Abstract
Angina or ischemia with no obstructive coronary disease (ANOCA/INOCA) is a common but under-treated condition due to poorly understood pathophysiologic mechanisms, limited diagnostic tools, and lack of proven targeted therapy. Coronary microvascular dysfunction (CMD) occurs when the microvasculature inadequately perfuses the myocardium under stress, or at rest in the case of microvascular spasm resulting in ANOCA/INOCA. Coronary functional angiography (CFA) measures endothelial independent microvascular dysfunction (coronary flow reduction <2.5) in response to adenosine and endothelial dependent microvascular dysfunction (lack of dilation and/or constriction) to acetylcholine testing as well as epicardial and microvascular spasm. Current treatment for coronary microvascular dysfunction is limited to renin-angiotensin system (RAS) inhibitors and statins as well as antianginal medications. Novel therapies targeting the underlying pathology are under development and include the coronary sinus reducer, CD34+ stem cell therapy, and novel pharmacologic agents such as sGC stimulators or endothelin-receptor blockers. We review the current understanding of pathophysiology, diagnostic tools, and novel therapies for coronary microvascular dysfunction in ANOCA/INOCA.
Keywords: INOCA, ANOCA, Coronary microvascular dysfunction, Coronary functional angiography, Coronary sinus reducer, CD34+ stem cell therapy
1. Introduction
Angina or ischemia with no obstructive coronary disease (ANOCA/INOCA) is an increasingly recognized problem with multiple potential etiologies [1]. Historically, patients with ANOCA/INOCA have been untreated, due to limited diagnostic tools and lack of proven targeted therapies [2]. Recently the CorMicA trial demonstrated that an accurate diagnosis of the underlying etiology of the angina (microvascular vs. vasospastic) with invasive coronary functional angiography (CFA) followed by targeted treatment strategies led to improvements in clinical outcomes [3]. These results led to the inclusion of CFA formally into the recent 2021 chest pain guidelines [4].
The Women's Ischemia Syndrome Evaluation (WISE) demonstrated that coronary microvascular dysfunction is not a benign diagnosis and is associated with significant increase in major adverse cardiovascular events (MACE) long-term [5]. We will discuss several novel therapies under development specifically designed to treat coronary microvascular dysfunction, an important cause of ANOCA/INOCA.
