Abstract
Background: Calcified coronary lesions can cause stent under-expansion, malapposition, and polymer degradation, hence increasing the risk of adverse clinical outcomes. Percutaneous coronary intervention (PCI) guided by intravascular ultrasound (IVUS) has been used regularly to improve outcomes. Our primary aim was to evaluate the clinical efficacy of IVUS-guided PCI in calcified coronary lesions. Methods: From August 2018 to December 2021, we prospectively included 300 patients in the CAPIRO study (CAlcified plaque in patients receiving Resolute Onyx®) at three educational hospitals in Jeonbuk Province. We studied 243 patients (265 lesions) who were followed up for over a year. Based on coronary calcification by IVUS analysis, the patient population was categorized into two groups (Group I: non/mild calcification; Group II: moderate/severe calcification (maximum calcium arc >180° and calcium length > 5 mm)). One-to-one Propensity Score Matching was used to match the baseline characteristics. The stent expansion rate was analyzed by recent criteria. The primary clinical outcome was Major Adverse Cardiac Events (MACE), which included Cardiac death, Myocardial Infarction (MI), and Target Lesion Revascularization (TLR). Results: After follow-up time, the MACE rate in Group I was 1.99%, comparable to Group II’s 1.09% (p = 0.594). The components of MACE did not significantly differ between the two groups. Based on absolute MSA or MSA/MVA at MSA site criteria, the stent expansion rate in Group II was lower than that of Group I. Nevertheless, based on recent relative criteria, the stent expansion rate in both groups was comparable. Conclusions: After more than a year of follow-up, IVUS-guided PCI in moderate/severe calcification lesions was associated with good clinical outcomes, which was comparable with non/mild calcification lesions. Future studies with a larger sample size and a more extended follow-up period are required to clarify our findings.
Keywords: percutaneous coronary intervention, drug-eluting stents, calcium, intravascular ultrasound
1. Introduction
Although ongoing technological advancements in drug-eluting stents (DES) and procedural techniques increasingly allow Percutaneous Coronary Intervention (PCI) for more high-risk and complex coronary lesions, there is still a sizable percentage of patients who experience stent-related major adverse cardiovascular events (MACE) following PCI [1]. A calcified plaque, extreme tortuosity, a large thrombus burden, and diffuse lesions with poor stent landing zones were all related to worse outcomes [2]. Lesions with a high calcification burden pose the most significant challenge and are most likely to have an adverse effect on PCI outcomes. Severe coronary calcification is independently associated with increased rates of MACE because calcified target lesions may result in reduced minimum stent area, stent under-expansion, stent malapposition, polymer degradation, and greater risks of restenosis and stent thrombosis [3,4,5,6,7,8].
In addition to coronary angiography, PCI procedures are increasingly being facilitated by intracoronary imaging modalities such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT). IVUS and OCT offer more accurate assessments of stenosis degree, lesion length, plaque burden, degree of calcification, and plaque features when compared to coronary angiography only [9]. Using IVUS during PCI did not increase the cost-effectiveness rate of the procedure [10]. For the calcification lesions, OCT and IVUS could measure calcium arc, clusters of microcalcifications, or densely calcified plaque, and OCT could measure calcium thickness [2,11,12]. This information could direct a strategy for lesion preparation before stenting, such as balloon angioplasty, mechanical atherectomy, laser atherectomy, or lithoplasty, and it could also optimize stent implantation, such as expansion rate, apposition, and complications [2,11,13,14,15,16,17]. According to certain studies, IVUS-guided PCI may produce better results than angiography guidance, especially in patients with complex coronary artery lesions [18,19,20], and suggest some criteria for optimal stent expansion [21,22,23,24,25].
However, the safety and effectiveness of IVUS-guided PCI in patients with calcified plaques are still uncertain. The CAPIRO (CAlcified Plaque in Patients Receiving Resolute Onyx®) study’s objective was to evaluate the role of IVUS-guided PCI in calcified coronary lesions and the clinical outcomes after at least one year of follow-up.
2. Materials and Methods
2.1. Study Designs
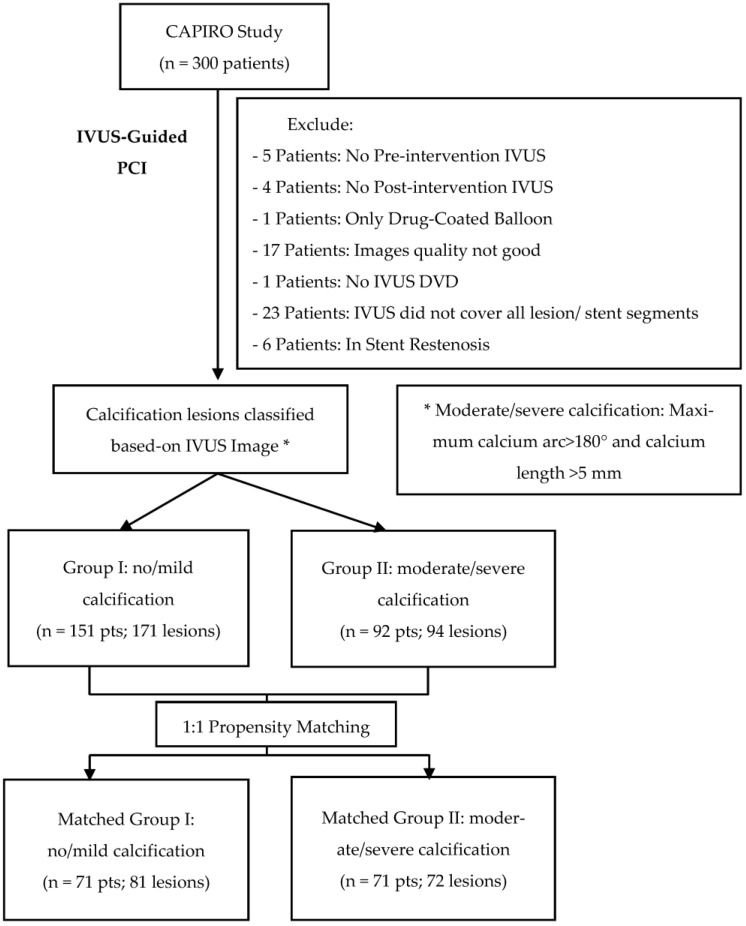
CAPIRO is a prospective, multi-center study designed to assess the impact of IVUS-guided PCI in patients with calcified lesions who received a Zotarolimus-Eluting Coronary Stent (Resolute OnyxTM DES, Medtronic, Minneapolis, MN, USA). From August 2018 to December 2021, the study was conducted in three educational hospitals in Jeonbuk Province, Korea (Jeonbuk National University Hospital, Wonkwang University Hospital, and Jeonju Presbyterian Medical Center). Each hospital included in the study approved the study protocol. All enrolled patients received the explanation of the study and signed an informed consent form. This study evaluated 243 patients (with 265 lesions) who were followed up for more than one year. The patient population was separated into two groups based on the degree of calcification detected by IVUS. In our study, moderate or severe calcification was categorized as lesions with a maximum calcium arc >180° and a calcium length of more than 5 mm (Group II: 92 patients, 94 lesions); otherwise, lesions were classified as non or mild calcification (Group I: 151 patients, 171 lesions). If a patient has numerous lesions, classification is based on the lesion with the most significant calcification.
Propensity score matching (PSM) was performed for age, male gender, history of diabetes mellitus, chronic kidney disease, current smoking, and LVEF with matching tolerance of 0.2. After the PSM, the patient population was categorized into matched-group I (n = 71 patients, 81 lesions) and matched-group II (n = 71 patients, 72 lesions) (Figure 1).
Figure 1.
Study Flowchart.
2.2. Study Criteria
Inclusion Criteria:
18 years old or older.
Patients with coronary artery disease who meet the criteria for index PCI according to current recommendations for myocardial revascularization [26,27].
Patients consented to participate and signed informed consent forms.
Exclusion Criteria:
Cardiogenic shock.
Contraindications for dual antiplatelet therapy for 12 months.
Major bleeding within three months or major surgery within two months.
Life expectancy is less than one year.
In-stent restenosis lesion.
IVUS image-related exclusions: lack of pre- or post-intervention IVUS, lack of coverage of all lesion or stent segments, and insufficient image quality for analysis.
2.3. IVUS-Guided PCI Procedure
All PCI procedures were carried out following current technological standards and guidelines [26,27]. All patients were administered a loading dose of antiplatelet medications (300 mg Aspirin and 600 mg Clopidogrel or 180 mg Ticagrelor) before the procedure. After obtaining vascular access via the radial or femoral artery, a loading dose of 70–100 IU/kg of Unfractionated Heparin was administered to achieve an Activated clotting time (ACT) of 250–300 seconds. If the procedure duration exceeds one hour, an extra 3000 IU of Unfractionated Heparin is administered, or the ACT findings are used to determine the amount of additional Unfractionated Heparin.
