 days. Erineum development was assessed by the change of color and trichome density. The density of the erineum and its color seem to be two important factors shaping mite population sizes among stages, varying from zero, in the initial infestation, to thousands, in the heavy infestation stage (Ataide et al. in preparation). To obtain erinea of different colors available for the experiments, the lychee plant infestation was repeated four times using plants of the same age. Infested plants were separated into four groups, covering four erinea stages, hereafter called initial infestation, intermediate infestation, heavy infestation, and overexploitation. Thus, five treatments were conducted for potted (infested) lychee plants (A): initial infestation (A1), intermediate infestation (A2), heavy infestation (A3), plant overexploitation (A4), and healthy, uninfested plant (A5) (Figure S1). In addition, uninfested lychee branches were cut from fully grown lychee trees ‘Mauritius’ at TREC and USDA-ARS Subtropical Horticulture Research Station, Miami, FL (25.64° N, 80.29° W). From these floral buds, open flowers, leaf buds and new leaf shoots were obtained. Thus, four treatments were conducted for field-collected (non-infested) lychee tissues (B): floral buds (B1), open flowers (B2), leaf buds (B3), and new leaf shoots (B4). LEM dual-choice bioassays and volatile collections were carried out at TREC’s biocontainment facility using potted plants and field-collected tissues, separately (see below).
2.2. Chemical Standards
Chemical standards were purchased from the following sources: (Z)-3-hexen-1-ol (Cas 928-96-1), α-pinene (Cas 80-56-8), sabinene (Cas 3387-41-5), β-pinene (Cas 127-91-3), 6-methyl-5-hepten-2-one (Cas 110-93-0), (+)-limonene (Cas 5989-27-5), mixture of ocimene isomers (Cas 13877-91-3), γ-terpinene (Cas 99-85-4), linalool oxide (Cas 60047-17-8), terpinolene (Cas 586-62-9), linalool (Cas 78-70-6), nonanal (Cas 124-19-6), estragole (Cas 140-67-0), decanal (Cas 112-31-2), (+)-cyclosativene (Cas 22469-52-9), tetradecane (Cas 629-59-4), methyl eugenol (Cas 93-15-2), β-caryophyllene (Cas 87-44-5), aromadendrene (Cas 489-39-4), α-humulene (Cas 6753-98-6), a mixture of farnesene isomers (Cas 502-61-4), pentadecane (Cas 629-62-9), hexadecane (Cas 544-76-3), heptadecane (Cas 629-78-7) from Sigma-Aldrich, St. Louis, MO, USA; α-copaene (Cas 3856-25-5) from Fluka Chemical Co., Buchs, SG, Switzerland), and (+)-ar-curcumene (Cas 4176-06-1), from BOC Sciences Shirley, NY, USA.
2.3. Headspace Volatile Collections
Volatiles were collected from potted plants (A) and field-collected tissues (B) from fully-grown trees of the same (‘Mauritius’) cultivar. Each potted plant (A1 to A5) was enclosed in an oven bag (406 mm × 444 mm, Reynolds, Richmond, VA, USA) and sealed with a cotton strip on the upper 5 cm above soil level to avoid soil emissions. Prior to use, oven bags were baked in an oven at 120 °C for 2 h, and inflated with clean air and deflated three times to remove any residual contamination [55]. Bags were then stored in the laboratory until use. VOCs from the lychee plants were collected by head-space-solid phase microextraction (HS-SPME). The medium polarity SPME fiber, polydimethylsiloxane/divinylbenzene (PDMS/DVB, 65 µm) (Supelco, Bellefonte, PA, USA) was used to extract volatile emissions from the samples. Before use, the SPME fiber was conditioned for 30 min at 225 °C in the gas-chromatograph injection port under the carrier gas, helium.
Volatiles from the potted plants were allowed to accumulate within the airspace of the bag for 10 min to equilibrate. The SPME device was affixed to a stand with a clamp. The fiber was introduced through the bag and exposed for 24 h at room temperature. After extraction, the fiber was removed from the sample; subsequently, the fiber was inserted into the injector port for gas chromatography-mass spectrometry (GC-MS) analysis. The desorption of the volatile compounds was performed at 220 °C for 2 min. Nine replicates were performed on different days. Blank measurements with empty oven bags and fibers were performed before each analysis to prevent the release of undesirable compounds. For field-collected samples (B1 to B4), a 30 mL beaker containing 1.003 g (±0.02 g) of each plant part was covered with aluminum foil and then subjected to saturate the headspace with the volatiles for 15 min at room temperature. Subsequently, a preconditioned fiber was pushed through the film and exposed to the headspace of each plant tissue for 30 min. Thereafter, volatile compounds were desorbed in the GC-MS injector at 220 °C for 2 min. Each plant sample was extracted by HS-SPME and GC-MS in three replicates. The blank SPME fiber was run before sampling to ensure that it was free of contaminants.
2.4. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
Volatile emissions were identified by gas chromatography/mass spectrometry (GC/MS) coupled to a 5975B quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with a DB-5 column (30 m × 0.25 mm × 0.25 µm). After sampling, the SPME fiber was introduced onto the GC injector in the splitless mode under the following conditions. The oven temperature program was set at 60 °C for 1.3 min and increased to 246 °C at 3 °C/min; the total time was 63.3 min. The carrier gas helium was set at 1.3 mL/min. The injector temperature was set at 220 °C and the detector temperature was 230 °C. Electron ionization mode (EI-MS; 70 eV) was used to acquire mass spectra in the 35-550 m/z at 2.8 scans/s. MassHunter software (B.07.06, Agilent Technologies, Santa Clara, CA, USA) was utilized to obtain the mass spectra and ion chromatogram. Volatile emissions of lychee plants were identified by a combination of 3 methods: (1) The individual peaks were identified by comparing their mass spectra with Wiley 12/National Institute of Standards and Technology (NIST) [56], MassFinder [57], Adams Library [58], Flavors and Fragrances of Natural and Synthetic Compounds 3 [59]; (2) in-house library “SHRS Essential Oil Constituents-DB-5” which was built up from authentic standards purchased from different sources, and components of known essential oils analyzed under the same conditions [60]; (3) to confirm the identification, retention index (RI) values calculated according to the RI van Den Dool and Kratz equation RIx = 100n + 100 (tx − tn)/(tn+1 − tn); tn+1 and tn retention times of the reference n-alkane hydrocarbons eluting immediately before and after compound “X”; tx retention time of compound “X” [61] to a series of n-alkanes (C9–C21, Sigma-Aldrich, St. Louis, MO, USA), and compared with their RIs available in the literature [58,62,63,64]. The relative percentages were obtained by total ion current (TIC) peak areas.
Principal Component Analysis (PCA) and Agglomerative Hierarchical Clustering (ACH) for GC-MS data were performed using XLSTAT 2021 (Addinsoft, New York, NY, USA). Only constituents in a concentration higher than 0.5% were used as variables for the PCA and ACH analysis. Data used for the PCA and ACH analyses were a 21 × 5 matrix (105 data) for samples A1 to A5, and 81 × 4 matrix (112 data) for samples B1 to B4. Pearson’s correlation model was used for PCA. Euclidean distance for measure and Ward’s method were used for ACH analysis.
2.5. Dual-Choice Test between Field-Collected (Non-Infested) Lychee Tissues
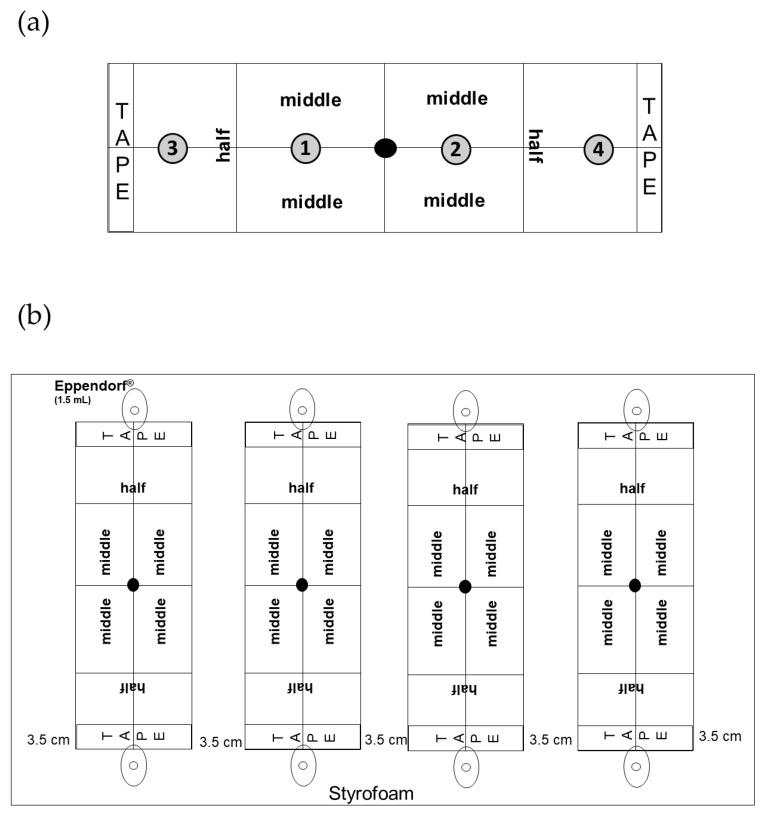
Considering the minute size of LEM (~200 nm), it was not possible to carry out conventional dual-choice bioassays using Y-tube olfactometers [65], petri-dish arenas [66], cages or greenhouses [67], as used for other mite species. All known experimental set-up were tested, but without yielding any mite response. Therefore, a new method to conduct dual-choice bioassays was developed. The experimental arena consisted of a microscope glass slide (75mm × 25mm) with markings dividing it into four equal sections (Figure 1a). Double-sided tape (1.25 cm wide) (Scotch®, 3M, Miami, FL, USA) was placed alongside the edges of the slide to keep the mites in the arena (Figure 1a). Then, an Eppendorf® tube filled with nutrient solution (10N-5P-14K) (MaxiGro®, General Hydroponics Inc., Sebastopol, CA, USA) and holding one plant tissue (flower buds, fruits, leaves, or new leaf shoots), was placed on two opposite sides of the arena. Four arenas were placed on a white Styrofoam board (20 × 10 cm) (Figure 1b) and each board was placed inside a custom-made acrylic cage (30 × 30 × 30 cm) with openings on two sides to allow airflow. The experimental units were kept at 25 ± 2 °C, 50% ± 10% RH, and a 12:12 h (L:D) photoperiod. Fluorescent lamps were placed above each cage to provide uniform light over the experimental arenas. A leafdisc (0.5 cm diam.), cut from an amber-colored erinea (i.e., fully infested with LEM) was placed in the middle of each arena (on the black dot Figure 1a), and after 24 h, the mites that moved towards one side and crossed the midline marking were considered attracted to the plant part offered on the same side. To test whether LEM was able to make reliable choices we offered the same plant tissue on both edges, as controls. The plant tissues tested were leaf, new leaf shoot, fruit, and quiescent flower bud. All possible combinations of plant tissues were offered to LEM in a factorial design (N = 8). Only LEM found in Sections 3 and 4 and TAPE (Figure 1a) were considered in the analyses. Differences in the proportion of mites between treatments were tested with generalized linear mixed-effects models (GLMM) using the lme4 package [68]. Binomial error distribution and including the position of the slide (left or right) and Styrofoam board (replicate) as a random factor [69]. Residual plots of data revealed no major deviation from the normality and variance homogeneity assumptions. Analyses were performed separately for each plant tissue/combination and using the R program version 4.2.1 [70].
Figure 1.
Schematic drawing of the bioassay used for assessing LEM preferences. Panel (a) shows the experimental arena used in the dual-choice bioassay. The drawing represents a microscope slide (75 mm × 25 mm) divided into Sections 1–4 and TAPE. Panel (b) shows the white Styrofoam board (20 cm × 10 cm) used to hold four slides and two Eppendorf® tubes (1.5 mL) per slide. Only LEM crossing half of the slide (Sections 3 and 4 and TAPE) were considered to make a choice between the two sources offered. The number of mites in each section was assessed after 24 h.
2.6. Dual-Choice Test on Single Volatile Compounds
Using the same experimental unit as above, dual-choice bioassays were used to evaluate LEM attraction to the six major volatile compounds identified in potted plants and field-collected tissues (nonanal, decanal, sabinene, limonene, β-caryophyllene and ar-curcumene; see Result Section 3.1). Eight concentrations were tested for each compound: 3%, 5%, 7%, 10%, 25%, 50%, 75% and 100%. Compounds were diluted in absolute EtOH, which was also used as a control. All dual-choice tests were made by offering a single compound concentration against the control (EtOH). To test whether LEM was able to make reliable choices we offered the clean filter paper or ethanol on both sides, as controls. Forty-five minutes prior to testing, 5 μL of each compound was applied to a circular filter paper (d = 1 cm), allowing enough time for the EtOH to evaporate. Then, the filter paper containing the test compound was placed on one end of the slide, and the filter paper containing EtOH (control) was placed on the other end (Figure 1a). The assessment of LEM choice between the two odor sources was performed as above. Replicates in which LEM made no choice (remained in the middle of the arena, Sections 1 and 2) were discarded. To correct for the potential positional bias, the treatment and the control sources were switched from left to right in all choice experiments. The number of replicates per compound concentration are shown in their respective figures (see Section 3). The same analysis described above was performed separately for each compound, as well as each concentration.
