Abstract
(1) Background: The objective of this analysis was to evaluate the device usage rates and patterns of use regarding Tumor-Treating Fields (TTFields) for patients with malignant pleural mesothelioma (MPM) throughout the US. (2) Methods: We evaluated de-identified data from 33 patients with MPM enrolled in FDA-required HDE protocols at 14 institutions across the US from September 2019 to March 2022. (3) Results: The median number of total TTFields usage days was 72 (range: 6–649 days), and the total treatment duration was 160 months for all patients. A low usage rate (defined as less than 6 h per day, 25%) was observed in 34 (21.2%) months. The median TTFields usage in the first 3 months was 12 h per day (range: 1.9–21.6 h), representing 50% (range: 8–90%) of the potential daily duration. The median TTFields usage after 3 months decreased to 9.1 h per day (range: 3.1–17 h), representing 38% (range: 13–71%) of the daily duration, and was lower than usage in the first 3 months (p = 0.01). (4) Conclusions: This study represents the first multicenter analysis of real-world TTFields usage based on usage patterns for MPM patients in clinical practice. The real-world usage level was lower than the suggested daily usage. Further initiatives and guidelines should be developed to evaluate the impact of this finding on tumor control.
Keywords: malignant pleural mesothelioma, real-world experience, tumor-treating fields (TTFields), usage
1. Introduction
Tumor-Treating Fields (TTFields) have emerged as a promising treatment approach for a variety of solid tumor malignancies [1,2,3,4,5]. TTFields function by delivering low-intensity alternating electric fields to tumor cells, disrupting their division and causing them to die [6]. The antimitotic effects of TTFields include the alignment of polar tubulin dimers and septin trimers with the electric field, which prevents proper mitosis. Additionally, the fields cause charged and polar molecules to accumulate at the cleavage furrow, which leads to abnormal mitosis and the formation of dysfunctional new cells. Preclinical studies have shown that human mesothelioma cells are highly responsive to TTFields at a frequency of 150 kHz, and when combined with certain chemotherapy agents, such as cisplatin and pemetrexed, a synergistic effect can be observed [7,8]. The single-arm phase 2 STELLAR study demonstrated the safety and preliminary efficacy of TTFields for the treatment of unresectable malignant pleural mesothelioma (MPM) with standard first-line pemetrexed and platinum-based chemotherapy [9]. This study reported a median overall survival (OS) of 18.2 months with modest device-related toxicity. In this prospective clinical study, a high median usage rate of TTFields in the first three months (16.3 h per day, which is 68% of the potential daily duration) was observed. Continued usage rates beyond the 3-month time point were not reported. Based on these results, TTFields therapy was made available for use under a Food-and-Drug-Administration (FDA)-approved Humanitarian Device Exemption (HDE) pathway in 2019 (H180002). The suggested device usage for patients is at least 18 h a day, which is 75% of the potential daily duration. However, in a recent real-world, single-institution study of five unresectable MPM patients treated with TTFields, median usage rates of 12.5 h per day (52%) for the first 3 months and 8.9 h per day (37%) after 3 months were reported [10]. Therefore, the objective of this analysis was to evaluate device usage rates and patterns of use throughout the United States (US) through a multi-institutional analysis and compare these with the usage rates and patterns for patients for the STELLAR trial.
