Abstract
Introduction: Klebsiella pneumoniae is a major pathogen implicated in healthcare-associated infections. Extended-spectrum β-lactamase (ESBL) and carbapenemase-producing K. pneumoniae isolates are a public health concern. This study investigated the existence of some ESBL and carbapenemase genes among clinical isolates of K. pneumoniae in Southwest Nigeria and additionally determined their circulating clones. Materials and Methods: Various clinical samples from 420 patients from seven tertiary hospitals within Southwestern Nigeria were processed between February 2018 and July 2019. These samples were cultured on blood agar and MacConkey agar, and the isolated bacteria were identified by Microbact GNB 12E. All K. pneumoniae were confirmed by polymerase chain reaction (PCR) using the 16s rRNA gene. Antibiotic susceptibility testing (AST) was done on these isolates, and the PCR was used to evaluate the common ESBL-encoding genes and carbapenem resistance genes. Genotyping was performed using multi-locus sequencing typing (MLST). Results: The overall prevalence of K. pneumoniae in Southwestern Nigeria was 30.5%. The AST revealed high resistance rates to tetracyclines (67.2%), oxacillin (61.7%), ampicillin (60.2%), ciprofloxacin (58.6%), chloramphenicol (56.3%), and lowest resistance to meropenem (43.0%). All isolates were susceptible to polymyxin B. The most prevalent ESBL gene was the TEM gene (47.7%), followed by CTX-M (43.8%), SHV (39.8%), OXA (27.3%), CTX-M-15 (19.5%), CTX-M-2 (11.1%), and CTX-M-9 (10.9%). Among the carbapenemase genes studied, the VIM gene (43.0%) was most detected, followed by OXA-48 (28.9%), IMP (22.7%), NDM (17.2%), KPC (13.3%), CMY (11.7%), and FOX (9.4%). GIM and SPM genes were not detected. MLST identified six different sequence types (STs) in this study. The most dominant ST was ST307 (50%, 5/10), while ST258, ST11, ST147, ST15, and ST321 had (10%, 1/10) each. Conclusion: High antimicrobial resistance in K. pneumoniae is a clear and present danger for managing infections in Nigeria. Additionally, the dominance of a successful international ST307 clone highlights the importance of ensuring that genomic surveillance remains a priority in the hospital environment in Nigeria.
Keywords: Klebsiella pneumoniae, extended spectrum β-lactamase, carbapenemase genes, multi-locus sequencing typing, polymerase chain reaction
1. Introduction
Klebsiella pneumoniae is one of the most important pathogenic bacteria in healthcare. It is a gram-negative, bacilli, nonmotile, and causative agent of many infectious diseases, such as pneumonia, sepsis, burns, wound infections, pyogenic liver abscesses, and urinary tract infections [1]. K. pneumoniae affects mainly patients who have predisposing debilitating backgrounds [2]. In Nigeria, K. pneumoniae is among the most common etiological agents of lower respiratory tract infections [3]. This bacterium has been reported to be the second most common cause of urinary tract infections [2], with an increasing rate of drug resistance to many commonly used antibiotics [4]. The emergence of carbapenem-resistant K. pneumoniae (CRKP) strains has become a critical challenge for public health worldwide due to their capacity to disseminate rapidly in the hospital environment [5]. The rapid spread of carbapenem-resistant K. pneumoniae (CRKP), listed by the WHO as a critical priority pathogen, has become a global threat to human health due to high morbidity and mortality [6].
K. pneumoniae utilizes different resistance mechanisms to counteract the effects of antibiotics, such as the production of destructive enzymes, target alteration, efflux pumps, and porin loss [2]. Therefore, hospital-associated infections with multidrug-resistant (MDR) strains of K. pneumoniae occur with high morbidity and mortality [7]. The emergence of extended-spectrum beta-lactamases (ESBL)-producing organisms was considered to be from the dissemination of clones of some epidemic strains along with the horizontal transmission of resistance gene-carrying plasmids among bacteria [8]. The development and selection of multiple drug-resistant bacteria, such as ESBL producers, have also been attributed to the rise in the use of second and third-generation cephalosporins to treat K. pneumonia infections [9].
Carbapenemases are enzymes that are capable of hydrolyzing the newer carbapenem antibiotics used in the treatment of MDRbacterial infections [7]. Among these, Klebsiella pneumoniae carbapenemase (KPC), metallo-β-lactamases (VIM, IMP, NDM), and OXA-48 types of enzymes are the most common. Mobile genetic elements, including plasmids, transposons, and integrons, are involved in disseminating related encoding genes [10]. ESBL and carbapenemase-producing organisms often acquire resistance to non-β-lactam antibiotics, including aminoglycosides and fluoroquinolones, resulting in multi-drug resistant properties.
In Nigeria, the existence of these resistance profiles has been established; however, little work has been done on the molecular identification and characterization of ESBLs and carbapenemase genes [11]. A study carried out in two tertiary hospitals in Northwest Nigeria showed that 58% of their K. pneumoniae and E.coli isolates were ESBL producers, while resistance to imipenem and meropenem was observed in 36.6% and40.3% of the isolates, respectively [12].
Factors known to promote the spread of ESBLs and carbapenemase-producing isolates include irrational use of antibiotics both in the hospital and community, suboptimal infection prevention and control practices, prolonged hospitalization, use of invasive devices (e.g., central venous lines, urinary catheters, and endotracheal tubes), stay in nursing homes, and presence of immunosuppressive conditions [13].
The main purpose of this study was to evaluate the antimicrobial resistance patterns and molecular mechanisms of ESBLs and carbapenem resistance among clinical isolates of K. pneumoniae from hospitalized patients in tertiary care hospitals in Southwestern Nigeria.
2. Materials and Methods
2.1. Study Site and Sample Collection
A total number of 420 clinical specimens that included urine, blood, sputum, wound swabs, high vaginal swabs (HVS), pus, stool, tracheal aspirate, and semen of patients that were diagnosed with various diseases were collected from hospitals in six states of Southwestern Nigeria. These included the Ladoke Akintola University of Technology Teaching Hospital, Osogbo, Osun State; the Obafemi Awolowo University Teaching Hospitals Complex, Ile—Ife, Osun State; the Lagos State University Teaching Hospital, Lagos State; the Federal Medical Centre Abeokuta, Ogun State; the University College Hospital, Ibadan Oyo State; the Federal Medical Centre Ido Ekiti, Ekiti State; and the Federal Medical Centre Owo, Ondo State between February 2018 and July 2019 and then transported to the medical microbiology and parasitology laboratory, the Ladoke Akintola University of Technology, Ogbomoso for microbiological and molecular analysis. Demographic and clinical information about the source of each clinical specimen were included in the data collection.
2.2. Isolation and Identification of Bacteria
Samples were cultured by inoculating into the blood and MacConkey agar and incubated at 37 °C for 18–24 h. Growth on blood agar and MacConkey (Oxoid Ltd., Basingstoke, Hampshire, UK) agar was identified by cultural characteristics, morphological appearance, and biochemical tests and confirmed by Microbact GNB 12E (Oxoid Ltd., Basingstoke, Hampshire, UK). All K. pneumonia isolates were further confirmed by polymerase chain reaction (PCR) using the 16s rRNA gene.
2.3. Antibiotic Susceptibility Testing
The antibiotic susceptibility testing was performed by the Kirby—Bauer Disc Diffusion and broth microdilution methods as modified by the Clinical and Laboratory Standards Institute [14]. The following antibiotic disks (Oxoid Ltd., Basingstoke, Hampshire, UK) were used: chloramphenicol (30 μg), ampicillin (10 μg), cefoxitin (30 μg), ceftriaxone (30 μg), cefuroxime (30 μg), cephalexin (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), levofloxacin (1 μg), imipenem (10 μg), meropenem (10 μg), and aztreonam (30 μg), tetracycline (30 μg), gentamicin (30 μg), ciprofloxacin (5 μg), cefepime (30 μg), amikacin (30 μg), ofloxacin (5 μg), amoxicillin/clavulanic acid (30 μg), oxacillin (5 μg), and polymyxin B (300 units).
All plates were incubated at 37 °C for 24 h. The diameters of inhibition zones were measured to the nearest millimeter using a ruler. Control strain K. pneumoniae ATCC 700603was used in the testing to validate the results of disc diffusion.
