Abstract
Chemotherapy resistance is still a serious problem in the treatment of most cancers. Many cellular and molecular mechanisms contribute to both inherent and acquired drug resistance. They include the use of unaffected growth-signaling pathways, changes in the tumor microenvironment, and the active transport of medicines out of the cell. The antioxidant capacity of polyphenols and their potential to inhibit the activation of procarcinogens, cancer cell proliferation, metastasis, and angiogenesis, as well as to promote the inhibition or downregulation of active drug efflux transporters, have been linked to a reduced risk of cancer in epidemiological studies. Polyphenols also have the ability to alter immunological responses and inflammatory cascades, as well as trigger apoptosis in cancer cells. The discovery of the relationship between abnormal growth signaling and metabolic dysfunction in cancer cells highlights the importance of further investigating the effects of dietary polyphenols, including their ability to boost the efficacy of chemotherapy and avoid multidrug resistance (MDR). Here, it is summarized what is known regarding the effectiveness of natural polyphenolic compounds in counteracting the resistance that might develop to cancer drugs as a result of a variety of different mechanisms.
Keywords: cancer, drug resistance, antioxidants, plant polyphenols, pharmaceutical uses
1. Introduction
The World Health Organization estimates that in 2020, around 10 million individuals lost their lives to cancer [1]. Cancers of the breast (2.26 million cases), lungs (2.21 million cases), colon/rectum (1.93 million cases), prostate (1.4 million cases), skin (non-melanoma) (1.2 million cases), and stomach (1.2 million cases) were the most prevalent types of cancer (1.09 million cases) [1]. It is worth noting that the cancer rate in the countries of the Middle East is rising at an alarming rate. It has been predicted that by the year 2030, the cancer rate will double [2,3]. Nonetheless, the rate of occurrence is significantly lower than in Western nations including the United States [3,4].
According to an age-standardized rate, Australia has the highest cancer rate in the world, with 452 new cases reported for every 100,000 people. Next comes New Zealand, with a rate of 422 per 100,000. The U.S.A. occupies a middle position in this list [4]. Yet, Middle Eastern countries report far lower numbers of cancer incidence. Egypt and Lebanon reported the highest rates, with 159 and 165 cases per 100,000 people, respectively [4]; Saudi Arabia and Sudan reported the lowest rates, with 96 and 95 cases per 100,000 people, respectively [3,4]
The prevalence of sedentary lifestyles (correlated with obesity, diabetes, and reduced physical activity) in Arab countries is considerable and is associated with an increased risk of cancer. The lack of exercise is likely due to a combination of factors, including cultural norms in some areas and the excessively hot weather that persists for many months of the year [5,6,7]. Having plenty of domestic help around the house can also play a role. Obese women, particularly post-menopausal women, have a relative risk of developing breast cancer that is more than twice as high as that of normal-weight women [8,9,10]. With the adoption of a Westernized lifestyle, smoking status and exposure to stress are projected to increase among Arabs. From 1990 to 2012, the estimated incidence of smoking was 12.5% higher than it had been in any GCC country, and this trend is projected to continue [11]. In addition, shisha is far worse than cigarettes since it contains significantly more nicotine [12]. For women who have smoked for more than ten years, the chance of developing breast cancer is roughly 10% higher than in never-smokers [7,13,14].
While cancer rates in Arab countries have risen somewhat in recent years, they are still significantly lower than those in the West. Fasting [15,16], food prepared according to specific recipes and rich with spices, vegetables, fruits, seeds, herbs, and olive oil; significantly lower rates of smoking and alcohol use, and genetic predisposition are all proposed explanations for the significantly reduced incidence of cancer in Arab countries [4,17,18,19,20,21,22,23,24,25].
Surgery is frequently used for the initial treatment of localized solid tumors. Nevertheless, targeted treatments, radiation, immunotherapy, and chemotherapy are used at later stages and/or after surgery [26]. Improvements in anticancer drugs have greatly enhanced patients’ quality of life and the length of time without relapse [27]. There are substantial limitations to the use of chemotherapeutic medicines in cancer treatment, including the medications’ solubility and instability, nonspecific drug distribution, and adverse effects related to systemic toxicity [28]. Although the initial therapy may be successful, some patients will experience a recurrence or relapse of their cancer. The failure of cancer treatment is now mostly attributable to acquired drug resistance [27]. Chemotherapy resistance in cancer cells can arise from either innate or acquired mechanisms [29]. The term “acquired drug resistance” describes a condition in which a patient develops immunity to a treatment that was successful in the past. Intrinsic chemoresistance occurs when there is something in the patient that makes the treatment ineffective from the start [30]. The heterogeneity of tumor cells is an underlying cause of resistance to chemotherapy. Heterogeneity is caused by self-renewing subpopulations of tumor cells, which have been identified as stem-like cancer cells. Several clones can be found within a single tumor, and all of them respond differently to chemotherapeutic drugs. Thus, a successful outcome may not be achieved by using a single drug to target tumor cells [31,32,33,34]. Cancer cells can become resistant to chemotherapeutic agents through a number of mechanisms, including upregulated drug efflux, altered drug target, apoptosis, and repair signaling pathways [35,36].
2. Therapeutic Potential of Polyphenols in Cancer
Polyphenols are a huge family of over 8000 plant compounds that share structural characteristics such as a three-membered flavan ring structure and numerous phenol units [36]. These natural substances (around 500 of them) can be found in a variety of foods, including fruits, green and black tea, coffee, red wine, chocolate, and seeds. Polyphenols are a group of organic compounds defined by the presence in them of numerous phenol structural units and found primarily in nature [36]. Subgroups of polyphenols can be established according to the number of phenol rings present and the structural factors that keep these rings together [36]. Phenolic acids, flavonoids, stilbenes, and lignans are the primary types of polyphenols. The concept of treating cancer patients with polyphenols is not novel. In the late 20th century, preliminary research on the anticancer effects of various polyphenols was conducted, and since then, our understanding of these advantageous agents has vastly improved [36,37]. These agents are highly advantageous and intriguing because they attack cancer cells in a variety of ways and target numerous cancer hallmarks. Initiation, promotion, and progression are the three major phases of cancer development [36,37]. The rapid and usual first stage in the development of cancer involves the ingestion of or exposure to a carcinogenic substance, which then interacts with chromatin and causes a mutation or epigenetic alteration [36,37].
The widespread severe side effects and subsequent toxicity generated by most conventional medicines have prompted cancer patients to turn their attention to natural products as a first line of defense [38,39,40]. Meanwhile, the pharmaceutical sector is researching and evaluating novel natural compounds for use in cancer therapy [41,42].
Despite attempts to promote cancer awareness, early detection, and new therapeutic treatments, progress in cancer therapy has been slowed by the emergence of drug resistance, the high costs of treatments, and the growing reports of secondary toxicity [43,44]. In addition, the expenses of treatment are inflated by the fact that most chemotherapeutic medications are known to cause unpleasant side effects such as nausea, vomiting, headache, musculoskeletal pain, anorexia, gastritis, oral ulcers, diarrhea, constipation, alopecia, neuropathy, and so on [45,46].
Natural supplements from different plants have been studied for their potential to treat cancer and its symptoms [47]. Here, natural products such as polyphenols may represent appropriate alternatives, especially when combined with other cancer medications to improve treatment efficacy and safety [48].
As stated earlier, resistance to chemotherapy in cancer cells can be intrinsic, resulting from pre-existing genetic abnormalities; such resistance typically becomes evident after the tumor has been exposed to anticancer medications (first leading to tumor regression) [49]. On the other hand, acquired resistance is created by the drug itself during treatment, leading to disease recurrence after initially successful chemotherapy [50,51]. Cancer cells can develop either intrinsic or acquired resistance when they undergo genomic or epigenomic alterations that promote signaling pathways other than the one targeted by the treatment in progress [52,53,54]. Hence, there are massive efforts to discover appropriate adjuvant drugs that aid in reversing the mechanisms of resistance, thereby favorably improving the efficacy of chemotherapies and, ultimately, disease remission in cancer patients.
3. Mechanisms of Cancer Chemoresistance
There are a number of hypotheses that have been put forward to describe the phenomenon of cancer chemoresistance as a reaction to both host-related factors that inhibit drug uptake across the plasma membrane and various genetic factors that lead to insensitivity to anticancer drugs. These hypotheses have been developed in response to both types of factors.
3.1. Cellular and Noncellular Mechanisms
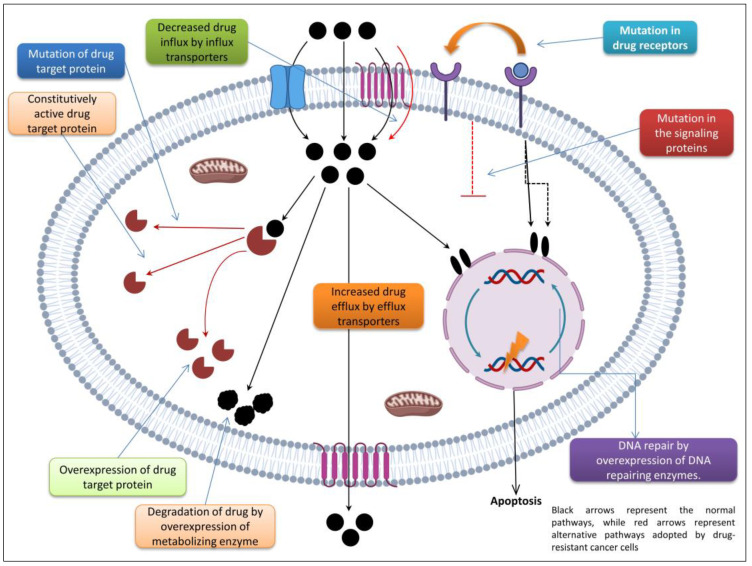
Inherent or natural resistance to chemotherapy upon initial drug exposure is due to noncellular mechanisms (such as low drug bioavailability and metabolic inactivation), while cellular mechanisms involve a wide range of enzymatic, signal transduction, and cellular transport systems [55]. The mechanism depicted in Figure 1 is just one way by which cancer cells become resistant to chemotherapy. The oxygenation status and tumor vascularization appear to play a significant role in the difficulty of successful therapy in cancer patients, as tumor-associated vascular, structural, and functional abnormalities create an acidic environment for cells that are located distal to the vasculature, resulting in poor oxygenation and cell cycle arrest [56]. It has been found that noncycling cells are less sensitive to most chemotherapies [57] and that many malignancies that have hypoxia as an associated feature experience environmental insufficiencies that influence a variety of cellular pathways in the emergence of resistance [58].
Figure 1.
The several ways in which cancer cells develop resistance to chemotherapy.
Enzymatic or non-transport-based mechanisms can also mediate cellular MDR phenotypic activity by altering the biotransformation of anticancer drugs, which is important because metabolic activation is a preliminary step for many of these drugs to exert their deadly effects. In the case of cytarabine [59], for instance, it is first phosphorylated by deoxycytidine kinase to generate cytarabine monophosphate and then further phosphorylated to become cytarabine triphosphate, its active form. Thus, cancer cells acquire resistance by decreasing drug activation through the downregulation or mutation of enzymes implicated in this metabolic pathway, thereby evading the effects of such medications [59]. By overexpressing drug-metabolizing enzymes including CYP-4503A, glutathione-S-transferase (GST), and aldehyde dehydrogenase, cancer cells can become resistant to chemotherapy and other cytotoxic treatments [60].
3.2. Active Membrane Transport (ATP-Dependent Multidrug Transporters)
One of the most common mechanisms of resistance to many standard antineoplastics chemicals is the upregulation of membrane transporters, which are responsible for the efflux of cytotoxic compounds and the maintenance of intracellular drug concentrations below the threshold required for effective cytotoxicity [61]. These ATP-dependent multidrug transporters are part of the ubiquitous ATP-binding cassette (ABC) protein family [62] and help regulate the body’s uptake, distribution, and elimination of several pharmacologically active substances.
There are more than 48 genes that code for ABC transporters, and they fall into seven different gene families. P-glycoprotein (ABCB1, MDR1) is the most understood member of the MDR/TAP family, which also includes the homologs ABCB1-11, while the breast cancer resistance protein (BCRP, ABCG2) is the best-understood member of the ABCG family of transporters [63,64,65]. Different in gene location, amino acid sequence, structure, and substrate selectivity, these resistance-conferring proteins are all a part of the ABC superfamily [66,67,68]. Many of these have been defined in humans and are naturally expressed in a wide variety of healthy tissues as well as neoplasms [69]. P-gp is ubiquitous in tissues involved in the absorption, secretion, and transport of substrates across cellular plasma membranes, but its low expression makes it difficult to detect. MDR-ABC transporters are found in a wide variety of tissues [70]. The epithelial lining of the gut, endothelial cells, bone marrow progenitor xenocells, peripheral blood lymphocytes, and natural killer cells all contain ABCB1 in mammals, while adrenal cortex cells include ABCB2.
The “multiple drug-resistant” “MDR” phenotype is characterized by the ability of cancer cells to develop resistance to a wide variety of drugs, including those that share no structural similarity [71]. Many studies suggest that kidney, colon, pancreatic, and liver cancers, as well as malignancies originating from other tissues with naturally high levels of P-gp expression, may be inherently drug-resistant [72].
3.3. DNA Repair-Mediated Mechanisms of Resistance
Nucleotide excision repair, mismatch repair (MMR), double-strand break repair, base excision repair, and direct repair are all important DNA damage repair processes involved in conferring MDR to cancer cells. A better response of cancer cells to anticancer treatments (e.g., cisplatin and methotrexate) that kill cells via DNA damage-induced apoptosis is achieved through the downregulation of these mechanisms. For instance, in ovarian and breast cancers, selective cancer cell toxicity can be achieved by targeting the DNA repair-associated poly(ADP-ribose) polymerase (PARP) protein [73,74]. Despite the success of PARP inhibitors (PARPi), research in animals and humans has shown that resistance can develop to these drugs, even when they are given in combination [75,76]. The capacity of cancer cells to restore the DNA repair function by reversing a mutation in the BRCA gene is the primary mechanism of resistance to PARPi [77].
The ultimate aim of anticancer medications is to activate cell death or cell cycle arrest pathways. Thus, alterations to these pathways play a significant role in the emergence of MDR, perhaps through the avoidance of apoptotic pathways triggered by the acquisition of inactivating mutations in genes encoding apoptotic proteins (such as p53) or activating mutations in genes encoding antiapoptotic proteins [such as B-cell lymphoma 2 (Bcl-2)]. As mutant p53-associated MDR has been documented in over 50% of all malignancies, p53 role in drug resistance has been studied extensively [78,79].
3.4. Other Facilitators of the MDR Phenotype
The ubiquitin–proteasome system, which helps break down damaged proteins and controls development and the stress response, is another factor influencing the cancer MDR phenotype. This system’s overactivity is linked to cancer cell resistance, whereas its suppression leads to cell death and increases the sensitivity to chemotherapeutic drugs [80]. In addition, recent research has demonstrated that autophagy (i.e., cell “self-digestion”) plays a crucial role in chemoresistance by degrading chemotherapeutic drug molecules, which enables cancer cells to avoid drug-induced apoptosis [81,82]. Hence, blocking autophagy has been found to improve the effectiveness of chemotherapies [83,84]. Apoptosis-to-autophagy conversion has also been identified as a key mechanism of resistance to paclitaxel-induced cytotoxicity in breast cancer cells [85].
Cancer cell defense against oxidative stress and the generation of reactive oxygen species (ROS) is another factor contributing to the MDR phenotype. This defense involves the induction of the overexpression of various antioxidant enzymes by ROS-sensitive transcription factors (e.g., Nrf2), which permits cancer cells to overcome apoptotic signals and, thus, results in chemoresistance and tumor progression [86,87]. Finally, “oncogene addiction” [88] describes how many cancer cells become over-reliant on an oncogene, providing a rationale for developing targeted therapies aimed at oncogene-encoded proteins [89]. Nevertheless, medication resistance due to mutation of the targeted protein may compromise the treatments’ long-term efficacy. Mutations at “gatekeeper” residues, which prevent drugs from entering the kinase back pocket active site, are a common cause of resistance to tyrosine kinase (BCR/ABL and EGFR) inhibitors [90,91,92]. Hence, under therapeutic pressure, the most adaptive or resistant heterogeneous subpopulations of cancer cells will be selected. Following this, the tumor becomes dominated and repopulated by these clones, which renders it extremely resistant to the treatment [93].
