INTRODUCTION:
Treatment of primary biliary cholangitis (PBC) can improve the GLOBE score. We aimed to assess the association between changes in the GLOBE score (ΔGLOBE) and liver transplantation (LT)–free survival in patients with PBC who were treated with ursodeoxycholic acid (UDCA).
METHODS:
Among UDCA-treated patients within the Global PBC cohort, the association between ΔGLOBE (ΔGLOBE0–1: during the first year of UDCA, ΔGLOBE1–2: during the second year) and the risk of LT or death was assessed through Cox regression analyses.
RESULTS:
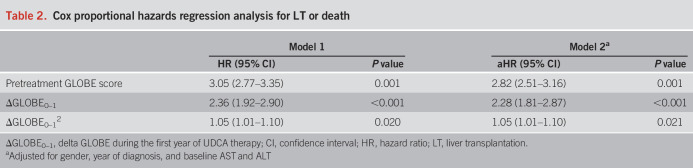
Overall, 3,775 UDCA-treated patients were included; 3,424 (90.7%) were female, the median age was 54.0 (interquartile range [IQR] 45.9–62.4) years, and the median baseline GLOBE score was 0.25 (IQR −0.47 to 0.96). During a median follow-up of 7.2 (IQR 3.7–11.5) years, 730 patients reached the combined end point of LT or death. The median ΔGLOBE0–1 was −0.27 (IQR −0.56 to 0.02). Cox regression analyses, adjusted for pretreatment GLOBE score and ΔGLOBE0–12, showed that ΔGLOBE was associated with LT or death (adjusted hazard ratio 2.28, 95% confidence interval 1.81–2.87, P < 0.001). The interaction between baseline GLOBE score and ΔGLOBE0–1 was not statistically significant (P = 0.296). The ΔGLOBE1–2 was associated with LT or death (adjusted hazard ratio 2.19, 95% confidence interval 1.67–2.86, P < 0.001), independently from the baseline GLOBE score and the change in GLOBE score during the first year of UDCA.
DISCUSSION:
UDCA-induced changes in the GLOBE score were significantly associated with LT-free survival in patients with PBC. While the relative risk reduction of LT or death was stable, the absolute risk reduction was heavily dependent on the baseline prognosis of the patient.
KEYWORDS: primary biliary cholangitis, ursodeoxycholic acid, GLOBE score, prognosis
INTRODUCTION
Primary biliary cholangitis (PBC) is a chronic and usually slowly progressive liver disease with autoimmune features (1). Managing patients with PBC is important because the disease may silently progress toward cirrhosis, and the survival of the affected patients is substantially impaired. Ursodeoxycholic acid (UDCA) is currently recommended as the first-line therapy (2,3), which is associated with an improved liver transplantation (LT)–free survival (4).
The GLOBE score is an externally validated continuous prognostic model, which includes age, bilirubin, alkaline phosphatase (ALP), albumin, and platelets, to accurately assess the absolute risk of LT or death among patients with PBC after 1 year of UDCA therapy (5,6). The GLOBE score also showed an adequate prognostic performance among patients who were not treated with UDCA, indicating it can be considered an objective predictor of the natural history of PBC (4).
Nowadays, the GLOBE score is frequently used to assess the potential impact of new drugs in development for PBC. Add-on treatment with obeticholic acid (OCA) or fibrates resulted in a treatment-induced decline of the GLOBE score, based on which clinical benefit was suggested (7–11). However, the change in GLOBE score (delta GLOBE: ΔGLOBE) has never been assessed in relation to the LT-free survival among patients with PBC who were treated with UDCA. Therefore, the aim of this study was to assess how UDCA-induced changes in GLOBE score were related to LT-free survival in PBC, also according to different risk groups based on the expected natural history.
METHODS
Study population and design
Patients were derived from the GLOBAL PBC Study Group database, an international and multicenter collaboration between liver centers across 10 countries in Europe and Northern America. All patients had an established diagnosis of PBC according to the international accepted guidelines (2,3). For this study, patients were excluded for analysis in case they were not treated with UDCA and in case of autoimmune overlap syndrome according to the Paris criteria (12), concomitant liver diseases, an insufficient follow-up (<1 year), unknown UDCA treatment start date, or unknown dates of clinical events. Data on liver histology were included only if the liver biopsy was performed within 1 year of study entry, except in case of an earlier biopsy showing cirrhosis. Further details about the study population and methodology of the data collection have been described in detail elsewhere (13).
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the international research board of the corresponding center and at each participating center, in accordance with their local regulations. During the observation period of this retrospective study, OCA was not available and alternative second-line treatment options for PBC were not generally prescribed, but data on off-label medication use were not available.
