Abstract
Modular megaprostheses (MPs) are commonly used after bone-tumor resection, but they can offer a limb salvage solution in massive bone defects. The aim of this systematic review of the Literature is to provide a comprehensive data collection concerning the use of MPs in non-oncologic cases, and to provide an overview of this topic, especially from an epidemiologic point of view. Three different databases (PubMed, Scopus, and Web of Science) were searched for relevant articles, and further references were obtained by cross-referencing. Sixty-nine studies met the inclusion criteria, reporting on cases of MP in non-oncologic cases. A total of 2598 MPs were retrieved. Among these, 1353 (52.1%) were distal femur MPs, 941 (36.2%) were proximal femur MPs, 29 (1.4%) were proximal tibia MPs and 259 (10.0%) were total femur MPs. Megaprostheses were most commonly used to treat periprosthetic fractures (1158 cases, 44.6%), in particular in the distal femur (859, 74.2%). Overall, complications were observed in 513 cases (19.7%). Type I (soft tissue failures) and type IV (infection) according to the Henderson classification were the most frequent (158 and 213, respectively). In conclusion, patients with severe post-traumatic deformities and/or significant bone loss who have had previous septic complications should be considered as oncologic patients, not because of the disease, but because of the limited therapeutic options available. The benefits of this treatment include relatively short operative times and immediate weight-bearing, thus making MP particularly attractive in the lower limb.
Keywords: severe bone loss, megaprosthesis, non-oncologic, pseudoarthrosis, fracture
1. Introduction
Reconstruction of massive defects of long bones is a demanding surgical procedure that poses multiple challenges for the treating orthopedic surgeon [1]. Several clinical scenarios can be associated with significant bone loss, which is comparable to the resection of a bone tumor. These can include severe trauma, failed osteosynthesis with a non-union or periprosthetic fracture, and multiple revisions of arthroplasty for either an aseptic loosening or a periprosthetic joint infection (PJI) [2,3,4,5,6]. Patients frequently have undergone a number of previous procedures which may limit the options of reconstruction or may involve a number of comorbidities.
There are various reconstructive strategies to treat bone defects such as autograft and allogeneic bone grafting, bone transport, and the use of standard prosthesis and megaprosthesis (MP). Modular MPs are commonly used after bone-tumor resection, but they can offer a limb-salvage solution in such difficult-to-manage situations [7]. A major advantage of MPs is their intraoperative flexibility, which enables the surgeon to reconstruct huge bone defects [7,8,9].
However, MPs have inherent disadvantages including implant costs and a lack of further revision options, increased risk of dislocation, and PJI [2,9]. Megaprosthesis may be preferable in elderly patients with loose implants and insufficient bone stock or in patients who require short hospitalization and rapid recovery because of low activity levels and multiple comorbidities [10,11,12].
Moreover, in such cases, bone and soft tissue conditions are completely different from the oncological patient group. The knee extensor mechanism is very often in a critical condition, particularly in post-traumatic septic patients who have undergone multiple surgeries. Tissue adhesion, scar interference, muscular and tendon impairment, soft tissue retractions, osteoporosis, and skin problems can lead to a reduced function of the knee and severe joint stiffness, and also create adverse conditions during the reconstructive step [13,14].
The aim of this systematic literature review is to provide a comprehensive data collection concerning the use of MP in non-oncologic cases and to provide an overview of this topic, especially from an epidemiologic point of view.
2. Materials and Methods
This systematic review was conducted in accordance with the 2020 PRISMA guidelines (Preferred Reporting Items of Systematic Reviews) [15].
All studies (randomized controlled trials (RCTs), prospective (PCCS) and retrospective comparative studies (RCCS), prospective (PCS) and retrospective case series (RCS)) reporting the use of megaprostheses in non-oncologic cases were included. Biomechanical studies, cadaveric studies, “in vitro” studies, and animal model studies were excluded. Only articles in English published in a peer-reviewed journal were included. Articles published before 1995 and those reporting on MP for oncologic reconstructions were also excluded.
The criteria used to select articles allowed us to extrapolate data about the use of an MP in non-oncologic cases. Studies eligible for this systematic review were identified through an electronic systematic search of PubMed, Scopus, and Web of Science, up to 30 April 2023. The search string used was as follows: (megaprosthesis OR endoprosthetic replacement) AND (pseudoarthrosis OR non-union OR non-oncologic OR fracture OR infection OR periprosthetic infection OR loosening). Articles without an abstract were excluded from the study. The articles were screened considering the relevance of titles and abstracts and looking for the full-text article when the abstract provided insufficient information about inclusion and exclusion criteria.
Articles that were considered relevant via electronic search were retrieved in full text, and a cross-referencing search of their bibliographies was performed to find further related articles. Reviews and meta-analyses were also analyzed in order to broaden the search to studies that might have been missed through the electronic search. All duplicates were removed, and all the articles retrieved were analyzed. After the first screening, records without eligibility criteria were excluded.
Remnant studies were categorized by type, according to the Oxford Centre for Evidence-Based Medicine (OCEBM).
Each study was assessed by two reviewers (SC.P. and R.Z.) independently and in duplicate; disagreement was resolved by the senior author (A.S.). All the included studies were analyzed, and data related to topics of interest were extracted and summarized.
In detail, the data extracted included study type, mean age, site, indication to implant an MP, mean follow-up, complications, and functional outcomes. Complications that required subsequent revision of the prosthesis were recorded and classified according to Henderson et al. [16]. Functional outcomes were reported according to the reported scoring systems used in each study analyzed in this review. Only homogeneous series which included only one MP site were considered to assess cumulative data on indication to implant an MP, complications, and functional results.
The study is descriptive, and data are presented as total frequencies and percentages. The heterogeneity of most of the included studies did not allow any statistical analysis.
3. Results
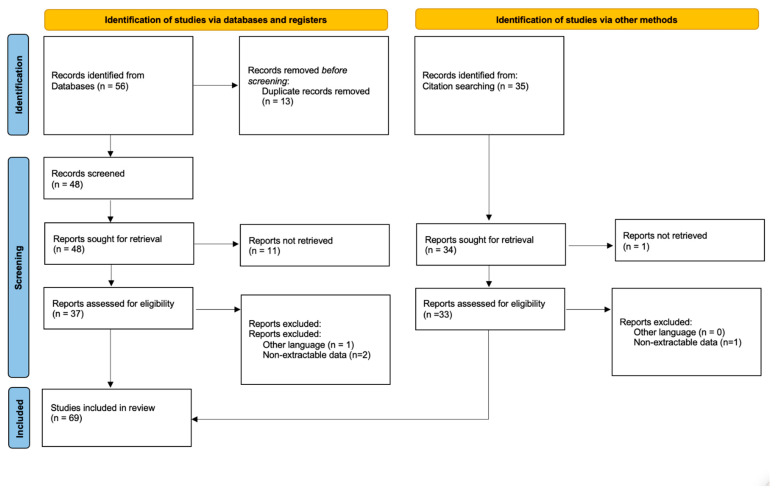
A total of 56 studies were found through the electronic search and 35 studies were added after the cross-referenced research on the bibliographies of the examined full-text articles (Figure 1).
Figure 1.
PRISMA flow diagram and the selection of studies.
After a preliminary analysis, a total of 69 studies reporting series of MPs in non-oncologic cases were included in this systematic review (6 prospective studies, 58 retrospective studies, 3 case reports, and 2 retrospective case series).
A total of 2598 MP were retrieved. Among these, 1353 (52.1%) were distal femur (DF) MPs, 941 (36.2%) proximal femur (PF) MPs, 29 (1.1%) proximal tibia (PT) MPs and 259 (10.0%) total femur MPs (Table 1).
Table 1.
