Abstract
In recent years, there has been a significant increase in the incidence of depression, which is related to, inter alia, the COVID-19 pandemic. Depression can be fatal if not treated or treated inappropriately; is the main cause of suicide attempts. The disease is multifactorial, and pharmacotherapy often fails to bring satisfactory results. Therefore, more and more importance is attached to the natural healing substances and nutrients contained in food. They can significantly affect the process of therapy and prevention of depressive disorders. A proper diet plays a very important role in the prevention of depression and can be a valuable addition to psychological and pharmacological treatment. In turn, an inadequate diet may reduce the effectiveness of antidepressants or may increase their side effects, leading to life-threatening symptoms. The work is a review of the literature on the pathogenesis of the development and treatment of depression, with particular emphasis on dietary supplements and the role of nutrition in the prevention and treatment of depressive disorders.
Keywords: depression, nutrition in depression, diet in depression, antidepressants, dietary supplements
Introduction
Depression is one of the most common mental illnesses. A significant increase in the incidence of this disease has been noted in recent years, which is related to the COVID-19 pandemic [1]. This disease has a significant impact on the deterioration of the quality of life of patients and also hinders their functioning in society. Untreated, it can also lead to death, as it is a major cause of suicidal attempts [2]. People suffering from the so-called major depressive disorder (MDD) are prone to relapses of depressive episodes throughout life. If remission is not observed for a minimum of two months over at least two years, it is termed persistent depressive disorder or dysthymia. On the other hand, when a patient has symptoms of depression, but these symptoms are mild, it is known as subthreshold depressive symptoms. In this case, it is important to take care to not let it turns into a major depression [3].
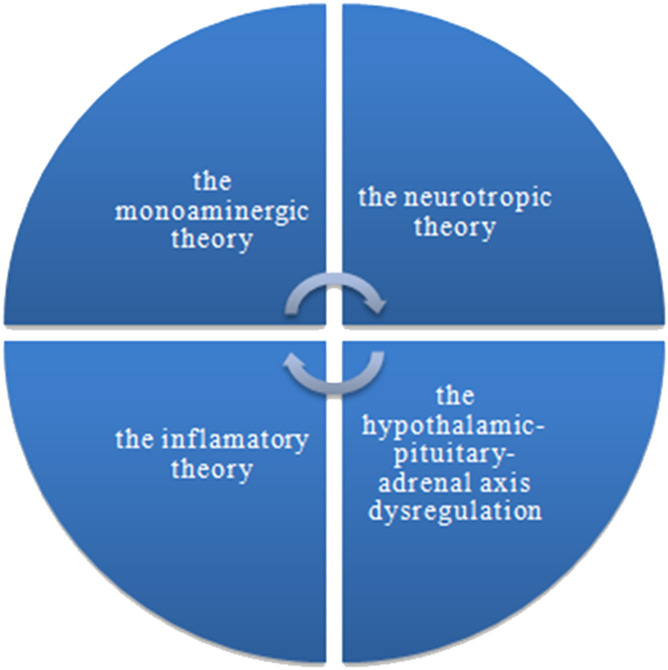
According to the Global Health Data Exchange (GDHx) catalog maintained by the Institute of Health Metrics and Evaluation, it is estimated that currently, about 280 million people worldwide suffer from depression, which is 3.8% of the total population. The data show that depression was diagnosed in 5% of people over 20 years of age, and in 5.7% of people over 60 years of age [4]. It is twice as common in women and often has a slightly different course [5]. Depression is a disease that requires drug treatment. Pharmacotherapy is effective in approximately 30% of patients. However, many patients experience relapses of depression and treatment modification is then required [6]. It is impossible to clearly define the causes and mechanism of depression, taking into account the studies conducted so far [3]. It is a complex disease with a multifactorial background [3, 7, 8]. There are several theses in the literature trying to explain the underlying causes of depression. The biological theories include, among others, the monoaminergic theory, the neurotrophic theory, the theory indicating dysregulation within the hypothalamic-pituitary-adrenal axis (HPA), and the inflammatory theory (Figure 1 ). Currently, the phenomenon of epigenesis is also of great importance. Whereas, psychological concepts include psychodynamic theories and theories of cognitive psychology. There are also attempts to holistically explain the causes of depression by integrating the above-mentioned theories [9].
Figure 1.
The most popular hypotheses of depression pathophysiology.
Depression is multifactorial desease. This is one of the reasons why pharmacotherapy of depressive disorders very often does not provide satisfactory results - treatment is ineffective and antidepressants have many side effects. Importantly, many patients suffer from drug-resistant depression for which currently available drugs are ineffective [3]. Therefore, in the fight against depression, other forms supporting pharmacological treatment are also used, such as psychotherapy, yoga, aromatherapy, and music therapy. In this context, what is significant, in recent years, more and more importance has been attached to natural medicinal substances as well as to food ingredients and the role of diet in the prevention and treatment of depressive disorders. They can significantly affect the process of therapy, or prevention of depressive disorders. An adequate diet can be a valuable complement to treatment or can modify the effects of antidepressants. In turn, improper nutrition may be one of the factors involved in the pathological mechanism of depression [10, 11]. Therefore, the aim of this paper is to review the literature on the pathogenesis and treatment of depression, with particular emphasis on dietary supplements and the role of nutrition in the prevention and treatment of depressive disorders.
Pathogenesis of depression
The monoamine theory
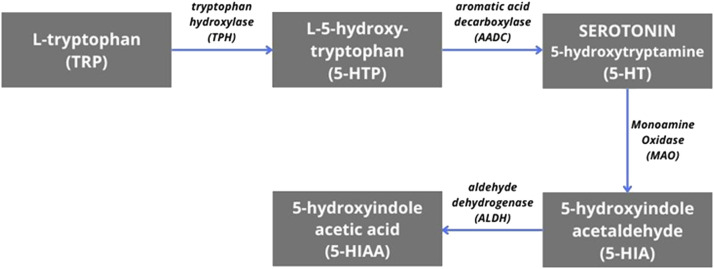
Already in the 1950s, it was observed that reserpine used in hypertension may induce depression in a certain group of patients [12]. A mentioned side effect of reserpine is related to the use of central monoamine stores. According to this theory, the cause of depression is a chemical imbalance caused by deficits in the neurotransmission of serotonin, norepinephrine, and dopamine - substances that affect mood, sleep, or appetite [13]. This theory may be confirmed by the effectiveness of tricyclic antidepressants and monoamine oxidase inhibitors, which enhance these transmissions [3, 9].The greatest role in the pathogenesis of depression is attributed to serotonin. Research indicates that patients diagnosed with depression have increased levels of L-tryptophan, its precursor. The concentration of the main metabolite of serotonin (5-Hydroxyindoleacetic acid, 5-HIAA) (Figure 2 ) in the cerebrospinal fluid was lower, especially in suicidal patients. Moreover, it has also been shown that lowering serotonin levels can cause recurrent depressive episodes in patients in remission of the disease [9]. However, this model does not explain many aspects of the disease, such as the variability of the clinical picture, and the ineffectiveness of the above-mentioned groups of drugs among some patients. It also does not answer the question of why these drugs have a delay of several weeks in their action [3].
Figure 2.
The biochemical pathway of serotonin synthesis and metabolism.
The neurotrophic theory
The studies carried out so far indicate a significant link between the occurrence of depression and abnormal processes in the prefrontal cortex (PFC) and the limbic system involving neuroplasticity disorders, which means the ability of neurons to reorganize as a result of environmental changes. The key factors that influence this remodeling are the so-called neurotrophins, which can bind to tyrosine kinase receptors. The best-known and most important factor in the phenomenon of neuroplasticity in the entire nervous system is BDNF (brain-derived neurotrophic factor) [14, 15]. The results of the researches indicate a decreased expression of BDNF in the limbic system and PFC in patients suffering from depression and an increase in this expression after the administration of antidepressants. This effect has been confirmed in animal models as well as in post-mortem studies of depressed people and suicides. Moreover, studies with fast-acting antidepressants such as ketamine indicate that the activity-dependent increased release of BDNF following the administration of ketamine is the basis of its rapid therapeutic response. Recent studies indicate that the presence of vascular endothelial growth factor (VEGF), which interacts with BDNF signaling, is also important. The action of BDNF was also confirmed by direct infusion of BDNF into the hippocampus or PFC, which resulted in an antidepressant effect after the first administration [16–18].
Participation of the hypothalamic-pituitary-adrenal axis
The hypothalamic-pituitary-adrenal axis (HPA) is the connection of these three organs, which is the result of feedback. In response to stress, this axis activates the sympathetic nervous system. The stress factor triggers the transmission of information to the paraventricular nuclei of the hypothalamus, initiating the functioning of the axis that regulates the stress response [19]. The first stage is the corticotropin-releasing hormone, which triggers production in the pituitary anterior lobe and transfers the secretion of the adrenocorticotropic hormone to the adrenal cortex together with the blood, producing glucocorticoids such as cortisol. They act on two types of receptors in the hippocampus, the amygdaloid body, and the adenohypophysis. The ratio of mineralocorticoid receptors to glucocorticoid receptors determines the predisposition for dealing with stress. Chronic stress causes an extremely high production of steroid hormones, which negatively influences neurogenesis and other processes. The regulation occurs while glucocorticoids block the axis by affecting the receptors in the hypothalamus and hippocampus. Chronic stress triggers higher levels of corticotropin-releasing factor. Receptors for corticotropin-releasing factor (CRF) are found not only in the brain but also in the gastrointestinal tract, which has a significant impact on the microbiota. It is also possible that CRF synthesis may take place in intestinal neurons. Stress affects the autonomic system, which affects the enteric nervous system. The released norepinephrine may influence the composition of the mi-crobiome. Chronic stress slows the anti-inflammatory effect of the vagus nerve on the intestine, which primarily controls the enteric nervous system (ENS). This also leads to an increase in pro-inflammatory cytokines [19, 20].
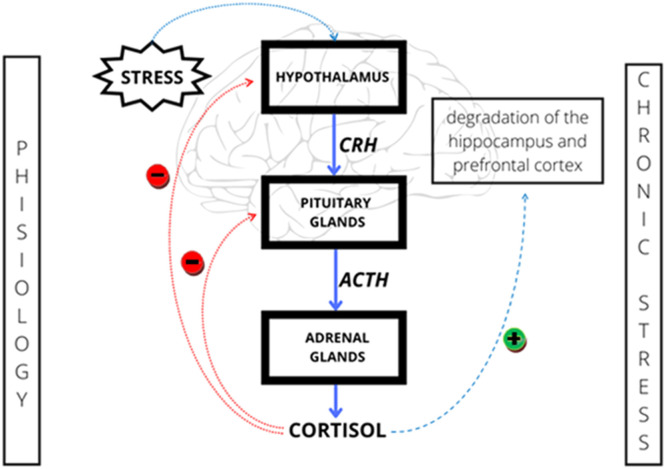
Disturbance in the functioning of the HPA axis may contribute to the development of many diseases of the nervous system [21, 22]. Its increased activity is observed, among others, during chronic stress. Research indicates that high childhood stress may induce permanent changes in the HPA axis adaptation to environmental changes and increase susceptibility to depression throughout life [23]. Stress disrupts the body's homeostasis by releasing a hormone that releases corticoliberin (CRH) from the hypothalamus. Adrenocorticotropic hormone (ACTH) is then secreted from the anterior pituitary gland and stimulates the release of cortisol into the bloodstream. The blood cortisol level is regulated by a negative feedback phenomenon and as a result, the above described reaction cascade is inhibited (Figure 3 ). Excessive secretion of cortisol in response to chronic stress may lead to a decreased sensitivity to glucocorticoids and a reduction in the efficiency of this regulation mechanism. Increased cortisol levels can cause the degradation of neurons in areas of the brain such as the hippocampus and prefrontal cortex, which can lead to depressive symptoms. In the conducted study, patients suffering from depression had significantly higher levels of cortisol than those in the control group [24]. A growing body of research suggests that intestinal microbiota may have a direct impact on the activity of the HPA axis. Microbiome plays an important role in the organization of the HPA axis, especially in childhood, which may affect the body's response to stressful situations in the future [25]. The microbiota influences the synthesis of neurotransmitters and the myelination of neurons in the prefrontal cortex (PFC). It is also involved in the development of the hippocampus and the amygdala. Its disturbance may also result in vitamin deficiencies, which may reduce the effectiveness of antidepressant treatment and exacerbate symptoms of the disease [26].
Figure 3.
Regulation of hypothalamic-pituitary-adrenal axis activity
Inflammatory theory
Chronic inflammation can correlate with poor physical and mental health in patients [27]. Long-term stress can result in the over-activation of pro-inflammatory factors that are capable of crossing the blood-brain barrier and activating microglia. As a consequence, neurodegenerative changes may occur, which are manifested, among others, by depressive disorders [28]. Released pro-inflammatory cytokines may also lead to dysregulation of the HPA axis mechanism, causing hypercortisolemia and contributing to the activation of the tryptophan-kynurenine (KYN) pathway. This triggers the synthesis of neurotoxins such as the N-methyl-d-aspartate (NMDA) agonist, quinolinic acid, and 3-hydroxykynurenine leading to the enhancement of oxidative stress and neurodegeneration [29]. Activation of the kynurenine pathway leads to a deficiency of serotonin and melatonin, which may also result in a depressed mood [30]. It is difficult to clearly determine whether neuroinflammation is the cause or the result of dysregulation of the HPA axis. Probably both of them affects the other, and the irregularities of these systems drive each other [28].
Microbiome-gut-brain axis
In recent years, more and more scientific studies have focused on the impact of the intestinal microbiome on health [31, 32]. The intestinal microbiome has a strong effect on the central nervous system, but it is a bilateral relationship because CNS regulates the work of intestines and secretion. The tryptophan mentioned above is metabolized by bacteria into metabolites in the intestine [20]. This occurs in three different ways, which lead to the synthesis of serotonin, kynurenine or indole. In stress situations, serotonin synthesis is reduced to kynurenine, a substrate for the formation of neuron-protective kynurenic acid, or quinoline acid, which has a neurotoxic effect. Lower microbiota may be observed in patients with known depression, a similar effect we could see in chronic stress [19].
Short-chain fatty acids (SCFAs) are the main communication mechanism between the intestine and the brain [33]. They regulate the process of serotonin synthesis in the intestine by pheochromocytoma. Short-chain fatty acids cross the blood-brain barrier to regulate microglia. The reduced continuity of the intestinal barrier may cause bacteria and metabolites to enter the bloodstream, from where it is close to the excessive synthesis of pro-inflammatory cytokines [34].This results in a predominance of the tryptophan kynurenine pathway, and thus much less serotonin is synthesized [20].
The intricate bidirectional communication between the gut microbiota and the central nervous system has gained increasing recognition in recent years [35]. The emerging field of psychobiotics explores the potential of certain probiotic strains and prebiotics to modulate the gut-brain axis and impact mental health.Psychobiotics are products that modify the composition of the microbiome. Psychobiotics, i.e. are non-digestible hydrocarbons, favoring the growth of bacteria in the intestines disturbed by dysbiosis, can modify the composition of the in-testinal microbiome. The main strains showing such an effect are Lactobacillus helveticus, and Lactobacillus rhamnosus, Bifidobacterium longum. Improvement of mood and alleviation of depression symptoms is, however, only noticeable in sick people, healthy participants of the research do not feel the improvement in well-being as a result of taking psychobiotics. As a result of the disease in question, the condition of the microbiome also deteriorates, so taking probiotics also aims to rebuild it. Nevertheless, the benefits of improving the function of the HPA axis are still believed to be the main reason for the use of probiotics [26]. In the literature is available the systematic review and meta-analysis to evaluate the effects of psychobiotics on depressive symptoms. Their study included randomized controlled trials (RCTs) that investigated the use of probiotics or prebiotics in individuals with depression. The findings suggested a modest but significant reduction in depressive symptoms with psychobiotic supplementation [36]. Another review emphasized the role of dysbiosis, an imbalance in gut microbial composition, in contributing to anxiety symptoms. There is discussed the potential of psychobiotics in modulating the gut microbiota and reducing anxiety. Furthermore, the authors underscored the need for further research to establish the specific strains and mechanisms involved in the anxiolytic effects of psychobiotics [37].
In 2021, the results of a study were published that investigated the potential of psychobiotics in improving cognitive function and reducing cognitive decline. The scientists investigated the potential of psychobiotics in improving depressive symptoms and overall well-being. Their study focused on the effects of probiotics on individuals with depression. The inclusion criteria for the studies required the assessment of subjects' mood or stress levels at the beginning of the study, followed by intervention through the consumption of probiotics, prebiotics, and/or synbiotics. The findings suggested that certain psychobiotics had a positive impact on depressive symptoms and could potentially serve as adjunctive treatments for depression. The study highlights the promising role of psychobiotics in the management of depression [38].
It is worth to mention about a comprehensive reviews of the effects of psychobiotics on depression and related factors [39]. Analysis encompassed preclinical and clinical studies, elucidating the mechanisms through which psychobiotics influence depression. The review discussed the modulation of neurotransmitter systems, inflammatory pathways, and the gut-brain axis. Overall, the findings suggested that psychobiotics may exert antidepressant effects, offering potential therapeutic avenues for depression [39]. Palepu et al. examined the role of psychobiotics in the management of depression. Their systematic review evaluated studies investigating the effects of psychobiotics on depressive symptoms and gut microbiota composition in individuals with depression. The review demonstrated preliminary evidence supporting the use of psychobiotics as adjunctive therapeutic approaches in depression, with improvements in depressive symptoms. However, further well-designed studies are needed to establish the efficacy and safety of psychobiotics in depression [40]. The findings underscore the significance of the gut-brain axis and highlight psychobiotics as a promising area of research for developing innovative interventions in depression. Further research is necessary to elucidate the mechanisms of action, optimal strains, dosages, and long-term effects of psychobiotics, ultimately paving the way for personalized psychobiotic interventions in depression.
Epigenetic theory
Severe childhood stress can significantly increase the risk of depression and even double the risk of longer and more long-lasting and difficult to treat depressive episodes compared to patients with depression without such a history [41]. In addition, it has been reported that up to a quarter to a third of abused children will experience severe depression in adulthood [23]. One of the mechanisms involved in this phenomenon may be epigenetic changes. They consist of the modification of the DNA structure and gene expression and, consequently, changes in behavior [42]. The human genome contains about 25,000 genes, and only part of them are expressed. Epigenetic changes are responsible for which genes are active. These transformations include DNA methylation, modification of histones and chromatin structure, and regulation by non-coding RNA. As a result, different phenotypes can arise on the basis of the same genome [43, 44]. Epigenetic mechanisms are sensitive to environmental changes, including strong emotional sensations and stress. These changes may potentially affect the hormonal changes of the fetus and result in susceptibility to depression for up to several generations [45]. Evidence of the association of epigenetic changes with the occurrence of depression may be an increase in marks in the brain and white cells of people who experienced childhood trauma. For example, this may result in a reduction in the number of glucocorticoid receptors in the hippocampus, which may predispose people to reduced stress resistance and increase the risk of suicidal thoughts [23].
