Abstract
IMiDs are a class of drugs approved for the treatment of multiple myeloma. These compounds exert their clinical effects by inducing interactions between the CRL4CRBN E3 ubiquitin ligase and a C2H2 zinc finger degron motif, resulting in degradation of degron-containing targets. However, although many cellular proteins feature the degron motif, only a subset of those are degradable via this strategy. Here, we demonstrated that FPFT-2216, a previously reported “molecular glue” compound, degrades PDE6D, in addition to IKZF1, IKZF3, and CK1α. We used FPFT-2216 as a starting point for a focused medicinal chemistry campaign and developed TMX-4100 and TMX-4116, which exhibit greater selectivity for degrading PDE6D and CK1α, respectively. We also showed that the region in PDE6D that interacts with the FPFT-2216 derivatives is not the previously pursued prenyl-binding pocket. Moreover, we found that PDE6D depletion by FPFT-2216 does not impede the growth of KRASG12C-dependent MIA PaCa-2 cells, highlighting the challenges of drugging PDE6D-KRAS. Taken together, the approach we described here represents a general scheme to rapidly develop selective degraders by reprogramming E3 ubiquitin ligase substrate specificity.
Graphical Abstract

1. INTRODUCTION
The immunomodulatory drugs (IMiDs), such as lenalidomide and pomalidomide, promote recruitment and ubiquitination of neo-substrate proteins to the CRL4CRBN (CUL4-DDB1-RBX1-CRBN) E3 ubiquitin ligase, leading to their subsequent degradation by the 26S proteasome.1–3 The validated neo-substrates of IMiDs include zinc finger transcription factors Ikaros (IKZF1), Aiolos (IKZF3), and spalt like transcription factor 4 (SALL4), as well as casein kinase 1α (CK1α), and G1 to S phase transition 1 protein (GSPT1). These neo-substrates are believed to be the efficacy targets of IMiDs in the treatment of multiple myeloma (MM, depletion of IKZF1 and IKZF3),4,5 5q-deletion-associated myelodysplastic syndrome (del(5q) MDS, depletion of CK1α),6 and acute myeloid leukemia (AML, depletion of GSPT1).7 However, degradation of SALL4 is associated with developmental malformations caused by thalidomide.8,9 Structurally, IMiDs share a common glutarimide moiety which is able to bind to a shallow tri-tryptophan pocket on the surface of CRBN,2,10 a substrate receptor of the CRL4CRBN E3 ligase complex. IMiDs feature an additional solvent-exposed moiety that protrudes out of the CRBN binding pocket and is unique to each IMiD. Upon binding, the exposed moiety modifies the surface of CRBN, causing it to interact with complementary neo-substrates, which in turn results in ‘neomorphic’ E3 ligase activity. IMiDs and other small molecules used for this purpose are commonly referred to as “molecular glues”, for their ability to mediate complex formation between two proteins that have not evolved to bind otherwise.11,12
Notably, different IMiDs (thalidomide, lenalidomide, pomalidomide, and CC-122, structures shown in Figure 1) mediate the degradation of overlapping but distinct sets of zinc finger proteins.13 This suggests that further chemical diversification of the protruding moiety may provide opportunities to improve selectivity and expand the range of degradable target proteins.14 For example, the urea-containing compound CC-885 mediates degradation of GSPT1,7 in addition to IKZF1 and IKZF3. Further medicinal chemistry campaigns resulted in the discovery of the selective GSPT1 degrader CC-90009,15 and the selective IKZF1/IKZF3 dual degraders CC-220 and CC-92480,16 highlighting the opportunity to improve selectivity through chemical derivatization of the basic IMiD scaffold.
Figure 1.
Chemical structures of IMiDs and IMiD derivatives.
Encouraged by the elegant efforts mentioned above, we set out to develop additional molecular glue compounds that divert CRL4CRBN E3 ubiquitin ligase towards new substrates. We discovered that the previously reported IMiD derivative FPFT-221617 degrades phosphodiesterase 6D (PDE6D), in addition to its known targets IKZF1, IKZF3, and CK1α. Further chemical derivatization of FPFT-2216 resulted in TMX-4100 and TMX-4116, with a greatly reduced ability to degrade IKZF1/IKZF3. Notably, the improved degradation preferences of both degraders were retained across multiple cell lines. In addition, we showed that the region in PDE6D that interacts with the FPFT-2216 derivatives is not the previously pursued prenyl-binding pocket, therefore providing a novel site for targeting PDE6D. Moreover, we found that PDE6D depletion by FPFT-2216 does not impede the growth of KRASG12C-dependent MIA PaCa-2 cells, which is contrary to the previous findings by others, thus highlighting the challenges of drugging PDE6D-KRAS. Collectively, our work demonstrates the feasibility of improving degradation selectivity of CRL4CRBN modulators for known targets through chemical diversification, and shows how a novel chemical tool molecule could help further cross-validate the therapeutic potential of the target.
2. RESULTS AND DISCUSSION
We initiated our efforts by synthesizing several previously reported IMiD derivatives and determined the range of targets degraded in MOLT4 cells using quantitative mass spectrometry (MS)-based proteomics.18 We found that the triazole compound FPFT-2216 was able to degrade PDE6D, in addition to its known targets IKZF1, IKZF3, and CK1α (Figure 2). PDE6D is a prenyl-protein chaperone that modulates the localization of farnesylated but not palmitoylated proteins such as KRAS in the cytosol.19,20 Given its critical role in the Ras-processing pathway, PDE6D has been explored as a potential therapeutic target using both reversible and irreversible inhibitors,21–24 as well as PROteolysis TArgeting Chimera (PROTAC)-based degrader molecules.25 We reasoned that a low-molecular weight, selective “molecular glue” type of PDE6D degraders could provide a versatile chemical probe to further interrogate its biological function and therapeutic potential.
Figure 2.
Proteome-wide degradation selectivity of FPFT-2216 at a dose of 1 µM in MOLT4 cells after 5 h treatment.
To evaluate the kinetics of PDE6D degradation, we performed a time course in MOLT4 cells using 1 µM of FPFT-2216. As shown in Figure 3A, degradation occurred rapidly, with complete degradation achieved within 2 h. In addition to being rapid, the degradation of PDE6D persisted for at least 24 h. To evaluate the effective concentration, we performed a dose response in MOLT4 cells treated with FPFT-2216 for 4 h. Over 50% degradation of PDE6D was achieved at a dose of 8 nM, while maximum degradation of PDE6D along with IKZF1, IKZF3, and CK1α was observed at a dose of 200 nM, indicating the potent degradation activity of FPFT-2216 (Figure 3B). No degradation of PDE6D and IKZF1 was detected in CRBN-null MOLT4 cells, confirming that the observed degradation is CRBN-dependent (Figure 3C).
Figure 3.
Immunoblot analysis of PDE6D, IKZF1, IKZF3, and CK1α in MOLT4 cells. (A) Immunoblot analysis of PDE6D treated with 1 µM of FPFT-2216 for the indicated time. (B) Immunoblot analysis of PDE6D, IKZF1, IKZF3, and CK1α treated with the indicated concentration of FPFT-2216 for 4 h. (C) Immunoblot analysis of PDE6D and IKZF1 in CRBN-null MOLT4 cells.
