Abstract
In COVID-19, critical disease and invasive mechanical ventilation (IMV) increase the risk of death, mainly in patients over 60 years of age. Objectives: To find the relationship between miR-21-5p and miR-146a-5p in terms of the severity, IMV, and mortality in hospitalized COVID-19 patients younger than 55 years of age. Methods: The patients were stratified according to disease severity using the IDSA/WHO criteria for severe and critical COVID-19 and subclassified into critical non-survivors and critical survivors. Results: Ninety-seven severe/critical COVID-19 patients were included; 81.3% of the deceased were male and 18.8% were female. Higher expression miR-21-5p levels were associated as follows: severe vs. critical disease (p = 0.007, FC = 0.498), PaO2/FiO2 index, mild vs. severe (p = 0.027, FC = 0.558), and survivors vs. non-survivors (p = 0.03, FC = 0.463). Moreover, we identified correlations with clinical variables: CRP (rho = −0.54, p < 0.001), D-dimer (rho = −0.47, p < 0.05), related to damage in the kidney (rho = 0.60, p < 0.001), liver (rho = 0.41, p < 0.05), and lung (rho = 0.54, p < 0.001). Finally, miR-21-5p thresholds were calculated according to severity (8.191), IMV (8.191), and mortality (8.237); these values increased the risk of developing a critical disease (OR = 4.19), the need for IMV (OR = 5.63), and death (OR = 6.00). Conclusion: Increased expression levels of miR-21-5p are related to worse outcome of COVID-19 in younger hospitalized patients.
Keywords: microRNAs, COVID-19, viral infections, mortality, mechanical ventilation, miR-21-5p
1. Introduction
The COVID-19 pandemic represents one of the most significant challenges in the history of public health. As of 5 October 2022, there were more than 620 million cases worldwide and more than 6.55 million deaths [1]. Approximately 20% of patients develop a severe disease requiring hospital admission, and more than 5% require intensive care unit (ICU) attention, with a variable ICU mortality rate ranging from 25 to 50% [2]. The main risk factors associated with more severe disease and death are patients older than 60 years [3], body mass index (BMI) > 30 kg/cm2 [4], type 2 diabetes mellitus (T2DM) [5], and systemic arterial hypertension (SAH) [6].
Between June–September 2021, some countries from Latin America and Europe showed a critical increase in infections, and a change in the demographics of patients who developed severe illness and/or death was evidenced; the median age decreased from 60 to 40 years [7,8,9,10]. Furthermore, most patients did not show the same risk factors as those older than 60 [7].
In this context, many studies have delved into less-studied risk factors associated with a high risk of developing severe forms of the disease and death. In addition to classical risk factors, genetic variants in several loci, such as TNFRSF1A [11], IFNAR2 [12], and SERPINE1 [13], have been described by our group. However, studies evaluating the participation of miRNAs in the severity and mortality of COVID-19 are scanty.
miRNAs are a relatively recently described group of small RNA molecules of 20 to 24 nucleotides that do not code for proteins but regulate more than 60% of all genes [14,15]. They can be part of complex regulatory networks in the gene expression of pathophysiological processes in progressing to severe forms of COVID-19 [16,17].
Therefore, we aimed to determine if alterations in miR-21-5p and miR-146a-5p levels are associated with severity and mortality in subjects under 55 years of age who were hospitalized for COVID-19. We selected these miRNAs due to a large number of target genes and pathways related to inflammation, heart, lung, and kidney damage, coagulopathy, pulmonary fibrosis, and sepsis, leading causes of severe illness, the need for mechanical ventilation, and death in COVID-19 patients, which we addressed in an extensive previous review [18]; the miRNA characteristics are addressed in the Supplementary Materials, Table S1: Characteristics on miR-25-5p and miR-146a-5p.
2. Results
The study population consisted of 128 peripheral blood samples from Mexican mestizo COVID-19 patients, and 31 patients older than 55 years were excluded, thus keeping 97 eligible samples. The selected samples were divided into two groups following the principle of proportionality according to the severity variable of the hospital population, leaving a group of 65 critical patients and 32 severe patients (Supplementary Materials, Figure S1: Stratification of the study population).
The description of the primary demographic data according to the severity of the disease, clinical characteristics, comorbidities, and pre-existing conditions is shown in Table 1. A total of 97 samples were obtained: 73 male and 24 female. For every woman in critical condition, there were 3.08 men, with a median age of 43 years.
Table 1.
Demographic and comorbid characteristics by severe and critical group type.
| Characteristics | Severe n = 32 |
Critical n = 65 |
p-Value |
|---|---|---|---|
| Age, years | 41.5 (31–46) | 43 (38–52) | 0.047 |
| Sex (n, %) | |||
| Female | 11 (34.4) | 13 (20) | 0.139 |
| Male | 21 (65.6) | 52 (80) | |
| BMI, kg/m2 | 27.91 (24.55–30.39) | 29.40 (27.10–34.16) | 0.027 |
| BMI classification (n, %) | |||
| Normal weight | 12 (37.5) | 13 (20) | 0.084 |
| Overweight | 9 (28.1) | 20 (30.8) | 0.974 |
| Obesity | 11 (34.4) | 32 (49.2) | 0.196 |
| Symptoms onset, days | 8 (7–10) | 8 (7–12) | 0.266 |
| PaO2/FiO2 ratio | 210 (161–248) | 129 (80–169) | 0.001 |
| Classification PaO2/FiO2 ratio (n, %) | |||
| Mild | 18 (56.2) | 12 (18.5) | 0.003 |
| Moderate | 14 (43.8) | 31 (47.7) | 0.829 |
| Severe | 0 (0) | 22 (33.8) | 0.001 |
| Comorbidities | |||
| T2DM | 10 (31.3) | 15 (23.1) | 0.461 |
| SAH | 8 (25) | 14 (21.5) | 0.797 |
| Cardiovascular disease | 1 (3.1) | 1 (1.5) | 0.533 |
| Respiratory disease | 3 (9.4) | 1 (1.5) | 0.115 |
| Tobacco smoking | 11 (34.4) | 22 (33.8) | 0.566 |
Continuous data are presented as median (interquartile range, IQR) and categorical data as number and frequency in percentage (%). Statistical tests employed for the comparisons: Mann–Whitney U test and Fisher’s exact test. BMI, body mass index; Classification of BMI: Normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), and obesity (BMI > 30); SAH, systemic arterial hypertension; and T2DM, Type 2 diabetes mellitus. PaO2/FiO2 ratio at hospital admission, mmHg.
On the other hand, when stratifying into survivors and non-survivors, it was found that 81.3% of the deceased patients were male and 18.8% were female, with a median age of 48.5 years (Table 2). Additionally, a generalized regression model was made, taking age and BMI as possible confounding variables; however, no significant results were found (p = 0.788, p = 0.769, respectively), so age or BMI did not modify the expression levels of miRNAs.
Table 2.
