Abstract
Coronavirus disease 2019 (COVID-19), the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which counts more than 650 million cases and more than 6.6 million of deaths worldwide, affects the respiratory system with typical symptoms such as fever, cough, sore throat, acute respiratory distress syndrome (ARDS), and fatigue. Other nonpulmonary manifestations are related with abnormal inflammatory response, the “cytokine storm”, that could lead to a multiorgan disease and to death. Evolution of effective vaccines against SARS-CoV-2 provided multiple options to prevent the infection, but the treatment of the severe forms remains difficult to manage. The cytokine storm is usually counteracted with standard medical care and anti-inflammatory drugs, but researchers moved forward their studies on new strategies based on cell therapy approaches. The perinatal tissues, such as placental membranes, amniotic fluid, and umbilical cord derivatives, are enriched in mesenchymal stromal cells (MSCs) that exert a well-known anti-inflammatory role, immune response modulation, and tissue repair. In this review, we focused on umbilical-cord-derived MSCs (UC-MSCs) used in in vitro and in vivo studies in order to evaluate the weakening of the severe symptoms, and on recent clinical trials from different databases, supporting the favorable potential of UC-MSCs as therapeutic strategy.
Keywords: COVID-19, SARS-CoV-2, Wharton’s jelly, mesenchymal stromal cells, umbilical-cord-derived mesenchymal stromal cells, extracellular vesicles, cytokine storm, inflammatory diseases, clinical trials, cell-based therapy, cell-free therapy
1. Introduction
After the three worldwide influenza outbreaks in the 20th century that were named in relation with the cite of origin (Spanish, 1918; Asian, 1957; Hong Kong, 1968), characterized by the infection of three different subtypes of influenza A virus (H1N1, H2N2, and H3N2, respectively) [1], the world recently faced the ongoing pandemic at the end of 2019, since Chinese health authorities informed of an outbreak of pneumonia of unknown etiology in Wuhan City, Hubei Province, on 31 December 2019 [2,3,4,5]. World Health Organization (WHO) established this as a public health emergency of international concern on 30 January 2020 [6]. The unknown origin was, thereafter, recognized as a possible zoonotic transmission, starting from bat and pangolin coronaviruses, spreading across other intermediate host species [7]. On 11 February 2020, the International Committee on Taxonomy of Viruses (ICTV) named this virus “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)” and the WHO named the related disease “Coronavirus Infectious Disease (COVID)-19” [8]. The SARS-CoV-2 genome consists of 29,903 nucleotides, and a phylogenetic analysis suggested that the virus is closely linked (89.1% nucleotide similarity) to a group of SARS-like coronaviruses (genus Betacoronavirus, subgenus Sarbecovirus) that had been reportedly found in animal hosts such as bats in China [9,10,11,12]. Following a global infection of SARS-CoV-2, COVID-19 was declared as a pandemic by WHO on 11 March 2020 [13]. Several clinical symptoms of COVID-19 are common to severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), such as fever, nonproductive cough, dyspnea, myalgia, fatigue, normal or decreased leukocyte counts, and radiographic evidence of pneumonia [2,14]. However, as described by WHO, the progression of the disease on vulnerable populations, such as pediatric patients, older people, and pregnant women, contributes to respiratory complication, multiorgan failure, the need for mechanical ventilation, and the admission to intensive care unit (ICU), where critical stage of disease could be potentially lethal [15]. According to the WHO data on 21 December 2022, the number of confirmed cases reached more than 650 million worldwide, with more than 6.6 million of counted deaths. These numbers are continuously updated on the dashboard of WHO (website: https://covid19.who.int/, accessed on 21 December 2022). Standard medical care for treating COVID-19 symptoms (mainly anti-inflammatory drugs) still awaits specific tools in order to counteract the most severe forms, which could lead to death. Research is moving along and it is supported by an enormous deployment of forces in terms of discovering new strategies and drugs. Importantly, the introduction of vaccines has reduced the spread of COVID-19 infection, even though the rapid increase in viral variants has reduced the effectiveness of vaccination, leading to the need for a booster dose against new viral strains. In addition, pharmacological approaches have been tested to treating COVID-19 patients, including anti-inflammatory and antiviral drugs. As a result, the damage caused by SARS-CoV-2 infection remains a long-term concern. Therapies based on the use of exogenous cells could represent an alternative and profitable strategy [16]. It is now known that mesenchymal stromal cells (MSCs) and their extracellular vesicles (EVs) represent a gold standard in regenerative medicine for several reasons, such as multipotent differentiation potential, immunomodulation and anti-inflammatory properties, mitochondrial transfer, and promotion of endogenous repair mechanisms [17]. MSCs can be isolated by adult tissues such as bone marrow (BM-MSCs) or adipose tissue (AT-MSCs), but also from perinatal tissues, including placenta (PL-MSCs), umbilical cord (UC-MSCs), umbilical cord blood (UCB-MSCs), and amniotic fluid (AF-MSCs). In recent years, consistent research has been carried out on EVs secreted by MSCs, which are enriched in proteins, lipids, and nucleic acids. Moreover, EVs can be used as a vehicle for drug delivery. Interestingly, it has been observed that UC-MSCs-derived EVs present a therapeutic potential for the treatment of different diseases, including COVID-19 [18,19,20]. Taking into account all these considerations, we focused this review on the recent in vitro and in vivo research in addition to the current clinical studies that involve the use of UC-MSCs in the treatment of the inflammatory status related to SARS-CoV-2 infection.
2. SARS-CoV-2 General Features and Mechanism of Infection
2.1. Genome, Structure, and the Variants of Concern behind the High Transmissibility
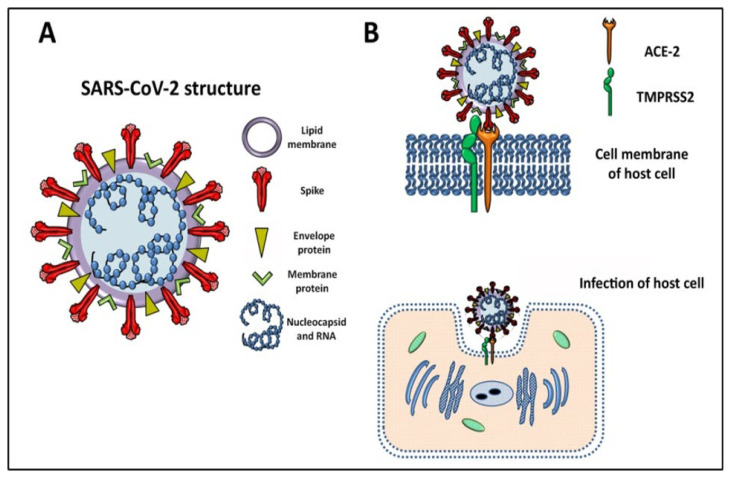
The first SARS-CoV-2 genome sequence was published on the community online resource virologial.org on 10 January 2020 (Wuhan-Hu-1, GenBank accession number MN908947) [21]. Based on the viral sequence database Global Initiative on Sharing All Influenza Data (GISAID, https://www.gisaid.org/, accessed on 21 December 2022), more than 12 million viral genomic sequences have been submitted. SARS-CoV-2 is an enveloped virus consisting of a single-stranded positive-sense RNA on which specific genes (5′ to 3′) are transduced for replicase ORF1a/b, and four structural components: spike (S), envelope, membrane, and nucleocapsid proteins [11]. A schematic structure of SARS-CoV-2 is depicted in Figure 1A, showing the proteins anchored to the lipid membrane. Similar to other SARS-CoVs, infected droplets or aerosols first target the respiratory mucosal epithelium, initiating viral infection, even if the reason for the fast spread of the infection worldwide and both asymptomatic and symptomatic patients demonstrated high levels of viral load in the lower-airway lung cells [10,12,22,23].
Figure 1.
(A) Schematic description of SARS-CoV-2 structures is characterized by the RNA genome containing viral information associated with nucleocapsid, while structural spike, envelope, and membrane proteins are anchored in the lipid membrane. (B) The infection of the host cell by SARS-CoV-2 is dependent on unique interaction between spike protein and the angiotensin-converting enzyme 2 (ACE-2). The presence of transmembrane serine protease 2 (TMPRSS2) guarantees the activation of viral fusion and internalization of the virus for the subsequent viral genome replication.
As expected, the diffusion of the virus and the increase of infections drive the generation of new mutations in the viral genome. Different mutations in SARS-CoV-2 S protein, nucleocapsid protein, and ORF3a have been found worldwide [24]. A phylogenetic network analysis conducted by Forster et al. showed three major variants of SARS-CoV-2 globally, named A, B, and C, diffused in United States and Australia, East Asia, and Europe, respectively [25]. Among the four structural genes, the S gene revealed a series of mutations which is, in turn, related with generation of variants of the virus in the S protein. Notably, the first SARS-CoV-2 variant of concern was identified on 16 December 2020, in the UK, consisting of 17 changes or mutations [26]. Variations occurring in the SARS-CoV-2 genome resulted, in some cases, in variants of concern, among which the most known are Alpha (B.1.1.7), Beta (B.1.351), Gamma (P1), Delta (B.1.617.2), and Omicron (B.1.1.529), giving rise to differences in symptoms, pathogenicity, viral load, increased rate in transmissibility, and reduced effectiveness of current diagnostic methods, therapies, and vaccines [27,28,29]. Even early and accurate detection of SARS-CoV-2 with efficient laboratory diagnostic tests has been thought necessary for facilitating public health interventions and interrupting the transmission chain [30,31].
2.2. Mechanism of Infection SARS-CoV-2, the Cytokine Storm, and Pathogenesis of COVID-19
The progression of COVID-19 in lung tissue on deceased donors revealed that viral RNAs were enriched in mononuclear phagocytic and endothelial lung cells which induced specific host response, while spatial analysis distinguished inflammatory host responses in lung regions with and without viral RNA [32]. As mentioned above, the SARS-CoV-2 S protein is necessary for infection, and this is related to its binding on the angiotensin-converting enzyme (ACE)-2 receptor expressed by host cells, and the receptor-mediated virus entry depends on a serine protease, transmembrane serine protease 2 (TMPRSS2) (Figure 1B) [9,33,34,35]. An scRNA-seq analysis of barrier tissues and model organisms aimed to identify the initial cellular targets of SARS-CoV-2 infection. The authors assessed that ACE-2 expression was a characteristic of certain cell types, such as type II pneumocytes in the lung [36,37], gut enterocytes, and goblet secretory cells of the nasal mucosa. On the other hand, the ACE-2/TMPRSS2 co-expression in respiratory tissues was limited to a rare subset of epithelial cells [32]. Moreover, ACE-2 has been identified in several tissues such as lungs, kidneys, heart, endothelial, and intestinal cells, as well as in liver, pancreas, reproductive tract, and central nervous system (CNS) [38,39,40,41,42]. For this reason, COVID-19 patients also present nonpulmonary or atypical manifestations, including headache, dizziness, olfactory and taste disorders, nausea, abdominal pain, vomiting, and diarrhea [39]. Not all of the cells express ACE-2. For example, bone marrow, lymph nodes, thymus, spleen, and immune cells, such as T and B lymphocytes and macrophages, are negative for ACE-2 [43]. Nevertheless, other receptors interacting with S protein, expressed even by ACE-2-negative cells, have been proposed for the entry of SARS-CoV-2 in cells expressing C-type leptin receptors (CLRs), such as CD209L (also known as L-SIGN) and CD209 (also known as DC-SIGN) [44,45]. The DC-SIGN/CD209 (dendritic-cell-specific intercellular adhesion molecule-3-grabbing nonintegrin) and the other CLRs can regulate immune responses via Toll-like receptors (TLRs), and TLR2 cooperates with the monocyte surface molecule CD14 in response to viral infection, leading to the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), inducing the production of inflammatory cytokines.
The structure of the SARS-CoV-2 S protein is similar to the SARS one, but its ACE-2-binding affinity is 10 to 20 times higher, and this could be a reason for the higher transmissibility of SARS-CoV-2 [46]. TMPRSS2 cleaves the S protein activating the virus to fuse with host lipid bilayer membrane, followed by the deposition and replication of the viral RNA genome into the host cell. The viral double-stranded RNA (dsRNA), created during replication, generates intermediates that can activate the cytoplasmic innate immune pathway, initiating a signaling cascade that leads to the production of type I and type III interferons (IFNs), the first antiviral function [47].
After internalization, the replication of the virus inside the host lung cells is followed by increased infiltration and activation of macrophages, as discovered in biopsy or autopsy specimens from patients with SARS-CoV-2 infection [48]. It has been established that the early response phase is characterized by the innate production of cytokines and the induction of emergency granulopoiesis, leading to the mobilization of neutrophils and monocytes [49,50]. Over 80% of COVID-19 patients present lymphopenia and an increased neutrophil–lymphocyte ratio [51], corresponding to low percentages of CD3+ T cells in peripheral blood [52]. All the events that follow virus entry lead to an imbalanced innate and adaptive host response, defined by low levels of type I and III interferons (IFNs), elevated chemokines, and high expression of interleukin (IL)-6, inducing a reduction of innate antiviral defenses [53], and generating the so-called “cytokine storm”, one of the main mechanisms for ARDS, which is the main cause of death in COVID-19 patients. The type II alveolar pneumocytes produce an innate proinflammatory response to SARS-CoV-2 infection, secreting proinflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-6, IL-1β, monocyte chemoattractant protein-1 (MCP-1), and granulocyte-macrophage colony-stimulating factor (GM-CSF) [36]. In addition, SARS-CoV-2 induces a rapid increase of pathogenic T helper (Th)1 lymphocytes expressing GM-CSF and IFN-γ. This is associated with an increased proliferation of inflammatory CD14+ CD16+ intermediate monocytes releasing both GM-CSF and IL-6, TNF-α, and IL-1β, thus contributing to cytokine storm [36,54]. Based on new nomenclature, the major population of human monocytes (90%) is described as the “classical” monocytes, which feature high levels of CD14 but are negative for CD16 (CD14++CD16−, or CD14+CD16−). The remaining group of human monocytes (10%) is further subdivided into the “intermediate” subset, in which CD16 is expressed alongside CD14 (CD14++CD16+, or CD14+CD16+), and the “nonclassical” subset, which features high expression of CD16 but a lower positivity to CD14 (CD14+CD16++ or CD14dimCD16+) [55]. Classical and intermediate monocytes are involved in inflammatory responses, while nonclassical ones are responsible for “patrolling behavior”, crawling the endothelium and supporting blood vessels integrity and antiviral functions [56]. The classical and intermediate monocytes observed in COVID-19 patients, including specimens such as plasma and bronchoalveolar lavage fluid, featured CD169+ expression [57]. In the lungs, the presence of macrophages is at multiple levels, i.e., alveolar and interstitial macrophages. Both alveolar and interstitial macrophages can be in two functional phenotypes, proinflammatory M1 macrophages and anti-inflammatory M2 macrophages, and since they express ACE-2, they produce proinflammatory cytokines/chemokines, leading to exacerbation of lung infection, resulting in ARDS. Thereafter, macrophages can migrate out of lungs contributing to systemic inflammations [58].
There is a natural temporal arc from induction to resolution of an immune response, with magnitude and duration that is finely orchestrated and balanced. Any disruption of this arc can lead to hyperimmune responses, or a delay in the resolution phase [49]. The impaired inflammatory response in COVID-19 patients may be due to a singular low expression of type I and III IFNs, resulting in reduction of antiviral defense, associated with elevated NF-kB-induced chemokines, leading to leukocytes recruitment and high expression of proinflammatory IL-6 [48]. Nevertheless, as described by Leisman and coworkers, the systemic inflammatory profile of COVID-19 patients was different compared with those having non-COVID-19 ARDS, sepsis, or chimeric antigen receptor T cell (CAR-T)-induced cytokine release syndrome. Several noncytokine biomarkers (including D-dimer, C-reactive protein, and ferritin) were elevated to a comparable or higher amount in patients with COVID-19 than in patients with the other disorders [59]. COVID-19 patients showed increased levels of D-dimer, fibrinogen, and profibrotic molecules such as platelet-derived growth factor-BB (PDGF-BB) and matrix metalloproteinase (MMPs) [60,61,62]. The disease duration in ICU patients positively correlated with an extremely increased of plasma protein levels of IL-13, IL-1β, GM-CSF, and the vascular endothelial growth factor (VEGF) beyond day 20 after symptom onset [63]. Ackermann et al. found a significant perturbation of angioarchitecture in the postmortem lung of seven patients who died due to COVID-19, showing variations in the caliber of the capillaries which exhibit cylindrical microstructures in the lumina and describing, for the first time, intussusceptive angiogenesis in the pathogenesis of COVID-19 [64]. The alveoli environment is also impaired with activation of endothelial cell death, platelet activation, exposure of extracellular matrix (ECM), the presence of active tissue factor, and extrinsic and intrinsic coagulation [47]. The formation of fibrin clot and platelet activation increase the risk of death [65].