Next, we synthesized a variety of FPFT-2216 analogs, with the aim of retaining PDE6D degradation activity while sparing other targets such as IKZF1, IKZF3, and CK1α. First, moving the 4-methoxythiophen group to the 5-position of triazole moiety, resulted in the generation of structural isomer compound 2. In turn, changing the triazole moiety attachment point from the 3-position to the 2-position of the thiophene group, yields a pair of isomers 3 and 4, respectively. Second, bioisosteric replacement of the 4-methoxythiophen group led to compounds 5–8. Finally, scaffold hopping strategy afforded compounds 9–13. The first round of derivatives were then subjected to immunoblot analysis in MOLT4 cells at a fixed dose of 1 µM, which was used as a quick method to filter out compounds based on their PDE6D degradation activities. As shown in Figure 4, all structural variations were surprisingly not tolerated, indicating a steep SAR as illustrated by the difference in activity of compounds 3 and 4. With the preliminary SAR information in mind, we turned our effort to prepare the second round of derivatives by attaching additional carbonyl groups to the neighboring position of the methoxy group (compounds 14–17) or by replacing the methoxy group with other substitutes (compounds 18–22), leaving the main scaffold of FPFT-2216 intact. It was found that compound 21 was the only molecule that potently degraded PDE6D without affecting IKZF1 and IKZF3, and had minimal degradation activity towards CK1α. Interestingly, compounds 16 and 17 selectively degraded CK1α with over 70% degradation achieved while sparing PDE6D, IKZF1, and IKZF3 under the conditions tested. Collectively, by performing two rounds of chemical derivatization on FPFT-2216, hit compounds 3 (TMX-4100) and 21 (TMX-4113), as well as hit compounds 16 (TMX-4116) and 17, were found to show a high degradation preference for PDE6D and CK1α, respectively.
Figure 4.
Degradation activities on PDE6D, IKZF1, IKZF3, and CK1α of FPFT-2216 derivatives in MOLT4 cells treated with 1 µM of degraders for 4 h.
Several investigations have noted cell-type dependent degradation profiles for degrader molecules26,27 and therefore it is critical to more fully characterize the degradation selectivity profile of the above-mentioned hit compounds in different cell lines. As shown in Figure 5, high degradation preference of TMX-4100 for PDE6D was retained across MOLT4, Jurkat, and MM.1S cells treated with doses up to 1 µM for 4 h with DC50 value less than 200 nM. In contrast, for TMX-4113, although it had lower DC50s for PDE6D than TMX-4100 in the three cell types and did not degrade IKZF1 and IKZF3, it clearly degraded over 50% of CK1α at a dose of 40 nM in MM.1S cells (Figure S1). Similar to TMX-4100, TMX-4116 retained the degradation preference for CK1α with DC50s less than 200 nM.
Figure 5.
Immunoblot analysis of PDE6D, IKZF1, IKZF3, and CK1α in specific cell types. (A) Immunoblot analysis in MOLT4 cells treated with the indicated concentration of TMX-4100 or TMX-4113, as well as (B) TMX-4116 or compound 17 for 4 h (with lenalidomide included as a control). (C) Immunoblot analysis in Jurkat cells and (D) MM.1S cells treated with the indicated concentration of TMX-4100 or TMX-4116 for 4 h.
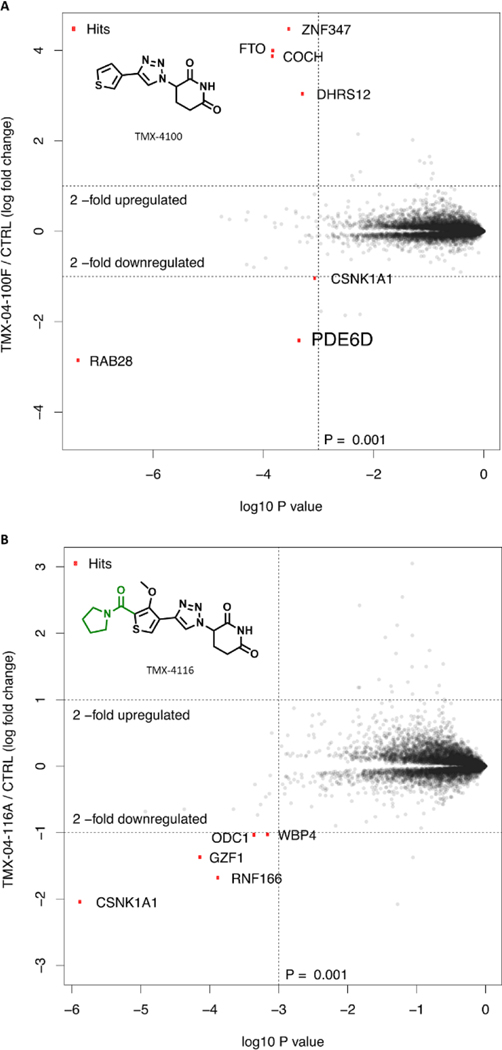
Next, to determine the range of targets degraded by TMX-4100 and TMX-4116 in MOLT4 cells, we used a quantitative proteomic method. As shown in Figure 6A, PDE6D was found to be effectively degraded, and the degradation selectivity of TMX-4100 for PDE6D was significantly improved compared to FPFT-2216. We also observed the pronounced change in levels of RAB28, a RAS GTPase and a client of PDE6D.28 However, at this point, we are unable to conclude whether its degradation is PDE6D-dependent or independent. As shown in Figure 6B, CK1α was found to be the primary target degraded by TMX-4116, while no down-regulation of PDE6D, IKZF1, and IKZF3 was detected. Taken together, compared with FPFT-2216, both TMX-4100 and TMX-4116 have better proteome-wide degradation selectivity in MOLT4 cells.
Figure 6.
Proteome-wide degradation selectivity of (A) TMX-4100 at a dose of 1 µM or (B) TMX-4116 at a dose of 250 nM in MOLT4 cells after 4 h treatment.
Prior to our work, the Waldmann’s laboratory developed compounds that bind to the prenyl-binding pocket and that disrupt the interaction of PDE6D-KRAS. These include reversible inhibitors (e.g., deltarasin and deltazinone),21–23 covalent inhibitors,24 and PROTACs.25 We confirmed that pretreatment of the MOLT4 cells with deltarasin did not result in protection of PDE6D from degradation by FPFT-2216, suggesting that the region interacting with FPFT-2216 is distinct from the prenyl-binding pocket (Figure S2). In addition, we found that PDE6D depletion by FPFT-2216, TMX-4100, or TMX-4113 does not impede the growth of MIA PaCa-2 cells, a KRASG12C-dependent human pancreatic ductal adenocarcinoma (PDAC) cell line previously reported to be sensitive to chemically-induced PDE6D knockdown or inhibition, nor did they impede the growth of NCI-H358 cells (a KRASG12C-dependent human non-small cell lung cancer (NSCLC) cell line), AGS cells (a KRASG12D-dependent human gastric adenocarcinoma (GAC) cell line), or PA-TU-8988T cells (a KRASG12V-dependent human PDAC cell line), suggesting that PDE6D is not essential to the proliferation of these KRAS-dependent cell lines (Figure 7, Figure S3, S4, S5, and S6).
Figure 7.
Growth inhibition of (A) MIA PaCa-2 cells, (B) NCI-H358 cells, (C) AGS cells, and (D) PA-TU-8988T cells after 3-day treatment with deltasonamide 2, MRTX849 (a KRASG12C inhibitor), FPFT-2216, TMX-4100, or TMX-4113. Both deltasonamide 2 and MRTX849 were dosed once, while FPFT-2216, TMX-4100, and TMX-4113 were dosed daily.
3. CHEMISTRY
Representative compounds 1, 2, and 9 were prepared as described in Scheme 1, respectively. Applying the copper(II)-catalysed azide-alkyne cycloaddition (CuAAC) reaction to alkyne R1 and azide R2 at rt afforded 1,4-disubstituted triazole derivative 1. In contrast, its alternate 1,5-regioisomer 2 was accomplished in 1,4-dioxane at 60 °C using a catalytic quantity of Cp*RuCl(PPh3)2.29 Compound 9 was prepared by treating triazole R3 with NaH and then reacting with 3-Bromopiperidine-2,6-dione R4 through SN2 substitution.
Scheme 1. Chemical Synthesis of Compounds 1, 2, and 9a.
aReagents and conditions: (a) CuSO4.5H2O, sodium ascorbate, DMF, H2O, rt, 6h, 21%; (b) Cp*RuCl(PPh3)2, 1,4-dioxane, 60 oC, 2 h, 18%; (c) NaH, DMF, 0 oC, 3 h, 31%.