It should be noticed that depression is a multifactorial and complex disease. None of the above-mentioned theories fully explains the mechanism of its pathophysiology. These theories complement each other, overlap, and influence each other, which is why a holistic approach to the problem of depression may be considered. It is difficult to clearly define what is the cause and what is the effect of these phenomena. For example, disturbance of the intestinal microflora may, by altering the permeability of the intestinal barrier, activate inflammatory factors, influence the release of monoamines, activate the HPA axis, and modify the release of BDNF, resulting in depression, affecting all the above-mentioned potential path-ways of its formation [25].
Treating Depression
Pharmacotherapy of depression
Tricyclic antidepressants (TCAs)
Tricyclic antidepressants are not used as a first-line treatment for major depression. TCAs have similar effectiveness in the treatment of major depression as SSRIs, but they cause more significant adverse effects. It is caused, by their anticholinergic activity and also a narrow therapeutic index. TCAs ingestion of 10-20 mg/kg is the situation potentially life-threatening [46, 47]. TCAs have a complex mechanism of action. They act on many (approximately 5) neurotransmitter pathways, which determines their therapeutic effects. First, they block the reuptake of serotonin and norepinephrine in the presynaptic terminals, increasing the concentration of these neurotransmitters in the synaptic cleft, and leading to the antidepressant effect. Another pathway of action of these drugs is the competitive antagonism of alpha-cholinergic, muscarinic and histaminergic receptors. The activity against these receptors depends on the structure of TCAs [48, 49]. Among the TCAs, a tricyclic form with a secondary or tertiary amine residue attached to it can be distinguished. Secondary amines have a lower tendency to block serotonin reuptake compared to tertiary amines, but in turn, they tend to more block norepinephrine reuptake [50]. The side effects that appear as a result of the use of TCAs are related to the structure of these drugs [51].The secondary amines include desipramine, nortriptyline, and protriptyline, while the tertiary amines include amitriptyline, clomipramine, doxepin, imipramine, and trimipramine. Due to their narrow therapeutic index, these groups of drugs are more commonly used by patients for suicidal purposes than other classes of antidepressants. Overdosing on older generations of TCAs like desipramine, nortriptyline, and trimipramine is easier than overdosing on newer generations like amitriptyline because of their greater toxicity [46]. Patients taking TCAs should be closely monitored for signs of toxicity such as widening of the QRS complex on the electrocardiogram (ECG), tremors, confusion and muscle stiffness [50]. When using TCAs, the patient should be careful about interactions with monoamine oxidase inhibitors (MAOIs) due to the risk of developing serotonin syndrome, which is an immediate life threat [52].
Monoamine oxidase inhibitors (MAOIs)
Monoaminoxidase is an enzyme that breaks down various neurotransmitters - serotonin, dopamine, tyramine, and norepinephrine. Monoamine oxidase inhibitors block the breakdown of these neurotransmitters, thus increasing their level in the brain, and showing an antidepressant effect [53]. Two types of monoamine oxidase are known: A and B. MAO-A is found mainly in the placenta, intestines, and liver, its substrates are serotonin, and noradrenaline. MAO-B is present in the brain, liver, and platelets. Its substrates are phenylethylamine, methylhistamine, and tryptamine. Tyramine and dopamine are degradation targets for both types of the enzyme [54]. This group of antidepressants is a last-line treatment for depression. The use of MAOIs potentially predisposes the patient to drug-drug and drug-food interactions. Patients should not use MAOIs with other classes of antidepressants such as SSRIs, SNRIs, TCAs, bupropion, mirtazapine, St. John's wort, and sympathomimetic amines (including stimulants) as serotonin syndrome is highly likely to occur [55]. Also, when switching antidepressant drugs from MAOIs to another group, patients should have a 14-day break between taking drugs [56, 57]. A particularly high risk of serotonin syndrome occurs in patients who use MAOI and drugs such as Tramadol, meperidine, dextromethorphan and methadone simultaneously. This risk is due to the pharmacological effects of these medications on serotonin levels in the brain. [55]. Since MAOIs inhibit the breakdown of tyramine, consumption of food containing high concentrations of this amine should be limited. High serum tyramine levels can cause a sharp increase in blood pressure, which can lead to a hypertensive crisis, leading to cerebral hemorrhage, and death [58, 59]. Tyramine content may increase in stale and deliberately aged foods such as cheese, sausage, and fish [59–61]. High levels of tyramine can also be found in overripe bananas, avocados, and other fruits. Foods high in this amine should be avoided by patients for up to two weeks after stopping the MAOI treatment. Currently, there are three generations of MAOI drugs related to their selectivity and reversibility of their action. First-generation drugs include iproniazide, phenelzine, and tranylcypro-mine. These drugs bind non-selectively to both types of MAO by a covalent bond and destroy the enzyme [62]. They are the most dangerous of the groups. Selegiline is considered a second generation drug. At low doses, it is a selective, irreversible MAO B inhibitor, but no longer selective at higher doses. Its antidepressant effect, is shown only in very high doses, but due to numerous side effects it is not used in the treatment of depression.The third generation drugs - selective and reversible MAO inhibitors - include moclobemide, amiflamine, and toloxatone, which act on MAO-A, and lazabemide, which inhibits MAO-B activity [53].
Serotonin-norepinephrine reuptake inhibitors (SNRIs)
Membrane proteins responsible for the reuptake of serotonin and norepinephrine are SERT (human serotonin transporter) and NET (norepinephrine transporter). It is proved, that balanced inhibition between monoamines reuptake can be better in the treatment of depression than other antidepressant groups, by limiting a wider variety of symptoms [63]. In a systematic review and meta-analysis from 2017 has been proved, that using SNRI by children and adolescents is more effective than a placebo, however occurred severe adverse effects, such as suicidal thoughts and suicide attempts, which appeared mainly in the first weeks of therapy [64]. SNRIs are more useful in the treatment of major depression than SSRIs, when they are used at doses that block both serotonin and noradrenaline reuptake. This this group of antidepressants belongs to venlafaxine, milnacipran, and duloxetine [65].
Serotonin antagonists and reuptake inhibitors (SARIs)
SARIs act as antagonists a 5HT2a receptor and block the function of serotonin transporter protein, thereby increasing its amount throughout in the brain [66]. A representative this antidepressant medications group is trazodone, a triazolopyridine derivative. Trazodone is widely used in psychiatry due to its wide pharmacodynamic profile. It is used in both monotherapy of depressive syndromes as well as in polytherapy to the potentialization activity of other drugs [67]. It is an antagonist of 5-HT2A, 5-HT2C serotonergic receptors, and an antagonist of alpha1 and alpha2 adrenergic receptors, and histaminergic H1 receptors. It is also noted to be a blocker of the SERT transporter at higher doses [68]. In clinical trials, the antidepressant effect was comparable to that of other groups of drugs (TCA, SSRI, SNRI) [69]. The advantage of using trazodone, compared to SSRI drugs, is the lack of side effects, such as sexual dysfunction or weight gain. It is related to a comprehensive effect on the serotonergic system. Additionally, the use of the extended release form reduces the occurrence of side effects. Trazodone is readily used in the elderly, especially in the course of depression with insomnia [70].
Selective serotonin reuptake inhibitors (SSRI)
One of the depression theories is the monoamine hypothesis. Lack of monoamines, especially serotonin, may predispose to severe depression disorder [71]. Unlike other classes of antidepressants, SSRIs don't have a significant effect on other neurotransmitters, such as dopamine or norepinephrine. SSRIs also have fewer side effects than TCAs and MAOIs due to fewer effects on adrenergic, cholinergic, and histaminergic receptors. This is the reason why SSRIs is the group of the most commonly prescribed antidepressants in many countries [72].
The mechanism of action of SSRIs involves inhibiting the reuptake of serotonin, resulting in increased serotonin availability in the synaptic cleft. This ultimately leads to enhanced serotonergic neurotransmission and therapeutic effects. SSRIs selectively bind to the serotonin transporter protein, which is responsible for the reuptake of serotonin from the synaptic cleft back into the presynaptic neuron. By binding to the serotonin transporter, SSRIs competitively block its activity, preventing the reuptake of serotonin. As a result, serotonin remains in the synaptic cleft for an extended period, increasing its concentration and availability for binding to postsynaptic receptors [3, 73].The prolonged presence of serotonin in the synaptic cleft leads to enhanced serotonergic neurotransmission. Serotonin is a key neurotransmitter involved in regulating mood, emotions, and cognition. By inhibiting its reuptake, SSRIs promote an increase in serotonin levels, which can have various effects on different brain regions and circuits. Increased serotonergic transmission is believed to contribute to the antidepressant and anxiolytic properties of SSRIs. Serotonin acts on several different subtypes of receptors, including 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT3 receptors, among others. Increasing the presence time of serotonin in the synaptic cleft due to SSRI inhibition of reuptake leads to increased activation of these postsynaptic receptors. The specific effects on different receptor subtypes can vary and contribute to the therapeutic actions and potential side effects. There are also exert long-term effects on neuronal structure and function. Chronic administration of SSRIs has been shown to promote neuroplasticity, including increased neurogenesis (generation of new neurons) and synaptic remodeling in certain brain regions. These neuroplastic changes are believed to play a role in the delayed onset of therapeutic effects of SSRIs and their long-term benefits in managing psychiatric disorders [74, 75]. This group of drugs includes Fluoxetine, Sertraline, Paroxetine, Escitalopram, Citalopram, Fluvoxamine, Vilazodone, Vortioxetine [76, 77]. Furthermore SSRIs are commonly prescribed as first-line pharmacotherapy for various psychiatric disorders, including anxiety disorders, obsessive-compulsive disorder (OCD), panic disorder, major depressive disorder (MDD), generalized anxiety disorder (GAD), bulimia nervosa, bipolar depression, premenstrual dysphoric disorder (PMDD), treatment-resistant depression, post-traumatic stress disorder (PTSD), and social anxiety disorder [78].
antidepressants
One of the subgroups of antidepressants is tetracyclic antidepressants. Chemically they are similar to the previous group – tricyclic antidepressants. Mirtazapine is an example of this group. It is structurally related to trazodone and mianserin [79]. Mirtazapine has an atypical pharmacological profile. It is a strong antagonist of alpha 2-adrenergic auto- and heteroreceptors This results in presynaptic autoreceptors being blocked, which triggers a cascade of reactions. Autoreceptor blockade enhances presynaptic autoreceptors, and thus increases norepinephrine release [80]. It also shows antagonism towards 5-HT2 and 5-HT3 receptors. Blockade of heteroreceptors on serotonergic neurons increases the release of serotonin [81]. It has a negligible effect on monoamine reuptake, which distinguishes it from venlafaxine and nefazodone. It has a low affinity for muscarinic, cholinergic, and dopaminergic receptors and therefore has fewer side effects. It has quite a high affinity for histamine H1 receptors, which results in a strong sedative and hypnotic effect of the drug [79].
NMDA receptor antagonists
Commercially available NMDA-receptor antagonists include ketamine, dextromethorphan, memantine, and amantadine [82]. The opioid methadone is also an antagonist at the NMDA receptor. A new approach to treating depression involves trying to act on NMDA receptors [83]. Direct stimulation of them may result in faster antidepressant effects. It was observed that a significant proportion of patients after 2 months did not achieve an effective improvement after using a typical antidepressant, in addition, patients struggled with numerous side effects. In an-imal models, NMDA receptor antagonists such as MK-801 (application-dependent channel blocker) and CGP 37849 (NMDA receptor antagonist) deserve considerable attention. In preclinical studies, both alone and in combination with traditional antidepressants, they showed a therapeutic potential . Additionally, ketamine has clinical applications in the treatment of resistant depression [84]. Esketamine is the enantiomer of ketamine, a drug approved by the FDA for the treatment of resistant depression [85].This drug is used as a nasal spray. It should be administered by medical institutions under the guidance of a doctor. Esketamine must be used in conjunction with a conventional antidepressant. Rapid drug action and reduction of depression symptoms are observed, even one week after administration in patients with depression not yet responsive to conventional anti-depressants [86]. Esketamine belongs to NMDA receptor antagonists. Headache, dizziness, and hypertension are the most frequent adverse effects reported [85].
Noradrenergic and specific serotoninergic antidepressant (NaSSA)
NaSSAs enhance adrenergic and serotonergic neurotransmission in the brain by the blockade of α2-adrenergic receptor and certain serotonin receptors such as 5-HT2A and 5-HT2C, 5-HT3, 5-HT6, and/or 5-HT7[59]. Mirtazapine has a novel mechanism of action related to antagonistic effects on alpha-2 autoreceptors and heteroreceptors and 5-HT2 receptors. Consequently, the release of norepinephrine and the indirect release of serotonin is increased. Several studies show that mirtazapine is effective in treating moderately to severely depressed patients and gives an improved condition as early as the first week of treatment [87, 88]. Common adverse effects include sedation, weight gain, dizziness, and less often mirtazapine causes nausea, vomiting, and sexual dysfunction [89, 90].
Serotonin partial agonist and reuptake inhibitors (SPARIs)
A representative of the SPARIs group is vilazodone, which was approved by the FDA in 2011 as a drug to treat major depression [91]. The mechanism of anti-depressive action of vilazodone involves inhibition of serotonin reuptake and agonist activity to the 5-HT1A receptor [92]. This double mechanism of action allows to increasing the concentration of serotonin in the synaptic cleft to a greater extent than in the case of classic antidepressants drugs, which translates into its effectiveness. A steady state is achieved after 3 days of taking the drug [93]. It is worth mentioning that vilazodone doesn't affect noradrenaline and dopamine up-take [89]. The most frequently reported side effects are gastrointestinal dysfunc-tion like diarrhea or nausea, headache, and xerostomia [94]. Vilazodone is well-tolerated and has less impairment of sexual function in patients compared to other antidepressants [95, 96]. Another SPARIs drug is vortioxetine. It is an innovative antidepressant with a multidirectional mechanism of action, used in the treatment of depression and anxiety disorders [68]. Vortioxetine is a SERT inhibitor, an antagonist of 5-HT3, and 5-HT7 receptors, and a partial agonist of 5-HT1b receptors [89]. Compared to duloxetine, it is less effective and it has similar side effects [97]. Nausea and vomiting are the most common [89]. Vortioxetine is an alternative drug and is not of choice for the first-line treatment of depression [97].
Other drugs
Tianeptine is a novel antidepressant, with a structure similar to TCAs. Unlike other drugs, tianeptine increases the reuptake of serotonin [98]. Thus, the action of the drug is not solely based on the monoaminergic theory. Additionally, the medicine normalizes glutamatergic transmission [99]. Tianeptine exhibits therapeutic properties in the treatment of severe depression, anxiety depression and depressive conditions in people who abuse alcohol. Due to the lack of cardiotoxic effects, it can be used in the elderly [98, 100].
Agomelatine is a novel antidepressant with an unique pharmacological profile characterized by agonism at the melatonergic MT1/MT2 receptors and antagonism at the 5-HT2C receptors. Agomelatine is an effectives anti-depressant drug with a quick onset of action. Agomelatine has been approved for the treatment of depression with insomnia due to its capability to regulate the circadian rhythm [101]. Contrary to classic antidepressants, agomelatine does not have a sedative effect and does not lead to weight gain or sexual dysfunction [102]. Agomelatine increases the release of norepinephrine and dopamine, especially in the frontal cortex, but does not affect the extracellular concentration of serotonin. Its therapeutic effectiveness lasts for a long time, so it is recommended to take it once a day before sleep. Dizziness, diarrhea, and feeling tired are among the more common side effects occurring during treatment with agomelatine [103].
Psychotheraphy in depressions treatment
Among the guidelines for the treatment of depression, it is recommended to combine psychotherapy with pharmacological treatment [104, 105]. There are many strands of psychotherapy offered by modern therapists. Among the best-known and researched are psychodynamic psychotherapy (PDP) and cognitive-behavioral therapy (CBT). The psychodynamic approach derives directly from Freudian psychoanalysis. PDP models are based mainly on the relationship between the patient and therapist. According to Gabbard, much of mental life is unconscious, childhood experiences and predispositions of the individual shape the psyche of the mature person [105, 106]. The therapeutic relationship created between patient and therapist is based on the concept of transference, which is concerned with transferring the patient's experiences to the psychotherapist and allowsing him to mature the patient's problems. Cognitive-behavioral psychotherapy is one of the most popular and researched methods of psychotherapy in terms of effectiveness [106]. It derives from behaviorism, the belief that behavioral disorders are the result of learned responses to various stimuli. The goal of therapy is to change abnormal reactions and develop new behaviors and a different way of thinking. Therapy is based on an educational approach - the patient acquires skills that will enable him to solve his problems on his own. Studies have been conducted focusing on demonstrating the effectiveness of psychotherapy in treating depressive disorders [106]. The cited meta-analysis showed that psychotherapy is about as effective as pharmacotherapy for depressive disorders [104]. Another meta-analysis based on 92 different randomized controlled trials (RCTs) showed the effectiveness of psychotherapy compared to pharmacotherapy - equal in the short term and better in the long term in preventing relapse. Different forms of psychotherapy were compared, with no clear differences observed, and if so, with some methodological specificities noted [107]. A comprehensive meta-analysis highlighted the effectiveness of interpersonal psychotherapy (which has its structure and theoretical roots in PDP) in depression compared to other psychotherapies and compared to combination treatment, as well as its role in preventing relapse or relapse after successful treatment, citing its efficacy and comparability with anti-depressant medications [108]. The significance of these findings and the possibility of publication bias have also received attention from the scientific community. A recent analysis found an excess of significant findings relative to what would be expected from studies of the effectiveness of psychotherapy in MDD [109]. Cuijpers et al. published a meta-analysis of the effects of psychotherapy on remission, recovery, and improvement of MDD in adults. The response rate to the psychotherapies analyzed was 48% (vs. 19% in the control condition), and there was no significant difference between the types of psychotherapy [110]. The efficacy of PDP has been demonstrated in various studies comparing it with other treatments. Empirical evidence supporting this efficacy has increased in recent years, and recent meta-analyses have confirmed the role of PDP in the treatment of depressive disorders [108–110].