2.4. Detection of Antimicrobial Resistance Determinants
DNA molecules were extracted by boiling method [15] and used to prepare the PCR reaction mixture. All isolates were analyzed for the presence of β-lactamase genes, including ESBL genes (blaTEM, blaSHV, blaOXA, blaCTX-M, blaCTX-M-2, blaCTX-M-9, blaCTX-M-15) and carbapenemases genes (blaFOX, blaCMY, blaKPC, blaIMP, blaVIM, blaGIM, blaSPM, blaNDM-1 and blaOXA-48) (Table 1). At the completion of the amplification, PCR products were resolved inl.2% agarose gel stained with 0.5 μL of ethidium bromide. The DNA bands were visualized and photographed using a gel bio-imaging system (UVP Imaging System, Upland, CA, USA). The type-specific PCR products were recognized clearly by their distinct band sizes.
Table 1.
Primers used to amplify genes encoding beta-lactamase in K. pneumoniae.
| Primer | Sequence 51–31 | Annealing Temperature | Product Size (bp) | Reference |
|---|---|---|---|---|
| 16 s rRNA F 16 s rRNA R |
ATTTGAAGAGGTTGCAAACGAT TTCACTCTGAAGTTTTCTTGTGTTC |
57 °C | 130 | [16] |
| TEM-H F TEM-H R |
CCCCGAAGAACGTTTTC ATCAGCAATAAACCAGC |
52 °C | 517 | [17] |
| SHV-1 F SHV-1 R |
AGGATTGACTGCCTTTTTG ATTTGCTGATTTCGCTCG |
57 °C | 393 | [17] |
| OXA F OXA R |
ATATCTCTACTGTTGCATCTCC AAACCCTTCAAACCATCC |
57 °C | 619 | [17] |
| CTX-M F CTX-M R |
CGATGTGCAGTACCAGTAA TTAGTGACCAGAACAGCGG |
57 °C | 585 | [18] |
| CTX-M-2 F CTX-M-2 R |
ATGATGACTCAGAGCATTCG GAAACCGTGGGTTACGATTT |
60 °C | 1400 | [19] |
| CTX-M-9 F CTX-M-9 R |
GTGACAAAGAGAGTGCAACGG ATGATTCTCGCCGCTGAAGCC |
60 °C | 857 | [20] |
| CTX-M-15 F CTX-M-15 R |
CCATGGTTAAAAAATCACTGCG TGGGTRAARTARGTSACCAGAAYSAGCGG |
60 °C | 805 | [21] |
| KPC F KPC R |
CATTCAAGGGCTTTCTTGCTGC ACGACGGCATAGTCATTTGC |
55 °C | 538 | [22] |
| NDM F NDM R |
CACCTCATGTTTGAATTCGCC CTCTGTCACATCGAAATCGC |
58 °C | 984 | [23] |
| VIM2004A VIM2004B |
GTTTGGTCGCATATCGCAAC AATGCGCAGCACCAGGATAG |
54 °C | 390 | [24] |
| IMP F IMP R |
CATGGTTTGGTGGTTCTTGT ATAATTTGGCGGACTTTGGC |
55 °C | 488 | [25] |
| SPM F SPM R |
CCTACAATCTAACGGCGACC TCGCCGTGTCCAGGTATAAC |
55 °C | 650 | [26] |
| GIM F GIM R |
AGAACCTTGACCGAACGCAG ACTCATGACTCCTCACGAGG |
55 °C | 599 | [27] |
| CMY F CMY R |
TGGCCAGAACTGACAGGCAAA TTTCTCCTGAACGTGGCTGG |
47 °C | 462 | [28] |
| FOXMF FOXMR |
AACATGGGGTATCAGGGAGATG CAAAGCGCGTAACCGGATTGG |
54 °C | 190 | [29] |
| OXA 48F OXA 48R |
TTGGTGGCATCGATTATCGG GAGCACTTCTTTTGTGATGGC |
55 °C | 743 | [30] |
Source: Inqaba Biotec, Pretoria, South Africa.
2.5. Genetic Diversity Assessment by Multi-locus Sequence Typing (MLST)
Ten isolates were randomly selected for multi-locus sequence typing (MLST). Primers, PCR reaction conditions, and detailed methodology were in accordance with those previously described by [31]. Determination of allele profiles and sequence types (STs) was conducted by comparing the obtained sequences to the documented data at Klebsiella Pasteur MLST database (https://bigsdb.web.pasteur.fr/Klebsiella/Klebsiella.html, (accessed on 25 October 2022). Table 2 shows the PCR Primers nucleotides, annealing temperatures, and product sizes.
Table 2.
Primer sequences, annealing temperatures, and PCR product sizes for MLST.
| Primer | Sequence 51–31 | Annealing Temperature | Product Size (bp) |
|---|---|---|---|
| rpoBF rpoBR |
GGCGAAATGGCWGAGAACCA GAGTCTTCGAAGTTGTAACC |
50 °C | 501 |
| gapA F gapA R |
TGAAATATGACTCCACTCACGG CTTCAGAAGCGGCTTTGATGGCTT |
60 °C | 450 |
| mdh F mdh R |
CCCAACTCGCTTCAGGTTCAG CCGTTTTTCCCCAGCAGCAG |
50 °C | 477 |
| pgi F pgi R |
GAGAAAAACCTGCCTGTACTGCTGGC CGCGCCACGCTTTATAGCGGTTAAT |
50 °C | 432 |
| phoE F phoE R |
ACCTACCGCAACACCGACTTCTTCGG TGATCAGAACTGGTAGGTGAT |
50 °C | 420 |
| infB F infB R |
CTCGCTGCTGGACTATATTCG CGCTTTCAGCTCAAGAACTTC |
50 °C | 318 |
| tonB F tonB R |
CTTTATACCTCGGTACATCAGGTT ATTCGCCGGCTGRGCRGAGAG |
45 °C | 414 |
2.6. Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences software (SPSS version 24), and statistical significance was set at p < 0.05. Data were presented as frequencies and percentages.
3. Results
3.1. Distribution of Socio-Demographic Data of Selected Variables and Number of Klebsiella pneumoniae Positive Isolates
Out of the 420 samples collected, 128 (30.5%) were positive for K. pneumoniae. The overall prevalence of K. pneumoniae in Southwestern Nigeria was 30.5%. Of the K. pneumoniae-positive samples, Lagos state had the highest prevalence of K.pneumoniae, 32/70 (45.7%), followed by Oyo state, 25/70 (35.7%), while the lowest prevalence was seen in samples from Ekiti state,16/70 (22.9%). The difference in these isolation rates was statistically significant (p = 0.027). The highest recovery rate of Klebsiella pneumoniae was from tracheal aspirate specimens (42.9%) though the highest number was seen in urine specimens (40). The differences in the proportion of recovery of K. pneumoniae from the various sample types were not significant (p = 0.540). Although there were no significant differences (p = 0.441) in the recovery rate of K. pneumoniae from wards and clinics, the highest recovery rate was from the intensive care unit, where almost half of their samples (43.7%) yielded K. pneumoniae (Table 3).
Table 3.
