Abstract
Objectives
Neuroimaging studies have confirmed that acupuncture can promote static functional reorganization in poststroke patients with motor dysfunction. But its effect on dynamic brain networks remains unclear. This study is aimed at investigating how acupuncture affected the brain's dynamic functional network connectivity (dFNC) after ischemic stroke.
Methods
We conducted a single-center, randomised controlled neuroimaging study in ischemic stroke patients. A total of 53 patients were randomly divided into the true acupoint treatment group (TATG) and the sham acupoint treatment group (SATG) at a ratio of 2 : 1. Clinical assessments and magnetic resonance imaging (MRI) scans were performed on subjects before and after treatment. We used dFNC analysis to estimate distinct dynamic connectivity states. Then, the temporal properties and strength of functional connectivity (FC) matrix were compared within and between the two groups. The correlation analysis between dynamic characteristics and clinical scales was also calculated.
Results
All functional network connectivity (FNC) matrices were clustered into 3 connectivity states. After treatment, the TATG group showed a reduced mean dwell time and found attenuated FC between the sensorimotor network (SMN) and the frontoparietal network (FPN) in state 3, which was a sparsely connected state. The FC between the dorsal attention network (DAN) and the default mode network (DMN) was higher after treatment in the TATG group in state 1, which was a relative segregated state. The SATG group preferred to increase the mean dwell time and FC within FPN in state 2, which displayed a local tightly connected state. In addition, we found that the FC value increased between DAN and right frontoparietal network (RFPN) in state 1 in the TATG group after treatment compared to the SATG group. Correlation analyses before treatment showed that the Fugl-Meyer Assessment (FMA) lower score was negatively correlated with the mean dwell time in state 3. FMA score showed positive correlation with FC in RFPN-SMN in state 3. FMA-lower score was positively correlated with FC in DAN-DMN and DAN-RFPN in state 1.
Conclusions
Acupuncture has the potential to modulate abnormal temporal properties and promote the balance of separation and integration of brain function. True acupoint stimulation may have a more positive effect on regulating the brain's dynamic function. Clinical Trial Registration. This trial is registered with Chinese Clinical Trials Registry (ChiCTR1800016263).
1. Introduction
Stroke remains the second leading cause of death and the third leading cause of disability worldwide, according to the latest Global Burden of Disease study [1]. In China, ischemic stroke is the leading cause of mortality and disability, resulting in an increasing annual disease burden [2]. Motor dysfunction is the most common symptom of ischemic stroke, with more than 70% of stroke survivors suffering from a varying degree of motor dysfunction [3]. Rehabilitation services, which promote spontaneous neurological recovery and experience-dependent plasticity, are the primary approaches for promoting functional recovery and independence of patients with stroke [4–6].
Acupuncture, as a vital part of Traditional Chinese Medicine, has gained increasing acceptance worldwide and has been applied in different forms to treat diseases in more than 180 countries around the world [7–10]. In China, acupuncture is widely used in the treatment of stroke patients with motor dysfunction [11]. American Heart Association/American Stroke Association Guideline also indicated that facilitating acupuncture may be effective as an adjunctive treatment for motor recovery and walking mobility [6]. Of note, Shou Zu Shi Er Zhen is the representative needling prescription in China for the treatment of poststroke patients with motor dysfunction, and its effectiveness has been identified by several randomised clinical trials [12–15]. However, its underlying mechanism remains unclear.
As a noninvasive neuroimaging technology, functional magnetic resonance imaging (fMRI) lays the foundation for real-time measurement of whole-brain activity and helps advance the understanding of neuroplasticity modulated by acupuncture [16, 17]. Neuroimaging evidence has demonstrated the facilitating effect of acupuncture in the regulation of brain functional reorganization, comprising activation of motor-related cortical areas [18–20], increasing motor-cognition connectivity and decreasing compensation of contralesional motor cortex, and modulating effective connectivity of resting-state networks in patients with poststroke hemiplegia [21, 22]. However, these studies mainly focus on instant acupuncture effects. Our previous work provided evidence for long-term acupuncture intervention effects using the voxel-mirrored homotopic connectivity method. We confirmed that Shou Zu Shi Er Zhen acupuncture prescription can integrate information among motor, vision, hearing processing, and cognitive brain regions, thereby promoting motor function recovery [23].
Despite such progress, the assessment of brain function is still limited by an assumption that the brain activity is constant throughout recording periods in resting state [24]. Recent studies have confirmed the time-varying properties of functional brain networks [25, 26]. Dynamic functional network connectivity (dFNC), which combines independent component analysis and sliding window approach, reflects temporal variations in functional network connectivity at much faster timescales (seconds-minutes) [24, 25]. Several studies have confirmed that acupuncture can affect the brain's dynamic functional connectivity (FC), such as decreasing temporal variability in chronic tinnitus and regulating dynamic alterations of brain activity in migraine patients [27, 28]. So far, no study has shown the acupuncture effect on dFNC in stroke patients. However, there is evidence suggesting that the brain's preference for distinct dynamic connection patterns was altered after ischemic stroke, and the specific patterns may be critical for neurological function recovery [29].
Therefore, the goal of the present study was to investigate how acupuncture affected the brain's dynamic connections following ischemic stroke based on dFNC analysis. We hypothesized that acupuncture might modulate dynamic properties of brain functional networks in poststroke patients, while true acupoint stimulation has a more positive effect.
2. Materials and Methods
2.1. Study Design
This was a single-center, randomised controlled neuroimaging study, with patients and assessors blinded for group allocation. According to the order of admission, the stroke patients were randomly divided into the true acupoint treatment group (TATG) and the sham acupoint treatment group (SATG) at a ratio of 2 : 1 using a random number table. Based on conventional treatment, the TATG group received Shou Zu Shi Er Zhen acupuncture treatment, while the SATG group received sham acupuncture treatment. All patients were evaluated using clinical scales and underwent MRI scan before and after acupuncture treatment. The trial was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR 1800016263). The study protocol had been published previously [30].
2.2. Participants
A total of 53 patients who matched the selection criteria were recruited from Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine (Beijing, China) from March 2018 to December 2021. The inclusion criteria were as follows: (1) patients with ischemic stroke; (2) within 6 weeks from onset; (3) single lesion of ischemic infarct restricted to the unilateral hemisphere involving the internal capsule, basal ganglia, corona radiata, and its neighboring regions; (4) right-handed before stroke; (5) 35-80 years of age; (6) consciousness and stable condition; (7) not taken any psychiatric medications in 1 month. The exclusion criteria were as follows: (1) severe primary diseases of heart, liver, kidneys, hematologic system, immune system disease, tumors, and psychiatric disorders; (2) pregnant or lactating women; (3) any MRI contraindications; (4) any brain abnormalities except infarction identified by MRI. Seven patients did not complete the second MRI scan for personal reasons, and six patients were excluded due to severe head motion during scanning. Therefore, a total of 40 patients were included in the final analysis. Figure 1 shows the flow chart of this study.
Figure 1.