2.3. The Role of Angiotensin II in Tissue Homeostasis Disruption and Multiorgan Failure
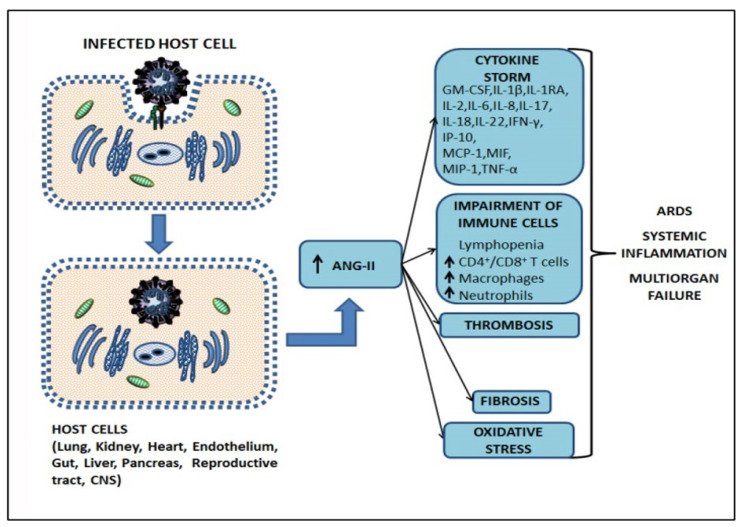
The ACE-2 receptor is also a negative regulator of the renin–angiotensin–aldosterone system (RAAS) and functionally lowers blood pressure by catalyzing angiotensin (ANG)-II into the vasodilator ANG (1–7) [37]. When ACE-2 is endocytosed together with SARS-CoV-2, this results in the reduction of ACE-2 on the cell surface and a consequent increase of serum ANG-II, leading to hypertension [66]. ANG-II has proinflammatory properties, including the increase of IL-6 through the activation of NF-kB and signal transducer and activator of transcription 3 (STAT3) pathways [67]. TNF-α and NF-kB trigger oxidative stress, and the interaction of ANG-II with angiotensin type 1 receptor (AT1R) activates NADPH oxidase (NOX), causes a reduction of nitric oxide (NO) bioavailability, and causes redox imbalance at mitochondrial level, determining the excess in reactive oxygen species (ROS), leading to vascular endothelial damage and prothrombotic events [68,69]. In addition, ANG-II causes a disintegrin and metalloproteinase 17 (ADAM17)-mediated shedding and activation of TNF-α-signaling [70]. It is involved in the increased expression of connective tissue growth factor (CTGF), a profibrotic factor involved in fibroblast proliferation, cellular adhesion, ECM deposition, and activation of transforming growth factor β1 (TGF-β1) signaling that stimulates myofibroblasts differentiation and ECM biosynthesis, along with the preservation of ECM proteins by regulation of MMPs and tissue inhibitors of metalloproteinases (TIMPs) [71,72]. ANG-II is also involved in reactive oxygen species (ROS) generation, resulting in endoplasmic reticulum stress, mitochondrial dysfunction, and vascular oxidative stress [67]. Consequently, a central role is played by the ANG-II, which increase in SARS-CoV-2 infection and lung dysfunction [73,74]. The increased level of ANG-II triggered by SARS-CoV-2 infection can result, in turn, in vasoconstriction as well as vasculopathy, coagulopathy, inflammation, and profibrotic effects, due to its effects in ECM remodeling, leading to multiorgan failure [66,71,75,76]. A descriptive sequence from host cell infection to severe COVID-19-related symptoms is shown in Figure 2.
Figure 2.
Schematic representation of the different aspects of COVID-19 pathogenesis. SARS-CoV-2 binds to the host cell, inducing ACE-2 endocytosis. The reduction of ACE-2 on cell surface determines an increase of serum angiotensin II (ANG-II,) triggering cytokine storm, impairment of immune cells, thrombosis, fibrosis, and oxidative stress contributing to the ARDS, systemic inflammation, and multiorgan failure. GM-CSF: Granulocyte-macrophage colony-stimulating factor; IL-: Interleukin (IL-1β, IL-1RA, IL-2, IL-6, IL-8, IL-17, IL-18, IL-22); IFN-γ: Interferon γ; IP-10: IFN-γ-induced protein 10; MCP-1: Monocyte chemoattractant protein-1; MIF: Macrophage migration inhibitory factor; MIP-1: Macrophage inflammatory protein 1; TNF-α: Tumor necrosis factor α.
3. From the First State of Emergency to the Current Standard Treatments of COVID-19 Patients
3.1. Social Distancing, Face Mask Wearing, and Convalescent Plasma
At the first signs of pandemic characteristics, in the absence of immediate protocols for treatment, emergency lockdowns, physical distancing, and face mask wearing have been mandatory across the world, since they have been thought to be the best first approach to prevent or reduce the rate of infection, but these have affected the health, business, and other aspects of daily life throughout societies. In a systematic review and meta-analysis, the authors suggested that transmission of virus was lower when maintaining a physical distancing of 1 m or more [77]. As confirmation, a large prospective U.S. cohort study (198,077 participants), showed that people living in communities with the greatest social distancing had a 31% lower risk of predicted COVID-19 compared with those living in communities with poor social distancing. It has to be noted that the use of face masks was associated with a 62% reduced risk of predicted COVID-19 even among individuals who lived in low social distancing conditions [78]. Nevertheless, the social behavior alone was not able to avoid the onset of pandemic, the increase of patients with moderate-to-severe form needing hospitalization, and the mechanical ventilation support for those with severe form of respiratory failure. Up to date, there is not a definitive therapeutic strategy for COVID-19 whose efficacy has been proven over the attempted solutions.
Even if it was seen as a promising strategy, the administration of plasma from convalescent patients, the hyperimmune plasma, did not exert any significant reduction in mortality and had minor impact on clinical improvement in individuals with moderate to severe disease [79]. A Chinese meta-analysis study [80] described that, in 32 randomized controlled trials and 21,478 patients, convalescent plasma therapy was not associated with significantly reduced 28-day mortality in COVID-19 patients, and it was not related to improvements in other survival outcomes (length of hospitalization, time without respiratory support, risk of symptoms progression, and requirement of mechanical ventilation). Moreover, in terms of safety, the treatment presented a trend with no statistical significance of higher incidence of adverse events, suggesting that the treatment could be recommended only in the context of clinical trials for severe COVID-19 patients, due to limited suppressive effect on inflammation and no significantly improved clinical outcomes [80].
3.2. IL-6 Receptor Blockers, Monoclonal Antibodies, and Antiviral Agents: The Recommendation of the WHO
Regarding drugs administration and therapeutics to treat COVID-19 patients, the WHO drafted and constantly updates living guidelines. Since COVID-19 is primarily related to increased IL-6 levels, WHO recommends both corticosteroids and IL-6 receptor blockers in patients with severe and critical COVID-19. However, corticosteroids, such as dexamethasone, are not recommended for nonsevere COVID-19 patients, but only for patients undergoing septic shock, or in critical cases [81,82]. Colchicine is not recommended, except in clinical trials, while other anti-inflammatory drugs (fluvoxamine or budesonide) did not provide sufficient evidence [83]. The potential role of IL-6 in COVID-19 pneumonia led to testing anti IL-6 receptor blockers, such as Tocilizumab and Sarilumab, humanized monoclonal antibodies used for other inflammatory diseases [84]. It has been proven by clinical studies that Tocilizumab and Sarilumab are both effective, as compared with the current standard of care, with reduced death rates in COVID-19 patients featuring severe illness, rapid deterioration, and increasing oxygen needs, and who had a significant inflammatory response [85,86]. Controversial results came from other studies: in a randomized trial involving hospitalized patients with severe COVID-19 pneumonia, the use of tocilizumab did not result in significant improvement of clinical status or lower mortality compared to placebo at 28 days [87]. In contrast, a prospective meta-analysis of clinical trials in patients hospitalized for COVID-19, conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, suggested that administration of IL-6 antagonists was associated with lower 28-day all-cause mortality [88]. Moreover, because of emergency situations, the heterogeneity of population, and different dosages administered, the first data on the safety and effectiveness of tocilizumab in severe COVID-19 were retrieved from observational retrospective studies [89]. Additionally, IL-6 receptor blockers cost is still a challenge. Up to date, the latest version of “Therapeutics and COVID-19: living guideline” (September 2022) regarding other monoclonal antibodies, such as casirivimab and imdevimab, demonstrated a lack of efficacy against the Omicron BA.1 variant. Therefore, casirivimab–imdevimab administration is no longer recommended for COVID-19 treatment except in cases where infection with a SARS-CoV-2 variant (such as Delta) is confirmed at sequencing level [90].
Other drugs with antiviral and anti-inflammatory mechanism of actions (such as remdesivir, lopinavir/ritonavir, chloroquine, and hydroxychloroquine) did not show evidence in reducing mortality or need for mechanical ventilation, and there was a risk of adverse events including diarrhea, nausea, and vomiting leading to the risk of hypovolemia, hypotension, and acute kidney injury [90]. In addition, convergent evolution on Omicron S protein has recently generated sublineages (such as the so called “Centaurus”, “Cerberus”, and “Gryphon”) which are able to escape anti-Spike monoclonal antibody therapies [91,92]. Fortunately, antiviral agents, such as remdesivir, molnupiravir, and nirmatrelvir, have been recently demonstrated as efficacious against both BQ.1.1 (“Cerberus”) and XBB (“Gryphon”) in vitro [93].
3.3. Vital Support: Prone Positioning, Mechanical Ventilation, and Extracorporeal Membrane Oxygenation (ECMO)
In individuals with low response to drugs, with a refractory respiratory failure and severe ARDS, lung function is seriously compromised, leading to severe impairment of gas exchange, hypoxemia, and impaired CO2 clearance in the alveolar space [94]. Therefore, in-hospital care is needed. Prone positioning for nonintubated patients has been widely applied and studied in COVID-19 patients. The results demonstrated that it exerted a reduction of lungs compression, since inducing a different gravitational-dependent redistribution of fluids. However, many questions are still unanswered, and randomized trials are ongoing in order to assess the clinical benefits of prone positioning in the management of COVID-19 patients [95].
Physicians need also to be able to critically evaluate the right conditions for giving supplemental oxygen, mechanical ventilation, and endotracheal intubation, due to the risks related with [96]. Mechanical ventilation is a double-edged sword because, on one hand, it is the usual treatment to support the respiration during ARDS, while, on the other, it is leading to ventilator-induced lung injury (VILI), causing lung fibrosis [97]. Moreover, ARDS shows that the deposition of additional ECM can result in a fibrotic remodeling of the lungs with related collapsed alveoli that need specific treatments for restoring alveolar space, such as mechanical ventilation PEEP (positive end expiratory pressure) [98].
Another invasive technique for blood reoxygenation is the venovenous extracorporeal membrane oxygenation (VV-ECMO), which can rest the lung functions, giving them time to recover, and it was revealed that ECMO is more effective in patients with more severe hypoxemia, even if the survival rate was not better compared with patients without ECMO [99]. Furthermore, ECMO could be beneficial but with higher risk of complication and mortality compared with influenza [100,101].
4. The Anti-SARS-CoV-2 Vaccines
In parallel with the definition of drug administration protocols, prevention through vaccination is the most effective way to reduce the spread of SARS-CoV-2 infection and the severe form of the disease. Based on “COVID-19—Landscape of novel coronavirus candidate vaccine development worldwide” distributed by the WHO (last publication, on 3 January 2023, available as a summary table) [102], more than 370 vaccines have been developed against SARS-CoV-2: 199 in preclinical and 176 in clinical development. The candidates in clinical development are 11 at phase IV, 50 at phase III, 15 at phase II/III, 14 at phase II, 31 at phase I/II, and 53 at phase I [102]. In particular, the mRNA-based vaccines BNT162b2 “Comirnaty” (produced by Pfizer-BioNTech) and mRNA-1273 Spikevax (produced by Moderna) have an efficacy rate near 95% to prevent COVID-19 disease and surprisingly with comparable outcomes [103,104,105], even considering the long-term effectiveness [106,107]. The effect has been proven also in frail individuals, such as immunocompromised solid organ transplant recipients, who featured improvements in both humoral and cellular-specific immune responses against the SARS-CoV-2 virus [108]. Another m-RNA-based vaccine is CVnCoV (CureVac, developed by CureVac N.V. and the Coalition for Epidemic Preparedness Innovations), while other vaccines are based on (i) viral vector (nonreplicating) (VVnr), such as ChAdOx1 (AstraZeneca), Ad26.COV2.S (Janssen, Johnson & Johnson), and Gam-COVID-Vac (the Sputnik V, developed by the Federal State Budgetary Institution N.F. Gamaleya Federal Research Centre for Epidemiology and Microbiology of the Ministry of Health of the Russian Federation); (ii) inactivated virus, such as CoronaVac (Sinovac), WIBP-CorV and BBIBP-CorV, BBV152 (COVILO) (Sinopharm), and BBV152 (Covaxin, Bharat Biotech); (iii) protein subunit, such as NVX-CoV2373 (Nuvaxovid, Novavax) and FINLAY-FR-2 (Soberana 02, produced by the Finlay Institute, a Cuban epidemiological research institute). It was described that there is high-certainty evidence that these vaccines reduce severe or critical disease, even if with important differences in vaccine efficacy, with little or no difference between most vaccines and placebo for serious adverse events (SAEs) [105]. Lower percentages of vaccine candidates are based on viral vector (replicating) (VVr), virus-like particle, VVr + antigen-presenting cell, live attenuated virus, VVnr + antigen-presenting cell, and bacterial antigen–spore expression vector [102]. The current vaccines become less effective the more the mutations occur, as in the case of seasonal flu vaccine, which is adjusted accordingly every year. Moreover, emerging evidence reported waning immunity after 6 months of completed vaccination [109]. Therefore, these issues drove the need to offer a booster dose of vaccine to restore the effectiveness of vaccination [110]. Another aspect worthy of attention is that the availability in large quantities and an effective campaign of vaccination of people are needed in lot of countries to reach a significant level of immunity.
However, despite the prevention of the severe form of the illness with vaccination, some difficulty in completing the vaccination campaign, placing an important pressure on ICUs for treating severe and critical stages of COVID-19 and ARDS in elderly patients, immunocompromised patients, and those with comorbidities (chronic obstructive pulmonary disease, cardiovascular diseases, diabetes), together with the risks of side effects after drug administration [90], multiorgan failure, and tissue damages, have made it necessary to continuously develop new therapeutic strategies. New approaches are recently focusing on the use of cells with high anti-inflammatory, regeneration, and tissue repair potency, such as the mesenchymal stromal cells (MSCs).
5. Characteristics of UC-MSCs in In Vitro and Preclinical Experimental Evidence Supporting Anti-Inflammatory, Immunomodulation, and Therapeutic Potential
5.1. Adult and Perinatal MSCs: General Features
Mesenchymal stromal cells (MSCs), which derive from the inner mass of the blastocyst, have a high capacity to self-renew, have fibroblastic-like shape when cultured in plastic surface, can differentiate into mesodermal derivatives such as osteoblasts, chondrocytes, and adipocytes, and show phenotype and characteristics in accordance with the minimal criteria of the International Society for Cellular Therapy (ISCT) [111]. They have, therefore, shed a new light on treatment of patients suffering from diseases and disorders that do not yet have a definite cure, and have a long history since their discovery to therapy applications [112]. MSCs are present in almost all post-natal/adult organs, i.e., bone marrow [113,114,115], adipose tissue [116,117], dental pulp [118,119], endometrium [120,121], menstrual blood [122,123], peripheral blood [124], salivary gland [125,126], skin and foreskin [127,128,129,130], synovial fluid [131,132], muscle [133,134,135], corneal stroma [136,137], heart [138,139], and lung [140]. Promising sources of MSCs are represented by the extraembryonic/perinatal tissues [141], among which there are the placenta, the chorionic and amniotic membranes [142,143,144], amniotic fluid [145], umbilical cord blood [146], and umbilical cord stroma [147]. Moreover, since MSCs derived from perinatal, as well as adipose tissue (AT-MSCs) and bone marrow (BM-MSCs), do not express ACE2 and TMPRSS2, this demonstrates that they are not permissive to SARS-CoV-2 infection, increasing the interest in the use of MSCs as potential therapy for COVID-19 [148]. However, the methods for obtaining adult tissues are invasive, and the yield of cells gained after isolation is scarce (e.g., 3.5 × 105 to 1 × 106 in 1 g of adipose tissue and from 500 to 5 × 104 from 1 g of bone marrow aspirate [149]). Perinatal tissues provide an interesting source of MSCs as they are usually wasted after birth and, therefore, the collection procedures are without risks for the donor and ethical issues [150]. Importantly, umbilical cord (UC) matrix is a better source of MSCs, in terms of yields, than the umbilical cord blood and adult tissues [151,152,153]. Furthermore, the adult MSCs displayed higher susceptibility to senescence and oxidative stress along with the passaging in culture compared to perinatal MSCs [154].
5.2. UC-MSCs Properties: Multilineage Differentiation, Immune Tolerance, Angiogenesis/Wound Healing, Matrix Remodeling, and Resistance to Hypoxia
Multilineage differentiation properties—The UC is consisted of two arteries and one vein included in a connective tissue called “Wharton’s jelly” (WJ), mainly composed of sponge-like structure woven with collagen fibers, proteoglycans, and embedded MSCs, and an outer layer of amniotic epithelium [147,155,156]. Our group and others focused research on molecular characterization of UC-MSCs, demonstrating that these cells express CD10, CD13, CD29, CD44, CD54, CD73, CD90, CD105, Stro-1, MHC class I (classical HLA-A, -B and -C), mesenchymal markers (vimentin, α-SMA), neuroectodermal markers (Nestin, NSE, GFAP), and early endoderaml markers (GATA-4,-5,-6, HNF4, cytokeratin-8,-18,-19), and lack the major costimulatory molecules responsible for T cell activation, specifically B7-1 (CD80) and B7-2 (CD86), hematopoietic and endothelial markers CD14, CD19, CD31, CD34, CD38, CD45, CD66b, CD80, CD86, CD106, and CD133, [147,157,158,159,160]. UC-MSCs multipotency is formally demonstrated by their in vitro differentiation capability towards cell types of mesodermal origin (chondrocytes, adipocytes, osteoblasts, odontoblast-like cells, dermal fibroblasts, smooth muscle cells, skeletal muscle cells, cardiomyocytes) and endodermal lineages (hepatocyte-like cells, pancreatic endocrine cells), as well as ectodermal and neuroectodermal (sweat gland cells, oligodendrocytes, and dopaminergic neurons) [147,157,161,162,163,164,165,166,167,168,169,170,171,172,173,174]. UC-MSCs also feature “primitive stemness” properties due to their close relation with the embryologic phase, also maintaining the length of telomeric ends even after around 60 population doublings, and having no chromosomal mutation acquisition [157].