Statistical analyses
The primary outcome measure of this study was defined as a composite end point of LT and all-cause mortality. Baseline was considered as the start of UDCA treatment. Patients were followed up until LT or death or censored at their last visit in case no event occurred. The GLOBE score was calculated with the following formula: 0.044378 × age at baseline + 0.93982 × LN (bilirubin) + 0.335648 × LN (ALP) + 2.266708 × albumin + 0.002581 × platelets (per 109/L) + 1.216865 (bilirubin and ALP in “times upper limit of normal” and albumin in “times lower limit of normal”). The change in GLOBE score during the first year (ΔGLOBE0–1) was calculated by subtracting the GLOBE score at baseline from the GLOBE score after 1 year of UDCA. The delta of the individual laboratory parameters within the GLOBE score was calculated accordingly. In line with the Rotterdam criteria, early biochemical disease stage was defined as normal albumin (≤1.0 × ULN) and normal bilirubin (≤1.0 × ULN) (14). Advanced biochemical disease stage was defined as abnormal albumin and/or abnormal bilirubin. Disease activity at baseline was defined according to the POISE criteria: below POISE (ALP <1.67 × ULN and bilirubin <1.0 × ULN) and above POISE (defined as ALP ≥1.67 × ULN or bilirubin >1.0 × ULN). In addition, the change in the GLOBE score during the second year of UDCA (ΔGLOBE1–2) was assessed.
Data are presented as mean values with SD, median with interquartile range (IQR), or as proportions. Biochemical markers were log-transformed in case of non-normality. Cox proportional hazards regression analyses were performed to assess the association between ΔGLOBE0–1 and the risk of LT or death, which was adjusted for the baseline GLOBE score and all other sufficiently available individual baseline parameters considering the power of the data set. Linearity was assessed by using polynomial terms of ΔGLOBE0–1, where appropriate. The ΔGLOBE1–2 was added to the model and thus assessed in the subgroup of patients with at least 2 years of follow-up. The cumulative LT-free survival rates were based on the Kaplan-Meier method and compared with a log-rank test. To visualize the relationship between ΔGLOBE0–1 and the LT-free survival, patients were stratified according to their baseline GLOBE score into a “low,” “medium,” and “high” group (based on the IQR of the GLOBE score before the start of UDCA therapy). For each group, cumulative LT-free survival curves were plotted based on categories of the ΔGLOBE0–1: no decrease and a moderate or large decrease (cutoff at the median GLOBE decline). A similar approach was used to visualize the relationship between the ΔGLOBE1–2 and clinical outcome, which was stratified for the IQR of the GLOBE score at 1 year of UDCA.
Missing biochemical data at baseline and during UDCA therapy were handled by means of multiple imputation (10 databases) using SAS software, version 9.4 (SAS Institute, Cary, NC), as described in previous reports from our study group (4,5,15). Rubin rules were used to obtain pooled parameters and corresponding SEs (16). For the cumulative LT-free survival estimates and survival figures, patients were categorized into specific subgroups based on their individual mean GLOBE score over the 10 imputed databases, either at baseline or at 1 year of UDCA therapy. In this data set that includes the mean of the 10 imputed values for each missing variable, the bootstrapping method with 5,000 replications was applied to the primary Cox model as a sensitivity analysis.
All statistical tests were 2-sided, and a P value <0.05 was considered statistically significant. Statistical analyses were performed in SPSS Statistics, version 25.0 (IBM, Armonk, NY).
RESULTS
Cohort characteristics
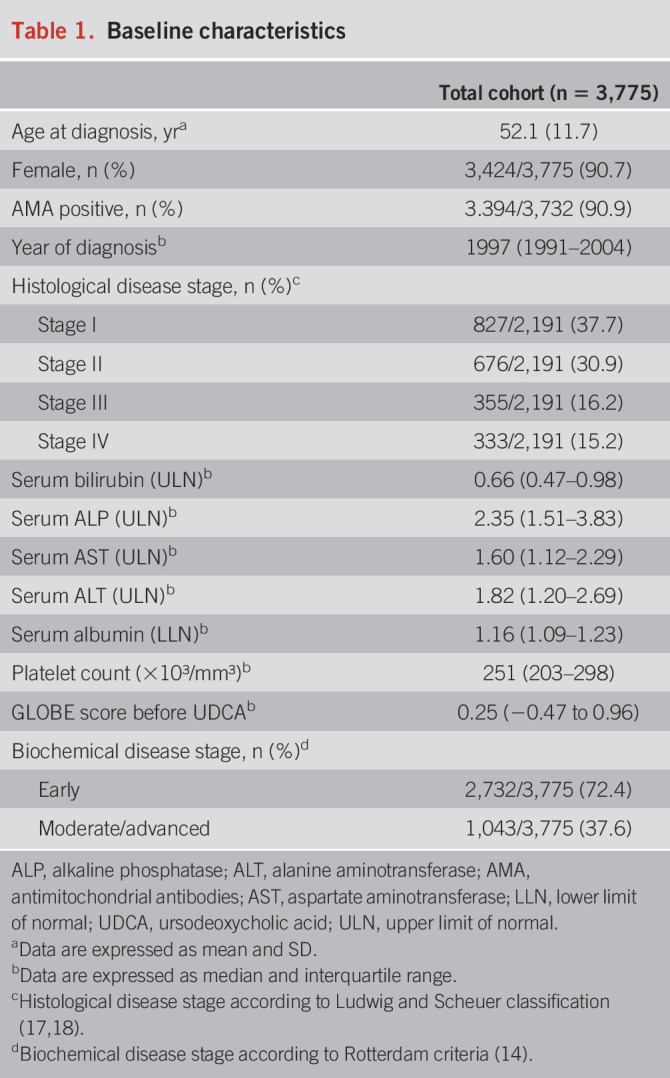
In total, 3,775 UDCA-treated patients with PBC were included. The mean (SD) age was 52.1 (11.7) years, and most of the patients were female (n = 3,424; 90.7%). Clinical and biochemical characteristics are summarized in Table 1 and were consistent with previous reports of PBC epidemiology. Patients were followed up for a median of 7.2 (IQR 3.7–11.5) years, during which a total of 253 patients underwent LT and 477 patients died (composite end point). The cumulative 10-year LT-free survival rate was 79.5% (95% confidence interval [CI] 77.9–81.1).
