Abstract
Vector-borne diseases cause significant financial and human loss, with billions of dollars spent on control. Arthropod vectors experience a complex suite of environmental factors that affect fitness, population growth and species interactions across multiple spatial and temporal scales. Temperature and water availability are two of the most important abiotic variables influencing their distributions and abundances. While extensive research on temperature exists, the influence of humidity on vector and pathogen parameters affecting disease dynamics are less understood. Humidity is often underemphasized, and when considered, is often treated as independent of temperature even though desiccation likely contributes to declines in trait performance at warmer temperatures. This Perspectives explores how humidity shapes the thermal performance of mosquito-borne pathogen transmission. We summarize what is known about its effects and propose a conceptual model for how temperature and humidity interact to shape the range of temperatures across which mosquitoes persist and achieve high transmission potential. We discuss how failing to account for these interactions hinders efforts to forecast transmission dynamics and respond to epidemics of mosquito-borne infections. We outline future research areas that will ground the effects of humidity on the thermal biology of pathogen transmission in a theoretical and empirical framework to improve spatial and temporal prediction of vector-borne pathogen transmission.
Keywords: climate change, ecophysiology, humidity, mosquito, parasite ecology, pathogen, temperature, vector-borne disease
INTRODUCTION
Vector-borne parasites are common, important biological enemies of humans, animals and plants, transmitted by one living organism to another. Despite the recent gains in reducing the overall global burden for parasites like malaria (Ashepet et al., 2021; Bhatt et al., 2015; Gething et al., 2010), vector-borne diseases still account for 17% of all infectious diseases and cause 700,000 deaths in humans annually (WHO, 2020). Livestock and crop systems are also plagued by vector-borne diseases, which place serious constraints on agricultural production globally (Döring, 2017; Garros et al., 2017), and vector-borne diseases can be devastating in wildlife populations, particularly when introduced to new areas. Collectively, tens of billions of dollars are spent every year on control, medical interventions and mitigating loss of productivity (Aguirre, 2017; George et al., 2015; Stuchin et al., 2016; Warner, 1968; Weaver et al., 2018).
The dependence of many pathogens on ectothermic arthropod vectors for transmission means that vector-borne diseases are highly sensitive to variation in the environment. Arthropod vectors experience a complex suite of environmental factors, both abiotic (e.g., temperature, rainfall, humidity, salinity) and biotic (e.g., biological enemies, inter- and intraspecific interactions and variation in habitat quality). These factors vary in their relative effects on organismal fitness, can synergize (Huxley et al., 2021, 2022; Kleynhans & Terblanche, 2011; Liu & Gaines, 2022), and exert their effects at different spatial scales (Cohen et al., 2016) with important consequences for the abundance and distribution of arthropod vectors (Evans et al., 2019; Ryan et al., 2015), vector population dynamics (Murdock et al., 2017) and pathogen transmission (Huber et al., 2018; Mordecai et al., 2013; Mordecai et al., 2017; Murdock et al., 2016; Murdock, Blanford, Hughes, et al., 2014a; Ngonghala et al., 2021; Samuel et al., 2011; Shocket, Vergara, et al., 2018b; Tesla et al., 2018; Wimberly et al., 2020).
In vector ecology, there has been a strong emphasis on studying the effects of temperature on mosquito-borne pathogen transmission (reviewed in Mordecai et al., 2019). In addition to temperature, water availability is another critical abiotic variable influencing ectotherm biology, and both play important roles determining the distribution and abundance of ectotherms (Chown & Nicolson, 2004; Deutsch et al., 2008; González-Tokman et al., 2020; Kearney & Porter, 2009; Lenhart et al., 2015; Roura-Pascual et al., 2011; Rozen-Rechels et al., 2019; Steiner et al., 2008; van Klink et al., 2020) and species richness (Beck et al., 2017; Calatayud et al., 2016; Cardoso et al., 2020; Hamann et al., 2021; Jamieson et al., 2012; Pilotto et al., 2020). Body temperature has important effects on the rates of enzymatic processes as well as the structural integrity of cellular membranes and proteins (Angilletta, 2009), while all cellular processes rely on water as a solvent for biochemical reactions and for trafficking nutrients into, within, and out of cells (Chaplin, 2006; Chown & Nicolson, 2004). Temperature also affects the amount of desiccation stress an organism experiences due to the fundamental relationship between ambient temperature and the amount of water the surrounding air can hold (Lawrence, 2005; Romps, 2021). Other fields at the climate-health interface have explored the effects of wet heat vs dry heat on the energy budgets of endotherms in the context of human heat stress and climate change (Buzan & Huber, 2020). We anticipate that variation in relative humidity is also an important force shaping the thermal performance of ectotherms, including mosquitoes. Whereas metabolic theory has been well developed and widely applied in ecology to understand temperature effects (Brown et al., 2004; Corkrey et al., 2016; Dell et al., 2011) we currently lack a similar framework for understanding how humidity and temperature interact to influence mosquitoes and their pathogens.
In this Perspectives, we explore the effects of humidity on the thermal performance of mosquito-borne pathogen transmission. We begin by summarizing what is currently known about how temperature and humidity affects mosquito fitness, population dynamics and pathogen transmission, whilst highlighting current knowledge gaps. We present a conceptual framework for understanding the interaction between temperature and humidity and how it shapes the range of temperatures across which mosquitoes persist and achieve high transmission potential. We then discuss how failing to account for these interactions across climate variables hinders efforts to forecast transmission dynamics and to respond to epidemics of mosquito-borne infections. We end by outlining future research areas that will ground the effects of humidity on thermal performance of pathogen transmission in a theoretical and empirical basis to improve spatial and temporal prediction of vector-borne pathogen transmission. Such a framework will inform multiple fields (thermal, disease and landscape ecology and epidemiology) and a diversity of vector-borne disease systems (human, wildlife, domestic animals and plants).
THE EFFECTS OF TEMPERATURE ON MOSQUITO POPULATION DYNAMICS AND PATHOGEN TRANSMISSION
Numerous studies have demonstrated that mosquito-borne pathogen transmission is both seasonally and geographically limited at various spatial scales by variation in ambient temperature (e.g., malaria (Ryan et al., 2015; Siraj et al., 2014; Villena et al., 2022), Zika (Ryan, Carlson, et al., 2020a; Siraj et al., 2018; Tesla et al., 2018), chikungunya (Johansson et al., 2014) and dengue (Mordecai et al., 2017)). The effects of temperature on ectotherm performance, including mosquito vectors, are typically non-linear, with performance steadily increasing from zero at a minimum critical temperature (CTmin) up to an optimum temperature (Topt), followed by a steep decline towards the critical thermal maximum (CTmax) (Figure 1). The CTmin and CTmax represent the operational limits for trait performance because temperatures that exceed their range are not permissive for ectotherm development, survival or reproduction (Brown et al., 2004; Corkrey et al., 2016; Deutsch et al., 2008; Hoffmann et al., 2013; Sinclair et al., 2016). These thermal limits in ectotherm performance are consistent with the metabolic theory of ecology, which posits that organismal physiological and enzymatic rates will increase predictably with temperature because of increased efficiency of biochemical reactions (Huey & Kingsolver, 2019) up to Topt. The steep decline in performance above the Topt is attributed to the declining efficiency of metabolic processes due to decreases in protein stability as temperatures increase, eventually resulting in organismal death at the Tmax. Collectively, this information gives us a Thermal Performance Curve (TPC), which can be used to infer ecological and evolutionary outcomes.
FIGURE 1.
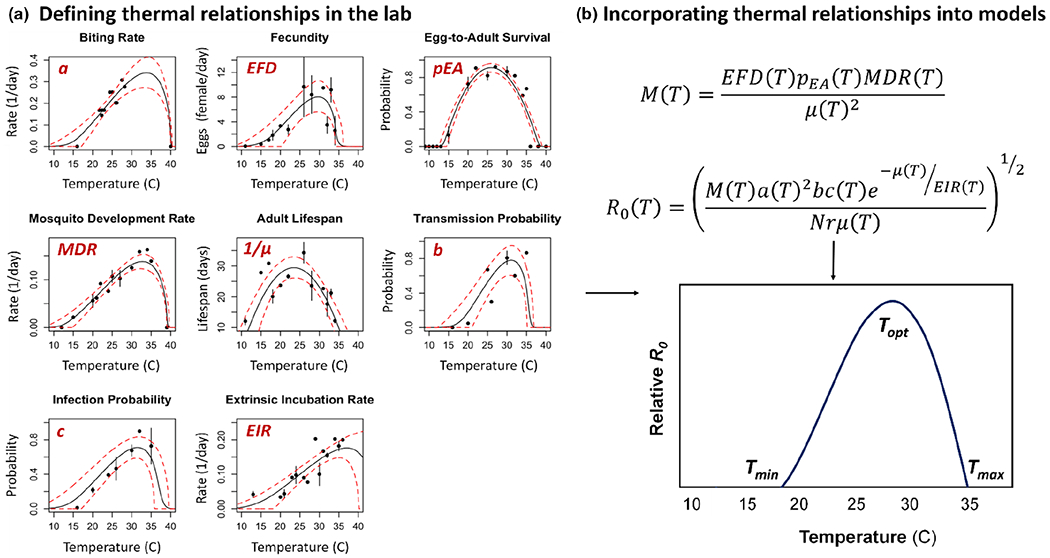
(a) Similar to other ectothermic organisms, the life history traits of mosquitoes and the pathogens they transmit typically exhibit non-linear relationships with environmental temperature, where trait performance is constrained by both cool and warm temperatures and optimized at some intermediate temperature. Further, the effect of temperature on these individual traits can vary qualitatively and quantitatively, resulting in different temperature ranges across which trait performance can occur, temperatures that maximize trait performance, and the overall shape of the temperature-trait relationship (e.g., symmetric vs. asymmetric). As a result, predicting the effects of temperature on mosquito fitness, population growth rates or pathogen transmission is complex. (b) Mathematical models of vector-borne pathogen transmission that incorporate these temperature-trait relationships generally predict transmission to also follow a non-linear relationship and to peak at some intermediate temperature, as depicted here with the temperature-dependent relative reproductive number R0 as a conceptual example. This model incorporates the effects of temperature on traits that drive mosquito population dynamics (e.g., per capita mosquito development rate (MDR), the probability of egg to adult survival (pEA) and the per capita number of eggs females produce per day (EFD)), host-vector contact rates (the per capita daily biting rate of female mosquitoes (a)) and the number of mosquitoes alive and infectious (transmission (b) and infection (c) probabilities, the extrinsic incubation period (1/EIR) and the per capita mosquito mortality rate (μ)). Where the predicted thermal minimum (Tmin), maximum (Tmax) and optimum (Topt) for transmission occur will be dependent upon the relative effect of each trait, the nature of the temperature-trait relationship, and how these factors combine to shape the transmission process. Adapted from Mordecai et al. (2017).
Mosquitoes, like other ectotherms, are highly susceptible to changes in ambient temperature, which demonstrably affects their growth rate (Alto & Juliano, 2001; Delatte et al., 2009; Evans, Newberry, & Murdock, 2018a; Huxley et al., 2022; Monteiro et al., 2007; Paaijmans et al., 2013; Tun-Lin et al., 2000), reproduction (Carrington et al., 2013; Miazgowicz et al., 2020), metabolic rate (Vorhees et al., 2013), lifespan (e.g., Alto & Juliano, 2001; Christofferson & Mores, 2016; Gunay et al., 2010; Miazgowicz et al., 2020), biting rate (Afrane et al., 2005; Lardeux et al., 2008; Miazgowicz et al., 2020; Shapiro et al., 2017), immunity (Adelman et al., 2013; Ferreira et al., 2020; Murdock et al., 2012, 2013; Murdock, Blanford, Luckhart, & Thomas, 2014b; Suwanchaichinda & Paskewitz, 1998), and ability to acquire, carry and transmit pathogens (Johnson et al., 2015; Lambrechts et al., 2011; Mordecai et al., 2013, 2017; Murdock et al., 2016; Murdock, Blanford, Luckhart, & Thomas, 2014b; Paaijmans et al., 2012; Shocket et al., 2020; Shocket, Ryan, & Mordecai, 2018a; Tesla et al., 2018) in a non-linear, unimodal fashion. These temperature-trait relationships can vary in overall shape (e.g., symmetric or asymmetric non-linear relationships) due to differences in the temperatures that optimize and constrain various traits, which in combination will determine the predicted thermal minimum, maximum and optimum for mosquito fitness, intrinsic growth rates of mosquito populations, and pathogen transmission (Figure 1).
Process-based models, which traditionally have relied upon temperature relationships grounded in metabolic theory, have enhanced our ability to predict the effects of environmental drivers on spatial and temporal dynamics of vector-borne disease. Several key biological insights have resulted from this general approach. First, temperate areas of the world that currently experience relatively cool temperatures are expected to increase in thermal suitability for many mosquito-borne diseases with future climate warming (Ryan et al., 2015; Ryan, Carlson, et al., 2020a; Siraj et al., 2014; Tesla et al., 2018), and, in temperate regions, mosquito-borne pathogens can invade or spread during the summer in seasonally varying environments (Huber et al., 2018; Ngonghala et al., 2021). Secondly, areas that are currently permissive (near the Topt) or warmer than the Topt for transmission are expected to experience a decline in thermal suitability with future warming (Murdock et al., 2016; Ryan et al., 2015; Ryan, Lippi, & Zermoglio, 2020b). Third, because mosquito and pathogen species can have different qualitative and quantitative relationships with temperature (resulting in different CTmin, CTmax and Topt) (Johnson et al., 2015; Miazgowicz et al., 2020; Mordecai et al., 2013, 2017, 2019; Shapiro et al., 2017; Shocket et al., 2020; Shocket, Ryan, & Mordecai, 2018a; Tesla et al., 2018; Villena et al., 2022), shifts in thermal suitability with climate and land-use change could also alter the prevalence and magnitude of mosquito-borne diseases in a given area (Tesla et al., 2018), such as sub-Saharan Africa (Mordecai et al., 2020). Fourth, small variations in ambient temperature at fine-spatial scales can contribute to high heterogeneity in predicted suitability for pathogen transmission across various environments (Afrane et al., 2005; Cator et al., 2013; Evans et al., 2019; Murdock et al., 2017; Okech et al., 2004; Paaijmans & Thomas, 2011; Pincebourde et al., 2016; Thomas et al., 2018; Verhulst et al., 2020; Wimberly et al., 2020), which can have important ramifications for predicting mosquito-borne pathogen transmission and targeting interventions (Thomas et al., 2018; Wimberly et al., 2020). Finally, disease intervention efforts can also be directly or indirectly affected by variation in ambient temperature. Various insecticides (Akinwande et al., 2021; Glunt et al., 2014), entomopathogenic fungi (Darbro et al., 2011; Kikankie et al., 2010) and Wolbachia transinfections (Foo et al., 2019; Gu et al., 2022; Murdock, Blanford, Hughes, et al., 2014a; Ross et al., 2017, 2019, 2020; Ulrich et al., 2016) are thermally sensitive, indicating that the efficacy and cost of these interventions could vary seasonally, across geographic regions and with future climate and land-use change (Parham & Hughes, 2015).