Our study utilized commercially available IVUS systems, including the Boston Scientific POLARIS Multi-Modality Guidance System with OPTICROSSTM and OPTICROSSTM HD Coronary Imaging Catheter and the Philips Volcano IVUS System with Eagle Eye Platinum Catheter. Lesions with total occlusion or severe stenosis that the IVUS catheter may not be able to cross were pre-dilated with a compliant balloon. After administering 100–200 ug of intracoronary Nitroglycerin, the IVUS catheter was advanced through the target lesion to a distance of at least 10 mm distal. The IVUS catheter was automatically pulled back at a rate of 0.5 mm/s for measurements on-site. On-site IVUS measures before stent implantation comprised minimal lumen diameter, minimal lumen area (MLA), reference lumen area, plaque burden, and lesion morphology (calcified, lipid-rich plaque). Utilization of a non-compliant balloon or adjunctive lesion preparation was left to the clinician’s discretion. The stent’s diameter, size, and landing zone were selected based on recent consensus documents [28]. In our study, every patient received the Zotarolimus-Eluting Coronary Stent system (Resolute OnyxTM DES, Medtronic). After stent implantation, IVUS images were acquired to evaluate stent deployment results (minimum stent area (MSA), expansion rate, apposition) and acute complications (dissection, thrombus, hematoma, and tissue protrusion). Using an additional non-compliant balloon for post-dilation was determined by clinical circumstances and the clinician’s discretion. The final IVUS image was recorded. All IVUS images were saved to DVD for offline analysis.
After PCI, patients were treated with guideline-directed medical therapy. This therapy included dual-antiplatelet therapy for 12 months, beta-blockers, statins, and other medications based on the patient’s specific clinical circumstances.
2.4. IVUS Images Analysis
Based on recent consensus documents, IVUS images were analyzed offline every 1 mm using planimetry software QIvus Research Edition 3.1. (Medis Medical Imaging System B.V., Leiden, The Netherlands) [29,30]. The IVUS image analyzers were blinded from the patient’s clinical information. The evaluated segments contained the target lesion (pre-PCI) or stent segment (post-PCI), as well as 5 mm proximal and distal reference segments with no intervening branches. Pre-PCI parameters include minimal luminal area, plaque burden at minimal luminal area site, proximal, distal, mean luminal reference area and plaque burden, remodeling index, and lesion length. The pre-PCI lesion morphology analysis includes calcium morphology, calcium nodule, maximal calcium arc, total calcium length, calcium index ((maximum calcium arc/360) × (calcium length/lesion length) × 100), attenuated plaque, and plaque rupture. Following the PCI, at the minimal stent area site, stent area (MSA), maximal stent diameter, minimal stent diameter, stent asymmetry, stent eccentricity, and stent length were examined. Stent expansion was assessed by absolute MSA, Conventional stent expansion (MSA/mean reference luminal area × 100%) [28], MSA/MVA at MSA site [25], IVUS-XPL (MSA > distal reference luminal area) [23], ULTIMATE criteria (MSA > 5.5 mm2 or >90% distal lumen reference area) [21,22]. Complications of PCI procedures such as stent edge dissection, malapposition, and tissue protrusion were also evaluated. Stent edge dissection was considered significant if the dissection flap opened more than 60°, if it reached the media, or if it was longer than 2 mm. Malapposition was considered major when the axial distance was >0.4 mm or >1 mm in length [28].
2.5. Quantitative Coronary Angiography (QCA)
Coronary angiography images from the intervention procedure were archived and evaluated by a different physician who was uninformed of IVUS images. QAngio XA 7.3.102.0 (Medis Medical Imaging System B.V., The Netherlands) was utilized for the QCA analysis. Using the catheter size for calibration was the first stage of the QCA process. Following this, a vessel line was drawn with 5 mm proximal and distal margins on both sides of the target lesion. The vessel contour was then automatically generated. The margin of the lesion, as well as the proximal or distal segment, was determined manually. From the QCA, it was possible to extract the following parameters: lesion diameter, lesion reference diameter, diameter stenosis, lesion area, lesion reference area, proximal reference diameter and area, distal reference diameter and area, plaque symmetry, and plaque area. In the case of total occlusion, the lesion length would be unavailable, and the reference diameter would be the diameter of the proximal reference segment [31].
2.6. Follow-Up and Endpoints
All patients should return for follow-up visits to the outpatient clinic after one, six, 12 months, and every six months; in some cases, patients were also contacted by phone at these intervals. The primary endpoint of the study is Major Adverse Cardiac Events (MACE) after the follow-up time, which is defined as the composite of Cardiac death, Myocardial Infarction (MI) of the target vessel, and Ischemia-driven Target lesion revascularization (TLR). If cardiac-related causes of death are discovered, a diagnosis of cardiac death is made. In all other cases, death was classed as non-cardiac death. Myocardial Infarction was defined according to the current definition of myocardial infarction [32]. Ichemia-driven target lesion revascularization consists of revascularization within the target lesion and 5 mm proximal and distal to the target lesion. Secondary endpoints include Patient-oriented composite endpoint (all-cause death, MI, and any revascularization), the individual components of MACE, all-cause death, stroke, target vessel revascularization (TVR), other vessel revascularization (OVR), chronic heart failure (CHF), and Stent thrombosis.
2.7. Statistical Analysis
All continuous variables were reported as means ± standard deviations or as median (interquartile range) and compared using Student’s t-test or Mann–Whitney U test, as appropriate. The Chi-square test or Fisher’s exact test, as appropriate, was used to compare categorical variables, represented as percentages (numbers). The baseline characteristics of both groups were matched by using Propensity score matching on a one-to-one basis. Before matching, age, male gender, diabetes mellitus history, chronic kidney disease, current smoking, and LVEF were established as predictors for the difference between the two groups. The tolerance for matching was set to 0.2. After at least one year of follow-up, the clinical outcomes were examined using the Kaplan–Meier method, and the difference between the two groups was determined using the log-rank test. All statistical analyses were conducted using version 26 of IBM SPSS Statistics (IBM Corporation, Armonk, NY, USA). Two-sided p values below 0.05 were regarded as statistically significant.
3. Results
3.1. Baseline Clinical Characteristics
Table 1 compares the baseline clinical characteristics of both groups before and after the PSM. Before the PSM, patients in Group II were older than those in Group I (mean age 67.71 ± 9.77 vs. 63.60 ± 9.75, p = 0.002). The proportion of patients over 65 years old in Group II was higher (61.96%, as opposed to 40.40% in Group I, p = 0.001). Group II also has more patients with diabetes mellitus (53.26% vs. 24.50%, p = 0.001), chronic kidney disease (8.70% vs. 1.32%, p = 0.005), and fewer patients who are male (65.22% vs. 82.12%, p = 0.003) and currently smoke (14.13% vs. 29.14%, p = 0.035). According to the findings of the common laboratory tests, patients in Group I have greater hemoglobin, EGFR, and LVEF values than patients in Group II. After the PSM, all clinical characteristics were comparable between the two groups, except that HbA1c was higher in Group II (6.15% (5.70,7.43) vs. 5.80% (5.50,6.30), p = 0.011) and there was a tendency for Group II to have more patients with diabetes mellitus (45.07% in Group II vs. 29.58% in Group I, p = 0.056).
Table 1.
Baseline Clinical Characteristics.