3. Results
3.1. Volatile Composition of Potted (Infested) Lychee Plants and Field-Collected (Non-Infested) Lychee Tissues
A total of 58 compounds were identified from potted lychee plants, accounting for 98.05% to 99.73% of total volatiles: 44 terpenoids, 2 esters, 1 alcohol, 1 ketone, 4 alkanes, and 6 aldehydes (Table 1). In lychee leaves with different LEM infestation levels (A1 to A5), monoterpene hydrocarbons (MHs), sesquiterpene hydrocarbons (SHs), aldehydes and alkanes were the major group of volatile components (Figure 2). Among the volatile terpenoids in samples analyzed, sabinene (1.92% to 11.67%), limonene (3.81% to 9.94%), β-caryophyllene (5.21% to 10.34%), ar-curcumene (10.25% to 38.54%) were the most abundant compounds, followed by α-copaene (1.08% to 2.82%) and α-zingiberene (0.24% to 2.03%). The aliphatic aldehydes were most characterized by higher relative amounts of nonanal (18.65% to 51.52%) and decanal (2.28% to 7.76%). Nonanal was more abundant in stage A1 (43.52%), followed by 24.06% in stage A2, 29.31% in stage A3, and 18.65% in stage A4. Nonanal concentration was higher in control plants (A5, 51.52%), and A1 stage plants, than in plants under higher levels of infestation (A2, A3, A4). A similar pattern was observed for decanal, 7.76% in control plants and 6.74% in stage A1. (E)-2-decanal (1.58%), undecanal (0.30%), and (E)-2-undecanal (0.12%) were higher in stage A1 than in other stages. Similar patterns were also observed with alkanes. Hexadecane (2.21%) and heptadecane (5.38%) were found in higher concentrations in the initial infestation stage than in the later infestation stages. The concentration of pentadecane was higher in early infestation stages (0.82% to 1.03%) than in progressed (0.21%) and over-exploitation stages (0.37%).
Table 1.
Percent (%) composition of volatile emissions from potted (infested) lychee plants and from field-collected (non-infested) lychee tissues (A1–A5; B1–B4).
| # | * RRIexp | ** RRIlit | Compounds | (Mean ± SE), n = 9 | (Mean ± SE), n = 3 | *** IM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | B1 | B2 | B3 | B4 | |||||
| 1 | 856 | 859 | (Z)-3-hexen-1-ol | - | - | - | - | - | 0.16 ± 0.06 | - | - | 0.25 ± 0.25 | MS, RI, Std |
| 2 | 927 | 930 | α-thujene | 0.27 ± 0.16 | 0.71 ± 0.69 | - | - | - | 1.71 ± 1.05 | 1.32 ± 1.22 | - | - | MS, RI |
| 3 | 933 | 939 | α-pinene | 0.70 ± 0.50 | 1.96 ± 1.84 | 0.30 ± 0.30 | 3.73 ± 2.40 | 0.09 ± 0.05 | 1.99 ± 1.17 | 1.45 ± 1.35 | - | 0.02 ± 0.02 | MS, RI, Std |
| 4 | 972 | 975 | sabinene | 5.29 ± 3.25 | 5.56 ± 4.54 | 1.92 ± 1.92 | 11.67 ± 4.94 | 4.50 ± 2.25 | 6.49 ± 2.97 | 4.23 ± 0.69 | 0.18 ± 0.10 | 0.20 ± 0.11 | MS, RI, Std |
| 5 | 980 | 979 | β-pinene | - | - | - | - | - | - | 0.43 ± 0.33 | - | - | MS, RI, Std |
| 6 | 983 | 985 | 6-methyl-5-hepten-2-one | - | - | - | - | - | 0.07 ± 0.06 | - | 0.01 ± 0.01 | 0.12 ± 0.12 | MS, RI, Std |
| 7 | 1005 | 1002 | (E)-3-hexenyl acetate | - | - | - | - | - | 0.54 ± 0.27 | - | - | 0.03 ± 0.03 | MS, RI |
| 8 | 1016 | 1013 | (E)-2-hexenyl acetate | - | - | - | - | - | 0.05 ± 0.04 | - | - | 0.02 ± 0.02 | MS, RI |
| 9 | 1027 | 1029 | limonene | 7.11 ± 2.88 | 3.81 ± 1.24 | 7.94 ± 4.77 | 4.00 ± 1.28 | 4.56 ± 1.72 | 0.02 ± 0.01 | 0.11 ± 0.10 | - | 0.02 ± 0.02 | MS, RI, Std |
| 10 | 1046 | 1050 | (E)-β-ocimene | 0.68 ± 0.48 | 2.00 ± 1.65 | 0.78 ± 0.32 | 0.04 ± 0.04 | 1.54 ± 1.08 | 0.59 ± 0.30 | 5.74 ± 1.14 | 0.03 ± 0.02 | 0.40 ± 0.20 | MS, RI, Std |
| 11 | 1057 | 1059 | γ-terpinene | 0.85 ± 0.44 | 0.83 ± 0.83 | 0.48 ± 0.48 | 1.46 ± 1.46 | 0.27 ± 0.21 | 0.98 ± 0.54 | 0.50± 0.25 | - | - | MS, RI, Std |
| 12 | 1071 | 1072 | cis-linalool oxide (cis-furanoid) | - | - | - | - | - | - | 0.74 ± 0.38 | - | - | MS, RI, Std |
| 13 | 1086 | 1086 | trans-linalool oxide (trans-furanoid) | - | - | - | - | - | - | 3.74 ± 1.90 | - | - | MS, RI, Std |
| 14 | 1088 | 1088 | terpinolene | - | 0.10 ± 0.10 | - | 0.24 ± 0.24 | - | - | - | - | - | MS, RI, Std |
| 15 | 1100 | 1096 | linalool | - | - | - | - | - | - | 1.72 ± 0.31 | - | - | MS, RI, Std |
| 16 | 1101 | 1100 | nonanal | 43.52 ± 8.13 | 24.06 ± 8.19 | 29.31 ± 8.17 | 18.65 ± 6.60 | 51.52 ± 8.97 | - | - | - | - | MS, RI, Std |
| 17 | 1165 | 1174 | cis-linalool oxide (cis-pyranoid) | - | - | - | - | - | 0.01 ± 0.00 | 0.11 ± 0.10 | - | - | MS, RI |
| 18 | 1170 | 1176 | trans-linalool oxide (trans-pyranoid) | - | - | - | - | - | 0.01 ± 0.00 | 1.15 ± 0.58 | - | - | MS, RI |
| 19 | 1194 | 1196 | estragole | 0.10 ± 0.10 | - | - | - | - | - | - | - | - | MS, RI, Std |
| 20 | 1202 | 1201 | decanal | 6.74 ± 2.27 | 2.83 ± 1.40 | 6.11 ± 2.03 | 2.28 ± 1.00 | 7.76 ± 2.66 | - | - | - | - | MS, RI, Std |
| 21 | 1255 | 1263 | (E)-2-decanal | 1.58 ± 0.83 | 0.45 ± 0.30 | 0.27 ± 0.20 | 0.48 ± 0.25 | - | - | - | - | - | MS, RI |
| 22 | 1300 | 1306 | undecanal | 0.30 ± 0.18 | 0.12 ± 0.08 | 0.18 ± 0.10 | 0.12 ± 0.07 | 0.28 ± 0.12 | - | - | - | - | MS, RI |
| 23 | 1330 | 1338 | δ-elemene | - | - | - | - | - | 0.01 ± 0.00 | - | - | 0.03 ± 0.03 | MS, RI |
| 24 | 1342 | 1348 | α-cubebene | - | - | - | - | - | 0.16 ± 0.12 | 0.22 ± 0.21 | 0.42 ± 0.02 | 0.08 ± 0.08 | MS, RI |
| 25 | 1353 | 1360 | (E)-2-undecanal | 0.12 ± 0.09 | 0.09 ± 0.09 | - | 0.06 ± 0.04 | - | - | - | - | - | MS, RI |
| 26 | 1359 | 1371 | cyclosativene | - | - | - | - | - | 1.15 ± 0.28 | 0.63 ± 0.33 | 1.47 ± 0.09 | 1.00 ± 0.02 | MS, RI, Std |
| 27 | 1369 | 1375 | α-ylangene | - | - | - | - | - | 0.11 ± 0.07 | 0.18 ± 0.17 | 0.18 ± 0.09 | 0.10 ± 0.03 | MS, RI |
| 28 | 1367 | 1376 | α-copaene | 1.08 ± 1.05 | 1.73 ± 0.51 | 2.82 ± 0.85 | 1.08 ± 0.26 | 2.23 ± 1.50 | 2.39 ± 0.36 | 2.04 ± 0.26 | 2.86 ± 0.16 | 2.44 ± 0.28 | MS, RI, Std |
| 29 | 1382 | 1391 | 7-epi-sesquithujene | 0.94 ± 0.62 | 3.69 ± 0.79 | 1.44 ± 0.59 | 2.82 ± 0.52 | 1.90 ± 0.38 | 6.30 ± 0.91 | 6.02 ± 1.06 | 6.45 ± 0.07 | 7.32 ± 1.54 | MS, RI |
| 30 | 1389 | 1400 | sibirene | - | - | - | - | - | 0.07 ± 0.03 | 0.30 ± 0.29 | - | 0.02 ± 0.02 | MS, RI |
| 31 | 1390 | 1400 | tetradecane | 3.30 ± 1.35 | 2.16 ± 0.68 | 1.04 ± 0.38 | 1.37 ± 0.67 | 1.85 ± 1.08 | - | - | - | - | MS, RI, Std |
| 32 | 1392 | 1403 | methyl eugenol | 1.41 ± 1.16 | - | - | - | - | - | - | - | - | MS, RI, Std |
| 33 | 1394 | 1405 | sesquithujene | - | - | - | - | - | 0.03 ± 0.02 | 0.20 ± 0.10 | 0.05 ± 0.05 | 0.03 ± 0.03 | MS, RI |
| 34 | 1396 | 1408 | dodecanal | 0.20 ± 0.11 | 0.10 ± 0.05 | 0.16 ± 0.09 | 0.04 ± 0.04 | 0.02 ± 0.02 | - | - | - | - | MS, RI |
| 35 | 1410 | 1419 | β-caryophyllene | 5.69 ± 3.08 | 8.10 ± 1.83 | 10.34 ± 2.89 | 6.23 ± 1.72 | 5.21 ± 2.12 | 12.60 ± 3.69 | 10.57 ± 0.81 | 13.98 ± 0.61 | 13.32 ± 1.53 | MS, RI, Std |
| 36 | 1411 | 1420 | β-cedrene | - | - | - | - | - | 1.04 ± 0.29 | 0.52 ± 0.42 | 1.25 ± 0.06 | 0.55 ± 0.32 | MS, RI |
| 37 | 1423 | 1432 | trans-α-bergamotene | 0.60 ± 0.26 | 1.45 ± 0.39 | 1.13 ± 0.58 | 1.16 ± 0.28 | 0.56 ± 0.25 | 1.75 ± 0.15 | 1.15 ± 0.19 | 2.15 ± 0.06 | 1.58 ± 0.16 | MS, RI |
| 38 | 1428 | 1441 | aromadendrene | - | - | - | - | - | 0.13 ± 0.12 | 0.09 ± 0.08 | 0.32 ± 0.11 | 0.27 ± 0.14 | MS, RI, Std |
| 39 | 1431 | 1444 | 6,9-guaiadiene | 0.02 ± 0.02 | 0.04 ± 0.04 | 0.02 ± 0.02 | 0.01 ± 0.01 | - | - | - | - | - | MS, RI |
| 40 | 1435 | 1450 | cis-muurola 3,5-diene | - | - | - | - | - | 0.11 ± 0.10 | 0.30 ± 0.29 | 0.21 ± 0.06 | 0.20 ± 0.11 | MS, RI |
| 41 | 1439 | 1454 | α-humulene | - | - | - | - | - | 1.66 ± 0.82 | 0.64 ± 0.54 | 2.19 ± 0.19 | 1.45 ± 0.24 | MS, RI, Std |
| 42 | 1442 | 1456 | (E)-β-farnesene | - | - | - | - | - | 0.50 ± 0.23 | 0.71 ± 0.33 | 1.43 ± 0.08 | 0.54 ± 0.27 | MS, RI |
| 43 | 1448 | 1463 | cis-cadina-1(6),4-diene | - | - | - | - | - | 0.07 ± 0.06 | 0 | 0.05 ± 0.04 | 0.07 ± 0.04 | MS, RI |
| 44 | 1453 | 1466 | α-acoradiene | - | - | - | - | - | 0.05 ± 0.02 | 0.35 ± 0.34 | 0.03 ± 0.02 | 0.06 ± 0.06 | MS, RI |
| 45 | 1462 | 1476 | trans-cadina-1(6),4-diene | - | - | - | - | - | 0.64 ± 0.29 | 0.56 ± 0.35 | 1.15 ± 0.06 | 0.55 ± 0.28 | MS, RI |
| 46 | 1466 | 1479 | γ-muurolene | - | - | - | - | - | 7.36 ± 4.41 | 4.85 ± 1.73 | 4.26 ± 0.68 | 7.70 ± 4.98 | MS, RI |
| 47 | 1468 | 1480 | ar-curcumene | 10.25 ± 3.18 | 32.51 ± 6.4 | 30.33 ± 6.38 | 38.54 ± 5.22 | 13.27 ± 3.97 | 16.78 ± 8.30 | 26.50 ± 4.07 | 11.64 ± 3.39 | 21.64 ± 5.33 | MS, RI, Std |
| 48 | 1476 | 1485 | germacrene D | - | - | - | - | - | 0.05 ± 0.04 | - | - | - | MS, RI |
| 49 | 1483 | 1493 | α-zingiberene | 0.24 ± 0.12 | 2.03 ± 0.77 | 1.50 ± 0.79 | 1.72 ± 0.49 | 1.34 ± 0.40 | 27.65 ± 4.89 | 16.50 ± 0.74 | 39.62 ± 1.43 | 33.73 ± 7.23 | MS, RI |
| 50 | 1486 | 1500 | pentadecane | 0.82 ± 0.45 | 1.03 ± 0.48 | 0.21 ± 0.12 | 0.37 ± 0.19 | 0.48 ± 0.32 | - | - | - | - | MS, RI, Std |
| 51 | 1489 | 1500 | α-muurolene | - | - | - | - | - | 0.86 ± 0.27 | 0.66 ± 0.36 | 1.01 ± 0.14 | 1.24 ± 0.06 | MS, RI |
| 52 | 1493 | 1505 | (E,E)-α-farnesene | - | - | - | - | - | 0.56 ± 0.10 | 0.99 ± 0.43 | 0.84 ± 0.02 | 0.60 ± 0.09 | MS, RI, Std |
| 53 | 1500 | 1515 | β-curcumene | - | - | - | - | - | 0.78 ± 0.31 | 0.37 ± 0.27 | 0.92 ± 0.10 | 0.21 ± 0.03 | MS, RI |
| 54 | 1507 | 1522 | β-sesquiphellandrene | - | - | - | - | - | 3.46 ± 0.97 | 2.02 ± 0.15 | 5.94 ± 0.04 | 3.13 ± 0.56 | MS, RI |
| 55 | 1509 | 1523 | δ-cadinene | 0.11 ± 0.07 | 1.12 ± 0.39 | 0.63 ± 0.35 | 2.03 ± 0.45 | 0.17 ± 0.10 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.71 ± 0.05 | 0.03 ± 0.01 | MS, RI |
| 56 | 1547 | 1561 | germacrene B | - | - | - | - | - | 0.07 ± 0.06 | 0.34 ± 0.24 | 0.12 ± 0.07 | 0.13 ± 0.07 | MS, RI |
| 57 | 1586 | 1600 | hexadecane | 2.21 ± 1.06 | 1.48 ± 0.71 | 0.88 ± 0.36 | 0.99 ± 0.52 | 1.29 ± 0.86 | - | - | - | - | MS, RI, Std |
| 58 | 1699 | 1700 | heptadecane | 5.38 ± 4.08 | 1.22 ± 0.81 | 0.85 ± 0.55 | 0.64 ± 0.37 | 0.89 ± 0.58 | - | - | - | - | MS, RI, Std |
| Total % | 99.51 ± 0.18 | 99.18± 0.32 | 98.64 ± 0.64 | 99.73 ± 0.11 | 99.73 ± 0.14 | 99.06 ± 0.91 | 98.05 ± 0.52 | 99.47 ± 0.23 | 99.08 ± 0.43 | ||||
* RIexp: Retention indices (RI) calculated from the current study. ** RRlit: Retention indices (RI) obtained from literature source [Adams Library, 2007]. *** IM: Identification method based on mass spectra (MS); Retention indices (RI) and authentic compounds (Std) on the DB-5 column. -: Not detected. Initial infestation (A1), intermediate infestation (A2), heavy infestation (A3), overexploitation (A4), and healthy, uninfested plant (A5). Floral buds (B1), open flowers (B2), leaf buds (B3), and new leaf shoots (B4).
