Abstract
Many reproductive physiological processes, such as folliculogenesis, ovulation, implantation, and fertilization, require the synthesis, remodeling, and degradation of the extracellular matrix (ECM). The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) family genes code for key metalloproteinases in the remodeling process of different ECM. Several genes of this family encode for proteins with important functions in reproductive processes; in particular, ADAMTS1, 4, 5 and 9 are genes that are differentially expressed in cell types and the physiological stages of reproductive tissues. ADAMTS enzymes degrade proteoglycans in the ECM of the follicles so that the oocytes can be released and regulate follicle development during folliculogenesis, favoring the action of essential growth factors, such as FGF-2, FGF-7 and GDF-9. The transcriptional regulation of ADAMTS1 and 9 in preovulatory follicles occurs because of the gonadotropin surge in preovulatory follicles, via the progesterone/progesterone receptor complex. In addition, in the case of ADAMTS1, pathways involving protein kinase A (PKA), extracellular signal regulated protein kinase (ERK1/2) and the epidermal growth factor receptor (EGFR) might contribute to ECM regulation. Different Omic studies indicate the importance of genes of the ADAMTS family from a reproductive aspect. ADAMTS genes could serve as biomarkers for genetic improvement and contribute to enhance fertility and animal reproduction; however, more research related to these genes, the synthesis of proteins encoded by these genes, and regulation in farm animals is needed.
Keywords: animal reproduction, ADAMTS, extracellular matrix, genetic improvement
1. Introduction
The enormous expansion in the production of food from livestock during the last 70 years has been possible, in great part, through genetic improvement [1]. Reproduction is one of the most relevant aspects in animal production, because of relevancy to productivity and sustainability of the different livestock systems [2]; however, these traits, as well as other economically important traits, are quantitative, multifactorial and have low heritability. The development of molecular and genomic technologies has contributed to the identification of major genes and genetic markers associated with phenotypes, leading to accelerated genetic improvement [3]. Nevertheless, it is important to elucidate how genes that encode for the proteins related to such traits are regulated and how the genetic variants affect their expression [4].
Extracellular matrix (ECM) remodeling is an important physiological process related to reproductive capacity in males and females. This restructuring of the ECM has been associated with the reproductive processes of folliculogenesis, ovulation, implantation and placentation [5,6] in females and in processes that induce correct testicular development, as well as the ongoing production and maturation of millions of spermatozoa in males [6,7]. In addition, the degradation of the ECM in the zona pellucida is an important physiological process for oocyte fertilization [8].
Different matrix metalloproteinases function in the degradation of extracellular matrices [9]. The metalloproteinases superfamily is composed of ADAM proteases (A Disintegrin-like and Metalloproteinase), MMPs (Matrix Metalloproteinases), astacins (BMP/tolloid proteases and meprins in mammals) and ADAMTS (A Disintegrin-like and Metalloproteinase Domain with Thrombospondin type 1 repeat) [10]. Both MMPs and ADAMTS are secretory proteins. ADAMTS are key proteases in the degradation of the ECM, particularly cleaving large proteoglycans, whereas MMPs recognize short peptides as substrates and thus have a wider range of protein targets and a role in many different physiological processes [11,12]. In contrast, the ADAM proteases are integral membrane enzymes that mainly cleave ectodomains of different secretory proteins [13].
In addition to the crucial role of the ADAMTS proteases in the ECM remodeling of the development of reproductive organs and processes, Etacin-related proteins also have an important function, activating growth factors necessary for the assembly of the ECM [14,15,16]. Thus, the study of these genes can contribute to the full understanding and improvement of the reproductive processes and can potentially impact the sustainability of animal production. In this article, we describe the general structure of the ADAMTS genes, the importance of ADAMTS proteases in reproduction biology and the biosynthesis regulation of these proteins, as determined in reproductive genomics studies.
2. Structural Features of ADAMTS Genes
ADAMTS proteinases are multidomain enzymes with highly conserved structures [17]. ADAMTS1 was the first gene of this family to be described in mice [18], and later, other genes were identified in other species. In mammalian genomes, 19 ADAMTS genes have been identified and named ADAMTS1 to ADAMTS20. It was later discovered that ADAMTS5 and ADAMTS11 are the same gene, and ADAMTS11 is no longer used [19,20]. The expansion in the number of ADAMTS genes in mammals seems to have occurred due to gene duplication, thus generating sub-functionalization or neo-functionalization regarding the physiological processes in which they participate [13]. Rose et al. [21], in their excellent review, explain that Gon-1 is the only ADAMTS orthologous gene in Caenorhabditis elegans, and it has similarity to ADAMTS9 and ADAMTS20 in humans. The six ADAMTS proteases in the ascidian Ciona intestinalis represent the central evolutionary clades in chordates from which gene expansion into vertebrates occurs [22], along with the evolution of the ECM. Phylogenetic analysis clearly suggests the gene duplication of the ADAMTS genes [21].
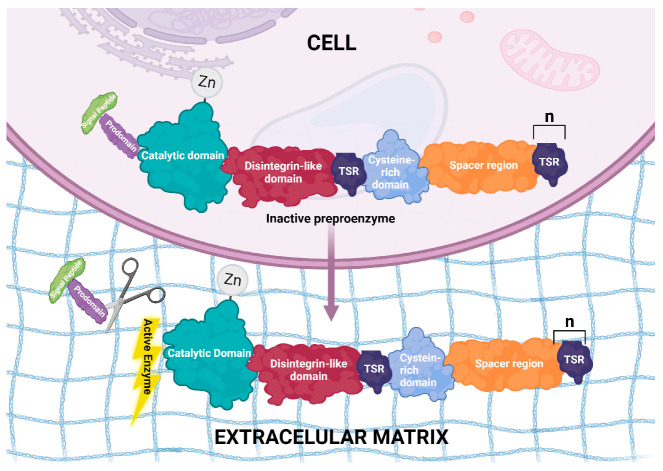
The signature domains of the ADAMTS proteins are: a signal peptide, necessary for protein trafficking and secretion, and an inhibitory prodomain that must be cleaved from the ADAMTS zymogens to render them catalytically active. Such cleavage occurs in Golgi, the cell surface or extracellularly. The size of this prodomain comprises about 200 residues in all ADAMTS proteases, with the exception of ADAMTS13, which has a short prodomain that does not need to be removed for the protease to be active [23]. Interestingly, the removal of the ADAMTS9 prodomain reduces the protease catalytic activity upon versican, its substrate; a disintegrin-like domain; a thrombospondin type 1 repeat (TSR-1) motif; and a cysteine-rich domain followed by a spacer region. The TSR-1 domains and the spacer domain appear to be involved in ECM anchoring. The description of the organization of these proteins is based on the structure of ADAMTS4, the other members of the family vary mainly at the C-terminus, with either, more or fewer repeats of the TSR-1 motifs [24]. Figure 1 illustrates the structure and localization of these proteins.
