Abstract
Simple Summary
The use of peripherally inserted central catheters (PICCs) or PICC lines has become an established part of daily practice due to their ease of insertion, maintenance, and removal. Their use has increased particularly in cancer patients treated with chemotherapy who are immunocompromised and, therefore, known to have an increased risk of infection. However, the risk of PICC-related infections in this population compared to noncancer patients remains poorly evaluated. We found that the PICC-related bloodstream infection rate was more than twice as high in cancer patients compared to noncancer patients. In addition, we confirmed that dual-lumen PICCs had a higher risk of PICC-related bloodstream infection than single-lumen PICCs. Our results encourage physicians to carefully implement infection-control measures in cancer patients receiving chemotherapy through a PICC and particularly to limit the number of catheter lumens in these patients.
Abstract
The use of peripherally inserted central catheters (PICCs) has increased in cancer patients. This study aimed to compare the incidence of PICC-related bloodstream infections (PICCR-BSIs) in cancer patients treated with chemotherapy and in noncancer patients. We performed a secondary analysis from a retrospective, single-center, observational cohort. The PICCR-BSI incidence rates in cancer and noncancer patients were compared after 1:1 propensity-score matching. Then, the factors associated with PICCR-BSI were assessed in a Cox model. Among the 721 PICCs (627 patients) included in the analysis, 240 were placed in cancer patients for chemotherapy and 481 in noncancer patients. After propensity-score matching, the PICCR-BSI incidence rate was 2.6 per 1000 catheter days in cancer patients and 1.0 per 1000 catheter days in noncancer patients (p < 0.05). However, after adjusting for variables resulting in an imbalance between groups after propensity-score matching, only the number of PICC lumens was independently associated with PICCR-BSI (adjusted hazard ratio 1.81, 95% confidence interval: 1.01–3.22; p = 0.04). In conclusion, the incidence rate of PICCR-BSI is higher in cancer patients treated with chemotherapy than in noncancer patients, but our results also highlight the importance of limiting the number of PICC lumens in such patients.
Keywords: PICC-line, CR-BSI, CRI, Gram-negative bacteria, catheter lumen, hematological malignancies, solid tumor
1. Introduction
Peripherally inserted central catheters (PICCs), also known as PICC lines, are venous catheters inserted into a peripheral vein in the upper arm, the distal tip of which is located in the territory of the superior vena cava [1,2]. They are easier to place and less prone to complications at the time of insertion than other central venous catheters (CVC) [1,2]. PICCs are mainly used for the administration of parenteral nutrition, prolonged antimicrobial therapy, or chemotherapy [1,2]. In some patients requiring simultaneous administration of these drugs, devices with two lumens (two separate tubings in the same catheter) can be inserted [3]. The different types of PICCs can be used for durations of up to 6 months and even longer [1,2].
Over the past decade, the use of PICCs has become more widespread in daily practice, leading at the same time to an increase in the number of PICC-related complications [1,4]. Complications include mechanical complications such as catheter dysfunction or accidental removal [5], thrombotic complications [6], and infectious complications [1,7]. PICC-related infections (PICCRIs) and, in particular, PICC-related bloodstream infections (PICCR-BSIs) are the most frequent complications [1,7]. However, PICCs would have a potential advantage over other central venous catheters since they would reduce the risk of catheter-related bloodstream infections compared to other CVCs [8,9,10].
In cancer patients treated with chemotherapy, the use of PICCs has increased dramatically [11] but studies provide conflicting data regarding the risk of CVC-related complications, with some reporting that implantable port catheters are safer [12], others that PICCs are safer [13], and finally, some authors report that both devices are equivalent [14]. Moreover, the risk of PICC-related complications in cancer patients compared with noncancer patients remains poorly assessed [15]. The complication rate appears to be higher in cancer patients than in noncancer patients, especially with regard to the PICC thrombosis rate [16,17]. In the literature, the incidence rates of PICCR-BSI in cancer patients range between 2 and 4 per 1000 catheter days [18,19,20,21,22] and between 1 and 2 per 1000 catheter days in noncancer patients [23,24], but few studies have compared the incidence rate of PICCR-BSI in cancer patients treated with chemotherapy and in noncancer patients.
We, therefore, conducted a study that aimed to compare the incidence rate of PICCR-BSI in cancer patients treated with chemotherapy and in noncancer patients and to describe the microorganisms involved in these patients.
2. Materials and Methods
2.1. Study Design and Settings
We performed a secondary analysis from a retrospective, single-center, observational cohort [1] that included consecutive adult inpatients and outpatients who had at least one PICC insertion at Nimes University Hospital from 1 April 2018 to 1 April 2019.
In this 2094-bed teaching hospital, ultrasound-guided insertions of single- or double-lumen PICC (Bard Access Systems, Salt Lake City, UT, USA) were performed 5 days per week in the medical imaging department under aseptic conditions according to the French Society of Infection Control (SF2H) guidelines [25]. The position of the PICC was then checked by chest X-ray and adjusted if necessary, and saline was used to avoid occlusion of the lumen(s) [25]. All PICCs were inserted by a trained radiologist or radiology technician in an interventional radiology room.
2.2. Patients
All patients of the Barrigah-Benissan et al. cohort [1] were screened. Only cancer patients treated with chemotherapy and noncancer patients were included in the current study. Cancer patients were defined as patients undergoing treatment for a solid tumor with or without metastasis or for a hematological malignancy (leukemia, lymphoma, or myeloma). Cancer patients not treated with chemotherapy (treatment completed or in palliative care) were excluded.
2.3. Data Collection
The following variables were collected: age, sex, body mass index (BMI), Charlson comorbidity index, type of cancer, ongoing immunosuppressive treatment (corticosteroids or other immunosuppressive drugs), the reason for PICC insertion, number of lumens, side of PICC insertion site, the reason for PICC removal, PICC duration, and vital status at PICC removal. The occurrence and timing of PICC colonization or PICCRI were also collected. The bacteria species involved in PICC colonization and PICCRI were recorded.