Literature data indicate that the effectiveness of psychotherapy depends to a large extent on the relationship between the therapist and the patient [107]. Psychodynamic therapy may be at least as effective as CBT in treating depression in other important aspects of patient functioning than reducing depressive symptoms [111]. In the cited study, there were no statistically significant differences between psychodynamic therapy and CBT in a large sample of patients treated for a major depressive episode, and less than a quarter of patients achieved remission within 22 weeks of treatment. Equivalence of psychodynamic therapy versus CBT was demonstrated for mean post-treatment depression scores, but could not be demonstrated for remission rates and follow-up measures [111]. The best results in the treatment of depression, are observed when psychotherapy and pharmacotherapy are combined. In chronic major depression, combination treatment has shown significant advantages over medication or psychotherapy alone [112, 113].
Mind-body medical interventions are commonly used to cope with depression and yoga is one of the most frequently used. Conventional pharmacotherapies and psychotherapies for major depression are associated with limited adherence to care, and relatively low remission rates. Yoga may offer an alternative treatment option, but rigorous studies are few [114]. Although, a systematic review of 19 studies (1,080 participants) and a meta-analysis of 13 studies (632 participants) show that yoga has a positive effect on reducing depressive symptoms. Depressive disorders, post-traumatic stress disorder, schizophrenia, anxiety, alcohol dependence and bipolar disorder were included. Yoga showed a greater reduction in depressive symptoms than waitlist, treatment as usual, and attention control (standardized mean difference=0.41; 95% CI -0.65 to -0.17; p<0.001). Greater reductions in depressive symptoms were associated with a greater frequency of yoga sessions per week (β=-0.44, p<0.01) [114, 115]. In addition, there are studies indicating a reduction in depressive symptoms in people who use music therapy. Music therapy provides short-term beneficial effects for people with depression. Music therapy added to treatment as usual (TAU) appears to alleviate symptoms of depression compared to TAU alone [116]. Additionally, music therapy plus TAU is not associated with more or fewer adverse events than TAU alone. Music therapy also shows efficacy in reducing anxiety and improving functioning in people with depression. The data are promising, but more research needs to be done in this direction. In addition, it is worth remembering that it is not a treatment for depression but only an adjunct to pharmacotherapy and psychotherapy [116].
The role of diet in depression
In recent years, nutrition has become increasingly important, both in the treatment and prevention of diseases. Improper nutrition harms health and can contribute to the development of depressive disorders. On the other hand, proper nutrition and a proper diet can be helpful in both preventing and treating depression. However, it should be emphasized that a healthy diet cannot replace pharmacotherapy or psychotherapy in patients suffering from depression. Neverthless, diagnosing nutritional deficiencies and adopting an appropriate diet to support the nervous system can accelerate the recovery of depressed patients and can significantly reduce the risk of relapse [117, 118].
Vitamins
While we may obtain it from the sun, often we have deficiencies. We can distinguish two types of this vitamin - ergocalciferol- D2 and cholecalciferol- D3. Along with food, vitamin D supplementation is small, but D2 is found mainly in mushrooms, especially in shitake, and yeast, while D3 is in animal products, mainly fish, cream, and milk. Despite this, cutaneous synthesis is its main source [119]. The amount of vitamin D in a balanced diet is insufficient to meet the needs of our body, products containing it are only a supplement to skin synthesis or supplementation. Vitamin D, through its receptors, regulates gene transcription by influencing the formation of neurotrophic factors such as BDNF and NGF (nerve growth factor) [120]. Vitamin D supplementation is especially recommended in seasonal depressions when there is not enough sun. Vitamin D probably lowers Ca2+ plasma levels, which are elevated in depressed patients by maintaining the expression of Ca2+ pumps and buffers - calbindins and parvalbumin in the cytoplasm of neurons, leading to a reduction in symptoms of depression [121–123]. Another important function of vitamin D is to control the proper level of serotonin. Vitamin D stimulates the expression of the tryptophan 2 hydroxylase gene and at the same time inhibits the expression of tryptophan 1 (nonneuronal) [124]. Tryptophan hydroxylase is essential at the stage of conversion from 5-hydroxytryptophan [121,122]. Research shows that in people with depression, the concentration of vitamin D is significantly reduced, so it is important to replenish deficiencies through increased sun exposure, supplementation, or diet [127]. Supplements containing vitamin D can complement the standard treatment of depression and are fairly safe, inexpensive and publicly available. The appropriate level of vitamin D improves the mood of patients struggling with depression [128]. Up to half of the population can be deficient in vitamin D. Its recommended adult dose is 800 U.I. up to 2000 U.I . However, before starting supplementation, it is worth taking blood tests to determine its concentration in the body. In the case of adults who use barrier creams in the summer, supplementation is recommended all year round [129].
B vitamins (B1 thiamine, B2 riboflavin, B3 niacin, B5 pantothenic acid, B6 pyridoxine, B7 biotin, B9 folic acid, and B12 cobalamin) are responsible for the regulation of metabolic processes, they are cofactors of many enzymes involved in DNA and protein synthesis and energy metabolism. Additionally, they are significantly related to the serotonergic, noradrenergic, dopamine, cholinergic, GABA, and glutamate systems [130]. The sources of these vitamins are animal products such as liver, beef, poultry, eggs, and dairy and plant products - nuts, legumes, and spinach. It should be mentioned that plant products do not provide vitamin B12, so vegans and vegetarians can struggle with its deficiency without proper supplementation. The intestinal microbiota must have the ability to syn-thesize B vitamins [131]. More and more studies prove that there is a connection between the deficiency of B vitamins and the development of mental diseases, including depression [132, 133]. This may be associated with a decrease in monoamines, a disturbance in serotonin transmission, and inappropriate dopaminergic and noradrenergic metabolism. Moreover, the deficiency of any B vitamin may contribute to the accumulation of homocysteine and its damaging effect on cells [130]. The role of B vitamins is correlated with each other. Vitamin B2 activates vitamin B6, which converts tryptophan into serotonin. Vitamin B6 and vitamin B12 are associated with the synthesis of S-adenosylmethionine, which alleviates the symptoms of depression [134, 135]. However, their deficiency causes an unfavorable accumulation of the above-mentioned homocysteine and an increased risk of the disease in question [135]. Studies have shown that vitamin B1 supplementation reduced symptoms of depression and improved cognitive function in geriatric patients with depression [136]. Also, the combined intake of tricyclic antidepressants with vitamins B1, B2, and B6 gives a similar therapeutic effect in this group of patients [137]. Providing vitamin B3 with a diet reduces the risk of depression. Probably vitamin B3 is converted into nicotinamide, which has an effect comparable to that of benzodiazepines [131]. Numerous studies have shown an association between low levels of folic acid (vitamin B9) in plasma and red blood cells and the occurrence of depression [138, 139]. Vitamin B9 deficiencies in patients correlated with depressive disorders compared to healthy controls, and were associated with greater severity and longer duration of disease. In addition, together with folic acid deficiencies, high levels of homocysteine have been observed [140]. In women struggling with depression, the simultaneous administration of folic acid and fluoxetine significantly enhanced the terapetic effect of the drug and decreased plasma homocysteine levels, which represents an advance in the treatment of depression [141].
Vitamin C (ascorbic acid) is a water-soluble, naturally occurring element. The main sources of vitamin C are fruits and vegetables such as blackcurrant, kiwi, citrus, broccoli, peppers, parsley and fermented cabbage [142]. Studies have shown that vitamin C has a beneficial effect on patients' mental health. Vitamin C supplementation (1000 mg/day) reduced mood disorders [143] and reduced symptoms of major depressive disorder (MDD) in both children and adults [144, 145]. In addition, people struggling with depression, whose plasma was found to be deficient in vitamin C, had worse symptoms of the disease [146]. The exact role of vitamin C in the treatment of depression is not fully understood, but a number of reports suggest that oxidative stress and thus the accumulation of free radicals may play a role in the development of depressive disorders [147]. Ascorbic acid is known for its powerful antioxidant action. It has redox properties and can therefore neutralize reactive oxygen species[148]. Vitamin C also has a neuroprotective function, because it is an antioxidant that is found in the largest amount in the brain and prevents damage by free radicals. Additionally, by modulating neurotransmission with dopamine and glutamate, it is a neuromodulator in the brain [149, 150]. Amr et al. studied the effectiveness of vitamin C as an adjunct to fluoxetine therapy in the treatment of major depressive disorder in children. The results showed a significant reduction in depressive symptoms and an increase in the effectiveness of fluoxetine compared to treatment with fluoxetine alone in pediatric patients. Vitamin C, due to its antioxidant properties, low cost of therapy, and small, insignificant side effects, is a promising strategy in the treatment of depression that requires further research [150].
Vitamin K is one of the fat-soluble vitamins known for its role in blood clotting. Under natural conditions, it occurs in two forms. Vitamin K1, or phylloquinone, is synthesised by plants and supplied to the body with food. The second form is vitamin K2 (menaquinone), which is produced by bacteria. There is extensive data proving that vitamin K is a brain nutrient that influences brain function. Through VKDF proteins, sphingolipids or menaquinone-4, this vitamin modulates the function of nerve cells and affects the brain vasculature [151]. In addition, it protects neurons from oxidative damage, thanks to its antioxidant properties [152]. Gancheva S et al. conducted a study on rats with metabolic syndrome and examined the anxiolytic and antidepressant effects of vitamin K2 in relation to blood glucose levels. The study showed that rats with elevated sugar levels exhibited anxiety, depressive behaviour and memory impairment. Vitamin K2 lowered sugar levels and reduced anxiety and depression. They did not establish a specific relationship between vitamin K2 effects and sugar levels in behavioural tests, so they concluded that the anti-anxiety effect of the vitamin partially correlates with its effect on blood sugar levels. In contrast, the antidepressant effect is not linked to the vitamin's ability to reduce glucose levels [153].
Minerals
Magnesium acts as a cofactor in many enzyme reactions. It participates in the regulation of energy metabolism, is responsible for DNA and RNA synthesis, cell growth, membrane structure and homeostasis. The main sources of the element are grains, spinach, broccoli, nuts, legumes, bananas and blueberries [154]. Magnesium deficiency can destroy neurons and reduce synaptic functions of nerve cells, thus contributing to the development of depression [155]. For many years, magnesium has been used in homeopathy for all kinds of mental disorders, including depression [156]. The mechanism of its antidepressant effect is not fully understood. It is known that it affects the activity of NMDA and GABA receptors and regulates the activity of the hippocampus. The main antidepressant effect of magnesium is related to blocking the NMDA receptor. Serotonin, dopamine and adrenergic receptors have also been shown to play an important role in antidepressant action. It is assumed that the dopaminergic reward system in the brain may be related to the antidepressant effect of magnesium, as the element stimulates this system in rats [157]. Magnesium supplements, considered safe and well-tolerated, can complement the pharmacotherapy of depression. However, it is not recommended to use magnesium aspartate and magnesium glutamate in depressed patients, who have worsened their symptoms, as excess glutamate and aspartate have a neurotoxic effect. The use of magnesium oxide is not effective because its bioavailability is very low [158].
There are three main forms of iron, non-heme iron in the form of iron salts and chelates, heme iron found in meat, and offal and non-heme iron in plant sources such as beetroot, legumes, parsley, pumpkin, and sunflower seeds. Heme iron is best absorbed, iron absorption can be increased by taking vitamin C supplements or by consuming vitamin C-rich foods [159]. In the human body, iron is mainly bound to protein-forming hemoglobin or myoglobin, heme enzymes, and non-heme compounds such as ferritin. Iron is necessary for the proper functioning of the organism. It is responsible for oxygen transport, DNA synthesis, and the formation of enzymes involved in redox reactions and electron transport [160]. In addition, iron is an important component of brain growth, and is responsible for myelination and thus the proper conduction of nerve impulses. Furthermore, iron is essential for enzymes that participate in the synthesis of serotonin, dopamine, and norepinephrine. Deficiencies of that element are associated with numerous health consequences such as anemia, excessive fatigue, cognitive and concentration disorders, cold intolerance, or drowsiness [161]. It has been found that the level of ferritin in patients with depression is lower than in healthy people. That may indicate that iron is associated with the development of depression due to its participation in the oxygenation of the brain and the synthesis of dopamine, a neurotransmitter whose decreased level is observed in depression [162]. Supplementing that element improved mood and reduced postpartum depression (PPD) symptoms. Moreover, taking iron supplements early in pregnancy significantly improved the PPD rate [160].
Zinc is a trace element that is supplied with food. It is found in foods such as liver, egg cheese, seafood, and sunflower seeds. People suffering from depression usually have zinc deficiency, its supplementation may improve the patient's well-being. This element is a natural inhibitor of the NMDA receptor, which reduces glutamatergic transmission [163]. According to the theory about the genesis of depression, the balance between this system and GABA ensures mental health. Zinc also increases the concentration of BDNF, a brain-derived neurotrophic factor with a neuroprotective effect. Zinc acts on the GPR39 metabotropic receptor located in the hippocampus, frontal cortex, and amygdala [164]. Low level of zinc is also the cause of an increase in the concentration of pro-inflammatory cytokines, which are one of the depression factors, but also af-fect the conversion of tryptophan to serotonin [165]. High cortisol levels can be caused by insufficient zinc level. Too high a concentration of this hormone, as we already know, is one of the causes of depression. Studies show that the use of zinc preparations in people with depression treated with antidepressants significantly reduces the symptoms of depression, especially in patients with major depression and over 40 years of age [165].
Copper is an essential trace element in the human body that participates in hematopoietic processes, and is responsible for energy metabolism, neurobehavioral disorders, and immune functions [166]. Products such as offal, nuts, legumes, crustaceans, and whole grains have a high content of copper [167]. Studies have shown that the level of copper in the blood of people struggling with depression was higher than that of healthy people [168]. Additionally, it has been shown that women with postpartum depression (PPD) or those who have struggled with PPD in the past also have elevated blood copper levels. This suggests that copper can be an indicator of identifying women with a PPD predisposition or helping to diagnose the condition [169]. There are several theories as to how copper may influence the pathogenesis of depression. One of them concerns the monoamine hypothesis, an increase in serum copper concentration causes a decrease in 5-HT [166]. In addition, studies have shown that the prion protein (PrPc) regulates the activity of the NMDA receptor in a copper-dependent manner [170]. The increase in the concentration of the element inhibits the NMDA receptor, and disrupts the transmission of glutamate, contributing to neurological disorders. Copper is also supposed to influence the BDNF and NGF, leading to changes in neuronal plasticity and the development of depression. There are insufficient studies to confirm any effect of antidepressants on copper levels [166].
Selenium in the human body is present in small amounts. The component is responsible for the proper functioning of proteins that are involved in antioxidant protection in the brain. It also plays a neuromodulatory role [171]. The main source of the element is cereals, and their content in plants largely depends on the selenium content in the soil [172]. Selenium protects cells against oxidative damage and thus against the de-velopment of depression due to its antioxidant properties [173]. In addition, selenium is key to the proper synthesis and function of thyroid hormones, and it is known that thyroid function is associated with mood or cognitive disorders [174, 175]. The antidepressant effect of selenium is also attributed to its modulating properties on serotonergic, dopaminergic and noradrenergic pathways [124]. There are no unambiguous studies that would show a relationship between serum selenium concentration and depression. The concentration of this element in healthy and depressed people does not differ significantly [176]. However, it has been proven that selenium supplementation can prevent the development of postpartum depression in women, alleviate the symptoms of the disease and improve the mood of patients. [177].
Manganese is an element found in air, drinking water and food products. It is involved in bone and tissue formation, fat and carbohydrate metabolism and influences the immune system. In addition, it prevents the development of cancer, because it is a component of manganese superoxide dismutase (MnSOD, SOD-2). The element mainly accumulates in the brain. Excessive accumulation of this element in the CNS can lead to neurological damage [178]. There are assumptions that low manganese levels may be related to the development of depression. As mentioned above, manganese is a component of SOD-2, a key antioxidant enzyme. Reduced levels of SOD-2 in the body have been observed in patients with depression. In addition, a reduction in the volume of the prefrontal cortex and hippocampus in major depressive patients correlates with changes in SOD-2 levels. On the other hand, excessive manganese levels have a neurotoxic effect and disrupt the glutamate-glutamine cycle, which contributes to the abnormal synthesis of glutamate and GABA. Dysfunction of these neurotransmitters is associated with the occurrence of psychiatric disorders, including anxiety and depression, so excess magnesium probably also plays a role in the development of depressive behavior [175].
Lithium is a chemical element, the lightest of metals., The main source of lithium in food are cereals and vegetables, lesser amounts are contained in animal products such as meat and dairy products[127]. Lithium salts stabilize the mood, reduce suicidal tendencies, and have antidepressant and antimanic properties. It has been used in the treatment of a bipolar disorder, depression, schizophrenia, and eating disorders[128]. The therapeutic effect of lithium salts is attributed to the inhibition of the GSK-3 signaling pathway, the abnormal activity of which is observed, among others, in the etiology of bipolar disorder [179]. In addition, lithium use is believed to increase BDNF levels by inhibiting the GSK-3 pathway. BDNF is involved in the maturation and differentiation of neurons and affects synaptic plasticity, and its low level correlates with the severity of mania and depression in patients with bipolar disorder [180]. Moreover, the inhibition of inositol monophosphatase by lithium causes a decrease in the concentration of intracellular myo-inositol, the abnormal level of which is observed in people with bipolar disorder. The anti-inflammatory effect of lithium by reducing the number of pro-inflammatory molecules may be another mechanism showing effectiveness in reducing affective disorders [181, 182].