2. Materials and Methods
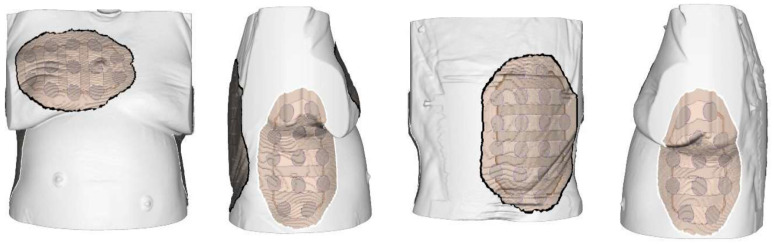
In this study, we evaluated data from 33 patients with histologically confirmed unresectable MPM enrolled in FDA-required HDE protocols at 14 institutions across the US from September 2019 to March 2022. For each patient, computed tomography (CT) scans of the chest were submitted to the device manufacturer (Novocure Ltd., Haifa, Israel) to generate a proposed layout of the arrays, which was ultimately approved by the treating physician prior to the application of TTFields according to the anatomical location of the disease and other clinical considerations (Figure 1). Patients and their caregivers were trained in the use of the device by at least one of the following: the treating physician, a designated health care provider (e.g., nurse), or a device technician (Device Support Specialist, DSS) trained by Novocure. All prescribing physicians underwent a training program and certification via Novocure. All patients were treated with a regimen of continuous TTFields at a frequency of 150 kHz [9]. The device was programmed to deliver the intended frequency in two sequential, perpendicular field directions at a maximal intensity of 1414 mA root mean square (RMS). Patients were advised to wear the device for a minimum of 18 h by the clinical team; breaks were allowed for personal needs (e.g., showering or array exchanges). Patients were instructed to replace the arrays approximately every 3 to 4 days with the help of a family member, caregiver, or DSS. During each replacement of the array, the set of arrays was moved approximately 2 cm from the previous position to ensure that the array discs were positioned in areas where there was no skin irritation. Shifting direction was determined by a trained professional (i.e., prescribing physician, designated healthcare provider, or DSS). The device logs for each patient were downloaded by the Novocure DSS every 4 weeks and compiled to assess patient usage with TTFields. No “dose” adjustments to the device were performed for adverse events. Treatment was continued until radiologic disease progression according to modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria for MPM [11]; unacceptable toxicity had been reached or clinical deterioration had occurred; the treated patient requested cessation; or death.
Figure 1.
A schematic illustration of TTFields arrays applied to the thoracic region for the treatment of malignant pleural mesothelioma.
Median and range values were used to describe continuous variables, while sample sizes and percentages were used to describe categorical variables. Comparisons of continuous variable distribution were performed using the nonparametric Mann–Whitney U test, for which values with p < 0.05 were considered significant. Statistical analyses were performed using SPSS version 27 (SPSS Inc., Chicago, IL, USA).
3. Results
Across all patients, the median number of total TTFields usage days was 72 (range: 6–649 days), and the total treatment duration was 160 months for all patients. A low usage rate (defined as less than 6 h per day, 25%) was observed in a total of 34 (21.2%) months. Four (12.1%) patients discontinued the treatment by the first month. The median TTFields device usage in the first three months was 12 h per day (range: 1.9–21.6 h), representing 50% (range: 8–90%) of the potential daily duration. Nineteen (57.5%) patients discontinued TTFields after three months. The median TTFields usage after three months decreased to 9.1 h per day (range: 3.1–17.0 h), representing 38% (range: 13–71%) of the daily duration, and was lower than usage in the first three months (p = 0.01). The overall usage was 10.3 h per day (range: 1.9–21.4 h), which was 43% (range: 8–89%) of the daily duration during all treatment courses. Three (9.1%) patients had a usage rate over 75% (manufacturer-suggested usage), and four (12.1%) patients had a usage rate over 68% (median usage rate in the STELLAR study). Six (18.2%) patients had a usage under the low usage rate.
