Abstract
A 15-year-old male terrier dog with symptoms of lethargy and severe abdominal distension was referred to the polyclinic hospital of the Ferdowsi University of Mashhad, Mashhad, Iran. In addition to numbness and abdominal distension, the dog also had anorexia and severe weakness and some skin masses were observed. Due to the enlarged abdomen, splenomegaly was diagnosed in ultrasonography. Fine needle aspiration was performed on the liver and skin mass and then, neoplastic lesions were reported based on cytology. On the necropsy, two masses were found on the liver and shoulder skin. These masses were well-encapsulated, soft and multi-lobulated. Samples taken from the liver and skin were prepared by Hematoxylin and Eosin staining and then, two different immunohistochemical markers were used to confirm the initial diagnosis. Histopathological examination of these two well-encapsulated, soft and multi-lobulated masses on the liver and skin showed lipid content and liposarcoma was indicated. Immunohistochemical staining using two markers, S100 and MDM2, made a definitive diagnosis and confirmed the diagnosis.
Key Words: Immunohistochemistry, Liposarcoma, Pathology, Terrier, Tumor
Introduction
Liposarcomas are uncommon neoplasms in dogs and are composed of malignant lipoblasts and mesenchymal tissues.1 These neoplasms occur in all domestic animals and are generally the most common soft-tissue sarcomas in humans. Most dogs with liposarcoma have skin lesions; but, the tumor can also occur in the abdominal cavity and other areas outside the skin. No breed or sexual predisposition has been identified in this neoplasm;2 but, the risk of developing a tumor increases with age.3 Like other soft-tissue sarcomas, liposarcomas tend to invade locally and even though the exact extent of their metastasis is unknown, metastasis of this neoplasm is rare. 4
However, there have been reports of liposarcoma metastasis to tissues such as bone. On the other hand, metastases to the liver, bone marrow and lungs have also been reported, 5 and these tumors have had variable growth rates and varying degrees of histopathological differentiation.6 Since liposarcomas and lipomas are often macroscopically indistinguishable, a histopathological examination would be necessary for a definitive diagnosis. Liposarcomas are histopathologically classified into several subtypes; 3 but, unlike humans, the behavioral differences between these subtypes have not been well established in dogs.6 Besides, human liposarcomas subtypes are still used to classify liposarcomas in animals accurately, and there is no precise classification in veterinary medicine. Animal liposarcomas are divided into three classes including well-differentiated, anaplastic (pleomorphic) and myxoid. Myxoid liposarcoma is the most apparent but rare type of liposarcoma.3
The location of liposarcomas depends on the amount of lipids they produce. Some liposarcomas behave like lipomas; but, other neoplasms are gray to white sub-cutaneous masses invading surrounding soft tissues and adjacent muscles. Liposarcomas are usually larger and have a higher degree of pleomorphism than lipomas. The anaplastic type of this tumor shows cells with variable morphology and irregular multi-nuclear cells. Identifiable intra-cytoplasmic fat vacuoles are usually present; but, only a small percentage of cells shows these vacuoles.3
The well-differentiated form contains polygonal cells with a sheet arrangement and has very limited collagen stroma (without stroma in some cases). In well- differentiated tumors, most cells represent normal adipocytes with a clear fat vacuole and a surrounding nucleus. Other cells have round to oval nuclei of varying size and abundant cytoplasm containing varying fat droplets. Compared to lipomas, these neoplasms are larger and show greater degrees of pleomorphism.7
Different morphological cells are seen in the anaplastic or pleomorphic forms, along with large, irregular and multi-nucleated cells. In this form, clear fat vacuoles are inside the cytoplasm; but, only a tiny percentage of the cells can be seen. Therefore, this tumor may be histologically very similar to histiocytic sarcoma and anaplastic sarcoma with giant cells (malignant fibrous histiocytoma). In the myxoid form, scattered spindle-shaped cells, lipocytes, and lipoblasts are located in a bubbly mucoid stroma. Liposarcoma, similar to myxosarcoma, can be associated with fatty vacuoles within the cytoplasm of neoplastic cells.7
The same treatment for this neoplasm is still unknown; but, surgery is the best choice for all soft tissue sarcomas; however, the effectiveness of chemotherapy and radiation therapy has not yet been well evaluated.8
Case Description
A 15-year-old male terrier dog was referred to the polyclinic hospital of the Ferdowsi University of Mashhad, Mashhad, Iran. Lethargy and severe abdominal distension were observed and the dog also had anorexia and severe weakness. Also, some skin masses were observed and due to the enlarged abdomen, splenomegaly was diagnosed in ultrasonography. Fine needle aspiration (FNA) was performed on the liver and skin masses and based on cytology, neoplastic lesions were diagnosed.