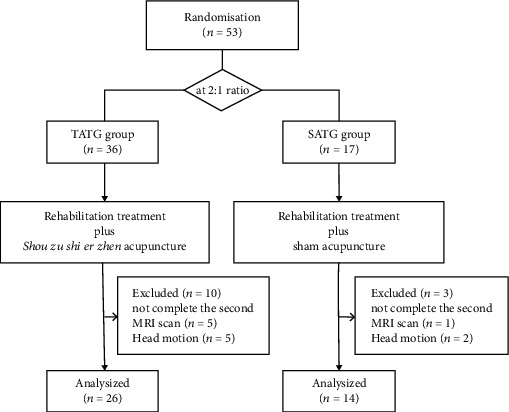
The study flow chart.
2.3. Interventions
Ande brand disposable acupuncture needles (size 0.25 × 40 mm) were used. Patients in the TATG group received Shou Zu Shi Er Zhen acupuncture treatment, which consist of bilateral Hegu (LI4), Quchi (LI11), Neiguan (PC6), Zusanli (ST36), Yanglingquan (GB34), and Sanyinjiao (SP6). After skin disinfection, acupuncture needles were inserted vertically into the skin to a depth of 20-30 mm. Following needle insertion, acupuncture manipulations of twirling and lifting were conducted on all needles to allow patients to obtain De qi, a combination of sensations including soreness, dullness, numbness, heaviness, and other feelings [31]. It was believed to play an important role in acupuncture efficacy. Needles were kept in situ for 30 minutes. The patients received five treatment sessions per week for 2 weeks (10 sessions in total). Patients in the SATG group received sham acupuncture treatment. The location of sham acupoints was referred to the method published in JAMA by Liu et al., with 20 mm beside the true acupoints [32]. The location of all acupoints is shown in Supplementary Material 1. We would avoid De qi sensations in the manipulation in the SATG group. Besides that, other treatment procedures were the same as the TATG group. And all the patients received routine medication and rehabilitation treatment.
2.4. Clinical Assessments
We used the National Institutes of Health Stroke Scale (NIHSS) to assess the severity of neurological deficit and the Fugl-Meyer Assessment (FMA) to evaluate the motor function at baseline and after the acupuncture treatment. SPSS 20 was applied for statistical analysis. Paired samples t-test was used for within-group comparisons. Independent sample t-test was used to compare the difference between the two groups. Statistical significance was set at P < 0.05.
2.5. MRI Data Acquisition
All MRI data were acquired from a 3.0 Tesla scanner (Siemens, Sonata, Germany) at Dongzhimen Hospital, Beijing, China. The parameters of gradient echoplanar imaging (EPI) were as follows: echo time (TE) = 30 ms, repetition time (TR) = 2000 ms, matrix = 64 × 64, field of vision (FOV) = 225 mm × 225 mm, slice thickness = 3.5 mm, and flip angle (FA) = 90°. The functional scan lasted for 6 minutes, and 179 image volumes were obtained. And the T1-weighted imaging (T1WI) scan lasted for 4 minutes and 10 seconds, with the following parameters: TE = 2.53 ms, TR = 1900 ms, matrix = 256 × 256, FOV = 250 mm × 250 mm, slice thickness = 1.0 mm, and FA = 9°.
2.6. Preprocessing of fMRI Data
The resting-state fMRI data were preprocessed using DPABI software [33] in MATLAB (version R2018a, MathWorks, Inc., Natick, MA, USA). For the left hemispheric lesions (n = 18), the functional images were flipped along the midsagittal plane in order to reduce the spatial heterogeneity of lesions. Thus, all lesions were located on the right hemisphere. The probability map and detailed information of the lesion location in each patient are shown in Supplementary Material 1. The first 10 time points were removed to stabilize MRI signal and remained 169 volumes. Then, the remaining volumes were slice-timing corrected to correct time differences between slices. Head motion correction was performed, and the patients were excluded when the maximum translation exceeded 3 mm and rotational parameters exceeded 3 degrees in any direction. Next functional images were normalized to the standard Montreal Neurological Institute space by using T1 image unified segmentation, resliced into 3 × 3 × 3 mm3. Afterward, images were smoothed using a Gaussian kernel with a 4 mm full width at half maximum, regressed with 24-parameter regression, white matter, and cerebrospinal fluid signal. Finally, the data were bandpass filtered with 0.01-0.1 Hz. Global signal regression was not performed.
2.7. Intrinsic Connectivity Networks
For all preprocessing data, spatial independent component analysis (ICA) was carried out to identify intrinsic connectivity network independent components (ICs) using the Group ICA Toolbox (GIFT version 4.0b, http://icatb.sourceforge.net). Firstly, the number of ICs was automatically estimated from the imaging data of all the patient, and 56 ICs were obtained. Following that, the principal component analysis was performed to reduce dimensionality. Secondly, Group ICA was conducted using the infomax algorithm to extract independent spatial maps and time courses of each component [34]. Lastly, the back-reconstruction [35] and Fisher's z-transformation were performed for each independent component of the subjects so that the transformed data approximately obeyed a normal distribution with standard deviation of the mean. Multiple regression analysis was applied to analyze the spatial correlation between the extracted components and the resting brain network templates [36]. The IC selection referred to previous criteria [37]: (1) peak coordinates of ICs located primarily in the gray matter; (2) almost no spatial overlap with blood vessels, ventricles, and suspicious artifacts; (3) dominated by low-frequency signals (<0.1 Hz). Following these steps, 11 ICs were kept for further analysis and arranged into different functional networks, including the default mode network (DMN), sensorimotor network (SMN), dorsal attention network (DAN), left frontoparietal network (LFPN), and right frontoparietal network (RFPN). And then, we performed the following postprocessing steps on the time courses of 11 ICs: (1) detrending linear, quadratic, and cubic trends; (2) removing spikes; (3) low-pass filtering with a cut-off frequency of 0.15 Hz. To estimate functional network connectivity, Pearson's correlations were performed using postprocessed time courses between ICs throughout the whole scanning and then converted to z values using Fisher's z-transformation to improve the normality [25, 38].
2.8. Dynamic Functional Network Connectivity
2.8.1. Sliding Window Analysis
Sliding window analysis, the most common method in dFNC analysis, was used to construct brain functional network in GIFT toolbox. Previous studies had confirmed that the window length of 20-30 TR can better reflect the dynamic characteristics of the brain [39, 40]. Thus, we selected a window length of 20TR, in step of 1TR, computing 149 individual windows. These time windows were convolved with a Gaussian value of σ = 3 TRs and computed via the L1-regularized precision matrix. To obtain z values and stabilize variance for further analyses, Fisher's z-transformation was applied to the functional connectivity matrices.
2.8.2. Clustering Analysis
Based on the windowed functional connectivity matrices of all participants, a k-means clustering algorithm was applied to detect reoccurring FC patterns. We used the city-block distance with 500 iterations and 150 replicates to assess the similarity between all time windows. The optimal number of clustering states was estimated by the elbow criterion. The number was determined to be 3, which meant 3 FC states, exhibiting different FC patterns.
