Abstract
A 35-year-old right hand dominant male sustained a high energy closed right distal radius fracture with associated generalized paresthesias. Following closed reduction, the patient was found to have an atypical low ulnar nerve palsy upon outpatient follow-up. After continued symptoms and an equivocal wrist MRI the patient underwent surgical exploration. Intraoperatively, the ulnar nerve as well as the ring and small finger flexor digitorum superficialis tendons were found to be translocated around the ulnar head. The nerve and tendons were reduced, the median nerve was decompressed, and the fracture was addressed with volar plating. Post-operatively, the patient continued to have sensory deficits and stiffness of the ring and small fingers. After one year, he reported substantial improvements as demonstrated by full sensation (4.0 mm two-point discrimination) and fixed flexion contractures at the proximal and distal interphalangeal joints of the small finger. The patient returned to work without functional limitations.
This case highlights a unique case of ulnar nerve and flexor tendon entrapment following a distal radius fracture. History, physical examination, and a high index of clinical suspicion is essential for proper management of this rare injury.
Level of Evidence: V
Keywords: distal radius fracture, wrist fracture, ulnar nerve entrapment, ulnar neuropraxia, guyon’s canal
Introduction
Distal radius fractures (DRF) are the most common type of fracture in the United States, accounting for 8-18% of all fractures.1,2 The majority of DRFs resolve uneventfully. However, a small percentage are complicated by an associated nerve injury. The most recognized of these is an acute carpal tunnel syndrome (ACTS), occurring in 3.3-8.6% of all DRFs.3,4
Recently, a growing body of literature has demonstrated an association between DRFs and ulnar nerve palsies.5,6 These nerve injuries are associated with higher-energy trauma, e.g. motor vehicle accidents.5,6 Similar to ACTS, these palsies are thought to be secondary to nerve contusion, fracture-induced traction, or compression from local edema. Rarely, the ulnar nerve may be lacerated.7 Ulnar nerve injuries in the presence of DRF remain a diagnostic challenge and their management remains undefined. We report a unique case of a DRF presenting with ulnar neuropraxia secondary to a dorsoulnar translocation of the nerve around the ulnar head, with concomitant entrapment of flexor digitorum superficialis (FDS) tendons. Based on the available evidence we propose a treatment algorithm for these injuries.
Case Report
A 35-year-old right-hand dominant male presented to the emergency department with a displaced closed left DRF secondary to a 35-mph rollover accident on an all-terrain vehicle (Fig. 1a and b). Upon initial examination, the wrist was grossly deformed with a 1.0 cm dorsal laceration between the long and ring finger webspace. Subjective dysesthesias were noted in all digits but were otherwise sensate to light touch. His motor exam revealed strong flexor pollicis longus (FPL) and extensor pollicis longus (EPL); weak FDS and extensor digitorum communis (EDC) of the index finger; and weak second dorsal/third palmar interossei muscles.
Figure 1A to 1F.
AP (1a) and lateral (1b) of a comminuted dorsally displaced left distal radius fracture with approximately 1 cm of shortening. AP (1c) and lateral (1d) post-reduction films. 2-week AP (1e) and lateral (1f) follow-up from open reduction internal fixation.
Closed reduction was then performed under fluoroscopic guidance, and a molded bi-valved short-arm fiberglass cast was applied (Fig 2 a and b). Post-reduction neurovascular exam demonstrated improved dysesthesias in the median nerve distribution but persistent symptoms in the ulnar nerve distribution. Motor examination was also notable for grade 2/5 strength of the second and third dorsal/palmar interossei. Passive flexion and extension of the ring and small finger elicited pain. After a period of observation, the patient’s pain and dysesthesias had modestly improved while the motor exam remained unchanged. The patient was discharged home.
Figure 2A to 2D.