Immune tolerance and anti-inflammatory properties—UC-MSCs exert immunomodulation properties [163,175,176], even related to the expression and release of specific factors, such as the nonclassical HLA class I antigen, HLA-G [157,177], HLA-E, CD276/B7-H3, leukemia inhibitory factor (LIF), indolamine 2,3-dioxygenase-1 (IDO-1), galectin-1 (Gal-1), and heat shock protein 10/Early Pregnancy Factor (HSP10/EPF), being able to modulate or inhibit lymphocyte proliferation [175]. These factors are involved in tolerogenic processes occurring at the fetal–maternal interface [178,179,180,181,182,183], permitting, in turn, the semi-allogeneic embryo to escape surveillance of the maternal immune system. Specifically, HLA-G is an inhibitory molecule involved in immune tolerance and exerts its inhibitory functions interacting with inhibitory receptors Ig-like transcript (ILT) receptors, such as ILT-2, ILT-3, and ILT-4, and killer cell immunoglobulin-like receptor (KIR), two Ig domains, and long cytoplasmic tail 4, KIR2DL4, differentially expressed by NK, T, and antigen-presenting cells [184,185]. Its expression is enhanced by Gal-1 [183]. HLA-G also interacts with leukocyte immunoglobulin-like receptor subfamily B member 1 (LILRB1) expressed by CD56brightCD16− natural killer (NK) cells that are enriched in the uterus during pregnancy, are poorly cytotoxic, and produce low amounts of IFN-γ as compared with peripheral blood CD56dimCD16+ NK cells [160]. HLA-E downregulates the immune response at the fetal–maternal interface, cooperating with classical HLA class I molecules in order to protect target cells from NK-cells-mediated cytotoxicity, interacting with CD94/NKG2A receptor [178]. CD276/B7-H3 expressed by UC-MSCs is able to inhibit T cell proliferation in a mixed lymphocyte reaction assay [175], and it is known to promote the survival of Th2 T cells over Th1 ones, together with indirect, noncontact-dependent mechanism mediated by IDO-1 and Gal-1 [186,187]. IDO-1 deprives effector T cells from tryptophan, reducing their proliferation and promoting apoptosis through O2 free radicals production, as well as inducing tolerogenic Tregs through interactions between naïve T cells and the products of tryptophan metabolism [188].
Studies show that LIF induces proliferation of CD4+ Foxp3+ Tregs, an anti-inflammatory phenotype in macrophages. In addition, it promotes survival of neurons and oligodendrocytes, and stimulates neurite outgrowth, all features that contribute to improving the clinical condition of experimental autoimmune encephalitis (EAE), a reliable mice model of human multiple sclerosis [189,190]. LIF production may play an important role in homeostasis and repair in the human lung tissues after induction by cytokines [191], and it could potentially support the use of UC-MSCs for restoring lung functions altered by SARS-CoV2 infection.
Lastly, HSP10, commonly known as a heat shock protein of 10 KDa, mainly expressed in the inner membrane of mitochondria, is also known as early pregnancy factor (EPF), discovered in the 1970s as a factor released during pregnancy in the serum of within 24 h after fertilization, preventing T cell-rosette formation and reduction of proinflammatory cytokines release, such as TNF-α (through interaction with TLR4 expressed by macrophages) (see in [192]). We found that Hsp10 was expressed by UC-MSCs both in vitro and in situ [175]. We were also the first to describe, in an in vitro COPD model, that Hsp10 showed a translocation from the cytoplasm to the nucleus, after exposure of the lung fibroblast cell line to cigarette smoke extract, but without direct interaction with DNA [193], thus suggesting a possible role of Hsp10 in gene expression modulation through chaperoning mechanism in response to pulmonary oxidative stress.
Although the underlying mechanism is still unknown, UC-MSCs secrete different prostanoids, such as PGD2, PGF2a, and PGE2, and several molecules, including IL-1R antagonist, IL-6, IL-10, M-CSF, VEGF, TGF-β1, and B7-H4, which could contribute to the differentiation of M2 macrophages [160]. UC-MSCs have been also involved in regulation of the monocyte/macrophage system. In particular, UC-MSCs can prevent the differentiation and maturation of monocytes toward DCs [194]. UC-MSCs also secrete other neuroprotective, angiogenic, and antiapoptotic factors, such as Neurotrophin 3 (NTF3), epidermal growth factor (EGF), neurite growth-promoting factor 2 (NEGF2/MDK), heparin binding EGF-like growth factor (HBEGF), Chemokine ligand 2 (CXCL2), CXCL5, and fibroblast growth factor 9 (FGF9) [195].
Angiogenesis/wound healing—Being mainly involved in WJ remodeling, UC-MSCs are not in contact with capillaries and small blood vessels, excluding the unique three vessels involved in umbilical blood circulation (the two arteries and the one vein), and they produce small amounts of angiogenic factor VEGF-A. Their support on endothelial cell proliferation and vasculogenesis could be related with other VEGF-independent factors, such as IL-8, hepatocyte growth factor (HGF), and MCP-1 [196].
Matrix remodeling—The UC-MSCs are involved in ECM composition of WJ that surrounds the umbilical vessels, expressing vimentin and collagen II [163]. Lo Iacono et al., demonstrated, using mass spectrometry analyses, that UC-MSCs co-cultured with umbilical cord blood–CD34+ hematopoietic stem/progenitor cells are able to secrete collagens, different proteases and their inhibitors, such as MMP-8, TIMP-1, ADAM with thrombospondin type 1 motif 9 (ADAMTS9), secreted protein acidic and cysteine rich (SPARC), plasminogen activator inhibitor-1 (PAI-1), and serine carboxypeptidase 1 involved in ECM remodeling, as well as α-2-HS glycoprotein, which is a TGF-β antagonist and prevents calcification by buffering excess matrix mineralization, in addition to than mimicking a hematopoietic niche [158]. Migration of cells on ECM, remodeling, and degradation of the ECM by MMPs are key regulators of wound repair, since wound healing requires the controlled activity of MMPs, and UC-MSCs may have a great potential in connective tissues and wounds [196,197,198].
Hypoxia resistance—Another aspect worthy of note about UC-MSCs is their high resistance to a stromal environment that is relatively hypoxic, therefore adapting to survive in limited nutrient and oxygen conditions [199]. To this regard, we recently demonstrated similar metabolism and survival capability in both normal and hypoxic conditions (in oxygen–glucose deprivation/reperfusion stroke model) exerted by the three different MSC populations isolated from the three different zones of umbilical stromal, Wharton’s jelly (WJ-MSCs), perivascular region (PV-MSCs), and cord lining (CL-MSCs) of human, suggesting that UC-MSCs are suitable for stem-cell-based therapy of ischemic diseases [200]. This provides the idea that tissue function support may be due to the transfer of healthy mitochondria [201], which has been demonstrated, improving oxidative phosphorylation (OXPHOS) and bioenergetics of recipient cells [202]. Moreover, our group demonstrated the secretion by UC-MSCs of the stress-induced chaperone hypoxia upregulated protein-1/GRP170, thought to have an important cytoprotective role in hypoxia-induced cellular perturbation [158].
The paracrine effects of UC-MSCs-derived molecules with immunomodulatory, anti-inflammatory, and regenerative properties are related to their release by MSCs on the extracellular environment not only through the secretion of soluble factors, but also as cargos of EVs, such as exosomes, which also contain lipids, metabolites, DNA fragments, miRNA fragments, and noncoding RNAs, acting locally and/or at distance as a cell-to-cell communication system, both in physiological and pathological conditions [203,204,205]. Due to their structure, and the opportunity to freely circulate into the body fluids, with low immunogenicity, and their bioavailability, they could be useful for drug delivery, and generate a great interest among scientists for cell-free therapies. The roles of perinatal MSCs and their conditioned media, enriched in EVs, were also explored in preventing lung injury across lung transplantation, described by Miceli and coworkers [206,207].
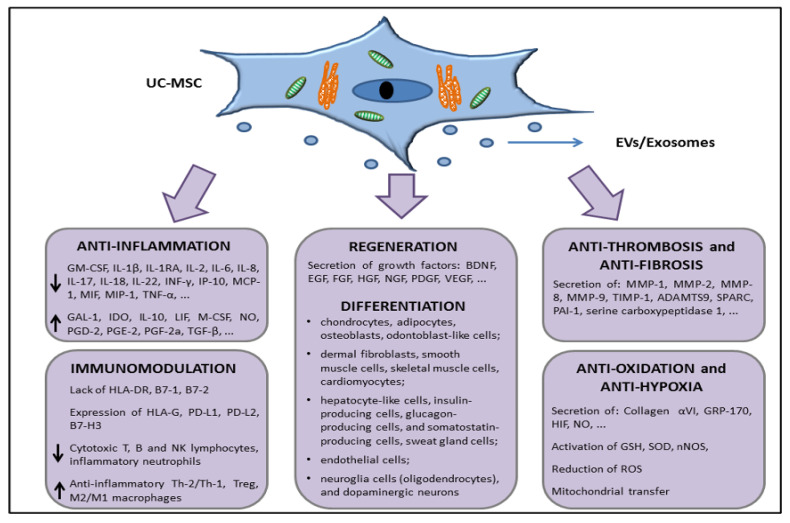
Taken together, all these features, characterized by the surface expression or secretion (through EVs/exosomes or in soluble form) of a series of factors with anti-inflammatory, immunomodulation, tissue repair, antifibrotic, and antihypoxic functions (summarized in Figure 3), support the interest in further studies about the use of UC-MSCs in in vivo experiments in order to move to allogeneic transplantation or develop novel cell-free products that are able to restore tissue functions, such as lung parenchymal functions dampening the damages exerted during COVID-19 disease.
Figure 3.
Schematic representation of the UC-MSCs effects related with molecules involved in direct cell-to-cell interaction or through the release of extracellular vesicles (EVs) and/or exosomes.
5.3. In Vivo Preclinical Data Supporting the Use of UC-MSCs to Treat Organ Dysfunctions
The researchers worldwide have been focused on xenogeneic UC-MSCs in in vivo preclinical trials for determining safety, tolerance, and efficacy.
It was found that UC-MSCs transplantation induced, in vivo, a weak activation of immune Th1 and Th2 cells, and they showed significantly longer survival times in immunocompetent Balb/c mice compared to BM-MSCs, with a survival time prolonged in immunodeficient SCID-beige mice that must be attributed to the compromised immune response of the host [208].
Transplantation by intravenous (IV) injection in female dark agouti rats, used for EAE model, revealed that UC-MSCs were detected in lung and spleen 2.5 weeks after transplantation in the chronic disease phase, but they were not observed in the lymph nodes, spinal cord, or brain of transplanted animals. However, they potently (even if transiently) ameliorated neurological symptoms [209]. As explained above, multiple sclerosis could be alleviated by neuroprotective and neuroregenerating properties of LIF released in the bloodstream by UC-MSCs [190].
Studies of the pathogenesis of autoimmune type I diabetes demonstrated that UC-MSCs reduced systemic and pancreatic levels of cell populations, such as Th1 and Th17 cells, and a shift toward the Th2 profile along with an increase in Treg cells was found in UC-MSCs-treated nonobese diabetic (NOD) mice [189]. Moreover, in diabetic rats it was shown that UC-MSCs could survive both in the pancreas and in the liver with improved hyperglycemia due to the support of damaged insulin-producing β cells [210].
In a myocardial infarction murine model, induced by left anterior descending (LAD) coronary artery ligation, delivery of UC-MSCs by intramyocardial injection resulted in a reduction of scar in the left ventricular wall thickness and stimulation of angiogenesis, preventing apoptosis and attenuating adverse tissue remodeling, compared to the vehicle control group [211].
Focusing our attention on lung dysfunction, it was found that in an E. coli-induced pneumonia rat model, IV injection of fresh or cryopreserved CD362+ UC-MSCs, with or without concomitant antibiotic administration, revealed a decrease in the severity of lung injury, increased static lung compliance, significantly reduced bronchoalveolar lavage (BAL) bacterial load and inflammatory cytokine secretion, and increased airspace induced by CD362+ UC-MSCs compared to the vehicle. The efficacy of CD362+ UC-MSCs was not impaired by cryopreservation and the effects on arterial oxygen pressure were more expressed by the cell-treatment group compared to treatment with antibiotic or vehicle alone, but it was reduced along with the passages in culture of the cells and needed a rescue with a second dose of cells [212].
Although UC-MSCs do not differentiate into any lung cell, in the bleomycin-induced murine model of lung injury, these cells show homing to the lung tissue at 14 days after injection (but not at 28 days) compared to healthy mice, and showed antifibrotic properties increasing MMP-2, inducing the reduction of endogenous inhibitors (TIMP-1 to -4), and decreasing inflammation by repressing the expression of TGF-β, IFN-γ, and the proinflammatory cytokines macrophage migratory inhibitory factor (MIF) and TNF-α [213].
Even UC-MSCs-derived EVs have been under the microscope for treating organ dysfunction. In fact, as reviewed by Lelek and Zuba-Surma, in vivo preclinical studies on EVs derived from UC-MSCs have been conducted in neurological, cardiovascular, liver, kidney, and skin diseases [214]. In a preclinical model of bronchopulmonary dysplasia (BPD), using neonatal mice exposed to hyperoxia (75% O2), the IV injection with UC-MSCs-derived exosomes restored lung morphology, postnatal development, pulmonary hypertension, and vascular remodeling, along with decreased lung fibrosis [215]. Since the IV injection of MSCs resulted in entrapment of the cells in the lungs as first site after injection into the bloodstream [216], and taking into account what was already described, it was not surprising that researchers thought about the use of UC-MSCs for treating COVID-19 in clinical studies.
6. MSCs in COVID-19 Patients: Are UC-MSCs Better than the “Gold-Standard” BM-MSCs?
Based on just-reported evidence about the shorter survival time of BM-MSCs compared with UC-MSCs [208], there is still the consensus about the use of BM-MSCs as a “gold-standard” for cell therapy. This is because it is relatively safer to use autologous BM-MSCs (or AT-MSCs) from the same patients, compared to allogeneic UC-MSCs, but the age of the patients, their gender, their health conditions, and the invasive procedure for isolating BM-MSCs (or AT-MSCs) must be taken into account in order to balance pros and cons. Even BM-MSCs have been studied in COVID-19 patients, as reviewed in Yao et al. [217]. BM-MSCs are able to produce and secrete soluble PD-1 ligands (sPD-L1 and sPD-L2) that are responsible for hyporesponsiveness in T cells, arresting the PD-1-mediated AKT pathway, thus inducing immune tolerance [218]. Further, in in vitro and in a humanized mouse model of graft versus host disease (GvHD), it was described that BM-MSCs affected T lymphocyte proliferation more than UC-MSCs, while the latter induced a higher increase of Tregs/Th17 ratio [219]. Since Treg/Th17 ratio imbalance correlates with immune thrombocytopenia (ITP) [220] and this imbalanced ratio was also observed in different cases of COVID-19 disease [221], the reactions of infused MSCs for correcting immune response in such condition should be absolutely taken in consideration. For example, the patients with ITP displayed abnormalities in BM-MSCs, due to defects in mRNA and miRNA that induced downregulation of genes involved in cellular stress machinery, such as the unfolded protein response (UPR), the nuclear protein transcriptional regulator 1 (Nupr1), involved in endoplasmic reticulum pathway, the TGF-β1 signaling, leading to a loss of immunosuppressive properties, and a breakdown of self-tolerance in ITP patients [222]. In this specific case, ITP patients are not eligible for autologous BM-MSCs.
In a pilot study involving liver allograft recipients with acute rejection, they also observed a significant increase of Treg/Th17 ratio after 4 weeks of UC-MSC infusion [223]. Moreover, the BM-MSCs revealed a donor’s age-related decrease in colony forming units-fibroblast (CFU-f) in growth rate, in differentiation potential, and in superoxide dismutase (SOD) activity (and an increase of reactive oxygen species production) that could, in turn, affect autologous cell-based therapy [224]. On the contrary, UC-MSCs derived from perinatal tissue of childbearing age population showed no sign of senescence over several passages [157]. An integrated transcriptome-proteome analysis, comparing MSCs from different sources (BM, AT, and UC) revealed that secretome derived from UC-MSCs had a predominantly anti-inflammatory effect enriched in T cell inhibitory interleukins, such as IL-4, IL-13, IL-6, IL-35, IL-2, IL-22, IL-1R1, and IL-25, as well as the colony-stimulating growth factor (CSF) 3, which promoted M2 macrophage polarization, compared with the adult MSCs, while BM-MSCs were more immunosuppressive [225]. The immunosuppression is probably initiated starting from the apoptotic events induced by cytotoxic cells against BM-MSCs infused in GvHD recipients, as a result of a bystander effect of CD56+ natural killer (NK) and CD8+ T cells [226]. Taken together, these findings could highlight that UC-MSCs are more effective in treating symptoms and inflammatory state during the COVID-19-related cytokine storm than BM-MSCs.
7. Clinical Trials for the Treatment of COVID-19 Patients with UC-MSCs
The first paper on the use of MSCs (without a specific description of the cell source) for treating COVID-19 patients was published on 3 March 2020 in the journal “Aging and Disease” by Leng and colleagues, with a pilot study enrolling seven patients (and three for placebo control) from 23 January 2020 to 31 January 2020 (according to the guidance of National Health Commission of China), and demonstrating that after IV injection of 1 × 106 cells/kg of weight, major symptoms (high fever, weakness, shortness of breath, and low oxygen saturation) disappeared in 2 to 4 days in all the patients, the oxygen saturations increased to ≥95% at rest, without or with oxygen uptake, the chest computer tomography (CT) scan showed that the ground-glass opacity (GGO) and pneumonia infiltration were reduced on the ninth day, there was a reduced amount of cytokine-secreting T cells, and two common and one severe patient were recovered and discharged in 10 days [227]. This study opened the path to further clinical trials.