2. Prevalence
ANOCA/INOCA, defined as angina or ischemia with <50 % epicardial stenosis, is a frequent clinical problem. Roughly 40 % of patients undergoing coronary angiography for stable angina have non-obstructive coronary artery disease including 51 % of women and 33 % of men [6]. The ISCHEMIA trial required evidence of moderate or severe ischemia documented by stress testing and 24 % of patients with ischemia were found to have non-obstructive coronary disease based on coronary computerized tomography (CT) angiogram [7]. These patients were therefore excluded from the trial, but this further illustrates the frequency of INOCA in patients with documented ischemia. ANOCA/INOCA patients with ongoing angina despite medical therapy are now considered to have a type of refractory angina [8,9].
3. Pathophysiology
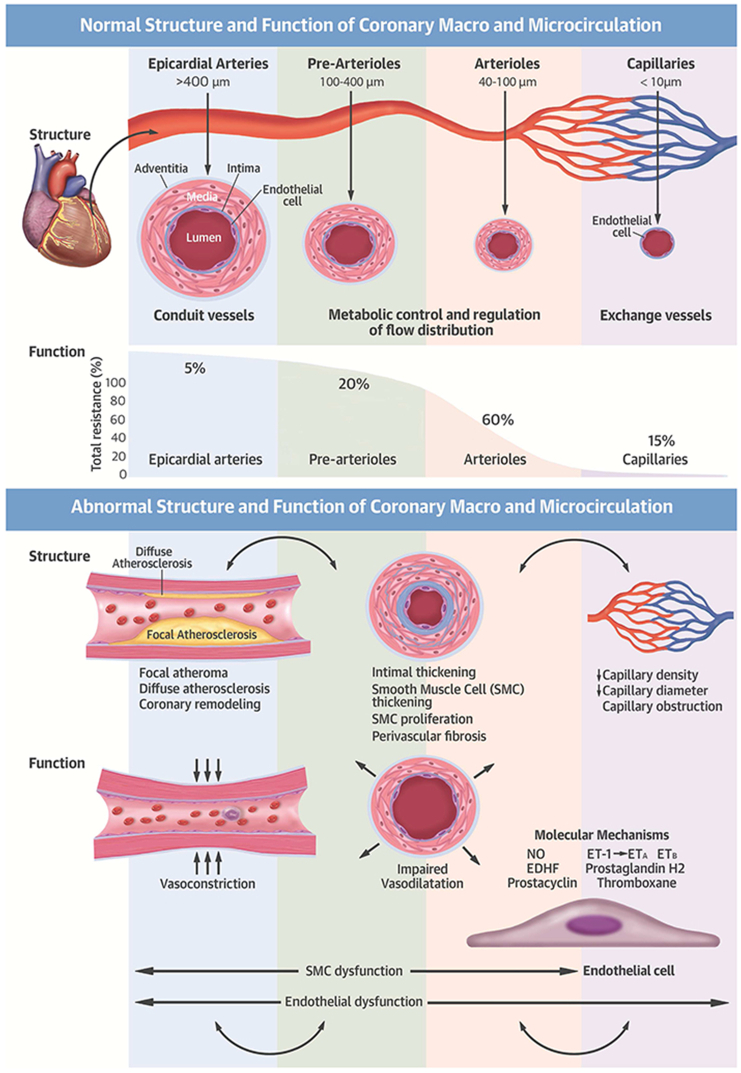
The epicardial arteries primary function is conductance, while the microvasculature is largely responsible for meeting demands of surrounding tissue (Fig. 1) by increasing coronary blow flow and vasodilation to match oxygen demand. Coronary microvascular dysfunction develops when the microvasculature is unable to adequately perfuse the myocardium under stress, or at rest in cases of microvascular spasm [10,11].
Fig. 1.
Normal and abnormal structure and function of the coronary macro- and microcirculation.
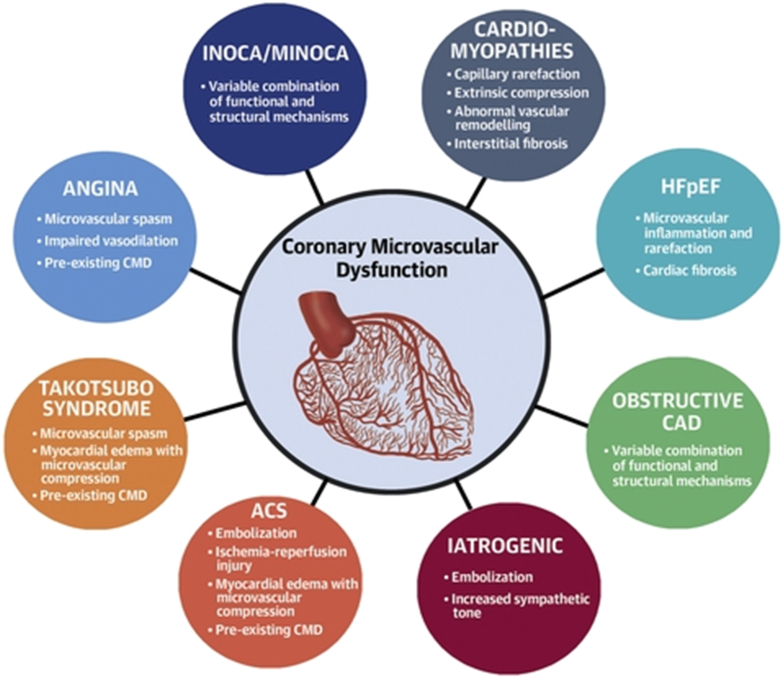
The microvasculature also plays a key role in many diseases beyond ANOCA/INOCA (Fig. 2). For example, 75 % of patients with heart failure with preserved ejection fraction (HFpEF) have abnormal coronary flow reserve (CFR) [12]. The highest risk patients with STEMI and non-STEMI are those with microvascular obstruction which correlates with a high index of microvascular resistance (IMR) [13]. About 20 to 25 % of patients post-PCI, (including post chronic total occlusion post percutaneous coronary intervention (PCI)) experience ongoing angina and many of these patients likely have microvascular dysfunction [14,15]. Microvascular disease predominantly affects women and frequently occurs without traditional cardiovascular risk factors but men and patients with traditional risk factors also may have microvascular dysfunction [16].