Table 1.
Baseline characteristics
Changes in GLOBE score during UDCA treatment and clinical outcome
The median GLOBE score was 0.25 (IQR −0.47 to 0.96) before treatment and −0.05 (IQR −0.72 to 0.66) after 1 year of UDCA therapy (P < 0.001). The calculated median ΔGLOBE0–1 was −0.27 (IQR −0.56 to 0.02). Cox proportional hazards regression analyses, adjusted for the pretreatment GLOBE score, showed that ΔGLOBE0–1 was associated with the risk of LT or death (hazard ratio [HR] 2.36, 95% CI 1.92–2.90, P < 0.001), which was not linear because ΔGLOBE0–12 was statistically associated with LT or death as well (Table 2). There was no statistically significant interaction between the pretreatment GLOBE score and ΔGLOBE0–1 for the occurrence of LT or death (P = 0.296). The association between ΔGLOBE0–1 and LT or death remained stable, when gender, year of diagnosis, baseline alanine aminotransferase, and aspartate aminotransferase were added to the model (adjusted HR [aHR] 2.28, 95% CI 1.81–2.87, P < 0.001). After applying the bootstrapping method with 5,000 replications as a sensitivity analysis, the ΔGLOBE0–1 remained independently associated with the primary end point (aHR 1.85, 95% CI 1.61–2.25, P < 0.001). When stratified by baseline disease severity according to the Rotterdam score, ΔGLOBE0–1 was associated with LT-free survival in both patients with early (aHR 2.20, 95% CI 1.57–3.07, P < 0.001) and advanced (aHR 2.31, 95% CI 1.76–3.03, P < 0.001) disease (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/C825). The ΔGLOBE0–1 also remained associated with the LT-free survival (aHR 2.67, 95% CI 1.54–4.63, P < 0.001) among patients who were already below the POISE criteria (ALP <1.67 × ULN and bilirubin <1.0 × ULN) before the start of UDCA therapy (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/C825).
Table 2.
Cox proportional hazards regression analysis for LT or death
As a sensitivity analysis, the baseline albumin and platelet levels were held stable for the calculation of the GLOBE score at 1 year of UDCA therapy, considering that the actual stage of disease is unlikely to change within 1 year. In this analysis, the ΔGLOBE0–1 showed a comparable independent association with the risk of LT or death (aHR 2.33, 95% CI 1.89–2.88, P < 0.001). In addition, allowing the age to increase by 1 year had no impact on the estimated association between ΔGLOBE0–1 and clinical outcome (aHR 2.28, 95% CI 1.80–2.88, P < 0.001).
After 1 year of UDCA, the median ΔALP was −0.79 × ULN (IQR −1.56 to −0.29), median Δbilirubin was −0.08 × ULN (IQR −0.21 to 0.05), median Δalbumin was 0.0 × LLN (IQR −0.05 to 0.06), and median Δplatelets was −6 × 109/L (IQR −25 to 11). Multivariate Cox regression analysis showed that ΔALP (aHR 1.32, 95% CI 1.05–1.66, P = 0.020), Δbilirubin (aHR 2.13, 95% CI 1.67–2.72, P < 0.001), Δalbumin (aHR 0.27, 95% CI 0.11–0.63, P = 0.004), and Δplatelets (aHR 0.54, 95% CI 0.32–0.91, P = 0.023) were all statistically significantly associated with the risk of LT or death.