Distribution of socio-demographic data of selected variables and number of Klebsiella pneumoniae-positive isolates.
| Variable | Number (%) |
Positive No(%) |
Pearson Chi-Square | df | p-Value | |
|---|---|---|---|---|---|---|
| Location | Ekiti Lagos Ogun Ondo Osun Oyo |
70 (16.7) 70 (16.7) 70 (16.7) 70 (16.7) 70 (16.7) 70 (16.7) |
16 (22.9) 32 (45.7) 17 (24.3) 20 (28.6) 18 (25.7) 25 (35.7) |
12.631 | 5 | 0.027 |
| Total | 420 | 128 (30.5) | ||||
| Age | ≤10 years | 19 (4.5) | 7 (36.8) | 0.634 | 3 | 0.889 |
| 11–25 years | 63 (15) | 19 (30.2) | ||||
| 26–50 years | 209 (49.8) | 61 (29.2) | ||||
| 51 years and above | 129 (30.7) | 41 (31.8) | ||||
| Sex | Female | 220 (52.4) | 69 (31.4) | 0.172 | 1 | 0.679 |
| Male | 200 (47.6) | 59 (29.5) | ||||
| Sample | Blood | 34 (8.0) | 7 (20.6) | 6.968 | 8 | 0.540 |
| High Vaginal Swab | 62 (14.8) | 20 (32.3) | ||||
| Pus | 65 (15.5) | 25 (38.5) | ||||
| Semen | 7 (1.7) | 1 (14.3) | ||||
| Sputum | 45 (10.7) | 12 (26.7) | ||||
| Stool | 14 (3.3) | 4 (28.6) | ||||
| Tracheal Aspirate | 7 (1.7) | 3 (42.9) | ||||
| Urine Wound Swab |
144 (34.3) 42 (10) |
40 (27.8) 16 (38.1) |
||||
| Ward | Accident and emergency | 10 (2.38) | 2 (20) | |||
| Intensive Care Unit | 16 (3.81) | 7 (43.7) | ||||
| Geriatrics | 25 (5.95) | 10 (40) | ||||
| Medical | 160 (38.1) | 41 (25.6) | ||||
| Obstetrics and Gynaecology | 96 (22.9) | 32 (33.3) | ||||
| Pediatrics | 13 (3.1) | 4 (30.8) | ||||
| Surgical | 100 (23.8) | 32 (32) | ||||
3.2. Antibiotic Resistance Patterns of K. pneumoniae Isolates
The highest levels of antibiotic resistance were displayed against tetracycline (67.2%), oxacillin (61.7%), ampicillin (60.2%), ciprofloxacin (58.6%), and chloramphenicol (56.3%), while drugs with the least antibiotic resistance (below 50%) were imipenem (48.4%), cefepime (44.5%), and meropenem (43.0%). All isolates were susceptible to polymyxin B (Table 4).
Table 4.
Antibiotic resistance patterns of K. pneumoniae isolates.
| S/N | Antibiotics | Resistant Number/Percentage |
|---|---|---|
| 1 | Levofloxacin | 66 (51.6) |
| 2 | Cefoxitin | 69 (53.9) |
| 3 | ceftazidime | 68 (53.1) |
| 4 | tetracycline | 86 (67.2) |
| 5 | aztreonam | 70 (54.7) |
| 6 | gentamicin | 69 (53.9) |
| 7 | Cefepime | 57 (44.5) |
| 8 | Imipenem | 62 (48.4) |
| 9 | Amikacin | 70 (54.7) |
| 10 | meropenem | 55 (43.0) |
| 11 | Ofloxacin | 69 (53.9) |
| 12 | cephalexin | 69 (53.9) |
| 13 | Amoxycillin/Clavulanic acid | 68 (53.1) |
| 14 | ciprofloxacin | 75 (58.6) |
| 15 | Cefuroxime | 74 (57.8) |
| 16 | ampicillin | 77 (60.2) |
| 17 | oxacillin | 79 (61.7) |
| 18 | Cefotaxime | 66 (51.6) |
| 19 | chloramphenicol | 72 (56.3) |
| 20 | Ceftriaxone | 69 (53.9) |
| 21 | Polymyxin B | 0 (0.0) |
3.3. The Distribution of the ESBL Genes Produced by the Multidrug-Resistant K. pneumoniae Isolates
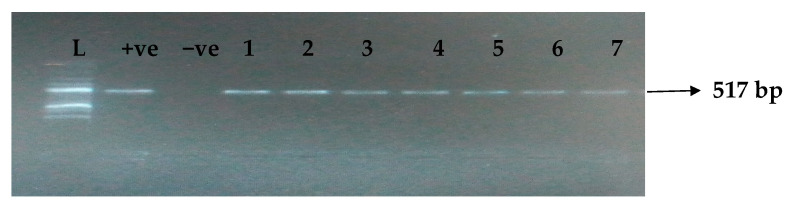
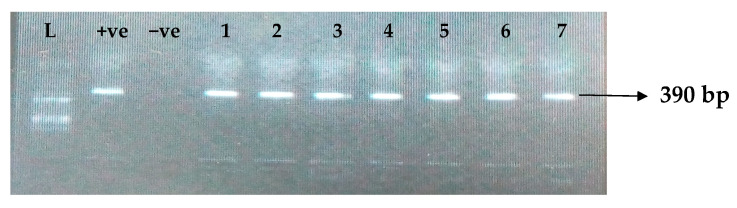
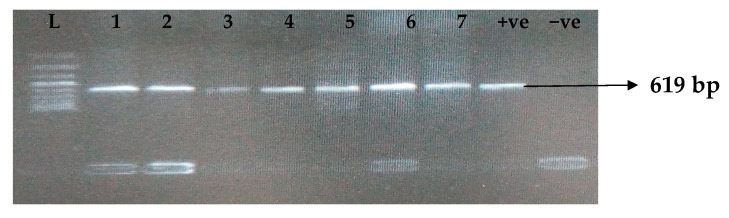
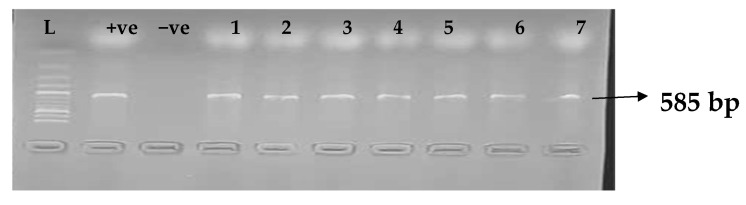
Table 5 depicts the prevalence of ESBL-associated genes in clinical isolates of K. pneumoniae. The TEM gene (47.7%) was recovered most, followed by CTX-M (43.8%), SHV (39.8%), OXA (27.3%), CTX-M-15 (19.5%), CTX-M-2 (11.1%), and CTX-M-9 (10.9%). The gel electrophoresis profiles of blaTEM, blaSHV, blaOXA, and blaCTXM genes are presented in Figure 1, Figure 2, Figure 3 and Figure 4, respectively.
Table 5.
Distribution of the ESBL genes produced by the multidrug-resistant K. pneumonia isolates.
| ESBL Genes | Frequency | Percent |
|---|---|---|
| TEM | 61 | 47.7 |
| SHV | 51 | 39.8 |
| OXA | 35 | 27.3 |
| CTX-M | 56 | 43.8 |
| CTX-M-2 | 15 | 11.1 |
| CTX-M-9 | 14 | 10.9 |
| CTX-M-15 | 25 | 19.5 |
Figure 1.
Electrophoresis gel picture of the blaTEM gene. L = 100 bp ladder, +ve = TEM positive control, −ve = TEM negative control, and 1–7 = sample representatives of blaTEM-positive isolates.
Figure 2.
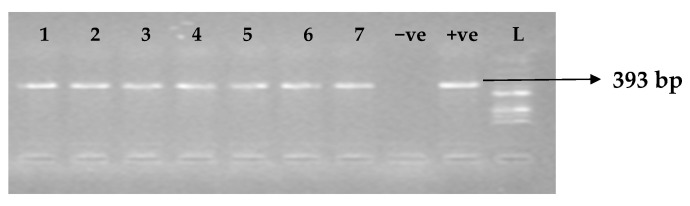
Electrophoresis gel picture of the blaSHV gene. L = 100 bp ladder, +ve = SHV positive control, −ve = SHV negative control, and 1–7 = sample representatives of blaSHV-positive isolates.
Figure 3.
Electrophoresis gel picture of the blaOXA gene. L = 100 bp ladder, +ve = OXA positive control, −ve = OXA negative control, and 1–7 = sample representatives of blaOXA-positive isolates.
Figure 4.
Electrophoresis gel picture of the blaCTX-M gene. L = 100 bp ladder, +ve = CTX-M positive control, −ve = CTX-M negative control, and 1–7 = sample representatives of blaCTX-M-positive isolates.
3.4. Distribution of Carbapenemase Genes among K. pneumoniae Isolates
Carbapenemases are a group of beta-lactamases that are able to breakdown the active core of carbapenems antibiotics. Table 6 depicts the distribution of different carbapenemase-associated genes from the clinical isolates of K. pneumoniae. The VIM gene (43.0%) was most detected among the clinical isolates, followed by OXA-48 (28.9%), IMP (22.7%), NDM (17.2%), KPC (13.3%), CMY (11.7%), and FOX (9.4%). GIM and SPM were not detected. The gel electrophoresis profiles of blaVIM, blaOXA-48, blaIMP, blaKPC, blaCMY, and blaFOX genes are presented in Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10, respectively.