4. Approaches to Overcome Drug Resistance
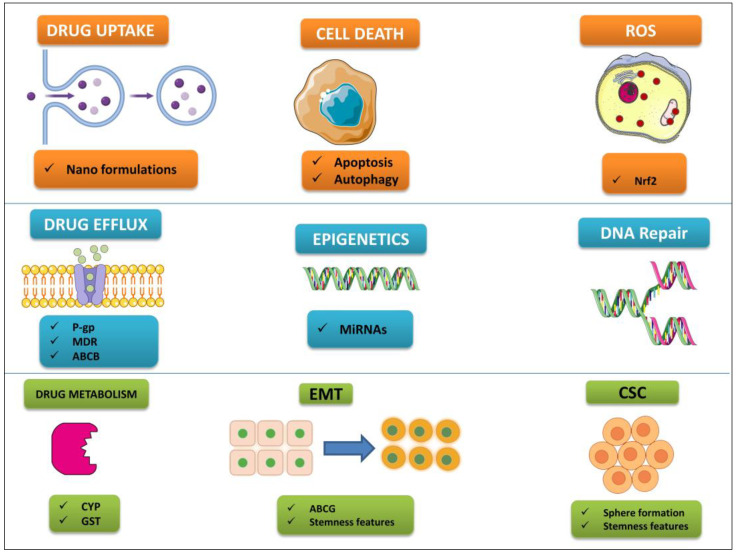
Traditional cancer treatments typically provoke an unfriendly cellular environment, which can result in organelle dysfunction and the development of drug resistance in the area surrounding the tumor. Studies have begun to concentrate more on the use of bioactive anticancer chemicals due to their multi-target specificity, selectivity, and cyto-friendly nature [94,95]. This is done in an effort to mitigate the negative effects that are caused by conventional cancer treatments. In this context, the mechanisms of action of polyphenols have been investigated, in particular with respect to overcoming the drug resistance of certain cancers (see Figure 2).
Figure 2.
Mechanisms of cancer drug resistance and potential involvement of polyphenols.
4.1. Combination Therapy
Disease progression is connected with a variety of processes, including inherent and acquired medication resistance, both of which are heavily regulated by signal transduction pathways. Over the years, numerous strategies have been documented that successfully sidestep resistance pathways to cytotoxic, hormonal, and biologic drugs. As both genetic and epigenetic alterations are dynamic, they cooperate to keep the malignant phenotype in “homeostasis” in the face of a selection pressure [96]. Combination therapy, in which two or more chemotherapeutic agents with different mechanisms of action and different pathways to drug resistance are used, is widely accepted as a means of reducing the impact of drug resistance by preventing the use of alternative intracellular escape pathways necessary for tumor survival [97]. This strategy seeks to induce complementary/synergistic rather than merely additive effects; however, it usually comes with a high price tag, which makes it unattractive to many patients [98,99].
4.2. Pharmacological Inhibition of Membrane Efflux Transporters
The MDR phenotype has been studied extensively because it presents the greatest barrier to successful chemotherapeutic intervention against cancer. Several medications have been discovered to act as pharmacological antagonists of drug exporters, most notably P-gp. Depending on their affinity for a specific transporter(s) and their relative toxicity toward normal cells, MDR antagonists are categorized as either first-, second-, or third-generation drugs. These compounds function as modulators, reversal agents, or inhibitors of drug efflux [100].
Most modulators, however, have the issue of being carried by P-gp itself. Hence, their inhibitory impact is through competition, necessitating extremely high concentrations to be effective in vivo, leading to intolerable side effects such as cardiotoxicity and immunosuppression for the P-gp inhibitors verapamil and cyclosporine A, respectively [101]. Yet, there is another class of modulators that P-gp does not carry. To this end, vinblastine efflux is severely inhibited by hydrophobic steroids such as progesterone, megestrol acetate, and medroxyprogesterone, in contrast to what is observed for their more transportable hydrophilic counterparts [102]. While RU486 (an antiprogestin) is a powerful modulator in the lab, its hormone characteristics make it potentially dangerous in humans [103]. Clinical trials have been conducted using hydrophobic antiestrogens such as tamoxifen and its variants to treat breast cancer; however, because these compounds act as growth agonists in some uterine cells, they may increase the risk of endometrial cancer [104,105]. Inhibitors of growth factors and protein kinase C are two novel experimental techniques that have the potential to prevent or postpone the development of membrane transport-associated resistance [106].
4.3. Reversal of Drug Resistance by Naturally Occuring Polyphenolic Compounds
The systemic toxicity of chemotherapeutic drugs can now be minimized, while treatment efficacy is maximized, thanks to the development of successful alternative techniques. Dietary supplements and other phytotherapeutic substances are being studied for their potential to enhance the effectiveness of anticancer medications. Polyphenolic compounds with an extraordinary capacity to reverse drug resistance in vitro and in vivo may be discovered with the help of recent investigations in various drug-resistant cancer cell lines [107], and these compounds may be viable candidates for future clinical applications in cancer treatment [107]. The most recently investigated polyphenols and the chemoresistance mechanisms involved are summarized in Table 1 and Table 2.
Table 1.
Summary of polyphenol and chemotherapeutic combination therapies in in vitro preclinical investigations.
| Polyphenol | Cancer | Chemotherapy Drug | Dosage | Assay | Molecular Effect | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| Curcumin | Lung cancer |
Cisplatin | 2–32 µM curcumin + 0.5–8 µg/mL cisplatin. | A549, H1299, NCI-H460 cell lines | Upregulating the levels of CTR1 and Sp1 to increase more Pt2+ uptake. | Enhancing sensitivity and antitumor effects of CIS in NSCLC | [108] |
| Colorectal cancer | Oxaliplatin | HCT116 and SW480 cells 0–8 µM curcumin + 0.5–32 µM oxaliplatin; HCT116/oxaliplatin cells 4 µM curcumin + 8 µM oxaliplatin | HCT116, SW480, HCT116/Oxaliplatin drug-resistant cell lines | Inhibition of TGF-β/Smad2/Smad3 signaling | Inhibition of cell proliferation and reduced tumor weight and volume | [109] | |
| Breast cancer | Doxorubicin | 25 µM curcumin + 5 µM doxorubicin | MCF-7/doxorubicin drug-resistant cell line | Reduced Aurora-A expression. Triggered p53 stabilization. Growth arrest and apoptosis induction | Reversed doxorubicin insensitivity and increased sensitivity in doxorubicin-resistant MCF-7 and MCF-7 cell lines | [110] | |
| Quercetin | Liver cancer | Doxorubicin; 5-fluorouracil (5-FU) | 40–160 µM quercetin + 0.2–125 µg/mL doxorubicin/5-FU | BEL-7402 and BEL-7402/5-FU drug-resistant cell lines | Inhibition of FZD7/β-catenin pathway and ABCB1, ABCC1, and ABCC2 efflux pump | Enhanced doxorubicin and 5-FU sensitivity | [111] |
| Colorectal cancer | Doxorubicin | 33 µM quercetin + 0.5 µM doxorubicin | SW620/ doxorubicin drug-resistant cell line and SW620/Ad300 cell line | Reversed P-gp-mediated drug resistance, increased intracellular doxorubicin accumulation; modulated glutamine metabolism in doxorubicin-resistant cells by inhibition of SLC1A5 | Reversed MDR, enhanced sensitivity to doxorubicin | [112] | |
| Resveratrol | Lung cancer | Gemcitabine | 10 µM RES + 1 µM gemcitabine | HCC827 cell lines and HCC827 | Downregulation of mRNA and protein levels of ENG, activation of ERK signaling pathway | RES promoted tumor microvessel growth, increased blood perfusion and drug delivery into tumor that resulted in enhanced anticancer effect of GEM | [113] |
| Gastric cancer | Cisplatin | 20 μM RES + 1 μg/mL cisplatin | AGS cell line | Upregulation of Bax and the cleaved form of PARP, downregulation of Bcl-2, increased PERK, p-eIF2α, and CHOP protein levels. Activation of PERK/eIF2α/ATF4/CHOP signaling pathway, induction of G2/M cell cycle arrest | Synergistically inhibited cell growth of cancer cell lines | [114] | |
| EGCG | Breast cancer | Arsenic trioxide and/or irradiation | 10–100 µM EGCG + 2 Gy radiation; 10–100 µM EGCG and 4 µM arsenic trioxide. 10–100 µM EGCG, 4 µM arsenic trioxide and 2 Gy radiation. | MCF-7 cell lines | Bax upregulation and Bcl-2 downregulation | Combination of EGCG and Arsenic trioxide with or without radiation showed synergistic effects in breast cancer treatment visible in the rise of cell death | [115] |
| Lung cancer | Doxorubicin | 0.5 μM EGCG + 0–100 μM doxorubicin | Nonresponsive A549 cell line | Decreased drug efflux, MDR signaling, and invasiveness. Increased drug internalization, cell cycle arrest, stress induced damage, and cell death | EGCG reversed the compromised functionality of doxorubicin in a nonresponsive A549 cell line and improved its oxidative damage-mediated antitumor effect by modulating redox signaling | [116] | |
| Apigenin | Colorectal cancer | 5-FU | 20 µM apigenin + 20 µM 5-FU | HCT116 and HT29 cell lines | Inhibited the upregulation of TS induced by 5-FU. Increased reactive oxygen species production, intracellular and intramitochondrial Ca2+ concentrations, and mitochondrial membrane potential | Apigenin enhanced the efficacy of 5-FU by potentiating HCT116 cell apoptosis and enhancing cell cycle disruption. Acquired resistance to 5-FU was reduced | [117] |
| Breast cancer | Cisplatin | 5–100 μg/mL apigenin + 5–100 μg/mL cisplatin | MDA-MB-231 and HCC1806 cell lines | Inhibition of telomerase activity. Down-regulation of hTERT, Hsp90, and p23 at transcriptional and translational levels | Apigenin and cisplatin synergistically inhibited telomerase activities by reducing the catalytic subunit of the enzyme | [118] |
Table 2.
Summary of polyphenol and chemotherapeutic combination therapies in in vivo preclinical investigations.
| Polyphenol | Cancer | Chemotherapy Drug | Dosage | Assay | Molecular Effect | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| Apigenin | Liver cancer | Paclitaxel | 1 mg/kg/day apigenin + 3.5 mg/kg/day paclitaxel | Balb/c nude mice | Suppressing the intratumoral expression of HIF-1a via inhibiting the AKT/p-AKT pathway and the expression of HSP90 simultaneously | Apigenin reduced hypoxia-induced paclitaxel resistance in hypoxic tumors | [119] |
| Lung cancer | Navitoclax | 25 mg/kg apigenin + 100 mg/kg ABT-263 | BALB/c nude mice | Upregulated the expression of Noxa by targeting the AKT–FoxO3a pathway and inhibited ERK | Apigenin synergized with ABT-263 by suppressing the growth and proliferation of tumor cells in vitro and in vivo | [120] | |
| EGCG | Lung cancer | Cisplatin | EGCG (1.5 mg/mouse/day IP) for 5 days and cisplatin (2 or 4 mg/kg IP) on day 5; EGCG (1.5 mg/mouse/day) and single-dose cisplatin (2 mg/mouse) on day 0 or 5 | A549 cell xenograft bearing BALB/c nude mice | Increased cisplatin concentration in tumor tissue and tumor growth delay due to EGCG-induced vascular normalization | EGCG synergistically potentiated cisplatin antitumor efficacy especially when cisplatin was applied during the vascular normalization window | [121] |
| Liver cancer | Sorafenib | 100 mg/kg EGCG + 10 mg/kg sorafenib | Diethyl nitrosamine-induced hepatocellular carcinoma in Wistar albino rats | Histopathological observations revealed a satisfying decline in tissue degeneration and hyperchromatism. Significantly lower alpha-fetoprotein and liver enzyme levels were detected, as well as a greater antioxidant capacity | EGCG and sorafenib combination had a comparable effect as that of standard-dose sorafenib. The combination resulted in enhanced chemoprotection and is considered effective against hepatocellular carcinoma | [122] | |
| Resveratrol | Colorectal cancer | 5-FU | 10 mg/kg b.w. resveratrol p.o./day + 12.5 mg/kg b.w. 5-FU i.p. injected on days 1, 3, and 5; repeated every 4 weeks for 4 months | Methyl nitrosourea-induced colon cancer in male albino rats | Decrease of NF-κB and reduction of COX-2, induced p53 gene expression | Resveratrol biochemically modulated and enhanced the therapeutic effects of 5-FU | [123] |
| Lung cancer | Gemcitabine | 25 mg/kg gemcitabine i.p. 2×/week + 1 µmol/kg resveratrol 5×/week | HCC827 xenografts in nude mice | Downregulation of mRNA and protein levels of ENG, activation of ERK signaling pathway | Resveratrol promoted tumor microvessel growth, increased blood perfusion and drug delivery into tumor that resulted in enhanced anticancer effect of gemcitabine | [124] | |
| Quercetin | Breast cancer | Cisplatin | 30 mg/kg quercetin + 7 mg/kg cisplatin | Breast tumor-bearing mouse model | Inhibited tumor growth and reduced renal toxicity | Synergistic effect; inhibited renal toxicity induced by cisplatin | [125] |
| Liver cancer | Sorafenib | 7.5 mg/kg/day sorafenib, 2 h later 50 mg/kg/day quercetin | Chemically induced HCC rat model | Suppressed proliferation, enhanced apoptosis and necrosis | Synergistically increased anticancer effect and increased liver recovery | [126] | |
| Curcumin | Lung cancer | Gefitinib | 1 g/kg CUR + 100 mg/kg gefitinib | BALBL/c mice | Inhibition of Sp1/EGFR activity to induce autophagy-mediated apoptosis | Reduction in tumor volume. Elevated the sensitivity to gefitinib in NSCLC patients with mutated EGFR | [127] |
| Liver cancer | 5-FU | 56.65 mg/kg curcumin + 10 mg/kg 5-FU | BALB/c nude mice | Decreased expression of NF-κB protein in the nucleus. Increased expression of NF-κB protein in the cytoplasm. Downregulation of COX-2 expression | Synergistic effects and in vivo tumor growth inhibition | [128] |
As a result of the drawbacks of existing pharmacological MDR modulators, researchers are looking for novel molecules that may be effective at manageable levels and have fewer side effects. Hence, recent studies have revealed that natural compounds such as plant polyphenols may be effective MDR modulators (Table 1 and Table 2) [107]. Most of these natural chemicals are necessary for human survival, and flavonoids, in particular, have demonstrated exceptional profiles of safety and tolerability [36] even when administered at extremely high dosages (e.g., 1.0 g dietary polyphenol/day). Clinical experiments including these substances have shown promising findings, indicating that polyphenols may have an anticancer effect; furthermore, multiple preclinical research suggests that increasing polyphenol consumption or their usage as adjuvant therapy for cancer treatment is warranted [36].
4.4. Flavonoid Antagonism of Drug Efflux Transporters
Vegetables, fruits, herbs, and drinks include flavonoids, which are a class of polyphenolic compounds found in plants and characterized by their structural diversity due to the presence of two or more aromatic rings in their molecular makeup [36]. Flavones, isoflavones, flavonols, flavanones, chalcones, catechins (flavan-3-ols), and procyanidins are subclasses based on the presence or absence of certain substitutions [36,37]. The relevance of dietary flavonoids in cancer prevention or treatment has been extensively debated due to their wide range of bioactivities, including antioxidant, anti-inflammatory, antiviral, antiproliferative, and proapoptotic properties [36,37]. Flavonoids are well-known for their ability to interact with ABC transporters [129], impeding the binding of drugs to the transporters in either a competitive or an allosteric manner, therefore preventing the export of antineoplastics [130]. By binding to members of the ABC transporter family, flavonoids can either increase or decrease the transporters’ ATPase activity [131].
Flavonoids may potentially affect MDR transporter protein expression [132,133]. Many P-gp inhibitors have been identified and investigated as potential MDR-reversing drugs, meaning they can turn chemoresistance into chemosensitivity [134]. In particular, quercetin has been shown to have antiproliferative effects in a wide variety of cancer cell lines and animal models; hence, it is a well-established anticancer chemical. Despite the lack of clinical evaluation of its chemo-sensitizing impact, preclinical studies have shown significant promise of the use of this compound as an adjuvant to standard chemotherapy (Table 1 and Table 2), which may improve the therapeutic results for various malignancies, including melanomas. Finally, researchers [101] showed that quercetin might sensitize temozolomide-resistant DB-1 melanoma (wild-type p53) and SK-Mel-28 (mutant p53) cell lines through the regulation of p53 family members. Multidrug-resistant MCF-7 human breast cancer cells showed enhanced Adriamycin accumulation in the presence of quercetin, according to preliminary studies of this chemical as a P-gp modulator [101].