2.8.3. Temporal Properties and Connectivity Strength of Dynamics States
Three parameters were measured to assess the temporal properties of FNC: (1) fraction time (the frequency of each state); (2) mean dwell time (the average time a subject spent in any state without converting to another state); (3) number of transitions (switching times between different states). Besides, the connectivity strength of 3 states within the TATG group and the SATG group was also calculated. Analysis of covariance (ANCOVA) was used to compare the mean changes from before treatment to after treatment between groups. Disease duration and lesion volume were taken as covariates. And paired samples t-test was adopted to compare the changes before and after treatment in each group. The P value for the above analysis was set to 0.05 and corrected for false discovery rate (FDR) correction.
2.9. Correlation Analysis
Pearson's correlation was carried out to assess the relationship between clinical characteristics and dFNC parameters at baseline in all subjects, as well as FC values showing significant changes in any state before and after treatment (level of significance: P < 0.05).
2.10. Validation Analysis
We used different sliding window lengths and number of clusters to verify the stability of our results. The numbers of clusters were set at 4 and 5, and the sliding window lengths were set at 22TRs and 30TRs (detailed information in Supplementary Material 2).
3. Results
3.1. Demographic Characteristics
A total of 40 patients completed the whole study, including 26 patients in the TATG group and 14 patients in the SATG group. There was no significant difference in age, gender, and duration of disease between the TATG group and the SATG group (P > 0.05). The detailed data is presented in Table 1.
Table 1.
Demographic features of stroke patients.
| The TATG group (n = 26) | The SATG group (n = 14) | P value | |
|---|---|---|---|
| Sex (male/female) | 18/8 | 10/4 | 0.885 |
| Age (years) | 59.38 ± 11.61 | 59.14 ± 8.77 | 0.946 |
| Disease duration (days) | 15.89 ± 20.70 | 20.07 ± 15.34 | 0.831 |
| Lesion side (left/right) | 12/14 | 6/8 | 0.842 |
| Lesion volume (ml) | 2.96 ± 2.85 | 4.75 ± 3.97 | 0.127 |
Values were expressed as mean ± standard deviation. TATG: true acupuncture treatment group; SATG: sham acupuncture treatment group. The chi-square test was used to compare the distribution of sex and lesion side between the two groups.
3.2. Clinical Information
For the clinical data, no statistical differences were observed in NIHSS and FMA scores pretreatment and posttreatment between the two groups (all P > 0.05). Intragroup comparisons showed that FMA-lower score, FMA total score, and NIHSS score improved after treatment compared with those before treatment in both the TATG group and the SATG group (all P < 0.05). In addition, the FMA-upper score increased after treatment in the TATG group (P < 0.05), while this result was not found in the SATG group (P > 0.05). The clinical information was provided in Table 2.
Table 2.
Clinical assessment before and after treatment in the two groups.
| Clinical scales | The TATG group | The SATG group | ||||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | P value | Before treatment | After treatment | P value | |
| FMA-UE | 41.23 ± 23.87 | 46.42 ± 22.57 | <0.0001∗ | 45.50 ± 24.49 | 49.42 ± 23.39 | 0.058 |
| FMA-LE | 27.50 ± 7.58 | 30.19 ± 5.06 | <0.0001∗ | 27.64 ± 7.08 | 30.64 ± 4.49 | 0.013∗ |
| FMA | 68.73 ± 37.38 | 76.61 ± 25.65 | <0.0001∗ | 73.14 ± 30.71 | 80.07 ± 27.56 | 0.016∗ |
| NIHSS | 3.46 ± 2.42 | 2.46 ± 2.24 | <0.0001∗ | 3.64 ± 3.24 | 2.50 ± 3.05 | 0.002∗ |
Values were expressed as mean ± standard deviation. TATG: true acupuncture treatment group; SATG: sham acupuncture treatment group. FMA: Fugl-Meyer. FMA-UE: FMA for the upper extremity. FMA-LE: FMA for the lower extremity. NIHSS: National Institute of Health Stroke Scale. The P value represents the difference before and after treatment in the TATG group or the SATG group. ∗ represents P < 0.05.
3.3. Intrinsic Connectivity Networks and Dynamic Functional Connectivity States
We identified five resting-state networks from the 11 ICs, including the default mode network (DMN: ICs 51, 56), the sensorimotor network (SMN: ICs 41, 48), the dorsal attention network (DAN: ICs 16, 27), the left frontoparietal network (LFPN: ICs 1, 53), and the right frontoparietal network (RFPN: ICs 12, 45, 50). Spatial maps of 11 ICs are shown in Figure 2. All FNC matrices were clustered into 3 connectivity states. State 1 presented a relatively complicated connectivity pattern, which was characterized by partly strong positive connections within networks (such as DMN and DAN) and predominantly negative and neutral internetwork connections (between DAN and LFPN, between SMN and other networks, etc.), along with less positive connections (between FPN and DMN). Therefore, we referred to this state as the relative segregated state. The overall frequency of state 1 accounted for 23.22% of all time windows. State 2 seemed to display a more densely intranetwork connection, especially in FPN. We called it the local tightly connected state. The frequency of state 2 was lower than state 1 at 19.60%. State 3 had the longest time window percentage of 57.18%. This state exhibited sparse intra-/internetwork connectivity, which meant no high positive or negative connections within or between networks. And we called this the sparsely connected state. All states are shown in Figure 3.
Figure 2.
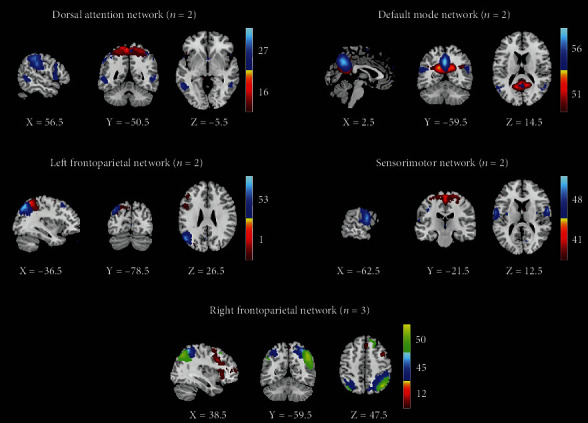
Spatial maps of all 11 independent components. They were assigned to 5 networks: the default mode network, the sensorimotor network, the dorsal attention network, the left frontoparietal network, and the right frontoparietal network. Different color represents the location of independent component of the peak point in each network.
Figure 3.
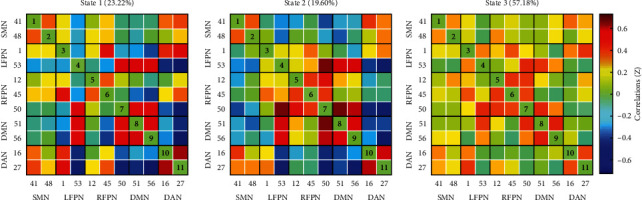
Three different dynamic functional connectivity states. Each matrix represents a stable connectivity state within the data. The percentage of occurrences in each state is also listed. The warm color indicates stronger positive connectivity, and the cold color implies stronger negative connectivity.
