Abstract
Anaplastic lymphoma kinase-positive (ALK-positive) lung adenocarcinoma with multiple liver metastases accounts for a relatively small number of cases of non-small cell lung cancer. Several ALK-tyrosine kinase inhibitors (ALK-TKIs) are available for the treatment of lung cancer. However, there is limited evidence on the treatment of multiple liver metastases in patients with lung cancer that are refractory to ALK-TKIs. We report the case of a 42-year-old male patient with ALK-positive lung adenocarcinoma who experienced rapid progression to multiple liver metastases while receiving treatment with alectinib. Biopsy of the liver metastases revealed echinoderm microtubule-associated protein-like 4-ALK (EML4-ALK) fusion and tumor protein p53 (TP53) mutation; notably, ALK secondary mutations were not detected. Despite the sequential administration of third-generation ALK-TKIs, the liver metastases did not respond, the serum levels of total bilirubin and biliary enzymes continued to increase, and the patient’s general appearance worsened. Finally, the patient exhibited a remarkable clinical response to treatment with a combination of atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP). ABCP is one of the optimal options for ALK-positive lung cancer with liver metastasis that is refractory to ALK-TKIs therapy.
Keywords: lung adenocarcinoma, anaplastic lymphoma kinase, ALK, liver metastasis, ABCP, TP53
Introduction
Chromosomal rearrangements involving the anaplastic lymphoma kinase (ALK) account for 3–5% of non-small cell lung cancer (NSCLC) cases.1 NSCLC harboring ALK rearrangements is more sensitive to ALK-tyrosine kinase inhibitors (ALK-TKIs) than cytotoxic chemotherapy. Currently, several ALK-TKIs are available for the treatment of lung cancer. Guidelines of the American Society of Clinical Oncology advocate that clinicians should offer alectinib or brigatinib to patients with previously untreated ALK-positive NSCLC, and long-term efficacy has been reported.2 The treatment of such tumors that are refractory to ALK-TKIs is complicated because their sensitivity to each ALK-TKI differs, and several resistance mechanisms are involved.3 A different mechanism of treatment options is an immune checkpoint inhibitor (ICI). The efficacy of ICI monotherapy for ALK-positive NSCLC is proved to be inferior to that of other driver mutations-positive NSCLC.4 As many clinical trials of ICIs plus chemotherapy excluded patients with ALK-positive NSCLC, the efficacy of chemotherapy plus ICIs in treating tumors that progressed after ALK-TKI therapy is not fully proven. In the IMpower150 trial, the combination of atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP) demonstrated a better efficacy against NSCLC with ALK or epidermal growth factor receptor (EGFR) alterations and with liver metastases than bevacizumab, carboplatin, and paclitaxel (BCP).5 ALK-positive patients with liver metastases were excluded from the primary analysis in the IMpower150 trial, and the mechanism of efficacy in these patients was unknown. In this article, we report a case of ALK-positive/tumor protein 53 (ALK-positive/TP53)-mutant lung adenocarcinoma with multiple liver metastases which exhibited a remarkable clinical response to treatment with ABCP after the failure of ALK-TKI therapy.
Case Report
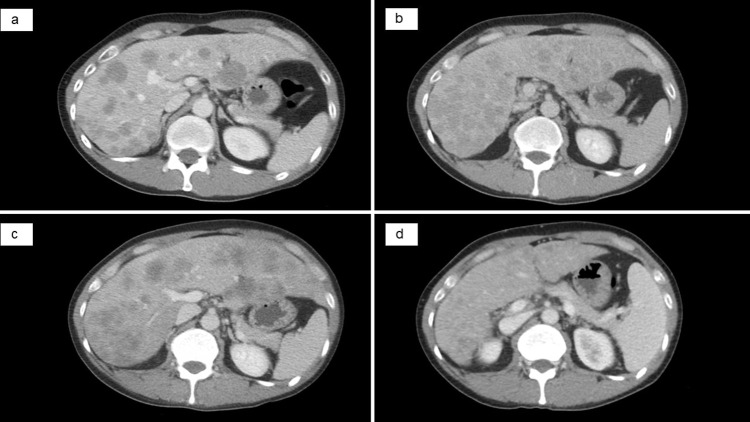
The patient was a 42-year-old male diagnosed with cT1N3M0 stage IIIB lung adenocarcinoma in 2009. Of note, he did not have a smoking history. He underwent chemoradiotherapy consisting of cisplatin, docetaxel, and 60 Gy radiation. Subsequently, the patient received six cycles of maintenance therapy with pemetrexed based on the attending physician’s discretion. In December 2012, an examination with computed tomography (CT) revealed recurrence in the right lung, and immunohistochemistry detected ALK expression. ALK rearrangement could not be detected by fluorescence in situ hybridization. The patient received two chemotherapeutic regimens, namely crizotinib as second-line therapy and carboplatin and nanoparticle albumin-bound-paclitaxel as third-line therapy. In 2014, fourth-line treatment with alectinib was initiated. The best overall response was partial response (PR) according to the Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1. PR continued until 2021. In August 2021, CT analysis showed multiple metastases in the liver (Figure 1a) and lymph nodes (ie, cervical, supraclavicular fossa, axillary, mediastinal, and abdominal lymph nodes). Brain magnetic resonance imaging did not reveal brain metastasis.