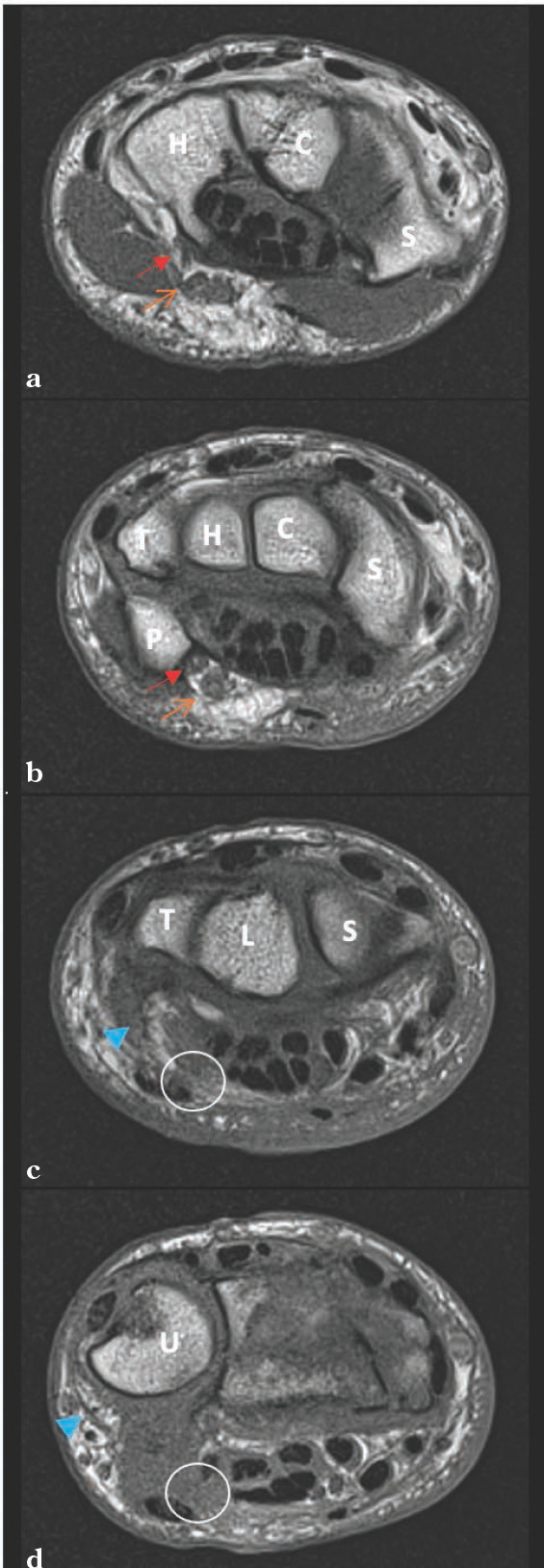
T1 Coronal of the left wrist (2a-2d). Visualized is the deep ulnar nerve becoming confluent with the superficial branch (2a) as the wrist is visualized more proximally (2b). The ulnar neurovascular bundle appears translated ulnarly away from its typical location just radial and deep to the flexor carpi ulnaris. Key: Red solid arrow = deep branch of the ulnar nerve, orange open arrow = superficial branch of the ulnar nerve, solid blue arrowhead = suspected ulnar neurovascular bundle, white circle = normal anatomic location of ulnar nerve, C = capitate, H = hamate, P = pisiform, R = radius, S = scaphoid, T = triquetrum, U = ulna.
After one week, the fracture reduction was maintained. However, his ulnar nerve dysesthesias persisted, and two-point discrimination (2PD) was diminished: 12 mm in the median distribution and indiscernible in the ulnar distribution. Interossei muscle function remained equivocal. The patient’s ring and small finger proximal interphalangeal joints remained in a partially flexed position. Passive extension of these fingers was limited by volar sided forearm pain, but the index and long fingers extended easily.