3.4. Temporal Properties
There were no statistical differences observed between the two groups in mean change of fraction time, mean dwell time, and number of transitions. The detailed results are listed in Table 3. The TATG group showed a significantly shorter mean dwell time in state 3 after treatment than that before treatment (P < 0.05, FDR correction), while the SATG group revealed a longer mean dwell time in state 2 after treatment (P < 0.05, FDR correction). Detailed information are shown in Table 4 and Figure 4.
Table 3.
Comparison in temporal properties between the two groups.
| Fraction time (%) | Mean dwell time (TR) | Number of transitions | |||||
|---|---|---|---|---|---|---|---|
| State 1 | State 2 | State 3 | State 1 | State 2 | State 3 | ||
| Mean difference (TATG-SATG) | 4.900 | -4.200 | -0.600 | 1.919 | -2.966 | -6.886 | 1.503 |
| 95% confidence interval (lower bound, upper bound) | -10.50020.200 | -17.500 9.000 | -15.300, 14.000 | -6.391, 10.230 | -10.107, 4.175 | -31.153, 17.380 | -1.335, 4340 |
| F | 0.417 | 0.428 | 0.008 | 0.221 | 0.716 | 0.334 | 1.164 |
| P | 0.523 | 0.518 | 0.931 | 0.641 | 0.404 | 0.567 | 0.289 |
TATG: true acupuncture treatment group; SATG: sham acupuncture treatment group.
Table 4.
Comparison in temporal properties within the two groups.
| Fraction time (%) | Mean dwell time (TR) | Number of transitions | ||||||
|---|---|---|---|---|---|---|---|---|
| State 1 | State 2 | State 3 | State 1 | State 2 | State 3 | |||
| The TATG group | Before treatment | 24.08 ± 19.70 | 21.73 ± 21.51 | 54.18 ± 29.54 | 12.38 ± 8.54 | 11.06 ± 9.55 | 35.08 ± 38.59 | 6.92 ± 3.42 |
| After treatment | 28.55 ± 15.55 | 24.96 ± 15.71 | 46.49 ± 15.94 | 14.61 ± 6.88 | 14.25 ± 9.03 | 21.35 ± 10.97 | 8.11 ± 2.59 | |
| t | -1.133 | -0.805 | 1.716 | -1.068 | -1.456 | 2.096 | -1.563 | |
| P | 0.268 | 0.428 | 0.098 | 0.296 | 0.158 | 0.046∗ | 0.131 | |
|
| ||||||||
| The SATG group | Before treatment | 21.62 ± 21.92 | 15.63 ± 18.23 | 62.75 ± 28.28 | 11.39 ± 10.63 | 8.74 ± 10.70 | 44.37 ± 46.14 | 6.5 ± 3.83 |
| After treatment | 22.82 ± 19.67 | 23.30 ± 23.09 | 53.88 ± 23.96 | 12.42 ± 8.53 | 15.14 ± 15.60 | 33.50 ± 36.39 | 6.5 ± 3.27 | |
| t | -0.189 | -1.588 | 1.622 | -0.296 | -2.313 | 1.113 | 0 | |
| P | 0.853 | 0.136 | 0.129 | 0.772 | 0.038∗ | 0.286 | 1 | |
Values were expressed as mean ± standard deviation. TATG: true acupuncture treatment group; SATG: sham acupuncture treatment group. The P value represents the difference before and after treatment in the TATG group or the SATG group. ∗ represents P < 0.05.
Figure 4.
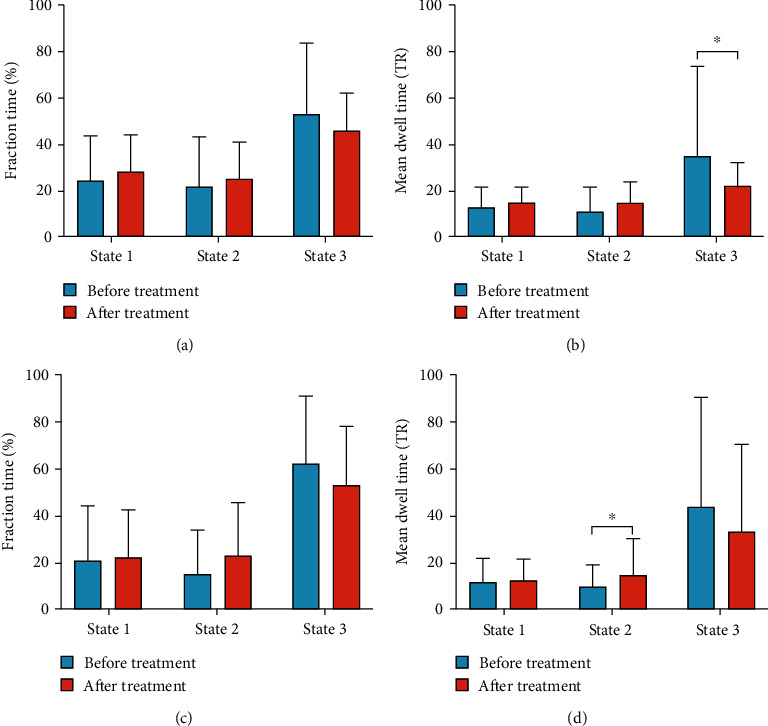
Changes in dynamic properties before and after treatment in the TATG group and the SATG group. (a) Change in fraction time before and after treatment in the TATG group. (b) Change in mean dwell time before and after treatment in the TATG group. (c) Change in fraction time before and after treatment in the SATG group. (d) Change in mean dwell time before and after treatment in the SATG group. TATG: true acupuncture treatment group. SATG: sham acupuncture treatment group. ∗ represents P < 0.05.
3.5. Strength of Dynamics States
We analyzed the alterations of FC within and between groups for each state. The TATG group showed increased FC between DAN and DMN in state 1 and reduced FC between RFPN and SMN in state 2 and FPN and SMN in state 3 after treatment (all P < 0.05). The SATG group had elevated FC in FPN (between LFPN and RFPN, within RFPN) in state 2 after treatment (P < 0.05).
Group difference showed positive effect between DAN and RFPN in state 1 (P < 0.05), which means increased FC between DAN and RFPN in state 1 in the TATG group compared to the SATG group. Altered connectivity pairs are shown in Figure 5.
Figure 5.