Characteristics of included studies. Site of megaprosthesis and reason to implant. MP: megaprosthesis; PJI: prosthetic joint infection; * Not detailed.
| Study | Study Design | Non Oncologic Recontructions (n) | Site | Age (Mean, Years) | Reason to Implant Megaprosthesis | Silver Coating (n) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distal Femur (n) | Proximal Tibia (n) | Proximal Femur (n) | Total Femur | Aseptic Loosening (n) | PJI (n) | Fracture (n) | Periprosthetic Fracture (n) | Non Union (n) | |||||
| Calori et al. [13] | Retrospective | 9 | 9 | 68 | 9 | No | |||||||
| Calori et al. [17] | Retrospective | 32 | 13 | 2 | 11 | 6 | 64 | 2 | 5 | 4 | 21 | 32 | |
| Corona et al. [18] | Retrospective | 29 | 12 | 14 | 3 | 75 | 29 | No | |||||
| De Marco et al. [19] | Case series | 4 | 4 | 77 | 4 | No | |||||||
| Aebischer et al. [20] | Retrospective | 306 | 306 | 76 | 306 | No | |||||||
| Vitiello et al. [12] | Retrospective | 12 | 6 | 6 | 73 | 1 | 1 | 1 | 5 | 4 | 12 | ||
| Calori et al. [7] | Retrospective | 72 | 31 | 7 | 21 | 13 | 68 | 22 | 5 | 11 | 34 | No | |
| Fram et al. [21] | Case series | 6 | 2 | 4 | 71 | 1 | 3 | 1 | 1 | No | |||
| Holl et al. [22] | Retrospective | 21 | 15 | 6 | 73 | 2 | 5 | 9 | 5 | No | |||
| Kar et al. [23] | Case report | 2 | 2 | 69 | 2 | No | |||||||
| Toepfer et al. [24] | Retrospective | 18 | 18 | 78 | 7 | 11 | No | ||||||
| Toepfer et al. [25] | Retrospective | 13 | 13 | 73 | 13 | No | |||||||
| Vitiello et al. [26] | Retrospective | 23 | 12 | 11 | 73 | 23 | 23 | ||||||
| Windhager et al. [27] | Retrospective | 11 | 10 | 1 | 81 | 11 | No | ||||||
| Zanchini et al. [28] | Retrospective | 11 | 11 | 86 | No | ||||||||
| Berend et al. [29] | Retrospective | 39 | 39 | 76 | 13 | 11 | 11 | 13 | 1 | No | |||
| Keenan et al. [30] | Retrospective | 7 | 7 | 78 | 1 | No | |||||||
| Springer et al. [31] | Retrospective | 26 | 26 | 72 | 8 | 13 | 5 | No | |||||
| Stancil et al. [32] | Retrospective | 90 | 90 | 77 | 14 | 58 | 18 | No | |||||
| Tandon et al. [33] | Retrospective | 21 | 21 | 78 | 14 | 21 | No | ||||||
| Chalmers et al. [34] | Retrospective | 49 | 49 | 76 | 49 | No | |||||||
| Darrith et al. [35] | Retrospective | 22 | 22 | 76 | 22 | No | |||||||
| Fountain et al. [36] | Retrospective | 14 | 14 | 64 | 3 | 9 | 2 | No | |||||
| Mortazavi et al. [37] | Retrospective | 20 | 22 | 70 | 22 | No | |||||||
| Friesecke et al. [38] | Retrospective | 96 | 96 | 68 | 31 | 65 | No | ||||||
| Berend et al. [39] | Retrospective | 59 | 59 | 74 | 13 | 14 | 31 | No | |||||
| Abolghasemian et al. [40] | Retrospective | 13 | 13 | 77.5 | No | ||||||||
| Cannon [41] | Retrospective | 27 | 27 | * | 1 | 22 | 4 | 27 | |||||
| Chen et al. [42] | Retrospective | 49 | 49 | 74.5 | 36 | 13 | No | ||||||
| Choi et al. [43] | Case report | 1 | 1 | 70 | 1 | No | |||||||
| Gan et al. [44] | Retrospective | 7 | 7 | 76 | 7 | No | |||||||
| Girgis et al. [45] | Retrospective | 14 | 14 | 82 | 14 | No | |||||||
| Hoellwarth et al. [46] | Retrospective | 53 | 53 | 80 | 53 | No | |||||||
| Jassim et al. [47] | Retrospective | 11 | 11 | 81 | 11 | No | |||||||
| Leino et al. [48] | Retrospective | 29 | 29 | 79 | 29 | No | |||||||
| Matar et al. [49] | Retrospective | 30 | 30 | 81 | 30 | No | |||||||
| Rahman et al. [50] | Retrospective | 17 | 17 | 76 | 17 | No | |||||||
| Rao et al. [51] | Retrospective | 12 | 12 | 78 | 12 | No | |||||||
| Saidi et al. [52] | Retrospective | 7 | 7 | 80 | 7 | No | |||||||
| Ruder et al. [53] | Retrospective | 23 | 23 | 80 | 23 | No | |||||||
| Ross et al. [54] | Retrospective | 27 | 27 | 79 | 27 | No | |||||||
| Haentjens et al. [55] | Retrospective | 16 | 16 | 78 | 16 | No | |||||||
| Klein et al. [4] | Retrospective | 21 | 21 | 78 | 21 | No | |||||||
| Parvizi et al. [5] | Retrospective | 43 | 43 | 74 | 13 | 15 | 3 | 22 | 3 | No | |||
| Shih et al. [56] | Prospective | 12 | 12 | 59 | 3 | 6 | 3 | No | |||||
| Shoenfeld et al. [57] | Retrospective | 19 | 19 | 76 | 10 | 9 | No | ||||||
| Rodriguez et al. [58] | Prospective | 97 | 97 | * | * | * | * | * | * | No | |||
| Gebert et al. [59] | Retrospective | 45 | 45 | 62 | 19 | 16 | 9 | No | |||||
| Sewell et al. [60] | Retrospective | 15 | 15 | 67 | 4 | 5 | 3 | 3 | No | ||||
| Al-Taki et al. [61] | Retrospective | 36 | 36 | 73 | * | * | * | * | * | No | |||
| McLean et al. [62] | Prospective | 20 | 20 | 72 | 9 | 11 | No | ||||||
| Dean et al. [63] | Prospective | 8 | 8 | 67 | 2 | 1 | 5 | No | |||||
| Grammatopoulos et al. [64] | Retrospective | 79 | 79 | 69 | 55 | 24 | No | ||||||
| Curtin et al. [65] | Prospective | 16 | 16 | 75 | 16 | No | |||||||
| Viste et al. [66] | Prospective | 44 | 44 | 79 | 17 | 12 | 15 | No | |||||
| Khajuria et al. [67] | Retrospective | 37 | 37 | 80 | 8 | 4 | 8 | 17 | No | ||||
| De Martino et al. [68] | Retrospective | 30 | 30 | 64 | * | * | * | * | * | No | |||
| Fenelon et al. [69] | Retrospective | 79 | 79 | 78 | 11 | 5 | 55 | 9 | No | ||||
| Döring et al. [70] | Retrospective | 28 | 28 | 67 | 6 | 11 | 10 | 1 | No | ||||
| Logoluso et al. [71] | Retrospective | 21 | 21 | 68 | 21 | 21 | |||||||
| Zanchini et al. [72] | Retrospective | 39 | 39 | 69 | 15 | 18 | 6 | No | |||||
| Dieckmann et al. [73] | Retrospective | 49 | 49 | 71 | 29 | 4 | 16 | 41 | |||||
| Theil et al. [74] | Retrospective | 70 | 59 | 11 | 73 | 70 | No | ||||||
| Theil et al. [75] | Retrospective | 41 | 41 | 73 | 41 | No | |||||||
| Sobol et al. [76] | Retrospective | 75 | 75 | 69 | 25 | 23 | 20 | 7 | No | ||||
| Barry et al. [77] | Retrospective | 22 | 22 | 63 | 6 | 7 | 9 | No | |||||
| Wiles et al. [78] | Retrospective | 144 | 144 | 72 | 28 | 40 | 11 | 55 | No | ||||
Three series reported the combined use of PT and DF MP in a few cases [7,21,22]. Only one case of proximal humerus MP was reported in an aseptic non-union case with proximal humerus arthrosis [79]. Regarding elbow MP, Capanna et al. [80] reported on five revision cases (failed elbow prosthesis or failed osteosynthesis) in a heterogeneous series which included a majority of oncologic MPs.
The mean age across all studies was 73.2 ± 8.2 years. The mean follow-up period was 39.7 months, ranging between 3 and 88 months. However, not all the included studies reported on the duration of follow-up.
