Abstract
Introduction
Dysregulation of the stress-regulatory hormone cortisol is associated with anxiety, but its potential impact on infertile women and in vitro fertilization (IVF) treatment remains unclear. This prospective cross-sectional study aimed at evaluating the dysregulation of cortisol and its correlation to anxiety in infertile women. The influence of stress on IVF outcomes was also investigated.
Methods
A point-of-care test was used for the measurement of morning serum cortisol in 110 infertile women and 112 age-matching healthy individuals. A Self-Rating Anxiety Scale (SAS) was used for the anxiety assessment of infertile women, and 109 of them underwent IVF treatment starting with the GnRH-antagonist protocol. If clinical pregnancy was not achieved, more IVF cycles were conducted with adjusted protocols until the patients got pregnant or gave up.
Results
Higher morning serum cortisol level was identified for infertile patients, especially for the elder. Women with no anxiety showed significant differences in cortisol levels, monthly income, and BMI compared with those with severe anxiety. A strong correlation was found between the morning cortisol level and the SAS score. When the cutoff value is 22.25 μg/dL, cortisol concentration could predict the onset of anxiety with high accuracy (95.45%) among infertile women. After IVF treatments, women with high SAS scores (>50) or cortisol levels (>22.25 μg/dL) demonstrated a lower rate of pregnancy (8.0%-10.3%) and more IVF cycles, although the impact of anxiety was not affirmative.
Conclusion
Hypersecretion of cortisol related to anxiety was prevalent among infertile women, but the influence of anxiety on multi-cycle IVF treatment was not affirmative due to the complicated treatment procedures. This study suggested that the assessment of psychological disorders and stress hormone dysregulation should not be overlooked. An anxiety questionnaire and rapid cortisol test might be included in the treatment protocol to provide better medical care.
Keywords: cortisol, anxiety, point-of-care testing, infertility, IVF outcome
Introduction
Infertility is a global burden that affects 10%–15% of couples of reproductive age (1, 2). The impairment of reproductive function and stigmatization have led to a significantly higher level of stress and a prevalence of mood disorders (3–5). The overall prevalence of anxiety among infertile women was 36.17% (4), and a higher rate (37.2-42.2%) has been reported in Chinese patients who visited the reproductive medical center for help (6, 7). Anxiety will not only affect mental health but also impair reproduction function through complicated mechanisms such as dysregulation of hormones and metabolisms (8, 9). Therefore, the diagnosis of anxiety should not be overlooked for infertility treatment.
In addition to the classical scale tests for psychological assessment (10, 11), efforts have been made to identify biomarkers that can facilitate the evaluation of anxiety in infertile women (12, 13). The hypothalamus–pituitary–adrenal (HPA) axis hormones, especially cortisol, are major stress and threat regulators (14, 15). As one of the most abundant hormones in the human serum, cortisol regulates energy metabolism in response to adverse stimulations (16) and shares a common synthetic pathway with sex hormones such as testosterone and progesterone (17, 18). The complicated role of cortisol makes it a good target for neuroendocrine studies among women of reproductive age.
The impact of cortisol dysregulation on reproductive function and in vitro fertilization (IVF) has been well established. In spontaneous fertilization, cortisol could act directly on granulosa lutein cells to inhibit the support of steroidogenesis by luteinizing hormone (19). It also plays an important role in follicular development and safeguards oogenesis by promoting follicular cell survival (20). Increased levels of cortisol and prolactin impair the menstrual cycle in females and reduce the chances of conception (21). Peripheral cortisol levels can reflect adrenal function, which indirectly interferes with sex hormone production in infertile patients (17). In studies of IVF treatments, long-term cortisol levels measured from hair negatively predict pregnancy, and the accumulation of salivary cortisol accounts for 26.7% of the variance of clinical pregnancy outcomes (22). Lower serum and follicular cortisol levels on the day of oocyte retrieval were found to be significantly associated with successful IVF treatment (23). Although the mechanism remained unclear, cortisol is likely an important neurohormone in the complex relationship between psychosocial stress and IVF outcome (24).
Studies of physiological biomarkers for anxiety, however, revealed heterogeneous conclusions about cortisol dysregulations (14, 25). Women with anxiety demonstrated a higher level of 24-hour urinary free cortisol (26), but hair cortisol concentration representing the long-term secretion was not related to self-reported anxiety (27). The non-invasive collection of hair or urine samples is convenient for the patients, but the operation protocol involved complicated steps and instruments, which are rarely available in clinical settings (28, 29). In the contrast, blood sample collection is invasive, but already widely used for sex hormone level determination of infertile patients in hospitals (30). Elevation of morning serum cortisol level has been reported in pregnant women with anxiety, as determined by an automated commercialized chemiluminescence analyzer (31). Thus, to better demonstrate the correlation between cortisol dysregulation and anxiety, the variation in sampling preparation and test operation should be minimized.
This study aimed to evaluate the potential of serum morning cortisol levels in facilitating the diagnosis of anxiety in infertile women and investigate the influence of physiological and psychological stress on the IVF treatment outcome. Since most studies focused on the cortisol level at different time points during treatment, we would like to investigate whether psychological and physiological stress occurred before the treatment and affect the outcome of multiple IVF cycles. A Self-Rating Anxiety Scale (SAS) with a well-established Chinese norm and a novel point-of-care test (POCT) for serum cortisol was adopted. The standardized sample collection and convenient operation of the test platform enabled the rapid onsite detection of serum cortisol, and provide insights for future clinical applications. With the retrieval of the pregnancy outcome after IVF treatment, the influence of anxiety and cortisol dysregulation was addressed. We hypothesized that dysregulation of cortisol might be prevalent and linked to anxiety or poor pregnancy outcome in infertile women.
Materials and methods
Study design
This prospective cross-sectional study was conducted in the Second Affiliated Hospital of Chongqing Medical University from December 2018 to March 2020. The study design and protocol were in accordance with the Declaration of Helsinki (1989) and approved by the institutional review board of the hospital. Two cohorts were recruited, including 120 infertile women from the reproductive medicine center (infertility group) and 112 healthy women from the medical center (control group). The sample size of the study was calculated with an online calculator for clinical studies developed by Wang and Ji (32).
After obtaining informed consent, basic information including age, blood glucose, blood pressure, and regular blood test result of patients were checked for inclusion or exclusion. The infertile patients in the reproductive medicine center were already diagnosed before they were asked to join the study, whereas the healthy patients in the medical center were visiting the hospital for regular annual examinations. Since all volunteers were planning to undergo serum sample collection for other assays, they acknowledged that the sample would be shared for the cortisol test to minimize the risk. The blood collection was conducted between 7:00 am and 9:00 am. The infertility group completed an additional background information collection form, a questionnaire for anxiety, and a blood test for antral follicle count (AFC), anti-Mullerian hormone (AMH), and follicle-stimulating hormone (FSH) (Roche Cobas E601., Germany).