Demographic and clinical characteristics of survivors and non-survivors.
| Characteristics | Survivors n = 65 |
Non-Survivors n = 32 |
p-Value |
|---|---|---|---|
| Age, years | 42 (32–48) | 48.5 (41–52) | 0.007 |
| Sex (n, %) | |||
| Female | 18 (27.7) | 6 (18.8) | 0.454 |
| Male | 47 (72.3) | 26 (81.2) | |
| PaO2/FiO2 ratio | 160 (106–225) | 143.65 (68.42–195) | 0.143 |
| Classification PaO2/FiO2 ratio (n, %) | |||
| Mild | 22 (33.8) | 8 (25) | 0.484 |
| Moderate | 31 (47.7) | 14 (43.8) | 0.829 |
| Severe | 12 (18.5) | 10 (31.3) | 0.198 |
| IMV | 32 (49.2) | 32 (100) | 0.001 |
| Length IMV, days | 6 (1–16) | 23.50 (15–34) | 0.001 |
Continuous data are presented as median (interquartile range, IQR) and categorical data as number and frequency in percentage (%). Statistical tests employed for the comparisons: Mann–Whitney U test and Fisher’s exact test. IMV, invasive mechanical ventilation. PaO2/FiO2 ratio at hospital admission, mmHg.
2.1. The miR-21-5p and miR-146a-5p and Their Relation with Mortality in Patients with COVID-19
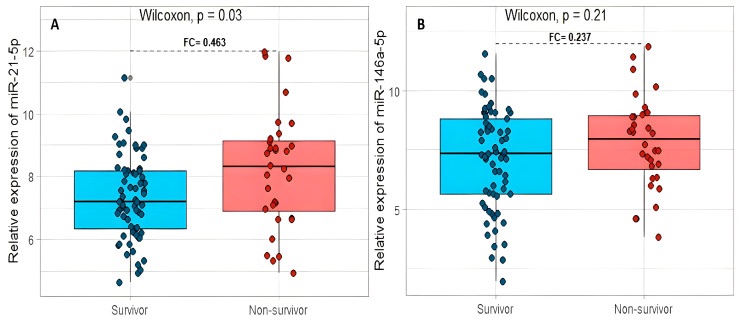
A significant difference in miR-21-5p expression was found in surviving patients compared with non-surviving patients (p = 0.03), with a difference in the expression between these two groups (or fold change, FC) of 0.463 in favor of the group of non-surviving patients (Figure 1A). For miR-146a-5p, the non-survivors group (FC = 0.237) was increased, but no significant difference was found (p = 0.21, Figure 1B).
Figure 1.
The relation between the relative expression levels of miR-21-5p (A) and miR-146a-5p (B) with the mortality in COVID-19 patients. FC: fold change.
Owing to the difference in the age observed among surviving patients (Table 2), we performed a Cox regression analysis, including age and expression levels of miR-21-5p as co-variables; however, no significance was found (Supplementary Materials, Table S2: Cox regression analysis for the multivariable assessment of mortality). Thus, these are independent variables affecting the mortality, which agrees with the lack of influence of age in the miRNA expression.
2.2. The miR-21-5p and miR-146a-5p Expression between Patients with Severe and Critical COVID-19
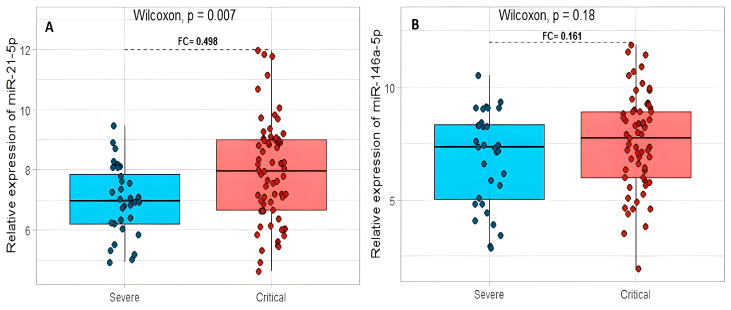
miR-21-5p had a statistically significant difference between these groups (p = 0.007) and an FC = 0.498 in favor of patients with a critical clinical disease (Figure 2A). The miR-146a-5p showed an increase in expression (FC = 0.161) and no statistically significant difference between the two study groups (p = 0.18, Figure 2B).
Figure 2.
The relation between the relative expression levels of miR-21-5p (A) and miR-146a-5p (B) with severe and critical COVID-19 patients. FC: fold change.
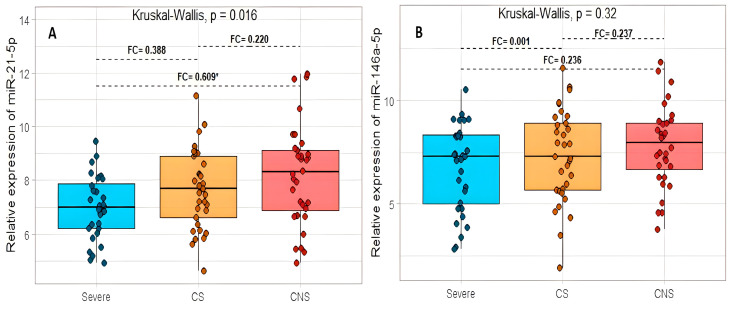
Subsequently, we stratified patients into three groups, severe, critical survivors (CS), and critical non-survivors (CNS). miR-21-5p presented significant differences between the three groups studied (p = 0.016). Subsequently, Dunn’s test revealed a significant difference in the expression levels between the severe and CNS groups (p = 0.005). However, no differences were found in the CS, CNS, or severe patients (p = 0.061, p = 0.329, respectively). (Figure 3A). The miR-146-5p did not express significant differences between the three groups studied (p = 0.320) nor Dunn’s subsequent test (Figure 3B).
Figure 3.
The relation between the relative expression levels of miR-21-5p (A) and miR-146a-5p (B) with the severe critical survivor and critical non-survivor COVID-19 patients. Critical survivor (CS); critical non-survivor (CNS). FC: fold change. * p < 0.05.
2.3. The miR-21-5p and miR-146a-5p and Their Relation to a Grade of Acute Respiratory Distress Syndrome in COVID-19
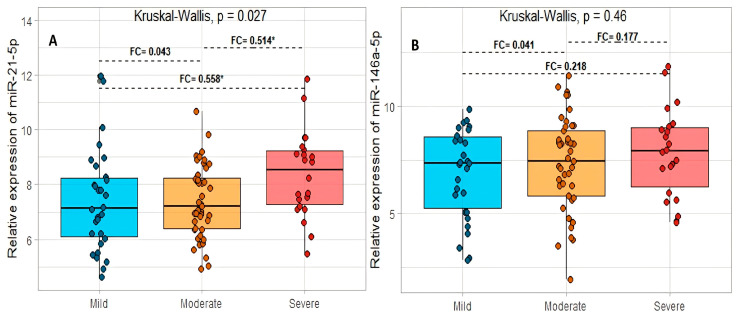
The miR-21-5p presented significant differences between the three groups studied (p = 0.027). Subsequently, in Dunn’s test, significant differences were found between the mild–severe and moderate–severe groups (p = 0.014 and p = 0.018, respectively), and for the mild–moderate groups, no significant differences were found (p = 0.748) (Figure 4A). Regarding miR-146-5p, no significant differences were found between the three groups studied (p = 0.460) or between pairs when performing Dunn’s tests (Figure 4B).
Figure 4.
The relation between the relative expression levels of miR-21-5p (A) and miR-146a-5p (B) and the grade of acute respiratory distress syndrome in COVID-19 patients. FC: fold change. * p < 0.05.
2.4. miRNA Relation to Comorbidities in Patients with Severe and Critical Disease in COVID-19
We compared the difference in the expression of miR-21-5p and miR-146a-5p between the main comorbidities of the patients regardless of the severity of COVID-19. The expression values of both miRNAs were raised in patients with comorbidities (T2DM, SAH, and tobacco smoking) compared with those without comorbidities; however, neither showed a significant difference between the groups; therefore, the relative expression of these miRNAs could not be affected by either comorbidity (Supplementary Materials, Table S3: miR-21-5p and miR-146a-5p and their relation with comorbidities in patients with severe and critical disease in COVID-19).