Figure 1.
Illustration of ADAMTS protein structure and localization. Cleavage can occur in trans-Golgi, on the cellular surface or extracellularly.
The ADAMTS genes are located on different chromosomes that vary depending on the species. These genes code for proteins with a theoretical weight ranging between 70.9 and 225.64 kDa, with 662 amino acid residues in species such as the rooster (Gallus gallus) or as many as 2028 in species such as the pig (Sus scrofa). Table 1 details the structural characteristics of these genes and their protein products in farm animals, comparing these with the mouse sequences. It is interesting to note that in sheep (Ovis aries), the ADAMTS1, 4 and 5 genes are found on the same chromosome (Table 1). These genes are thought to encode for proteins involved in the regulation of reproductive functions. In the case of cattle, humans and mice, genes 1 and 5 are located in the same chromosome, as reported by Dubail and Apte [13].
Table 1.
Characteristics of ADAMTS genes in livestock and mice.
| Gene | Species | Chr | Exon No | Size (bp) | AA No | Gene | Species | Chr | Exon No | Size (bp) | AA No |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADAMTS1 | Mus musculus | 16 | 9 | 9287 | 968 | ADAMTS9 | M. musculus | 6 | 40 | 171,752 | 1931 |
| Bos taurus | 1 | 9 | 8698 | 970 | B. taurus | 22 | 41 | 163,000 | 1937 | ||
| Ovis aries | 1 | 9 | 9452 | 968 | O. aries | 19 | 41 | 162,411 | 1935 | ||
| Equus caballus | 26 | 9 | 9002 | 949 | E. caballus | 16 | 40 | 166,329 | 1936 | ||
| Sus scrofa | 13 | 9 | 8783 | 947 | S. scrofa | 13 | 40 | 175,486 | 2028 | ||
| Gallus gallus | 1 | 9 | 6915 | 923 | G. gallus | 12 | 41 | 67,065 | 1928 | ||
| ADAMTS2 | M. musculus | 11 | 22 | 205,489 | 1213 | ADAMTS10 | M. musculus | 17 | 26 | 30,137 | 1104 |
| B. taurus | 7 | 22 | 245,248 | 1205 | B. taurus | 7 | 26 | 21,940 | 1103 | ||
| O. aries | 5 | 23 | 255,023 | 1206 | O. aries | 5 | 27 | 21,873 | 1103 | ||
| E. caballus | 14 | 23 | 215,470 | 1211 | E. caballus | 7 | 27 | 21,061 | 1191 | ||
| S. scrofa | 2 | 23 | 223,979 | 1208 | S. scrofa | 2 | 24 | 16,972 | 1103 | ||
| G. gallus | 13 | 27 | 236,034 | 1187 | G. gallus | 28 | 25 | 47,963 | 1109 | ||
| ADAMTS4 | M. musculus | 1 | 9 | 12,139 | 833 | ADAMTS12 | M. musculus | 15 | 24 | 284,541 | 1600 |
| B. taurus | 3 | 9 | 8057 | 839 | B. taurus | 20 | 25 | 394,653 | 1607 | ||
| O. aries | 1 | 9 | 8091 | 872 | O. aries | 16 | 24 | 402,782 | 1605 | ||
| E. caballus | 5 | 9 | 7009 | 837 | E. caballus | 21 | 24 | 314,874 | 1598 | ||
| S. scrofa | 4 | 9 | 8885 | 872 | S. scrofa | 16 | 24 | 373,778 | 1599 | ||
| G. gallus | 25 | 9 | 7976 | 662 | G. gallus | Z | 23 | 170,964 | 1580 | ||
| ADAMTS5 | M. musculus | 16 | 9 | 42,969 | 930 | ADAMTS16 | M. musculus | 13 | 24 | 114,040 | 1222 |
| B. taurus | 1 | 9 | 50,445 | 934 | B. taurus | 20 | 23 | 193,779 | 1246 | ||
| O. aries | 1 | 8 | 57,118 | 934 | O. aries | 16 | 23 | 183,599 | 1226 | ||
| E. caballus | 26 | 9 | 49,839 | 929 | E. caballus | 21 | 23 | 154,339 | 1249 | ||
| S. scrofa | 13 | 10 | 60,092 | 929 | S. scrofa | 16 | 24 | 164,333 | 1238 | ||
| G. gallus | 1 | 8 | 46,807 | 877 | G. gallus | - | - | - | - | ||
| ADAMTS6 | M. musculus | 13 | 28 | 210,178 | 1117 | ADAMTS18 | M. musculus | 8 | 24 | 152,221 | 1219 |
| B. taurus | 20 | 26 | 286,175 | 1117 | B. taurus | 18 | 24 | 145,453 | 1223 | ||
| O. aries | 16 | 28 | 289,952 | 1117 | O. aries | 14 | 21 | 147,254 | 1050 | ||
| E. caballus | 21 | 25 | 358,367 | 1117 | E. caballus | 3 | 23 | 125,706 | 1199 | ||
| S. scrofa | 16 | 24 | 308,302 | 1117 | S. scrofa | 6 | 23 | 164,005 | 1224 | ||
| G. gallus | Z | 26 | 159,448 | 1119 | G. gallus | 11 | 23 | 69,793 | 1228 |
Chr = chromosome location, AA = amino acids. Data from NCBI (https://www.ncbi.nlm.nih.gov/) accessed on 10 March 2023.
According to different studies, the ADAMTS family proteins have functions in tissue remodeling during the development of organs and reproductive processes [6]. The reproductive function of proteins encoded by these genes has been extensively studied in mice, while studies in farm animals are scarce; however, as shown in Table 2, there is a high ADAMTS protein similarity among species.
Table 2.