On the necropsy, two masses were found on liver and shoulder skin. These masses were well-encapsulated, soft and multi-lobulate masses (4.00 × 4.00 × 4.00 cm). In section, the tumors had a homogeneous white appearance and a greasy texture. After necropsy, all samples were paraffin-embedded and Hematoxylin and Eosin (H&E) staining was performed as usual. Fine needle aspiration as well as histopathological and immunohistochemical examinations can diagnose liposarcoma. Therefore, in this study, samples taken from the liver and skin were prepared by H&E staining and then, two different immunohistochemical markers were used to confirm the initial diagnosis.
A 3.00 μm section was used for immunohistochemistryand after dehydration and waxing, the sections were washed in saline solution (pH = 7). The sections were then immersed in citrate buffer (pH = 6) to preserve antigens. Then, 750 watt oven heat was used for two 5 min cycles for MDM2 marker (Mouse Anti-MDM2; Medaysis, Livermore, USA) and finally, for cooling, the sections were placed at room temperature for about 20 min. For confirmation, it was necessary to observe at least one positive nucleus in the power field of optical light microscope (400×).
Results
Liposarcomas are generally seen with varying degrees of malignancy and different types of malignancies may occur. In the present study, the myxoid form of liposarcoma was observed, in which scattered spindle-shaped cells, lipocytes and lipoblasts were located in a bubbly mucoid stroma. In histopathological examination, all masses were similar. The splenomegaly observed in this case is probably not related to the tumor and its metastasis to the liver. However, more research is required to investigate the relationship between these two issues. In this case, no such connection can be found. Liposarcoma, similar to myxosarcoma, can be associated with fatty vacuoles within the cytoplasm of some neoplastic cells. There was a cystic pattern with different myxoid content, and amorphous tumor cells with spindle to oval nuclei were seen in a myxoid background (Figs. 1 and 2).
Fig. 1.

Histopathological characteristics of myxoid liposarcoma. Lipoblasts (red arrow) and spindle-shaped and stellate cells (black arrow) inter-spersed in a myxoid background with small number of collagen fibrils (H & E staining, bar = 100 μm).
Fig. 2.

Histological features of canine myxoid liposarcoma. Mixture of lipocytes (red arrow), with a single vast and clear cyto-plasmic lipid vacuole compressing the nuclei, variably cellular mass of spindle cells (black arrow) in a pale basophilic matrix with lipoblasts (H & E staining, bar = 50.00 μm).
The MDM2 and S100 markers were used to confirm liposarcoma, and these immunohistochemical examinations also confirmed the histological findings. In most well-differentiated liposarcoma, expression of the MDM2 in nucleus occurs. This reaction can be seen in lipocytes and spindle-shaped cells. The positive and negative cells following MDM2 immunohistochemical staining were seen as mixed; but, the tumor cells were stained focally following S100 immunohistochemical staining (Figs. 3 and 4).
Fig. 3.

Strong and diffuse immunoreactivity of neoplastic cells to MDM2 (immunohistochemical staining, bar = 100 μm).
Fig. 4.