THE EFFECTS OF HUMIDITY ON MOSQUITO FITNESS, POPULATION DYNAMICS AND PATHOGEN TRANSMISSION
Spatial and temporal variation in atmospheric moisture has important implications for an organism’s ability to hydroregulate (Box 1). Hydroregulation is defined as the suite of physiological and behavioural responses organisms utilize to regulate water balance and tolerate dehydrating environmental conditions (Benoit, 2010; Chown et al., 2011; Chown & Nicolson, 2004; Edney, 2012; Lucio et al., 2013). The relationship between organismal fitness and optimal hydroregulation is complex, with significant costs to fitness (e.g., decreased survival and reproduction) occurring when organisms become dehydrated (Anderson & Andrade, 2017; Mitchell & Bergmann, 2016) or overhydrated (Chown & Nicolson, 2004). Insects have a suite of adaptations to conserve water, like physiological changes in skin or cuticular permeability (Rajpurohit et al., 2008; Wu & Wright, 2015), differential regulation of urine and faeces production (Durant et al., 2021; Durant & Donini, 2019; Lajevardi et al., 2021; Weihrauch et al., 2012), and behavioural changes in activity (Kühnholz & Seeley, 1997; Ostwald et al., 2016). Insects also can mitigate water loss by regulating water intake via changes in water utilization, food sources and selection of specific habitats (Benoit, 2010; Bezerra Da Silva et al., 2019; Hagan et al., 2018)). Finally, insects can also produce water via metabolic processes (Chown et al., 2011; Jindra & Sehnal, 1990). Maintaining water balance is a particular challenge for blood-feeding (haematophagic) vectors (Chappuis et al., 2013; Kleynhans & Terblanche, 2011), like mosquitoes (Edney, 2012), where the act of taking a blood meal results in overhydration that requires specialized adaptations for the excretion of water, which, in turn, enhances susceptibility to desiccation overall (Benoit & Denlinger, 2010).
Box 1. Different measurements of humidity.
In the most general sense, humidity is a measure of water vapour in the atmosphere, which is one important component defining an organism’s risk of desiccation. In practice, humidity can be quantified in multiple ways. A fundamental characteristic is the vapour pressure, which is defined in meteorology as the partial pressure of water vapour in the atmosphere. Vapour pressure affects the movement of atmospheric moisture, which diffuses from higher to lower vapour pressures (Allen et al., 1998; Wexler & Greenspan, 1971). The saturation vapour pressure is the maximum vapour pressure of a completely saturated parcel of air and is a non-linear increasing function of temperature (Figure 1). The dew point temperature is defined as the temperature to which air would need to be cooled for the saturation vapour pressure to equal the actual vapour pressure and is related to the total amount of moisture in the air. Absolute humidity is the mass of water vapour per unit volume of air, whereas relative humidity is the ratio of the actual water vapour pressure divided by saturation vapour pressure (at ambient temperature and pressure) (Lawrence, 2005). The vapour pressure deficit is the difference between saturation vapour pressure and the actual vapour pressure. Vapour pressure deficit is a key term in the numerator of the Penman-Monteith equation (used for calculating evaporation and evapotranspiration, see Allen et al., 1998; Chiew et al., 1995). Thus, if other environmental factors are constant, the rates of evaporative water loss from open water bodies and vegetated surfaces are expected to increase as a linear function of vapour pressure deficit.
Of these humidity measures, relative humidity is most commonly provided in meteorological datasets. Relative humidity is a unitless ratio in which the influence of temperature on the saturation vapour pressure has been removed, which has several important implications. Even if the total amount of water in the air is constant, relative humidity will vary with temperature. This effect is most commonly observed in the diurnal cycle of humidity, which reaches a minimum during the warmest time of day and a maximum at the coolest times of night. When comparing two observations with similar relative humidities but different temperatures, the observation with the higher temperature will have a higher vapour pressure, saturation vapour pressure and vapour pressure deficit. Thus, the potential for evaporative water loss at a given relative humidity is inherently sensitive to changes in temperature (Figure 1).
Instead of measuring humidity directly (Box 1), many studies use related variables, like seasonal precipitation or land cover to predict mosquito population dynamics or pathogen transmission (Abdelrazec & Gumel, 2017; Chandy et al., 2013; Chaves & Kitron, 2011; Johansson et al., 2009; Nosrat et al., 2021; Sang et al., 2017; Soti et al., 2012). Mosquito-borne diseases generally peak during, or following, periods of highest rainfall (Chowdhury et al., 2018; Karim et al., 2012; Magombedze et al., 2018; McLaughlin et al., 2019). Rainfall can matter as a standalone variable, since standing water is essential for mosquitoes’ ontogenetic development. However, the effect of precipitation on mosquito population dynamics and disease transmission can operate through other factors that covary with precipitation, such as increased humidity and shifts in temperature that impact mosquito development rates, adult survival and reproduction, parasite development rates and mosquito-human contact rates. The relationship between mosquitoes and precipitation is even more difficult to discern for mosquito species that develop in artificial, human watered containers, where complex interactions can occur between amount of rainfall and access to piped water (Brown et al., 2014; Hayden et al., 2010; Lippi et al., 2018; Padmanabha et al., 2010; Schmidt et al., 2011; Stewart Ibarra et al., 2013). Similarly, measures of land cover such as the normalized difference vegetation index (NDVI) have been used to account for areas too dry for widespread mosquito habitat (Ryan et al., 2015). Ultimately, the use of these proxy measures obscures our understanding of how relative humidity and other environmental variables affect transmission, which, in turn, constrains our ability to predict how mosquito-borne pathogens will respond to future climate and land-use change.
Several studies have demonstrated statistical associations between humidity and mosquito abundance, as well as vector-borne disease incidence and prevalence (Althouse et al., 2015; Asigau & Parker, 2018; Azil et al., 2010; Buckner et al., 2011; Chen et al., 2010; Davis et al., 2018; Diallo et al., 2019; Evans et al., 2019; Jemal & Al-Thukair, 2018; Karim et al., 2012; Lega et al., 2017; Mayne, 1930; Santos-Vega et al., 2022). For example, the sizes of seasonal malaria epidemics in two cities in India exhibit a clear association with relative humidity (Figure 2), with a higher correlation than for temperature or rainfall (Santos-Vega et al., 2016). A semi-mechanistic epidemiological model that incorporates this effect of relative humidity on the transmission rate parameter accurately predicts the temporal dynamics of the disease, including the multiyear cycles in the size of seasonal epidemics (Santos-Vega et al., 2016, 2022). Such predictions can inform mosquito control efforts and targeting prophylaxes. However, the underlying biology of the relationships that exist between humidity and these response variables are often assumed and based on a limited number of empirical studies (summarized in Table 1). Experimental work has thus far shown generally positive effects of increased relative humidity on mosquito survival and desiccation tolerance, production and development of eggs and mosquito activity (up to 90% relative humidity). In contrast, biting rates exhibited increases when conditions are drier and the effect of humidity on vector competence is less clear (Table 1).
FIGURE 2.
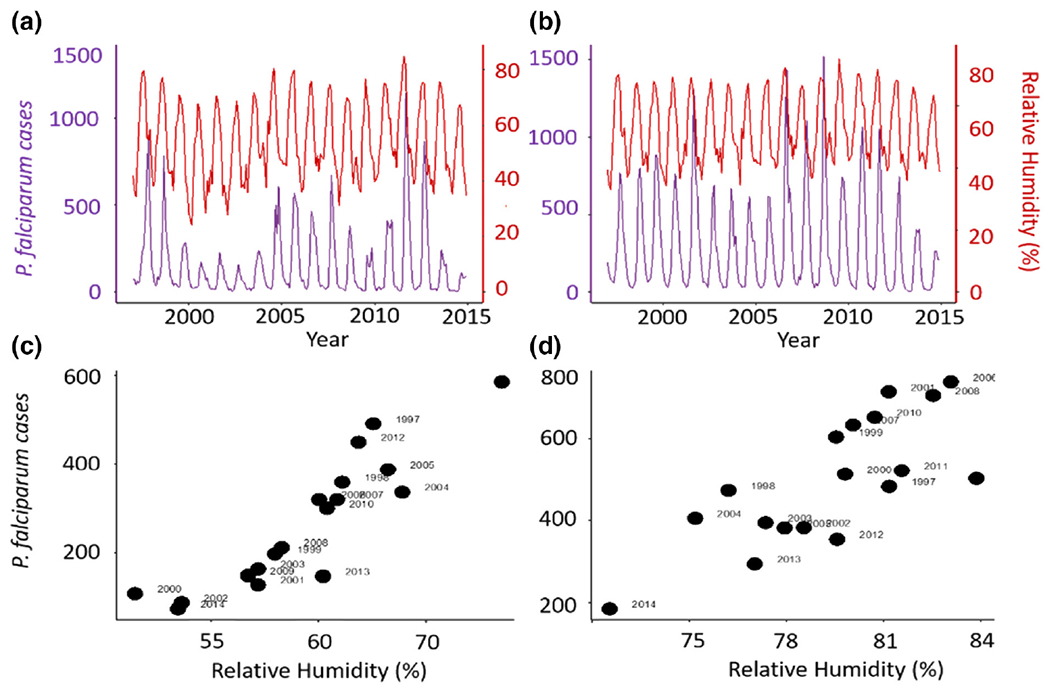
Monthly malaria case data for Plasmodium falciparum shown (in purple) with a corresponding time series for relative humidity (RH, red) for two cities in India, Ahmedabad (a) and Surat (b). Total cases during the transmission season from August to November are shown as a function of mean RH in a critical time window preceding this season and including the monsoons from May to July for Ahmedabad (c) and March to July for Surat (d). Figure is taken from Santos-Vega et al. (2022) Nature Communications doi: 10.1038/s41467-022-28,145-7. Figure is reproduced under Creative Commons Attribution 4.0 International Licence.
TABLE 1.
Summary of the published literature that investigated the effects of relative humidity on mosquitoes, organized by life history trait, presented with a summary of the effect of RH.
| Life history trait | Range explored (RH%) | Effect of RH | Mosquito species | References |
|---|---|---|---|---|
| Longevity/Survival/Desiccation tolerance | 5–100 | Increased RH significantly increased female longevity; tendency to survive longest at low temp—high humidity combinations and females to survive longer than males | Anopheles gambiae, An. stephensi, An. subpictus, An. culicifacies, An. pharoensis, An. arabiensis, An.funestus, Ae. aegypti, Ae. albopictus, Ae. togoi, Ae. paullusi, Culex pipiens, Eretmapodites chrysogaster | Alto et al. (2015), Bayoh (2001), Canyon et al. (2013), Costa et al. (2010), Gaaboub et al. (1971), Gray and Bradley (2005), Hylton (1969), Lega et al. (2017), Lewis (1933), Lomax (1968), Lyons et al. (2014), Mayne (1930), Mogi et al. (1996), Reiskind and Lounibos (2009) Schmidt et al. (2018) and Urbanski et al. (2010) |
| Egg production | 34–95 | Increased RH increased egg production; significantly lower numbers of eggs and delayed oviposition in dry conditions | An. pharoensis, Ae. aegypti, Ae. taeniorhynchus, Ae. vexans, Ae. trivittatus | Canyon et al. (1999, 2013), Costa et al. (2010), Gaaboub et al. (1971), Lega et al. (2017) and Mcgaughey and Knight (1967) |
| Activity/Behaviour | 10–30, up to 100 | Mosquito activity increases with increasing relative humidity to a point (~90%) and then precipitously declines, also has a general lower limit at ~40%. | An. quadrimaculatus, An. earlei, An. walkeri, An. crucians, Ae. aegypti, Ae. sollicitans, Ae. cantator, Ae. vexans, Ae. trivittatus, Ae. canadensis, Ae. communis, Ae. sticticus, Ae. taeniorhynchus, Ae. vigilax, Cx. pipiens, Cx. fatigans, Cx. restuans, Cx. nigripalpus, Cx. sitiens, Coquillettidia perturbans, Psorophora confinnis, Uranotaenia sapphirina | Bidlingmayer (1974, 1985), Dow and Gerrish (1970), Grimstad and DeFoliart (1975), Hagan et al. (2018), Johnson et al. (2020), Platt et al. (1957, 1958), Provost (1973), Rowley & Graham (1968), Rudolfs (1923, 1925), Thomson (1938), Witter et al. (2012) and Wright & Knight (1966) |
| Plasmodium infection | 39–100 | Mixed or unclear effects of humidity | An. stephensi, An. subpictus, An. culicifacies, An. fuliginosus, Cx quinquefasciatus, Cx. fatigans | Knowles and Basu (1943) and Mayne (1930) |
| Egg hatching | Real-world RH data; 0–100 | Adding RH data to a predictive model focused on egg hatching made the model more accurate. Egg hatching worse at lower humidities | Ae. aegypti | Albernaz et al. (2009), Bar-Zeev (1957) and Lega et al. (2017) |
| Microclimate preference upon emergence | 75, 86 | Newly emerged adults with no access to water or sugar preferred cooler, more humid refugia. | An. gambiae, An. stephensi, Ae. aegypti and Cx. pipiens | Kessler and Guerin (2008) |
Despite the existing body of research, we still do not have a sufficient knowledge base for incorporating the effects of humidity into the current temperature-trait modelling framework. Results from observations studies cannot necessarily be extrapolated to new locations or into the future. Further, the effects of humidity on mosquito and pathogen fitness described by experimental / causation studies are of limited resolution, typically exploring a limited number of humidity levels and encompassing only a handful of mosquito species. The need to better incorporate humidity effects is not unique to vector-borne diseases, but parallels trends seen in the larger body of ecological work on the effects of climate variability and climate change on heat health in ectotherms (Gunderson & Stillman, 2015; van Heerwaarden & Sgrò, 2014). In the following section, we outline how variation in relative humidity interacts with temperature to change the thermal performance of ectothermic vectors and, consequently, pathogen transmission.