| Non/Mild Calcification (n = 151) | Moderate/Severe Calcification (n = 92) | p | Matched Non/Mild Calcification (n = 71) | Matched Moderate/Severe Calcification (n = 71) | p | |
|---|---|---|---|---|---|---|
| Baseline Characteristics | ||||||
| Age (years) | 63.60 ± 9.75 | 67.71 ± 9.77 | 0.002 | 66.48 ± 10.14 | 66.96 ± 9.05 | 0.767 |
| Age > 65 years old | 40.40% (61) | 61.96% (57) | 0.001 | 49.30% (35) | 59.15% (42) | 0.238 |
| Male | 82.12% (124) | 65.22% (60) | 0.003 | 76.06% (54) | 73.24% (52) | 0.700 |
| BMI (g/m2) | 24.96 ± 3.19 | 25.25 ± 3.11 | 0.498 | 24.78 ± 3.44 | 25.23 ± 2.87 | 0.393 |
| Hypertension | 69.54% (105) | 80.43% (74) | 0.061 | 76.06% (54) | 76.06% (54) | 1 |
| Diabetes Mellitus | 24.50% (37) | 53.26% (49) | 0.001 | 29.58% (21) | 45.07% (32) | 0.056 |
| Current Smoking | 29.14% (44) | 14.13% (13) | 0.035 | 18.31% (13) | 16.90% (12) | 0.714 |
| Chronic Kidney Disease | 1.32% (2) | 8.70% (8) | 0.005 | 2.82% (2) | 4.23% (3) | 0.649 |
| Clinical Presentation | 0.176 | 0.647 | ||||
| STEMI NSTEMI UAP Non-ACS |
1.99% (3) 11.92% (18) 54.30% (82) 31.79% (48) |
2.17% (2) 20.65% (19) 55.43% (51) 21.74% (20) |
0.921 0.066 0.864 0.091 |
4.23% (3) 14.08% (10) 50.70% (36) 30.99% (22) |
2.82% (2) 15.49% (11) 59.15% (42) 22.54% (16) |
0.649 0.813 0.312 0.255 |
| Multi-lesions PCI | 7.28% (11) | 10.87% (10) | 0.335 | 5.63% (4) | 8.45% (6) | 0.512 |
| Family history of CAD | 5.96% (9) | 2.17% (2) | 0.221 | 2.82% (2) | 2.82% (2) | 0.508 |
| Previous PCI | 5.96% (9) | 13.04% (12) | 0.057 | 4.23% (3) | 8.45% (6) | 0.301 |
| TIA | 8.61% (13) | 9.78% (9) | 0.757 | 7.04% (5) | 8.45% (6) | 0.754 |
| Laboratory Findings | ||||||
| Hemoglobin (103/μL) | 14.10 (13.10,15.00) | 13.15 (12.00,14.60) | <0.001 | 13.80 (12.70,14.70) | 13.30 (12.00,14.60) | 0.257 |
| Creatinine (mg/dL) | 0.88 (0.74,1.01) | 0.88 (0.73,1.17) | 0.295 | 0.87 (0.71,1.06) | 0.87 (0.73,1.17) | 0.485 |
| EGFR (mL/min/m2) | 85.80 ± 22.55 | 76.66 ± 31.38 | 0.016 | 82.95 ± 25.68 | 79.35 ± 28.86 | 0.434 |
| EGFR < 60 (mL/min/m2) | 7.95% (12) | 25.00% (23) | <0.001 | 11.27% (8) | 22.54% (16) | 0.073 |
| Dyslipidemia * | 58.28% (88) | 71.74% (66) | 0.035 | 66.20% (47) | 71.83% (51) | 0.468 |
| LVEF (%) | 61.00 (58.00,66.00) | 58.00 (55.00,62.00) | 0.001 | 60.00 (57.00,64.00) | 58.00 (56.00,63.00) | 0.108 |
| HbA1C (%) | 5.80 (5.40,6.40) | 6.30 (5.78,7.50) | <0.001 | 5.80 (5.50,6.30) | 6.15 (5.70,7.43) | 0.011 |
| Pre-PCI Medications | ||||||
| Aspirin Betablocker RAS Statin P2Y12 |
22.52% (34) 19.21% (29) 36.42% (55) 38.41% (58) 84.11% (127) |
29.35% (27) 26.09% (24) 52.17% (48) 59.78% (55) 69.57% (64) |
0.234 0.187 0.020 0.001 0.007 |
26.76% (19) 22.54% (16) 38.03% (27) 49.30% (35) 81.69% (58) |
30.99% (22) 19.72% (14) 52.11% (37) 59.15% (42) 74.65% (53) |
0.579 0.565 0.127 0.238 0.310 |
Abbreviations: BMI = Body Mass Index; STEMI = ST-Elevation Myocardial Infarction; NSTEMI = Non-ST-Elevation Myocardial Infarction; UAP = Unstable Angina; Non-ACS = Non-Acute Coronary Syndrome; PCI = Percutaneous Coronary Intervention; CAD = Coronary Artery Disease; TIA = Transient Ischemic Attacks; EGFR = Estimates Glomerular Filtration Rate, based on MDRD equation; LVEF = Left Ventricular Ejection Fraction. * Dyslipidemia: LDL cholesterol more than 140 mg/dl or Total cholesterol more than 220 mg/dl or treated with medications.
3.2. Coronary Angiography Analysis
Before the PSM, Table 2 showed that most of the lesions in the two groups were in the LAD artery (68.42% in Group I and 70.21% in Group II, p = 0.763). Group II had a more significant percentage of lesions classified as type C according to the AHA/ACC classification (54.26% vs. 35.67%, p = 0.003). Group I had significantly more lesions with TIMI-2 (21.05% vs. 10.64%, p = 0.032) and significantly fewer lesions with TIMI-3 than Group II (63.16% vs. 76.60%, p = 0.025). Based on QCA analysis, the lesion length was longer in Group II compared to Group I (27.30 ± 9.94 vs. 23.95 ± 8.56, p = 0.005). However, compared to Group II, Group I has a greater lesion reference diameter (2.94 ± 0.59 vs. 2.67 ± 0.63, p = 0.001), proximal mean diameter (3.28 ± 0.56 vs. 3.04 ± 0.60, p = 0.002), and distal mean diameter (2.92 ± 0.64 vs. 2.75 ± 0.79, p = 0.047). Plaque symmetry and plaque area were comparable between the two groups. After the PSM, all QCA analysis parameters were equal across the two groups, except for the lesion reference diameter, which remained significantly smaller in Group II (2.70 ± 0.63 vs. 2.91 ± 0.58 in Group I, p = 0.049).
Table 2.
Coronary Angiography Findings.
| Non/Mild Calcification (n = 171) | Moderate/Severe Calcification (n = 94) | p | Matched Non/Mild Calcification (n = 81) | Matched Moderate/Severe Calcification (n = 72) | p | |
|---|---|---|---|---|---|---|
| Artery | 0.879 | 0.616 | ||||
| LAD | 68.42% (117) | 70.21% (66) | 0.763 | 67.90% (55) | 75.00% (54) | 0.333 |
| LCX | 11.11% (19) | 11.70% (11) | 0.884 | 9.88% (8) | 8.33% (6) | 0.741 |
| RCA | 19.30% (33) | 18.09% (17) | 0.809 | 22.22% (18) | 16.67% (12) | 0.388 |
| Left Main | 0.58% (1) | 0% (0) | 0.458 | 0% (0) | 0% (0) | N/A |
| Ramus | 0.58% (1) | 0% (0) | 0.458 | 0% (0) | 0% (0) | N/A |
| AHA/ACC Classification | 0.010 | 0.374 | ||||
| A | 1.17% (2) | 0% (0) | 0.293 | 1.23% (1) | 0% (0) | 0.344 |
| B1 | 14.04% (24) | 5.32% (5) | 0.030 | 12.35% (10) | 5.56% (4) | 0.146 |
| B2 | 49.12% (84) | 40.43% (38) | 0.174 | 41.98% (34) | 44.44% (32) | 0.758 |
| C | 35.67% (61) | 54.26% (51) | 0.003 | 44.44% (36) | 50.0% (36) | 0.492 |
| TIMI Grade | 0.091 | 0.398 | ||||
| TIMI-0 | 4.68% (8) | 2.13% (2) | 0.297 | 4.94% (4) | 2.78% (2) | 0.492 |
| TIMI-1 | 11.11% (19) | 10.64% (10) | 0.906 | 7.41% (6) | 12.50% (9) | 0.290 |
| TIMI-2 | 21.05% (36) | 10.64% (10) | 0.032 | 18.52% (15) | 11.11% (8) | 0.201 |
| TIMI-3 | 63.16% (108) | 76.60% (72) | 0.025 | 69.14% (56) | 73.61% (53) | 0.542 |
| Total Occlusion | 4.09% (7) | 6.38% (6) | 0.409 | 4.94% (4) | 8.33% (6) | 0.396 |
| Chronic total occlusion | 3.51% (6) | 5.32% (5) | 0.480 | 3.70% (3) | 6.94% (5) | 0.369 |
| Ostium Lesion | 1.75% (3) | 2.13% (2) | 0.831 | 2.47% (2) | 2.78% (2) | 0.905 |
| QCA Analysis | ||||||
| Lesion Diameter (mm) | 1.03 ± 0.38 | 0.95 ± 0.37 | 0.094 | 0.97 ± 0.37 | 0.94 ± 0.38 | 0.611 |
| Lesion Reference Diameter (mm) | 2.94 ± 0.59 | 2.67 ± 0.63 | 0.001 | 2.91 ± 0.58 | 2.70 ± 0.63 | 0.049 |
| Diameter Stenosis (%) | 66.23 ± 12.76 | 66.85 ± 14.02 | 0.718 | 67.66 ± 13.96 | 68.17 ± 14.86 | 0.828 |
| Lesion Area (mm2) | 0.82 (0.43,1.24) | 0.70 (0.35,1.13) | 0.157 | 0.74 (0.38,1.11) | 0.62 (0.32,1.16) | 0.603 |
| Lesion Reference Area (mm2) | 6.45 (5.20,8.49) | 5.50 (3.98,7.23) | 0.001 | 6.27 (5.20,7.37) | 5.70 (3.88,7.26) | 0.077 |
| Area Stenosis (%) | 87.98 ± 7.76 | 87.07 ± 7.97 | 0.931 | 87.61 ± 8.20 | 87.69 ± 8.28 | 0.956 |
| Lesion Length (mm) | 23.95 ± 8.56 | 27.30 ± 9.94 | 0.005 | 24.83 ± 9.12 | 27.16 ± 9.18 | 0.132 |
| Proximal Mean Diameter (mm) | 3.28 ± 0.56 | 3.04 ± 0.60 | 0.002 | 3.25 ± 0.54 | 3.08 ± 0.61 | 0.072 |
| Lesion Mean Diameter (mm) | 2.30 ± 0.47 | 2.10 ± 0.44 | 0.001 | 2.24 ± 0.43 | 2.14 ± 0.44 | 0.160 |
| Distal Mean Diameter (mm) | 2.92 ± 0.64 | 2.75 ± 0.69 | 0.047 | 2.89 ± 0.64 | 2.76 ± 0.72 | 0.257 |
| Proximal Mean Area (mm2) | 3.27 ± 0.65 | 3.10 ± 0.64 | 0.049 | 3.28 ± 0.58 | 3.15 ± 0.66 | 0.212 |
| Lesion Mean Area (mm2) | 2.31 ± 0.49 | 2.12 ± 0.45 | 0.003 | 2.27 ± 0.45 | 2.16 ± 0.45 | 0.178 |
| Distal Mean Area (mm2) | 2.95 ± 0.77 | 2.84 ± 0.84 | 0.288 | 2.99 ± 0.89 | 2.89 ± 0.90 | 0.503 |
| Plaque Symmetry | 0.70 (0.49,0.87) | 0.67 (0.56,0.82) | 0.856 | 0.76 (0.50,0.87) | 0.65 (0.56,0.81) | 0.211 |
| Plaque Area (mm2) | 13.44 (9.95,18.75) | 13.53 (10.25,18.65) | 0.766 | 13.79 (9.77,20.15) | 12.97 (10.35,18.14) | 0.592 |
Abbreviations: LAD = Left Anterior Descending, LCX = Left Circumflex, RCA = Right Coronary Artery, AHA/ACC = American Heart Association/American College of Cardiology, TIMI = Thrombolysis In Myocardial Infarction, QCA = Quantitative Coronary Angiography.