All but three studies detailed the indication to MP. Megaprostheses were most commonly used to treat periprosthetic fractures (1158 cases, 44.6%), in particular in DF (859, 74.2%). Another common indication to implant an MP was a fracture. In 137 cases (5.3%), an MP was used as the primary treatment, whereas in 325 (12.5%) cases it was a salvage procedure to treat a non-union. Megaprostheses were also reported for the treatment of standard prosthesis failure, with 251 (9.9%) cases described after aseptic loosening and 371 (14.3%) to treat a PJI. The majority of MPs in PJI cases were reported in proximal femur (166) compared to DF (83) and total femur (23). Nonetheless, only a few series specifically focus on PJI treatment [13,71,73,75], thus making any evaluation of the efficacy of MP to treat PJI extremely difficult. On the other hand, most of the series were heterogeneous either on the site or the reason to implant an MP. Only five series reported on the use of silver-coated MPs [12,17,26,41,73].
Overall, complications were observed in 513 cases (19.7%) (Table 2). Type I (soft tissue failures) and type IV (infection) were the most frequent (158 and 213, respectively). However, data on infections are difficult to analyze as most of the series did not distinguish between infected/non-infected cases at baseline. Limiting the analysis to series reporting on a single site MP, complications (dislocation in particular) were more commonly observed in TF (34.5%) and PF (26.7%) MPs than in DF MPs (14.7%).
Table 2.
Characteristics of included studies. Complications and functional outcomes. HHS: Harris Hip Score; MSTS: Musculoskeletal Tumor Society Scoring System; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; OKS: Oxford Knee Score; KSS: Knee Society Score; OHS: Oxford Hip Score; TESS: Toronto Extremity Salvage Score.
| Study | Study Design | Non Oncologic Recontructions (n) | Follow-Up (Mean, Months) | Complications | Functional Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type I (n) | Type II (n) | Type III (n) | Type IV (n) | HHS | MSTS | WOMAC | OKS | KSS | Bristol Knee Score | OHS | TESS | ||||
| Calori et al. [13] | Retrospective | 9 | 18 | 1 | 78.2 at 6 months 76.4 at 1 year 74.8 at 18 months |
||||||||||
| Calori et al. [17] | Retrospective | 32 | 18 | 1 | 1 | ||||||||||
| Corona et al. [18] | Retrospective | 29 | 48 | 4 | 5 | ||||||||||
| De Marco et al. [19] | Case series | 4 | 3 | 33.5 | |||||||||||
| Aebischer et al. [20] | Retrospective | 306 | 24 | 9 | 8 | 10 | |||||||||
| Vitiello et al. [12] | Retrospective | 12 | 33 | ||||||||||||
| Calori et al. [7] | Retrospective | 72 | 18 | 3 | |||||||||||
| Fram et al. [21] | Case series | 6 | 33 | ||||||||||||
| Holl et al. [22] | Retrospective | 21 | 34 | 2 | 2 | 6 | |||||||||
| Kar et al. [23] | Case report | 2 | 12 | 75 | |||||||||||
| Toepfer et al. [24] | Retrospective | 18 | 80 | 5 | 2 | 8 | 40.5 | 15.5 | |||||||
| Toepfer et al. [25] | Retrospective | 13 | 62 | 2 | 1 | 4 | 35.4 | 15.3 | |||||||
| Vitiello et al. [26] | Retrospective | 23 | 24 | 1 | |||||||||||
| Windhager et al. [27] | Retrospective | 11 | 40 | 1 | 2 | ||||||||||
| Zanchini et al. [28] | Retrospective | 11 | 23 | ||||||||||||
| Berend et al. [29] | Retrospective | 39 | 24 | ||||||||||||
| Keenan et al. [30] | Retrospective | 7 | 12 | 80.1 | |||||||||||
| Springer et al. [31] | Retrospective | 26 | 59 | 1 | 5 | 75.5 | |||||||||
| Stancil et al. [32] | Retrospective | 90 | 24 | 2 | 2 | ||||||||||
| Tandon et al. [33] | Retrospective | 21 | 72 | 28 | 70 | ||||||||||
| Chalmers et al. [34] | Retrospective | 49 | 48 | 6 | 1 | 5 | |||||||||
| Darrith et al. [35] | Retrospective | 22 | 66 | 3 | 1 | 84 | |||||||||
| Fountain et al. [36] | Retrospective | 14 | 89 | 5 | 3 | 17.7 | |||||||||
| Mortazavi et al. [37] | Retrospective | 20 | 59 | 5 | |||||||||||
| Friesecke et al. [38] | Retrospective | 96 | 59 | 6 | 3 | 12 | |||||||||
| Berend et al. [39] | Retrospective | 59 | 56 | 10 | 8 | 79 | |||||||||
| Abolghasemian et al. [40] | Retrospective | 13 | 31 | 1 | 1 | 82 | |||||||||
| Cannon [41] | Retrospective | 27 | NR | 1 | 88 | ||||||||||
| Chen et al. [42] | Retrospective | 49 | 37 | 5 | 5 | ||||||||||
| Choi et al. [43] | Case report | 1 | 12 | ||||||||||||
| Gan et al. [44] | Retrospective | 7 | 44 | ||||||||||||
| Girgis et al. [45] | Retrospective | 14 | 27 | 1 | 27 | ||||||||||
| Hoellwarth et al. [46] | Retrospective | 53 | 12 | 1 | |||||||||||
| Jassim et al. [47] | Retrospective | 11 | 33 | 22.6 | |||||||||||
| Leino et al. [48] | Retrospective | 29 | 35 | 3 | 3 | ||||||||||
| Matar et al. [49] | Retrospective | 30 | 48 | 1 | 3 | 78 | |||||||||
| Rahman et al. [50] | Retrospective | 17 | 34 | 1 | 1 | 1 | 67.2 | ||||||||
| Rao et al. [51] | Retrospective | 12 | 20 | 72 | |||||||||||
| Saidi et al. [52] | Retrospective | 7 | 6 | 74 | |||||||||||
| Ruder et al. [53] | Retrospective | 23 | 30 | ||||||||||||
| Ross et al. [54] | Retrospective | 27 | 44 | 1 | 2 | 1 | |||||||||
| Haentjens et al. [55] | Retrospective | 16 | 60 | 7 | 1 | 3 | 2 | ||||||||
| Klein et al. [4] | Retrospective | 21 | 38 | 3 | 1 | 1 | 1 | 71 | |||||||
| Parvizi et al. [5] | Retrospective | 43 | 36 | 8 | 4 | 1 | 65 | ||||||||
| Shih et al. [56] | Prospective | 12 | 68 | 5 | 1 | 4 | 83 | ||||||||
| Shoenfeld et al. [57] | Retrospective | 19 | 44 | 2 | 1 | ||||||||||
| Rodriguez et al. [58] | Prospective | 97 | 38 | 9 | 2 | 3 | 1 | 84 | |||||||
| Gebert et al. [59] | Retrospective | 45 | 38 | 1 | 2 | 5 | 78 | ||||||||
| Sewell et al. [60] | Retrospective | 15 | 60 | 69 | |||||||||||
| Al-Taki et al. [61] | Retrospective | 36 | 38 | 3 | 1 | 1 | 1 | 70 | |||||||
| McLean et al. [62] | Prospective | 20 | 48 | 3 | 1 | 2 | 68 | ||||||||
| Dean et al. [63] | Prospective | 8 | 18 | 71 | |||||||||||
| Grammatopoulos et al. [64] | Retrospective | 79 | 60 | 3 | 3 | 5 | 9 | ||||||||
| Curtin et al. [65] | Prospective | 16 | 19 | 2 | 40 | ||||||||||
| Viste et al. [66] | Prospective | 44 | 72 | 6 | 1 | 6 | 68 | ||||||||
| Khajuria et al. [67] | Retrospective | 37 | 32 | 1 | 3 | 31 | |||||||||
| De Martino et al. [68] | Retrospective | 30 | 60 | 2 | 2 | 2 | 3 | ||||||||
| Fenelon et al. [69] | Retrospective | 79 | 31 | 12 | 1 | 3 | |||||||||
| Döring et al. [70] | Retrospective | 28 | 88 | 8 | 5 | 5 | 6 | ||||||||
| Logoluso et al. [71] | Retrospective | 21 | 64 | 8 | 2 | 2 | |||||||||
| Zanchini et al. [72] | Retrospective | 39 | 60 | 2 | 2 | 3 | |||||||||
| Dieckmann et al. [73] | Retrospective | 49 | 52 | 6 | 2 | 1 | 2 | 69 | |||||||
| Theil et al. [74] | Retrospective | 70 | 50 | 11 | 2 | 16 | |||||||||
| Theil et al. [75] | Retrospective | 41 | 59 | 19 | |||||||||||
| Sobol et al. [76] | Retrospective | 75 | 60 | 5 | 10 | 4 | 16 | ||||||||
| Barry et al. [77] | Retrospective | 22 | 60 | 1 | 12 | ||||||||||
| Wiles et al. [78] | Retrospective | 144 | 60 | 1 | 6 | 2 | 10 | 71 | |||||||
Functional results were reported only by a few series, with great variability in reported outcome scores. Most of the series focusing on PF used the Harris Hip score (HHS), with a mean value of 72.8, whereas two series reported an Oxford hip score (OHS) of 40 and 30. Only two series reporting only on TF MPs reported a functional assessment, with a mean HHS value of 38.4. These series reported also on knee function in TF with a mean Oxford Knee Score (OKS) of 15.4. Another TF series used the Knee Society Score (KSS) to report functional outcomes (79). Series focused on DF reported a mean OKS of 27.5 and a mean KSS of 77.1.
