Abstract
A universal calibrator for the determination of all anti-Xa inhibitors would support laboratory processes. We aimed to test the clinical performance of an anti-Xa assay utilizing a universal edoxaban calibrator to determine clinically relevant concentrations of all anti-Xa inhibitors. Following a pilot study, we enrolled 553 consecutive patients taking rivaroxaban, edoxaban, or apixaban from nine study centers in a prospective cross-sectional study. The Technochrom® anti-Xa assay was conducted using the Technoview® edoxaban calibrator. Using ultra-high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS), anti-Xa inhibitor drug concentrations were determined. Sensitivities and specificities to detect three clinically relevant drug concentrations (30 µgL−1, 50 µgL−1, 100 µgL−1) were determined. Overall, 300 patients treated with rivaroxaban, 221 with apixaban, and 32 with edoxaban were included. The overall correlation coefficient (rs) was 0.95 (95% CI 0.94, 0.96). An area under the receiver operating characteristic curve of 0.96 for 30 µgL−1, 0.98 for 50 µgL−1, and 0.99 for 100 µgL−1 was found. The sensitivities were 92.3% (95% CI 89.2, 94.6), 92.7% (89.4, 95.1), and 94.8% (91.1, 97.0), respectively (specificities 82.2%, 93.7%, and 94.4%). In conclusion, the clinical performance of a universal, edoxaban-calibrated anti-Xa assay was solid and most drug concentrations were predicted correctly.
Keywords: diagnostic accuracy, anti-Xa assay, laboratory monitoring, rivaroxaban, edoxaban, apixaban, edoxaban, anticoagulants introduction
1. Introduction
A growing population of patients using direct oral anticoagulants (DOAC) are subject to critical situations, including major bleeding, urgent surgery, and planned thrombolysis [1,2,3,4,5,6]. In these situations, the risk of significant bleeding and subsequent complications is high, and relevant drug concentrations will increase this risk even further [7]. Therefore, knowledge of DOAC drug concentrations supports physicians on whether or not reversal agents should be given [8]. Moreover, the time point of surgical or other interventions can be planned [7,8]. Notably, a decision can be made as to whether or not acute thrombolysis can be conducted safely [9,10,11]. Furthermore, the determination of drug levels is also important to detect accumulation in case of renal or hepatic failure or accidental poisoning [12,13]. Furthermore, in specific populations, such as patients with renal impairment, there might potentially be an application to monitor DOAC and adjust dosages [13]. Thus, the accurate and rapid measurement of DOAC drug levels supports the management of patients in various clinical situations [1].
To be useful in daily clinical practice, laboratory tests must be rapidly available. However, standard hemostasis tests such as activated partial thromboplastin time or prothrombin time are not sensitive [14,15]. High-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) detects DOAC drug levels with high accuracy, but this test is not available in routine clinical practice [16]. Chromogenic anti-Xa assays, commonly used to monitor unfractionated and low-molecular-weight heparin, also demonstrated high accuracy and good reproducibility when measuring rivaroxaban, edoxaban, and apixaban. [17]. However, widespread implementation is hampered by elaborate laboratory procedures: separate calibration and quality controls are necessary for rivaroxaban, edoxaban, and apixaban. A low-molecular-weight heparin calibration was suggested as a universal calibrator to solve this problem [16,17]. Recently, we studied unfractionated heparin as a potential calibrator in this situation [18]. The disadvantage of this approach is that results are not directly given in the correct unit (mg L−1). Preliminary data from a pilot study suggest that a universal edoxaban calibration would determine drug levels of all three anti-Xa inhibitors with high accuracy [19].
We conducted a prospective, multicenter study in routine clinical practice to assess the diagnostic accuracy of an anti-Xa assay utilizing a universal edoxaban calibrator to determine critical plasma concentrations of all anti-Xa inhibitors (rivaroxaban, edoxaban, and apixaban). This study therefore represents a proof-of-principle study.
2. Methods
2.1. Study Design, Setting, and Population
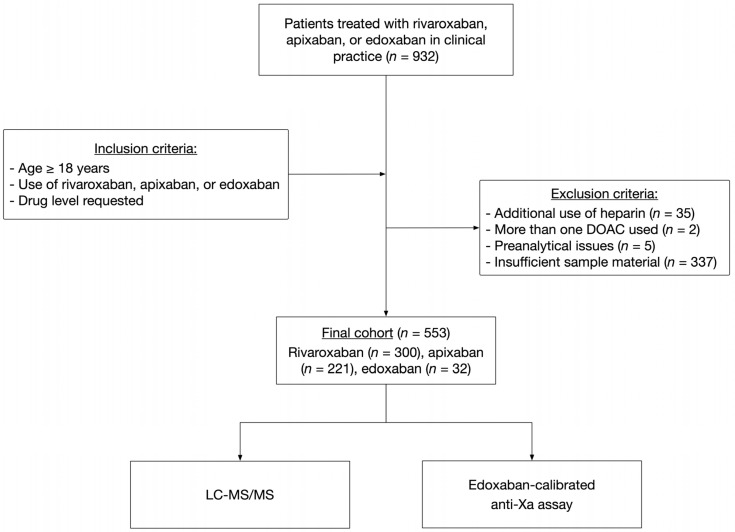
Following a pilot study using spiked samples, we conducted a multicenter cross-sectional study, enrolling consecutive patients from 9 Swiss tertiary hospitals [16,20]. The following inclusion criteria were applied: (a) anticoagulant treatment with either rivaroxaban, edoxaban, or apixaban, (b) drug concentration ordered, (c) age > 18 years, and (d) signed general informed consent if requested by the local authorities. Exclusion criteria were (a) refused informed consent, (b) heparin use, (c) preanalytical problems such as hemolytic samples, (d) more than one drug detected, and (e) not enough sample material. To cover the full range of drug concentrations in clinical practice, samples were collected regardless of the time of last drug consumption. Figure 1 illustrates the study design (CONSORT flow diagram). On all samples, ultra-high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) was conducted as a reference standard. [16]. We followed previous recommendations to define the clinically relevant drug concentrations: 30 mgL−1, 50 mgL−1, and 100 mgL−1 [1]. The study was approved by the ethical committees of all institutions. The study was conducted in accordance with the declaration of Helsinki.