Identity and similarity percentage of ADAMTS proteins between M. musculus and farm animals.
| Species | ADAMTS | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 5 | 6 | 9 | 10 | 12 | 16 | 18 | |||||||||||
| I | S | I | S | I | S | I | S | I | S | I | S | I | S | I | S | I | S | I | S | |
| B. taurus | 81 | 87 | 89 | 93 | 88 | 92 | 88 | 90 | 96 | 97 | 88 | 93 | 95 | 96 | 78 | 85 | 78 | 85 | 86 | 91 |
| O. aries | 80 | 87 | 89 | 93 | 88 | 92 | 88 | 90 | 95 | 97 | 88 | 93 | 95 | 96 | 79 | 85 | 78 | 86 | 87 | 93 |
| E. caballus | 86 | 91 | 87 | 91 | 89 | 92 | 91 | 93 | 96 | 97 | 90 | 94 | 96 | 97 | 79 | 86 | 80 | 86 | 89 | 93 |
| S. scrofa | 86 | 91 | 88 | 92 | 89 | 92 | 91 | 93 | 95 | 97 | 89 | 94 | 95 | 96 | 80 | 86 | 79 | 86 | 87 | 91 |
| G. gallus | 74 | 81 | 74 | 84 | 60 | 71 | 79 | 86 | 91 | 95 | 74 | 85 | 73 | 83 | 57 | 70 | - | - | 75 | 84 |
I = identity percentage; S = similarity percentage. Alignments performed with BLAST tool at the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) accessed on 10 March 2023.
3. ADAMTS and Fertility in Females
3.1. Folliculogenesis
The process of development and maturation of follicles, termed folliculogenesis, is necessary for ovulation to occur [25]. The ADAMTS family genes seem to be related to this process, inferred by the relative abundance of mRNA transcripts in the follicles and corpus lutea of several mammalian species. ADAMTS1 have been reported to be expressed in granulosa cells in cows [26], horses [27] and pigs [28].
According to Brown et al. [29], ADAMTS1 functions are necessary for the structural changes of the ECM to occur during follicular development. The proteoglycans present in the ECM can inhibit the action of certain growth factors, such as FGF-2, FGF-7 and GDF-9 [30], which are essential for various exquisite reproductive processes to occur. For example, FGF-2 stimulates angiogenesis and granulosa cell proliferation and function in cattle [31] and buffalo [32]. FGF-2 also stimulates the initiation and development of follicular growth in sheep and goats [33,34].
Thus, the functions of ADAMTS1 and 4 are thought to enhance these processes by controlling the amount and location of various proteoglycans [35]. Shozu et al. [36] inactivated the ADAMTS1 gene and reported that the absence of ADAMTS1 led to follicular atresia. Versican is an abundant ECM proteoglycan that is hormonally regulated by the ovary. Versican abundance varies throughout the several stages of follicular growth but particularly during ovulation in rodents [37]. The presence of versican in bovine and porcine follicular basement membrane [38,39], suggests that ADAMTS1 may also regulate the development of follicles during folliculogenesis.
3.2. Ovulation
The preovulatory surge of gonadotropins induces a series of biochemical processes within the dominant follicle that culminate in ovulation and, subsequently, in the formation of the corpus luteum. Ovulation is associated with the degradation of the follicular basement membrane and the fragmentation of the ECM at the apex of the follicle wall, resulting in the release of the oocyte [40]. Metalloproteinases enzymes are responsible for the degradation of the follicular ECM during ovulation [9]. ADAMTS1 degrades versican, aggrecan and brevican, proteoglycans present in the ECM of the follicle. Such degradation of the follicular wall allows oocyte release [41,42]. Indeed, ADAMTS1 was reported to have a fundamental function in ovulation, as reported by Mittaz et al. [43], based on results from a study with female mice lacking the ADAMTS1. In this study, exon 2 was deleted to disrupt the ADAMTS1 gene and a selectable marker gene was inserted in intron 1. The modified ADAMTS1 allele was functionally null. The authors reported that these females were subfertile due to impaired ovulation, resulting in the mature oocytes not being released from the follicles, as would typically occur during ovulation. Likewise, Brown et al. [44] reported that the ovulation rate was 77% less in female mice lacking the ADAMTS1 enzyme, compared to the wild-type animals. The finding was explained by the lack of versican degradation during the matrix expansion of the cumulus–oocyte complex.
ADAMTS1 and ADAMTS9 mRNA abundance was increased in the granulosa [45] and theca cells [46] of periovulatory follicles compared with the preovulatory follicles of cattle. This was also observed in vitro in response to LH stimulation in theca cells and to LH or FSH stimulation in granulosa cells [47], indicating the importance of the ADAMTS proteins in ovulation, as these enzymes are necessary for the disruption of the ECM integrity of the follicles and the subsequent release of the oocytes [46]. In mice, there is a marked increase in the mRNA expression of the ADAMTS4 gene in granulosa cells before ovulation and in different cell types after ovulation, including the site where follicle wall degradation occurs [16]; however, when studying expression in cattle, Madan et al. [26] did not observe changes in the ADAMTS4 mRNA transcript abundance in granulosa or theca cells in periovulatory compared to preovulatory follicles. On the other hand, in a transcriptome sequencing study, ADAMTS4 was one of the genes with the highest relative expression, based on mRNA transcript abundance in the ovarian tissue of prolific compared to non-prolific sheep [48].
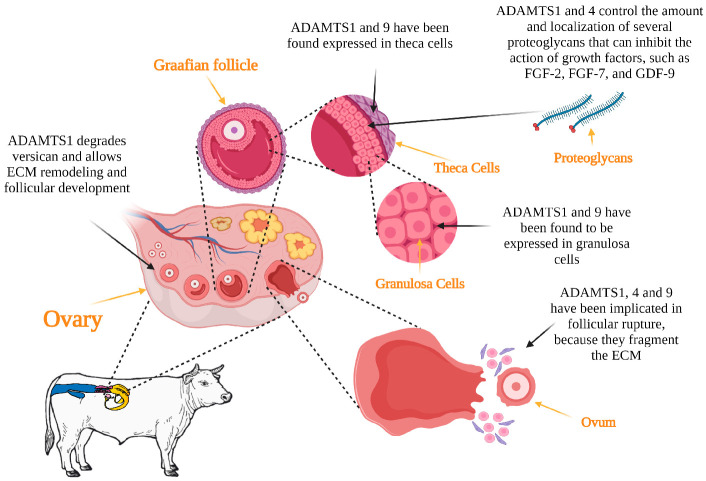
Hu et al. [49] reported that SNP-type polymorphisms in ADAMTS1 are related to litter size in goats; therefore, they could be used as molecular markers for the selection of litter size. ADAMTS1, 4 and 9 are considered to be of great importance for animal production, where reproductive prolificacy is a determinant for sustainability. Figure 2 depicts the possible functions of ADAMTS proteins in folliculogenesis and ovulation in livestock.