Table 6.
Distribution of carbapenemase genes among K. pneumoniae isolates.
| Frequency | Percent | |
|---|---|---|
| VIM | 55 | 43.0 |
| OXA-48 | 37 | 28.9 |
| IMP | 29 | 22.7 |
| NDM | 22 | 17.2 |
| KPC | 17 | 13.3 |
| CMY | 15 | 11.7 |
| FOX | 12 | 9.4 |
| SPM | 0 | 0.0 |
| GIM | 0 | 0.0 |
Figure 5.
Electrophoresis gel picture of the blaVIM gene. L = 100 bp ladder, +ve = VIM positive control (K. pneumoniae ATCC 13883 strain), −ve = VIM negative control, and 1–7 = sample representatives of blaVIM-positive isolates.
Figure 6.
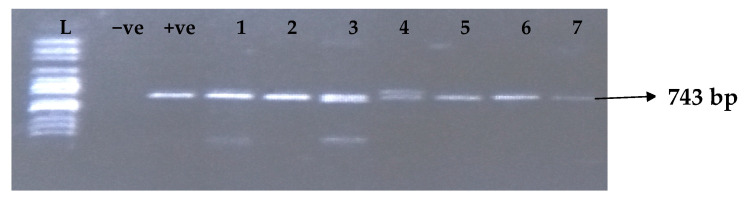
Electrophoresis gel picture of the blaOXA-48gene.L = 100 bp ladder, +ve = OXA-48positive control, −ve = OXA-48 negative control, and 1–7 = sample representatives of blaOXA-48-positive isolates.
Figure 7.
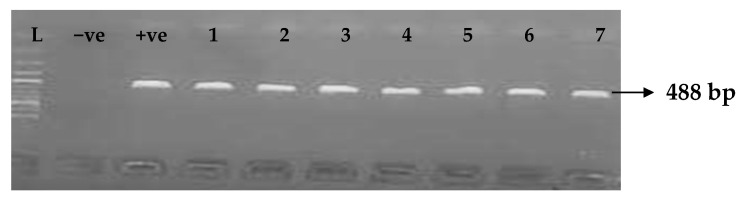
Electrophoresis gel picture of the blaIMP gene. L = 100 bp ladder, +ve = IMP positive control, −ve = IMP negative control, and 1–7 = sample representatives of blaIMP-positive isolates.
Figure 8.
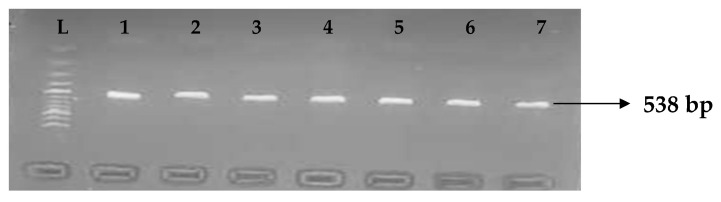
Electrophoresis gel picture of the blaKPC gene. L = 100 bp ladder and1–7 = sample representatives of blaIMP-positive isolates.
Figure 9.
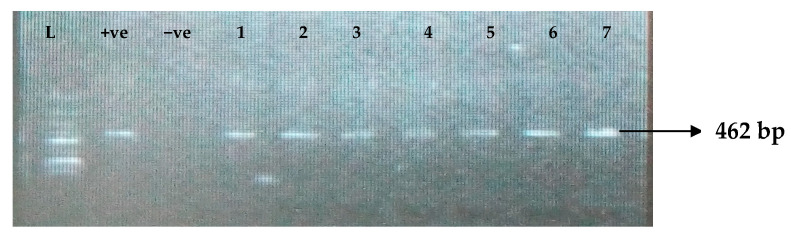
Electrophoresis gel picture of the blaCMY gene. L = 100 bp ladder, +ve = CMY positive control, −ve = CMY negative control, and 1–7 = sample representatives of blaCMY-positive isolates.
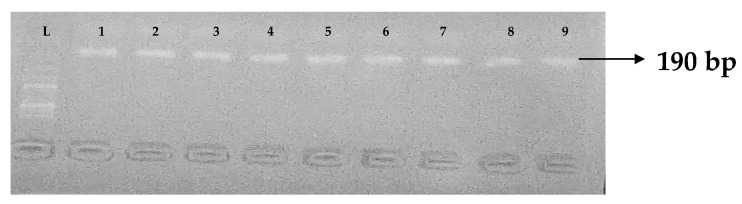
Figure 10.
Electrophoresis gel picture of the blaFOX gene. L = 100 bp ladder and 1–9 = sample representatives of blaFOX-positive isolates.
Table 7 shows relationship between phenotypic and genotypic genes of K. pneumoniae. From the table, carbapenem phenotype showed a significant relationship with KPC, IMP, and VIM genes.
Table 7.
Analysis of carbapenem phenotypes and carbapenem genes.
| Antibiotics | Number/% Resistant (Phenotype) |
KPC Gene (17) | IMP Gene (29) | VIM Gene (55) | |||
|---|---|---|---|---|---|---|---|
| Number (%) | p Value | Number/% | p Value | Number/% | p Value | ||
| Levofloxacin | 66 (51.6) | 2 (11.8) | 0.505 | 16 (55.2) | 0.062 | 29 (52.7) | 0.480 |
| Cefoxitin | 69 (53.9) | 4 (23.5) | 0.447 | 11 (37.9) | 0.510 | 32 (58.2) | 0.254 |
| Ceftazidime | 68 (53.1) | 1 (5.9) | 0.274 | 14 (48.3) | 0.107 | 29 (52.7) | 0.540 |
| Tetracycline | 86 (67.2) | 6 (35.2) | 0.381 | 17 (58.6) | 0.403 | 37 (67.3) | 0.570 |
| Aztreonam | 70 (54.7) | 5 (29.4) | 0.325 | 12 (41.4) | 0.406 | 33 (60.0) | 0.193 |
| Gentamicin | 69 (53.9) | 4 (23.5) | 0.447 | 14 (48.3) | 0.130 | 31 (56.4) | 0.380 |
| Cefepime | 57 (44.5) | 2 (11.8) | 0.315 | 8 (27.6) | 0.395 | 27 (49.1) | 0.235 |
| Imipenem | 62 (48.4) | 5 (29.4) | 0.041 * | 16 (55.2) | 0.000 * | 30 (54.5) | 0.036 * |
| Amikacin | 70 (54.7) | 4 (23.5) | 0.486 | 8 (27.6) | 0.181 | 32 (58.2) | 0.305 |
| Meropenem | 55 (43.0) | 8 (47.1) | 0.001 * | 16 (55.2) | 0.000 * | 35 (63.6) | 0.000 * |
| Ofloxacin | 69 (53.9) | 7 (41.2) | 0.081 | 12 (41.4) | 0.361 | 33 (60.0) | 0.154 |
| Cephalexin | 69 (53.9) | 3 (17.6) | 0.553 | 13 (44.8) | 0.230 | 32 (58.2) | 0.254 |
| Amoxycillin/Clavulanic acid | 68 (53.1) | 1 (5.9) | 0.274 | 6 (20.7) | 0.076 | 28 (50.9) | 0.398 |
| Ciprofloxacin | 75 (58.6) | 7 (41.2) | 0.209 | 14 (48.3) | 0.342 | 36 (65.4) | 0.118 |
| Cefuroxime | 74 (57.8) | 3 (17.6) | 0.359 | 12 (41.4) | 0.556 | 31 (56.4) | 0.457 |
| Ampicillin | 77 (60.2) | 7 (41.2) | 0.270 | 11 (37.9) | 0.271 | 34 (61.8) | 0.441 |
| Oxacillin | 79 (61.7) | 10 (58.8) | 0.041 | 15 (51.7) | 0.377 | 41 (74.5) | 0.080 |
| Cefotaxime | 66 (51.6) | 1 (5.9) | 0.343 | 12 (41.4) | 0.273 | 31 (56.4) | 0.222 |
| Chloramphenicol | 72 (56.3) | 3 (17.6) | 0.436 | 9 (31.0) | 0.221 | 28 (50.9) | 0.190 |
| Ceftriaxone | 69 (53.9) | 4 (23.5) | 0.447 | 9 (31.0) | 0.342 | 31 (56.4) | 0.380 |
NOTE *: Carbapenem phenotype showed a significant relationship with KPC, IMP, and VIM.