3.3. Percutaneous Coronary Intervention-Related Findings
Table 3 demonstrates that before the PSM, femoral artery access was utilized in more Group II lesions than Group I (30.85% vs. 14.62%, with p = 0.002). Concerning the type of balloon utilized for pre-dilation, Group II had more patients using scoring balloons (3.19% vs. 0% in Group I with p = 0.019). In Group I, pre-dilation balloons were longer but had a lower maximum pressure than in Group II (17.51 ± 2.53 vs. 16.61 ± 2.68 with p = 0.008 and 11.62 ± 3.03 vs. 12.48 ± 2.93 with p = 0.029, respectively). Plaque modification was utilized by 4.26% of lesions in Group II, compared to 0% in Group I with p = 0.007. After the PSM, the utilization rate of femoral vascular access was greater in Group II (30.56%) than in Group I (14.81%) with p = 0.019. There were no differences in balloon type, length, or maximum pressure for pre-dilation balloons between the two groups following PSM.
Table 3.
PCI-related findings.
| Non/Mild Calcification (n = 171) | Moderate/ Severe Calcification (n = 94) |
p | Matched Non/Mild Calcification (n = 81) | Matched Moderate/Severe Calcification (n = 72) | p | |
|---|---|---|---|---|---|---|
| Vascular Access | 0.002 | 0.019 | ||||
| Radial | 85.38% (146) | 69.15% (65) | 85.19% (69) | 69.44% (50) | ||
| Femoral | 14.62% (25) | 30.85% (29) | 14.81% (12) | 30.56% (22) | ||
| Pre-dilation | 97.08% (166) | 97.87% (92) | 0.699 | 98.77% (80) | 98.61% (71) | 0.933 |
| Pre-Stent-Balloon type | 0.103 | 0.269 | ||||
| Semi-compliant balloon | 94.74% (162) | 90.43% (85) | 0.182 | 97.53% (79) | 90.28% (65) | 0.057 |
| Non-compliant balloon | 2.34% (4) | 3.19% (3) | 0.679 | 1.23% (1) | 2.78% (2) | 0.492 |
| Stent-balloon | 0% (0) | 1.06% (1) | 0.177 | 0% (0) | 1.39% (1) | 0.287 |
| Scoring balloon | 0% (0) | 3.19% (3) | 0.019 | 0% (0) | 4.17% (3) | 0.064 |
| Pre-dilation balloon diameter size (mm) | 2.57 ± 0.37 | 2.64 ± 0.38 | 0.208 | 2.58 ± 0.35 | 2.62 ± 0.39 | 0.457 |
| Pre-dilation balloon length (mm) | 17.51 ± 2.53 | 16.61 ± 2.68 | 0.008 | 17.24 ± 2.52 | 17.01 ± 2.88 | 0.612 |
| Pre-dilation balloon maximum pressure (atm) | 11.62 ± 3.03 | 12.48 ± 2.93 | 0.029 | 11.96 ± 3.12 | 12.28 ± 3.11 | 0.531 |
| Pre-dilation balloon maximum diameter (mm) | 2.68 ± 0.37 | 2.76 ± 0.38 | 0.101 | 2.69 ± 0.32 | 2.74 ± 0.39 | 0.420 |
| Plaque modification | 0% (0) | 4.26% (4) | 0.007 | 0% (0) | 4.17% (3) | 0.064 |
| Rotablator | 0% (0) | 1.06% (1) | 0.177 | 0% (0) | 0% (0) | N/A |
| Scoring balloon | 0% (0) | 3.19% (3) | 0.019 | 0% (0) | 4.17% (3) | 0.064 |
| Stent diameter size (mm) * | 3.35 ± 0.51 | 3.21 ± 0.50 | 0.034 | 3.28 ± 0.47 | 3.25 ± 0.53 | 0.673 |
| Stent length size (mm) * | 26.00 (22.00,34.00) | 30.00 (22.00,34.00) | 0.062 | 26.00 (22.00,34.00) | 30.00 (26.00,37.00) | 0.113 |
| Number of stents per lesion | 1.07 ± 0.26 | 1.16 ± 0.37 | 0.038 | 1.09 ± 0.28 | 1.14 ± 0.35 | 0.306 |
| Total stent length (mm) | 26.00 (22.00,34.00) | 30.00 (22.00,38.00) | 0.010 | 26.00 (22.00,34.00) | 30.00 (26.00,38.00) | 0.062 |
| Stent-balloon maximum pressure at deployment (atm) * | 13.99 ± 2.84 | 13.18 ± 2.58 | 0.022 | 13.62 ± 2.85 | 13.35 ± 2.77 | 0.554 |
| Stent maximum diameter at deployment (mm) * | 3.42 ± 0.52 | 3.24 ± 0.52 | 0.008 | 3.34 ± 0.47 | 3.28 ± 0.56 | 0.487 |
| Post-dilation | 62.57% (107) | 77.66% (73) | 0.012 | 65.43% (53) | 80.56% (58) | 0.036 |
| Post-dilation balloon type | 0.072 | 0.151 | ||||
| Semi-compliant balloon | 1.75% (3) | 3.19% (3) | 0.452 | 1.23% (1) | 1.39% (1) | 0.933 |
| Non-compliant balloon | 57.89% (99) | 72.34% (68) | 0.020 | 64.20% (52) | 77.78% (56) | 0.066 |
| Stent-balloon | 2.92% (5) | 2.13% (2) | 0.699 | 0% (0) | 1.39% (1) | 0.287 |
| Post-dilation balloon diameter size (mm) | 3.61 ± 0.53 | 3.39 ± 0.44 | 0.005 | 3.58 ± 0.51 | 3.43 ± 0.44 | 0.100 |
| Post-dilation balloon length (mm) | 12.32 ± 3.92 | 12.84 ± 3.76 | 0.377 | 11.42 ± 3.55 | 12.83 ± 3.82 | 0.047 |
| Post-dilation balloon maximum pressure (atm) | 16.70 ± 3.67 | 16.38 ± 3.31 | 0.554 | 16.72 ± 4.00 | 16.34 ± 2.93 | 0.575 |
| Post-dilation balloon maximum diameter (mm) | 3.71 ± 0.52 | 3.48 ± 0.44 | 0.003 | 3.69 ± 0.50 | 3.52 ± 0.45 | 0.072 |
* If a lesion was implanted with multiple stents, stent diameter size, stent length size, stent maximum pressure at deployment, and stent maximum diameter at deployment were derived from the parameters of the stent with the largest diameter size.