Coenzyme Q10 (CoQ10), also known as ubiquinone, is a component found in living cells [183]. Participates in the production of adenosine triphosphate (ATP) by acting as a cofactor in the oxidative phosphorylation process in the mitochondria. Additionally, it neutralizes free radicals and serves as an antioxidant [184]. It also has neuroprotective properties[185]. In a healthy organism, CoQ10 is synthesized from tyrosine and mevalonate. It is also available in foods including beef, pork, poultry, fish, fruit (oranges) and vegetables (tomatoes, spinach, cauliflower) [186]. There is evidence that depression is associated with induction of oxidative/nitrosative stress pathways and reduced antioxidant capacity of the body [187]. Due to the antioxidant properties of coenzyme Q10, many researchers are interested in its role in terms of antidepressant effects. Maes et al. investigated the association between plasma coenzyme Q10 levels and the occurrence of treatment-resistant depression (TRD) and the presence of chronic fatigue. The results showed significantly reduced CoQ10 levels in depressed patients compared to healthy subjects. In addition, the above parameter was lower in those with TRD and chronic fatigue than in the rest of the depressed subjects [188]. Sanoobar and co-authors determined the effect of coenzyme Q10 supplementation (500 mg / day) on fatigue and depression in multiple sclerosis patients by conducting a randomized, double-blind, placebo-controlled trial. Patients with multiple sclerosis and depressive symptoms were observed to have oxidative and nitrosative stress and less effective antioxidant defenses, so it was concluded that CoQ10, due to its properties, could be useful in the treatment of depression. After 12 weeks of coenzyme Q10 supplementation, the results of the study showed a reduction in symptoms of fatigue according to the Fatigue Severity Scale (FSS) and a reduction in symptoms of depression based on the Beck Depression Inventory (BDI) [189]. Another group of researchers, Forester et al. investigated the antidepressant effect of coenzyme Q10 in the treatment of bipolar depression in geriatric patients. Patients were administered CoQ10 at doses of 400 mg and 800 mg for 4 weeks while continuing psychotropic treatment. At the end of the study, there was a reduction in the Montgomery Asberg Depression Rating Scale score. Additionally, there was a reduction in symptoms such as fatigue, sadness and impaired concentration. These results suggest that CoQ10 may be an adjunctive therapy for the treatment of depression. However, further research is required [190].
3.3.3. Macronutriens
Of all the food that we eat, many of them include serotonin, however by the existence of the blood-brain barrier, it is not available as a neurotransmitter. Part of the products in the daily diet contain tryptophan, which is a precursor of 5-HT. Tryptophan is transported much more through the barrier so it can be used for synthesis, the reaction always occurs in the presence of pyridoxal phosphate. Which increases the main neurotransmitter level [191]. This amino acid cannot be produced by our body, so the only source is food. Tryptophan in combination with serotonergic drugs can cause serotonin syndrome, a situation that can be life-threatening. To diagnose it, we can observe symptoms such as spontaneous epileptic seizure, higher temperature, profuse sweating, and disturbance of consciousness. Tryptophan withdrawal is usually sufficient if this occurs [192]. The amino acid mentioned in this chapter is found in products such as chia seeds, chicken, cheese, beef, wheat flour, soybeans, and cocoa. Other inert amino acids limit the absorption of tryptophan in the intestine [193].
People's diets have changed over the years. In the past, there has been a greater balance between the various nutrients, which has resulted in a lower incidence of various diseases, including depression. Fatty acids are an important component of our diet, necessary for the proper functioning of the brain, they are responsible for neurogenesis, nerve impulse conduction, as well as the formation of synapses and myelin [194]. We currently eat a lot of processed foods that are rich in saturated fatty acids and unsaturated fatty acids in the trans configuration. Their excess leads to disorders of lipid metabolism, negatively affects the functioning of the nervous system by reducing the plasticity of synapses and increasing oxidative stress [195]. In addition, a high-fat diet leads to weight gain, worsened self-esteem, and depressed mood. The effect of this is a significant increase in the incidence of depression and other diseases related to the nervous system in recent years [196]. In addition to the imbalance between the amount of saturated and unsaturated fatty acids, an excessive increase in the amount of omega-6 fatty acids consumed and changes in the ratio of omega-6 to omega-3 acids are observed, which also adversely affects the functioning of the nervous system and contributes to depression [197].
Omega-3 and omega-6 acids, despite the fact that they differ only in the location of the multiple bonds in the chain, have a different effects on the functioning of the nervous system. They are exogenous substances supplied to the body with food [196]. The most common omega-3 acids include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and omega-6 acid is arachidonic acid (ARA). The central nervous system contains a large amount of omega-3 fatty acids, necessary for the proper development and functioning of neurons [198, 199]. Nowadays, the ratio of omega-6 to omega-3 fatty acids has changed, and it is 20:1 or even more, where the correct value is 1:1. The imbalance between these acids promotes obesity, thrombosis and inflammation. When the amount of omega-3 insufficient it significantly increases the risk of depression, schizophrenia, Alzheimer's disease, attention deficit disorder, and hyperactivity [200]. Serotonin receptor activity is governed by DHA acids, seeing that have a major influence on the fluidity of cell membrane. It is built with cholesterol which reduces the movement of neighboring phospholipids, but its synthesis is under the control of feedback. Phospholipids are the main component of the cytoplasmic membrane, taking into account that they are made of fatty acids, and their quantity is relative to the diet. As the membrane's fluency decreases, the serotonin affinity is shrinking [201]. Omega 3 fatty acids contribute to neurogenesis [202].
Eicosapentaenoic acid is a precursor for the synthesis of eicosanoids - tissue hormones with a broad spectrum of activity. These compounds are physiologically and pharmacologically active. These include prostaglandins, prostacyclins, thromboxane, leukotrienes, and lipoxins [199]. Eicosapentaenoic acid is an important anti-inflammatory factor involved in the reduction of pro-inflammatory cytokines. It lowers the level of inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6), and TNF-α [203]. Omega-3 acids are also responsible for increasing the level of BDNF - a substance that supports the production of new connections in the brain, and thus improves cognitive functions [202]. The unsaturated acid deficiency may result in decreased expression of the tryptophan hydroxylase enzyme involved in the production of serotonin [204]. For this reason, people suffering from depression should ensure that they consume an adequate amount of omega-3 fatty acids. They can be found primarily in fish: perch, tuna, carp, cod, and other animal products, such as lard, butter, and pork fat. Walnuts are also a good source of these acids, as they contain many other ingredients that have a beneficial effect on our bodies. The presence of omega-3 fatty acids in the diet has a positive effect on mood improvement, reduces cognitive disorders, and lowers the risk of depression [205, 206].
γ-linolenicacid, one of the omega-6 fatty acids, also plays an important role in preventing depression.Together with eicosapentaenoic and arachidonoic acids, it is a source of eicosanoids. It is also a direct precursor of prostaglandin 1 (PGE1) with anti-inflammatory effects, thromboxane (TBX3) and leukotrienes (LT3) [207]. γ-linolenic acid can be supplied to the body with food. They are found in the oil of Borago officinalis, Oenothera biennis, Oenothera paradoxa, Cannabis sativa seeds and others [208]. In addition, the body can produce it from linoleic acid with the participation of the Δ-6-desaturase enzyme. It shows the greatest activity in the liver, brain, lung parenchyma cells and retinal cells. Many factors influence the expression of Δ-6-desaturase. These include: gender, age, diet, hormonal disorders and pathological conditions of the body. Insulin has a positive effect on this enzyme, while a high-fat and low-protein diet, as well as the presence of corticosteroid hormones, glucagon and adrenaline, have an unfavorable effect. Low Δ-6-desaturase activity leads to various diseases, including pathological conditions of the brain and peripheral nervous tissue [207, 209]. Research from 2017 conducted by Yara T and colleagues confirmed the relationship between the risk of depression and the concentration of γ -linoleic acid in the body. Increasing the amount of this acid reduces the likelihood of developing depression [210]. n-3 and n-6 polyunsaturated fatty acids (PUFA), including y-linoleic acid, participate in several physiological processes, including proper neurotransmission and the functioning of the monoamine system. Deficiency of γ-linolenic acid leads to disorders in the dopaminergic system, which is responsible for regulating emotions. One of the possible pathomechanisms of depression assumes the presence of inflammation. γ-linolenic acid and other PUFA compounds have anti-inflammatory properties, therefore they have a positive effect on the functioning of the nervous system and brain [210, 211].
There is ample evidence of the negative effects of a high-fat diet on health and the proper functioning of the body. Excess lipids lead to obesity and hyperlipidemia, which is a major factor in many cardiovascular diseases, metabolic disorders, and diabetes, as well as an increased risk of mood disorders and depression [212].The excess of saturated fatty acids disturbs the homeostasis of the body, it results in a decrease in the volume of the hippocampus, impairment of psychomotor efficiency, memory, attention, and an increase in susceptibility to depression [213]. One of the pathogenic mechanisms of depression is the excess of inflammatory factors that are secreted by adipose tissue as a result of the increased immune activity associated with a high-fat diet [214]. The excess of lipids also increases oxidative stress, leading to damage to nerve cells and reduction of synaptic plasticity. In response, the body activates autophagy to maintain homeostasis. It is responsible for the regulation of lipid metabolism, reduction of oxidative stress, degradation of damaged proteins and organelles, and protection of nerve cells. The molecule that regulates this process is mTOR. Its activity is inhibited by the phosphorylation that occurs during the use of a high-fat diet, and the autophagy level is additionally lowered. This results in an increase in inflammation, disturbances in lipid metabolism, and the occurrence of anxiety and depression [194].
Carnitine is also important in the treatment of depression. A compound synthesized in the liver, kidneys and brain from the amino acids lysine and methionine. However, the main source of this compound in the body is the diet (about 75%), primarily meals rich in protein, beef and pork [215]. It exists in the form of two isomers: L-carnitine and D-carnitine. The greatest therapeutic potential is shown by acetyl-L-carnitine (ALC), which has neurobiological properties. It affects the metabolism of phospholipids, increases the activity of neurotrophic factors, especially in the hippocampus and prefrontal cortex, modulates serotonergic and GABAergic neurotransmission. It also plays an important role in the β-oxidation of fatty acids and energy production by transporting them from the cytoplasm to the mitochondria, and its deficiency increases the risk of insulin resistance [216, 217]. In addition, ALC has a neuroprotective effect, increases the number of dendrites, improves cognitive functions and regulates the release of glutamate, the deficiency of which leads to dysfunction in the hippocampus and the development of depression. Numerous studies have confirmed the positive effect of carnitine on the treatment of depression and dysthymia [215, 217, 218]. Levels of acetyl-L-carnitine are lower in patients with depressive disorders. The degree of ALC deficiency reflects the severity as well as the age of onset of major depression. For this reason, L-carnitine supplementation has a positive effect on the treatment of this disease. It leads to the improvement of mood and symptoms, giving effects similar to antidepressants. This led to further research on L-carnitine as a new drug for depression, not only as a support for the pharmacotherapy of this disease [218]. Confirmation of the relationship between depression and low ALC levels suggests its participation in the pathomechanism of this disease. For this reason, it can be used as a biomarker in the diagnosis and treatment of depression and other mental illnesses such as bipolar disorder or schizophrenia [215, 219].
The excitatory neurotransmitters in the brain that can be supplied with food are glutamine (Glu) and glutamic acid (Gln) [220]. These amino acids are found in protein-rich foods such as fish, dairy products, eggs, beef also in the vegetables such as beets, spinach, cabbage, carrots, kaleand others [221]. Astrocytes are responsible for maintaining the right amount of these compounds and homeostasis between them, which regulate the Glu-Gln cycle, that is the conversion of glutamine into gluta mate [222]. The loss of astrocytes or the alteration of the levels of these amino acids in the brain is crucial for glutamatergic neurotransmission. Decreased activity in the cerebral cortex occurs during depression and is a consequence of lowering glutamate levels [220]. Its deficiency in neurons, despite the correct amount of astrocytes, leads to depressive disorders. To prevent this, exogenous glutamate should be provided in the form of a supplement or nutritional products mentioned earlier. Then there is an improvement in well-being, increased activity and an overall improvement in psychosocial status [222–224]. This allows for better control of depressive behavior and suggests the possibility of using glutamate as an effective antidepressant with fewer side effects than traditional antidepressants [222].
The compound obtained from the combination of glutamate, cysteine and glycine is glutathione (GSH) [225]. It occurs in the cells of all organs, has antioxidant and neuroprotective properties. It protects cells and organs, including the brain, from oxidative stress and damage caused by free radicals [226]. GSH deficiency plays an important role in the pathological mechanism of depression, schizophrenia, bipolar disorder, ADHD, autism and non-psychiatric diseases such as type 2 diabetes, hereditary optic neuropathy or Huntington's disease. The dysfunction of the mitochondria as the main source of reactive oxygen species (ROS) causes oxidative damage [227]. Increased total oxidation state in the serum of depressed patients has been confirmed by scientific research. For this reason, the appropriate level of GSH in the brain is important as the main antioxidant substance of this organ. Numerous enzymes are responsible for its maintenance, for example glutathione peroxidase, the level of which decreases in people struggling with depression [187]. Substrates for the production of glutathione are also important, that is amino acids, the production of which requires an adequate supply of protein in the diet [228].
Sugar, when used in excess, as in a high-fat diet it leads to obesity, depression, and other serious diseases. There is an inverse relationship between blood sugar and hippocampal volume, therefore hyperglycemia or large fluctuations in sugar levels contribute to hippocampal atrophy and neuronal damage [229]. This is due to the activation of the polyol pathway, which causes oxidative stress and excessive glucose glycation [230]. The end products of these processes are responsible for the occurrence of both somatic (e.g. sleep problems, fatigue, appetite problems) and cognitive symptoms of depression (e.g. low mood, negative feelings towards oneself, problems with concentration) [231]. Persistent stress leads directly or via the hypothalamic-pituitary-adrenal (HPA) axis to the excretion of more inflammatory cytokines that interfere with the neuronal transmission and neurotransmitter signaling, which is characteristic of the pathophysiology of depression [230]. In addition, a large amount of pro-inflammatory cytokines affects the microcirculation and the uptake of glucose and insulin. This results in insulin resistance as a result of the association of inflammatory factors with the beta cells of the pancreas. There are also hormonal disorders, resulting in peripheral hypercortisolemia, which also adversely affects mood. This causes changes in the effectiveness of negative feedback and dysfunction of glucocorticoid receptors, leading to abnormal carbohydrate metabolism. Excessive lipolysis results in the release of large amounts of free fatty acids into the bloodstream. They accumulate in the pancreas, causing beta-cell failure, and resulting in insulin resistance [232]. Research has confirmed the relationship between cortisol concentration and glycemia and metabolic disorders, and thus the relationship between depressive disorders and insulin resistance and diabetes, resulting from disturbances in HPA and inflammatory processes [233].
Antidepressant treatment and lifestyle changes can be effective in reversing these disorders. Poor eating habits, a high-fat diet, excessive sugar consumption, and insulin resistance in combination with a lack of physical activity are the causes of obesity, weight gain, decreased self-esteem, and mood changes, which indirectly contribute to depression. On the other hand, hyperlipidemia, hypoglycemia, and obesity have a direct influence on its development and the occurrence of other metabolic disorders [234].
Plant components
Considering the important role of oxidative stress and inflammation in the pathogenesis of depression, the potential role of polyphenols and anthocyanins in reducing the risk and progression of depression can be considered. Both of these ingredients have antioxidant, anti-radical and anti-inflammatory properties [235]. They can also act by inhibiting neuronal apoptosis and activating neurogenesis. The richest sources of polyphenols include fruits (e.g. berries, apples, grapes), vegetables (e.g. eggplant, peppers) as well as juices, nuts, dark chocolate, coffee, tea and red wine [236, 237]. On the other hand, the sources of anthocyanins include fruits, mainly blueberries, cherries, grapes and raspberries, as well as red cabbage, flowers, and roots of some plants [11]. Studies have shown a significant effect of anthocyanins on the suppression of monoamine oxidases and, as a result, inhibition of the degradation of neurotransmitters [238]. The promising results of studies in animals were obtained for anthocyanins contained in hibiscus flowers (Hibiscus rosa-Sinensis L.). They had a positive effect on the level of monoamines and, as a result, an antidepressant effect [239]. The anthocyanins contained in berries show a similar effect. However, the risk of an interaction resulting from the consumption of large amounts of anthocyanins and drugs affecting MAO, which may lead to a dangerous serotonin syndrome, should be considered [238].
One of the most promising representatives of polyphenols is resveratrol. It is contained in products such as grape skins, red wine, and some nuts. Resveratrol has been shown to have antioxidant, neuroprotective, anti-inflammatory, and anti-cancer properties. It also has a positive effect on the quality of sleep and reduces fatigue, anxiety, and depressive symptoms. Its mechanisms of action includes the regulation of the HPA axis, beneficial effects on neurogenesis, reduction of oxidative stress and inflammation, and increases in the production of monoamines [240, 241]. Studies conducted in rats show that resveratrol inhibits the reuptake of norepinephrine and serotonin and increases the levels of these substances in the hippocampus. At the same time, its inhibitory effect on monoamine oxidase was found. Thus, it has two very important effects in the treatment of depression. This fact gives researchers high hopes for both the use of natural resveratrol and its use as a model for the design of new antidepressants [242].
Another plant that can be used to support the treatment and prevention of depression is turmeric (Curcuma longa). The positive effect on human health is attributed to the intensely yellow polyphenol contained in it - curcumin. Curcumin has anti-inflammatory, antioxidant, neuroprotective, and anti-cancer properties. It is considered as a natural remedy for the prevention and treatment of neurological diseases, diabetes, and heart disease [243]. It is widely used in natural medicine in Asian countries - in Ayurveda and traditional Chinese medicine. Turmeric has a fairly low bioavailability which can be increased by the addition of piperine [244].
Green tea (Camellia sinensis L.) also has a strong antioxidant and anti-inflammatory effect. It is a rich source of catechins, flavonols, and other biologically active ingredients. Population studies among Japanese over 70 years of age have shown a strong correlation between drinking more than 4 cups of green tea a day and fewer symptoms of depression compared to those who drink less than one cup a day [245]. In vitro studies on mouse brain cell lines have shown that catechins and polyphenols inhibit the activity of MAO-A [246]. Animal studies also show the effect of green tea on the inhibition of cyclooxygenase (COX), which affects the formation of neuroinflammation, as well as the regulation of the HPA axis [241]. On the other hand, theanine contained in tea increases the level of monoamines, such as dopamine and serotonin. This ingredient also increases the level of BDNF [247]. An anxiolytic and calming effect of both black and green tea was also found due to the affinity of epigallocatechin contained in them to the GABAa receptor [248].
Studies conducted using animal models, provide evidence suggesting that ellagic acid possesses antidepressant-like properties [249, 250]. The compound has ability to modulate neurotransmitter systems, reduce oxidative stress, and mitigate inflammation indicates its potential as a natural antidepressant intervention. Badel et al. (2018) demonstrated that ellagic acid exhibits antidepressant-like effects in animal models of depression. The study revealed that ellagic acid administration resulted in the reversal of depressive-like behaviors and restored neurotransmitter levels implicated in depression. Furthermore, ellagic acid demonstrated antioxidant and anti-inflammatory properties, which are relevant factors in the pathophysiology of depression. Similarly, another study investigated the antidepressant effects of ellagic acid in animal models. There was reported that ellagic acid administration led to improvements in behavioral paradigms associated with depression. Moreover, ellagic acid exhibited modulatory effects on various neurotransmitter systems, including serotonin and dopamine, which are known to play crucial roles in mood regulation [250].