3.5. Genetic Diversity Assessment by MLST
Table 8 shows the results of MLST conducted on 10 K. pneumoniae to determine the extent of genotypic diversity among the K. pneumoniae isolates. Results from the table revealed that six different sequence types (STs) were identified in this study. The most dominant ST was ST307 (50%, 5/10), while ST258, ST11, ST147, ST15, and ST321 had (10%, 1/10) each.
Table 8.
Sequence types of multidrug-resistant and hypervirulent Klebsiella pneumoniae.
| Isolate | State | Sample | Beta-Lactamase Genes | Allelic Profile | MLST | Cloner Cluster (CC) |
|---|---|---|---|---|---|---|
| LK16 | Lagos | Urine | CTX-M-15, TEM, SHV, KPC | 3-3-1-1-1-1-4 | ST11 | 258 |
| LK23 | Lagos | Urine | CTX-M-15, VIM, OXA-48, KPC | 3-4-6-1-7-4-38 | ST147 | 147 |
| LK27 | Lagos | HVS | CTX-M-15, VIM, OXA-48, KPC, NDM | 4-1-2-52-1-1-7 | ST307 | 307 |
| OGK1032 | Ogun | Pus | CTX-M-15, VIM, KPC, NDM | 4-1-2-52-1-1-7 | ST307 | 307 |
| OYK39 | Oyo | Urine | CTX-M-15, VIM, OXA-48, KPC | 1-1-1-1-1-1-1 | ST15 | 15 |
| OYK24 | Oyo | Sputum | CTX-M-15, VIM, OXA-48, KPC, NDM | 4-1-2-52-1-1-7 | ST307 | 307 |
| EK55 | Ekiti | Urine | CTX-M-15, VIM, OXA-48, KPC, NDM | 4-1-2-52-1-1-7 | ST307 | 307 |
| ONK74 | Ondo | Wound swab | TEM, CTX-M-15, KPC, NDM | 3-3-1-1-1-1-79 | ST258 | 258 |
| OSK12 | Osun | Urine | CTX-M-15, KPC, OXA-48, NDM | 4-1-2-52-1-1-7 | ST307 | 307 |
| OSK16 | Osun | Urine | CTX-M-15, OXA-48 | 4-16-2-1-28-3-40 | ST321 | 321 |
4. Discussion
K. pneumoniae has been reported as one of the main pathogens causing nosocomial and community-acquired infections in humans over a long period of time. Due to antimicrobial resistance, treatment of K. pneumonia infections has become complicated and difficult to treat [32]. The 30.5% prevalence of K. pneumoniae in this study is similar to the 34% reportedin Lagos state and also in the southwest [33]. Hence, it can be inferred that K. pneumoniae is associated with clinical infections in Southwest Nigeria. Similar findings have been reported in other parts of the country, such as 30.0% recorded in Kano State [34]; it is, however, higher than 12.8%reported in Kaduna State [35]. The high prevalence rate of K. pneumoniae observed in this study could be explained by the fact that all isolates investigated in this study were sourced from hospitalized patients, which may underscore the lack of proper infection control practices [36], showing that K. pneumoniae is a common nosocomial pathogen [37]. There was an association between isolated K. pneumoniae and the selected six states showing that isolation of K. pneumoniae depends on the hospital or its site.
The result of this study revealed that K. pneumoniae infection was seen more in females than males. The higher occurrence of these isolates among females might result from the higher prevalence of urogenital K. pneumoniae isolates in our study. The major proportion of samples used in this study were urine samples. Hence, the highest number of K. pneumoniae was observed in the urine sample, and this is in agreement with the finding of [38].
A high rate of antimicrobial resistance was observed in our study, as more than half of the isolates were resistant to most antibiotics tested. The 48.4% and 43.0% resistance rates of clinical K. pneumoniae isolates to imipenem and meropenem in this study are similar to the observation in Ebonyi, Nigeria, where [39] reported 41.1% for imipenem and 43.3% for meropenem. However, our observed resistance rate to imipenem is higher than 24% in Oyo State [40] and 19.05% in Kaduna State [41], also in Nigeria. According to previous studies, imipenem and meropenem have shown good activity against Enterobacteriaceae [42]; therefore, the findings of this study show that there has been a steady increase in resistance to these antibiotics over the years. This may be as a result of their increasing use among the populace.
In the current study, we observed that ESBL-KP isolated from different clinical samples harbor multiple ESBL genes (blaCTX-M, blaTEM, blaOXA, and blaSHV), which is similar to other studies, including a study from India [43].The most prevalent ESBL gene in this study was blaTEM, with a 47.7% prevalence rate comparable to 49.3% reported in India [44] and 52% reported in Southwestern Nigeria [45]. However, this prevalence is in sharp contrast to the 100% reported in Port Harcourt [46] and 14.28% in Sokoto State [35]. The 39.8% prevalence of the blaSHV gene among clinical K. pneumonia isolates reported in this study is comparable to the 35% reported in Pakistan [47]. This current prevalence is lower than 58.33% reported in Port Harcourt [46] and 48% in Southwestern Nigeria [45]. In addition, the 27.3% prevalence rate of the blaOXA gene among clinical K. pneumoniae isolates is lower than the 65% reported in Pretoria [48] and 41.67% in Port Harcourt [46].
It has been proven that the blaCTX-M-15 among humans has increased outstandingly over time in most countries. The 43.8% prevalence of the blaCTX-M gene among our clinical K. pneumoniae isolates is slightly higher than the 41.67% reported in Port Harcourt two years earlier [46] and also higher than 35.71% in Sokoto State [35] and the 32%in Southwestern Nigeria four years ago [45]. The 19.5% prevalence of the blaCTX-M-15 among clinical K. pneumoniae isolates is comparable to the 12.5% prevalence in China [49] and 14.54% in Iran [50]. However, [47] reported a 46% prevalence of the CTX-M-15 gene in Pakistan. Our study adds to the body of evidence that the CTX-M-15 remains the most important CTX-M enzyme in K. pneumoniae as a result of its large diffusion and relation to infections in humans. Similarly, this particular genotype is widely disseminated in Africa [51].
Moreover, 13.3% of the clinical K. pneumoniae isolates possessed the CTX-M-2 gene, which is lower than the 45.7% reported in Argentina [52]. Additionally, the 10.9% prevalence of the blaCTX-M-9 among clinical K. pneumoniae isolates is comparable to the 9.69% in China [53]. However, [54] reported a 40% prevalence of the CTX-M-9 gene in Saudi Arabia, which is significantly greater than the study’s prevalence rate. The coexistence of ESBL genes in these isolates may have also contributed to the observed high rate of antimicrobial drug resistance [55]. These data have clinical applications for selecting empiric antibiotic therapy when infections caused by ESBL-producing K. pneumoniae are suspected [55].
The present work corroborates the findings of [56], who reported that blaVIM was frequently involved in causing carbapenem resistance in humans. Similarly, [56] also reported that blaVIM (69.2%) was the predominant gene in hospitalized patients in Egypt. The 43.0% prevalence rate of the blaVIM gene among carbapenemase-producing K. pneumoniae in this study is higher than the 33.3% reported in Iran [57] but lower than the 84.62% in Egypt [58]. However, in contrast to our findings, no clinical isolate of K. pneumonia harbored the VIM in a Brazilian study [7].
We report a lower prevalence of the blaKPC gene among carbapenemase-producing K. pneumonia compared to the blaVIM gene. Similar low proportions have been reported by [59] in Jos, Plateau state, and even a much lower prevalence of 2.7%in Port Harcourt, Nigeria [60]. Our findings were contrary to the zero prevalence reported in South Africa, which could be because these were mainly surveillance studies conducted among asymptomatic persons [61]. It could also be attributed to the restricted use of antibiotics in those countries as opposed to Nigeria, where antibiotics are easily available over-the-counter. We did not find any blaSPM or blaGIM genes in K. pneumoniae isolates. This is in agreement with other studies reporting that these genes are limited to distinct geographical regions such as Germany and Brazil [62].