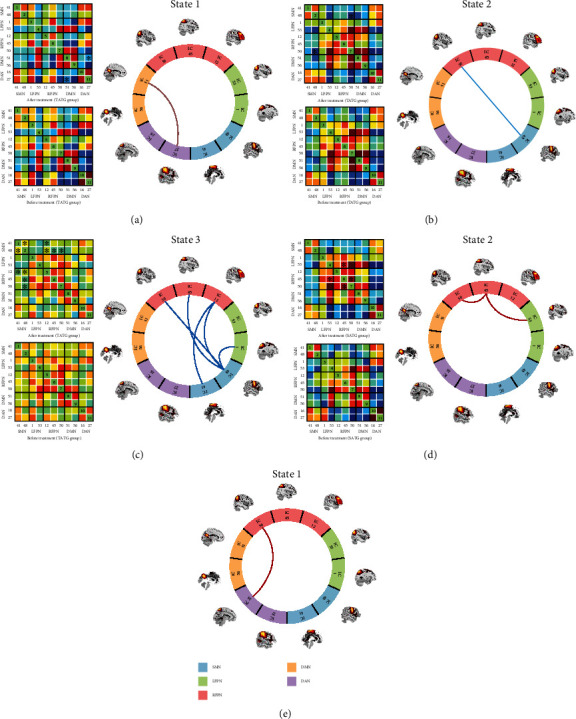
Differences in functional connectivity among distinct states. Specific connectivity matrix (left) and circle plots of significant difference in connectivity states (right) (a–d). The significant difference in specific state is marked by asterisk (∗ represents P < 0.05). In circle plots, red lines indicate increased functional connectivity (FC) between or within networks. Blue lines indicate decreased FC between or within networks. (a–d) The significant connectivity differences after treatment compared to before treatment in the TATG group or the SATG group. (a) Increased FC between DAN and DMN in state 1 in the TATG group. (b) Reduced FC between RFPN and SMN in state 2 in the TATG group. (c) Reduced FC between FPN and SMN in state 3 in the TATG group. (d) Increased FC in FPN in state 2 in the SATG group. (e) Positive effect between DAN and RFPN in state 1 between the two groups, which means increased FC between DAN and RFPN in state 1 in the TATG group compared to the SATG group. SMN: sensorimotor network. LFPN: left frontoparietal network. RFPN: the right frontoparietal network. DMN: default mode network. DAN: dorsal attention network.
3.6. Correlation between Clinical Measures and dFNC Parameters
Correlation analyses were carried out to detect whether dFNC parameters were related to clinical characteristics. The mean dwell time was negatively correlated with FMA-lower score in state 3 (r = −0.266, P = 0.049). We speculate that better motor recovery may be associated with short time spent in state 3 to some extent. We also found a negative correlation trend between mean dwell time and NIHSS score in state 1 (r = −0.236, P = 0.071). In addition, FMA score showed positive correlation with FC in RFPN-SMN in state 3 (r = 0.331, P = 0.037). FMA-lower score was positively correlated with FC in DAN-DMN and DAN-RFPN in state 1 (r = 0.405, P = 0.016 and r = 0.394, P = 0.012) (see in Figure 6).
Figure 6.
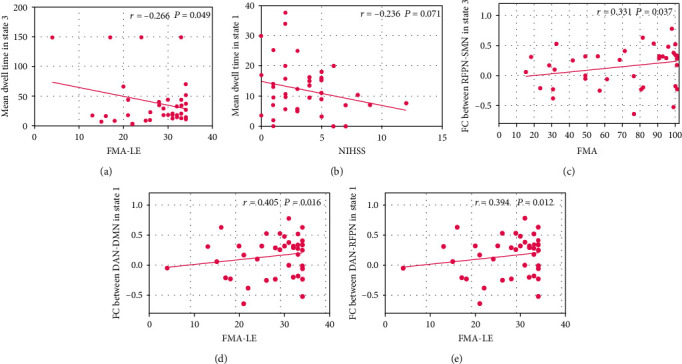
Correlation between clinical measures and dFNC parameter. (a) Negative correlation between mean dwell time and FMA-LE score in state 3. (b) Negative correlation trend between mean dwell time and NIHSS score in state 1. (c) Positive correlation between FMA score and FC in RFPN-SMN in state 3. (d) Positive correlation between FMA-LE score and FC in DAN-DMN in state 1. (e) Positive correlation between FMA-LE score and FC in DAN-RFPN in state 1. FMA: Fugl-Meyer. FMA-LE: FMA for the lower extremity. NIHSS: National Institute of Health Stroke Scale.
3.7. Validation Analysis
Due to the different settings of dynamic analysis parameters, the results are different to some extent. But most of our main results were well replicated when using different parameters. These observations indicated that acupuncture can indeed improve the abnormal temporal properties and functional network connectivity in stroke patients. One interesting finding was that the number of transitions significantly increased after treatment in the TATG group when the number of clusters k = 3 and the length of sliding time windows W = 22TRs. Previous studies reported that such variability may lead to greater cognitive and behavioral flexibility [41, 42]. Thus, we speculated that true acupuncture has the potential to improve the brain's flexibility.
4. Discussion
4.1. Acupuncture Improves Motor Function in Stroke Patients with Hemiplegia
From Traditional Chinese Medicine, Shou Zu Shi Er Zhen has the effect of harmonizing qi and blood, balancing yin and yang, harmonizing the spleen and stomach, and regulating body constituent and spirit [30, 43]. Current evidence suggested that conventional treatment combined with Shou Zu Shi Er Zhen therapy is more effective in improving FMA score and lowering NIHSS score compared to conventional treatment in patients with poststroke hemiplegia, exerting the effectiveness of acupuncture [13–15]. In this study, we found significant improvements in the FMA score (FMA-upper, FMA-lower, and FMA total) and NIHSS score in the TATG group after treatment, which is in accordance with previous studies.
4.2. Acupuncture Alters Brain's Dynamic Functional Network Connectivity
After treatment, the TATG group showed a reduced mean dwell time in state 3, as manifested sparse connections within and between brain functional networks. Previous studies demonstrated that such weakly connected state resembled stationary functional connectivity, representing the average of a great number of additional states that were infrequent to be separated or insufficiently distinct [25]. Furthermore, recent studies suggested that a significantly longer mean dwell time of this weakly connected state was observed in stroke patients compared to healthy individuals and may prevent the recovery of neurological function [29, 44–46]. With disease remission, the mean dwell time in weakly state decreases and tends to normal time [44]. Our correlation analysis revealed that the motor dysfunction improved with a reduction of mean dwell time in state 3. The stroke patients who received Shou Zu Shi Er Zhen treatment displayed a significant decrease in mean dwell time in state 3, indicating the positive effects of acupuncture in ameliorating the abnormal temporal properties. One study considered that a weakly connected pattern was more likely to be altered and converted to another connection pattern or to construct a new function matrix [29]. Hence, we speculate that acupuncture has the potential to enhance such alteration and promote abnormal whole-brain functional connectivity toward normal patterns.
In addition, we found attenuated connectivity between SMN and FPN in state 3 in the TATG group after treatment. SMN is involved in perceptual and motor function, and FPN participates in many cognitive tasks consisting of attention, working memory, and motor control [22, 47]. Several studies report that increased FC between SMN and FPN at earlier time after stroke contributed to complete motor planning and execution by integrating information related to the motor-related goal and spatial information, reflecting a compensation strategy for the damaged motor system [22, 47, 48]. As stroke patients recover, the higher FC between brain networks will be decreased. However, prolonged compensatory brain activity is believed to a maladaptive plasticity, which leads to poorer motor performance [49, 50]. Therefore, acupuncture may facilitate aberrant functional network normalization during stroke rehabilitation.