Fig. 2.
Coronary microvascular dysfunction across CVD.
4. Testing for coronary microvascular dysfunction
CFA is used in the diagnosis of the multiple abnormal pathways that contribute to ANOCA/INOCA presentation. Endothelial independent coronary microvascular dysfunction is diagnosed using adenosine-mediated coronary flow reserve (CFR) <2.5, index of microvascular resistance (IMR) >25, and hyperemic microvascular resistance (HMR) > 2.5 [1,17,18]. Endothelial dependent microvascular dysfunction is defined by the lack of dilation and/or constriction of the vessel during the mid-dose acetylcholine testing. It is important to note, that while the terms endothelial-independent and endothelial-dependent are frequently used, the pathways are more complicated. For example, L-Name blocks endothelial nitric oxide production and reduces CFR by 25 % only. Therefore, some prefer to use adenosine-mediated and acetylcholine mediated. Microvascular spasm is defined as <90 % epicardial vasoconstriction associated with chest pain and ischemic electrocardiographic changes in response to acetylcholine. The Coronary Vasomotion Disorders International Study Group uses the term microvascular angina to include endothelial dependent and independent coronary microvascular dysfunction and microvascular spasm [19]. Coronary vasospasm is defined as clinically significant epicardial vasoconstriction (>90 %), reproduction of chest pain and ischemic electrocardiographic changes, however spasm may also involve the microvasculature in approximately 48 % of patients [1,17,20].
5. Contemporary management of ANOCA/INOCA
To date, treatment of coronary microvascular and vasospastic angina has been limited to small studies on medications already in use for treatment of other cardiac disorders. The only medications shown to improve coronary microvascular dysfunction are renin-angiotensin system (RAS) inhibitors which improve CFR, and statins which have been shown to improve CFR and endothelial function likely related to pleotropic effects of statins, although the exact mechanisms remain unknown [1,3]. Calcium channel blockers (CCB) induce vascular smooth muscle relaxation and are indicated for vasospastic angina and have been shown to reduce the prevalence of epicardial spasm [21]. This frequently requires titration of the CCB and/or an additional CCB. In the Efficacy of Diltiazem to Improve Coronary Microvascular Dysfunction (EDIT-CMD) trial, patients were randomized to receive diltiazem (CCB) or placebo. Despite no significant differences in microvascular dysfunction improvement between the two groups, patients who received Diltiazem had improvement in their epicardial spasm, that either improved to microvascular spasm or completely subsided [21]. Beta-blockers improve anginal symptoms by decreasing heart rate and thereby cardiovascular demand but do not directly improve microvascular function. The EXAMINE–CAD (Clinical Trial#NCT05294887) is an ongoing multicenter, crossover clinical trial that compares the efficacy of beta-blockers and CCB after CFA for either the presence of abnormal microvascular vasodilation or abnormal vasoconstriction in patients with non-obstructive CAD. The Ilias ANOCA trial is a global registry assessing the potential therapeutic impact of combined pressure and flow measurements in evaluating epicardial stenosis and microvascular function (Clinical Trial#NCT04485234).
While nitroglycerin is indicated in patients with epicardial spasm, its efficacy in microvascular spasm may be limited. The Acetylcholine Rechallenge study investigated the response to nitrates in patients with epicardial or microvascular spasm. The study found that while nitrates were successful in reducing epicardial spasm, 80 % (44/55) of patients with microvascular spasm continued to experience symptoms to a second dose of acetylcholine despite the prior the administration of intracoronary nitroglycerin. These findings conflict with current treatment guidelines and suggest the need for a more personalized approach to oral nitrate treatment in microvascular spasm [20].
New oral therapies are under investigation to relieve symptoms of microvascular dysfunction. Ivabradine, indicated for heart failure, reduces heart rate similar to beta blockers without the associated blood pressure side effects. It has been shown to increase CFR in ANOCA patients leading to improvement in ischemic and anginal symptoms [22,23]. Furthermore, heightened diastolic tone seen in diastolic dysfunction can exacerbate microvascular dysfunction by increasing microvascular resistance. Ranolazine targets intracellular calcium overload, improving diastolic function along with microvascular dysfunction [8,9,24]. Additionally, Vericiguat, a soluble guanylate cyclase (sGC) simulator, increases intracellular cGMP levels causing vasodilation and inhibition of smooth muscle relaxation and therefore shows promise in the treatment of underlying microvascular dysfunction [25,26].