CONSIDERING THE COMBINED EFFECTS OF TEMPERATURE AND HUMIDITY ON TRANSMISSION
The optimal regulation of both body temperature and water balance is crucial for organismal performance and fitness (Bradshaw, 2003). Due to the fundamental relationship that exists between temperature and the amount of moisture the air can hold (Figure 3), variations in both relative humidity and temperature will alter the degree of moisture stress ectothermic organisms, like mosquitoes, experience. For a given amount of atmospheric moisture, warmer temperatures result in higher saturation vapour pressures that reduce relative humidity and increase vapour pressure deficit (Figure 3). Depending on the ambient temperature, variation in relative humidity can exacerbate or buffer the negative effects of higher temperature on mosquito fitness and pathogen transmission. The current manner in which thermal performance of vector-borne pathogen transmission is conceptualized and empirically measured does not explicitly account for these effects. Even when relative humidity is held constant, increases in temperature will increase the vapour pressure deficit and the evaporative stress an adult mosquito experiences. Thus, it is currently unclear if the thermal maximum of a given trait, which is typically an upper lethal limit (Chown & Nicolson, 2004), is really being driven by temperature effects on metabolic function or rather is a function of dehydration and water stress on the organism. Understanding the physiological mechanisms underpinning mosquito responses to these abiotic constraints will be critical for predicting how transmission will shift with future anthropogenic change (Chown & Gaston, 2008; Deutsch et al., 2008; Dillon et al., 2010; Pörtner & Farrell, 2008).
FIGURE 3.
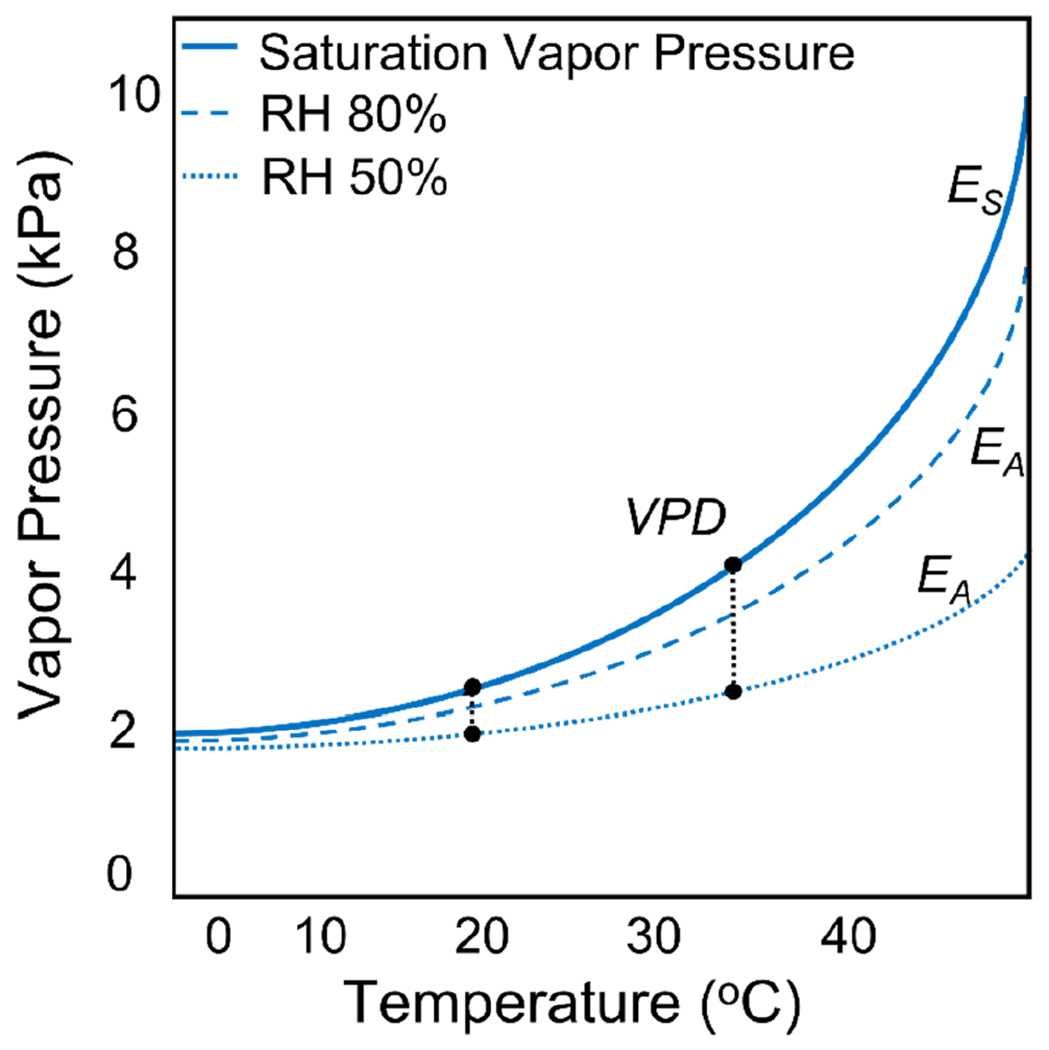
The total amount of water the air can hold, expressed here as saturation vapour pressure (Es), increases exponentially with temperature and is estimated as a function of temperature using the Tetens equation. The actual amount of water in the air, expressed here as vapour pressure (Ea), can be derived from relative humidity (RH) as Ea = RH/100 * Es. The vapour pressure deficit (VPD) is the absolute difference between Es and Ea and is an important metric of atmospheric moisture because it has a near linear relationship with evaporative potential. Thus, as temperature warms, for a given decrease in RH, there will be a larger increase in VPD and the amount of water stress mosquitoes experience.
We utilize a trait-based approach that leverages a widely used relative R0 model (Mordecai et al., 2013, 2017, 2019; Murdock et al., 2017; Ryan, Lippi, & Zermoglio, 2020b; Shocket et al., 2020; Shocket, Ryan, & Mordecai, 2018a; Tesla et al., 2018; Villena et al., 2022; Wimberly et al., 2020) to present a framework that outlines the manner in which variation in relative humidity could influence the thermal performance of vector-borne pathogen transmission (Figures 4 and 5). Overall, we anticipate that variation in relative humidity could result in significant shifts in the qualitative shape of the temperature-trait relationship and cause these effects to vary with mosquito traits. Drawing from the literature on other ectotherms, insects and what little we do know for mosquitoes, we outline several hypotheses for how variation in relative humidity may affect the thermal performance of mosquito and pathogen traits (Table 2). We anticipate variation in relative humidity will be important throughout the mosquito life cycle, with the largest effects at temperatures that approach the upper thermal limit (Tmax) for a given trait, with little to no effect of variation in relative humidity on the predicted thermal minimum (Tmin) (Table 2). This hypothesis is based on the observation that for a given change in relative humidity, the corresponding change in vapour pressure deficit and evaporative stress will be greater at higher temperatures (Figures 3 and 4). How variation in relative humidity affects the predicted thermal optimum (Topt) of a given trait will be somewhat dependent on the specific trait as well as the magnitude of the effect at warmer temperatures.
FIGURE 4.
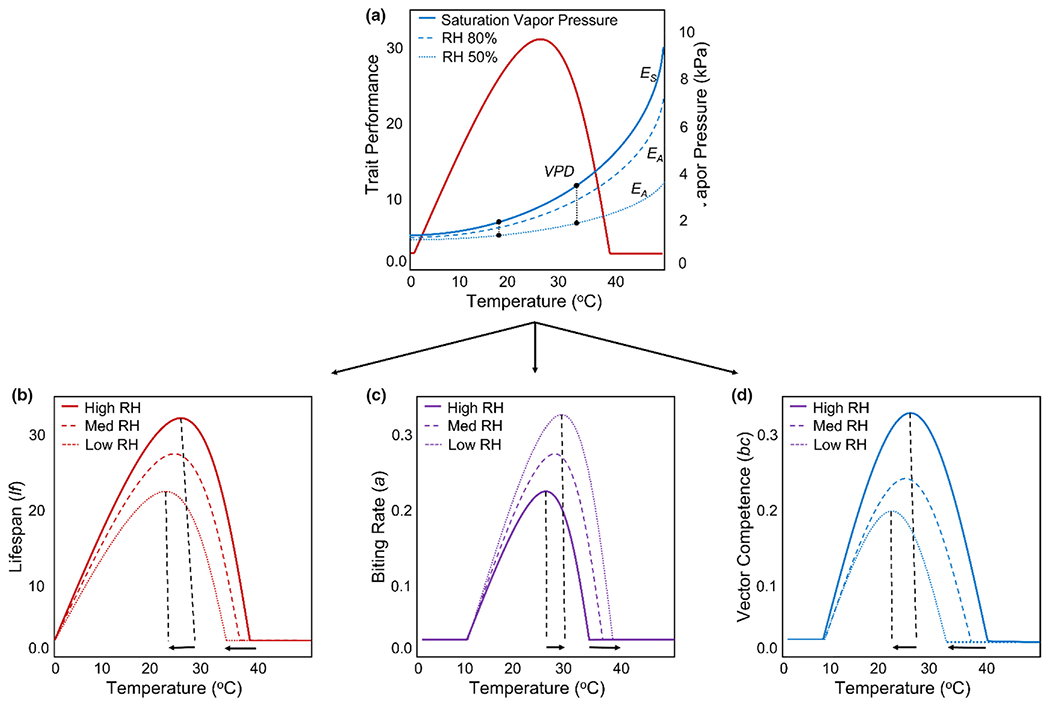
(a) Thermal performance is often measured by placing mosquitoes in different life stages and infection stages across a range of constant temperatures at a set relative humidity (typically between 70–90% RH). However, despite holding relative humidity constant, as temperatures warm there will be a corresponding increase in the vapour pressure deficit (VPD) and the amount of water stress mosquitoes experience. Overlaying these relationships (from Figure 1) on a given temperature-trait relationship demonstrates that the sensitivity of trait performance to variation in relative humidity should be highest on the descending limb of this relationship. Es = saturation vapour pressure, which increases exponentially with temperature and is estimated as a function of temperature using the Tetens equation. Ea = vapour pressure, meaning the actual amount of water in the air and can be derived from relative humidity (RH) as Ea = RH/100 * Es. (B–D) represent the hypothetical responses of three temperature-trait relationships to variation in relative humidity. These shifts are predicted to both decrease the thermal optimum and maximum for some traits (e.g., (b) lifespan and (d) vector competence) or increase them for others (e.g., (c) per capita biting rate).
FIGURE 5.
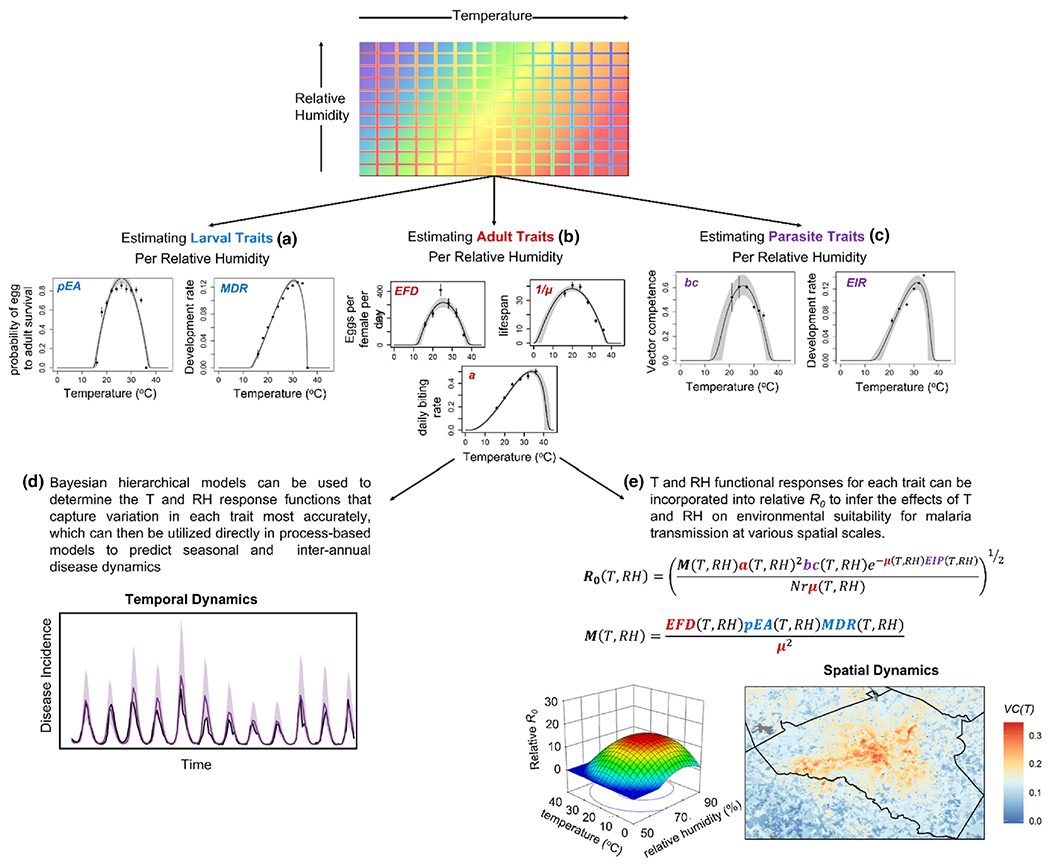
Laboratory work with field derived mosquitoes can be conducted to estimate the effect of multiple environmental variables on mosquito fitness, population dynamics and pathogen transmission. For example, mosquitoes could be housed across a range of constant temperature (T) and relative humidity (RH) conditions that are reflective of monthly field conditions. From these experiments, one can estimate the effects of variation in these environmental variables on key larval traits (a: mosquito development rate (MDR) and the probability of egg to adult survival (pEA)), adult traits (b: per capita mortality rate (μ), per capita eggs laid per day (EFD) and per capita daily biting rate (a)) and parasite / pathogen traits (c: vector competence (bc) and the extrinsic incubation period (EIP)). (d) Bayesian hierarchical models can be used to develop T and RH response surfaces for each trait, which can either be incorporated in process-based modelling approaches to infer effects on seasonal and interannual variation in vector-borne pathogen transmission dynamics. (e) Bayesian models can also be used to generate a T and RH dependent, relative R0 model that can be used to predict environmental suitability for pathogen transmission at various spatial scales. A crucial detail for modelling approaches, based on the evidence presented in Box 2, is that the effects of T and RH will be interactive, not additive. (Inset on temporal dynamics in (D) is from Santos-Vega et al. (2022) Nature Communications; doi: 10.1038/s41467-022-28,145-7. Figure is reproduced under Creative Commons Attribution 4.0 International Licence).
TABLE 2.
