Abstract
Background: The vast majority of electrohypersensitive (EHS) patients present headaches on contact with an electromagnetic source. Clinical features suggest that the headaches of these patients could be a variant of the migraine disease and could be treated as such. We aimed to assess the prevalence of migraine disease in EHS patients using a validated questionnaire. Methods: Patients with EHS defined according to WHO criteria were contacted through EHS patient support associations. They were required to answer a self-questionnaire including clinical data and the extended French version of the ID Migraine questionnaire (ef-ID Migraine) to screen for the migraine disease. Migraine prevalence and its 95% confidence interval (CI) were reported. Patients’ characteristics, symptoms (rheumatology, digestive, cognitive, respiratory, cardiac, mood, cutaneous, headache, perception, genital, tinnitus and tiredness) and impact on daily life were compared between migraineur and non-migraineur patients. Results: A total of 293 patients were included (97% women, mean age 57 ± 12 years). Migraine was diagnosed in 65% (N = 191; 95% CI: 60–71%) with the ef-ID Migraine. The migraine diagnosis was accompanied by nausea/vomiting in 50% of cases, photophobia in 69% or visual disturbances in 38%. All of the 12 symptoms assessed were of higher intensity in migraineurs than in non-migraineurs. The symptoms prevented social life in 88% of migraineurs and 75% of non-migraineurs (p < 0.01). Conclusions: Our work encourages us to consider the headaches of these patients as a possible variant of the migraine disease and, possibly, to manage them according to the current recommendations.
Keywords: migraine, headache, electrohypersensitive, electromagnetic fields, prevalence, multiple chemical sensitivity
1. Background
Electrohypersensitivity (EHS), or idiopathic environmental intolerance to electromagnetic fields, is defined by the World Health Organization (WHO) by three criteria: the perception by the subject of various non-specific functional symptoms, the absence of clinical and biological evidence to explain the symptoms and the attribution by the subjects themselves of these symptoms to exposure to electromagnetic fields, which are themselves diverse [1]. EHS affects between 3 and 5% of the French population [2].
The cause and scientific basis of this syndrome are widely debated [3,4,5,6,7]. The relationship between exposure to electromagnetic fields and patients’ symptoms has not yet been formally and reproducibly demonstrated in provocative studies [8,9,10]. However, in their 2020 Joint report, the US National Academies of Medicine, Science and Engineering officially recognized the existence of non-thermal effects of non-ionizing electromagnetic radiation in humans and, in particular, headaches [11]. Moreover, a 2020 study found that the electromagnetic radiation emitted by smartphones was one of the main triggers of migraines in a population of Thai adolescents [12].
One of the symptoms most frequently reported by EHS patients is headaches, which were present in 98% of cases in the largest series reported [13]. The collective expertise report on electromagnetic hypersensitivity of the French national agency for food, environmental and occupational health safety (ANSES) recommends defining whether the headaches of EHS people are in whole or in part migraines and whether these people are more prone to migraines than the rest of the population [2].
Headaches occurring in EHS patients share common features with the migraine disease, such as demographic characteristics of EHS patients, family history, description of the headaches, accompanying signs (photophobia, osmophobia, nausea) and, sometimes, clinical improvement with triptan. This suggests that the headaches of EHS patients could be a variant of the migraine disease and that they could be treated as such.
The aim of this study was to determine the prevalence of the migraine disease using the extended French version of the ID Migraine (ef-ID Migraine) questionnaire in a population of EHS patients [14,15]. Secondary objectives were to compare the characteristics and symptoms of migraineurs versus non-migraineurs.
2. Methods
2.1. Study Design and Population
The study was validated by the local ethics committee of Montpellier (IRB-MTP_2021_04_202100828 19 April 2021) in accordance with French regulations and registered on the ClinicalTrials.org study registration platform (NCT04845152).
Any French-speaking adult patient meeting the WHO criteria for EHS could participate [1]. The non-inclusion criteria concerned patients refusing to participate.
Patients were informed of the existence of the study through specific French associations of patients with an information letter and a questionnaire available online or by mail by request. Every volunteer could participate from 1 May to 1 December 2021 by answering and sending the completed questionnaire.
2.2. Collected Data
The questionnaire was composed of four parts detailing:
family history: allergy, food intolerance, asthma, epilepsy, migraines, intolerance to noise, light, smells or vibrations, fibromyalgia, electro-sensitivity and multiple chemical sensitivity (MCS),
personal history included the same items as well as a history of head trauma and dental care with amalgam placement,
characteristics of the pathology included the year of the symptoms onset, sources of electromagnetic radiation involved, triggering factor, medical diagnosis, existence of a file at the Departmental House for the Disabled and recognition as a disabled worker,
EHS symptoms, evaluated from 0 (no symptom) to 10 (very intense and disabling symptoms) in twelve categories. Rheumatology symptoms included pain, cramps and stiffness or weakness of muscles or joints. Digestive symptoms included abdominal pain or cramps, bloating, nausea, diarrhea or constipation. Cognitive symptoms included difficulty in concentrating, memory problems, feeling of disconnection, lack of words or difficulty in making decisions. Respiratory symptoms included irritation of the eyes, shortness of breath, chest tightness or cough. Cardiac symptoms included accelerated or irregular heartbeat, extrasystoles palpitations or discomfort in the chest. Feeling tense or nervousness, irritability, depression, crying or angry outbursts or disinterest in activities that usually motivate were in the mood category. Rash, hives or dry skin concerned cutaneous. Headache symptoms included headaches or feeling of a heavy head or congested face. Perception symptoms included balance disorder or coordination disorder, numbness or tingling in extremities or blurred visual blur. Genital symptoms included pelvic pain or frequent urination. Tinnitus was defined by the sentence “noises in the ears”. Tiredness symptoms corresponded to fatigue or sleep disorders.