Recent studies indicate there are genetic mutations on the endothelin-1 pathway which can elevate levels of endothelin-1 (ET-1) in the bloodstream. This potent vasoconstrictor peptide has been linked to coronary microvascular dysfunction. Specifically, the rs-9349379-G allele has been found to raise plasma serum ET-1 levels (p = 0.005) and doubled the likelihood of developing microvascular disease (0.027) [27]. Zibotentan, a novel oral ETA receptor antagonist currently in phase 3 oncology trials, has been shown to reverse endothelian-1 vasoconstriction. The Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial is an ongoing randomized, double-blind, placebo-controlled trial examining the efficacy of Zibotentan in patients with microvascular angina. This also illustrates the potential role of precision medicine for individuals with coronary microvascular disease [27].
Enhanced external counter pulsation (EECP) is a non-invasive treatment for angina that involves applying external inflatable cuffs to the lower extremities to increase blood flow to the vasculature and is a potential therapeutic option for refractory angina including patients with microvascular dysfunction [8,9,28]. Cardiac rehabilitation is indicated in all patients and can improve vasodilation and potential growth of new micro-vessels [29]. Secondary prevention including smoking cessation, management of hypertension, diabetes and hyperlipidemia are also warranted.
The CorMicA trial provides insight into the importance of treatment based on the endotype [3]. Nearly 50 % of ANOCA/INOCA patients enrolled in the trial had microvascular dysfunction, and another 20 % had both microvascular dysfunction and vasospastic angina. Therefore, a total of 70 % of the patients had abnormal microvascular function. In addition, 10 to 15 % of patients had solely vasospastic angina and 10 % had non-cardiac related chest pain. For patients with microvascular angina beta-blockers were used as first line anti-anginals and nitrates were avoided whereas for vasospastic angina calcium-channel blockers and nitrates were utilized. The CorMicA study showed that patients randomized to stratified medical therapy guided by CFA results had marked and sustained angina improvement and better quality of life at 1 year following CFA compared to standard control group which was blinded to CFA results and received standard of care [3].
6. Novel invasive therapies for INOCA
Current treatment strategies for INOCA focused on treating the underlying cause are limited. Two novel therapies that appear to directly improve coronary microvascular function specifically are the coronary sinus reducer and infusion of CD34+ stem cells [8,9].
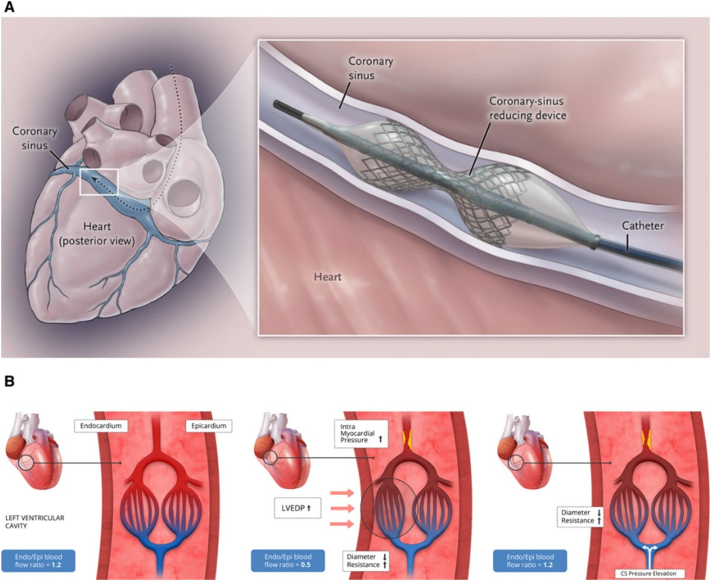
The idea for the coronary sinus reducer in the treatment of angina was based on the Beck surgical procedure which reduced the coronary sinus to 3 mm and provided relief of angina prior to the development of coronary artery bypass surgery [8,9]. The reducer is deployed via catheter using the right internal jugular vein accessing the coronary sinus to implant an hourglass shaped stent (Fig. 3). The stent is designed to create a controlled area of restenosis which creates a gradient in the venous outflow from the myocardium. The therapeutic mechanism is based on changing the endocardial to epicardial blood flow ratio in the heart [8,9]. A normal endocardial to epicardial blood flow ratio is 1.2, but in ischemia, the ratio drops, largely due to an increase in LVEDP. It is not unusual to see an endocardial to epicardial blood flow ratio of 0.5 in ischemic myocardium. By implanting the coronary sinus reducer and restricting outflow, the pressure in the coronary sinus is increased and the endocardial to epicardial blood flow ratio approaches normal limits.