Predictions for the interactive effects of relative humidity & temperature on different mosquito traits.
| Trait | Definition | T min | T opt | T max |
|---|---|---|---|---|
| MDR | Mosquito development rate (1/days) | No change | ? | evaporation ↓ with ↑ RH = ↑ or no Δ in Tmax no evaporation with ↑ RH = ↓Tmax |
| pEA | Probability of egg to adult survival | No change | ? | evaporation ↓ with ↑ RH = ↑ Tmax no evaporation with ↑ RH = ↓ Tmax |
| EFD | Per capita no. of eggs produced daily per female (1/days) | No change | ? | RH ↑ = ↑ or ↓ Tmax |
| a | Per capita female biting rate (1/days) | No change | ? | RH ↑ = ↑ or ↓ Tmax |
| μ | Per capita mosquito mortality rate (1/days) | No change | ? | RH ↑ = ↑ Tmax |
| bc | Probability of becoming infectious | No change | ? | RH ↑ =? Tmax |
| EIR | Extrinsic incubation rate (1/EIP or 1/days) | No change | ? | RH ↑ = ↑ Tmax |
The nature and magnitude of the effects of relative humidity and temperature variation on mosquito and pathogen traits important for transmission could differ depending on mosquito life stage. One way in which relative humidity and temperature interact to affect developing mosquitoes is through the evaporation rate of larval habitat, which is also determined by the size and surface area of the larval habitat and rate of water replenishment (Juliano & Stoffregen, 1994). A second type of interaction could involve altering some intrinsic factor of the larval environment such as surface tension, microbial growth or solute concentration (Juliano & Stoffregen, 1994; Pérez-Díaz et al., 2012). Causal evidence from semi-field experiments shows negative effects of high relative humidity at temperatures near or above the predicted thermal optimum for Aedes albopictus (Mordecai et al., 2017; Murdock et al., 2017) on larval survival and the probability of adult emergence (Murdock et al., 2017). One possibility is that both temperature and water vapour in the atmosphere will affect the surface tension of aquatic larval habitats. Warm temperatures and high humidity may cause larval habitats to have too little surface tension, while cool and dry larval environments may have too high surface tension (Pérez-Díaz et al., 2012; Singh & Micks, 1957), negatively affecting the ability of larval mosquitoes to breath, access nutrients and emerge from the pupal stage. In all likelihood, both types of effect could be important in the field. Thus, the effects of relative humidity on the rate of evaporation relative to larval development or shifts in intrinsic conditions of larval habitats could have substantial effects on the thermal performance curves for both mosquito development rate (MDR), the probability of egg to adult survival (pEA) and consequently the intrinsic growth rate of mosquito populations.
Once adults emerge from the larval environment, variation in relative humidity could potentially increase or decrease the predicted upper thermal limit for adult traits that are critical for mosquito population dynamics and transmission (Figure 4, Table 2). For example, decreases in relative humidity at warm temperatures could decrease mosquito survival (by increasing the per capita daily mortality rate (μ)) via increasing desiccation stress (Gaaboub et al., 1971; Lyons et al., 2014; Mayne, 1930). This, in turn, will decrease the temperatures at which mosquitoes can survive to become infected and to transmit vector-borne pathogens. Evidence from other insect systems (Chown and Nicolson (2004); Edney and Barrass (1962); Shelford (1918); Yu et al. (2010)) would predict that decreases in relative humidity at warm temperatures could also decrease the per capita daily biting rate (a) and production of eggs (EFD) by altering mosquito activity and blood feeding due to shifts in behaviour (e.g., utilization of specific habitats, times of day or times of season; Canyon et al. (1999); Drakou et al. (2020); Dow and Gerrish (1970); Gaaboub et al. (1971); Provost (1973)) and physiological responses (e.g., decreased metabolic rate) to increase desiccation resistance or tolerance (Chown & Davis, 2003; Marron et al., 2003). However, the evidence that does exist for mosquitoes suggests decreases in relative humidity can actually increase biting rates on hosts (e.g., Culex pipiens, Ae. aegypti, An. quadramaculatus; Hagan et al. (2018)). It remains unclear if this pattern would persist in the field for mosquito species that utilize sugar sources for hydration and nutrition, because nectar-feeding mosquitoes can increase sugar feeding behaviour when environmental conditions are dry (Fikrig et al., 2020).
Finally, we also anticipate that the development of mosquito-borne pathogens and parasites, and potentially mosquito susceptibility to infection, should be affected by variation in relative humidity under different ambient temperature conditions based on physiological acclimation responses (Beitz, 2006; Liu et al., 2016). Aquaporin water channels allow organisms to rapidly move water (aquaporins) or water and glycerol (aquaglyceroporins) across cellular membranes to promote cellular function. Mosquitoes utilize aquaporins and aquaglyceroporins to minimize water loss in desiccating environments (Liu et al., 2011) and to maintain glycerol concentrations to stabilize proteins when mosquitoes are exposed to high heat (Deocaris et al., 2006; Diamant et al., 2001; Liu et al., 2016; Tatzel et al., 1996). The physiological responses of mosquitoes to optimally thermo- and hydroregulate under sub-optimal temperature and relative humidity environments could also have consequences for the energy available to developing pathogen (Liu et al., 2016).
IMPLICATIONS FOR UNDERSTANDING PATHOGEN TRANSMISSION AND CONTROL IN A CHANGING WORLD
Understanding the respective effects of variation in temperature and humidity, as well as any interaction between variables, will be critical for addressing how the regional and global distributions of mosquito vectors, and the seasonality and intensity of vector-borne pathogen transmission, will shift in response to future climate and land-use change. Based on the importance of maintaining optimal temperature and water balance in other organisms, we also argue that variation in temperature, humidity and water availability are important selective determinants driving local adaptation of mosquitoes to various environments as well as their capacity to respond to future environmental change. Finally, variation in temperature and humidity will also likely affect the efficacy, coverage and cost of disease control programs.
Human-mediated environmental change
Human-mediated climate change is resulting in widespread and uneven changes in global temperature, humidity and precipitation patterns and more frequent extreme weather events (IPCC, 2021). In addition to climate warming, regional changes in humidity and precipitation will result in increased drought in some areas, while others become wetter (Konapala et al., 2020). If mosquitoes and their transmission cycles are more sensitive to humidity at higher temperatures, then future increases in wet vs. dry heat may have very different implications for mosquito populations and pathogen risk. Regional variation in temperature and relative humidity could have important implications for both the seasonal timing and peak of vector-borne disease (Santos-Vega et al., 2016, 2022) as well as pathogen persistence or emergence. For example, it has been suggested that future temperatures in tropical Africa will exceed the thermal optimum for malaria and result in reduced transmission (Mordecai et al., 2020). However, these tropical regions are characterized by humid heat, and malaria may persist if the maximum temperature for transmission increases at high humidity. Similarly, the potential for arboviruses to expand into warming temperate climates may be greater in regions with increasing humid heat vs. dry heat, which has not been considered in current mechanistic model projections of disease risk with various climate change scenarios [e.g., (Caldwell et al., 2021; Ryan, Carlson, et al., 2020a; Ryan, Lippi, & Zermoglio, 2020b)].
Land-use change is another key human driver affecting mosquito-borne disease transmission (Baeza et al., 2017). For example, urban landscapes are one of the most rapidly growing land cover types across the globe (United Nations, 2019), with the proportion of people living in urban environment projected to increase from 55% to 68% between now and 2050. High environmental heterogeneity in urban areas creates substantial variation in the local microclimates mosquitoes experience, through differences in temperature, moisture and wind speed (Stewart & Oke, 2012). These differences are mediated by the extent of impervious surfaces, the distribution of vegetation and the three-dimensional structure created by buildings and trees. Together, these changes result in urban heat and dry islands (Heaviside et al., 2017) with higher land surface (Yuan & Bauer, 2007) and near-surface air temperatures (Coseo & Larsen, 2014) and lower relative humidity (Hao et al., 2018; Heaviside et al., 2017; Lokoshchenko, 2017; Yang et al., 2017) compared to more vegetated landscapes. This fine-scale variation in mosquito microclimate can have significant implications for multiple mosquito species (e.g., Aedes aegypti, Ae. albopictus, Anopheles stephensi) that drive urban outbreaks of diseases (e.g., dengue, chikungunya, Zika and malaria) (Beebe et al., 2009; Heinisch et al., 2019; Li et al., 2014; Murdock et al., 2017; Stoddard et al., 2009; Takken & Lindsay, 2019; Thomas et al., 2016, 2017).
Small-scale variation in temperature and relative humidity could also have important implications for the spatial distribution of risk in urban environments (Figure 5). Recent studies that combine field experimentation with direct monitoring of urban microclimates and mosquito abundance demonstrate that fine-scale variation (e.g., individual neighbourhoods or city blocks) in both temperature and relative humidity can have important implications for mosquito life history, population dynamics and disease transmission within urban environments (Evans et al., 2019; Evans, Shiau, et al., 2018b; Murdock et al., 2017; Wimberly et al., 2020). Thus, neighbourhoods with a high proportion of impervious surfaces that experience mean temperatures near or exceeding the thermal optimum for transmission could experience even higher decreases in vectorial capacity than what models would predict from temperature alone, if drier conditions increase desiccation stress and reduce mosquito survival.
To generalize the effects of changing temperature and humidity across diverse locations and into the future, it will be necessary to develop a conceptual framework that incorporates the psychometrics of temperature and atmospheric moisture with mosquito biology and the natural and built environments in which transmission occurs. Incorporating the effects of humidity into hierarchical models and assessment of mosquito population dynamics and disease transmission will increase the precision of mapping environmental suitability, both globally and regionally with human-mediated environmental change, as well as across heterogeneous human-modified landscapes.
Local adaptation and capacity to adapt in the future
There is growing interest in the factors driving adaptation of mosquitoes to local environmental conditions for providing insights into the long-term responses of mosquito species to future warming. Mosquito species are composed of an array of locally adapted populations across their respective ranges. Substantial genetic variation exists in mosquito species (Fouet et al., 2017; Holt et al., 2002; Kang et al., 2021; Maffey et al., 2020; Pless et al., 2020; Yurchenko et al., 2020) and at fine-spatial scales (Ayala et al., 2020; Carvajal et al., 2020; Gutiérrez et al., 2010; Jasper et al., 2019; Matowo et al., 2019), with significant consequences for transmission potential (Azar et al., 2017; Palmer et al., 2018; Vega-Rúa et al., 2020). This genetic variation can interact with local environmental conditions to impact the capacity of mosquito vectors to transmit human pathogens (e.g., dengue; Gloria-Soria et al. (2017) and chikungunya; Zouache et al. (2014)). Yet, we still do not have a clear understanding of what environmental factors are driving this differentiation.
The work that has been done in this area to date has largely focused on the effects of temperature variation in driving local adaptation of current mosquito populations (Couper et al., 2021; Sternberg & Thomas, 2014). However, research from the broader field of ectotherms [e.g., reviewed in Rozen-Rechels et al., 2019, vertebrates; Chown et al., 2011, insects] suggests that selection on thermal response curves are constrained by other metabolic stressors, like desiccation stress, as temperatures warm. For example, a study on 94 Drosophila species from diverse climates found substantial variation in the upper thermal limits among species. Further, the species specific CTmax correlated positively with increasing temperature in dry environments, with species from hot and dry environments exhibiting higher heat tolerance. However, this relationship completely disappeared for species inhabiting wet environments suggesting temperature as a selective force is less important when humidity is high (Kellermann et al., 2012). A similar study in ectothermic vertebrates (400 lizards), found the thermal optimum to be more strongly related to ambient precipitation than to average temperature (Clusella-Trullas et al., 2011). Environmental mean temperature was only found to be predictive of the lower thermal limit (CTmin) (Clusella-Trullas et al., 2011).
Both common garden and experimental evolution studies, two standard approaches to measure local adaptation and evolutionary potential of a particular species, could be incorrectly attributing observed phenotypic responses to temperature selection when they could be responding to a combination of energetic effects and moisture stress. This impacts our ability to accurately characterize thermal response curves of mosquitoes, as well as their capacity to adapt to future environmental change. From our conceptual framework outlined above (Figure 5), we would predict that the current approach to studying local adaptation, steeped in metabolic theory of ecology, will be most predictive of mosquito population responses to future warming in regions of the world that currently exist below the species specific thermal optima (Topt). However, for mosquito populations that inhabit environments above their thermal optima, humidity will be an important determinant of their capacity to respond to future environmental change. For example, mosquito populations in warm and wet, humid environments may have less capacity to adapt to future climate change in a warming and drying environment than what would be predicted from evolutionary models that consider the effects of temperature alone. Conversely, mosquito populations that currently live in warm and dry environments may have a greater capacity to adapt to warming conditions if they exhibit higher heat tolerance than their counterparts inhabiting wetter areas of the geographic distribution.
Controlling mosquito populations and disease transmission
There have been several mechanistic modelling efforts to understand how regional and seasonal environmental variation will impact the relative reproductive number of a pathogen, the intensity of human transmission and the efficacy of key disease interventions (e.g., Zika; Ngonghala et al. (2021), schistosomiasis; Nguyen et al. (2021)). These studies have, again, focused largely on the effects of ambient temperature. However, seasonal and regional variation in humidity and precipitation could extend or shorten the transmission season and magnify or depress the intensity of epidemics as predicted from models incorporating the effects of temperature alone (Huber et al., 2018; Ngonghala et al., 2021). For example, this is likely to be the case in seasonally dry environments where mosquito-borne disease transmission tends to be highest during or just after the rainy season and lowest during the hottest / driest parts of the season due to seasonal shifts in mosquito habitat, as well as the effects of temperature and humidity on mosquito and pathogen traits relevant for transmission.
How variation in humidity affects the efficacy of current and novel mosquito control interventions also needs to be considered. Many novel mosquito control technologies involve the mass release of males that have been sterilized or genetically engineered to pass on traits that confer either severe fitness costs (i.e., population suppression approaches; Alphey et al., 2010; Wilke & Marrelli, 2012; Wang et al., 2021) or enhanced resistance to human pathogens (i.e., population replacement approaches (Carballar-Lejarazú & James, 2017; Hegde & Hughes, 2017; Wilke & Marrelli, 2015)). For example, the wMel strain of the symbiont Wolbachia can prevent dengue, chikungunya and Zika transmission in Ae. aegypti (Aliota, Peinado, et al., 2016a; Aliota, Walker, et al., 2016b; Moreira et al., 2009; Ye et al., 2015). Experimental work has determined that wMel infections are temperature sensitive, with high temperatures causing reductions in Wolbachia density (Foo et al., 2019; Gu et al., 2022; Ross et al., 2017, 2019, 2020; Ulrich et al., 2016) and temperature variation affects the host-pathogen interaction and the outcome of infection in Wolbachia-infected mosquitoes (Murdock, Blanford, Hughes, et al., 2014a). Based on the relationship between temperature and water balance laid out in this paper, further experiments should examine whether Wolbachia infections are limited by temperature alone or by cellular water availability, and examine what role mosquito desiccation stress plays in limiting Wolbachia abundance within mosquitoes at varying temperature.
Furthermore, thermal performance differs between insecticide resistant vectors and their susceptible counterparts, with important implications for assessing fitness costs associated with insecticide resistance (Akinwande et al., 2021). Thus, insecticide resistant mosquitoes may have to optimize temperature and water needs across environmental constraints differently and, therefore, be affected by changes in humidity, with potentially important consequences for population dynamics, mosquito-pathogen interactions and transmission. Identifying these environmental constraints on efficacy and coverage will be critical for the successful implementation of current and future control programs (Parham & Hughes, 2015).
