Abstract
Introduction
Cardiovascular disease (CVD) is the foremost killer disease worldwide. ASCVD is one of the most common types of CVD. It is mainly associated with a condition called atherosclerosis. Its occurrence is linked to several risk factors. Hypertension, diabetes, dyslipidemia, smoking, genetic factors, and so on are examples. The presence of ASCVD, as well as its risk factors, causes a variety of disruptions in the body’s physiological and biological functions. The presence of abnormal physiological and biological functions, for example, tends to disrupt hematological parameters.
Purpose
The study’s aim was to assess and compare the pattern of hematological parameters in people with established atherosclerotic cardiovascular disease (ASVD) versus people with ASCVD risks alone who attended TASH Addis Ababa, Ethiopia, as well as to correlate hematological parameters with the novel inflammatory marker hs-CRP.
Methods
A prospective cross-sectional comparative study with 100 study participants was conducted during where October 2019-March 2020 proposal development, sample collection, and lab analysis period, and from March 2020-June to 2021 data entry, analysis, and writing period. A serum sample was collected from each study participant for the lipid and hsCRP analyses and whole blood for hematological parameter determination. The socio-demographic characteristics of the study participants were obtained through a well-structured questionnaire.
Results
The ASCVD-risk group had significantly higher mean platelet volume (MPV), which was associated with the presence of the risk. Furthermore, hs-CRPs show a significant correlation with MPV in a correlation analysis of highly sensitive C-reactive protein (hs-CRP) with hematological parameters. Thus, using these affordable, routinely tested, and easily available tests may help to infer future ASCVD risk as well as the presence of ASCVD morbidity while hsCRP level in comparison group vs cases requires further study.
Keywords: ASCVD, hematological parameters, hs-CRP, hypertension, diabetes
Introduction
Cardiovascular disease is the foremost reason for many deaths worldwide which is accountable for around 17·8 million deaths in 20171 and atherosclerotic cardiovascular disease (ASCVD) is one of the most epidemic types of CVD which is becoming a huge burden in the population.2
ASCVD is related to a condition called atherosclerosis, the deposition of fat in the artery due to endothelium dysfunction, which is commonly manifested as coronary heart disease and stroke.2
Numerous factors are implicated as risk factors for atherosclerosis and its consequences, although the traditional risk factors—hypertension, diabetes, dyslipidemia, age, smoking, drinking, eating poorly, and not exercising—account for between 70% and 90% of the risk.3 Metabolic syndrome (Mets) is another risk factor that has been identified.4 The primary elements of this MetS are obesity, insulin resistance, dyslipidemia, and hypertension.5
Hypertension is defined as systolic or diastolic blood pressure above 140/90 mm Hg. Systolic pressure is the highest pressure in the artery when the heart beats and fills the artery, while diastolic pressure is the lowest pressure in the artery when the heart relaxes between beats.6
Diabetes is a group of chronic metabolic diseases characterized by hyperglycemia as a result of defects in insulin secretion, insulin action, or both (.7 Both hypertension and diabetes are known to be substantial risk factors for macrovascular and microvascular complications, including atherosclerotic cardiovascular disease (ASCVD) and peripheral vascular disease, including retinopathy, nephropathy, and possibly neuropathy.8,9
Several metabolic diseases, including cardiovascular disease, have been linked to hematological parameters that can be used as predictors of vascular complications.10 For example, hemoglobin, hematocrit, white blood cell (WBC), red blood cell (RBC), and blood platelet (PLT) counts are elevated in adults with MetS,11 and increased platelet-leukocyte conjugates in MetS contribute to the pathophysiological processes that increase risk for atherosclerotic disorders.12,13 Furthermore, RDW has been linked to an increased risk of mortality and CVD events in patients with established coronary artery disease (CAD).14
In patients with metabolic disorders, there are increased inflammatory markers (eg, C-reactive protein, chemokines, adhesion molecules, etc.).15 C-reactive protein is a novel inflammatory biomarker that is promptly elevated during inflammation.16
The C- reactive protein (CRP); is a serum protein that was discovered >80 years ago and it is also known to be associated with metabolic diseases (.17 It has also been demonstrated to be a useful predictor of disease development and therapeutic efficacy for a variety of illnesses including cancer, diabetes, infection, and inflammation.18 Studies on the associations of hematological parameters in people with atherosclerotic cardiovascular disease in contrast with ASCVD risk alone are very few or scarce. Therefore, the present study aimed to assess and compare complete blood cell counts of people with atherosclerotic cardiovascular disease, and thus with risk alone without ASCVD, and correlate them to CRP to describe it as a cost-effective indicator of increased risk for ASCVD or morbidity from ASCVD. The primary goal of this study was to examine the pattern of hematological parameters in people with pre-existing atherosclerotic cardiovascular disease and those with ASCVD risk alone, as well as their relationship with hs-CRP. The study was part of an MSc thesis uploaded on Addis Ababa University’s (AAU) library websites19 (unpublished work).
Methods and Materials
Study Design and Place
A prospective cross-sectional study was conducted at Tikur Anbessa Specialized Hospital (TASH), Department of Biochemistry, in collaboration with internal medicine, the renal clinic, diabetes, neurology, and the cardiac clinic at Addis Ababa University, Addis Ababa, Ethiopia. The source population of the study was all patients visiting or attending the renal, diabetic, neurology, and cardiac clinics at TASH. The study population was all volunteer patients who attended these clinics.
Inclusion and Exclusion Criteria
The study included patients with HTN and/or DM who were willing to give their informed consent, as well as those with no known hematological abnormalities or acute infections. Patients with known hematological abnormalities, patients with acute infections, embolic ischemic stroke patients, and patients with hematological complications—retinopathy, nephropathy, and neuropathy—were all excluded from the study.
Sample Size and Sampling Techniques
The study population was chosen using a convenient sampling technique, and the sample size was calculated using the double population formula. The prevalence of hypertension (0.28)20 and CVD (0.08)21 were considered.
Where;
Zα/2- is the critical value of the Normal distribution at α/2 (eg for a confidence level of 95%, α is 0.05 and the critical value is 1.96),
Zβ- is the critical value of the Normal distribution at β (eg for a power of 90%, β is 0.1 and the critical value is 1.28) and
P1 and P2 - are the expected sample proportions of the two groups.
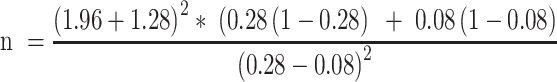 |
n =72.22 ≈ 72 plus 10% non-response rate 82 rounded to 100 (50 participants in each group) to increase the power of the study.