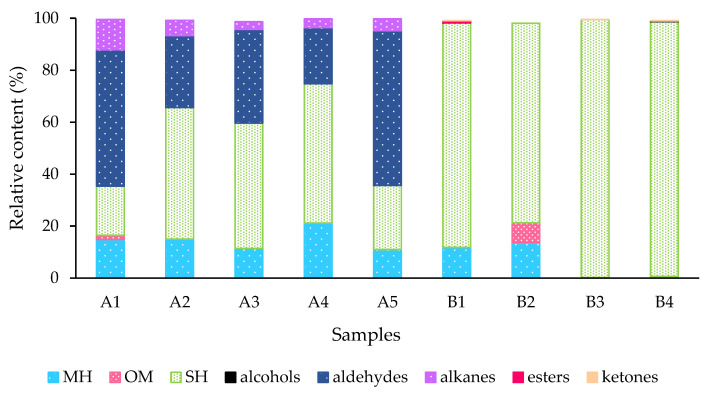
Figure 2.
Abundance (%) of the chemical classes in volatiles from potted (infested) lychee plants and from field-collected (non-infested) lychee tissues. MH- monoterpene hydrocarbons, OM- oxygenated monoterpenes, SH- sesquiterpene hydrocarbons, alcohols, aldehydes, alkanes, esters, and ketones. Initial infestation (A1), intermediate infestation (A2), heavy infestation (A3), overexploitation (A4), and healthy, uninfested plant (A5). Floral buds (B1), open flowers (B2), leaf buds (B3), and new leaf shoots (B4).
Regarding the volatiles from field-collected tissues (B1 to B4), sesquiterpene hydrocarbons were the highest among all categories and represented approximately 77% to 99% of the total volatile profile in flower buds (86.44%), open flowers (76.81%), leaf buds (99.25%), and new leaf shoot (98.02%, Figure 2). Major SHs identified were zingiberene (16.50% to 39.62%), ar-curcumene (11.64% to 26.50%), β-caryophyllene (10.57% to 13.98%), γ-muurolene (4.26% to 7.70%), β-sesquiphellandrene 2.02% to 5.94%), α-copaene (2.04% to 2.86%), α-humulene (0.64% to 2.19%) and trans-α-bergamotene (1.15% to 2.15%). Monoterpene hydrocarbons (MHs), α-thujene (1.71% and 1.2%), α-pinene (1.99% and 1.45%), sabinene (6.49% and 4.23%), and (E)-β-ocimene (0.59% and 5.74%) were present mostly in flower buds and open flowers, respectively, while β-pinene (0.43%) was only found in open flowers. Limonene was about 5.5 times more abundant in open flowers than flower buds and new leaf shoots. From the group of oxygenated monoterpenes (OMs), OM linalool and its cis- and trans-linalool oxides (furanoid) were only detected in open flowers (Table 1).
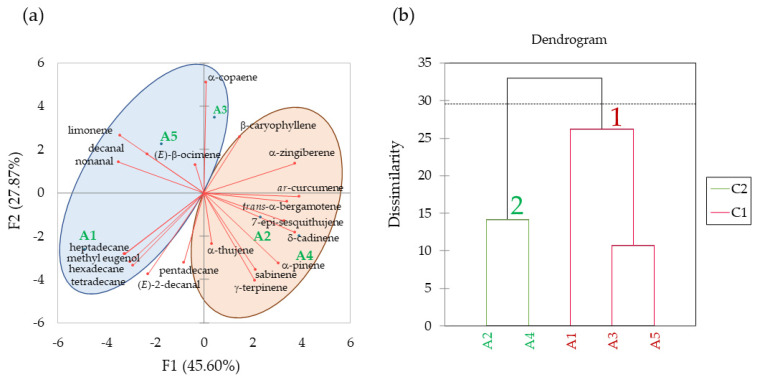
Principal component analysis (PCA) was carried out to identify the grouping pattern among potted (infested) lychee plants (Figure 3) and field-collected (non-infested) samples (Figure 4) based on volatile compounds obtained from GC-MS data (Table 1). For better distinction between the groups, we focused on the proportions of the volatile compounds. Based on the Eigenvectors criterion, principal components with an eigenvalue greater than one are considered important. First, PCA analysis was applied based on the GC-MS data from samples A1 to A5. Principal components F1 and F2 had higher Eigenvectors of 9.58 and 5.85, respectively (Table 2). Two macro principal components accounted for 45.60% (F1) and 27.87% (F2) representing 73.47% of the total variance, indicating that the F1 and F2 had good support for the difference in the plant tissues (Figure 3a).
Figure 3.
Panel (a) shows the Biplot Analysis for Principal Component Analysis (PCA) of volatile compounds emitted from potted lychee plants under different levels of LEM infestation (A1 to A5). Panel (b) shows the Agglomerative Hierarchical Cluster (AHC) analysis for LEM infestation samples A1 to A5 based on selected volatile active compounds. Initial infestation (A1), intermediate infestation (A2), heavy infestation (A3), overexploitation (A4), and healthy, uninfested plant (A5).
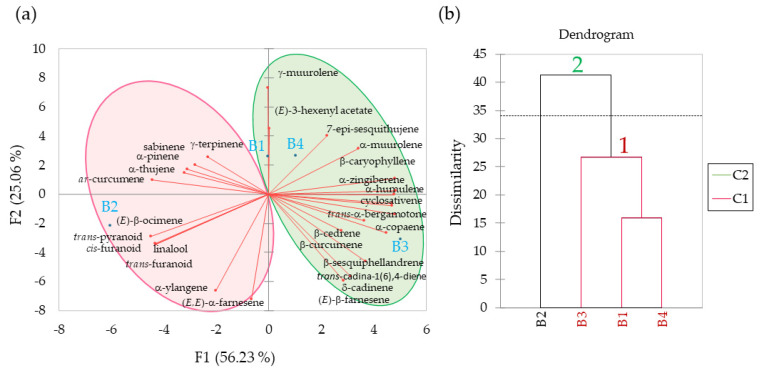
Figure 4.
Panel (a) shows the Biplot Analysis for Principal Component Analysis (PCA) of volatile compounds emitted from field-collected (non-infested) lychee tissues (B1 to B4). Panel (b) shows the Agglomerative Hierarchical Cluster (AHC) analysis for samples B1 to B4 based on selected volatile active compounds. Floral buds (B1), open flowers (B2), leaf buds (B3), and new leaf shoots (B4).
Table 2.
Eigenvalues for principal components F1 to F4 (A1 to A5).
| Principal Components | Eigenvalue | Proportion of Total Variance (%) | Cumulative Proportion of Total Variance (%) |
|---|---|---|---|
| F1 | 9.575 | 45.597 | 45.597 |
| F2 | 5.852 | 27.868 | 73.465 |
| F3 | 3.424 | 16.307 | 89.772 |
| F4 | 2.148 | 10.228 | 100.00 |
Table 3 shows the percent contribution and squared cosine values (cos2) of each principal component. The percent contribution of each variable shows the most important variables. Also, the cos2 values indicate the potential importance of components for a given observation. Taking the values of cos2 and the percent contribution, nonanal (7.63%; 0.73), decanal (7.44%; 0.71), ar-curcumene (9.57%; 0.92), α-zingiberene (8.59%; 0.82) and δ-cadinene (8.60%; 0.82) showed a strong impact on the F1, while α-copaene (16.13%; 0.94) showed the strongest impact on the F2. Cos2 for F1 was the highest impact on the observation of initial infestation A1 (0.758) and overexploitation A4 (0.614); heavy infestation A3 (0.724) was the highest impact on F2.
Table 3.
Relative contributions and squared cosines of each principal component to samples A1 to A5.
| Variables | Contribution of the Variables (%) | Squared Cosines of the Variables * | ||||||
|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F1 | F2 | F3 | F4 | |
| α-thujene | 0.059 | 3.461 | 20.992 | 3.395 | 0.006 | 0.203 | 0.719 | 0.073 |
| α-pinene | 5.847 | 6.635 | 1.363 | 0.241 | 0.560 | 0.388 | 0.047 | 0.005 |
| sabinene | 2.758 | 7.930 | 3.957 | 6.345 | 0.264 | 0.464 | 0.136 | 0.136 |
| limonene | 3.377 | 1.984 | 3.633 | 20.304 | 0.323 | 0.116 | 0.124 | 0.436 |
| (E)-β-ocimene | 0.075 | 1.052 | 26.445 | 1.193 | 0.007 | 0.062 | 0.906 | 0.026 |
| γ-terpinene | 2.717 | 10.184 | 3.936 | 0.424 | 0.260 | 0.596 | 0.135 | 0.009 |
| nonanal | 7.633 | 1.236 | 0.217 | 8.815 | 0.731 | 0.072 | 0.007 | 0.189 |
| decanal | 7.440 | 4.369 | 0.172 | 1.209 | 0.712 | 0.256 | 0.006 | 0.026 |
| (E)-2-decanal | 3.320 | 8.718 | 0.547 | 7.133 | 0.318 | 0.510 | 0.019 | 0.153 |
| α-copaene | 0.005 | 16.126 | 0.816 | 1.298 | 0.000 | 0.944 | 0.028 | 0.028 |
| 7-epi-sesquiterpene | 6.753 | 1.033 | 7.608 | 1.507 | 0.647 | 0.060 | 0.261 | 0.032 |
| tetradecane | 5.307 | 6.955 | 2.478 | 0.000 | 0.508 | 0.407 | 0.085 | 0.000 |
| methyl eugenol | 6.544 | 5.017 | 0.560 | 2.819 | 0.627 | 0.294 | 0.019 | 0.061 |
| β-caryophyllene | 1.347 | 4.182 | 0.449 | 28.441 | 0.129 | 0.245 | 0.015 | 0.611 |
| trans-aα-bergamotene | 7.245 | 0.099 | 2.819 | 9.497 | 0.694 | 0.006 | 0.097 | 0.204 |
| ar-curcumene | 9.565 | 0.020 | 0.217 | 3.519 | 0.916 | 0.001 | 0.007 | 0.076 |
| α-zingiberene | 8.588 | 1.147 | 2.987 | 0.385 | 0.822 | 0.067 | 0.102 | 0.008 |
| pentadecane | 0.422 | 6.462 | 16.937 | 0.067 | 0.040 | 0.378 | 0.580 | 0.001 |
| δ-cadinene | 8.596 | 2.092 | 1.590 | 0.002 | 0.823 | 0.122 | 0.054 | 0.000 |
| hexadecane | 5.762 | 6.300 | 2.239 | 0.136 | 0.552 | 0.369 | 0.077 | 0.003 |
| heptadecane | 6.640 | 5.000 | 0.040 | 3.269 | 0.636 | 0.293 | 0.001 | 0.070 |
| Total | 100.000 | 100.000 | 100.000 | 100.000 | 9.575 | 5.852 | 3.424 | 2.148 |
* Values in bold correspond for each variable to the factor for which the squared cosine is the largest.
