Abstract
Contemporary scientific findings revealed that our daily food stuffs are enriched by encrypted bioactive peptides (BPs), evolved by peptide linkage of amino acids or encrypted from the native protein structures. Remarkable to these BPs lies in their potential health benefiting biological activities to serve as nutraceuticals or a lead addition to the development of functional foods. The biological activities of BPs vary depending on the sequence as well as amino acid composition. Existing database records approximately 3000 peptide sequences which possess potential biological activities such as antioxidants, antihypertensive, antithrombotic, anti-adipogenics, anti-microbials, anti-inflammatory, and anti-cancerous. The growing evidences suggest that BPs have very low toxicity, higher accuracy, less tissue accretion, and are easily degraded in the disposed environment. BPs are nowadays evolved as biologically active molecules with potential scope to reduce microbial contamination as well as ward off oxidation of foods, amend diverse range of human diseases to enhance the overall quality of human life. Against the clinical and health perspectives of BPs, this review aimed to elaborate current evolution of nutritional potential of BPs, studies pertaining to overcome limitations with respect to special focus on emerging extraction, protection and delivery tools of BPs. In addition, the nano-delivery mechanism of BP and its clinical significance is detailed. The aim of current review is to augment the research in the field of BPs production, identification, characterisation and to speed up the investigation of the incredible potentials of BPs as potential nutritional and functional food ingredient.
Keywords: Bioactive peptides, Nutritional, Nano-delivery, Peptide sequence, Enzyme activity, Functional food
Introduction
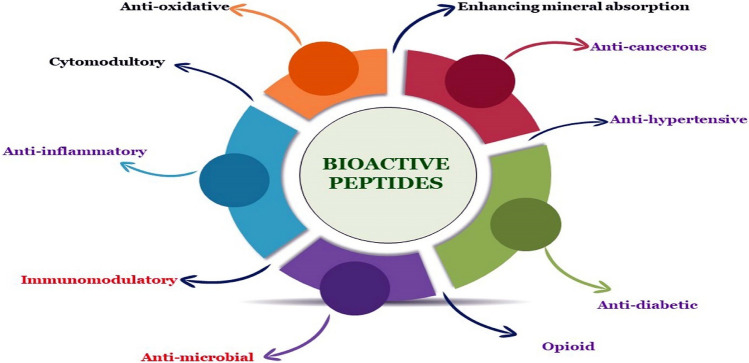
Food safety is an emerging concern for humans on global scale to guard the health of end-use consumers. The ever-growing consumption of processed foods laced with chemical preservatives and synthetic food additives is raising threat to human health (Gizaw 2019). This concern has forced to shift the focus on consuming natural foods and reducing the intake of processed food items. Consequently, scientists are showing growing interest to explore nutraceutical agents mostly bioactive peptides (BPs) found in natural foods to serve as a gross component in our daily food intake (Thomas and Latha 2022). BPs are built by amino acids bound by peptide bonds and comprises of a peptide sequence between 2 and 20 amino acids (Minkiewicz et al. 2019). BPs have found beneficial effects on the proper functioning of the metabolism and overall health status of human beings (Redha et al. 2022). Therefore, the beneficial effects of various BPs are increasing with regards to health promotion and disease prevention and show a wide range of biological activities both in vivo and in-vitro. BPs production has opened up new opportunities to discover treatments for various diseases therefore, strengthening the standard of life. BPs have also been regarded as the recent procreation to inhibit microbial oxidation and degradation of food (Daliri et al. 2017). Presently, almost 3000 BPs have been documented in the existing database and have been entitled as BIOPEP-UWM™ formerly known as ‘Biopep’ (Minkiewicz et al. 2019). Inevitably, significant interest has been dedicated to the preparation of BPs (Manzoor et al. 2022) which are found to exhibit a range of nutraceutical properties such as anti-microbial, anti-hypertensive, opioid, anti-cancerous, immune-modulatory, and anti-oxidative as depicted in Fig. 1. The source of BPs can be endogenous or exogenous if taken through the diet or from an outside origin respectively and are, therefore, isolated from different sources such as plants, animals, and microorganisms to serve as promising biomolecules and possess a wide range of nutraceutical properties as described in Table 1. Protein and peptide-based drugs are accessible from separate origins for therapeutic uses, but along with many drugs, they have diverse beneficial effects as compared to synthetic drugs. Peptide drugs are successful and specified to the biological targets and tremendous developments in clinical strategies have unlocked modern day possibilities for drug discovery in the field of peptide and protein drugs (Ilangala et al. 2021). The target host defines ultimately the selection of techniques for the extraction and purifications of BPs. Recent advances in analytical techniques have paved new opportunities in the field of peptide and protein drugs for the production of pharmaceuticals against various health diseases. A lot of improvements have been reported in the purification and analytical techniques to characterise these compounds which aim to attain further understanding of the complexity of different molecular structures of BPs (Lafarga et al. 2020). Although BPs have been identified and extracted from different natural sources, their undertaking is examined in various disciplines and the current review typically relates BP to the theme of distinct food matrices. However, we are still at infancy to have a composite study providing an updated account on BPs with respect to their existence in across plant and animal kingdoms and technical know-how regarding their production and characterization. This prompted the authors to mention the current extraction techniques for generation of bioactive peptides from different sources. Moreover, we have also emphasised their role as natural therapeutic agents for a diverse range of diseases and their delivery systems. The current review is believed to augment the research in the field of BP’s production, nutraceutical potential, identification, and characterization and speeds up investigating the incredible potentials of BPs as a potential nutraceutical and new addition to the list of functional foods (Verma and Chandel 2019).
Fig. 1.