medical treatments ongoing,
classification of the symptoms’ impact on daily life defined by our clinical experience: stage 1, the symptoms do not modify daily life; stage 2, the symptoms oblige the patient to implement avoidance measures and stage 3, the symptoms prevent a normal social life,
the extended French version of thew ID Migraine questionnaire. The ef-ID Migraine is a brief, practical and easy-to-use diagnostic tool for migraines. This self-administered questionnaire composed of four items assesses disabling headaches occurring in the past year associated with nausea or vomiting and/or photophobia and/or prodromal visual signs [14,15]. The association should lead to the consideration of a migraine disease, which should be confirmed by a specialized consultation according to the criteria of the international classification of headaches (ICHD-3) [16].
In this study, headache was defined as pain in any region of the head.
The main outcome was the proportion of patients suffering from migraine according to the ef-ID Migraine questionnaire.
2.3. Sample Size
According to our clinical practice, we estimated that 60% of EHS patients seen in consultation suffered from the migraine disease. To estimate this prevalence with an accuracy of ±6% and an alpha error of 5%, 256 patients were required. This sample size was increased by 20% in anticipation of incomplete or unusable questionnaires, yielding a number of subjects to be included that is close to 312.
2.4. Statistical Analysis
All the patients included were analyzed. The prevalence of migraine was reported with its 95% confidence interval. For the characteristics of the patients, data were expressed as the number and percentage for qualitative variables. Continuous variables were expressed as the mean and standard deviation when the distribution was Gaussian and as median and quartiles (Q25; Q75) otherwise. Characteristics of migraineurs versus non-migraineurs were compared using the Student or Wilcoxon Mann–Whitney test for continuous variables and the Chi-square or Fisher test for categorical ones.
All analyses were two-tailed, with a p value of <0.05 considered statistically significant. The statistical analyses were carried out using SAS® (SAS Institute, Cary, NC, USA).
3. Results
During the recruitment period, 317 questionnaires were received, 23 patients were not eligible because they did not report symptoms related to EHS and 1 patient was excluded from the analysis because of an a posteriori refusal to use his data. Thus, 293 patients were analyzed.
Patients’ characteristics are presented in Table 1. They were mainly women, with a mean age of 57 years and a healthy weight. Histories of allergy, food intolerance, multiple chemical sensitivity (MCS) and migraine were present in the majority of patients. All patients reported an onset of symptoms following exposure to electromagnetic radiation, which disappeared at the end of the exposure in 210 patients (81%) and reappeared systematically at a new exposure in 271 patients (97%). EHS-related symptoms were reported a decade earlier. Finally, most patients interviewed reported an impact of EHS on their daily life according to the proposed classification, through a change in the way they live (99%), the implementation of avoidance measures (99%) and/or an impact on social life (83%).
Table 1.
Characteristics of patients.
| Patients’ Description and History | n | Values |
|---|---|---|
| Women | 292 | 283 (96.9) |
| Age, years, mean ± SD | 291 | 56.5 ± 12.3 |
| BMI, kg/m2, median [IQ25-75] | 292 | 21.9 [19.7; 24.3] |
| History of allergy | 293 | 197 (67.2) |
| History of food intolerance | 293 | 188 (64.2) |
| History of asthma | 293 | 45 (15.4) |
| History of seizure | 293 | 10 (3.4) |
| History of migraine | 293 | 178 (60.7) |
| History of noise intolerance | 293 | 184 (62.8) |
| History of light intolerance | 293 | 150 (51.2) |
| History of vibrations intolerance | 293 | 123 (42,0) |
| History of smells intolerance | 293 | 185 (63.1) |
| History of MCS | 293 | 159 (54.3) |
| History of fibromyalgia | 293 | 61 (20.8) |
| History of dental care | 293 | 242 (82.6) |
| History of brain injury | 293 | 60 (20.5) |
| History of Lyme disease | 293 | 58 (19.8) |
| Electrohypersensitivity characteristics | ||
| Duration between first EHS symptoms and study, years, median [IQ25-75] | 293 | 10 [5; 16] |
| Diagnosis of EHS made by a doctor | 293 | 217 (74.1) |
| Sick leave for EHS | 293 | 64 (21.8) |
| Medication for EHS | 293 | 97 (33.1) |
| Departmental House for the Disabled file | 293 | 141 (48.1) |
| Recognition as a disabled worker | 131 | 94 (71.8) |
| Trigger identified | 293 | 204 (69.6) |
| Symptoms appear during exposure to a source of electromagnetic radiation | 293 | 293 (100) |
| Symptoms stop after exposure is stopped | 258 | 210 (81.4) |
| Symptoms appear systematically in response to new exposure | 280 | 271 (96.8) |
| During the past 12 months, symptoms have been occurring more and more frequently | 247 | 157 (63.6) |
| Symptoms triggered by new sources | 220 | 141 (64.1) |
| Headaches | 293 | 230 (78.5) |
| Headaches and nausea/vomiting | 226 | 112 (49.6) |
| Headaches and photophobia | 228 | 158 (69.3) |
| Headaches and visual disturbances | 224 | 84 (37.5) |
| Migraine | 191 (65.2) | |
| Migraine with aura | 84 (28.7) | |
| Number of electromagnetic field sources identified, mean ± SD | 293 | 12 (±5) |
| Electrohypersensitivity impact on daily life | ||
| Stage 1: Symptoms do not change the way of living | 292 | 1 (0.3) |
| Stage 2: Symptoms force putting in place avoidance measures | 291 | 47 (16) |
| Stage 3: Symptoms prevent social life | 290 | 241 (83) |
| Intensity of symptoms in EHS patients, median [IQ25-75] | ||
| Rheumatology | 287 | 7 [4–8] |
| Digestive | 284 | 6 [3–8] |
| Cognitive | 289 | 8 [7–10] |
| Respiratory | 286 | 6 [3–8] |
| Cardiac | 290 | 6 [4–8] |
| Mood | 284 | 8 [5–9] |
| Cutaneous | 273 | 4 [0–7] |
| Headache | 287 | 8 [7–10] |
| Perception | 284 | 7 [4–8] |
| Genital | 282 | 4.5 [0–7] |
| Tinnitus | 286 | 7 [2–9] |
| Tiredness | 286 | 9 [8–10] |
BMI: Body mass index; EHS: Electrohypersensitivity; MCS: Multiple chemical sensibility; IQ: interquartile. Values are n (%) unless otherwise stated.