Using the WHO International Clinical Trial Registry Platform (https://www.who.int/clinical-trials-registry-platform), accessed on 23 September 2022, 61 studies were documented in regards to the treatment of COVID-19 with UC-MSCs (or WJ-MSCs) and they are listed in Table 1.
Table 1.
Clinical trials using MSCs derived from umbilical cord.
| N° | Trial ID | Recruitment Status | Study Status | Treatment | Phase | Country |
|---|---|---|---|---|---|---|
| 1 | NCT04573270 | Completed | Completed | UC-MSCs | 1 | USA |
| 2 | NCT04288102 | Completed | Completed | UC-MSCs | 2 | China |
| 3 | NCT04625738 | Completed | Completed | WJ-MSCs | 2 | France |
| 4 | NCT04355728 | Completed | Completed | UC-MSCs | 1–2 | USA |
| 5 | NCT04392778 | Completed | Completed | UC-MSCs | 1–2 | Turkey |
| 6 | NCT04400032 | Completed | Completed | UC-MSCs | 1–2 | Canada |
| 7 | NCT04333368 | Completed | Completed | WJ-MSCs | 1–2 | France |
| 8 | NCT04252118 | Completed | Completed | UC-MSCs | 1 | China |
| 9 | NCT04457609 | Completed | Completed | UC-MSCs | 1 | Indonesia |
| 10 | NCT05286255 | Recruiting | Ongoing | UC-MSCs | 1 | USA |
| 11 | NCT04896853 | Recruiting | Ongoing | WJ-MSCs | 1 | Sweden |
| 12 | NCT05387278 | Recruiting | Ongoing | UC-MSCs and PL-derived exosomes | 1 | USA |
| 13 | NCT04869397 | Recruiting | Ongoing | WJ-MSCs | 2 | Canada |
| 14 | NCT04865107 | Recruiting | Ongoing | UC-MSCs | 2 | Canada |
| 15 | NCT04390139 | Recruiting | Ongoing | WJ-MSCs | 1–2 | Spain |
| 16 | NCT04390152 | Recruiting | Ongoing | WJ-MSCs | 1–2 | Colombia |
| 17 | NCT04494386 | Recruiting | Ongoing | CL-MSCs | 1–2 | USA |
| 18 | NCT04399889 | Recruiting | Ongoing | UC-MSCs | 1–2 | USA |
| 19 | NCT03042143 | Recruiting | Ongoing | CD362 enriched UC-MSCs | 1–2 | UK |
| 20 | NCT05132972 | Recruiting | Ongoing | UC-MSCs | 2–3 | Indonesia |
| 21 | NCT05240430 | Recruiting | Ongoing | UC-MSCs | N/A | Turkey |
| 22 | NCT04313322 | Recruiting | Unknown | WJ-MSCs | 1 | Jordan |
| 23 | NCT04437823 | Recruiting | Unknown | UC-MSCs | 2 | Pakistan |
| 24 | NCT04269525 | Recruiting | Unknown | UC-MSCs | 2 | China |
| 25 | NCT04339660 | Recruiting | Unknown | UC-MSCs | 1–2 | China |
| 26 | NCT04371601 | Not yet recruiting | Active, not recruiting | UC-MSCs | Early 1 | China |
| 27 | NCT04456361 | Not yet recruiting | Active, not recruiting | WJ-MSCs | Early 1 | Mexico |
| 28 | NCT04452097 | Not yet recruiting | Active, not recruiting | UC-MSCs | 1–2 | USA |
| 29 | NCT05501418 | Not yet recruiting | Active, not recruiting | UC-MSCs | 1–2 | Taiwan |
| 30 | NCT04398303 | Not yet recruiting | Unknown | UC-MSCs | 1–2 | USA |
| 31 | NCT04429763 | Not yet recruiting | Unknown | UC-MSCs | 2 | Colombia |
| 32 | NCT04273646 | Not yet recruiting | Unknown | UC-MSCs | N/A | China |
| 33 | EUCTR2020-002772-12 | Completed | Completed | WJ-MSCs | 2 | France |
| 34 | EUCTR2020-001505-22 | Recruiting | Ongoing | WJ-MSCs | 1–2 | Spain |
| 35 | EUCTR2020-001577-70 | Recruiting | Ongoing | UC-MSCs and others MSCs | 1–2 | Italy |
| 36 | ChiCTR2000030173 | Completed | Completed | UC-MSCs | Early 1 | China |
| 37 | ChiCTR2000030088 | Completed | Completed | WJ-MSCs | Early 1 | China |
| 38 | ChiCTR2000030866 | Completed | Completed | UC-MSCs | Early 1 | China |
| 39 | ChiCTR2000030261 | Completed | Completed | WJ-MSCs-derived exosomes | Early 1 | China |
| 40 | ChiCTR2000030944 | Completed | Completed | UC-MSCs | 1 | China |
| 41 | ChiCTR2000030138 | Completed | Completed | UC-MSCs | 2 | China |
| 42 | ChiCTR2000031430 | Completed | Completed | UC-MSCs | 2 | China |
| 43 | ChiCTR2000030116 | Completed | Completed | UC-MSCs | N/A | China |
| 44 | ChiCTR2000030835 | Completed | Completed | UC-MSCs | N/A | China |
| 45 | ChiCTR2000030484 | Not yet recruiting | Active, not recruiting | UC-MSCs and exosomes | N/A | China |
| 46 | ChiCTR2000031494 | Recruiting | Ongoing | UC-MSCs | 1 | China |
| 47 | IRCT20190717044241N2 | Completed | Completed | WJ-MSCs | 1 | Iran |
| 48 | IRCT20200217046526N2 | Completed | Completed | UC-MSCs | 2–3 | Iran |
| 49 | IRCT20190101042197N2 | Completed | Unknown | UC-MSCs-derived exosomes | 1–3 | Iran |
| 50 | IRCT20201202049568N3 | Completed | Unknown | UC-MSCs-derived exosomes | 1–2 | Iran |
| 51 | IRCT20160809029275N1 | Completed | Unknown | UC-MSCs | 2–3 | Iran |
| 52 | IRCT20200421047150N1 | Completed | Unknown | WJ-MSCs | 2–3 | Iran |
| 53 | IRCT20200426047206N2 | Completed | Unknown | UC-MSCs | 3 | Iran |
| 54 | IRCT20140528017891N8 | Completed | Unknown | UC-MSCs | 3 | Iran |
| 55 | IRCT20211012052743N1 | Recruiting | Ongoing | UC-MSCs | 3 | Iran |
| 56 | JPRN-JapicCTI-205465 | Recruiting | Ongoing | UC-MSCs | 1 | Japan |
| 57 | CTRI/2020/08/027043 | Not yet recruiting | Unknown | UC-MSCs | 1 | India |
| 58 | CTRI/2021/09/036645 | Recruiting | Ongoing | UC-MSCs | 1–2 | India |
| 59 | RBR-3fz9yr | Completed | Ongoing | UC-MSCs | N/A | Brazil |
| 60 | RBR-4jh63b | Not yet recruiting | Unknown | UC-MSCs | 1–2 | Brazil |
| 61 | RBR-8zg5rg7 | Recruiting | Ongoing | UC-MSCs | 1–2 | Brazil |
Recruitment status: Completed—the participants are no longer being examined or treated; Recruiting—the study is currently recruiting participants; Not yet recruiting—the study has not started recruiting participant. Study status: Completed—the study has ended normally; Ongoing—ongoing recruitment; Unknown—the status has not updated or verified within the past 2 years; Active, not recruiting: ongoing study but the recruitment has not started. N/A: trials without FDA-defined phases. MScs: mesenchymal stromal cells; UC-MSCs: umbilical cord MSCs; PL-derived: placental derived; CL-MSCs: cord lining-derived MSCs; WJ-MSCs: Wharton’s Jelly-derived MSCs.
As listed, the majority of the trials resulted in phase 1, but in some countries the UC-MSCs treatment for COVID-19 passed through phase 2 and only in Iran and Indonesia reached phase 3. Five clinical trials are testing the safety and efficacy of UC-MSCs- and WJ-MSCs-derived exosomes for the treatment of COVID-19 patients, and two of these trials are testing EVs even from other MSCs. Most of the clinical trials are ongoing, while 21 were completed, and the data were published for 15 of them [228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245], while 6 were case reports [246,247,248,249,250,251]. Results of these studies and their outcomes are listed and specified in Table 2.
Table 2.
Results of published clinical studies based of UC-MSCs and WJ-MSCs (or derived exosomes) therapy administered to COVID-19 patients.
| Type of Study | Phase | Number of Patients | COVID Symptoms | Treatment | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Pilot trial | Early 1 | 7 | Mild 5 Severe 2 |
Nebulization ranged from 7.66 × 100.8 to 7.00 × 100.7 WJ-MSCs-derived exosomes/mL, twice a day, up to discharge. | Reduction of pulmonary lesions and period of hospitalization in mild cases and reduction in cellular residue in severe cases. No adverse events were observed. | Chu et al., 2022 [228] |
| Parallel assigned controlled, nonrandomized trial | 1 | 18 | Moderate 10 Severe 8 |
Moderate = 5; Severe = 4; Infusion of 3 × 107 UC-MSCs for 3 times on days 0, 3, and 6. |
Reduced trend in plasma levels of inflammatory cytokines IFN-γ, TNF-α, MCP-1, IP-10, IL-1RA, IL-6, IL-8, IL-18, IL-22 and MIP-1. No serious adverse events were observed. | Meng et al., 2020 [229] |
| Double-blind, multicenter, randomized controlled trial | 1 | 40 | Critical | N = 20 patients; Infusion of 1 × 106 UC-MSCs/kg in single dose. | Increased survival rate. Decrease trend in IL-6 levels and increase trend in IL-10, LIF and VEGF levels in plasma. No adverse events were observed. | Dilogo et al., 2021 [230] |
| Single-center open-label, individually randomized, standard treatment-controlled trial | 1 | 41 | Severe | N = 12 patients; Infusion of 2 × 106 UC-MSCs/kg in single dose. |
No progression from severe to critical illness. Reduction of weakness, fatigue, shortness of breath, and low oxygen saturation. Significant decreased in CRP and IL-6 plasma levels. Faster normalization in lymphocyte count and reduction of lung inflammation. No adverse events were observed. A 3-month follow-up of 28 patients (treated = 8, control = 20) revealed reduction of partial pulmonary function recovery time, ameliorated HRQL, and no adverse events were observed after 3 months. | Shu et al., 2020 [231] and Feng et al., 2021 [232] |
| Open-label, single-center trial | 1 | 5 | Severe | Injection of 150 × 106 WJ-MSCs for 3 times on days 0, 3, and 6. | Increase in IL-10 and SDF-1 and decrease of VEGF, TGF-β, IFN-γ, IL-6, and TNF-α plasma levels. Improvement in hematology, myocardial enzyme, inflammation, and biochemical tests. No adverse events were observed. | Saleh et al., 2021 [233] |
| Single-center, open-label, placebo-controlled trial | 1 | 20 | Mild-to-moderate | N = 10 patients; Infusion of 1 × 106 UC-MSCs/kg for 3 times on days 1, 3 and 5. |
Significant improvement in SpO2/FiO2 ratio. Significant reduction in cytokine IL-6, IFN-γ, TNF-α, IL-17 A, and CRP levels and increase in cytokine levels of TGF-β, IL-1B, and IL-10. No serious adverse events were observed. | Kaffash Farkhad et al., 2022 [234] |
| Single-arm, pilot trial | 2 | 16 | Severe 9 Critical 7 |
Infusion of 1 × 108 UC-MSCs for 4 rounds of transplantation. | Amelioration of oxygenation index. Increase of CD4+ T, CD8+ T, and NK lymphocytes. IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, and CRP have returned in the normal range. No adverse events were observed. | Feng et al., 2020 [235] |
| Single-blind, randomized, placebo-controlled trial | 2 | 58 | Mild 31 Severe 21 Critical 6 |
N = 29 patients; Mild 15 Severe 11 Critical 3 Infusion of 1 × 106 UC-MSCs/kg in single dose. |
Shorter hospital stay and symptoms remission. Improved CT scans. Reduction of CD14+ monocytes, CRP, NETs and proinflammatory cytokines IL-1RA, IL-18, IL-27, IL-17E/IL-25, IL-17F, GRO-alpha (CXCL-1), and IL-5. High expression of genes involved in chemotaxis, telomerase assembly and maturation, angiopoiesis, HSCs mobilization, and fetal extramedullary hematopoiesis, including VNN2. Stimulation of SARS-CoV-2 specific antibodies production. No adverse events were observed. | Zhu et al., 2021 [236] |
| Double-blind, randomized, placebo-controlled trial | 2 | 100 | Severe | N = 65 patients; Infusion of 4 × 107 UC-MSCs for 3 times on days 0, 3, and 6. |
Improvement in lung lesion volume, improved restoration of the integrated reserve capability, and normal CT scans after 1 year. Similar incidence of adverse events and tumor markers to placebo group after 1 year. | Shi et al., 2021 [237] and Shi et al., 2022 [238] |
| Multicenter, double-blind, randomized, placebo-controlled trial | 2 | 45 | Mild 31.1%; Moderate 48.9%; Severe 20% |
N = 21 patients; Infusion of 0.9 ± 0.1 × 106 UC-MSCs/kg per dose over 5 days (on day 1, day 3 ± 1, and day 5 ± 1) N = 17 − 3 doses N = 2 − 2 doses N = 2 − 1 dose. |
UC-MSC-treated patients’ greater PaO2/FiO2-ratio increased between D0 and D7, with lack of statistically significant differences, compared to controls. No adverse events were observed. | Monsel et al., 2022 [239] |
| Double-blind, randomized, controlled trial | 1–2 | 24 | Mild-to-moderate 6; Moderate-to-severe 18 |
N = 12 patients; Mild-to-moderate 3 Moderate-to-severe 9 Infusion of 100 ± 20 × 106 UC-MSCs for 2 times on days 0 and 3. |
Significantly improved SAE-free survival and time to recovery. Significant reduction in plasma levels of inflammatory cytokines, chemokines, and growth factors GM-CSF, IFN-γ, IL-5, IL-6, IL-7, TNFα, TNF-β, PDGF-BB, RANTES, and sTNFR2. No difference in adverse events among both groups. | Lanzoni et al., 2021 [240] and Kouroupis et al., 2021 [241] |
| Randomized trial | 1–2 | 210 | Severe 111; Critical 99 |
Infusion of 1–2 × 106 UC-MSCs/kg in single dose. | After 2–3 weeks after transplantation, improvement oxygen saturation and high survival rate, especially before intubation. No adverse events observed. | O Ercelen et al., 2021 [242] |
| Prospective double controlled trial | 1–2 | 30 | Moderate 10; Critical 20 |
N = 10 critical patients; Infusion of 3 × 106 WJ-MSCs/kg for 3 times on days 0, 3, and 6. |
Decrease in proinflammatory and profibrotic factors IL-6, CRP, IFNγ, IL-2, IL-12, IL-17A, MMP-9, and MMP-3 plasma levels. Increase in anti-inflammatory and angiogenesis promoting factors IL-10, TGF-β, VEGF, KGF, and NGF plasma levels. Reduction of the mechanical ventilation period and high survival rate. No adverse events were observed. | Adas et al., 2021 [243] |
| Prospective, single-center, randomized, double-blind, placebo-controlled trial | 1–2 | 17 | Critical | N = 11 patients; Infusion of 5 × 105 UC-MSCs/kg every 48 h for 3 times. |
Decrease in ferritin, IL-6 and MCP-1-CCL2, CRP, D-dimer, and neutrophils levels and reduction of lung damage. Increase in the numbers of lymphocytes T CD3+, CD4+ and NK. All these values are maintained until 4 months. No serious adverse events were observed. | Rebelatto et al., 2022 [244] |
| Primary report of a two-center, open-label, single-arm trial | 2–3 | 11 | Critical | Infusion of 200 × 106 UC-MSCs (N = 6) and PL-MSCs (N = 5) every other day for 3 times. | Increased of SpO2. Significant reductions in serum levels of TNF-α, IL-8, and CRP. No adverse events were observed. | Hashemian et al., 2021 [245] |
| Case report | N/A | 1 | Severe | Infusion of 5 × 107 UC-MSCs for 2 times on days 30 and 32. | Increased PaO2/FiO2 ratio. Decrease of inflammatory monocytes and increase of patrolling monocytes, CD4+ T lymphocytes, and cDC2. Reduction of lung infiltrates and fibrosis. No adverse events were observed. | da Silva et al., 2021 [246] |
| Case report | N/A | 1 | Critical | Infusion of 1 × 106 UC-MSCs/kg in single dose. | Increase of SpO2 and absolute number of the lymphocytes. Reduction of GGO, lung infiltration, and plasma levels of CRP and D-dimer. No adverse events were observed. | Zhu et al., 2020 [247] |
| Case report | N/A | 1 | Severe | Infusion of 1 × 106 WJ-MSCs/kg in single dose. | Reduction of GGO, lung infiltration and plasma levels of CRP, IL-6 and TNF-α. Increase of CD3+, CD4+, and CD8+ T lymphocytes. No adverse events were observed. | Zhang Y. et al., 2020 [248] |
| Case report | N/A | 1 | Critical | Infusion of 1 × 106 UC-MSCs/kg for 8 times divided in 3 rounds. | Reduction of fiber strands and GGO. Reduction in IL-6, IL-10, WBCs, CRP, and D-dimer levels. Increase of CD4+ T lymphocytes and decrease of NK cells. No adverse events were observed. | Zhang Q. et al., 2021 [249] |
| Case report | N/A | 1 | Critical | Infusion of 5 × 107 UC-MSCs for 3 times on days 13, 16, and 19. | Reduction of GGO, D-dimer, WBCs, neutrophils, and lymphocytes/neutrophils ratio. Increase of CD3+, CD4+, and CD8+ T lymphocytes. No adverse events were observed. | Liang et al., 2020 [250] |
| Case report | N/A | 1 | Severe | Infusion of 0.5 × 106 UC-MSCs/kg for 3 times. | Reduction of GGO, plasmablasts, creatinine, GOT, ferritin, D-dimer, and CRP levels. Increase in the absolute number of total lymphocytes, CD4+ T, and Treg lymphocytes. No adverse events were observed. | Senegaglia et al., 2021 [251] |
CRP: C-reactive protein; CT: computed tomography; GGO: ground-glass opacity; HRQL: health-related quality of life; HSCs: hematopoietic stem cells; NETs: neutrophil extracellular traps; PaO2/FiO2: partial pressure arterial of oxygen to fractional inspired oxygen; PL-MSCs: placental MSCs; SAE: serious adverse event; SDF-1: stromal cell-derived growth factor 1; KGF: keratinocyte growth factor; NGF: nerve growth factor; SpO2/FiO2: peripheral arterial oxygen saturation to FiO2; GOT: glutamic-oxaloacetic transaminase; VNN2: vascular noninflammatory molecule 2; WBCs: white blood cells.