Fig. 3.
Effect of the coronary sinus reducer on myocardial blood flow.
An initial clinical trial in refractory angina due to obstructive disease showed significant reduction in Canadian Cardiovascular Society (CCS) angina score 6 months after coronary sinus reducer placement with improvement in angina in 12/14 participants [30]. This was followed by the COSIRA trial which randomized 52 patients with refractory angina to receive a coronary sinus reducer and 52 to a sham procedure. Patients treated with the coronary sinus reducer had ≥2 CCS class improvement in angina and reduction in the overall CCS Angina Score compared to controls [31]. The coronary sinus reducer is now approved in many European countries and a large phase 3 trial in COSIRA-2 is underway in the U.S (Clinical Trial# NCT05102019). Recent data presented at the 2022 TCT meeting demonstrated improvement in CFR following implantation of the coronary sinus reducer in ANOCA/INOCA patients with microvascular dysfunction. COSIRA-2 will include a registry that will allow enrollment of patients with microvascular disease alone and a double blind, placebo-controlled trial specifically for microvascular dysfunction is under development.
Regenerative therapy with stem cells or gene therapy [32] is another novel therapy that appears promising for coronary microvascular disease. Similar to the coronary sinus reducer, this therapy may directly treat the underlying etiology of abnormal endothelial function. Preclinical studies have shown the infusion of CD34+ stem cells promote vascular repair, enhances angiogenesis, and increases capillary density [32]. CD34+ stem cell therapy was initially tested in patients with refractory angina due to obstructive coronary disease and the trials indicated persistent improvements in symptoms, decrease in mortality, and MACE [33]. In the REPAIR-AMI trial, patients that received intracoronary bone marrow mononuclear stem cells after an acute myocardial infarction had significant improvement in CFR compared to placebo [34]. These results laid the groundwork for the Phase One ESCaPE-CMD Trial [35]. A total of 20 patients with ongoing angina and CFR <2.5 were given G-CSF for five days and underwent apheresis to isolate the CD34+ stem cells, which were delivered by intra-coronary injection. There was substantial improvement in CFR from baseline to six months (CFR 2.08 to 2.68 p = 0.0045). Patients experienced decreased angina frequency, improvement in angina class, and Seattle Angina Questionnaire. This was the first study of CD34+ cells in patients with coronary microvascular dysfunction showing encouraging results and no cell-related adverse events [35].
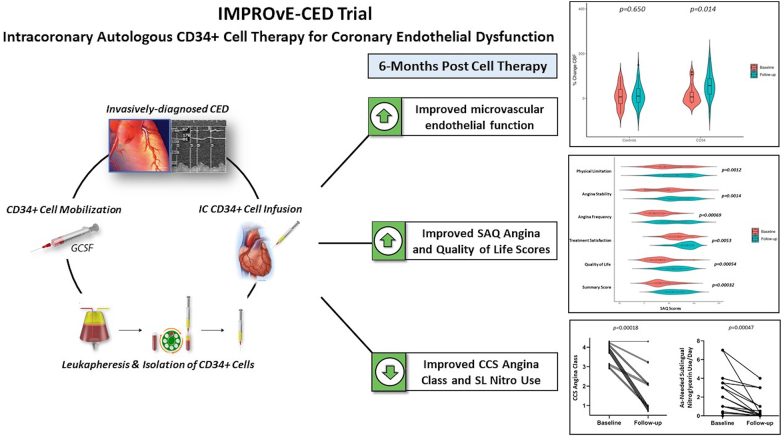
Another trial testing CD34+ stem cell therapy was the IMPROvE-CED trial which enrolled patients with endothelial dependent microvascular dysfunction [36] (Fig. 4). The results were similar to the ESCaPE-CMD trial with significant symptom and angina class improvement. In addition, the trial showed a percentage increase in acetylcholine mediated coronary blood flow from 7.2 % to 57.6 % and reduced daily sublingual nitroglycerin use [36]. Patients with abnormal endothelial function have reduced functioning CD34+ cells, and theoretically the infusion of vasoprotective stem cells balanced the impaired progenitor cells and resulted in improved endothelial function. Both trials have prompted a larger placebo-controlled trial to test stem cell therapy in the treatment of coronary microvascular dysfunction, the Freedom trial (Clinical trial# NCT04614467).