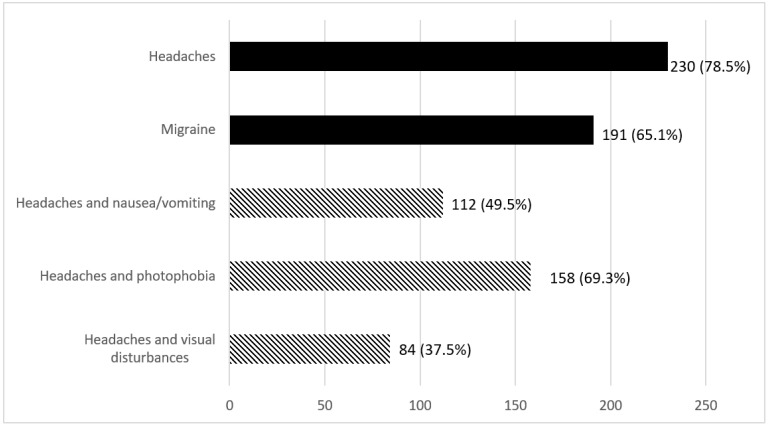
According to the results of the ef-ID Migraine questionnaire presented in Figure 1, 230 (78.5%) patients reported suffering from headaches, of which 191 (83%) could be suspected of having a migraine. The estimated prevalence of migraine was therefore 65% (95% CI 60–71%) in our population of 293 participants. A history of migraine was reported in 142 of the 191 patients identified as migraineurs by the questionnaire (74%) and in 36 of the 102 non-migraineurs (35%). Conversely, 142 of the 178 patients reporting a history of migraine were identified as migraineurs by the questionnaires (80%).
Figure 1.
Description of migraine with the ef-ID Migraine tool. Hatched values represent associated symptoms in headaches sufferers. Black values represent all patients studied.
The comparison of patients identified as migraineurs with non-migraineurs is presented in Table 2. Migraine patients were significantly younger, while the gender and BMI were comparable between the two groups.
Table 2.
Comparison between EHS and migraineur patients identified with the ef-ID Migraine tool and EHS non-migraineurs.
| Migraineur n = 191 |
Non-Migraineur n = 102 |
p | |
|---|---|---|---|
| Age, years, mean ± SD | 54.6 ± 11.9 | 59.97 ± 12.2 | <0.01 |
| Women | 183 (96.3) | 100 (98.0) | 0.68 |
| BMI, kg/cm2, median (IQ25-75) | 21.8 (19.5–24.5) | 22.0 (20.1–23.9) | 0.55 |
| History of allergy | 133 (69.6) | 64 (62.7) | 0.23 |
| History of food intolerance | 130 (68.1) | 58 (56.9) | 0.06 |
| History of asthma | 35 (18.3) | 10 (9.8) | 0.05 |
| History of seizure | 9 (4.7) | 1 (0.9) | 0.17 |
| History of migraine | 142 (74.3) | 36 (35.3) | <0.01 |
| History of noise intolerance | 130 (68.1) | 54 (52.9) | 0.01 |
| History of light intolerance | 120 (62.8) | 30 (29.4) | <0.01 |
| History of vibrations intolerance | 90 (47.1) | 33 (32.3) | 0.01 |
| History of smells intolerance | 132 (69.1) | 53 (51.9) | <0.01 |
| History of MCS | 116 (60.7) | 43 (42.2) | <0.01 |
| History of fibromyalgia | 49 (25.6) | 12 (11.8) | <0.01 |
| History of dental care | 162 (84.8) | 80 (78.4) | 0.17 |
| History of brain injury | 45 (23.6) | 15 (14.7) | 0.07 |
| History of Lyme disease | 41 (21.5) | 17 (16.7) | 0.33 |
| Electrohypersensitivity characteristics | |||
| Symptoms appear during exposure to a source of electromagnetic radiation | 191 (100.0) | 102 (100.0) | . |
| Symptoms stop after exposure is stopped | 134 (81.2) | 76 (81.7) | 0.92 |
| Symptoms appear systematically in response to new exposure | 178 (97.3) | 93 (95.9) | 0.50 |
| During the past 12 months, symptoms have been occurring more and more frequently | 111 (68.1) | 46 (54.7) | 0.04 |
| Symptoms triggered by new sources | 99 (68.7) | 42 (55.3) | 0.05 |
| Number of electromagnetic field sources identified, mean ± SD | 12.92 ± 4.7 | 10.33 ± 5.0 | <0.01 |
| Electrohypersensitivity impact on daily life | |||
| Symptoms changed the way of living | 188 (98.9) | 100 (98.0) | 0.61 |
| Symptoms forced to put in place avoidance measures | 188 (99.8) | 99 (97.0) | 0.13 |
| Symptoms prevent social life | 165 (87.8) | 76 (74.5) | <0.01 |
BMI: Body mass index; EHS: Electrohypersensitivity; MCS: Multiple chemical sensibility; IQ: interquartile. Values are n (%) unless otherwise stated.