Clinical outcome according to pretreatment GLOBE score and ΔGLOBE0–1
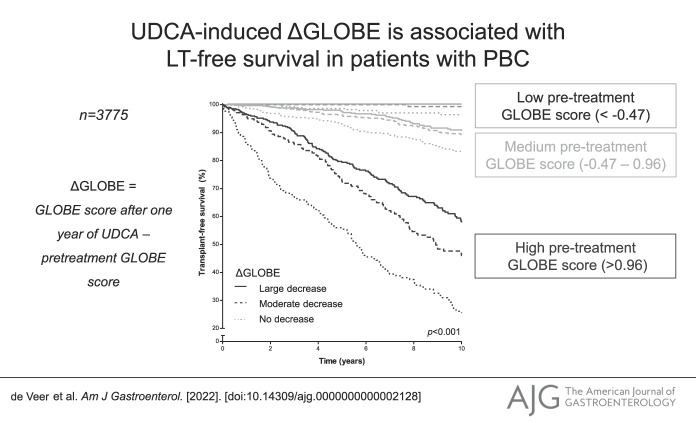
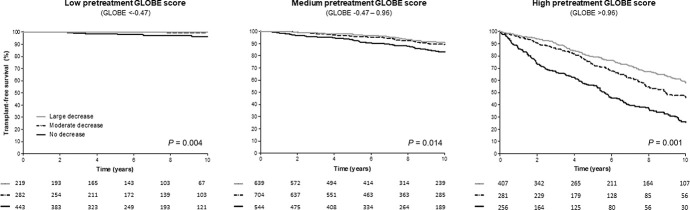
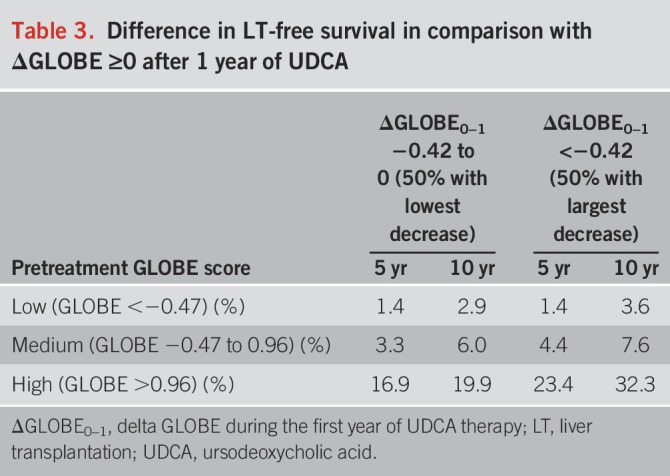
Patients were categorized into 3 groups according to the IQR of the pretreatment GLOBE score. The low GLOBE score group included 944 (25%) patients with a median-predicted 10-year LT-survival at baseline of 93.1% (IQR 91.4–94.9); the medium GLOBE score group included 1,887 (50%) patients with a median-predicted 10-year LT-free survival of 80.3% (IQR 73.8–85.6); and the high GLOBE score group included 944 (25%) patients with a median-predicted 10-year LT-free survival of 43.3% (IQR 23.0–55.4). Among patients in the low GLOBE score group, the cumulative 10-year LT-free survival was 100% for those with a ΔGLOBE0–1 <−0.42 (large decrease, n = 219; 23.2%), 99.3% (95% CI 97.9–100) for patients with a ΔGLOBE0–1 between −0.42 and 0 (moderate decrease, n = 282; 29.9%), and 96.4% (95% CI 94.0–98.8) for those with a ΔGLOBE0–1 ≥0 (no decrease, n = 443; 46.9%) (P = 0.004) (Figure 1). Patients in the high GLOBE score group showed the largest differences in the absolute cumulative LT-free survival over the 3 ΔGLOBE0–1 groups. The cumulative survival rates were 58.4% (95% CI 52.3–64.5), 46.0% (95% CI 38.6–53.4), and 26.1% (95% CI 19.2–33.0) for those with a large decrease (n = 407; 43.1%), moderate decrease (n = 281; 29.8%), and no decrease (n = 256; 27.1%) in GLOBE score at 1 year of UDCA therapy, respectively (P < 0.001). Table 3 summarizes the differences in cumulative 5-year and 10-year LT-free survival in comparison with a stable/increased GLOBE score, according to the baseline prognosis and change in GLOBE score at 1 year of UDCA therapy.
Figure 1.
Transplant-free survival in patients with a low, medium, and high pretreatment GLOBE score, stratified according to ΔGLOBE0–1 category. A ΔGLOBE0–1 ≥0 was included in the group of no decrease. Patients with ΔGLOBE0–1 <0 were separated into 2 groups based on their median ΔGLOBE0–1 of −0.42 as cutoff. Patients with a ΔGLOBE0–1 between −0.42 and 0 were considered to have a moderate decrease, whereas patients with a ΔGLOBE0–1 <−0.42 were considered to have a large decrease.
Table 3.
Difference in LT-free survival in comparison with ΔGLOBE ≥0 after 1 year of UDCA
Changes in GLOBE score during the second year of UDCA in relation to clinical outcome
Two years after the start of UDCA therapy, 3,524 (93.4%) patients were still in follow-up without LT. Among these patients, the median GLOBE score was −0.55 (IQR −0.72 to 0.66) at 1 year of UDCA therapy and −0.10 (IQR −0.80 to 0.67) at 2 years. The median ΔGLOBE1–2 was −0.04 (IQR −0.34 to 0.28). Adjusted for the baseline GLOBE score and the ΔGLOBE0–1, the ΔGLOBE1–2 was independently associated with the LT-free survival (aHR 2.19, 95% CI 1.67–2.86, P < 0.001). This association remained similar in the full Cox model (aHR 2.11, 95% CI 1.58–2.82, P < 0.001). Supplementary Figure S1 (see Supplementary Digital Content 1, http://links.lww.com/AJG/C825) visualizes these results according to the IQR of the GLOBE score at 1 year and the predefined ΔGLOBE1–2 categories.