To secure the diagnosis of PICCRI [26], all medical records were reviewed by an adjudication committee composed of an intensivist, an infection control specialist, and an infectious disease physician. If there was a discrepancy, the PICCRI diagnosis was discussed among the committee members until a consensus was achieved.
2.4. Microbiology
The department of microbiology performed quantitative culture of the distal segment of intravascular catheters as described by Brun-Buisson. BD BACTEC™ Aerobic Plus and BD BACTEC™ Anaerobic Plus blood-culture bottles were placed in the BD BACTEC™ FX system (Becton-Dickinson, Franklin Lakes, NJ, USA) for incubation up to 5 and 7 days, respectively, or until automatic detection of positivity. In cases of suspected endocarditis, the total incubation period was 14 days. If the bottle was not positive during the incubation period, it was considered negative. For bottles that detected positive, the detection time was recorded; then, Gram strain and subculture for incubation for 24 h at 35 °C were performed. Bacterial and fungal identification were performed using mass spectrometry Vitek® MS (bioMérieux, Marcy-l’Etoile, France) and antimicrobial susceptibility testing (AST) using disk diffusion method on Mueller–Hinton agar (Bio-Rad, Hercules, CA, USA), according to the European committee on antimicrobial susceptibility testing (EUCAST) guidelines [27].
2.5. Study Definitions
PICC colonization were defined as a quantitative culture ≥103 CFU/mL, according to Brun-Buisson, without bacteremia or clinical signs [26].
No-bacteremia PICCRI (NB-PICCRI), were defined, in the absence of bacteremia, as a combination of (i) PICC culture ≥103 CFU/mL and (ii) (a) signs of local infection (purulent discharge from the PICC insertion site); and/or (b) systemic signs, with complete or partial resolution of systemic signs of infection within 48 h after PICC removal [26].
PICCR-BSI were defined as an association of (i) the occurrence of either bacteremia or fungaemia during the 48-h period surrounding PICC removal (or a suspected diagnosis of PICCRI when the PICC is not removed immediately); (ii) and either a positive culture with the same microorganism on one of the following samples: insertion site culture, or PICC culture ≥103 CFU/mL or positive central and peripheral blood cultures with the same microorganism, with a central/peripheral positive blood culture time lag > 2 h with central blood cultures being positive earlier than the peripheral ones [26].
PICCRI were defined as NB- PICCRI and PICCR-BSI [26].
2.6. Statistical Analysis
PICC insertion was the unit for statistical analyses. The categorical data were described as numbers and percentages, and continuous data as medians with 25th and 75th percentiles (interquartile range: IQR). Patients were segregated according to cancer (yes or no). The categorical variables were compared by Chi-square or Fisher’s exact test, and the continuous variables were compared by Student’s t test or Wilcoxon’s rank-sum test, as appropriate.
Propensity-score matching was performed to compare the incidence of PICCR-BSI in cancer patients treated with chemotherapy with those in noncancer patients. Patients were matched (1:1) with the algorithm for nearest-neighbor matching without replacement, using a maximum tolerance distance between the matched subjects of 0.1 standard deviation. The confounding variables used to calculate the propensity scores were age, BMI, number of PICC lumens, and Charlson comorbidity index.
We performed survival analyses to consider the time dimension. The observation time was the time from PICC insertion to the occurrence of the event (PICCR-BSI) and/or PICC removal. We identified the variables resulting in an imbalance between groups after propensity score matching by calculating the standardized mean difference; then, we included them in the subsequent Cox proportional hazards model as covariates to assess the effect of chemotherapy-treated cancer on the incidence of PICCR-BSI. Cumulative incidence curves of PICCR-BSI were obtained by the Kaplan–Meier methodology and compared using the log-rank test.
All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant. Analyses were performed using the R software version 4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Patients and Peripherally Inserted Central Venous Catheters
Of the 901 PICCs inserted in 783 patients in the initial cohort [1], 721 inserted PICCs in 627 patients were included in the analysis corresponding to 31,831 catheter days. Among the PICCs included, 240 were placed in cancer patients for chemotherapy and 481 in noncancer patients, corresponding to 15,108 and 16,723 catheter days, respectively (Figure S1). The median age of the study population was 69 years (IQR: 57, 79) and 55% of PICCs were inserted in male patients. Two thirds of the cancer patients had a solid tumor and one third had hematological malignancies (Table 1).
Table 1.
Characteristics of the study population and peripherally inserted central catheters (PICC).
| Characteristics | Overall n = 721 1 |
Cancer Patients n = 240 1 |
Noncancer Patients n = 481 1 |
p-Value 2 |
|---|---|---|---|---|
| Demographics: | ||||
| Age | 69 (57, 79) | 66 (54, 74) | 72 (60, 82) | <0.001 |
| Male | 399 (55%) | 129 (54%) | 270 (56%) | 0.5 |
| Body Mass Index (BMI) | 24 (21, 29) | 23 (21, 27) | 25 (21, 30) | <0.001 |
| Charlson comorbidity index | 6 (3, 8) | 6 (4, 9) | 5 (3, 7) | <0.001 |
| Cancer type: | ||||
| Solid tumor | 161 (22%) | 161 (67%) | - | |
| Localized solid tumor | 55 (7.6%) | 55 (23%) | - | |
| Metastatic solid tumor | 106 (15%) | 106 (44%) | - | |
| Hematological malignancies | 79 (11%) | 79 (33%) | - | |
| Leukemia | 43 (6.0%) | 43 (18%) | - | |
| Lymphoma | 18 (2.5%) | 18 (7.5%) | - | |
| Myeloma | 18 (2.5%) | 18 (7.5%) | - | |
| Main reason for PICC placement: | ||||
| Cancer chemotherapy | 240 (33%) | 240 (100%) | - | |
| Antimicrobial therapy | 306 (42%) | - | 306 (64%) | |
| Limited peripheral access | 109 (15%) | - | 109 (23%) | |
| Long-term venous access | 31 (4.3%) | - | 31 (6.4%) | |
| Parenteral nutrition | 35 (4.9%) | - | 35 (7.3%) | |
| Double-lumen PICC | 155 (21%) | 104 (43%) | 51 (11%) | <0.001 |
| Right side PICC insertion site | 167 (23%) | 51 (21%) | 116 (24%) | 0.4 |
| Reason for PICC removal: | ||||
| End of intravenous therapy | 426 (59%) | 89 (37%) | 337 (70%) | <0.001 |
| Port implantation | 23 (3.2%) | 18 (7.5%) | 5 (1.0%) | <0.001 |
| Mechanical complication | 67 (9.3%) | 20 (9.8%) | 47 (9.8%) | >0.9 |
| PICCRI 3 (suspected or confirmed) | 123 (17%) | 77 (32%) | 46 (9.6%) | <0.001 |
| Death | 82 (11%) | 36 (15%) | 46 (9.6%) | 0.03 |
| PICC duration (days) | 21 (10, 46) | 32 (15, 76) | 17 (8, 35) | <0.001 |
| Number of catheter days | 31,831 | 15,108 | 16,723 |
1 Median (interquartile range) or n (%); 2 Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test as appropriate; 3 PICCRI: PICC-related infection.