The studied hematological parameters were white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet count (PLT), and platelet distribution width (PDW). Inflammatory markers; highly sensitive C-reactive protein (CRP) Socio-demographic characteristics include age, sex, height, weight, BMI, waist circumference, alcohol consumption, cigarette smoking, exercise, and types of diet. Clinical variables include types of diabetes, hypertension, systolic pressure, diastolic pressure, and types of atherosclerotic cardiovascular disease.
Sample Collection
Five (5) milliliters of fasting venous blood were drawn from each participant. Following an aseptic condition, 2.5 mL was transferred to EDTA-containing tubes, and 2.5 mL was transferred to a serum separator tube. When the collection was completed, it was placed in an ice box and transported to the laboratory. For the serum sample, after being left at room temperature for a minimum of 30 minutes to allow coagulation, the coagulated blood was centrifuged for 10 minutes at 3500 rpm to separate serum from clotted blood. Then serum was collected into an Eppendorf tube. Samples that were not assayed within 24 hours of the collection were stored at a-20 °. The whole blood sample is placed in the ice box and transported to EPHI for analysis.
Data Collection
Socio-demographic characteristics and other related data were collected from the participants by the principal investigator via face-to-face interviews using an Amharic-version structured questionnaire, reviewing the patient’s record, and direct measurement of height, weight, and blood pressure. A pilot studies on 10% of study participants validated the questionnaire ahead of actual sample collection.
Laboratory Methods
Determination of Hematological Parameters
To determine a complete blood count, SYSMEX was used, which provides accurate and precise results through high-quality technology. This method accurately counts and sizes cells by using measurable charges in electric resistance produced by nonconductive particles suspended in electrolytes.23
Principles of Hematological Parameters Determination
A suspension of blood cells passes through a small orifice simultaneously with an electric current. A small opening (aperture) between electrodes is the sensing zone through which suspended particles pass. In the sensing zone, each particle displaces its own volume of electrolyte. Sysmex measures the displaced volume as a voltage pulse, with the height of each pulse being proportional to the volume of the particle.24
hs-CRP Determination
The hs-CRP was determined using a Roche/Hitachi Cobas 6000 analyzer particle-enhanced immunoturbidimetric assay. Human CRP agglutinates with latex particles coated with monoclonal anti-CRP antibodies. The precipitate is determined turbidimetrically at the wavelength of 546 nm.
Data Processing and Analysis
All the statistical analysis was performed using the Statistical Package for Social Science (SPSS) version 25. The variable distribution was tested for normality by using the Kolmogorov test and transformed if necessary. Simple descriptive statistics such as mean, standard deviations, median, and IQR were calculated for continuous variables, and percentages were calculated for categorical variables. To compare socio-demographic, anthropometric, and clinical characteristics, the Chi-square test was used, and to compare normally distributed quantitative data, the independent t-test was used, while the Mann–Whitney U-test was used for non-normally distributed data. Pearson or Spearman correlation tests were also used as required. Multinomial logistic regression was done. Tables and/or charts were used to interpret the output data, and a P value of 0.05 was considered statistically significant for all analyses.
Result
Socio-Demographic, Anthropometric, and Clinical Characteristics
This study enrolled a total of 100 participants (50 cases, (74% female)) (Table 1) and only a quarter (24%) of the cases are in the age category of above 55yr while two-thirds (66%) of the comparison group were in this age category, having a significant P value of 0.001.
Table 1.
Comparison of Sociodemographic, Anthropometric Characteristics and Clinical Characteristics (N=100)
| Variables | Category | Comparison Group (n=50) | Case(n=50) | p-value |
|---|---|---|---|---|
| Gender | Female | 20(40%) | 13(26%) | 0.137 |
| Male | 30(60%) | 37(74%) | ||
| Age (yr.) | 15–35 | 1(2%) | 20(40%) | 0.001 |
| 36–55 | 16(32%) | 18(36%) | ||
| >=56 | 33(66%) | 12(24%) | ||
| BMI (kg/cm2) | <18.5 | 0(0%) | 11(22) | 0.003 |
| 18.6–24.9 | 23(46%) | 21(42) | ||
| >25 | 27(54%) | 18(36) | ||
| WC (cm) | 41.5±12.1 | 36.91±9.94 | 0.241 | |
| Exercise | Occasionally | 5(10%) | 1(2%) | 0.739 |
| 2–3 days/week | 4(8%) | 4(8%) | ||
| 4–5 days/week | 38(76%) | 41(82%) | ||
| Never | 3(6%) | 4(8%) | ||
| Red meat eating | Occasionally | 30(60%) | 34(68%) | 0.037 |
| 1days/week | 3(6%) | 7(14%) | ||
| 2–5 days/week | 4(8%) | 6(12%) | ||
| Never | 13(26%) | 3(6%) | ||
| Vegetable eating | Occasionally | 7(14%) | 4(8%) | 0.716 |
| Days/week | 4(8%) | 4(8%) | ||
| 45days/week | 37(74%) | 41(82%) | ||
| Never | 2(4%) | 1(2%) | ||
| Fruit-eating | Occasionally | 21(42%) | 20(40%) | 0.929 |
| Days/week | 7(14%) | 7(14%) | ||
| 4–5days/ week | 20(40%) | 22(44%) | ||
| Never | 1(2%) | 2(4%) | ||
| Type of oil | Palm oil | 31(62%) | 36(72%) | 0.202 |
| Sunflower | 6(12%) | 9(18%) | ||
| Non-particular | 12(24%) | 4(8%) | ||
| Don’t know | 1(2%) | 1(2%) | ||
| Cigarette smoking | Never | 50(100%) | 50(100%) | |
| Alcohol consumption | Occasionally | 31(62%) | 36(72%) | 0.554 |
| 1 bottle/day | 18(36%) | 13(26%) | ||
| Never | 1(2%) | 1(2%) | ||
| DM | 12(24%) | 2(4%) | 0.001 | |
| Type 1 | 11(22%) | 2(4%) | ||
| Type 2 | 1(2%) | 0(0%) | ||
| HTN | 20(40%) | 10(20%) | 0.001 | |
| HTN and DM | 18(36%) | 8(16%) | 0.001 | |
| Type 1 | 8(16%) | 0(0%) | 0.121 | |
| Type2 | 10(20%) | 8(16%) | ||
| SBP (mmHg) | <120 | 14(28%) | 22(44%) | 0.128 |
| 120–140 | 28(64%) | 25(50%) | ||
| >140 | 8(16%) | 3(6%) | ||
| DBP (mmHg) | <70 | 4(8%) | 6(12%) | 0.128 |
| 70–80 | 36(64%) | 38(76%) | ||
| >80 | 14(28%) | 6(12%) | ||
| ASCVD | Ischemic | - | 38(76%) | |
| CHD | Stroke | - | 12(24%) |
The case group (36%), and the comparison group (54%), were in the body mass index category of >25 kg/cm2 (p = 0.003), and the mean waist circumference in the case group was 41.5±12.16 while it was 36.91±9.94 in the comparison group (Table 1). None of the participants in either group smoked, and 76% and 82% of the comparison and case groups, respectively, exercised 4–5 days per week (Table 1). About 68% of the comparison group and 60% of the case group had the habit of eating meat occasionally (p = 0.037). Out of 50 cases included in the study, 76% had a history of coronary heart disease, and 24% had an ischemic stroke (Table 1). Out of the 50 members of the comparison group, 11 (22%) were diabetic (2% type 1, 20% type 2), 21 (42%) were hypertensive, and 18 (42%) were both hypertensive and diabetic (8 (16%) type 1, 10 (20%) type 2) (Table 1). The other socio-demographic, anthropometric, and clinical characteristics of both groups are summarized in Table 1 and Table 2.