DISCUSSION
In this large retrospective international cohort study, we showed how treatment-induced changes in the GLOBE score were associated with the LT-free survival among UDCA-treated patients with PBC. This association remained in patients who already had a beneficial biochemical profile at the start of UDCA therapy. The relative reduction of the hazard of LT or death per UDCA-induced change in GLOBE score was stable over the baseline prognosis of patients. However, with a similar GLOBE score reduction, the absolute LT-free survival improved substantially more in patients with a higher baseline risk of LT or death (indicated by higher pretreatment GLOBE scores). The decline of the GLOBE score after 1 year of UDCA therapy was mainly due to a reduction in ALP, but the deltas of the individual biochemical parameters in the GLOBE score were all independently related to the time to LT or death. These findings support the hypothesis of long-term clinical gain with therapeutic agents for PBC based on their short-term impact on the GLOBE score. In addition, the results are helpful for physicians to counsel patients with PBC on their projected clinical benefit with UDCA therapy, for instance, to improve compliance in case of mild symptoms during UDCA use, especially in case of an unclear causal relation.
The GLOBE score has proven to be an accurate continuous prognostic model to predict the overall LT-free survival of patients with PBC treated with UDCA (link to online calculator in the acknowledgments) (5). Subsequently, authors have concluded on the clinical benefit of new PBC treatment options based on the drug-induced GLOBE score improvements (9,10,19,20). While changes in prediction scores (i.e., deltas) are often used to assess clinical benefit, which is indeed helpful for patients, physicians, and policymakers, it is important to assess whether this approach is valid. For example, there has been conflicting results regarding the predictive value of MELD score changes in relation to clinical outcome among patients with liver cirrhosis (21,22). For PBC, several studies reported on the change in GLOBE score with second-line therapy. In their open-label prospective trial, Gomez et al (7) found that the mean GLOBE score decreased from 0.31 to 0.14 among the 78 patients who completed 1 year of OCA. According to the GLOBE score estimates, this translated into an improvement of the projected 10-year risk of LT or death from 20.8% to 17.9% (risk reduction of approximately 14%). This is in line with our estimated 9% risk reduction per 0.1 decline of the GLOBE score. For this long-term clinical benefit, the recently shown maintenance of biochemical response to OCA over many years of combination therapy is likely to be an important precondition (23). In a smaller Spanish cohort of 47 patients, the mean GLOBE score had reduced from 0.13 to −0.16 (ΔGLOBE −0.29) at the end of 1 year of OCA add-on therapy (24). The 23.4% relative reduction of the projected risk of LT or death was again in line with our estimates regarding the ΔGLOBE. For bezafibrate (BZF), cohort reports from Spain and Japan showed mean ΔGLOBE scores of −0.36 and −0.38, respectively, after 1 year of add-on therapy (8,24). These estimates were remarkably similar despite the differences in background population and mean baseline GLOBE scores (0.5 and −0.2, respectively). Because of this difference, however, the absolute risk reduction was twice as high in the Japanese cohort (6.9%) as opposed to the Spanish cohort (3.7%). Our study also highlights that the relative reduction of the risk of LT or death was stable over the baseline prognosis (P = 0.296 for the interaction between ΔGLOBE0–1 and pretreatment GLOBE score), while the absolute risk reduction was strongly dependent of the GLOBE score at baseline. Besides the biochemical response of new treatment options, our study indicates that it is important to consider the potential survival gain and side effects of these new drugs in relation to the target population. For instance, in real-life cohorts, OCA seems to be prescribed to those with more severe PBC-related liver disease when compared with BZF (24,25), perhaps because currently OCA is the only approved second-line treatment option and there were toxicity concerns with fibrates. The absolute survival gain with these treatment options should therefore be assessed separately from the biochemical response.
The change in GLOBE score during the second year of UDCA therapy (ΔGLOBE1–2) was associated with the risk of LT or death independently from the GLOBE score at baseline and the change in GLOBE score during the first year of treatment. This is relevant considering the previously described biphasic biochemical response to UDCA; an initial steep ALP decline is followed by a more gradual reduction of this cholestatic parameter to a maximum response at approximately 2 years of therapy (26,27). While it is frequently suggested to evaluate the need of adding second-line therapy such as OCA or BZF after 1 year of UDCA, our results indicate that a continued biochemical improvement after the first year of UDCA is clinically relevant (2). This may argue to postpone the decision to add second-line drugs in selected patients, especially considering the potential side effects and associated costs. This could be relevant, for instance, for patients not in urgent need of add-on treatment because of slowly progressive PBC who are just above the biochemical threshold for add-on therapy at 1 year of UDCA. Further validation of these results is needed, however, before such an approach can be generally advised.
It is important to consider that all GLOBE score–related estimates for clinical outcome are based on follow-up among patients with PBC treated with UDCA (5). Activation of the farnesoid X receptor or peroxisome proliferator–activated receptor, however, is considered to have multiple favorable effects besides improvement of cholestasis (28,29). Therefore, not all potential long-term clinical benefits of peroxisome proliferator–activated receptor and farnesoid X receptor agonism may be captured by the reduction of the GLOBE score (8). For instance, OCA therapy led to substantial alanine aminotransferase declines (which was also more pronounced when compared with the BZF therapy) (25). Despite being relevant prognostic markers, the hepatic transaminases are not included in the GLOBE score. More recently, the first long-term data on BZF add-on therapy showed an even greater reduction in mortality (aHR 0.33) as what may have been based on the BZF-associated GLOBE score decline (described above) (30). This may also hint toward additional benefit beyond the decline in ALP or bilirubin, which remains our main surrogate markers in clinical trials. Future cohort studies are needed to assess the validity of the GLOBE score LT-free survival estimates in long-term combination treatments. A benefit beyond the improvement of cholestasis has been shown for UDCA as well because patients with PBC without any reduction in ALP or bilirubin with UDCA therapy still had favorable long-term outcome compared with those untreated (31). Thus, even in case the GLOBE score is not reduced with UDCA, patients should generally continue treatment.