Certain flavonoids have the ability to reverse MDR, just like the well-known but highly lethal P-gp inhibitors verapamil and cyclosporine A [135]. By modulation of death receptor 3, a receptor for tumor necrosis factor (TNF), fisetin induces apoptosis, inhibits proliferation, and inhibits invasion in chemoresistant pancreatic cancer cells [101]. Several studies looked at the structure–activity connections of various flavonoids that were shown to impair P-gp-mediated transport. Flavanone (naringenin), flavone (baicalein), flavonols (flavanols), kaempferol, quercetin, myricetin, morin, fisetin, and two additional glycosides of quercetin were among the substances studied [101]. Drug buildup was observed in P-gp-overexpressing human epidermal carcinoma KB-C2 cells due to a suppression of the P-gp-mediated efflux of daunomycin, as demonstrated by the aforementioned studies. In terms of promotion of daunomycin accumulation, kaempferol was followed by quercetin, baicalein, myricetin, fisetin, and morin, among flavonoids [101]. Flavonols not only lowered P-gp expression but also impeded P-gp-mediated drug transport [101].
Flavonoids have been demonstrated to interact with the ATP-binding domains of P-gp and to reduce its ATPase activity, which is essential for drug translocation. P-gp has been well recognized as an ATP-driven drug export pump. Both the ATP-binding site and the steroid-interacting sections of P-gp cytosolic domain are targets for these drugs [136]. Certain flavonoids, in contrast to those that impede organic anion transport by binding to and activating MRP1, increase the transport of reduced glutathione (GSH) [137]. The largest (six-fold) increase in GSH transport by apigenin was observed at a dose of 30 mM, when multiple flavonoids (apigenin, naringenin, genistein, and quercetin) were employed to stimulate MRP1-mediated GSH transport. Flavonoids have been hypothesized to promote GSH transport by elevating MRP1 apparent affinity for GSH [138]. In addition, quercetin was shown to inhibit a heat shock factor in a P-gp-independent manner, reducing MDR [139]. The results of this study were corroborated by another that found flavonoid-rich foods caused tumor cells to accumulate more doxorubicin, allowing MDR to be reversed [140]. Research on this topic has yielded mixed results, although the vast bulk of the information points in a positive direction. Regarding this, various studies disproved the hypothesis that the isoflavone genistein has no effect on cancer cell P-gp-mediated MDR [141,142,143].
Research into the structural basis of MDR activity modulation by flavonoids and the establishment of a strong structure–activity relationship for the ultimate selection of a polyphenolic lead molecule have also been intensively pursued. P-gp active site has been used to test the binding affinities of several synthetic analogues [144]. It was also revealed [145] that several flavonoids may selectively revert BCRP-mediated drug resistance in various chemoresistant leukemia cell lines. The possible clinical benefits include enhanced efficacy and reduced toxicity of cancer chemotherapies made possible by these flavonoid BCRP inhibitors [145]. In addition to enhancing the bioavailability of doxorubicin, quercetin was demonstrated to decrease drug resistance by competitively inhibiting P-gp, MRP1, BRCP, and the metabolizing enzyme cytochrome P4503A4 [146]. As an added bonus, quercetin was found to exert antiproliferative and apoptosis-inducing actions on doxorubicin-resistant human gastric cancer cells [147].
As an ABC transporter, the BCRP protein plays a crucial role in healthy bodily processes [148]. Moreover, BCRP is crucial in regulating access to pharmaceuticals [149]. Currently, there are not many studies looking for particular inhibitors of this transporter. Several tamoxifen derivatives and the hormones estrone and estradiol have been found to overcome BCRP-mediated medication resistance in cancer [150]. There is also strong evidence that flavonoids can act as powerful BCRP-specific inhibitors. The accumulation of BCRP substrates is stimulated by silymarin, hesperetin, quercetin, and daidzein in resistant but not wild-type breast cancer cell lines [151]. The polyphenols genistein and naringenin were shown to interact with BCRP at higher concentrations than estrone [101]. Certain flavonols, such as kaempferide and kaempferol, and several flavones, such as acacetin, apigenin, chrysin, diosmetin, and luteolin, have great potential to reverse BCRP-mediated drug resistance, and they are safe enough to employed in clinical practice [101].
4.5. Dietary Polyphenol Inhibition of Oncogenic Signaling Pathways and Enhancement of Tumor-Suppressive Pathways
Dietary polyphenols have been found to act as chemopreventive agents by disrupting signal transduction pathways involved in carcinogenesis [152]. Cell cycle arrest, induction of apoptosis, antioxidant and anti-inflammatory actions, and suppression of angiogenesis are some of the outcomes of their interference with the cell’s natural processes [153]. A comparable effect of resveratrol on ovarian cancer cells’ sensitivity to cisplatin was observed through its ability to modulate cancer cell growth via MAPK inhibition [101]. There are many NF-kB-controlled genes implicated in tumorigenesis that have been shown to be downregulated when NF-kB activity is suppressed. These genes include tumor necrosis factor alpha (TNF-a), cyclooxygenase-2 (COX-2), cyclin D1 (cyclin D1), matrix metalloproteinase (MMP)-9 [154,155]. Curcumin and other polyphenols have inhibitory effects on NF-kB, which is important because most anticancer medicines activate NF-kB, leading to resistance [101].
Curcumin, like other phenolic chemopreventive agents, reduces the activity of the oncoprotein NF-kB and induces a proapoptotic cellular state by upregulating proapoptotic signaling proteins (p53 and p21) and downregulating cell survival proteins (phosphatidylinositol 3-kinase, protein kinase B, nuclear factor kappa B, and AP-1) [101].
Epigallocatechin gallate (EGCG), a component of green tea, inhibits the proliferation of human breast cancer cells by reducing the production of survivin, a member of the inhibitor of apoptosis protein family that is highly expressed in a wide variety of cancer cells and tissues [101].
4.6. Polyphenol Resensitization of Drug-Resistant Cancer Cells and Tumors
Milk thistle seed silibinin was proven in numerous in vitro and in vivo investigations to successfully sensitize cancer cells to apoptotic processes generated by a wide variety of chemotherapy drugs [156,157]. The invasiveness of human ovarian cancer cells resistant to paclitaxel was reduced in vitro after treatment with silibinin [158]. If this effect is replicated in vivo, silibinin would be a great contender as an adjuvant for paclitaxel, suggesting a potentially favorable chemotherapeutic regimen, particularly for patients with tumors that are resistant to paclitaxel alone. Cell growth inhibition by cisplatin or carboplatin in hormone-refractory DU145 prostate carcinoma cells was increased to 100% when silibinin was added, according to another study. This was due to a more robust G2-M arrest brought about by the reduced expression of Cdc2, cyclin B1, and Cdc25C, all of which are crucial for the transition from the G2 to the M phase [159]. Similarly, A549 lung cancer tumor xenografts were suppressed in growth, cell proliferation, and angiogenesis, and apoptosis was triggered when nude mice were orally fed silibinin (200 mg/kg) after being pretreated with doxorubicin (4 mg/kg). This anticancer impact was accompanied by less doxorubicin-induced systemic toxicity, which was a notable finding when the medicines were taken together [154]. As for methotrexate, silibinin dihemisuccinate increased the cytotoxic activity of methotrexate in a concentration-dependent manner, making methotrexate-resistant human rhabdomyosarcoma cell lines more sensitive to the drug [160].
Therapeutic approaches that target DNA repair pathways are an attractive option for making chemoresistant cancers more sensitive to treatment [161]. Inhibiting DNA repair and inducing death in MDR cells via modulation of autophagy were described following a short-term treatment with curcumin. Tumor angiogenesis and metastasis can be stifled by curcumin because it reduces the levels of vascular endothelial growth factor (VEGF) [162]. Downregulation of MMPs and VEGF is a possible mechanism by which quercetin, genistein, resveratrol, and other phenolic acids are able to suppress angiogenesis in in vitro cell-based systems and animal models [163,164]. The metastatic process may be slowed by the fact that polyphenolic chemicals also suppress the expression of vascular adhesion molecules [165,166].
Researchers found that the polyphenols in red raspberries can slow the expansion of a variety of cancer cell lines by inducing cell cycle arrest, apoptosis, or autophagy-associated cell death in a dose-dependent way [167]. At greater concentrations, berry extracts can also resensitize fulvestrant-resistant cells to treatment (1 mM). This suggests that berry extracts not only improve the response of resistant breast epithelial cells to the antiestrogen fulvestrant, but also raise the cell death response in susceptible cell lines [168].
4.7. Polyphenols and Cellular Metabolism in Cancer
Recent research suggests that regardless of their tissue of origin, nearly all cancer cells have a common trait of poor cellular energy due to uncontrolled metabolism [36,169,170,171]. There are significant differences between the metabolic features of cancer cells and those of normal cells when it comes to the generation of cellular energy [172]. Cancer cells, in contrast to normal cells, rely abnormally on anaerobic glycolysis and substrate-level phosphorylation to meet their energy demands, with glucose and/or glutamine as substrates [173]. Increased fatty acid production and elevated glutamine metabolism are just two examples of tumors’ unusual metabolic features [174,175]. Cancer cells, in contrast to normal cells, which typically rely on growth factor signaling to make use of energy resources [176], break down these resources via glycolysis and glutaminolysis to divert the intermediate products that are required for biosynthetic pathways and the maintenance of cellular growth and proliferation [177]. As a result of this metabolic transition, cells are no longer limited in their ability to grow and divide.
Damage to mitochondrial respiration, a possible cause of cancer, leads to fermentation (glycolysis) and dysregulated cellular metabolic processes [178]. In light of these hallmarks of cancer cells, the disease is increasingly thought of as an interchangeable process involving metabolic abnormalities and genetic mutability. Metabolic problems associated with genomic instability are commonly preceded by hypoxia and mitochondrial malfunction, which have been linked to cancer and resistance [179,180]. If left untreated, this issue can progress to the point of somatic genomic instability, which in turn can cause additional mitochondrial abnormalities and metabolic rigidity in tumor cells [181,182]. Recent studies have found that energy restriction mediated by interfering with glucose or glutamine metabolic pathways is closely related to improvement in cancer therapeutic response and tumor regression [183,184,185], lending credence to the hypothesis that dysregulated cellular metabolism is linked to drug resistance in cancer therapy. Therefore, compounds that affect particular metabolic components and enzymes (such as glucose transporters, hexokinase, pyruvate kinase M2, lactate dehydrogenase A, glutaminase, and fatty acid synthase) associated with dysregulated cellular glycolysis, glutaminolysis, and fatty acid synthesis may improve the therapeutic response or decrease resistance to conventional anticancer agents [186]. Combining chemotherapeutics with inhibitors of cellular metabolism is also regarded to be promising; however, this approach to overcoming chemoresistance has not been thoroughly investigated [187].
The majority of bioactive chemicals with medicinal potential are found in nature. Flavonoids and dietary polyphenolic chemicals are just two examples of these food components that have been shown to have anticancer action [36,180]. Dietary polyphenols have been linked in numerous studies to controlling glucose and lipid metabolism via a variety of mechanisms and pathways [188]. In several types of cancer cells, polyphenols were shown to regulate glucose transporter activity. Well-known inhibitors of glucose absorption include myricetin, quercetin, genistein, cyanidin, hesperetin, naringenin, and catechin [189,190,191]. To destroy cancer cells and make them more sensitive to chemotherapy, polyphenols are used to inhibit their basal glucose transport [192]. EGCG, curcumin, and resveratrol are three of the most researched and potentially cancer-preventing polyphenols. These compounds’ chemopreventive effects can be explained in a number of different ways, including the fact that they interfere with or even reverse the carcinogenic process by influencing molecules in the intracellular signaling network that play a role in the development and/or maintenance of cancer [193,194,195].
In addition to mediating hypoxia-induced MDR in cancer cells by preventing drug-induced apoptosis and lowering intracellular drug accumulation, polyphenols can inhibit the activation of hypoxia-inducible factor-1 (HIF-1a), the central molecule responsible for controlling the expression of glucose transporters and the other key glycolytic enzymes [196,197].
By blocking the production of HIF-1 and its target genes GLUT-1, hexokinase II (HKII), and VEGF, polyphenols are thought to sensitize cancer cells to chemotherapy [198]. Additionally, many polyphenols can inhibit the mitochondrial metabolism, a key mechanism in cancer development [199,200]. Reduced mitochondrial membrane potential and an imbalance in the cellular energy molecule (ATP/ADP) as a result of ATP synthase inhibition are two telltale signs of mitochondrial malfunction. Alterations in the expression of apoptotic markers such as Bax and Bcl-2 are also linked to mitochondrial malfunction [201]. Polyphenols found in tea and curcumin have been shown to trigger apoptosis in a variety of cancer cell types by disrupting mitochondrial metabolism and function [202,203,204]. Several studies point to mitochondrial dysfunction in cancer as a result of redox changes. Examples include a new mechanism for the regulatory role of curcumin in this phenomenon [205]. Last but not least, one intriguing approach to inhibiting cancer cell survival and resistance is to interfere with their lipid metabolism. Many natural polyphenols’ anticancer effects are thought to stem from their capacity to regulate the lipid metabolism [206], specifically by blocking the overexpression and activation of the enzyme fatty acid synthase [207].
4.8. Anti-Metastatic and Epigenetic Effects Exerted by Polyphenols
The term “metastasis” refers to the process by which cancer cells spread from their original location to other parts of the body, where they might cause even more damage. Microenvironmental components, such as stromal fibroblasts and immune cells, influence tumor cell behavior and promote metastasis. The key processes that prime tumor cells for infiltration are cellular motility, hypoxia, epithelial–mesenchymal transition (EMT), and angiogenesis [208,209,210]. Studies have shown that matrix metalloproteinases (MMPs), transforming growth factor beta (TGF-β), and TP53 all play critical roles in metastasis management [208,209,210].
Multiple stages of this process have been demonstrated to be susceptible to modification by polyphenols. For example, curcumin reduces the metastatic properties of cancer cells by influencing EMT-related proteins such as vimentin, fibronectin, β-catenin, and E-cadherin as well as genes expressed in cancer stem cells such as Oct4, Nanog, and Sox2 [211]. Additionally, quercetin and its derivatives are able to block EMT, MMP secretion, NF-kB, and cancer cell migration and metastasis [212,213,214,215]. Resveratrol inhibits metastasis by reversing EMT via AKT/GSK-3β/Snail signaling and reducing MMP-2 and 9 as well as Smad2 and 3 levels [216].
Tumor development, metastasis, and therapeutic resistance stem from epigenetic dysregulations and aberrations [217]. Cancer is characterized by epigenetic abnormalities including, but not limited to, DNA methylation, histone modifications, chromatin/nucleosome remodeling, and miRNA regulation [217].
Among the polyphenols, curcumin is one of the most effective at preventing these modifications from helping cancer cells. The enzymes known as histone deacetylases (HDACs) remove acetyl groups from histones, thereby contributing to gene silencing [218]. Curcumin has been shown to inhibit these enzymes and hence control the growth and death of cancer cells [219]. Curcumin has been shown to block the activity of histone deacetylases 1 (HDAC1), 2 (HDAC2), 3 (HDAC3), 4 (HDAC4), and 8 (HDAC8) [219,220,221]. Histone acetyltransferases (HATs) are another group of enzymes that can be used to foretell whether or not cancer cells will proliferate and survive. Some studies have found that curcumin inhibits these enzymes, and one of them is p300 [222,223]. This inhibition might occur directly or indirectly.
In addition, curcumin inhibits DNA methylation in the promoter region of numerous cancer-related genes by lowering the amount of DNA methyltransferase 1 (DNMT1) [224,225]. This includes the tumor suppressor gene Wnt inhibitory factor-1 (WIF-1) [226], FANCF [227], Nrf2 [228], Neurog1 [229], and RARβ2 [230].
Curcumin regulates microRNAs (miRNAs) including miR-125-5p, miR-19a, miR-9, and miR-145 in nasopharyngeal, breast, and ovarian cancer and leukemia [231]. Comparatively, resveratrol can affect the expression of miR-200, miR-122-5p, miR-20, and miR-633, just like other polyphenols can [232,233,234,235]. In cancer cells, quercetin controls the expression of many microRNAs [236,237,238,239], including miR-16, miR-22, miR-200b-3p, and miR-146a. In addition to EGCG, genistein regulates a number of other microRNAs [240,241] that play a role in correcting epigenetic changes in cancer cells.