Another group of compounds worth mentioning in the context of potential antidepressant activity are cannabinoids (CBD). Scarante et al. (2021) conducted a comprehensive review of preclinical and clinical studies investigating the potential of CBD as an adjunctive therapy for antidepressant treatment resistance [251]. The authors critically analyzed the existing literature and summarized the findings to evaluate CBD's effectiveness in enhancing the response to conventional antidepressants. The study highlights the potential of CBD as a promising adjunctive therapy for overcoming antidepressant treatment resistance. The authors found that CBD possesses unique pharmacological properties that may interact with the endocannabinoid system and various neurotransmitter systems implicated in depression. CBD have been shown to modulate the release of serotonin, a neurotransmitter often targeted by conventional antidepressants, and to exert neuroprotective and anti-inflammatory effects. Additionally, CBD may mitigate the adverse effects associated with antidepressant treatment, such as anxiety and cognitive impairment. Although there is a need for further research to elucidate the optimal dosing, treatment duration, and long-term safety profile of CBD as an adjunctive therapy. Authors acknowledge the limited number of clinical trials available and emphasize the importance of rigorous investigations to establish CBD's efficacy and safety in a larger patient population [251].
A noteworthy plant in this topic is crocus (Crocus sativus), popularly known as a saffron. Saffron and its active compound, crocin, present a promising natural approach to address depressive disorders. There was performed a study demonstrated that crocin administration led to a significant reduction in depressive-like behaviors, including despair and anxiety, in animal models [252, 253]. Crocin's impact on key neurotransmitter systems implicated in depression, such as serotonin, norepinephrine, and dopamine, was also observed. Furthermore, crocin exhibited neuroprotective and antioxidant properties, which may contribute to its antidepressant effects by counteracting oxidative stress and promoting neuroplasticity. The study also shed light on crocin's potential to modulate neuroinflammation, a process closely associated with the pathophysiology of depression. The findings from Siddiqui et al.'s study provide valuable insights into this compound's potential as an antidepressant agent. Its multifaceted effects on neurotransmitter systems, neuroprotection, and antioxidant activities suggest its promising role in alleviating depressive symptoms. However, it is crucial to recognize that these results were obtained from preclinical models, necessitating further investigation through rigorous clinical trials to validate crocin's effectiveness in humans. Additionally, optimal dosages, treatment durations, and potential interactions or side effects of crocin warrant thorough exploration [252].
The plant that can be counted among the factors delaying and alleviating depressive symptoms is cocoa. After processing its beans, cocoa and chocolate are obtained. Cocoa is rich in polyphenols, and contains a unique blend of catechins, epicatechins and procyanidins. Both animal studies and population studies show a positive effect of consuming cocoa polyphenols on well-being and alleviating depressive symptoms. An anxiolytic effect of cocoa polyphenols was also noted, which may be caused by the affinity of these compounds for GABAa receptors [248].
Panax ginseng is one of the most popular herbs used in China. This root contains ginsenosides, saponins, polypeptides, and polysaccharides. Ginsenoside Rb1 shows significantly raising serotonin, noradrenaline, and dopamine level. Saponins set out in Ginseng could reduce the 5‐HT2A receptor expression [254]. Although ginsenosides themselves break down quickly in the hydrochloric acid environment, their metabolites are also active substances. Panax ginseng increases norepinephrine and 5-hydroxytryptamine, and also increases BDNF levels. The action of regulating pro-inflammatory cytokines is also attributed to; IL-6 and TNF [255]. Ginsenoside Rg3 also reduces the levels of ACTH and corticosteroid, which helps in the work of the HPA axis. Confirmation of the antidepressant effect may be the forced swimming test, one of preclinical animal study [256].
The gut microbiota plays a key role in the pathomechanism of depression. Its disturbance can lead to inflammation and organism malfunction in many ways, including mood and nervous system disorders. An ingredient that has a significant impact on the correct composition of the microbiome is fiber. Fiber includes non-digestible carbohydrates and plant-derived lignins [221]. Fiber passes through the digestive tract intact and is fermented inside the large intestine by intestinal bacteria. As a result of fermentation, among others, short-chain fatty acids (SCFA) are formed. Animal studies also suggest that this process increases the production of tryptophan, which may be important in the prevention and alleviation of de-pression [257]. During fermentation, however, bicarbonates are produced, which lower the intestinal pH, preventing or limiting the growth and development of pathogenic bacteria. People who eat low amounts of fiber have higher inflammatory markers than people who eat high amounts of fiber [258]. Cytokines produced as a result of inflammation may increase the reuptake and decrease the synthesis of serotonin and dopamine. Cytokines can also activate the HPA axis leading to increases in cortisol levels [259]. The resulting SCFAs, especially butyrate and propionate, can also inhibit histone deacetylases, which are involved in the epigenetic regulation of genes. Inhibiting deacetylation can lead to an increase in DNA availability, increasing transcription. Animal studies show that fiber fermentation may also stimulate tissue G Protein-Coupled Receptor (GPCR) activation, mediating the immune response [257].
3.3.5. Lifestyle diets
The diet used can affect not only a person's physical health but can also play an important role in his mental health. Nowadays, people pay more and more attention to food and nutrition. More and more often they decide to change their eating habits and introduce a specific diet permanently. Among many environments and social groups, the so-called lifestyle diets. Vegetarian or vegan, Mediterranean, and ketogenic diets are the most popular. Among people using the above diets, there is a common belief that they have a positive effect on health. However, research shows that this impact is not unambiguous. On the one hand, they may have a beneficial effect on the body, preventing many diseases or alleviating their symptoms, but also they may predispose to diseases, including depression [10, 260–263].
Vegetarian and vegan diets
Vegan and vegetarian diets have a significant impact on reducing the risk of cardiovascular events, cholesterol levels, and the risk of cancer [264]. Nevertheless, along with the restriction of meat consumption, they may be accompanied by a deficiency of vitamin B12, creatine and unsaturated fatty acids [265]. In addition, these diets can lead to zinc and iron deficiency. The reason for this may be not only the low content in the diet, but also the content in the food of a large number of absorption inhibitors, for example phytates or polyphenols, blocking access to nutrients. All of these can cause disorders in the nervous system, like cognitive impairment, an increased risk of neurodegeneration, or a general drop in the mood [264, 266]. A study of adolescents suffering from anorexia showed that those who were on a vegetarian diet showed a lower frame of mind than omnivores. Therefore, an ambiguous conclusion can be drawn about the relationship between the occurrence of mental disorders and the deficiency of fatty acids in the diet [260]. However, not all studies show a clear negative impact of vegetarian, and vegan diets on mental health [267]. It is probably dependent on the lifestyle of the person on the diet, their age, health condition, diet variation and duration of its application. The reason for switching to a meatless diet may also be the reason for the differences. If not eating meat is the result of economic status, then the person may not be satisfied with their situation. In addition, he may not be able to eat a balanced, varied plant-based diet by eating only low-value foods, low in protein and fat [267]. In conclusion, there is no conclusive evidence that vegan and vegetarian diets have a negative effect on occurrence, nevertheless, most studies point to this association. . Meals should include key ingredients such as iron, calcium, magnesium, and potassium, vitamins, especially B12, B2 and D, as well as high-quality proteins and unsaturated fatty acids [268].
The Mediterranean diet
The traditional Mediterranean diet is characterized by a high proportion of plant products in the diet. They are mainly vegetables, nuts, legumes, seeds, olives, and whole grains A moderate amount of fish and seafood rich in omega-3 fats is also consumed, while the main cooking fat is extra-virgin olive oil. Red meat, confectionery, and processed foods are avoided or restricted in this diet. Studies show a lower risk of depression in people on a Mediterranean diet [269]. Nutrients such as vitamins and minerals as well as unsaturated fatty acids are essential for the proper functioning of neurons. This is due to their key role as, among others, enzyme cofactors, elements of metabolic pathways or the structure of the myelin sheath, as well as protection against oxidation [269].The positive effect of this diet is therefore related to the high content of the ingredients discussed in this article. It should be emphasized, however, that the effects of this diet are not noticeable after a short period of use [270, 271]. It may also be important that people who use this diet for a long time do not usually have problems with overweight and obesity, which may also be associated with better well-being [10].
The ketogenic diet
The ketogenic diet includes high-fat meals, very low carbohydrate levels, and a moderate amount of protein. Such a proportion of macronutrients leads to sugar savings and increased fat consumption, then it becomes the main source of energy. This condition is called nutritional ketosis [272]. It can probably increase the level of GABA in our body and also lowers the level of excitatory neurotransmitters [273]. Therefore, it can affect communication between neurons and reduce neuronal hyperactivity. Moreover, this diet has an anti-inflammatory effect as it lowers the level of inflammatory cytokines and influences the condition of the intestinal microbiome [261]. Tests carried out on rats showed that rats with depressive symptoms, fed according to the principles of a ketogenic diet, spent less time motionless. These results were comparable to the effects of antidepressant treatment [262, 263].
3.4. Diet and antidepressants
In recent years, more and more attention has been paid to the issue of proper nutrition during the treatment of diseases. Diet also significantly influences the pharmacotherapy of depression - it can weaken it, strengthen it, and it can also lead to the intensification of side effects of drugs, and even to drug poisoning and the occurrence of symptoms posing a threat to the patient's life. Hence, there is a need to carefully study and analyze some of the possible interactions between antidepressants and food ingredients, and dietary supplements. The time of taking medication in relation to meals is also important. Interactions between antidepressants and food ingredients may occur during the pharmacokinetic phase as well as pharmacodynamic interactions [274, 275]. While drug interactions are commonly discussed at the pharmacokinetic level, with an emphasis on alterations in drug absorption, distribution, metabolism, and excretion, the importance of exploring pharmacodynamic interactions should not be overlooked. Pharmacodynamic interactions involve changes in drug response and efficacy resulting from the concurrent administration of drugs and various substances, including food ingredients. While the pharmacokinetic aspects of drug-food interactions have traditionally received more attention in publications, understanding pharmacodynamic interactions is crucial for comprehensive drug management and patient safety. By exploring the interactions between food ingredients and antidepressants at the pharmacodynamic level, researchers and healthcare professionals can gain insights into the potential consequences and develop appropriate guidelines to optimize patient outcomes [274, 275].
Pharmacokinetic interactions may occur during the absorption, distribution, metabolism, and elimination of the drug. They should be kept in mind during pharmacotherapy, especially when using antidepressants, as this is of great importance for improving the patient's health [274]. Antidepressants should be taken according to the doctor's recommendation. Some antidepressants should be taken after the meal for example moclobemide, and other drugs with food such as duloxetine or venlafaxine [276]. Trazodone in the form of prolonged-release tablets is taken on an empty stomach. However, for most antidepressants, it does not matter whether they are taken before, after or during the meal. It is more important, what is eaten during treatment [275, 276]. The type of drink we use while taking medications is also very important. The antidepressant drug may be adversely affected by the liquid used to drink it - too hot water may cause premature dissolution of the enteric coating of the tablet or capsule. Antidepressants should be washed down with lukewarm, boiled water [275, 277]. The tannins and polyphenols contained in coffee or tea reduce the absorption of these drugs and their effectiveness [277]. Food-drug interactions can occur at the drug absorption stage in the gastrointestinal tract. Antacids, plants with coating properties such as flax seed (Linum usitatissimum), psyllium plantain (Plantago Psyllium) and plantain ovoid (Plantago ovata) reduce the effectiveness of antidepressants, especially TCAs for example amitriptyline, doxepin [278]. Dietary fiber (bran, oatmeal, legumes, barley and buckwheat) adsorbs tricyclic antidepressants (amitriptyline and imipramine) on its surface. In order to prevent this, it is advisable to keep a time interval (1-2 h) between a meal containing these ingredients and taking medications [278, 279]. . Absorption of lipophilic antidepressants is altered when larger amounts of lipids are supplied with food. High-fat meals (such as fried eggs, bacon, butter, full-fat milk, and lard) increase the absorption of these drugs from the gastrointestinal tract [274]. There is an increase in the concentration of the drug and its metabolites in the blood. As a result of increased absorption of the drug substance, side effects may be intensified: disturbance of consciousness, sleep problems, disorders of the nervous system, tachycardia, hypotension, convulsion, agitation and mydriasis [279].
Antidepressants are metabolized in the liver. They also influence the activity of cytochrome P450 isoenzymes. At the stage of hepatic metabolism, the greatest number of interactions between different drugs taken at the same time occurs [275]. Taking antidepressants together with cytochrome P450 inducers increases their metabolism and reduces the effectiveness of the therapy. The antidepressants with the greatest inhibitory effect on the activity of CYP450 isoenzymes, and thus the greatest risk of adversely affecting the metabolism of other medications taken by the patient, are TCAs, fluoxetine, paroxetine, and fluvoxamine. Cytochrome P450 inhibitors increase the concentration of the drug, extend the time of its me-tabolism and lead to numerous side effects [277]. Nutrients and stimulants can be both inhibitors and inducers of one or more cytochrome P 450 isoenzymes. The inducers include: some vegetables from the Cabbage family for example cabbage, broccoli, brussels sprouts, radish also meat roasted over the fire, soybeans, ginger and spices: black pepper, cinnamon, cloves, nutmeg. The active ingredients contained in this food affect various isoenzymes and intensify the breakdown of drugs, which reduces the therapeutic effect of drugs. The exception are pro-drugs, whose metabolism and conversion to the active form is possible only in the presence of cytochrome P450 enzymes, especially CYP3A4 and CYP2D6 isoenzymes [280]. An important inducer of cytochrome P450 isoenzymes is tobacco smoke. Treatment of patients who smoke large amounts of cigarettes is less effective [275, 277]. A large number of cytochrome P450 enzyme inhibitors interact with antidepressants. These include grapefruit juice, citrus and their juices, cranberry juice, legumes, tomatoes, parsley, thyme, garlic, licorice root and the food in which it is found, natural honey, peppers, chilli peppers, turmeric, black pepper and long pepper. Cytochrome P450 inhibitors, with the exception of pro-drugs, reduce the metabolism of antidepressants, potentially leading to enhanced therapeutic effects or adverse effects. Treatment with antidepressants requires restriction or refraining from eating the above-mentioned foods and maintaining an appropriate time interval between drugs and meals containing cytochrome P450 inhibitors and inducers [275, 277]. Particular care should be taken when reaching for grapefruit juice, other citrus fruits and chili peppers. They contain bergamotin, naringin, narigin (citrus), and capseaicin (peppers), which strongly inhibit the activity of cytochrome P450 and increase the concentration of the drug in the body. Grapefruit juice can be drunk at least 4 hours before or after taking your medications [275, 281, 282]. An important issue in the treatment of depression is the use of plant-based dietary supplements and vitamin supplementation [278]. St. John's Wort (Hypericiperforatum) extract is a component of natural medicines and dietary supplements. It has an antidepressant effect, therefore it has been used as a safer drug for mild and moderate depression. The basis of its effectiveness in the treatment of depression is the inhibition of the reuptake of serotonin, dopamine and norepinephrine [283]. St. John's Wort (Hypericum perforatum) should not be used with selective serotonin reuptake inhibitors (SSRIs) and tricyclic antide-pressants (TCAs). The combination of these drugs and dietary supplements leads to the health-threatening "serotonin syndrome" [283]. The effect of anti-depressants is enhanced by dietary supplements containing Ginkgo biloba, Kava kava (Piper methysicum), Ginseng (Panax ginseng), and Valeriana officinalis. The interaction of these drugs with active ingredients in plants increases the risk of side effects. Ginkgo biloba increases the side effects of phenelzine and trazodone so much that in some cases the patient may fall into a coma. Kava kava enhances the effects of fluoxetine, amitriptyline, clomipramine, and sedatives [278, 283].
In addition to fluoxetine and amitriptyline, Valeriana officinalis also inhibits the metabolism of duloxetine, increasing the risk of side effects such as nausea, con-stipation, weight gain, and increased heart rate [276]. Ginseng interacts with monoamine oxidase inhibitors (MAOIs). The psychoactive stimulation increases and flu-like symptoms occur [278]. .
MAOI (moclobemide) by blocking the activity of monoamine oxidase inhibits the metabolism of tyramine present in food [274, 275]. During therapy with these drugs, products containing tyramine should be eliminated from the diet, such as salami, pepperoni, cheeses (cheddar, emmentaler, camembert, brie, blue, mozzarella, parmesan, roquefort, stilton, gruyere), fish (marinated, smoked), beef liver (stored), chicken liver (stored), soy sauce, caviar, Bolognese sausage, meat concentrate (in sauces and soups), avocado, bananas (overripe), chocolate, figs (canned or overripe), broad beans, yeast supplements, yeast extract, caffeine (in large amounts), vermouth wines, chianti wine, chartreuse liqueurs. The interaction of MAOIs with tyramine-rich foods may result in psychomotor agitation, subsequent vasoconstriction tachycardia and hypertension, which is a life- and health-threatening (sometimes hypertensive crisis) [278, 279].
Summary
Depression is a multifactorial disease and its pharmacological treatment is not satisfactory. MAOIs and TCAs are effective in treatment, but they have many side effects. The latest generation of antidepressants in most cases do not activate side effects, but they are ineffective in 30 to 40% of treated patients. Proper nutrition or taking appropriate doses of dietary supplements can be a very important factor both in the prevention of depressive disorders and can be a valuable supplement to the treatment of depression. In turn, improper nutrition can contribute to the development of depressive disorders, as well as significantly interfere with the treatment of depression. By affecting the pharmacokinetics of antidepressants in the body, it may reduce their therapeutic effect, and may increase their side effects, leading to poisoning or life-threatening symptoms. Food ingredients, through interactions in the pharmaco-dynamic phase, may also modify the action of antidepressants.
Author Contributions: conceptualization,methodology, project administration: MH, writing: W.M., J.S., K.S., A.Sk,. A.Sy., M.H., review and editing:I.P.Ch., M.H. All authors have read and agreed to the published version of the manuscript..
Funding: This research received no external funding.