2.5. Correlation between miRNA Relative Expression and Clinical and Laboratory Variables
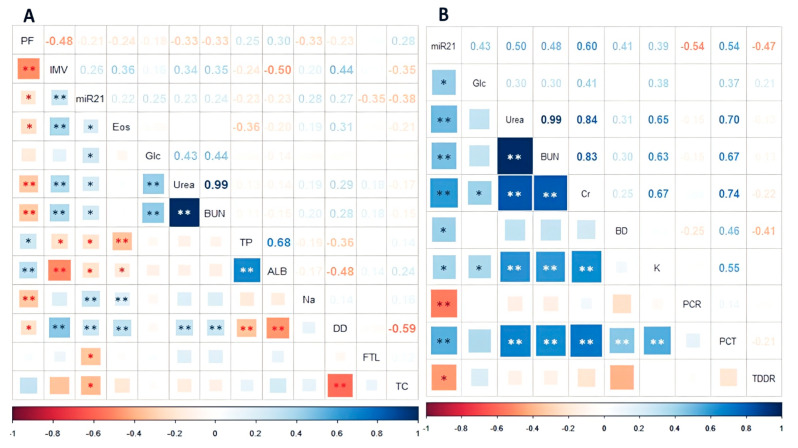
Correlations were sought using more than 32 clinical and laboratory datasets. The miR-21-5p showed significant correlations with variables related to coagulation, lung damage, kidney impairment, lipids, and glucose metabolism. In contrast, miR-146a only showed significant correlations with variables related to coagulation pathways. A summary of the statistically significant correlations can be found in Figure 5. A complete list of included variables can be found in the Supplementary Materials, Table S4: Clinical and laboratory variables included in the correlation tests.
Figure 5.
The miR-21-5p correlation with clinical and laboratory variables in all patients (A), and the miR-21-5p correlation in thirty-two non-survivor patients (B), with the PaO2/FiO2 ratio at hospital admission (PF), days of invasive mechanical ventilation (IMV), eosinophils count (Eos), glucose (Glc), urea, blood urea nitrogen (BUN), total proteins (TP), albumin (ALB), sodium (Na), D-dimer (DD), thromboplastin/D-dimer ratio (TDDR), ferritin (FTL), total cholesterol (TC), bilirubin direct (BD), protein C reactive (PCR), and procalcitonin (PCT) in COVID-19. * p < 0.05. ** p < 0.01.
In the ninety-seven subjects, the most representative correlations with the miR-21-5p were with D-dimer (DD), ferritin (FTL), total cholesterol (TC), sodium (Na), glucose, and blood urea nitrogen (BUN). However, they did not show a strong correlation (rho = 0.24–0.38) but displayed statistical significance (Figure 5). For miR-146a-5p, only platelets and prothrombin were correlated (p = 0.026 and 0.048, respectively; Supplementary Materials, Figure S2: Statistically significant correlations between miR-146a-5p and clinical variables). Among clinical variables, the notable correlations and statistically significant (p < 0.01) were urea/BUN (rho = 0.99), total proteins/albumin (rho = 0.68), DD/total cholesterol (rho = −0.59), and PaO2/FiO2 ratio/days of IMV (rho = 0.48); these and other correlations are summarized in Figure 5A.
In addition, we found that in the non-survivor patients, the miR-21-5p correlations with variables such as glucose, urea, and BUN were stronger than in the general group (rho = 0.43, 0.50, and 0.48, respectively), and C reactive protein (CRP) and procalcitonin (PCT) showed a statistically and strong correlation in this group (rho = −0.54 p < 0.01, rho = 0.54 p < 0.01, respectively). These and other correlations are summarized in Figure 5B.
2.6. Receiver Operating Characteristic Curve Analysis of miR-21-5p and miR-146a-5p and Mortality in COVID-19 Patients
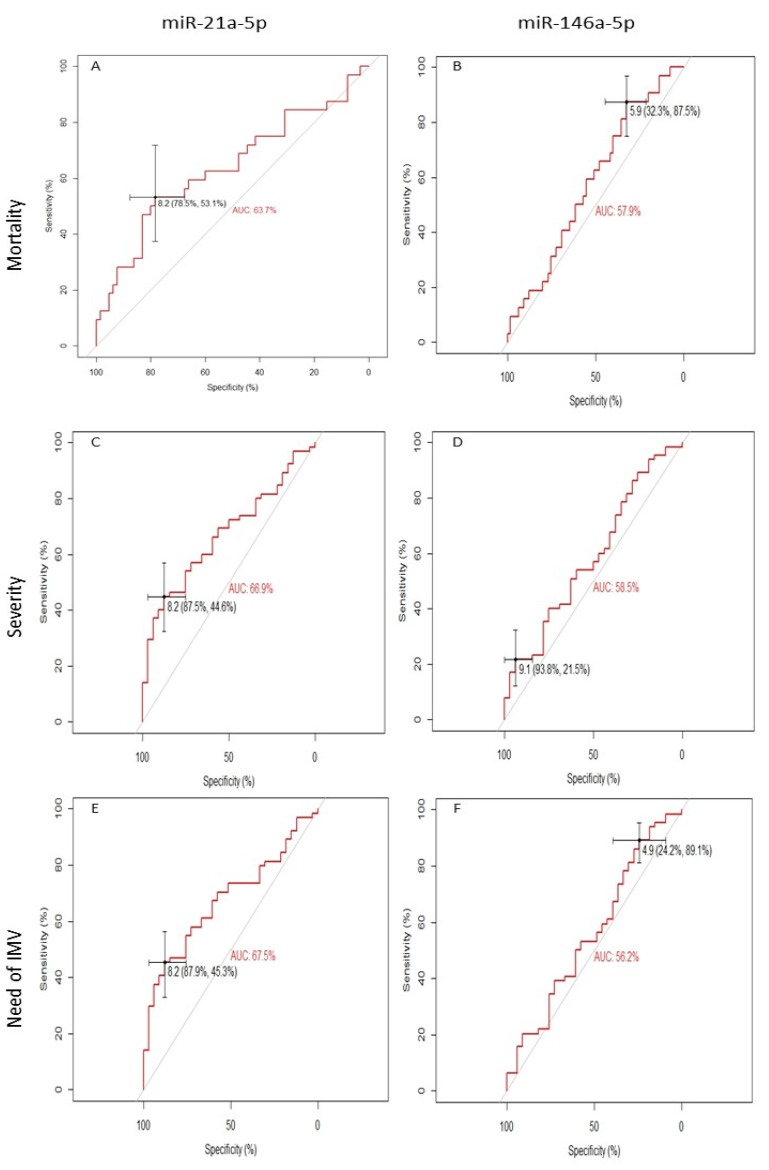
ROC curve analyses showed a high ability to distinguish non-surviving patients based on miR-21-5p expression levels (cut-off = 8.237, AUC = 63.3%, 95% CI = 0.512–0.761, p = 0.03) (Figure 6A). However, miR-146a-5p and mortality showed no statistical significance to distinguish non-survivors (cut-off = 5.884, AUC = 57.9%, 95% CI = 0.462–0.671, p = 0.206) (Figure 6B).
Figure 6.