Cancer patients were younger, had lower BMIs, and higher Charlson score than noncancer patients. Double-lumen PICCs were more frequently placed in cancer patients who also have longer PICC indwelling time than noncancer patients, 32 days (IQR: 15, 76) versus 17 days (IQR: 8, 35).
In noncancer patients, PICCs were removed primarily at the end of treatment because they were no longer useful (70%). On the contrary, in more than half of cancer patients (57%), PICCs were removed due to a suspected or confirmed complication.
3.2. Incidence of PICC-Related Complications
The incidence of PICC-related complications was similar between cancer and noncancer patients, except for PICCR-BSI (Table 2).
Table 2.
Peripherally inserted central catheter (PICC) related complications (rates and incidences).
| PICC Related Complications | Overall 1 n = 721 |
Cancer Patients 1 n = 240 |
Noncancer Patients 1 n = 481 |
p-Value 2 |
|---|---|---|---|---|
| Accidental removal (rate) | 47 (6.5%) | 10 (4.2%) | 37 (7.7%) | 0.071 |
| Accidental removal per 1000 catheter days | 1.5 | 0.7 | 2.2 | |
| Vein thrombosis (rate) | 14 (1.9%) | 8 (3.3%) | 6 (1.2%) | 0.082 |
| Vein thrombosis per 1000 catheter days | 0.4 | 0.5 | 0.4 | |
| Catheter dysfunction (rate) | 6 (0.8%) | 2 (0.8%) | 4 (0.8%) | >0.9 |
| Catheter dysfunction per 1000 catheter days | 0.2 | 0.1 | 0.2 | |
| PICC colonization (rate) | 33 (4.6%) | 15 (6.2%) | 18 (3.7%) | 0.13 |
| PICC colonization per 1000 catheter days | 1.0 | 0.9 | 1.1 | |
| NB-PICCRI 3 (rate) | 11 (1.5%) | 5 (2.1%) | 6 (1.2%) | 0.5 |
| NB-PICCRI per 1000 catheter days | 0.3 | 0.3 | 0.4 | |
| PICCR-BSI 4 (rate) | 58 (8.0%) | 40 (17%) | 18 (3.7%) | <0.001 |
| PICCR-BSI per 1000 catheter days | 1.8 | 2.6 | 1.1 |
1 Median (IQR) or n (%); 2 Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test as appropriate; 3 NB-PICCRI: nonbacteremia PICC-related infection; 4 PICCR-BSI: PICC-related bloodstream infection.
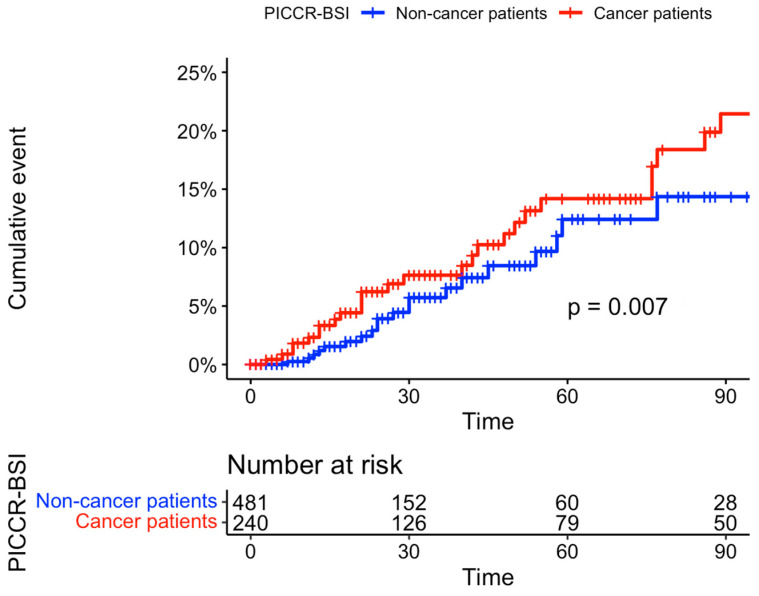
The incidence of PICCR-BSI was 2.6 per 1000 catheter days in cancer patients and 1.1 per 1000 catheter days in noncancer patients (p = 0.07), see Figure 1.
Figure 1.
Cumulative incidence curves of peripherally inserted central catheter-related bloodstream infection (PICCR-BSI) in cancer (red) and noncancer patients (blue).
3.3. Microbiology
Cancer patients mainly had PICCR-BSI caused by Gram-negative bacteria, especially Enterobacterales, and nonfermenters, whereas noncancer patients more frequently had PICCR-BSI caused by Gram-positive bacteria, mainly coagulase-negative staphylococci, and PICC-related fungemia. It should be noted that no cancer patients had PICC-related fungemia and noncancer patients did not have PICCR-BSI caused by nonfermenters.
Bacterial species involved in PICCR-BSI are presented in Table 3 (see also Table S1).
Table 3.