Besides, we found both fraction time and mean dwell time in state 1 that had an increasing tendency after Shou Zu Shi Er Zhen acupuncture treatment. State 1 was characterized by partially high intranetwork connectivity, as well as low internetwork connectivity with few positive connections. We referred to this state as the relative segregated state, which can be explained by the concept of functional segregation [51, 52]. Functional segregation reflects better cognitive abilities and information processing for specific tasks [53]. Recent evidence shows that the mean dwell time of such state significantly decreased in stroke patients, suggesting reduced functional segregation to be a sign of impaired function [45, 54]. In our study, there was a negative trend between NIHSS score and the mean dwell time in state 1, meaning reduced mean dwell time in segregated state in parallel to worse neurological deficits. Nevertheless, this interpretation should be viewed with caution due to limited statistical power. Notably, the connectivity between DAN and DMN in state 1 was positively correlated with FMA score and was higher in the TATG group after treatment than before. DAN is related to motor attention task, especially in spatial attention and pointing movements [55]. DMN is associated with self-referential and spontaneous cognition, and this network is anticorrelated with DAN in the resting state [56]. Neuroimaging evidence revealed that the enhanced negative connectivity may compensate for decreased cognitive function in Parkinson's disease [57]. Prior studies suggested that acupuncture therapy could help patients improve their cognitive function [58, 59]. Our result showed an increased FC between DAN and DMN after true acupuncture treatment; that is, the negative FC between the two networks decreased. Thus, we presume that acupuncture promotes higher-order cognitive information integration without the need for functional compensation induced by increased negative FC between DAN and DMN. And the result also revealed that decreased FC between DAN and DMN was positively related to good motor recovery, supporting our speculation to some extent.
In terms of sham acupuncture treatment, we found increased mean dwell time, as well as elevated FC within FPN in state 2 after treatment. FPN is mainly involved in the cognitive control process such as attention and memory [60]. Fu et al. approved that FPN was the causal hub and inputted most information from other brain networks in the resting state for stroke patients [22]. Interestingly, after needling stimulation, they found that LFPN converted into the network outputting most information to other brain networks, reflecting that acupuncture may help transmit information by thinking and decision-making. Considering our results, we speculated that sham acupuncture also had a certain effect on brain functional activity, which may help to strengthen communication within FPN to support cognitive process and motor preparation.
4.3. Differences in Functional Connectivity between True and Sham Acupuncture
Acupuncture points are distributed on the meridians, with specific names, definite locations and clear therapeutic ranges. Acupoint specificity is considered the core scientific issue in the practice of acupuncture at the Society for Acupuncture Research International Symposium held in 2007 [31]. Several studies have demonstrated that acupoint stimulation in stroke patients induces significant activation of more brain areas and stronger effective connections between functional networks in comparison to sham acupuncture [61]. We found that the FC value increased between DAN and RFPN in state 1 in the TATG group compared to the SATG group. And further analysis showed that stronger FC between DAN and RFPN was associated with better motor performance. Accordingly, we supposed that true acupuncture may enhance the integration of movement-related and cognitive formation. In terms of temporal properties, there was a trend toward a greater decline in mean dwell time in the weakly connected state (state 3), as well as a larger increase in mean dwell time in the relative segregated state (state 1) in patients treated with true acupuncture compared to sham acupuncture, and these alterations were closely related with motor recovery. Interestingly, the mean dwell time in state 2 increased significantly after sham acupuncture, with elevated FC within FPN in state 2. However, no clinical relevance was observed for these indicators in state 2. Such changes suggest that sham acupoint stimulation also has some impact on brain function but may not be sufficient to achieve therapeutic effects.
4.4. Limitations
This work has several limitations. In this study, we failed to confirm the advantages of true acupuncture from the clinical perspective. Some evidence suggests that sham acupuncture also creates some effects, which may underestimate the true acupuncture treatment effect [11]. And although the sample size met the requirement of a minimum sample size for statistical power in neuroimaging research [62, 63], it was still relatively small for a clinical study. Our neuroimaging results also need to be validated via independent datasets with larger sample sizes. Besides, due to the limitation of the objective conditions, we only performed two weeks of acupuncture intervention for stroke patients, which is not the optimal duration of treatment for rehabilitation. Further studies should provide long-course acupuncture treatment to explore the mechanism underlying how acupuncture promotes brain neuroplasticity. In addition, there is evidence suggesting that stroke severity is related to temporal properties and dynamic connectivity of the brain. In the present study, most patients were mild to moderate, and our conclusions may not be adapted to patients with severe motor dysfunction.
5. Conclusion
This is the first study to investigate the alteration of dynamic brain function induced by acupuncture treatment in stroke patients with motor dysfunction based on dFNC analysis. We identified the relationship between dynamic properties and motor function in poststroke patients. Our findings demonstrated that acupuncture has the potential to modulate abnormal temporal properties and promote the balance of separation and integration of brain function. True acupoint stimulation may have a more positive effect on regulating the brain's dynamic function. Our findings provide additional evidence for exploring the association between acupuncture therapy and neuroplasticity.
Acknowledgments
We are indebted to our patients for generously supporting our study. We would like to thank Dr. Mingsheng Lyu for providing picture processing help and thank Lekai Luo for his guidance in data processing. This work was supported by the Natural Science Foundation of China (Grant No. 81873257) and the Natural Science Foundation of Beijing (Grant No. 7182104).
Data Availability
The data used to support the findings of this study will be available upon reasonable request, which can be directed to the corresponding author.
Ethical Approval
This study has been approved by the ethics committee of Dongzhimen Hospital affiliated to Beijing University of Chinese Medicine (DZMEC-KY-2018-04), Beijing, China. All participants signed informed consent before the beginning of this study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
YHW wrote the manuscript. YHZ conceptualized and designed the study. MXL, RYL, LPW, and YW collected the clinical and fMRI data. YHW and KSL analyzed the fMRI data. MXL, KW, and CC completed the evaluation of the clinical scale. TZC, LLX, and XYS performed the acupuncture manipulation. Yahui Wang, Mengxin Lu, and Ruoyi Liu contributed equally to this work and share the first authorship.
Supplementary Materials
The supplementary materials provide more detailed information. The probability map and detailed information of the lesion location in each patient are shown in Supplementary Material 1. The location of all acupoints is also shown in Supplementary Material 1. The results of validation analysis are shown in Supplementary Material 2.