4. Discussion
Several studies on MP for non-tumor reconstruction have been published, but their quality was mainly undermined by heterogeneous populations including different sites and indications. Moreover, some studies reported also on the use of revision arthroplasties mixed with MPs [81,82].
Indication to MP has been described particularly in periprosthetic fractures around a total knee arthroplasty (TKA). Chen et al. [42] compared primary versus secondary DF MP for the treatment of TKA periprosthetic fractures. If ORIF fails, these patients could be revised to a DF MP, but this might expose patients to repeat surgery, and may increase the risk of further complications. Megaprosthesis is a viable treatment option also for DF fractures in the elderly or patients with similarly poor-quality bone. It represents a good alternative to the more commonly used option of distal femoral ORIF/retrograde femoral nails, especially in those patients with radiological evidence of existing osteoarthritis and in the very distal fractures where reconstruction is difficult [52,83]. This can prevent patients from being bedridden and its outcomes such as thrombosis, worsening of dementia, negative impact on independence and autonomy, and the quality of life [84,85]. Similar functional outcomes between ORIF and MP were reported [33,86]. The cost of the implant is higher than that of ORIF but the time to start fully weight-bearing is less. Thus, the higher cost of implants in MP is recouped in the much shorter hospital stay in this procedure [33]. The complication rate of DF MP in non-oncologic cases (9.8%) seems to be generally lower compared to DF MP implanted for oncologic reconstructions (14.6%) [16]. This might be due to several causes which include different ages of populations, comorbidities, and different follow-ups of the studies.
Most PF and TF MPs have been reported as a salvage option for patients with extreme bone loss, once reconstruction with revision stems is no longer feasible, in cases of either aseptic loosening or PJI. Even though they allow for improvement in pain which is comparable to that achieved after revision hip arthroplasty using a conventional hip revision system [61], dislocation is a common complication [64,66,68,87]. Soft tissue failures in PF and TF occurred much more frequently in non-oncologic populations (11.9% and 14.0%, respectively), compared to 5.2% and 8.9%, respectively, in oncologic reconstructions. To reduce the risk of dislocation, attention should be focused on the anatomical reconstruction of muscles such as gluteus and extrarotator of the hip or the iliopsoas. These muscles have to be preserved, where possible, with their bone insertion and linked with the prosthesis in their specific anchoring sites. Moreover, the use of bipolar prostheses, larger femoral heads, constrained liners, or dual mobility cups is advisable [88,89]. Theil et al. [74] reported a high risk of dislocation even among patients treated with dual-mobility acetabular components as part of a two-stage revision for PJI of the hip, with an even higher risk among TF MP than PF MP. The use of bipolar cups had already been suggested by Abdelaziz et al. [90], who observed that revision THA for PJI using a PF MP and a constrained liner or a cemented dual-mobility cup had a comparable dislocation rate with patients treated with a standard THA. However, even though the use of additional constraints (liners or cups) might appear tempting, published results vary tremendously [91,92] and it is unclear whether constrained liners or cups will reduce the risk of instability in patients with a PFR or TFR after a two-stage exchange.
Artificial ligaments can also be used to reduce the dislocation rate [89]. Post-operative care is of paramount importance with immobilization of the limb operated on in abduction for various post-operative durations, and protected weight-bearing thereafter [70].
In the setting of massive segmental defects of the proximal tibia (PT) with loss of collateral ligamentous support and lack of bone to support prosthetic augments or metaphyseal cones or sleeves, a PT MP may create the most biomechanically stable construct. Nonetheless, Henderson et al. [93] found PT MPs to have the highest failure rates of all megaprostheses in oncologic reconstructions, with infection as the leading cause at 16%. It is critical to ensure adequate tissue coverage during closure to prevent infection and enable healing, which may necessitate a flap. Moreover, functional outcomes generally vary based on the extensor mechanism status. In non-oncologic cases, the tibial tubercle can be preserved and healing of the diaphyseal bone has been demonstrated. Thus, it is recommended to preserve the anterolateral column of the proximal tibia including the tubercle when possible to optimize the extension mechanism function. However, only one series specifically focused on PT MPs, thus making any analysis not feasible [13].
In the case of the upper limb, it is impossible to draw any conclusions as there are only two small series available on the topic [79,80]. This lack of evidence for the upper limb is probably due to two main reasons: (1) non-oncological etiologies for massive bone loss are considerably more uncommon; (2) in the case of complex reconstructions, the absence of weight-bearing probably leads to a preference for ORIF or alternative solutions for end-stage scenarios (e.g., proximal humerus permanent spacer, elbow arthrodesis).
Using MPs is undoubtedly an attractive option in end-stage infection scenarios, to avoid amputation. However, concerns over the risk of infection relapse or reinfection remain a reality within the orthopedic community. In cases of post-traumatic septic non-union or prosthetic joint infection (PJI), surgical treatment should be conducted in two steps [7]. In the case of PJI managed with a modular MP, Corona et al. [18] found an overall infection eradication rate of 82.8%, similar to other treatment options. Similar infection control after staged PJI treatment has been reported by Theil et al. [74,75] Despite the greater metallic surface of MP possibly being a significant risk factor for relapse [18], there is the option of performing extensive bone resections—allowing much more aggressive debridement than in normal surgeries—and so eliminating possible osteomyelitis foci that might otherwise have perpetuated the infection.
In cases of revision after infection, the antibiotics added to the cement may have a positive effect on infection control [94,95]. Moreover, even though the cement-free method is particularly advantageous in younger patients [96], in older patients (such as most of those with an MP for non-oncologic indications) with multiple comorbidities, by contrast, the use of the cemented technique can allow immediate full weight-bearing. Nonetheless, the optimal stem fixation for revision remains unknown.
There is a growing trend toward using MPs with surface modifications to reduce the risk of implant colonization. Studies in the literature have reported on three different silver-coated MPs [97], with most of the data coming from oncologic patient series. Fiore et al. [98] highlighted that silver-coated implants are particularly useful in two-stage revisions for infection and in patients with incidental positive cultures at the time of prosthesis implantation [64,98,99,100,101,102]. On the other hand, the results of silver-coated MPs in PJI prevention are extremely heterogeneous. Only a few series described the use of silver-coated MPs in non-oncologic settings [12,17,26,41,73]. Even though they were mainly heterogeneous, including both silver-coated and standard titanium-coated MPs, they were in agreement on the protective role against reinfection when dealing with PJI.
Functional results of megaprotheses seem to be encouraging, in particular in the DF, where similar functional outcomes between ORIF and MP were reported [33,86,103]. On the other hand, functional results in PF and TF can be severely compromised in cases of dislocation or muscle insufficiency. However, functional results in PF MPs used in non-oncologic scenarios seem to be comparable to those observed after revision total hip arthroplasty for a periprosthetic fracture [104] and after hip reimplantation in staged treatments for a PJI [105,106].
There are several limitations to this study. Many of the included series were heterogeneous both in terms of site and reason to implant an MP. There is a real lack of long-term data on MPs in non-oncologic settings, with many series not reporting the outcome. Additionally, many series used different outcome measurements. Moreover, the heterogeneity of most of the series regarding both sites and indications would make any pooled results unsubstantiated. Thus, it is not possible to draw any correlation between the indication to MP and complications.
Megaprosthesis is an attractive option in the management of extreme cases of severe bone loss and prosthetic failure. Benefits of this treatment include relatively short operative times and immediate weight-bearing and resumption of activity. This is highly advantageous in the avoidance of postoperative complications in elderly patients with multiple comorbidities.