4.9. Targeting Cancer Stem Cells Using Polyphenols
Less than one percent of the cells in a tumor are cancer stem cells (CSCs), a subtype of tumorigenic cells that are distinguished by their capacity for self-renewal and multipotency [242,243]. Researchers found that CSCs are to blame for the poor response of tumors to chemotherapy and radiation in virtually all cases [244,245,246,247,248,249,250,251,252]. The main reasons why CSCs provide resistance to our common therapies are their stemness features and the fact that they slow down the cell cycle, possess an anti-apoptotic machinery, have a high capacity for repairing DNA damage, and have the power to establish a proper environment for cancer growth [242,243,253,254]. Other factors that aid CSCs in this process include the Notch, Wnt, STAT3, PI3K/Akt, and NF-kB signaling pathways, protective autophagy, metabolic flexibility, and oxidative modulators [254,255]. It may be said that CSC targeting is a viable strategy for lowering tumor resistance and raising the efficacy of our standard medicines.
Recently, a novel idea for treating resistant tumors has emerged: using polyphenols to specifically target these cells. Curcumin has been shown to have anti-CSC properties in a variety of cancer types, including colon [256], pancreatic [257], liver [258], breast [259], and brain [260]. Colon cancer cells treated with curcumin had reduced levels of CSC markers such as CD44, CD133, and CD24 and a diminished ability to form a sphere [256]. Curcumin, either alone or in combination with irinotecan (CPT-11), induced apoptosis in CSCs, leading to less resistance to a chemotherapeutic medication [256]. Curcumin was also linked to suppressing CSC stemness by blocking the EZH2 polycomb repressive complex 2 (PRC2) subunit [257,261]. Curcumin increased the sensitivity to gemcitabine in pancreatic cancer cells by inhibiting a long non-coding RNA called PVT1 [257]. Curcumin can also influence genes including Nanog, Sox2, and Oct4 that are involved in stemness [259]. Curcumin’s capacity to reduce antiapoptotic protein levels and raise proapoptotic protein levels in CSCs is another method by which it reduces chemoresistance [262]. Bcl-2 and Bcl-w are examples of the former, while Bax, Bak, Bad, Bik, and Bim are examples of the latter. By doing so, mitomycin C resistance in breast cancer can be lowered [262]. Curcumin has shown promise in the treatment of brain cancer when used in conjunction with nanomedicine [260]. Anti-aldehyde dehydrogenase-grafted curcumin-loaded nanoparticles not only improved curcumin’ capacity to cross the blood–brain barrier but also released the polyphenol steadily over time [260]. Curcumin’s ability to make breast cancer cells more vulnerable to mitomycin C was also shown in an in vivo investigation [263]. Breast cancer stem cells were inhibited by a combination of curcumin and paclitaxel [263]. This action was mediated through the ATP-binding cassette (ABC) transporters ABCG2 and ABCC1.
The polyphenol EGCG can also inhibit CSC features including proliferation and migration in nasopharyngeal cancer cells [264]. In an osteosarcoma cell line, baicalin, a flavone, was tested for its role in EMT and was found to diminish anoikis-related resistance by inhibiting the EMT-inducing transcription factors Snail1 and Slug [265]. In addition to reversing EMT and decreasing the cisplatin resistance of lung cancer cells (in vivo and in vitro), baicalin also suppressed the PI3K/Akt/NF-kB pathway [266].
The anti-resistance effects of EGCG in cancer stem cells were further supported by an in vivo study. Researchers found that by inhibiting colorectal cancer stem cells with EGCG, they were able to increase the levels of tumor-suppressing microRNAs and make the cells more sensitive to the chemotherapy drug 5-fluorouracil (5-FU) [267].
4.10. Clinical Studies of Polyphenols with Promising Anticancer Effects
Polyphenols have emerged as a promising new class of therapeutic agents, and their usage in clinical studies to investigate potential health benefits in various cancers has increased dramatically in recent years [268]. Curcumin, EGCG, resveratrol, quercetin, apigenin, and kaempferol were all shown to have strong anticancer effects, but most of these research was conducted in preclinical models [269]. Experimental results in cellular/animal models cannot be considered to be applicable to people, mainly because of variations in genetics and metabolism; hence the bioactivities of polyphenols must also be explored in detail in humans. Most of these investigations will focus on learning more about the substances’ pharmacokinetics, pharmacodynamics, and safety and the processes by which they manifest their effects. Table 3 summarizes the results of some recent clinical trials involving a variety of polyphenols for the treatment of various cancers.
Table 3.
Recent clinical trials using polyphenols against cancers (adapted from ref. [268]).
| Polyphenol | Type of Cancer | Outcome | Status |
|---|---|---|---|
| Resveratrol | Liver Cancer | Improvement of the Metabolic Profile of Liver Cells | Withdrawn |
| Caffeic Acid | Esophagus Cancer | 1-Year Overall Survival (OS) | Ongoing |
| Quercetin | Primary Prevention of Prostate Cancer | Log2-Transformed PSA Measurements | Ongoing |
| Retinol | Lung Cancer | Prevention Of Lung Cancer | Completed |
| Alpha-Tocopherol | Head and Neck Neoplasms | Prevention of Second Primary Cancers | Completed |
| Grapes | Colon Cancer | Localization of β-Catenin and Wnt Target Gene Expression in Intestinal Mucosa | Withdrawn |
| Retinol | Lung Cancer | Lung Cancer Incidence | Completed |
Moreover, polyphenols, when given as an adjuvant, were demonstrated to increase chemosensitivity in tumor cells, as reported in Table 4.
Table 4.
Polyphenols that make tumor cells more sensitive to chemotherapeutic drugs (adapted from ref. [107]).
| Polyphenol | Chemotherapy Drug | Result |
|---|---|---|
| Apigenin | Cisplatin | Inhibits growth of drug-resistant colon cancer cells while inducing autophagy |
| Resveratrol | Cisplatin | Induces autophagic and apoptotic death in drug-resistant oral cancer cells |
| Curcumin | 5-Fluorouracil | Exerts synergistic effects with the chemotherapeutic drug by impairing AMPK/ULK1-dependent autophagy |
| EGCG | Cisplatin | Increases sensitivity of CAR cells, apoptosis, and autophagy by AKT/STAT3 pathway |
| Curcumin | Docetaxel | Leads to induction of apoptosis and autophagy through PI3K/AKT/mTOR pathway |
| Resveratrol | Gefitinib | Overcomes drug resistance while inducing apoptosis, autophagy, and senescence in PC9/G NSCLC cells |
| Curcumin | Gefitinib | Enhances the efficacy of the drug and overcomes EGFR-TKI resistance in NSCLC patients with wild-type EGFR and/or KRAS mutation |
Polyphenols have the potential to be employed either alone or in combination with other cancer therapies. Exploring the mechanisms of action of these compounds could lead to greater therapeutic success in the treatment of cancer [268,269]. Prior to their usage as prescription medications, however, the safety of these substances must be established by additional research, which may include human subjects and various pharmacokinetic parameters. Moreover, more clinical trials could investigate the development of a standardized extract or dosage to be used as a successful cancer treatment regime.
5. Polyphenol Bioavailability and Metabolism
Recent research on the bioavailability of polyphenols has shown that even when consumed in relatively large concentrations, these compounds have a low bioavailability. Their poor bioavailability is a major roadblock to their application in pharmacology because of the molecular structure changes that occur during digestion, absorption, and distribution as a result of interactions with food, digestive enzymes, and transporters in the intestine and with blood proteins [270].
It is considered that polyphenols are bioavailable and can efficiently reach the target tissue. Knowing how they are ingested, processed, and eliminated from the body is crucial. The chemical complexity of polyphenol-rich diet, as well as other characteristics including the degree of polyphenol polymerization and conjugation with other compounds and phenols, makes absorption studies difficult to conduct [270]. Most polyphenols in food are not digestible because they are bound to esters, glycosides, or polymers. As the body treats polyphenols as foreign invaders after they have been ingested, their bioavailability is limited compared to that of micro- and macronutrients [270,271].
Similar to metabolic detoxification, polyphenol metabolism can lessen their cytotoxicity by increasing their hydrophilicity and making them easier to eliminate via the urinary or the biliary system [270]. The rate and degree of absorption and the kind of circulating metabolites in the plasma are determined by the structure of polyphenols, not by their content. These chemicals are either easily absorbed in the small intestine (monomeric and dimeric polyphenols) or reach the large intestine almost unchanged (oligomeric and polymeric polyphenols) [270,271].
Only about 5–10% of polyphenols that are consumed are absorbed in the small intestine, according to some literature. Less complex polyphenolic chemicals are able to undergo hydrolysis and biotransformation in enterocytes and subsequently in hepatocytes following absorption. This process leads to a cascade of hydrophilic conjugated metabolites (methyl, glucuronide, and sulfate derivatives) that are rapidly absorbed in the bloodstream and either transported to various organs or eliminated in the urine [270]. Because each phenolic molecule may give rise to ten other compounds throughout metabolism [270], it is challenging to trace the fate of each component in the body. Metabolic activities typically transform phenolic antioxidants into entirely new compounds, making the parent phenol nearly impossible to identify [270]. Phase I metabolism occurs in enterocytes when xenobiotics are oxidized, reduced, or hydrolyzed to introduce or expose a functional group, such as a hydroxyl group, particularly for conjugation (phase II metabolism) [270,271]. However, the hydroxyl groups on the phenolic aromatic ring are successfully conjugated to glucuronide, sulfate, and/or methylated metabolites through the action of uridine-5-diphosphate glucuronosyltransferase (UGT), sulfotransferase (SULT), and catechol-O-methyltransferase (COMT) [270,271]. The meta position is the preferred conjugation site for catechol antioxidants [270]. Para-position conjugation with a glucuronic acid or sulfate group is less common than meta-position conjugation with a methyl group, although it does happen [270,271,272].
The poor knowledge available regarding the absorption of polyphenols in humans is a major hurdle that needs to be addressed to increase the efficacy of these compounds as chemopreventive and chemotherapeutic agents for the benefit of health. Nanoparticle encapsulation is a possibility that may allow for more precise targeting and controllable release. However, further research is required to put this idea into general practice for polyphenol-based cancer therapies.
6. Conclusions and Future Perspectives
New approaches are being considered to improve the therapeutic outcomes as the incidence of cancer rises and standard treatments lose their efficacy. There has been a rise in interest in alternative medicines because of the negative outcomes that can result from conventional treatments. Scientists advocate for the use of polyphenolic chemicals found in nature, which can spare healthy tissues while killing cancers. Polyphenols such as curcumin, quercetin, EGCG, resveratrol, and apigenin have shown efficacy as potential cancer therapy agents, especially when paired with chemotherapeutic drugs such as cisplatin, 5-fluorouracil, docetaxel, paclitaxel, gefitinib, and others to induce synergistic effects. They are highly effective as antioxidants and also reduce inflammation, slow cell proliferation, inhibit angiogenesis, inhibit metastasis, and promote apoptosis. They were shown to perform exceptionally well in tests involving several different kinds of cancer, including those of the lung, breast, liver, colon, and stomach. They were able to trigger apoptosis and block the development and division of numerous cell lines. They can also be preloaded into certain biomaterials to provide a steady release and a simultaneous therapeutic and regenerative effect. In order to fully understand the mechanism involved in the polyphenol-induced regulation of cancer, additional research is needed, particularly on in vivo models and in additional clinical trials. The immunomodulatory impact of polyphenols, which can boost the efficacy of treatments such as immune checkpoint blockade, also needs a thorough evaluation. The potential for polyphenol treatment to reduce toxicity and adverse effects has contributed to its increased prominence in cancer therapy. However, this technique must be used with caution, as it can lead to the development of dormant tumor cells, which can cause the disease to recur.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. 3640].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arafa M.A., Rabah D.M., Farhat K.H. Rising cancer rates in the Arab World: Now is the time for action. East. Mediterr. Health J. 2020;26:638–640. doi: 10.26719/emhj.20.073. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., Parkin D.M., African Cancer Registry N. Cancer in sub-saharan africa in 2020: A review of current estimates of the national burden, data gaps, and future needs. Lancet. Oncol. 2022;23:719–728. doi: 10.1016/S1470-2045(22)00270-4. [DOI] [PubMed] [Google Scholar]
- 4.Arafa M.A., Farhat K.H. Why cancer incidence in the Arab counties is much lower than other parts of the world? J. Egypt. Natl. Cancer Inst. 2022;34:41. doi: 10.1186/s43046-022-00142-3. [DOI] [PubMed] [Google Scholar]
- 5.Noorwali E.A., Aljaadi A.M., Al-Otaibi H.H. Change in Growth Status and Obesity Rates among Saudi Children and Adolescents Is Partially Attributed to Discrepancies in Definitions Used: A Review of Anthropometric Measurements. Healthcare. 2023;11:1010. doi: 10.3390/healthcare11071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AlMarri F., Al Sabah S., Al Haddad E., Vaz J.D. A Call for More Research from the Arabian Gulf. Obes. Surg. 2017;27:2034–2043. doi: 10.1007/s11695-017-2588-7. [DOI] [PubMed] [Google Scholar]
- 7.Basudan A.M. Breast Cancer Incidence Patterns in the Saudi Female Population: A 17-Year Retrospective Analysis. Medicina. 2022;58:1617. doi: 10.3390/medicina58111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan Z.A., Prabhu N., Issrani R., Albulayhid A.A.S., Mlih Alruwaili S.M., Gadoe Alruwaili R.H., Alsiyat B.M., Bader A.K., Sghaireen M.G., Rao K., et al. Oral Health-Related Quality of Life in Breast Cancer Patients in the Northern Region of Saudi Arabia. Healthcare. 2023;11:1189. doi: 10.3390/healthcare11081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen K. The importance of food, nutrition and physical activity in cancer prevention: An interview with Dr Kate Allen. Future Oncol. 2018;14:1427–1429. doi: 10.2217/fon-2018-0230. [DOI] [PubMed] [Google Scholar]