Figure 1.
Abdominal CT showing multiple liver metastases. (a) Before the administration of brigatinib: detection of multiple liver lesions. (b) After 13 days of treatment with brigatinib. (c) After 25 days of treatment with lorlatinib and before the administration of ABCP: progression of multiple liver lesions. (d) After four cycles of ABCP: partial response of multiple liver lesions.
Abbreviations: ABCP, atezolizumab, bevacizumab, carboplatin, and paclitaxel; CT, computed tomography.
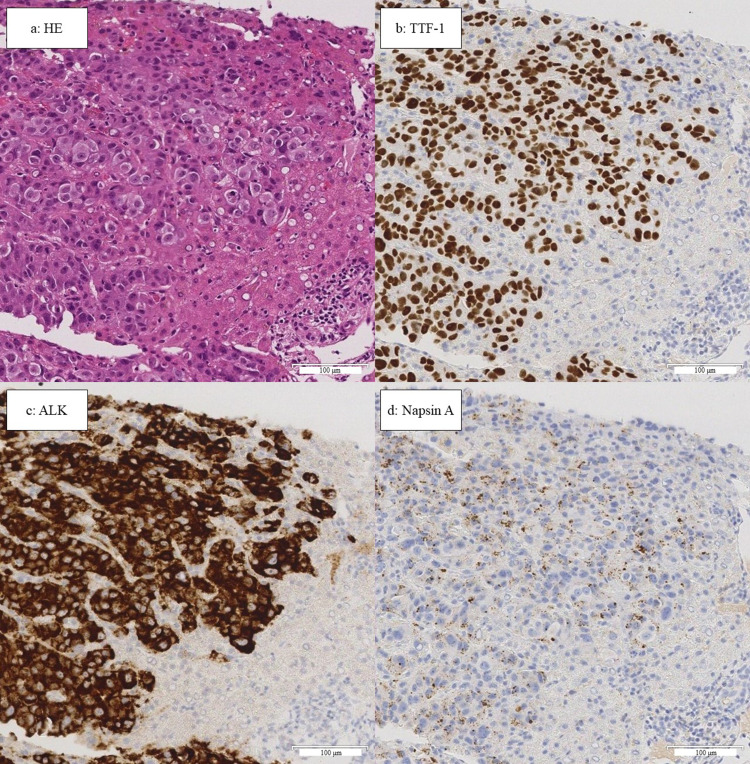
The patient underwent a biopsy of the liver metastases to examine for ALK second mutation and programmed death-ligand 1 (PD-L1) expression. Pathological tissue analysis revealed lung adenocarcinoma (Figure 2a). The examination yielded positive results for thyroid transcription factor-1 (TTF-1) (Figure 2b), ALK (Figure 2c), and Napsin A (Figure 2d). Further analysis using FoundationOne® (Foundation Medicine, Cambridge, MA, USA) showed EML4-ALK fusion, but no emergence of ALK-TKI resistance mutations. The results were positive for TP53 and set domain containing 2 (SETD2) mutations. The tumor mutation burden (TMB) score was 1.26. Microsatellite instability analysis indicated stability. According to the findings of the 22C3 assay, PD-L1 expression was 5–10%.
Figure 2.
Results of liver biopsy. The scale bars below each image are equivalent to 100μm. (a) Hematoxylin and eosin staining showing atypical cell hyperplasia and formation of glandular luminal structures. (b–d) Immunohistochemical staining of TTF-1, ALK, and Napsin A showing diffuse staining of tumor cells.
Abbreviations: ALK, anaplastic lymphoma kinase; HE, hematoxylin and eosin; TTF-1, thyroid transcription factor-1.