Patients with severe post-traumatic deformities and/or significant bone loss who have had previous septic complications should be considered as an oncologic patient, not because of the disease, but because of the limited therapeutic options available.
Author Contributions
Conceptualization, A.S. and M.F.; methodology, S.C.P., L.D.P. and L.M.; data curation: R.Z., M.B., G.L. and A.M.; writing—original draft preparation, S.C.P. and M.F.; writing—review and editing, A.S. and R.D.C.; supervision, A.S. and M.D.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Giannoudis P.V. Treatment of bone defects: Bone transport or the induced membrane technique? Injury. 2016;47:291–292. doi: 10.1016/j.injury.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Rasouli M.R., Porat M.D., Hozack W.J., Parvizi J. Proximal femoral replacement and allograft prosthesis composite in the treatment of periprosthetic fractures with significant proximal bone loss. Orthop. Surg. 2012;4:203–210. doi: 10.1111/os.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer B.D., Berry D.J., Lewallen D.G. Treatment of periprosthetic femoral fractures following total hip Arthroplasty with femoral component revision. J. Bone Jt. Surg. Am. 2003;85:2156–2162. doi: 10.2106/00004623-200311000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Klein G.R., Parvizi J., Rapuri V., Wolf C.F., Hozack W.J., Sharkey P.F., Purtill J.J. Proximal femoral replacement for the treatment of periprosthetic fractures. J. Bone Jt. Surg. Am. 2005;87:1777–1781. doi: 10.2106/JBJS.D.02420. [DOI] [PubMed] [Google Scholar]
- 5.Parvizi J., Tarity T.D., Slenker N., Wade F., Trappler R., Hozack W.J., Sim F.H. Proximal femoral replacement in patients with non-neoplastic conditions. J. Bone Jt. Surg. Am. 2007;89:1036–1043. doi: 10.2106/00004623-200705000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Sakellariou V.I., Babis G.C. Management bone loss of the proximal femur in revision hip arthroplasty: Update on reconstructive options. World J. Orthop. 2014;5:614–622. doi: 10.5312/wjo.v5.i5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calori G.M., Colombo M., Malagoli E., Mazzola S., Bucci M., Mazza E. Megaprosthesis in post-traumatic and periprosthetic large bone defects: Issues to consider. Injury. 2014;45((Suppl. S6)):S105–S110. doi: 10.1016/j.injury.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Bernthal N.M., Greenberg M., Heberer K., Eckardt J.J., Fowler E.G. What are the functional outcomes of endoprosthestic reconstructions after tumor resection? Clin. Orthop. Relat. Res. 2015;473:812–819. doi: 10.1007/s11999-014-3655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capanna R., Scoccianti G., Frenos F., Vilardi A., Beltrami G., Campanacci D.A. What was the survival of megaprostheses in lower limb reconstructions after tumor resections? Clin. Orthop. Relat. Res. 2015;473:820–830. doi: 10.1007/s11999-014-3736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apprich S.R., Nia A., Schreiner M.M., Jesch M., Böhler C., Windhager R. Modular megaprostheses in the treatment of periprosthetic fractures of the femur. Wien. Klin. Wochenschr. 2021;133:550–559. doi: 10.1007/s00508-021-01838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitiello R., Bellieni A., Oliva M.S., Di Capua B., Fusco D., Careri S., Colloca G.F., Perisano C., Maccauro G., Lillo M. The importance of geriatric and surgical co-management of elderly in muscoloskeletal oncology: A literature review. Orthop. Rev. 2020;12:8662. doi: 10.4081/or.2020.8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitiello R., Ziranu A., Oliva M.S., Meluzio M.C., Cauteruccio M., Maccauro G., Liuzza F., Saccomanno M.F. The value of megaprostheses in non-oncological fractures in elderly patients: A short-term results. Injury. 2022;53:1241–1246. doi: 10.1016/j.injury.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Calori G.M., Mazza E.L., Vaienti L., Mazzola S., Colombo A., Gala L., Colombo M. Reconstruction of patellar tendon following implantation of proximal tibia megaprosthesis for the treatment of post-traumatic septic bone defects. Injury. 2016;47((Suppl. S6)):S77–S82. doi: 10.1016/S0020-1383(16)30843-9. [DOI] [PubMed] [Google Scholar]
- 14.Lynch A.F., Rorabeck C.H., Bourne R.B. Extensor mechanism complications following total knee arthroplasty. J. Arthroplast. 1987;2:135–140. doi: 10.1016/S0883-5403(87)80020-7. [DOI] [PubMed] [Google Scholar]
- 15.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson E.R., Groundland J.S., Pala E., Dennis J.A., Wooten R., Cheong D., Windhager R., Kotz R.I., Mercuri M., Funovics P.T., et al. Failure mode classification for tumor endoprostheses: Retrospective review of five institutions and a literature review. J. Bone Jt. Surg. Am. 2011;93:418–429. doi: 10.2106/JBJS.J.00834. [DOI] [PubMed] [Google Scholar]
- 17.Calori G.M., Colombo M., Ripamonti C., Malagoli E., Mazza E., Fadigati P., Bucci M. Megaprosthesis in large bone defects: Opportunity or chimaera? Injury. 2014;45:388–393. doi: 10.1016/j.injury.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Corona P.S., Vicente M., Lalanza M., Amat C., Carrera L. Use of modular megaprosthesis in managing chronic end-stage periprosthetic hip and knee infections: Is there an increase in relapse rate? Eur. J. Orthop. Surg. Traumatol. 2018;28:627–636. doi: 10.1007/s00590-018-2127-9. [DOI] [PubMed] [Google Scholar]
- 19.De Marco D., Messina F., Meschini C., Oliva M.S., Rovere G., Maccagnano G., Noia G., Maccauro G., Ziranu A. Periprosthetic knee fractures in an elderly population: Open reduction and internal fixation vs. distal femur megaprostheses. Orthop. Rev. 2022;14:33772. doi: 10.52965/001c.33772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aebischer A.S., Hau R., de Steiger R.N., Holder C., Wall C.J. Distal Femoral Replacement for Periprosthetic Fractures After TKA: Australian Orthopaedic Association National Joint Replacement Registry Review. J. Arthroplast. 2022;37:1354–1358. doi: 10.1016/j.arth.2022.02.115. [DOI] [PubMed] [Google Scholar]
- 21.Fram B., Smith E.B., Deirmengian G.K., Abraham J.A., Strony J., Cross M.B., Ponzio D.Y. Proximal tibial replacement in revision knee arthroplasty for non-oncologic indications. Arthroplast. Today. 2020;6:23–35. doi: 10.1016/j.artd.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Höll S., Schlomberg A., Gosheger G., Dieckmann R., Streitbuerger A., Schulz D., Hardes J. Distal femur and proximal tibia replacement with megaprosthesis in revision knee arthroplasty: A limb-saving procedure. Knee Surg. Sports Traumatol. Arthrosc. 2012;20:2513–2518. doi: 10.1007/s00167-012-1945-2. [DOI] [PubMed] [Google Scholar]
- 23.Kar B.K., Ojha M.M., Yadav S.K., Agrawal A.C., Kowshik S. Distal Femur Tumor Megaprosthesis for Non-union of Supracondylar Femur Fracture after Failed Osteosynthesis. An Ingenious Solution. J. Orthop. Case Rep. 2021;11:16–19. doi: 10.13107/jocr.2021.v11.i09.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toepfer A., Harrasser N., Petzschner I., Pohlig F., Lenze U., Gerdesmeyer L., Pförringer D., Toepfer M., Beirer M., Crönlein M., et al. Short- to long-term follow-up of total femoral replacement in non-oncologic patients. BMC Musculoskelet. Disord. 2016;17:498. doi: 10.1186/s12891-016-1355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toepfer A., Harrasser N., Petzschner I., Pohlig F., Lenze U., Gerdesmeyer L., von Eisenhart-Rothe R., Mühlhofer H., Suren C. Is total femoral replacement for non-oncologic and oncologic indications a safe procedure in limb preservation surgery? A single center experience of 22 cases. Eur. J. Med. Res. 2018;23:5. doi: 10.1186/s40001-018-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitiello R., Smimmo A., De Fazio A., Bocchi M.B., Oliva M.S., Perna A., Maccauro G., Ziranu A. Megaprosthesis in articular fractures of the lower limbs in fragile patients: A proposal for the therapeutic algorithm. Eur. Rev. Med. Pharmacol. Sci. 2022;26:84–91. doi: 10.26355/eurrev_202211_30286. [DOI] [PubMed] [Google Scholar]