Table 2.
Comparison of Anthropometric, Demographic, and Clinical Characteristics in ASCVD Sub-Types (N=50)
| Variables | Category | CHD (38) | Ischemic Stroke (n =12) | P-value |
|---|---|---|---|---|
| Gender | Male | 8(21.05%) | 6(50%) | |
| Female | 30(78.9%) | 6(50%) | ||
| Age (year) | 13–35 | 18(47%) | 1(2%) | 0.011 |
| 36–55 | 12(32%) | 6(50%) | ||
| >56 | 8(21%) | 5(41.6%) | ||
| BMI (kg/m2) | <18.5 | 11(28.9%) | - | 0.005 |
| 18.6–24.9 | 16 (42.1%) | 4(10.5%) | ||
| >25 | 11(28.9%) | 8(21.05%) | ||
| WC (cm) | 38.67±3.27 | 36.41±11.33 | 0.287 | |
| Systolic blood Pressure | <120 | 21(55.26) | - | 0.013 |
| 120–140 | 13(34.2%) | 12(31.5%) | ||
| >140 | 4(1.05%) | - | ||
| Diastolic blood Pressure | <70 | 4(1.05%) | 2(1%) | 1.000 |
| 70–80 | 29(76.3%) | 8(6.6%) | ||
| 5(13.1) | 2(1%) | |||
| Hypertension >80 | 13(35.1%) | 5(13.1%) | ||
| Diabetes | 10(26.3%) | 2(5.26%) | ||
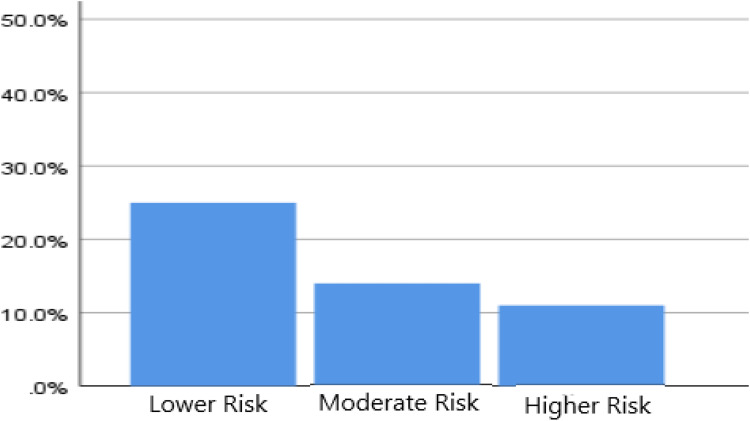
According to the Framingham risk score classification, 25 (50%), 14 (28%), and 11 (22% of the comparison group) are in the low, moderate, and high-risk groups, respectively (Figure 1).
Figure 1.
Classification of the comparison group according to the Framingham risk score.
Comparison of Clinical Laboratory Results
Hematological Parameters
When the hematological parameters of the two groups (case and comparison) are compared (Table 3), the mean± SD of MCHC (33.32±1.01 vs 33.7±0.68, p=0.031) and MPV (8.44±1.03 vs.9.32+1.16, p=0.00) were lower and the MD (IQR) of MCV (89.52 (5.50) vs 87.5 (4.25), p=0.006) and MONO (7.85 (2.77) vs 6.25 (2.70), p=0.00) were higher in the case group, while values of RBC, HCT, MCHC, HGB, NEUT, and lymphocytes were lower but not significantly. But WBC, MCH, RDW, and PLT counts were higher in the case group but not significantly. Additionally, the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio were compared and shown to be higher in the case group than in the comparison group but not significant (Table 3).
Table 3.
Comparison of Hematological Parameters in Comparison and Case Group (N=100)
| Hematological Parameter | Comparison Group | Case Group | P-value | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ±SD | Median (IQR) | ||
| Hgb (g/dl) | 15.23±1.47 | 14.94±2.22 | 0.436 | ||
| Hct (%) | 45.19±1.47 | 44.77±6.63 | 0.707 | ||
| RBC (10^6/ul) | 5.16±0.53 | 5.03±0.67 | 0.275 | ||
| WBC (10^3/ul) | 6.41±2.75 | 7.17±2.42 | 0.086 | ||
| MCHC (g/dl) | 33.70±0.68 | 33.32±1.01 | 0.031 | ||
| RDW (%) | 13.69±0.95 | 14.15±1.71 | 0.106 | ||
| PLT (10^3/ul) | 245±67.60 | 268±68 | 0.142 | ||
| MPV (fl) | 9.32±1.16 | 8.44±1.03 | 0.00 | ||
| Neutrophil (%) | 54.31±10.45 | 53.48±12.97 | 0.726 | ||
| Lymphocyte (%) | 33.74±9.69 | 33.14±11.36 | 0.776 | ||
| MCV (fl) | _ | 87.5(4.25) | 89.52(5.50) | 0.006 | |
| Monocyte (%) | _ | 6.25(2.70) | 7.85(2.77) | 0.001 | |
| Eosinophil (%) | _ | 2.75(2.97) | 2.35(4.78) | 0.926 | |
| MCH (pg) | _ | 29.65(1.80) | 29.95(1.77) | 0.155 | |
| Basophil (%) | _ | 0.80(0.70) | 0.65(0.50) | 0.426 | |
| NLR (%) | 1.82± 0.88 | 2.05 ±1.52 | 0.350 | ||
| PLR (%) | 7.97±3.53 | 9.53± 5.98 | 0.117 | ||
NB: For parameters expressed in mean ± SD, the p-value is derived from the independent T-test, while it is derived from the Mann–Whitney U-test for parameters expressed in median (interquartile range); the p-value of 0.05 is significant.