4. CONCLUSIONS
Selectively targeting a protein for degradation with IMiD derivatives has been a long-standing challenge in drug development, and a major limitation for clinical development of next-generation IMiDs. In the search for new IMiD derivatives to selectively redirect the CRL4CRBN E3 ubiquitin ligase to new substrates, we found that the triazole compound, FPFT-2216, showed degradation activity towards PDE6D, in addition to its known targets IKZF1, IKZF3, and CK1α. Despite its high activity, the low degradation preference for PDE6D reduces the applicability of FPFT-2216 as a PDE6D-targeted molecule. By performing varied chemical derivatization approaches, we confirmed a steep SAR around the main scaffold of FPFT-2216, consistent with the mode of action of the “molecular glues”. Further careful exploration of structural modifications resulted in the discovery of a PDE6D degrader TMX-4100, and a CK1α degrader TMX-4116, both of which retained high degradation preference across multiple cell types, including MOLT4, Jurkat, and MM.1S cells. Notably, we also demonstrated that the region in PDE6D that interacts with the FPFT-2216 series is not the not the previously pursued prenyl-binding pocket. More importantly, TMX-4100 represents a useful addition to the set of chemical probes available for interrogating the biological and potential therapeutic value of PDE6D in RAS- and other signaling pathways. Collectively, our work demonstrates the feasibility of improving E3 ligase substrate specificity through a chemical reprogramming approach, and shows how a novel chemical tool molecule could help further cross-validate the therapeutic potential of the target.
5. EXPERIMENTAL SECTION
5.1. Cell lines.
MOLT-4 (ATCC, #CRL-1582), Jurkat (ATCC, #TIB-152), MM.1S (ATCC, #CRL-2974), MIA PaCa-2 cells (ATCC, #CRL-142), AGS (ATCC, #CRL-1739), and NCI-H358 (ATCC, #CRL-5807) were obtained from American Type Culture Collection (ATCC). PA-TU-8988T cells were gifts from the Abu-Remaileh lab at Stanford university. Cells were cultured according to ATCC recommendations in presence with 10% fetal bovine serum (ThermoFischer, #26140079), 100 units/mL of penicillin and 100 μg/mL streptomycin (ThermoFischer, #15140–122). Mycoplasma was routinely checked using Lonza mycoplasma detection kit (Lonza, #LT07–218).
5.2. Immunoblotting analysis.
Cell samples were harvested, washed with PBS thrice and lysed in RIPA buffer with Halt Protease and phosphatase inhibitor (ThermoFischer, #78442) for 30 min on ice. Then samples were centrifugated and the supernatant was subjected to BCA analysis. In total, 20 μg protein for each sample was loaded for SDS-PAGE separation. Protein was then transferred to 0.2 μm nitrocellulose membrane at 10 V for 60 min. After wet transfer, the membrane was blocked at rt for 60 min using Intercept (TBS) blocking buffer (LICOR, #927–60001). The following antibodies were used in this study: PDE6D (Novus, #NBP1–32730, 1:1000), CK1α (Abcam, #ab108296), IKZF1 (Cell Signaling, #14859S, 1:1000), IKZF3 (Cell Signaling, #15103S, 1:1000), phospho-MEK1/2 (Ser217/221) (Cell Signaling, #9121S, 1:1000), MEK1/2 (Cell Signaling, #4694S, 1:1000), phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (Cell Signaling, #4370S, 1:1000), p44/42 MAPK (Erk1/2) (Cell Signaling, #4696S, 1:1000), β-actin (Cell Signaling, #3700S, 1:2000), IRDye 800CW Goat anti-Rabbit IgG secondary antibody (LICOR, #926–32211, 1:5000), and IRDye 680RD Goat anti-Mouse IgG secondary antibody (LICOR, #926–68070, 1:5000). For rescue experiments, cells were pre-treated with Carfilzomib (MedChemExpress, #HY-10455), MLN7243 (MedChemExpress, #HY-100487), MLN4924 (MedChemExpress, #HY-70062), thalidomide (Selleckchem, #S1193), or deltarasin (MedChemExpress, #HY-15747) at indicated concentrations for 2 h and then co-treated with FPFT-2216 for 4 h. Samples were harvested and then subjected to immunoblot analysis.
5.3. Cell proliferation.
800 cells were seeded in 384 well plate (Corning, #3570) with a total volume of 50 μL. Next day, cells were treated with Deltasonamide 2 (MedChemExpress, #HY-122641A) or FPFT-2216 at indicated concentrations/frequency. After three-day treatment, cell viability was measured using CellTiter-Glo kits (Promega, #G7572) according to manufacturer instructions. After 20 min incubation, luminescence signal was collected using EnVision plate reader (PerkinElmer).
5.4. Sample preparation for tandem mass tag liquid chromatography coupled with multistage mass spectrometry (TMT LC-MS3).
MOLT4 cells were treated with DMSO (biological triplicate) or 1 µM (or 250 nM) of TMX compounds for 4 h (or 5 h), and cells were collected by centrifugation at 4 oC. Cells were lysed by the addition of urea lysis buffer (8 M urea, 50 mM NaCl, 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (EPPS) pH 8.5, and protease and phosphatase inhibitors (Roche)) followed by manual homogenization by 20 passes through a 21-gauge (3.175-cm long) needle. Cell lysate was clarified by centrifugation, and protein concentration was measured by micro-BCA assay (Pierce). Protein (50–200 µg) for each sample was reduced and alkylated as previously described.7
Proteins were precipitated using methanol/chloroform, resuspended in 4 M urea, 50 mM HEPES pH 7.4 and diluted to 1 M urea with the addition of 200 mM EPPS, pH 8. Proteins were digested with LysC (1:50, enzyme:protein) for 12 h at rt followed by dilution to 0.5 M urea with 200 mM EPPS pH 8 for digestion with trypsin (1:50, enzyme:protein) for 6 h at 37 °C. Anhydrous acetonitrile was added to each peptide sample to a final concentration of 30% (vol/vol), TMT labeling reagent (Thermo Fisher Scientific) was added to each sample at a ratio of 1:4 (peptide:TMT label), and labeling occurred over 1.5 h at rt. The labeling reaction was quenched by the addition of hydroxylamine to a final concentration of 0.3% for 15 min at rt. Each of the sample channels was then combined in a 1:1 ratio and desalted using in-house C18 stage tips, followed by LC–MS analysis for the assessment of sample quality and channel ratio comparison. Samples were then combined using the adjusted volumes determined in the channel ratio analysis, dried down in a speed vacuum and desalted using C18 SPE columns (Sep-Pak, Waters). Samples were then offline fractionated into 96 fractions by high pH reverse-phase HPLC (Agilent LC1260) through an Aeris peptide XB-C18 column (phenomenex) with mobile phase A containing 5% acetonitrile and 10 mM NH4HCO3 in LC-MS-grade water and mobile phase B containing 90% acetonitrile and 5 mM NH4HCO3 in LC-MS grade water (both pH 8.0). The 96 resulting fractions were then combined in a non-contiguous manner into 24 fractions, and these fractions were used for subsequent MS analysis.
Data were collected using either an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific) coupled with a Proxeon EASY-nLC 1200 LC pump or an Orbitrap Eclipse Tribrid mass spectrometer (Thermo Fisher Scientific) coupled with an UltiMate 3000 RSLCnano system. Peptides were separated on an EasySpray ES803 or ES903 75-μm inner diameter microcapillary column (Thermo Fisher Scientific). Peptides were separated using a 190-min gradient of 6–27% acetonitrile in 1.0% formic acid with a flow rate of 300 nL/min.
Each analysis used an MS3-based TMT method as described previously.30 The data were acquired using a mass range of m/z 340–1,350, resolution 120,000, AGC target 5 × 105, maximum injection time 100 ms and dynamic exclusion of 120 s for the peptide measurements in the Orbitrap. Data-dependent MS2 spectra were acquired in the ion trap with a normalized collision energy set at 35%, AGC target set to 1.8 × 104 and a maximum injection time of 120 ms. MS3 scans were acquired in the Orbitrap with an HCD collision energy set to 55%, AGC target set to 2 × 105, maximum injection time of 150 ms, resolution at 50,000 and with a maximum synchronous precursor selection (SPS) precursors set to 10. The advanced peak detection algorithm was disabled.