An MRI of the wrist without contrast was obtained (Fig. 2). The ulnar nerve was visualized at Guyon’s canal but obscured at the fracture site. Given the concern for underlying nerve injury, surgical intervention was pursued. The patient underwent open reduction internal fixation of the distal radius, carpal tunnel release, and exploration of the ulnar nerve 10 days after his injury.
A longitudinal midline incision was made along the distal forearm. A carpal tunnel release revealed a contused but intact median nerve. Attention was then directed to Guyon’s canal. After identifying the ulnar neurovascular bundle, the structures were traced proximally and appeared to be traveling dorsoulnarly around the ulnar head under moderate tension. The bundle was then re-identified within the forearm and traced distally which confirmed the dorsoulnar translocation. Additionally, the ring and small finger FDS tendinous slips were also found translocated in a similar manner (Fig 3).
Figure 3A to 3B.

Surgical images demonstrating translocation of the ulnar nerve traveling dorsally to the ulnar head when traced proximally (3a). Similarly, slips of the FDS are visualized coursing ulnarly and superficially relative to the ulnar head. UH = Ulnar head, green open arrow = neurovascular bundle, purple bracket = triangular fibrocartilage complex, solid blue arrowhead = FDS tendons, white arrowhead = proximal ulnar nerve.
Tenolysis and reduction of the entrapped tendons was performed. Unrestricted tendon gliding of the ring and small fingers was visualized. Further exploration revealed a complete transection of the ulnar artery, which had thrombosed. The ulnar nerve was then reduced without undue tension. The nerve and its deep motor branch were contused and hemorrhagic but were in continuity.
Lastly, the DRF was addressed with an Acu-Loc 2 volar plate (Acumed, Hillsboro, Oregon, USA). Stability of the DRUJ was confirmed under fluoroscopy. A standard closure was performed, and the patient was discharged home in a volar wrist splint.
During the two-week follow-up visit, the patient presented with a flexible claw deformity of the ring and small finger, absent function of all interossei muscles, and diminished light touch with absent 2PD of the ring and small finger. His finding was consistent with a low ulnar nerve palsy. His exam and radiographic imaging were otherwise unremarkable (Fig. 3). The patient was enrolled in hand therapy to maintain flexibility of the claw hand deformity and a thermoplastic splint was fabricated. At his six-weeks, three-months, and four and a half-months follow-ups, he reported subjective improvement, but objective physical exam findings were unchanged. The patient returned to work (car audio installation specialist) three months post-operatively.
At eight months, his claw deformity persisted. The ring finger remained partially flexible while his small finger stiffness worsened secondary to poor adherence to his stretching regimen. His 2PD was 6.0 mm in the ring finger, with negligible small finger 2PD. His first dorsal interosseous muscle improved to 4/5 strength.
At one-year, interossei muscle strength and ring finger sensorimotor function had returned to baseline. Small finger 2PD improved to 4.0 mm. Due to continued difficulties with hand therapy adherence the patient developed a fixed flexion contracture of the small finger distal and proximal interphalangeal joints of 20-30 degrees, and metacarpophalangeal joints of 45-50 degrees.
The patient reported continued frustrations with his activities of daily living due to his flexion contractures. Two years postoperatively, the patient elected to undergo a reoperation. Dense adhesions encased all of the flexor tendons necessitating a radical flexor tenosynovectomy extending from the distal forearm and to the palmar arch. Due to the persistence of a flexion contractures of the small and ring finger, the FDP and FDS were fractionally lengthened utilizing the pie crusting technique allowing the fingers to achieve full extension. Six months post-operatively the patient's flexion contracture was reduced to 10-15 degrees at the distal and proximal interphalngeal joints with complete resolution at the metacarpophalangeal joints.