Receiver operating characteristic curve analysis and area under the curve of miR-21-5p and mortality (A); miR-21-5p and disease severity (B); and miR-21-5p and the need for IMV (C). In addition, miR-146a-5p and mortality (D); miR-146a-5p and disease severity (E); miR-146a-5p and the need for IMV (F) data are shown. Each variable’s cut-offs and sensitivity and specificity between parentheses are shown. AUC: area under the curve.
Similarly, miR-21-5p showed a high ability to distinguish patients with more severe disease (cut-off = 8.191, AUC:66.9%, 95% CI: 0.562–0.777, p = 0.007) (Figure 6C) and the need for IMV (cut-off = 8.191, AUC:67.5%, 95% CI: 0.568–0.781, p = 0.005) (Figure 6E). In contrast, miR-146a-5p did not show significant results in distinguishing patients with more severe disease or the need for IMV (cut-off = 9.148, AUC: 58.5%, 95% CI: 0.464–0.705, p = 0.177, cut-off = 4.851, AUC: 56.2%, 95% CI: 0.440–0.685, p = 0.315, respectively) (Figure 6D,F). In addition, the best cut-off of the expression levels for miR-21-5p and miR-146a-5p for mortality, disease severity, and the need for IMV were calculated following the coordinates of the ROC curves and using the tallest index Youden.
With the previously selected cut-off, miR-21-5p was shown to increase mortality risk by 4.1-fold, the risk of developing critical illness by 5.6-fold, and the need for IMV by 6.0-fold. However, miR-146a-5p did not show significant results (Table 3).
Table 3.
Odds ratios of miR-21-5p and miR-146a-5p and the patient outcomes.
| miR-21-5p | ||||
| Variable | Cut-Off | Odds Ratio | 95% CI | p -Value |
| Mortality | 8.237 | 4.129 | 1.658–10.227 | 0.020 |
| Severity | 8.191 | 5.639 | 1.774–17.914 | 0.002 |
| Need of IMV | 8.191 | 6.007 | 1.892–19.075 | 0.001 |
| miR-146a-5p | ||||
| Variable | Cut-Off | Odds Ratio | 95% CI | p -Value |
| Mortality | 5.884 | 2.577 | 0.869–7.640 | 0.081 |
| Severity | 9.148 | 2.654 | 0.703–10.010 | 0.138 |
| Need of IMV | 4.851 | 2.480 | 0.755–6.696 | 0.140 |
IMV: invasive mechanical ventilation. Statistical tests were employed for the comparisons. Fisher’s exact test.
3. Discussion
Our study aimed to identify the expression and relationship between miR-21-5p and miR-146a-5p, which have been linked to multiple inflammatory pathways and antiviral immune responses in patients with varying degrees of COVID-19 severity [19,20,21,22].
We found that miR-21-5p levels are associated with mortality, severity, and PaO2/FiO2 index, as well as some correlations with clinical variables in the severity and mortality risk of COVID-19. Moreover, miR-21-5p could distinguish patients with a higher risk of death, severe disease, and patients needing an IMV.
In the current study, the patients with severe or critical disease were younger than 55 years of age, and the critical disease patients were older than the severe group; moreover, most of the critical patients were overweight or obese, agreeing with previous reports [23,24,25]. Interestingly, comorbidities such as T2DM and SAH were more frequent in severe patients than in critical. In addition, all the non-survivor patients required IMV and used it for a longer time.
In this study, we show that the relative expression levels of miR-146a-5p (although not statistically significant) are elevated in COVID-19 patients with a worse clinical outcome. In a smaller population, Tang and collaborators found that miR-146a-5p expression was significantly decreased in severe patients [26].
For miR-21-5p, we found that higher expression levels were associated with mortality, worst critical disease, and a lower PaO2/FiO2 index. Garg et al. mention that miR-21-5p expression is increased in the COVID-19 group compared with healthy controls and patients with influenza ARDS, suggesting that the upregulation of miR-21 in COVID-19 survivors could be a predictor of inflammation and chronic myocardial damage [22].
Our results could be explained by the significant miR-21-5p relation with the pro-inflammatory effect and its ability to promote the activation of the NLR family pyrin domain containing 3 (NLRP3) NLRP3 inflammasome, thus enabling the secretion of IL-1β and the cleavage of caspase-1, as well as the activation of gasdermin D that potentiates the activation of inflammasomes and the production of IL-6 and TNF-α [27,28,29]. Moreover, the miR-21 controls mRNAs such as the programmed cell death receptor 4 (PDCD4), E-cadherin, tissue inhibitor of metalloproteinase 2, which is responsible for regulating IL-1B, IL-18, IL-6, IL-33, NF-κB, TNF-α, and STAT 3/5, and Caspase-1/4/5/11 [29,30,31]. Together, these molecules are intensely involved in lung inflammation, repair, regeneration, and remodeling of the injured respiratory tissue, including stressed or injured epithelium, increased smooth muscle mass, subepithelial fibrosis, enlargement of goblet cells and submucosal glands, hyperemia with increased vascularization of subepithelial tissues, thickening of the basement membrane, and extracellular matrix deposition [28,32,33,34,35]. These data support our results that upregulated miR-21-5p expression levels lead to the most critical disease evidenced by a lower PaO2/FiO2 index, more days of IMV, and death.
Almost all risk factors could result in different expressions of specific miRNAs in peripheral blood, including those older than 60 years, obesity, SAH, and T2DM [36,37]. Our results show that these comorbidities did not modify the miR-21-5p expression levels. On the other hand, although the sex of the patients could significantly influence the expression of these miRNAs, we did not find significant differences in the comparisons made.
Clinical variables such as D-dimer [38], procalcitonin [39], glucose [40], creatinine [41], and urea [42] have demonstrated an association with coagulation pathways and lung, kidney, and liver damage, which could lead to more severe disease and death in COVID-19 patients. Upregulated miR-21-5p expression levels were statistically correlated with procalcitonin, creatinine, urea, and thromboplastin-D-dimer index in non-survivor patients; albumin, D-dimer, and ferritin levels were correlated in the whole population. On the other hand, Giannella et al. described that the upregulated miR-21-5p correlated with variables such as IL-6 and CPK, which were involved in an inflammatory process. Of note, the CRP levels show an inverse correlation with the miR-21-5p compared with other publications [43]. In this sense, we found an association with clinical variables related to inflammation, coagulation, kidney, hepatic, and pulmonary damage pathways.
Different miRNA cut-offs in COVID-19 can predict hospitalized patients’ outcomes [20]. We found that miR-21-5p expression levels around 8.2 could distinguish more severe disease, the requirement for IMV, and death among COVID-19 patients. It should be noted that these similar cut-offs between our three main variables further demonstrate that miR-21-5p expression is maintained during the progression to more severe disease and provide the ability to predict which patients could develop a more critical illness. Even though miR-21 has been evaluated as a specific predictive or prognostic biomarker in many diseases [44], the results are inconclusive. Still, many other investigations have suggested a close relation with lung involvement in the COVID-19 pathophysiology [45].
In a recent clinical study, our group identified that the age >65 years has an increased death risk (OR = 2.7) [46]. In the current study, the miR-21-5p cut-off >8 in patients under 55 showed a higher risk of developing a worse condition requiring IMV. Interestingly, the odds ratios are lower in the mortality (OR = 4.1229) compared with the severity of the disease (OR = 5.639) and the need for IMV (OR = 6.007); therefore, we suggest that early intervention could modify (decrease) the risk of death.