Figure 2.
ADAMTS genes are involved in folliculogenesis and ovulation. The specific gene, site of expression and function is shown.
3.3. Implantation, Placentation and Parturition
Similar to the constant remodeling of the ECM required for the cyclical transformations of the ovary, the uterus also undergoes the cyclic development and remodeling of the endometrial tissue matrix. This remodeling is necessary for implantation and placentation [50]. Implantation is a critical process for the establishment of pregnancy and begins with essential signaling from the blastocyst to the endometrium, which must be prepared to respond [51,52]. The attachment of the blastocyst to the uterus and subsequent trophoblast cell invasion occurs through ECM remodeling [53]. Uterine tissue remodeling is also required for placental cotyledon formation and angiogenesis near trophoblast tissue in sheep, as well as a decrease in endometrial thickness during implantation [54,55]. Shindo et al. [56] reported that non-ADAMTS1 female mice had thicker uteri compared to wildtype females, and such uterine thickening negatively affected fertilization. In addition, ADAMTS1 is expressed in the endometrium during the estrous cycle of cattle and at the time of uterine remodeling for implantation and placental development. Mishra et al. [57] reported that ADAMTS1 was more abundant in the last stage of gestation in the cotyledonary tissues of the placenta. This could be because the remodeling of the endometrial ECM leads to the modification of the composition in response to implantation signals. ADAMTS5 and ADAMTS6 genes are expressed in the mouse placenta and ADAMTS5 is expressed in the 7-day embryo but not in the later stages of development [17]. The remodeling of the uterine ECM, therefore, was proposed to be essential for embryonic/fetal development.
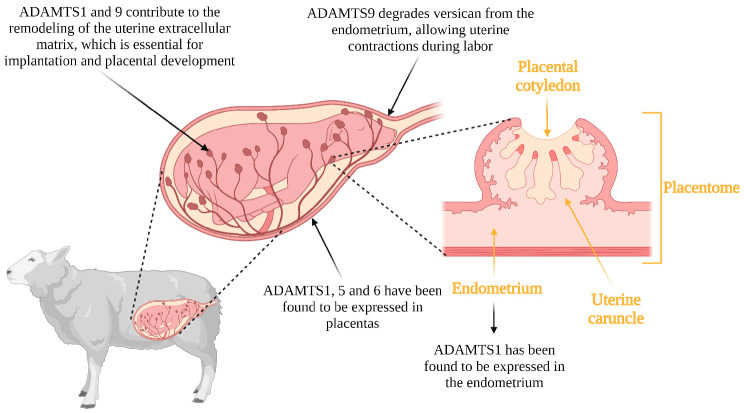
Another member of this family of metalloproteinases, ADAMTS9, has been reported to contribute to remodeling in the uterus at the time of parturition. The extracellular matrix undergoes remodeling during late gestation to allow smooth muscle cells to connect to each other and effect uterine contractions at the time of parturition [58]. ADAMTS9 is present in all reproductive states and contributes to uterine tissue remodeling. The accumulation of versican from the extracellular matrix in the uterus leads to abnormal contractions. Mead et al. [59] reported that there are abnormally large concentrations of versican in mice that do not produce ADAMTS9, leading to abnormal parturition processes. This abnormality was due to a reduction in focal adhesions between cells that interact with one another to generate uterine contractions. Thus, ADAMTS9 contributes to the remodeling of the uterine extracellular matrix through the degradation of versican, and its null or poor functionality disrupts parturition processes. The possible functions of the ADAMTS proteases in implantation, placentation and parturition in livestock is illustrated in Figure 3.
Figure 3.
ADAMTS genes are involved in implantation, placentation and parturition. The specific gene and its site of expression and function is shown.
4. ADAMTS and Fertility in Males
4.1. Testicular Development
The expression of ADAMTS family genes, in addition to being related to fertility in females, has also been linked to reproductive capacity in males, specifically to testicular development [60]. The testicles develop in the abdomen during the embryonic and fetal stages. Subsequently, the testes pass through the inguinal canal into the scrotum [7], with this process requiring the tissue remodeling of the ECM [6]; however, regarding this process, the literature is limited to only a few studies of rodents. Jacobi et al. [61] reported there was expression of ADAMTS16 in the testes of mouse embryos. Likewise, alterations in ADAMTS16 led to cryptorchidism and infertility in male rats [60,62]. Nevertheless, as reported by Livermore et al. [63], the silencing of ADAMTS16 via CRISPR/Cas9 gene editing led to the production of mice that were fertile, although with smaller testicles. On the other hand, Carré et al. [64] reported that there was a greater abundance of ADAMTS12 expressed in developing testicular cords at the time of sex determination in poultry. Only these two genes of the ADAMTS family (12 and 16) have been linked to testicular development, and more research is needed to elucidate ADAMTS functions during the development of gonads in different species.
4.2. Spermatogenesis
Spermatogenesis is the process through which germ cells multiply and differentiate to produce sperm in the seminiferous tubules [65]. The presence of ADAMTS10 expression in the testis, epididymis and ejaculated spermatozoa of Asian buffalo (Bubalus bubalis) [66] suggests a possible function in the sperm maturation process [67]. ADAMTS2 has also been linked to sperm maturation in mice, as shown by Li et al. [68]. In transgenic mice homozygous for the inactive alleles of ADAMTS2, there was less sperm maturation and activity compared to those in the control group; however, more research is needed to determine the functions of these proteins in sperm maturation.
In bulls with besnoitiosis compared to healthy bulls, ADAMTS1 mRNA abundance is lower in scrotal skin, the pampiniform plexus and testicular parenchyma [69]. This disease causes the fibrosis and thickening of the skin of the scrotum, which leads to failure in thermoregulation and to the inhibition of spermatogenesis, ultimately causing infertility [70].
Wu et al. [71] performed a transcriptomic analysis of yak and cattleyak testes to investigate the genetic causes of hybrid animal sterility, and several ADAMTS genes were differentially expressed. ADAMTS1, 10, 12, 3, 5 and 14 were upregulated, whereas ADAMTS16, 20, 6 and 18 were downregulated; thus, these proteins could be involved in cattleyak sterility. However, how these proteins are associated with hybrid animal sterility is still unclear.