Groups of patients enrolled for these trials were diagnosed with COVID-19-related ARDS, showing clinical symptoms from mild to critical, following the WHO severity classification described in the “Living guidance for clinical management of COVID-19” [15]. In particular, the clinical trials were mainly in early phases 1 to 2. Only one, a primary report of a two-center, open-label single-arm trial, arrived at a phase 2/3, using UC-MSCs or placental-derived MSCs [240]. The main administration route was a time-controlled IV infusion, while the pilot study, using exosomes, described an administration through nebulization [228], to easily reach the respiratory tract. Up to 210 patients were recruited and received IV infusion of 1–2 × 106 UC-MSCs/kg in a single dose performed in several hospitals in Turkey [242]. In many cases, the dosage was repeated for two or three times (48-72 h after the first dose). In all the studies, no SAEs relative to UC-MSCs or WJ-MSCs-derived exosomes transplantation were observed (even with repeated infusions), supporting the safety of this treatment. The other visible ameliorations were related to the increased rate of survival and reduction of hospitalization times, improvement of all COVID-19 symptoms, including oxygen saturation that returned to normal ranges as shown by improved PaO2/FiO2 (partial pressure arterial of oxygen to fractional inspired oxygen) and SpO2/FiO2 (peripheral arterial oxygen saturation to FiO2), reduction of lung lesions and fibrosis, reduction of GGO, and mechanical ventilation need [231,232,234,235,236,237,238,240,242,243,245,246,247,248,249,250,251]. In the Monsel et al. study, the PaO2/FiO2 ratio change between day 0 and day 7 increased in the treated group, but it was not significant compared to the control group, and there were no differences in 28-day mortality [239]. However, a possible explanation of this contrasting result could be the small number of participants, the different doses of cells received (one, two, or three injections of 0.9 ± 0.1 × 106 UC-MSCs/kg per dose) not considered in the final outcome, and the minor specific stratification based on the severity of their disease. Interestingly, Shu et al. also observed no progression from severe to critical illness with a faster restoration of symptoms of weakness, fatigue, shortness of breath, and low oxygen saturation after the treatment [231], together with significant decrease in CRP and IL-6 plasma level, normalization of lymphocyte count, and reduction of lung inflammation [232]. Reduced inflammatory state was determined by decreased levels of proinflammatory cytokines, such as IFN-γ, TNF-α, TNF-β, PDGF-BB, RANTES, sTNFR2, MCP-1, IP-10, IL-1RA, IL-5, IL-6, IL-8, IL-12, IL-17A, IL-17E/IL-25, IL-22, MIP-1, CXCL-1, reduction of CRP, D-dimer, ferritin, liver aminotransferase (GOT), MMPs, and an increase of IL-1β IL-10, LIF, SDF-1, KGF, and NGF [229,230,231,232,233,234,235,236,240,241,243,244,248,249,251]. Proangiogenic factor VEGF shows differences in plasma level when comparing different studies. For example, it increased in Dilogo et al. [230] and Adas et al. [243], but Saleh et al. [233] described a decreased level. The importance of VEGF expression in MSCs is linked to its biological action, in that VEGF is an angiogenic factor that promotes capillary regeneration and recovery of lung damage. Therefore, VEGF-expressing MSCs are viewed as a key factor protecting pulmonary vascular permeability [252]. On the other hand, a synergic action of hypoxia-induced angiogenic factors (VEGF, SDF-1, and ANG-II), hyperinflammation and cytokine storm, thrombosis, associated hemodynamic changes, and renin–angiotensin–aldosterone system dysregulation will trigger intussusceptive angiogenesis [253]. Moreover, COVID-19 patients showed high levels of SDF-1 associated with intussusceptive angiogenesis and T-lymphocytes infiltrates in lungs [64]. However, SDF-1 is also a key factor in facilitating MSCs homing and repair [254]. Nevertheless, the treatments with UC-MSCs resulted in normalization of immune cells infiltration with reduction of CD14+ monocytes, neutrophils, and NETs, and lymphocytes/neutrophils ratio, increase of CD3+, CD4+, CD8+, and Tregs [230,239,241,242,243,244,246], while NK cells were increased based on some studies [224,233] and decreased in another one [249]. In the case report published by da Silva et al., an increase of type-2 conventional dendritic cells (cDC2s) was found after UC-MSCs treatment [246]. This supports the immune modulating role of UC-MSCs, since it is known that type 1 DCs (DC1s) promote actions of CD8+, NK, and Th1 lymphocytes, while DC2s activate CD4+, Th1, Th2, Th17, and Treg lymphocytes [255]. Even the case report described by Senegaglia et al. showed a high absolute number of CD4+ and Treg, activated B-cells and plasmablasts (stimulating the production of specific SARS-CoV-2 antibodies), which is crucial in acute viral infections, but also a progressively decreased number of plasmablast at day 14 after cell treatment, demonstrating the patient’s recovery [251]. Even the use of the EVs alone, through nebulization, resulted in reduction of pulmonary lesions and a shorter hospitalization period in mild cases, and a twofold decrease was observed for the NK cells after exosome treatments [228].
Since UC-MSCs are HLA-G-expressing cells, they may contribute to modulate immune response in COVID-19 patients, even if the interaction with specific receptors in T cells, B cells, and macrophages (ILT-2, ILT-4, and KIR2DL4) in peripheral blood followed a high–low–high pattern, which may reflect the three subsequent stages of “infection”, “replication”, and “clearance” of SARS-CoV-2, as described in a single-patient study [256]. Further studies on HLA-G dynamics in a larger number of COVID-19 patients are necessary to better understand the potential role of this signaling cascade. UC-MSCs secrete LIF, which is able to directly oppose IL-6 activity and protect the lung function from injury [257]. Additionally, further studies are needed to better understand, for example, the pathways underlying VEGF and SDF-1 regulated by UC-MSCs to explain the contrasting functions of these molecules and to determine the meaning of some contradictory results found in the clinical trials.
Collectively, the use of UC-MSCs for treating patients affected by COVID-19, exhibiting ARDS-related symptoms and the risk of multiorgan failure have shown common evidence of amelioration of the critical signs that may lead to death, but limitations exist because of different severity of the illness of enrolled patients among the studies, and the concomitant use or not of other antiviral and/or inflammatory drugs, that could make it difficult to compare the results.
8. Discussion
The beginning of the zoonotic-related pandemic due to SARS-CoV-2 opened the door for the fragile healthcare system to face critical situations. The overload of the ICUs during COVID-19, the high rate of infectivity, the high number of deaths, and the lack of beds, led governments to declare lockdowns necessary worldwide, with all their economic and social consequences. SARS-CoV-2 will not be the last virus responsible for a pandemic, but what has been addressed during these last years has challenged the effectiveness of standard therapies, using well-known drugs. This aspect could be taken into account even to address both emerging and re-emerging infections. The beneficial properties of UC-MSCs (immunomodulation, immune modulatory, tissue repair, organ function recovery) exerted in COVID-19 patients may suggest their indication for the treatment of diseases which feature viral infection, inflammation/cytokine storm, and hypoxic microenvironmental conditions, present not only in lungs of patients infected by SARS-CoV-2, but also by other respiratory pathogens, or present in organs with failed functions due to other infectious diseases. Moreover, umbilical cord, even being one of the most MSCs-enriched tissues, is still considered a waste after childbirth, being therefore easily and painlessly obtained from young donors, weakening the concerns related to ethical issues. Nevertheless, it could be necessary to fill the gaps between the cure and the compliance with the regulations for the manufacture and clinical application of allogeneic UC-MSCs. In fact, UC-MSC-based products have been classified as advanced therapy medicinal products (ATMPs) since 2007, according to the European Regulation 1394/2007/EC [258]. Despite a series of completed clinical trials, only seven cord-blood-based cell therapies have received international approval from the Food and Drug Administration (FDA) [259]. However, UC-MSCs have been approved as interventional new drug (IND) in subjects with type 1 diabetes and Alzheimer’s disease [240]. In December 2014, the European Medicines Agency (EMA) recommended the first ATMP containing stem cells for approval in the European Union, regarding ex vivo expanded autologous human corneal epithelial cells containing stem cells [260]. Moreover, on 20 March 2017, orphan designation (EU/3/17/1852) was granted by the European Commission to Regulatory Resources Group Ltd., United Kingdom, for NiCord, allogeneic ex vivo-expanded umbilical-cord-blood-derived hematopoietic CD34+ progenitor cells and allogeneic nonexpanded umbilical-cord-blood-derived hematopoietic mature myeloid and lymphoid cells, for treatment in hematopoietic stem cell transplantation [261]. Up to date, most of the MSC-based ATMPs are composed of autologous BM-MSCs or AT-MSCs, and only a French manufacturing process of the UC-MSC-based ATMP was qualified and authorized by the French regulatory agency for clinical use [262]. However, even if all the findings reported in this review shed new light on the use of allogeneic UC-MSCs as ATMP or cell-free therapies (through EVs/exosomes), no UC-MSC-derived medicine has yet been authorized for marketing authorization by EMA. For these reasons, UC-MSC-based ATMP authorizations still need (i) to increase the number of studies related with safety and efficacy; (ii) to increase the number of participants enrolled in randomized controlled studies; (iii) to correctly stratify the patients; (iv) to standardize the dose of cell infused, the route of infusion, and the number of infusions at specific time points, following the long-term effects after the treatment; and (v) to standardize all the procedures regarding the investigational product, from cell isolation to the authorized final product, passing thorough the scale-up procedures in bioreactors under GMP regulations, and the choice of the best cell passage, all in order to reduce the discrepancies of outcomes between the trials, reduce contradictory results, and obtain authorizations. The development and manufacture of ATMPs are, at present, difficult, slow, and high in cost compared with production of traditional drugs, and UC-MSC-based ATMPs’ authorization, availability, assessment, and affordability are still the great challenges for both academic institutions and pharmaceutical companies.
9. Conclusions
It is now known that COVID-19 has become a global pandemic for which there is no specific cure for serious and/or critical patients. Initially, the clinical trials involved therapies with antiviral or immunomodulatory molecules, as well as neutralizing antibodies and immune plasma from convalescent patients for hospitalized patients with signs of ARDS and pathologies resulting from SARS-CoV-2. Nevertheless, these strategies resulted in poor outcomes and reproducibility. In this regard, MSCs could represent excellent candidates for their intrinsic immunomodulatory, anti-inflammatory, and reparative (antifibrosis and angiogenesis) properties, mediated through direct cell–cell interaction, the secretion of specific molecules, and the release of EVs. To date, encouraging results have been obtained from ongoing clinical trials involving the treatment of COVID-19 patients with adult and perinatal MSCs. However, it is important to underline that, compared to adult MSCs, perinatal MSCs possess a higher proliferative capacity with a lower risk of rejection, as well as ready availability through noninvasive procedures and without ethical issue. These are reasons that led us to discuss in this review the promising results obtained in therapies based on the infusion of UC-MSCs in patients affected with COVID-19. As we have observed, there are numerous advantages that can be acquired through the treatment with UC-MSCs or their derived EVs, such as the decrease in the levels of proinflammatory cytokines and the increase in the survival rate, combined with the robust safety and efficacy of the therapy. In conclusion, we can state that UC-MSCs could be a potent tool against phlogistic status, not only during the last pandemic caused by SARS-CoV-2, but also for other future pandemic or respiratory tract inflammatory diseases. However, a series of challenges still lies ahead, comprising the accomplishment of all the tests required by the regulatory bodies.
Author Contributions
Draft, original writing preparation: G.A., E.R., S.C., F.D.G. and G.L.R.; draft, illustrative material preparation: P.K. (Peter Kruzliak), V.M., M.C., P.G.C. and F.D.G.; writing, revision, and editing: G.A., E.R., S.C., P.K. (Peter Kubatka), V.M., P.G.C., F.D.G., C.V.B. and G.L.R.; supervision: P.G.C., F.D.G., C.V.B. and G.L.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
G.L.R. is a member of the scientific board of Auxocell Laboratories Inc. The other authors declare no conflict of interest.
Funding Statement
The authors’ research was supported by the University of Palermo (FFR 2022-2023) to G.L.R. and by the Italian Ministry of Research and University (MIUR) (PRIN 2017 program; grant number: 2017RSAK7_003) to G.L.R.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kilbourne E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006;12:9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.She J., Jiang J., Ye L., Hu L., Bai C., Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: Emerging attack and management strategies. Clin. Transl. Med. 2020;9:19. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Novel Coronavirus (2019-nCoV): Situation Report, 1. [(accessed on 21 December 2022)]. Available online: https://apps.who.int/iris/handle/10665/330760.
- 5.WHO Novel Coronavirus (2019-nCoV) Report-1. 2020. [(accessed on 21 December 2022)]. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4.
- 6.WHO Statement on the Second Meeting of the International Health Regulations. Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV) 2005. [(accessed on 21 December 2022)]. Available online: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- 7.Nie J., Li Q., Zhang L., Cao Y., Zhang Y., Li T., Wu J., Liu S., Zhang M., Zhao C., et al. Functional comparison of SARS-CoV-2 with closely related pangolin and bat coronaviruses. Cell Discov. 2021;7:21. doi: 10.1038/s41421-021-00256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Naming the Coronavirus Disease (COVID-19) and the Virus that Causes It. [(accessed on 21 December 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- 9.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. [(accessed on 21 December 2022)]. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Living Guidance for Clinical Management of COVID-19. [(accessed on 22 December 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf.