Discussion
Ulnar nerve palsy following DRFs is a rare phenomenon, but its association is becoming increasingly apparent in the literature.3,4 Despite a paucity of literature on this topic, it appears there is no single predictable cause for ulnar nerve injury after a DRF. There have been reports of neurogenic edema, scar formation, and local mass-effect as relatively common sources for ulnar nerve injury, while cases of transection or translocation, such as in this report, are far less common. Appreciating the possible mechanisms of injury can heighten clinical suspicions and allow for quicker identification of a nerve injury.
Anatomy of the Ulnar Nerve
To better understand the etiology and rarity of ulnar nerve injuries following DRFs, a comparison may be made with the median nerve – the most commonly injured nerve associated with DRFs.3,4 This higher incidence is due to its anatomic location: lying volar to the distal radius before traveling through the carpal tunnel, which is bordered by the rigid osseous walls of the carpal bones, roofed by an unyielding transverse carpal ligament, and its static volume is further limited by the presence of the flexor tendons.8,9 Nerve injury may stem from injuries that increase pressure within the carpal tunnel. These include local tissue edema, hemorrhage, osseous deformity, improper wrist immobilization, and/ or direct nerve contusion.5,6,9
Conversely, the ulnar nerve travels volarly over the ulna, distant from the DRF site, before entering Guyon’s canal.10 Through the fibro-osseous tunnel (Guyon’s canal), the ulnar nerve is embedded fibrofatty tissue alongside the ulnar artery. MRI studies estimate the cross-sectional area of Guyon’s canal as 32 ± 11 mm2 with a coinciding ulnar nerve diameter of 3.0 ± 1 mm (cross section 7.01 mm2).10 At the level of the wrist the ulnar nerve also remains mobile relative to the median nerve.11
The small footprint of the ulnar nerve within Guyon’s canal, large excursion potential, and location away from the fracture site are likely protective attributes. Previous reports have hypothesized that ulnar nerve related injuries are secondary to nerve tethering at Guyon’s canal, resulting in a stretch neuropraxia.5,12-15 Younger age and significantly dorsally displaced DRFs appear to elevate risk for associated acute ulnar nerve injury.5-7,13,15-18
Presentation
The presentation of ulnar injury can be separated by its onset. For acute phase injuries, symptoms presenting at the time of injury, or shortly after closed reduction.5,12-15 Conversely, patients with chronic phase injuries present weeks to months after the injury, and are typically the result of local scar tissue.16
Acute Phase Injuries
In a case series by Soong and Ring, 280 DRFs were reviewed within a two-year period.5 Five cases of complete ulnar-sensorimotor-deficits following acute DRF were identified. All five underwent internal fixation (locked plating), 3/5 underwent ulnar nerve exploration due to concomitant ACTS and 1/3 demonstrated ulnar nerve entrapment within the fracture. Post-operatively, 80% demonstrated complete ulnar recovery while only one had persistent sensorimotor deficits of the ulnar nerve. The average time to clinical signs of nerve recovery was five months, with maximum recovery around seven months.5
In more severe cases, partial or complete transection of the ulnar nerve may be observed. Pogetti et al. reported a 47-year-old male who suffered a subtotal ulnar nerve transection after an open DRF that was acutely repaired. After six months the patient’s sensation returned to baseline, with an intrinsic strength deficit of 4/5.15
There have been reported cases of ulnar nerve injury and translocation of the ulnar nerve dorsal to the distal radioulnar joint (DRUJ) after a DRF. In one case, a 19-year-old male sustained a DRF following an MVA treated with external fixation and delayed internal fixation eight weeks later. Post-operatively an EMG conveyed ulnar denervation. At exploration the nerve was found translocated through the DRUJ. The patient progressively recovered over an additional nine months with residual ulnar hypoesthesias.18 Pientka II et al. described an open DRF/DRUJ dislocation requiring external fixation and DRUJ pinning with post-surgical deficits. During internal fixation eight weeks later, the ulnar neurovascular bundle was noted to have translocated through the DRUJ and wrapped around the ulnar head.19 Follow-up at two months demonstrated improved ulnar nerve sensation but persistent motor deficits.