5.5. Liquid chromatography-mass spectrometry data analysis.
Proteome Discoverer 2.2 (Thermo Fisher Scientific) was used for .RAW file processing and controlling peptide and protein level false discovery rates, assembling proteins from peptides, and protein quantification from peptides. MS/MS spectra were searched against a Uniprot human database (September 2016) with both the forward and reverse sequences. Database search criteria are as follows: tryptic with two missed cleavages, a precursor mass tolerance of 20 ppm, fragment ion mass tolerance of 0.6 Da, static alkylation of cysteine (57.02146 Da), static TMT labelling of lysine residues and N-termini of peptides (229.16293 Da), and variable oxidation of methionine (15.99491 Da). TMT reporter ion intensities were measured using a 0.003 Da window around the theoretical m/z for each reporter ion in the MS3 scan. Peptide spectral matches with poor quality MS3 spectra were excluded from quantitation (summed signal-to-noise across 11 channels < 100 and precursor isolation specificity < 0.5), and resulting data was filtered to only include proteins that had a minimum of 2 unique peptides identified. Reporter ion intensities were normalised and scaled using in-house scripts in the R framework (version 4.0.2). Statistical analysis was carried out using the limma package (version 3.44.3) within the R framework.31 Significant changes were assessed using a moderated t-test as implemented in Bioconductor’s limma package.
5.6. Synthesis.
Unless otherwise noted, reagents and solvents were obtained from commercial suppliers and were used without further purification. 1H NMR spectra were recorded on 500 MHz Bruker Avance III spectrometer, and chemical shifts are reported in parts per million (ppm, d) downfield from tetramethylsilane (TMS). Coupling constants (J) are reported in Hz. Spin multiplicities are described as s (singlet), brs (broad singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Mass spectra were obtained on a Waters Acquity UPLC. Preparative HPLC was performed on a Waters Sunfire C18 column (19 mm X 50 mm, 5 μM) using a gradient of 15–95% methanol in water containing 0.05% trifluoroacetic acid (TFA) over 60 min at a flow rate of 43 mL/min. Flash column chromatography was carried out using prepacked silica cartridges (from 4 g up to 24 g) from Redisep TM and eluted using an Isco Companion system. Purities of assayed compounds were in all cases greater than 95%, as determined by reverse-phase HPLC analysis.
5.6.1. 3-(4-(4-methoxythiophen-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (1, FPFT-2216).
To a solution of 3-ethynyl-4-methoxythiophene R1 (18.0 mg, 0.13 mmol) and 3-azidopiperidine-2,6-dione R2 (20 mg, 0.13 mmol) in DMF/H2O (v/v=2/1, 1.0 mL) was added CuSO4.5H2O (3.3 mg, 0.013 mmol) and sodium ascorbate (13 mg, 0.065 mmol). The reaction mixture was stirred at rt for 6 h. The reaction mixture was purified directly via prep HPLC to give FPFT-2216 (8.0 mg, 21% yield) as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.22 (s, 1H), 8.34 (s, 1H), 7.88 (d, J = 8.5 Hz, 1H), 6.74 (d, J = 3.5 Hz, 1H), 5.87 (dd, J = 8.5, 5.0 Hz, 1H), 3.88 (s, 3H), 2.91–2.83 (m, 1H), 2.80–2.73 (m, 1H), 2.72–2.65 (m, 1H), 2.34–2.27 (m, 1H). MS (ESI) for C12H13N4O3S [M+H]+: m/z calcd, 293.07; found, 293.25. HRMS (ESI+): [M+H]+ m/z found 293.0705.
5.6.2. 3-(5-(4-methoxythiophen-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (2).
To a solution of 3-ethynyl-4-methoxythiophene R1 (20.0 mg, 0.14 mmol) and 3-azidopiperidine-2,6-dione R2 (22 mg, 0.14 mmol) in 1,4-dioxane (1.0 mL) was added Cp*RuCl(PPh3)2 (6.0 mg, 7.23 µmol). The reaction mixture was stirred at 60 oC for 2 h. The reaction mixture was purified directly via prep HPLC to give compound 2 (7.8 mg, 18% yield) as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.15 (s, 1H), 7.82 (s, 1H), 7.71 (d, J = 3.0 Hz, 1H), 6.86 (d, J = 3.5 Hz, 1H), 5.50 (dd, J = 12.0, 5.5 Hz, 1H), 3.82 (s, 3H), 2.91–2.82 (m, 1H), 2.80–2.70 (m, 1H), 2.70–2.64 (m, 1H), 2.40–2.33 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 173.1, 170.0, 155.5, 133.2, 133.0, 127.1, 117.8, 99.5, 58.3, 57.8, 31.2, 24.9. MS (ESI) for C12H13N4O3S [M+H]+: m/z calcd, 293.07; found, 293.25. HRMS (ESI+): [M+H]+ m/z found 293.0706.
5.6.3. 3-(4-(thiophen-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (3).
Following the same procedure as compound 1 by using 3-ethynylthiophene instead of R1, compound 3 (20.0 mg, 76% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.53 (s, 1H), 7.86 (dd, J = 3.0, 1.0 Hz, 1H), 7.66 (dd, J = 5.0, 3.0 Hz, 1H), 7.51 (dd, J = 5.0, 1.0 Hz, 1H), 5.85 (dd, J = 12.5, 5.0 Hz, 1H), 2.94–2.85 (m, 1H), 2.74–2.62 (m, 2H), 2.39–2.33 (m, 1H). ). 13C NMR (125 MHz, DMSO-d6): 172.9, 170.0, 143.4, 132.4, 127.7, 126.2, 121.9, 121.4, 59.6, 31.2, 24.9. MS (ESI) for C11H11N4O2S [M+H]+: m/z calcd, 263.06; found, 263.26. HRMS (ESI+): [M+H]+ m/z found 263.0599.
5.6.4. 3-(4-(thiophen-2-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (4).
Following the same procedure as compound 1 by using 2-ethynylthiophene instead of R1, compound 4 (15.0 mg, 57% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.27 (s, 1H), 8.60 (s, 1H), 7.55 (dd, J = 5.0, 1.0 Hz, 1H), 7.44 (dd, J = 3.0, 1.0 Hz, 1H), 7.14 (dd, J = 5.0, 3.5 Hz, 1H), 5.86 (dd, J = 12.5, 5.0 Hz, 1H), 2.94–2.85 (m, 1H), 2.74–2.63 (m, 2H), 2.39–2.33 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 142.2, 133.3, 128.4, 126.0, 124.7, 121.5, 59.7, 31.2, 24.8. MS (ESI) for C11H11N4O2S [M+H]+: m/z calcd, 263.06; found, 263.23. HRMS (ESI+): [M+H]+ m/z found 263.0598.
5.6.5. 3-(4-(2-methoxyphenyl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (5).
Following the same procedure as compound 1 by using 1-ethynyl-2-methoxybenzene instead of R1, compound 5 (16.0 mg, 57% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.23 (s, 1H), 8.51 (s, 1H), 8.17 (dd, J = 7.5, 1.0 Hz, 1H), 7.35 (td, J = 7.5, 2.0 Hz, 1H), 7.14 (d, J = 8.0 Hz, 1H), 7.07 (td, J = 7.5, 1.0 Hz, 1H), 5.87 (dd, J = 12.5, 5.0 Hz, 1H), 3.92 (s, 3H), 2.94–2.85 (m, 1H), 2.83–2.73 (m, 1H), 2.73–2.66 (m, 1H), 2.36–2.29 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 173.0, 170.0, 155.8, 142.3, 129.5, 127.0, 124.6, 121.1, 119.4, 112.0, 59.6, 55.9, 31.3, 24.8. MS (ESI) for C14H15N4O3 [M+H]+: m/z calcd, 287.11; found, 287.29. HRMS (ESI+): [M+H]+ m/z found 287.1141.
5.6.6. methyl 2-(1-(2,6-dioxopiperidin-3-yl)-1H-1,2,3-triazol-4-yl)benzoate (6).