References
- 1.GBD. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Neurology . 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L., Peng B., Zhang H., Wang Y. Brief report on stroke prevention and treatment in China, 2020. Chinese Journal of Cerebrovascular Diseases . 2022;19:136–144. [Google Scholar]
- 3.Mayo N. E., Wood-Dauphinee S., Ahmed S., et al. Disablement following stroke. Disability and Rehabilitation . 1999;21(5-6):258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- 4.Kim W. S., Cho S., Ku J., et al. Clinical application of virtual reality for upper limb motor rehabilitation in stroke: review of technologies and clinical evidence. Journal of Clinical Medicine . 2020;9(10):p. 9. doi: 10.3390/jcm9103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinkensmeyer D. J., Boninger M. L. Technologies and combination therapies for enhancing movement training for people with a disability. Journal of Neuroengineering and Rehabilitation . 2012;9(1):p. 17. doi: 10.1186/1743-0003-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gittler M., Davis A. M. Guidelines for adult stroke rehabilitation and recovery. Journal of the American Medical Association . 2018;319(8):820–821. doi: 10.1001/jama.2017.22036. [DOI] [PubMed] [Google Scholar]
- 7.Pan J. X., al ZNZe P. L. J. Strategic thinking of evidence-based medicine in promoting the internationalization of acupuncture and moxibustion from the perspective of belt and road initiative. Chinese Journal of traditional Chinese Medicine . 2020;35:3287–3289. [Google Scholar]
- 8.Yang A., Wu H. M., Tang J. L., et al. Acupuncture for stroke rehabilitation. Cochrane Database of Systematic Reviews . 2016;2016(8):p. CD004131. doi: 10.1002/14651858.CD004131.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen J., Chen X., Yang Y., et al. Acupuncture medical therapy and its underlying mechanisms: a systematic review. The American Journal of Chinese Medicine . 2021;49(1):1–23. doi: 10.1142/S0192415X21500014. [DOI] [PubMed] [Google Scholar]
- 10.NIH Consensus Conference. Acupuncture. JAMA . 1998;280(17):1518–1524. doi: 10.1001/jama.280.17.1518. [DOI] [PubMed] [Google Scholar]
- 11.Fei Y. T., Cao H. J., Xia R. Y., et al. Methodological challenges in design and conduct of randomised controlled trials in acupuncture. BMJ . 2022;376, article e064345 doi: 10.1136/bmj-2021-064345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medicine BhoTC. 'Gold Needle' WangLeting . Beijing: Beijing Publishing House; 1984. [Google Scholar]
- 13.Sun Z. Clinical observation of cerebral infarction treated with shou zu shi er zhen in 150 cases. China Modern Medicine . 2009;16:82–83. [Google Scholar]
- 14.Li H., Tian L., Du X. Wang's 12 needle acupuncture combined with Western medicine for 30 cases of hemiplegia after ischemic stroke. Traditional Chinese Medicinal Research . 2016;29:56–58. [Google Scholar]
- 15.Wang X., Zhang H., Ding X., Yangyang The effect of “hands and feet twelve acupuncture” on the quality of life of patients. World Journal of Integrated Traditional and Western Medicine . 2020;15:1684–1687. [Google Scholar]
- 16.Zhang J., Li Z., Li Z., et al. Progress of acupuncture therapy in diseases based on magnetic resonance image studies: a literature review. Frontiers in Human Neuroscience . 2021;15, article 694919 doi: 10.3389/fnhum.2021.694919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z., Chen S., Li Y., et al. Comparison of sensory observation and somatosensory stimulation in mirror neurons and the sensorimotor network: a task-based fMRI study. Frontiers in Neurology . 2022;13, article 916990 doi: 10.3389/fneur.2022.916990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y., Chen J. Q., Lai X. S., et al. Lateralisation of cerebral response to active acupuncture in patients with unilateral ischaemic stroke: an fMRI study. Acupuncture in Medicine . 2013;31(3):290–296. doi: 10.1136/acupmed-2012-010299. [DOI] [PubMed] [Google Scholar]
- 19.Li M. K., Li Y. J., Zhang G. F., et al. Acupuncture for ischemic stroke: cerebellar activation may be a central mechanism following Deqi. Neural Regeneration Research . 2015;10(12):1997–2003. doi: 10.4103/1673-5374.172318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaechter J. D., Connell B. D., Stason W. B., et al. Correlated change in upper limb function and motor cortex activation after verum and sham acupuncture in patients with chronic stroke. Journal of Alternative and Complementary Medicine . 2007;13(5):527–532. doi: 10.1089/acm.2007.6316. [DOI] [PubMed] [Google Scholar]
- 21.Chen X., Zhang H., Zou Y. A functional magnetic resonance imaging study on the effect of acupuncture at GB34 (Yanglingquan) on motor-related network in hemiplegic patients. Brain Research . 2015;1601:64–72. doi: 10.1016/j.brainres.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Fu C. H., Li K. S., Ning Y. Z., et al. Altered effective connectivity of resting state networks by acupuncture stimulation in stroke patients with left hemiplegia: a multivariate granger analysis. Medicine (Baltimore) . 2017;96(47, article e8897) doi: 10.1097/MD.0000000000008897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L. Mechanism based on bilateral cerebral connections of acupuncture effect on hemiplegia after cerebral infarction . Beijing University of Chinese Medcine; 2020. [DOI] [Google Scholar]
- 24.Hutchison R. M., Womelsdorf T., Allen E. A., et al. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage . 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen E. A., Damaraju E., Plis S. M., Erhardt E. B., Eichele T., Calhoun V. D. Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex . 2014;24(3):663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang C., Glover G. H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage . 2010;50(1):81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y., Zhang W., Li Y., et al. Acupuncture treatment decreased temporal variability of dynamic functional connectivity in chronic tinnitus. Frontiers in Neuroscience . 2022;15, article 737993 doi: 10.3389/fnins.2021.737993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y., Kang Y., Luo S., et al. The cumulative therapeutic effect of acupuncture in patients with migraine without aura: evidence from dynamic alterations of intrinsic brain activity and effective connectivity. Frontiers in Neuroscience . 2022;16, article 925698 doi: 10.3389/fnins.2022.925698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonkhoff A. K., Espinoza F. A., Gazula H., et al. Acute ischaemic stroke alters the brain's preference for distinct dynamic connectivity states. Brain . 2020;143(5):1525–1540. doi: 10.1093/brain/awaa101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L., Geng H., Lu M., et al. Acupuncture for poststroke hemiplegia focusing on cerebral bilateral connections: study protocol for a randomised controlled neuroimaging trial. BMJ Open . 2020;10(4, article e034548) doi: 10.1136/bmjopen-2019-034548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing J. J., Zeng B. Y., Li J., Zhuang Y., Liang F. R. Acupuncture point specificity. International Review of Neurobiology . 2013;111:49–65. doi: 10.1016/B978-0-12-411545-3.00003-1. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z., Liu Y., Xu H., et al. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence: a randomized clinical trial. Journal of the American Medical Association . 2017;317(24):2493–2501. doi: 10.1001/jama.2017.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan C. G., Wang X. D., Zuo X. N., Zang Y. F. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics . 2016;14(3):339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- 34.Bell A. J., Sejnowski T. J. An information-maximization approach to blind separation and blind deconvolution. Neural Computation . 