The types of ASCVD (ischemic stroke and coronary heart disease) were also compared in terms of hematological parameters (Table 4), and it was discovered that the mean ± SD of total WBC count (5.98±2.05 vs 7.55±2.43, p = 0.048) and neutrophil count (46.4+9.36 vs 55.72 ± 13.23, p = 0.028) were higher in the CHD group, while lymphocytes were lower (40. 03±8.78 vs.30.96±11.30, p=0.024, whereas the RBC count, HCT, MCHC, MCH, Hgb, RDW, PLT, P/L, N/L, BASO, and EOS count did not show significant differences between the two groups.
Table 4.
Comparison of Hematological Parameters in ASCVD Sub Types (N=100)
| Variables | Ischemic Stroke (n=12) | Coronary Heart Disease (n=38) | P-value | ||
|---|---|---|---|---|---|
| Mean± SD | Median (IQR) | Mean ±SD | Median (IQR) | ||
| WBC (10^3/ul) | 5.98±2.05 | 7.55±2.43 | 0.048 | ||
| RBC (10^6/ul) | 5.3±0.45 | 4.97±0.72 | 0.243 | ||
| Hgb (g/dl) | 15.27±2.89 | 14.83±1.99 | 0.552 | ||
| HCT (%) | 45.45±7.48 | 44.55±6.43 | 0.685 | ||
| MCHC (g/dl) | 33.39±1.63 | 33.30±0.96 | 0.800 | ||
| RDW (%) | 14.30±2.85 | 14.09±1.20 | 0.711 | ||
| PLT (10^3/ul) | 271.92±69 | 267.39±91.1 | 0.875 | ||
| MPV (fl) | 8.59 ±1.26 | 8.39± 0.96 | 0.570 | ||
| NEU (%) | 46.4±9.36 | 55.72±13.23 | 0.028 | ||
| LYM (%) | 40.03±8.78 | 30.96±11.30 | 0.024 | ||
| MCV (fl) | 88.75(6.77) | 90.4(5.47) | 0.369 | ||
| MONO (%) | 7.65(3.10) | 7.85(2.80) | 0.682 | ||
| EOS (%) | 2.90(2.72) | 2.35(3.16) | 0.601 | ||
| MCH (pg) | 29.95(2.52) | 30(1.63) | 0.927 | ||
| BASO (%) | 0.75(1.80) | 0.6(0.43) | 0.946 | ||
| N/L | 1.24±0.44 | 2.34±1.67 | 0.001 | ||
| P/L | 7.01±1.08 | 10.44±6.7 | 0.007 | ||
Notes: NB: For parameters expressed in mean ± SD, the p-value is derived from the independent T-test, while it is derived from the Mann–Whitney test for parameters expressed in median (interquartile range).
The hs-CRP
The comparison group, or the group without established ASCVD but with risk factors for ASCVD, has significantly higher levels of highly sensitive C-reactive Protein (hs-CRP) (2.38 (2.98) vs 0.95 (1.98); P = 0.005) (Figure 2).
Figure 2.
Comparison of hs-CRP (N=100).
Abbreviations: NB, hs-CRP- highly sensitive C-reactive protein; MD, median; IQR, interquartile range.
Correlation of Hematological Parameters with hs-CRP
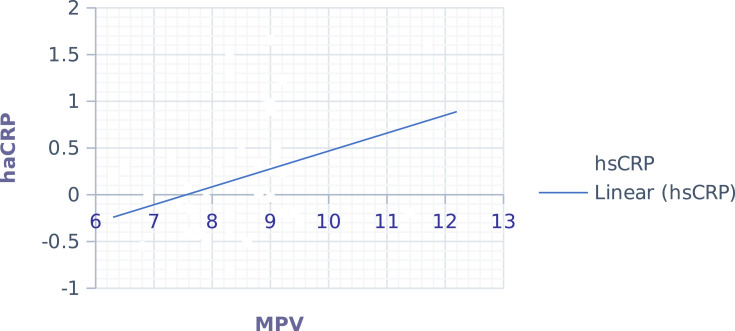
While we analyze the correlation of hematological parameters with hsCRP (Table 5), only MPV (0.341 (0.016)) (Figure 3) had a significant positive association in the comparison group. But the rest of the hematological parameters had no significant association. Additionally, the correlation of NLR and PLR with hsCRP (Table 5) was also done, but significance was not spotted.
Table 5.
Correlation of Haematological Profiles with hsCRP (N=100)
| Variables | Comparison Group | Case |
|---|---|---|
| WBC (10^3/ul) | 0.025(0.867) | 0.176(0.125) |
| RBC (10^6/ul) | 0.122(0.403) | 0.221(0.128) |
| Hgb (g/dl) | 0.121(0.406) | 0.162(0.265) |
| Hct (%) | 0.223(0.123) | 0.183(0.194) |
| MCV (fl) | 0.132(0.367) | −0.006(0.969) |
| MCH (pg) | 0.007(0.962) | −0.062(0.673) |
| MCHC (g/dl) | −0.218(0.128) | −0.133(0.364) |
| RDW (%) | 0.241(0.092) | −0.017(0.906) |
| PLT (10^3/ul) | 0.143(0.326) | −0.258(0.073) |
| Neutrophil (%) | −0.097(0.506) | 0.221(0.124) |
| Lymphocyte (%) | −0.019(0.896) | −0.237(0.101) |
| Monocyte (%) | 0.163(0.264) | 0.020(0.893) |
| Basophil (%) | 0.135(0.351) | −0.198(0.172) |
| Eosinophil (%) | 0.236(0.102) | −0.134(0.360) |
| Neutrophil lymphocyte ratio | −0.002(0.992) | 0.195(0.174) |
| Platelet lymphocyte ratio | 0.187(0.193) | −0.004(0.978) |
Notes: P<0.05 (2-tailed), derived from Pearson and Spearman rank correlation.
Figure 3.
Correlation of hs-CRP and MPV. In the case group, p=0.016, r =0.341, derived from the Pearson correlation test.
Abbreviations: Hs-CRP, highly sensitive C-reactive protein; MPV, mean platelet volume.
Prognostic Value of the Hematological Parameters
All the hematological parameters related to CVD and its risk were used in multinomial logistic regression (Table 6). WBC, MCV, RDW, and MONO are significantly associated with pre-existing ASCVD, while MPV is significantly associated with the presence of ASCVD risk. Additionally, hs-CRP is significantly associated (odds ratio (B) =0.437, 95% CI 0.1988–0.966, P =0.041) with the presence of ASCVD risk factors.
Table 6.