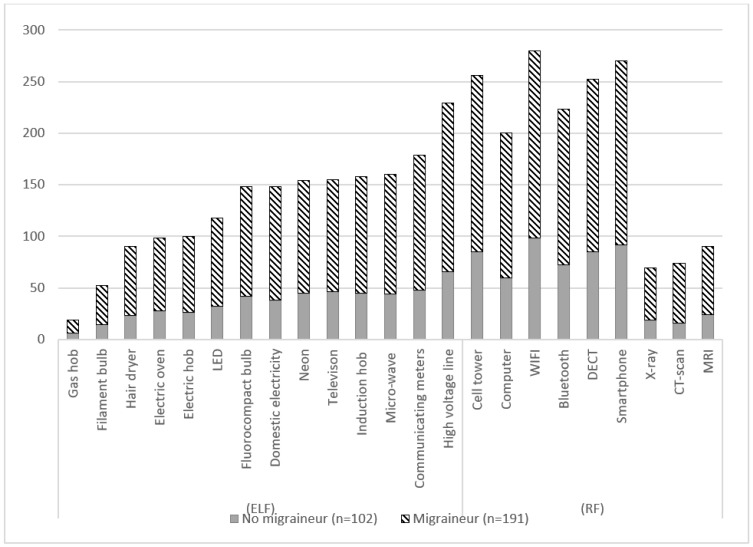
Histories of asthma, migraine, fibromyalgia and MCS were more frequent among migraineurs than non-migraineurs. Migraineur patients reported more intolerance to noise, light, vibrations and smells. Furthermore, a greater proportion of migraineurs patients reported discomfort in social life, although the proportion of patients reporting a change in lifestyle or the implementation of avoidance measures was comparable in the two groups. Migraineurs reported more electromagnetic (EM) field sources responsible for EHS symptoms than non-migraineurs (13 ± 5 vs. 10 ± 5, p < 0.01). The details of the sources of EM fields are presented in Figure 2.
Figure 2.
Description of electrohypersensitivity sources expressed according to the number of declarations in each category: all participants, migraineurs identified with the ef-ID-Migraine tool and non-migraineurs. RF: Radio frequencies; ELF: Extremely low frequencies.
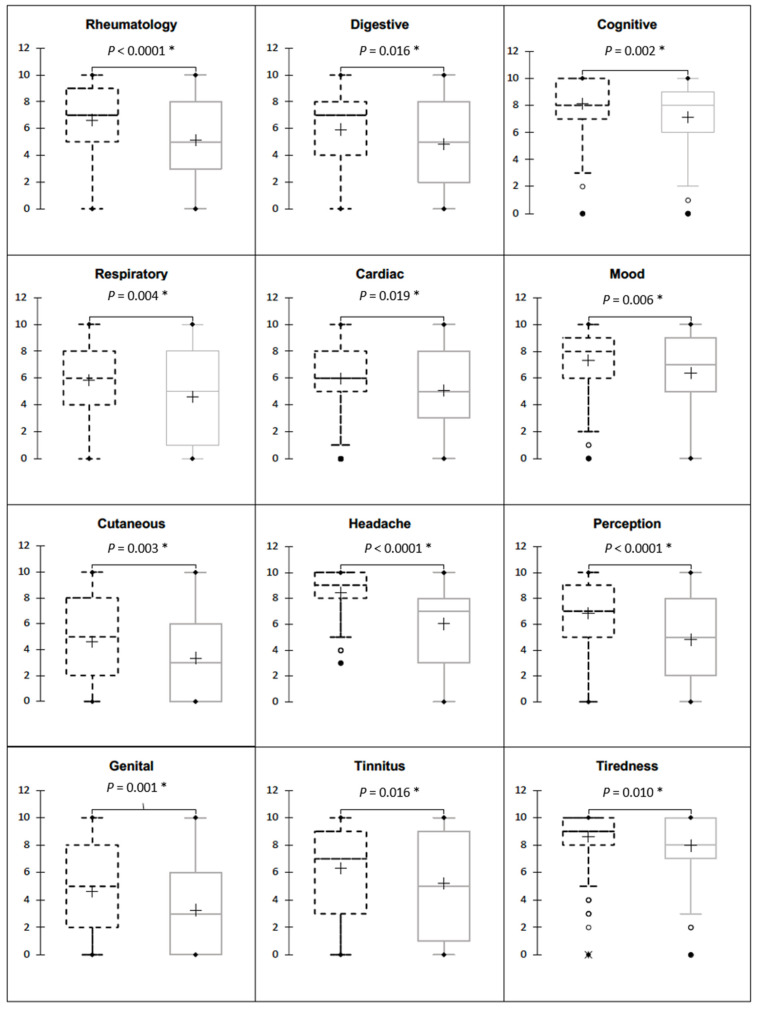
The total population described symptoms with a median intensity greater than or equal to four out of ten (Figure 3). Tiredness, headache, mood disturbance and cognitive symptoms were the symptoms with intensities greater than or equal to five out of ten in 75% of the patients studied (Table 1).