- 10.Lathigara D., Kaushal D., Wilson R.B. Molecular Mechanisms of Western Diet-Induced Obesity and Obesity-Related Carcinogenesis—A Narrative Review. Metabolites. 2023;13:675. doi: 10.3390/metabo13050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennji S.M., Jayakrishnan B., Al-Kindi A.H., Al-Jahdhami I., Al-Hashami Z. Lung cancer screening in the gulf: Rationale and recommendations. Ann. Thorac. Med. 2022;17:189–192. doi: 10.4103/atm.atm_69_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monshi S.S., Ibrahim J. Implementation of tobacco control measures in the Gulf Cooperation Council countries, 2008–2020. Subst. Abus. Treat. Prev. Policy. 2021;16:57. doi: 10.1186/s13011-021-00393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakur C., Qiu Y., Fu Y., Bi Z., Zhang W., Ji H., Chen F. Epigenetics and environment in breast cancer: New paradigms for anti-cancer therapies. Front. Oncol. 2022;12:971288. doi: 10.3389/fonc.2022.971288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J.W., Charkhchi P., Adekunte S., Akbari M.R. What Is Known about Breast Cancer in Young Women? Cancers. 2023;15:1917. doi: 10.3390/cancers15061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marinac C.R., Nelson S.H., Breen C.I., Hartman S.J., Natarajan L., Pierce J.P., Flatt S.W., Sears D.D., Patterson R.E. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol. 2016;2:1049–1055. doi: 10.1001/jamaoncol.2016.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandhorst S., Longo V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Metab. Cancer. 2016;207:241–266. doi: 10.1007/978-3-319-42118-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawczak-Dębicka A., Kufel-Grabowska J., Bartoszkiewicz M., Perdyan A., Jassem J. Complementary and Alternative Therapies in Oncology. Int. J. Environ. Res. Public Health. 2022;19:5071. doi: 10.3390/ijerph19095071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhasawi M.A.I., Aatif M., Muteeb G., Alam M.W., Oirdi M.E., Farhan M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules. 2022;27:7410. doi: 10.3390/molecules27217410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadeghi S., Davoodvandi A., Pourhanifeh M.H., Sharifi N., ArefNezhad R., Sahebnasagh R., Moghadam S.A., Sahebkar A., Mirzaei H. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur. J. Med. Chem. 2019;178:131–140. doi: 10.1016/j.ejmech.2019.05.067. [DOI] [PubMed] [Google Scholar]
- 20.Ashokkumar K., Murugan M., Dhanya M.K., Warkentin T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020;246:112244. doi: 10.1016/j.jep.2019.112244. [DOI] [PubMed] [Google Scholar]
- 21.Mir M.A., Ganai S.A., Mansoor S., Jan S., Mani P., Masoodi K.Z., Amin H., Rehman M.U., Ahmad P. Isolation, purification and characterization of naturally derived crocetin beta-d-glucosyl ester from crocus sativus l. Against breast cancer and its binding chemistry with er-alpha/hdac2. Saudi J. Biol. Sci. 2020;27:975–984. doi: 10.1016/j.sjbs.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim E.Y., Choi H.J., Park M.J., Jung Y.S., Lee S.O., Kim K.J., Choi J.H., Chung T.W., Ha K.T. Myristica fragrans suppresses tumor growth and metabolism by inhibiting lactate dehydrogenase a. Am. J. Chin. Med. 2016;44:1063–1079. doi: 10.1142/S0192415X16500592. [DOI] [PubMed] [Google Scholar]
- 23.Rahim M.A., Shoukat A., Khalid W., Ejaz A., Itrat N., Majeed I., Koraqi H., Imran M., Nisa M.U., Nazir A., et al. A Narrative Review on Various Oil Extraction Methods, Encapsulation Processes, Fatty Acid Profiles, Oxidative Stability, and Medicinal Properties of Black Seed (Nigella sativa) Foods. 2022;11:2826. doi: 10.3390/foods11182826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhuqail A.J., Alzahrani A., Almubarak H., Al-Qadheeb S., Alghofaili L., Almoghrabi N., Alhussaini H., Park B.H., Colak D., Karakas B. High prevalence of deleterious brca1 and brca2 germline mutations in arab breast and ovarian cancer patients. Breast Cancer Res. Treat. 2018;168:695–702. doi: 10.1007/s10549-017-4635-4. [DOI] [PubMed] [Google Scholar]
- 25.AlHarthi F.S., Qari A., Edress A., Abedalthagafi M. Familial/inherited cancer syndrome: A focus on the highly consanguineous Arab population. NPJ Genom. Med. 2020;5:3. doi: 10.1038/s41525-019-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elshimali Y.I., Wu Y., Khaddour H., Wu Y., Gradinaru D., Sukhija H., Chung S.S., Vadgama J.V. Optimization Of Cancer Treatment Through Overcoming Drug Resistance. J. Cancer Res. Oncobiol. 2018;1:107. doi: 10.31021/jcro.20181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolaou M., Pavlopoulou A., Georgakilas A.G., Kyrodimos E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis. 2018;35:309–318. doi: 10.1007/s10585-018-9903-0. [DOI] [PubMed] [Google Scholar]
- 28.Du M., Ouyang Y., Meng F., Ma Q., Liu H., Zhuang Y., Pang M., Cai T., Cai Y. Nanotargeted agents: An emerging therapeutic strategy for breast cancer. Nanomedicine. 2019;14:1771–1786. doi: 10.2217/nnm-2018-0481. [DOI] [PubMed] [Google Scholar]
- 29.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 30.Ramos A., Sadeghi S., Tabatabaeian H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021;22:9451. doi: 10.3390/ijms22179451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan C., Yu M., Xu J., Li B.Y., Zhao Y., Kankala R.K. Overcoming Cancer Multi-drug Resistance (MDR): Reasons, mechanisms, nanotherapeutic solutions, and challenges. Biomed. Pharmacother. 2023;162:114643. doi: 10.1016/j.biopha.2023.114643. [DOI] [PubMed] [Google Scholar]
- 32.Al Bitar S., El-Sabban M., Doughan S., Abou-Kheir W. Molecular mechanisms targeting drug-resistance and metastasis in colorectal cancer: Updates and beyond. World J. Gastroenterol. 2023;29:1395–1426. doi: 10.3748/wjg.v29.i9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alisi A., Cho W.C., Locatelli F., Fruci D. Multidrug resistance and cancer stem cells in neuroblastoma and hepatoblastoma. Int. J. Mol. Sci. 2013;14:24706–24725. doi: 10.3390/ijms141224706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z., Wu X., Chen H.N., Wang K. Amino acid metabolic reprogramming in tumor metastatic colonization. Front. Oncol. 2023;13:1123192. doi: 10.3389/fonc.2023.1123192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vafadar A., Shabaninejad Z., Movahedpour A., Mohammadi S., Fathullahzadeh S., Mirzaei H.R., Namdar A., Savardashtaki A., Mirzaei H. Long non-coding rnas as epigenetic regulators in cancer. Curr. Pharm. Des. 2019;25:3563–3577. doi: 10.2174/1381612825666190830161528. [DOI] [PubMed] [Google Scholar]
- 36.Farhan M., Rizvi M., Aatif M., Ahmad A. Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites. 2023;13:481. doi: 10.3390/metabo13040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farhan M., Rizvi A. Understanding the Prooxidant Action of Plant Polyphenols in the Cellular Microenvironment of Malignant Cells: Role of Copper and Therapeutic Implications. Front. Pharmacol. 2022;13:929853. doi: 10.3389/fphar.2022.929853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye Y., Ma Y., Kong M., Wang Z., Sun K., Li F. Effects of Dietary Phytochemicals on DNA Damage in Cancer Cells. Nutr. Cancer. 2023;75:761–775. doi: 10.1080/01635581.2022.2157024. [DOI] [PubMed] [Google Scholar]
- 39.Cragg G.M., Pezzuto J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016;25((Suppl. S2)):41–59. doi: 10.1159/000443404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majrashi T.A., Alshehri S.A., Alsayari A., Muhsinah A.B., Alrouji M., Alshahrani A.M., Shamsi A., Atiya A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients. 2023;15:1704. doi: 10.3390/nu15071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mushtaq S., Abbasi B.H., Uzair B., Abbasi R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018;17:420–451. doi: 10.17179/excli2018-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atanasov A.G., Zotchev S.B., Dirsch V.M., Supuran C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasan N., Baselga J., Hyman D.M. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marotta G., Muto T., Benincasa G., De Monaco A. Formyl peptide receptor 2 mediated chemotherapeutics drug resistance in colon cancer cells. Point of view from pharmacogenetics field. Eur. Rev. Med. Pharmacol. Sci. 2018;22:1178–1179. doi: 10.26355/eurrev_201803_14455. [DOI] [PubMed] [Google Scholar]
- 45.Van Den Boogaard W.M.C., Komninos D.S.J., Vermeij W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers. 2022;14:627. doi: 10.3390/cancers14030627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milliron B.-J., Packel L., Dychtwald D., Klobodu C., Pontiggia L., Ogbogu O., Barksdale B., Deutsch J. When Eating Becomes Torturous: Understanding Nutrition-Related Cancer Treatment Side Effects among Individuals with Cancer and Their Caregivers. Nutrients. 2022;14:356. doi: 10.3390/nu14020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin S.R., Chang C.H., Hsu C.F., Tsai M.J., Cheng H., Leong M.K., Sung P.J., Chen J.C., Weng C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020;177:1409–1423. doi: 10.1111/bph.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vladu A.F., Ficai D., Ene A.G., Ficai A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022;23:10244. doi: 10.3390/ijms231810244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaidya F.U., Sufiyan Chhipa A., Mishra V., Gupta V.K., Rawat S.G., Kumar A., Pathak C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022;5:e1291. doi: 10.1002/cnr2.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitola G., Falvo P., Bertolini F. New Insight to Overcome Tumor Resistance: An Overview from Cellular to Clinical Therapies. Life. 2021;11:1131. doi: 10.3390/life11111131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rueff J., Rodrigues A.S. Cancer Drug Resistance: A Brief Overview from a Genetic Viewpoint. Methods Mol. Biol. 2016;1395:1–18. doi: 10.1007/978-1-4939-3347-1_1. [DOI] [PubMed] [Google Scholar]
- 52.Wajapeyee N., Gupta R. Epigenetic Alterations and Mechanisms That Drive Resistance to Targeted Cancer Therapies. Cancer Res. 2021;81:5589–5595. doi: 10.1158/0008-5472.CAN-21-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X., Zhang H., Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–160. doi: 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin-Rahardja K., Weaver D.T., Scarborough J.A., Scott J.G. Evolution-Informed Strategies for Combating Drug Resistance in Cancer. Int. J. Mol. Sci. 2023;24:6738. doi: 10.3390/ijms24076738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar G., Virmani T., Sharma A., Pathak K. Codelivery of Phytochemicals with Conventional Anticancer Drugs in Form of Nanocarriers. Pharmaceutics. 2023;15:889. doi: 10.3390/pharmaceutics15030889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goenka A., Khan F., Verma B., Sinha P., Dmello C.C., Jogalekar M.P., Gangadaran P., Ahn B.C. Tumor microenvironment signaling and therapeutics in cancer progression. Cancer Commun. 2023;43:525–561. doi: 10.1002/cac2.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yahiro S., Kawamoto T., Fujiwara S., Hara H., Fukase N., Sawada R., Takemori T., Miyamoto T., Mifune Y., Kakutani K., et al. Identification and characterization of slow-cycling cells in ewing sarcoma. Int. J. Oncol. 2022;61:138. doi: 10.3892/ijo.2022.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi R., Liao C., Zhang Q. Hypoxia-Driven Effects in Cancer: Characterization, Mechanisms, and Therapeutic Implications. Cells. 2021;10:678. doi: 10.3390/cells10030678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida-Sakai N., Watanabe T., Yamamoto Y., Ureshino H., Kamachi K., Kurahashi Y., Fukuda-Kurahashi Y., Kimura S. Adult T-cell leukemia-lymphoma acquires resistance to DNA demethylating agents through dysregulation of enzymes involved in pyrimidine metabolism. Int. J. Cancer. 2022;150:1184–1197. doi: 10.1002/ijc.33901. Erratum in Int. J. Cancer 2022, 150, E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh S. Cytoprotective and regulatory functions of glutathione S-transferases in cancer cell proliferation and cell death. Cancer Chemother. Pharmacol. 2015;75:1–15. doi: 10.1007/s00280-014-2566-x. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Z., Mei Y., Wang Z., He W. The Effect of Oxidative Phosphorylation on Cancer Drug Resistance. Cancers. 2022;15:62. doi: 10.3390/cancers15010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z., Xu Y., Meng X., Watari F., Liu H., Chen X. Suppression of c-Myc is involved in multi-walled carbon nanotubes’ down-regulation of ATP-binding cassette transporters in human colon adenocarcinoma cells. Toxicol. Appl. Pharmacol. 2015;282:42–51. doi: 10.1016/j.taap.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Agarwal V., Naskar P., Agasti S., Khurana G.K., Vishwakarma P., Lynn A.M., Roche P.A., Puri N. The cysteine-rich domain of synaptosomal-associated protein of 23 kDa (SNAP-23) regulates its membrane association and regulated exocytosis from mast cells. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:1618–1633. doi: 10.1016/j.bbamcr.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keogh J.P. Membrane transporters in drug development. Adv. Pharmacol. 2012;63:1–42. doi: 10.1016/B978-0-12-398339-8.00001-X. [DOI] [PubMed] [Google Scholar]
- 65.Dean M., Moitra K., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Hum. Mutat. 2022;43:1162–1182. doi: 10.1002/humu.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibrahim M.A.A., Abdeljawaad K.A.A., Jaragh-Alhadad L.A., Oraby H.F., Atia M.A.M., Alzahrani O.R., Mekhemer G.A.H., Moustafa M.F., Shawky A.M., Sidhom P.A., et al. Potential drug candidates as P-glycoprotein inhibitors to reverse multidrug resistance in cancer: An in silico drug discovery study. J. Biomol. Struct. Dyn. 2023;41:1–16. doi: 10.1080/07391102.2023.2176360. [DOI] [PubMed] [Google Scholar]
- 67.Karthika C., Sureshkumar R., Zehravi M., Akter R., Ali F., Ramproshad S., Mondal B., Tagde P., Ahmed Z., Khan F.S., et al. Multidrug Resistance of Cancer Cells and the Vital Role of P-Glycoprotein. Life. 2022;12:897. doi: 10.3390/life12060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim T.H., Shin S., Yoo S.D., Shin B.S. Effects of Phytochemical P-Glycoprotein Modulators on the Pharmacokinetics and Tissue Distribution of Doxorubicin in Mice. Molecules. 2018;23:349. doi: 10.3390/molecules23020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yhee J.Y., Song S., Lee S.J., Park S.G., Kim K.S., Kim M.G., Son S., Koo H., Kwon I.C., Jeong J.H., et al. Cancer-targeted MDR-1 siRNA delivery using self-cross-linked glycol chitosan nanoparticles to overcome drug resistance. J. Control. Release. 2015;198:1–9. doi: 10.1016/j.jconrel.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 70.da Costa K.M., Freire-de-Lima L., da Fonseca L.M., Previato J.O., Mendonça-Previato L., Valente R.d.C. ABCB1 and ABCC1 Function during TGF-β-Induced Epithelial-Mesenchymal Transition: Relationship between Multidrug Resistance and Tumor Progression. Int. J. Mol. Sci. 2023;24:6046. doi: 10.3390/ijms24076046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhadwal P., Randhawa V., Vaiphei K., Dahiya D., Agnihotri N. Clinical relevance of CERK and SPHK1 in breast cancer and their association with metastasis and drug resistance. Sci. Rep. 2022;12:18239. doi: 10.1038/s41598-022-20976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gameiro M., Silva R., Rocha-Pereira C., Carmo H., Carvalho F., Bastos M.D.L., Remião F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules. 2017;22:600. doi: 10.3390/molecules22040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bryant H.E., Schultz N., Thomas H.D., Parker K.M., Flower D., Lopez E., Kyle S., Meuth M., Curtin N.J., Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 74.Farmer H., McCabe N., Lord C.J., Tutt A.N., Johnson D.A., Richardson T.B., Santarosa M., Dillon K.J., Hickson I., Knights C., et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 75.Riffell J.L., Lord C.J., Ashworth A. Tankyrase-targeted therapeutics: Expanding opportunities in the PARP family. Nat. Rev. Drug Discov. 2012;11:923–936. doi: 10.1038/nrd3868. [DOI] [PubMed] [Google Scholar]
- 76.Zhou P., Wang J., Mishail D., Wang C.Y. Recent advancements in PARP inhibitors-based targeted cancer therapy. Precis. Clin. Med. 2020;3:187–201. doi: 10.1093/pcmedi/pbaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dias M.P., Moser S.C., Ganesan S., Jonkers J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2021;18:773–791. doi: 10.1038/s41571-021-00532-x. [DOI] [PubMed] [Google Scholar]
- 78.Brown D.W., Beatty P.H., Lewis J.D. Molecular Targeting of the Most Functionally Complex Gene in Precision Oncology: P53. Cancers. 2022;14:5176. doi: 10.3390/cancers14215176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen L., Zhao Y., Halliday G.C., Berry P., Rousseau R.F., Middleton S.A., Nichols G.L., Del Bello F., Piergentili A., Newell D.R., et al. Structurally diverse MDM2-p53 antagonists act as modulators of MDR-1 function in neuroblastoma. Br. J. Cancer. 2014;111:716–725. doi: 10.1038/bjc.2014.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mir R.H., Mir P.A., Uppal J., Chawla A., Patel M., Bardakci F., Adnan M., Mohi-ud-din R. Evolution of Natural Product Scaffolds as Potential Proteasome Inhibitors in Developing Cancer Therapeutics. Metabolites. 2023;13:509. doi: 10.3390/metabo13040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farhan M., Silva M., Li S., Yan F., Fang J., Peng T., Hu J., Tsao M.S., Little P., Zheng W. The role of FOXOs and autophagy in cancer and metastasis-Implications in therapeutic development. Med. Res. Rev. 2020;40:2089–2113. doi: 10.1002/med.21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu Q., Sharma D. Autophagy and Breast Cancer: Connected in Growth, Progression, and Therapy. Cells. 2023;12:1156. doi: 10.3390/cells12081156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo X.L., Hu F., Wang H., Fang J.M., Zhu Z., Wei L., Xu Q. Inhibition of autophagy in hepatocarcinoma cells promotes chemotherapeutic agent-induced apoptosis during nutrient deprivation. Oncol. Rep. 2018;39:773–783. doi: 10.3892/or.2017.6115. [DOI] [PubMed] [Google Scholar]