Fig. 4.
IMPROvE-CED TRIAL.
7. Conclusion
In summary, ANOCA and INOCA are frequent clinical challenges currently with limited treatment options. Progress will be made as we improve our diagnostic testing and develop specifically designed therapies to improve microvascular dysfunction. Hopefully the ongoing clinical trials provide much needed novel treatment options.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: NIH K23 HL151867 (OQ).
References
- 1.Kunadian V., Chieffo A., Camici P.G., et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020;41:3504–3520. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bairey Merz C.N., Pepine C.J., Walsh M.N., Fleg J.L. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford T.J., Stanley B., Sidik N., et al. 1-year outcomes of angina management guided by invasive coronary function testing (CorMicA) JACC Cardiovasc. Interv. 2020;13:33–45. doi: 10.1016/j.jcin.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001030. [DOI] [PubMed] [Google Scholar]
- 5.Merz C.N., Kelsey S.F., Pepine C.J., et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J. Am. Coll. Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 6.Shaw L.J., Shaw R.E., Merz C.N., et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 7.Maron D.J., Hochman J.S., Reynolds H.R., et al. Initial invasive or conservative strategy for stable coronary disease. N. Engl. J. Med. 2020;382:1395–1407. doi: 10.1056/NEJMoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Povsic T.J., Henry T.D., Ohman E.M. Therapeutic approaches for the no-option refractory angina patient. Circ. Cardiovasc. Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.009002. [DOI] [PubMed] [Google Scholar]
- 9.Gallone G., Baldetti L., Tzanis G., et al. Refractory angina: from pathophysiology to new therapeutic nonpharmacological technologies. JACC Cardiovasc. Interv. 2020;13:1–19. doi: 10.1016/j.jcin.2019.08.055. [DOI] [PubMed] [Google Scholar]
- 10.Taqueti V.R., Di Carli M.F. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72:2625–2641. doi: 10.1016/j.jacc.2018.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Buono M.G., Montone R.A., Camilli M., et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021;78:1352–1371. doi: 10.1016/j.jacc.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srivaratharajah K., Coutinho T., deKemp R., et al. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ. Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 13.Fearon W.F., Low A.F., Yong A.S., et al. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izzo P., Macchi A., De Gennaro L., Gaglione A., Di Biase M., Brunetti N.D. Recurrent angina after coronary angioplasty: mechanisms, diagnostic and therapeutic options. Eur. Heart J. Acute Cardiovasc. Care. 2012;1:158–169. doi: 10.1177/2048872612449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong P., Athanasiadis A., Perne A., et al. Coronary vasomotor abnormalities in patients with stable angina after successful stent implantation but without in-stent restenosis. Clin. Res. Cardiol. 2014;103:11–19. doi: 10.1007/s00392-013-0615-9. [DOI] [PubMed] [Google Scholar]
- 16.Kothawade K., Bairey Merz C.N. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr. Probl. Cardiol. 2011;36:291–318. doi: 10.1016/j.cpcardiol.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widmer R.J., Samuels B., Samady H., et al. The functional assessment of patients with non-obstructive coronary artery disease: expert review from an international microcirculation working group. EuroIntervention. 2019;14:1694–1702. doi: 10.4244/EIJ-D-18-00982. [DOI] [PubMed] [Google Scholar]
- 18.Feenstra R.G.T., Seitz A., Boerhout C.K.M., et al. Reference values for intracoronary Doppler flow velocity-derived hyperaemic microvascular resistance index. Int. J. Cardiol. 2023;371:16–20. doi: 10.1016/j.ijcard.2022.09.054. [DOI] [PubMed] [Google Scholar]