4.3. Fertilization
ADAMTS is apparently involved in sperm and egg fertilization processes. In a study conducted by Dun et al. [8] in mice, ADAMTS10 was expressed during the late stages of spermatogenesis, and the protein was incorporated into the acrosome of developing spermatids. ADAMTS10 presence in the acrosome is thought to function by inducing sperm adhesion to the zona pellucida. The zona pellucida is an ECM that surrounds the oocytes and must be crossed by spermatozoa to penetrate the oocyte and carry out fertilization. ADAMTS10 has important functions in this process by acting in the degradation of the zona pellucida [72]; however, further research in farm animals is needed to understand the role of ADAMTS10 in fertilization.
5. Regulation of ADAMTS Genes Involved in Reproduction
The ADAMTS proteases family is formed of 19 members that have actions on a huge range of substrates and in different tissues. In an excellent review about the ADAMTS regulation [21], the authors highlight the importance of the regulation of these metalloproteinases in different human pathologies, such as arthritis, where pro-inflammatory molecules promote the transcriptional overexpression of the ADAMTS genes.
The transcription of the ADAMTS genes in reproduction-related processes is hormonally regulated. The gonadotropin surge that induces ovulation leads to a series of drastic changes in the follicles. ADAMTS1, 2, 7 and 9 are regulated in vivo by the LH/FSH surge in the ovarian theca cells of cattle, in a time-dependent manner, whereas results from in vitro studies confirmed the effect of LH on ADAMTS1 and 9 but not on ADAMTS2; however, using the same in vitro cell model, the treatment with mifepristone, a progesterone receptor inhibitor, blocked the up-regulatory effect of LH in periovulatory theca cells [46]. These results confirm that the gonadotropin surge regulates the ADAMTS1 and 9 transcription through the progesterone/progesterone receptor (PGR) complex, as was also reported for ADAMTS1 in follicle granulosa cells of cattle [47], horses [27] and pigs [28].
In vitro studies with cattle granulosa cells stimulated by forskolin showed a greater abundance of ADAMTS1, thus providing a good model to study the regulation of this gene. Using different fragments of the ADAMTS1 promoter region and luciferase as a reporter gene, Sayasith et al. [73] determined that the deletion between −330 and −177 led to a marked reduction in both basal promoter activity and forskolin-stimulated promoter activity, with the Ebox elements located in this region appearing to play a key role in the regulation of ADAMTS1 transcription. Investigating the possible pathway for transcription regulation, it was determined that protein kinase A (PKA), extracellular signal-regulated protein kinase (ERK1/2), epidermal growth factor receptor (EGFR) and progesterone receptors (PGR) have a potential role in the activation of ADAMTS promoter in cattle.
Little is known about the regulation of ADAMTS genes related to reproductive biology in farm animals; therefore, further research should be conducted. In addition, from what is described in this review article, it is important to consider that these genes have a large number of post-transcriptional and post-translational modifications, some of which have already been described in humans and mice and excellently compiled by Rose et al. [21].
6. ADAMTS in Omics Studies
The genomics era has evolved throughout the past two decades. Genomics and transcriptomic studies have been conducted in different animal species to determine what genes are present in these genomes, to identify genetic variations and their possible association with traits of interest and to determine differences in transcription levels in different cell types, environmental conditions, physiological stages, etc.
Wu et al. [71] performed a transcriptomic analysis of yak and cattleyak testes to investigate the genetic causes of hybrid sterility. The mRNA abundance in several ADAMTS family genes was different: ADAMTS1, 10, 12, 3 and 5 were over-expressed and ADAMTS16, 20, 6 and 18 were under-expressed in cattleyaks in comparison to yaks. In transcriptomes analyzed in uterine tissue at day 6 post-insemination, genes related to ECM remodeling had differential mRNA transcript abundance in pregnant and non-pregnant cows, 30 days post-insemination. ADAMTS1 was among these genes, indicating the importance of this protein in the uterine receptivity for the embryo to ensure the sustaining of pregnancy [74]. In an in vitro study, ADAMTS20 was one of the 20 over-expressed genes in the granulosa cells of cattle in response to severe heat stress [75]. In sheep, where prolificacy is a very important aspect, ADAMTS4 and ADAMTS14 were overexpressed in the whole ovarian transcriptomes of Pelibuey ewes with greater prolificacy compared with ewes with less prolificacy [48]. Furthermore, Gootwine [76] suggests further investigation on the possible relationship between ADAMTS9 and uterine capacity in sheep, considering the relative abundances of these gene-transcripts in different genomic studies in pigs and sheep. Transcriptomic studies and genome scans can be conducted to identify genes for the further analysis of functionality and regulation.
7. Conclusions
The results of many studies indicate the ADAMTS family genes are involved in the reproductive biology of both males and females in farm animals; however, further research is required to elucidate the specific functions of the products of these genes and the regulation of their expression and effects of environmental conditions, as well as the genotype:environment:phenotype integration. With such integrative understanding, more strategies can emerge that will allow genetic improvement and gradually enhance the sustainability of animal production systems.
Acknowledgments
The authors thank Consejo Nacional de Ciencia y Tecnología, México (CONACYT) by the scholarship 1097563 to Pamela Hernández-Delgado.