At the reproductive medicine center, 120 patients confirmed with infertility (failed to become pregnant after 1 year of preparation) were recruited. The inclusion criteria were as follows (1): 20 - 45 years old (2), able to understand and complete the SAS test, and (3) able to visit the reproductive medicine center before 7:30 am and finish blood collection on time (4), no hypertension or hyperglycemia, and (5) no visible abnormality in the blood sample. The exclusion criteria were as follows (1): under hormone treatment such as dexamethasone in the past 3 months (2); having endocrine diseases such as hypothyroidism, hyperprolactinemia, adrenal cortical hyperplasia, and diabetes (3), a history of psychological disorder (4), history of smoking or alcohol abuse. Patients in the control group have no known medical history of infertility treatments. The same inclusion criteria were adopted for these healthy individuals, except that they were only asked for the detection of morning serum cortisol. For the infertile women, those with severe negative life events within 1 year and known gynecological diseases such as uterine malformation and severe endometriosis were also excluded.
After completing all procedures, the date was matched and checked. Women who lacked background information or failed to complete any tests were excluded (n = 10), resulting in a final number of 110 (91.7%) and 112 (100%) for infertility and control groups, respectively. Among the 110 infertile patients, 1 didn’t undergo IVF treatment in our reproductive medicine center for personal reasons. A GnRH-antagonist protocol for IVF treatment was applied for all patients during the first cycle, which started within 1 month of the stress evaluation. More IVF cycles were conducted if the first one failed. The treatment continued until successful clinical pregnancy or the patient decided to give up. Finally, the number of IVF cycles performed and treatment outcomes were retrieved for the 109 participants.
Measurement of cortisol
The collection of serum samples was conducted before breakfast with a standard extraction protocol and equipment. After arrival and resting for > 30 min in the morning, 5 mL of peripheral blood was taken between 7:00 am and 9:00 am using a standard coagulation blood collection tube. The sample was centrifuged at 3500 rpm for 6 min, and the serum was isolated for testing and storage. Serum cortisol was either measured immediately after separation or stored at 4°C before being tested within 8 h. The samples were kept at −20°C for long-term storage.
A quantum-dot immunochromatography assay for cortisol was used in this study (Jiangsu NepQD Biotech Ltd.). The quantitative result of serum cortisol concentration was measured by a portable immunofluorescence analyzer designed for this POCT (NepQD-Infinity-V1, Jiangsu NepQD Biotech Ltd., China). To perform the assay, 20 μL of serum sample was mixed with the diluent, and 60 μL of diluted sample was added to the sample loading well of the test card. After 10-min incubation, the test card was inserted into the analyzer for fluorescent signal reading. The detection range of this platform is 1.00–60.00 μg/dL, with an intra- and inter-assay coefficient of variation < 15%. The performance correlation between this POCT platform and the chemiluminescent assay is > 0.975.
Measurement of SAS
A Chinese version of the widely employed SAS by Zung was used for the psychological evaluation of infertile patients (33). Basic instructions were provided by trained researchers using the same wording, and then the patients were asked to complete the 20-item questionnaire independently. The raw score was multiplied by 1.25 according to the Chinese norm, with the cutoff being 50. Scores of 50–59, 60–69, and above 70 indicate mild, medium, and severe levels of anxiety, respectively.
IVF treatment
Patients involved in this study underwent GnRH-antagonist protocol for the first cycle of IVF treatment. Embryos transfers were performed on day 3 (2 cleavage stage embryos) or day 5 (1 blastocyst). Most patients finished the first IVF cycle within 3 months. Those who were not pregnant continued with other treatment protocols. The IVF treatment continued until successful clinical pregnancy or the patient decided to give up. In the end, the number of IVF cycles performed and treatment outcomes were retrieved for each individual.
Statistical analysis
The data of 110 patients from the reproductive medicine center (infertility group) and 112 patients from the medical center (control group) were used for statistical analysis using SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA). The distributions of age, serum cortisol level, BMI, SAS score, AFC, AMH, and FSH concentrations were examined using the Shapiro–Wilk test. Since the data were nonparametric, the differences in age and cortisol concentration between infertility and control groups were verified using the Mann–Whitney U test. The anxiety status of women with primary or secondary infertility was also compared with this method.
To investigate the level of cortisol across different ages, the participants were divided into different age groups (≤25, 26–28, 29–31, 32–34, and ≥35), and the difference was verified using the two-tailed Mann–Whitney U test. The correlation between variables was evaluated using the non-parametric hypothesis test Kendall test, with the significance level set at 0.05 and 0.01. Further comparison between age, cortisol level, monthly income, year of infertility, level of education, and BMI was performed between four anxiety groups (no, mild, medium, and severe) among the infertility participants using the Kruskal–Wallis test, which is a non-parametric test for median comparison between multiple groups.
The onset of anxiety among infertile patients was assessed based on the SAS questionnaire. Binary logistic regression was conducted to illustrate the contributing factors of anxiety. “Anxiety” and “no anxiety” was set as the dichotomous dependent variables, and age, cortisol level, year of infertility, BMI, monthly income, and education level were set as covariates. Monthly income and education level were categorical covariates, and others were numerical variants. The Forward: Likelihood Ratio model was selected for the construction of the regression equation.
To investigate the potential role of morning serum cortisol concentration in the evaluation of anxiety in the target population, we constructed the receiver operating characteristic (ROC) curve and determined the Youden index and cutoff value for cortisol. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for anxiety were calculated based on the cutoff concentration. A Pearson’s Chi-square analysis was used to evaluate the difference in clinical pregnancy outcomes regarding the physiological and psychological approach for anxiety assessment, and the average number of IVF cycles conducted for each subgroup was calculated. The influence of infertility types and factors on pregnancy outcomes were also compared with the same method.
Results
Demographic characterization and cortisol levels
The basic information of individuals in the infertility group (N = 110) and control group (N = 112) is summarized in Table 1 . The age of the infertility group ranged from 23 to 44 years with an average of 30.67 years, whereas that of the control group ranged from 21 to 44 years with an average of 31.37 years. In the infertility group, 20 (18.2%) patients were at the advanced maternal age as defined by the Chinese standard (≥35), and 15 (13.6%) were overweight according to local standards (BMI > 24.0) (34, 35). Patients at advanced maternal age generally have a decreased likelihood of natural pregnancy and a higher incidence of pregnancy complications (35). The year of infertility ranged from 1 to 8 years. Most of the patients (58.2%) that joined this study had a history of infertility of 2-4 years.