- 19.Ong P., Camici P.G., Beltrame J.F., et al. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 20.Seitz A., Feenstra R., Konst R.E., et al. Acetylcholine rechallenge: a first step toward tailored treatment in patients with coronary artery spasm. JACC Cardiovasc. Interv. 2022;15:65–75. doi: 10.1016/j.jcin.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Jansen T.P.J., Konst R.E., de Vos A., et al. Efficacy of diltiazem to improve coronary vasomotor dysfunction in ANOCA: the EDIT-CMD randomized clinical trial. JACC Cardiovasc. Imaging. 2022;15:1473–1484. doi: 10.1016/j.jcmg.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Borer J.S., Fox K., Jaillon P., Lerebours G., Ivabradine Investigators G. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebo-controlled trial. Circulation. 2003;107:817–823. doi: 10.1161/01.cir.0000048143.25023.87. [DOI] [PubMed] [Google Scholar]
- 23.McChord J., Ong P. Use of pharmacology in the diagnosis and management of vasomotor and microcirculation disorders. Heart. 2023:2022–321267. doi: 10.1136/heartjnl-2022-321267. [DOI] [PubMed] [Google Scholar]
- 24.Tagliamonte E., Rigo F., Cirillo T., et al. Effects of ranolazine on noninvasive coronary flow reserve in patients with myocardial ischemia but without obstructive coronary artery disease. Echocardiography. 2015;32:516–521. doi: 10.1111/echo.12674. [DOI] [PubMed] [Google Scholar]
- 25.Oettrich J.M., Dao V.T., Frijhoff J., et al. Clinical relevance of cyclic GMP modulators: a translational success story of network pharmacology. Clin. Pharmacol. Ther. 2016;99:360–362. doi: 10.1002/cpt.336. [DOI] [PubMed] [Google Scholar]
- 26.Martinez Pereyra V., Seitz A., Hubert A., et al. Repurposing riociguat for treatment of refractory angina resulting from coronary spasm. JACC Case Rep. 2021;3:392–396. doi: 10.1016/j.jaccas.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford T.J., Corcoran D., Padmanabhan S., et al. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur. Heart J. 2020;41:3239–3252. doi: 10.1093/eurheartj/ehz915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C., Liu X., Wang X., Wang Q., Zhang Y., Ge Z. Efficacy of enhanced external counterpulsation in patients with chronic refractory angina on Canadian Cardiovascular Society (CCS) angina class: an updated meta-analysis. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanza G.A., Golino M., Villano A., et al. Cardiac rehabilitation and endothelial function. J. Clin. Med. 2020:9. doi: 10.3390/jcm9082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannini F., Baldetti L., Ponticelli F., et al. Coronary sinus reducer implantation for the treatment of chronic refractory angina: a single-center experience. JACC Cardiovasc. Interv. 2018;11:784–792. doi: 10.1016/j.jcin.2018.01.251. [DOI] [PubMed] [Google Scholar]
- 31.Verheye S., Jolicoeur E.M., Behan M.W., et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N. Engl. J. Med. 2015;372:519–527. doi: 10.1056/NEJMoa1402556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rai B., Shukla J., Henry T.D., Quesada O. Angiogenic CD34 stem cell therapy in coronary microvascular repair-a systematic review. Cells. 2021:10. doi: 10.3390/cells10051137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry T.D., Losordo D.W., Traverse J.H., et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: a patient-level pooled analysis of randomized double-blinded trials. Eur. Heart J. 2018;39:2208–2216. doi: 10.1093/eurheartj/ehx764. [DOI] [PubMed] [Google Scholar]
- 34.Schachinger V., Assmus B., Erbs S., et al. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur. J. Heart Fail. 2009;11:973–979. doi: 10.1093/eurjhf/hfp113. [DOI] [PubMed] [Google Scholar]
- 35.Henry T.D., Bairey Merz C.N., Wei J., et al. Autologous CD34+ stem cell therapy increases coronary flow reserve and reduces angina in patients with coronary microvascular dysfunction. Circ. Cardiovasc. Interv. 2022;15 doi: 10.1161/CIRCINTERVENTIONS.121.010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corban M.T., Toya T., Albers D., et al. IMPROvE-CED Trial: intracoronary autologous CD34+ cell therapy for treatment of coronary endothelial dysfunction in patients with angina and nonobstructive coronary arteries. Circ. Res. 2022;130:326–338. doi: 10.1161/CIRCRESAHA.121.319644. [DOI] [PubMed] [Google Scholar]