CONCLUSIONS AND FUTURE DIRECTIONS
Sufficiently understanding the performance of insect vectors within the natural environmental mosaics where they occur will require substantially more data on the spatial and temporal complexities in microclimate, behavioural responses to temperature and humidity change, plasticity in thermal tolerance traits and the eco-physiological mechanisms of vector water balance, coupled with broader understanding of the general relationships between water and temperature described in this paper. We have collated these goals into a general framework incorporating humidity into research questions and temperature-dependent mechanistic models (Figure 5 & Box 2). We intend for the evidence and theory presented here to be signposts for future research, leading to a collective broadening in our understanding of insect vectors and how their responses to climate variables will affect parasite transmission.
Box 2. Future Questions.
- Direct effects of humidity
- To what extent will humidity affect parasite and pathogen development within mosquitoes, and what are the underlying mechanisms?
- How does humidity affect thermal performance curves of both mosquitoes and pathogens? Does it impact the variation in the thermal optimum, limits, operative range or performance magnitude?
- How do experimental conditions (e.g., constant vs. diurnally fluctuating environments, different humidity values used to assess thermal performance) influence the interpretation and variation in thermal performance, including in semi-field experiments?
- Does humidity affect different mosquito populations and species similarly?
- How do background humidity conditions influence the interpretation and variation in thermal performance characterized in laboratory experiments or in semi-field experiments?
- How much of the decrease in thermal performance at thermal extremes (CTmin, CTmax) can be explained by mosquito water balance, rather than temperature alone?
- Does the environmental niche, defined by temperature and water constraints, explain current distributions and abundances of key mosquito vectors and vector-borne diseases?
- At what spatial or temporal scales should measurement, inference and prediction be implemented for mosquito population dynamics, disease transmission and control, in the context of temperature and humidity? Do these scales differ between the two climate variables?
- Consequences of interactive effects of temperature and humidity
- How do humidity and desiccation stress shape the evolution of thermal performance? Which traits and elements of thermal performance (e.g., thermal optimum, thermal limits, operative range) will experience, and respond to, selection?
- How will changes in humidity resulting from future climate and land-use change affect the potential for mosquito thermal performance to adapt to warming temperatures?
- Do differences in the ability to thermo-hydroregulate across mosquito species help explain evolutionary differences in life history and how these species utilize their environments (e.g., day, night or dawn/dusk host seeking; circadian rhythms in the production of malaria infectious stages in human hosts; propensity to sugar feed; microhabitat selection of resting mosquitoes; oviposition site selection; seasonal and diurnal timing of mating; duration of dry diapause or overwintering)
- How will population genetic variation impact mosquito adaptability to local microclimates? And to what extent will phenotypic plasticity in traits affecting thermal tolerance and water balance influence persistence of mosquito populations, communities and species?
- How will current and novel (e.g., Wolbachia) vector control strategies be impacted by variation in humidity and temperature? How will this affect the timing, distribution, duration and efficacy of a given intervention?
Funding information
National Institutes of Health, Grant/Award Number: 5R01AI153444-03
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no known conflict of interest regarding the publication of this manuscript.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- Abdelrazec A & Gumel AB (2017) Mathematical assessment of the role of temperature and rainfall on mosquito population dynamics. Journal of Mathematical Biology, 74, 1351–1395 [DOI] [PubMed] [Google Scholar]
- Adelman ZN, Anderson MAE, Wiley MR, Murreddu MG, Samuel GH, Morazzani EM et al. (2013) Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Neglected Tropical Diseases, 7, e2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrane YA, Lawson BW, Githeko AK & Yan G (2005) Effects of microclimatic changes caused by land use and land cover on duration of Gonotrophic cycles of Anopheles gambiae (Diptera: Culicidae) in Western Kenya highlands. Journal of Medical Entomology, 42, 974–980. [DOI] [PubMed] [Google Scholar]
- Aguirre AA (2017) Changing patterns of emerging zoonotic diseases in wildlife, domestic animals, and humans linked to biodiversity loss and globalization. ILAR Journal, 58, 315–318. [DOI] [PubMed] [Google Scholar]
- Akinwande KL, Arotiowa AR & Ete AJ (2021) Impacts of changes in temperature and exposure time on the median lethal concentrations (LC50) of a combination of organophosphate and pyrethroid in the control of Culex quinquefasciatus, say (Diptera: Culicidae). Scientific African, 12, e00743. [Google Scholar]
- Allen RG, Pereira LS, Raes D & Smith M (1998) Crop evapotranspiration-Guidelines for computing crop water requirements. FAO Irrigation and Drainage Paper 56, 17, 50–53. [Google Scholar]
- Albernaz D. a. S., Tai MHH & Luz C (2009). Enhanced ovicidal activity of an oil formulation of the fungus Metarhizium anisopliae on the mosquito Aedes aegypti. Medical and Veterinary Entomology, 23, 141–147. [DOI] [PubMed] [Google Scholar]
- Aliota MT, Peinado SA, Velez ID & Osorio JE (2016a) The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Scientific Reports, 6, 28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Walker EC, Yepes AU, Velez ID, Christensen BM & Osorio JE (2016b) The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Neglected Tropical Diseases, 10, e0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW et al. (2010) Sterile-insect methods for control of mosquito-Borne diseases: an analysis. Vector-Borne Zoonotic Disease, 10, 295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althouse BM, Hanley KA, Diallo M, Sall AA, Ba Y, Faye O et al. (2015) Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in Senegal. The American Journal of Tropical Medicine and Hygiene, 92, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Bettinardi DJ & Ortiz S (2015) Interspecific larval competition differentially impacts adult survival in dengue vectors. Journal of Medical Entomology, 52, 163–170. [DOI] [PubMed] [Google Scholar]
- Alto BW & Juliano SA (2001) Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. Journal of Medical Entomology, 38, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RCO & Andrade DV (2017) Trading heat and hops for water: dehydration effects on locomotor performance, thermal limits, and thermoregulatory behavior of a terrestrial toad. Ecology and Evolution, 7, 9066–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: OUP Oxford. [Google Scholar]
- Ashepet MG, Jacobs L, Van Oudheusden M & Huyse T (2021) Wicked solution for wicked problems: citizen science for vector-Borne disease control in Africa. Trends in Parasitology, 37, 93–96. [DOI] [PubMed] [Google Scholar]
- Asigau S & Parker PG (2018) The influence of ecological factors on mosquito abundance and occurrence in Galápagos. Journal of Vector Ecology, 43, 125–137. [DOI] [PubMed] [Google Scholar]
- Ayala AM, Vera NS, Chiappero MB, Almirón WR & Gardenal CN (2020) Urban populations of Aedes aegypti (Diptera: Culicidae) from Central Argentina: dispersal patterns assessed by Bayesian and multivariate methods. Journal of Medical Entomology, 57, 1069–1076. [DOI] [PubMed] [Google Scholar]
- Azar SR, Roundy CM, Rossi SL, Huang JH, Leal G, Yun R et al. (2017) Differential vector competency of Aedes albopictus populations from the Americas for Zika virus. The American Journal of Tropical Medicine and Hygiene, 97, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azil AH, Long SA, Ritchie SA & Williams CR (2010) The development of predictive tools for pre-emptive dengue vector control: a study of Aedes aegypti abundance and meteorological variables in North Queensland, Australia. Tropical Medicine & International Health, 15, 1190–1197. [DOI] [PubMed] [Google Scholar]
- Baeza A, Santos-Vega M, Dobson AP & Pascual M (2017) The rise and fall of malaria under land-use change in frontier regions. Nature Ecology and Evolution, 1, 1–7. [DOI] [PubMed] [Google Scholar]
- Bar-Zeev M (1957) The effect of extreme temperatures on different stages of Aädes aegypti (L.). Bulletin of Entomological Research, 48, 593–599. [Google Scholar]
- Bayoh MN (2001) Studies on the development and survival of Anopheles gambiae sensu stricto at various temperatures and relative humidities. Doctoral. Durham, UK: Durham University. [Google Scholar]
- Beck J, McCain CM, Axmacher JC, Ashton LA, Bärtschi F, Brehm G et al. (2017) Elevational species richness gradients in a hyperdiverse insect taxon: a global meta-study on geometrid moths. Global Ecology and Biogeography, 26, 412–424. [Google Scholar]
- Beebe NW, Cooper RD, Mottram P & Sweeney AW (2009) Australia’s dengue risk driven by human adaptation to climate change. PLoS Neglected Tropical Diseases, 3, e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz E (2006) Aquaporin water and solute channels from malaria parasites and other pathogenic protozoa. ChemMedChem, 1, 587–592. [DOI] [PubMed] [Google Scholar]
- Benoit JB (2010) Water management by dormant insects: comparisons between dehydration resistance during summer aestivation and winter diapause. In: Arturo Navas C & Carvalho JE (Eds.) Aestivation: molecular and physiological aspects, Progress in molecular and subcellular biology. Berlin, Heidelberg: Springer, pp. 209–229. [DOI] [PubMed] [Google Scholar]
- Benoit JB & Denlinger DL (2010) Meeting the challenges of on-host and off-host water balance in blood-feeding arthropods. Journal of Insect Physiology, 56, 1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra Da Silva CS, Price BE & Walton VM (2019) Water-deprived parasitic wasps (Pachycrepoideus vindemmiae) kill more pupae of a Pest (Drosophila suzukii) as a water-intake strategy. Scientific Reports, 9, 3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U et al. (2015) The effect of malaria control on plasmodium falciparum in Africa between 2000 and 2015. Nature, 526, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmayer WL (1974) The influence of environmental factors and physiological stage on flight patterns of mosquitoes taken in the vehicle aspirator and truck, suction, bait and New Jersey light traps. Journal of Medical Entomology, 11, 119–146. [DOI] [PubMed] [Google Scholar]
- Bidlingmayer WL (1985) The measurement of adult mosquito population changes—some considerations. Journal of the American Mosquito Control Association, 1, 328–248. [PubMed] [Google Scholar]
- Bradshaw D (2003) Vertebrate ecophysiology: an introduction to its principles and applications. Cambridge: Cambridge University Press. [Google Scholar]
- Brown JH, Gillooly JF, Allen AP, Savage VM & West GB (2004) Toward a metabolic theory of ecology. Ecology, 85, 1771–1789. [Google Scholar]
- Brown L, Medlock J & Murray V (2014) Impact of drought on vector-borne diseases – how does one manage the risk? Public Health, 128, 29–37. [DOI] [PubMed] [Google Scholar]
- Buckner EA, Blackmore MS, Golladay SW & Covich AP (2011) Weather and landscape factors associated with adult mosquito abundance in southwestern Georgia, U.S.A. Journal of Vector Ecology, 36, 269–278. [DOI] [PubMed] [Google Scholar]
- Buzan JR & Huber M (2020) Moist heat stress on a hotter earth. Annual Review of Earth and Planetary Sciences, 48, 623–655. [Google Scholar]
- Calatayud J, Hortal J, Medina NG, Turin H, Bernard R, Casale A et al. (2016) Glaciations, deciduous forests, water availability and current geographical patterns in the diversity of European Carabus species. Journal of Biogeography, 43, 2343–2353. [Google Scholar]
- Caldwell JM, LaBeaud AD, Lambin EF, Stewart-Ibarra AM, Ndenga BA, Mutuku FM et al. (2021) Climate predicts geographic and temporal variation in mosquito-borne disease dynamics on two continents. Nature Communications, 12, 1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canyon DV, Hii JLK & Müller R (1999) Adaptation of Aedes aegypti (Diptera: Culicidae) oviposition behavior in response to humidity and diet. Journal of Insect Physiology, 45, 959–964. [DOI] [PubMed] [Google Scholar]
- Canyon DV, Müller R & Hii JLK (2013) Aedes aegypti disregard humidity-related conditions with adequate nutrition. Tropical Biomedicine, 30, 1–8. [PubMed] [Google Scholar]
- Carballar-Lejarazú R & James AA (2017) Population modification of Anopheline species to control malaria transmission. Pathogens and Global Health, 111, 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T et al. (2020) Scientists’ warning to humanity on insect extinctions. Biological Conservation, 242, 108426. [Google Scholar]
- Carrington LB, Armijos MV, Lambrechts L, Barker CM & Scott TW (2013) Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS One, 8, e58824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal TM, Ogishi K, Yaegeshi S, Hernandez LFT, Viacrusis KM, Ho HT et al. (2020) Fine-scale population genetic structure of dengue mosquito vector, Aedes aegypti, in metropolitan Manila, Philippines. PLOS Neglected Tropical Diseases, 14, e0008279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Thomas S, Paaijmans KP, Ravishankaran S, Justin JA, Mathai MT et al. (2013) Characterizing microclimate in urban malaria transmission settings: a case study from Chennai, India. Malaria Journal, 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy S, Ramanathan K, Manoharan A, Mathai D & Baruah K (2013) Assessing effect of climate on the incidence of dengue in Tamil Nadu. Indian Journal of Medical Microbiology, 31, 283–286. [DOI] [PubMed] [Google Scholar]
- Chaplin M (2006) Do we underestimate the importance of water in cell biology? Nature Reviews. Molecular Cell Biology, 7, 861–866. [DOI] [PubMed] [Google Scholar]
- Chappuis CJ, Béguin S, Vlimant M & Guerin PM (2013) Water vapour and heat combine to elicit biting and biting persistence in tsetse. Parasites & Vectors, 6, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves LF & Kitron UD (2011) Weather variability impacts on oviposition dynamics of the southern house mosquito at intermediate time scales. Bulletin of Entomological Research, 101, 633–641. [DOI] [PubMed] [Google Scholar]
- Chen S-C, Liao C-M, Chio C-P, Chou H-H, You S-H & Cheng Y-H (2010) Lagged temperature effect with mosquito transmission potential explains dengue variability in southern Taiwan: insights from a statistical analysis. Science of the Total Environment, 408, 4069–4075. [DOI] [PubMed] [Google Scholar]
- Chiew FHS, Kamaladasa NN, Malano HM & McMahon TA (1995) Penman-Monteith, FAO-24 reference crop evapotranspiration and class-Apan data in Australia. Agricultural Water Management, 28, 9–21. [Google Scholar]
- Chowdhury FR, Ibrahim QSU, Bari MS, Alam MMJ, Dunachie SJ, Rodriguez-Morales AJ et al. (2018) The association between temperature, rainfall and humidity with common climate-sensitive infectious diseases in Bangladesh. PLoS One, 13, e0199579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown SL & Davis ALV (2003) Discontinuous gas exchange and the significance of respiratory water loss in scarabaeine beetles. The Journal of Experimental Biology, 206, 3547–3556. [DOI] [PubMed] [Google Scholar]