Author Contributions
Conceptualization, J.A.M.-Q. and M.F.-P.; writing—review and editing, P.H.-D., J.A.M.-Q. and M.F.-P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hill W.G. Is continued genetic improvement of livestock sustainable? Genetics. 2016;202:877–881. doi: 10.1534/genetics.115.186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor J.F., Schnabel R.D., Sutovsky P. Genomics of bull fertility. Animal. 2018;12:172–183. doi: 10.1017/S1751731118000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Notter D. Genetic aspects of reproduction in sheep. Reprod. Domest. Anim. 2008;43:122–128. doi: 10.1111/j.1439-0531.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 4.Albert F.W., Kruglyak L. The role of regulatory variation in complex traits and disease. Nat. Rev. Genet. 2015;16:197–212. doi: 10.1038/nrg3891. [DOI] [PubMed] [Google Scholar]
- 5.Demircan K., Comertoglu I., Akyol S., Yigitoglu B.N., Sarikaya E. A new biological marker candidate in female reproductive system diseases: Matrix metalloproteinase with thrombospondin motifs (ADAMTS) J. Turk. Ger. Gynecol. Assoc. 2014;15:250–255. doi: 10.5152/jtgga.2014.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell D.L., Brown H.M., Dunning K.R. ADAMTS proteases in fertility. Matrix Biol. 2015;44–46:54–63. doi: 10.1016/j.matbio.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Copp H.L., Shortliffe L.D. Ashcraft’s Pediatric Surgery. Elsevier; Amsterdam, The Netherlands: 2010. Undescended testes and testicular tumors; pp. 676–686. [Google Scholar]
- 8.Dun M.D., Anderson A.L., Bromfield E.G., Asquith K.L., Emmett B., McLaughlin E.A., Aitken R.J., Nixon B. Investigation of the expression and functional significance of the novel mouse sperm protein, a disintegrin and metalloprotease with thrombospondin type 1 motifs number 10 (ADAMTS10) Int. J. Androl. 2012;35:572–589. doi: 10.1111/j.1365-2605.2011.01235.x. [DOI] [PubMed] [Google Scholar]
- 9.Fata J., Ho A.-V., Leco K., Moorehead R., Khokha R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: Functions of metalloproteinases and their inhibitors. CMLS. 2000;57:77–95. doi: 10.1007/s000180050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blobel C.P., Apte S. Encyclopedia of Respiratory Medicine. Elsevier; Amsterdam, The Netherlands: 2020. ADAMs and ADAMTSs; pp. 568–573. [Google Scholar]
- 11.Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apte S.S., Parks W.C. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015;44–46:1–6. doi: 10.1016/j.matbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Dubail J., Apte S.S. Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biol. 2015;44:24–37. doi: 10.1016/j.matbio.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Menke D.B., Koubova J., Page D.C. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. J. Dev. Biol. 2003;262:303–312. doi: 10.1016/S0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 15.Doyle K.M., Russell D.L., Sriraman V., Richards J.S. Coordinate transcription of the ADAMTS-1 gene by luteinizing hormone and progesterone receptor. Mol. Endocrinol. 2004;18:2463–2478. doi: 10.1210/me.2003-0380. [DOI] [PubMed] [Google Scholar]
- 16.Richards J.S., Hernandez-Gonzalez I., Gonzalez-Robayna I., Teuling E., Lo Y., Boerboom D., Falender A.E., Doyle K.H., LeBaron R.G., Thompson V. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: Evidence for specific and redundant patterns during ovulation. Biol. Reprod. 2005;72:1241–1255. doi: 10.1095/biolreprod.104.038083. [DOI] [PubMed] [Google Scholar]
- 17.Hurskainen T.L., Hirohata S., Seldin M.F., Apte S.S. ADAM-TS5, ADAM-TS6, and ADAM-TS7, Novel Members of a New Family of Zinc Metalloproteases. J. Biol. Chem. 1999;274:25555–25563. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- 18.Kuno K., Kanada N., Nakashima E., Fujiki F., Ichimura F., Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J. Biol. Chem. 1997;272:556–562. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- 19.Porter S., Clark I.M., Kevorkian L., Edwards D.R. The ADAMTS metalloproteinases. Biochem. J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apte S.S. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: Functions and mechanisms. J. Biol. Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose K.W., Taye N., Karoulias S.Z., Hubmacher D. Regulation of ADAMTS proteases. Front. Mol. Biosci. 2021;8:701959. doi: 10.3389/fmolb.2021.701959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huxley-Jones J., Apte S.S., Robertson D.L., Boot-Handford R.P. The characterisation of six ADAMTS proteases in the basal chordate Ciona intestinalis provides new insights into the vertebrate ADAMTS family. Int. J. Biochem. Cell Biol. 2005;37:1838–1845. doi: 10.1016/j.biocel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Longpré J.-M., Leduc R. Identification of prodomain determinants involved in ADAMTS-1 biosynthesis. J. Biol. Chem. 2004;279:33237–33245. doi: 10.1074/jbc.M313151200. [DOI] [PubMed] [Google Scholar]
- 24.Kelwick R., Desanlis I., Wheeler G.N., Edwards D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015;16:113. doi: 10.1186/s13059-015-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gershon E., Dekel N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int. J. Mol. Sci. 2020;21:4565. doi: 10.3390/ijms21124565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madan P., Bridges P.J., Komar C.M., Beristain A.G., Rajamahendran R., Fortune J.E., MacCalman C.D. Expression of messenger RNA for ADAMTS subtypes changes in the periovulatory follicle after the gonadotropin surge and during luteal development and regression in cattle. Biol. Reprod. 2003;69:1506–1514. doi: 10.1095/biolreprod.102.013714. [DOI] [PubMed] [Google Scholar]
- 27.Boerboom D., Russell D.L., Richards J.S., Sirois J. Regulation of transcripts encoding ADAMTS-1 (a disintegrin and metalloproteinase with thrombospondin-like motifs-1) and progesterone receptor by human chorionic gonadotropin in equine preovulatory follicles. J. Mol. Endocrinol. 2003;31:473–485. doi: 10.1677/jme.0.0310473. [DOI] [PubMed] [Google Scholar]
- 28.Shimada M., Nishibori M., Yamashita Y., Ito J., Mori T., Richards J.S. Down-regulated expression of A disintegrin and metalloproteinase with thrombospondin-like repeats-1 by progesterone receptor antagonist is associated with impaired expansion of porcine cumulus-oocyte complexes. Endocrinology. 2004;145:4603–4614. doi: 10.1210/en.2004-0542. [DOI] [PubMed] [Google Scholar]