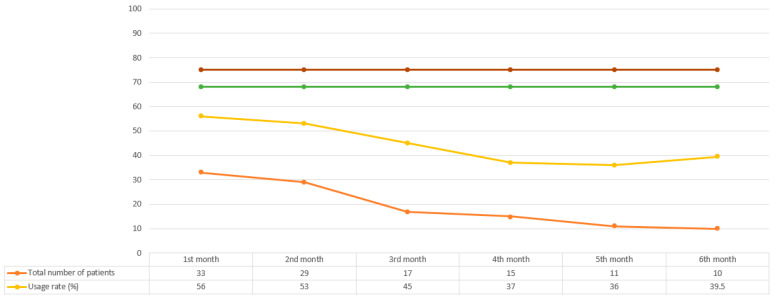
Significant differences were observed in usage across months, with the highest percentage of usage observed in the first treatment month at 56% (range: 8–90%) and the lowest percentage of usage observed in the last treatment month at 32% (range: 1–74%) (p < 0.05). We observed discontinuation for 23 (69.7%) patients, which most commonly occurred in the first three months. The median time of TTFields treatment was 2 months (range: 1–7 months) for the physicians who treated one to four patients and 5 months (range: 1–23 months) for the physicians who treated five or more patients (p = 0.037). However, the median usage rate per month did not differ according to the physicians’ experience (44.5% for the physicians who treated one to four patients vs. 42% for the physicians who treated more than four patients, p = 0.57). The average daily TTFields device usage percentages and the total number of patients receiving therapy by month in the first 6 months are shown in Figure 2.
Figure 2.
Average daily TTFields device usage percentages and the total number of patients receiving therapy by month in the first 6 months. The device usage rates recommended by the company and recorded by the STELLAR study are indicated by the brown and green lines, respectively.
4. Discussion
This study is the first multicenter analysis of the real-world usage of TTFields—an emerging treatment approach for several solid tumor malignancies—based on usage patterns for MPM patients in clinical practice. We observed that this is a feasible treatment strategy for appropriately selected patients. The median TTFields usage rate in patients with MPM was 43%, with a low usage rate observed in a significant proportion of the treatment duration. The highest usage rate was observed in the first treatment month, with a decline in usage observed over subsequent months. Our study also highlights the importance of a physician’s experience in determining the duration of TTFields treatment, with longer treatment durations observed among physicians who treated more patients. Additionally, the real-world usage level was lower than the suggested daily usage and the results reported in the prior STELLAR study. As we know, clinical trials are generally accepted as a standard approach for generating clinical evidence for the implementation of a new treatment method. However, real-world studies can better reflect the feasibility of a treatment and the clinical scenarios affecting treatment outcomes, such as patient demographics, comorbidities, compliance, toxicities, patients’ motivation, and concurrent treatments. Additionally, as seen in this study, usage after the first three months significantly declines; therefore, the long-term usage rates for those who are responding to treatment remain a concern.
Previous studies concerning the treatment of central nervous system (CNS) malignancies indicated a significant median OS advantage with daily TTFields usage rates of ≥75% versus <75% [1,12,13]. Regarding MPM, Ceresoli et al. reported a 68% device usage rate in the first three months of the phase 2 STELLAR study [9]. This study, on the other hand, showed a median 50% device usage rate during the first three months. In addition, this study also reported device usage rates beyond the initial 3-month period, which were even lower at 9.1 h per day (38% usage rate). Similar to this study, Rivera et al. found that the median usage rates of TTFields with gemcitabine alone and TTFields plus gemcitabine and nab-paclitaxel in pancreatic cancer patients were 58% and 51%, respectively [3]. Vergote et al. also reported a median usage rate of 58% in recurrent ovarian cancer patients during the first three months [4]. Additionally, a recent case series of MPM patients treated with TTFields in combination with pemetrexed and platin-based chemotherapy also showed a median usage rate of 52% for the first three months and 37% after these three months [10]. These findings highlight the need for strategies to improve patient adherence to TTFields therapy, especially after the first three months of treatment.
Understanding device usage rates and patterns of use is critical for assessing the feasibility and effectiveness of TTFields therapy in real-world clinical settings. Our study reflects the usage rates for the real-world implementation of TTFields therapy in the treatment of MPM patients and represents an opportunity for further study and evaluation. At this point, it is crucial to identify the reasons for decreasing usage that may help further inform strategies to address issues and improve device usage rates. In a recent study, one suggested possible strategy is a fractionation approach to allow time for the sublethal repair of normal epithelial cells [10]. Another potential strategy is to customize the treatment plan and array layouts that compute the fields’ intensity with respect to the different tumor volumes to maximize treatment according to the extent of the disease, even if delivered during shorter time intervals. Finally, we found that treatment continuity was higher for the patients who received treatment from more experienced physicians and healthcare teams. Interestingly, daily usage did not differ; however, this might be related to the experience of managing side effects from the device. To overcome this issue, guidelines have recently been proposed to help improve the management of device-related skin adverse events [14,15].