In terms of stent information, prior to the PSM, Group I had greater stent diameter size (3.35 ± 0.51 vs. 3.21 ± 0.50, p = 0.034), stent-balloon maximum pressure (13.99 ± 2.84 vs. 13.18 ± 2.58, p = 0.022), and stent maximum diameter at deployment (3.42 ± 0.52 vs. 3.24 ± 0.52, p = 0.008), but Group I had shorter total stent length (26.00 (22.00,34.00) vs. 30.00 (22.00,38.00), p = 0.010) and fewer number of stents per lesion (1.07 ± 0.26 vs. 1.16 ± 0.37, p = 0.038). Post-dilation was performed on 77.66% of lesions in Group II, which is a significantly higher percentage than the 62.57% in Group I (p = 0.012). The percentage of lesions in Group II, which used an N.C. balloon for post-dilation (72.34%), was significantly higher than those in Group I (57.89%) with p = 0.020. The post-dilation balloon diameter size and the maximum diameter were found to be significantly higher in Group I (3.61 ± 0.53 vs. 3.39 ± 0.44, and 3.71 ± 0.52 vs. 3.48 ± 0.44, respectively, with p < 0.01 for both comparisons). Following the PSM, all of the parameters associated with the stent were comparable between the two groups. All post-dilation-related parameters were comparable in both groups, except that the lesions in Group II used post-dilation more frequently (80.56% vs. 65.43% in Group I, p = 0.036), and post-dilation balloon length in Group II was significantly longer (12.83 ± 3.82 vs. 11.42 ± 3.55 in Group I, p = 0.047).
3.4. IVUS Images Analysis
Table 4 shows all IVUS measurements pre- and post-intervention. Prior to the PSM, pre-intervention IVUS revealed that the minimum luminal area was smaller in Group II (2.44 ± 0.67 mm2 vs. 2.67 ± 0.90 mm2, p = 0.019). Group II also exhibited a more significant plaque burden (81.34% (78.45,85.29) vs. 79.83% (74.57,83.41), p = 0.006) and a longer lesion length (30.88 ± 11.09 vs. 26.76 ± 9.73, p = 0.002) than Group I. At the reference sites, Group I had a greater luminal area and a lower plaque burden than Group II. All calcium morphology-related measures, including maximum calcium arc, calcium length, calcium index, calcium nodule, super calcium, and deep calcium were greater in Group II than in Group I. After the PSM, both groups exhibited equivalent luminal area at the minimal luminal area site (2.69 ± 0.91 vs. 2.49 ± 0.69, p = 0.120) and the proximal reference (11.73 ± 4.68 vs. 10.85 ± 4.54, p = 0.244). Group II also had greater plaque burdens at the minimal luminal area site (81.43% (78.40,85.59) vs. 80.27% (73.67,84.00), p = 0.041), the proximal reference (45.15 ± 13.47 vs. 38.86 ± 12.82, p = 0.004), and the distal reference sites (35.89 ± 14.14 vs. 30.45 ± 11.14, p = 0.009) than Group I. Group II continued to have greater values for all morphological calcification criteria compared to Group I even after the PSM was applied.
Table 4.
IVUS findings.
| Non/Mild Calcification (n = 171) | Moderate/Severe Calcification (n = 94) | p | Matched Non/Mild Calcification (n = 81) | Matched Moderate/Severe Calcification (n = 72) | p | |
|---|---|---|---|---|---|---|
| Pre-PCI IVUS | ||||||
| Minimum luminal area site | ||||||
| Luminal Area (mm2) | 2.67 ± 0.90 | 2.44 ± 0.67 | 0.019 | 2.69 ± 0.91 | 2.49 ± 0.69 | 0.120 |
| Vessel Area (mm2) | 13.56 ± 4.92 | 13.74 ± 4.59 | 0.772 | 13.94 ± 4.65 | 14.23 ± 4.80 | 0.702 |
| Plaque Burden (%) | 79.83 (74.57,83.41) | 81.34 (78.45,85.29) | 0.006 | 80.27 (73.67,84.00) | 81.43 (78.40,85.59) | 0.041 |
| Remodeling Index | 0.86 ± 0.23 | 0.89 ± 0.21 | 0.294 | 0.88 ± 0.22 | 0.90 ± 0.21 | 0.565 |
| Lesion Length (mm) | 26.76 ± 9.73 | 30.88 ± 11.09 | 0.002 | 27.88 ± 9.44 | 31.56 ± 11.27 | 0.029 |
| Proximal reference site Luminal Area (mm2) Vessel Area (mm2) Plaque Burden (%) |
11.82 ± 4.53 18.75 ± 5.7 36.98 ± 12.40 |
10.41 ± 4.23 18.76 ± 5.35 44.78 ± 12.98 |
0.014 0.985 0.001 |
11.73 ± 4.68 19.23 ± 6.19 38.86 ± 12.82 |
10.85 ± 4.54 19.49 ± 5.29 45.15 ± 13.47 |
0.244 0.780 0.004 |
| Distal reference site Luminal Are (mm2) Vessel Area (mm2) Plaque burden (%) |
8.21 (6.27,11.03) 13.20 ± 5.70 30.46 ± 10.97 |
7.04 (5.33,10.18) 12.64 ± 5.43 35.05 ± 14.46 |
0.012 0.435 0.004 |
8.15 (6.41,10.62) 13.18 ± 5.42 30.45 ± 11.14 |
7.04 (5.18,10.38) 12.83 ± 5.70 35.89 ± 14.14 |
0.034 0.702 0.009 |
| Mean Reference Luminal Area (mm2) | 10.45 ± 3.88 | 9.19 ± 3.49 | 0.010 | 10.40 ± 3.92 | 9.43 ± 3.71 | 0.119 |
| Volumetric analysis | ||||||
| Mean luminal area (mm3/mm) | 5.89 (4.70,7.68) | 5.04 (4.00,5.96) | <0.001 | 5.82 (4.77,7.27) | 5.16 (4.04,6.26) | 0.028 |
| Mean vessel area (mm3/mm) | 14.98 ± 4.98 | 14.87 ± 4.35 | 0.856 | 14.97 ± 4.47 | 15.39 ± 4.48 | 0.567 |
| Plaque burden (%) | 56.80 ± 8.44 | 63.47 ± 7.28 | <0.001 | 57.56 ± 9.28 | 63.91 ± 7.25 | <0.001 |
| Maximum calcium arc (o) | 86.70 (43.90,139.20) | 266.80 (226.28,360.00) | <0.001 | 96.30 (67.20,148.05) | 263.95 (222.58,360.00) | <0.001 |
| Calcium length (mm) | 6.10 (2.10,13.10) | 20.30 (14.23,26.55) | <0.001 | 7.50 (3.30,12.75) | 21.65 (15.20,26.50) | <0.001 |
| Calcium Index * | 10.06 ± 11.22 | 56.63 ± 24.24 | <0.001 | 11.61 ± 12.23 | 55.90 ± 23.34 | <0.001 |
| Superficial Calcium | 80.12% (137) | 100.0% (94) | <0.001 | 88.89% (72) | 100.0% (72) | 0.004 |
| Deep Calcium | 7.60% (13) | 26.60% (25) | <0.001 | 7.41% (6) | 25.00% (18) | 0.003 |
| Calcium nodule | 6.43% (11) | 23.40% (22) | <0.001 | 9.88% (8) | 25.00% (18) | 0.013 |
| Plaque rupture | 15.20% (26) | 39.36% (37) | <0.001 | 18.52% (15) | 45.83% (33) | <0.001 |
| Attenuate Plaque | 71.35% (122) | 79.79% (75) | 0.132 | 74.07% (60) | 79.17% (57) | 0.459 |
| POST-PCI IVUS | ||||||
| Minimum stent area site | ||||||
| Minimum stent area (mm2) | 6.68 ± 2.34 | 5.71 ± 2.04 | 0.001 | 6.42 ± 2.10 | 5.89 ± 2.15 | 0.126 |
| Vessel area at MSA (mm2) | 15.02 ± 5.07 | 14.71 ± 5.06 | 0.643 | 14.83 ± 4.91 | 14.95 ± 5.30 | 0.891 |
| Maximum stent diameter (mm) | 3.17 ± 0.56 | 2.96 ± 0.54 | 0.04 | 3.11 ± 0.51 | 3.02 ± 0.56 | 0.286 |
| Minimum stent diameter (mm) | 2.58 ± 0.46 | 2.36 ± 0.44 | 0.001 | 2.54 ± 0.43 | 2.39 ± 0.44 | 0.028 |
| Stent asymmetry | 0.17 (0.12,0.23) | 0.17 (0.13,0.25) | 0.248 | 0.17 (0.12,0.22) | 0.19 (0.14,0.26) | 0.060 |
| Stent eccentricity | 0.83 (0.77,0.88) | 0.83 (0.75,0.87) | 0.248 | 0.83 (0.78,0.88) | 0.81 (0.74,0.86) | 0.060 |
| Mean stent area (mm2) | 8.16 (6.63,11.07) | 7.39 (5.87,9.85) | 0.055 | 7.70 (6.47,11.21) | 8.22 (5.94,10.33) | 0.642 |
| Conventional stent expansion | 65.91 ± 14.71 | 64.38 ± 13.86 | 0.127 | 63.94 ± 13.83 | 65.11 ± 14.19 | 0.607 |
| MSA/MVA at MSA site (%) | 45.42 ± 9.07 | 38.83 ± 8.89 | <0.001 | 44.56 ± 9.44 | 40.41 ± 8.67 | 0.006 |
| IVUS-XPL * trial stent expansion criteria | 11.70% (20) | 11.70% (11) | 0.999 | 6.17% (5) | 13.89% (10) | 0.109 |
| ULTIMATE * trial stent expansion criteria | 74.27% (127) | 57.45% (54) | 0.005 | 70.37% (57) | 63.89% (46) | 0.394 |
| MSA > 5.5 mm2 | 66.08% (113) | 41.49% (39) | 0.006 | 65.43% (53) | 45.83% (33) | 0.015 |
| MSA/Average reference lumen area > 80% | 17.54% (30) | 13.83% (13) | 0.433 | 13.58% (11) | 15.28% (11) | 0.765 |
| Stent malapposition | 24.56% (42) | 45.74% (43) | <0.001 | 24.69% (20) | 52.78% (38) | <0.001 |
| Major Stent Malapposition | 12.28% (21) | 18.09% (17) | 0.197 | 11.11% (9) | 22.22% (16) | 0.064 |
| Minor Stent Malapposition | 12.28% (21) | 27.66% (26) | 0.002 | 13.58% (11) | 30.56% (22) | 0.011 |
| Tissue protrusion | 1.75% (3) | 1.06% (1) | 0.659 | 2.47% (2) | 1.39% (1) | 0.630 |
| Stent Edge Dissection | 1.17% (2) | 1.06% (1) | 0.938 | 1.23% (1) | 1.39% (1) | 0.933 |
Abbreviations: MSA = Minimum Stent Area, ULTIMATE criteria = MSA > 5.5 mm2 or >90% distal lumen reference area, IVUS-XPL criteria = MSA > distal lumen reference area, Conventional stent expansion = MSA/(Average reference lumen area) × 100, Calcium Index = (maximum calcium arc/360) × (total calcium length/lesion length) × 100, Major Stent Malapposition: axial distance > 0.4 mm or >1 mm in length.