Chronic Phase Injury
A few case reports of ulnar neuropraxia symptoms presenting months after the initial injury have been reported as well. Cho et al. reported two cases of ulnar nerve palsy following a DRF attributed to surrounding tissue fibrosis at the level of Guyon’s canal.6 Each patient was found to have a progressive ulnar nerve palsy and claw hand deformity eight weeks post operatively confirmed on EMG. Both patients underwent decompression with complete motor recovery 3-12 months post-operatively. Another case by Yang et al., described a new claw hand deformity five weeks after ORIF for a closed DRF.16 After ultrasound examination, a decompression was performed. Six months thereafter the patient’s sensation resolved but a mild claw hand deformity persisted.
Diagnostic Work-up
There are no formalized diagnostic guidelines for ulnar nerve evaluation after a DRF. Advanced imaging modalities like ultrasound6,16 or MRI can assess for nerve continuity in equivocal cases. However, these studies provide limited utility in ruling out ulnar nerve pathology. Previous reports utilized EMG to functionally assess ulnar nerve injury.6,7,17,18 These are often performed weeks after presentation and appear limited in the acute phase. Diagnostic work-up relies heavily on clinical exam findings. Negative advanced imaging does not exclude injury and operative intervention should not be delayed if clinical suspicion remains high.
Timing of Operative Intervention and Recovery
Ideally, nerve injuries should be addressed early; however, multiple reports have demonstrated robust sensorimotor recovery with delayed exploration ranging from a few days up to eight weeks.5,18 We recommend urgent and early surgical intervention for acute phase ulnar nerve injuries in the setting of closed DRF, however, cases of chronic presentation should be offered operative intervention too. Appropriate counseling regarding the duration of sensorimotor recovery is necessary. Sequelae such as claw hand, stiffness, numbness, and dysesthesias should also be addressed.
Conclusion
This report offers a unique case of ulnar nerve and flexor tendon translocation dorsoulnarly relative to the ulnar head. Our review also emphasizes the importance of close monitoring of patients with atypical exams. Current literature suggests persistent ulnar nerve palsy, independent of acuity, can be an indication for surgical exploration following a DRF with a high likelihood of functional recovery. Advanced imaging may be helpful for presurgical planning but remains inadequate for excluding an ulnar nerve injury. Functional testing modalities such as EMG can also be considered in the appropriate setting.
References
- 1.Kakar S, Noureldin M, Van Houten HK, Mwangi R, Sangaralingham LR. Trends in the Incidence and Treatment of Distal Radius Fractures in the United States in Privately Insured and Medicare Advantage Enrollees. Hand. 2020. Published online. doi: [DOI] [PMC free article] [PubMed]
- 2.Nellans KW, Kowalski E, Chung KC. The Epidemiology of Distal Radius Fractures. Hand Clin. 2012;28(2):113. doi: 10.1016/J.HCL.2012.02.001. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch AC, Lipscomb PR. The Carpal Tunnel Syndrome and Colles’ Fractures. JAMA. 1963;185(5):363366. doi: 10.1001/jama.1963.03060050041018. doi: [DOI] [PubMed] [Google Scholar]
- 4.Pope D, Tang P. Carpal Tunnel Syndrome and Distal Radius Fractures. Hand Clin. 2018;34(1):27–32. doi: 10.1016/j.hcl.2017.09.003. doi: [DOI] [PubMed] [Google Scholar]