- 84.Li X., He S., Ma B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer. 2020;19:12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao S., Tang Y., Wang R., Najafi M. Mechanisms of cancer cell death induction by paclitaxel: An updated review. Apoptosis. 2022;27:647–667. doi: 10.1007/s10495-022-01750-z. [DOI] [PubMed] [Google Scholar]
- 86.Rizvi A., Farhan M., Nabi F., Khan R.H., Adil M., Ahmad A. Transcriptional Control of the Oxidative Stress Response and Implications of Using Plant Derived Molecules for Therapeutic Interventions in Cancer. Curr. Med. Chem. 2021;28:8480–8495. doi: 10.2174/0929867328666210218110550. [DOI] [PubMed] [Google Scholar]
- 87.Khodakarami A., Adibfar S., Karpisheh V., Abolhasani S., Jalali P., Mohammadi H., Gholizadeh Navashenaq J., Hojjat-Farsangi M., Jadidi-Niaragh F. The molecular biology and therapeutic potential of Nrf2 in leukemia. Cancer Cell Int. 2022;22:241. doi: 10.1186/s12935-022-02660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petroni G., Buque A., Coussens L.M., Galluzzi L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat. Rev. Drug Discov. 2022;21:440–462. doi: 10.1038/s41573-022-00415-5. [DOI] [PubMed] [Google Scholar]
- 89.Girish V., Lakhani A.A., Scaduto C.M., Thompson S.L., Brown L.M., Hagenson R.A., Sausville E.L., Mendelson B.E., Lukow D.A., Yuan M.L., et al. Oncogene-like addiction to aneuploidy in human cancers. bioRxiv. 2023 doi: 10.1101/2023.01.09.523344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moreira-Silva F., Henrique R., Jerónimo C. From Therapy Resistance to Targeted Therapies in Prostate Cancer. Front. Oncol. 2022;12:877379. doi: 10.3389/fonc.2022.877379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao C., Zhang L., Xu Y., Ma X., Chen P., Chen Z.S., Wei L. I13 overrides resistance mediated by the T315I mutation in chronic myeloid leukemia by direct BCR-ABL inhibition. Front. Pharmacol. 2023;14:1183052. doi: 10.3389/fphar.2023.1183052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Șandor A., Ionuț I., Marc G., Oniga I., Eniu D., Oniga O. Structure–Activity Relationship Studies Based on Quinazoline Derivatives as EGFR Kinase Inhibitors (2017–Present) Pharmaceuticals. 2023;16:534. doi: 10.3390/ph16040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boddy A.M. The need for evolutionary theory in cancer research. Eur. J. Epidemiol. 2022. Epub ahead of printing . [DOI] [PMC free article] [PubMed]
- 94.Subramaniam S., Selvaduray K.R., Radhakrishnan A.K. Bioactive Compounds: Natural Defense Against Cancer? Biomolecules. 2019;9:758. doi: 10.3390/biom9120758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asma S.T., Acaroz U., Imre K., Morar A., Shah S.R.A., Hussain S.Z., Arslan-Acaroz D., Demirbas H., Hajrulai-Musliu Z., Istanbullugil F.R., et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers. 2022;14:6203. doi: 10.3390/cancers14246203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yardley D.A. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int. J. Breast Cancer. 2013;2013:137414. doi: 10.1155/2013/137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weber A.M., Ryan A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol. Ther. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 98.Bukowski K., Kciuk M., Kontek R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020;21:3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pavlović Mavić M., Šeparović R., Tečić Vuger A., Vazdar L. Difference in Estimation of Side Effects of Chemotherapy between Physicians and Patients with Early-Stage Breast Cancer: The Use of Patient Reported Outcomes (PROs) in the Evaluation of Toxicity in Everyday Clinical Practice. Cancers. 2021;13:5922. doi: 10.3390/cancers13235922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ullah M.F. Cancer multidrug resistance (MDR): A major impediment to effective chemotherapy. Asian Pac. J. Cancer Prev. 2008;9:1–6. [PubMed] [Google Scholar]
- 101.Hussain S.A., Sulaiman A.A., Balch C., Chauhan H., Alhadidi Q.M., Tiwari A.K. Natural polyphenols in cancer chemoresistance. Nutr. Cancer. 2016;68:879–891. doi: 10.1080/01635581.2016.1192201. [DOI] [PubMed] [Google Scholar]
- 102.Fedotcheva T.A., Fedotcheva N.I., Shimanovsky N.L. Progestins as Anticancer Drugs and Chemosensitizers, New Targets and Applications. Pharmaceutics. 2021;13:1616. doi: 10.3390/pharmaceutics13101616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bluthgen N., Castiglioni S., Sumpter J.P., Fent K. Effects of low concentrations of the antiprogestin mifepristone (RU486) in adults and embryos of zebrafish (Danio rerio): 1. Reproductive and early developmental effects. Aquat. Toxicol. 2013;144–145:83–95. doi: 10.1016/j.aquatox.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 104.Alameh G., Emptoz-Bonneton A., Rolland de Ravel M., Matera E.L., Mappus E., Balaguer P., Rocheblave L., Lomberget T., Dumontet C., Le Borgne M., et al. In vitro modulation of multidrug resistance by pregnane steroids and in vivo inhibition of tumour development by 7α-OBz-11α(R)-OTHP-5β-pregnanedione in K562/R7 and H295R cell xenografts. J. Enzym. Inhib. Med. Chem. 2019;34:684–691. doi: 10.1080/14756366.2019.1575825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee M., Piao J., Jeon M.J. Risk Factors Associated with Endometrial Pathology in Premenopausal Breast Cancer Patients Treated with Tamoxifen. Yonsei Med. J. 2020;61:317–322. doi: 10.3349/ymj.2020.61.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shiota M., Yokomizo A., Takeuchi A., Imada K., Kashiwagi E., Song Y., Inokuchi J., Tatsugami K., Uchiumi T., Naito S. Inhibition of protein kinase C/Twist1 signaling augments anticancer effects of androgen deprivation and enzalutamide in prostate cancer. Clin. Cancer Res. 2014;20:951–961. doi: 10.1158/1078-0432.CCR-13-1809. [DOI] [PubMed] [Google Scholar]
- 107.Dana P.M., Sadoughi F., Asemi Z., Yousefi B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022;27:1. doi: 10.1186/s11658-021-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang W., Shi H., Chen C., Ren K., Xu Y., Liu X., He L. CUR Enhances Cisplatin Sensitivity of Human NSCLC Cell Lines through Influencing Cu-Sp1-CTR1 Regulatory Loop. Phytomedicine. 2018;48:51–61. doi: 10.1016/j.phymed.2018.04.058. [DOI] [PubMed] [Google Scholar]
- 109.Yin J., Wang L., Wang Y., Shen H., Wang X., Wu L. CUR Reverses Oxaliplatin Resistance in Human Colorectal Cancer via Regulation of TGF-β/Smad2/3 Signaling Pathway. OncoTargets Ther. 2019;12:3893–3903. doi: 10.2147/OTT.S199601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Biswas S., Mahapatra E., Ghosh A., Das S., Roy M., Mukherjee S. CUR Rescues Doxorubicin Responsiveness via Regulating Aurora a Signaling Network in Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2021;22:957–970. doi: 10.31557/APJCP.2021.22.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Z., Huang C., Ma T., Jiang L., Tang L., Shi T., Zhang S., Zhang L., Zhu P., Li J., et al. Reversal Effect of Quercetin on Multidrug Resistance via FZD7/β-Catenin Pathway in Hepatocellular Carcinoma Cells. Phytomedicine. 2018;43:37–45. doi: 10.1016/j.phymed.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 112.Zhou Y., Zhang J., Wang K., Han W., Wang X., Gao M., Wang Z., Sun Y., Yan H., Zhang H., et al. Quercetin Overcomes Colon Cancer Cells Resistance to Chemotherapy by Inhibiting Solute Carrier Family 1, Member 5 Transporter. Eur. J. Pharmacol. 2020;881:173185. doi: 10.1016/j.ejphar.2020.173185. [DOI] [PubMed] [Google Scholar]
- 113.Qin S.-H., Lau A.T.Y., Liang Z.-L., Tan H.W., Ji Y.-C., Zhong Q.-H., Zhao X.-Y., Xu Y.-M. Resveratrol Promotes Tumor Microvessel Growth via Endoglin and Extracellular Signal-Regulated Kinase Signaling Pathway and Enhances the Anticancer Efficacy of Gemcitabine against Lung Cancer. Cancers. 2020;12:974. doi: 10.3390/cancers12040974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ren M., Zhou X., Gu M., Jiao W., Yu M., Wang Y., Liu S., Yang J., Ji F. Resveratrol Synergizes with Cisplatin in Antineoplastic Effects against AGS Gastric Cancer Cells by Inducing Endoplasmic Reticulum Stressmediated Apoptosis and G2/M Phase Arrest. Oncol. Rep. 2020;44:1605–1615. doi: 10.3892/or.2020.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Changizi V., Azariasl S., Motevaseli E., Jafari Nodooshan S. Assessment Synergistic Effects of Integrated Therapy with Epigallocatechin-3-Gallate (EGCG) & Arsenic Trioxide and Irradiation on Breast Cancer Cell Line. Iran. J. Public Health. 2020;49:1555–1563. doi: 10.18502/ijph.v49i8.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Datta S., Sinha D. Low Dose Epigallocatechin-3-Gallate Revives Doxorubicin Responsiveness by a Redox-Sensitive Pathway in A549 Lung Adenocarcinoma Cells. J. Biochem. Mol. Toxicol. 2022;36:e22999. doi: 10.1002/jbt.22999. [DOI] [PubMed] [Google Scholar]
- 117.Yang C., Song J., Hwang S., Choi J., Song G., Lim W. Apigenin Enhances Apoptosis Induction by 5-Fluorouracil through Regulation of Thymidylate Synthase in Colorectal Cancer Cells. Redox Biol. 2021;47:102144. doi: 10.1016/j.redox.2021.102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aziz N.A., Froemming G.R.A., Kadir S.H.S.A., Ibahim M.J. Apigenin Increases Cisplatin Inhibitory Effects on the Telomerase Activity of Triple Negative Breast Cancer Cells. J. Teknol. 2018;80:123–132. doi: 10.11113/jt.v80.10378. [DOI] [Google Scholar]
- 119.Li K., Li M., Luo Z., Mao Y., Yu Y., He Y., Zhou J., Fei Y., Pei Y., Cai K. Overcoming the Hypoxia-Induced Drug Resistance in Liver Tumor by the Concurrent Use of Apigenin and Paclitaxel. Biochem. Biophys. Res. Commun. 2020;526:321–327. doi: 10.1016/j.bbrc.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 120.Zhan Y., Wang Y., Qi M., Liang P., Ma Y., Li T., Li H., Dai C., An Z., Qi Y., et al. BH3 Mimetic ABT-263 Enhances the Anticancer Effects of Apigenin in Tumor Cells with Activating EGFR Mutation. Cell Biosci. 2019;9:60. doi: 10.1186/s13578-019-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deng P., Hu C., Xiong Z., Li Y., Jiang J., Yang H., Tang Y., Cao L., Lu R. Epigallocatechin-3-gallate-induced vascular normalization in A549-cell xenograft-bearing nude mice: Therapeutic efficacy in combination with chemotherapy. Cancer Manag. Res. 2019;11:2425–2439. doi: 10.2147/CMAR.S187750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neelamegam U., Muthuvel R., Suganthi V. Hepatoprotective Effect of Epigallocatechin-Gallate (Egcg) And Sorafenib Against Den Induced Hepato Cellular Carcinoma in Experimental Animals. J. Pharm. Negat. Results. 2022;13:921–929. [Google Scholar]
- 123.Abdel Latif Y., El-Bana M., Hussein J., El-Khayat Z., Farrag A.R. Effects of Resveratrol in Combination with 5-Fluorouracil on N-Methylnitrosourea-Induced Colon Cancer in Rats. Comp. Clin. Pathol. 2019;28:1351–1362. doi: 10.1007/s00580-019-02967-2. [DOI] [Google Scholar]
- 124.Kiamehr P., Shahidi M., Samii A., Zaker F. Dual effects of resveratrol on the expression and secretion of angiogenic factors. Int. J. Mol. Cell Med. 2022;11:16–30. doi: 10.22088/IJMCM.BUMS.11.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu H., Lee J.I., Ahn T.-G. Effect of Quercetin on the Anti-Tumor Activity of Cisplatin in EMT6 Breast Tumor-Bearing Mice. Obstet. Gynecol. Sci. 2019;62:242. doi: 10.5468/ogs.2019.62.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abdu S., Juaid N., Amin A., Moulay M., Miled N. Effects of Sorafenib and Quercetin Alone or in Combination in Treating Hepatocellular Carcinoma: In Vitro and In Vivo Approaches. Molecules. 2022;27:8082. doi: 10.3390/molecules27228082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen P., Huang H.-P., Wang Y., Jin J., Long W.-G., Chen K., Zhao X.-H., Chen C.-G., Li J. CUR Overcome Primary Gefitinib Resistance in Non-Small-Cell Lung Cancer Cells through Inducing Autophagy-Related Cell Death. J. Exp. Clin. Cancer Res. 2019;38:254. doi: 10.1186/s13046-019-1234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu T., Guo P., Pi C., He Y., Yang H., Hou Y., Feng X., Jiang Q., Wei Y., Zhao L. Synergistic Effects of CUR and 5-Fluorouracil on the Hepatocellular Carcinoma In Vivo and Vitro through Regulating the Expression of COX-2 and NF-ΚB. J. Cancer. 2020;11:3955–3964. doi: 10.7150/jca.41783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang Q., Cai T., Bai L., Huang Y., Li Q., Wang Q., Chiba P., Cai Y. State of the art of overcoming efflux transporter mediated multidrug resistance of breast cancer. Transl. Cancer Res. 2019;8:319–329. doi: 10.21037/tcr.2019.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saeed M., Kadioglu O., Khalid H., Sugimoto Y., Efferth T. Activity of the dietary flavonoid, apigenin, against multidrug-resistant tumor cells as determined by pharmacogenomics and molecular docking. J. Nutr. Biochem. 2015;26:44–56. doi: 10.1016/j.jnutbio.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 131.Tran V.H., Marks D., Duke R.K., Bebawy M., Duke C.C., Roufogalis B.D. Modulation of P-glycoprotein-mediated anticancer drug accumulation, cytotoxicity, and ATPase activity by flavonoid interactions. Nutr. Cancer. 2011;63:435–443. doi: 10.1080/01635581.2011.535959. [DOI] [PubMed] [Google Scholar]
- 132.Ye Q., Liu K., Shen Q., Li Q., Hao J., Han F., Jiang R.W. Reversal of Multidrug Resistance in Cancer by Multi-Functional Flavonoids. Front. Oncol. 2019;9:487. doi: 10.3389/fonc.2019.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim K.H., Ki M.-R., Min K.H., Pack S.P. Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy. Antioxidants. 2023;12:1048. doi: 10.3390/antiox12051048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Laberge R.M., Ambadipudi R., Georges E. P-glycoprotein mediates the collateral sensitivity of multidrug resistant cells to steroid hormones. Biochem. Biophys. Res. Commun. 2014;447:574–579. doi: 10.1016/j.bbrc.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 135.Ke A.B., Eyal S., Chung F.S., Link J.M., Mankoff D.A., Muzi M., Unadkat J.D. Modeling cyclosporine A inhibition of the distribution of a P-glycoprotein PET ligand, 11C-verapamil, into the maternal brain and fetal liver of the pregnant nonhuman primate: Impact of tissue blood flow and site of inhibition. J. Nucl. Med. 2013;54:437–446. doi: 10.2967/jnumed.112.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taldaev A., Terekhov R., Nikitin I., Zhevlakova A., Selivanova I. Insights into the Pharmacological Effects of Flavonoids: The Systematic Review of Computer Modeling. Int. J. Mol. Sci. 2022;23:6023. doi: 10.3390/ijms23116023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee P.J., Shin I., Seo S.Y., Kim H., Kim H.P. Upregulation of both heme oxygenase-1 and ATPase inhibitory factor 1 renders tumoricidal activity by synthetic flavonoids via depleting cellular ATP. Bioorganic Med. Chem. Lett. 2014;24:4845–4849. doi: 10.1016/j.bmcl.2014.08.055. [DOI] [PubMed] [Google Scholar]
- 138.Poku V.O., Iram S.H. A critical review on modulators of Multidrug Resistance Protein 1 in cancer cells. PeerJ. 2022;10:e12594. doi: 10.7717/peerj.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koltai T. The complex relationship between multiple drug resistance and the tumor pH gradient: A review. Cancer Drug Resist. 2022;5:277–303. doi: 10.20517/cdr.2021.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oh J.-W., Muthu M., Pushparaj S.S.C., Gopal J. Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules. 2023;28:2151. doi: 10.3390/molecules28052151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Versantvoort C.H., Schuurhuis G.J., Pinedo H.M., Eekman C.A., Kuiper C.M., Lankelma J., Broxterman H.J. Genistein modulates the decreased drug accumulation in non-P-glycoprotein mediated multidrug resistant tumour cells. Br. J. Cancer. 1993;68:939–946. doi: 10.1038/bjc.1993.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Castro A.F., Altenberg G.A. Inhibition of drug transport by genistein in multidrug-resistant cells expressing P-glycoprotein. Biochem. Pharmacol. 1997;53:89–93. doi: 10.1016/S0006-2952(96)00657-0. [DOI] [PubMed] [Google Scholar]