- Chown SL & Gaston KJ (2008) Macrophysiology for a changing world. Proceedings of the Royal Society B: Biological Sciences, 275, 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown SL & Nicolson S (2004) Insect physiological ecology: mechanisms and patterns. Oxford, UK: OUP Oxford. [Google Scholar]
- Chown SL, Sørensen JG & Terblanche JS (2011) Water loss in insects: an environmental change perspective. Journal of Insect Physiology, 57, 1070–1084. [DOI] [PubMed] [Google Scholar]
- Christofferson RC & Mores CN (2016) Potential for extrinsic incubation temperature to Alter interplay between transmission potential and mortality of dengue-infected Aedes aegypti. Environmental Health Insights, 10, EHI.S38345–EHI.S38123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusella-Trullas S, Blackburn TM & Chown SL (2011) Climatic predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. The American Naturalist, 177, 738–751. [DOI] [PubMed] [Google Scholar]
- Cohen JM, Civitello DJ, Brace AJ, Feichtinger EM, Ortega CN, Richardson JC et al. (2016) Spatial scale modulates the strength of ecological processes driving disease distributions. Proceedings of the National Academy of Sciences of the United States of America, 113, E3359–E3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkrey R, McMeekin TA, Bowman JP, Ratkowsky DA, Olley J & Ross T (2016) The biokinetic spectrum for temperature. PLoS One, 11, e0153343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coseo P & Larsen L (2014) How factors of land use/land cover, building configuration, and adjacent heat sources and sinks explain urban Heat Islands in Chicago. Landscape and Urban Planning, 125, 117–129. [Google Scholar]
- Costa, E.A.P.d.A., Santos E.M.d.M., Correia JC & Albuquerque C.M.R.d. (2010) Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae). Revista Brasileira de Entomologia, 54, 488–493. [Google Scholar]
- Couper LI, Farner JE, Caldwell JM, Childs ML, Harris MJ, Kirk DG et al. (2021) How will mosquitoes adapt to climate warming? eLife, 10, e69630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbro JM, Graham RI, Kay BH, Ryan PA & Thomas MB (2011) Evaluation of entomopathogenic fungi as potential biological control agents of the dengue mosquito, Aedes aegypti (Diptera: Culicidae). Biocontrol Science and Technology, 21, 1027–1047. [Google Scholar]
- Davis JK, Vincent GP, Hildreth MB, Kightlinger L, Carlson C & Wimberly MC (2018) Improving the prediction of arbovirus outbreaks: a comparison of climate-driven models for West Nile virus in an endemic region of the United States. Acta Tropica, 185, 242–250. [DOI] [PubMed] [Google Scholar]
- Delatte H, Gimonneau G, Triboire A & Fontenille D (2009) Influence of temperature on immature development, survival, longevity, fecundity, and Gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. Journal of Medical Entomology, 46, 33–41. [DOI] [PubMed] [Google Scholar]
- Dell AI, Pawar S & Savage VM (2011) Systematic variation in the temperature dependence of physiological and ecological traits. Proceedings of the National Academy of Sciences of the United States of America, 108, 10591–10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deocaris CC, Shrestha BG, Kraft DC, Yamasaki K, Kaul SC, Rattan SIS et al. (2006) Geroprotection by glycerol. Annals of the new York Academy of Sciences, 1067, 488–492. [DOI] [PubMed] [Google Scholar]
- Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC et al. (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences of the United States of America, 105, 6668–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo D, Diagne CT, Buenemann M, Ba Y, Dia I, Faye O et al. (2019) Biodiversity pattern of mosquitoes in southeastern Senegal, epidemiological implication in arbovirus and malaria transmission. Journal of Medical Entomology, 56, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant S, Eliahu N, Rosenthal D & Goloubinoff P (2001) Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses*. The Journal of Biological Chemistry, 276, 39586–39591. [DOI] [PubMed] [Google Scholar]
- Dillon ME, Wang G & Huey RB (2010) Global metabolic impacts of recent climate warming. Nature, 467, 704–706. [DOI] [PubMed] [Google Scholar]
- Döring TF (2017) Vector-Borne diseases. In: Plant diseases and their Management in Organic Agriculture. The American Phytopathological Society: IPM, pp. 107–116. [Google Scholar]
- Dow RP & Gerrish GM (1970) Day-to-day change in relative humidity and the activity of Culex nigripalpus (Diptera: Culicidae)1. Annals of the Entomological Society of America, 63, 995–999. [DOI] [PubMed] [Google Scholar]
- Drakou K, Nikolaou T, Vasquez M, Petric D, Michaelakis A, Kapranas A et al. (2020) The effect of weather variables on mosquito activity: a snapshot of the Main point of entry of Cyprus. International Journal of Environmental Research and Public Health, 17, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant AC & Donini A (2019) Development of Aedes aegypti (Diptera: Culicidae) mosquito larvae in high ammonia sewage in septic tanks causes alterations in ammonia excretion, ammonia transporter expression, and osmoregulation. Scientific Reports, 9, 19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant AC, Grieco Guardian E, Kolosov D & Donini A (2021) The transcriptome of anal papillae of Aedes aegypti reveals their importance in xenobiotic detoxification and adds significant knowledge on ion, water and ammonia transport mechanisms. Journal of Insect Physiology, 132, 104269. [DOI] [PubMed] [Google Scholar]
- Edney EB (2012) Water balance in land arthropods. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- Edney EB & Barrass R (1962) The body temperature of the tsetse fly, Glossina morsitans Westwood (Diptera, Muscidae). Journal of Insect Physiology, 8, 469–481. [Google Scholar]
- Evans MV, Hintz CW, Jones L, Shiau J, Solano N, Drake JM et al. (2019) Microclimate and larval habitat density predict adult Aedes albopictus abundance in urban areas. The American Journal of Tropical Medicine and Hygiene, 101, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MV, Newberry PM & Murdock CC (2018a) Carry-over effects of the larval environment in mosquito-borne disease systems. Population biology of vector-borne diseases. Oxford, UK: Oxford University Press. [Google Scholar]
- Evans MV, Shiau JC, Solano N, Brindley MA, Drake JM & Murdock CC (2018b) Carry-over effects of urban larval environments on the transmission potential of dengue-2 virus. Parasites & Vectors, 11, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira PG, Tesla B, Horácio ECA, Nahum LA, Brindley MA, de Oliveira Mendes TA et al. (2020) Temperature dramatically shapes mosquito gene expression with consequences for mosquito–Zika virus interactions. Frontiers in Microbiology, 11, 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig K, Peck S, Deckerman P, Dang S, Fleur KS, Goldsmith H et al. (2020) Sugar feeding patterns of New York Aedes albopictus mosquitoes are affected by saturation deficit, flowers, and host seeking. PLoS Neglected Tropical Diseases, 14, e0008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo IJ-H, Hoffmann AA & Ross PA (2019) Cross-generational effects of heat stress on fitness and Wolbachia density in Aedes aegypti mosquitoes. Tropical Medicine and Infectious Disease, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouet C, Kamdem C, Gamez S & White BJ (2017) Extensive genetic diversity among populations of the malaria mosquito anopheles moucheti revealed by population genomics. Infection, Genetics and Evolution, 48, 27–33. [DOI] [PubMed] [Google Scholar]
- Gaaboub IA, El-Sawaf SK & El-Latif MA (1971) Effect of different relative Humidities and temperatures on egg-production and longevity of adults of anopheles (Myzomyia) pharoensis Theob.1. Zeitschrift für Angewandte Entomologie, 67, 88–94. [Google Scholar]
- Garros C, Bouyer J, Takken W & Smallegange RC (2017) Control of vector-borne diseases in the livestock industry: new opportunities and challenges. In: Pests and vector-borne diseases in the livestock industry, ecology and control of vector-borne diseases. Wageningen, Netherlands: Wageningen Academic Publishers, pp. 575–580. [Google Scholar]
- George TL, Harrigan RJ, LaManna JA, DeSante DF, Saracco JF & Smith TB (2015) Persistent impacts of West Nile virus on north American bird populations. Proceedings of the National Academy of Sciences of the United States of America, 112, 14290–14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething PW, Smith DL, Patil AP, Tatem AJ, Snow RW & Hay SI (2010) Climate change and the global malaria recession. Nature, 465, 342–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloria-Soria A, Armstrong PM, Powell JR & Turner PE (2017) Infection rate of Aedes aegypti mosquitoes with dengue virus depends on the interaction between temperature and mosquito genotype. Proceedings of the Royal Society B: Biological Sciences, 284, 20171506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glunt KD, Paaijmans KP, Read AF & Thomas MB (2014) Environmental temperatures significantly change the impact of insecticides measured using WHOPES protocols. Malaria Journal, 13, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Tokman D, Córdoba-Aguilar A, Dáttilo W, Lira-Noriega A, Sánchez-Guillén RA & Villalobos F (2020) Insect responses to heat: physiological mechanisms, evolution and ecological implications in a warming world. Biological Reviews, 95, 802–821. [DOI] [PubMed] [Google Scholar]
- Gray EM & Bradley TJ (2005) Physiology of desiccation resistance in Anopheles gambiae and anopheles arabienses. The American Journal of Tropical Medicine and Hygiene, 73, 553–559. [PubMed] [Google Scholar]
- Grimstad PR & DeFoliart GR (1975) Mosquito nectar feeding in Wisconsin in relation to twilight and microclimate. Journal of Medical Entomology, 11, 691–698. [DOI] [PubMed] [Google Scholar]
- Gu X, Ross PA, Rodriguez-Andres J, Robinson KL, Yang Q, Lau M-J et al. (2022) A wMel Wolbachia variant in Aedes aegypti from field-collected Drosophila melanogaster with increased phenotypic stability under heat stress. Environmental Microbiology, 24, 2119–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunay F, Alten B & Ozsoy ED (2010) Estimating reaction norms for predictive population parameters, age specific mortality, and mean longevity in temperature-dependent cohorts of Culex quinquefasciatus say (Diptera: Culicidae). Journal of Vector Ecology, 35, 354–362. [DOI] [PubMed] [Google Scholar]
- Gunderson AR & Stillman JH (2015) Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proceedings of the Royal Society B: Biological Sciences, 282, 20150401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez LA, Gómez GF, González JJ, Castro MI, Luckhart S, Conn JE et al. (2010) Microgeographic genetic variation of the malaria vector Anopheles darlingi root (Diptera: Culicidae) from Córdoba and Antioquia, Colombia. American Journal of Tropical Medicine and Hygiene, 83, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan RW, Didion EM, Rosselot AE, Holmes CJ, Siler SC, Rosendale AJ et al. (2018) Dehydration prompts increased activity and blood feeding by mosquitoes. Scientific Reports, 8, 6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann E, Blevins C, Franks SJ, Jameel MI & Anderson JT (2021) Climate change alters plant–herbivore interactions. The New Phytologist, 229, 1894–1910. [DOI] [PubMed] [Google Scholar]
- Hao L, Huang X, Qin M, Liu Y, Li W & Sun G (2018) Ecohydrological processes explain urban Dry Island effects in a wet region, Southern China. Water Resources Research, 54, 6757–6771. [Google Scholar]
- Hayden MH, Uejio CK, Walker K, Ramberg F, Moreno R, Rosales C et al. (2010) Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, U.S./Sonora, MX Border. EcoHealth, 7, 64–77. [DOI] [PubMed] [Google Scholar]
- Heaviside C, Macintyre H & Vardoulakis S (2017) The urban Heat Island: implications for health in a changing environment. Current Environmental Health Reports, 4, 296–305. [DOI] [PubMed] [Google Scholar]
- Hegde S & Hughes GL (2017) Population modification of anopheles mosquitoes for malaria control: pathways to implementation. Pathogens and Global Health, 111, 401–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch MRS, Diaz-Quijano FA, Chiaravalloti-Neto F, Menezes Pancetti FG, Rocha Coelho R, dos Santos Andrade P et al. (2019) Seasonal and spatial distribution of Aedes aegypti and Aedes albopictus in a municipal urban park in São Paulo, SP, Brazil. Acta Tropica, 189, 104–113. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Chown SL & Clusella-Trullas S (2013) Upper thermal limits in terrestrial ectotherms: how constrained are they? Functional Ecology, 27, 934–949. [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, Nusskern DR et al. (2002) The genome sequence of the malaria mosquito Anopheles gambiae. Science, 298, 129–149. [DOI] [PubMed] [Google Scholar]
- Huber JH, Childs ML, Caldwell JM & Mordecai EA (2018) Seasonal temperature variation influences climate suitability for dengue, chikungunya, and Zika transmission. PLoS Neglected Tropical Diseases, 12, e0006451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey RB & Kingsolver JG (2019) Climate warming, resource availability, and the metabolic meltdown of ectotherms. The American Naturalist, 194, E140–E150. [DOI] [PubMed] [Google Scholar]
- Huxley PJ, Murray KA, Pawar S & Cator LJ (2021) The effect of resource limitation on the temperature dependence of mosquito population fitness. Proceedings of the Royal Society B: Biological Sciences, 288, 20203217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley PJ, Murray KA, Pawar S & Cator LJ (2022) Competition and resource depletion shape the thermal response of population fitness in Aedes aegypti. Communications Biology, 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylton AR (1969) Studies on longevity of adult Eretmapodites chrysogaster, Aedes togoi and Aedes (Stegomyia) albopictus females (Diptera: Culicidae). Journal of Medical Entomology, 6, 147–149. [DOI] [PubMed] [Google Scholar]
- IPCC. (2021) Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Geneva, Switzerland: Cambridge University Press. [Google Scholar]
- Jamieson MA, Trowbridge AM, Raffa KF & Lindroth RL (2012) Consequences of climate warming and altered precipitation patterns for plant-insect and multitrophic interactions. Plant Physiology, 160, 1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper M, Schmidt TL, Ahmad NW, Sinkins SP & Hoffmann AA (2019) A genomic approach to inferring kinship reveals limited intergenerational dispersal in the yellow fever mosquito. Molecular Ecology Resources, 19, 1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal Y & Al-Thukair AA (2018) Combining GIS application and climatic factors for mosquito control in Eastern Province, Saudi Arabia. Saudi Journal of Biological Sciences, 25, 1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindra M & Sehnal F (1990) Linkage between diet humidity, metabolic water production and heat dissipation in the larvae of galleria mellonella. Insect Biochemistry, 20, 389–395. [Google Scholar]
- Johansson MA, Dominici F & Glass GE (2009) Local and global effects of climate on dengue transmission in Puerto Rico. PLoS Neglected Tropical Diseases, 3, e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MA, Powers AM, Pesik N, Cohen NJ & Staples JE (2014) Nowcasting the spread of chikungunya virus in the Americas. PLoS One, 9, e104915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BJ, Manby R & Devine GJ (2020) What happens on islands, doesn’t stay on islands: patterns of synchronicity in mosquito nuisance and host-seeking activity between a Mangrove Island and adjacent coastal development. Urban Ecosystem, 23, 1321–1333. [Google Scholar]