Following the same procedure as compound 1 by using methyl 2-ethynylbenzoate instead of R1, compound 6 (21.3 mg, 73% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.46 (s, 1H), 7.76 (dd, J = 7.5, 0.5 Hz, 1H), 7.66 (dd, J = 8.0, 1.0 Hz, 1H), 7.63 (td, J = 7.5, 1.0 Hz, 1H), 7.49 (td, J = 7.5, 1.5 Hz, 1H), 5.87 (dd, J = 12.5, 5.0 Hz, 1H), 3.72 (s, 3H), 2.94–2.85 (m, 1H), 2.76–2.66 (m, 2H), 2.38–2.32 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.8, 169.3, 145.1, 131.7, 131.2, 129.6, 129.4, 129.4, 128.6, 123.8, 59.7, 52.6, 31.2, 24.9. MS (ESI) for C15H15N4O4 [M+H]+: m/z calcd, 315.11; found, 315.30. HRMS (ESI+): [M+H]+ m/z found 315.1089.
5.6.7. 3-(4-(3-methoxypyridin-2-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (7).
Following the same procedure as compound 1 by using 2-ethynyl-3-methoxypyridine instead of R1, compound 7 (14.0 mg, 37% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.24 (s, 1H), 8.65 (s, 1H), 8.26 (d, J = 4.5 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.38 (dd, J = 8.5, 4.5 Hz, 1H), 5.89 (dd, J = 13.0, 5.0 Hz, 1H), 3.93 (s, 3H), 2.94–2.86 (m, 1H), 2.83–2.73 (m, 1H), 2.73–2.67 (m, 1H), 2.38–2.31 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 170.0, 152.9, 143.5, 141.4, 139.1, 126.3, 124.1, 119.5, 59.6, 56.1, 31.3, 24.8. MS (ESI) for C13H14N5O3 [M+H]+: m/z calcd, 288.11; found, 288.27. HRMS (ESI+): [M+H]+ m/z found 288.1093.
5.6.8. 3-(4-(2-aminopyridin-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (8).
Following the same procedure as compound 1 by using 3-ethynylpyridin-2-amine instead of R1, compound 8 (13.6 mg, 38% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.33 (s, 1H), 9.01 (s, 1H), 8.46–8.30 (m, 2H), 8.35 (dd, J = 7.0, 0.5 Hz, 1H), 8.07 (d, J = 3.5 Hz, 1H), 7.03 (t, J = 7.0 Hz, 1H), 5.97 (dd, J = 13.0, 5.5 Hz, 1H), 2.96–2.87 (m, 1H), 2.78–2.65 (m, 2H), 2.45–2.38 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.8, 169.6, 152.3, 143.8, 143.4, 140.1, 138.8, 124.5, 113.0, 60.1, 31.2, 24.8. MS (ESI) for C12H13N6O2 [M+H]+: m/z calcd, 273.11; found, 273.30. HRMS (ESI+): [M+H]+ m/z found 273.1096.
5.6.9. 3-(5-methoxy-1H-benzo[d][1,2,3]triazol-1-yl)piperidine-2,6-dione (9).
To a solution of 6-methoxy-1H-benzo[d][1,2,3]triazole R3 (20.0 mg, 0.13 mmol) and 3-Bromopiperidine-2,6-dione R4 (25 mg, 0.13 mmol) in DMF (1.0 mL) was added NaH (60% dispersion in mineral oil, 9.0 mg, 0.40 mmol) at 0 oC. The reaction mixture was stirred at 0 oC for 3 h. The reaction mixture was purified directly via prep HPLC to give compound 9 (11.0 mg, 31% yield) as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.25 (s, 1H), 7.83 (d, J = 9.5 Hz, 1H), 7.26 (d, J = 2.0 Hz, 1H), 7.12 (dd, J = 9.5, 2.0 Hz, 1H), 6.06 (dd, J = 13.0, 4.5 Hz, 1H), 3.85 (s, 3H), 2.94–2.85 (m, 1H), 2.83–2.74 (m, 1H), 2.74–2.68 (m, 1H), 2.48–2.42 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.5, 159.0, 145.2, 140.2, 121.9, 119.3, 95.7, 64.9, 56.1, 30.8, 24.9. MS (ESI) for C12H13N4O3 [M+H]+: m/z calcd, 261.10; found, 261.33. HRMS (ESI+): [M+H]+ m/z found 261.0984.
5.6.10. 3-(3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione (10).
Following the same procedure as compound 9 by using 1-Methyl-2-benzimidazolinone instead of R3, compound 10 (11.2 mg, 32% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.09 (s, 1H), 7.18 (d, J = 7.5 Hz, 1H), 7.14–7.02 (m, 3H), 5.37 (dd, J = 13.0, 5.5 Hz, 1H), 3.34 (s, 3H), 2.95–2.85 (m, 1H), 2.77–2.66 (m, 1H), 2.66–2.59 (m, 1H), 2.05–1.98 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 173.3, 170.5, 153.7, 130.2, 128.6, 121.7, 121.5, 109.0, 108.5, 52.1, 31.6, 27.4, 22.5. MS (ESI) for C13H14N3O3 [M+H]+: m/z calcd, 260.10; found, 260.30. HRMS (ESI+): [M+H]+ m/z found 260.1031.
5.6.11. 3-(4-(hydroxy(thiophen-3-yl)methyl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (11).
Following the same procedure as compound 1 by using 1-(thiophen-3-yl)prop-2-yn-1-ol instead of R1, compound 11 (13.0 mg, 44% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.18 (s, 1H), 8.01 (d, J = 3.5 Hz, 1H), 7.49–7.45 (m, 1H), 7.37–7.34 (m, 1H), 7.10 (d, J = 5.0 Hz, 1H), 6.00 (dd, J = 5.0, 3.0 Hz, 1H), 5.88 (d, J = 5.5 Hz, 1H), 5.80–5.74 (m, 1H), 2.89–2.79 (m, 1H), 2.69–2.60 (m, 2H), 2.29–2.21 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 151.5 (151.5), 145.9, 127.2, 126.6, 122.8 (122.7), 121.8 (121.8), 65.0, 59.5, 31.2 (31.2), 24.9 (24.8). MS (ESI) for C12H13N4O3S [M+H]+: m/z calcd, 293.07; found, 293.25. HRMS (ESI+): [M+H]+ m/z found 293.0705.
5.6.12. 3-(4-benzyl-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (12).
Following the same procedure as compound 1 by using prop-2-yn-1-ylbenzene instead of R1, compound 12 (11.2 mg, 32% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.17 (s, 1H), 7.93 (s, 1H), 7.33–7.25 (m, 4H), 7.21 (t, J = 7.0 Hz, 1H), 5.74 (dd, J = 13.0, 5.5 Hz, 1H), 4.01 (s, 2H), 2.88–2.79 (m, 1H), 2.68–2.57 (m, 2H), 2.29–2.22 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 170.0, 146.5, 139.9, 129.1, 128.9, 126.7, 123.2, 59.4, 31.8, 31.2, 24.8. MS (ESI) for C14H15N4O2 [M+H]+: m/z calcd, 271.12; found, 271.32. HRMS (ESI+): [M+H]+ m/z found 271.1192.
5.6.13. 3-(4-(tetrahydro-2H-pyran-4-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (13).
Following the same procedure as compound 1 by using 4-ethynyltetrahydro-2H-pyran instead of R1, compound 13 (9.3 mg, 35% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.19 (s, 1H), 7.99 (s, 1H), 5.74 (dd, J = 12.5, 5.0 Hz, 1H), 3.92–3.87 (m, 2H), 3.48–3.42 (m, 2H), 2.99–2.91 (m, 1H), 2.90–2.81 (m, 1H), 2.69–2.56 (m, 2H), 2.30–2.23 (m, 1H), 1.87 (dt, J = 13.0, 2.0 Hz, 2H), 1.67–1.57 (m, 2H). 13C NMR (125 MHz, DMSO-d6): 172.9, 170.0, 151.4, 121.5, 67.2, 59.4, 32.8, 32.4, 31.2, 24.9. MS (ESI) for C12H17N4O3 [M+H]+: m/z calcd, 265.13; found, 265.32. HRMS (ESI+): [M+H]+ m/z found 265.1297.