- 27.Windhager R., Schreiner M., Staats K., Apprich S. Megaprostheses in the treatment of periprosthetic fractures of the knee joint: Indication, technique, results and review of literature. Int. Orthop. 2016;40:935–943. doi: 10.1007/s00264-015-2991-4. [DOI] [PubMed] [Google Scholar]
- 28.Zanchini F., Piscopo A., Cipolloni V., Fusini F., Cacciapuoti S., Piscopo D., Pripp C., Nasto L.A., Pola E. Distal femur complex fractures in elderly patients treated with megaprosthesis: Results in a case series of 11 patients. World J. Orthop. 2022;13:454–464. doi: 10.5312/wjo.v13.i5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berend K.R., Lombardi A.V. Distal femoral replacement in nontumor cases with severe bone loss and instability. Clin. Orthop. Relat. Res. 2009;467:485–492. doi: 10.1007/s11999-008-0329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keenan J., Chakrabarty G., Newman J.H. Treatment of supracondylar femoral fracture above total knee replacement by custom made hinged prosthesis. Knee. 2000;7:165–170. doi: 10.1016/S0968-0160(00)00041-7. [DOI] [PubMed] [Google Scholar]
- 31.Springer B.D., Sim F.H., Hanssen A.D., Lewallen D.G. The modular segmental kinematic rotating hinge for nonneoplastic limb salvage. Clin. Orthop. Relat. Res. 2004;421:181–187. doi: 10.1097/01.blo.0000126306.87452.59. [DOI] [PubMed] [Google Scholar]
- 32.Stancil R., Romm J., Lack W., Bohnenkamp F., Sems S., Cross W., Cass J., Keeney J., Nam D., Nunley R., et al. Distal Femoral Replacement for Fractures Allows for Early Mobilization with Low Complication Rates: A Multicenter Review. J. Knee Surg. 2023;36:146–152. doi: 10.1055/s-0041-1731353. [DOI] [PubMed] [Google Scholar]
- 33.Tandon T., Tadros B.J., Avasthi A., Hill R., Rao M. Management of periprosthetic distal femur fractures using distal femoral arthroplasty and fixation-Comparative study of outcomes and costs. J. Clin. Orthop. Trauma. 2020;11:160–164. doi: 10.1016/j.jcot.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalmers B.P., Syku M., Gausden E.B., Blevins J.L., Mayman D.J., Sculco P.K. Contemporary Distal Femoral Replacements for Supracondylar Femoral Fractures Around Primary and Revision Total Knee Arthroplasties. J. Arthroplast. 2021;36:S351–S357. doi: 10.1016/j.arth.2020.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Darrith B., Bohl D.D., Karadsheh M.S., Sporer S.M., Berger R.A., Levine B.R. Periprosthetic Fractures of the Distal Femur: Is Open Reduction and Internal Fixation or Distal Femoral Replacement Superior? J. Arthroplast. 2020;35:1402–1406. doi: 10.1016/j.arth.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 36.Fountain J.R., Dalby-Ball J., Carroll F.A., Stockley I. The use of total femoral arthroplasty as a limb salvage procedure: The Sheffield experience. J. Arthroplast. 2007;22:663–669. doi: 10.1016/j.arth.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Mortazavi S.M., Kurd M.F., Bender B., Post Z., Parvizi J., Purtill J.J. Distal femoral arthroplasty for the treatment of periprosthetic fractures after total knee arthroplasty. J. Arthroplast. 2010;25:775–780. doi: 10.1016/j.arth.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Friesecke C., Plutat J., Block A. Revision arthroplasty with use of a total femur prosthesis. J. Bone Jt. Surg. Am. 2005;87:2693–2701. doi: 10.2106/00004623-200512000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Berend K.R., Lombardi A.V., Mallory T.H., Adams J.B., Dodds K.L. Total femoral arthroplasty for salvage of end-stage prosthetic disease. Clin. Orthop. Relat. Res. 2004;427:162–170. doi: 10.1097/01.blo.0000142351.88039.e8. [DOI] [PubMed] [Google Scholar]
- 40.Abolghasemian M., Kim C., Soever L., Backstein D. Megaprostheses for well-fixed TKA femoral fractures. Semin. Arthroplast. 2015;26:95–99. doi: 10.1053/j.sart.2015.08.013. [DOI] [Google Scholar]
- 41.Cannon S.R. The use of megaprosthesis in the treatment of periprosthetic knee fractures. Int. Orthop. 2015;39:1945–1950. doi: 10.1007/s00264-015-2969-2. [DOI] [PubMed] [Google Scholar]
- 42.Chen A.F., Choi L.E., Colman M.W., Goodman M.A., Crossett L.S., Tarkin I.S., McGough R.L. Primary versus secondary distal femoral arthroplasty for treatment of total knee arthroplasty periprosthetic femur fractures. J. Arthroplast. 2013;28:1580–1584. doi: 10.1016/j.arth.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Choi H.S., Nho J.H., Kim C.H., Kwon S.W., Park J.S., Suh Y.S. Revision arthroplasty Using a MUTARS® Prosthesis in Comminuted Periprosthetic Fracture of the Distal Femur. Yonsei Med. J. 2016;57:1517–1522. doi: 10.3349/ymj.2016.57.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gan G., Teo Y.H., Kwek E.B.K. Comparing Outcomes of Tumor Prosthesis Revision and Locking Plate Fixation in Supracondylar Femoral Periprosthetic Fractures. Clin. Orthop. Surg. 2018;10:174–180. doi: 10.4055/cios.2018.10.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girgis E., McAllen C., Keenan J. Revision knee arthroplasty using a distal femoral replacement prosthesis for periprosthetic fractures in elderly patients. Eur. J. Orthop. Surg. Traumatol. 2018;28:95–102. doi: 10.1007/s00590-017-2009-6. [DOI] [PubMed] [Google Scholar]
- 46.Hoellwarth J.S., Fourman M.S., Crossett L., Goodman M., Siska P., Moloney G.B., Tarkin I.S. Equivalent mortality and complication rates following periprosthetic distal femur fractures managed with either lateral locked plating or a distal femoral replacement. Injury. 2018;49:392–397. doi: 10.1016/j.injury.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 47.Jassim S.S., McNamara I., Hopgood P. Distal femoral replacement in periprosthetic fracture around total knee arthroplasty. Injury. 2014;45:550–553. doi: 10.1016/j.injury.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 48.Leino O.K., Lempainen L., Virolainen P., Sarimo J., Pölönen T., Mäkelä K.T. Operative Results of Periprosthetic Fractures of The Distal Femur In A Single Academic Unit. Scand. J. Surg. 2015;104:200–207. doi: 10.1177/1457496914552343. [DOI] [PubMed] [Google Scholar]
- 49.Matar H.E., Bloch B.V., James P.J. Distal Femoral Replacements for Acute Comminuted Periprosthetic Knee Fractures: Satisfactory Clinical Outcomes at Medium-Term Follow-up. Arthroplast. Today. 2021;7:37–42. doi: 10.1016/j.artd.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman W.A., Vial T.A., Backstein D.J. Distal Femoral Arthroplasty for Management of Periprosthetic Supracondylar Fractures of the Femur. J. Arthroplast. 2016;31:676–679. doi: 10.1016/j.arth.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 51.Rao B., Kamal T., Vafe J., Moss M. Distal femoral replacement for selective periprosthetic fractures above a total knee arthroplasty. Eur. J. Trauma. Emerg. Surg. 2014;40:191–199. doi: 10.1007/s00068-013-0347-6. [DOI] [PubMed] [Google Scholar]
- 52.Saidi K., Ben-Lulu O., Tsuji M., Safir O., Gross A.E., Backstein D. Supracondylar periprosthetic fractures of the knee in the elderly patients: A comparison of treatment using allograft-implant composites, standard revision components, distal femoral replacement prosthesis. J. Arthroplast. 2014;29:110–114. doi: 10.1016/j.arth.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Ruder J.A., Hart G.P., Kneisl J.S., Springer B.D., Karunakar M.A. Predictors of Functional Recovery Following Periprosthetic Distal Femur Fractures. J. Arthroplast. 2017;32:1571–1575. doi: 10.1016/j.arth.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 54.Ross L.A., Keenan O.J.F., Magill M., Brennan C.M., Clement N.D., Moran M., Patton J.T., Scott C.E.H. Management of low periprosthetic distal femoral fractures. Bone Jt. J. 2021;103-B:635–643. doi: 10.1302/0301-620X.103B4.BJJ-2020-1710.R1. [DOI] [PubMed] [Google Scholar]