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 35.Erhardt E. B., Rachakonda S., Bedrick E. J., Allen E. A., Adali T., Calhoun V. D. Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping . 2011;32(12):2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo B. T., Krienen F. M., Sepulcre J., et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology . 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordes D., Haughton V. M., Arfanakis K., et al. Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology . 2000;21(9):1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y., Wu K., Hu X., et al. Altered effective connectivity of resting-state networks by tai chi chuan in chronic fatigue syndrome patients: a multivariate granger causality study. Frontiers in Neurology . 2022;13, article 858833 doi: 10.3389/fneur.2022.858833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leonardi N., Van De Ville D. On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage . 2015;104:430–436. doi: 10.1016/j.neuroimage.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Wang S., Cai H., Cao Z., et al. More than just static: dynamic functional connectivity changes of the thalamic nuclei to cortex in Parkinson's disease with freezing of gait. Frontiers in Neurology . 2021;12, article 735999 doi: 10.3389/fneur.2021.735999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marusak H. A., Calhoun V. D., Brown S., et al. Dynamic functional connectivity of neurocognitive networks in children. Human Brain Mapping . 2017;38(1):97–108. doi: 10.1002/hbm.23346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchison R. M., Morton J. B. Tracking the brain’s functional coupling dynamics over development. The Journal of Neuroscience . 2015;35(17):6849–6859. doi: 10.1523/JNEUROSCI.4638-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Wang L., Lu M., Wang Y., Zou Y. Exploring the clinical value and effect mechanism of hand-foot twelve needles in the treatment of stroke based on the theory of “harmonization of form and spirit”. Journal of Traditional Chinese Medicine . 2022;63:327–331. [Google Scholar]
- 44.Xiao R., Zuo L., Zhou Y., Chen Y. Functional magnetic resonance imaging study on the changes of dynamic brain functional. Chinese Journal of Stroke . 2021;16:996–1005. [Google Scholar]
- 45.Wang Y., Wang C., Miao P., et al. An imbalance between functional segregation and integration in patients with pontine stroke: a dynamic functional network connectivity study. NeuroImage: Clinical . 2020;28, article 102507 doi: 10.1016/j.nicl.2020.102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonkhoff A. K., Schirmer M. D., Bretzner M., et al. Abnormal dynamic functional connectivity is linked to recovery after acute ischemic stroke. Human Brain Mapping . 2021;42(7):2278–2291. doi: 10.1002/hbm.25366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam T. K., Dawson D. R., Honjo K., et al. Neural coupling between contralesional motor and frontoparietal networks correlates with motor ability in individuals with chronic stroke. Journal of the Neurological Sciences . 2018;384:21–29. doi: 10.1016/j.jns.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Inman C. S., James G. A., Hamann S., Rajendra J. K., Pagnoni G., Butler A. J. Altered resting-state effective connectivity of fronto-parietal motor control systems on the primary motor network following stroke. NeuroImage . 2012;59(1):227–237. doi: 10.1016/j.neuroimage.2011.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei C., Ni J., Shan C. Progress in the study of mechanisms of brain area recovery and functional connectivity after motor impairment in stroke. Chinese Journal of Rehabilitation Medicine . 2018;33:239–243. [Google Scholar]
- 50.Dromerick A. W., Lang C. E., Birkenmeier R., Hahn M. G., Sahrmann S. A., Edwards D. F. Relationships between upper-limb functional limitation and self-reported disability 3 months after stroke. Journal of Rehabilitation Research and Development . 2006;43(3):401–408. doi: 10.1682/jrrd.2005.04.0075. [DOI] [PubMed] [Google Scholar]
- 51.Friston K. J. Functional and effective connectivity: a review. Brain Connectivity . 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 52.Wang R., Liu M., Cheng X., Wu Y., Hildebrandt A., Zhou C. Segregation, integration, and balance of large-scale resting brain networks configure different cognitive abilities. Proceedings of the National Academy of Sciences of the United States of America . 2021;118(23) doi: 10.1073/pnas.2022288118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wig G. S. Segregated Systems of human brain networks. Trends in Cognitive Sciences . 2017;21(12):981–996. doi: 10.1016/j.tics.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 54.Siegel J. S., Seitzman B. A., Ramsey L. E., et al. Re-emergence of modular brain networks in stroke recovery. Cortex . 2018;101:44–59. doi: 10.1016/j.cortex.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mengotti P., Käsbauer A. S., Fink G. R., Vossel S. Lateralization, functional specialization, and dysfunction of attentional networks. Cortex . 2020;132:206–222. doi: 10.1016/j.cortex.2020.08.022. [DOI] [PubMed] [Google Scholar]
- 56.Raichle M. E. The brain's default mode network. Annual Review of Neuroscience . 2015;38(1):433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 57.Yu Q., Li Q., Fang W., et al. Disorganized resting-state functional connectivity between the dorsal attention network and intrinsic networks in Parkinson's disease with freezing of gait. The European Journal of Neuroscience . 2021;54(7):6633–6645. doi: 10.1111/ejn.15439. [DOI] [PubMed] [Google Scholar]
- 58.He W., Li M., Han X., Zhang W. Acupuncture for mild cognitive impairment and dementia: an overview of systematic reviews. Frontiers in Aging Neuroscience . 2021;13, article 647629 doi: 10.3389/fnagi.2021.647629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou L., Wang Y., Qiao J., Wang Q. M., Luo X. Acupuncture for improving cognitive impairment after stroke: a meta-analysis of randomized controlled trials. Frontiers in Psychology . 2020;11, article 549265 doi: 10.3389/fpsyg.2020.549265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Witt S. T., van Ettinger-Veenstra H., Salo T., Riedel M. C., Laird A. R. What executive function network is that? An image-based meta-analysis of network labels. Brain Topography . 2021;34(5):598–607. doi: 10.1007/s10548-021-00847-z. [DOI] [PubMed] [Google Scholar]
- 61.Liu L., Li X., Wang F., Ji Y., Qu B., Cao D. Activating the brain fMRI cerebral functional imaging by needling gall bladder channel of foot- Shaoyang meridian point and he sea point. Acta Chinese Medicine and Pharmacology . 2014;42:74–77. [Google Scholar]
- 62.Szucs D., Ioannidis J. P. Sample size evolution in neuroimaging research: an evaluation of highly-cited studies (1990-2012) and of latest practices (2017-2018) in high-impact journals. Neuroimage . 2020;221, article 117164 doi: 10.1016/j.neuroimage.2020.117164. [DOI] [PubMed] [Google Scholar]
- 63.Desmond J. E., Glover G. H. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. Journal of Neuroscience Methods . 2002;118(2):115–128. doi: 10.1016/S0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary materials provide more detailed information. The probability map and detailed information of the lesion location in each patient are shown in Supplementary Material 1. The location of all acupoints is also shown in Supplementary Material 1. The results of validation analysis are shown in Supplementary Material 2.
Data Availability Statement
The data used to support the findings of this study will be available upon reasonable request, which can be directed to the corresponding author.