Figure 1.
The flow of the patients.
2.2. Pilot Study
Commercially available calibrators for the individual DOACs (Technoclone, Vienna, Austria) were tested in the anti-Xa assay using three different sample pre-dilutions (1:5, 1:10, and 1:20 in assay dilution buffer). All calibrators had known concentrations of the respective DOAC, which was traceable to the LC-MS/MS method of the developing company. In a second set of experiments, using the calibrators for apixaban and rivaroxaban, the edoxaban-containing calibrators were assigned values for the other DOACs. Using these assigned values, an anti-Xa calibration curve was established for apixaban and rivaroxaban using the edoxaban calibrators. Commercially available DOAC-specific controls were tested using the calibrated anti-Xa assay.
2.3. Data Collection and Handling of Samples
All data were anonymized and collected in a secured RedCAP database. The following data were recorded: sex, age, and drug used. To ensure appropriate pre-analytic conditions, detailed protocols were put in place at all institutions [21]. Venous blood samples were drawn in plastic syringes containing 1 ml trisodium citrate (0.106 mol L−1) per mL of blood. To avoid contamination with tissue factor, the citrated samples were collected after obtaining syringes containing different anticoagulants. The application of tourniquet was minimized and removed before transport and processing. Aliquots were frozen at −80 °C, kept frozen during a delivery time of 3 to 4 h (on dry ice) to Inselspital, central laboratory, and kept continuously without any freeze–thaw cycle. The samples were stored for 1 to 13 months before analysis.
2.4. Determination of the Edoxaban-Calibrated Anti-Xa Assay
Following the pilot study mentioned above, we selected the TECHNOCHROM® anti-Xa assay using an edoxaban calibrator (Technoclone, Vienna, Austria). The Ceveron® s100 analyzer was applied (Technoclone, Vienna, Austria). Samples were analyzed immediately after thawing (15 min; 37 °C) in a 1:20 dilution following the manufacturer’s instructions. Briefly, reagent 2 (bovine factor Xa) and reagent 3 (chromogenic substrate) were reconstituted separately in 4 mL distilled water and preheated to 37 °C. Reagent 1 consisted of 20 mL anti-Xa buffer and TRIS-EDTA buffer (pH 8.4). The samples were incubated in a cuvette of the analyzer kept at 37 °C (50 μL of the diluted sample, 50 μL of bovine factor Xa, 50 μL of Xa substrate). The kinetic absorbance was measured at 405 nm. Neither clinical information nor LC-MS/MS results were known to the performer of the anti-Xa assay.
2.5. Determination of LC-MS/MS
To quantify the concentrations of all anti-Xa inhibitors (apixaban, rivaroxaban, and edoxaban—including edoxaban metabolite M4), ultra-high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) was conducted as a reference standard. The analytical procedures have been described previously [16,18,20]. Briefly, for analyte extraction and protein precipitation, 10 µL acetonitrile/water 1:1 (v/v), 25 µL extraction buffer (MassTox TDM Series A, Chromsystems Instruments & Chemicals GmbH, Gräfelfing, Germany), and 240 µL precipitation reagent (MassTox TDM Series A, Chromsystems Instruments & Chemicals GmbH, Gräfelfing, Germany) including the internal standards (apixaban 13C,D3, rivaroxaban 13C6, edoxaban 13C,D2) were added to 50 µL plasma. After vortexing, the samples were centrifuged at 14,000× g rcf and 20 °C for 4 min. An amount of 20 µL of the supernatant material was diluted with 80 µL of water/methanol 8:2 (v/v) and stored at 10 °C. Pooled plasma was used to prepare calibrators and QC (Innovative Research, Novi, MI, USA). Three microliters of the specimen were measured by reversed-phase chromatography (Cortecs UPLC C18 column, 2.1 × 75 mm, 1.7 µm, Waters, Milford, MA, USA) on a triple quadrupole mass spectrometer (Xevo TQ-S, Waters) coupled to a UPLC Acquity I-Class system (Waters). Rivaroxaban, apixaban, edoxaban, and edoxaban M4 were separated at 0.4 mL/min with a gradient using water (A) and methanol (B) acidified with 0.1% (v/v) formic acid (mobiles phase) (0.0–0.5 min; 20% B; 0.5–2.5 min, 20–99% B; 2.5–3.5 min, 99% B; 3.5–3.51 min, 99–20% B; 3.51–4.5 min, 20% B). We optimized source offset and transition parameters for each analyte. TargetLynx of the MassLynx software was used to process the raw data (version 4.1, Waters). For edoxaban analysis, edoxaban M4 metabolites were added to edoxaban. Apixaban, rivaroxaban, and edoxaban pure substances were provided by Bristol Myers Squibb Company (New Brunswick, NJ, USA), Bayer AG (Wuppertal, Germany), and Daichi Sankyo Co, Ltd. (Tokyo, Japan). The LC-MS/MS assay was validated according to international guidelines and fulfilled their acceptance criteria [22,23]. Inter-/intra-day imprecision and inaccuracy are given in Table S1 of the Supplemental Materials. Clinical information and index test results were not available to the performer of LC-MS/MS.