Bacterial species involved in a peripherally inserted central catheter (PICC) related bloodstream infections.
| Bacterial Species | Overall 1 n = 58 |
Cancer Patients 1 n = 40 |
Noncancer Patients 1 n = 18 |
|---|---|---|---|
| Gram-negative bacteria | 38 (66%) | 31 (78%) | 7 (39%) |
| Enterobacterales 2 | 29 (50%) | 22 (55%) 2 | 7 (39%) |
| Escherichia coli | 8 (14%) | 5 (13%) | 3 (17%) |
| Enterobacter cloacae | 7 (12%) | 6 (15%) | 1 (6%) |
| Klebsiella pneumoniae | 5 (9%) | 4 (10%) | 1 (6%) |
| Klebsiella oxytoca | 3 (5%) | 3 (8%) | 0 (0%) |
| Serratia marcescens | 2 (3%) | 1 (3%) | 1 (6%) |
| Citrobacter koseri | 1 (2%) | 0 (0%) | 1 (6%) |
| Hafnia alvei | 1 (2%) | 1 (3%) | 0 (0%) |
| Klebsiella aerogenes | 1 (2%) | 1 (3%) | 0 (0%) |
| Proteus mirabilis | 1 (2%) | 1 (3%) | 0 (0%) |
| Nonfermenters | 9 (16%) | 9 (23%) | 0 (0%) |
| Acinetobacter baumannii | 3 (5%) | 3 (8%) | 0 (0%) |
| Acinetobacter ursingii | 1 (2%) | 1 (3%) | 0 (0%) |
| Stenotrophomonas maltophilia | 2 (3%) | 2 (5%) | 0 (0%) |
| Achromobacter xylosoxidans | 1 (2%) | 1 (3%) | 0 (0%) |
| Pseudomonas aeruginosa | 1 (2%) | 1 (3%) | 0 (0%) |
| Rhizobium radiobacter | 1 (2%) | 1 (3%) | 0 (0%) |
| Gram-positive bacteria | 26 (45%) | 17 (43%) | 9 (50%) |
| Staphylococcus epidermidis | 13 (22%) | 7 (18%) | 6 (30%) |
| Staphylococcus aureus | 4 (7%) | 4 (10%) | 0 (0%) |
| Staphylococcus haemolyticus | 1 (2%) | 0 (0%) | 1 (6%) |
| Streptococcus pasteurianus | 3 (5%) | 2 (5%) | 1 (6%) |
| Streptococcus mitis | 1 (2%) | 1 (3%) | 0 (0%) |
| Enterococcus faecium | 2 (3%) | 1 (3%) | 1 (6%) |
| Enterococcus faecalis | 1 (2%) | 1 (3%) | 0 (0%) |
| Bacillus licheniformis | 1 (2%) | 1 (3%) | 0 (0%) |
| Fungi | 4 (8%) | 0 (0%) | 4 (22%) |
| Candida glabrata | 2 (3%) | 0 (0%) | 2 (11%) |
| Candida parapsilosis | 2 (3%) | 0 (0%) | 2 (11%) |
1 n (%); 2 including 10 AmpC beta-lactamase-producing Enterobacterales, 6 extended spectrum beta-lactamase-producing Enterobacterales and 5 carbapenem-resistant Enterobacterales.
3.4. Incidence of PICC-Related Complications after Propensity Score Matching
After propensity-score matching on age, BMI, number of PICC lumens, and Charlson comorbidity index, the rate of PICCR-BSI remains higher in cancer patients than in noncancer patients at 17% and 2.9%, respectively (p < 0.001), corresponding to a PICCR-BSI incidence rate of 2.6 per 1000 catheter days in cancer patients treated with chemotherapy and 1 per 1000 catheter days in noncancer patients (Table 4).
Table 4.
Characteristics of the study population and peripherally inserted central catheters (PICC) related complications (rates and incidences).
| Characteristics | Overall n = 480 1 |
Cancer Patients n = 240 1 |
Noncancer Patients n = 240 1 |
p-Value 2 |
|---|---|---|---|---|
| Age | 68 (54, 77) | 66 (54, 74) | 71 (54, 82) | 0.003 |
| Male | 276 (57%) | 129 (54%) | 147 (61%) | 0.10 |
| Body Mass Index (BMI) | 23 (20, 28) | 23 (21, 27) | 24 (20, 28) | 0.9 |
| Charlson comorbidity index | 6 (3, 9) | 6 (4, 9) | 6 (3, 9) | 0.12 |
| Double-lumen PICC | 154 (32%) | 104 (43%) | 50 (21%) | <0.001 |
| PICC duration (days) | 24 (11, 54) | 32 (15, 76) | 16 (8, 34) | <0.001 |
| Number of catheter days | 22,432 | 15,108 | 7324 | |
| Accidental removal (rate) | 27 (5.6%) | 10 (4.2%) | 17 (7.1%) | 0.2 |
| Accidental removal per 1000 catheter days | 1.2 | 0.7 | 2.3 | |
| Vein thrombosis (rate) | 11 (2.3%) | 8 (3.3%) | 3 (1.3%) | 0.13 |
| Vein thrombosis per 1000 catheter days | 0.5 | 0.5 | 0.4 | |
| Catheter dysfunction (rate) | 6 (1.3%) | 2 (0.8%) | 4 (1.7%) | 0.7 |
| Catheter dysfunction per 1000 catheter days | 0.3 | 0.1 | 0.5 | |
| PICC colonization 3 (rate) | 23 (4.8%) | 15 (6.2%) | 8 (3.3%) | 0.13 |
| PICC colonization per 1000 catheter days | 1.0 | 0.9 | 1.1 | |
| NB-PICCRI 4 (rate) | 10 (2.1%) | 5 (2.1%) | 5 (2.1%) | >0.9 |
| NB-PICCRI per 1000 catheter days | 0.4 | 0.3 | 0.7 | |
| PICCR-BSI 5 (rate) | 47 (9.8%) | 40 (17%) | 7 (2.9%) | <0.001 |
| NB-PICCRI per 1000 catheter days | 2.1 | 2.6 | 1.0 |
1 Median (IQR) or n (%); 2 Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test as appropriate; 3 PICCRI: PICC-related infection; 4 NB-PICCRI: nonbacteremia PICC-related infection; 5 PICCR-BSI: PICC-related bloodstream infection.