Figure 3.
Comparison intensity of the twelve symptoms studied in migraineurs (dashed lines) versus non-migraineurs (grey lines). The cross represents the mean value, the medium line represents the median value, the box represents the first and third quartiles, whiskers represent minimum and maximum adjacent values and circles represent outliers. *: a p-value < 0.5 was considered statistically significant.
The comparison of these symptoms in the identified migraineur population versus the non-migraineur population showed a significantly greater intensity in migraine patients (Figure 3). The difference was clinically and statistically relevant for all visceral symptoms. The intensity of symptoms classically attributed to EHS, tiredness, headaches, perceptual disturbances, mood disturbances and cognitive impairment intensity was higher in migraineur patients.
4. Discussion
The results of this study show that EHS patients present nonspecific and varied symptoms which meet the current definition of EHS. Similarly, all the patients who responded to the questionnaire reported that symptoms appeared following exposure to electromagnetic fields and disappeared rapidly after exposure for 81% of them and reappeared systematically on re-exposure in almost all patients. Importantly, in 74% of the cases, the diagnosis was made by a physician, particularly by ruling out any other pathology that might explain the clinical signs.
The symptoms affecting the nervous system were the most frequent and the noisiest. In particular, 78.5% of our patients suffered from headaches, whereas their prevalence was estimated at 52% in a literature review [17]. Symptoms were of moderate to severe intensity and seemed to be concomitant with signs of allergy and intolerance to chemical products evocative of a multiple chemical sensitivity, described in 54% of our patients [18]. This observation is relayed in a large study that shows that EHS is associated with MCS patients in 30% of cases and could be a good clinical criterion for the diagnosis of EHS [13].
We observed that the sources involved were mostly related to radiofrequency and that the mean duration of the pathology was about ten years. These observations suggest a symptoms onset in the 2010s, corresponding to the explosion of “wireless” technologies with the generalization of smartphones and WIFI. These findings are in line with the current literature [19]. Of note, these symptoms prevented a normal social life in most patients.
Thus, all these elements make us think that the population studied corresponds well with patients suffering from EHS in accordance with the definitions of the WHO and Belpomme et al.: absence of a known pathology explaining the observed clinical symptoms, association of symptoms with headache, tinnitus, hyperacusis, vertigo, immediate memory loss and attention deficit/concentration, reproducibility of symptoms under the said influence of electromagnetic fields, regression or disappearance of symptoms in the case of said avoidance of electromagnetic fields and association with multiple chemical sensitivity [1,13].
However, the current definition of EHS relies on subjective criteria, which is a source of bias for studies dealing with this population [4]. In order to obtain the most homogeneous population possible to be able to answer our question, we asked the EHS patient associations to help us disseminate the existence of the study, although it has been shown that the patients recruited by this mean had more marked symptoms than people with EHS recruited by a call for participation aimed at the general population [20]. We also note that our sample differs from those in the literature by the preponderance of women, who usually make up about 70% of the subjects, while they represented nearly 97% of our sample [21]. There are additional biases to consider: the number of EHS patients who did not participate in the study cannot be estimated, and each data item studied is derived from the participants’ declarations. In addition, the diagnosis of migraine is not validated by a medical specialist, although the ef-ID-Migraine questionnaire is relatively reliable in the literature [15]. As our study focused on the prevalence of migraine disease in electrosensitive patients, we did not study the characteristics of migraine pathology in depth. Indeed, we have not supplemented the ef-ID Migraine screening questionnaire with more specific questions such as the frequency of headaches, therefore preventing us from determining the proportion of patients suffering from chronic migraine [22]. Nevertheless, our work allowed us to determine that 65% of the interviewed persons were likely to present a migraine disease, with 56% of these patients presenting migraines without aura and 44% presenting migraines with aura.
Taking these reflections into consideration, the important message is that the prevalence of migraine disease identified by the ef-ID-Migraine in our sample was 65% (95% CI 60–71%). This prevalence seems to be much higher than that in the general female French population, where it ranged from 11 to 30% [23,24]. In a Belgian study, the prevalence was 26% in the whole sample and 33.4% in women with the same tool, and among the potential migraineurs, 41% had visual signs, which is close to our findings (44%) [25].