2.6. Statistical Analysis
Descriptive statistics (numbers/percentages or median/range) were used as appropriate. Spearman correlation coefficients (rs), Deming regressions, and Bland and Altman difference plots were used to describe the relationship between anti-Xa measurements and drug concentrations (LC-MS/MS). Correlation coefficients of 0.9 were considered accurate, and those of 0.6 were considered inadequate. Cut-off levels of the edoxaban-calibrated anti-Xa assay in relation to clinically relevant drug concentrations (30 mgL−1, 50 mgL−1, and 100 mgL−1) were established by receiver operating characteristics curve analysis (ROC). Two-by-two tables were created, and sensitivities and specificities were calculated. Analyses were performed using Stata 14.1 (Stata-Corp. 2015. Stata Statistical Software: Release 14. Stata-Corp LP, College Station, TX, USA). Prism 8 was used to generate all figures (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Pilot Study
When plotting the raw data from calibrators for apixaban, edoxaban, and rivaroxaban in an anti-Xa assay, very similar patterns could be observed. Using different sample pre-dilutions typically employed in anti-Xa assays, the different DOACs behaved similarly, exhibiting a steep discrepancy between 0 and approx. 100 µgL−1 using a low sample dilution (e.g., 1:5) and a broad variation between 50 and 500 µgL−1 using a high sample dilution (e.g., 1:20).
In a small calibration exercise, ten samples of the edoxaban calibrator set were assigned values for apixaban and rivaroxaban using a calibrated anti-Xa assay (Table 1).
Table 1.
Results of a pilot study assigning values for apixaban and rivaroxaban to an edoxaban calibrator set.
| Calibrator | Apixaban (µgL−1) | Edoxaban (µgL−1) | Rivaroxaban (µgL−1) |
|---|---|---|---|
| CAL 1 | 0 | 0 | 0 |
| CAL 2 | 26 | 50 | 38 |
| CAL 3 | 119 | 144 | 124 |
| CAL 4 | 255 | 316 | 234 |
| CAL 5 | 447 | 506 | 385 |
Then, using these newly assigned values, DOAC-specific calibration curves were generated, and quality control samples for apixaban and rivaroxaban were measured. All controls recovered within the acceptable ranges from the manufacturer.
3.2. Patient Characteristics
Eventually, 932 patients were included from nine study centers (Figure 1). We excluded 375 for several reasons: additional application of unfractionated or low-molecular-weight heparin (n = 35), more than one drug was detected (n = 2), pre-analytical interfering factors (n = 5), and not enough specimen material (n = 337). Overall, 553 were considered for the present analysis. In total, 221 patients were treated with apixban (40.0%), 300 with rivaroxaban (54.3%), and 32 with edoxaban (5.7%). The median age was 74 years (IQR 63 to 83), and 40.1% of the individuals were female. Detailed characteristics can be found in Table 2.
Table 2.
Patient characteristics (n = 553).