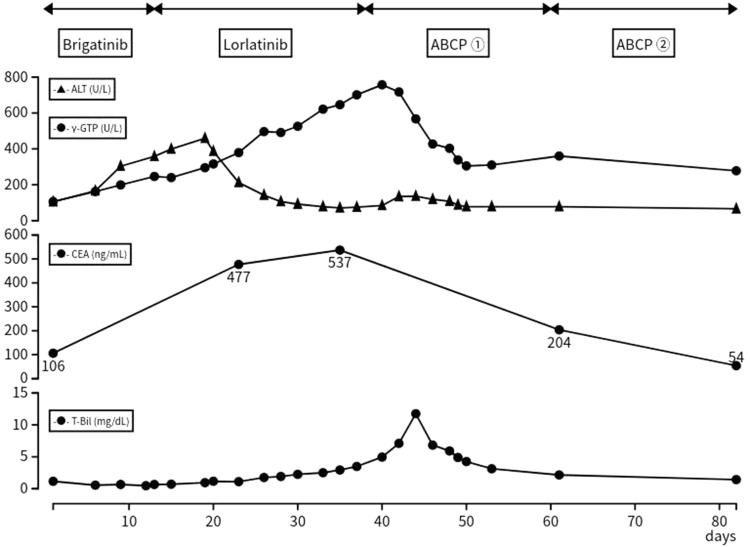
In September 2021, fifth-line treatment with brigatinib was initiated. However, the serum levels of hepatobiliary enzymes continued to increase (Figure 3). Coagulation system, the serum albumin level and platelet count were intact. A CT scan was performed after 13 days to identify potential liver injury induced by the drug or progression of liver metastases. The results showed the progression of the liver metastases and occurrence of new lesions (Figure 1b), and his treatment was switched from brigatinib to lorlatinib (sixth-line treatment). However, biliary enzymes and total bilirubin (T-bil) levels continued to rise (Figure 3). Prior to the initiation of treatment, the patient had an Eastern Cooperative Oncology Group performance status (PS) score of 1; after 1 month of treatment, this score was 3. The administration of lorlatinib was discontinued on day 25. A CT scan showed a continued increase in the size of multiple liver metastases (Figure 1c), but shrinkage of multiple lymph node metastases.
Figure 3.
Clinical course of the patient and transition of hematologic examination. Timeline after the administration of brigatinib, Day 1 was defined as the day brigatinib was administered. Serum levels of γ-GPT, ALT, T-bil, and CEA, the transition of the hematologic examination, and treatment regimen.
Abbreviations: ALT, alanine aminotransferase; CEA, carcinoembryonic antigen; γ-GPT, gamma-glutamyl transpeptidase, T-bil, total bilirubin; ABCP, atezolizumab, bevacizumab, carboplatin, and paclitaxel.
The patient received seventh-line treatment with atezolizumab (1200mg/body weight), bevacizumab (7.5 mg/kg), carboplatin (area under the curve 5), and paclitaxel (140 mg/m2). Owing to liver injury and poor PS, carboplatin, and paclitaxel were administered at a reduced dose. After one cycle of therapy, the levels of γ-glutamyl transpeptidase (γ-GPT), T-bil, and carcinoembryonic antigen (CEA) showed remarkable improvement. The maximum serum levels of γ-GPT, T-bil, and CEA were 757 U/L, 11.75 mg/dL, and 537 ng/mL, respectively. After administrating the first cycle of ABCP, these levels improved to 360 U/L, 2.15 mg/dL, and 204 ng/mL, respectively (Figure 3). The PS recovered from 3 to 1. Grade 3 febrile neutropenia and grade 3 thrombocytopenia according to the Common Terminology Criteria for Adverse Events (CTCAE) were observed but were manageable without treatment interruption. Following four cycles, a CT scan showed a reduction of the liver metastases (Figure 1d), and the serum levels of biliary enzymes, bilirubin, and CEA continued to improve. The patient received three cycles of maintenance therapy with atezolizumab and bevacizumab; nevertheless, there was the progression of multiple liver metastases. The antitumor effect of the treatment was sustained for 5 months after the administration of ABCP.
Discussion
In our case, there was progression of liver metastases despite treatment with several ALK-TKIs.
EML4-ALK fusion and TP53 were detected from re-biopsy of liver metastases, but ALK secondary mutations were not proved. Although prior treatment with carboplatin and nanoparticle albumin-bound-paclitaxel, ABCP was effective against the liver metastases and the patient’s PS score recovered to baseline.