Prior to the PSM, compared to Group I, the IVUS examination after PCI revealed that Group II had a smaller MSA (5.71 ± 2.04 vs. 6.68 ± 2.34, p = 0.001), minimum stent diameter (2.36 ± 0.44 vs. 2.58 ± 0.46, p = 0.001), and maximum stent diameter (2.96 ± 0.54 vs. 3.17 ± 0.56, p = 0.04). After PSM, only Group II’s minimum stent diameter remained smaller (2.39 ± 0.44 vs. 2.54 ± 0.43, p = 0.028).
Regarding the stent expansion rate, before the PSM, Group II had a smaller stent expansion rate based on the MSA/MVA at the MSA site (38.83 ± 8.89 vs. 45.42 ± 9.07, p < 0.001), absolute MSA > 5.5 mm2 (41.49% vs. 66.08%, p = 0.006), and ULTIMATE criteria (57.45% vs. 74.27%, p = 0.005). Recent criteria, such as conventional criteria or IVUS-XPL criteria, revealed no difference in the expansion rate of stents between the two groups. Following the PSM, while the percentage of lesions achieving the MSA/MVA at the MSA site and MSA > 5.5 mm2 criteria was still lower in Group II (40.41 ± 8.67 vs. 44.56 ± 9.44 with p = 0.006 and 45.83% vs. 65.43% with p = 0.015, respectively), the percentage of lesions achieving the other criteria was comparable between the two groups.
Before the PSM, the percentage of lesions with malapposition was greater in Group II (45.74% vs. 24.56%, p < 0.001). Still, the proportion of lesions with major malapposition did not significantly differ between the two groups (12.28% vs. 18.09%, p = 0.197). After the PSM, the percentage of lesions with major malapposition was not significantly different between the two groups (11.11% vs. 22.22%, p = 0.064). Other complications, including tissue protrusion and stent edge dissection, were comparable across the two groups both before and after the PSM.
3.5. Clinical Endpoints
The cardiac events that occurred in both Groups during the follow-up period are detailed in Table 5. The mean follow-up duration was 18.6 ± 5.18 months. Before the PSM, the MACE (composite of cardiac death, MI, and TLR) was 1.99% in Group I, which was not significantly different from Group II with 1.09% with p = 0.594. Secondary outcomes, including Patient-oriented composite endpoint, all-cause mortality or components of MACE (cardiac death, MI, and TLR), TVR, Stroke, and Stent thrombosis, were comparable between the two groups. After the PSM, the MACE rates in Group I were 0%, comparable with 1.41% in Group II with p = 0.317. The secondary endpoints remained not significantly different between the two groups.
Table 5.
MACE after one-year follow-up.
| Non/Mild Calcification (n = 151) | Moderate/Severe Calcification (n = 92) | p | Matched Non/Mild Calcification (n = 71) | Matched Moderate/Severe Calcification (n = 71) | p | |
|---|---|---|---|---|---|---|
| MACE | 1.99% (3) | 1.09% (1) | 0.594 | 0% (0) | 1.41% (1) | 0.317 |
| Cardiac Death | 0.66% (1) | 0% (0) | 0.435 | 0% (0) | 0% (0) | N/A |
| MI | 0% (0) | 0% (0) | N/A | 0% (0) | 0% (0) | N/A |
| TLR | 1.32% (2) | 1.09% (1) | 0.870 | 0% (0) | 1.41% (1) | 0.317 |
| POCE | 5.96% (9) | 3.26% (3) | 0.303 | 7.04% (5) | 2.82% (2) | 0.244 |
| All-cause Death | 4.64% (7) | 2.17% (2) | 0.276 | 7.04% (5) | 1.41% (1) | 0.092 |
| Stroke | 1.32% (2) | 0% (0) | 0.269 | 1.41% (1) | 0% (0) | 0.317 |
| TVR | 1.32% (2) | 0% (0) | 0.266 | 0% (0) | 0% (0) | N/A |
| OVR | 1.99% (3) | 4.35% (4) | 0.343 | 4.23% (3) | 4.23% (3) | 0.965 |
| CHF | 0.66% (1) | 1.09% (1) | 0.723 | 0% (0) | 1.41% (1) | 0.317 |
| Stent Thrombosis | 0% (0) | 0% (0) | N/A | 0% (0) | 0% (0) | N/A |
Abbreviations: MI = Myocardial Infarction, TLR = Target Lesion Revascularization, TVR = Target Vessel Revascularization, OVR = Other Vessel Revascularization, CHF = Chronic Heart Failure, MACE = Cardiac death or MI or TLR, POCE = All-cause death or MI or TLR or TVR.
4. Discussion
After exclusion, 243 patients with 265 lesions in the CAPIRO study population were evaluated. In our investigation, we categorized the patients into two groups, with Group II having moderate/severe calcification based on a maximum calcium arc >180° and calcium length >5 mm. Maximum calcium arc >180° and calcium length >5 mm were independent indicators of the stent under expansion and worse clinical outcomes based on intravascular imaging [13,28,33]. Regarding the baseline characteristics, there were substantial differences between the two groups in terms of age, male gender, diabetes mellitus, current smoking, and chronic kidney disease. We performed the PSM for these factors to eliminate the bias of baseline features since these characteristics could affect the rate of calcified lesions as well as cardiac events [7]. All of the baseline characteristics of the two groups were equivalent following the PSM.
In this prospective, multi-center study, the proportion of patients with moderate/severe calcification lesions was 37.86%. With IVUS-guided PCI, the major adverse cardiac events (MACE) rate was comparable between the two groups (1.99% vs. 1.09%, p = 0.594 before the PSM and 0% vs. 1.41%, p = 0.317 after the PSM) at a mean follow-up of 18.6 months. There was no difference in MACE between patients with moderate/severe calcification lesions and those with non/mild calcification lesions. Both groups had similar rates of MACE components such as cardiac death, stroke, MI, TLR, and TVR. These findings show that IVUS-guided PCI could improve intervention outcomes in patients with calcified lesions, which is not inferior to lesions without calcifications. Recent studies comparing IVUS-guided PCI to angiography-guided PCI have revealed favorable outcomes. Several observational studies and meta-analyses involving a greater number of patients indicated that IVUS-guided PCI in patients with complex lesions was linked with a decreased mortality rate and major adverse cardiac events [18,34]. The use of IVUS in PCI with CTO lesions also showed that patients with IVUS-guided post-stent optimization had a lower rate of TLR/reocclusion than patients without IVUS [19]. In 2023, a prospective, multi-center study showed that in PCI with complex lesions, imaging-guided PCI was associated with a lower incidence of target lesion failure, including cardiac death, myocardial infarction, and target vessel revascularization [35]. In 2019, the MACE-trial study, which is a prospective study, evaluated the impact of calcified lesions on the outcomes of PCI. According to this study’s findings, moderate calcification had similar outcomes compared with non/mild calcification; however, severe calcification still had a negative impact on PCI outcomes [6]. Therefore, using a new-generation stent system and new devices to prepare lesions before implanting the stent could improve the outcomes of PCI. The main results of our study showed that IVUS-guided PCI in calcified lesions could lead to better outcomes, which is comparable with the outcomes in patients with non-complex lesions treated with IVUS-guided PCI.