| Rivaroxaban | Apixaban | Edoxaban | All | |
|---|---|---|---|---|
| Patients (n/%) | 300 (54.3) | 221 (40.0) | 32 (5.7) | 553 (100) |
| Age (median/IQR) | 74 (63–83) | 78 (67–82) | 75 (58–81) | 76 (65–82) |
| Female sex (n/%) | 126 (40.1) | 86 (39.8) | 10 (32.3) | 222 (40.1) |
3.3. Accuracy of the Edoxaban-Calibrated Anti-Xa Assay
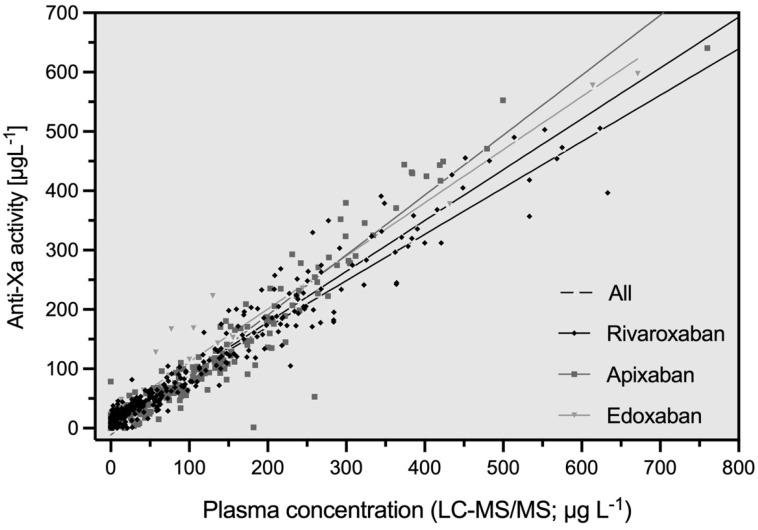
The associations between edoxaban-calibrated anti-Xa results and apixaban, rivaroxaban, and edoxaban drug concentrations are shown in Figure 2. The correlation coefficient (rs) of all measurements was 0.94 (95% confidence interval [CI] 0.93, 0.95). It was 0.95 for individuals treated with rivaroxaban (95% CI 0.94, 0.96), 0.93 with apixaban (95% CI 0.91, 0.95), and 0.93 with edoxaban (95% CI 0.86, 0.97). The coefficients of the regression equations (slope and Y-intercept) are reported in Table 3.
Figure 2.
Scattergram showing the association between edoxaban-calibrated anti-Xa activity and the measurements of LC-MS/MS by drug. The overall correlation (rs) was 0.94 (95% CI 0.93, 0.95). It was 0.95 for rivaroxaban (95% CI 0.94, 0.96), 0.93 for apixaban (95% CI 0.91, 0.95), and 0.93 for edoxaban (95% CI 0.86, 0.97).
Table 3.
Accuracy of an edoxaban-calibrated anti-Xa assay for measuring all anti-Xa inhibitors: apixaban, rivaroxaban, and edoxaban. Patients treated in clinical practice were enrolled in a multicenter cross-sectional study (n = 553). An LC-MS/MS analysis was performed as a reference standard.
| Rivaroxaban | Apixaban | Edoxaban | All | |
|---|---|---|---|---|
| Spearman’s correlation coefficient (95% CI) | 0.95 (0.94, 0.96) |
0.93 (0.91, 0.95) |
0.93 (0.86, 0.97) |
0.94 (0.93, 0.95) |
|
Deming regression slope (95% CI) |
0.78 (0.70, 0.87) |
1.01 (0.94, 1.08) |
0.90 (0.83, 0.96) |
0.86 (0.79, 0.92) |
| Y-Intercept | 14.08 (6.28, 21.87) |
−11.39 (−19.25, −3.53) |
21.73 (10.69, 32.77) |
6.97 (0.74, 13.19) |
3.4. Detection of Clinically Significant Drug Levels
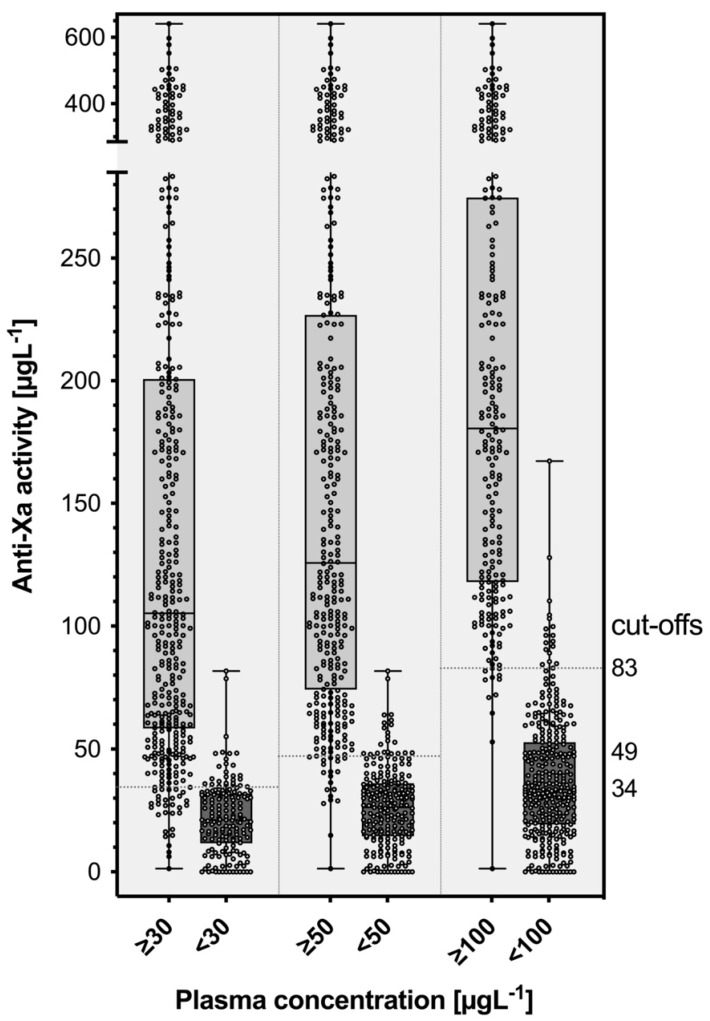
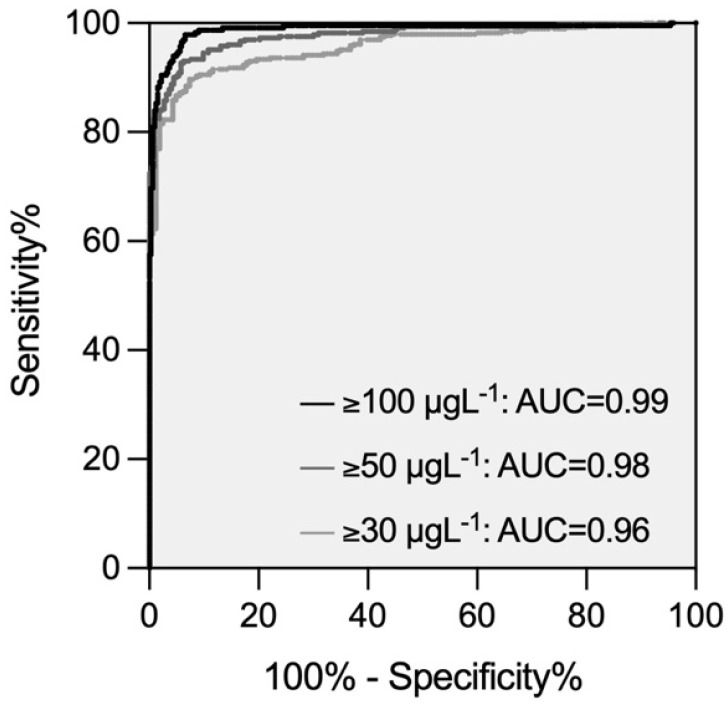
Figure 3 shows the distribution of edoxaban-calibrated anti-Xa measurements in individuals with and without clinically relevant drug concentrations (30 µgL−1, 50 µgL−1, and 100 µgL−1, respectively). The optimal cut-off values derived from receiver operating characteristics (ROC) analysis were 34 µg/L−1 for 30 µgL−1, 49 µg/L−1 for 50 µgL−1, and 83 µg/L−1 for 100 µgL−1. The performance in terms of areas under the ROC curve was 0.96, 0.98, and 0.99, respectively (Figure 4). The edoxaban-calibrated anti-Xa assay detected clinically relevant drug concentrations with sensitivities of 92.3% (30 µgL−1; 95% CI 89.2, 94.6), 92.7% (50 µgL−1; 95% CI 89.4, 95.1), and 94.8% (100 µgL−1; 95% CI 91.1, 97.0). The specificity was 82.2% (95% CI 75.6, 87.3), 93.7% (95% CI 89.7, 96.2), and 94.4% (95% CI 91.3, 96.4).