A small number of patients with ALK-positive lung cancer have been treated with ICIs and chemotherapy.6 The most frequent metastatic sites of lung cancer are the nervous system, bones, liver, and adrenal glands. Liver metastases are found in 17% of patients with lung adenocarcinoma and associated with worse prognosis versus other metastases.7 Analysis of the liver metastases using FoundationOne® revealed EML4-ALK fusion, TP53, and SETD2 mutations, but no ALK-secondary mutation. ALK secondary mutations, bypass signaling activation through other oncogenes, and small-cell lung cancer transformations have been reported as the mechanisms of resistance to ALK-TKIs.8–10 EML4-ALK fusion has been reported several variants and more than 80% of variants are variant 1 and variant 3a/b. Frequency of resistance mutations such as G1202R and its compound mutations, and prognosis differ from each variant.11 However, resistance mutations and small-cell lung cancer transformations were not detected in our case. We considered that genetic and pharmacological factors contributed to the remarkable response to treatment with ABCP.
TP53 and SETD2 mutations have been identified as tumor suppressor genes. Functional TP53 proteins induce cell-cycle arrest, DNA repair, and apoptosis in response to chemotherapy.12 TP53 mutations occur commonly in patients with ALK-positive NSCLC (23.8%).13 These patients are linked to a higher incidence of liver metastases and worse outcomes versus those with TP53 wild type.8 SETD2 is a tumor suppressor gene in lung adenocarcinoma, and its mutation co-occurs with that of TP53.14 Patients with ALK-positive/TP53-mutant NSCLC who have been treated with ALK-TKIs exhibited worse progression-free survival than those having wild-type TP53.15 However, the overall survival of patients with TP53 or SETD2 mutation receiving treatment with ICIs was longer than that of patients with the wild-type genes.16–18 Also, TP53 mutations have been reported to relieve the transcriptional repression of vascular endothelial growth factor (VEGF), and high expression of VEGF led to the high efficacy of bevacizumab.19 TP53 mutations promoted the resistance of ALK-TKIs. On the other hand, in this case they likely contributed to clinical response of ABCP against ALK-TKI-resistant lung cancer.
We consider response of ABCP in this case was also related to differences in the tumor microenvironment in the liver. The tissue-specific factors of the liver, which may modulate the sensitivity of the tumor to ICIs.20 This implies that liver metastases of lung cancer may respond to ICIs in a similar manner to hepatocellular carcinoma that is associated with high VEGF expression. VEGF induces immunosuppression in the tumor microenvironment and promotes factors of anti-tumor immunity.21 Moreover, VEGF induces the proliferation of regulatory T cells, increases the recruitment of myeloid-derived suppressor cells, and elevates the expression of programmed cell death-1 (PD-1) on CD8(+) cytotoxic T lymphocytes and regulatory T cells.22–24 We consider that the liver metastases detected in this case resembled more closely the microenvironment of hepatocellular carcinoma than that of lung cancer. This may explain the response of the multiple lymph node metastases to treatment with ALK-TKIs, as well as the absence of a response by the liver metastases.
The multiple liver metastases exhibited a good response to treatment with ABCP, accompanied by improvement in the serum levels of bilirubin and biliary enzymes. Moreover, the PS score improved from 3 to 1. Generally, ABCP is used as first-line treatment and administered to patients with a PS score of 0–1. The patient was 42 years old, and his baseline PS was good. The high serum levels of bilirubin and biliary enzymes were attributed to biliary obstruction caused by the liver metastases. We estimated that following response of the liver metastases to treatment with ABCP, hyperbilirubinemia and the PS score would return to the baseline levels. The optimal treatment for ALK-positive lung cancer after the failure of treatment with ALK-TKIs has not been determined. The relationship between efficacy of ABCP and ALK-rearranged/TP53-mutant lung cancer remains unclear; hence further research is warranted. Examining genetic status leads to developing optimal therapies. Thus, clinicians consider performing a re-biopsy to determine the status of these genes.
Conclusion
ABCP may be a favorable treatment option for ALK-positive lung cancer with liver metastasis that is refractory to ALK-TKIs therapy.
Consent for Publication
Institutional approval was not required to publish the case details.
Written informed consent was provided by the patient for publication of this case report, including the patient’s details and accompanying images.
Disclosure
Dr Seike reported personal fees from Chugai, Takeda, and Pfizer. Dr Kubota reported personal fees from BMS, Chugai, Nihon Kayaku, Taiho, MSD and Pfizer outside the submitted work. Dr Miynaga reported honoraria from AstraZeneca, and Pfizer. Dr Tozuka reported honoraria from CHUGAI PHARMACEUTICAL CO., LTD and AstraZeneca K.K., outside the submitted work. There are no conflicts for other coauthors.