Table 1.
Demographic characteristics of participants.
| Parameters | Mean (SD) | Levels | Counts |
|---|---|---|---|
| Age | |||
| Control group | 31.38 ± 5.62* | ≤25 | 17 (15.2%) |
| 26–34 | 63 (56.2%) | ||
| Above 35 | 32 (28.6%) | ||
| Infertility group | 30.67 ± 4.48* | ≤25 | 10 (9.1%) |
| 26–34 | 80 (72.7%) | ||
| Above 35 | 20 (18.2%) | ||
| Cortisol (μg/dL) | |||
| Control group | 14.38 ± 4.84 | ||
| Infertility group | 18.39 ± 7.79* | ||
| AFC | 12.03 ± 5.26* | ||
| AMH (ng/ml) | 3.65 ± 2.51* | ||
| FSH (mIU/mL) | 7.81 ± 2.18 | ||
| SAS Score | 38.45 ± 22.32* | 0-49 | 64 (58.1%) |
| 50-59 | 17 (15.5%) | ||
| 60-69 | 19 (17.3%) | ||
| Above 70 | 10 (9.1%) | ||
| BMI | 21.51 ± 2.04* | <18.5 | 6 (5.5%) |
| 18.5–24.0 | 89 (80.9%) | ||
| >24.0 | 15 (13.6%) | ||
| Years of infertility | 3.74 ± 2.12* | 1 Y | 13 (11.8%) |
| 2–4 Y | 64 (58.2%) | ||
| Above 5 Y | 33 (30.0%) | ||
| Monthly income (CNY) | Below 2000 | 17 (15.4%) | |
| 2000–5000 | 51(46.4%) | ||
| Above 5000 | 42 (38.2%) | ||
| Education level | Middle school/below | 22 (20.0%) | |
| High school | 41 (37.3%) | ||
| Bachelor/above | 47 (42.7%) | ||
*Test for normal distribution, p < 0.05 (two-tailed).
As shown in Table 1 , the serum cortisol level of the control group followed a normal distribution, but that of the infertility group did not. A comparison of the two groups revealed a significant difference in stress regulatory hormone concentration (p = 0.000), but not in age (p = 0.337). The level of morning cortisol was significantly higher in the infertility group (18.39 μg/dL), with a larger standard deviation ( ± 7.79 μg/dL).
Correlation of variables
A positive correlation between cortisol level and age was found in the infertility group (Kendell’s tau = 0.201, p = 0.003), but not in the control group (Kendell’s tau = −0.064, p = 0.329). To further investigate the association between cortisol level and age, participants were divided into subgroups. The concentration gap between the two cohorts increased as their age increased, and a significant difference was observed for the 32-34 (Z = −2.725, p = 0.006) and >35 (Z = −3.630, p = 0.000) subgroups ( Figure 1 ). The morning cortisol concentration was relatively stable across the age of interest (20–45) for the control patients.
Figure 1.
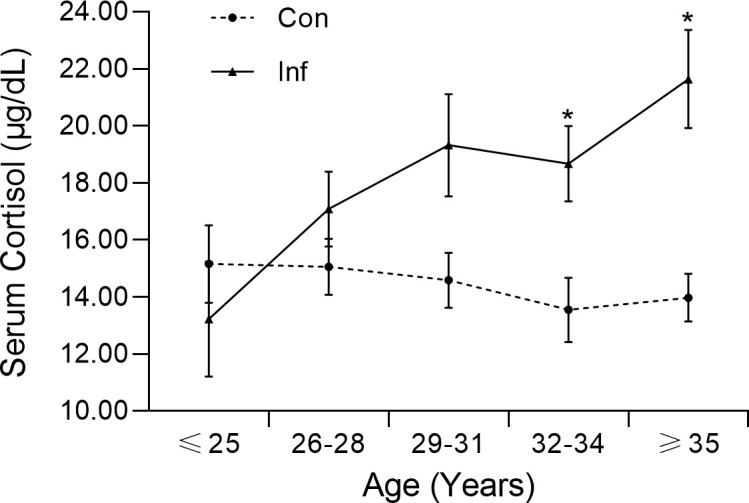
Morning serum cortisol level of infertile patients (Inf) and controls (Con) in different age groups. The error bar represents the standard error mean.
Pairwise correlation analysis was conducted between different factors of the infertile patients ( Table 2 ). The SAS score was strongly correlated to morning serum cortisol level, monthly income, and BMI (all p < 0.01). Cortisol concentration was positively related to age, SAS score, BMI (all p < 0.01), and year of infertility (p < 0.05), but was negatively related to monthly income (p < 0.01). Many demographic characters were correlated with each other, including age, year of infertility, BMI, and education level (all p < 0.01). Higher monthly income was linked to higher education levels but lower BMI (p < 0.01), which might be a reflection of healthier dietary habits. Interestingly, education level was negatively correlated to BMI and year of infertility (p < 0.01) in the infertility group. The number of cycles for IVF treatment was strongly related to age and year of infertility (p < 0.01), but negatively correlated to the pregnancy outcome (p < 0.05), which is because the treatment will continue until the patient gets pregnant or give up.
Table 2.
Correlations between demographic and clinical variables of infertility group.
| Correlation | SAS score | Cortisol level | Age | Monthly income | Year of infertility | BMI | Education level | IVF cycle |
|---|---|---|---|---|---|---|---|---|
| Cortisol level | 0.624** | |||||||
| Age | 0.124 | 0.201** | ||||||
| Monthly income | −0.232** | −0.248** | −0.150 | |||||
| Year of Infertility | 0.070 | 0.156* | 0.495** | −0.153 | ||||
| BMI | 0.233** | 0.299** | 0.215** | −0.283** | 0.152* | |||
| Education level | −0.024 | −0.075 | −0.275** | 0.303** | −0.240** | −0.258** | ||
| IVF cycle | 0.071 | 0.050 | 0.356** | -0.117 | 0.250** | 0.123 | −0.236** | |
| Pregnancy | -0.047 | -0.087 | -0.041 | -0.033 | -0.040 | 0.060 | 0.000 | −0.212* |
*p < 0.05 (two-tailed); **p < 0.01 (two-tailed).