Limitations of this study mostly relate to the nature of this cohort. The Global PBC cohort is a largely retrospectively constructed data set, in which (biochemical) data are not always fully complete. Nonetheless, by using the multiple imputation method, we were able to correct for these missing laboratory data. Second, data collection in our cohort did not exceed beyond 2015. Further efforts are needed to assess the relationship between biochemical status and long-term solid clinical end points in those with combination therapy. Third, we lacked detailed data on the cause of mortality, and therefore, we were unable to repeat our analysis with the change in the UK-PBC score. It may be anticipated, however, that the results with this alternative continuous risk model will be similar. Last, we recognize that this cohort is predominated by patients treated in tertiary liver centers, which may have led to a potential selection bias. A strength of this study is that it was conducted with the use of a large, internationally, well-characterized cohort with a long-term follow-up and many clinical end points, resulting in substantial statistical power.
In conclusion, we showed how short-term UDCA-induced changes in GLOBE score were related to the long-term risk of LT or death among patients with PBC. Importantly, among patients with an unfavorable prognosis at baseline, a similar reduction in the GLOBE score resulted in a larger absolute improvement of their LT-free survival. This should be considered when assessing the value of treatment for PBC.
CONFLICTS OF INTEREST
Guarantor of the article: Rozanne C. de Veer, MD, and Adriaan J. van der Meer, MD, PhD.
Specific author contributions: R.C.d.V., M.H.H., J.C.G., B.E.H., and A.J.v.d.M.: study concept and design. All authors: acquisition of data. R.C.d.V., M.C.v.H., M.H.H., and A.J.v.d.M.: analysis and interpretation of data, drafting of the manuscript. All authors: critical revision of the manuscript for important intellectual content. B.E.H.: obtained funding. B.E.H. and A.J.v.d.M.: study supervision.
Financial support: This investigator-initiated study was supported by an unrestricted grant from Intercept Pharmaceuticals and was funded by the Foundation for Liver and Gastrointestinal Research (a not-for-profit foundation) in Rotterdam, the Netherlands. The supporting parties had no influence on the study design, data collection and analyses, writing of the manuscript, or on the decision to submit the manuscript for publication.
Potential competing interests: The following authors declared that they have no conflicts of interest: R.C.v.H., M.C.v.H., and P.M.B. M.H.H. reports a speaker fee from Zambon Nederland B.V. C.C. reports receiving consulting fees from Intercept Pharmaceuticals, Cymabay, Genkyotex, and Inventiva, grant support from Arrow and Intercept Pharmaceuticals, and fees for teaching from GlaxoSmithKline. D.T. reports consulting activities for Intercept Pharmaceuticals. P.I. reports personal fees from Intercept and nonfinancial support from Bruschettini and Menarini Diagnostics. H.L.J. reports grants from and consulting work for AbbVie Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, Innogenetics, Merck, Novartis, Roche, Intercept Pharmaceuticals, and Janssen. F.N. reports Advisory boards for Astellas, Janssen-Cilag, AbbVie, Gilead, CAF, Intercept, Gore, BMS, Novartis, MSD, Janssen-Cilag, Promethera Biosciences, Ono Pharma, Durect, Roche, and Ferring and research grants from Roche, Ferring, and Novartis. K.D.L. reports that he is an unpaid advisor for Intercept Pharmaceuticals and Shire. A.F. reports consulting activities for Intercept Pharmaceuticals. C.Y.P. has received grant support from Takeda, speaker's fees from Tillotts and Takeda, consultancy fee from Pliant, and served as consultant for Takeda. M.J.M. reports being on advisory committees or review panels for GSK, Target, and Regeneron and grant/research support from Cymabay, Intercept, Mallinckrodt, Target, GSK, and Salix. A.P. reports consulting services for Intercept Pharmaceuticals and Novartis Pharma. A.L.M. reports advisory services for Intercept Pharmaceuticals, AbbVie, and Novartis and research funding resources from the Canadian Institutes of Health Research, Canadian Liver Foundation, American Kennel Club, Intercept Pharmaceuticals, AbbVie, and Gilead Sciences. K.V.K. reports personal fees from Gilead Sciences, Intercept Pharmaceuticals, and Novartis and grants from Gilead Sciences and Intercept Pharmaceuticals. P.J.T. receives institutional salary support from the NIHR Birmingham Liver Biomedical Research Center. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. P.J.T. has received research grant funding from the Wellcome trust, the Core Digestive Diseases Charity, Intercept Pharmaceuticals, Bristol Myers' Squibb, Innovate UK, the Medical Research Foundation, EASL, and PSC Support. He has also received speaker fees from Dr. Falk Pharma, Intercept Pharmaceuticals, and Perspectum Diagnostics. He has received consulting and advisory board fees from Dr. Falk Pharma, Intercept Pharmaceuticals, and GSK. G.M.H. reports advisory services for Intercept Pharmaceuticals, Novartis, and GlaxoSmithKline Pharmaceuticals. T.B. has received honoraria from Intercept Pharmaceuticals, Falk Foundation, Abbvie, and Norgine, and travel expenses from Gilead. G.N.D. is an advisor or lecturer for Ipsen, Pfizer, Genkyotex, Novartis, and Sobi, received research grants from Abbvie and Gilead, and has served as PI in studies for Abbvie, Novartis, Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics, Tiziana Life Sciences, Bayer, Astellas, Pfizer, Amyndas Pharmaceuticals, CymaBay Therapeutics, and Intercept Pharmaceuticals. N.K.G. is an advisor or lecturer for Gilead and has acted as a coinvestigator in studies for Abbvie, Novartis, Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics, Tiziana Life Sciences, Bayer, Astellas, Pfizer, Amyndas Pharmaceuticals, CymaBay Therapeutics, and Intercept Pharmaceuticals. X.V. received grants from Gilead, Abbvie, Dr. Phalk Pharma, and MSD and acted as a consultant for Gilead, Abbvie, and MSD. W.J.L. reports consulting services for Intercept Pharmaceuticals. B.E.H. reports grants from Intercept Pharmaceuticals, Cymabay Therapeutics, and Zambon Nederland B.V. and consulting work for Intercept Pharmaceuticals, Cymabay Therapeutics, Albireo AB, and Novartis. A.J.v.d.M. reports speakers fees from MSD, Gilead Sciences, AbbVie Pharmaceuticals, and Zambon Nederland B.V., received an unrestricted grant from Gilead Sciences, and reports travel expenses covered by Dr. Falk Pharma.
Supplementary Material
ACKNOWLEDGMENT
Link to online GLOBE score calculator of the GLOBAL PBC Study Group: https://www.globalpbc.com/globe.
Study Highlights.
WHAT IS KNOWN
✓ The GLOBE score is a validated and accurate continuous prognostic model to determine the patients' individual long-term liver transplantation (LT)–free survival.
✓ New drugs in development for primary biliary cholangitis (PBC) have shown to reduce the GLOBE score shortly after treatment initiation.
WHAT IS NEW HERE
✓ Ursodeoxycholic acid–induced changes in the GLOBE score were associated with an improved clinical outcome.
✓ The relative risk reduction of the risk of LT or death was stable over the predicted prognosis before treatment, while the absolute risk reduction was strongly dependent of the GLOBE score at baseline.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C825
Contributor Information
Rozanne C. de Veer, Email: r.deveer@erasmusmc.nl.
Maria C. van Hooff, Email: m.vanhooff@erasmusmc.nl.
Christophe Corpechot, Email: christophe.corpechot@sat.aphp.fr.
Douglas Thorburn, Email: douglas.thorburn@nhs.net.
Pietro Invernizzi, Email: pietro.invernizzi@unimib.it.
Willem J. Lammers, Email: w.lammers@erasmusmc.nl.
Harry L.A. Janssen, Email: h.janssen@erasmusmc.nl.
Pier M. Battezzati, Email: piermaria.battezzati@unimi.it.
Frederik Nevens, Email: frederik.nevens@uzleuven.be.
Keith D. Lindor, Email: Keith.Lindor@asu.edu.
Annarosa Floreani, Email: annarosa.floreani@unipd.it.
Cyriel Y. Ponsioen, Email: c.y.ponsioen@amsterdamumc.nl.
Marlyn J. Mayo, Email: marlyn.mayo@utsouthwestern.edu.
Albert Parés, Email: pares@ub.edu.
Andrew L. Mason, Email: andrew.mason@ualberta.ca.
Kris V. Kowdley, Email: Kris.Kowdley@swedish.org.
Palak J. Trivedi, Email: p.j.trivedi@bham.ac.uk.
Gideon M. Hirschfield, Email: Gideon.Hirschfield@uhn.ca.
Jorn C. Goet, Email: j.c.goet@gmail.com.
Tony Bruns, Email: tbruns@ukaachen.de.
George N. Dalekos, Email: georgedalekos@gmail.com.
Nikolaos K. Gatselis, Email: gatselis@me.com.
Xavier Verhelst, Email: Xavier.Verhelst@uzgent.be.
Bettina E. Hansen, Email: b.hansen@erasmusmc.nl.
Maren H. Harms, Email: mhharms@gmail.com.