- 5.Soong M, Ring D. Ulnar nerve palsy associated with fracture of the distal radius. J Orthop Trauma. 2007;21(2):113–116. doi: 10.1097/BOT.0b013e31802f7335. doi: [DOI] [PubMed] [Google Scholar]
- 6.Cho CH, Kang CH, Jung JH. Ulnar nerve palsy following closed fracture of the distal radius: A report of 2 cases. Clin Orthop Surg. 2010;2(1):55–58. doi: 10.4055/cios.2010.2.1.55. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vance RM, Gelberman RH. Acute ulnar neuropathy with fractures at the wrist. J Bone Joint Surg Am. 1978;60(7):962–965. doi: 10.2106/00004623-197860070-00015. doi: [DOI] [PubMed] [Google Scholar]
- 8.Rotman MB, Donovan JP. Practical anatomy of the carpal tunnel. Hand Clin. 2002;18(2):219–230. doi: 10.1016/S0749-0712(01)00003-8. doi: [DOI] [PubMed] [Google Scholar]
- 9.Schnetzler KA. Acute carpal tunnel syndrome. J Am Acad Orthop Surg. 2008;16(5):276–282. doi: 10.5435/00124635-200805000-00006. doi: [DOI] [PubMed] [Google Scholar]
- 10.Zeiss J, Jakab E, Khimji T, Imbriglia J. The ulnar tunnel at the wrist (Guyon’s canal): normal MR anatomy and variants. Am J Roentgenol. 1992;158(5):1081–1085. doi: 10.2214/ajr.158.5.1566671. doi: [DOI] [PubMed] [Google Scholar]
- 11.Wright TW, Glowczewskie F, Cowin D, Wheeler DL. Ulnar nerve excursion and strain at the elbow and wrist associated with upper extremity motion. J Hand Surg Am. 2001;26(4):655–662. doi: 10.1053/jhsu.2001.26140. doi: [DOI] [PubMed] [Google Scholar]
- 12.Dauzere F, Delclaux S, Pham TT, Rongières M, Mansat P. Combined median and ulnar nerve palsy complicating distal radius fractures. Orthop Traumatol Surg Res. 2018;104(6):871–875. doi: 10.1016/j.otsr.2018.04.026. doi: [DOI] [PubMed] [Google Scholar]
- 13.Clarke AC, Spencer RF. Ulnar Nerve Palsy Following Fractures of the Distal Radius: Clinical and Anatomical Studies. J Hand Surg Am. 1991;16(4):438–440. doi: 10.1016/0266-7681(91)90022-G. doi: [DOI] [PubMed] [Google Scholar]
- 14.Pazart F, Stindel E, Le Nen D. Fracture of the distal part of the radius associated with severed ulnar nerve. Ann Chir la Main du Memb Supérieur. 1999;18(3):197201. doi: 10.1016/S1153-2424(99)80005-3. doi: [DOI] [PubMed] [Google Scholar]
- 15.Poggetti A, Nucci AM, Baluganti A, Pfanner S. Jammed ulnar nerve after distal radius fracture: A case report. JPRAS Open. 2020;24:20–24. doi: 10.1016/j.jpra.2020.02.005. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jing-Jing Y, Wei Q, Yu-Xuan W, Hua-Jun J. Ulnar Nerve Injury associated with displaced distal radius fracture: Two case reports. World J Clin Cases. 2021;9(23):6956–6963. doi: 10.3280/PU2021-003007. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin MS, Gokkus K, Sargin MB. Ulnar Nerve and Ulnar Artery Injury Caused by Comminuted Distal Radius Fracture. J Orthop case reports. 2020;10(4):25–30. doi: 10.13107/jocr.2020.v10.i04.1786. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Ruiter GCW, den HP. Ulnar Nerve Palsy with Dislocation of the Nerve around the Ulna Following a Fracture of the Distal Radius. J Spine Neurosurg. 2016;5(6) doi: 10.4172/2325-9701.1000243. doi: [DOI] [Google Scholar]
- 19.Pientka W, Hoelscher S, Bates C, Pipkin W. Traumatic Transposition of the Ulnar Nerve Through the Distal Radioulnar Joint A Case Report. JBJS Case Connect. 2022;12(1) doi: 10.2106/JBJS.CC.21.00180. doi: [DOI] [PubMed] [Google Scholar]