Despite matching, the population remained unbalanced in terms of age and number of PICC lumens. After adjustment on these confounders, the risk for PICCR-BSI in patients treated with chemotherapy for cancer remained higher, but the difference was no longer statistically significant: adjusted hazard ratio (aHR) 1.83 confidence interval at 95% (95%CI): 0.8699–3.858 (p = 0.11). On the contrary, double-lumen PICC placement was independently associated with an increased incidence of PICCR-BSI, aHR 1.81, 95%CI: 1.01–3.22 (p = 0.04), see Table 5.
Table 5.
Multivariable Cox model for peripherally inserted central catheters (PICC) related bloodstream infection after propensity score matching.
| Variables | aHR 1 | 95%CI 2 | p-Value |
|---|---|---|---|
| Cancer | 1.83 | 0.86–3.86 | 0.11 |
| Age (year) | 1.003 | 0.98–1.02 | 0.75 |
| Double-lumen PICC | 1.81 | 1.01–3.22 | 0.04 |
1 aHR: adjusted hazard ratio; 2 95%CI: confidence interval at 95%.
4. Discussion
We reported herein the results of a large cohort of 721 PICC placements in 627 patients (31,831 catheter days) showing a PICCR-BSI incidence rate of 1.8 per 1000 catheter days. In cancer patients, the PICCR-BSI incidence rate was 2.6 per 1000 catheter days and PICCR-BSIs were mainly caused by Gram-negative bacteria, whereas in noncancer patients, the PICCR-BSI incidence rate was 1.1 per 1000 catheter days and PICCR-BSIs were mostly caused by Gram-positive bacteria. After propensity-score matching, the PICCR-BSI incidence rate remained more than twofold higher in cancer patients (2.6 versus 1 per 1000 catheter days). However, after adjusting the variables resulting in an imbalance between groups after propensity score matching, only the number of PICC lumens was independently associated with PICCR-BSI.
The incidence rates of PICCR-BSI reported in the literature range widely, from 1.0 to 2.1 per 1000 catheter days in noncancer patients [23,24] and from 2.0 to 4.0 per 1000 catheter days in patients with hematological malignancies [20,21,22] or solid tumors [8]. We found that the incidence rate of PICCR-BSI was twice as high in cancer patients treated with chemotherapy as in noncancer patients, which is consistent with previous reports [8,20,21,22,23,24]. Among cancer patients, those with hematological malignancies, especially those with leukemia or high-grade lymphoma, are at higher risk for PICCRI compared with patients with solid tumors [11]. In addition, neutropenic patients with bloodstream infections are at higher risk of mortality compared with nonneutropenic patients [11].
Our results confirm that hematological malignancies and solid cancers with ongoing chemotherapy are risk factors associated with PICCR-BSI [3]. However, they also highlighted the importance of limiting the number of PICC lumens to the minimum required. The use of multilumen PICCs or the presence of another central venous catheter at the time of PICC placement have already been reported as risk factors for PICCR-BSI [3,28]. The cancer patient remains fragile and susceptible to infections; therefore, maximum precautions should be taken to limit PICCRI. It seems also important to limit the catheter dwell time [9], particularly in patients with hematological malignancies [20]. Although this risk factor remains debated [22], many studies [1,20,29] encouraged clinicians to limit the PICC indwelling time to approximately 4 weeks. Especially since the risk of PICCR-BSI does not seem to increase with multiple PICC insertions [20]. Moreover, our results confirmed that the PICCR-BSI rate was not influenced by the side of the PICC insertion site [21]. In addition to limiting the number of PICC lumens and catheter dwell time, it is necessary to perform dressing changes every 4 to 7 days in aseptic conditions, to disinfect the administration sites at each use, to improve hand-hygiene compliance, and to monitor clinical signs of infection [25]. Some authors have also reported the interest of self-management to reduce complications in cancer patients receiving chemotherapy through a PICC [30]. In particular, the importance of self-monitoring for clinical signs of infection should be emphasized [1].
As the use of PICCs is booming, some authors have suggested placing PICCs at the patient’s bedside to facilitate access to these devices [31,32]. This practice does not seem to increase PICC-related complications, including infections, but studies [31,32] are mainly carried out in intensive care units and include few or no cancer patients. As cancer patients have a higher risk for PICCR-BSI, a careful evaluation of this type of practice will be required before it becomes routine in the oncology and hematology departments. Importantly, no PICCs were inserted at the bedside in our cohort. Thus, our results encourage physicians in charge of cancer patients treated with chemotherapy to favor PICC placement in an interventional radiology room that is a controlled environment with air filtration systems capable of delivering clean, filtered, and contaminant-free air into the room, and in which biocleaning is performed between each PICC placement. To prevent PICCRI, a trained operator must observe appropriate infection-control measures, such as hand hygiene, skin preparation with an alcohol antiseptic, maximal sterile-barrier precautions, and an aseptic technique during PICC placement [25]. In addition to the particular attention that must be paid to limiting catheter dwelling time and the number of lumens, patients and caregivers must be educated to apply strict hand hygiene and skin antisepsis during PICC care and dressing management and to detect and recognize signs of PICCRI and other PICC-related complications [1].
Another striking finding of our study highlighting the peculiarities of cancer patients is that Gram-negative bacteria are an increasing cause of PICCR-BSI in this population. Over the last decade, Gram-negative bacteria have become the main etiological microorganisms of catheter-related bloodstream infections [33,34,35]. The immunocompromised patients are at the greatest risk of being infected by their own enterobacteria [36]; accordingly, more than half of the bacteria involved in PICCR-BSI of cancer patients are Gram-negative bacilli [34,35]. In contrast, Gram-positive bacteria remained responsible for most catheter-related infections in noncancer patients, followed by gram-negative bacteria and fungi [24,28], although a change in this trend has been suggested [37,38,39].