Predictive Value of Hematological Parameters (N=100)
| Variables | Regression Coefficient (B) | Adjusted Odd Ratio (B) | P-value | 95% CI (Confidence Interval) | |
|---|---|---|---|---|---|
| Upper Bound | Lower Bound | ||||
| WBC | 0.281 | 0.755 | 0.033 | 0.583 | 0.977 |
| MONO | 0.500 | 0.607 | 0.000 | 0.463 | 0.795 |
| RDW | 0.231 | 1.512 | 0.034 | 1.037 | 2.203 |
| MPV | −0.828 | 2.289 | 0.008 | 1.245 | 4.206 |
| MCV | 0.122 | 1.135 | 0.013 | 1.029 | 1.252 |
| HsCRP | −0.828 | 0.437 | 0.041 | 0.198 | 0.966 |
Note: Reference category comparison group. P<0.05.
Abbreviations: WBC, white blood cells; MONO, monocyte; RDW, red cell distribution; MPV, mean platelet volume; MCV, mean cell volume; hs-CRP, highly sensitive C-reactive protein.
Discussion
To the best of our knowledge, this was Ethiopia’s first study on the role of a complete blood count in the presence (case group) and absence (comparison group) of established ASCVD. We tried to discuss this by referring to various related studies, as follows: There was an association of total leukocyte count with pre-existing ASCVD in the current study, which is similar to a previous study done by Kim et al in Korea and Waterhouse et al in Ireland, respectively,25,26 and different scholars have shown that there is a higher total leukocyte count in CVD diseased groups than non-diseased groups.27 There are also additional supportive studies, like a study conducted by Lasek-Bal et al that showed a positive association between high leukocyte counts and a worse prognosis regarding the course of acute stroke, and WBC was higher in the group with symptomatic atherosclerosis than in patients with no clinical features of atherosclerosis26 Studies by Lee et al also revealed that a higher WBC is associated with an increased incidence of the two prominent ASCVD types (ischemic stroke and CHD).28
About the WBC subtypes, this study revealed that it was only the monocyte that was associated with pre-existing ASCVD. This is consistent with previous research,29–31 and some review articles confirmed this by analyzing previous research.32,33 This would be due to the nature of the monocyte, which is involved in both innate and adaptive immunity and has high trafficking and plasticity abilities, is highly recruited to the site of inflammation, and differentiates into lipid phagocytizing cells and macrophages. These macrophages aggravate the formation of atherosclerotic lesions by secreting the metalloproteinase enzyme and ROS in the atherosclerotic lesion.34
On the other hand, Waterhouse et al discovered that monocytes are associated with CVD risk.25 And also, some studies have found out the role of other WBC subtypes concerning CVD. For instance, a study by Fan et al showed that higher NLR and decreased eosinophils are associated with ischemic stroke mortality rather than monocytes.35 Verdoia et al also showed that NLR is independently associated with the prevalence and severity of coronary artery disease (CAD).36
In this study, higher RDW was associated with ASCVD morbidity rather than risk, which is consistent with various studies like the Lappé et al study that was done on 1489 patients with CAD and a population of 449 normal subjects (no CAD), in which RDW was associated with mortality in patients with stable angina pectoris or normal coronary subjects.37 Similarly, other studies found that an elevated RDW was linked to death in patients with stable angina pectoris and MI, respectively.38,39 Furthermore, Ani and Ovbiagele’s study on adults in the United States revealed that the mean RDW was significantly higher among people who had a known stroke compared to those who did not have a stroke.40 The explanation for this may be due to the presence of inflammation, in which pro-inflammatory cytokines are produced or released continuously. Those cytokines can interact with erythropoietin in the bone marrow and result in the reduction of RBC synthesis and also result in an elevation in the number of immature RBCs by suppressing RBC maturation, which is detected by higher RDW levels.39,41
In contrast, the epidemiological study done in Taiwan found that elevated RDW is associated with increased risk rather than with the development of the disease.42
In our study, MCV was significantly higher in the case group than in the comparison group. Similarly, Solak et al discovered that MCV is a predictor of composite CV events independent of major confounding factors and that it is linked to endothelial dysfunction.43 Consistently, Myojo et al also showed that a higher MCV is significantly associated with all-cause and cardiac mortality.44 According to Tulubas et al, who studied “MCV and MCH values in coronary artery patients with a positive Gensini score”, the angiographic positive CAD group has a significantly higher MCV than the angiographic negative.45
An MPV is one of the platelet indices that estimate the size of individual platelets, and it is higher expressively in the comparison group as compared to cases. Many studies done on the role of MPV in various inflammatory diseases revealed that it has an inverse correlation with the level of inflammation.46–49 Ihara et al discovered that MPV is lower in the angiographic positive group than in the angiographic negative group.50
The longitudinal study done in Brazil on the association of platelet volume with the Framingham risk score pointed out that elevated MPV is significantly related to Framingham risk. That means it increases as the Framingham risk score increases.51 This contradicts many studies; for instance, high MPV was indicated to be correlated with the severity of coronary atherosclerosis in the study done by Murat et al on 395 patients with ACS.52 Similarly, the study conducted by Henning et al on a total of 518 patients conveyed that high MPV is associated with coronary heart disease.53
The lower MPV in the case group might be due to the use of antiplatelet drugs, while the comparison groups might not be taking them. But we did not investigate the treatment status of our study participants, and it could be a new insight for future studies.
Highly sensitive C-reactive Protein (hs-CRP) had a significant association with the presence of the risk in the comparison group and was significantly higher in these groups compared to cases in this study. The decrement in the presence of the disease is also seen in the cohort study done by Taheri et al, in which the level of hs-CRP is lower in patients with CVD than in those without CVD.54 According to some studies, determining hs-CRP is beneficial in individuals with intermediate cardiovascular risk (10–20% risk at 10 years) to improve risk stratification and clinical management.55,56 This is also confirmed by the result of the Seo et al study, in which the levels of hs-CRP significantly increased from the low to the very high-risk group57 and were also shown to have a significant association with CVD (CHD, MI, and stroke risk).58 In reverse, the cross-sectional study done by Chowta et al revealed that there was a higher mean hsCRP level in patients with CVD than in those without CVD.59
The lower hs-CRP in the already established ASCVD group could be due to the fact that people in this group had the opportunity to take drugs like statins, which can affect or reduce CRP levels, or it could be due to the fact that they began to change their lifestyle after learning that they had ASCVD. Unfortunately, this study did not go through the types of drugs that the study participants were taking, and this might require further investigation.