5.6.14. 4-(1-(2,6-dioxopiperidin-3-yl)-1H-1,2,3-triazol-4-yl)-3-methoxythiophene-2-carboxylic acid (14).
Following the same procedure as compound 1 by using 4-ethynyl-3-methoxythiophene-2-carboxylic acid instead of R1, compound 14 (30.0 mg, 28% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 13.08 (s, 1H), 11.24 (s, 1H), 8.45 (s, 1H), 8.16 (s, 1H), 5.88 (dd, J = 12.5, 5.0 Hz, 1H), 3.96 (s, 3H), 2.93–2.84 (m, 1H), 2.83–2.73 (m, 1H), 2.73–2.66 (m, 1H), 2.36–2.29 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 162.3, 158.5, 140.0, 127.5, 126.3, 122.1, 118.3, 62.4, 59.7, 31.2, 24.8. MS (ESI) for C13H13N4O5S [M+H]+: m/z calcd, 337.06; found, 337.23. HRMS (ESI+): [M+H]+ m/z found 337.0603.
5.6.15. methyl 4-(1-(2,6-dioxopiperidin-3-yl)-1H-1,2,3-triazol-4-yl)-3-methoxythiophene-2-carboxylate (15).
Following the same procedure as compound 1 by using methyl 4-ethynyl-3-methoxythiophene-2-carboxylate instead of R1, compound 15 (6.8 mg, 31% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.48 (s, 1H), 8.23 (s, 1H), 5.89 (dd, J = 12.5, 5.0 Hz, 1H), 3.97 (s, 3H), 3.82 (s, 3H), 2.93–2.84 (m, 1H), 2.83–2.73 (m, 1H), 2.73–2.66 (m, 1H), 2.36–2.29 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 161.2, 159.1, 139.8, 127.4, 127.1, 122.2, 116.5, 62.5, 59.7, 52.5, 31.2, 24.8. MS (ESI) for C14H15N4O5S [M+H]+: m/z calcd, 351.08; found, 351.25. HRMS (ESI+): [M+H]+ m/z found 351.0758.
5.6.16. 3-(4-(4-methoxy-5-(pyrrolidine-1-carbonyl)thiophen-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (16).
Following the same procedure as compound 1 by using (4-ethynyl-3-methoxythiophen-2-yl)(pyrrolidin-1-yl)methanone instead of R1, compound 16 (8.7 mg, 26% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.24 (s, 1H), 8.41 (s, 1H), 7.99 (s, 1H), 5.87 (dd, J = 12.5, 5.0 Hz, 1H), 3.84 (s, 3H), 3.46 (s, 4H), 2.93–2.84 (m, 1H), 2.81–2.72 (m, 1H), 2.72–2.66 (m, 1H), 2.35–2.28 (m, 1H), 1.88 (s, 4H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 161.7, 152.1, 140.3, 125.3, 122.0, 121.8, 118.8, 61.0, 59.6, 48.5, 46.5, 31.2, 26.0, 24.8, 24.5. MS (ESI) for C17H20N5O4S [M+H]+: m/z calcd, 390.12; found, 390.36. HRMS (ESI+): [M+H]+ m/z found 390.1229.
5.6.17. N-cyclopentyl-4-(1-(2,6-dioxopiperidin-3-yl)-1H-1,2,3-triazol-4-yl)-3-methoxythiophene-2-carboxamide (17).
Following the same procedure as compound 1 by using N-cyclopentyl-4-ethynyl-3-methoxythiophene-2-carboxamide instead of R1, compound 17 (11.1 mg, 34% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.46 (s, 1H), 8.04 (s, 1H), 7.70 (d, J = 8.0 Hz, 1H), 5.89 (dd, J = 12.5, 5.0 Hz, 1H), 4.27–4.18 (m, 1H), 3.82 (s, 3H), 2.94–2.85 (m, 1H), 2.82–2.66 (m, 2H), 2.38–2.30 (m, 1H), 1.95–1.86 (m, 2H), 1.74–1.65 (m, 2H), 1.62–1.50 (m, 4H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 160.4, 153.6, 140.1, 126.3, 124.5, 124.3, 122.3, 62.0, 59.7, 51.2, 32.8, 31.2, 24.8, 23.8. MS (ESI) for C18H22N5O4S [M+H]+: m/z calcd, 404.14; found, 404.42. HRMS (ESI+): [M+H]+ m/z found 404.1386.
5.6.18. 3-(4-(4-(pyrrolidine-1-carbonyl)thiophen-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (18).
Following the same procedure as compound 1 by using (4-ethynylthiophen-3-yl)(pyrrolidin-1-yl)methanone instead of R1, compound 18 (1.6 mg, 18% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.21 (s, 1H), 8.13 (s, 1H), 7.95 (d, J = 3.5 Hz, 1H), 7.76 (d, J = 3.0 Hz, 1H), 5.85 (dd, J = 12.5, 5.0 Hz, 1H), 3.52–3.46 (m, 2H), 3.06 (t, J = 6.5 Hz, 2H), 2.90–2.81 (m, 1H), 2.77–2.64 (m, 2H), 2.35–2.27 (m, 1H), 1.86–1.78 (m, 2H), 1.75–1.68 (m, 2H). 13C NMR (125 MHz, DMSO-d6): 173.1, 169.8, 165.2, 141.7, 136.8, 129.5, 126.2, 124.1, 122.1, 59.6, 48.4, 46.0, 31.1, 25.8, 24.6, 24.4. MS (ESI) for C16H18N5O3S [M+H]+: m/z calcd, 360.11; found, 360.30. HRMS (ESI+): [M+H]+ m/z found 360.1125.
5.6.19. 3-(4-(4-(azetidine-1-carbonyl)thiophen-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (19).
Following the same procedure as compound 1 by using azetidin-1-yl(4-ethynylthiophen-3-yl)methanone instead of R1, compound 19 (4.4 mg, 27% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.23 (s, 1H), 8.30 (s, 1H), 7.97 (d, J = 3.5 Hz, 1H), 7.88 (d, J = 3.5 Hz, 1H), 5.87 (dd, J = 12.0, 5.0 Hz, 1H), 4.02 (t, J = 7.5 Hz, 2H), 3.96 (t, J = 7.5 Hz, 2H), 2.91–2.82 (m, 1H), 2.78–2.66 (m, 2H), 2.37–2.30 (m, 1H), 2.22–2.14 (m, 2H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 165.9, 141.7, 133.3, 130.6, 128.6, 124.7, 123.0, 59.5, 51.8, 48.4, 31.3, 24.8, 15.4. MS (ESI) for C15H16N5O3S [M+H]+: m/z calcd, 346.10; found, 346.31. HRMS (ESI+): [M+H]+ m/z found 346.0968.
5.6.20. N-cyclopropyl-4-(1-(2,6-dioxopiperidin-3-yl)-1H-1,2,3-triazol-4-yl)thiophene-3-carboxamide (20).
Following the same procedure as compound 1 by using N-cyclopropyl-4-ethynylthiophene-3-carboxamide instead of R1, compound 20 (12.0 mg, 55% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.22 (s, 1H), 8.52 (d, J = 4.0 Hz, 1H), 8.32 (s, 1H), 7.96 (d, J = 3.5 Hz, 1H), 7.88 (d, J = 3.5 Hz, 1H), 5.87 (dd, J = 12.0, 5.0 Hz, 1H), 2.90–2.77 (m, 2H), 2.76–2.66 (m, 2H), 2.36–2.30 (m, 1H), 0.69–0.64 (m, 2H), 0.56–0.51 (m, 2H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 165.9, 141.8, 136.4, 130.7, 129.3, 124.9, 123.5, 59.5, 31.3, 24.8, 23.3, 6.2, 6.2. MS (ESI) for C15H16N5O3S [M+H]+: m/z calcd, 346.10; found, 346.31. HRMS (ESI+): [M+H]+ m/z found 346.0971.