According to the ef-ID-Migraine tool, migraineur patients showed a more pronounced hypersensitivity, with a more frequent intolerance to noise, vibrations, light and odors and symptoms of higher intensity than non-migraineur patients. The results also showed that nearly 61% of the respondents declared a history of migraine. This proportion does not correspond exactly to the proportion identified with the questionnaire (65.2%). It seems that part of the patients studied did not consider themselves as migraine sufferers, and vice versa, highlighting a potential bias in understanding the questions. Patients with a history of migraine and migraineur patients did not take any specific treatment for migraine. Indeed, it is known that migraine sufferers are generally reluctant to take medication. Furthermore, 60% of these patients have elements suggestive of multiple chemical sensitivity; thus, we can assume that they are not taking any treatment because of intolerance and numerous side effects that prevent the benefits. However, it seems that our sample of patients complaining about headaches and probably migraines could at least be offered management of headaches in accordance with the recommendations of the French Society for the Study of Migraines and Headaches [26,27,28].
All these data can suggest a central sensitization syndrome [29]. It would have been interesting to specify this in the course of this investigation, taking the example of our Japanese colleagues who consider a link between migraine, multiple chemical sensitivity and central sensitization syndrome [30]. Regarding migraineurs’ prevalence in this studied population and the link between migraine diseases and central sensitization syndrome, it could be interesting to explore the allodynia phenomenon, related to both diseases but not studied in this survey [31,32,33].
As our work does not specifically investigate the sources of electromagnetic fields most often associated with negative health effects by electrosensitive patients, we can only observe that the impact of electromagnetic fields varies according to the frequency, distance and type of electrical appliance.
This study does not pretend to establish the responsibility of electromagnetic fields in the occurrence of migraines in the patients who answered the questionnaire; however, the fact that electromagnetic fields may be a trigger for migraines is not new. There are many hypotheses for an explanation of headaches in EHS [22,34]. Many migraine patients find that the change in weather is a trigger for their headaches. In the region of Giessen, Germany, Vaitl et al. found a correlation in autumn between sferic activity and the occurrence of migraine attacks [35]. Panangopoulos et al. describe this phenomenon of meteoropathy related to the extremely low frequencies of electromagnetic pulses in thunderstorms and propose a mechanism by which voltage-dependent cationic channels, called electrosensitive, are activated by the polarized and pulsed electromagnetic signal generated by lightning [36]. Moreover, this sensitivity mechanism is also used in tumor reduction or control therapy by administering 27.12 MHz amplitude-modulated radiofrequency electromagnetic fields that are thought to act on cancer cells via certain voltage-dependent calcium channels [37,38]. A review of the literature on the effect of electromagnetic radiation on neuronal ion channels provides insight into the magnitude of the phenomenon and concludes that ion channels represent a major transducer of the effects of electromagnetic fields on the central nervous system [39].
5. Conclusions
Our work seems to indicate that the prevalence of migraine disease in EHS individuals is much higher than that in the general population and constitutes the beginning of an answer to the questioning of the French national agency for food environmental and occupational health safety. It incites the continuation of research work and encourages practitioners confronted with electrohypersensitive patients to look for headaches and manage them in accordance with the recommendations of medical societies.
Acknowledgments
We would like to thank not only all the patients for their collaboration but also the associations of help and support to electrohypersensitive patients, which allowed us to contact them: Robin des toits (https://www.robindestoits.org, accessed on 15 June 2023), Association zone blanches (asso-zonesblanches.org, accessed on 15 June 2023), POEM 26 (https://poem26.com, accessed on 15 June 2023), Cœur d’EHS (http://coeursdehs.fr, accessed on 15 June 2023) and SOS MCS (https://sosmcs.fr, accessed on 15 June 2023). We would also like to thank Anne Ducros from the Neurology Department of the Montpellier University Hospital for giving us a better understanding of migraine disease.
Abbreviations
EHS: Electrohypersensitive; WHO: World Health Organization; ANSES: Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail; MCS: Multiple chemical sensitivity; ICDH: International classification of headaches; BMI: Body mass index; EM: Electromagnetic; RF: Radio frequencies; ELF: Extremely low frequencies.
Author Contributions
F.G. and M.C.P. designed the study. O.G. collected and analyzed the data. M.C.P. performed an independent statistical analysis. F.G. and O.G. drafted the manuscript. V.M. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of UNIVERSITY HOSPITAL CENTER OF MONTPELLIER (IRB-MTP_2021_04_202100828 19/04/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study according to the French law.
Data Availability Statement
Data will be available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors of this manuscript declare no conflicts of interest. They did not receive funding or compensation of any kind for this study.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Prague C.R., Mild K.H., Repacholi M.H., van Deventer E. World Health Organization Electromagnetic hypersensitivity; Proceedings of the International Workshop on Electromagnetic Field Hypersensitivity; Prague, Czech Republic. 25–27 October 2004; [(accessed on 15 August 2022)]. Available online: https://apps.who.int/iris/handle/10665/43435. [Google Scholar]
- 2.Hypersensibilité Électromagnétique ou Intolérance Environnementale Idiopathique Attribuée aux Champs Électromagnétiques. 2018. [(accessed on 12 June 2023)]. Available online: https://www.anses.fr/fr/content/avis-et-rapport-de-lanses-relatif-à-l’expertise-sur-l’hypersensibilité-electromagnétique-ehs.