We found that miR-146a-5p had a statistically positive correlation with platelet count in patients with COVID-19; mir-146a-5p has previously been described to have interactions with molecules such as platelet-derived growth factor receptor alpha (PDGFRA), which increases the transduction of platelet signals and stimulates adhesion between platelets and the endothelium. In addition, it has been linked to patients with severe COVID-19 and thromboembolic events, as well as in patients who required admission to the intensive care unit (ICU) [47,48].
On the other hand, miR-21-5p had significant correlations with markers related to kidney damage, and it has been previously described that this miRNA plays an important role in regulating pathways related to programmed cell death protein 4 (PDCD4) that inhibit the translation of initiation factor eIF4A, a helicase responsible for regulating the cell cycle and apoptosis. This promotes NF-kB activation and suppresses IL-10 translation and the activation of caspase 3 [49,50], which together are related to atrophy, dilation, and swelling of the renal tubules, accompanied by expressed tubular necrosis, initially expressed as elevation in the increase in BUN and creatinine levels in serum [51]. Similar results were reported by Chen et al., where higher urinary levels of miR-21 were related to creatinine elevations, tubulointerstitial injury, and renal fibrosis at eight days in mice models with renal-induced damage [52]. On the other hand, miR-21 overexpression was related to increased plasma creatinine levels, progressively increasing protein and albumin excretion rates, with low serum levels, proximal tubular damage including pronounced dilation, the formation of apical bullae, and the loss of the brush border of renal cells [53].
Although there is little information on the relationships of miR-21-5p with other laboratory variables, miR-21 has previously been shown to bind to the 5′-UTR of IκB Kinase (IKKB) protein and TNF-α by the interaction between the maternally expressed protein 3 (MEG3) and miR-21. IKKB phosphorylates the inhibitor of NF-κB (IκBα) and promotes its degradation, thus activating NF-κB p65 and promoting its translocation into the nucleus, which acts as a transcription factor to activate the transcription of inflammatory-related genes, such as IL-1 and IL-6, and TNF-α, thus promoting mitochondrial activity and NADPH oxidase expression, which are the primary source of endogenous ROS [54]. In addition to elevating acute phase proteins such as C-reactive protein [55] and fibroblast growth factor 18 (FGF18), an increased concentration of fibroblast growth factor, granulocyte colony-stimulating factor (G-CSF), granulocytes, and monocytes colony-stimulating factor (GM-CSF) has been linked to hospitalized COVID-19 patients compared with healthy controls [56]. Moreover, COVID-19 patients who died exhibited significantly elevated levels of IFN-α, IFN-λ, and IL-1Ra, as well as chemokines associated with monocytes and T cell recruitment and survival such as CCL1, CLL2, macrophage colony-stimulating factor (M-CSF), IL-2, IL-16, and CCL21, within the first 12 days from symptom onset [57].
Our study is not exempt from limitations; firstly, it was elaborated in a third-level hospital that only received patients with severe or critical COVID-19 disease and not patients with mild or asymptomatic disease. Moreover, while other studies evaluated a large number of miRNAs, we studied two miRNAs that, in a previous review, showed significant differences in COVID-19 patients with extensive associations with molecules that support the development of severe forms of COVID-19 [18]. On the other hand, we did not collect data from current healthy controls in this study since we had to demonstrate that the controls had had a positive test for SARS-CoV-2 to compare under similar circumstances (differences in viral variants mainly). Moreover, laboratory testing was limited to people with severe or critical illnesses at recruitment time. Furthermore, our study focuses on a single biological sample obtained at the disease onset so that the treatment did not change the interpretation of the results. We will consider the above limitations for future studies and in the development of a new longitudinal study design.
As far as we know, this is the first study with a large number of recruited patients that seeks to recognize the relations between the studied miRNAs in the plasma of patients with COVID-19 not older than 55 years. Moreover, we included an important number of critical patients, identifying associations with disease severity, the need for IMV, and mortality. Moreover, we established cut-offs that could distinguish patients with more risk of developing severe disease and calculated the odds ratios that show the critical role of the studied miRNAs in COVID-19.
4. Materials and Methods
4.1. Study Population
This study included Mexican mestizo men and women between 22 to 55 years admitted to the hospital with a diagnosis of SARS-CoV-2 via a nasopharyngeal swab PCR test at the Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas (INER) during the third wave of COVID-19 in Mexico (July–August 2021). All patients were evaluated by specialist pulmonologists using clinical and laboratory criteria to stratify them according to the severity of the disease using the Infection Disease Society of America (IDSA) [58] complemented with the WHO criteria [59] in the following groups:
Severe: Clinical signs of pneumonia (fever, cough, dyspnea, and tachypnea) plus any of the following: respiratory rate > 30 inspirations/min, severe respiratory distress, or SpO2 < 90% on room air, but no requirement for IMV.
Critical: The patient presented acute respiratory distress syndrome (ARDS) and required immediate IMV. Moreover, the patient presented signs of sepsis, septic shock, or life-threatening multi-organ damage.
The ARDS is classified as follows.
Mild ARDS level: 200 mmHg < PaO2/FiO2 ≤ 300 mmHg.
Moderate ARDS: 100 mmHg < PaO2/FiO2 ≤ 200 mmHg.
Severe ARDS: PaO2/FiO2 ≤ 100 mmHg.
Subsequently, the patients received follow-up assessments during their hospital stay to report the severity of the disease until their death or recovery and discharge from the hospital. Complete demographic, clinical, and laboratory data were manually extracted from electronic medical records. As exclusion criteria, patients who decided to discharge themselves voluntarily, whose biological sample did not contain the necessary quantity and quality, patients with coinfection of another virus proven by PCR tests, and patients diagnosed with cancer or undergoing treatment were excluded to avoid potential conflicts when reading the results of the miRNAs studied, which have been previously studied in cancer and other diseases.
4.2. Statement of Ethics
The study was conducted in full compliance with the Declaration of Helsinki. The institutional ethics committee approved the study protocol (approbation number: C53-20). The participants, or their legal representatives, gave their written informed consent.
4.3. Obtaining Blood Samples and RNA Isolation
On the patient’s arrival at the hospital for admission, blood samples were collected in ethylenediaminetetraacetic acid (EDTA) tubes. Subsequently, the plasma was separated, and the presence of hemolysis in the plasma was evaluated through a first visual inspection and a second review based on spectrophotometry with the NanoDrop™ 2000/2000c instrument (Id: ND-2000, Thermo Scientific™, Foster City, CA, USA) with readings at wavelengths from 350 to 650 nm. Samples are considered hemolyzed if the reading at 414 nm exceeds a value of 0.2 [60,61]. Samples that did not meet this requirement were discarded for this study. Afterward, RNA extraction was performed with the miRNeasy serum/plasma Advanced Kit (Qiagen, Hilden, Germany). For normalization, synthetic Caenorhabditis elegans 39-3p (cel-miR-39-3p) was added as an external reference miRNA (1.6 × 108 copies/μL). The next step was quantifying the RNA obtained via spectrophotometry at 260 nm wavelength using the NanoDrop™ 2000/2000c instrument (Id: ND-2000, Thermo Scientific™, Foster City, CA, USA). The contamination with organic compounds and proteins was established using the ratio of the readings 260/230 and 260/280, respectively; the samples were considered free of contaminants when the relationship was between 1.7–2.0. Then, they were stored at −20 °C until use.