5.6.21. 3-(4-(4-(methylthio)thiophen-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (21).
Following the same procedure as compound 1 by using 3-ethynyl-4-(methylthio)thiophene instead of R1, compound 21 (6.0 mg, 33% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.23 (s, 1H), 8.52 (s, 1H), 7.99 (d, J = 3.5 Hz, 1H), 7.32 (d, J = 3.0 Hz, 1H), 5.89 (dd, J = 12.0, 5.5 Hz, 1H), 2.92–2.83 (m, 1H), 2.81–2.65 (m, 2H), 2.49 (s, 3H), 2.37–2.30 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.9, 141.6, 133.0, 130.7, 124.9, 122.2, 120.1, 59.7, 31.3, 24.8, 16.9. MS (ESI) for C12H13N4O2S2 [M+H]+: m/z calcd, 309.05; found, 309.25. HRMS (ESI+): [M+H]+ m/z found 309.0476.
5.6.22. 3-(4-(4-(methylsulfonyl)thiophen-3-yl)-1H-1,2,3-triazol-1-yl)piperidine-2,6-dione (22).
Following the same procedure as compound 1 by using 3-ethynyl-4-(methylsulfonyl)thiophene instead of R1, compound 22 (7.4 mg, 20% yield) was prepared as a white solid. 1H NMR (500 MHz, DMSO-d6) δ 11.25 (s, 1H), 8.50 (s, 1H), 8.50 (d, J = 3.5 Hz, 1H), 8.08 (d, J = 3.5 Hz, 1H), 5.90 (dd, J = 13.0, 5.5 Hz, 1H), 3.32 (s, 3H), 2.92–2.83 (m, 1H), 2.79–2.67 (m, 2H), 2.40–2.33 (m, 1H). 13C NMR (125 MHz, DMSO-d6): 172.9, 169.8, 140.2, 139.1, 136.6, 129.5, 128.8, 124.4, 59.7, 43.9, 31.2, 24.7. MS (ESI) for C12H13N4O4S2 [M+H]+: m/z calcd, 341.04; found, 341.26. HRMS (ESI+): [M+H]+ m/z found 341.0373.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Milka Kostic (Twitter: @MilkaKostic) for assistance with critical reading and scientific editing of the manuscript. We thank Dr. Lyn H. Jones for helpful discussions. We thank Dr. Zhen-Yu Jim Sun of the Dana-Farber Cancer Institute NMR Core Facility for NMR assistance. This work was supported in part by the National Institutes of Health (R01CA214608 awarded to E.S.F.).
The authors declare the following competing financial interest(s): N.S.G. is a founder, science advisory board member (SAB) and equity holder in Syros, Jengu, Inception, C4, B2S, Voronoi, EoCys Larkspur (board member), and Soltego (board member). The Gray lab receives or has received research funding from Novartis, Takeda, Astellas, Taiho, Janssen, Kinogen, Voronoi, Arbella, Epiphanes, Deerfield and Sanofi. E.S.F. is a founder, science advisory board (SAB) member, and equity holder in Civetta Therapeutics, Jengu Therapeutics (board member), Neomorph Inc and an equity holder in C4 Therapeutics. E.S.F. is a consultant to Novartis, Sanofi, EcoR1 capital, Avilar, and Deerfield. The Fischer lab receives or has received research funding from Astellas, Novartis, Voronoi, Ajax, and Deerfield. B.L.E. has received research funding from Celgene and Deerfield. He has received consulting fees from GRAIL, and he serves on the scientific advisory boards for Skyhawk Therapeutics, Exo Therapeutics, and Neomorph Inc. K.A.D. is a consultant to Kronos Bio. T. Z. is a founder, equity holder, and consultant in EoCys.
ABBREVIATIONS
- oC
Celsius
- 1H NMR
proton nuclear magnetic resonance
- Cp*RuCl(PPh3)2
pentamethylcyclopentadienylbis(triphenylphosphine)ruthenium(II) chloride
- CRBN
cereblon
- CRL4
cullin ring ligase 4
- CuSO4.5H2O
copper(II) sulfate pentahydrate
- DMF
N,N-dimethylformamide
- DMSO-d6
deuterated dimethyl sulfoxide
- H2O
water
- h
hour
- min
minute
- mL
milliliter
- MS
mass spectrometry
- NaH
sodium hydride
- nM
nanomolar
- rt
room temperature
- SAR
structure-activity relationship
- µM
micromolar
Footnotes
ASSOCIATED CONTENT
Supporting Information.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.xxxxxxx.
1H NMR spectra of compounds 2–22 and UPLC trace of compounds 1–22 (PDF)
Molecular formula strings (CSV)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.jmedchem.xxxxxxx
Contributor Information
Mingxing Teng, Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States.
Wenchao Lu, Department of Chemical and Systems Biology, ChEM-H, Stanford Cancer Institute, School of Medicine, Stanford University, Stanford, CA 94305, United States.
Katherine A. Donovan, Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115, United States..
Jialin Sun, Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States; Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115, United States..
Noah M. Krupnick, Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States.
Radosław P. Nowak, Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115, United States..
Yen-Der Li, Broad Institute of MIT and Harvard, Cambridge, Massachusetts 02142, United States; Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States..
Adam S. Sperling, Broad Institute of MIT and Harvard, Cambridge, Massachusetts 02142, United States Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States..
Tinghu Zhang, Department of Chemical and Systems Biology, ChEM-H, Stan-ford Cancer Institute, School of Medicine, Stanford University, Stanford, CA 94305, United States..
Benjamin L. Ebert, Broad Institute of MIT and Harvard, Cambridge, Massachusetts 02142, United States Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States; Howard Hughes Medical Institute, Boston, Massachusetts, United States..
Eric S. Fischer, Department of Cancer Biology, Dana-Farber Cancer Institute, Boston, Massachusetts 02215, United States Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts 02115, United States.
Nathanael S. Gray, Department of Chemical and Systems Biology, ChEM-H, Stanford Cancer Institute, School of Medicine, Stanford University, Stanford, CA 94305, United States.