Figure 3.
Distribution of edoxaban-calibrated anti-Xa measurements in patients with and without clinically relevant drug concentrations (30 µgL−1, 50 µgL−1, 100 µgL−1). The cut-off thresholds were determined using a receiver operating characteristics curve (ROC) analysis. The sensitivities were 92.3% (30 µgL−1; 95% CI 89.2, 94.6), 92.7% (50 µgL−1; 95% CI 89.4, 95.1), and 94.8% (100 µgL−1; 95% CI 91.1, 97.0). The specificities were 82.2% (95% CI 75.6, 87.3), 93.7% (95% CI 89.7, 96.2), and 94.4% (95% CI 91.3, 96.4).
Figure 4.
Receiver operating characteristics curves demonstrating the diagnostic accuracy of an edoxaban-calibrated anti-Xa assay for the prediction of clinically relevant drug concentrations (30 µgL−1, 50 µgL−1, 100 µgL−1).
4. Discussion
Using a specimen of 553 patients enrolled in a large prospective study, we assessed the clinical performance of an edoxaban-calibrated anti-Xa assay to determine critical apixaban, rivaroxaban, or edoxaban drug concentrations in clinical practice. All drugs showed solid accuracy concerning correlation coefficients (rs) and areas under ROC curves. In addition, clinically relevant drug concentrations were captured with a high sensitivity and specificity.
These results align with previous studies of various study designs using other reagents. In different analyses of the same study, we demonstrated recently that both a low-molecular-weight heparin-calibrated anti-Xa assay [16] and an assay calibrated to unfractionated heparin can accurately measure apixaban, rivaroxaban, and edoxaban drug concentrations [18]. High accuracy and consistency of a rivaroxaban-calibrated anti-Xa assay with rivaroxaban plasma concentrations was demonstrated in 20 healthy individuals [17]. A cross-sectional study analyzing samples of 30 patients taking rivaroxaban showed a high correlation between LC-MS/MS and heparin anti-factor Xa activity [24]. A strong correlation between heparin-calibrated anti-Xa activity and LC-MS/MS measurements was also found in a retrospective cohort of 24 patients taking rivaroxaban or apixaban [25]. Moreover, 241 left-over specimens were used to determine the correlation between UFH-calibrated and rivaroxaban-/apixaban-calibrated anti-Xa measurements, concluding that there was a high correlation. Several additional studies assessed anti-Xa results’ association with LC-MS/MS measurements in spiked samples [26,27,28,29].