- 29.Brown H.M., Dunning K.R., Robker R.L., Pritchard M., Russell D.L. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev. Biol. 2006;300:699–709. doi: 10.1016/j.ydbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Park P.W., Reizes O., Bernfield M. Cell surface heparan sulfate proteoglycans: Selective regulators of ligand-receptor encounters. J. Biol. Chem. 2000;275:29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- 31.Berisha B., Sinowatz F., Schams D. Expression and localization of fibroblast growth factor (FGF) family members during the final growth of bovine ovarian follicles. Mol. Reprod. Dev. 2004;67:162–171. doi: 10.1002/mrd.10386. [DOI] [PubMed] [Google Scholar]
- 32.Mishra S.R., Thakur N., Somal A., Parmar M.S., Reshma R., Rajesh G., Yadav V.P., Bharti M.K., Bharati J., Paul A., et al. Expression and localization of fibroblast growth factor (FGF) family in buffalo ovarian follicle during different stages of development and modulatory role of FGF2 on steroidogenesis and survival of cultured buffalo granulosa cells. Res. Vet. Sci. 2016;108:98–111. doi: 10.1016/j.rvsc.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Matos M.H., Lima-Verde I.B., Bruno J.B., Lopes C.A., Martins F.S., Santos K.D., Rocha R.M., Silva J.R., Bao S.N., Figueiredo J.R. Follicle stimulating hormone and fibroblast growth factor-2 interact and promote goat primordial follicle development in vitro. Reprod. Fertil. Dev. 2007;19:677–684. doi: 10.1071/RD07021. [DOI] [PubMed] [Google Scholar]
- 34.Santos J.M., Menezes V.G., Barberino R.S., Macedo T.J., Lins T.L., Gouveia B.B., Barros V.R., Santos L.P., Goncalves R.J., Matos M.H. Immunohistochemical localization of fibroblast growth factor-2 in the sheep ovary and its effects on pre-antral follicle apoptosis and development in vitro. Reprod. Domest. Anim. 2014;49:522–528. doi: 10.1111/rda.12322. [DOI] [PubMed] [Google Scholar]
- 35.Richards J.S., Russell D.L., Ochsner S., Espey L.L. Ovulation: New dimensions and new regulators of the inflammatory-like response. Annu. Rev. Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 36.Shozu M., Minami N., Yokoyama H., Inoue M., Kurihara H., Matsushima K., Kuno K. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J. Mol. Endocrinol. 2005;35:343–355. doi: 10.1677/jme.1.01735. [DOI] [PubMed] [Google Scholar]
- 37.Russell D.L., Ochsner S.A., Hsieh M., Mulders S., Richards J.S. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 2003;144:1020–1031. doi: 10.1210/en.2002-220434. [DOI] [PubMed] [Google Scholar]
- 38.McArthur M.E., Irving-Rodgers H.F., Byers S., Rodgers R.J. Identification and immunolocalization of decorin, versican, perlecan, nidogen, and chondroitin sulfate proteoglycans in bovine small-antral ovarian follicles. Biol. Reprod. 2000;63:913–924. doi: 10.1095/biolreprod63.3.913. [DOI] [PubMed] [Google Scholar]
- 39.Rodgers R.J., Irving-Rodgers H.F. Formation of the ovarian follicular antrum and follicular fluid. Biol. Reprod. 2010;82:1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 40.Richards J.S., Russell D.L., Robker R.L., Dajee M., Alliston T.N. Molecular mechanisms of ovulation and luteinization. Mol. Cell. Endocrinol. 1998;145:47–54. doi: 10.1016/S0303-7207(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 41.Russell D.L., Doyle K.M., Ochsner S.A., Sandy J.D., Richards J.S. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J. Biol. Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- 42.Curry T.E., Smith M.F. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin. Reprod. Med. 2006;24:228–241. doi: 10.1055/s-2006-948552. [DOI] [PubMed] [Google Scholar]
- 43.Mittaz L., Russell D., Wilson T., Brasted M., Tkalcevic J., Salamonsen L.A., Hertzog P.J., Pritchard M.A. Adamts-1 is essential for the development and function of the urogenital system. Biol. Reprod. 2004;70:1096–1105. doi: 10.1095/biolreprod.103.023911. [DOI] [PubMed] [Google Scholar]
- 44.Brown H.M., Dunning K.R., Robker R.L., Boerboom D., Pritchard M., Lane M., Russell D.L. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol. Reprod. 2010;83:549–557. doi: 10.1095/biolreprod.110.084434. [DOI] [PubMed] [Google Scholar]
- 45.Lussier J.G., Diouf M.N., Lévesque V., Sirois J., Ndiaye K. Gene expression profiling of upregulated mRNAs in granulosa cells of bovine ovulatory follicles following stimulation with hCG. Reprod. Biol. Endocrinol. 2017;15:88. doi: 10.1186/s12958-017-0306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willis E.L., Bridges P.J., Fortune J.E. Progesterone receptor and prostaglandins mediate luteinizing hormone-induced changes in messenger RNAs for ADAMTS proteases in theca cells of bovine periovulatory follicles. Mol. Reprod. Dev. 2017;84:55–66. doi: 10.1002/mrd.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fortune J.E., Willis E.L., Bridges P.J., Yang C.S. The periovulatory period in cattle: Progesterone, prostaglandins, oxytocin and ADAMTS proteases. Anim. Reprod. 2009;6:60–71. [PMC free article] [PubMed] [Google Scholar]
- 48.Hernández-Montiel W., Collí-Dula R.C., Ramón-Ugalde J.P., Martínez-Núñez M.A., Zamora-Bustillos R. RNA-seq transcriptome analysis in ovarian tissue of pelibuey breed to explore the regulation of prolificacy. Genes. 2019;10:358. doi: 10.3390/genes10050358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu W., Tang J., Zhang Z., Tang Q., Yan Y., Wang P., Wang X., Liu Q., Guo X., Jin M., et al. Polymorphisms in the ASMT and ADAMTS1 gene may increase litter size in goats. Vet. Med. Sci. 2020;6:775–787. doi: 10.1002/vms3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guillomot M. Changes in extracellular matrix components and cytokeratins in the endometrium during goat implantation. Placenta. 1999;20:339–345. doi: 10.1053/plac.1998.0385. [DOI] [PubMed] [Google Scholar]
- 51.Nardo L.G., Li T.C., Edwards R.G. Introduction: Human embryo implantation failure and recurrent miscarriage: Basic science and clinical practice. Reprod. Biomed. Online. 2006;13:11–12. doi: 10.1016/S1472-6483(10)62010-X. [DOI] [PubMed] [Google Scholar]
- 52.Guzeloglu-Kayisli O., Basar M., Arici A. Basic aspects of implantation. Reprod. Biomed. Online. 2007;15:728–739. doi: 10.1016/S1472-6483(10)60541-X. [DOI] [PubMed] [Google Scholar]