Nuclear expression of MDM2 in neoplastic cells in myxoid liposarcoma. Nuclear expression of MDM2 was present in both spindle cells and lipocytic areas. Positive and negative cells were inter-mixed (immunohistochemical staining, bar = 50.00 μm).
Immunoreactivity of neoplastic cells to MDM2 was strong, diffuse and cytoplasmic, and the reactivity was seen in both cytoplasm and nucleous. Nuclear expression was present in both spindle-shaped cells and lipocytic areas. Positive and negative cells were inter-mixed in the MDM2 marker immunohistochemical analysis (Figs. 3 and 4). In the immunohistochemical staining for S100, most neoplastic cells had a diffuse cytoplasmic positive response (Fig. 5).
Fig. 5.
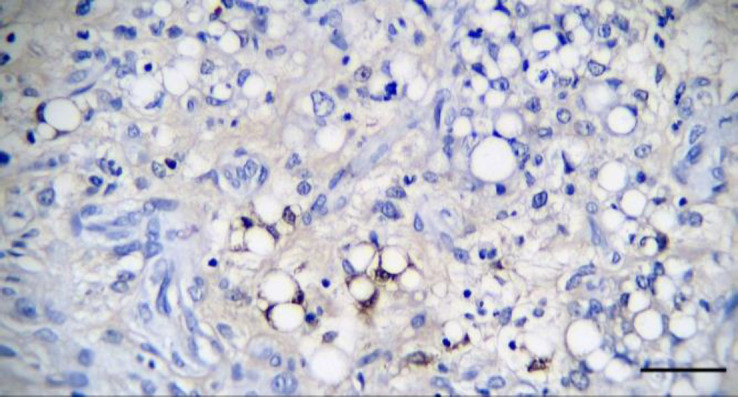
The majority of neoplastic cells had relatively solid and diffuse cytoplasmic positive response to S100 (immuno-histochemical staining, bar = 50.00 μm).
Discussion
The myxoid variant of liposarcoma is not a common type of liposarcoma. This subtype is well-encapsulated and shows lipocytes with a single large cytoplasmic lipid vacuole. There are scattered spindle-shaped and stellate cells within a myxoid background. Collagen fibrils are rarely present, and anastomosing capillary vasculature can sometimes be seen (Table 1). Myxoid liposarcoma is similar to myxosarcoma; but, cytoplasmic lipid vacuoles in myxoid liposarcoma can be differentiated with this characteristic.7
Table 1.
Different subtypes of liposarcoma.7
| Liposarcoma subtype | Prevalence | Histological features | Diagnostic differentiation |
|---|---|---|---|
| Well-differentiated variant | Most common subtype | Multi-lobular, fairly well-circumscribed but unencapsulated tumor. Round to polygonal cells arranged in solid sheets with a single clear fat vacuole and peripheral nuclear displacement. Low mitotic activity. | Infiltrative lipomas (which lack mitotic figures and show no evidence of anaplasia) |
| Myxoid variant | Uncommon subtype | Multi-lobular and non-encapsulated with poorly defined mass margins. Mixture of lipocytes, with a single large and clear cytoplasmic lipid vacuole compressing the nuclei. Lipoblasts and scattered spindle and stellate cells inter-spersed in a myxoid background with small number of collagen fibrils. | Myxosarcoma (which lacks cytoplasmic lipid-filled vacuoles) |
| Pleomorphic liposarcoma | Medium subtype | Pleomorphic cells of variable size and shape, and large bizarre multi-nucleated cells. Abundant eosinophilic cytoplasmthat may appear glassy or foamy (intra-cytoplasmic distinct fat vacuoles in a few cells). | Pleomorphic mesenchymal malignancies |
Although these subtypes have been associated with different biological behaviors in humans, there is no difference in the subtypes of dog liposarcoma. There is a possible tumor recurrence because of the invasive nature of sarcomas,1 which is more likely in the pleomorphic variant and not in the other subtypes. Metastasis is also rare; but, it usually occurs in the lung, bone or liver,3 as it was observed in the liver in this case.