REFERENCES
- 1.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005;353(12):1261–73. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver, Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67(1):145–72. [DOI] [PubMed] [Google Scholar]
- 3.Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2019;69(1):394–419. [DOI] [PubMed] [Google Scholar]
- 4.Harms MH, van Buuren HR, Corpechot C, et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol 2019;71(2):357–65. [DOI] [PubMed] [Google Scholar]
- 5.Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 2015;149(7):1804–12.e4. [DOI] [PubMed] [Google Scholar]
- 6.Efe C, Tascilar K, Henriksson I, et al. Validation of risk scoring systems in ursodeoxycholic acid-treated patients with primary biliary cholangitis. Am J Gastroenterol 2019;114(7):1101–8. [DOI] [PubMed] [Google Scholar]
- 7.Gomez E, Garcia Buey L, Molina E, et al. Effectiveness and safety of obeticholic acid in a Southern European multicentre cohort of patients with primary biliary cholangitis and suboptimal response to ursodeoxycholic acid. Aliment Pharmacol Ther 2021;53(4):519–30. [DOI] [PubMed] [Google Scholar]
- 8.Honda A, Tanaka A, Kaneko T, et al. Bezafibrate improves GLOBE and UK-PBC scores and long-term outcomes in patients with primary biliary cholangitis. Hepatology 2019;70(6):2035–46. [DOI] [PubMed] [Google Scholar]
- 9.Carbone M, Harms MH, Lammers WJ, et al. Clinical application of the GLOBE and United Kingdom-primary biliary cholangitis risk scores in a trial cohort of patients with primary biliary cholangitis. Hepatol Commun 2018;2(6):683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trauner M, Nevens F, Shiffman ML, et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol 2019;4(6):445–53. [DOI] [PubMed] [Google Scholar]
- 11.Corpechot C, Chazouilleres O, Lemoinne S, et al. Letter: Reduction in projected mortality or need for liver transplantation associated with bezafibrate add-on in primary biliary cholangitis with incomplete UDCA response. Aliment Pharmacol Ther 2019;49(2):236–8. [DOI] [PubMed] [Google Scholar]
- 12.Chazouilleres O, Wendum D, Serfaty L, et al. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: Clinical features and response to therapy. Hepatology 1998;28(2):296–301. [DOI] [PubMed] [Google Scholar]
- 13.Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology 2014;147(6):1338–49.e5; quiz e15. [DOI] [PubMed] [Google Scholar]
- 14.Kuiper EM, Hansen BE, de Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009;136(4):1281–7. [DOI] [PubMed] [Google Scholar]
- 15.Murillo Perez CF, Harms MH, Lindor KD, et al. Goals of treatment for improved survival in primary biliary cholangitis: Treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase. Am J Gastroenterol 2020;115(7):1066–74. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc 1996;91(434):473–89. [Google Scholar]
- 17.Ludwig J, Dickson ER, McDonald GSA. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol 1978;379(2):103–12. [DOI] [PubMed] [Google Scholar]
- 18.Scheuer PJ. Primary biliary cirrhosis. Proc R Soc Med 1967;60(12):1257–60. [PMC free article] [PubMed] [Google Scholar]
- 19.Corpechot C, Chazouilleres O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med 2018;378(23):2171–81. [DOI] [PubMed] [Google Scholar]
- 20.Schattenberg JM, Pares A, Kowdley KV, et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol 2021;74(6):1344–54. [DOI] [PubMed] [Google Scholar]
- 21.D'Amico G. Developing concepts on MELD: Delta and cutoffs. J Hepatol 2005;42(6):790–2. [DOI] [PubMed] [Google Scholar]
- 22.Krassenburg LAP, Maan R, Ramji A, et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J Hepatol 2021;74(5):1053–63. [DOI] [PubMed] [Google Scholar]
- 23.Hansen B, Jones D, Carbone M, et al. Long-term efficacy and safety of obeticholic acid in primary biliary cholangitis: Responder analysis of over 5 years of treatment in the POISE trial. Hepatology 2020;72(1):752A. [PMC free article] [PubMed] [Google Scholar]
- 24.Reig A, Alvarez-Navascues C, Vergara M, et al. Obeticholic acid and fibrates in primary biliary cholangitis: Comparative effects in a multicentric observational study. Am J Gastroenterol 2021;116(11):2250–7. [DOI] [PubMed] [Google Scholar]
- 25.Abbas N, Culver E, Thorburn D, et al. Real-world evaluation of second line therapy in primary biliary cholangitis: A multicenter nationwide study. J Hepatol 2021;75:S426–7. [Google Scholar]
- 26.Poupon RE, Balkau B, Eschwege E, et al. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med 1991;324(22):1548–54. [DOI] [PubMed] [Google Scholar]
- 27.Leuschner M, Dietrich CF, You T, et al. Characterisation of patients with primary biliary cirrhosis responding to long term ursodeoxycholic acid treatment. Gut 2000;46(1):121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beuers U, Trauner M, Jansen P, et al. New paradigms in the treatment of hepatic cholestasis: From UDCA to FXR, PXR and beyond. J Hepatol 2015;62(1 Suppl):S25–37. [DOI] [PubMed] [Google Scholar]
- 29.Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology 2015;62(2):635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka A, Hirohara J, Nakano T, et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J Hepatol 2021;75(3):565–71. [DOI] [PubMed] [Google Scholar]
- 31.Harms M, Van Buuren H, Lammers WJ, et al. Ursodeoxycholic acid treatment is associated with prolonged transplant-free survival in primary biliary cholangitis—Even in patients without biochemical improvements. J Hepatol 2018;68:S8. [Google Scholar]