One of the limitations of our study is its retrospective nature, which means there was no standardization for prior treatments, such as chemotherapy and radiotherapy regimens, across all participants. Another limitation is that the number of patients enrolled in the HDE protocols over the two-year period was limited due to the rarity of MPM diagnoses and the specific indications for TTFields treatment. In addition, given the de-deidentified nature of the initial data, clinical outcomes and patterns of failure could not be evaluated in this study.
5. Conclusions
In conclusion, this real-world analysis provides valuable insights into the usage patterns and feasibility of TTFields therapy for MPM patients in clinical practice. While the results indicate that TTFields therapy is a feasible treatment strategy for appropriately selected patients, the declining usage rates after the first 3 months highlight the need for further evaluation and optimization of treatment strategies. Future research should focus on identifying factors that contribute to the declining usage rates, such as patient compliance and device-related toxicities, and developing interventions to address these factors, including educational resources for physicians.
Acknowledgments
Part of the results of this study were presented at the IASLC 2022 World Conference on Lung Cancer in Vienna, Austria on 6–9 August 2022.
Author Contributions
Conceptualization, methodology, writing—original draft preparation and writing—review and editing: T.K., J.M.W., M.T.B., R.B.C., J.B.A., S.C., E.L., D.B., A.D.P., N.M., T.R., A.S., C.S., J.Z. and R.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All data were collected under Institutional Review Board of Miami Cancer Institute (IRB protocol code 1855079-1) for retrospective data analysis.
Informed Consent Statement
Informed consent was waived due to de-identification of the dataset and lack of any PHI used.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflicts of Interest
Matthew T. Ballo: Consultant for Novocure, LLC, and ZaiLab. Deepti Behl: Research support—Natera; served on advisory boards for Merck, AstraZeneca, Novartis, Eli Lilly, Janssen, and Oncocyte; speaker’s bureau—Janssen and Guardant. Jun Zhang: JZ has served as a scientific advisor/consultant for AstraZeneca, Biodesix, Novocure, Bayer, Daiichi Sankyo, Hengrui, Eli Lilly, Mirati, Novartis, Cardinal Health, Bristol-Myers Squibb, Nexus Health, Takeda, Regeneron, and Sanofi. JZ is on the speakers’ bureau for AstraZeneca, Regeneron, Sanofi, and MJH Life Sciences. JZ has also received research funding/support from AstraZeneca, Biodesix, Novartis, Genentech/Roche, Mirati, AbbVie, Hengrui, BeiGene, InnoCare Pharma, and Nilogen. Rupesh Kotecha: Honoraria from Accuray Inc., Elekta AB, ViewRay Inc., Novocure Inc., Elsevier Inc., and Brainlab, Kazia Therapeutics. Institutional research funding was provided by Medtronic Inc., Blue Earth Diagnostics Ltd., Novocure Inc., GT Medical Technologies, AstraZeneca, Exelixis, ViewRay Inc., Brainlab, Kazia Therapeutics, and Cantex Pharmaceuticals.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Toms S.A., Kim C.Y., Nicholas G., Ram Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: A subgroup analysis of the EF-14 phase III trial. J. Neurooncol. 2019;141:467–473. doi: 10.1007/s11060-018-03057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R., Taillibert S., Kanner A., Read W., Steinberg D., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivera F., Benavides M., Gallego J., Guillen-Ponce C., Lopez-Martin J., Kung M. Tumor treating fields in combination with gemcitabine or gemcitabine plus nab-paclitaxel in pancreatic cancer: Results of the PANOVA phase 2 study. Pancreatology. 2019;19:64–72. doi: 10.1016/j.pan.