- 55.Haentjens P., De Boeck H., Opdecam P. Proximal femoral replacement prosthesis for salvage of failed hip arthroplasty: Complications in a 2-11 year follow-up study in 19 elderly patients. Acta Orthop. Scand. 1996;67:37–42. doi: 10.3109/17453679608995606. [DOI] [PubMed] [Google Scholar]
- 56.Shih S.T., Wang J.W., Hsu C.C. Proximal femoral megaprosthesis for failed total hip arthroplasty. Chang Gung Med. J. 2007;30:73–80. [PubMed] [Google Scholar]
- 57.Schoenfeld A.J., Leeson M.C., Vrabec G.A., Scaglione J., Stonestreet M.J. Outcomes of modular proximal femoral replacement in the treatment of complex proximal femoral fractures: A case series. Int. J. Surg. 2008;6:140–146. doi: 10.1016/j.ijsu.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez J.A., Fada R., Murphy S.B., Rasquinha V.J., Ranawat C.S. Two-year to five-year follow-up of femoral defects in femoral revision treated with the link MP modular stem. J. Arthroplast. 2009;24:751–758. doi: 10.1016/j.arth.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Gebert C., Wessling M., Götze C., Gosheger G., Hardes J. The Modular Universal Tumour And Revision System (MUTARS®) in endoprosthetic revision surgery. Int. Orthop. 2010;34:1261–1265. doi: 10.1007/s00264-010-1007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sewell M.D., Hanna S.A., Carrington R.W., Pollock R.C., Skinner J.A., Cannon S.R., Briggs T.W. Modular proximal femoral replacement in salvage hip surgery for non-neoplastic conditions. Acta Orthop. Belg. 2010;76:493–502. [PubMed] [Google Scholar]
- 61.Al-Taki M.M., Masri B.A., Duncan C.P., Garbuz D.S. Quality of life following proximal femoral replacement using a modular system in revision THA. Clin. Orthop. Relat. Res. 2011;469:470–475. doi: 10.1007/s11999-010-1522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLean A.L., Patton J.T., Moran M. Femoral replacement for salvage of periprosthetic fracture around a total hip replacement. Injury. 2012;43:1166–1169. doi: 10.1016/j.injury.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 63.Dean B.J., Matthews J.J., Price A., Stubbs D., Whitwell D., Gibbons C.M. Modular endoprosthetic replacement for failed internal fixation of the proximal femur following trauma. Int. Orthop. 2012;36:731–734. doi: 10.1007/s00264-011-1332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grammatopoulos G., Alvand A., Martin H., Whitwell D., Taylor A., Gibbons C.L. Five-year outcome of proximal femoral endoprosthetic arthroplasty for non-tumour indications. Bone Jt. J. 2016;98-B:1463–1470. doi: 10.1302/0301-620X.98B11.BJJ-2016-0244.R1. [DOI] [PubMed] [Google Scholar]
- 65.Curtin M., Bryan C., Murphy E., Murphy C.G., Curtin W. Early results of the LPS™ limb preservation system in the management of periprosthetic femoral fractures. J. Orthop. 2017;14:34–37. doi: 10.1016/j.jor.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viste A., Perry K.I., Taunton M.J., Hanssen A.D., Abdel M.P. Proximal femoral replacement in contemporary revision total hip arthroplasty for severe femoral bone loss: A review of outcomes. Bone Jt. J. 2017;99-B:325–329. doi: 10.1302/0301-620X.99B3.BJJ-2016-0822.R1. [DOI] [PubMed] [Google Scholar]
- 67.Khajuria A., Ward J., Cooper G., Stevenson J., Parry M., Jeys L. Is endoprosthetic replacement of the proximal femur appropriate in the comorbid patient? HIP Int. 2018;28:68–73. doi: 10.5301/hipint.5000520. [DOI] [PubMed] [Google Scholar]
- 68.De Martino I., D’Apolito R., Nocon A.A., Sculco T.P., Sculco P.K., Bostrom M.P. Proximal femoral replacement in non-oncologic patients undergoing revision total hip arthroplasty. Int. Orthop. 2019;43:2227–2233. doi: 10.1007/s00264-018-4220-4. [DOI] [PubMed] [Google Scholar]
- 69.Fenelon C., Murphy E.P., Kearns S.R., Curtin W., Murphy C.G. Cemented Proximal Femoral Replacement for the Management of Non-Neoplastic Conditions: A Versatile Implant but Not Without Its Risks. J. Arthroplast. 2020;35:520–527. doi: 10.1016/j.arth.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Döring K., Vertesich K., Martelanz L., Staats K., Böhler C., Hipfl C., Windhager R., Puchner S. Proximal femoral reconstruction with modular megaprostheses in non-oncological patients. Int. Orthop. 2021;45:2531–2542. doi: 10.1007/s00264-021-05080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Logoluso N., Pedrini F.A., Morelli I., De Vecchi E., Romanò C.L., Pellegrini A.V. Megaprostheses for the revision of infected hip Arthroplasties with severe bone loss. BMC Surg. 2022;22:68. doi: 10.1186/s12893-022-01517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zanchini F., Piscopo A., Cipolloni V., Vitiello R., Piscopo D., Fusini F., Cacciapuoti S., Panni A.S., Pola E. The major proximal femoral defects: Megaprosthesis in non oncological patients-A case series. Orthop. Rev. 2023;15:38432. doi: 10.52965/001c.38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dieckmann R., Schmidt-Braekling T., Gosheger G., Theil C., Hardes J., Moellenbeck B. Two stage revision with a proximal femur replacement. BMC Musculoskelet. Disord. 2019;20:58. doi: 10.1186/s12891-019-2442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Theil C., Schwarze J., Smolle M.A., Pützler J., Moellenbeck B., Schneider K.N., Schulze M., Klingebiel S., Gosheger G. What Is the Risk of Dislocation and Revision in Proximal Femoral Replacement with Dual-mobility Articulation After Two-stage Revision for Periprosthetic Hip Infection? Clin. Orthop. Relat. Res. 2023:10–1097. doi: 10.1097/CORR.0000000000002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theil C., Schneider K.N., Gosheger G., Schmidt-Braekling T., Ackmann T., Dieckmann R., Frommer A., Klingebiel S., Schwarze J., Moellenbeck B. Revision TKA with a distal femoral replacement is at high risk of reinfection after two-stage exchange for periprosthetic knee joint infection. Knee Surg. Sport. Traumatol. Arthrosc. 2022;30:899–906. doi: 10.1007/s00167-021-06474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sobol K.R., Fram B.R., Strony J.T., Brown S.A. Survivorship, complications, and outcomes following distal femoral arthroplasty for non-neoplastic indications. Bone Jt. Open. 2022;3:173–181. doi: 10.1302/2633-1462.33.BJO-2021-0202.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barry J.J., Thielen Z., Sing D.C., Yi P.H., Hansen E.N., Ries M. Length of Endoprosthetic Reconstruction in Revision Knee arthroplasty Is Associated With Complications and Reoperations. Clin. Orthop. Relat. Res. 2017;475:72–79. doi: 10.1007/s11999-016-4836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wyles C.C., Tibbo M.E., Yuan B.J., Trousdale R.T., Berry D.J., Abdel M.P. Long-Term Results of Total Knee arthroplasty with Contemporary Distal Femoral Replacement. J. Bone Jt. Surg. Am. 2020;102:45–51. doi: 10.2106/JBJS.19.00489. [DOI] [PubMed] [Google Scholar]