Prediction of anxiety with cortisol levels
As determined by the SAS score, 58.1% (n = 64) of infertile patients had no anxiety, whereas. Comparison between the anxiety (n = 46) and not anxiety (n = 64) groups of the infertility patients revealed that all numerical data (age, cortisol, years of infertility, BMI) of these two groups were in a skewed distribution. Further investigation of patients with different levels of anxiety (mild 15.5%, n = 17; medium 17.3%, n = 19; severe 9.1%, n = 10) demonstrated the pairwise difference in cortisol level, monthly income, and BMI ( Table 3 ). The level of serum cortisol in patients with no anxiety was significantly different from that in patients with anxiety; meanwhile, patients with mild, medium, and severe anxiety had similar serum cortisol levels. The severe group had a significantly lower monthly income than other groups, implying that poverty might be a factor that exacerbates the disease. As the average BMI increased with the severity of anxiety, patients with no anxiety had a significantly lower BMI compared with the severe groups. This finding was generally in agreement with the percentage of overweight patients with no, mild, medium, and severe anxiety (4.7%, 17.6%, 21.1%, and 50.0%).
Table 3.
Assessment of parameters among infertile patients with different levels of anxiety.
| Pair of groups | Cortisol level | Age | Monthly income | Year of infertility | BMI | Education level |
|---|---|---|---|---|---|---|
| All groups | 0.000* | 0.071 | 0.007* | 0.315 | 0.001* | 0.292 |
| No-mild | 0.000* | 1.000 | 0.073 | |||
| No-medium | 0.000* | 1.000 | 0.071 | |||
| No-severe | 0.000* | 0.003* | 0.007* | |||
| Mild-medium | 1.000 | 1.000 | 1.000 | |||
| Mild-severe | 1.000 | 0.112 | 1.000 | |||
| Medium-severe | 1.000 | 0.060 | 1.000 |
*Kruskal–Wallis test p < 0.05 (two-tailed); no, mild, medium, and severe represent the group of patients whose SAS score ranges from <50, 50–59, 60–69, and >70, n = 110.
To identify the factors that contributed to anxiety in the infertility group, we first conducted a binary logistic regression using the Forward: Likelihood Ratio model. Among all input factors, only cortisol level was significantly related to anxiety (χ 2 = 93.307, p < 0.000). The regression analysis gave a sensitivity, specificity, and accuracy of 93.8%, 95.7%, and 94.5% respectively. This finding was in agreement with the significant difference (Z = −3.666, p = 0.000) in cortisol concentration between infertility and control groups ( Table 1 ; Figure 1 ). In addition, the pairwise correlation test and binary logistic regression indicated a significant correlation between SAS score and morning serum cortisol concentration.
The prediction of anxiety with morning serum cortisol levels of the infertile women was achieved through the construction of the ROC curve. As shown in Figure 2 , the area under the curve (AUC) was calculated to be 0.960, with a 95% confidence interval (CI) between 0.917 and 1.000. When the cutoff value for serum cortisol concentration was set to 22.25 μg/dL, the maximum value of the Youden index (0.910) was reached. Based on the threshold calculated, the performance of the cortisol test was summarized ( Table 4 ). A PPV of 93.6%, NPV of 96.8%, sensitivity of 95.6%, and specificity of 95.3% were obtained. The overall diagnostic accuracy for the morning serum cortisol test to the SAS score was 95.4% for infertile patients.
Figure 2.
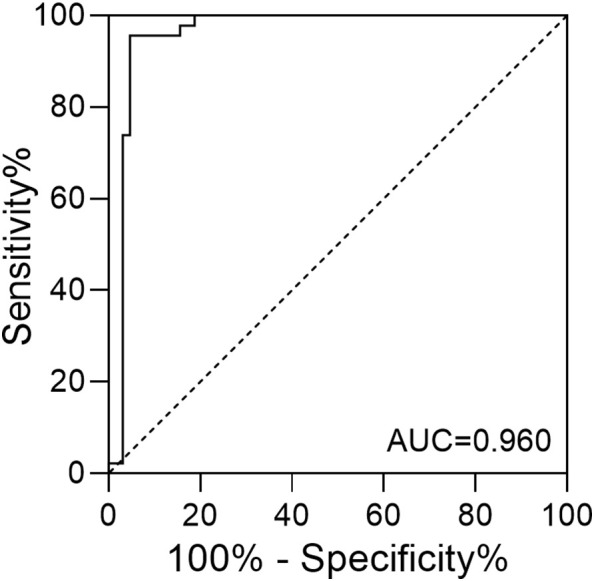
The ROC curve of serum cortisol concentration in predicting anxiety among infertile patients.
Table 4.
Performance of the serum cortisol level test in anxiety assessment.
| SAS score | |||||
|---|---|---|---|---|---|
| Anxiety | No anxiety | ||||
| Serum cortisol | Anxiety | 44 | 3 | PPV | Sensitivity |
| 40.00% | 2.73% | 93.62% | 95.65% | ||
| Not anxiety | 2 | 61 | NPV | Specificity | |
| 1.82% | 55.45% | 96.83% | 95.31% | ||
| Total | 46 | 64 | |||
SAS cutoff value >50; Serum cortisol cutoff value > 22.25 μg/dL.
IVF Treatment outcomes
Among the 110 infertile patients involved in the anxiety assessment, 109 underwent IVF treatment in our reproductive medicine center and 63.3% became clinically pregnant. Miscarriage was documented for one patient with primary infertility and two with secondary infertility. After 1 IVF cycle, 44.9% of the participants conceived, and 19.3% failed even with 2 cycles or more. As shown in Table 5 , no significant difference regarding the final treatment outcome was found between patients with primary or secondary infertility, or between patients with various infertility factors. The pregnancy rate was similar between women with anxiety (58.7% and 57.4%) and without anxiety (66.7% and 67.7%), regardless of the method used for disease evaluation. When the cortisol levels and SAS scores of women who conceived during the first treatment cycle were compared, no significant differences were found. It should be noticed that after the first IVF cycle, the IVF treatment procedure varied between individuals to achieve the best outcome. Although a decrease in pregnancy rate (8.0%-10.3%) was observed for patients with high perceived anxiety (SAS > 50) or level of serum cortisol (concentration > 22.25 μg/dL), the impact of anxiety to multi-cycle IVF treatment was inconclusive.
Table 5.