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Vergote I., von Moos R., Manso L., Van Nieuwenhuysen E., Concin N., Sessa C. Tumor Treating Fields in combination with paclitaxel in recurrent ovarian carcinoma: Results of the INNOVATE pilot study. Gynecol. Oncol. 2018;150:471–477. doi: 10.1016/j.ygyno.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Pless M., Droege C., von Moos R., Salzberg M., Betticher D. A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer. 2013;81:445–450. doi: 10.1016/j.lungcan.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Kirson E.D., Gurvich Z., Schneiderman R., Dekel E., Itzhaki A., Wasserman Y., Schatzberger R., Palti Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–3295. doi: 10.1158/0008-5472.CAN-04-0083. [DOI] [PubMed] [Google Scholar]
- 7.Giladi M., Weinberg U., Schneiderman R.S., Porat Y., Munster M., Voloshin T., Blatt R., Cahal S., Itzhaki A., Onn A., et al. Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin. Oncol. 2014;41((Suppl. S6)):S35–S41. doi: 10.1053/j.seminoncol.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Mumblat H., Martinez-Conde A., Braten O., Munster M., Dor-On E., Schneiderman R.S., Porat Y., Voloshin T., Davidi S., Blatt R., et al. Tumor Treating Fields (TTFields) downregulate the Fanconi Anemia-BRCA pathway and increase the efficacy of chemotherapy in malignant pleural mesothelioma preclinical models. Lung Cancer. 2021;160:99–110. doi: 10.1016/j.lungcan.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Ceresoli G.L., Aerts J.G., Dziadziuszko R., Ramlau R., Cedres S., van Meerbeeck J.P., Mencoboni M., Planchard D., Chella A., Crino L., et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): A multicentre, single-arm phase 2 trial. Lancet Oncol. 2019;20:1702–1709. doi: 10.1016/S1470-2045(19)30532-7. [DOI] [PubMed] [Google Scholar]
- 10.Kutuk T., Appel H., Avendano M.C., Albrecht F., Kaywin P., Ramos S., Suarez-Murias M.E., Mehta M.P., Kotecha R. Feasibility of Tumor Treating Fields with Pemetrexed and Platinum-Based Chemotherapy for Unresectable Malignant Pleural Mesothelioma: Single-Center, Real-World Data. Cancers. 2022;14:2020. doi: 10.3390/cancers14082020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne M.J., Nowak A.K. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann. Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 12.Mrugala M.M., Engelhard H.H., Dinh Tran D., Kew Y., Cavaliere R., Villano J.L., Annenelie Bota D., Rudnick J., Love Sumrall A., Zhu J.J., et al. Clinical practice experience with NovoTTF-100A system for glioblastoma: The Patient Registry Dataset (PRiDe) Semin. Oncol. 2014;41((Suppl. S6)):S4–S13. doi: 10.1053/j.seminoncol.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Kanner A.A., Wong E.T., Villano J.L., Ram Z., Investigators E.F. Post Hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A system versus best physician’s choice chemotherapy. Semin. Oncol. 2014;41((Suppl. S6)):S25–S34. doi: 10.1053/j.seminoncol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Lacouture M.E., Anadkat M.J., Ballo M.T., Iwamoto F., Jeyapalan S.A., La Rocca R.V., Schwartz M., Serventi J.N., Glas M. Prevention and Management of Dermatologic Adverse Events Associated with Tumor Treating Fields in Patients with Glioblastoma. Front. Oncol. 2020;10:1045. doi: 10.3389/fonc.2020.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anadkat M.J., Lacouture M., Friedman A., Horne Z.D., Jung J., Kaffenberger B., Kalmadi S., Ovington L., Kotecha R., Abdullah H.I., et al. Expert guidance on prophylaxis and treatment of dermatologic adverse events with Tumor Treating Fields (TTFields) therapy in the thoracic region. Front. Oncol. 2022;12:975473. doi: 10.3389/fonc.2022.975473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.