An important strength of our investigation was the large number of patients analyzed, which increased precision and statistical power. As a multicenter study conducted in clinical practice, the study adequately reflects the potential clinical application of the test. LC-MS/MS was also used as a reference standard, considered the most appropriate method for measuring DOAC [16,30]. However, our study is associated with limitations as well. First, only one edoxaban-calibrated reagent was tested, and we cannot be sure that other assays behave similarly. Thus, our results must be repeated in other settings using other reagents as well. In addition, fewer individuals received edoxaban treatment compared to rivaroxaban and edoxaban. We believe that the large sample size compensated for this, however.
Our results suggest that an edoxaban-calibrated anti-Xa assay is a feasible laboratory test to detect clinically relevant DOAC concentrations in clinical practice. The most straightforward way to apply this test in clinical practice would be to use the above-mentioned cut-off values of 30, 50, and 100 µgL−1. As an alternative, the actual values could also be calculated with the help of regression equations. However, caution must be taken here, as there is a certain degree of dispersion. Of note, other reagents and tests must be evaluated in additional studies before implementation. One might argue that the edoxaban-calibrated anti-Xa test could also be used to monitor DOAC treatment. However, this is a controversial topic, and no clinical guideline currently recommends this outside of clinical trials. The present study aimed to assess the diagnostic accuracy of an anti-Xa assay with regard to clinically relevant cut-off concentrations, that support decision making with regard to (a) reversal agents in case of acute bleeding, (b) scheduling urgent surgery, and (c) thrombolysis in case of acute stroke. We believe that the current study does not support the monitoring of DOAC with the help of uni-calibrated anti-Xa assays.
5. Conclusions
In a large prospective clinical study including 553 patients treated in real-life clinical practice, the accuracy of an edoxaban-calibrated anti-Xa assay with apixaban, rivaroxaban, and edoxaban drug levels was solid and most clinically relevant drug concentrations were predicted correctly. However, the accuracy of other edoxaban-calibrated reagents must be confirmed in future studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics13122128/s1, Table S1: Intra- and inter-day (n = 6) imprecision (CV) and inaccuracy (bias) for the different analytes (LC-MS/MS).
Author Contributions
Conceptualization, M.N., C.B. and N.B.B.; methodology, M.N., C.B. and N.B.B.; formal analysis, A.B. and M.N.; data curation, J.-D.S., A.M., L.A., P.F., W.A.W., A.S., L.G., C.B., T.C.S. and N.B.B.; resources, M.N., writing—original draft, A.B., N.B.B., C.B. and M.N.; writing—review and editing, A.B., J.-D.S., A.M., L.A., P.F., W.A.W., A.S., L.G., B.G., C.B., T.C.S., N.B.B. and M.N.; visualization, M.N.; supervision, M.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee (Kantonale Ethikkommission Bern (2017-00919; 28 June 2017).
Informed Consent Statement
General informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset can be requested from the authors upon reasonable request.
Conflicts of Interest
MN reports research grants from Bayer Healthcare, outside of the submitted work, lecture honoraria from Bayer Healthcare, and Daiichi Sankyo. LA reports research grants from Bayer, CSL-Behring, Novartis, Novo Nordisk, Roche, Sobi, and Takeda. WAW reports research grants from Bayer Healthcare, BMS-Pfizer, Daiichi Sankyo, and Sanofi, and honoraria for participating in scientific advisory boards from Bayer, Pfizer, and from Alexion Pharma GmbH, all outside the submitted work. JDS reports lecture fees and advisory honoraria from Bayer Healthcare, Pfizer, Takeda, Siemens, and Sanofi. TCS holds an endowed professorship supported by the Touring Club Switzerland. BG reports non-financial support and funding for an accredited continuing medical education program from Axonlab, and from Thermo Fisher Scientific, during the conduct of the study; personal fees and funding for an accredited continuing medical education program from Alnylam; grants, personal fees and funding for accredited continuing medical education program from Pfizer; funding for an accredited continuing medical education program from Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Takeda, Octapharma, SOBI, Janssen, Novo Nordisk, and Mitsubishi Tanabe Pharma, outside the submitted work. NBB is an employee and stakeholder of Technoclone.