- 53.Das S.K., Yano S., Wang J., Edwards D.R., Nagase H., Dey S.K. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse uterus during the peri-implantation period. Dev. Genet. 1997;21:44–54. doi: 10.1002/(SICI)1520-6408(1997)21:1<44::AID-DVG5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Smith S.E., Cullen W.C., Godkin J.D. Ultrastructural morphometric analysis of the uterine epithelium during early pregnancy in the sheep. J. Reprod. Fertil. 1990;89:517–525. doi: 10.1530/jrf.0.0890517. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds L.P., Redmer D.A. Growth and microvascular development of the uterus during early pregnancy in ewes. Biol. Reprod. 1992;47:698–708. doi: 10.1095/biolreprod47.5.698. [DOI] [PubMed] [Google Scholar]
- 56.Shindo T., Kurihara H., Kuno K., Yokoyama H., Wada T., Kurihara Y., Imai T., Wang Y., Ogata M., Nishimatsu H., et al. ADAMTS-1: A metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J. Clin. Investig. 2000;105:1345–1352. doi: 10.1172/JCI8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mishra B., Koshi K., Kizaki K., Ushizawa K., Takahashi T., Hosoe M., Sato T., Ito A., Hashizume K. Expression of ADAMTS1 mRNA in bovine endometrium and placenta during gestation. Domest. Anim. Endocrinol. 2013;45:43–48. doi: 10.1016/j.domaniend.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Riemer R.K., Heymann M.A. Regulation of uterine smooth muscle function during gestation. Pediatr. Res. 1998;44:615–627. doi: 10.1203/00006450-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Mead T.J., Du Y., Nelson C.M., Gueye N.A., Drazba J., Dancevic C.M., Vankemmelbeke M., Buttle D.J., Apte S.S. ADAMTS9-Regulated Pericellular Matrix Dynamics Governs Focal Adhesion-Dependent Smooth Muscle Differentiation. Cell Rep. 2018;23:485–498. doi: 10.1016/j.celrep.2018.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdul-Majeed S., Mell B., Nauli S.M., Joe B. Cryptorchidism and infertility in rats with targeted disruption of the Adamts16 locus. PLoS ONE. 2014;9:e100967. doi: 10.1371/journal.pone.0100967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacobi C.L., Rudigier L.J., Scholz H., Kirschner K.M. Transcriptional regulation by the Wilms tumor protein, Wt1, suggests a role of the metalloproteinase Adamts16 in murine genitourinary development. J. Biol. Chem. 2013;288:18811–18824. doi: 10.1074/jbc.M113.464644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarila G., Bao T., Abeydeera S.A., Li R., Mell B., Joe B., Catubig A., Hutson J. Interplay between collagenase and undescended testes in Adamts16 knockout rats. J. Pediatr. Surg. 2020;55:1952–1958. doi: 10.1016/j.jpedsurg.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 63.Livermore C., Warr N., Chalon N., Siggers P., Mianne J., Codner G., Teboul L., Wells S., Greenfield A. Male mice lacking ADAMTS-16 are fertile but exhibit testes of reduced weight. Sci. Rep. 2019;9:17195. doi: 10.1038/s41598-019-53900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carré G.-A., Couty I., Hennequet-Antier C., Govoroun M.S. Gene expression profiling reveals new potential players of gonad differentiation in the chicken embryo. PLoS ONE. 2011;6:e23959. doi: 10.1371/journal.pone.0023959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staub C., Johnson L. Review: Spermatogenesis in the bull. Animal. 2018;12:27–35. doi: 10.1017/S1751731118000435. [DOI] [PubMed] [Google Scholar]
- 66.Gurupriya V.S., Roy S.C., Javvaji P.K., Dhali A., Badami S., Rahim F., Divyashree B.C., Panda A.P. Expression of ADAMTS10 in male reproductive tract of buffaloes (Bubalus bubalis) J. Exp. Biol. Agric. Sci. 2018;6:800–807. doi: 10.18006/2018.6(5).800.807. [DOI] [Google Scholar]
- 67.Cornwall G.A. New insights into epididymal biology and function. Hum. Reprod. Update. 2009;15:213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S.W., Arita M., Fertala A., Bao Y., Kopen G.C., Langsjo T.K., Hyttinen M.M., Helminen H.J., Prockop D.J. Transgenic mice with inactive alleles for procollagen N-proteinase (ADAMTS-2) develop fragile skin and male sterility. Biochem. J. 2001;355:271–278. doi: 10.1042/bj3550271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.González-Barrio D., Diezma-Díaz C., Gutiérrez-Expósito D., Tabanera E., Jiménez-Meléndez A., Pizarro M., González-Huecas M., Ferre I., Ortega-Mora L.M., Álvarez-García G. Identification of molecular biomarkers associated with disease progression in the testis of bulls infected with Besnoitia besnoiti. Vet. Res. 2021;52:106. doi: 10.1186/s13567-021-00974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olias P., Schade B., Mehlhorn H. Molecular pathology, taxonomy and epidemiology of Besnoitia species (Protozoa: Sarcocystidae) Infect. Genet. Evol. 2011;11:1564–1576. doi: 10.1016/j.meegid.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Wu S., Mipam T., Xu C., Zhao W., Shah M.A., Yi C., Luo H., Cai X., Zhong J. Testis transcriptome profiling identified genes involved in spermatogenic arrest of cattleyak. PLoS ONE. 2020;15:e0229503. doi: 10.1371/journal.pone.0229503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le Goff C., Cormier-Daire V. The ADAMTS(L) family and human genetic disorders. Hum. Mol. Genet. 2011;20:R163–R167. doi: 10.1093/hmg/ddr361. [DOI] [PubMed] [Google Scholar]
- 73.Sayasith K., Lussier J., Sirois J. Molecular characterization and transcriptional regulation of a disintegrin and metalloproteinase with thrombospondin motif 1 (ADAMTS1) in bovine preovulatory follicles. Endocrinology. 2013;154:2857–2869. doi: 10.1210/en.2013-1140. [DOI] [PubMed] [Google Scholar]
- 74.Binelli M., Scolari S.C., Pugliesi G., Van Hoeck V., Gonella-Diaza A.M., Andrade S.C., Gasparin G.R., Coutinho L.L. The transcriptome signature of the receptive bovine uterus determined at early gestation. PLoS ONE. 2015;10:e0122874. doi: 10.1371/journal.pone.0122874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sammad A., Luo H., Hu L., Zhu H., Wang Y. Transcriptome reveals granulosa cells coping through redox, inflammatory and metabolic mechanisms under acute heat stress. Cells. 2022;11:1443. doi: 10.3390/cells11091443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gootwine E. Invited review: Opportunities for genetic improvement toward higher prolificacy in sheep. Small Rumin. Res. 2020;186:106090. doi: 10.1016/j.smallrumres.2020.106090. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.