Molecular studies showed the prevalence of AmpC genes were 11.7% and 9.4% for blaCMY and blaFOX, respectively. Similarly, in the Zorgani study in Tripoli, the majority of AmpC-positive isolates (66.6%) were found to carry the CMY-encoding gene [63]. The possible reason for this prevalence may be due to excessive usage of extended-spectrum cephalosporin in the treatment of gram-negative infections [64].
In this study, MLST showed that the K. pneumoniae strains belonged to six different sequence types (STs), revealing clonal diversity. ST307 was the most concentrated, accounting for 5 (50%). This finding is in accordance with the report of [65], who reported ST307 as the most prevalent ST in Southwestern Nigeria. All five isolates having ST307 were obtained from the urine. These isolates were also found to have similar genotypes regarding ESBLs (CTX-M-15) and carbapenemase (KPC). Several countries, such as Italy, Korea, the USA, Mexico, and China, have reported carbapenem-resistant K. pneumoniae ST307 with ESBL production [66]. The ST307 identified in our study were present in five tertiary hospitals in Southwestern Nigeria, indicating the role of immigration in the transmission of these successful international clones from diverse geographical settings.
It is of note in Africa that the problem of carbapenem-resistant Enterobacteriaceae (CRE) is becoming increasing on a daily basis, especially in Nigeria, where the usage of carbapenem is on the increase in our clinical settings, as expressed from our data. It is of note that other factors contributed to this aggravated increase by other factors such as the issue of poor diagnostic tools in our tertiary health care settings, poor sanitation and dirty environment linked to the high rate of infections, sub-optimal disease surveillance, and incessant over-the-counter abuse and usage of antibiotics. It is of note that the burden and the problem of CRE in Africa are underreported [11,67].
5. Conclusions
A total number of 128 non-duplicate K. pneumoniae were isolated and characterized from hospitalized patients in Southwestern Nigeria. The high MDR K. pneumoniae observed in this study is worrisome and calls for action. Factors such as the frequent use of carbapenems and cephalosporins, as well as the lack of antibiotic therapy policies and guidelines in most healthcare facilities in the country, should be addressed as these could be responsible for the observed high level of resistance. This study also demonstrates that although there is considerable diversity among the K. pneumoniae in Nigerian hospitals, a high proportion of the isolates belonged to one clonal group; therefore, molecular epidemiological surveillance and control can effectively reduce the occurrence and spread of drug-resistant bacterial infections in hospitalized patients.
Author Contributions
O.A.O. and G.O. conceived the study. O.A.O., G.O. and R.A.O. designed the study and wrote the literature review and the method. G.O. collected the data M.Y.J.-S. and R.A.O. performed the data analysis, and G.O. wrote the initial draft. O.O. and O.A.O. Edited the manuscripts and supervised the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Research Ethics Committee, Uniosun Teaching Hospital, Osogbo, Osun State, Nigeria. (UTH/REC/2023/04/762).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are within the manuscript. The datasets used and/or during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The Alexander von Humboldt Foundation provided part of the funding for this work within the scope of the alumni sponsorship program titled ‘Overcoming the pandemic with science—Humboldt Research Hubs in Africa’ financed by the Bayer Science Foundation. Olusola Ojurongbe is an alumnus of Alexander von Humboldt Foundation.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gorrie C.L., Mirceta M., Wick R.R., Judd L.M., Wyres K.L., Thomson N.R. Antimicrobial resistant Klebsiella pneumoniae carriage and infection in specialized geriatric care wards linked to acquisition in the referring hospital. Clin. Infect. Dis. 2018;67:161–170. doi: 10.1093/cid/ciy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caneiras C., Lito L., Melo-Cristino J., Duarte A. Community- and Hospital-Acquired Klebsiella pneumoniae Urinary Tract Infections in Portugal: Virulence and Antibiotic Resistance. Microorganisms. 2019;7:138. doi: 10.3390/microorganisms7050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uzoamaka M., Ngozi O., Johnbull O.S., Martin O. Bacterial Etiology of lower respiratory tract infections and their antimicrobial susceptibility. Am. J. Med. Sci. 2017;354:471–475. doi: 10.1016/j.amjms.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Jafari Z., Harati A.A., Haeili M., Kardan-Yamchi J., Jafari S., Jabalameli F. Molecular epidemiology and drug resistance pattern of carbapenemresistant Klebsiella pneumoniae isolates from Iran. Microb. Drug Resist. 2019;25:336–343. doi: 10.1089/mdr.2017.0404. [DOI] [PubMed] [Google Scholar]
- 5.Rossolini G.M. Extensively drug-resistant carbapenemase-producing Enterobacteriaceae: An emerging challenge for clinicians and healthcare systems. J. Intern. Med. 2015;277:528–531. doi: 10.1111/joim.12350. [DOI] [PubMed] [Google Scholar]
- 6.WHO Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. [(accessed on 20 February 2021)]. Available online: http://remed.org/wp-content/uploads/2017/03/lobal-priority-list-of-antibiotic-resistant-bacteria-2017.pdf.
- 7.Ferreira R.L., da Silva B.C.M., Rezende G.S., Nakamura-Silva R., Pitondo-Silva A., Campanini E.B. High prevalence of multidrug-resistant Klebsiella pneumoniae harboring several virulence and β-lactamase encoding genes in a Brazilian intensive care unit. Front. Microbiol. 2019;9:3198. doi: 10.3389/fmicb.2018.03198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriquez-Bano J., Navarro M.D., Romero L., Martinez-Martinez L., Muniain M.A., Perea E.J. Epidemiology and clinical features of infections Caused by extended spectrum beta-lactamase producing Escherishia coli in non Hospitalized patients. J. Clin. Microbiol. 2004;42:1089–1094. doi: 10.1128/JCM.42.3.1089-1094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitout J.D.D., Laupland K.B. Extended-spectrum β-lactamase-producing enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 10.Nasiri M.J., Mirsaeidi M., Mousavi S.M.J., Arshadi M., Fardsanei F., Deihim B. Prevalence and mechanisms of carbapenem resistance in Klebsiella pneumoniae and Escherichia coli: A systematic review and meta-analysis of cross-sectional studies from Iran. Microb. Drug Resist. 2020;26:1491–1502. doi: 10.1089/mdr.2019.0440. [DOI] [PubMed] [Google Scholar]
- 11.Olowe O.A., Aboderin B.W., Motayo B.O., Ibeh O., Adegboyega T.T., Ogiowa I.J. Detection of Extented Spectrum BetaLactamase producing strains of Escherichia coli and Klebsiella species in a tertiary health center in Ogun state, Nigeria. Int. J. Trop. Med. 2010;5:62–64. [Google Scholar]
- 12.Ibrahim Y., Sani Y., Saleh Q., Saleh A., Hakeem G. Phenotypic detection of extended-spectrum beta-lactamase and carbapenemase co-producing clinical isolates from two tertiary hospitals in Kano, northwest Nigeria. Ethiop. J. Health Sci. 2017;27:3–10. doi: 10.4314/ejhs.v27i1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freire M.P., Pierrotti L.C., Filho H.H.C., Ibrahim K.Y., Magri A.S., Bonazzi P.R. Infections with Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae in cancer patients. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:277–286. doi: 10.1007/s10096-014-2233-5. [DOI] [PubMed] [Google Scholar]
- 14.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. CLSI supplement M100. [Google Scholar]