Agglomerative Hierarchical Clustering (AHC) shows the similarity between existing and merged clusters. Different LEM infestation levels were classified into 2 clusters (Figure 3b). Cluster 1 includes initial infestation (A1), heavy infestation (A3), and uninfested plant (A5), and the second cluster included intermediate infestation (A2) and overexploitation (A4). Compounds limonene, nonanal, decanal, β-caryophyllene, ar-curcumene showed a strong impact on cluster 1, while compounds α-pinene, sabinene, limonene, β-caryophyllene, ar-curcumene showed a strong impact on the cluster 2 (Table 4).
Table 4.
Results of cluster analysis for samples A1 to A5.
| Results by Cluster | Cluster 1 | Cluster 2 |
|---|---|---|
| Objects | A1, A3, A5 | A2, A4 |
| Sum of weights | 3 | 2 |
| Within-class variance | 270.81 | 59.25 |
| Maximum distance to centroid |
17.97 | 5.44 |
| Cluster centroids > 1.0 | sabinene (3.90), limonene (6.54), (E)-β-ocimene (1.00), α-copaene (2.04), nonanal (41.45), decanal (6.87), 7-epi-sesquithujene (1.43), tetradecane (2.06), β-caryophyllene (7.08), trans-α-bergamotene (0.76), ar-curcumene (17.95), α-zingiberene (1.03), hexadecane (1.46), heptadecane (2.37) |
α-pinene (2.84), sabinene (8.62), limonene (3.91) (E)-β-ocimene (1.02), γ-terpinene (1.15), nonanal (21.36), decanal (2.56), α-copaene (1.41), 7-epi-sesquithujene (3.26), tetradecane (1.77), β-caryophyllene (7.12), trans-α-bergamotene (1.31), ar-curcumene (35.53), α-zingiberene (1.88), δ-cadinene (1.58), hexadecane (1.24) |
PCA analysis was applied based on the GC-MS data from samples B1 to B4. The results showed three best eigenvectors of 15.74 (F1), 7.02 (F2), and 5.24 (F3), describing 56.23%, 25.06%, and 18.71% of the total variance, respectively (Table 5). Most variables were accumulated at 81.29% in the principal components F1 and F2 (Figure 4a).
Table 5.
Eigenvalues for principal components F1 to F3 (B1 to B4).
| Principal Components | Eigenvalue | Proportion of Total Variance (%) | Cumulative Proportion of Total Variance (%) |
|---|---|---|---|
| F1 | 15.743 | 56.227 | 56.227 |
| F2 | 7.018 | 25.065 | 81.292 |
| F3 | 5.238 | 18.708 | 100.000 |
Cos2 values from field-collected (non-infested) lychee tissues (B1 to B4) on the F1 axis were strongly correlated with (E)-β-ocimene (0.84), cyclosativene (0.92), α-copaene (0.96), β-caryophyllene (0.97), trans-α-bergamotene (0.92), α-humulene (0.95), ar-curcumene (0.82), α-zingiberene (0.97) and β-sesquiphellandrene (0.84), which contributed 5.34%, 5.85%, 6.12%, 6.15%, 5.85%, 6.03%, 5.23%, 6.19%, and 5.34%, respectively, while cos2 values on the F2 axis strongly correlated with α-ylangene (0.98), γ-muurolene (0.99), (E,E)-α-farnesene (0.82), which contributed 13.90%, 14.16%, and 11.64%, respectively (Table 6).
Table 6.
Relative contributions and squared cosines of each principal component to samples B1 to B4.
| Variables | Contribution of the Variables (%) | Squared Cosines of the Variables * | ||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F1 | F2 | F3 | |
| α-thujene | 2.737 | 0.580 | 10.088 | 0.431 | 0.041 | 0.528 |
| α-pinene | 2.540 | 0.766 | 10.428 | 0.400 | 0.054 | 0.546 |
| sabinene | 2.087 | 1.079 | 11.372 | 0.329 | 0.076 | 0.596 |
| (E)-3-hexenyl acetate | 0.000 | 5.361 | 11.907 | 0.000 | 0.376 | 0.624 |
| (E)-β-ocimene | 5.340 | 2.221 | 0.066 | 0.841 | 0.156 | 0.003 |
| γ-terpinene | 1.412 | 1.709 | 12.555 | 0.222 | 0.120 | 0.658 |
| cis-linalool oxide | 4.916 | 3.121 | 0.136 | 0.774 | 0.219 | 0.007 |
| trans-linalool oxide | 4.916 | 3.121 | 0.136 | 0.774 | 0.219 | 0.007 |
| linalool | 4.916 | 3.121 | 0.136 | 0.774 | 0.219 | 0.007 |
| trans-linalool oxide | 4.944 | 3.072 | 0.114 | 0.778 | 0.216 | 0.006 |
| cyclosativene | 5.852 | 0.104 | 1.363 | 0.921 | 0.007 | 0.071 |
| α-ylangene | 0.117 | 13.896 | 0.121 | 0.018 | 0.975 | 0.006 |
| α-copaene | 6.123 | 0.499 | 0.019 | 0.964 | 0.035 | 0.001 |
| 7-epi-sesquithujene | 1.313 | 4.287 | 9.402 | 0.207 | 0.301 | 0.493 |
| β-caryophyllene | 6.146 | 0.300 | 0.216 | 0.968 | 0.021 | 0.011 |
| β-cedrene | 3.488 | 0.880 | 7.428 | 0.549 | 0.062 | 0.389 |
| trans-α-bergamotene | 5.854 | 0.160 | 1.281 | 0.922 | 0.011 | 0.067 |
| α-humulene | 6.027 | 0.000 | 0.976 | 0.949 | 0.000 | 0.051 |
| (E)-β-farnesene | 2.140 | 9.446 | 0.005 | 0.337 | 0.663 | 0.000 |
| trans-cadina-1(6),4-diene | 3.593 | 5.625 | 0.756 | 0.566 | 0.395 | 0.040 |
| γ-muurolene | 0.000 | 14.161 | 0.118 | 0.000 | 0.994 | 0.006 |
| ar-curcumene | 5.230 | 0.259 | 3.023 | 0.823 | 0.018 | 0.158 |
| α-zingiberene | 6.186 | 0.013 | 0.482 | 0.974 | 0.001 | 0.025 |
| α-muurolene | 3.120 | 2.628 | 6.191 | 0.491 | 0.184 | 0.324 |
| (E,E)-α-farnesene | 1.071 | 11.638 | 0.281 | 0.169 | 0.817 | 0.015 |
| β-curcumene | 2.018 | 1.685 | 10.766 | 0.318 | 0.118 | 0.564 |
| β-sesquiphellandrene | 5.343 | 1.860 | 0.539 | 0.841 | 0.131 | 0.028 |
| δ-cadinene | 2.571 | 8.410 | 0.096 | 0.405 | 0.590 | 0.005 |
| Total | 100.000 | 100.000 | 100.000 | 15.743 | 7.018 | 5.238 |
* Values in bold correspond for each variable to the factor for which the squared cosine is the largest.
AHC was performed on the volatile composition obtained from field-collected samples B1 to B4. Samples of floral buds (B1), leaf buds (B3), and new leaf shoots (B4) were grouped into the same cluster, in agreement with the PCA results, while the open flower sample (B2) was clustered alone (Figure 4b). Maximum distance to centroid was found for cluster 1 of 8.73 between two clusters, indicating the similarity of chemical profiles among the B1, B3 and B4 samples (Table 7). Cluster 2 showed maximum distances to centroid was zero, indicating it was closed to cluster 1. Compounds α-zingiberene, ar-curcumene and β-caryophyllene showed a strong impact on cluster 1 and 2. This finding was also in accordance with the experimental data detected by GC-MS (Table 1).
Table 7.
Results of cluster analysis for samples B1 to B4.
| Results by Cluster | Cluster 1 | Cluster 2 |
|---|---|---|
| Objects | B1, B3, B4 | B2 |
| Sum of weights | 3 | 1 |
| Within-class variance | 84.80 | 0.00 |
| Maximum distance to centroid | 8.73 | 0.00 |
| Cluster centroids > 1.0 | sabinene (2.29), cyclosativene (1.21), α-copaene (2.56), 7-epi-sesquithujene (6.69), β-caryophyllene (13.30), α-humulene (1.77), γ-muurolene (6.44), ar-curcumene (16.69), α-zingiberene (33.67), α-muurolene (1.04),β-sesquiphellandrene (4.18) | α-thujene (1.32), α-pinene (1.45), sabinene (4.23), (E)-β-ocimene (5.74), trans-linalool oxide (3.74), linalool (1.72), trans-linalool oxide (1.15), α-copaene (2.04), 7-epi-sesquithujene (6.02), β-caryophyllene (10.57), trans-α-bergamotene (1.15), γ-muurolene (4.85), ar-curcumene (26.50), α-zingiberene (16.50), β-sesquiphellandrene (2.02) |
3.2. LEM Behavioral Responses to Field-Collected (Non-Infested) Lychee Tissues and Single Volatile Compounds
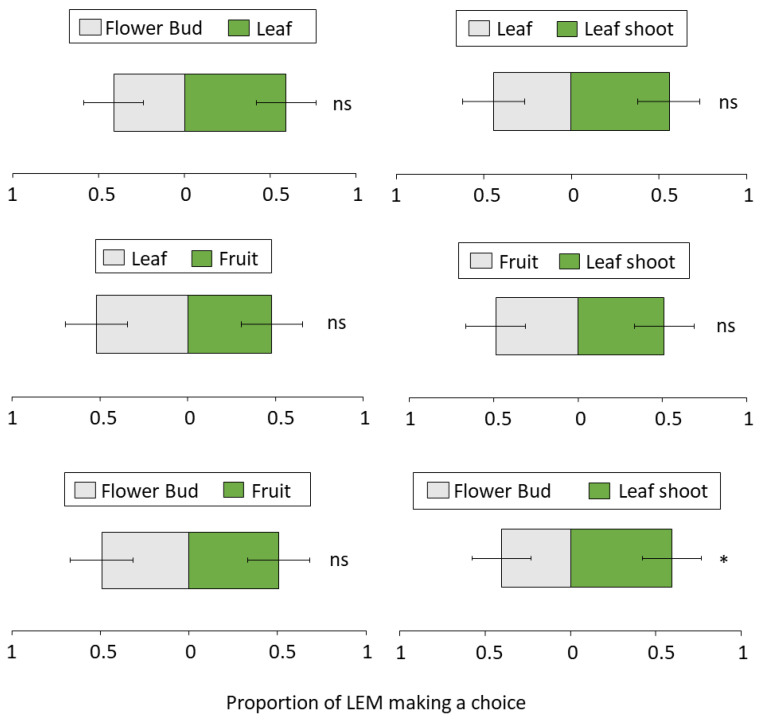
In almost all cases, when given a choice between two field-collected (non-infested) lychee tissues (control groups), there was no significant difference in the number of LEM that reached both sources. Except when presented with a choice between leaf shoot vs. leaf shoot there was a slightly significant difference (p = 0.04) towards one of the sides (Figure S2). For the other groups tested, fruit vs. fruit (p = 0.1), leaf vs. leaf (p = 0.1) and bud vs. bud (p = 0.31) there was no difference between choices made by LEM. When different plant tissues were offered, LEM showed a preference for leaf shoot over buds (p = 0.04, Figure 5). No clear choice was observed for the other treatments, leaf vs. fruit (p = 0.61), leaf vs. bud (p = 0.12), leaf vs. leaf shoot (p = 0.31), fruit vs. bud (p = 0.31) and fruit vs. leaf shoot (p = 0.1).
Figure 5.
Preference of LEM when offered different field-collected (non-infested) lychee tissues. Panel shows LEM choice when offered different combinations of plant tissues (leaf vs. fruit, leaf vs. bud, leaf vs. leaf shoot, fruit vs. bud, fruit vs. leaf shoot, leaf shoot vs. leaf shoot, N = 8). * GLMM: p < 0.05; ns, not significant. Bars indicate proportion (±SE) of mites reaching the end section of the slide (Sections 3 and 4 and TAPE) towards each plant tissue in 24 h.