Conflicts of Interest: The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference
1. Pérez-Cano HJ, Moreno-Murguía MB, Morales-López O, et al (2020) Anxiety, depression, and stress in response to the coronavirus disease-19 pandemic. Cir Cir 88:562–568. https://doi.org/10.24875/CIRU.20000561
2. Ghosh M, Chatterjee S, Das D, et al An Analytical Study for Predicting Suicidal Tendencies Using Machine Learning Algorithms
3. Malhi GS, Mann JJ (2018) Depression. The Lancet (British edition) 392:2299-. https://doi.org/10.1016/S0140-6736(18)31948-2
4. Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b. Accessed 30 Dec 2021
5. Kuehner C (2017) Why is depression more common among women than among men? The Lancet Psychiatry 4:146–158. https://doi.org/10.1016/S2215-0366(16)30263-2
6. Ceskova E (2016) Current pharmacotherapy of depression - focused on multimodal/multifunctional antidepressants. Expert Opin Pharmacother 17:1835–1837. https://doi.org/10.1080/14656566.2016.1219340
7. Saveanu RV, Nemeroff CB (2012) Etiology of Depression: Genetic and Environmental Factors. Psychiatric Clinics of North America 35:51–71. https://doi.org/10.1016/j.psc.2011.12.001
8. Talarowska M (2020) Epigenetic Mechanisms in the Neurodevelopmental Theory of Depression. Depression Research and Treatment 2020:e6357873. https://doi.org/10.1155/2020/6357873
9. Yohn CN, Gergues MM, Samuels BA (2017) The role of 5-HT receptors in depression. Mol Brain 10:28. https://doi.org/10.1186/s13041-017-0306-y
10. Bremner JD, Moazzami K, Wittbrodt MT, et al (2020) Diet, Stress and Mental Health. Nutrients 12:E2428. https://doi.org/10.3390/nu12082428
11. Nabavi SM, Daglia M, Braidy N, Nabavi SF (2017) Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr Neurosci 20:180–194. https://doi.org/10.1080/1028415X.2015.1103461
12. Baumeister AA, Hawkins MF, Uzelac SM (2003) The myth of reserpine-induced depression: role in the historical development of the monoamine hypothesis. J Hist Neurosci 12:207–220. https://doi.org/10.1076/jhin.12.2.207.15535
13. Segal DS, Kuczenski R, Mandell AJ (1974) Theoretical implications of drug-induced adaptive regulation for a biogenic amine hypothesis of affective disorder. Biol Psychiatry 9:147–159
14. Koo JW, Chaudhury D, Han M-H, Nestler EJ (2019) Role of mesolimbic brain-derived neurotrophic factor in depression. Biological psychiatry 86:738–748
15. Levy MJF, Boulle F, Steinbusch HW, et al (2018) Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology (Berl) 235:2195–2220. https://doi.org/10.1007/s00213-018-4950-4
16. Duman RS, Deyama S, Fogaça MV (2021) Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. European Journal of Neuroscience 53:126–139
17. Deyama S, Bang E, Wohleb ES, et al (2019) Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry 176:388–400. https://doi.org/10.1176/appi.ajp.2018.17121368
18. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. The FASEB Journal 13:9–22. https://doi.org/10.1096/fasebj.13.1.9
19. Herselman MF, Bailey S, Bobrovskaya L (2022) The Effects of Stress and Diet on the “Brain–Gut” and “Gut–Brain” Pathways in Animal Models of Stress and Depression. International Journal of Molecular Sciences 23:2013. https://doi.org/10.3390/ijms23042013
20. Roth W, Zadeh K, Vekariya R, et al (2021) Tryptophan Metabolism and Gut-Brain Homeostasis. Int J Mol Sci 22:2973. https://doi.org/10.3390/ijms22062973
21. Juruena MF (2014) Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav 38:148–159. https://doi.org/10.1016/j.yebeh.2013.10.020
22. Gerritsen L, Milaneschi Y, Vinkers CH, et al (2017) HPA Axis Genes, and Their Interaction with Childhood Maltreatment, are Related to Cortisol Levels and Stress-Related Phenotypes. Neuropsychopharmacol 42:2446–2455. https://doi.org/10.1038/npp.2017.118
23. Juruena MF, Gadelrab R, Cleare AJ, Young AH (2021) Epigenetics: A missing link between early life stress and depression. Prog Neuropsychopharmacol Biol Psychiatry 109:110231. https://doi.org/10.1016/j.pnpbp.2020.110231
24. Mikulska J, Juszczyk G, Gawrońska-Grzywacz M, Herbet M (2021) HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain sciences 11:1298-. https://doi.org/10.3390/brainsci11101298
25. Du Y, Gao X-R, Peng L, Ge J-F (2020) Crosstalk between the microbiota-gut-brain axis and depression. Heliyon 6:e04097. https://doi.org/10.1016/j.heliyon.2020.e04097
26. Trzeciak P, Herbet M (2021) Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 13:927. https://doi.org/10.3390/nu13030927
27. Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186. https://doi.org/10.1097/PSY.0b013e3181907c1b
28. Troubat R, Barone P, Leman S, et al (2021) Neuroinflammation and depression: A review. Eur J Neurosci 53:151–171. https://doi.org/10.1111/ejn.14720
29. Leonard BE (2018) Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr 30:1–16. https://doi.org/10.1017/neu.2016.69
30. Gałecki P, Talarowska M (2018) Inflammatory theory of depression. Psychiatr Pol 52:437–447. https://doi.org/10.12740/PP/76863
31. Dash S, Clarke G, Berk M, Jacka FN (2015) The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry 28:1–6. https://doi.org/10.1097/YCO.0000000000000117
32. Peirce JM, Alviña K (2019) The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res 97:1223–1241. https://doi.org/10.1002/jnr.24476
33. Dicks LMT, Hurn D, Hermanus D (2021) Gut Bacteria and Neuropsychiatric Disorders. Microorganisms 9:2583. https://doi.org/10.3390/microorganisms9122583
34. Ortega MA, Alvarez-Mon MA, García-Montero C, et al (2022) Gut Microbiota Metabolites in Major Depressive Disorder—Deep Insights into Their Pathophysiological Role and Potential Translational Applications. Metabolites 12:50. https://doi.org/10.3390/metabo12010050
35. Kelly JR, Kennedy PJ, Cryan JF, et al (2015) Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9:392. https://doi.org/10.3389/fncel.2015.00392
36. Cheng L-H, Liu Y-W, Wu C-C, et al (2019) Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. Journal of Food and Drug Analysis 27:632–648. https://doi.org/10.1016/j.jfda.2019.01.002
37. Misra S, Mohanty D (2019) Psychobiotics: A new approach for treating mental illness? Crit Rev Food Sci Nutr 59:1230–1236. https://doi.org/10.1080/10408398.2017.1399860
38. Smith KS, Greene MW, Babu JR, Frugé AD (2021) Psychobiotics as treatment for anxiety, depression, and related symptoms: a systematic review. Nutr Neurosci 24:963–977. https://doi.org/10.1080/1028415X.2019.1701220
39. Zou R, Tian P, Xu M, et al (2021) Psychobiotics as a novel strategy for alleviating anxiety and depression. Journal of Functional Foods 86:104718. https://doi.org/10.1016/j.jff.2021.104718
40. Palepu MSK, Dandekar MP (2022) Remodeling of microbiota gut-brain axis using psychobiotics in depression. Eur J Pharmacol 931:175171. https://doi.org/10.1016/j.ejphar.2022.175171
41. Nanni V, Uher R, Danese A (2012) Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry 169:141–151. https://doi.org/10.1176/appi.ajp.2011.11020335
42. Park C, Rosenblat JD, Brietzke E, et al (2019) Stress, epigenetics and depression: A systematic review. Neurosci Biobehav Rev 102:139–152. https://doi.org/10.1016/j.neubiorev.2019.04.010
43. Gruber BM (2011) Epigenetics and etiology of neurodegenerative diseases. Postepy Hig Med Dosw (Online) 65:542–551. https://doi.org/10.5604/17322693.956497
44. Kader F, Ghai M, Maharaj L (2018) The effects of DNA methylation on human psychology. Behavioural brain research 346:47–65
45. Gałecki P, Talarowska M (2018) Neurodevelopmental theory of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 80:267–272
46. Moraczewski J, Aedma KK (2022) Tricyclic Antidepressants. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
47. von Wolff A, Hölzel LP, Westphal A, et al (2013) Selective serotonin reuptake inhibitors and tricyclic antidepressants in the acute treatment of chronic depression and dysthymia: a systematic review and meta-analysis. J Affect Disord 144:7–15. https://doi.org/10.1016/j.jad.2012.06.007
48. Fanoe S, Kristensen D, Fink-Jensen A, et al (2014) Risk of arrhythmia induced by psychotropic medications: a proposal for clinical management. Eur Heart J 35:1306–1315. https://doi.org/10.1093/eurheartj/ehu100
49. Hillhouse TM, Porter JH (2015) A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol 23:1–21. https://doi.org/10.1037/a0038550
50. Gillman PK (2007) Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol 151:737–748. https://doi.org/10.1038/sj.bjp.0707253
51. Hiemke C, Baumann P, Bergemann N, et al (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44:195–235
52. Schipper P, Vanmolkot L, Peeters FPML (2016) Combining a classic monoamine oxidase inhibitor with a tricyclic antidepressant in therapy-resistant depression: a case report and literature review. Tijdschr Psychiatr 58:886–890
53. Sub Laban T, Saadabadi A (2023) Monoamine Oxidase Inhibitors (MAOI). In: StatPearls. StatPearls Publishing, Treasure Island (FL)
54. Müller T, Riederer P, Grünblatt E (2017) Determination of Monoamine Oxidase A and B Activity in Long-Term Treated Patients With Parkinson Disease. Clin Neuropharmacol 40:208–211. https://doi.org/10.1097/WNF.0000000000000233
55. Thomas SJ, Shin M, McInnis MG, Bostwick JR (2015) Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy 35:433–449. https://doi.org/10.1002/phar.1576
56. Fiedorowicz JG, Swartz KL (2004) The Role of Monoamine Oxidase Inhibitors in Current Psychiatric Practice. J Psychiatr Pract 10:239–248
57. Flockhart DA (2012) Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry 73 Suppl 1:17–24. https://doi.org/10.4088/JCP.11096su1c.03
58. Brown C, Taniguchi G, Yip K (1989) The Monoamine Oxidase Inhibitor—Tyramine Interaction. The Journal of Clinical Pharmacology 29:529–532. https://doi.org/10.1002/j.1552-4604.1989.tb03376.x
59. Sathyanarayana Rao TS, Yeragani VK (2009) Hypertensive crisis and cheese. Indian Journal of Psychiatry 51:65. https://doi.org/10.4103/0019-5545.44910
60. Walker SE, Shulman KI, Tailor SA, Gardner D (1996) Tyramine content of previously restricted foods in monoamine oxidase inhibitor diets. J Clin Psychopharmacol 16:383–388. https://doi.org/10.1097/00004714-199610000-00007
61. Sullivan EA, Shulman KI (1984) Diet and Monoamine Oxidase Inhibitors: A Re-Examination. Can J Psychiatry 29:707–711. https://doi.org/10.1177/070674378402900814
62. Grabowski Ł (2021) Inhibitory monoaminooksydazy (IMAO): farmakologia, metabolizm i zastosowanie w terapii depresji o różnej etiologii. Postępy Biochemii 67:130–140
63. Cashman JR, Ghirmai S (2009) Inhibition of serotonin and norepinephrine reuptake and inhibition of phosphodiesterase by multi-target inhibitors as potential agents for depression. Bioorg Med Chem 17:6890–6897. https://doi.org/10.1016/j.bmc.2009.08.025
64. Locher C, Koechlin H, Zion SR, et al (2017) Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents: A Systematic Review and Meta-analysis. JAMA Psychiatry 74:1011–1020. https://doi.org/10.1001/jamapsychiatry.2017.2432
65. Stahl SM, Grady MM, Moret C, Briley M (2005) SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr 10:732–747. https://doi.org/10.1017/s1092852900019726
66. Ayipo YO, Alananzeh WA, Ahmad I, et al (2022) Structural modelling and in silico pharmacology of β-carboline alkaloids as potent 5-HT1A receptor antagonists and reuptake inhibitors. J Biomol Struct Dyn 1–17. https://doi.org/10.1080/07391102.2022.2104376
67. Härter M, Klesse C, Bermejo I, et al (2010) Unipolar depression: diagnostic and therapeutic recommendations from the current S3/National Clinical Practice Guideline. Dtsch Arztebl Int 107:700–708. https://doi.org/10.3238/arztebl.2010.0700
68. Khouzam HR (2017) A review of trazodone use in psychiatric and medical conditions. Postgrad Med 129:140–148. https://doi.org/10.1080/00325481.2017.1249265
69. Fagiolini A, Comandini A, Catena Dell'Osso M, Kasper S (2012) Rediscovering trazodone for the treatment of major depressive disorder. CNS Drugs 26:1033–1049. https://doi.org/10.1007/s40263-012-0010-5
70. Jarema M, Dudek D, Landowski J, et al (2011) Trazodon : lek przeciwdepresyjny : mechanizm działania i miejsce w leczeniu depresji. Trazodon : the antidepressant : mechanism of action and its position in the treatment of depression 45:
71. Li Y-F (2020) A hypothesis of monoamine (5-HT) - Glutamate/GABA long neural circuit: Aiming for fast-onset antidepressant discovery. Pharmacol Ther 208:107494. https://doi.org/10.1016/j.pharmthera.2020.107494
72. Chu A, Wadhwa R (2022) Selective Serotonin Reuptake Inhibitors. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
73. Blier P, El Mansari M (2013) Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci 368:20120536. https://doi.org/10.1098/rstb.2012.0536
74. Andrade C (2010) Stahl's Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Mens Sana Monogr 8:146–150. https://doi.org/10.4103/0973-1229.58825
75. Wong DT, Bymaster FP, Engleman EA (1995) Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci 57:411–441. https://doi.org/10.1016/0024-3205(95)00209-o
76. Bandelow B, Michaelis S (2015) Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci 17:327–335
77. Cipriani A, Furukawa TA, Salanti G, et al (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391:1357–1366. https://doi.org/10.1016/S0140-6736(17)32802-7
78. Baldwin DS, Anderson IM, Nutt DJ, et al (2014) Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol 28:403–439. https://doi.org/10.1177/0269881114525674
79. Kent JM (2000) SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet 355:911–918. https://doi.org/10.1016/S0140-6736(99)11381-3
80. de Boer TH, Maura G, Raiteri M, et al (1988) Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacology 27:399–408. https://doi.org/10.1016/0028-3908(88)90149-9
81. de Boer T (1995) The effects of mirtazapine on central noradrenergic and serotonergic neurotransmission. Int Clin Psychopharmacol 10 Suppl 4:19–23. https://doi.org/10.1097/00004850-199512004-00004
82. Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. https://doi.org/10.1038/mp.2017.255
83. Adell A (2020) Brain NMDA Receptors in Schizophrenia and Depression. Biomolecules 10:947. https://doi.org/10.3390/biom10060947
84. Ates-Alagoz Z, Adejare A (2013) NMDA Receptor Antagonists for Treatment of Depression. Pharmaceuticals (Basel) 6:480–499. https://doi.org/10.3390/ph6040480
85. Kaur U, Pathak BK, Singh A, Chakrabarti SS (2021) Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci 271:417–429. https://doi.org/10.1007/s00406-019-01084-z
86. Fantasia HC (2020) Esketamine Nasal Spray for Treatment-Resistant Depression. Nurs Womens Health 24:228–232. https://doi.org/10.1016/j.nwh.2020.03.004
87. Thase ME, Nierenberg AA, Vrijland P, et al (2010) Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta-analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. Int Clin Psychopharmacol 25:189–198. https://doi.org/10.1097/YIC.0b013e328330adb2
88. Lavergne F, Berlin I, Gamma A, et al (2005) Onset of improvement and response to mirtazapine in depression: a multicenter naturalistic study of 4771 patients. Neuropsychiatr Dis Treat 1:59–68
89. Schwasinger-Schmidt TE, Macaluso M (2019) Other Antidepressants. Handb Exp Pharmacol 250:325–355. https://doi.org/10.1007/164_2018_167
90. Watanabe N, Omori IM, Nakagawa A, et al (2011) Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev CD006528. https://doi.org/10.1002/14651858.CD006528.pub2
91. Iranikhah M, Wensel TM, Thomason AR (2012) Vilazodone for the treatment of major depressive disorder. Pharmacotherapy 32:958–965. https://doi.org/10.1002/j.1875-9114.2012.01121
92. van Amsterdam C, Seyfried CA (2014) Mechanism of action of the bimodal antidepressant vilazodone: evidence for serotonin1A-receptor-mediated auto-augmentation of extracellular serotonin output. Psychopharmacology (Berl) 231:2547–2558. https://doi.org/10.1007/s00213-013-3428-7
93. Wysokiński A, Kłoszewska I (2013) Wilazodon — nowy wielofunkcyjny lek przeciwdepresyjny. Psychiatria 10:72–75
94. Wang S-M, Han C, Lee S-J, et al (2016) Vilazodone for the Treatment of Depression: An Update. Chonnam Med J 52:91–100. https://doi.org/10.4068/cmj.2016.52.2.91
95. Khan A (2009) Vilazodone, a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin Investig Drugs 18:1753–1764. https://doi.org/10.1517/13543780903286396
96. Hellerstein DJ, Flaxer J (2015) Vilazodone for the treatment of major depressive disorder: an evidence-based review of its place in therapy. Core Evid 10:49–62. https://doi.org/10.2147/CE.S54075
97. Koesters M, Ostuzzi G, Guaiana G, et al (2017) Vortioxetine for depression in adults. Cochrane Database Syst Rev 7:CD011520. https://doi.org/10.1002/14651858.CD011520.pub2
98. Wilde MI, Benfield P (1995) Tianeptine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depression and coexisting anxiety and depression. Drugs 49:411–439. https://doi.org/10.2165/00003495-199549030-00007
99. Alamo C, García-Garcia P, Lopez-Muñoz F, Zaragozá C (2019) Tianeptine, an atypical pharmacological approach to depression. Rev Psiquiatr Salud Ment (Engl Ed) 12:170–186. https://doi.org/10.1016/j.rpsm.2018.09.002