- 15.Mentasti M., Prime K., Sands K., Khan S., Wootton M. Rapid detection of IMP, NDM, VIM, KPC and OXA-48-like carbapenemases from Enterobacteriales and Gram-negative non-fermenter bacteria by real-time PCR and melt-curve analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:2029–2036. doi: 10.1007/s10096-019-03637-5. [DOI] [PubMed] [Google Scholar]
- 16.Ghasemnejad A., Doudi M.A., Mirmozafari N. Evaluation of modified hodge test as a non-molecular assay for accurate detection of KPC-producing Klebsiella pneumoniae. Pol. J. Microbiol. 2018;67:291–295. doi: 10.21307/pjm-2018-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma M., Pathak S., Srivastava P. Prevalence and antibiogram of Extended Spectrum β-Lactamase (ESBL) producing Gram negative bacilli and further molecular characterization of ESBL producing Escherichia coli and Klebsiella spp. J. Clin. Diagn. Res. 2013;7:2173–2177. doi: 10.7860/JCDR/2013/6460.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batchelor M., Hopkins K., Threlfall E.J., Clifton-Hadley F.A., Stallwood A.D., Davies R.H., Liebana E. Bla (CTXM) genes in clinical Salmonella isolates recovered from humans in England and Wales from 1992 to 2003. Antimicrob. Agents Chemother. 2005;49:1319–1322. doi: 10.1128/AAC.49.4.1319-1322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park Y.J., Lee S., Kim Y.R., Oh E.J., Woo G.J., Lee K. Occurrence of extended-spectrum (beta)-lactamases and plasmid-mediated AmpC (beta)-lactamases among Korean isolates of Proteus mirabilis. J. Antimicrob. Chemother. 2006;57:156–158. doi: 10.1093/jac/dki408. [DOI] [PubMed] [Google Scholar]
- 20.Briñas L., Moreno M.A., Zarazaga M., Porrero C., Sáenz Y., García M., Dominguez L., Torres C. Detection of CMY-2, CTX-M-14, and SHV-12 β-lactamases in Escherichia coli fecal-sample isolates from healthy chickens. Antimicrob. Agents Chemother. 2003;47:2056–2058. doi: 10.1128/AAC.47.6.2056-2058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendriksen R.S., Mikoleit M., Kornschober C., Rickert R.L., Duyne S.V., Kjelsø C., Hasman H., Cormican M., Mevius D., Threlfall J., et al. Emergence of Multidrug-Resistant Salmonella Concord Infections in Europe and the United States in Children Adopted from Ethiopia, 2003–2007. Pediatr. Infect. Dis. J. 2009;28:814–818. doi: 10.1097/INF.0b013e3181a3aeac. [DOI] [PubMed] [Google Scholar]
- 22.Mushi M.F., Mshana S.E., Imirzalioglu C., Bwanga F. Carbapenemase Genes among Multidrug Resistant Gram Negative Clinical Isolates from a Tertiary Hospital in Mwanza, Tanzania. BioMed Res. Int. 2014;2014:303104. doi: 10.1155/2014/303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordmann P., Gniadkowski M., Giske C., Poirel L., Woodford N., Miriagou V. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 2012;5:432–438. doi: 10.1111/j.1469-0691.2012.03815.x. [DOI] [PubMed] [Google Scholar]
- 24.Mendes R.E., Kiyota K.A., Monteiro J. Rapid detection and identification of metallo-β-lactamase-encoding genes by multiplex real-time PCR assay and melt curve analysis. J. Clin. Microbiol. 2007;45:544–547. doi: 10.1128/JCM.01728-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Queenan A.M., Bush K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007;20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirel L., Walsh T.R., Cuvillier V., Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011;70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Yong D., Lee K., Yum J.H., Shin H.B., Rossolini G.M., Chong Y. Imipenem-EDTA disk method for differentiation of metallo-beta-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2002;40:3798–3801. doi: 10.1128/JCM.40.10.3798-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van T.T., Chin J., Chapman T., Tran L.T., Coloe P.J. Safety of raw meat and shellfish in Vietnam: An analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food Microbiol. 2008;124:217–223. doi: 10.1016/j.ijfoodmicro.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Perez F.J., Hanson N.D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 2002;40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poirel L., Héritier C., Tolün V., Nordmann P. Emergence of Oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2004;48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diancourt L., Passet V., Verhoef J., Grimont P.A., Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 2005;43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moradigarav J., Martin D.V., Peacock S.J., Parkhill J. Evolution and epidemiology of multidrugresistant Klebsiella pneumoniae in the United Kingdom and Ireland. MBio. 2017;8:1–13. doi: 10.1128/mBio.01976-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akinyemi K.O., Abegunrin R.O., Iwalokun B.A., Fakorede C.O., Makarewicz O., Neubauer H., Pletz M.W., Wareth G. The Emergence of Klebsiella pneumoniae with Reduced Susceptibility against Third Generation Cephalosporins and Carbapenems in Lagos Hospitals, Nigeria. Antibiotics. 2021;10:142. doi: 10.3390/antibiotics10020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olayemi O.T., Oyebanji A.A., Bashir M.A., Achancho A.E., Mih T.M., Daniel E.T., Nghonjuyi N.W. Prevalence and Antimicrobial Susceptibility Pattern of Klebsiella Pneumoniae in Sputum Samples of Patients Attending Aminu Kano Teaching Hospital of Kano State, Nigeria. Acta Sci. Pharmacol. 2020;1:7–11. [Google Scholar]
- 35.Olowo-okere A., Ibrahim Y.K.E., Olayinka B.O., Ehinmidu J.O., Mohammed Y., Nabti L.Z., Rolain J.M., Diene S.M. Phenotypic and genotypic characterization of clinical carbapenem-resistant Enterobacteriaceae isolates from Sokoto, Northwest Nigeria. New Microbe New Infect. 2020;37:100727. doi: 10.1016/j.nmni.2020.100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta Y., Gupta A., Todi S., Myatra S., Samaddar D.P., Patil V. Guidelines for prevention of hospital acquired infections. Indian J. Crit. Care Med. 2014;18:149–163. doi: 10.4103/0972-5229.128705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onori R., Gaiarsa S., Comandatore F., Pongolini S., Brisse S., Colombo A., Cassani G., Marone P., Grossi P., Minoja G., et al. Tracking nosocomial Klebsiella pneumoniae infections and outbreaks by whole-genome analysis: Small-scale Italian scenario within a single hospital. J. Clin. Microbiol. 2015;53:2861–2868. doi: 10.1128/JCM.00545-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurung S., Kafle S., Dhungel B., Adhikari N., Thapa Shrestha U., Adhikari B., Banjara M.R., Rijal K.R., Ghimire P. Detection of OXA-48 gene in carbapenem-resistant Escherichia coli and Klebsiella pneumoniae from urine samples. Infect. Drug Resist. 2020;13:2311–2321. doi: 10.2147/IDR.S259967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ejikeugwu C., Nworie O., Saki M., Al-Dahmoshi H.O.M., Al-Khafaji N.S.K., Ezeador C. Metallo-β-lactamase and AmpC genes in Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa isolates from abbatoir and poultry origin in Nigeria. BMC Microbiol. 2021;21:124. doi: 10.1186/s12866-021-02179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okunlola A.T., Olowe O.A., Taiwo S.S. Healthcare associated infections caused by plasmid-encoded blaKPC and blaNDM strains of Klebsiella pneumoniae in Ibadan, Nigeria. Pan Afr. J. Life Sci. 2019;1:46–53. doi: 10.36108/pajols/8102/10(0180). [DOI] [Google Scholar]
- 41.Mukail A., Tytler B.A., Adeshina G.O., Igwe J.C. Incidence of carbapenemase production among antibiotic resistant Klebsiella isolates in Zaria, Nigeria. Niger. J. Biotechnol. 2019;36:138–145. doi: 10.4314/njb.v36i1.18. [DOI] [Google Scholar]