- 16.Samanipour R., Tabatabaee S., Delyanee M., Tavakoli A. The promising approach of MSCs therapy for COVID-19 treatment. Cell Tissue Bank. 2022;16:1–16. doi: 10.1007/s10561-022-10060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cozene B.M., Russo E., Anzalone R., Rocca G., Borlongan C.V. Mitochondrial activity of human umbilical cord mesenchymal stem cells. Brain. Circ. 2021;7:33–36. doi: 10.4103/bc.bc_15_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaghoubi Y., Movassaghpour A., Zamani M., Talebi M., Mehdizadeh A., Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. doi: 10.1016/j.lfs.2019.116733. [DOI] [PubMed] [Google Scholar]
- 19.Alzahrani F.A., Saadeldin I.M., Ahmad A., Kumar D., Azhar E.I., Siddiqui A.J., Kurdi B., Sajini A., Alrefaei A.F., Jahan S. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes as Immunomodulatory Agents for COVID-19 Patients. Stem Cells Int. 2020;2020:8835986. doi: 10.1155/2020/8835986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo E., Caprnda M., Kruzliak P., Conaldi P.G., Borlongan C.V., La Rocca G. Umbilical Cord Mesenchymal Stromal Cells for Cartilage Regeneration Applications. Stem Cells Int. 2022;2022:2454168. doi: 10.1155/2022/2454168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y.Z., Holmes E.C. A Genomic Perspective on the Origin and Emergence of SARS-CoV-2. Cell. 2020;181:223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison C.B., Edwards C.E., Shaffer K.M., Araba K.C., Wykoff J.A., Williams D.R., Asakura T., Dang H., Morton L.C., Gilmore R.C., et al. SARS-CoV-2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL-13. Proc. Natl. Acad. Sci. USA. 2022;119:e2119680119. doi: 10.1073/pnas.2119680119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra D., Suri G.S., Kaur G., Tiwari M. A comparative insight into genomic landscape of SARS-CoV-2 and identification of mutations associated with origin of infection and diversity. J. Med. Virol. 2020;93:2406–2419. doi: 10.1002/jmv.26744. [DOI] [PubMed] [Google Scholar]
- 25.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. USA. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise J. Covid-19: New coronavirus variant is identified in UK. BMJ. 2020;371:m4857. doi: 10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]
- 27.Scovino A., Dahab E., Vieira G., Freire-de-Lima L., Freire-de-Lima C., Morrot A. SARS-CoV-2’s Variants of Concern: A Brief Characterization. Front. Immunol. 2022;13:834098. doi: 10.3389/fimmu.2022.834098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanelli P., Trentini F., Guzzetta G., Marziano V., Mammone A., Sane Schepisi M., Poletti P., Molina Grane C., Manica M., Del Manso M., et al. Co-circulation of SARS-CoV-2 Alpha and Gamma variants in Italy, February and March 2021. Euro. Surveill. 2022;27:2100429. doi: 10.2807/1560-7917.ES.2022.27.5.2100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefanelli P., Trentini F., Petrone D., Mammone A., Ambrosio L., Manica M., Guzzetta G., d’Andrea V., Marziano V., Zardini A., et al. Tracking the progressive spread of the SARS-CoV-2 Omicron variant in Italy, December 2021 to January 2022. Euro. Surveill. 2022;27:2200125. doi: 10.2807/1560-7917.ES.2022.27.45.2200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Domenico M., De Rosa A., Di Gaudio F., Internicola P., Bettini C., Salzano N., Castrianni D., Marotta A., Boccellino M. Diagnostic Accuracy of a New Antigen Test for SARS-CoV-2 Detection. Int. J. Env. Res. Public Health. 2021;18:6310. doi: 10.3390/ijerph18126310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Gaudio F., Brunacci G., Contino F., Gallo A., Centineo F. Technical and health governance aspects of the External Quality Assessment Scheme for the SARS-CoV-2 molecular tests: Institutional experience performed in all clinical laboratories of a Regional Health Service. Clin. Chem. Lab. Med. 2023;61:173–179. doi: 10.1515/cclm-2022-0780. [DOI] [PubMed] [Google Scholar]
- 32.Delorey T.M., Ziegler C.G.K., Heimberg G., Normand R., Yang Y., Segerstolpe A., Abbondanza D., Fleming S.J., Subramanian A., Montoro D.T., et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021;595:107–113. doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liskova A., Samec M., Koklesova L., Samuel S.M., Zhai K., Al-Ishaq R.K., Abotaleb M., Nosal V., Kajo K., Ashrafizadeh M., et al. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021;138:111430. doi: 10.1016/j.biopha.2021.111430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S. SARS-CoV-2 Cell Entry Depends on ACE2 aTMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hussman J.P. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front. Pharmacol. 2020;11:1169. doi: 10.3389/fphar.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016–1035.e1019. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albini A., Di Guardo G., McClain Noonan D., Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: Implications for ACE-inhibitor-and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020;15:759–766. doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abobaker A., Raba A.A., Alzwi A. Extrapulmonary and atypical clinical presentations of COVID-19. J. Med. Virol. 2020;92:2458–2464. doi: 10.1002/jmv.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zirpe K.G., Dixit S., Kulkarni A.P., Sapra H., Kakkar G., Gupta R., Bansal A.R. Pathophysiological Mechanisms and Neurological Manifestations in COVID-19. Indian J. Crit. Care Med. 2020;24:975–980. doi: 10.5005/jp-journals-10071-23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jothimani D., Venugopal R., Abedin M.F., Kaliamoorthy I., Rela M. COVID-19 and the liver. J. Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuschieria S., Grech S. COVID-19 and diabetes: The why, the what and the how. J. Diabetes Complicat. 2020;34:107637. doi: 10.1016/j.jdiacomp.2020.107637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amraei R., Yin W., Napoleon M.A., Suder E.L., Berrigan J., Zhao Q., Olejnik J., Chandler K.B., Xia C., Feldman J., et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2. bioRxiv. 2021;7:1156–1165. doi: 10.1021/acscentsci.0c01537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao C., Zeng J., Jia N., Stavenhagen K., Matsumoto Y., Zhang H., Li J. SARS-CoV-2 Spike Protein Interacts with Multiple Innate Immune Receptors. BioRxiv. 2020 doi: 10.1101/2020.07.29.227462. preprint . [DOI] [Google Scholar]
- 46.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona A., Graham B.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamers M.M., Haagmans B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022;20:270–284. doi: 10.1038/s41579-022-00713-0. [DOI] [PubMed] [Google Scholar]
- 48.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients with Lung Cancer. J. Thorac. Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangalmurti N., Hunter C.A. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M.A., Endeman H., van den Akker J.P.C. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020;5:eabd2071. doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonaventura A., Vecchié A., Wang T., Lee E., Cremer P., Carey B., Rajendram P. Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies. Front. Immunol. 2020;11:1625. doi: 10.3389/fimmu.2020.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D.N., Leenen P.J., Liu Y.J., MacPherson G., Randolph G.J., et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 56.Thomas G., Tacke R., Hedrick C.C., Hanna R.N. Nonclassical patrolling monocyte function in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2015;35:1306–1316. doi: 10.1161/ATVBAHA.114.304650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Affandi A.J., Olesek K., Grabowska J., Nijen Twilhaar M.K., Rodriguez E., Saris A., Zwart E.S., Nossent E.J., Kalay H., de Kok M., et al. CD169 Defines Activated CD14(+) Monocytes With Enhanced CD8(+) T Cell Activation Capacity. Front. Immunol. 2021;12:697840. doi: 10.3389/fimmu.2021.697840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abassi Z., Knaney Y., Karram T., Heyman S. The Lung Macrophage in SARS-CoV-2 Infection: A Friend or a Foe? Front. Immunol. 2020;11:1312. doi: 10.3389/fimmu.2020.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., Hirayama A.V., Mastroiani F., Turtle C.J., Harhay M.O., et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rostami M., Mansouritorghabeh H. D-dimer level in COVID-19 infection: A systematic review. Expert Rev. Hematol. 2020;13:1265–1275. doi: 10.1080/17474086.2020.1831383. [DOI] [PubMed] [Google Scholar]
- 61.Gelzo M., Cacciapuoti S., Pinchera B., De Rosa A., Cernera G., Scialo F., Comegna M., Mormile M., Fabbrocini G., Parrella R., et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci. Rep. 2022;12:1212. doi: 10.1038/s41598-021-04677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ong S.W.X., Fong S.W., Young B.E., Chan Y.H., Lee B., Amrun S.N., Chee R.S., Yeo N.K., Tambyah P., Pada S., et al. Persistent Symptoms and Association With Inflammatory Cytokine Signatures in Recovered Coronavirus Disease 2019 Patients. Open Forum Infect. Dis. 2021;8:ofab156. doi: 10.1093/ofid/ofab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruhl L., Pink I., Kuhne J.F., Beushausen K., Keil J., Christoph S., Sauer A., Boblitz L., Schmidt J., David S., et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct. Target. Ther. 2021;6:418. doi: 10.1038/s41392-021-00819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackermann M., Mentzer S.J., Kolb M., Jonigk D. Inflammation and intussusceptive angiogenesis in COVID-19: Everything in and out of flow. Eur. Respir. J. 2020;56:2003147. doi: 10.1183/13993003.03147-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eslamifar Z., Behzadifard M., Soleimani M., Behzadifard S. Coagulation abnormalities in SARS-CoV-2 infection: Overexpression tissue factor. Thromb. J. 2020;18:38. doi: 10.1186/s12959-020-00250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miesbach W. Pathological Role of Angiotensin II in Severe COVID-19. TH Open. 2020;4:e138–e144. doi: 10.1055/s-0040-1713678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eguchi S., Kawai T., Scalia R., Rizzo V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension. 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beltran-Garcia J., Osca-Verdegal R., Pallardo F.V., Ferreres J., Rodriguez M., Mulet S., Sanchis-Gomar F., Carbonell N., Garcia-Gimenez J.L. Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants. 2020;9:936. doi: 10.3390/antiox9100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montiel V., Lobysheva I., Gerard L., Vermeersch M., Perez-Morga D., Castelein T., Mesland J.B., Hantson P., Collienne C., Gruson D., et al. Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients. EBioMedicine. 2022;77:103893. doi: 10.1016/j.ebiom.2022.103893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murakami M., Kamimura D., Hirano T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity. 2019;50:812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 71.Rupérez M., Lorenzo O., Blanco-Colio L.M., Esteban V., Egido J., Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003;108:1499–1505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- 72.Mauviel A. Transforming growth factor-beta: A key mediator of fibrosis. Methods Mol. Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- 73.Hirano T., Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hrenak J., Simko F. Renin–Angiotensin System: An Important Player in the Pathogenesis of Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020;21:8038. doi: 10.3390/ijms21218038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murphy A.M., Wong A.L., Bezuhlly M. Modulation of angiotensin II signaling in the prevention of fibrosis. Fibrogenesis Tissue Repair. 2015;8:7. doi: 10.1186/s13069-015-0023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao W., Li H., Li J., Xu B., Xu J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J. Med. Virol. 2022;94:1886–1892. doi: 10.1002/jmv.27627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schunemann H.J., COVID-19 Systematic Urgent Review Group Effort (SURGE) Study Authors Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon S., Joshi A.D., Lo C.H., Drew D.A., Nguyen L.H., Guo C.G., Ma W., Mehta R.S., Warner E.T., Astley C.M., et al. Association of social distancing and masking with risk of COVID-19. medRxiv. 2020;12:3737. doi: 10.1101/2020.11.11.20229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Piechotta V., Iannizzi C., Chai K.L., Valk S.J., Kimber C., Dorando E., Monsef I., Wood E.M., Lamikanra A.A., Roberts D.J., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qian Z., Zhang Z., Ma H., Shao S., Kang H., Tong Z. The efficiency of convalescent plasma in COVID-19 patients: A systematic review and meta-analysis of randomized controlled clinical trials. Front. Immunol. 2022;13:964398. doi: 10.3389/fimmu.2022.964398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang C., Wang Y., Lv H., Guan Z., Gu J. Caution against corticosteroid-based COVID-19 treatment. Lancet. 2020;395:1759–1760. doi: 10.1016/S0140-6736(20)30749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shuto H., Komiya K., Yamasue M., Uchida S., Ogura T., Mukae H., Tateda K., Hiramatsu K., Kadota J.I. A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19. Sci. Rep. 2020;10:20935. doi: 10.1038/s41598-020-78054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minkoff J.M., tenOever B. Innate immune evasion strategies of SARS-CoV-2. Nat. Rev. Microbiol. 2023;21:178–194. doi: 10.1038/s41579-022-00839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubbert-Roth A., Furst D.E., Nebesky J.M., Jin A., Berber E. A Review of Recent Advances Using Tocilizumab in the Treatment of Rheumatic Diseases. Rheumatol. Ther. 2018;5:21–42. doi: 10.1007/s40744-018-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.WHO WHO Prequalifies First Monoclonal Antibody—Tocilizumab—To Treat COVID-19. [(accessed on 21 December 2022)]. Available online: https://www.who.int/news/item/11-02-2022-who-prequalifies-first-monoclonal-antibody---tocilizumab-to-treat-covid-19.
- 86.Brown M.J., Alazawi W., Kanoni S. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021;385:1147. doi: 10.1056/NEJMc2108482. [DOI] [PubMed] [Google Scholar]
- 87.Rosas I.O., Brau N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N. Engl. J. Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shankar-Hari M., Vale C.L., Godolphin P.J., Fisher D., Higgins J.P.T., Spiga F., Savovic J., Tierney J., Baron G., The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campochiaro C., Tomelleri A., Matucci-Cerinic M., Dagna L. One year later: The case of tocilizumab in COVID-19. Eur. J. Intern. Med. 2022;95:5–6. doi: 10.1016/j.ejim.2021.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.WHO Therapeutics and COVID-19: Living Guideline, 16 September 2022. [(accessed on 21 December 2022)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.5.
- 91.Focosi D., McConnell S., Casadevall A. The Omicron variant of concern: Diversification and convergent evolution in spike protein, and escape from anti-Spike monoclonal antibodies. Drug. Resist. Updat. 2022;65:100882. doi: 10.1016/j.drup.2022.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.WHO TAG-VE Statement on Omicron Sublineages BQ.1 and XBB. [(accessed on 28 December 2022)]. Available online: https://www.who.int/news/item/27-10-2022-tag-ve-statement-on-omicron-sublineages-bq.1-and-xbb.
- 93.Imai M., Ito M., Kiso M., Yamayoshi S., Uraki R., Fukushi S., Watanabe S., Suzuki T., Maeda K., Sakai-Tagawa Y., et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023;388:89–91. doi: 10.1056/NEJMc2214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Radermacher P., Maggiore S.M., Mercat A. Fifty Years of Research in ARDS. Gas Exchange in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017;196:964–984. doi: 10.1164/rccm.201610-2156SO. [DOI] [PubMed] [Google Scholar]
- 95.Touchon F., Trigui Y., Prud’homme E., Lefebvre L., Giraud A., Dols A.M., Martinez S., Bernardi M., Begne C., Granier P., et al. Awake prone positioning for hypoxaemic respiratory failure: Past, COVID-19 and perspectives. Eur. Respir. Rev. 2021;30:210022. doi: 10.1183/16000617.0022-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cabrera-Benitez N.E., Laffey J.G., Parotto M., Spieth P.M., Villar J., Zhang H., Slutsky A.S. Mechanical Ventilation–associated Lung Fibrosis in Acute Respiratory Distress Syndrome A Significant Contributor to Poor Outcome. Anesthesiology. 2014;121:189–198. doi: 10.1097/ALN.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pierrakos C., Karanikolas M., Scolletta S., Karamouzos V., Velissaris D. Acute Respiratory Distress Syndrome: Pathophysiology and Therapeutic Options. J. Clin. Med. Res. 2012;4:7–16. doi: 10.4021/jocmr761w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hajage D., Combes A., Guervilly C., Lebreton G., Mercat A., Pavot A., Nseir S., Mekontso-Dessap A., Mongardon N., Mira J.P., et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome Associated with COVID-19: An Emulated Target Trial Analysis. Am. J. Respir. Crit. Care Med. 2022;206:281–294. doi: 10.1164/rccm.202111-2495OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bertini P., Guarracino F., Falcone M., Nardelli P., Landoni G., Nocci M., Paternoster G. ECMO in COVID-19 Patients: A Systematic Review and Meta-analysis. J. Cardiothorac. Vasc. Anesth. 2022;36:2700–2706. doi: 10.1053/j.jvca.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fanelli V., Giani M., Grasselli G., Mojoli F., Martucci G., Grazioli L., Alessandri F., Mongodi S., Sales G., Montrucchio G., et al. Extracorporeal membrane oxygenation for COVID-19 and influenza H1N1 associated acute respiratory distress syndrome: A multicenter retrospective cohort study. Crit. Care. 2022;26:34. doi: 10.1186/s13054-022-03906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.WHO The COVID-19 Vaccine Tracker and Landscape Compiles Detailed Information of Each COVID-19 Vaccine Candidate in Development by Closely Monitoring Their Progress through the Pipeline. [(accessed on 5 January 2023)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 103.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020. online ahead of print . [DOI] [PMC free article] [PubMed]