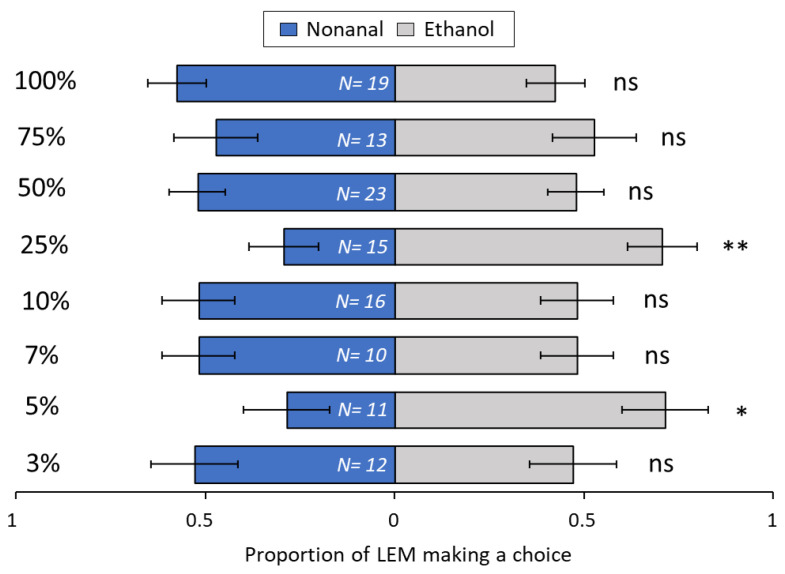
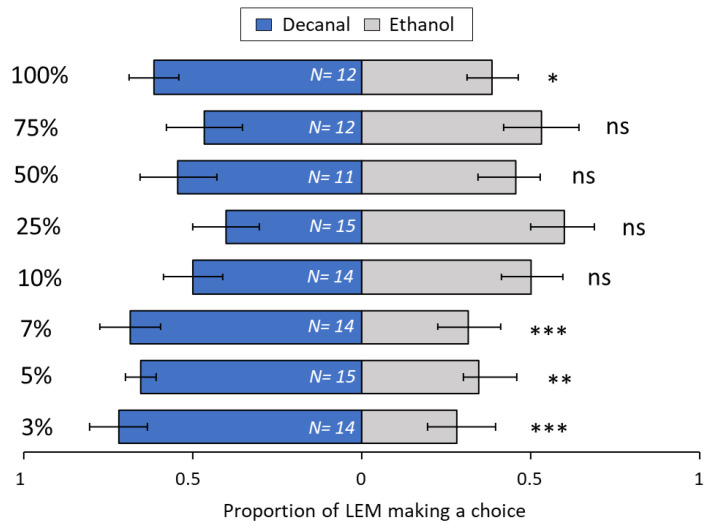
In dual-choice assays between single volatile compounds, the concentration was the most important factor determining LEM attraction or repellence. The most repellent compound, aliphatic aldehyde nonanal (Figure 6), repelled over 70% of LEM at 5% (p = 0.02) and 25% nonanal (p = 0.003) concentrations. For the other concentrations of nonanal, 3% (p = 0.10), 7% (p = 0.65), 10% (p = 0.28), 50% (p = 0.13), 75% (p = 0.10) and 100% (p = 0.17), LEM choice was not significantly different between nonanal and the solvent control (Ethanol). Several concentrations of aliphatic aldehyde decanal (Figure 7), were attractive to LEM: 3% (p ≤ 0.001), 5% (p = 0.009), 7% (p ≤ 0.001) and 100% (p ≤ 0.04). However, no choice was observed between the control and decanal at 10% (p = 0.69), 25% (p = 0.14), 50% (p = 0.1) and 75% (p = 0.68).
Figure 6.
Preference of LEM to the volatile compound nonanal and its solvent ethanol (control), in a dual-choice experimental unit. Panel shows eight Nonanal concentrations tested: 3%, 5%, 7%, 10%, 25%, 50%, 75% and 100%. Numbers inside bars represent number of replicates; GLMM: * p < 0.05; ** p < 0.01; ns, not significant. Bars indicate the proportion (±SE) of mites reaching the end section of the slide towards nonanal and ethanol in 24 h.
Figure 7.
Preference of LEM to the volatile compound decanal and its solvent ethanol (control), in a dual-choice experimental unit. Panel shows all eight Decanal concentrations tested: 3%, 5%, 7%, 10%, 25%, 50%, 75% and 100%. Numbers inside bars represent number of replicates; GLMM: * p < 0.05; ** p < 0.01, *** p < 0.001; ns, not significant. Bars indicate proportion (±SE) of mites reaching the end section of the slide towards decanal and ethanol in 24 h.
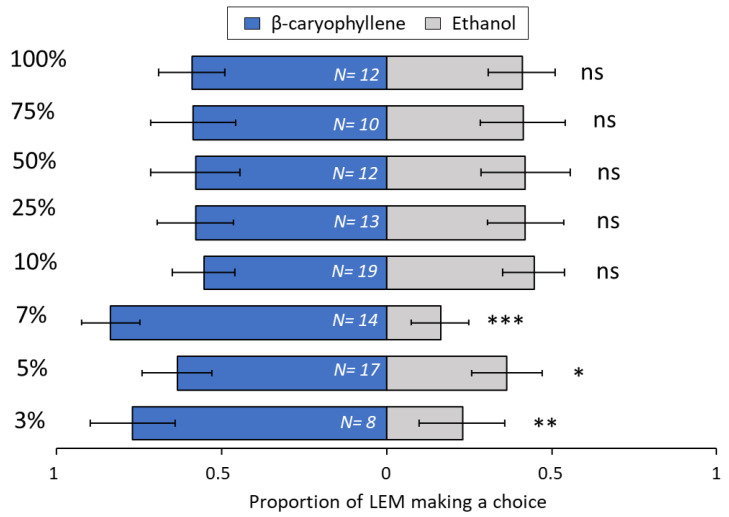
Among the terpenoids tested, the sesquiterpene β-caryophyllene (Figure 8) was very attractive to LEM in lower concentrations, at 3% (p = 0.008), 5% (p = 0.01) and 7% (p ≤ 0.001), but attractiveness was lost with increased concentrations, i.e., 10% (p = 0.19), 25% (p = 0.69), 50% (p = 0.36), 75% (p = 0.34) and 100% (p = 0.09). For the sesquiterpene ar-curcumene (Figure 9), only two concentrations were attractive to LEM, 10% (p = 0.001) and 100% (p = 0.002). All other concentrations were not attractive: 3% (p = 0.27), 5% (p = 0.1), 7% (p = 0.1), 25% (p = 0.27), 50% (p = 0.1) and 75% (p = 0.31). The monoterpene limonene (Figure 10) at 3% concentration (p = 0.008) was repellent to LEM i.e., more mites chose EtOH over the limonene. By contrast, three concentrations, 10% (p = 0.04), 25% (p = 0.05) and 75% (p = 0.01), were attractive to LEM, while all other tested concentrations were not attractive nor repellent: 5% (p = 0.10), 7% (p = 0.10), 50% (p = 0.69) and 100% (p = 0.14). For the monoterpene sabinene (Figure 11), the concentrations of 5% (p = 0.03), 7% (p ≤ 0.001), 10% (p = 0.02) and 50% (p = 0.003) were attractive, the concentration of 75% was repellent (p = 0.02), and the concentrations 3% (p = 0.28), 25% (p = 0.71) and 100% (p = 0.47) were not attractive nor repellent. When given a choice between same odor sources in both ends of the slide (clean vs. clean or ethanol vs. ethanol), there was no significant difference in the number of LEM that reached each source (p = 0.15 and p = 1, respectively) (Figure S3).
Figure 8.
Preference of LEM to the volatile compound β-caryophyllene and its solvent ethanol (control), in a dual-choice experimental unit. Panel shows all eight β-caryophyllene concentrations tested: 3%, 5%, 7%, 10%, 25%, 50%, 75% and 100%. Numbers inside bars represent number of replicates; GLMM: * p < 0.05; ** p < 0.01, *** p < 0.001; ns, not significant. Bars indicate proportion (±SE) of mites reaching the end section of the slide towards β-caryophyllene and ethanol in 24 h.
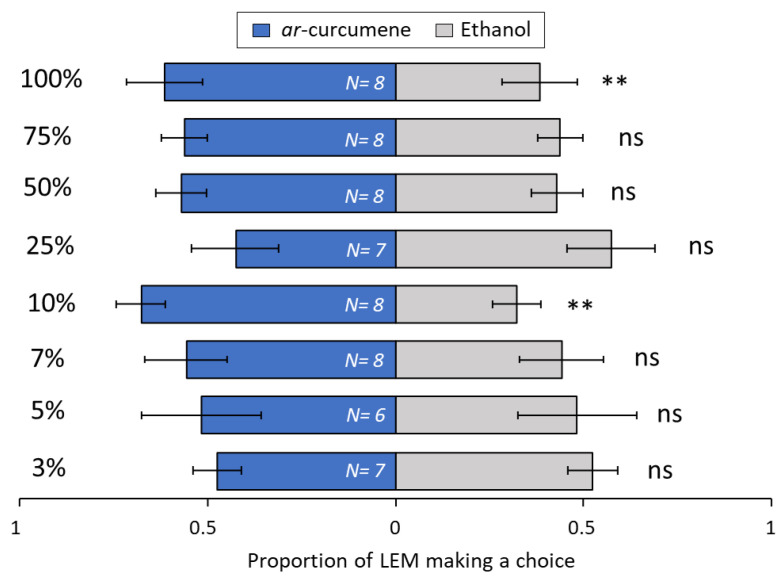
Figure 9.
Preference of LEM to the volatile compound ar-curcumene and its solvent ethanol (control), in a dual-choice experimental unit. Panel shows all eight ar-curcumene concentrations tested: 3%, 5%, 7%, 10%, 25%, 50%, 75% and 100%. Numbers inside bars represent number of replicates; GLMM: ** p < 0.01; ns, not significant. Bars indicate proportion (±SE) of mites reaching the end section of the slide towards ar-curcumene and ethanol in 24 h.
Figure 10.
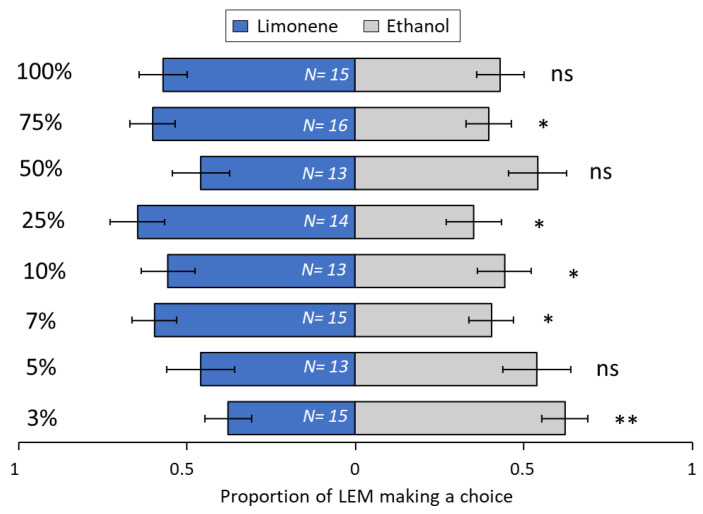
Preference of LEM to the volatile compound limonene and its solvent ethanol (control), in a dual-choice experimental unit. Panel shows all eight Limonene concentrations tested: 3%, 5%, 7%, 10%, 25%, 50%, 75% and 100%. Numbers inside bars represent number of replicates; GLMM: * p < 0.05; ** p < 0.01; ns, not significant. Bars indicate proportion (±SE) of mites reaching the end section of the slide towards limonene and ethanol in 24 h.
Figure 11.
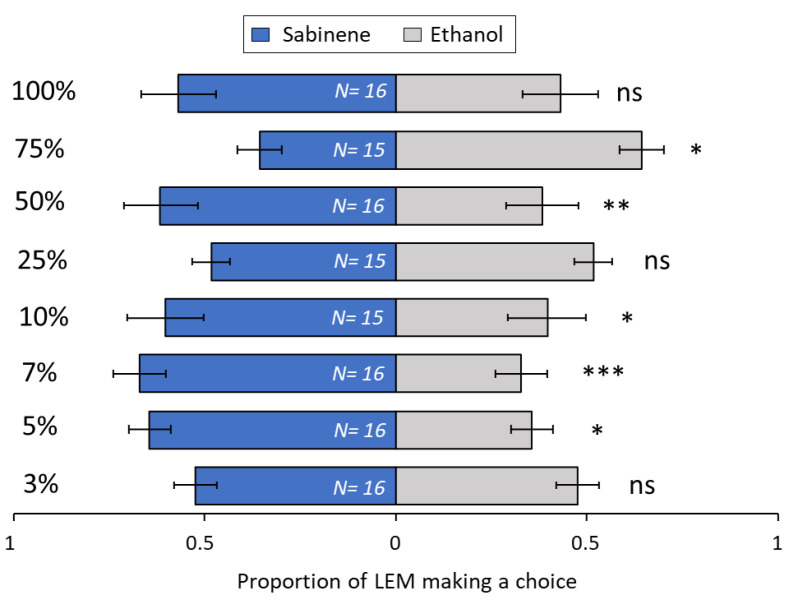
Preference of LEM to the volatile compound sabinene and its solvent ethanol (control), in a dual-choice experimental unit. Panel shows all eight Sabinene concentrations tested: 3%, 5%, 7%, 10%, 25%, 50%, 75% and 100%. Numbers inside bars represent number of replicates; GLMM: * p < 0.05; ** p < 0.01, *** p < 0.001; ns, not significant. Bars indicate proportion (±SE) of mites reaching the end section of the slide towards sabinene and ethanol in 24 h.