- 42.Hoban D.J., Bouchillon S.K., Hawser S.P., Badal R.E. Trends in the frequency of multiple drug-resistant Enterobacteriaceae and their susceptibility to ertapenem, imipenem, and other antimicrobial agents: Data from the Study for Monitoring Antimicrobial Resistance Trends 2002 to 2007. Diagn. Microbiol. Infect. Dis. 2010;66:78–86. doi: 10.1016/j.diagmicrobio.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Asir J., Nair S., Devi S., Prashanth K., Saranathan R., Kanungo R. Simultaneous gut colonisation and infection by ESBLproducing Escherichia coli in hospitalised patients. Australas. Med. J. 2015;8:200–207. doi: 10.4066/AMJ.2015.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan E.R., Aung M.S., Paul S.K., Ahmed S., Haque N., Ahamed F. Prevalence and molecular epidemiology of clinical isolates of Escherichia coli and Klebsiella pneumoniae harboring extended-spectrum beta-lactamase and carbapenemase genes in Bangladesh. Microb. Drug Resist. 2018;24:1568–1579. doi: 10.1089/mdr.2018.0063. [DOI] [PubMed] [Google Scholar]
- 45.Akinbami O.R., Olofinsae S., Ayeni F.A. Prevalence of extended spectrum beta lactamase and plasmid mediated quinolone resistant genes in strains of Klebsiella pneumoniae, Morganella morganii, Leclercia adecarboxylata and Citrobacter freundii isolated from poultry in South Western Nigeria. PeerJ. 2018;6:e5053. doi: 10.7717/peerj.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okogeri E.I., Amala S.E., Nwokah E.G., Monsi T.P. Cross-sectional study of extended spectrum β-lactamase genes (SHV, TEM, CTX-M and OXA) in Klebsiella species from clinical specimens in Port Hacourt, Nigeria. Am. J. Med. Sci. Med. 2020;8:180–186. [Google Scholar]
- 47.Imtiaz W., Syed Z., Rafaque Z., Andrews S.C., Dasti J.I. Analysis of antibiotic resistance and virulence traits (genetic and phenotypic) in Klebsiella pneumoniae clinical isolates from Pakistan: Identification of significant levels of carbapenem and colistin resistance. Infect. Drug Resist. 2021;14:227–236. doi: 10.2147/IDR.S293290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopotsa K., Mbelle N.M., Sekyere J.O. Epigenomics, genomics, resistome, mobilome, virulome and evolutionary phylogenomics of carbapenem-resistant Klebsiella pneumoniae clinical strains. Microb. Genom. 2020;6:1–19. doi: 10.1099/mgen.0.000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Zhang J., Li Y., Shen Q., Jiang W., Zhao K. Diversity and frequency of resistance and virulence genes in blaKPC and blaNDM co-producing Klebsiella pneumoniae strains from China. Infect. Drug Resist. 2019;12:2819–2826. doi: 10.2147/IDR.S214960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azargun R., Sadeghi M.R., Hossein M., Barhaghi S., Kafil H.S., Yeganeh F. The prevalence of plasmid-mediated quinolone resistance and ESBL-production in Enterobacteriaceae isolated from urinary tract infections. Infect. Drug Resist. 2019;11:1007–1014. doi: 10.2147/IDR.S160720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nouria L., Djamel E., Hassaine H., Frderic R., Richard B. First characterization of CTX-M-15 and DHA-1-lactamases among clinical isolates of Klebsiella pneumoniae in Laghouat Hospital, Algeria. Afr. J. Microbiol. Res. 2014;8:1221–1227. doi: 10.5897/AJMR2013.6229. [DOI] [Google Scholar]
- 52.Vargas J.M., Mochi M.P.M., Nunez J.M., Caceres M., Mochi S., Moreno R.D., Jure M.A. Virulence Factors and Clinical Patterns of Multiple-Clone Hypermucoviscous KPC-2 Producing K. Pneumoniae. Heliyon. 2019;5:e01829. doi: 10.1016/j.heliyon.2019.e01829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J., Zhou K., Zheng B., Zhao L., Shen P., Ji J., Wei Z., Li L., Zhou J., Xiao Y. High prevalence of ESBL-producing Klebsiella pneumoniae causing community-onset infections in China. Front. Microbiol. 2016;7:1830. doi: 10.3389/fmicb.2016.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Agamy M.H.M., Shibl A.M., Tawfik A.F. Prevalence and molecular characterization of extended spectrum β- lactamase -producing Klebsiella pneumonia in Riyadh, Saudi Arabia. Ann. Saudi Med. 2009;29:253–257. doi: 10.4103/0256-4947.55306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G., Huang T., Surendraiah P.K.M., Wang K., Komal R., Zhuge J., Chern C.R., Kryszuk A.A., King C., Wormser G.P. CTX-M ß-lactamase-producing Klebsiella pneumoniae in suburban New York City, New York, USA. Emerg. Infect. Dis. 2013;19:1803–1810. doi: 10.3201/eid1911.121470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elmonir W., Abd El-Aziz N.K., Tartor Y.H., Moustafa S.M., Abo Remela E.M., Eissa R., Saad H.A., Tawab A.A. Emergence of Colistin and Carbapenem Resistance in Extended-Spectrum β-Lactamase Producing Klebsiella pneumoniae Isolated from Chickens and Humans in Egypt. Biology. 2021;10:373. doi: 10.3390/biology10050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kazemian H., Heidari H., Ghanavati R., Ghafourian S., Yazdani F., Sadeghifard N. Phenotypic and genotypic characterization of ESBL-, AmpC-, and carbapenemase-producing Klebsiella pneumoniae and Escherichia coli isolates. Med. Princ. Pract. 2019;28:547–551. doi: 10.1159/000500311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ragheb S.M., Tawfick M.M., El-Kholy A.A., Abdulall A.K. Phenotypic and genotypic features of Klebsiella pneumoniae harboring carbapenemases in Egypt: OXA-48-like carbapenemases as an investigated model. Antibiotics. 2020;9:852. doi: 10.3390/antibiotics9120852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Onyedibe K.I., Bode-Thomas F., Nwadike V., Afolaranmi T., Okolo M.O., Uket O. High Rates of Bacteria Isolates of Neonatal sepsis with Multidrug Resistance patterns in Jos Nigeria. Ann. Pediatr. Child Health. 2015;3:1052. [Google Scholar]
- 60.Jeremiah I.A., Nne A.C., Laura O.I. Genotypic Determination of Carbapenamase Gene Production in Clinical Isolates of Klebsiella Pneumoniae in the University of Port-Harcourt Teaching Hospital. Am. J. Lab. Med. 2020;5:70–75. doi: 10.11648/j.ajlm.20200503.12. [DOI] [Google Scholar]
- 61.Vasaikar S., Obi L., Morobe I., Bisi-Johnson M. Molecular Characteristics and Antibiotic Resistance Profiles of Klebsiella Isolates in Mthatha, Eastern Cape Province, South Africa. Int. J. Microbiol. 2017;2017:8486742. doi: 10.1155/2017/8486742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castanheira M., Toleman M.A., Jones R.N., Schmidt F.J., Walsh T.R. Molecular characterization of a β-lactamase gene, blaGIM-1, encoding a new subclass of metallo-β-lactamase. Antimicrob. Agents Chemother. 2004;12:4654–4661. doi: 10.1128/AAC.48.12.4654-4661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zorgani A., Daw H., Sufya N. Cooccurrence of plasmid-mediated AmpC β-lactamase activity among Klebsiella pneumoniae and Escherichia coli. Open Microbiol. J. 2017;11:195. doi: 10.2174/1874285801711010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park Y.S., Yoo S., Seo M.-R., Kim J.Y., Cho Y.K., Pai H. Risk factors and clinical features of infections caused by plasmid-mediated AmpC β-lactamase-producing Enterobacteriaceae. Int. J. Antimicrob. Agents. 2009;34:38–43. doi: 10.1016/j.ijantimicag.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Ayorinde A.O., Oaikhena A.O., Aboderin A.O., Olabisi O.F., Amupitan A.A., Abiri O.V., Ogunleye V.O., Odih E.E., Adeyemo A.T., Adeyemo A.T., et al. Clones and Clusters of Antimicrobial-Resistant Klebsiella from Southwestern Nigeria. Clin. Infect. Dis. 2021;73:S308–S315. doi: 10.1093/cid/ciab769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bocanegra-Ibarias P., Garza-González E., Padilla-Orozco M., Mendoza-Olazarán S., Pérez-Alba E., Flores-Treviño S., Garza-Ramos U., Silva-Sánchez J., Camacho-Ortiz A. The successful containment of a hospital outbreak caused by NDM-1-producing Klebsiella pneumoniae ST307 using active surveillance. PLoS ONE. 2019;14:e0209609. doi: 10.1371/journal.pone.0209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manenzhe R.I., Zar H.J., Nicol M.P., Kaba M. The spread of carbapenemase-producing bacteria in Africa: A systematic review. J. Antimicrob. Chemother. 2015;70:23–40. doi: 10.1093/jac/dku356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript. The datasets used and/or during the current study are available from the corresponding author upon reasonable request.