Funding Statement
The study was supported by a research grant of the Research Fund Haematology Cantonal Hospital Lucerne. MN was supported by a research grant of the Swiss National Science Foundation (#179334). Implementation of the LC-MS/MS measurements was supported by the Gottfried & Julia Bangerter-Rhyner-Stiftung. The edoxaban calibrator was provided by Technoclone.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Levy J.H., Ageno W., Chan N.C., Crowther M., Verhamme P., Weitz J.I. for the Subcommittee on Control of Anticoagulation. When and how to use antidotes for the reversal of direct oral anticoagulants: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016;14:623–627. doi: 10.1111/jth.13227. [DOI] [PubMed] [Google Scholar]
- 2.Cuker A., Siegal D. Monitoring and reversal of direct oral anticoagulants. Hematology/the Education Program of the American Society of Hematology. Am. Soc. Hematol. Educ. Program. 2015;2015:117–124. doi: 10.1182/asheducation.V2015.1.117.3916182. [DOI] [PubMed] [Google Scholar]
- 3.Adcock D.M., Gosselin R. Direct Oral Anticoagulants (DOACs) in the Laboratory: 2015 Review. Thromb. Res. 2015;136:7–12. doi: 10.1016/j.thromres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Cuker A., Burnett A., Triller D., Crowther M., Ansell J., Van Cott E.M., Wirth D., Kaatz S. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am. J. Hematol. 2019;94:697–709. doi: 10.1002/ajh.25475. [DOI] [PubMed] [Google Scholar]
- 5.Chan N., Sobieraj-Teague M., Eikelboom J.W. Direct oral anticoagulants: Evidence and unresolved issues. Lancet. 2020;396:1767–1776. doi: 10.1016/S0140-6736(20)32439-9. [DOI] [PubMed] [Google Scholar]
- 6.Douxfils J., Adcock D.M., Bates S.M., Favaloro E.J., Gouin-Thibault I., Guillermo C., Kawai Y., Lindhoff-Last E., Kitchen S., Gosselin R.C. 2021 Update of the International Council for Standardization in Haematology Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb. Haemost. 2021;121:1008–1020. doi: 10.1055/a-1450-8178. [DOI] [PubMed] [Google Scholar]
- 7.Sauter T.C., Eberle B., Wuillemin W.A., Thiele T., Angelillo-Scherrer A., Exadaktylos A.K., Erdoes G., Cuker A., Nagler M. How I manage patients with anticoagulation-associated bleeding or urgent surgery. Swiss. Med. Wkly. 2018;148:w14598. doi: 10.4414/smw.2018.14598. [DOI] [PubMed] [Google Scholar]
- 8.Pernod G., Albaladejo P., Godier A., Samama C.M., Susen S., Gruel Y., Blais N., Fontana P., Cohen A., Llau J.V., et al. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: Proposals of the working group on perioperative haemostasis (GIHP)—March 2013. Arch. Cardiovasc. Dis. 2013;106:382–393. doi: 10.1016/j.acvd.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Seiffge D.J., Wilson D., Wu T.Y. Administering Thrombolysis for Acute Ischemic Stroke in Patients Taking Direct Oral Anticoagulants: To Treat or How to Treat. JAMA Neurol. 2021;78:515–516. doi: 10.1001/jamaneurol.2021.0287. [DOI] [PubMed] [Google Scholar]
- 10.Touze E., Gruel Y., Gouin-Thibault I., De Maistre E., Susen S., Sie P., Derex L. Intravenous thrombolysis for acute ischaemic stroke in patients on direct oral anticoagulants. Eur. J. Neurol. 2018;25:747-e52. doi: 10.1111/ene.13582. [DOI] [PubMed] [Google Scholar]
- 11.Shahjouei S., Tsivgoulis G., Goyal N., Sadighi A., Mowla A., Wang M., Seiffge D.J., Zand R. Safety of Intravenous Thrombolysis Among Patients Taking Direct Oral Anticoagulants: A Systematic Review and Meta-Analysis. Stroke J. Cereb. Circ. 2020;51:533–541. doi: 10.1161/STROKEAHA.119.026426. [DOI] [PubMed] [Google Scholar]
- 12.Lindhoff-Last E. Direct oral anticoagulants (DOAC)—Management of emergency situations. Hamostaseologie. 2017;37:257–266. doi: 10.5482/HAMO-16-11-0043. [DOI] [PubMed] [Google Scholar]
- 13.Grandone E., Aucella F., Barcellona D., Brunori G., Forneris G., Gresele P., Marietta M., Poli D., Testa S., Tripodi A., et al. Position paper on the safety/efficacy profile of Direct Oral Anticoagulants in patients with Chronic Kidney Disease: Consensus document of Societa Italiana di Nefrologia (SIN), Federazione Centri per la diagnosi della trombosi e la Sorveglianza delle terapie Antitrombotiche (FCSA) and Societa Italiana per lo Studio dell’Emostasi e della Trombosi (SISET) J. Nephrol. 2021;34:31–38. doi: 10.1007/s40620-020-00768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett Y.C., Wang Z., Frost C., Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: Anti-Xa assay is preferable to prothrombin time assay. Thromb. Haemost. 2010;104:1263–1271. doi: 10.1160/TH10-05-0328. [DOI] [PubMed] [Google Scholar]