4.4. RT-PCR and Polymerase Chain Reaction for miRNA Analysis
The cDNA synthesis was performed using a TaqMan® Advanced miRNA cDNA Synthesis Kit (Id: A28007, Applied Biosystems™ Foster City, CA, USA) in 10 μL following the manufacturer’s specifications. Afterward, miRNAs were amplified with the following TaqMan probes: hsa-miR-21-5p (Id: A25576 477975_mir, Applied Biosystems™ Foster City, CA, USA) and hsa-miR-146a-5p (A25576 478399_mir, Applied Biosystems™ Foster City, CA, USA) using the Cel-miR-39-3p (Id: 4427975 000200, Applied Biosystems™, Foster City, CA, USA) probe as an endogenous gene. The reaction mixture was carried out in a final volume of 25 µL in a StepOne™ Real-Time PCR System (Id: 4376357, Thermo Scientific™, Foster City, CA, USA) following the manufacturer’s instructions. In addition, the expression value of Cel-miR-39 was obtained as the normalization factor. The relative quantification of each miRNA was calculated using the formula 2–∆Ct, and the value of change or fold change was calculated using the formula 2–∆∆Ct.
4.5. Statistical Analysis
All statistical analyses were performed with SPSS 21.0 software and RStudio 4.2.0 (libraries: ggplot2, corrplot, pROC, Hmisc, tidyverse, rstatix, and stringi). The normality of the data distribution was calculated using the Kolmogorov–Smirnov test; for the quantitative variables, the median and interquartile ranges were used. Then, to compare the expression levels of the miRNAs between the groups of the two categories, a Mann–Whitney–Wilcoxon U test was conducted; and the Kruskal–Wallis test was conducted in three categories. A Cox regression analysis was performed for the multivariable assessment of mortality. In addition, Dunn’s multiple comparisons test was carried out to compare pairs; both tests had a statistical significance of 0.05. Spearman’s rank correlation coefficient was calculated to assess the relationship between plasma miRNA levels with different clinical variables and laboratory values with a statistical significance of 0.05. For the discrimination of groups by selected miRNA levels, receptor operating characteristic (ROC) curves were used, and the area under the curve (AUC) was calculated. Subsequently, the chosen miRNA cut-off was calculated using Youden’s indices, and odds ratios (OR) and 95% confidence intervals (CI) were estimated.
5. Conclusions
The increased expression levels of miR-21-5p are related to worse outcome of COVID-19 in younger hospitalized Mexican mestizo patients.
Acknowledgments
The authors acknowledge the support received from physicians and technicians from the clinical services at INER to confirm the study participants’ diagnoses and clinical care. Brandon Bautista-Becerril is an MD student from the Posgrado de Ciencias de la Salud, Instituto Politécnico Nacional, and recipient of a fellowship from CONACYT (scholarship number: 1073434).
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241210112/s1.
Author Contributions
Conceptualization, B.B.-B., Á.C. and R.F.-V.; data curation, K.J.N.-Q.; formal analysis, B.B.-B., I.F.-G., G.P.-R. and R.F.-V.; funding acquisition, R.F.-V.; Investigation, B.B.-B., E.M.-S., I.F.-G., I.B.-R., G.P.-R., L.C.-G., K.P.-T., F.T.-Q., E.M.-G., A.M.-M., R.d.J.H.-Z., M.E.J.-C. and R.F.-V.; methodology, B.B.-B., K.J.N.-Q., E.M.-S., I.F.-G., I.B.-R., G.P.-R., L.C.-G., K.P.-T., F.T.-Q., E.M.-G., A.M.-M., R.d.J.H.-Z. and M.E.J.-C.; project administration, I.B.-R., G.P.-R., L.C.-G. and R.F.-V.; resources, I.B.-R., G.P.-R., L.C.-G., and R.F.-V.; software, B.B.-B., K.J.N.-Q., E.M.-S., I.F.-G., G.P.-R., F.T.-Q., E.M.-G., A.M.-M. and M.E.J.-C.; supervision, G.P.-R. and R.F.-V.; validation, K.J.N.-Q., E.M.-S., I.F.-G. and G.P.-R.; visualization, I.F.-G., I.B.-R. and G.P.-R.; writing—original draft, B.B.-B. and R.F.-V.; writing—review and editing, B.B.-B. and R.F.-V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in full compliance with the Declaration of Helsinki. The institutional ethics committee approved the study protocol (approbation number: C53-20) of the Instituto Nacional de Enfermedades Respiratorias, Ismael Cosío Villegas. The participants, or their legal representatives, gave their written informed consent.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors confirm that the raw data to support this study’s conclusions are included in the manuscript. The corresponding author will provide more information to any qualified researcher upon a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The research in this study was supported by an allocated budget (RFV-HLA Laboratory) from the Instituto Nacional de Enfermedades Respiratorias Ismael Cosio Villegas (INER).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mathieu E., Ritchie H., Rodés-Guirao L., Appel C., Gavrilov D., Giattino C., Hasell J., Macdonald B., Dattani S., Beltekian D., et al. Coronavirus Pandemic (COVID-19). Our World in Data 2020. [(accessed on 6 October 2022)]. Available online: https://ourworldindata.org/coronavirus.
- 2.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., Bonanomi E., Cabrini L., Carlesso E., Castelli G., et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim L., Garg S., O’halloran A., Whitaker M., Pham H., Anderson E.J., Armistead I., Bennett N.M., Billing L., Como-Sabetti K., et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality Among Hospitalized Adults Identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET) Clin. Infect. Dis. 2020;72:e206–e214. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., Stachel A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra R., Kumar A., Ingle H., Kumar H. The Interplay Between Viral-Derived miRNAs and Host Immunity During Infection. Front. Immunol. 2020;10:3079. doi: 10.3389/fimmu.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverio A., Di Maio M., Citro R., Esposito L., Iuliano G., Bellino M., Baldi C., De Luca G., Ciccarelli M., Vecchione C., et al. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: Systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc. Disord. 2021;21:23. doi: 10.1186/s12872-020-01816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Secretaría de Salud Comunicados Técnicos Diarios COVID-19. 2021. [(accessed on 24 July 2022)]. Available online: https://www.gob.mx/salud/documentos/comunicados-tecnicos-diarios-covid19.
- 8.Secetarias Municipais e Estaduais de Saúde Painel CoronaVirus. Ministério de Saúde. [(accessed on 24 August 2022)];2022 Available online: https://covid.saude.gov.br/
- 9.Ministerio de Salud Chile Plan de acción Coronavirus COVID-19. Red Pública de Salud Gratuita. 2022. [(accessed on 23 August 2022)]. Available online: https://www.minsal.cl/nuevo-coronavirus-2019-ncov/casos-confirmados-en-chile-covid-19/
- 10.Baruch P.J., Aubert R., Ferrer M., Sanchez L., Dagorn G., Breteau P. COVID-19: Le Tableau de Bord de l’epidémie. Les Décoderus 2022. [(accessed on 24 August 2022)]. Available online: https://www.lemonde.fr/les-decodeurs/article/2022/09/14/covid-19-le-tableau-de-bord-de-l-epidemie_6038751_4355775.html.