To diagnose liposarcomas, FNA can be helpful and mesenchymal cells with a variable amount of lipid vacuoles may be present. It has been suggested that Oil Red O staining can be valuable in diagnosing liposarcoma.7 The CD34 immunoreactivity for spindle cell lipoma and myxoid liposarcoma was also suggested.9
The S100 protein is another valuable approach for cartilaginous components in various tumors, atypical adipocytes, and lipoblasts. So, it can be used to diagnose liposarcomas. The expression of S-100 protein is suggestive of liposarcoma. It was previously described in canine liposarcoma;9 so, it was used here to confirm the diagnosis. The MDM2 is also a marker distinguishing between ordinary lipoma and well-differentiated liposarcoma. Identifying MDM2 amplification by immunohisto-chemistry can be valuable; so, it is suggested in our study.
Wide surgical excision is suggested to treat animals with liposarcoma as a soft tissue sarcoma. The successful use of chemotherapy or radiation therapy is not proven.7 Chemotherapy is not a routine treatment for the human patient either because liposarcomas are not sensitive enough to chemotherapy. Further studies are needed to identify the efficacy of chemotherapy or radiation therapy in dogs. Although radiation therapy is currently used for dogs and cats with soft tissue sarcomas,1 further studies are needed to determine whether it has a role in liposarcomas.
Conflict of interest
The authors declare no probable conflicts of interest with respect to the authorship, research, and publication of this paper.
Acknowledgments
The authors express their thanks to the reviewers for their valuable insight and expertise that greatly assisted the research.
References
- 1.Baez JL, Hendrick MJ, Shofer FS, et al. Liposarcomas in dogs: 56 cases (1989-2000) J Am Vet Med Assoc. 2004;224(6):887–891. doi: 10.2460/javma.2004.224.887. [DOI] [PubMed] [Google Scholar]
- 2.Doster AR, Tomlinson MJ, Mahaffey EA, et al. Canine liposarcoma. Vet Pathol. 1986;23(1):84–87. doi: 10.1177/030098588602300117. [DOI] [PubMed] [Google Scholar]
- 3.Hendrick MJ. Mesenchymal tumors of the skin and soft tissues. In: Meuten DJ, editor. Tumors in domestic animals. 5th ed. Ames, USA: John Wiley and Sons ; 2017. pp. 142–175. [Google Scholar]
- 4.Saik JE, Diters RE, Wortman JA. Metastasis of a well-differentiated liposarcoma in a dog and a note on nomenclature of fatty tumours. J Comp Pathol. 1987;97(3):369–373. doi: 10.1016/0021-9975(87)90104-6. [DOI] [PubMed] [Google Scholar]
- 5.Davis PE, Dixon RT, Johnson JA, et al. Multiple liposarcoma of bone marrow origin in a Greyhound. J Small Anim Pract. 1974;15(7):445–456. doi: 10.1111/j.1748-5827.1974.tb06522.x. [DOI] [PubMed] [Google Scholar]
- 6.Messick JB, Radin MJ. Cytologic, histologic, and ultrastructural characteristics of a canine myxoid liposarcoma. Vet Pathol. 1989;26: 520–522. doi: 10.1177/030098588902600612. [DOI] [PubMed] [Google Scholar]
- 7.Doria-Torra G, Martínez J, Domingo M, et al. Liposarcoma in animals: literature review and case report in a domestic pig (Sus scrofa) J Vet Diagn Invest. 2015;27(2):196–202. doi: 10.1177/1040638714567190. [DOI] [PubMed] [Google Scholar]
- 8.Kuntz CA, Dernell WS, Powers BE, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986-1996) J Am Vet Med Assoc. 1997;211(9):1147–1151. [PubMed] [Google Scholar]
- 9.Frase R, Freytag M, Baumgärtner W, et al. Metastasising liposarcoma of bone in a young dog. Vet Rec. 2009;164: 372–373. doi: 10.1136/vr.164.12.372. [DOI] [PubMed] [Google Scholar]