4. Discussion
Using HS-SPME collections and GC-MS analyses, 58 volatile compounds were identified from lychee, of which 44 were terpenoids (Table 1). There were large quantitative and qualitative differences in the blend of volatiles released from distinct plant tissues (B1–B4) and among plants with varying LEM infestation levels (A1–A5). Uninfested field-collected samples obtained from floral buds (B1), open flowers (B2), leaf buds (B3), and new leaf shoots (B4) had 30 unique compounds, mostly terpenoids (Table 1, Figure 2), that were not detected in LEM infested lychee plants. The PCA analysis applied based on the GC-MS data from samples B1 to B4 showed that these plant tissues can be clustered in two groups (Figure 4). The sesquiterpenes α-zingiberene, ar-curcumene and β-caryophyllene were the most important volatiles separating floral buds (B1), leaf buds (B3) and new leaf shoots (B4) from the open flowers (B2). Although sesquiterpene hydrocarbons were the most abundant compounds detected in these plant tissues, the monoterpene alcohol linalool and its cis- and trans-linalool oxides were only detected in open flowers (B2). The monoterpene hydrocarbons α-thujene, α-pinene, sabinene and (E)-β-ocimene were present in higher quantities in the floral buds (B1) and open flowers (B2) than in the other plant tissues. The sesquiterpene hydrocarbons β-caryophyllene and α-zingiberene were mostly detected in leaf buds (B3) and new leaf shoots (B4).
In agreement with our findings, previous studies have also found that the most abundant compounds present in lychee samples were terpenoids. Wu et al. [44] identified 43 volatiles in lychee fruit from nine lychee cultivars and found remarkable qualitative and quantitative differences among cultivars. Nonetheless, lychee cultivars held different terpene profiles that played an important role in discriminating them. For instance, only 11 terpenoids were found in ‘Xiangli’ and ‘Jizuili’, while ‘Guangxi Huaizhi’ and ‘Guangdong Huaizhi’ had 30 and 27 terpenoids, respectively. Linalool, cis-rose oxide, α-terpineol, β-citronellol, geraniol, and p-cymene were identified in all lychee cultivars, while α-copaene and cis-pyran linalool oxide were found only in ‘Nuomici’ and ‘Guiwei’, respectively. Besides terpenoids, they also found ester aldehydes and alcohols in less abundance. By using a solvent-assisted flavor evaporation (SAFE) coupled with gas chromatography-olfactometry/mass spectrometry (GC-O/MS), Feng et al. [45] identified 31 volatiles in lychee fruit with methional, geraniol, furaneol, nerol and linalool pointed as the most abundant compounds. Similarly, Chyau et al. [52] reported alcohols and terpenoids as major components of lychee, and Sivakumar et al. [53] reported that nearly all the volatiles from lychee fruit cv. Mauritius and McLean’s Red were terpenoids or derivatives. Nerol, citronellol, geraniol, linalool, limonene, terpinolene, myrcene, germacrene D, β-ocimene, β-myrcene, α-muurolene and curcumene were the compounds identified as responsible for the characteristic odor of ‘Mauritius’ fruit [47,53]. By using simultaneous distillation extraction (SDE), Wang et al. [48] extracted 13 terpenoids, 3 aldehydes, 1 acid, 2 alcohols and 1 ester from young and old lychee leaves. Major terpenoids found were zingiberene, trans-caryophyllene, α-sesquiphellandrene, α-humulene, α-bergamotene and α-copaene. The terpenoids δ-elemene and L-linalool were only detected in young leaves.
In our subsequent experiment using potted plants (A1–A5), 14 unique compounds were found, mostly aldehydes and alkanes (Table 1, Figure 2). In the PCA analysis from samples A1 to A5, the abundant aldehydes nonanal and decanal were found to have a strong discrimination effect among the LEM infestation levels, separating them into two clusters (Figure 3). The first cluster included the initial infestation (A1), heavy infestation (A3) and uninfested plant (A5) treatments, and the second cluster included the intermediate infestation (A2) and overexploitation (A4) treatments. In addition, the terpenoids limonene, β-caryophyllene, ar-curcumene and α-zingiberene also contributed greatly to discriminate among LEM infestation levels. Hence, the terpenoids β-caryophyllene, ar-curcumene and α-zingiberene were found to play an important role in the discrimination among both the infested and uninfested lychee plants (A), besides their participation in the discrimination among the field-collected tissues (B). Importantly, while the levels of the most abundant aldehydes (nonanal, decanal) decreased with the progression of the LEM infestation, the most abundant terpenoids (sabinene, limonene, ar-curcumene and β-caryophyllene) and alkanes (tetradecane, hexadecane, heptadecane) increased in the presence of LEM, suggesting that those major compounds may play an important role in lychee plant defense and LEM attraction/repellence for these compounds might differ.
Recently, Gunpal and Patni [54] showed that lychee leaflets infested by LEM were rich in sugars, terpenoids, linoleic acid esters, vitamins, antioxidants, glycerol, and steroids. Our results were different from those reported by Gunpal and Patni [54] and we attribute these differences to the sampling methods. They used dried, powdered leaves extracted by Soxhlet apparatus with methanol. Our analyses using the HS-SPME method possess unsurpassed sensitivity and specificity in volatile sampling. In addition, we used authentic standards to confirm the identity of the compounds, while Gunpal and Patni [54] based their identification solely on the mass spectra of peaks. Second, our study offers further knowledge, demonstrating for the first time that the volatile profile of LEM infested lychee plants changes according to LEM infestation level. It has been shown by Popitanu et al. [71] that the erineum-forming mite Aceria erinea induced significant emissions of terpenoids in Persian walnut leaves (Juglans regia) and these emissions scaled positively with the percentage of the infested leaf area. In agreement with these findings, our study showed that terpenoids correlated positively with the LEM progression of infestation. These findings raised the hypothesis that LEM responses to the main terpenoids emitted by lychee are dose dependent. Aiming to address this question, we designed a dual-choice arena (Figure 1) to investigate the LEM preferences when offering the terpenoids β-caryophyllene, ar-curcumene, limonene and sabinene on different concentrations. In fact, we confirmed our hypothesis by showing that key terpenoids played an important role in LEM attraction or repellence, depending on their concentration (Figure 8, Figure 9, Figure 10 and Figure 11).
Terpenoid VOCs play an important role in direct and indirect plant defenses [3]. These compounds can be toxic and repellent to the attacking insects [72] and mites [73], and can attract natural enemies of herbivores [72,74]. For example, the terpenoids α-pinene, eugenol, limonene, and terpinolene repel several mosquito species [75,76], and α-pinene repels the tick Ixodes ricinus [77]. Additionally, the sesquiterpene β-caryophyllene has been shown to repel mosquitoes [78] and the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae) [79]. Terpenoids can also act as elicitors that trigger plant defense mechanisms to target pests. For instance, tomato plants exposed to the volatiles of transgenic tobacco enriched in β-ocimene decreased aphid, Macrosiphum euphorbiae, development and reproduction (direct defense) and increased attraction of its natural enemy, the parasitoid Aphidius ervi (indirect defense) [72]. Similarly, by inhibiting the emission of terpenoid HPIVs, Mumm et al. [74] demonstrated that the inhibition of those terpenoids in mite-infested plants decreased the attractiveness of the predatory mite Phytoseiulus persimilis.
Like terpenoids, aldehydes can also act as specific elicitor molecules emitted after herbivory, pathogen infection, or oxidative stresses [80,81]. For example, Yi et al. [81] showed that Lima bean plants growing adjacent to plants chemically induced with benzothiadiazole up-regulated the production of nonanal and were more resistant to infection by the pathogen Pseudomonas syringae. In insect-plant systems, infestation by the peach aphid (Myzus persicae) and the Colorado potato beetle (CPB), (Leptinotarsa decemlineata) drastically increased the amounts of nonanal in attacked plants [82]. The increased amounts of nonanal in potato plants infested with CPB played an important role in the attraction of the two predators, Podisum maculiventris and Perillus bioculatus [80]. However, nonanal has also been shown to increase the attraction and oviposition of Helicoverpa assulta (Lepidoptera: Noctuidae) moths in tobacco plants [83]. In the current study, the emissions of nonanal and decanal decreased with increasing LEM infestation (Table 1). Although the results from the behavioral assays were inconsistent with increasing doses of nonanal and decanal (Figure 6 and Figure 7), in the trials with lower concentrations of decanal (3, 5, and 7%) the treated-side was more attractive. Taken together, these data may suggest that decanal may initially elicit attraction to LEM, and as infestation progresses and decanal emissions increase, infested tissues may become less attractive to LEM.
The attractiveness of volatile odors to most insects and mites is dose dependent [84,85,86,87]. Similarly, in the present study, we found that the compound concentration was the most important factor determining attraction or repellence of LEM. A trend towards attraction of LEM at low and intermediate concentration and repellence at high concentration was found in three out of the six volatiles tested. Decanal (Figure 7), β-caryophyllene (Figure 8) and limonene (Figure 10) were mostly attractive to LEM at low and intermediate concentrations. Sabinene (Figure 11) promoted attraction of LEM in almost all concentrations tested, and nonanal (Figure 6) promoted repellence or had no effect on LEM preferences. Importantly, since we tested individual compounds, the outcome might be different when testing them in a mixture. Further research should investigate if volatile emissions of these compounds are associated with natural enemies of LEM.
Several studies have shown that the ratio of odor blends are important in insect attraction [88,89,90]. Blends of volatiles in the proper ratios rather than single compounds can mimic natural conditions. Thus, the next step in understanding the attractive role of the lychee volatiles identified in this study to LEM is testing different odor blends at different ratios. Nevertheless, the findings that the LEM in this study significantly preferred some volatile compounds at lower concentrations is an important first step in understanding the attractive behavior of this pest. Further, to our knowledge, this is the first description of a bioassay for testing LEM odor preferences. It is important to note that because LEM hides in the erineum it was not possible to assess the number of mites released in the arena. In an erineum disc of 0.5 mm dimeter the LEM population may vary from 0 to more than 8000 mites. This information, however, can only be confirmed after the mites exit the erineum. In addition, most LEM cannot survive outside the erinea for more than 24 h [39], meaning that mites that did not make their decision within the period of observation (24 h), ended up dead in the middle of the arena. As this method allows mites to walk freely, it is uncertain how long a mite stayed on either side of the slide. Hence, we did not evaluate the activity of the mites, but the proportion of mites choosing one specific odor over another and whether the compound repelled or attracted those mites. By assessing only their final choice, this experimental set-up might have decreased the chances of having undecided mites influencing the results. One may wonder why there was a variation in LEM response when offered the choice between leaf shoot vs. leaf shoot (control group). The non-infested lychee tissue used was field-collected. Individual lychee trees may be exposed to different biotic and abiotic conditions that in turn may influence nutrition or synthesis of defensive compounds. These factors can also affect mite behavior. In our study we were not able to control for this variation.
Finally, LEM is a host specific eriophyid mite known to attack several lychee tissues [32]. Consistent with what has been observed in the field, here we showed that LEM’s most preferred tissue were the new leaf shoots (Figure 5). The attacked young tissue and the erinea progress simultaneously, meaning that the infested old plant tissue only dies months after exploitation by the mite (Ataide et al. in preparation). Once new growth starts to form, mite infestation in new tissue prevails and soon the whole plant is infested. This is important information for the management of LEM. It is known that lychee trees undergo two to three waves of flushing each year, usually in the summer and early in the autumn in southern China [91]. Thus, this is a critical period for prophylactic treatments with pesticides. Once the new leaf shoots are protected, LEM spread within and between plants and therefore yield losses are expected to be suppressed.
Here, we showed that there is the potential to use key terpenoids and aldehydes in the attraction or repellence of LEM to lychee plants. In the future, lychee VOCs or HIPV can be used to develop volatile-based management strategies against LEM. HIPVs, especially elicitors such as the plant hormones salicylic acid and jasmonic acid, could induce the plant defense system before insect attack [91,92,93,94,95]. In addition, a “semiochemically assisted trap cropping”, which consists of the use of trap crops supplemented with lures, such as pheromones or kairomones, can enhance the effectiveness of pest control [96]. All these strategies have contributed to the detection, monitoring, and manipulation of populations of native and invasive insect species around the world. However, these strategies have been barely investigated for mite control [97] and there are still significant knowledge gaps in the chemical communication in the acari compared to insects. As they are wingless organisms, we envision a management system for LEM focused on the use of attractant semiochemicals to lure and target them outside the erinea. Alternatively, repellents could potentially be used to protect new shoots from LEM infestation. Overall, by addressing LEM preferences to plant volatiles and specific plant tissues, we hope that new control strategies can be developed.