- 143.Hooijberg J.H., Broxterman H.J., Heijn M., Fles D.L., Lankelma J., Pinedo H.M. Modulation by (iso)flavonoids of the ATPase activity of the multidrug resistance protein. FEBS Lett. 1997;413:344–348. doi: 10.1016/S0014-5793(97)00940-X. [DOI] [PubMed] [Google Scholar]
- 144.Mora Lagares L., Pérez-Castillo Y., Minovski N., Novič M. Structure–Function Relationships in the Human P-Glycoprotein (ABCB1): Insights from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2022;23:362. doi: 10.3390/ijms23010362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahn-Jarvis J.H., Parihar A., Doseff A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants. 2019;8:202. doi: 10.3390/antiox8070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Choi J.S., Piao Y.J., Kang K.W. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition by quercetin. Arch. Pharmacal Res. 2011;34:607–613. doi: 10.1007/s12272-011-0411-x. [DOI] [PubMed] [Google Scholar]
- 147.Borska S., Chmielewska M., Wysocka T., Drag-Zalesinska M., Zabel M., Dziegiel P. In vitro effect of quercetin on human gastric carcinoma: Targeting cancer cells death and MDR. Food Chem. Toxicol. 2012;50:3375–3383. doi: 10.1016/j.fct.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 148.Yuan J., Wong I.L., Jiang T., Wang S.W., Liu T., Wen B.J., Chow L.M., Wan Sheng B. Synthesis of methylated quercetin derivatives and their reversal activities on P-gp- and BCRP-mediated multidrug resistance tumour cells. Eur. J. Med. Chem. 2012;54:413–422. doi: 10.1016/j.ejmech.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 149.Robey R.W., To K.K., Polgar O., Dohse M., Fetsch P., Dean M., Bates S.E. ABCG2: A perspective. Adv. Drug Deliv. Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang Y., Cole S.P., Cai T., Cai Y.U. Applications of nanoparticle drug delivery systems for the reversal of multidrug resistance in cancer. Oncol. Lett. 2016;12:11–15. doi: 10.3892/ol.2016.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Noguchi K., Katayama K., Mitsuhashi J., Sugimoto Y. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv. Drug Deliv. Rev. 2009;61:26–33. doi: 10.1016/j.addr.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 152.Li Y.W., Zhang Y., Zhang L., Li X., Yu J.B., Zhang H.T., Tan B.B., Jiang L.H., Wang Y.X., Liang Y., et al. Protective effect of tea polyphenols on renal ischemia/reperfusion injury via suppressing the activation of TLR4/NF-kappaB p65 signal pathway. Gene. 2014;542:46–51. doi: 10.1016/j.gene.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 153.Maraldi T., Vauzour D., Angeloni C. Dietary polyphenols and their effects on cell biochemistry and pathophysiology 2013. Oxidative Med. Cell. Longev. 2014;2014:576363. doi: 10.1155/2014/576363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jafari S., Heydarian S., Lai R., Mehdizadeh Aghdam E., Molavi O. Silibinin induces immunogenic cell death in cancer cells and enhances the induced immunogenicity by chemotherapy. Bioimpacts. 2023;13:51–61. doi: 10.34172/bi.2022.23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tyagi A., Agarwal C., Dwyer-Nield L.D., Singh R.P., Malkinson A.M., Agarwal R. Silibinin modulates TNF-alpha and IFN-gamma mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol. Carcinog. 2012;51:832–842. doi: 10.1002/mc.20851. [DOI] [PubMed] [Google Scholar]
- 156.Sadava D., Kane S.E. Silibinin reverses drug resistance in human small-cell lung carcinoma cells. Cancer Lett. 2013;339:102–106. doi: 10.1016/j.canlet.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tyagi A.K., Agarwal C., Chan D.C., Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol. Rep. 2004;11:493–499. doi: 10.3892/or.11.2.493. [DOI] [PubMed] [Google Scholar]
- 158.Zhou L., Liu P., Chen B., Wang Y., Wang X., Chiriva Internati M., Wachtel M.S., Frezza E.E. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res. 2008;28:1119–1127. [PubMed] [Google Scholar]
- 159.Dhanalakshmi S., Agarwal P., Glode L.M., Agarwal R. Silibinin sensitizes human prostate carcinoma DU145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptotic death. Int. J. Cancer. 2003;106:699–705. doi: 10.1002/ijc.11299. [DOI] [PubMed] [Google Scholar]
- 160.Hussain S.A., Marouf B.H. Silibinin improves the cytotoxicity of methotrexate in chemo resistant human rhabdomyosarcoma cell lines. Saudi Med. J. 2013;34:1145–1150. [PubMed] [Google Scholar]
- 161.Bonneau C., Rouzier R., Geyl C., Cortez A., Castela M., Lis R., Darai E., Touboul C. Predictive markers of chemoresistance in advanced stages epithelial ovarian carcinoma. Gynecol. Oncol. 2015;136:112–120. doi: 10.1016/j.ygyno.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 162.Lu J.J., Cai Y.J., Ding J. The short-time treatment with curcumin sufficiently decreases cell viability, induces apoptosis and copper enhances these effects in multidrug-resistant K562/A02 cells. Mol. Cell. Biochem. 2012;360:253–260. doi: 10.1007/s11010-011-1064-2. [DOI] [PubMed] [Google Scholar]
- 163.Haraguchi T., Kayashima T., Okazaki Y., Inoue J., Mineo S., Matsubara K., Sakaguchi E., Yanaka N., Kato N. Cecal succinate elevated by some dietary polyphenols may inhibit colon cancer cell proliferation and angiogenesis. J. Agric. Food Chem. 2014;62:5589–5594. doi: 10.1021/jf501142k. [DOI] [PubMed] [Google Scholar]
- 164.Walter A., Etienne-Selloum N., Sarr M., Kane M.O., Beretz A., Schini-Kerth V.B. Angiotensin II induces the vascular expression of VEGF and MMP-2 in vivo: Preventive effect of red wine polyphenols. J. Vasc. Res. 2008;45:386–394. doi: 10.1159/000121408. [DOI] [PubMed] [Google Scholar]
- 165.Zeng Y., Zhou W., Yu J., Zhao L., Wang K., Hu Z., Liu X. By-Products of Fruit and Vegetables: Antioxidant Properties of Extractable and Non-Extractable Phenolic Compounds. Antioxidants. 2023;12:418. doi: 10.3390/antiox12020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ali M., Wani S.U.D., Salahuddin M., Manjula S.N., Mruthunjaya K., Dey T., Zargar M.I., Singh J. Recent advance of herbal medicines in cancer- a molecular approach. Heliyon. 2023;9:e13684. doi: 10.1016/j.heliyon.2023.e13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brown E.M., Gill C.I., McDougall G.J., Stewart D. Mechanisms underlying the anti-proliferative effects of berry components in in vitro models of colon cancer. Curr. Pharm. Biotechnol. 2012;13:200–209. doi: 10.2174/138920112798868773. [DOI] [PubMed] [Google Scholar]
- 168.Woode D.R., Aiyer H.S., Sie N., Zwart A.L., Li L., Seeram N.P., Clarke R. Effect of Berry Extracts and Bioactive Compounds on Fulvestrant (ICI 182,780) Sensitive and Resistant Cell Lines. Int. J. Breast Cancer. 2012;2012:147828. doi: 10.1155/2012/147828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Diaz L.A., Cantley L.C. The Hallmarks of a Cancer Discovery. Cancer Discov. 2023;13:797–798. doi: 10.1158/2159-8290.CD-23-0171. [DOI] [PubMed] [Google Scholar]
- 170.Luo N. Editorial: Tumor microenvironment in cancer hallmarks and therapeutics. Front. Mol. Biosci. 2022;9:1019830. doi: 10.3389/fmolb.2022.1019830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ravi S., Alencar A.M., Jr., Arakelyan J., Xu W., Stauber R., Wang C.I., Papyan R., Ghazaryan N., Pereira R.M. An Update to Hallmarks of Cancer. Cureus. 2022;14:e24803. doi: 10.7759/cureus.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pal S., Sharma A., Mathew S.P., Jaganathan B.G. Targeting cancer-specific metabolic pathways for developing novel cancer therapeutics. Front. Immunol. 2022;13:955476. doi: 10.3389/fimmu.2022.955476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma G., Zhang Z., Li P., Zhang Z., Zeng M., Liang Z., Li D., Wang L., Chen Y., Liang Y., et al. Reprogramming of glutamine metabolism and its impact on immune response in the tumor microenvironment. Cell Commun. Signal. 2022;20:114. doi: 10.1186/s12964-022-00909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aghakhani S., Zerrouk N., Niarakis A. Metabolic Reprogramming of Fibroblasts as Therapeutic Target in Rheumatoid Arthritis and Cancer: Deciphering Key Mechanisms Using Computational Systems Biology Approaches. Cancers. 2020;13:35. doi: 10.3390/cancers13010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Penkert J., Ripperger T., Schieck M., Schlegelberger B., Steinemann D., Illig T. On metabolic reprogramming and tumor biology: A comprehensive survey of metabolism in breast cancer. Oncotarget. 2016;7:67626–67649. doi: 10.18632/oncotarget.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yin C., Qie S., Sang N. Carbon source metabolism and its regulation in cancer cells. Crit. Rev. Eukaryot. Gene Expr. 2012;22:17–35. doi: 10.1615/CritRevEukarGeneExpr.v22.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fatima Z., Abonofal A., Stephen B. Targeting Cancer Metabolism to Improve Outcomes with Immune Checkpoint Inhibitors. J. Immunother. Precis. Oncol. 2023;6:91–102. doi: 10.36401/JIPO-22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lu W., Hu Y., Chen G., Chen Z., Zhang H., Wang F., Feng L., Pelicano H., Wang H., Keating M.J., et al. Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol. 2012;10:e1001326. doi: 10.1371/journal.pbio.1001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wieder R. Fibroblasts as Turned Agents in Cancer Progression. Cancers. 2023;15:2014. doi: 10.3390/cancers15072014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim M.C., Hwang S.H., Yang Y., Kim N.Y., Kim Y. Reduction in mitochondrial oxidative stress mediates hypoxia-induced resistance to cisplatin in human transitional cell carcinoma cells. Neoplasia. 2021;23:653–662. doi: 10.1016/j.neo.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kubicka A., Matczak K., Łabieniec-Watała M. More Than Meets the Eye Regarding Cancer Metabolism. Int. J. Mol. Sci. 2021;22:9507. doi: 10.3390/ijms22179507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jones R.G., Thompson C.B. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Oh K.S., Nam A.R., Bang J.H., Seo H.R., Kim J.M., Yoon J., Kim T.Y., Oh D.Y. A synthetic lethal strategy using PARP and ATM inhibition for overcoming trastuzumab resistance in HER2-positive cancers. Oncogene. 2022;41:3939–3952. doi: 10.1038/s41388-022-02384-w. [DOI] [PubMed] [Google Scholar]
- 184.Gandhi N., Das G.M. Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells. 2019;8:89. doi: 10.3390/cells8020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nguyen T.T., Katt W.P., Cerione R.A. Alone and together: Current approaches to targeting glutaminase enzymes as part of anti-cancer therapies. Future Drug Discov. 2023;4:FDD79. doi: 10.4155/fdd-2022-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jang M., Kim S.S., Lee J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013;45:e45. doi: 10.1038/emm.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhao Y., Butler E.B., Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rathod N.B., Elabed N., Punia S., Ozogul F., Kim S.-K., Rocha J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants. 2023;12:1217. doi: 10.3390/plants12061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Câmara J.S., Locatelli M., Pereira J.A.M., Oliveira H., Arlorio M., Fernandes I., Perestrelo R., Freitas V., Bordiga M. Behind the Scenes of Anthocyanins—From the Health Benefits to Potential Applications in Food, Pharmaceutical and Cosmetic Fields. Nutrients. 2022;14:5133. doi: 10.3390/nu14235133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Farrell T.L., Ellam S.L., Forrelli T., Williamson G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with SGLT1 and GLUT2 transporters. Biofactors. 2013;39:448–456. doi: 10.1002/biof.1090. [DOI] [PubMed] [Google Scholar]
- 191.Williamson G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013;57:48–57. doi: 10.1002/mnfr.201200511. [DOI] [PubMed] [Google Scholar]
- 192.Nong S., Han X., Xiang Y., Qian Y., Wei Y., Zhang T., Tian K., Shen K., Yang J., Ma X. Metabolic reprogramming in cancer: Mechanisms and therapeutics. MedComm. 2023;4:e218. doi: 10.1002/mco2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kumar S., Gupta S. Dietary phytochemicals and their role in cancer chemoprevention. J. Cancer Metastasis Treat. 2021;7:51. doi: 10.20517/2394-4722.2021.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liskova A., Stefanicka P., Samec M., Smejkal K., Zubor P., Bielik T., Biskupska-Bodova K., Kwon T.K., Danko J., Büsselberg D., et al. Dietary phytochemicals as the potential protectors against carcinogenesis and their role in cancer chemoprevention. Clin. Exp. Med. 2020;20:173–190. doi: 10.1007/s10238-020-00611-w. [DOI] [PubMed] [Google Scholar]
- 195.Kapinova A., Kubatka P., Golubnitschaja O., Kello M., Zubor P., Solar P., Pec M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018;23:36. doi: 10.1186/s12199-018-0724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li D.W., Dong P., Wang F., Chen X.W., Xu C.Z., Zhou L. Hypoxia induced multidrug resistance of laryngeal cancer cells via hypoxia-inducible factor-1alpha. Asian Pac. J. Cancer Prev. 2013;14:4853–4858. doi: 10.7314/APJCP.2013.14.8.4853. [DOI] [PubMed] [Google Scholar]
- 197.Li D., Xu D., Chen P., Xie J. Notch1 signaling modulates hypoxia-induced multidrug resistance in human laryngeal cancer cells. Mol. Biol. Rep. 2022;49:6235–6240. doi: 10.1007/s11033-022-07421-1. [DOI] [PubMed] [Google Scholar]
- 198.Wang R., Zhou S., Li S. Cancer therapeutic agents targeting hypoxia-inducible factor-1. Curr. Med. Chem. 2011;18:3168–3189. doi: 10.2174/092986711796391606. [DOI] [PubMed] [Google Scholar]
- 199.O’Neill E.J., Termini D., Albano A., Tsiani E. Anti-Cancer Properties of Theaflavins. Molecules. 2021;26:987. doi: 10.3390/molecules26040987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pan H., Kim E., Rankin G.O., Rojanasakul Y., Tu Y., Chen Y.C. Theaflavin-3,3′-Digallate Enhances the Inhibitory Effect of Cisplatin by Regulating the Copper Transporter 1 and Glutathione in Human Ovarian Cancer Cells. Int. J. Mol. Sci. 2018;19:117. doi: 10.3390/ijms19010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tasyriq M., Najmuldeen I.A., In L.L., Mohamad K., Awang K., Hasima N. 7α-Hydroxy-β-Sitosterol from Chisocheton tomentosus Induces Apoptosis via Dysregulation of Cellular Bax/Bcl-2 Ratio and Cell Cycle Arrest by Downregulating ERK1/2 Activation. Evid.-Based Complement. Altern. Med. 2012;2012:765316. doi: 10.1155/2012/765316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Singh M., Singh R., Bhui K., Tyagi S., Mahmood Z., Shukla Y. Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear factor-kappaB and Akt activation in human cervical cancer cells. Oncol. Res. 2011;19:245–257. doi: 10.3727/096504011X13021877989711. [DOI] [PubMed] [Google Scholar]
- 203.Liu S.M., Ou S.Y., Huang H.H. Green tea polyphenols induce cell death in breast cancer MCF-7 cells through induction of cell cycle arrest and mitochondrial-mediated apoptosis. J. Zhejiang Univ. Sci. B. 2017;18:89–98. doi: 10.1631/jzus.B1600022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Guo L.D., Chen X.J., Hu Y.H., Yu Z.J., Wang D., Liu J.Z. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytother. Res. 2013;27:422–430. doi: 10.1002/ptr.4731. [DOI] [PubMed] [Google Scholar]
- 205.Hao M., Chu Y., Lei J., Yao Z., Wang P., Chen Z., Wang K., Sang X., Han X., Wang L., et al. Pharmacological Mechanisms and Clinical Applications of Curcumin: Update. Aging Dis. 2023;14:716–749. doi: 10.14336/AD.2022.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Crew K.D., Ho K.A., Brown P., Greenlee H., Bevers T.B., Arun B., Sneige N., Hudis C., McArthur H.L., Chang J., et al. Effects of a green tea extract, Polyphenon E, on systemic biomarkers of growth factor signalling in women with hormone receptor-negative breast cancer. J. Hum. Nutr. Diet. 2015;28:272–282. doi: 10.1111/jhn.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen J., Zhuang D., Cai W., Xu L., Li E., Wu Y., Sugiyama K. Inhibitory effects of four plants flavonoids extracts on fatty acid synthase. J. Environ. Sci. 2009;21((Suppl. S1)):S131–S134. doi: 10.1016/S1001-0742(09)60056-5. [DOI] [PubMed] [Google Scholar]