Comparison of the treatment outcome between patients with different types of infertility and infertility factors, as well as patients evaluated with SAS or morning serum cortisol.
| Group | Pregnant | Not pregnant | Total | IVF cycle | χ2 | p-value | |
|---|---|---|---|---|---|---|---|
| Type of infertility | Secondary | 43 (61.43) | 27 (38.57%) | 70 (64.22%) | 1.90 | 0.296 | 0.587 |
| Primary | 26 (66.66%) | 13 (33.33%) | 39 (35.78%) | 1.28 | |||
| Infertility factors | Husband | 12 (57.14%) | 9 (42.86%) | 21 (19.27%) | 1.33 | 1.303 | 0.728 |
| Wife | 29 (60.42%) | 19 (39.58%) | 48 (44.04%) | 1.58 | |||
| Both | 26 (70.27%) | 11 (29.73%) | 37 (33.94%) | 2.03 | |||
| Unknown | 2 (66.66%) | 1 (33.33%) | 3 (2.75%) | 1.33 | |||
| SAS | High SAS | 27 (58.7%) | 19 (41.3%) | 46 (42.2%) | 1.76 | 0.727 | 0.394 |
| Low SAS | 42 (66.7%) | 21 (33.3%) | 63 (57.8%) | 1.62 | |||
| Cortisol | High cortisol | 27 (57.4%) | 20 (42.6%) | 47 (43.1%) | 1.77 | 1.220 | 0.269 |
| Low cortisol | 42 (67.7%) | 20 (32.3%) | 62 (56.9%) | 1.61 |
IVF cycle represents the average IVF cycle performed for the group of patients; SAS cutoff value > 50; Cortisol cutoff value > 22.25 μg/dL.
When comparing the number of IVF cycles performed, a significant difference was found between patients with primary and secondary infertility (1.28 VS 1.90). We also identified that women with secondary infertility were elder, and their SAS score, morning cortisol level, and BMI were significantly higher (p < 0.05) compared with those of primary infertility. However, if the anxiety statues were used as the independent variables, the average number of IVF cycles conducted for the anxiety groups was similar to that of the not anxiety ones (1.76-1.77 VS 1.61-1.62).
Discussion
Demographic analysis between infertility and control groups revealed a similar age range and a skewed distribution. The morning serum cortisol demonstrated a normal distribution in that of the control group, which was similar to the distribution in the general population. Compare with hair cortisol which is also frequently evaluated in anxiety studies, serum sample requires fewer sample preparation steps and could be tested with commercialized platforms. More importantly, reference ranges have been proposed for serum/plasma cortisol levels of healthy individuals. As reported by the Roche Diagnostic GmbH (Roche Cobas platform, Elecsys Cortisol II), the 5th-95th percental of the morning (6-10 am) serum cortisol level in healthy adults ranges from 6.02-18.4 μg/dL, and the reference range reported by Beckman Coulter cortisol test is 6.7-22.6 μg/dL. The test results of the control patients in our study generally followed a similar distribution within the same reference range. However, the POCT method enabled the onsite evaluation of serum cortisol with a share of the serum sample, and the result can be obtained within 15 min. A significant increase in morning cortisol levels was identified among infertile participants, which might be due to higher stress (i.e., anxiety) or other factors related to endocrine dysregulation.
Despite the similarity in age, the infertile patients demonstrated a higher average serum cortisol concentration and an increased cortisol level with age, especially in those over 32 years old ( Figure 1 ). Previous studies have also documented higher cortisol levels in women with anxiety or patients with depression (31, 36). The gap in cortisol levels between the same age groups was possibly due to physiological or psychological differences between the two populations, as women might feel more stressed about their infertility issues in their 30s.
The correlation analysis for the primary variables of infertile patients revealed complicated relationships between variables ( Table 2 ). Our result demonstrated a significant correlation between the SAS score and morning serum cortisol level, which has also been reported in pregnant women with anxiety (31). These findings implied that women with a stress-induced mood disorder were likely suffered from dysregulation of the stress-regulatory hormone as well.
The negative correlation between monthly income and SAS score was in agreement with several studies involving the socioeconomic status of patients with anxiety and depression (6, 10). A previous investigation conducted by Xu et al. in China revealed that higher education in infertile women could be a protective factor against anxiety (7). Although we did not observe a strong correlation between SAS score and education level, the significant correlation between education level and monthly income supported the same finding indirectly.
Based on the SAS score, the prevalence of anxiety in the infertility group was 41.9%, which is within the range of that of low- and middle-income countries (95% CI: 31.86%–78.62%) reported by Kiani et al. (4). The monthly income represents a typical medium income level in China, but the percentages of overweight patients in the mild, medium, and severe anxiety groups were much higher than that in the no-anxiety group, which was not related to the age difference ( Table 3 ). The percentage of overweight women in the severe anxiety group was much higher than that of local women of childbearing age (37). Further investigation in a larger sample size is needed to identify the mechanism.
Due to the complicated physiological conditions of infertile patients, we could not determine whether the significant correlation between anxiety and BMI was caused by any minor malfunction of the endocrine system such as cortisol hypersecretion (p < 0.01) or the oily diet popular in the Chongqing region. Infertile anxiety women are generally prone to chronic pressure and are more likely to have a worse diet and unhealthy lifestyles, which exacerbate the issue. Similar to our result, a positive correlation between BMI and morning cortisol level has also been reported in a previous study (38). We postulate that hypercortisolemia could potentially contribute to increasing adiposity in the setting of caloric excess, as cortisol also regulates energy metabolism. Compared with serum cortisol level, which showed an increasing trend in the infertility group ( Figure 1 ), the SAS score was independent of age. These findings suggest that the development of anxiety disorder in our patients might be attributed to both physiological and socioeconomic factors.
Our analysis also identified a positive connection between the year of infertility, age, and BMI. These findings were in agreement with the idea that being overweight (BMI > 24.0) might cause many health issues such as infertility (39). Neither the SAS score nor the cortisol level was associated with the year of infertility, suggesting that stress or anxiety may not exacerbate with time. However, the onset of mood disorder and the impairment of reproductive function might be connected through complicated endocrine issues.
A previous study involving both sides of infertility couples indicated that sex, education level, infertility duration, and treatment failure would all contribute significantly to anxiety (4). However, the binary logistic regression analysis in the present work suggested that only the morning serum cortisol level contributes significantly to anxiety (p = 0.000). The difference might be due to the lack of cortisol level as a parameter, the usage of different evaluation instruments (SAS vs Generalized Anxiety Disorder-7), the selection of the target population (diagnosed female vs couples), and the social or environmental difference. Our study suggested that morning serum cortisol levels yielded a high AUC for the ROC curve and predictive accuracy comparable to the binary logistic model ( Figure 2 ; Table 4 ). The application of the POCT method suggested that morning serum cortisol level could serve as a potential biomarker to facilitate the screening of anxiety among infertile women.
The effect of stress on IVF outcomes has been investigated intensively in the field. It has been reported that both anxiety and concentrations of cortisol rose during IVF treatment (40), and lower blood cortisol at the time of oocyte retrieval is associated with successful treatment (24). Since our anxiety evaluation was conducted before any sex hormone administration and IVF treatment, it should be considered as a baseline for the psychological and physiological condition of patients. At this stage, the physiological condition of patients was not affected by external hormones, and they were not influenced by the excitement or stress of IVF treatment.