- 15.Fontana P., Alberio L., Angelillo-Scherrer A., Asmis L.M., Korte W., Mendez A., Schmid P., Stricker H., Studt J.D., Tsakiris D.A., et al. Impact of rivaroxaban on point-of-care assays. Thromb. Res. 2017;153:65–70. doi: 10.1016/j.thromres.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Willekens G., Studt J.D., Mendez A., Alberio L., Fontana P., Wuillemin W.A., Schmidt A., Graf L., Gerber B., Bovet C., et al. A universal anti-Xa assay for rivaroxaban, apixaban, and edoxaban measurements: Method validation, diagnostic accuracy and external validation. Br. J. Haematol. 2021;193:1203–1212. doi: 10.1111/bjh.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studt J.D., Alberio L., Angelillo-Scherrer A., Asmis L.M., Fontana P., Korte W., Mendez A., Schmid P., Stricker H., Tsakiris D.A., et al. Accuracy and consistency of anti-Xa activity measurement for determination of rivaroxaban plasma levels. J. Thromb. Haemost. 2017;15:1576–1583. doi: 10.1111/jth.13747. [DOI] [PubMed] [Google Scholar]
- 18.Meihandoest T., Studt J.D., Mendez A., Alberio L., Fontana P., Wuillemin W.A., Schmidt A., Graf L., Gerber B., Amstutz U., et al. Accuracy of a Single, Heparin-Calibrated Anti-Xa Assay for the Measurement of Rivaroxaban, Apixaban, and Edoxaban Drug Concentrations: A Prospective Cross-Sectional Study. Front. Cardiovasc. Med. 2022;9:817826. doi: 10.3389/fcvm.2022.817826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiter S., Unterberger M., Binder N.B. Use of a Universal Calibrator for Direct FXa inhibitor DOACs; Proceedings of the 65th Annual Meeting of the Society of Thrombosis and Haemostasis Research; Lausanne, Switzerland. 22–26 February 2021. [Google Scholar]
- 20.Meihandoest T., Studt J.D., Mendez A., Alberio L., Fontana P., Wuillemin W.A., Schmidt A., Graf L., Gerber B., Maeder G.M., et al. Automated Thrombin Generation Assay for Rivaroxaban, Apixaban, and Edoxaban Measurements. Front. Cardiovasc. Med. 2021;8:717939. doi: 10.3389/fcvm.2021.717939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CLSI . Collection, Transport, and Processing of Blood Specimens for Testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays. 5th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008. Approved Guideline. CLSI Document H21-A5. [Google Scholar]
- 22.Center for Drug Evaluation and Research. Center for Veterinary Medicine . Bioanalytical Method Validation Guidance for Industry. Food and Drug Administration; Silver Spring, MD, USA: 2018. [Google Scholar]
- 23.Clarke W., Molinaro R.J., Bachmann L.M., Cook Botelho J., Cao Z., French D., Garg S., Gawoski J.M., Grant R.P. Liquid Chromatography-Mass Spectrometry Methods. Vol. 34 Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2014. Approved Guideline. [Google Scholar]
- 24.Gosselin R.C., Francart S.J., Hawes E.M., Moll S., Dager W.E., Adcock D.M. Heparin-Calibrated Chromogenic Anti-Xa Activity Measurements in Patients Receiving Rivaroxaban: Can This Test Be Used to Quantify Drug Level? Ann. Pharmacother. 2015;49:777–783. doi: 10.1177/1060028015578451. [DOI] [PubMed] [Google Scholar]
- 25.Beyer J., Trujillo T., Fisher S., Ko A., Lind S.E., Kiser T.H. Evaluation of a Heparin-Calibrated Antifactor Xa Assay for Measuring the Anticoagulant Effect of Oral Direct Xa Inhibitors. Clin. Appl. Thromb./Hemost. 2016;22:423–428. doi: 10.1177/1076029616629759. [DOI] [PubMed] [Google Scholar]
- 26.Asmis L.M., Alberio L., Angelillo-Scherrer A., Korte W., Mendez A., Reber G., Seifert B., Stricker H., Tsakiris D.A., Wuillemin W.A. Rivaroxaban: Quantification by anti-FXa assay and influence on coagulation tests: A study in 9 Swiss laboratories. Thromb. Res. 2012;129:492–498. doi: 10.1016/j.thromres.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Samama M.M., Contant G., Spiro T.E., Perzborn E., Le Flem L., Guinet C., Gourmelin Y., Rohde G., Martinoli J.L. Laboratory assessment of rivaroxaban: A review. Thromb. J. 2013;11:11. doi: 10.1186/1477-9560-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardy G., Fischer F., Appert A., Baldin B., Steve M., Spreux A., Lavrut T., Drici M.D. Is anti-factor Xa chromogenic assay for Rivaroxaban appropriate in clinical practice? Advantages and comparative drawbacks. Thromb. Res. 2015;136:396–401. doi: 10.1016/j.thromres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz E.M., Boonen K., van den Heuvel D.J., van Dongen J.L., Schellings M.W., Emmen J.M., van der Graaf F., Brunsveld L., van de Kerkhof D. Determination of dabigatran, rivaroxaban and apixaban by ultra-performance liquid chromatography—Tandem mass spectrometry (UPLC-MS/MS) and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J. Thromb. Haemost. 2014;12:1636–1646. doi: 10.1111/jth.12702. [DOI] [PubMed] [Google Scholar]
- 30.Schellings M.W., Boonen K., Schmitz E.M., Jonkers F., van den Heuvel D.J., Besselaar A., Hendriks J.G., van de Kerkhof D. Determination of dabigatran and rivaroxaban by ultra-performance liquid chromatography-tandem mass spectrometry and coagulation assays after major orthopaedic surgery. Thromb. Res. 2016;139:128–134. doi: 10.1016/j.thromres.2016.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset can be requested from the authors upon reasonable request.