This study has limitations. First, its single-center design could limit the extrapolation of the results, as PICC-related complication rates vary between hospitals [15]. However, our cohort included a large number of patients from four different oncology departments and one hematology department, representing a mix of different practices. In addition, this study is one of the few to assess the risk of PICCR-BSI in cancer and noncancer patients in a recent period. Second, we studied only one type of device, whereas the rate of complications differed across the PICC types [18]. Nonetheless, the rate of PICCR-BSI did not [18]. Third, the retrospective design of the study limits our analyses to the available data in medical records and may induce bias in data collection and results interpretation. Some risk factors such as the receipt of total parenteral nutrition through the PICC [3], the neutrophil count at the time of PICC placement or infection [11], or the degree of dependence of patients could not be assessed, nor could the outpatient/inpatient status. However, the data suggested that using PICCs in outpatients is not associated with an elevated risk of complications [40], including in cancer patients [41].
5. Conclusions
In a large French retrospective cohort study, we found that the incidence rate of PICCR-BSI was 2.6 per 1000 patient days in cancer patients treated with chemotherapy and 1.1 per 1000 patient days in noncancer patients. We showed that PICCR-BSIs were most often caused by Gram-negative bacteria in cancer patients whereas they were mainly caused by Gram-positive bacteria in noncancer patients. The incidence rate of PICCR-BSI remained higher in cancer patients treated with chemotherapy than in noncancer patients after propensity-score matching. However, our results suggest that the use of double-lumen PICCs in cancer patients may be a higher risk of PICCR-BSI than the immunosuppression induced by cancer treatment with chemotherapy.
Further multicenter studies are mandatory to better understand the reasons for the increase in PICCRI caused by Gram-negative bacteria, to assess the risk of PICCR-BSI in cancer patients, and to determine whether measures to prevent PICCRI, such as limiting the number of PICC lumens, improve outcomes in these patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15123253/s1, Figure S1: Flow chart of the study population and peripherally inserted central catheters (PICC); Table S1: Bacterial species involved in peripherally inserted central catheter (PICC) colonization and nonbacteremia PICC-related infection.
Author Contributions
Conceptualization, R.L. and A.S.; methodology, R.L.; software, R.L.; validation, K.B.-B., J.O. and A.S.; formal analysis, R.L.; investigation, K.B.-B.; resources, C.S. and J.-P.B.; data curation, R.L.; writing—original draft preparation, R.L.; writing—review and editing, J.O. and A.S.; visualization, R.L.; supervision, A.S.; project administration, A.S.; funding acquisition, J.-P.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Institutional Review Board of Nimes University Hospital approved the study protocol (protocol code 22.03.06, date of approval: 6 March 2022) and waived the need for signed patient consent. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
The need for patient written consent was waived by the Institutional Review Board of Nimes University Hospital in accordance with the national legislation and institutional requirements because this observational study did not modify existing diagnostic or therapeutic strategies. However, patients were informed of their inclusion in the study.
Data Availability Statement
The authors consent to share the collected data with others. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Data will be available immediately after the main publication and indefinitely.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Barrigah-Benissan K., Ory J., Simon C., Loubet P., Martin A., Beregi J.-P., Lavigne J.-P., Sotto A., Larcher R. Clinical Factors Associated with Peripherally Inserted Central Catheters (PICC) Related Bloodstream Infections: A Single Centre Retrospective Cohort. Antimicrob. Resist. Infect. Control. 2023;12:5. doi: 10.1186/s13756-023-01209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopra V., Flanders S.A., Saint S., Woller S.C., O’Grady N.P., Safdar N., Trerotola S.O., Saran R., Moureau N., Wiseman S., et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann. Intern. Med. 2015;163:S1–S40. doi: 10.7326/M15-0744. [DOI] [PubMed] [Google Scholar]
- 3.Herc E., Patel P., Washer L.L., Conlon A., Flanders S.A., Chopra V. A Model to Predict Central-Line-Associated Bloodstream Infection Among Patients with Peripherally Inserted Central Catheters: The MPC Score. Infect. Control Hosp. Epidemiol. 2017;38:1155–1166. doi: 10.1017/ice.2017.167. [DOI] [PubMed] [Google Scholar]
- 4.Piredda A., Radice D., Zencovich C., Cerri M., Aventino L., Naccarato F., Magon G., Biffi R. Safe Use of Peripherally Inserted Central Catheters for Chemotherapy of Solid Malignancies in Adult Patients: A 1-Year Monocentric, Prospectively-Assessed, Unselected Cohort of 482 Patients. J. Vasc. Access. 2021;22:873–881. doi: 10.1177/1129729820962905. [DOI] [PubMed] [Google Scholar]
- 5.Vidal V., Muller C., Jacquier A., Giorgi R., Le Corroller T., Gaubert J.Y., Champsaur P., Bartoli J.M., Moulin G. Évaluation prospective des complications des PICCs. J. Radiol. 2008;89:495–498. doi: 10.1016/S0221-0363(08)71453-7. [DOI] [PubMed] [Google Scholar]
- 6.Al-Asadi O., Almusarhed M., Eldeeb H. Predictive Risk Factors of Venous Thromboembolism (VTE) Associated with Peripherally Inserted Central Catheters (PICC) in Ambulant Solid Cancer Patients: Retrospective Single Centre Cohort Study. Thromb. J. 2019;17:2. doi: 10.1186/s12959-019-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madabhavi I., Patel A., Sarkar M., Kataria P., Kadakol N., Anand A. A Study of the Use of Peripherally Inserted Central Catheters in Cancer Patients: A Single-Center Experience. J. Vasc. Nurs. 2018;36:149–156. doi: 10.1016/j.jvn.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Nakaya Y., Imasaki M., Shirano M., Shimizu K., Yagi N., Tsutsumi M., Yoshida M., Yoshimura T., Hayashi Y., Nakao T., et al. Peripherally Inserted Central Venous Catheters Decrease Central Line-Associated Bloodstream Infections and Change Microbiological Epidemiology in Adult Hematology Unit: A Propensity Score-Adjusted Analysis. Ann. Hematol. 2022;101:2069–2077. doi: 10.1007/s00277-022-04908-6. [DOI] [PubMed] [Google Scholar]