To further investigate the potential influence of anxiety on multi-cycle IVF treatment, the pregnancy outcome for 109 patients was retrieved. We found that the number of IVF cycles conducted was positively correlated with age (p < 0.01) and year of infertility (p < 0.01), but negatively correlated with pregnancy outcome ( Table 2 ). This suggested that as the patients grow older, the difficulty of treatment increases, even if multiple procedures were tried. As shown in Table 5 , the types and factors for infertility did not show a strong impact on the outcomes of the multi-cycle IVF treatment. However, patients with secondary infertility underwent more IVF cycles (1.90 VS 1.28), probably because the average age of these women was elder (31.6 VS 29.2). The significantly severe anxiety status of the elder, secondary infertility women evaluated through SAS and cortisol level also supports the idea that psychological evaluation and support should become incorporated into the medical service provided by reproductive medical centers.
The pregnancy rate for the “anxiety groups” either determined through SAS or cortisol level was 8.0%-10.3% lower, but the result did not reach a significant level or affirmative conclusion. Fortunately, the pregnancy rate of the anxiety groups and no anxiety groups all lay within the success rate reported by our hospital in the past few years (53% to 64%). Compared with other reproductive medical centers in China (success rate 50% to 65%), the outcomes of the IVF treatments conducted in this study were also reasonable. The same result was confirmed for the pregnancy rate after the first IVF cycle, where the same GnRH-antagonist protocol was adopted. Previous studies involving only one treatment cycle revealed a strong correlation between lower morning cortisol levels on the transplant day and successful pregnancy (41). We speculate that the anxiety level might change during treatment, and the stress regulation on the operation day should have a stronger effect. In addition, variables such as the number of oocytes retrieved, mature oocytes, and the number of embryos transferred were not strictly controlled between the IVF cycles and individuals. Consequently, no affirmation could be done on the effect of anxiety on the outcome of multi-cycle IVF treatment. A slightly higher average number of IVF cycle (1.76-1.77 VS 1.61-1.62) was also observed for the anxiety patients, in agreement with the fact that more IVF cycle was required until the patient was conceived or finally gave up. Most patients who failed after the first IVF cycle were willing to try multiple times, which might lead to a higher success rate. As we observed in this study, an alternative IVF protocol might be effective and result in clinical pregnancy. It is also possible that the medical operations of the women during IVF, especially the injection of sex hormones, had overcome the influence of anxiety and cortisol dysregulation to a certain degree (42). The expectation of a positive outcome from the treatment (43), and the encouragement from doctors and nurses could also help to release the stress.
Generally, our study has several strengths such as the novel application of onset serum cortisol detection and the fast entrance to IVF treatment. The evaluation of anxiety before any IVF treatment enabled the quantitative comparison of morning cortisol concentration between the healthy control group and the infertile women, which was missing from previous studies (24, 41, 44). The analysis between these two groups of participants provided a better demonstration of morning serum cortisol levels as a reflection of physiological stress. The SAS questionnaire could be easily applied in the reproductive medical center and has a well-defined Chinese norm. Both the SAS and cortisol tests revealed the baseline psychological and physiological condition of the patients without the influence of hormone injection during treatment. However, the limitations of this study should also be mentioned. Cortisol as one of the most important stress and metabolism regulatory hormones might also relate to other stress-related mood disorders such as depression. Since the reason for infertility is often complicated, the causation between anxiety, cortisol dysregulation, and infertility is unclear. We observed a high prevalence of overweight participants with no hypertension or hyperglycemia in this study. Detailed information on the endocrine function and metabolism of these women remained unclear. Future studies with larger population sizes and better control IVF treatment protocols should be conducted to address these issues.
Conclusion
The investigation of physiological and psychological stress revealed a high prevalence of anxiety and a significant increase in morning serum cortisol levels related to the SAS score of infertile women. Although the effect of anxiety on multi-cycle IVF treatment was inconclusive due to the variations in treatment protocols, we observed a slightly lower rate of clinical pregnancy for the anxiety patients, who also showed higher levels of morning serum cortisol. A study with a larger scale, identical infertility factors, or standardized IVF treatment between patients might be needed for better evaluation of the potential effect of anxiety.
Despite that anxiety before treatment could not be viewed as a direct drawback for infertility treatment, given the high prevalence of the disease and dysregulation of cortisol secretion, evaluation and intervention of the psychological and physiological issues of anxiety are preferred. Some relaxation methods such as music therapy could be recommended, and patients with severe symptoms can be referred to a psychologist (45, 46). With the convenience of sampling and the POCT method, morning serum cortisol measurement could be a feasible approach to promote the primary evaluation of anxiety and related endocrine dysregulations among infertile women. Such assessment before IVF treatment might be beneficial to the relief of stress, restoration of the endocrine regulatory system, and improve the well-being of patients.
Trial registration
This trial was approved by the institutional review board of The Second Affiliated Hospital of Chongqing Medical University, identifier: No. 2018 (100).
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Ethics statement
This trial was approved by the institutional review board of The Second Affiliated Hospital of Chongqing Medical University, identifier: No. 2018 (100). The patients/participants provided their written informed consent to participate in this study.
Author contributions
HZ and YC conceived and designed the study. QL prepared the cortisol test platform and wrote the first draft of the paper with YC. YW, BN, and TF performed the data retrieving, processing, and analysis. HC and XK helped with questionnaire collecting, data recording, and blood sample collection in the reproductive medicine center. HZ performed the cortisol measurement and supervised all data collected in the hospital. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank all women who participated in this study. The authors thank our colleagues in the Second Affiliated Hospital of Chongqing Medical University for their advice on study design and assistance with background information collection.
Funding Statement
This study was supported by Shenzhen Overseas Talent Program (827–000511); SZU Top Ranking Project (86000000210); SZU Start-up Grant (860-000002110806); Guangdong province Young Innovative Talents Project (2022KQNCX067); Innovation and Entrepreneurship Training Program for College Students (202210590017X); Traditional Chinese Medicine research joint project of Chongqing Health Commission and Chongqing Science and Technology Bureau (2019ZY3152) and Guangdong Province Innovation Team “Intelligent Management and Interdisciplinary Innovation” (2021WCXTD002).