Only MPV showed a significant positive association with hs-CRP in the group with pre-existing ASCVD. This result was concordant with a study that was conducted on 84 IHD patients and found that there was a significant correlation between MPV and hs-CRP levels.60 The Kaya et al study on the relationship between MPV and hs-CRP in dippers and non-dippers with hypertension found a significant positive association in non-dippers with hypertension, who have approximately three times the risk of atherosclerotic events.61 This relationship was also demonstrated in the study piloted by Yazici et al and Yarlioglues et al on-platelet indices in rheumatoid arthritis and the relationship between mean platelet volume levels and subclinical target organ damage in newly diagnosed hypertensive patients, respectively.60,62
There are many studies in controversy to this; in 2001, Kapsoritakis et al conveyed that there is a significant inverse relationship between MPV and hs-CRP63 and a cross-sectional study done on 100 children with a diagnosis of infectious and inflammatory disease in 2014 revealed that MPV and CRP have negative relation.49
According to the analysis of hematological parameters in ASCVD subtypes, a higher WBC is observed in coronary heart disease than in ischemic stroke. But there is no ample evidence from other studies to discuss this. Many studies on ischemic and coronary heart disease have found elevated leukocytes in the presence of both coronary heart disease and ischemic stroke,64,65 but why and how WBC is higher in CHD than in ischemic stroke is still unknown.
With regard to the WBC subtype, even though it is in the normal range, neutrophil, lymphocyte, NLR, and PLR were higher in CHD; this may be due to the presence of a large number of participants as well as a large number of participants with diabetes, hypertension, and other clinical variables in a group with CHD than ischemic stroke, which may result in higher inflammation and stress.66 This could also be an explanation for the above result.
Generally, in addition to inflammation and other clinical factors, the presence of different age distribution, BMI, and lifestyle factors such as eating habits, drinking habits, and exercising could also result in the presence of a different pattern of hematological and biochemical parameters between the comparison group and the case group.67–69
Strength and Limitation
This study has strength by being the first to give a new insight into the pattern of hematological parameters in pre-existing ASCVD and ASCVD risk factors, and it also gives new information on the existing proofs. It has limitations in that the type of medication that the participants were taking was not assessed, which may affect the pattern of hematological parameters and could result in the occurrence of bias. There may also be selection and misclassification biases regarding the participants.
Conclusion and Recommendation
The purpose of this prospective comparative cross-sectional study was to compare the pattern of hematological parameters in the confirmed ASCVD group versus the risk group. WBC, monocyte, RDW, and MCV were associated with ASCVD morbidity, while MPV and hs-CRP were associated with the presence of ASCVD risk factors. Furthermore, in the group with ASCVD risks, MPV and hsCRP had a significant positive correlation. Therefore, using these affordable, routinely tested, and easily available tests might help to identify future ASCVD risks as well as the current ASCVD morbidity.
The current study also showed research directions by revealing that hs-CRP and some CBC parameters were higher in the comparison group than the group with already established ASCVD, which is different from pre-existing proofs and needs further intensive investigation.
Acknowledgments
We are incredibly appreciative to Addis Ababa University for supporting the study. Additionally, it gives us great pleasure to express our appreciation to the Department of Biochemistry at Addis Ababa University for its crucial coordination and assistance in the successful completion of this research. We also want to thank the research participants from the bottom of our hearts. Additionally, we acknowledge all who support the study directly and indirectly and staff members of the hematology laboratory at the Ethiopian Public Health Institute. This manuscript was part of an MSc thesis titled” Cross-sectional comparative study of hematological parameters in people with atherosclerotic cardiovascular disease and those with cardiovascular risk alone”. It is not published by any publisher yet but the thesis is available online on the AAU library website, http://etd.aau.edu.et/handle/123456789/28597.
Funding Statement
The study was funded by Addis Ababa University. The funder had no role in designing the study, collection, analysis, and interpretation of data as well as the writing of the manuscript.
Abbreviations
ASCVD, Atherosclerotic cardiovascular disease; CHD, Coronary Heart Diseases; CRP, C - reactive protein; CVD, Cardiovascular Disease; Hs-CRP, Highly Sensitive C - reactive protein; MCH, Mean Corpuscular Haemoglobin; MCHC, Mean Corpuscular Haemoglobin Concentration; MCV, Mean Corpuscular Volume; MPV, Mean Platelet Volume; NLR, Neutrophil Lymphocyte Ratio; PDW, Platelet Distribution Width; PLR, Platelet Lymphocyte Ratio; PLT, Platelet; RBC, Red Blood Cells; RDW, Red Cell Distribution Width; WBC, White Blood Cells.
Data Sharing Statement
All relevant information and materials related to the article are included in the manuscript, and when additional information is required, the authors are available to provide it.
Ethics Approval and Consent to Participate
The study was authorized by the research and ethics committee of the Biochemistry Department of the College of Health Sciences at Addis Ababa University. Participants in the study were fully informed before they provided their information. The information that was received from the study participants was kept confidential.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report that they do not have any competing interests.
References
- 1.Kaptoge S, Pennells L, De Bacquer D, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7(10):e1332–e45. doi: 10.1016/S2214-109X(19)30318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barquera S, Pedroza-Tobías A, Medina C, et al. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46(5):328–338. doi: 10.1016/j.arcmed.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 3.Maisch B, Oelze R. Cardiovascular Benefits of Omega-3 Polyunsaturated Fatty Acids. IOS Press; 2006. [Google Scholar]
- 4.Ninomiya J, Criqui MH, Whyte JL, Gamst A, Chen RS, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the third national health and nutrition examination survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C [DOI] [PubMed] [Google Scholar]