In order to minimize the impact of diverse stent systems on the outcomes, our study utilized a single type of second-generation DES for each patient. Second-generation DES was linked with a decreased rate of patient-oriented endpoint or target lesion failure in individuals with coronary artery calcification compared to first-generation DES [5]. However, the rate of target lesion failure and stent thrombosis was greater in patients with moderate or severe calcification lesions than in patients with non-calcified or mild calcification lesions [6,36]. In our study, the outcomes of patients with moderate/severe calcification were equivalent to those of patients with no/mild calcification, indicating that IVUS guidance with second-generation DES could improve PCI outcomes, even in calcified lesions.
Concerning the stent expansion rate, the MSA in Group I (6.68 ± 2.34 mm2) was larger than in Group II (5.71 ± 2.04 mm2) with p < 0.001; however, following the PSM, this difference (6.42 ± 2.10 vs. 5.89 ± 2.15) was not significant with p = 0.126. Group II had a smaller minimum stent diameter than Group I before and after PSM with p < 0.05. Comparing the stent expansion rate by recent expansion criteria, based on MSA > 5.5 mm2 and MSA/MVA at the MSA site criteria, Group I had more lesions that achieved these criteria than Group II. Fujimura et al. (2019) compared numerous stent expansion rate criteria. They suggested that compared to absolute MSA and other expansion indexes, the MSA/MVA at the MSA site could be independently associated with an increased rate of TLR and stent thrombosis after two years [25]. However, in our study, more than 30% of lesions had moderate to severe calcification, and the ultrasound could not penetrate the calcium; therefore, the measurement of MVA in calcified lesions by IVUS could be imprecise. Therefore, the MSA/MVA at the MSA site criterion was not appropriate for comparing the expansion rate in our investigation. Based on IXUS-XPL criteria, the number of lesions that achieved the criteria was comparable between both groups. In the IVUS-XPL trial, 54% of patients achieved the expansion criteria, and these patients had a significantly lower rate of MACE compared to patients who did not meet the criteria [23]. The proportion of lesions achieved IVUS-XPL in our study was lower than in the IVUS-XPL trial but comparable with results from the study of Fujimura et al. (2021) [25]. In our study, we analyzed all “all-comer” patients, so we think the ULTIMATE criteria should be suitable to compare the expansion rate. Based on the ULTIMATE criteria, before PSM, Group I had more lesions that achieved this criterion compared to Group II, but after PSM, there was no significant difference. Although the expansion rate based on different expansion criteria was heterogeneous, in our study, with the guidance of IVUS and the use of second-generation DES, the expansion rate and the outcome of patients with moderate/severe calcification were comparable with patients with non/mild calcification. Moreover, in our study, a significant proportion of procedures that use non-compliant balloons for optimizing the stent expansion contribute to a better stent expansion rate and clinical outcomes.
However, interpreting the results of our study should be concerned with these limitations: (1) Although the prospective, multi-center study, the CAPIRO study patient population was small; therefore, the number of cardiac events was still low compared to other randomized trials. (2) In our study, we included “all-comer” patients, including all ACS or non-ACS patients, with complex or non-complex lesions. Although this patient recruitment method is suitable for real clinical practice, the heterogeneity in baseline and lesion characteristics should be considered. (3) In 300 patients from the CAPIRO study, we excluded 50 patients who met the exclusion criteria for IVUS image analysis. The exclusion of a significant proportion of patients could have a considerable effect on the results of our study. (4) In patients with moderate/severe calcification, only about 4% were treated with plaque modification methods before implanting the stent. Therefore, in our study, we could not assess the role of plaque modification methods in the procedure outcomes. (5) Although we address the cardiac events for more than one-year follow-up, to assess the long-term benefits of IVUS-guided PCI, the follow-up time will be increased to 3 years.
5. Conclusions
Through this prospective, multi-center study, IVUS-guided PCI in calcified lesions was not inferior compared to PCI in non/mild calcified lesions. Although the expansion rate based on some criteria was lower in groups with moderate/severe calcification, other criteria showed that the expansion rate was comparable between both groups. The clinical outcomes after mid-term follow-up were similar between patients with moderate/severe calcification and patients with none/mild calcification. However, future study with a larger population and longer follow-up time is needed to confirm the role of IVUS-guided PCI in calcified lesions.
Author Contributions
Conceptualization, S.-R.L.; methodology, K.H.Y., J.-P.P., S.K.O. and S.-R.L.; software, K.-H.D., T.-L.L. and W.-S.Y.; formal analysis, K.-H.D., T.-L.L., W.-S.Y., S.K.O. and J.Y.R.; investigation, Y.-S.K., S.K.O. and J.Y.R.; data curation, K.-H.D. and W.-S.Y.; writing—original draft preparation, K.-H.D.; writing—review and editing, Y.-S.K., K.H.Y., J.-P.P. and S.-R.L.; supervision, K.H.Y., J.Y.R. and S.-R.L.; project administration, S.-R.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of Jeonbuk National University Hospital Institutional Review Board, number 2018-03-050; and registered to Cris.nih.go.kr (accessed on 7 April 2021), number KCT0006069.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
This article presents most of the study’s data in tables and figures. Additional data related to this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Madhavan M.V., Kirtane A.J., Redfors B., Généreux P., Ben-Yehuda O., Palmerini T., Benedetto U., Biondi-Zoccai G., Smits P.C., von Birgelen C., et al. Stent-Related Adverse Events >1 Year After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2020;75:590–604. doi: 10.1016/j.jacc.2019.11.058. [DOI] [PubMed] [Google Scholar]
- 2.De Maria G.L., Scarsini R., Banning A.P. Management of Calcific Coronary Artery Lesions: Is it Time to Change Our Interventional Therapeutic Approach? JACC Cardiovasc. Interv. 2019;12:1465–1478. doi: 10.1016/j.jcin.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 3.Lee M.S., Yang T., Lasala J., Cox D. Impact of coronary artery calcification in percutaneous coronary intervention with paclitaxel-eluting stents: Two-year clinical outcomes of paclitaxel-eluting stents in patients from the ARRIVE program. Catheter. Cardiovasc. Interv. 2016;88:891–897. doi: 10.1002/ccd.26395. [DOI] [PubMed] [Google Scholar]
- 4.Kim M.C., Ahn Y., Sim D.S., Hong Y.J., Kim J.H., Jeong M.H., Gwon H.C., Kim H.S., Rha S.W., Yoon J.H., et al. Impact of calcified bifurcation lesions in patients undergoing percutaneous coronary intervention using drug-eluting stents: Results from the COronary BIfurcation Stent (COBIS) II registry. EuroIntervention. 2017;13:338–344. doi: 10.4244/EIJ-D-16-00264. [DOI] [PubMed] [Google Scholar]
- 5.Guedeney P., Claessen B.E., Mehran R., Mintz G.S., Liu M., Sorrentino S., Giustino G., Farhan S., Leon M.B., Serruys P.W., et al. Coronary Calcification and Long-Term Outcomes According to Drug-Eluting Stent Generation. JACC Cardiovasc. Interv. 2020;13:1417–1428. doi: 10.1016/j.jcin.2020.03.053. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S.K., Bolduan R.W., Patel M.R., Martinsen B.J., Azemi T., Giugliano G., Resar J.R., Mehran R., Cohen D.J., Popma J.J., et al. Impact of calcification on percutaneous coronary intervention: MACE-Trial 1-year results. Catheter. Cardiovasc. Interv. 2019;94:187–194. doi: 10.1002/ccd.28099. [DOI] [PubMed] [Google Scholar]
- 7.Copeland-Halperin R.S., Baber U., Aquino M., Rajamanickam A., Roy S., Hasan C., Barman N., Kovacic J.C., Moreno P., Krishnan P., et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES: Findings from a large multiethnic registry. Catheter. Cardiovasc. Interv. 2018;91:859–866. doi: 10.1002/ccd.27204. [DOI] [PubMed] [Google Scholar]