Abbreviations
AUC, Area under the curve; BMI, Body mass index; CI, Confidence interval; GAD-7, 7-item self-administered scale; HPA, Hypothalamus–pituitary–adrenal; IVF, In vitro fertilization; NPV, Negative predictive value; POCT, Point-of-care test; PPV, Positive predictive value; ROC, Receiver operating characteristic; SAS, Self-Rating Anxiety Scale; SPSS, Statistical product and service solutions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY) (2019) 11(23):10952–91. doi: 10.18632/aging.102497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cong J, Li P, Zheng L, Tan J. Prevalence and risk factors of infertility at a rural site of northern China. PloS One (2016) 11(5):e0155563. doi: 10.1371/journal.pone.0155563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fallahzadeh H, Zareei Mahmood Abadi H, Momayyezi M, Malaki Moghadam H, Keyghobadi N. The comparison of depression and anxiety between fertile and infertile couples: a meta-analysis study. Int J Reprod Biomed (2019) 17(3):153–62. doi: 10.18502/ijrm.v17i3.4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiani Z, Simbar M, Hajian S, Zayeri F, Shahidi M, Saei Ghare Naz M, et al. The prevalence of anxiety symptoms in infertile women: a systematic review and meta-analysis. Fertil Res Pract (2020) 6:7. doi: 10.1186/s40738-020-00076-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang B, Zhang J, Qi Y, Wang P, Jiang R, Li H. Assessment on occurrences of depression and anxiety and associated risk factors in the infertile Chinese men. Am J Mens Health (2017) 11(3):767–74. doi: 10.1177/1557988317695901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui C, Wang L, Wang X. Effects of self-esteem on the associations between infertility-related stress and psychological distress among infertile Chinese women: a cross-sectional study. Psychol Res Behav Manage (2021) 14:1245–55. doi: 10.2147/PRBM.S326994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu H, Ouyang N, Li R, Tuo P, Mai M, Wang W. The effects of anxiety and depression on in vitro fertilisation outcomes of infertile Chinese women. Psychol Health Med (2017) 22(1):37–43. doi: 10.1080/13548506.2016.1218031 [DOI] [PubMed] [Google Scholar]
- 8. Valsamakis G, Chrousos G, Mastorakos G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology (2019) 100:48–57. doi: 10.1016/j.psyneuen.2018.09.031 [DOI] [PubMed] [Google Scholar]
- 9. Hoff HS, Crawford NM, Mersereau JE. Mental health disorders in infertile women: prevalence, perceived effect on fertility, and willingness for treatment for anxiety and depression. Fertility Sterility (2015) 104(3):e357. doi: 10.1016/j.fertnstert.2015.07.1113 [DOI] [Google Scholar]
- 10. Gui W, Yang X, Jiang H, Wu H, Zeng M, Wen Y, et al. Prevalence of anxiety and its associated factors among infertile patients after 'two-child' policy in chongqing, China: a cross-sectional study. Reprod Health (2021) 18(1):193. doi: 10.1186/s12978-021-01140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omani-Samani R, Ghaheri A, Navid B, Sepidarkish M, Maroufizadeh S. Prevalence of generalized anxiety disorder and its related factors among infertile patients in Iran: a cross-sectional study. Health Qual Life Outcomes (2018) 16(1):129. doi: 10.1186/s12955-018-0956-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demyttenaere K, Nijs P, Evers-Kiebooms G, Koninckx PR. The effect of a specific emotional stressor on prolactin, cortisol, and testosterone concentrations in women varies with their trait anxiety. Fertility Sterility (1989) 52(6):942–8. doi: 10.1016/S0015-0282(16)53156-2 [DOI] [PubMed] [Google Scholar]
- 13. Sanders KA, Bruce NW. A prospective study of psychosocial stress and fertility in women. Hum Reprod (1997) 12(10):2324–9. doi: 10.1093/humrep/12.10.2324 [DOI] [PubMed] [Google Scholar]
- 14. Fischer S, Cleare AJ. Cortisol as a predictor of psychological therapy response in anxiety disorders-systematic review and meta-analysis. J Anxiety Disord (2017) 47:60–8. doi: 10.1016/j.janxdis.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 15. Rincon-Cortes M, Herman JP, Lupien S, Maguire J, Shansky RM. Stress: influence of sex, reproductive status and gender. Neurobiol Stress (2019) 10:100155. doi: 10.1016/j.ynstr.2019.100155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther (2014) 94(12):1816–25. doi: 10.2522/ptj.20130597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gleicher N, Seier K, Kushnir VA, Weghofer A, Wu YG, Wang Q, et al. Associations between peripheral androgens and cortisol in infertile women. J Steroid Biochem Mol Biol (2016) 158:82–9. doi: 10.1016/j.jsbmb.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 18. Dolfing JG, Tucker KE, Lem CM, Uittenbogaart J, Verzijl JC, Schweitzer DH. Low 11-deoxycortisol to cortisol conversion reflects extra-adrenal factors in the majority of women with normo-gonadotrophic normo-estrogenic infertility. Hum Reprod (2003) 18(2):333–7. doi: 10.1093/humrep/deg082 [DOI] [PubMed] [Google Scholar]
- 19. Michael AE, Pester LA, Curtis P, Shaw RW, Edwards CR, Cooke BA. Direct inhibition of ovarian steroidogenesis by cortisol and the modulatory role of 11 beta-hydroxysteroid dehydrogenase. Clin Endocrinol (Oxf) (1993) 38(6):641–4. doi: 10.1111/j.1365-2265.1993.tb02147.x [DOI] [PubMed] [Google Scholar]
- 20. Xiao H, Xu Z, Zhu X, Wang J, Zheng Q, Zhang Q, et al. Cortisol safeguards oogenesis by promoting follicular cell survival. Sci China Life Sci (2022) 65(8):1563–77. doi: 10.1007/s11427-021-2051-0 [DOI] [PubMed] [Google Scholar]
- 21. Wdowiak A, Raczkiewicz D, Janczyk P, Bojar I, Makara-Studzinska M, Wdowiak-Filip A. Interactions of cortisol and prolactin with other selected menstrual cycle hormones affecting the chances of conception in infertile women. Int J Environ Res Public Health (2020) 17(20):7537. doi: 10.3390/ijerph17207537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Massey AJ, Campbell BK, Raine-Fenning N, Pincott-Allen C, Perry J, Vedhara K. Relationship between hair and salivary cortisol and pregnancy in women undergoing IVF. Psychoneuroendocrinology (2016) 74:397–405. doi: 10.1016/j.psyneuen.2016.08.027 [DOI] [PubMed] [Google Scholar]
- 23. An Y, Wang Z, Ji H, Zhang Y, Wu K. Pituitary-adrenal and sympathetic nervous system responses to psychiatric disorders in women undergoing in vitro fertilization treatment. Fertil Steril (2011) 96(2):404–8. doi: 10.1016/j.fertnstert.2011.05.092 [DOI] [PubMed] [Google Scholar]