- 5.Cornier M-A, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan NM. Kaplan’s Clinical Hypertension. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 7.Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034. doi: 10.2337/dc14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y, Ali F. Atherosclerotic cardiovascular disease: a review of initiators and protective factors. Inflammopharmacology. 2016;24:1–10. doi: 10.1007/s10787-015-0255-y [DOI] [PubMed] [Google Scholar]
- 9.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabet. 2008;26(2):77–82. doi: 10.2337/diaclin.26.2.77 [DOI] [Google Scholar]
- 10.Nebeck K, Gelaye B, Lemma S, et al. Hematological parameters and metabolic syndrome: findings from an occupational cohort in Ethiopia. Diabetes Metab Syndr. 2012;6(1):22–27. doi: 10.1016/j.dsx.2012.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aypak C, Türedi Ö, Bircan MA, Yüce A. Could mean platelet volume among complete blood count parameters be a surrogate marker of metabolic syndrome in pre-pubertal children? Platelets. 2014;25(6):393–398. doi: 10.3109/09537104.2013.827783 [DOI] [PubMed] [Google Scholar]
- 12.Arteaga RB, Chirinos JA, Soriano AO, et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98(1):70–74. doi: 10.1016/j.amjcard.2006.01.054 [DOI] [PubMed] [Google Scholar]
- 13.Harrison CN. Platelets and thrombosis in myeloproliferative diseases. ASH Educ Program Book. 2005;2005(1):409–415. [DOI] [PubMed] [Google Scholar]
- 14.Lohsoonthorn V, Jiamjarasrungsi W, Williams MA. Association of hematological parameters with clustered components of metabolic syndrome among professional and office workers in Bangkok, Thailand. Diabetes Metab Syndr. 2007;1(3):143–149. doi: 10.1016/j.dsx.2007.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefanadi E, Tousoulis D, Androulakis E, et al. Inflammatory markers in essential hypertension: potential clinical implications. Curr Vasc Pharmacol. 2010;8(4):509–516. doi: 10.2174/157016110791330870 [DOI] [PubMed] [Google Scholar]
- 16.Abo El Ainin H, Attya A, Tabl M, Samir M. Assessment of the diagnostic accuracy of C-reactive protein and pentraxin3 in acute coronary syndrome compared with cardiac troponin-1. Benha J Appl Sci. 2020;5(6 part (1)):127–132. doi: 10.21608/bjas.2020.137126 [DOI] [Google Scholar]
- 17.Timpson NJ, Lawlor DA, Harbord RM, et al. C-reactive protein and its role in metabolic syndrome: Mendelian randomisation study. Lancet. 2005;366(9501):1954–1959. doi: 10.1016/S0140-6736(05)67786-0 [DOI] [PubMed] [Google Scholar]
- 18.Stone PA, Kazil J. The relationships between serum C-reactive protein level and risk and progression of coronary and carotid atherosclerosis. In: Seminars in Vascular Surgery. Elsevier; 2014. [DOI] [PubMed] [Google Scholar]
- 19.Abate E. Cross-Sectional Comparative Study of Hematological Parameters in People with Atherosclerotic Cardiovascular Disease and Those with Cardiovascular Risk Alone. Addis Ababa, Ethiopia: Addia Ababa University; 2021. [Google Scholar]
- 20.Anteneh ZA, Yalew WA, Abitew DB. Prevalence and correlation of hypertension among adult population in Bahir Dar city, northwest Ethiopia: a community based cross-sectional study. Int J Gen Med. 2015;175–185. doi: 10.2147/IJGM.S81513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angaw DA, Ali R, Tadele A, Shumet S. The prevalence of cardiovascular disease in Ethiopia: a systematic review and meta-analysis of institutional and community-based studies. BMC Cardiovasc Disord. 2021;21(1):1–9. doi: 10.1186/s12872-020-01828-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickey GL, Grant SW, Dunning J, Siepe M. Statistical primer: sample size and power calculations—why, when and how? Eur J Cardiothorac Surg. 2018;54(1):4–9. doi: 10.1093/ejcts/ezy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scoffin K. Hematology analyzers-from complete blood counts to cell morphology. Am Lab. 2014;46(5):26–28. [Google Scholar]
- 24.Davis BH, Barnes PW. Automated cell analysis: principles. Laboratory hematology practice. Lab Hematol Pract. 2012;2012:26–32. [Google Scholar]
- 25.Waterhouse DF, Cahill RA, Sheehan F, McCreery C. Prediction of calculated future cardiovascular disease by monocyte count in an asymptomatic population. Vasc Health Risk Manag. 2008;4(1):177–187. doi: 10.2147/VHRM.S2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasek-Bal A, Jedrzejowska-Szypulka H, Student S, et al. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J Physiol Pharmacol. 2019;70(2):209–217. [DOI] [PubMed] [Google Scholar]
- 27.Yan X-N, Jin J-L, Zhang M, et al. Differential leukocyte counts and cardiovascular mortality in very old patients with acute myocardial infarction: a Chinese cohort study. BMC Cardiovasc Disord. 2020;20(1):1–12. doi: 10.1186/s12872-020-01743-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke, and mortality from cardiovascular disease in African-American and white men and women: the atherosclerosis risk in communities study. Am Heart Assoc. 2001;2001:1357–1358. [DOI] [PubMed] [Google Scholar]
- 29.Swirski FK, Libby P, Aikawa E, et al. Ly-6C hi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8(10):802–815. doi: 10.1038/nri2415 [DOI] [PubMed] [Google Scholar]
- 32.Combadière C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117(13):1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091 [DOI] [PubMed] [Google Scholar]
- 33.Rahman K, Fisher EA. Insights from pre-clinical and clinical studies on the role of innate inflammation in atherosclerosis regression. Front Cardiovasc Med. 2018;5:32. doi: 10.3389/fcvm.2018.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fayad ZA, Swirski FK, Calcagno C, Robbins CS, Mulder W, Kovacic JC. Monocyte and macrophage dynamics in the cardiovascular system: JACC macrophage in CVD series (Part 3). J Am Coll Cardiol. 2018;72(18):2198–2212. doi: 10.1016/j.jacc.2018.08.2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan L, Gui L, Chai EQ, Wei CJ. Routine hematological parameters are associated with short‐and long‐term prognosis of patients with ischemic stroke. J Clin Lab Anal. 2018;32(2):e22244. doi: 10.1002/jcla.22244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdoia M, Barbieri L, Di Giovine G, Marino P, Suryapranata H, De Luca G. Neutrophil to lymphocyte ratio and the extent of coronary artery disease: results from a large cohort study. Angiology. 2016;67(1):75–82. doi: 10.1177/0003319715577529 [DOI] [PubMed] [Google Scholar]
- 37.Lappé JM, Horne BD, Shah SH, et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta. 2011;412(23–24):2094–2099. doi: 10.1016/j.cca.2011.07.018 [DOI] [PubMed] [Google Scholar]
- 38.Sangoi MB, Rödel A, Zorzo P, et al. Prognostic value of red blood cell distribution width in prediction of in-hospital mortality in patients with acute myocardial infarction. Clin Lab. 2014;60(8):1351–1356. doi: 10.7754/Clin.Lab.2013.130907 [DOI] [PubMed] [Google Scholar]
- 39.Uyarel H, Işık T, Ayhan E, Ergelen M. Red cell distribution width (RDW): a novel risk factor for cardiovascular disease. Int J Cardiol. 2012;154:351–352. doi: 10.1016/j.ijcard.2011.10.126 [DOI] [PubMed] [Google Scholar]