- 9.Lv Y., Huang X., Lan Y., Xia Q., Chen F., Wu J., Li W., Cao H., Xie C., Li L., et al. Peripherally Inserted Central Catheters Have a Protective Role and the Effect of Fluctuation Curve Feature in the Risk of Bloodstream Infection Compared with Central Venous Catheters: A Propensity-Adjusted Analysis. BMC Infect. Dis. 2022;22:289. doi: 10.1186/s12879-022-07265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitiriga V., Bakalis J., Theodoridou K., Kanellopoulos P., Saroglou G., Tsakris A. Lower Risk of Bloodstream Infections for Peripherally Inserted Central Catheters Compared to Central Venous Catheters in Critically Ill Patients. Antimicrob. Resist. Infect. Control. 2022;11:137. doi: 10.1186/s13756-022-01180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Böll B., Schalk E., Buchheidt D., Hasenkamp J., Kiehl M., Kiderlen T.R., Kochanek M., Koldehoff M., Kostrewa P., Claßen A.Y., et al. Central Venous Catheter–Related Infections in Hematology and Oncology: 2020 Updated Guidelines on Diagnosis, Management, and Prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO) Ann. Hematol. 2020;100:239–259. doi: 10.1007/s00277-020-04286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pu Y.-L., Li Z.-S., Zhi X.-X., Shi Y.-A., Meng A.-F., Cheng F., Ali A., Li C., Fang H., Wang C. Complications and Costs of Peripherally Inserted Central Venous Catheters Compared with Implantable Port Catheters for Cancer Patients: A Meta-Analysis. Cancer Nurs. 2020;43:455–467. doi: 10.1097/NCC.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 13.Cotogni P., Mussa B., Degiorgis C., De Francesco A., Pittiruti M. Comparative Complication Rates of 854 Central Venous Access Devices for Home Parenteral Nutrition in Cancer Patients: A Prospective Study of Over 169,000 Catheter-Days. J. Parenter. Enter. Nutr. 2021;45:768–776. doi: 10.1002/jpen.1939. [DOI] [PubMed] [Google Scholar]
- 14.Picardi M., Della Pepa R., Cerchione C., Pugliese N., Mortaruolo C., Trastulli F., Giordano C., Grimaldi F., Zacheo I., Raimondo M., et al. A Frontline Approach with Peripherally Inserted Versus Centrally Inserted Central Venous Catheters for Remission Induction Chemotherapy Phase of Acute Myeloid Leukemia: A Randomized Comparison. Clin. Lymphoma Myeloma Leuk. 2019;19:e184–e194. doi: 10.1016/j.clml.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Mitbander U.B., Geer M.J., Taxbro K., Horowitz J.K., Zhang Q., O’Malley M.E., Ramnath N., Chopra V. Patterns of Use and Outcomes of Peripherally Inserted Central Catheters in Hospitalized Patients with Solid Tumors: A Multicenter Study. Cancer. 2022;128:3681–3690. doi: 10.1002/cncr.34410. [DOI] [PubMed] [Google Scholar]
- 16.Yuen H.L.A., Zhao J., Tran H., Chunilal S.D. Development of a Risk Score to Predict Peripherally Inserted Central Catheter Thrombosis in Active Cancer. Intern. Med. J. 2022;52:1733–1740. doi: 10.1111/imj.15557. [DOI] [PubMed] [Google Scholar]
- 17.Taxbro K., Hammarskjöld F., Thelin B., Lewin F., Hagman H., Hanberger H., Berg S. Clinical Impact of Peripherally Inserted Central Catheters vs Implanted Port Catheters in Patients with Cancer: An Open-Label, Randomised, Two-Centre Trial. Br. J. Anaesth. 2019;122:734–741. doi: 10.1016/j.bja.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Scrivens N., Sabri E., Bredeson C., McDiarmid S. Comparison of Complication Rates and Incidences Associated with Different Peripherally Inserted Central Catheters (PICC) in Patients with Hematological Malignancies: A Retrospective Cohort Study. Leuk. Lymphoma. 2020;61:156–164. doi: 10.1080/10428194.2019.1646908. [DOI] [PubMed] [Google Scholar]
- 19.Sapkota S., Sannur R., Naik R. Analysis of Peripherally Inserted Central Catheter Line in Cancer Patients: A Single-Center Experience. South Asian J. Cancer. 2020;9:253–256. doi: 10.1055/s-0040-1721175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto Y., Hosoda R., Omura H., Tanaka T. Catheter-Related Bloodstream Infection Associated with Multiple Insertions of the Peripherally Inserted Central Catheter in Patients with Hematological Disorders. Sci. Rep. 2021;11:12209. doi: 10.1038/s41598-021-91749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mollee P., Okano S., Looke D., Kennedy G., Harper J., Clouston J., Van Kuilenburg R., Geary A., Joubert W., Eastgate M., et al. Catheter-Associated Bloodstream Infections (CA-BSI) in Adults with Cancer: A Prospective Randomized Controlled Trial. Blood. 2017;130:4729. doi: 10.1182/blood.V130.Suppl_1.4729.4729. [DOI] [PubMed] [Google Scholar]
- 22.Caris M.G., de Jonge N.A., Punt H.J., Salet D.M., de Jong V.M.T., Lissenberg-Witte B.I., Zweegman S., Vandenbroucke-Grauls C.M.J.E., van Agtmael M.A., Janssen J.J.W.M. Indwelling Time of Peripherally Inserted Central Catheters and Incidence of Bloodstream Infections in Haematology Patients: A Cohort Study. Antimicrob. Resist. Infect. Control. 2022;11:37. doi: 10.1186/s13756-022-01069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swaminathan L., Flanders S., Horowitz J., Zhang Q., O’Malley M., Chopra V. Safety and Outcomes of Midline Catheters vs Peripherally Inserted Central Catheters for Patients with Short-Term Indications: A Multicenter Study. JAMA Intern. Med. 2022;182:50. doi: 10.1001/jamainternmed.2021.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safdar N., Maki D.G. Risk of Catheter-Related Bloodstream Infection with Peripherally Inserted Central Venous Catheters Used in Hospitalized Patients. Chest. 2005;128:489–495. doi: 10.1378/chest.128.2.489. [DOI] [PubMed] [Google Scholar]
- 25.Société Française d’hygiène Hospitalière (SF2H) Recommendations from a Formalized Expert Consensus: Good Practice and Risk Management for the Use of PICC. Dec, 2013. [(accessed on 23 April 2023)]. Available online: https://sf2h.net/wp-content/uploads/2016/04/SF2H_Recos_PICC_Def.pdf.