- 3.Stein Y., Udasin I.G. Electromagnetic hypersensitivity (EHS, microwave syndrome)—Review of mechanisms. Environ. Res. 2020;186:109445. doi: 10.1016/j.envres.2020.109445. [DOI] [PubMed] [Google Scholar]
- 4.Leszczynski D. Review of the scientific evidence on the individual sensitivity to electromagnetic fields (EHS) Rev. Environ. Health. 2021;37:423–450. doi: 10.1515/reveh-2021-0038. [DOI] [PubMed] [Google Scholar]
- 5.Belpomme D., Carlo G.L., Irigaray P., Carpenter D.O., Hardell L., Kundi M., Belyaev I., Havas M., Adlkofer F., Heuser G., et al. The Critical Importance of Molecular Biomarkers and Imaging in the Study of Electrohypersensitivity. A Scientific Consensus International Report. Int. J. Mol. Sci. 2021;22:7321. doi: 10.3390/ijms22147321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieudonné M. Electromagnetic hypersensitivity: A critical review of explanatory hypotheses. Environ. Health. 2020;19:48. doi: 10.1186/s12940-020-00602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belpomme D., Irigaray P. Why the psychogenic or psychosomatic theories for electrohypersensitivity causality should be abandoned, but not the hypothesis of a nocebo-associated symptom formation caused by electromagnetic fields conditioning in some patients. Environ. Res. 2022:114839. doi: 10.1016/j.envres.2022.114839. Online ahead of print . [DOI] [PubMed] [Google Scholar]
- 8.Rubin G.J., Hillert L., Nieto-Hernandez R., van Rongen E., Oftedal G. Do people with idiopathic environmental intolerance attributed to electromagnetic fields display physiological effects when exposed to electromagnetic fields? A systematic review of provocation studies. Bioelectromagnetics. 2011;32:593–609. doi: 10.1002/bem.20690. [DOI] [PubMed] [Google Scholar]
- 9.Schmiedchen K., Driessen S., Oftedal G. Methodological limitations in experimental studies on symptom development in individuals with idiopathic environmental intolerance attributed to electromagnetic fields (IEI-EMF)—A systematic review. Environ. Health. 2019;18:88. doi: 10.1186/s12940-019-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bordarie J., Dieudonné M., Ledent M., Prignot N. A qualitative approach to experiential knowledge identified in focus groups aimed at co-designing a provocation test in the study of electrohypersensitivity. Ann. Med. 2022;54:2362–2374. doi: 10.1080/07853890.2022.2114605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Academies of Sciences, Engineering, and Medicine . Standing Committee to Advise the Department of State on Unexplained Health Effects on U.S. Government Employees and Their Families at Overseas Embassies; Health and Medicine Division; Division on Engineering and Physical Sciences. In: Relman D.A., Pavlin J., editors. An Assessment of Illness in U.S. Government Employees and Their Families at Overseas Embassies. National Academies Press; Washington, DC, USA: 2020. [(accessed on 12 June 2023)]. p. 25889. Available online: https://www.nap.edu/catalog/25889. [PubMed] [Google Scholar]
- 12.Chongchitpaisan W., Wiwatanadate P., Tanprawate S., Narkpongphan A., Siripon N. Trigger of a migraine headache among Thai adolescents smartphone users: A time series study. Environ. Anal. Health Toxicol. 2021;36:e2021006. doi: 10.5620/eaht.2021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belpomme D., Irigaray P. Electrohypersensitivity as a Newly Identified and Characterized Neurologic Pathological Disorder: How to Diagnose, Treat, and Prevent It. Int. J. Mol. Sci. 2020;21:1915. doi: 10.3390/ijms21061915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streel S., Donneau A.-F., Dardenne N., Hoge A., Bruyère O., Albert A., Guillaume M., Schoenen J. Validation of an extended French version of ID MigraineTM as a migraine-screening tool. Cephalalgia. 2015;35:437–442. doi: 10.1177/0333102414544910. [DOI] [PubMed] [Google Scholar]
- 15.Lipton R.B., Dodick D., Sadovsky R., Kolodner K., Endicott J., Hettiarachchi J., Harrison W. ID Migraine validation study A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology. 2003;61:375–382. doi: 10.1212/01.WNL.0000078940.53438.83. [DOI] [PubMed] [Google Scholar]
- 16.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 17.Stovner L.J., Hagen K., Linde M., Steiner T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain. 2022;23:34. doi: 10.1186/s10194-022-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Multiple chemical sensitivity: A 1999 consensus. Arch Environ. Health. 1999;54:147–149. doi: 10.1080/00039899909602251. [DOI] [PubMed] [Google Scholar]
- 19.Kacprzyk A., Kanclerz G., Rokita E., Tatoń G. Which sources of electromagnetic field are of the highest concern for electrosensitive individuals?—Questionnaire study with a literature review. Electromagn. Biol. Med. 2021;40:33–40. doi: 10.1080/15368378.2020.1839489. [DOI] [PubMed] [Google Scholar]
- 20.van Dongen D., Smid T., Timmermans D.R.M. Symptom attribution and risk perception in individuals with idiopathic environmental intolerance to electromagnetic fields and in the general population. Perspect. Public Health. 2014;134:160–168. doi: 10.1177/1757913913492931. [DOI] [PubMed] [Google Scholar]
- 21.Belpomme D., Campagnac C., Irigaray P. Reliable disease biomarkers characterizing and identifying electrohypersensitivity and multiple chemical sensitivity as two etiopathogenic aspects of a unique pathological disorder. Rev. Environ. Health. 2015;30:251–271. doi: 10.1515/reveh-2015-0027. [DOI] [PubMed] [Google Scholar]