REFERENCES
- (1).Ito T; Ando H; Suzuki T; Ogura T; Hotta K; Imamura Y; Yamaguchi Y; Handa H.Identification of a primary target of thalidomide teratogenicity. Science 2010, 327, 1345–1350. [DOI] [PubMed] [Google Scholar]
- (2).Fischer ES; Bohm K; Lydeard JR; Yang H; Stadler MB; Cavadini S; Nagel J; Serluca F; Acker V; Lingaraju GM; Tichkule RB; Schebesta M; Forrester WC; Schirle M; Hassiepen U; Ottl J; Hild M; Beckwith RE; Harper JW; Jenkins JL; Thoma NH Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 2014, 512, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jan M; Sperling AS; Ebert BL Cancer therapies based on targeted protein degradation - lessons learned with lenalidomide. Nat. Rev. Clin. Oncol 2021, 18, 401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kronke J; Udeshi ND; Narla A; Grauman P; Hurst SN; McConkey M; Svinkina T; Heckl D; Comer E; Li X; Ciarlo C; Hartman E; Munshi N; Schenone M; Schreiber SL; Carr SA; Ebert BL Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lu G; Middleton RE; Sun H; Naniong M; Ott CJ; Mitsiades CS; Wong KK; Bradner JE; Kaelin WG Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014, 343, 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Kronke J; Fink EC; Hollenbach PW; MacBeth KJ; Hurst SN; Udeshi ND; Chamberlain PP; Mani DR; Man HW; Gandhi AK; Svinkina T; Schneider RK; McConkey M; Jaras M; Griffiths E; Wetzler M; Bullinger L; Cathers BE; Carr SA; Chopra R; Ebert BL Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature 2015, 523, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Matyskiela ME; Lu G; Ito T; Pagarigan B; Lu CC; Miller K; Fang W; Wang NY; Nguyen D; Houston J; Carmel G; Tran T; Riley M; Nosaka L; Lander GC; Gaidarova S; Xu S; Ruchelman AL; Handa H; Carmichael J; Daniel TO; Cathers BE; Lopez-Girona A; Chamberlain PP A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature 2016, 535, 252–257. [DOI] [PubMed] [Google Scholar]
- (8).Matyskiela ME; Couto S; Zheng X; Lu G; Hui J; Stamp K; Drew C; Ren Y; Wang M; Carpenter A; Lee CW; Clayton T; Fang W; Lu CC; Riley M; Abdubek P; Blease K; Hartke J; Kumar G; Vessey R; Rolfe M; Hamann LG; Chamberlain PP SALL4 mediates teratogenicity as a thalidomide-dependent cereblon substrate. Nat. Chem. Biol 2018, 14, 981–987. [DOI] [PubMed] [Google Scholar]
- (9).Donovan KA; An J; Nowak RP; Yuan JC; Fink EC; Berry BC; Ebert BL; Fischer ES Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. Elife 2018, 7, e38430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Chamberlain PP; Lopez-Girona A; Miller K; Carmel G; Pagarigan B; Chie-Leon B; Rychak E; Corral LG; Ren YJ; Wang M; Riley M; Delker SL; Ito T; Ando H; Mori T; Hirano Y; Handa H; Hakoshima T; Daniel TO; Cathers BE Structure of the human cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol 2014, 21, 803–809. [DOI] [PubMed] [Google Scholar]
- (11).Schreiber SL The rise of molecular glues. Cell 2021, 184, 3–9. [DOI] [PubMed] [Google Scholar]
- (12).Hanan EJ; Liang J; Wang X; Blake RA; Blaquiere N; Staben ST Monomeric targeted protein degraders. J. Med. Chem 2020, 63, 11330–11361. [DOI] [PubMed] [Google Scholar]
- (13).Sievers QL; Petzold G; Bunker RD; Renneville A; Slabicki M; Liddicoat BJ; Abdulrahman W; Mikkelsen T; Ebert BL; Thoma NH Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 2018, 362, eaat0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ito T; Yamaguchi Y; Handa H.Exploiting ubiquitin ligase cereblon as a target for small-molecule compounds in medicine and chemical biology. Cell Chem. Biol 2021, 28, 987–999. [DOI] [PubMed] [Google Scholar]
- (15).Hansen JD; Correa M; Alexander M; Nagy M; Huang D; Sapienza J; Lu G; LeBrun LA; Cathers BE; Zhang W; Tang Y; Ammirante M; Narla RK; Piccotti JR; Pourdehnad M; Lopez-Girona A.CC-90009: a cereblon E3 ligase modulating drug that promotes selective degradation of GSPT1 for the treatment of acute myeloid leukemia. J Med. Chem 2021, 64, 1835–1843. [DOI] [PubMed] [Google Scholar]
- (16).Hansen JD; Correa M; Nagy MA; Alexander M; Plantevin V; Grant V; Whitefield B; Huang D; Kercher T; Harris R; Narla RK; Leisten J; Tang Y; Moghaddam M; Ebinger K; Piccotti J; Havens CG; Cathers B; Carmichael J; Daniel T; Vessey R; Hamann LG; Leftheris K; Mendy D; Baculi F; LeBrun LA; Khambatta G; Lopez-Girona A.Discovery of CRBN E3 ligase modulator CC-92480 for the treatment of relapsed and refractory multiple myeloma. J. Med. Chem 2020, 63, 6648–6676. [DOI] [PubMed] [Google Scholar]
- (17).Gemechu Y; Millrine D; Hashimoto S; Prakash J; Sanchenkova K; Metwally H; Gyanu P; Kang S; Kishimoto T.Humanized cereblon mice revealed two distinct therapeutic pathways of immunomodulatory drugs. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 11802–11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).An J; Ponthier CM; Sack R; Seebacher J; Stadler MB; Donovan KA; Fischer ES pSILAC mass spectrometry reveals ZFP91 as IMiD-dependent substrate of the CRL4(CRBN) ubiquitin ligase. Nat. Commun 2017, 8, 15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Chandra A; Grecco HE; Pisupati V; Perera D; Cassidy L; Skoulidis F; Ismail SA; Hedberg C; Hanzal-Bayer M; Venkitaraman AR; Wittinghofer A; Bastiaens PI The GDI-like solubilizing factor PDEdelta sustains the spatial organization and signalling of Ras family proteins. Nat. Cell Biol 2011, 14, 148–158. [DOI] [PubMed] [Google Scholar]
- (20).Dharmaiah S; Bindu L; Tran TH; Gillette WK; Frank PH; Ghirlando R; Nissley DV; Esposito D; McCormick F; Stephen AG; Simanshu DK Structural basis of recognition of farnesylated and methylated KRAS4b by PDEdelta. Proc. Natl. Acad. Sci. U. S. A 2016, 113, E6766–E6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Papke B; Murarka S; Vogel HA; Martin-Gago P; Kovacevic M; Truxius DC; Fansa EK; Ismail S; Zimmermann G; Heinelt K; Schultz-Fademrecht C; Al Saabi A; Baumann M; Nussbaumer P; Wittinghofer A; Waldmann H; Bastiaens PI Identification of pyrazolopyridazinones as PDEdelta inhibitors. Nat. Commun 2016, 7, 11360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zimmermann G; Papke B; Ismail S; Vartak N; Chandra A; Hoffmann M; Hahn SA; Triola G; Wittinghofer A; Bastiaens PI; Waldmann H.Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature 2013, 497, 638–642. [DOI] [PubMed] [Google Scholar]
- (23).Martin-Gago P; Fansa EK; Klein CH; Murarka S; Janning P; Schurmann M; Metz M; Ismail S; Schultz-Fademrecht C; Baumann M; Bastiaens PI; Wittinghofer A; Waldmann H.A PDE6delta-KRas inhibitor chemotype with up to seven H-bonds and picomolar affinity that prevents efficient inhibitor release by Arl2. Angew. Chem. Int. Ed. Engl 2017, 56, 2423–2428. [DOI] [PubMed] [Google Scholar]
- (24).Martin-Gago P; Fansa EK; Winzker M; Murarka S; Janning P; Schultz-Fademrecht C; Baumann M; Wittinghofer A; Waldmann H.Covalent protein labeling at glutamic acids. Cell Chem. Biol 2017, 24, 589–597. [DOI] [PubMed] [Google Scholar]
- (25).Winzker M; Friese A; Koch U; Janning P; Ziegler S; Waldmann H.Development of a PDEdelta-targeting PROTACs that impair lipid metabolism. Angew. Chem. Int. Ed. Engl 2020, 59, 5595–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Teng M; Jiang J; He Z; Kwiatkowski NP; Donovan KA; Mills CE; Victor C; Hatcher JM; Fischer ES; Sorger PK; Zhang T; Gray NS Development of CDK2 and CDK5 dual degrader TMX-2172. Angew. Chem. Int. Ed. Engl 2020, 59, 13865–13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Huang HT; Dobrovolsky D; Paulk J; Yang G; Weisberg EL; Doctor ZM; Buckley DL; Cho JH; Ko E; Jang J; Shi K; Choi HG; Griffin JD; Li Y; Treon SP; Fischer ES; Bradner JE; Tan L; Gray NS A chemoproteomic approach to query the degradable kinome using a multi-kinase degrader. Cell Chem. Biol 2018, 25, 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ying G; Boldt K; Ueffing M; Gerstner CD; Frederick JM; Baehr W.The small GTPase RAB28 is required for phagocytosis of cone outer segments by the murine retinal pigmented epithelium. J. Biol. Chem 2018, 293, 17546–17558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhang L; Chen X; Xue P; Sun HH; Williams ID; Sharpless KB; Fokin VV; Jia G.Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc 2005, 127, 15998–15999. [DOI] [PubMed] [Google Scholar]
- (30).McAlister GC, Nusinow DP, Jedrychowski MP, Wuhr M, Huttlin EL, Erickson BK, Rad R, Haas W, Gygi SP, MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem 2014, 86, 7150–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK, limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.