100. Agabio R, Trogu E, Pani PP (2018) Antidepressants for the treatment of people with co-occurring depression and alcohol dependence. Cochrane Database Syst Rev 4:CD008581. https://doi.org/10.1002/14651858.CD008581.pub2
101. Kasper S, Hajak G, Wulff K, et al (2010) Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry 71:109–120. https://doi.org/10.4088/JCP.09m05347blu
102. Landowski J (2012) Agomelatine - A Novel Antidepressive Drug. Psychiatria 9:11–20
103. Kennedy SH, Rizvi SJ (2010) Agomelatine in the treatment of major depressive disorder. CNS Drugs 24:479–499. https://doi.org/10.2165/11534420-000000000-00000
104. Ribeiro Â, Ribeiro JP, von Doellinger O (2017) Depression and psychodynamic psychotherapy. Braz J Psychiatry 40:105–109. https://doi.org/10.1590/1516-4446-2016-2107
105. Sivertsen H, Bjørkløf GH, Engedal K, et al (2015) Depression and Quality of Life in Older Persons: A Review. Dement Geriatr Cogn Disord 40:311–339. https://doi.org/10.1159/000437299
106. van Bentum JS, van Bronswijk SC, Sijbrandij M, et al (2021) Cognitive therapy and interpersonal psychotherapy reduce suicidal ideation independent from their effect on depression. Depress Anxiety 38:940–949. https://doi.org/10.1002/da.23151
107. Leichsenring F, Luyten P, Abbass A, Steinert C (2021) Psychodynamic therapy of depression. Aust N Z J Psychiatry 55:1202–1203. https://doi.org/10.1177/00048674211031481
108. Cuijpers P, Donker T, Weissman MM, et al (2016) Interpersonal Psychotherapy for Mental Health Problems: A Comprehensive Meta-Analysis. Am J Psychiatry 173:680–687. https://doi.org/10.1176/appi.ajp.2015.15091141
109. Flint J, Cuijpers P, Horder J, et al (2015) Is there an excess of significant findings in published studies of psychotherapy for depression? Psychol Med 45:439–446. https://doi.org/10.1017/S0033291714001421
110. Cuijpers P, Karyotaki E, Weitz E, et al (2014) The effects of psychotherapies for major depression in adults on remission, recovery and improvement: a meta-analysis. J Affect Disord 159:118–126. https://doi.org/10.1016/j.jad.2014.02.026
111. Driessen E, Van HL, Don FJ, et al (2013) The efficacy of cognitive-behavioral therapy and psychodynamic therapy in the outpatient treatment of major depression: a randomized clinical trial. Am J Psychiatry 170:1041–1050. https://doi.org/10.1176/appi.ajp.2013.12070899
112. Munder T, Flückiger C, Leichsenring F, et al (2019) Is psychotherapy effective? A re-analysis of treatments for depression. Epidemiol Psychiatr Sci 28:268–274. https://doi.org/10.1017/S2045796018000355
113. Arnow BA, Constantino MJ (2003) Effectiveness of psychotherapy and combination treatment for chronic depression. Journal of Clinical Psychology 59:893–905. https://doi.org/10.1002/jclp.10181
114. Brinsley J, Schuch F, Lederman O, et al (2021) Effects of yoga on depressive symptoms in people with mental disorders: a systematic review and meta-analysis. Br J Sports Med 55:992–1000. https://doi.org/10.1136/bjsports-2019-101242
115. Cramer H, Lauche R, Langhorst J, Dobos G (2013) Yoga for depression: a systematic review and meta-analysis. Depress Anxiety 30:1068–1083. https://doi.org/10.1002/da.22166
116. Aalbers S, Fusar-Poli L, Freeman RE, et al (2017) Music therapy for depression. Cochrane Database Syst Rev 11:CD004517. https://doi.org/10.1002/14651858.CD004517.pub3
117. Bamber DJ, Stokes CS, Stephen AM (2007) The role of diet in the prevention and management of adolescent depression. Nutrition Bulletin 32:90–99. https://doi.org/10.1111/j.1467-3010.2007.00608.x
118. Marx W, Lane M, Hockey M, et al (2021) Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry 26:134–150. https://doi.org/10.1038/s41380-020-00925-x
119. Casseb GAS, Kaster MP, Rodrigues ALS (2019) Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs 33:619–637. https://doi.org/10.1007/s40263-019-00640-4
120. Roy NM, Al-Harthi L, Sampat N, et al (2021) Impact of vitamin D on neurocognitive function in dementia, depression, schizophrenia and ADHD. Front Biosci (Landmark Ed) 26:566–611. https://doi.org/10.2741/4908
121. de Viragh PA, Haglid KG, Celio MR (1989) Parvalbumin increases in the caudate putamen of rats with vitamin D hypervitaminosis. Proc Natl Acad Sci U S A 86:3887–3890
122. Alexianu ME, Robbins E, Carswell S, Appel SH (1998) 1Alpha, 25 dihydroxyvitamin D3-dependent up-regulation of calcium-binding proteins in motoneuron cells. J Neurosci Res 51:58–66. https://doi.org/10.1002/(SICI)1097-4547(19980101)51:1<58::AID-JNR6>3.0.CO;2-K
123. Pérez AV, Picotto G, Carpentieri AR, et al (2008) Minireview on regulation of intestinal calcium absorption. Emphasis on molecular mechanisms of transcellular pathway. Digestion 77:22–34. https://doi.org/10.1159/000116623
124. Berridge MJ (2017) Vitamin D and Depression: Cellular and Regulatory Mechanisms. Pharmacol Rev 69:80–92. https://doi.org/10.1124/pr.116.013227
125. Chen G-L, Miller GM (2012) Advances in tryptophan hydroxylase-2 gene expression regulation: new insights into serotonin-stress interaction and clinical implications. Am J Med Genet B Neuropsychiatr Genet 159B:152–171. https://doi.org/10.1002/ajmg.b.32023
126. Israelyan N, Del Colle A, Li Z, et al (2019) Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology 157:507-521.e4. https://doi.org/10.1053/j.gastro.2019.04.022
127. Schneider B, Weber B, Frensch A, et al (2000) Vitamin D in schizophrenia, major depression and alcoholism. J Neural Transm (Vienna) 107:839–842. https://doi.org/10.1007/s007020070063
128. Wong SK, Chin K-Y, Ima-Nirwana S (2018) Vitamin D and Depression: The Evidence from an Indirect Clue to Treatment Strategy. Current Drug Targets 19:888–897. https://doi.org/10.2174/1389450118666170913161030
129. Buczkowski K, Chlabicz S, Dytfeld J, et al (2013) Wytyczne dla lekarzy rodzinnych dotyczące suplementacji witaminy D. In: Forum Medycyny Rodzinnej. pp 55–58
130. Peterson CT, Rodionov DA, Osterman AL, Peterson SN (2020) B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 12:3380. https://doi.org/10.3390/nu12113380
131. Nguyen HD, Oh H, Hoang NHM, et al (2022) Environmental science and pollution research role of heavy metal concentrations and vitamin intake from food in depression: a national cross-sectional study (2009-2017). Environ Sci Pollut Res Int 29:4574–4586. https://doi.org/10.1007/s11356-021-15986-w
132. Hvas A-M, Juul S, Bech P, Nexø E (2004) Vitamin B6 level is associated with symptoms of depression. Psychother Psychosom 73:340–343. https://doi.org/10.1159/000080386
133. Sánchez-Villegas A, Doreste J, Schlatter J, et al (2009) Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet 22:122–133. https://doi.org/10.1111/j.1365-277X.2008.00931.x
134. Cuomo A, Beccarini Crescenzi B, Bolognesi S, et al (2020) S-Adenosylmethionine (SAMe) in major depressive disorder (MDD): a clinician-oriented systematic review. Ann Gen Psychiatry 19:50. https://doi.org/10.1186/s12991-020-00298-z
135. Wu Y, Zhang L, Li S, Zhang D (2022) Associations of dietary vitamin B1, vitamin B2, vitamin B6, and vitamin B12 with the risk of depression: a systematic review and meta-analysis. Nutr Rev 80:351–366. https://doi.org/10.1093/nutrit/nuab014
136. Jong Baw PG, van Veen MM, Hoek HW (2008) [Thiamine deficiency caused by malnutrition: a rare cause?]. Tijdschr Psychiatr 50:611–615
137. Bell IR, Edman JS, Morrow FD, et al (1992) Brief communication. Vitamin B1, B2, and B6 augmentation of tricyclic antidepressant treatment in geriatric depression with cognitive dysfunction. J Am Coll Nutr 11:159–163
138. Shorvon SD, Carney MW, Chanarin I, Reynolds EH (1980) The neuropsychiatry of megaloblastic anaemia. Br Med J 281:1036–1038. https://doi.org/10.1136/bmj.281.6247.1036
139. Carney MW, Chary TK, Laundy M, et al (1990) Red cell folate concentrations in psychiatric patients. J Affect Disord 19:207–213. https://doi.org/10.1016/0165-0327(90)90093-n
140. Coppen A, Bolander-Gouaille C (2005) Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol 19:59–65. https://doi.org/10.1177/0269881105048899
141. Coppen A, Bailey J (2000) Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord 60:121–130. https://doi.org/10.1016/s0165-0327(00)00153-1
142. Doseděl M, Jirkovský E, Macáková K, et al (2021) Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 13:615. https://doi.org/10.3390/nu13020615
143. Evans-Olders R, Eintracht S, Hoffer LJ (2010) Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition 26:1070–1074. https://doi.org/10.1016/j.nut.2009.08.015
144. Cocchi P, Silenzi M, Calabri G, Salvi G (1980) Antidepressant effect of vitamin C. Pediatrics 65:862–863
145. Zhang M, Robitaille L, Eintracht S, Hoffer LJ (2011) Vitamin C provision improves mood in acutely hospitalized patients. Nutrition 27:530–533. https://doi.org/10.1016/j.nut.2010.05.016
146. Gariballa S (2014) Poor vitamin C status is associated with increased depression symptoms following acute illness in older people. Int J Vitam Nutr Res 84:12–17. https://doi.org/10.1024/0300-9831/a000188
147. Sarandol A, Sarandol E, Eker SS, et al (2007) Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol 22:67–73. https://doi.org/10.1002/hup.829
148. Pehlivan FE (2017) Vitamin C: An Antioxidant Agent. In: Hamza AH (ed). InTech
149. Rebec GV, Pierce RC (1994) A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog Neurobiol 43:537–565. https://doi.org/10.1016/0301-0082(94)90052-3
150. Amr M, El-Mogy A, Shams T, et al (2013) Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. Nutr J 12:31. https://doi.org/10.1186/1475-2891-12-31
151. Ferland G (2013) Vitamin K and Brain Function. Semin Thromb Hemost 849–855. https://doi.org/10.1055/s-0033-1357481
152. Westhofen P, Watzka M, Marinova M, et al (2011) Human Vitamin K 2,3-Epoxide Reductase Complex Subunit 1-like 1 (VKORC1L1) Mediates Vitamin K-dependent Intracellular Antioxidant Function. J Biol Chem 286:15085–15094. https://doi.org/10.1074/jbc.M110.210971
153. Gancheva SM, Zhelyazkova-Savova MD (2016) Vitamin K2 Improves Anxiety and Depression but not Cognition in Rats with Metabolic Syndrome: a Role of Blood Glucose? Folia Med (Plovdiv) 58:264–272. https://doi.org/10.1515/folmed-2016-0032
154. Cazzola R, Della Porta M, Manoni M, et al (2020) Going to the roots of reduced magnesium dietary intake: A tradeoff between climate changes and sources. Heliyon 6:e05390. https://doi.org/10.1016/j.heliyon.2020.e05390
155. Derom M-L, Sayón-Orea C, Martínez-Ortega JM, Martínez-González MA (2013) Magnesium and depression: a systematic review. Nutr Neurosci 16:191–206. https://doi.org/10.1179/1476830512Y.0000000044
156. Eby GA, Eby KL (2006) Rapid recovery from major depression using magnesium treatment. Med Hypotheses 67:362–370. https://doi.org/10.1016/j.mehy.2006.01.047
157. Lawley SI, Kantak KM (1990) Magnesium-induced conditioned place preference in mice. Pharmacology Biochemistry and Behavior 36:539–545. https://doi.org/10.1016/0091-3057(90)90253-E
158. Serefko A, Szopa A, Wlaź P, et al (2013) Magnesium in depression. Pharmacol Rep 65:547–554. https://doi.org/10.1016/s1734-1140(13)71032-6
159. Theil EC (2011) Iron homeostasis and nutritional iron deficiency. J Nutr 141:724S-728S. https://doi.org/10.3945/jn.110.127639
160. Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19:164–174
161. Khedr E, Hamed SA, Elbeih E, et al (2008) Iron states and cognitive abilities in young adults: neuropsychological and neurophysiological assessment. Eur Arch Psychiatry Clin Neurosci 258:489–496. https://doi.org/10.1007/s00406-008-0822-y
162. Vahdat Shariatpanaahi M, Vahdat Shariatpanaahi Z, Moshtaaghi M, et al (2007) The relationship between depression and serum ferritin level. Eur J Clin Nutr 61:532–535. https://doi.org/10.1038/sj.ejcn.1602542
163. Mlyniec K (2015) Zinc in the Glutamatergic Theory of Depression. Curr Neuropharmacol 13:505–513. https://doi.org/10.2174/1570159 × 13666150115220617
164. Mlyniec K (2021) Interaction between Zinc, GPR39, BDNF and Neuropeptides in Depression. Curr Neuropharmacol 19:2012–2019. https://doi.org/10.2174/1570159 × 19666210225153404
165. da Silva LEM, de Santana MLP, Costa PR de F, et al (2021) Zinc supplementation combined with antidepressant drugs for treatment of patients with depression: a systematic review and meta-analysis. Nutr Rev 79:1–12. https://doi.org/10.1093/nutrit/nuaa039
166. Ni M, You Y, Chen J, Zhang L (2018) Copper in depressive disorder: A systematic review and meta-analysis of observational studies. Psychiatry Res 267:506–515. https://doi.org/10.1016/j.psychres.2018.05.049
167. Ma J, Betts NM (2000) Zinc and copper intakes and their major food sources for older adults in the 1994-96 continuing survey of food intakes by individuals (CSFII). J Nutr 130:2838–2843. https://doi.org/10.1093/jn/130.11.2838
168. Narang RL, Gupta KR, Narang AP, Singh R (1991) Levels of copper and zinc in depression. Indian J Physiol Pharmacol 35:272–274
169. Crayton JW, Walsh WJ (2007) Elevated serum copper levels in women with a history of post-partum depression. J Trace Elem Med Biol 21:17–21. https://doi.org/10.1016/j.jtemb.2006.10.001
170. Stys PK, You H, Zamponi GW (2012) Copper-dependent regulation of NMDA receptors by cellular prion protein: implications for neurodegenerative disorders. J Physiol 590:1357–1368. https://doi.org/10.1113/jphysiol.2011.225276
171. Pasco JA, Jacka FN, Williams LJ, et al (2012) Dietary selenium and major depression: a nested case-control study. Complement Ther Med 20:119–123. https://doi.org/10.1016/j.ctim.2011.12.008
172. Wang J, Um P, Dickerman BA, Liu J (2018) Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 10:584. https://doi.org/10.3390/nu10050584
173. Duntas LH (2009) Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res 41:443–447. https://doi.org/10.1055/s-0029-1220724
174. Köhrle J, Jakob F, Contempré B, Dumont JE (2005) Selenium, the thyroid, and the endocrine system. Endocr Rev 26:944–984. https://doi.org/10.1210/er.2001-0034
175. Młyniec K, Gaweł M, Doboszewska U, et al (2015) Essential elements in depression and anxiety. Part II. Pharmacol Rep 67:187–194. https://doi.org/10.1016/j.pharep.2014.09.009
176. Sajjadi SS, Foshati S, Haddadian-Khouzani S, Rouhani MH (2022) The role of selenium in depression: a systematic review and meta-analysis of human observational and interventional studies. Sci Rep 12:1045. https://doi.org/10.1038/s41598-022-05078-1
177. Mokhber N, Namjoo M, Tara F, et al (2011) Effect of supplementation with selenium on postpartum depression: a randomized double-blind placebo-controlled trial. J Matern Fetal Neonatal Med 24:104–108. https://doi.org/10.3109/14767058.2010.482598
178. Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, et al (2013) Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ 454–455:562–577. https://doi.org/10.1016/j.scitotenv.2013.03.047
179. de Sousa RT, Zanetti MV, Talib LL, et al (2015) Lithium increases platelet serine-9 phosphorylated GSK-3β levels in drug-free bipolar disorder during depressive episodes. J Psychiatr Res 62:78–83. https://doi.org/10.1016/j.jpsychires.2015.01.016
180. Yasuda S, Liang M-H, Marinova Z, et al (2009) The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 14:51–59. https://doi.org/10.1038/sj.mp.4002099
181. Won E, Kim Y-K (2017) An Oldie but Goodie: Lithium in the Treatment of Bipolar Disorder through Neuroprotective and Neurotrophic Mechanisms. Int J Mol Sci 18:2679. https://doi.org/10.3390/ijms18122679
182. Pacholko AG, Bekar LK (2021) Lithium orotate: A superior option for lithium therapy? Brain Behav 11:e2262. https://doi.org/10.1002/brb3.2262
183. Hidaka T, Fujii K, Funahashi I, et al (2008) Safety assessment of coenzyme Q10 (CoQ10). Biofactors 32:199–208. https://doi.org/10.1002/biof.5520320124
184. Raizner AE (2019) Coenzyme Q10. Methodist Debakey Cardiovasc J 15:185–191. https://doi.org/10.14797/mdcj-15-3-185
185. Chaturvedi RK, Beal MF (2008) Mitochondrial approaches for neuroprotection. Ann N Y Acad Sci 1147:395–412. https://doi.org/10.1196/annals.1427.027
186. Crane FL (2001) Biochemical functions of coenzyme Q10. J Am Coll Nutr 20:591–598. https://doi.org/10.1080/07315724.2001.10719063
187. Maes M, Galecki P, Chang YS, Berk M (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 35:676–692. https://doi.org/10.1016/j.pnpbp.2010.05.004
188. Maes M, Mihaylova I, Kubera M, et al (2009) Lower plasma Coenzyme Q10 in depression: a marker for treatment resistance and chronic fatigue in depression and a risk factor to cardiovascular disorder in that illness. Neuro Endocrinol Lett 30:462–469
189. Sanoobar M, Dehghan P, Khalili M, et al (2016) Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr Neurosci 19:138–143. https://doi.org/10.1179/1476830515Y.0000000002
190. Forester BP, Harper DG, Georgakas J, et al (2015) Antidepressant effects of open label treatment with coenzyme Q10 in geriatric bipolar depression. J Clin Psychopharmacol 35:338–340. https://doi.org/10.1097/JCP.0000000000000326
191. Shabbir F, Patel A, Mattison C, et al (2013) Effect of diet on serotonergic neurotransmission in depression. Neurochem Int 62:324–329. https://doi.org/10.1016/j.neuint.2012.12.014
192. Simon LV, Keenaghan M (2023) Serotonin Syndrome. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