- 79.Manzotti A., Brioschi D., Grassi M., Biazzo A., Cerveri P. Humeral head necrosis associated to shaft non-union with massive bone loss: A case report. Acta Biomed. 2020;91:e2020076. doi: 10.23750/abm.v91i3.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Capanna R., Muratori F., Campo F.R., D’Arienzo A., Frenos F., Beltrami G., Scoccianti G., Cuomo P., Piccioli A., Müller D.A. Modular megaprosthesis reconstruction for oncological and non-oncological resection of the elbow joint. Injury. 2016;47((Suppl. S4)):S78–S83. doi: 10.1016/j.injury.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 81.Atrey A., Hussain N., Gosling O., Giannoudis P., Shepherd A., Young S., Waite J. A 3 year minimum follow up of Endoprosthetic replacement for distal femoral fractures-An alternative treatment option. J. Orthop. 2017;14:216–222. doi: 10.1016/j.jor.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aebischer A.S., Hau R., de Steiger R.N., Holder C., Wall C.J. Distal femoral arthroplasty for native knee fractures: Results from the Australian Orthopaedic Association National Joint Replacement Registry. Bone Jt. J. 2022;104-B:894–901. doi: 10.1302/0301-620X.104B7.BJJ-2021-1136.R3. [DOI] [PubMed] [Google Scholar]
- 83.Pour A.E., Parvizi J., Slenker N., Purtill J.J., Sharkey P.F. Rotating hinged total knee replacement: Use with caution. J. Bone Jt. Surg. Am. 2007;89:1735–1741. doi: 10.2106/JBJS.F.00893. [DOI] [PubMed] [Google Scholar]
- 84.Rorabeck C.H., Angliss R.D., Lewis P.L. Fractures of the femur, tibia, and patella after total knee arthroplasty: Decision making and principles of management. Instr. Course Lect. 1998;47:449–458. [PubMed] [Google Scholar]
- 85.Norrish A.R., Jibri Z.A., Hopgood P. The LISS plate treatment of supracondylar fractures above a total knee replacement: A case-control study. Acta Orthop. Belg. 2009;75:642–648. [PubMed] [Google Scholar]
- 86.Mortazavi S.M., Vegari D., Ho A., Zmistowski B., Parvizi J. Two-stage exchange arthroplasty for infected total knee arthroplasty: Predictors of failure. Clin. Orthop. Relat. Res. 2011;469:3049–3054. doi: 10.1007/s11999-011-2030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korim M.T., Esler C.N., Ashford R.U. Systematic review of proximal femoral arthroplasty for non-neoplastic conditions. J. Arthroplast. 2014;29:2117–2121. doi: 10.1016/j.arth.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 88.Levin J.M., Sultan A.A., O’Donnell J.A., Sodhi N., Khlopas A., Piuzzi N.S., Mont M.A. Modern Dual-Mobility Cups in Revision Total Hip arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2018;33:3793–3800. doi: 10.1016/j.arth.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 89.Du Z., Tang S., Yang R., Tang X., Ji T., Guo W. Use of an Artificial Ligament Decreases Hip Dislocation and Improves Limb Function After Total Femoral Prosthetic Replacement Following Femoral Tumor Resection. J. Arthroplast. 2018;33:1507–1514. doi: 10.1016/j.arth.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 90.Abdelaziz H., Schröder M., Shum Tien C., Ibrahim K., Gehrke T., Salber J., Citak M. Resection of the proximal femur during one-stage revision for infected hip arthroplasty: Risk factors and effectiveness. Bone Jt. J. 2021;103-B:1678–1685. doi: 10.1302/0301-620X.103B11.BJJ-2021-0022.R1. [DOI] [PubMed] [Google Scholar]
- 91.Derksen A., Kluge M., Wirries N., Budde S., Schwarze M., Windhagen H., Floerkemeier T. Constrained tripolar liner in patients with high risk of dislocation-Analysis of incidence and risk of failure. J. Orthop. 2021;25:288–294. doi: 10.1016/j.jor.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Unter Ecker N., Kocaoğlu H., Zahar A., Haasper C., Gehrke T., Citak M. What Is the Dislocation and Revision Rate of Dual-mobility Cups Used in Complex Revision THAs? Clin. Orthop. Relat. Res. 2021;479:280–285. doi: 10.1097/CORR.0000000000001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henderson E.R., O’Connor M.I., Ruggieri P., Windhager R., Funovics P.T., Gibbons C.L., Guo W., Hornicek F.J., Temple H.T., Letson G.D. Classification of failure of limb salvage after reconstructive surgery for bone tumours: A modified system Including biological and expandable reconstructions. Bone Jt. J. 2014;96-B:1436–1440. doi: 10.1302/0301-620X.96B11.34747. [DOI] [PubMed] [Google Scholar]
- 94.Wang C., Pfitzner T., von Roth P., Mayr H.O., Sostheim M., Hube R. Fixation of stem in revision of total knee arthroplasty: Cemented versus cementless-a meta-analysis. Knee Surg. Sport. Traumatol. Arthrosc. 2016;24:3200–3211. doi: 10.1007/s00167-015-3820-4. [DOI] [PubMed] [Google Scholar]
- 95.Medellin M.R., Fujiwara T., Clark R., Stevenson J.D., Parry M., Jeys L. Mechanisms of failure and survival of total femoral endoprosthetic replacements. Bone Jt. J. 2019;101-B:522–528. doi: 10.1302/0301-620X.101B5.BJJ-2018-1106.R1. [DOI] [PubMed] [Google Scholar]
- 96.Gosheger G., Gebert C., Ahrens H., Streitbuerger A., Winkelmann W., Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin. Orthop. Relat. Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 97.Fiore M., Sambri A., Zucchini R., Giannini C., Donati D.M., De Paolis M. Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: A systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery. Eur. J. Orthop. Surg. Traumatol. 2021;31:201–220. doi: 10.1007/s00590-020-02779-z. [DOI] [PubMed] [Google Scholar]
- 98.Sambri A., Bianchi G., Parry M., Frenos F., Campanacci D., Donati D., Jeys L. Is Arthrodesis a Reliable Salvage Option following Two-Stage Revision for Suspected Infection in Proximal Tibial Replacements? A Multi-Institutional Study. J. Knee Surg. 2018;32:911–918. doi: 10.1055/s-0038-1672121. [DOI] [PubMed] [Google Scholar]
- 99.Sambri A., Zucchini R., Giannini C., Zamparini E., Viale P., Donati D.M., De Paolis M. Correction to: Silver-coated (PorAg®) endoprosthesis can be protective against reinfection in the treatment of tumor prostheses infection. Eur. J. Orthop. Surg. Traumatol. 2020;30:1355. doi: 10.1007/s00590-020-02725-z. [DOI] [PubMed] [Google Scholar]
- 100.Sambri A., Zucchini R., Giannini C., Zamparini E., Viale P., Donati D.M., De Paolis M. Silver-coated (PorAg) Eur. J. Orthop. Surg. Traumatol. 2020;30:1345–1353. doi: 10.1007/s00590-020-02705-3. [DOI] [PubMed] [Google Scholar]
- 101.Wafa H., Grimer R.J., Reddy K., Jeys L., Abudu A., Carter S.R., Tillman R.M. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: Case-control study. Bone Jt. J. 2015;97-B:252–257. doi: 10.1302/0301-620X.97B2.34554. [DOI] [PubMed] [Google Scholar]
- 102.Zajonz D., Birke U., Ghanem M., Prietzel T., Josten C., Roth A., Fakler J.K.M. Silver-coated modular Megaendoprostheses in salvage revision arthroplasty after periimplant infection with extensive bone loss-a pilot study of 34 patients. BMC Musculoskelet. Disord. 2017;18:383. doi: 10.1186/s12891-017-1742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verma N., Jain A., Pal C., Thomas S., Agarwal S., Garg P. Management of periprosthetic fracture following total knee arthroplasty-a retrospective study to decide when to fix or when to revise? J. Clin. Orthop. Trauma. 2020;11:S246–S254. doi: 10.1016/j.jcot.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schreiner A.J., Steidle C., Schmidutz F., Gonser C., Hemmann P., Stöckle U., Ochs G. Hip Revision arthroplasty of Periprosthetic Fractures Vancouver B2 and B3 with a Modular Revision Stem: Short-Term Results and Review of Literature. Z. Orthopädie Unf. 2022;160:40–48. doi: 10.1055/a-1209-4002. [DOI] [PubMed] [Google Scholar]
- 105.Fiore M., Rondinella C., Paolucci A., Morante L., De Paolis M., Sambri A. Functional Outcome after Reimplantation in Patients Treated with and without an Antibiotic-Loaded Cement Spacers for Hip Prosthetic Joint Infections. Hip Pelvis. 2023;35:32–39. doi: 10.5371/hp.2023.35.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sandiford N.A., Bolam S.M., Afzal I., Radha S. Clinical and Functional Outcomes of the Exeter V40 Short Stem in Primary and Revision Arthroplasty: Does the Indication Affect Outcomes in the Short Term? Hip Pelvis. 2023;35:40–46. doi: 10.5371/hp.2023.35.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.