- 26.Timsit J.-F., Baleine J., Bernard L., Calvino-Gunther S., Darmon M., Dellamonica J., Desruennes E., Leone M., Lepape A., Leroy O., et al. Expert Consensus-Based Clinical Practice Guidelines Management of Intravascular Catheters in the Intensive Care Unit. Ann. Intensive Care. 2020;10:118. doi: 10.1186/s13613-020-00713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EUCAST: Clinical Breakpoints and Dosing of Antibiotics. [(accessed on 1 March 2023)]. Available online: https://www.eucast.org/clinical_breakpoints/
- 28.Zhang S., Sun X., Lei Y. The Microbiological Characteristics and Risk Factors for PICC-Related Bloodstream Infections in Intensive Care Unit. Sci. Rep. 2017;7:15074. doi: 10.1038/s41598-017-10037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S., Moon S., Pai H., Kim B. Appropriate Duration of Peripherally Inserted Central Catheter Maintenance to Prevent Central Line-Associated Bloodstream Infection. PLoS ONE. 2020;15:e0234966. doi: 10.1371/journal.pone.0234966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X., Tao S., Ji H., Chen S., Gu Y., Jin X. Risk Factors for Peripherally Inserted Central Catheter (PICC)-Associated Infections in Patients Receiving Chemotherapy and the Preventive Effect of a Self-Efficacy Intervention Program: A Randomized Controlled Trial. Ann. Palliat. Med. 2021;10:9398–9405. doi: 10.21037/apm-21-1848. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y.O., Chung C.R., Gil E., Park C.-M., Suh G.Y., Ryu J.-A. Safety and Feasibility of Ultrasound-Guided Placement of Peripherally Inserted Central Catheter Performed by Neurointensivist in Neurosurgery Intensive Care Unit. PLoS ONE. 2019;14:e0217641. doi: 10.1371/journal.pone.0217641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon K.W., Wi W., Choi M.S., Gil E., Park C.-M., Yoo K. Feasibility of Ultrasound-Guided, Peripherally Inserted Central Catheter Placement at the Bedside in a Communicable-Disease Isolation Unit. J. Pers. Med. 2023;13:863. doi: 10.3390/jpm13050863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laporte-Amargos J., Sastre E., Bergas A., Pomares H., Paviglianiti A., Rodriguez-Arias M., Pallares N., Badia-Tejero A.M., Pons-Oltra P., Carratalà J., et al. Increasing Gram-Negative Catheter-Related Bloodstream Infection in Cancer Patients. Pathogens. 2023;12:228. doi: 10.3390/pathogens12020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaftari A.M., Hachem R., Jiang Y., Shah P., Hussain A., Hamal Z.A., Yousif A., Jordan M., Michael M., Raad I. Changing Epidemiology of Catheter-Related Bloodstream Infections in Cancer Patients. Infect. Control Hosp. Epidemiol. 2018;39:727–729. doi: 10.1017/ice.2018.75. [DOI] [PubMed] [Google Scholar]
- 35.Lendak D., Puerta-Alcalde P., Moreno-García E., Chumbita M., García-Pouton N., Cardozo C., Morata L., Suárez-Lledó M., Hernández-Meneses M., Ghiglione L., et al. Changing Epidemiology of Catheter-Related Bloodstream Infections in Neutropenic Oncohematological Patients. PLoS ONE. 2021;16:e0251010. doi: 10.1371/journal.pone.0251010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dropulic L.K., Lederman H.M. Overview of Infections in the Immunocompromised Host. Microbiol. Spectr. 2016;4:1–43. doi: 10.1128/microbiolspec.DMIH2-0026-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun E., Hussein K., Geffen Y., Rabino G., Bar-Lavie Y., Paul M. Predominance of Gram-Negative Bacilli among Patients with Catheter-Related Bloodstream Infections. Clin. Microbiol. Infect. 2014;20:O627–O629. doi: 10.1111/1469-0691.12565. [DOI] [PubMed] [Google Scholar]
- 38.Badia-Cebada L., Peñafiel J., Saliba P., Andrés M., Càmara J., Domenech D., Jiménez-Martínez E., Marrón A., Moreno E., Pomar V., et al. Trends in the Epidemiology of Catheter-Related Bloodstream Infections; towards a Paradigm Shift, Spain, 2007 to 2019. Eurosurveillance. 2022;27:2100610. doi: 10.2807/1560-7917.ES.2022.27.19.2100610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ripa M., Morata L., Rodríguez-Núñez O., Cardozo C., Puerta-Alcalde P., Hernández-Meneses M., Ambrosioni J., Linares L., Bodro M., Valcárcel A., et al. Short-Term Peripheral Venous Catheter-Related Bloodstream Infections: Evidence for Increasing Prevalence of Gram-Negative Microorganisms from a 25-Year Prospective Observational Study. Antimicrob. Agents Chemother. 2018;62:e00892-18. doi: 10.1128/AAC.00892-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mielke D., Wittig A., Teichgräber U. Peripherally Inserted Central Venous Catheter (PICC) in Outpatient and Inpatient Oncological Treatment. Support. Care Cancer. 2020;28:4753–4760. doi: 10.1007/s00520-019-05276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cotogni P., Barbero C., Garrino C., Degiorgis C., Mussa B., De Francesco A., Pittiruti M. Peripherally Inserted Central Catheters in Non-Hospitalized Cancer Patients: 5-Year Results of a Prospective Study. Support. Care Cancer. 2015;23:403–409. doi: 10.1007/s00520-014-2387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors consent to share the collected data with others. The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Data will be available immediately after the main publication and indefinitely.