- 11.Palacios Y., Ruiz A., Ramón-Luing L.A., Ocaña-Guzman R., Barreto-Rodriguez O., Sánchez-Monciváis A., Tecuatzi-Cadena B., Regalado-García A.G., Pineda-Gudiño R.D., García-Martínez A., et al. Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality. Int. J. Mol. Sci. 2021;22:8423. doi: 10.3390/ijms22168423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fricke-Galindo I., Martínez-Morales A., Chávez-Galán L., Ocaña-Guzmán R., Buendía-Roldán I., Pérez-Rubio G., Hernández-Zenteno R.D.J., Verónica-Aguilar A., Alarcón-Dionet A., Aguilar-Duran H., et al. IFNAR2 relevance in the clinical outcome of individuals with severe COVID-19. Front. Immunol. 2022;13:4031. doi: 10.3389/fimmu.2022.949413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fricke-Galindo I., Buendia-Roldan I., Chavez-Galan L., Pérez-Rubio G., Hernández-Zenteno R.D.J., Ramos-Martinez E., Zazueta-Márquez A., Reyes-Melendres F., Alarcón-Dionet A., Guzmán-Vargas J., et al. SERPINE1 rs6092 Variant Is Related to Plasma Coagulation Proteins in Patients with Severe COVID-19 from a Tertiary Care Hospital. Biology. 2022;11:595. doi: 10.3390/biology11040595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman R.C., Farh K.K.-H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 16.Keikha R., Hashemi-Shahri S.M., Jebali A. The relative expression of miR-31, miR-29, miR-126, and miR-17 and their mRNA targets in the serum of COVID-19 patients with different grades during hospitalization. Eur. J. Med. Res. 2021;26:75. doi: 10.1186/s40001-021-00544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Gonzalo-Calvo D., Dávalos A., Montero A., García-González A., Tyshkovska I., González-Medina A., Soares S.M.A., Martínez-Camblor P., Casas-Agustench P., Rabadán M., et al. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J. Appl. Physiol. 2015;119:124–134. doi: 10.1152/japplphysiol.00077.2015. [DOI] [PubMed] [Google Scholar]
- 18.Bautista-Becerril B., Pérez-Dimas G., Sommerhalder-Nava P.C., Hanono A., Martínez-Cisneros J.A., Zarate-Maldonado B., Muñoz-Soria E., Aquino-Gálvez A., Castillejos-López M., Juárez-Cisneros A., et al. miRNAs, from Evolutionary Junk to Possible Prognostic Markers and Therapeutic Targets in COVID-19. Viruses. 2021;14:41. doi: 10.3390/v14010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donyavi T., Bokharaei-Salim F., Baghi H.B., Khanaliha K., Janat-Makan M.A., Karimi B., Nahand J.S., Mirzaei H., Khatami A., Garshasbi S., et al. Acute and post-acute phase of COVID-19: Analyzing expression patterns of miRNA-29a-3p, 146a-3p, 155–5p, and let-7b-3p in PBMC. Int. Immunopharmacol. 2021;97:107641. doi: 10.1016/j.intimp.2021.107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayyad-Kazan M., Makki R., Skafi N., El Homsi M., Hamade A., El Majzoub R., Hamade E., Fayyad-Kazan H., Badran B. Circulating miRNAs: Potential diagnostic role for coronavirus disease 2019 (COVID-19) Infect. Genet. Evol. 2021;94:105020. doi: 10.1016/j.meegid.2021.105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S., Amahong K., Sun X., Lian X., Liu J., Sun H., Lou Y., Zhu F., Qiu Y. The miRNA: A small but powerful RNA for COVID-19. Brief. Bioinform. 2021;22:1137–1149. doi: 10.1093/bib/bbab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg A., Seeliger B., Derda A.A., Xiao K., Gietz A., Scherf K., Sonnenschein K., Pink I., Hoeper M.M., Welte T., et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur. J. Heart Fail. 2021;23:468–475. doi: 10.1002/ejhf.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alizadehsani R., Sani Z.A., Behjati M., Roshanzamir Z., Hussain S., Abedini N., Hasanzadeh F., Khosravi A., Shoeibi A., Roshanzamir M., et al. Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J. Med. Virol. 2020;93:2307–2320. doi: 10.1002/jmv.26699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dessie Z.G., Zewotir T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y.-D., Ding M., Dong X., Zhang J.-J., Azkur A.K., Azkur D., Gan H., Sun Y.-L., Fu W., Li W., et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 26.Tang H., Gao Y., Li Z., Miao Y., Huang Z., Liu X., Xie L., Li H., Wen W., Zheng Y., et al. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 2020;10:e200. doi: 10.1002/ctm2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang C., Guo Y., Yu H., Lu S., Meng L. Pleiotropic microRNA-21 in pulmonary remodeling: Novel insights for molecular mechanism and present advancements. Allergy Asthma Clin. Immunol. 2019;15:33. doi: 10.1186/s13223-019-0345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheedy F.J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015;6:19. doi: 10.3389/fimmu.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue Z., Xi Q., Liu H., Guo X., Zhang J., Zhang Z., Li Y., Yang G., Zhou D., Yang H., et al. miR-21 promotes NLRP3 inflammasome activation to mediate pyroptosis and endotoxic shock. Cell Death Dis. 2019;10:461. doi: 10.1038/s41419-019-1713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekar D. Implications of microRNA 21 and its involvement in the treatment of different type of arthritis. Mol. Cell. Biochem. 2020;476:941–947. doi: 10.1007/s11010-020-03960-y. [DOI] [PubMed] [Google Scholar]
- 31.Choi B., Kim H.-A., Suh C.-H., Byun H.O., Jung J.-Y., Sohn S. The Relevance of miRNA-21 in HSV-Induced Inflammation in a Mouse Model. Int. J. Mol. Sci. 2015;16:7413–7427. doi: 10.3390/ijms16047413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumarswamy R., Volkmann I., Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8:706–713. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peacock O., Lee A.C., Cameron F., Tarbox R., Vafadar-Isfahani N., Tufarelli C., Lund J.N. Inflammation and MiR-21 Pathways Functionally Interact to Downregulate PDCD4 in Colorectal Cancer. PLoS ONE. 2014;9:e110267. doi: 10.1371/journal.pone.0110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hur J., Rhee C.K., Lee S.Y., Kim Y.K., Kang J.Y. MicroRNA-21 inhibition attenuates airway inflammation and remodelling by modulating the transforming growth factor β–Smad7 pathway. Korean J. Intern. Med. 2021;36:706–720. doi: 10.3904/kjim.2020.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bejerano T., Etzion S., Elyagon S., Etzion Y., Cohen S. Nanoparticle Delivery of miRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018;18:5885–5891. doi: 10.1021/acs.nanolett.8b02578. [DOI] [PubMed] [Google Scholar]