References
- 1.Chevallier M, Borgeaud M, Addeo A, Friedlaender A. Oncogenic driver mutations in non-small cell lung cancer: past, present and future. World J Clin Oncol. 2021;12(4):217–237. doi: 10.5306/wjco.v12.i4.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39(9):1040–1091. doi: 10.1200/JCO.20.03570 [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Seto Y, Okada K, et al. Overcoming resistance by ALK compound mutation (I1171S + G1269A) after sequential treatment of multiple ALK inhibitors in non-small cell lung cancer. Thorac Cancer. 2020;11(3):581–587. doi: 10.1111/1759-7714.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oya Y, Kuroda H, Nakada T, Takahashi Y, Sakakura N, Hida T. Efficacy of immune checkpoint inhibitor monotherapy for advanced non-small-cell lung cancer with ALK rearrangement. Int J Mol Sci. 2020;21(7):2623. doi: 10.3390/ijms21072623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 6.Von CE, Fuang HG. A case of remarkable response to atezolizumab in ALK-translocated metastatic lung adenocarcinoma. Respir Med Case Rep. 2021;34:101478. doi: 10.1016/j.rmcr.2021.101478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Huang J, Wang T, et al. Decoding the evolutionary response to ensartinib in patients with ALK-positive NSCLC by dynamic circulating tumor DNA sequencing. J Thorac Oncol. 2021;16(5):827–839. doi: 10.1016/j.jtho.2021.01.1615 [DOI] [PubMed] [Google Scholar]
- 9.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–1133. doi: 10.1158/2159-8290.CD-16-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamagata A, Yokoyama T, Fukuda Y, Ishida T. Alectinib re-challenge in small cell lung cancer transformation after chemotherapy failure in a patient with ALK-positive lung cancer: a case report. Respir Med Case Rep. 2021;33:101440. doi: 10.1016/j.rmcr.2021.101440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36(12):1199–1206. doi: 10.1200/JCO.2017.76.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speidel D. The role of DNA damage responses in p53 biology. Arch Toxicol. 2015;89(4):501–517. doi: 10.1007/s00204-015-1459-z [DOI] [PubMed] [Google Scholar]
- 13.Kron A, Alidousty C, Scheffler M, et al. Impact of TP53 mutation status on systemic treatment outcome in ALK-rearranged non-small-cell lung cancer. Ann Oncol. 2018;29(10):2068–2075. doi: 10.1093/annonc/mdy333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter DM, Venancio OS, Buza EL, et al. Systematic in vivo inactivation of chromatin-regulating enzymes identifies Setd2 as a potent tumor suppressor in lung adenocarcinoma. Cancer Res. 2017;77(7):1719–1729. doi: 10.1158/0008-5472.CAN-16-2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanimoto A, Matsumoto S, Takeuchi S, et al. Proteasome inhibition overcomes ALK-TKI resistance in ALK-rearranged/TP53-mutant NSCLC via noxa expression. Clin Cancer Res. 2021;27(5):1410–1420. doi: 10.1158/1078-0432.CCR-20-2853 [DOI] [PubMed] [Google Scholar]
- 16.Assoun S, Theou-Anton N, Nguenang M, et al. Association of TP53 mutations with response and longer survival under immune checkpoint inhibitors in advanced non-small-cell lung cancer. Lung Cancer. 2019;132:65–71. doi: 10.1016/j.lungcan.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 17.Dong ZY, Zhong WZ, Zhang XC, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–3024. doi: 10.1158/1078-0432.CCR-16-2554 [DOI] [PubMed] [Google Scholar]
- 18.Lu M, Zhao B, Liu M, et al. Pan-cancer analysis of SETD2 mutation and its association with the efficacy of immunotherapy. NPJ Precis Oncol. 2021;5(1):51. doi: 10.1038/s41698-021-00193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie KK, Filiaci VL, Mallen AR, et al. Mutated p53 portends improvement in outcomes when bevacizumab is combined with chemotherapy in advanced/recurrent endometrial cancer: an NRG Oncology study. Gynecol Oncol. 2021;161(1):113–121. doi: 10.1016/j.ygyno.2021.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pao W, Ooi CH, Birzele F, et al. Tissue-specific immunoregulation: a call for better understanding of the “immunostat” in the context of cancer. Cancer Discov. 2018;8(4):395–402. doi: 10.1158/2159-8290.CD-17-1320 [DOI] [PubMed] [Google Scholar]
- 21.Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52(9):1475–1485. doi: 10.1038/s12276-020-00500-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2):539–549. doi: 10.1158/0008-5472.CAN-12-2325 [DOI] [PubMed] [Google Scholar]
- 23.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105–115. doi: 10.1111/imm.12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559 [DOI] [PMC free article] [PubMed] [Google Scholar]