193. Kałużna-Czaplińska J, Gątarek P, Chirumbolo S, et al (2019) How important is tryptophan in human health? Crit Rev Food Sci Nutr 59:72–88. https://doi.org/10.1080/10408398.2017.1357534
194. Li Y, Cheng Y, Zhou Y, et al (2022) High fat diet-induced obesity leads to depressive and anxiety-like behaviors in mice via AMPK/mTOR-mediated autophagy. Exp Neurol 348:113949. https://doi.org/10.1016/j.expneurol.2021.113949
195. Tsaluchidu S, Cocchi M, Tonello L, Puri BK (2008) Fatty acids and oxidative stress in psychiatric disorders. BMC Psychiatry 8:S5. https://doi.org/10.1186/1471-244X-8-S1-S5
196. Majkutewicz P, Tyszko P, Okręglicka K (2014) Leczenie żywieniowe depresji. Family Medicine & Primary Care Review 48–50
197. Wilczynska A (2012) Kwasy tłuszczowe w diecie człowieka a jego funkcjonowanie poznawcze i emocjonalne. Neuropsychiatria i Neuropsychologia 7:35–42
198. Cunnane SC, Plourde M, Pifferi F, et al (2009) Fish, docosahexaenoic acid and Alzheimer's disease. Prog Lipid Res 48:239–256. https://doi.org/10.1016/j.plipres.2009.04.001
199. Marciniak-Lukasiak K (2011) Rola i znaczenie kwasów tłuszczowych omega-3. Żywność Nauka Technologia Jakość 18:
200. Simopoulos AP (2013) Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome, brain metabolic abnormalities, and non-alcoholic fatty liver disease. Nutrients 5:2901–2923. https://doi.org/10.3390/nu5082901
201. Patrick RP, Ames BN (2015) Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J 29:2207–2222. https://doi.org/10.1096/fj.14-268342
202. Tang W, Wang Y, Xu F, et al (2020) Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav Immun 88:529–534. https://doi.org/10.1016/j.bbi.2020.04.034
203. Zhang C, Fang X, Yao P, et al (2017) Metabolic adverse effects of olanzapine on cognitive dysfunction: A possible relationship between BDNF and TNF-alpha. Psychoneuroendocrinology 81:138–143. https://doi.org/10.1016/j.psyneuen.2017.04.014
204. Farioli-Vecchioli S, Sacchetti S, di Robilant NV, Cutuli D (2018) The Role of Physical Exercise and Omega-3 Fatty Acids in Depressive Illness in the Elderly. Curr Neuropharmacol 16:308–326. https://doi.org/10.2174/1570159 × 15666170912113852
205. Liao Y, Xie B, Zhang H, et al (2019) Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl Psychiatry 9:190. https://doi.org/10.1038/s41398-019-0515-5
206. Giles GE, Mahoney CR, Kanarek RB (2013) Omega-3 fatty acids influence mood in healthy and depressed individuals. Nutr Rev 71:727–741. https://doi.org/10.1111/nure.12066
207. Białek M, Rutkowska J (2015) [The importance of γ-linolenic acid in the prevention and treatment]. Postepy Hig Med Dosw (Online) 69:892–904. https://doi.org/10.5604/17322693.1162991
208. Flider F (2005) GLA: Uses and new sources. 16:
209. Horrobin DF (1993) Fatty acid metabolism in health and disease: the role of delta-6-desaturase. Am J Clin Nutr 57:732S-736S; discussion 736S-737S. https://doi.org/10.1093/ajcn/57.5.732S
210. Yary T, Tolmunen T, Lehto SM, et al (2017) Serum dihomo-γ-linolenic acid level is inversely associated with the risk of depression. A 21-year follow-up study in general population men. J Affect Disord 213:151–155. https://doi.org/10.1016/j.jad.2017.02.022
211. Chalon S, Vancassel S, Zimmer L, et al (2001) Polyunsaturated fatty acids and cerebral function: focus on monoaminergic neurotransmission. Lipids 36:937–944. https://doi.org/10.1007/s11745-001-0804-7
212. O'Brien PD, Hinder LM, Callaghan BC, Feldman EL (2017) Neurological consequences of obesity. Lancet Neurol 16:465–477. https://doi.org/10.1016/S1474-4422(17)30084-4
213. Zemdegs J, Quesseveur G, Jarriault D, et al (2016) High-fat diet-induced metabolic disorders impairs 5-HT function and anxiety-like behavior in mice. Br J Pharmacol 173:2095–2110. https://doi.org/10.1111/bph.13343
214. Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34. https://doi.org/10.1038/nri.2015.5
215. Liu T, Deng K, Xue Y, et al (2022) Carnitine and Depression. Front Nutr 9:853058. https://doi.org/10.3389/fnut.2022.853058
216. Evans AM, Fornasini G (2003) Pharmacokinetics of L-carnitine. Clin Pharmacokinet 42:941–967. https://doi.org/10.2165/00003088-200342110-00002
217. Zanardi R, Smeraldi E (2006) A double-blind, randomised, controlled clinical trial of acetyl-L-carnitine vs. amisulpride in the treatment of dysthymia. Eur Neuropsychopharmacol 16:281–287. https://doi.org/10.1016/j.euroneuro.2005.10.005
218. Nasca C, Bigio B, Lee FS, et al (2018) Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc Natl Acad Sci U S A 115:8627–8632. https://doi.org/10.1073/pnas.1801609115
219. Tashiro K, Kaida Y, Yamagishi S-I, et al (2017) L-Carnitine Supplementation Improves Self-Rating Depression Scale Scores in Uremic Male Patients Undergoing Hemodialysis. Lett Drug Des Discov 14:737–742. https://doi.org/10.2174/1570180814666170216102632
220. Son H, Baek JH, Go BS, et al (2018) Glutamine has antidepressive effects through increments of glutamate and glutamine levels and glutamatergic activity in the medial prefrontal cortex. Neuropharmacology 143:143–152. https://doi.org/10.1016/j.neuropharm.2018.09.040
221. Trumbo P, Schlicker S, Yates AA, et al (2002) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 102:1621–1630. https://doi.org/10.1016/s0002-8223(02)90346-9
222. Lee Y, Son H, Kim G, et al (2013) Glutamine deficiency in the prefrontal cortex increases depressive-like behaviours in male mice. J Psychiatry Neurosci 38:183–191. https://doi.org/10.1503/jpn.120024
223. Hasler G, van der Veen JW, Tumonis T, et al (2007) Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. https://doi.org/10.1001/archpsyc.64.2.193
224. Young LS, Bye R, Scheltinga M, et al (1993) Patients receiving glutamine-supplemented intravenous feedings report an improvement in mood. JPEN J Parenter Enteral Nutr 17:422–427. https://doi.org/10.1177/0148607193017005422
225. Morris G, Anderson G, Dean O, et al (2014) The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol 50:1059–1084. https://doi.org/10.1007/s12035-014-8705-x
226. Gawryluk JW, Wang J-F, Andreazza AC, et al (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14:123–130. https://doi.org/10.1017/S1461145710000805
227. Gibson SA, Korade Ž, Shelton RC (2012) Oxidative stress and glutathione response in tissue cultures from persons with major depression. Journal of Psychiatric Research 46:1326–1332. https://doi.org/10.1016/j.jpsychires.2012.06.008
228. Maes M, Mihaylova I, Kubera M, et al (2011) Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis / chronic fatigue syndrome: another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuro Endocrinol Lett 32:133–140
229. Gold SM, Dziobek I, Sweat V, et al (2007) Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50:711–719. https://doi.org/10.1007/s00125-007-0602-7
230. Habib S, Sangaraju SL, Yepez D, et al (2022) The Nexus Between Diabetes and Depression: A Narrative Review. Cureus 14:. https://doi.org/10.7759/cureus.25611
231. van Dooren FEP, Pouwer F, Schalkwijk CG, et al (2017) Advanced Glycation End Product (AGE) Accumulation in the Skin is Associated with Depression: The Maastricht Study. Depress Anxiety 34:59–67. https://doi.org/10.1002/da.22527
232. Witek L, Kowalska I, Adamska A (2019) The association between depression and diabetes — the role of the hypothalamo- -pituitary-adrenal axis and chronic inflammation. Clinical Diabetology 8:127–131. https://doi.org/10.5603/DK.2019.0007
233. Oltmanns KM, Dodt B, Schultes B, et al (2006) Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur J Endocrinol 154:325–331. https://doi.org/10.1530/eje.1.02074
234. Knüppel A, Shipley MJ, Llewellyn CH, Brunner EJ (2017) Sugar intake from sweet food and beverages, common mental disorder and depression: prospective findings from the Whitehall II study. Sci Rep 7:6287. https://doi.org/10.1038/s41598-017-05649-7
235. Smith DF (2013) Benefits of flavanol-rich cocoa-derived products for mental well-being: A review. Journal of Functional Foods 5:10–15. https://doi.org/10.1016/j.jff.2012.09.002
236. Kontogianni MD, Vijayakumar A, Rooney C, et al (2020) A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients 12:E2445. https://doi.org/10.3390/nu12082445
237. Godos J, Castellano S, Ray S, et al (2018) Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules 23:E999. https://doi.org/10.3390/molecules23050999
238. Dreiseitel A, Korte G, Schreier P, et al (2009) Berry anthocyanins and their aglycons inhibit monoamine oxidases A and B. Pharmacol Res 59:306–311. https://doi.org/10.1016/j.phrs.2009.01.014
239. Shewale PB, Patil RA, Hiray YA (2012) Antidepressant-like activity of anthocyanidins from Hibiscus rosa-sinensis flowers in tail suspension test and forced swim test. Indian J Pharmacol 44:454–457. https://doi.org/10.4103/0253-7613.99303
240. Quincozes-Santos A, Bobermin LD, Latini A, et al (2013) Resveratrol Protects C6 Astrocyte Cell Line against Hydrogen Peroxide-Induced Oxidative Stress through Heme Oxygenase 1. PLoS One 8:e64372. https://doi.org/10.1371/journal.pone.0064372
241. Moore A, Beidler J, Hong MY (2018) Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules 23:E2197. https://doi.org/10.3390/molecules23092197
242. Yáñez M, Fraiz N, Cano E, Orallo F (2006) Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem Biophys Res Commun 344:688–695. https://doi.org/10.1016/j.bbrc.2006.03.190
243. Rahmani AH, Alsahli MA, Aly SM, et al (2018) Role of Curcumin in Disease Prevention and Treatment. Adv Biomed Res 7:38. https://doi.org/10.4103/abr.abr_147_16
244. Kocaadam B, Şanlier N (2017) Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr 57:2889–2895. https://doi.org/10.1080/10408398.2015.1077195
245. Niu K, Hozawa A, Kuriyama S, et al (2009) Green tea consumption is associated with depressive symptoms in the elderly. Am J Clin Nutr 90:1615–1622. https://doi.org/10.3945/ajcn.2009.28216
246. Bandaruk Y, Mukai R, Kawamura T, et al (2012) Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. J Agric Food Chem 60:10270–10277. https://doi.org/10.1021/jf303055b
247. Lardner AL (2014) Neurobiological effects of the green tea constituent theanine and its potential role in the treatment of psychiatric and neurodegenerative disorders. Nutr Neurosci 17:145–155. https://doi.org/10.1179/1476830513Y.0000000079
248. Pase MP, Scholey AB, Pipingas A, et al (2013) Cocoa polyphenols enhance positive mood states but not cognitive performance: a randomized, placebo-controlled trial. J Psychopharmacol 27:451–458. https://doi.org/10.1177/0269881112473791
249. Bedel HA, Kencebay Manas C, Özbey G, Usta C (2018) The antidepressant-like activity of ellagic acid and its effect on hippocampal brain derived neurotrophic factor levels in mouse depression models. Nat Prod Res 32:2932–2935. https://doi.org/10.1080/14786419.2017.1385021
250. Lorigooini Z, Salimi N, Soltani A, Amini-Khoei H (2019) Implication of NMDA-NO pathway in the antidepressant-like effect of ellagic acid in male mice. Neuropeptides 76:101928. https://doi.org/10.1016/j.npep.2019.04.003
251. Scarante FF, Lopes VD, Fusse EJ, et al (2021) Cannabidiol as an add-on therapy to overcome the slow-onset and, possibly, resistance to antidepressant treatment: involvement of NAPE-PLD in the medial prefrontal cortex. bioRxiv 2021–04
252. Siddiqui SA, Ali Redha A, Snoeck ER, et al (2022) Anti-Depressant Properties of Crocin Molecules in Saffron. Molecules 27:2076. https://doi.org/10.3390/molecules27072076
253. Lopresti AL, Smith SJ, Malvi H, Kodgule R (2019) An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine (Baltimore) 98:e17186. https://doi.org/10.1097/MD.0000000000017186
254. Jin Y, Cui R, Zhao L, et al (2019) Mechanisms of Panax ginseng action as an antidepressant. Cell Prolif 52:e12696. https://doi.org/10.1111/cpr.12696
255. Xie W, Meng X, Zhai Y, et al (2018) Panax Notoginseng Saponins: A Review of Its Mechanisms of Antidepressant or Anxiolytic Effects and Network Analysis on Phytochemistry and Pharmacology. Molecules 23:940. https://doi.org/10.3390/molecules23040940
256. Zhang H, Li Z, Zhou Z, et al (2016) Antidepressant-like effects of ginsenosides: A comparison of ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, compound K, and 20(S)-protopanaxadiol in mice models of despair. Pharmacol Biochem Behav 140:17–26. https://doi.org/10.1016/j.pbb.2015.10.018
257. Swann OG, Kilpatrick M, Breslin M, Oddy WH (2020) Dietary fiber and its associations with depression and inflammation. Nutr Rev 78:394–411. https://doi.org/10.1093/nutrit/nuz072
258. Ma Y, Griffith JA, Chasan-Taber L, et al (2006) Association between dietary fiber and serum C-reactive protein-. Am J Clin Nutr 83:760–766
259. Harsanyi S, Kupcova I, Danisovic L, Klein M (2022) Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int J Mol Sci 24:578. https://doi.org/10.3390/ijms24010578
260. Stokes N, Gordon CM, DiVasta A (2011) Vegetarian diets and mental health in adolescents with anorexia nervosa. Journal of Adolescent Health 48:S50
261. Ricci A, Idzikowski MA, Soares CN, Brietzke E (2020) Exploring the mechanisms of action of the antidepressant effect of the ketogenic diet. Rev Neurosci 31:637–648. https://doi.org/10.1515/revneuro-2019-0073
262. Murphy P, Likhodii S, Nylen K, Burnham WM (2004) The antidepressant properties of the ketogenic diet. Biological psychiatry 56:981–983
263. Chmiel I (2021) Ketogenic diet in therapy of bipolar affective disorder - case report and literature review. Psychiatr Pol 1–19. https://doi.org/10.12740/PP/OnlineFirst/136356
264. Iguacel I, Huybrechts I, Moreno LA, Michels N (2021) Vegetarianism and veganism compared with mental health and cognitive outcomes: a systematic review and meta-analysis. Nutr Rev 79:361–381. https://doi.org/10.1093/nutrit/nuaa030
265. Kris-Etherton PM, Petersen KS, Hibbeln JR, et al (2021) Nutrition and behavioral health disorders: depression and anxiety. Nutr Rev 79:247–260. https://doi.org/10.1093/nutrit/nuaa025
266. Li Z, Li B, Song X, Zhang D (2017) Dietary zinc and iron intake and risk of depression: A meta-analysis. Psychiatry Res 251:41–47. https://doi.org/10.1016/j.psychres.2017.02.006
267. Lavallee K, Zhang XC, Michalak J, et al (2019) Vegetarian diet and mental health: Cross-sectional and longitudinal analyses in culturally diverse samples. J Affect Disord 248:147–154. https://doi.org/10.1016/j.jad.2019.01.035
268. Weikert C, Trefflich I, Menzel J, et al (2020) Vitamin and Mineral Status in a Vegan Diet. Dtsch Arztebl Int 117:575–582. https://doi.org/10.3238/arztebl.2020.0575
269. Parletta N, Zarnowiecki D, Cho J, et al (2019) A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr Neurosci 22:474–487. https://doi.org/10.1080/1028415X.2017.1411320
270. O'Neill S, Minehan M, Knight-Agarwal CR, Turner M (2022) Depression, Is It Treatable in Adults Utilising Dietary Interventions? A Systematic Review of Randomised Controlled Trials. Nutrients 14:1398. https://doi.org/10.3390/nu14071398
271. Oddo VM, Welke L, McLeod A, et al (2022) Adherence to a Mediterranean Diet Is Associated with Lower Depressive Symptoms among U.S. Adults. Nutrients 14:278. https://doi.org/10.3390/nu14020278
272. Pietrzak D, Kasperek K, Rękawek P, Piątkowska-Chmiel I (2022) The Therapeutic Role of Ketogenic Diet in Neurological Disorders. Nutrients 14:1952. https://doi.org/10.3390/nu14091952
273. Włodarczyk A, Cubała WJ, Stawicki M (2021) Ketogenic diet for depression: A potential dietary regimen to maintain euthymia? Prog Neuropsychopharmacol Biol Psychiatry 109:110257. https://doi.org/10.1016/j.pnpbp.2021.110257
274. Gezmen-Karadağ M, Çelik E, Kadayifçi FZ, et al (2018) Role of food‑drug interactions in neurological and psychological diseases. Acta Neurobiol Exp (Wars) 78:187–197
275. Wasik A, Krupa A, Siwek M (2019) Interactions of antidepressants, mood-stabilisers and antipsychotics with food. Pharmacotherapy in Psychiatry and Neurology 35:51–74. https://doi.org/10.33450/fpn.2019.04.002
276. Rizea-Savu S, Duna SN, Ghita A, et al (2020) The Effect of Food on the Single-Dose Bioavailability and Tolerability of the Highest Marketed Strength of Duloxetine. Clin Pharmacol Drug Dev 9:797–804. https://doi.org/10.1002/cpdd.759
277. Siwek M (2020) Interactions of antidepressants in primary care practice. Lekarz POZ 6:142–148
278. Bojarowicz H, Dźwigulska P (2012) Suplementy diety. Część III. Interakcje suplementów diety z lekami. 6
279. Woroń J, Siwek M, Wasik A (2019) Interakcje leków w psychiatrii. Gdańsk : AsteriaMed
280. López-Muñoz F, Álamo C (2013) Active Metabolites as Antidepressant Drugs: The Role of Norquetiapine in the Mechanism of Action of Quetiapine in the Treatment of Mood Disorders. Front Psychiatry 4:102. https://doi.org/10.3389/fpsyt.2013.00102
281. Won CS, Oberlies NH, Paine MF (2012) Mechanisms underlying food-drug interactions: inhibition of intestinal metabolism and transport. Pharmacol Ther 136:186–201. https://doi.org/10.1016/j.pharmthera.2012.08.001
282. Commissioner O of the (2021) Grapefruit Juice and Some Drugs Don't Mix. FDA
283. Singh YN (2005) Potential for interaction of kava and St. John's wort with drugs. J Ethnopharmacol 100:108–113. https://doi.org/10.1016/j.jep.2005.05.014