- 104.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020;383:920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grana C., Ghosn L., Evrenoglou T., Jarde A., Minozzi S., Bergman H., Buckley B.S., Probyn K., Villanueva G., Henschke N., et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022;12:CD015477. doi: 10.1002/14651858.CD015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Busa R., Miele M., Sorrentino M.C., Amico G., Timoneri F., Miceli V., Di Bella M., Russelli G., Gallo A., Zito G., et al. Long-Term Effectiveness of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine on B Cell Compartment: Efficient Recall of SARS-CoV-2-Specific Memory B Cells. Int. J. Mol. Sci. 2022;23:15046. doi: 10.3390/ijms232315046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ailsworth S.M., Keshavarz B., Richards N.E., Workman L.J., Murphy D.D., Nelson M.R., Platts-Mills T.A.E., Wilson J.M. Enhanced SARS-CoV-2 IgG durability following COVID-19 mRNA booster vaccination and comparison of BNT162b2 with mRNA-1273. Ann. Allergy Asthma Immunol. 2023;130:67–73. doi: 10.1016/j.anai.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Busa R., Russelli G., Miele M., Sorrentino M.C., Di Bella M., Timoneri F., Di Mento G., Mularoni A., Vitulo P., Conaldi P.G., et al. Immune Response after the Fourth Dose of SARS-CoV-2 mRNA Vaccine Compared to Natural Infection in Three Doses’ Vaccinated Solid Organ Transplant Recipients. Viruses. 2022;14:2299. doi: 10.3390/v14102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Emary K., Golubchik T., Aley P., Ariani C., Angus B., Bibi S., Blane B. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krause P., Fleming T., Peto R., Longini I., Figueroa J., Sterne J., Cravioto A. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398:1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 112.Hoang D.M., Pham P.T., Bach T.Q., Ngo A.T.L., Nguyen Q.T., Phan T.T.K., Nguyen G.H., Le P.T.T., Hoang V.T., Forsyth N.R., et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022;7:272. doi: 10.1038/s41392-022-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Friedenstein A.J., Gorskaja J.F., Kulagina N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 114.Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 115.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 116.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 117.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang G.T., Gronthos S., Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chan R.W., Schwab K.E., Gargett C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 121.Lenero C., Bowles A.C., Correa D., Kouroupis D. Characterization and response to inflammatory stimulation of human endometrial-derived mesenchymal stem/stromal cells. Cytotherapy. 2022;24:124–136. doi: 10.1016/j.jcyt.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 122.Meng X., Ichim T.E., Zhong J., Rogers A., Yin Z., Jackson J., Wang H., Ge W., Bogin V., Chan K.W., et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen L., Qu J., Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res. Ther. 2019;10:1. doi: 10.1186/s13287-018-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pang Q.M., Yang R., Zhang M., Zou W.H., Qian N.N., Xu Q.J., Chen H., Peng J.C., Luo X.P., Zhang Q., et al. Peripheral Blood-Derived Mesenchymal Stem Cells Modulate Macrophage Plasticity through the IL-10/STAT3 Pathway. Stem Cells Int. 2022;2022:5181241. doi: 10.1155/2022/5181241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rotter N., Oder J., Schlenke P., Lindner U., Bohrnsen F., Kramer J., Rohwedel J., Huss R., Brandau S., Wollenberg B., et al. Isolation and characterization of adult stem cells from human salivary glands. Stem Cells Dev. 2008;17:509–518. doi: 10.1089/scd.2007.0180. [DOI] [PubMed] [Google Scholar]
- 126.Moon J.H., Kim H.R., Lim J.Y., Lim Y.C. Single clonal glandular stem cells derived from human parotid glands do not attain malignant phenotype during long-term in vitro culture. Neoplasma. 2021;68:1139–1146. doi: 10.4149/neo_2021_210302N272. [DOI] [PubMed] [Google Scholar]
- 127.Tappenbeck N., Schroder H.M., Niebergall-Roth E., Hassinger F., Dehio U., Dieter K., Kraft K., Kerstan A., Esterlechner J., Frank N.Y., et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy. 2019;21:546–560. doi: 10.1016/j.jcyt.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vander Beken S., de Vries J.C., Meier-Schiesser B., Meyer P., Jiang D., Sindrilaru A., Ferreira F.F., Hainzl A., Schatz S., Muschhammer J., et al. Newly Defined ATP-Binding Cassette Subfamily B Member 5 Positive Dermal Mesenchymal Stem Cells Promote Healing of Chronic Iron-Overload Wounds via Secretion of Interleukin-1 Receptor Antagonist. Stem Cells. 2019;37:1057–1074. doi: 10.1002/stem.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Najar M., Lagneaux L. Foreskin as a source of immunotherapeutic mesenchymal stromal cells. Immunotherapy. 2017;9:207–217. doi: 10.2217/imt-2016-0093. [DOI] [PubMed] [Google Scholar]
- 130.Najar M., Merimi M., Faour W.H., Lombard C.A., Moussa Agha D., Ouhaddi Y., Sokal E.M., Lagneaux L., Fahmi H. In Vitro Cellular and Molecular Interplay between Human Foreskin-Derived Mesenchymal Stromal/Stem Cells and the Th17 Cell Pathway. Pharmaceutics. 2021;13:1736. doi: 10.3390/pharmaceutics13101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neybecker P., Henrionnet C., Pape E., Mainard D., Galois L., Loeuille D., Gillet P., Pinzano A. In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018;9:329. doi: 10.1186/s13287-018-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Amemiya M., Tsuji K., Katagiri H., Miyatake K., Nakagawa Y., Sekiya I., Muneta T., Koga H. Synovial fluid-derived mesenchymal cells have non-inferior chondrogenic potential and can be utilized for regenerative therapy as substitute for synovium-derived cells. BioChem. Biophys. Res. Commun. 2020;523:465–472. doi: 10.1016/j.bbrc.2019.12.068. [DOI] [PubMed] [Google Scholar]
- 133.Asakura A., Komaki M., Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 134.Kang J.S., Krauss R.S. Muscle stem cells in developmental and regenerative myogenesis. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:243–248. doi: 10.1097/MCO.0b013e328336ea98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elashry M.I., Kinde M., Klymiuk M.C., Eldaey A., Wenisch S., Arnhold S. The effect of hypoxia on myogenic differentiation and multipotency of the skeletal muscle-derived stem cells in mice. Stem Cell Res. Ther. 2022;13:56. doi: 10.1186/s13287-022-02730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Funderburgh J.L., Funderburgh M.L., Du Y. Stem Cells in the Limbal Stroma. Ocul. Surf. 2016;14:113–120. doi: 10.1016/j.jtos.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eslani M., Putra I., Shen X., Hamouie J., Afsharkhamseh N., Besharat S., Rosenblatt M.I., Dana R., Hematti P., Djalilian A.R. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investig. Ophthalmol. Vis. Sci. 2017;58:5507–5517. doi: 10.1167/iovs.17-22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Messina E., De Angelis L., Frati G., Morrone S., Chimenti S., Fiordaliso F., Salio M., Battaglia M., Latronico M.V., Coletta M., et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ. Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 139.Anzalone R., Corrao S., Lo Iacono M., Loria T., Corsello T., Cappello F., Di Stefano A., Giannuzzi P., Zummo G., Farina F., et al. Isolation and characterization of CD276+/HLA-E+ human subendocardial mesenchymal stem cells from chronic heart failure patients: Analysis of differentiative potential and immunomodulatory markers expression. Stem Cells Dev. 2013;22:1–17. doi: 10.1089/scd.2012.0402. [DOI] [PubMed] [Google Scholar]
- 140.Rolandsson Enes S., Andersson Sjöland A., Skog I., Hansson L., Larsson H., Le Blanc K., Eriksson L. MSC from fetal and adult lungs possess lung-specific properties compared to bone marrow-derived MSC. Sci. Rep. 2016;6:29160. doi: 10.1038/srep29160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hass R., Kasper C., Bohm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soncini M., Vertua E., Gibelli L., Zorzi F., Denegri M., Albertini A., Wengler G.S., Parolini O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007;1:296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- 143.Pampalone M., Corrao S., Amico G., Vitale G., Alduino R., Conaldi P.G., Pietrosi G. Human Amnion-Derived Mesenchymal Stromal Cells in Cirrhotic Patients with Refractory Ascites: A Possible Anti-Inflammatory Therapy for Preventing Spontaneous Bacterial Peritonitis. Stem Cell Rev. Rep. 2021;17:981–998. doi: 10.1007/s12015-020-10104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miceli V., Chinnici C.M., Bulati M., Pampalone M., Amico G., Schmelzer E., Gerlach J.C., Conaldi P.G. Comparative study of the production of soluble factors in human placenta-derived mesenchymal stromal/stem cells grown in adherent conditions or as aggregates in a catheter-like device. BioChem. Biophys. Res. Commun. 2020;522:171–176. doi: 10.1016/j.bbrc.2019.11.069. [DOI] [PubMed] [Google Scholar]
- 145.De Coppi P., Bartsch G., Jr., Siddiqui M.M., Xu T., Santos C.C., Perin L., Mostoslavsky G., Serre A.C., Snyder E.Y., Yoo J.J., et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 146.Lee O.K., Kuo T.K., Chen W.M., Lee K.D., Hsieh S.L., Chen T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 147.Corrao S., La Rocca G., Lo Iacono M., Corsello T., Farina F., Anzalone R. Umbilical cord revisited: From Wharton’s jelly myofibroblasts to mesenchymal stem cells. Histol. Histopathol. 2013;28:1235–1244. doi: 10.14670/HH-28.1235. [DOI] [PubMed] [Google Scholar]
- 148.Avanzini M.A., Mura M., Percivalle E., Bastaroli F., Croce S., Valsecchi C., Lenta E., Nykjaer G., Cassaniti I., Bagnarino J., et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl. Med. 2021;10:636–642. doi: 10.1002/sctm.20-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Ugarte D.A., Morizono K., Elbarbary A., Alfonso Z., Zuk P.A., Zhu M., Dragoo J.L., Ashjian P., Thomas B., Benhaim P., et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 150.Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells—Current trends and future prospective. BioSci. Rep. 2015;35:e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zeddou M., Briquet A., Relic B., Josse C., Malaise M.G., Gothot A., Lechanteur C., Beguin Y. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol. Int. 2010;34:693–701. doi: 10.1042/CBI20090414. [DOI] [PubMed] [Google Scholar]
- 152.Vangsness C.T., Jr., Sternberg H., Harris L. Umbilical Cord Tissue Offers the Greatest Number of Harvestable Mesenchymal Stem Cells for Research and Clinical Application: A Literature Review of Different Harvest Sites. Arthroscopy. 2015;31:1836–1843. doi: 10.1016/j.arthro.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 153.Arutyunyan I., Elchaninov A., Makarov A., Fatkhudinov T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016;2016:6901286. doi: 10.1155/2016/6901286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Facchin F., Bianconi E., Romano M., Impellizzeri A., Alviano F., Maioli M., Canaider S., Ventura C. Comparison of Oxidative Stress Effects on Senescence Patterning of Human Adult and Perinatal Tissue-Derived Stem Cells in Short and Long-term Cultures. Int. J. Med. Sci. 2018;15:1486–1501. doi: 10.7150/ijms.27181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Watson N., Divers R., Kedar R., Mehindru A., Mehindru A., Borlongan M.C., Borlongan C.V. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy. 2015;17:18–24. doi: 10.1016/j.jcyt.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Davies J.E., Walker J.T., Keating A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2017;6:1620–1630. doi: 10.1002/sctm.16-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.La Rocca G., Anzalone R., Corrao S., Magno F., Loria T., Lo Iacono M., Di Stefano A., Giannuzzi P., Marasà L., Cappello F., et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: Differentiation potential and detection of new markers. Histochem. Cell Biol. 2009;131:267–282. doi: 10.1007/s00418-008-0519-3. [DOI] [PubMed] [Google Scholar]
- 158.Lo Iacono M., Russo E., Anzalone R., Baiamonte E., Alberti G., Gerbino A., Maggio A. Wharton’s Jelly Mesenchymal Stromal Cells Support the Expansion of Cord Blood–derived CD34+ Cells Mimicking a Hematopoietic Niche in a Direct Cell–cell Contact Culture System. Cell Transpl. 2018;27:117–129. doi: 10.1177/0963689717737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Silini A.R., Di Pietro R., Lang-Olip I., Alviano F., Banerjee A., Basile M., Borutinskaite V., Eissner G., Gellhaus A., Giebel B., et al. Perinatal Derivatives: Where Do We Stand? A Roadmap of the Human Placenta and Consensus for Tissue and Cell Nomenclature. Front. Bioeng. Biotechnol. 2020;8:610544. doi: 10.3389/fbioe.2020.610544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Magatti M., Abumaree M.H., Silini A.R., Anzalone R., Saieva S., Russo E., Trapani M.E., La Rocca G., Parolini O. Chapter 6. The Immunomodulatory Features of Mesenchymal Stromal Cells Derived from Wharton’s Jelly, Amniotic Membrane, and Chorionic Villi: In Vitro and In Vivo Data. In: Parolini O., editor. Placenta: The Tree of Life. CRC Press; Boca Raton, FL, USA: 2016. pp. 91–128. [Google Scholar]
- 161.Anzalone R., Lo Iacono M., Loria T., Di Stefano A., Giannuzzi P., Farina F., La Rocca G. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: Extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. 2011;7:342–363. doi: 10.1007/s12015-010-9196-4. [DOI] [PubMed] [Google Scholar]
- 162.Bharti D., Shivakumar S.B., Park J.K., Ullah I., Subbarao R.B., Park J.S., Lee S.L., Park B.W., Rho G.J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018;372:51–65. doi: 10.1007/s00441-017-2699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.La Rocca G., Lo Iacono M., Corsello T., Corrao S., Farina F., Anzalone R. Human Wharton’s jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: New perspectives for cellular therapy. Curr. Stem Cell Res. Ther. 2013;8:100–113. doi: 10.2174/1574888X11308010012. [DOI] [PubMed] [Google Scholar]
- 164.Corrao S., La Rocca G., Lo Iacono M., Zummo G., Gerbino A., Farina F., Anzalone R. New frontiers in regenerative medicine in cardiology: The potential of Wharton’s jelly mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2013;8:39–45. doi: 10.2174/1574888X11308010006. [DOI] [PubMed] [Google Scholar]
- 165.Kwon A., Kim Y., Kim M., Kim J., Choi H., Jekarl D.W., Lee S., Kim J.M., Shin J.C., Park I.Y. Tissue-specific Differentiation Potency of Mesenchymal Stromal Cells from Perinatal Tissues. Sci. Rep. 2016;6:23544. doi: 10.1038/srep23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Baksh D., Yao R., Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 167.Han Y., Chai J., Sun T., Li D., Tao R. Differentiation of human umbilical cord mesenchymal stem cells into dermal fibroblasts in vitro. BioChem. Biophys. Res. Commun. 2011;413:561–565. doi: 10.1016/j.bbrc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 168.Conconi M.T., Burra P., Di Liddo R., Calore C., Turetta M., Bellini S., Bo P., Nussdorfer G.G., Parnigotto P.P. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int. J. Mol. Med. 2006;18:1089–1096. doi: 10.3892/ijmm.18.6.1089. [DOI] [PubMed] [Google Scholar]
- 169.Wu K.H., Mo X.M., Zhou B., Lu S.H., Yang S.G., Liu Y.L., Han Z.C. Cardiac potential of stem cells from whole human umbilical cord tissue. J. Cell Biochem. 2009;107:926–932. doi: 10.1002/jcb.22193. [DOI] [PubMed] [Google Scholar]
- 170.Campard D., Lysy P.A., Najimi M., Sokal E.M. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 171.Anzalone R., Iacono M.L., Corrao S., Magno F., Loria T., Cappello F., Zummo G., Farina F., La Rocca G. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: Immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010;19:423–438. doi: 10.1089/scd.2009.0299. [DOI] [PubMed] [Google Scholar]
- 172.Belame Shivakumar S., Bharti D., Baregundi Subbarao R., Park J.M., Son Y.B., Ullah I., Choe Y.H., Lee H.J., Park B.W., Lee S.L., et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton’s jelly mesenchymal stem cells using small molecules. J. Cell Physiol. 2019;234:3933–3947. doi: 10.1002/jcp.27184. [DOI] [PubMed] [Google Scholar]
- 173.Sarang S., Viswanathan C. Umbilical Cord Derived Mesenchymal Stem Cells Useful in Insulin Production—Another Opportunity in Cell Therapy. Int. J. Stem Cells. 2016;9:60–69. doi: 10.15283/ijsc.2016.9.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mitchell K.E., Weiss M.L., Mitchell B.M., Martin P., Davis D., Morales L., Helwig B., Beerenstrauch M., Abou-Easa K., Hildreth T., et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 175.Corsello T., Amico G., Corrao S., Anzalone R., Timoneri F., Lo Iacono M., Russo E. Wharton’s Jelly Mesenchymal Stromal Cells from Human Umbilical Cord: A Close-up on Immunomodulatory Molecules Featured In Situ and In Vitro. Stem Cell Rev. Rep. 2019;15:900–918. doi: 10.1007/s12015-019-09907-1. [DOI] [PubMed] [Google Scholar]
- 176.Tesarova L., Jaresova K., Simara P., Koutna I. Umbilical Cord-Derived Mesenchymal Stem Cells Are Able to Use bFGF Treatment and Represent a Superb Tool for Immunosuppressive Clinical Applications. Int. J. Mol. Sci. 2020;21:5366. doi: 10.3390/ijms21155366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Weiss M.L., Anderson C., Medicetty S., Seshareddy K.B., Weiss R.J., VanderWerff I., Troyer D., McIntosh K.R. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865–2874. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 178.Yang Y., Wang W., Weng J., Li H., Ma Y., Liu L., Ma W. Advances in the study of HLA class Ib in maternal-fetal immune tolerance. Front. Immunol. 2022;13:976289. doi: 10.3389/fimmu.2022.976289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhao Y., Zheng Q., Jin L. The Role of B7 Family Molecules in Maternal-Fetal Immunity. Front. Immunol. 2020;11:458. doi: 10.3389/fimmu.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sawai K., Matsuzaki N., Kameda T., Hashimoto K., Okada T., Shimoya K., Nobunaga T., Taga T., Kishimoto T., Saji F. Leukemia inhibitory factor produced at the fetomaternal interface stimulates chorionic gonadotropin production: Its possible implication during pregnancy, including implantation period. J. Clin. Endocrinol. Metab. 1995;80:1449–1456. doi: 10.1210/jcem.80.4.7714123. [DOI] [PubMed] [Google Scholar]
- 181.Hamelin-Morrissette J., Dallagi A., Girouard J., Ravelojaona M., Oufqir Y., Vaillancourt C., Van Themsche C., Carrier C., Reyes-Moreno C. Leukemia inhibitory factor regulates the activation of inflammatory signals in macrophages and trophoblast cells. Mol. Immunol. 2020;120:32–42. doi: 10.1016/j.molimm.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 182.Kudo Y., Koh I., Sugimoto J. Localization of Indoleamine 2,3-Dioxygenase-1 and Indoleamine 2,3-Dioxygenase-2 at the Human Maternal-Fetal Interface. Int. J. Tryptophan. Res. 2020;13:1178646920984163. doi: 10.1177/1178646920984163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tirado-Gonzalez I., Freitag N., Barrientos G., Shaikly V., Nagaeva O., Strand M., Kjellberg L., Klapp B.F., Mincheva-Nilsson L., Cohen M., et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013;19:43–53. doi: 10.1093/molehr/gas043. [DOI] [PubMed] [Google Scholar]
- 184.Rouas-Freiss N., Goncalves R.M., Menier C., Dausset J., Carosella E.D. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA. 1997;94:11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.LeMaoult J., Zafaranloo K., Le Danff C., Carosella E.D. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005;19:662–664. doi: 10.1096/fj.04-1617fje. [DOI] [PubMed] [Google Scholar]