- Johnson LR, Ben-Horin T, Lafferty KD, McNally A, Mordecai E, Paaijmans KP et al. (2015) Understanding uncertainty in temperature effects on vector-borne disease: a Bayesian approach. Ecology, 96, 203–213. [DOI] [PubMed] [Google Scholar]
- Juliano SA & Stoffregen TL (1994) Effects of habitat drying on size at and time to metamorphosis in the tree hole mosquito Aedes triseriatus. Oecologia, 97, 369–376. [DOI] [PubMed] [Google Scholar]
- Kang DS, Kim S, Cotten MA & Sim C (2021) Transcript assembly and quantification by RNA-Seq reveals significant differences in gene expression and genetic variants in mosquitoes of the Culex pipiens (Diptera: Culicidae) complex. Journal of Medical Entomology, 58, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MDN, Munshi SU, Anwar N & Alam MDS (2012) Climatic factors influencing dengue cases in Dhaka city: a model for dengue prediction. The Indian Journal of Medical Research, 136, 32–39. [PMC free article] [PubMed] [Google Scholar]
- Kearney M & Porter W (2009) Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecology Letters, 12, 334–350. [DOI] [PubMed] [Google Scholar]
- Kellermann V, Overgaard J, Hoffmann AA, Fløjgaard C, Svenning J-C & Loeschcke V (2012) Upper thermal limits of drosophila are linked to species distributions and strongly constrained phylogenetically. Proceedings of the National Academy of Sciences of the United States of America, 109, 16228–16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S & Guerin PM (2008) Responses of Anopheles gambiae, Anopheles stephensi, Aedes aegypti, and Culex pipiens mosquitoes (Diptera: Culicidae) to cool and humid refugium conditions. Journal of Vector Ecology, 33, 145–149. [DOI] [PubMed] [Google Scholar]
- Kikankie CK, Brooke BD, Knols BG, Koekemoer LL, Farenhorst M, Hunt RH et al. (2010) The infectivity of the entomopathogenic fungus Beauveria bassiana to insecticide-resistant and susceptible anopheles arabiensis mosquitoes at two different temperatures. Malaria Journal, 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleynhans E & Terblanche J (2011) Complex interactions between temperature and relative humidity on water balance of adult tsetse (Glossinidae, Diptera): implications for climate change. Frontiers in Physiology, 2, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R & Basu BC (1943) Laboratory studies on the infectivity of Anopheles stephensi. Journal of Malaria Institute India, 5, 1–29. [Google Scholar]
- Konapala G, Mishra AK, Wada Y & Mann ME (2020) Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nature Communications, 11, 3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnholz S & Seeley TD (1997) The control of water collection in honey bee colonies. Behavioral Ecology and Sociobiology, 41, 407–422. [Google Scholar]
- Lajevardi A, Sajadi F, Donini A & Paluzzi J-PV (2021) Studying the activity of neuropeptides and other regulators of the excretory system in the adult mosquito. Journal of Visualized Experiments, e61849. 10.3791/61849 [DOI] [PubMed] [Google Scholar]
- Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB et al. (2011) Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proceedings of the National Academy of Sciences of the United States of America, 108, 7460–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardeux FJ, Tejerina RH, Quispe V & Chavez TK (2008) A physiological time analysis of the duration of the gonotrophic cycle of Anopheles pseudopunctipennis and its implications for malaria transmission in Bolivia. Malaria Journal, 7, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MG (2005) The relationship between relative humidity and the Dewpoint temperature in moist air: a simple conversion and applications. Bulletin of the American Meteorological Society, 86, 225–234. [Google Scholar]
- Lega J, Brown HE & Barrera R (2017) Aedes aegypti (Diptera: Culicidae) abundance model improved with relative humidity and precipitation-driven egg hatching. Journal of Medical Entomology, 54, 1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart PA, Eubanks MD & Behmer ST (2015) Water stress in grasslands: dynamic responses of plants and insect herbivores. Oikos, 124, 381–390. [Google Scholar]
- Lewis DJ (1933) Observations on Aädes aegypti, L. (Dipt. Culic.) under controlled atmospheric conditions. Bulletin of Entomological Research, 24, 363–372. [Google Scholar]
- Li Y, Kamara F, Zhou G, Puthiyakunnon S, Li C, Liu Y et al. (2014) Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Neglected Tropical Diseases, 8, e3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi CA, Stewart-Ibarra AM, Muñoz ÁG, Borbor-Cordova MJ, Mejía R, Rivero K et al. (2018) The social and spatial ecology of dengue presence and burden during an outbreak in Guayaquil, Ecuador, 2012. International Journal of Environmental Research and Public Health, 15, 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Tsujimoto H, Cha S-J, Agre P & Rasgon JL (2011) Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation. Proceedings of the National Academy of Sciences of the United States of America, 108, 6062–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Tsujimoto H, Huang Y, Rasgon JL & Agre P (2016) Aquaglyceroporin function in the malaria mosquito Anopheles gambiae. Biology of the Cell, 108, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu OR & Gaines SD (2022) Environmental context dependency in species interactions. Proceedings of the National Academy of Sciences of the United States of America, 119, e2118539119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokoshchenko MA (2017) Urban Heat Island and urban Dry Island in Moscow and their centennial changes. Journal of Applied Meteorology and Climatology, 56, 2729–2745. [Google Scholar]
- Lomax JL (1968) Proceedings. Fifty-fifth annual meeting. New Jersey mosquito extermination association. A study of mosquito mortality relative to temperature and relative humidity in an overwintering site. Humidity Overwintering Site, 230, 81–85. [Google Scholar]
- Lucio PS, Degallier N, Servain J, Hannart A, Durand B, de Souza RN et al. (2013) A case study of the influence of local weather on Aedes aegypti (L.) aging and mortality. Journal of Vector Ecology, 38, 20–37. [DOI] [PubMed] [Google Scholar]
- Lyons CL, Coetzee M, Terblanche JS & Chown SL (2014) Desiccation tolerance as a function of age, sex, humidity and temperature in adults of the African malaria vectors anopheles arabiensis and Anopheles funestus. The Journal of Experimental Biology, 217, 3823–3833. [DOI] [PubMed] [Google Scholar]
- Maffey L, Garzón MJ, Confalonieri V, Chanampa MM, Hasson E & Schweigmann N (2020) Genome-wide screening of Aedes aegypti (Culicidae: Diptera) populations from northwestern Argentina: active and passive dispersal shape genetic structure. Journal of Medical Entomology, 57, 1930–1941. [DOI] [PubMed] [Google Scholar]
- Magombedze G, Ferguson NM & Ghani AC (2018) A trade-off between dry season survival longevity and wet season high net reproduction can explain the persistence of anopheles mosquitoes. Parasites & Vectors, 11, 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron MT, Markow TA, Kain KJ & Gibbs AG (2003) Effects of starvation and desiccation on energy metabolism in desert and Mesic drosophila. Journal of Insect Physiology, 49, 261–270. [DOI] [PubMed] [Google Scholar]
- Matowo NS, Abbasi S, Munhenga G, Tanner M, Mapua SA, Oullo D et al. (2019) Fine-scale spatial and temporal variations in insecticide resistance in Culex pipiens complex mosquitoes in rural South-Eastern Tanzania. Parasites & Vectors, 12, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne B (1930) A study of the influence of relative humidity on the life and infectibility of the mosquito. Indian Journal of Medical Research, 17, 1119–1137. [Google Scholar]
- Mcgaughey WH & Knight KL (1967) Preoviposition activity of the black salt-marsh mosquito, Aedes taeniorhynchus (Diptera: Culicidae)1. Annals of the Entomological Society of America, 60, 107–115. [DOI] [PubMed] [Google Scholar]
- McLaughlin K, Russell TL, Apairamo A, Bugoro H, Oscar J, Cooper RD et al. (2019) Smallest Anopheles farauti occur during the peak transmission season in the Solomon Islands. Malaria Journal, 18, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miazgowicz KL, Shocket MS, Ryan SJ, Villena OC, Hall RJ, Owen J et al. (2020) Age influences the thermal suitability of plasmodium falciparum transmission in the Asian malaria vector Anopheles stephensi. Proceedings of the Royal Society B: Biological Sciences, 287, 20201093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A & Bergmann PJ (2016) Thermal and moisture habitat preferences do not maximize jumping performance in frogs. Functional Ecology, 30, 733–742. [Google Scholar]
- Mogi M, Miyagi I, Abadi K & syafruddin. (1996) Inter- and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. Journal of Medical Entomology, 33, 53–57. [DOI] [PubMed] [Google Scholar]
- Monteiro LCC, de Souza JRB & de Albuquerque CMR (2007) Eclosion rate, development and survivorship of Aedes albopictus (Skuse) (Diptera: Culicidae) under different water temperatures. Neotropical Entomology, 36, 966–971. [DOI] [PubMed] [Google Scholar]
- Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M et al. (2019) Thermal biology of mosquito-borne disease. Ecology Letters, 22, 1690–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA, Cohen JM, Evans MV, Gudapati P, Johnson LR, Lippi CA et al. (2017) Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Neglected Tropical Diseases, 11, e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, Moor E. de de Moor E., McNally A, Pawar S, Ryan SJ, Smith TC, Lafferty KD (2013). Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecology Letters, 16, 22–30. [DOI] [PubMed] [Google Scholar]
- Mordecai EA, Ryan SJ, Caldwell JM, Shah MM & LaBeaud AD (2020) Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planetary Health, 4, e416–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell, 139, 1268–1278. [DOI] [PubMed] [Google Scholar]
- Murdock CC, Blanford S, Hughes GL, Rasgon JL & Thomas MB (2014a) Temperature alters plasmodium blocking by Wolbachia. Scientific Reports, 4, 3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock CC, Blanford S, Luckhart S & Thomas MB (2014b) Ambient temperature and dietary supplementation interact to shape mosquito vector competence for malaria. Journal of Insect Physiology, 67, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock CC, Evans MV, McClanahan TD, Miazgowicz KL & Tesla B (2017) Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Neglected Tropical Diseases, 11, e0005640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock CC, Moller-Jacobs LL & Thomas MB (2013) Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proceedings of the Royal Society B: Biological Sciences, 280, 20132030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock CC, Paaijmans KP, Bell AS, King JG, Hillyer JF, Read AF et al. (2012) Complex effects of temperature on mosquito immune function. Proceedings of the Royal Society B: Biological Sciences, 279, 3357–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock CC, Sternberg ED & Thomas MB (2016) Malaria transmission potential could be reduced with current and future climate change. Scientific Reports, 6, 27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngonghala CN, Ryan SJ, Tesla B, Demakovsky LR, Mordecai EA, Murdock CC et al. (2021) Effects of changes in temperature on Zika dynamics and control. Journal of the Royal Society Interface, 18, 20210165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KH, Boersch-Supan PH, Hartman RB, Mendiola SY, Harwood VJ, Civitello DJ et al. (2021) Interventions can shift the thermal optimum for parasitic disease transmission. Proceedings of the National Academy of Sciences of the United States of America, 118, e2017537118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrat C, Altamirano J, Anyamba A, Caldwell JM, Damoah R, Mutuku F et al. (2021) Impact of recent climate extremes on mosquito-borne disease transmission in Kenya. PLoS Neglected Tropical Diseases, 15, e0009182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okech BA, Gouagna LC, Knols BGJ, Kabiru EW, Killeen GF, Beier JC et al. (2004) Influence of indoor microclimate and diet on survival of Anopheles gambiae s.s. (Diptera: Culicidae) in village house conditions in western Kenya. International Journal of Tropical Insect Science, 24, 207–212. [Google Scholar]
- Ostwald MM, Smith ML & Seeley TD (2016) The behavioral regulation of thirst, water collection and water storage in honey bee colonies. The Journal of Experimental Biology, 219, 2156–2165. [DOI] [PubMed] [Google Scholar]
- Paaijmans KP, Blanford S, Chan BHK & Thomas MB (2012) Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biology Letters, 8, 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Heinig RL, Seliga RA, Blanford JI, Blanford S, Murdock CC et al. (2013) Temperature variation makes ectotherms more sensitive to climate change. Global Change Biology, 19, 2373–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP & Thomas MB (2011) The influence of mosquito resting behaviour and associated microclimate for malaria risk. Malaria Journal, 10, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabha H, Soto E, Mosquera M, Lord CC & Lounibos LP (2010) Ecological links between water storage behaviors and Aedes aegypti production: implications for dengue vector control in variable climates. EcoHealth, 7, 78–90. [DOI] [PubMed] [Google Scholar]
- Palmer WH, Varghese FS & Van Rij RP (2018) Natural variation in resistance to virus infection in dipteran insects. Viruses, 10, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham PE & Hughes DA (2015) Climate influences on the cost-effectiveness of vector-based interventions against malaria in elimination scenarios. Philosophical Transactions of the Royal Society B: Biological Sciences, 370, 20130557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Díaz JL, Álvarez-Valenzuela MA & García-Prada JC (2012) The effect of the partial pressure of water vapor on the surface tension of the liquid water–air interface. Journal of Colloid and Interface Science, 381, 180–182. [DOI] [PubMed] [Google Scholar]
- Pilotto F, Kühn I, Adrian R, Alber R, Alignier A, Andrews C et al. (2020) Meta-analysis of multidecadal biodiversity trends in Europe. Nature Communications, 11, 3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincebourde S, Murdock CC, Vickers M & Sears MW (2016) Fine-scale microclimatic variation can shape the responses of organisms to global change in both natural and urban environments. Integrative and Comparative Biology, 56, 45–61. [DOI] [PubMed] [Google Scholar]
- Platt RB, Collins CL & Witherspoon JP (1957) Reactions of Anopheles quadrimaculatus say to moisture, temperature, and light. Ecological Monographs, 27, 303–324. [Google Scholar]
- Platt RB, Love GJ & Williams EL (1958) A positive correlation between relative humidity and the distribution and abundance of Aedes vexans. Ecology, 39, 167–169. [Google Scholar]
- Pless E, Hopperstad KA, Ledesma N, Dixon D, Henke JA & Powell JR (2020) Sunshine versus gold: the effect of population age on genetic structure of an invasive mosquito. Ecology and Evolution, 10, 9588–9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner HO & Farrell AP (2008) Physiology and climate change. Science, 322, 690–692. [DOI] [PubMed] [Google Scholar]
- Provost MW (1973) Mosquito flight and night relative humidity in Florida. Florida Scientist, 36, 217–225. [Google Scholar]
- Rajpurohit S, Parkash R & Ramniwas S (2008) Body melanization and its adaptive role in thermoregulation and tolerance against desiccating conditions in drosophilids. Entomological Research, 38, 49–60. [Google Scholar]