- 8.Généreux P., Madhavan M.V., Mintz G.S., Maehara A., Palmerini T., Lasalle L., Xu K., McAndrew T., Kirtane A., Lansky A.J., et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes: Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trials. J. Am. Coll. Cardiol. 2014;63:1845–1854. doi: 10.1016/j.jacc.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Matsumura M., Mintz G.S., Lee T., Zhang W., Cao Y., Fujino A., Lin Y., Usui E., Kanaji Y., et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc. Imaging. 2017;10:869–879. doi: 10.1016/j.jcmg.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J., Liew D., Duffy S.J., Shaw J., Walton A., Chan W., Gerber R., Stub D. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: A Health Economic Analysis. Circ. Cardiovasc. Qual. Outcomes. 2021;14:E006789. doi: 10.1161/CIRCOUTCOMES.120.006789. [DOI] [PubMed] [Google Scholar]
- 11.Mehanna E., Dawn Abbott J., Bezerra H.G. Optimizing percutaneous coronary intervention in calcified lesions: Insights from optical coherence tomography of atherectomy. Circ. Cardiovasc. Interv. 2018;11:e006813. doi: 10.1161/CIRCINTERVENTIONS.118.006813. [DOI] [PubMed] [Google Scholar]
- 12.Mintz G.S. Intravascular Imaging of Coronary Calcification and its Clinical Implications. JACC Cardiovasc. Imaging. 2015;8:461–471. doi: 10.1016/j.jcmg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Fujino A., Mintz G.S., Matsumura M., Lee T., Kim S.Y., Hoshino M., Usui E., Yonetsu T., Haag E.S., Shlofmitz R.A., et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13:e2182–e2189. doi: 10.4244/EIJ-D-17-00962. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M., Matsumura M., Usui E., Noguchi M., Fujimura T., Fall K.N., Zhang Z., Nazif T.M., Parikh S.A., Rabbani L.R.E., et al. Intravascular Ultrasound-Derived Calcium Score to Predict Stent Expansion in Severely Calcified Lesions. Circ. Cardiovasc. Interv. 2021;14:e010296. doi: 10.1161/CIRCINTERVENTIONS.120.010296. [DOI] [PubMed] [Google Scholar]
- 15.Kereiakes D.J., Hill J.M., Shlofmitz R.A., Klein A.J., Riley R.F., Price M.J., Herrmann H.C., Bachinsky W., Waksman R., Stone G.W. Intravascular Lithotripsy for Treatment of Severely Calcified Coronary Lesions: 1-Year Results from the Disrupt CAD III Study. J. Soc. Cardiovasc. Angiogr. Interv. 2022;1:100001. doi: 10.1016/j.jscai.2021.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aksoy A., Salazar C., Becher M.U., Tiyerili V., Weber M., Jansen F., Sedaghat A., Zimmer S., Leick J., Grube E., et al. Intravascular Lithotripsy in Calcified Coronary Lesions: A Prospective, Observational, Multicenter Registry. Circ. Cardiovasc. Interv. 2019;12:e008154. doi: 10.1161/CIRCINTERVENTIONS.119.008154. [DOI] [PubMed] [Google Scholar]
- 17.Galougahi K., Shlofmitz E., Jeremias A., Gogia S., Kirtane A., Hill J., Karmpaliotis D., Mintz G., Meahara A., Stone G., et al. Therapeutic Approach to Calcified Coronary Lesions: Disruptive Technologies. Curr. Cardiol. Rep. 2021;23:33. doi: 10.1007/s11886-021-01458-7. [DOI] [PubMed] [Google Scholar]
- 18.Choi K.H., Song Y.B., Lee J.M., Lee S.Y., Park T.K., Yang J.H., Choi J.H., Choi S.H., Gwon H.C., Hahn J.Y. Impact of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention on Long-Term Clinical Outcomes in Patients Undergoing Complex Procedures. JACC Cardiovasc. Interv. 2019;12:607–620. doi: 10.1016/j.jcin.2019.01.227. [DOI] [PubMed] [Google Scholar]
- 19.Kwon O., Lee P.H., Lee S.W., Brilakis E.S., Lee J.Y., Yoon Y.H., Lee K., Park H., Kang S.J., Kim Y.H., et al. Clinical outcomes of post-stent intravascular ultrasound examination for chronic total occlusion intervention with drug-eluting stents. EuroIntervention. 2021;17:E639–E646. doi: 10.4244/EIJ-D-20-00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn J., Kang S., Yoon S., Park H., Kang S., Lee Y., Lee S., Kim Y., Lee C., Park S., et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am. J. Cardiol. 2014;113:1338–1347. doi: 10.1016/j.amjcard.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 21.Gao X.F., Ge Z., Kong X.Q., Kan J., Han L., Lu S., Tian N.L., Lin S., Lu Q.H., Wang X.Y., et al. 3-Year Outcomes of the ULTIMATE Trial Comparing Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021;14:247–257. doi: 10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J., Gao X., Kan J., Ge Z., Han L., Lu S., Tian N., Lin S., Lu Q., Wu X., et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J. Am. Coll. Cardiol. 2018;72:3126–3137. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Hong S.J., Kim B.K., Shin D.H., Nam C.M., Kim J.S., Ko Y.G., Choi D., Kang T.S., Kang W.C., Her A.Y., et al. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: The IVUS-XPL randomized clinical trial. JAMA J. Am. Med. Assoc. 2015;314:2155–2163. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 24.Lee Y.J., Zhang J.J., Mintz G.S., Hong S.J., Ahn C.M., Kim J.S., Kim B.K., Ko Y.G., Choi D., Jang Y., et al. Impact of Intravascular Ultrasound-Guided Optimal Stent Expansion on 3-Year Hard Clinical Outcomes. Circ. Cardiovasc. Interv. 2021;14:e011124. doi: 10.1161/CIRCINTERVENTIONS.121.011124. [DOI] [PubMed] [Google Scholar]
- 25.Fujimura T., Matsumura M., Witzenbichler B., Metzger D.C., Rinaldi M.J., Duffy P.L., Weisz G., Stuckey T.D., Ali Z.A., Zhou Z., et al. Stent Expansion Indexes to Predict Clinical Outcomes: An IVUS Substudy From ADAPT-DES. JACC Cardiovasc. Interv. 2021;14:1639–1650. doi: 10.1016/j.jcin.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Neumann F.J., Sousa-Uva M., Ahlsson A., Alfonso F., Banning A.P., Benedetto U., Byrne R.A., Collet J.P., Falk V., Head S.J., et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 27.Lawton J.S., Tamis-Holland J.E., Bangalore S., Bates E.R., Beckie T.M., Bischoff J.M., Bittl J.A., Cohen M.G., DiMaio J.M., Don C.W., et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022;79:e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Räber L., Mintz G.S., Koskinas K.C., Johnson T.W., Holm N.R., Onuma Y., Radu M.D., Joner M., Yu B., Jia H., et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14:656–677. doi: 10.4244/EIJY18M06_01. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y., Kobayashi Y., Fujii K., Sonoda S., Tsujita K., Hibi K., Morino Y., Okura H., Ikari Y., Honye J. Clinical expert consensus document on standards for measurements and assessment of intravascular ultrasound from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc. Interv. Ther. 2020;35:1–12. doi: 10.1007/s12928-019-00625-6. [DOI] [PubMed] [Google Scholar]
- 30.Mintz G.S., Nissen S.E., Co-Chairs W.D., Anderson S.R., Bailey R., Erbel F., Fitzgerald P.J., Pinto F.J., Rosenfield K., Siegel R.J., et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS) A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents Developed in Collaboration with the European Society of Cardiology Endorsed by the Society of Cardiac Angiography and Interventions Writing Committee Members Task Force Members. J. Am. Coll. Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki N., Asano T., Nakazawa G., Aoki J., Tanabe K., Hibi K., Ikari Y., Kozuma K. Clinical expert consensus document on quantitative coronary angiography from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc. Interv. Ther. 2020;35:105–116. doi: 10.1007/s12928-020-00653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Mickley H., Crea F., Van De Werf F., et al. Fourth universal definition of myocardial infarction (2018) Eur. Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 33.Sonoda S., Hibi K., Okura H., Fujii K., Honda Y., Kobayashi Y. Current clinical use of intravascular ultrasound imaging to guide percutaneous coronary interventions. Cardiovasc. Interv. Ther. 2020;35:30–36. doi: 10.1007/s12928-019-00603-y. [DOI] [PubMed] [Google Scholar]
- 34.Hannan E.L., Zhong Y., Reddy P., Jacobs A.K., Ling F.S.K., King S.B., Berger P.B., Venditti F.J., Walford G., Tamis-Holland J. Percutaneous Coronary Intervention with and Without Intravascular Ultrasound for Patients with Complex Lesions: Utilization, Mortality, and Target Vessel Revascularization. Circ. Cardiovasc. Interv. 2022;15:E011687. doi: 10.1161/CIRCINTERVENTIONS.121.011687. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.M., Choi K.H., Song Y.B., Lee J.-Y., Lee S.-J., Lee S.Y., Kim S.M., Yun K.H., Cho J.Y., Kim C.J., et al. Intravascular Imaging–Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023;388:1668–1679. doi: 10.1056/NEJMoa2216607. [DOI] [PubMed] [Google Scholar]
- 36.Hemetsberger R., Abdelghani M., Toelg R., Mankerious N., Allali A., Garcia-Garcia H.M., Windecker S., Lefèvre T., Saito S., Slagboom T., et al. Impact of coronary calcification on clinical outcomes after implantation of newer-generation drug-eluting stents. J. Am. Heart Assoc. 2021;10:e019815. doi: 10.1161/JAHA.120.019815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article presents most of the study’s data in tables and figures. Additional data related to this study are available upon request from the corresponding author.