- 24. An Y, Sun Z, Li L, Zhang Y, Ji H. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment: psychological and neurohormonal assessment. J Assist Reprod Genet (2013) 30(1):35–41. doi: 10.1007/s10815-012-9904-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petrowski K, Schmalbach I, Strunk A, Hoyer J, Kirschbaum C, Joraschky P. Cortisol reactivity in social anxiety disorder: a highly standardized and controlled study. Psychoneuroendocrinology (2021) 123:104913. doi: 10.1016/j.psyneuen.2020.104913 [DOI] [PubMed] [Google Scholar]
- 26. Conde A, Costa R, Figueiredo B. Anxiety and depressive symptoms effects on cortisol trajectories from pregnancy to postpartum: differences and similarities between women and men. Horm Behav (2021) 128:104917. doi: 10.1016/j.yhbeh.2020.104917 [DOI] [PubMed] [Google Scholar]
- 27. Braig S, Grabher F, Ntomchukwu C, Reister F, Stalder T, Kirschbaum C, et al. The association of hair cortisol with self-reported chronic psychosocial stress and symptoms of anxiety and depression in women shortly after delivery. Paediatric Perinatal Epidemiol (2016) 30(2):97–104. doi: 10.1111/ppe.12255 [DOI] [PubMed] [Google Scholar]
- 28. Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int (2010) 196(1-3):32–7. doi: 10.1016/j.forsciint.2009.12.040 [DOI] [PubMed] [Google Scholar]
- 29. Chai Y, Li Q, Wang Y, Tao E, Asakawa T. The value of HPA axis hormones as biomarkers for screening and early diagnosis of postpartum depression: updated information about methodology. Front Endocrinol (Lausanne) (2022) 13:916611. doi: 10.3389/fendo.2022.916611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu L, Bu Z, Huang G, Sun H, Deng C, Sun Y. Assisted reproductive technology in China: results generated from data reporting system by CSRM from 2013 to 2016. Front Endocrinol (Lausanne) (2020) 11:458. doi: 10.3389/fendo.2020.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leff-Gelman P, Flores-Ramos M, Carrasco AEÁ, Martínez ML, Takashima MFS, Coronel FMC, et al. Cortisol and DHEA-s levels in pregnant women with severe anxiety. BMC Psychiatry (2020) 20(1):393. doi: 10.1186/s12888-020-02788-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Ji X. Sample size estimation in clinical research: from randomized controlled trials to observational studies. Chest (2020) 158(1S):S12–20. doi: 10.1016/j.chest.2020.03.010 [DOI] [PubMed] [Google Scholar]
- 33. Zung WWK. A rating instrument for anxiety disorders. Psychosomatics (1971) 12(6):371–9. doi: 10.1016/S0033-3182(71)71479-0 [DOI] [PubMed] [Google Scholar]
- 34. Liang S, Chen Y, Wang Q, Chen H, Cui C, Xu X, et al. Prevalence and associated factors of infertility among 20-49 year old women in henan province, China. Reprod Health (2021) 18(1):254. doi: 10.1186/s12978-021-01298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Z, Li L, Lei XY, Xue J, Mi HY. Effect of advanced maternal age on birth defects and postnatal complications of neonates. Zhongguo Dang Dai Er Ke Za Zhi (2016) 18(11):1084–9. doi: 10.7499/j.issn.1008-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kahl KG, Bens S, Ziegler K, Rudolf S, Dibbelt L, Kordon A, et al. Cortisol, the cortisol-dehydroepiandrosterone ratio, and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biol Psychiatry (2006) 59(7):667–71. doi: 10.1016/j.biopsych.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 37. Chen Y, Wan K, Gong Y, Zhang X, Liang Y, Wang X, et al. Assessing the relationship between pregravid body mass index and risk of adverse maternal pregnancy and neonatal outcomes: prospective data in southwest China. Sci Rep (2021) 11(1):7591. doi: 10.1038/s41598-021-87135-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schorr M, Lawson EA, Dichtel LE, Klibanski A, Miller KK. Cortisol measures across the weight spectrum. J Clin Endocrinol Metab (2015) 100(9):3313–21. doi: 10.1210/JC.2015-2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Best D, Avenell A, Bhattacharya S. How effective are weight-loss interventions for improving fertility in women and men who are overweight or obese? a systematic review and meta-analysis of the evidence. Hum Reprod Update (2017) 23(6):681–705. doi: 10.1093/humupd/dmx027 [DOI] [PubMed] [Google Scholar]
- 40. Harlow CR, Fahy UM, Talbot WM, Wardle PG, Hull MG. Stress and stress-related hormones during in-vitro fertilization treatment. Hum Reprod (1996) 11(2):274–9. doi: 10.1093/HUMREP/11.2.274 [DOI] [PubMed] [Google Scholar]
- 41. Yu TC Z. Correlation analysis of plasma cortisol levels with fertility stress and pregnancy outcome in patients with IVF/ICSI. Chin J Reprod Contracep (2022) 42(1):30–5. doi: 10.3760/cma.j.cn101441-20200623-00354 [DOI] [Google Scholar]
- 42. Evans-Hoeker EA, Eisenberg E, Diamond MP, Legro RS, Alvero R, Coutifaris C, et al. Major depression, antidepressant use, and male and female fertility. Fertility Sterility (2018) 109(5):879–87. doi: 10.1016/j.fertnstert.2018.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Capuzzi E, Caldiroli A, Ciscato V, Zanvit FG, Bollati V, Barkin JL, et al. Is in vitro fertilization (IVF) associated with perinatal affective disorders? J Affect Disord (2020) 277:271–8. doi: 10.1016/j.jad.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 44. Santa-Cruz DC, Caparros-Gonzalez RA, Romero-Gonzalez B, Peralta-Ramirez MI, Gonzalez-Perez R, Garcia-Velasco JA. Hair cortisol concentrations as a biomarker to predict a clinical pregnancy outcome after an IVF cycle: a pilot feasibility study. Int J Environ Res Public Health (2020) 17(9):3020. doi: 10.3390/ijerph17093020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Witte M, Pinho ADS, Stams GJ, Moonen X, Bos AER, van Hooren S. Music therapy for stress reduction: a systematic review and meta-analysis. Health Psychol Rev (2022) 16(1):134–59. doi: 10.1080/17437199.2020.1846580 [DOI] [PubMed] [Google Scholar]
- 46. Mahmoud MY, Labib K, Sileem SA, Mustafa FA, Hamed WM, Abd Elhamid A, et al. The impact of music therapy on anxiety and pregnancy rate among infertile women undergoing assisted reproductive technologies: a systematic review and meta-analysis. J Psychosom Obstet Gynaecol (2022) 43(2):205–13. doi: 10.1080/0167482X.2021.1977277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.