Acknowledgments
We thank James C. Colee (UF/IFAS/Statistics) for statistical advice. We also thank Jully A. R. P. Dutra, and Wendy P. V. Romero for technical support during the experiments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom13060933/s1, Figure S1: Images of the four erineum stages associated with different levels of LEM infestation. Photos of the leaflets showing the erinea were taken using an Apple iPhone 11 (f/1.8; 1/120 s; ISO-125). Photo background has been removed using Adobe® Photoshop CS 5.0. Figure S2: Preference of LEM to same lychee plant tissues. Panel shows LEM choice when offered similar plant tissues in both ends of the experimental arena (leaf vs. leaf, fruit vs. fruit, flower bud vs. flower bud and leaf shoot vs. leaf shoot, N = 8). GLMM: * p < 0.05; ns, not significant. Bars indicate proportion (±SE) of mites reaching the end section of the slide (Sections 3 and 4 and TAPE) towards each plant tissue in 24 h. Figure S3: Preference of LEM to same odor sources in a dual-choice experimental unit. Panel shows LEM choice when offered same odor sources in both ends of the experimental arena (clean filter paper vs. clean filter paper, N = 16; ethanol vs. ethanol, N = 15). GLMM: ns, not significant. Bars indicate proportion (±SE) of mites reaching the end section of the slide (Sections 3 and 4 and TAPE) towards each source in 24 h.
Author Contributions
Conceptualization, methodology and data collection were performed by L.M.S.A., A.M.R., M.A.C., T.I.N., E.Q.S., P.E.K., N.T. and D.C. Formal analyses of the data was performed by L.M.S.A., A.M.R. and N.T. The first draft of the manuscript was written by L.M.S.A., N.T. and A.M.R.; D.C., K.R.C. and P.E.K. reviewed and edited the manuscript. Supervision and project administration was done by D.C. and A.M.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available in the figshare repository under the DOI 10.6084/m9.figshare.23269553.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by USDA ARS-UF Non-Assistance Cooperative Agreement No. 58-6038-8-004. The findings and conclusions in this preliminary publication have not been formally disseminated by the U.S. Department of Agriculture and should not be construed to represent any Agency determination or policy. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA; USDA is an equal opportunity provider and employer.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Howe G.A., Jander G. Plant Immunity to Insect Herbivores. Annu. Rev. Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 2.Dudareva N., Negre F., Nagegowda D.A., Orlova I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006;25:417–440. doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 3.Boncan D.A.T., Tsang S.S., Li C., Lee I.H., Lam H.-M., Chan T.-F., Hui J.H. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020;21:7382. doi: 10.3390/ijms21197382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dicke M., Baldwin I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Dicke M., Loon J.J.A. Multitrophic Effects of Herbivore-induced Plant Volatiles in an Evolutionary Context. Entomol. Exp. Appl. 2000;97:237–249. doi: 10.1046/j.1570-7458.2000.00736.x. [DOI] [Google Scholar]
- 6.Janssen A., Sabelis M.W., Bruin J. Evolution of herbivore-induced plant volatiles. Oikos. 2002;97:134–138. doi: 10.1034/j.1600-0706.2002.970114.x. [DOI] [Google Scholar]
- 7.Schuman M.C., Barthel K., Baldwin I.T. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. Elife. 2012;1:e00007. doi: 10.7554/eLife.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dicke M., Van Beek T.A., Posthumus M.A., Ben Dom N., Van Bokhoven H., De Groot A. Isolation and identification of volatile kairomone that affects acarine predatorprey interactions Involvement of host plant in its production. J. Chem. Ecol. 1990;16:381–396. doi: 10.1007/BF01021772. [DOI] [PubMed] [Google Scholar]
- 9.Takabayashi J., Dicke M., Posthumus M.A. Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: Relative influence of plant and herbivore. Chemoecology. 1991;2:bf01240659. doi: 10.1007/BF01240659. [DOI] [Google Scholar]
- 10.Takabayashi J., Dicke M., Posthumus M.A. Volatile herbivore-induced terpenoids in plant-mite interactions: Variation caused by biotic and abiotic factors. J. Chem. Ecol. 1994;20:1329–1354. doi: 10.1007/BF02059811. [DOI] [PubMed] [Google Scholar]
- 11.Van Den Boom C.E.M., Van Beek T.A., Posthumus M.A., De Groot A., Dicke M. Qualitative and Quantitative Variation Among Volatile Profiles Induced by Tetranychus urticae Feeding on Plants from Various Families. J. Chem. Ecol. 2004;30:69–89. doi: 10.1023/B:JOEC.0000013183.72915.99. [DOI] [PubMed] [Google Scholar]
- 12.van den Boom C.E.M., van Beek T.A., Dicke M. Attraction of Phytoseiulus persimilis (Acari: Phytoseiidae) towards volatiles from various Tetranychus urticae-infested plant species. Bull. Èntomol. Res. 2002;92:539–546. doi: 10.1079/BER2002193. [DOI] [PubMed] [Google Scholar]
- 13.Bernasconi M.L., Turlings T.C.J., Ambrosetti L., Bassetti P., Dorn S. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Èntomol. Exp. Appl. 1998;87:133–142. doi: 10.1046/j.1570-7458.1998.00315.x. [DOI] [Google Scholar]
- 14.Farmer E.E. Surface-to-air signals. Nature. 2001;411:854–856. doi: 10.1038/35081189. [DOI] [PubMed] [Google Scholar]
- 15.Frost C.J., Appel H.M., Carlson J.E., De Moraes C.M., Mescher M.C., Schultz J.C. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol. Lett. 2007;10:490–498. doi: 10.1111/j.1461-0248.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 16.Heil M., Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 2010;25:137–144. doi: 10.1016/j.tree.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Bruin J., Dicke M., Sabelis M.W. Plants are better protected against spider-mites after exposure to volatiles from infested conspecifics. Experientia. 1992;48:525–529. doi: 10.1007/BF01928181. [DOI] [Google Scholar]
- 18.Dicke M., Bruin J. Chemical information transfer between plants: Back to the Future. Biochem. Syst. Ecol. 2001;29:981–994. doi: 10.1016/S0305-1978(01)00045-X. [DOI] [Google Scholar]
- 19.Baldwin I.T., Halitschke R., Paschold A., von Dahl C.C., Preston C.A. Volatile Signaling in Plant-Plant Interactions: “Talking Trees” in the Genomics Era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 20.Kessler A., Baldwin I.T. Plant Responses to Insect Herbivory: The Emerging Molecular Analysis. Annu. Rev. Plant Biol. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- 21.Heil M., Silva Bueno J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmer E.E., Ryan C.A. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beale M.H., Birkett M.A., Bruce T.J.A., Chamberlain K., Field L.M., Huttly A.K., Martin J.L., Parker R., Phillips A.L., Pickett J.A., et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc. Natl. Acad. Sci. USA. 2006;103:10509–10513. doi: 10.1073/pnas.0603998103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kos M., Houshyani B., Overeem A.-J., Bouwmeester H.J., Weldegergis B.T., van Loon J.J., Dicke M., Vet L.E. Genetic engineering of plant volatile terpenoids: Effects on a herbivore, a predator and a parasitoid. Pest Manag. Sci. 2012;69:302–311. doi: 10.1002/ps.3391. [DOI] [PubMed] [Google Scholar]
- 25.Dudareva N., Pichersky E. Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008;19:181–189. doi: 10.1016/j.copbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Degenhardt J., Gershenzon J., Baldwin I.T., Kessler A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 2003;14:169–176. doi: 10.1016/S0958-1669(03)00025-9. [DOI] [PubMed] [Google Scholar]
- 27.Bruce T.J., Aradottir G.I., Smart L.E., Martin J.L., Caulfield J.C., Doherty A., Sparks C.A., Woodcock C.M., Birkett M.A., Napier J.A., et al. The first crop plant genetically engineered to release an insect pheromone for defence. Sci. Rep. 2015;5:11183. doi: 10.1038/srep11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q.-H., Schlyter F. Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric. For. Èntomol. 2004;6:1–20. doi: 10.1111/j.1461-9555.2004.00202.x. [DOI] [Google Scholar]
- 29.Menzel C. The Lychee Crop in Asia and the Pacific. Volume 16 RAP Publication; Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific; Bangkok, Thailand: 2002. [Google Scholar]
- 30.Carrillo D., Cruz L.F., Revynthi A.M., Duncan R.E., Bauchan G.R., Ochoa R., Kendra P.E., Bolton S.J. Detection of the Lychee Erinose Mite, Aceria litchii (Keifer) (Acari: Eriophyidae) in Florida, USA: A Comparison with Other Alien Populations. Insects. 2020;11:235. doi: 10.3390/insects11040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oldfield G.N. 1.4.3 diversity and host plant specificity. In: Lindquist E.E., Sabelis M.W., Bruin J., editors. World Crop Pests. Volume 6. Elsevier; Amsterdam, The Netherlands: 1996. pp. 199–216. [Google Scholar]
- 32.Nishida T., Holdaway F.G. Hawaii Agriculture Experiment Station. University of Hawai; Honolulu, HI, USA: 1955. The Erinose Mite of Lychee. Circular No. 48. [Google Scholar]
- 33.Menzel C.M., Waite G.K. Litchi and Longan: Botany, Production and Uses. CABI Publishing; Oxfordshire, UK: 2005. [Google Scholar]
- 34.Castro B.M.d.C.e., Plata-Rueda A., Silva W.M., de Menezes C.W.G., Wilcken C.F., Zanuncio J.C. Manejo Del Ácaro Aceria litchii (Acari: Eriophyidae) En Litchi Chinensis. Rev. Colomb. De Entomol. 2018;44:2–7. doi: 10.25100/socolen.v44i1.6528. [DOI] [Google Scholar]
- 35.Waite G.K., Hwang J.S. Tropical Fruit Pests and Pollinators: Biology, Economic Importance, Natural Enemies, and Control. Volume 331 CABI Publishing; Oxfordshire, UK: 2002. 11 Pests of Litchi and Longan. [Google Scholar]
- 36.Navia D., Júnior A.M., Gondim M.G.C., Jr., de Mendonza R.S., da Silva Pereira P.R.V. Recent mite invasions in South America. In: Peña J.E., editor. Potential Invasive Pests Agric Crop. 1st ed. CABI Publishing; Oxfordshire, UK: 2013. pp. 251–287. [Google Scholar]
- 37.Prasad V.G., Singh R.K. Prevalence and Control of Litchi Mite, Aceria litchii Kiefer in Bihar. Indian J. Entomol. 1981;43:67–75. [Google Scholar]
- 38.Azevedo L.H., Moraes G.J., Yamamoto P.T., Zanardi O.Z. Development of a Methodology and Evaluation of Pesticides Against Aceria litchii and Its Predator Phytoseius intermedius (Acari: Eriophyidae, Phytoseiidae) J. Econ. Èntomol. 2013;106:2183–2189. doi: 10.1603/EC13026. [DOI] [PubMed] [Google Scholar]
- 39.Revynthi A.M., Cruz L.F., Canon M.A., Crane J.H., Kendra P.E., Mannion C., Carrillo D. Evaluation of Abamectin as a Potential Chemical Control for the Lychee Erinose Mite (Acari: Eriophyidae), a New Invasive Pest in Florida. Fla. Èntomol. 2022;105:1–5. doi: 10.1653/024.105.0101. [DOI] [Google Scholar]
- 40.Waite G., Gerson U. The predator guild associated withAceria litchii (Acari: Eriophyidae) in Australia and China. Biocontrol. 1994;39:275–280. doi: 10.1007/BF02373032. [DOI] [Google Scholar]
- 41.Ferreira Picoli P.R., Vieira M.R., da Silva E.A., de Oliveira da Mota M.S. Predator Mites Associated with the Litchi Erinose Mite. Pesqui. Agropecu. Bras. 2010;45:1246–1252. doi: 10.1590/s0100-204x2010001100003. [DOI] [Google Scholar]
- 42.Azevedo L.H., Castilho R.d.C., de Moraes G.J. Suitability of the Litchi Erineum Mite, Aceria litchii (Keifer), as Prey for the Mite Phytoseius intermedius Evans & MacFarlane (Acari: Eriophyidae, Phytoseiidae) Syst. Appl. Acarol. 2016;21:270–278. [Google Scholar]
- 43.Ferraz C., Ataide L., Gondim M., Pallini A. Arthropods associated with the lychee erinose mite, Aceria litchii (Acari: Eriophyidae) on lychee trees in Minas Gerais, Brazil. Exp. Appl. Acarol. 2022;88:289–300. doi: 10.1007/s10493-022-00762-3. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y., Pan Q., Qu W., Duan C. Comparison of Volatile Profiles of Nine Litchi (Litchi chinensis Sonn.) Cultivars from Southern China. J. Agric. Food Chem. 2009;57:9676–9681. doi: 10.1021/jf902144c. [DOI] [PubMed] [Google Scholar]