- 208.Hao Y., Baker D., Ten Dijke P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019;20:2767. doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zeeshan R., Mutahir Z. Cancer metastasis—Tricks of the trade. Bosn. J. Basic Med. Sci. 2017;17:172–182. doi: 10.17305/bjbms.2017.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hu C., Li M., Guo T., Wang S., Huang W., Yang K., Liao Z., Wang J., Zhang F., Wang H. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine. 2019;58:152740. doi: 10.1016/j.phymed.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 212.Yu D., Ye T., Xiang Y., Shi Z., Zhang J., Lou B., Zhang F., Chen B., Zhou M. Quercetin inhibits epithelial-mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial-mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. OncoTargets Ther. 2017;10:4719–4729. doi: 10.2147/OTT.S136840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hoca M., Becer E., Kabadayi H., Yucecan S., Vatansever H.S. The Effect of Resveratrol and Quercetin on Epithelial-Mesenchymal Transition in Pancreatic Cancer Stem Cell. Nutr. Cancer. 2020;72:1231–1242. doi: 10.1080/01635581.2019.1670853. [DOI] [PubMed] [Google Scholar]
- 214.Lee Y.H., Tuyet P.T. Synthesis and biological evaluation of quercetin-zinc (II) complex for anti-cancer and anti-metastasis of human bladder cancer cells. Vitr. Cell Dev. Biol. Anim. 2019;55:395–404. doi: 10.1007/s11626-019-00363-2. [DOI] [PubMed] [Google Scholar]
- 215.Shafabakhsh R., Asemi Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019;12:55. doi: 10.1186/s13048-019-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sun Y., Zhou Q.M., Lu Y.Y., Zhang H., Chen Q.L., Zhao M., Su S.B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-beta1-Induced Epithelial-Mesenchymal Transition. Molecules. 2019;24:1131. doi: 10.3390/molecules24061131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 217.Ponnusamy L., Mahalingaiah P.K.S., Singh K.P. Epigenetic reprogramming and potential application of epigenetic-modifying drugs in acquired chemotherapeutic resistance. Adv. Clin. Chem. 2020;94:219–259. doi: 10.1016/bs.acc.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 218.Xu W.S., Parmigiani R.B., Marks P.A. Histone deacetylase inhibitors: Molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 219.Bouyahya A., El Hachlafi N., Aanniz T., Bourais I., Mechchate H., Benali T., Shariati M.A., Burkov P., Lorenzo J.M., Wilairatana P., et al. Natural Bioactive Compounds Targeting Histone Deacetylases in Human Cancers: Recent Updates. Molecules. 2022;27:2568. doi: 10.3390/molecules27082568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rajendran P., Abdelsalam S.A., Renu K., Veeraraghavan V., Ben Ammar R., Ahmed E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022;23:11712. doi: 10.3390/ijms231911712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang J., Song C., Zhan X. The role of protein acetylation in carcinogenesis and targeted drug discovery. Front. Endocrinol. 2022;13:972312. doi: 10.3389/fendo.2022.972312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu L., Fu Y., Zheng Y., Ma M., Wang C. Curcumin inhibits proteasome activity in triple-negative breast cancer cells through regulating p300/miR-142-3p/PSMB5 axis. Phytomedicine. 2020;78:153312. doi: 10.1016/j.phymed.2020.153312. [DOI] [PubMed] [Google Scholar]
- 223.Sen G.S., Mohanty S., Hossain D.M.S., Bhattacharyya S., Banerjee S., Chakraborty J., Saha S., Ray P., Bhattacharjee P., Mandal D., et al. Curcumin enhances the efficacy of chemotherapy by tailoring p65NFκB-p300 cross-talk in favor of p53–p300 in breast cancer. J. Biol. Chem. 2011;286:42232–42247. doi: 10.1074/jbc.M111.262295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sun W., Shahrajabian M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules. 2023;28:1845. doi: 10.3390/molecules28041845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bhattacharya T., Dutta S., Akter R., Rahman M.H., Karthika C., Nagaswarupa H.P., Murthy H.C.A., Fratila O., Brata R., Bungau S. Role of Phytonutrients in Nutrigenetics and Nutrigenomics Perspective in Curing Breast Cancer. Biomolecules. 2021;11:1176. doi: 10.3390/biom11081176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tong R., Wu X., Liu Y., Liu Y., Zhou J., Jiang X., Zhang L., He X., Ma L. Curcumin-Induced DNA Demethylation in Human Gastric Cancer Cells Is Mediated by the DNA-Damage Response Pathway. Oxid. Med. Cell. Longev. 2020;2020:2543504. doi: 10.1155/2020/2543504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang Z., Wang G., Li Y., Lei D., Xiang J., Ouyang L., Wang Y., Yang J. Recent progress in DNA methyltransferase inhibitors as anticancer agents. Front. Pharmacol. 2022;13:1072651. doi: 10.3389/fphar.2022.1072651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Khor T.O., Huang Y., Wu T.Y., Shu L., Lee J., Kong A.N. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011;82:1073–1078. doi: 10.1016/j.bcp.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 229.Shu L., Khor T.O., Lee J.H., Boyanapalli S.S., Huang Y., Wu T.Y., Saw C.L., Cheung K.L., Kong A.N. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. AAPS J. 2011;13:606–614. doi: 10.1208/s12248-011-9300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ghazi T., Arumugam T., Foolchand A., Chuturgoon A.A. The Impact of Natural Dietary Compounds and Food-Borne Mycotoxins on DNA Methylation and Cancer. Cells. 2020;9:2004. doi: 10.3390/cells9092004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hassan F.U., Rehman M.S.U., Khan M.S., Ali M.A., Javed A., Nawaz A., Yang C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019;10:514. doi: 10.3389/fgene.2019.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Michaille J.J., Piurowski V., Rigot B., Kelani H., Fortman E.C., Tili E. MiR-663, a MicroRNA Linked with Inflammation and Cancer That Is under the Influence of Resveratrol. Medicines. 2018;5:74. doi: 10.3390/medicines5030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Hayakawa S., Ohishi T., Oishi Y., Isemura M., Miyoshi N. Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol. Antioxidants. 2022;11:2352. doi: 10.3390/antiox11122352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang W., Jiang H., Chen Y., Ren F. Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J. Cell. Biochem. 2019;120:16283–16292. doi: 10.1002/jcb.28910. [DOI] [PubMed] [Google Scholar]
- 235.Fu J., Shrivastava A., Shrivastava S.K., Srivastava R.K., Shankar S. Triacetyl resveratrol upregulates miRNA-200 and suppresses the Shh pathway in pancreatic cancer: A potential therapeutic agent. Int. J. Oncol. 2019;54:1306–1316. doi: 10.3892/ijo.2019.4700. [DOI] [PubMed] [Google Scholar]
- 236.Zhao J., Fang Z., Zha Z., Sun Q., Wang H., Sun M., Qiao B. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur. J. Pharmacol. 2019;847:11–18. doi: 10.1016/j.ejphar.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 237.Zhang C., Hao Y., Sun Y., Liu P. Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/beta-catenin axis. J. Pharmacol. Sci. 2019;140:128–136. doi: 10.1016/j.jphs.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 238.Nwaeburu C.C., Abukiwan A., Zhao Z., Herr I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol. Cancer. 2017;16:23. doi: 10.1186/s12943-017-0589-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 239.Tao S.F., He H.F., Chen Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015;402:93–100. doi: 10.1007/s11010-014-2317-7. [DOI] [PubMed] [Google Scholar]
- 240.Bhardwaj V., Mandal A.K.A. Next-Generation Sequencing Reveals the Role of Epigallocatechin-3-Gallate in Regulating Putative Novel and Known microRNAs Which Target the MAPK Pathway in Non-Small-Cell Lung Cancer A549 Cells. Molecules. 2019;24:368. doi: 10.3390/molecules24020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ahmed F., Ijaz B., Ahmad Z., Farooq N., Sarwar M.B., Husnain T. Modification of miRNA Expression through plant extracts and compounds against breast cancer: Mechanism and translational significance. Phytomedicine. 2020;68:153168. doi: 10.1016/j.phymed.2020.153168. [DOI] [PubMed] [Google Scholar]
- 242.Garcia-Mayea Y., Mir C., Masson F., Paciucci R., LLeonart M.E. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020;60:166–180. doi: 10.1016/j.semcancer.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 243.Lytle N.K., Barber A.G., Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer. 2018;18:669–680. doi: 10.1038/s41568-018-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cao H.Z., Liu X.F., Yang W.T., Chen Q., Zheng P.S. LGR5 promotes cancer stem cell traits and chemoresistance in cervical cancer. Cell Death Dis. 2017;8:e3039. doi: 10.1038/cddis.2017.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chaudhary A.K., Mondal G., Kumar V., Kattel K., Mahato R.I. Chemosensitization and inhibition of pancreatic cancer stem cell proliferation by overexpression of microRNA-205. Cancer Lett. 2017;402:1–8. doi: 10.1016/j.canlet.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cheng S., Huang Y., Lou C., He Y., Zhang Y., Zhang Q. FSTL1 enhances chemoresistance and maintains stemness in breast cancer cells via integrin beta3/Wnt signaling under miR-137 regulation. Cancer Biol. Ther. 2019;20:328–337. doi: 10.1080/15384047.2018.1529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lu H., Ju D.D., Yang G.D., Zhu L.Y., Yang X.M., Li J., Song W.W., Wang J.H., Zhang C.C., Zhang Z.G., et al. Targeting cancer stem cell signature gene SMOC-2 Overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma. EBioMedicine. 2019;40:276–289. doi: 10.1016/j.ebiom.2018.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang T., Fahrmann J.F., Lee H., Li Y.J., Tripathi S.C., Yue C., Zhang C., Lifshitz V., Song J., Yuan Y., et al. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27:1357. doi: 10.1016/j.cmet.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ward Rashidi M.R., Mehta P., Bregenzer M., Raghavan S., Fleck E.M., Horst E.N., Harissa Z., Ravikumar V., Brady S., Bild A., et al. Engineered 3D Model of Cancer Stem Cell Enrichment and Chemoresistance. Neoplasia. 2019;21:822–836. doi: 10.1016/j.neo.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chi H.C., Tsai C.Y., Wang C.S., Yang H.Y., Lo C.H., Wang W.J., Lee K.F., Lai L.Y., Hong J.H., Chang Y.F., et al. DOCK6 promotes chemo- and radioresistance of gastric cancer by modulating WNT/beta-catenin signaling and cancer stem cell traits. Oncogene. 2020;39:5933–5949. doi: 10.1038/s41388-020-01390-0. [DOI] [PubMed] [Google Scholar]
- 251.Troschel F.M., Bohly N., Borrmann K., Braun T., Schwickert A., Kiesel L., Eich H.T., Gotte M., Greve B. miR-142-3p attenuates breast cancer stem cell characteristics and decreases radioresistance in vitro. Tumour Biol. 2018;40:1010428318791887. doi: 10.1177/1010428318791887. [DOI] [PubMed] [Google Scholar]
- 252.Tsao T., Beretov J., Ni J., Bai X., Bucci J., Graham P., Li Y. Cancer stem cells in prostate cancer radioresistance. Cancer Lett. 2019;465:94–104. doi: 10.1016/j.canlet.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 253.Eun K., Ham S.W., Kim H. Cancer stem cell heterogeneity: Origin and new perspectives on CSC targeting. BMB Rep. 2017;50:117–125. doi: 10.5483/BMBRep.2017.50.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Najafi M., Mortezaee K., Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. doi: 10.1016/j.lfs.2019.116781. [DOI] [PubMed] [Google Scholar]
- 255.Galoczova M., Coates P., Vojtesek B. STAT3, stem cells, cancer stem cells and p63. Cell. Mol. Biol. Lett. 2018;23:12. doi: 10.1186/s11658-018-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Su P., Yang Y., Wang G., Chen X., Ju Y. Curcumin attenuates resistance to irinotecan via induction of apoptosis of cancer stem cells in chemoresistant colon cancer cells. Int. J. Oncol. 2018;53:1343–1353. doi: 10.3892/ijo.2018.4461. [DOI] [PubMed] [Google Scholar]
- 257.Yoshida K., Toden S., Ravindranathan P., Han H., Goel A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis. 2017;38:1036–1046. doi: 10.1093/carcin/bgx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Marquardt J.U., Gomez-Quiroz L., Arreguin Camacho L.O., Pinna F., Lee Y.H., Kitade M., Dominguez M.P., Castven D., Breuhahn K., Conner E.A., et al. Curcumin effectively inhibits oncogenic NF-kappaB signaling and restrains stemness features in liver cancer. J. Hepatol. 2015;63:661–669. doi: 10.1016/j.jhep.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nan Y., Su H., Zhou B., Liu S. The function of natural compounds in important anticancer mechanisms. Front. Oncol. 2022;12:1049888. doi: 10.3389/fonc.2022.1049888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kuo Y.C., Wang L.J., Rajesh R. Targeting human brain cancer stem cells by curcumin-loaded nanoparticles grafted with anti-aldehyde dehydrogenase and sialic acid: Colocalization of ALDH and CD44. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:362–372. doi: 10.1016/j.msec.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 261.Toden S., Okugawa Y., Jascur T., Wodarz D., Komarova N.L., Buhrmann C., Shakibaei M., Boland C.R., Goel A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36:355–367. doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhou Q.M., Sun Y., Lu Y.Y., Zhang H., Chen Q.L., Su S.B. Curcumin reduces mitomycin C resistance in breast cancer stem cells by regulating Bcl-2 family-mediated apoptosis. Cancer Cell Int. 2017;17:84. doi: 10.1186/s12935-017-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhou Q., Ye M., Lu Y., Zhang H., Chen Q., Huang S., Su S. Curcumin Improves the Tumoricidal Effect of Mitomycin C by Suppressing ABCG2 Expression in Stem Cell-Like Breast Cancer Cells. PLoS ONE. 2015;10:e0136694. doi: 10.1371/journal.pone.0136694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Li Y.J., Wu S.L., Lu S.M., Chen F., Guo Y., Gan S.M., Shi Y.L., Liu S., Li S.L. (-)-Epigallocatechin-3-gallate inhibits nasopharyngeal cancer stem cell self-renewal and migration and reverses the epithelial-mesenchymal transition via NF-kappaB p65 inactivation. Tumour Biol. 2015;36:2747–2761. doi: 10.1007/s13277-014-2899-4. [DOI] [PubMed] [Google Scholar]
- 265.Wang Y., Wang H., Zhou R., Zhong W., Lu S., Ma Z., Chai Y. Baicalin inhibits human osteosarcoma cells invasion, metastasis, and anoikis resistance by suppressing the transforming growth factor-beta1-induced epithelial-to-mesenchymal transition. Anticancer. Drugs. 2017;28:581–587. doi: 10.1097/CAD.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 266.Yu M., Qi B., Xiaoxiang W., Xu J., Liu X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-kappaB pathway. Biomed. Pharmacother. 2017;90:677–685. doi: 10.1016/j.biopha.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 267.Toden S., Tran H.M., Tovar-Camargo O.A., Okugawa Y., Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. 2016;7:16158–16171. doi: 10.18632/oncotarget.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Rudzińska A., Juchaniuk P., Oberda J., Wiśniewska J., Wojdan W., Szklener K., Mańdziuk S. Phytochemicals in Cancer Treatment and Cancer Prevention—Review on Epidemiological Data and Clinical Trials. Nutrients. 2023;15:1896. doi: 10.3390/nu15081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jakobušić Brala C., Karković Marković A., Kugić A., Torić J., Barbarić M. Combination Chemotherapy with Selected Polyphenols in Preclinical and Clinical Studies—An Update Overview. Molecules. 2023;28:3746. doi: 10.3390/molecules28093746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bešlo D., Golubić N., Rastija V., Agić D., Karnaš M., Šubarić D., Lučić B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants. 2023;12:1141. doi: 10.3390/antiox12061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gonzales G.B.M., Smagghe G., Grootaert C., Zotti M., Raes K., Van Camp J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure -activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015;47:175–190. doi: 10.3109/03602532.2014.1003649. [DOI] [PubMed] [Google Scholar]
- 272.Scott M.B., Styring A.K., McCullagh J.S.O. Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals. Pathogens. 2022;11:770. doi: 10.3390/pathogens11070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.