- 36.Hijmans J.G., Diehl K.J., Bammert T.D., Kavlich P.J., Lincenberg G.M., Greiner J.J., Stauffer B.L., DeSouza C.A. Association between hypertension and circulating vascular-related microRNAs. J. Hum. Hypertens. 2018;32:440–447. doi: 10.1038/s41371-018-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roganović J. Downregulation of microRNA-146a in diabetes, obesity and hypertension may contribute to severe COVID-19. Med. Hypotheses. 2020;146:110448. doi: 10.1016/j.mehy.2020.110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lian H., Cai H., Zhang H., Ding X., Wang X., Zhang S. The Prediction Value of D-Dimer on Prognosis in Intensive Care Unit among Old Patients (≥65 Years): A 9-Year Single-Center Retrospective Study of 9261 Cases. Oxidative Med. Cell. Longev. 2022;2022:2238985. doi: 10.1155/2022/2238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar A., Karn E., Trivedi K., Kumar P., Chauhan G., Kumari A., Pant P., Munisamy M., Prakash J., Sarkar P.G., et al. Procalcitonin as a predictive marker in COVID-19: A systematic review and meta-analysis. PLoS ONE. 2022;17:e0272840. doi: 10.1371/journal.pone.0272840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minata M., Harada K.H., Yamaguchi T., Fujitani T., Nakagawa H. Diabetes Mellitus May Exacerbate Liver Injury in Patients with COVID-19: A Single-Center, Observational, Retrospective Study. Diabetes Ther. 2022;13:1847–1860. doi: 10.1007/s13300-022-01318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smadja D.M., Fellous B.A., Bonnet G., Hauw-Berlemont C., Sutter W., Beauvais A., Fauvel C., Philippe A., Weizman O., Mika D., et al. D-dimer, BNP/NT-pro-BNP, and creatinine are reliable decision-making biomarkers in life-sustaining therapies withholding and withdrawing during COVID-19 outbreak. Front. Cardiovasc. Med. 2022;9:935333. doi: 10.3389/fcvm.2022.935333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pál K., Molnar A.A., Huțanu A., Szederjesi J., Branea I., Timár Á., Dobreanu M. Inflammatory Biomarkers Associated with In-Hospital Mortality in Critical COVID-19 Patients. Int. J. Mol. Sci. 2022;23:10423. doi: 10.3390/ijms231810423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giannella A., Riccetti S., Sinigaglia A., Piubelli C., Razzaboni E., Di Battista P., Agostini M., Molin E.D., Manganelli R., Gobbi F., et al. Circulating microRNA signatures associated with disease severity and outcome in COVID-19 patients. Front. Immunol. 2022;13:968991. doi: 10.3389/fimmu.2022.968991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenike A.E., Halushka M.K. miR-21: A non-specific biomarker of all maladies. Biomark. Res. 2021;9:18. doi: 10.1186/s40364-021-00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Visacri M.B., Nicoletti A.S., Pincinato E.C., Loren P., Saavedra N., Saavedra K., Salazar L.A., Moriel P. Role of miRNAs as biomarkers of COVID-19: A scoping review of the status and future directions for research in this field. Biomark. Med. 2021;15:1785–1795. doi: 10.2217/bmm-2021-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutiérrez-Pérez I.A., Buendía-Roldán I., Pérez-Rubio G., Chávez-Galán L., Hernández-Zenteno R.D.J., Aguilar-Duran H., Fricke-Galindo I., Zaragoza-García O., Falfán-Valencia R., Guzmán-Guzmán I.P. Outcome predictors in COVID-19: An analysis of emergent systemic inflammation indices in Mexican population. Front. Med. 2022;9:1000147. doi: 10.3389/fmed.2022.1000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gómez-Gómez B., Leopoldo Rodríguez-Weber F., Díaz-Greene E.J. Fisiología plaquetaria, agregometría plaquetaria y su utilidad clínica. Med. Interna Mex. 2018;34:244–263. [Google Scholar]
- 48.Amezcua-Guerra L.M. Anotaciones breves sobre el síndrome de liberación de citocinas y el bloqueo terapéutico de la interleucina-6 en SARS-CoV-2/COVID-19. Arch. Cardiol. Mex. 2021;90:84–87. doi: 10.24875/ACM.M20000067. [DOI] [PubMed] [Google Scholar]
- 49.Dorrello N.V., Peschiaroli A., Guardavaccaro D., Colburn N.H., Sherman N.E., Pagano M. S6K1- and βTRCP-Mediated Degradation of PDCD4 Promotes Protein Translation and Cell Growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 50.Glasgow A., Vencken S., De Santi C., Raoof R., Henshall D., Linnane B., McNally P., Greene C. An investigation of miR-21 and the TLR4/PDCD4 axis in CF bronchial epithelium. Mol. Pathol. Funct. Genom. Eur. Respir. Soc. 2020;56:915. doi: 10.1183/13993003.congress-2020.915. [DOI] [Google Scholar]
- 51.Huang G., Yao Q., Ye Z., Huang Y., Zhang C., Jiang Y., Xi X. Gender Differential Expression of AR/miR-21 Signaling Axis and Its Protective Effect on Renal Ischemia-Reperfusion Injury. Front. Cell Dev. Biol. 2022;10:861327. doi: 10.3389/fcell.2022.861327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C., Lu C., Qian Y., Li H., Tan Y., Cai L., Weng H. Urinary miR-21 as a potential biomarker of hypertensive kidney injury and fibrosis. Sci. Rep. 2017;7:17737. doi: 10.1038/s41598-017-18175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chuppa S., Liang M., Liu P., Liu Y., Casati M.C., Cowley A.W., Patullo L., Kriegel A.J. MicroRNA-21 regulates peroxisome proliferator–activated receptor alpha, a molecular mechanism of cardiac pathology in Cardiorenal Syndrome Type 4. Kidney Int. 2017;93:375–389. doi: 10.1016/j.kint.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J., Bai J., Tuerdi N., Liu K. Long non-coding RNA MEG3 promotes tumor necrosis factor-alpha induced oxidative stress and apoptosis in interstitial cells of cajal via targeting the microRNA-21 /I-kappa-B-kinase beta axis. Bioengineered. 2022;13:8676–8688. doi: 10.1080/21655979.2022.2054501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Na L., Ding H., Xing E., Zhang Y., Gao J., Liu B., Yu J., Zhao Y. The predictive value of microRNA-21 for sepsis risk and its correlation with disease severity, systemic inflammation, and 28-day mortality in sepsis patients. J. Clin. Lab. Anal. 2019;34:e23103. doi: 10.1002/jcla.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgos-Blasco B., Güemes-Villahoz N., Santiago J.L., Fernandez-Vigo J.I., Espino-Paisán L., Sarriá B., García-Feijoo J., Martinez-De-La-Casa J.M. Hypercytokinemia in COVID-19: Tear cytokine profile in hospitalized COVID-19 patients. Exp. Eye Res. 2020;200:108253. doi: 10.1016/j.exer.2020.108253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lucas C., Wong P., Klein J., Castro T.B.R., Silva J., Sundaram M., Ellingson M.K., Mao T., Oh J.E., Israelow B., et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhimraj A., Morgan R.L., Hirsch Shumaker A., Baden L., Chi-Chung Cheng V., Edwards K.M., Gallagher J.C., Gandhi R.T., Muller W.J., Nakamura M.M., et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. IDSA 2022. [(accessed on 2 October 2022)]. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
- 59.World Health Organization WHO Characterizes COVID-19 as a Pandemic. Pan American Health Organization 2020. [(accessed on 19 March 2021)]. Available online: https://www.paho.org/en/news/11-3-2020-who-characterizes-covid-19-pandemic.
- 60.Kirschner M.B., Edelman J.B., Kao S.C.-H., Vallely M.P., Van Zandwijk N., Reid G. The Impact of Hemolysis on Cell-Free microRNA Biomarkers. Front. Genet. 2013;4:94. doi: 10.3389/fgene.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirschner M.B., Kao S.C., Edelman J.J., Armstrong N.J., Vallely M.P., van Zandwijk N., Reid G. Haemolysis during Sample Preparation Alters microRNA Content of Plasma. PLoS ONE. 2011;6:e24145. doi: 10.1371/journal.pone.0024145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the raw data to support this study’s conclusions are included in the manuscript. The corresponding author will provide more information to any qualified researcher upon a reasonable request.