- 45.Feng S., Huang M., Crane J., Wang Y. Characterization of key aroma-active compounds in lychee (Litchi chinensis Sonn.) J. Food Drug Anal. 2018;26:497–503. doi: 10.1016/j.jfda.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong P.K.C., Acree T.E. Gas Chromatography/Olfactory Analysis of Lychee (Litchi chinesis Sonn.) J. Agric. Food Chem. 1998;46:2282–2286. doi: 10.1021/jf9801318. [DOI] [Google Scholar]
- 47.Mahattanatawee K., Perez-Cacho P.R., Davenport T., Rouseff R. Comparison of Three Lychee Cultivar Odor Profiles Using Gas Chromatography−Olfactometry and Gas Chromatography−Sulfur Detection. J. Agric. Food Chem. 2007;55:1939–1944. doi: 10.1021/jf062925p. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Zhao D., Gao J., Peng Z. Volatile Components of Litchi chinensis Sonn. Leaf Oil Extracts Obtained by Simultaneous Distillation Extraction. J. Essent. Oil Bear. Plants. 2013;16:161–165. doi: 10.1080/0972060X.2013.794048. [DOI] [Google Scholar]
- 49.Chen D., Liu S.-Q. Chemical and volatile composition of lychee wines fermented with four commercial Saccharomyces cerevisiae yeast strains. Int. J. Food Sci. Technol. 2013;49:521–530. doi: 10.1111/ijfs.12332. [DOI] [Google Scholar]
- 50.Tang Z.-S., Zeng X.-A., Brennan M.A., Han Z., Niu D., Huo Y. Characterization of aroma profile and characteristic aromas during lychee wine fermentation. J. Food Process. Preserv. 2019;43:e14003. doi: 10.1111/jfpp.14003. [DOI] [Google Scholar]
- 51.Ong P.K.C., Acree T.E. Similarities in the Aroma Chemistry of Gewürztraminer Variety Wines and Lychee (Litchi chinesis Sonn.) Fruit. J. Agric. Food Chem. 1999;47:665–670. doi: 10.1021/jf980452j. [DOI] [PubMed] [Google Scholar]
- 52.Chyau C.-C., Ko P.-T., Chang C.-H., Mau J.-L. Free and glycosidically bound aroma compounds in lychee (Litchi chinensis Sonn.) Food Chem. 2003;80:387–392. doi: 10.1016/S0308-8146(02)00278-9. [DOI] [Google Scholar]
- 53.Sivakumar D., Naudé Y., Rohwer E., Korsten L. Volatile compounds, quality attributes, mineral composition and pericarp structure of South African litchi export cultivars Mauritius and McLean’s Red. J. Sci. Food Agric. 2008;88:1074–1081. doi: 10.1002/jsfa.3201. [DOI] [Google Scholar]
- 54.Gunpal D., Patni V. Comparative Analysis of Volatile Compounds in Normal Leaves and Mite Induced Leaf Galls of Litchi chinensis Sonn. Int. J. Bot. Stud. 2021;6:631–636. [Google Scholar]
- 55.Stewart-Jones A., Poppy G.M. Comparison of Glass Vessels and Plastic Bags for Enclosing Living Plant Parts for Headspace Analysis. J. Chem. Ecol. 2006;32:845–864. doi: 10.1007/s10886-006-9039-6. [DOI] [PubMed] [Google Scholar]
- 56.Wiley Science Solution . NIST 2020 Mass Spectral Library. Solutions, Scientific Instrument Services Inc.; Ringoes, NJ, USA: 2020. [Google Scholar]
- 57.MassFinder; MassFinder Software 2004; Dr. Hochmuth Scientific Consulting: Hamburg, Germany. [(accessed on 1 February 2022)]. Available online: https://www.massfinder.org/
- 58.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Allured Publishing Corporation; Carol Steam, IL, USA: 2007. [Google Scholar]
- 59.Mass Spectral Database. Scientific Instrument Services Inc.; Hoboken, NJ, USA: 2015. FFNSC3 Flavors and Fragrances of Natural and Synthetic Compounds. [Google Scholar]
- 60.Kendra P.E., Tabanca N., Cruz L.F., Menocal O., Schnell E.Q., Carrillo D. Volatile Emissions and Relative Attraction of the Fungal Symbionts of Tea Shot Hole Borer (Coleoptera: Curculionidae) Biomolecules. 2022;12:97. doi: 10.3390/biom12010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 62.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data. 2011;40:43101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 63.Acree T., Arn H. Flavornet and Human Odor Space. [(accessed on 1 November 2022)]. Available online: http://www.flavornet.org/
- 64.The Pherobase Database. [(accessed on 1 November 2022)]. Available online: http://www.pherobase.com/database/kovats/kovatsdetailsulcatone.php.
- 65.Sarmento R.A., Lemos F., Bleeker P.M., Schuurink R.C., Pallini A., Oliveira M.G.A., Lima E.R., Kant M., Sabelis M.W., Janssen A. A herbivore that manipulates plant defence. Ecol. Lett. 2011;14:229–236. doi: 10.1111/j.1461-0248.2010.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arlian L.G., Vyszenski-Moher D.L. Response of Sarcoptes scabiei var. canis (Acari: Sarcoptidae) to Lipids of Mammalian Skin. J. Med. Èntomol. 1995;32:34–41. doi: 10.1093/jmedent/32.1.34. [DOI] [PubMed] [Google Scholar]
- 67.Pallini A., Janssen A., Sabelis M.W. Odour-mediated responses of phytophagous mites to conspecific and heterospecific competitors. Oecologia. 1997;110:179–185. doi: 10.1007/s004420050147. [DOI] [PubMed] [Google Scholar]
- 68.Bates D., Mächler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67:48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 69.Crawley M.J. The R Book. John Wiley & Sons; Hoboken, NJ, USA: 2012. [Google Scholar]
- 70.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2022. [Google Scholar]
- 71.Popitanu C., Lupitu A., Copolovici L., Bungău S., Niinemets Ü., Copolovici D.M. Induced Volatile Emissions, Photosynthetic Characteristics, and Pigment Content in Juglans regia Leaves Infected with the Erineum-Forming Mite Aceria erinea. Forests. 2021;12:920. doi: 10.3390/f12070920. [DOI] [Google Scholar]
- 72.Cascone P., Iodice L., Maffei M.E., Bossi S., Arimura G.-I., Guerrieri E. Tobacco overexpressing β-ocimene induces direct and indirect responses against aphids in receiver tomato plants. J. Plant Physiol. 2015;173:28–32. doi: 10.1016/j.jplph.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Tak J.-H., Isman M.B. Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae) Ind. Crops Prod. 2017;108:786–792. doi: 10.1016/j.indcrop.2017.08.003. [DOI] [Google Scholar]
- 74.Mumm R., Posthumus M.A., Dicke M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008;31:575–585. doi: 10.1111/j.1365-3040.2008.01783.x. [DOI] [PubMed] [Google Scholar]
- 75.Jaenson T.G.T., Pålsson K., Borg-Karlson A.-K. Evaluation of Extracts and Oils of Mosquito (Diptera: Culicidae) Repellent Plants from Sweden and Guinea-Bissau. J. Med. Èntomol. 2006;43:113–119. doi: 10.1093/jmedent/43.1.113. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y.-C., Lee E.-H., Lee H.-S., Lee D.-K., Ahn Y.-J. Repellency of aromatic medicinal plant extracts and a steam distillate to Aedes aegypti. J. Am. Mosq. Control Assoc. 2004;20:146–149. [PubMed] [Google Scholar]
- 77.Tunón H., Thorsell W., Mikiver A., Malander I. Arthropod repellency, especially tick (Ixodes ricinus), exerted by extract from Artemisia abrotanum and essential oil from flowers of Dianthus caryophyllum. Fitoterapia. 2006;77:257–261. doi: 10.1016/j.fitote.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 78.Gillij Y.G., Gleiser R.M., Zygadlo J.A. Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Bioresour. Technol. 2008;99:2507–2515. doi: 10.1016/j.biortech.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 79.Alquézar B., Volpe H.X.L., Magnani R.F., de Miranda M.P., Santos M.A., Wulff N.A., Bento J.M.S., Parra J.R.P., Bouwmeester H., Peña L. β-caryophyllene emitted from a transgenic Arabidopsis or chemical dispenser repels Diaphorina citri, vector of Candidatus Liberibacters. Sci. Rep. 2017;7:5639. doi: 10.1038/s41598-017-06119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dickens J.C. Predator-prey interactions: Olfactory adaptations of generalist and specialist predators. Agric. For. Èntomol. 1999;1:47–54. doi: 10.1046/j.1461-9563.1999.00007.x. [DOI] [Google Scholar]
- 81.Yi H.-S., Heil M., Adame-Álvarez R.M., Ballhorn D.J., Ryu C.-M. Airborne Induction and Priming of Plant Defenses against a Bacterial Pathogen. Plant Physiol. 2009;151:2152–2161. doi: 10.1104/pp.109.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gosset V., Harmel N., Gobel C., Francis F., Haubruge E., Wathelet J.-P., Du Jardin P., Feussner I., Fauconnier M.-L. Attacks by a piercing-sucking insect (Myzus persicae Sultzer) or a chewing insect (Leptinotarsa decemlineata Say) on potato plants (Solanum tuberosum L.) induce differential changes in volatile compound release and oxylipin synthesis. J. Exp. Bot. 2009;60:1231–1240. doi: 10.1093/jxb/erp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang C., Li G., Miao C., Zhao M., Wang B., Guo X. Nonanal modulates oviposition preference in female Helicoverpa assulta (Lepidoptera: Noctuidae) via the activation of peripheral neurons. Pest Manag. Sci. 2020;76:3159–3167. doi: 10.1002/ps.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seybold S.J., Bentz B.J., Fettig C.J., Lundquist J.E., Progar R.A., Gillette N.E. Management of Western North American Bark Beetles with Semiochemicals. Annu. Rev. Èntomol. 2018;63:407–432. doi: 10.1146/annurev-ento-020117-043339. [DOI] [PubMed] [Google Scholar]
- 85.Ogah E.O., Smart L.E., Woodcock C.M., Caulfield J.C., Birkett M.A., Pickett J.A., Nwilene F.E., Bruce T.J. Electrophysiological and behavioral responses of female African rice gall midge, Orseolia oryzivora Harris and Gagné, to host plant volatiles. J. Chem. Ecol. 2017;43:13–16. doi: 10.1007/s10886-016-0788-6. [DOI] [PubMed] [Google Scholar]
- 86.Mishra S., Sihag R. Efficacy of Some Chemicals as Repellents against Two Honeybee Species, Apis mellifera L. and Apis florea F. in Semi-Field Trials. J. Apic. Sci. 2009;53:53–66. [Google Scholar]
- 87.Nieuwenhuizen N.J., Wang M.Y., Matich A.J., Green S.A., Chen X., Yauk Y.-K., Beuning L.L., Nagegowda D.A., Dudareva N., Atkinson R.G. Two terpene synthases are responsible for the major sesquiterpenes emitted from the flowers of kiwifruit (Actinidia deliciosa) J. Exp. Bot. 2009;60:3203–3219. doi: 10.1093/jxb/erp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Visser J.H., Avé D.A. General green leaf volatiles in the olfactory orientation of the colorado beetle, Leptinotarsa decemlineata. Èntomol. Exp. Appl. 1978;24:738–749. doi: 10.1111/j.1570-7458.1978.tb02838.x. [DOI] [Google Scholar]
- 89.Bruce T.J., Wadhams L.J., Woodcock C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005;10:269–274. doi: 10.1016/j.tplants.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 90.Fraser A.M., Mechaber W.L., Hildebrand J.G. Electroantennographic and behavioral responses of the sphinx moth Manduca sexta to host plant headspace volatiles. J. Chem. Ecol. 2003;29:1813–1833. doi: 10.1023/A:1024898127549. [DOI] [PubMed] [Google Scholar]
- 91.Wei Y., Zhang H., Li W., Xie J., Wang Y., Liu L., Shi S. Phenological growth stages of lychee (Litchi chinensis Sonn.) using the extended BBCH-scale. Sci. Hortic. 2013;161:273–277. doi: 10.1016/j.scienta.2013.07.017. [DOI] [Google Scholar]
- 92.Doughty K.J., Kiddle G.A., Pye B.J., Wallsgrove R.M., Pickett J.A. Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry. 1995;38:347–350. doi: 10.1016/0031-9422(94)00653-B. [DOI] [Google Scholar]
- 93.Thaler J.S., Stout M.J., Karban R., Duffey S.S. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 1996;22:1767–1781. doi: 10.1007/BF02028503. [DOI] [PubMed] [Google Scholar]
- 94.Ataide L.M., Pappas M.L., Schimmel B.C., Lopez-Orenes A., Alba J.M., Duarte M.V., Pallini A., Schuurink R.C., Kant M.R. Induced plant-defenses suppress herbivore reproduction but also constrain predation of their offspring. Plant Sci. 2016;252:300–310. doi: 10.1016/j.plantsci.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Landolt P.J., Tumlinson J.H., Alborn D.H. Attraction of Colorado Potato Beetle (Coleoptera: Chrysomelidae) to Damaged and Chemically Induced Potato Plants. Environ. Èntomol. 1999;28:973–978. doi: 10.1093/ee/28.6.973. [DOI] [Google Scholar]
- 96.Shelton A., Badenes-Perez F. Concepts and applications of trap cropping in pest management. Annu. Rev. Èntomol. 2006;51:285–308. doi: 10.1146/annurev.ento.51.110104.150959. [DOI] [PubMed] [Google Scholar]
- 97.Carr A.L., Roe M. Acarine attractants: Chemoreception, bioassay, chemistry and control. Pestic. Biochem. Physiol. 2016;131:60–79. doi: 10.1016/j.pestbp.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available in the figshare repository under the DOI 10.6084/m9.figshare.23269553.