- 186.Gieseke F., Bohringer J., Bussolari R., Dominici M., Handgretinger R., Muller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–3779. doi: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- 187.McGuirk J.P., Smith J.R., Divine C.L., Zuniga M., Weiss M.L. Wharton’s Jelly-Derived Mesenchymal Stromal Cells as a Promising Cellular Therapeutic Strategy for the Management of Graft-versus-Host Disease. Pharmaceuticals. 2015;8:196–220. doi: 10.3390/ph8020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dunavin N., Dias A., Li M., McGuirk J. Mesenchymal Stromal Cells: What Is the Mechanism in Acute Graft-Versus-Host Disease? Biomedicines. 2017;5:39. doi: 10.3390/biomedicines5030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cheng J.G., Chen J.R., Hernandez L., Alvord W.G., Stewart C.L. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc. Natl. Acad. Sci. USA. 2001;98:8680–8685. doi: 10.1073/pnas.151180898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Janssens K., Van den Haute C., Baekelandt V., Lucas S., van Horssen J., Somers V., Van Wijmeersch B. Leukemia inhibitory factor tips the immune balance towards regulatory T cells in multiple sclerosis. Brain. Behav. Immun. 2015;45:180–188. doi: 10.1016/j.bbi.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 191.Elias J.A., Zheng T., Whiting N.L., Marcovici A., Trow T.K. Cytokine-cytokine synergy and protein kinase C in the regulation of lung fibroblast leukemia inhibitory factor. Am. J. Physiol. 1994;266:L426–L435. doi: 10.1152/ajplung.1994.266.4.L426. [DOI] [PubMed] [Google Scholar]
- 192.Corrao S., Campanella C., Anzalone R., Farina F., Zummo G., Conway de Macario E., Macario A.J., Cappello F., La Rocca G. Human Hsp10 and Early Pregnancy Factor (EPF) and their relationship and involvement in cancer and immunity: Current knowledge and perspectives. Life Sci. 2010;86:145–152. doi: 10.1016/j.lfs.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 193.Corrao S., Anzalone R., Lo Iacono M., Corsello T., Di Stefano A., D’Anna S.E., Balbi B., Carone M., Sala A., Corona D., et al. Hsp10 nuclear localization and changes in lung cells response to cigarette smoke suggest novel roles for this chaperonin. Open Biol. 2014;4:140125. doi: 10.1098/rsob.140125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tsai P.J., Wang H.S., Lin G.J., Chou S.C., Chu T.H., Chuan W.T., Lu Y.J. Undifferentiated Wharton’s Jelly Mesenchymal Stem Cell Transplantation Induces Insulin-Producing Cell Differentiation and Suppression of T-Cell-Mediated Autoimmunity in Nonobese Diabetic Mice. Cell Transpl. 2015;24:1555–1570. doi: 10.3727/096368914X683016. [DOI] [PubMed] [Google Scholar]
- 195.Hsieh J.Y., Wang H.W., Chang S.J., Liao K.H., Lee I.H., Lin W.S., Wu C.H., Lin W.Y., Cheng S.M. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE. 2013;8:e72604. doi: 10.1371/journal.pone.0072604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Arutyunyan I., Fatkhudinov T., Kananykhina E., Usman N., Elchaninov A., Makarov A., Bolshakova G., Goldshtein D., Sukhikh G. Role of VEGF-A in angiogenesis promoted by umbilical cord-derived mesenchymal stromal/stem cells: In vitro study. Stem Cell Res. Ther. 2016;7:46. doi: 10.1186/s13287-016-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Caley M.P., Martins V.L., O’Toole E.A. Metalloproteinases and Wound Healing. Adv. Wound. Care. 2015;4:225–234. doi: 10.1089/wound.2014.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Edwards S.S., Zavala G., Prieto C.P., Elliott M., Martinez S., Egana J.T., Bono M.R., Palma V. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton’s jelly in dermal regeneration. Angiogenesis. 2014;17:851–866. doi: 10.1007/s10456-014-9432-7. [DOI] [PubMed] [Google Scholar]
- 199.Lavrentieva A., Majore I., Kasper C., Hass R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010;8:18. doi: 10.1186/1478-811X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Russo E., Lee J.Y., Nguyen H., Corrao S., Anzalone R., La Rocca G., Borlongan C.V. Energy Metabolism Analysis of Three Different Mesenchymal Stem Cell Populations of Umbilical Cord Under Normal and Pathologic Conditions. Stem Cell Rev. Rep. 2020;16:585–595. doi: 10.1007/s12015-020-09967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Russo E., Napoli E., Borlongan C.V. Healthy mitochondria for stroke cells. Brain Circ. 2018;4:95–98. doi: 10.4103/bc.bc_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lin H.Y., Liou C.W., Chen S.D., Hsu T.Y., Chuang J.H., Wang P.W., Huang S.T. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion. 2015;22:31–44. doi: 10.1016/j.mito.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 203.Alberti G., Russo E., Corrao S., Anzalone R., Kruzliak P., Miceli V., Conaldi P.G., Di Gaudio F., La Rocca G. Current Perspectives on Adult Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Biological Features and Clinical Indications. Biomedicines. 2022;10:2822. doi: 10.3390/biomedicines10112822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Alberti G., Sánchez-López C.M., Andres A., Santonocito R., Campanella C., Cappello F., Marcilla A. Molecular Profile Study of Extracellular Vesicles for the Identification of Useful Small “Hit” in Cancer Diagnosis. Appl. Sci. 2021;11:10787. doi: 10.3390/app112210787. [DOI] [Google Scholar]
- 205.Stefano F., Mariantonia L., Giusi A., Claudia C. Exosomal Hsp60: A tumor biomarker? In: Asea A., Kaur P., editors. Heat Shock Protein 60 in Human Diseases and Disorders. Springer; Berlin/Heidelberg, Germany: 2020. pp. 107–116. [Google Scholar]
- 206.Miceli V., Bertani A., Chinnici C.M., Bulati M., Pampalone M., Amico G., Carcione C., Schmelzer E., Gerlach J.C., Conaldi P.G. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2021;22:510. doi: 10.3390/ijms22020510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Miceli V., Bertani A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells. 2022;11:826. doi: 10.3390/cells11050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deuse T., Stubbendorff M., Tang-Quan K., Phillips N., Kay M.A., Eiermann T., Phan T.T., Volk H.D., Reichenspurner H., Robbins R.C., et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transpl. 2011;20:655–667. doi: 10.3727/096368910X536473. [DOI] [PubMed] [Google Scholar]
- 209.Donders R.M., Vanheusden R.M., Bogie J.F., Ravanidis S., Thewissen K., Stinissen P., Gyselaers W. Human Wharton’s jelly-derived stem cells display immunomodulatory properties and transiently improve rat experimental autoimmune encephalomyelitis. Cell Transpl. 2015;24:2077–2098. doi: 10.3727/096368914X685104. [DOI] [PubMed] [Google Scholar]
- 210.Wang H., Qiu X., Ni P., Qiu X., Lin X., Wu W., Xie L. Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. Int. J. Mol. Med. 2014;33:263–270. doi: 10.3892/ijmm.2013.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Santos Nascimento D., Mosqueira D., Sousa L.M., Teixeira M., Filipe M., Resende T.P., Araujo A.F., Valente M., Almeida J., Martins J.P., et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res. Ther. 2014;5:5. doi: 10.1186/scrt394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Horie S., Masterson C., Brady J., Loftus P., Horan E., O’Flynn L., Elliman S. Umbilical cord-derived CD362+ mesenchymal stromal cells for E. coli pneumonia: Impact of dose regimen, passage, cryopreservation, and antibiotic therapy. Stem Cell Res. Ther. 2020;11:116. doi: 10.1186/s13287-020-01624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Moodley Y., Atienza D., Manuelpillai U., Samuel C.S., Tchongue J., Ilancheran S., Boyd R. Human Umbilical Cord Mesenchymal Stem Cells Reduce Fibrosis of Bleomycin-Induced Lung Injury. Am. J. Pathol. 2009;175:303–313. doi: 10.2353/ajpath.2009.080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lelek J., Zuba-Surma E.K. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int. J. Mol Sci. 2020;21:799. doi: 10.3390/ijms21030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Willis G.R., Fernandez-Gonzalez A., Anastas J., Vitali S.H., Liu X., Ericsson M., Kwong A. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am. J. Respir. Crit. Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fischer U., Harting M., Jimenez F., Monzon-Posadas W., Xue H., Savitz S., Laine G., Cox C.J. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yao W., Shi L., Zhang Y., Dong H., Zhang Y. Mesenchymal stem/stromal cell therapy for COVID-19 pneumonia: Potential mechanisms, current clinical evidence, and future perspectives. Stem Cell Res. Ther. 2022;13:124. doi: 10.1186/s13287-022-02810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Davies L.C., Heldring N., Kadri N., Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017;35:766–776. doi: 10.1002/stem.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Grégoire C., Ritacco C., Hannon M., Seidel L., Delens L., Belle L., Dubois S., Vériter S., Lechanteur C., Briquet A., et al. Comparison of Mesenchymal Stromal Cells from Different Origins for the Treatment of Graft-vs.-Host-Disease in a Humanized Mouse Model. Front. Immunol. 2019;10:619. doi: 10.3389/fimmu.2019.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ji L., Zhan Y., Hua F., Li F., Zou S., Wang W., Song D., Min Z., Chen H., Cheng Y. The ratio of Treg/Th17 cells correlates with the disease activity of primary immune thrombocytopenia. PLoS ONE. 2012;7:e50909. doi: 10.1371/journal.pone.0050909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lapietra G., Ferretti A., Baldacci E., Chistolini A., Santoro C. Immune thrombocytopenia management during COVID-19 pandemic: An Italian monocentric experience. EJHaem. 2022;3:453–456. doi: 10.1002/jha2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang J.M., Zhu X.L., Xue J., Liu X., Long Zheng X., Chang Y.J., Liu K.Y., Huang X.J., Zhang X.H. Integrated mRNA and miRNA profiling revealed deregulation of cellular stress response in bone marrow mesenchymal stem cells derived from patients with immune thrombocytopenia. Funct. Integr. Genom. 2018;18:287–299. doi: 10.1007/s10142-018-0591-2. [DOI] [PubMed] [Google Scholar]
- 223.Shi M., Liu Z., Wang Y., Xu R., Sun Y., Zhang M., Yu X., Wang H., Meng L., Su H., et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl. Med. 2017;6:2053–2061. doi: 10.1002/sctm.17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stolzing A., Jones E., McGonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 225.Ganguly A., Swaminathan G., Garcia-Marques F., Regmi S., Yarani R., Primavera R., Chetty S., Bermudez A., Pitteri S.J., Thakor A.S. Integrated transcriptome-proteome analyses of human stem cells reveal source-dependent differences in their regenerative signature. Stem Cell Rep. 2023;18:190–204. doi: 10.1016/j.stemcr.2022.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., von Bonin M., Barbieri L., Halai K., Ward S., et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017;9:eaam7828. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 227.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., et al. Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chu M., Wang H., Bian L., Huang J., Wu D., Zhang R., Fei F. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. Stem Cell Rev. Rep. 2022;18:2152–2163. doi: 10.1007/s12015-022-10398-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., Yang T. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduct. Target. Ther. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dilogo I., Aditianingsih D., Sugiarto A., Burhan E., Damayanti T., Sitompul P., Mariana N. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Transl. Med. 2021;10:1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shu L., Niu C., Li R., Huang T., Wang Y., Huang M., Ji N. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020;11:361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Feng G., Shi L., Huang T., Ji N., Zheng Y., Lin H., Niu C. Human Umbilical Cord Mesenchymal Stromal Cell Treatment of Severe COVID-19 Patients: A 3-Month Follow-Up Study Following Hospital Discharge. Stem Cells Dev. 2021;30:773–781. doi: 10.1089/scd.2021.0015. [DOI] [PubMed] [Google Scholar]
- 233.Saleh M., Vaezi A., Aliannejad R., Sohrabpour A., Kiaei S., Shadnoush M., Siavashi V. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: A phase 1 clinical trial. Stem Cell Res. Ther. 2021;12:410. doi: 10.1186/s13287-021-02483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kaffash Farkhad N., Sedaghat A., Reihani H., Adhami Moghadam A., Bagheri Moghadam A., Khadem Ghaebi N., Khodadoust M. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: A successful phase 1, control-placebo group, clinical trial. Stem Cell Res. Ther. 2022;13:283. doi: 10.1186/s13287-022-02920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Feng Y., Huang J., Wu J., Xu Y., Chen B., Jiang L., Xiang H. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Prolif. 2020;53:e12947. doi: 10.1111/cpr.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhu R., Yan T., Feng Y., Liu Y., Cao H., Peng G., Yang Y. Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Res. 2021;31:1244–1262. doi: 10.1038/s41422-021-00573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., Wang S. Treatment with human umbilical cord-1 derived mesenchymal stem cells for COVID-19 patients with lung damage: A randomised, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shi L., Yuan X., Yao W., Wang S., Zhang C., Zhang B., Song J. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75:103789. doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Monsel A., Hauw-Berlemont C., Mebarki M., Heming N., Meaux J., Tchoumba N., Diehl J. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: A multicenter randomized double-blind trial. Crit. Care. 2022;26:48. doi: 10.1186/s13054-022-03930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lanzoni G., Linetsky E., Correa D., Messinger Cayetano S., Alvarez R., Kouroupis D., Alvarez Gil A. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021. online ahead of print. [DOI] [PMC free article] [PubMed]
- 241.Kouroupis D., Lanzoni G., Linetsky E., Messinger Cayetano S., Wishnek Metalonis S., Leñero C., Stone L. Umbilical Cord-derived Mesenchymal Stem Cells modulate TNF and soluble TNF Receptor 2 (sTNFR2) in COVID-19 ARDS patients. Eur. Rev. Med. Pharm. Sci. 2021;25:4435–4438. doi: 10.26355/eurrev_202106_26156. [DOI] [PubMed] [Google Scholar]
- 242.O Ercelen N., Pekkoc-Uyanik K., Alpaydin N., Gulay G., Simsek M. Clinical experience on umbilical cord mesenchymal stem cell treatment in 210 severe and critical COVID-19 cases in Turkey. Stem Cell Rev. Rep. 2021;17:1917–1925. doi: 10.1007/s12015-021-10214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Adas G., Cukurova Z., Yasar K., Yilmaz R., Isiksacan N., Kasapoglu P., Yesilbag Z. The Systematic Effect of Mesenchymal Stem Cell Therapy in Critical COVID-19 Patients: A Prospective Double Controlled Trial. Cell Transpl. 2021;30:9636897211024942. doi: 10.1177/09636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rebelatto C., Senegaglia A., Franck C., Daga D., Shigunov P., Stimamiglio M., Marsaro D. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: A randomized clinical trial. Stem Cell Res. Ther. 2022;13:122. doi: 10.1186/s13287-022-02796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hashemian S., Aliannejad R., Zarrabi M., Soleimani M., Vosough M., Hosseini S., Hossieni H. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: A case series. Stem Cell Res. Ther. 2021;12:91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.da Silva K., Pinheiro P., Gobatto A., Passos R., Paredes B., França L., Nonaka C. Immunomodulatory and Anti-fibrotic Effects Following the Infusion of Umbilical Cord Mesenchymal Stromal Cells in a Critically Ill Patient With COVID-19 Presenting Lung Fibrosis: A Case Report. Front. Med. 2021;8:767291. doi: 10.3389/fmed.2021.767291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhu Y., Zhu R., Liu K., Li X., Chen D., Bai D., Luo J. Human Umbilical Cord Mesenchymal Stem Cells for Adjuvant Treatment of a Critically Ill COVID-19 Patient: A Case Report. Infect. Drug. Resist. 2020;13:3295–3300. doi: 10.2147/IDR.S272645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang Y., Ding J., Ren S., Wang W., Yang Y., Li S., Meng M. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res. Ther. 2020;11:207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhang Q., Huang K., Lv J., Fang X., He J., Lv A., Sun X. Case Report: Human Umbilical Cord Mesenchymal Stem Cells as a Therapeutic Intervention for a Critically Ill COVID-19 Patient. Front. Med. 2021;8:691329. doi: 10.3389/fmed.2021.691329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., Li J. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine. 2020;99:e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Senegaglia A., Rebelatto C., Franck C., Lima J., Boldrini-Leite L., Daga D., Leitão C. Combined Use of Tocilizumab and Mesenchymal Stromal Cells in the Treatment of Severe Covid-19: Case Report. Cell Transpl. 2021;30:9636897211021008. doi: 10.1177/09636897211021008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yang Y., Hu S., Xu X., Li J., Liu A., Han J., Liu S., Liu L., Qiu H. The Vascular Endothelial Growth Factors-Expressing Character of Mesenchymal Stem Cells Plays a Positive Role in Treatment of Acute Lung Injury In Vivo. Mediat. Inflamm. 2016;2016:2347938. doi: 10.1155/2016/2347938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Madureira G., Soares R. The misunderstood link between SARS-CoV-2 and angiogenesis. A narrative review. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2021.08.004. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhao F.Y., Cheng T.Y., Yang L., Huang Y.H., Li C., Han J.Z., Li X.H., Fang L.J., Feng D.D., Tang Y.T., et al. G-CSF Inhibits Pulmonary Fibrosis by Promoting BMSC Homing to the Lungs via SDF-1/CXCR4 Chemotaxis. Sci. Rep. 2020;10:10515. doi: 10.1038/s41598-020-65580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Macri C., Pang E.S., Patton T., O’Keeffe M. Dendritic cell subsets. Semin. Cell Dev. Biol. 2018;84:11–21. doi: 10.1016/j.semcdb.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 256.Zhang S., Gan J., Chen B.G., Zheng D., Zhang J.G., Lin R.H., Zhou Y.P. Dynamics of peripheral immune cells and their HLA-G and receptor expressions in a patient suffering from critical COVID-19 pneumonia to convalescence. Clin. Transl. Immunol. 2020;9:e1128. doi: 10.1002/cti2.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Metcalfe S. COVID-19 lockdown: De-risking exit by protecting the lung with leukaemia inhibitory factor (LIF) Med. Drug. Discov. 2020;6:100043. doi: 10.1016/j.medidd.2020.100043. [DOI] [Google Scholar]
- 258.Mebarki M., Abadie C., Larghero J., Cras A. Human umbilical cord-derived mesenchymal stem/stromal cells: A promising candidate for the development of advanced therapy medicinal products. Stem Cell Res. Ther. 2021;12:152. doi: 10.1186/s13287-021-02222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.FDA Approved Cellular and Gene Therapy Products. [(accessed on 16 March 2023)]; Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.
- 260.EMA First Stem-Cell Therapy Recommended for Approval in EU. [(accessed on 22 March 2023)]. Available online: https://www.ema.europa.eu/en/news/first-stem-cell-therapy-recommended-approval-eu.
- 261.EMA EU/3/17/1852: Orphan Designation for the Treatment in Haematopoietic Stem Cell Transplantation. [(accessed on 25 March 2023)]. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu-3-17-1852.
- 262.Mebarki M., Iglicki N., Marigny C., Abadie C., Nicolet C., Churlaud G., Maheux C., Boucher H., Monsel A., Menasche P., et al. Development of a human umbilical cord-derived mesenchymal stromal cell-based advanced therapy medicinal product to treat immune and/or inflammatory diseases. Stem Cell Res. Ther. 2021;12:571. doi: 10.1186/s13287-021-02637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.