- Reiskind MH & Lounibos LP (2009) Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Medical and Veterinary Entomology, 23, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romps DM (2021) The Rankine–Kirchhoff approximations for moist thermodynamics. Quarterly Journal of the Royal Meteorological Society, 147, 3493–3497. [Google Scholar]
- Ross PA, Axford JK, Yang Q, Staunton KM, Ritchie SA, Richardson KM et al. (2020) Heatwaves cause fluctuations in wMel Wolbachia densities and frequencies in Aedes aegypti. PLoS Neglected Tropical Diseases, 14, e0007958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PA, Ritchie SA, Axford JK & Hoffmann AA (2019) Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Neglected Tropical Diseases, 13, e0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM & Hoffmann AA (2017) Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathogens, 13, e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura-Pascual N, Hui C, Ikeda T, Leday G, Richardson DM, Carpintero S et al. (2011) Relative roles of climatic suitability and anthropogenic influence in determining the pattern of spread in a global invader. Proceedings of the National Academy of Sciences of the United States of America, 108, 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley WA & Graham CL (1968) The effect of temperature and relative humidity on the flight performance of female Aedes aegypti. Journal of Insect Physiology, 14, 1251–1257. [DOI] [PubMed] [Google Scholar]
- Rozen-Rechels D, Dupoué A, Lourdais O, Chamaillé-Jammes S, Meylan S, Clobert J et al. (2019) When water interacts with temperature: ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecology and Evolution, 9, 10029–10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolfs W (1923) Observations on the relations between atmospheric conditions and the behavior of mosquitoes. New Jersey Agricultural Experiment Stations, 1–32. [Google Scholar]
- Rudolfs W (1925) Relation between temperature, humidity and activity of house mosquitoes. Journal of the New York Entomological Society, 33, 163–169. [Google Scholar]
- Ryan SJ, Carlson CJ, Tesla B, Bonds MH, Ngonghala CN, Mordecai EA et al. (2020a) Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Global Change Biology, 27, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Lippi CA & Zermoglio F (2020b) Shifting transmission risk for malaria in Africa with climate change: a framework for planning and intervention. Malaria Journal, 19, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, McNally A, Johnson LR, Mordecai EA, Ben-Horin T, Paaijmans K et al. (2015) Mapping physiological suitability limits for malaria in Africa under climate change. Vector-Borne Zoonotic Disease, 15, 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MD, Hobbelen PHF, DeCastro F, Ahumada JA, LaPointe DA, Atkinson CT et al. (2011) The dynamics, transmission, and population impacts of avian malaria in native Hawaiian birds: a modeling approach. Ecological Applications, 21, 2960–2973. [Google Scholar]
- Sang R, Lutomiah J, Said M, Makio A, Koka H, Koskei E et al. (2017) Effects of irrigation and rainfall on the population dynamics of Rift Valley fever and other arbovirus mosquito vectors in the epidemic-prone Tana River county, Kenya. Journal of Medical Entomology, 54, 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Vega M, Bouma MJ, Kohli V & Pascual M (2016) Population density, climate variables and poverty synergistically structure spatial risk in urban malaria in India. PLoS Neglected Tropical Diseases, 10, e0005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Vega M, Martinez PP, Vaishnav KG, Kohli V, Desai V, Bouma MJ et al. (2022) The neglected role of relative humidity in the interannual variability of urban malaria in Indian cities. Nature Communications, 13, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CA, Comeau G, Monaghan AJ, Williamson DJ & Ernst KC (2018) Effects of desiccation stress on adult female longevity in Aedes aegypti and ae. Albopictus (Diptera: Culicidae): results of a systematic review and pooled survival analysis. Parasites & Vectors, 11, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W-P, Suzuki M, Thiem VD, White RG, Tsuzuki A, Yoshida L-M et al. (2011) Population density, water supply, and the risk of dengue fever in Vietnam: cohort study and spatial analysis. PLoS Medicine, 8, e1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LLM, Whitehead SA & Thomas MB (2017) Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biology, 15, e2003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelford VE (1918) A comparison of the responses of animals in gradients of environmental factors with particular reference to the method of reaction of representatives of the various groups from protozoa to mammals. Science, 48, 225–230. [DOI] [PubMed] [Google Scholar]
- Shocket MS, Ryan SJ & Mordecai EA (2018a) Temperature explains broad patterns of Ross River virus transmission. eLife, 7, e37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shocket MS, Vergara D, Sickbert AJ, Walsman JM, Strauss AT, Hite JL et al. (2018b) Parasite rearing and infection temperatures jointly influence disease transmission and shape seasonality of epidemics. Ecology, 99, 1975–1987. [DOI] [PubMed] [Google Scholar]
- Shocket MS, Verwillow AB, Numazu MG, Slamani H, Cohen JM, El Moustaid F et al. (2020) Transmission of West Nile and five other temperate mosquito-borne viruses peaks at temperatures between 23°C and 26°C. eLife, 9, e58511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair BJ, Marshall KE, Sewell MA, Levesque DL, Willett CS, Slotsbo S et al. (2016) Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecology Letters, 19, 1372–1385. [DOI] [PubMed] [Google Scholar]
- Singh KEP & Micks DW (1957) The effects of surface tension on mosquito development. Mosquito News, 17, 70–73. [Google Scholar]
- Siraj AS, Rodriguez-Barraquer I, Barker CM, Tejedor-Garavito N, Harding D, Lorton C et al. (2018) Spatiotemporal incidence of Zika and associated environmental drivers for the 2015-2016 epidemic in Colombia. Scientific Data, 5, 180073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraj AS, Santos-Vega M, Bouma MJ, Yadeta D, Carrascal DR & Pascual M (2014) Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science, 343, 1154–1158. [DOI] [PubMed] [Google Scholar]
- Soti V, Tran A, Degenne P, Chevalier V, Seen DL, Thiongane Y et al. (2012) Combining hydrology and mosquito population models to identify the drivers of Rift Valley fever emergence in semi-arid regions of West Africa. PLoS Neglected Tropical Diseases, 6, e1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner FM, Schlick-Steiner BC, VanDerWal J, Reuther KD, Christian E, Stauffer C et al. (2008) Combined modelling of distribution and niche in invasion biology: a case study of two invasive Tetramorium ant species. Diversity and Distributions, 14, 538–545. [Google Scholar]
- Sternberg ED & Thomas MB (2014) Local adaptation to temperature and the implications for vector-borne diseases. Trends in Parasitology, 30, 115–122. [DOI] [PubMed] [Google Scholar]
- Stewart ID & Oke TR (2012) Local climate zones for urban temperature studies. Bulletin of the American Meteorological Society, 93, 1879–1900. [Google Scholar]
- Stewart Ibarra AM, Ryan SJ, Beltrán E, Mejía R, Silva M & Muñoz Á (2013) Dengue vector dynamics (Aedes aegypti) influenced by climate and social factors in Ecuador: implications for targeted control. PLoS One, 8, e78263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard ST, Morrison AC, Vazquez-Prokopec GM, Soldan VP, Kochel TJ, Kitron U et al. (2009) The role of human movement in the transmission of vector-borne pathogens. PLoS Neglected Tropical Diseases, 3, e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchin M, Machalaba CC & Karesh WB (2016) VECTOR-BORNE DISEASES: ANIMALS AND PATTERNS. Global health impacts of vector-borne diseases: Workshop summary. Washington DC: National Academies Press (US). [PubMed] [Google Scholar]
- Suwanchaichinda C & Paskewitz SM (1998) Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to Melanize Sephadex beads. Journal of Medical Entomology, 35, 157–161. [DOI] [PubMed] [Google Scholar]
- Takken W & Lindsay S (2019) Increased threat of urban malaria from Anopheles stephensi mosquitoes, Africa. Emerging Infectious Diseases, 25, 1431–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatzel J, Prusiner SB & Welch WJ (1996) Chemical chaperones interfere with the formation of scrapie prion protein. The EMBO Journal, 15, 6363–6373. [PMC free article] [PubMed] [Google Scholar]
- Tesla B, Demakovsky LR, Mordecai EA, Ryan SJ, Bonds MH, Ngonghala CN et al. (2018) Temperature drives Zika virus transmission: evidence from empirical and mathematical models. Proceedings of the Royal Society B: Biological Sciences, 285, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Ravishankaran S, Justin JA, Asokan A, Mathai MT, Valecha N et al. (2016) Overhead tank is the potential breeding habitat of Anopheles stephensi in an urban transmission setting of Chennai, India. Malaria Journal, 15, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Ravishankaran S, Justin NAJA, Asokan A, Kalsingh TMJ, Mathai MT et al. (2018) Microclimate variables of the ambient environment deliver the actual estimates of the extrinsic incubation period of plasmodium vivax and plasmodium falciparum: a study from a malaria-endemic urban setting, Chennai in India. Malaria Journal, 17, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Ravishankaran S, Justin NAJA, Asokan A, Mathai MT, Valecha N et al. (2017) Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malaria Journal, 16, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RCM (1938) The reactions of mosquitoes to temperature and humidity. Bulletin of Entomological Research, 29, 125–140. [Google Scholar]
- Tun-Lin W, Burkot TR & Kay BH (2000) Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in North Queensland, Australia. Medical and Veterinary Entomology, 14, 31–37. [DOI] [PubMed] [Google Scholar]
- Ulrich JN, Beier JC, Devine GJ & Hugo LE (2016) Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Neglected Tropical Diseases, 10, e0004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, United Nations, Department of Economic and Social Affairs, Population Division. (2019) World urbanization prospects: the 2018 revision (ST/ESA/SER.A/420). New York, NY: United Nations. [Google Scholar]
- Urbanski JM, Benoit JB, Michaud MR, Denlinger DL & Armbruster P (2010) The molecular physiology of increased egg desiccation resistance during diapause in the invasive mosquito, Aedes albopictus. Proceedings of the Royal Society B: Biological Sciences, 277, 2683–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerwaarden B & Sgrò CM (2014) Is adaptation to climate change really constrained in niche specialists? Proceedings of the Royal Society B: Biological Sciences, 281, 20140396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klink R, Bowler DE, Gongalsky KB, Swengel AB, Gentile A & Chase JM (2020) Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science, 368, 417–420. [DOI] [PubMed] [Google Scholar]
- Vega-Rúa A, Marconcini M, Madec Y, Manni M, Carraretto D, Gomulski LM et al. (2020) Vector competence of Aedes albopictus populations for chikungunya virus is shaped by their demographic history. Communications Biology, 3, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst NO, Brendle A, Blanckenhorn WU & Mathis A (2020) Thermal preferences of subtropical Aedes aegypti and temperate ae. Japonicus mosquitoes. Journal of Thermal Biology, 91, 102637. [DOI] [PubMed] [Google Scholar]
- Villena OC, Ryan SJ, Murdock CC & Johnson LR (2022) Temperature impacts the transmission of malaria parasites by Anopheles gambiae and Anopheles stephensi mosquitoes. Ecology, 103, e3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees AS, Gray EM & Bradley TJ (2013) Thermal resistance and performance correlate with climate in populations of a widespread mosquito. Physiological and Biochemical Zoology, 86, 73–81. [DOI] [PubMed] [Google Scholar]
- Wang G-H, Gamez S, Raban RR, Marshall JM, Alphey L, Li M et al. (2021) Combating mosquito-borne diseases using genetic control technologies. Nature Communications, 12, 4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RE (1968) The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. The Condor, 70, 101–120. [Google Scholar]
- Weaver SC, Charlier C, Vasilakis N & Lecuit M (2018) Zika, chikungunya, and other emerging vector-Borne viral diseases. Annual Review of Medicine, 69, 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihrauch D, Donini A & O’Donnell MJ (2012) Ammonia transport by terrestrial and aquatic insects. J. Insect Physiol., molecular physiology of epithelial transport in insects—a tribute to William R. Harvey, 58, 473–487. [DOI] [PubMed] [Google Scholar]
- Wexler A & Greenspan L (1971) Vapor Pressure Equation for Water in the Range 0 to 100 °C. Journal of Research of the National Bureau of Standards. Section A, Physics and Chemistry, 75A, 213–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2020) World malaria report 2020: 20 years of global progress and challenges. Geneva: World Health Organization. [Google Scholar]
- Wilke ABB & Marrelli MT (2012) Genetic control of mosquitoes: population suppression strategies. Revista do Instituto de Medicina Tropical de São Paulo, 54, 287–292. [DOI] [PubMed] [Google Scholar]
- Wilke ABB & Marrelli MT (2015) Paratransgenesis: a promising new strategy for mosquito vector control. Parasites & Vectors, 8, 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly MC, Davis JK, Evans MV, Hess A, Newberry PM, Solano-Asamoah N et al. (2020) Land cover affects microclimate and temperature suitability for arbovirus transmission in an urban landscape. PLoS Neglected Tropical Diseases, 14, e0008614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter LA, Johnson CJ, Croft B, Gunn A & Poirier LM (2012) Gauging climate change effects at local scales: weather-based indices to monitor insect harassment in caribou. Ecological Applications, 22, 1838–1851. [DOI] [PubMed] [Google Scholar]
- Wright RE & Knight KL (1966) Effect of environmental factors on biting activity of Aedes vexans (Meigen) and Aedes trivittatus (Coquillett). Mosquito News, 26, 565–578. [Google Scholar]
- Wu GC & Wright JC (2015) Exceptional thermal tolerance and water resistance in the mite Paratarsotomus macropalpis (Erythracaridae) challenge prevailing explanations of physiological limits. Journal of Insect Physiology, 82, 1–7. [DOI] [PubMed] [Google Scholar]
- Yang P, Ren G & Hou W (2017) Temporal–spatial patterns of relative humidity and the urban dryness Island effect in Beijing City. Journal of Applied Meteorology and Climatology, 56, 2221–2237. [Google Scholar]
- Ye YH, Carrasco AM, Frentiu FD, Chenoweth SF, Beebe NW, van den Hurk AF et al. (2015) Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Neglected Tropical Diseases, 9, e0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H-P, Shao L, Xiao K, Mu L-L & Li G-Q (2010) Hygropreference behaviour and humidity detection in the yellow-spined bamboo locust, Ceracris kiangsu. Physiological Entomology, 35, 379–384. [Google Scholar]
- Yuan F & Bauer ME (2007) Comparison of impervious surface area and normalized difference vegetation index as indicators of surface urban heat Island effects in Landsat imagery. Remote Sensing of Environment, 106, 375–386. [Google Scholar]
- Yurchenko AA, Masri RA, Khrabrova NV, Sibataev AK, Fritz ML & Sharakhova MV (2020) Genomic differentiation and intercontinental population structure of mosquito vectors Culex pipiens pipiens and Culex pipiens molestus. Scientific Reports, 10, 7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge J-M, Lourenco-De-Oliveira R et al. (2014) Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proceedings of the Royal Society B: Biological Sciences, 281, 20141078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