- 40.Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277(1–2):103–108. doi: 10.1016/j.jns.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 41.Lassale C, Curtis A, Abete I, van der Schouw Y, Verschuren W, Lu Y. Elements of the complete blood count associated with cardiovascular disease incidence: findings from the EPIC-NL cohort study. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-21661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen P-C, Sung F-C, Chien K-L, Hsu H-C, T-C S, Lee Y-T. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am J Epidemiol. 2010;171(2):214–220. doi: 10.1093/aje/kwp360 [DOI] [PubMed] [Google Scholar]
- 43.Solak Y, Yilmaz MI, Saglam M, et al. Mean corpuscular volume is associated with endothelial dysfunction and predicts composite cardiovascular events in patients with chronic kidney disease. Nephrology. 2013;18(11):728–735. doi: 10.1111/nep.12130 [DOI] [PubMed] [Google Scholar]
- 44.Myojo M, Iwata H, Kohro T, et al. Prognostic implication of macrocytosis on adverse outcomes after coronary intervention. Atherosclerosis. 2012;221(1):148–153. doi: 10.1016/j.atherosclerosis.2011.11.044 [DOI] [PubMed] [Google Scholar]
- 45.Tülübaş F, Gürel A, Akkoyun DC, et al. MCV and MCH values in coronary artery patients with positive Gensini score. Eur J Gen Med. 2013;10(3):131–135. doi: 10.29333/ejgm/82240 [DOI] [Google Scholar]
- 46.Douda T, Bures J, Rejchrt S, Kopácová M, Pecka M, Malý J. Mean platelet volume (MPV) in Crohn’s disease patients. Cas Lek Cesk. 2006;145(11):870–873. [PubMed] [Google Scholar]
- 47.Gunluoglu G, Ertan E, Veske NS, Seyhan EC, Altin S. Mean platelet volume as an inflammation marker in active pulmonary tuberculosis. Multidiscip Respir Med. 2014;9(1):1–5. doi: 10.1186/2049-6958-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Safak S, Uslu AU, Serdal K, Turker T, Sonar S, Lutfi A. Association between mean platelet volume levels and inflammation in SLE patients presented with arthritis. Afr Health Sci. 2014;14(4):919–924. doi: 10.4314/ahs.v14i4.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zareifar S, Farahmand Far MR, Golfeshan F, Cohan N. Changes in platelet count and mean platelet volume during infectious and inflammatory disease and their correlation with ESR and CRP. J Clin Lab Anal. 2014;28(3):245–248. doi: 10.1002/jcla.21673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ihara A, Kawamoto T, Matsumoto K, et al. Relationship between platelet indexes and coronary angiographic findings in patients with ischemic heart disease. Pathophysiol Haemost Thromb. 2006;35(5):376–379. doi: 10.1159/000097692 [DOI] [PubMed] [Google Scholar]
- 51.Maluf CB, Barreto SM, Dos Reis Rc R, Vidigal PG. Platelet volume is associated with the Framingham risk score for cardiovascular disease in the Brazilian longitudinal study of adult health (ELSA-Brasil). Clin Chem Lab Med. 2016;54(5):879–887. doi: 10.1515/cclm-2015-0686 [DOI] [PubMed] [Google Scholar]
- 52.Murat SN, Duran M, Kalay N, et al. Relation between mean platelet volume and severity of atherosclerosis in patients with acute coronary syndromes. Angiology. 2013;64(2):131–136. doi: 10.1177/0003319711436247 [DOI] [PubMed] [Google Scholar]
- 53.Henning BF, Zidek W, Linder B, Tepel M. Mean platelet volume and coronary heart disease in hemodialysis patients. Kidney Blood Press Res. 2002;25(2):103–108. doi: 10.1159/000063516 [DOI] [PubMed] [Google Scholar]
- 54.Taheri S, Baradaran A, Aliakbarian M, Mortazavi M. Level of inflammatory factors in chronic hemodialysis patients with and without cardiovascular disease. J Res Med Sci. 2017;22. doi: 10.4103/jrms.JRMS_282_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2010;122(25):e584–e636. doi: 10.1161/CIR.0b013e3182051b4c [DOI] [PubMed] [Google Scholar]
- 56.Silva D, de Lacerda AP. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev Port Cardiol. 2012;31(11):733–745. doi: 10.1016/j.repc.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 57.Seo SM, Baek SH, Jeon HK, et al. Correlations between the level of high-sensitivity C-reactive protein and cardiovascular risk factors in Korean adults with cardiovascular disease or diabetes mellitus: the CALLISTO study. J Atheroscler Thromb. 2013;20(7):616–622. doi: 10.5551/jat.16089 [DOI] [PubMed] [Google Scholar]
- 58.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.CIR.0000055014.62083.05 [DOI] [PubMed] [Google Scholar]
- 59.Chowta MN, Adhikari PM, Sinha R, Acharya SD, Gopalakrishna H, Ramapuram JT. Highly sensitive C reactive protein in patients with metabolic syndrome and cardiovascular disease. Ann Trop Med Public Health. 2012;5(2):98–102. doi: 10.4103/1755-6783.95960 [DOI] [Google Scholar]
- 60.Yarlioglues M, Kaya MG, Ardic I, et al. Relationship between mean platelet volume levels and subclinical target organ damage in newly diagnosed hypertensive patients. Blood Press. 2011;20(2):92–97. doi: 10.3109/08037051.2010.532317 [DOI] [PubMed] [Google Scholar]
- 61.Kaya MG, Yarlioglues M, Gunebakmaz O, et al. Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis. 2010;209(1):278–282. doi: 10.1016/j.atherosclerosis.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 62.Yazici S, Yazici M, Erer B, et al. The platelet indices in patients with rheumatoid arthritis: mean platelet volume reflects disease activity. Platelets. 2010;21(2):122–125. doi: 10.3109/09537100903474373 [DOI] [PubMed] [Google Scholar]
- 63.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96(3):776–781. doi: 10.1111/j.1572-0241.2001.03621.x [DOI] [PubMed] [Google Scholar]
- 64.Koren-Morag N, Tanne D, Goldbourt U. White blood cell count and the incidence of ischemic stroke in coronary heart disease patients. Am J Med. 2005;118(9):1004–1009. doi: 10.1016/j.amjmed.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 65.Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol. 2004;44(10):1945–1956. doi: 10.1016/j.jacc.2004.07.056 [DOI] [PubMed] [Google Scholar]
- 66.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2(2):82–104. doi: 10.1089/met.2004.2.82 [DOI] [PubMed] [Google Scholar]
- 67.Ebrahimi M, Heidari-Bakavoli AR, Shoeibi S, et al. Association of serum hs-CRP levels with the presence of obesity, diabetes mellitus, and other cardiovascular risk factors. J Clin Lab Anal. 2016;30(5):672–676. doi: 10.1002/jcla.21920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shamai L, Lurix E, Shen M, et al. Association of body mass index and lipid profiles: evaluation of a broad spectrum of body mass index patients including the morbidly obese. Obes Surg. 2011;21(1):42–47. doi: 10.1007/s11695-010-0170-7 [DOI] [PubMed] [Google Scholar]
- 69.Singh P, Singh SK, Reddy VK, Sharma S, Chandra S, Vijay P. A study on association of age, gender, and body mass index with hematological parameters. J Indian Assoc Public Health Dent. 2021;19(2):109. doi: 10.4103/jiaphd.jiaphd_114_20 [DOI] [Google Scholar]