- 22.Toffa D.H., Sow A.D. The enigma of headaches associated with electromagnetic hyperfrequencies: Hypotheses supporting non-psychogenic algogenic processes. Electromagn. Biol. Med. 2020;39:196–205. doi: 10.1080/15368378.2020.1762638. [DOI] [PubMed] [Google Scholar]
- 23.A Nationwide Survey of Migraine in France: Prevalence and Clinical Features in Adults—Patrick Henry, Philippe Michel, Bruno Brochet, Jean François Dartigues, Sylvie Tison, Roger Salamon. 1992. [(accessed on 27 June 2022)]. Available online: https://journals.sagepub.com/doi/10.1046/j.1468-2982.1992.1204229.x. [DOI] [PubMed]
- 24.Henry P., Auray J.P., Gaudin A.F., Dartigues J.F., Duru G., Lantéri-Minet M., Lucas C., Pradalier A., Chazot G., El Hasnaoui A. Prevalence and clinical characteristics of migraine in France. Neurology. 2002;59:232–237. doi: 10.1212/WNL.59.2.232. [DOI] [PubMed] [Google Scholar]
- 25.Streel S., Donneau A.-F., Hoge A., Albert A., Schoenen J., Guillaume M. One-year prevalence of migraine using a validated extended French version of the ID MigraineTM: A Belgian population-based study. Rev. Neurol. 2015;171:707–714. doi: 10.1016/j.neurol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Demarquay G., Moisset X., Lantéri-Minet M., de Gaalon S., Donnet A., Giraud P., Guégan-Massardier E., Lucas C., Mawet J., Roos C., et al. Revised guidelines of the French Headache Society for the diagnosis and management of migraine in adults. Part 1: Diagnosis and assessment. Rev. Neurol. 2021;177:725–733. doi: 10.1016/j.neurol.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Ducros A., de Gaalon S., Roos C., Donnet A., Giraud P., Guégan-Massardier E., Lantéri-Minet M., Lucas C., Mawet J., Moisset X., et al. Revised guidelines of the French headache society for the diagnosis and management of migraine in adults. Part 2: Pharmacological treatment. Rev. Neurol. 2021;177:734–752. doi: 10.1016/j.neurol.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Demarquay G., Mawet J., Guégan-Massardier E., de Gaalon S., Donnet A., Giraud P., Lantéri-Minet M., Lucas C., Moisset X., Roos C., et al. Revised guidelines of the French headache society for the diagnosis and management of migraine in adults. Part 3: Non-pharmacological treatment. Rev. Neurol. 2021;177:753–759. doi: 10.1016/j.neurol.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Woolf C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki K., Okamura M., Haruyama Y., Suzuki S., Shiina T., Kobashi G., Hirata K. Exploring the contributing factors to multiple chemical sensitivity in patients with migraine. J. Occup. Health. 2022;64:e12328. doi: 10.1002/1348-9585.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burstein R., Yarnitsky D., Goor-Aryeh I., Ransil B.J., Bajwa Z.H. An association between migraine and cutaneous allodynia. Ann. Neurol. 2000;47:614–624. doi: 10.1002/1531-8249(200005)47:5<614::AID-ANA9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Bigal M.E., Ashina S., Burstein R., Reed M.L., Buse D., Serrano D., Lipton R.B., On behalf of the AMPP Group Prevalence and characteristics of allodynia in headache sufferers: A population study. Neurology. 2008;70:1525–1533. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altay H., Celenay S.T. An investigation of the relationship between cutaneous allodynia and kinesiophobia, gastrointestinal system symptom severity, physical activity and disability in individuals with migraine. Korean J. Pain. 2023;36:137–146. doi: 10.3344/kjp.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pall M.L. Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. J. Chem. Neuroanat. 2016;75:43–51. doi: 10.1016/j.jchemneu.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Vaitl D., Propson N., Stark R., Walter B., Schienle A. Headache and Sferics. Headache J. Head Face Pain. 2001;41:845–853. doi: 10.1111/j.1526-4610.2001.01155.x. [DOI] [PubMed] [Google Scholar]
- 36.Panagopoulos D.J., Balmori A. On the biophysical mechanism of sensing atmospheric discharges by living organisms. Sci. Total Environ. 2017;599–600:2026–2034. doi: 10.1016/j.scitotenv.2017.05.089. [DOI] [PubMed] [Google Scholar]
- 37.Costa F.P., de Oliveira A.C., Meirelles R., Machado M.C.C., Zanesco T., Surjan R., Chammas M.C., de Souza Rocha M., Morgan D., Cantor A., et al. Treatment of advanced hepatocellular carcinoma with very low levels of amplitude-modulated electromagnetic fields. Br. J. Cancer. 2011;105:640–648. doi: 10.1038/bjc.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez H., Wang M., Zimmerman J.W., Pennison M.J., Sharma S., Surratt T., Xu Z.X., Brezovich I., Absher D., Myers R.M., et al. Tumour-specific amplitude-modulated radiofrequency electromagnetic fields induce differentiation of hepatocellular carcinoma via targeting Cav3.2 T-type voltage-gated calcium channels and Ca2+ influx. eBioMedicine. 2019;44:209–224. doi: 10.1016/j.ebiom.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertagna F., Lewis R., Silva S.R.P., McFadden J., Jeevaratnam K. Effects of electromagnetic fields on neuronal ion channels: A systematic review. Ann. N. Y. Acad. Sci. 2021;1499:82–103. doi: 10.1111/nyas.14597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon reasonable request to the corresponding author.