Bioactive properties of food protein-derived peptides related to their promotion of human health and disease prevention. Here we have highlighted the role of BP as anti-oxidative, anti-inflammatory, anti-hypertensive, anti-microbial, anti-diabetic etc. The BPs act upon the AT1 receptors as well as act on renin—angiotensinogen converting enzymes as inhibitors to impart there in vivo anti-hypertensive benefits. BPs induce apoptosis by caspase independent as well as caspase dependent pathways in the mitochondria, hence anti-cancerous effects
Table 1.
Details of bioactive peptides and their major sources, extraction methods and sequences employed as potential nutraceuticals
| Nutraceutical properties | Sources | Extraction method | Peptide sequence/s | Reference/s |
|---|---|---|---|---|
| Anti-oxidant | Plant-based bioactive peptides | |||
| Triticum aestivum | Enzymatic hydrolysis |
Seven peptides- • NL • QL • FL • HAL • AAVL • AKTVF • TPLTR |
Zou et al. (2020) | |
| Oryza sativa | Enzymatic hydrolysis | • YSK | Wang et al. (2017a, b) | |
| Cicer arietinum | Enzymatic hydrolysis | • ALEPDHR | Faridy et al. (2020) | |
| Brassica napus | Enzymatic hydrolysis | • WDHHAPQLR | Xu et al. (2018) | |
| Glycine max | Enzymatic hydrolysis | • L/IVPK | Chen et al. (2019) | |
| Oryza sativa | Enzymatic hydrolysis | • MLHYGTP | Zaky et al. (2020) | |
| Arachis hypogaea | Fermentation | • QIPQQFLRTLPM SVNVPL | Karami et al. (2019) | |
| Animal-based bioactive peptides | ||||
| Pollock skin collagen | Enzymatic hydrolysis | • GPAGPHGPPG | Guo et al. (2015) | |
| Duck skin gelatin | Enzyme hydrolysates | • HTVQCMFQ | ||
| Marine based bioactive peptides | ||||
| Pyropia columbina | Enzymatic hydrolysis | • AF | Cian et al. (2015) | |
| Palmaria palmate (Dulse) Algae | Enzymatic hydrolysis | • VECYGPNRPQF | Harnedy et al. (2017) | |
| Fungal-based bioactive peptides | ||||
| Agaricus bisporus | Enzymatic hydrolysis | – | Farzaneh et al. (2018) | |
| Morchella esculenta | Enzymatic hydrolysis | – | Zhang et al. (2018) | |
| Cordyceps sinensis | Enzymatic hydrolysis | – | Mishra et al. (2019) | |
| Terfezia claveryi | Enzymatic hydrolysis | – | Farzaneh et al. (2018) | |
| Anti-hypertensive | Plant-based bioactive peptide | |||
| Cicer arietinum | Enzymatic extraction | – | Felix et al. (2019) | |
| Zea mays | – | • FNQLAALNSAAYLQQQQLLPFSQLA, MI or LPP | Díaz-Gomez et al. (2022) | |
| Oryza sativa | Enzyme treatment | – | Wang et al. (2017a, b) | |
| Animal-based bioactive peptides | ||||
| Bovine collagen | Enzymatic hydrolysis | • SDNRNQGY, IQVPL& KGLWE | Fu et al. (2016) | |
| Cheese | Ultra filtration | – | Pontonio et al. (2021) | |
| Marine-based bioactive peptide | ||||
| Undaria pinnatifida | Enzymatic hydrolysis and | • GPRGF | Wang et al. (2018) | |
| Palmaria palmata | Enzymatic hydrolysis | • MVGSAPGVL | ||
| Rhopilema esculentum | Enzymatic hydrolysis | • IW | Furuta et al. (2016) | |
| Oncorhynchus gorbuscha | Enzymatic hydrolysis and chromatography | • VYRT, LDY, LRY & FEQDWAS | Borawska-Dziadkiewicz et al. (2021) | |
| Fungal-based bioactive peptides | ||||
| Ganoderma lucidum (mushroom) | Enzymatic hydrolysis | • EPGP & TGDIGY | Wu et al. (2019) | |
| Tricholoma matsutake | Enzymatic hydrolysis | • AHEPVK, RIGLF, PSSNK | Geng et al. (2016) | |
| Anti-diabetic | Plant-based bioactive peptides | |||
| Amaranthus | – | • LTFPGSAED | Ayala-Niño et al. (2020) | |
| Triticum aestivum | Fermentation | • VH & ALGP | Budhwar et al. (2020) | |
| Glycine max | Enzyme treatment | • RCMAFLLSDGAAAAQQLLPQYW | De Brucker et al. (2014) | |
| Phaseolus vulgaris cv | – | • LDAFDPLR & DWLLAGDDY | Lammi et al. (2018) | |
| Soybean | Enzymatic hydrolysis | – | Tamam et al. (2019) | |
| Cumin seed | Enzyme treatment | • KLPGF | Wang et al. (2018) | |
| Oat | Fermentation | • GPAGL | ||
| Animal-based bioactive peptides | ||||
| Meat | Enzymatic digestion |
• LPIIDI, • APGPAGP |
Kęska et al. (2019) | |
| Sheep milk caseins | Enzymatic digestion | • GGSK ELS | El-Sayed and Awad (2019) | |
| Bovine and camel milk | – | – | Ayyash et al. (2018) | |
| Egg | – | – | Liao et al. (2018) | |
| Marine-based bioactive peptide | ||||
| Micromesistius poutassou | Enzymatic treatment | – | Harnedy et al. (2018) | |
| Salmon | – | – | Harnedy et al. (2018) | |
| Porphyra species | – | – | Admassu et al. (2018) | |
| Anti-cancerous | Plant-based bioactive peptides | |||
| Rice bran | Enzymatic extraction | • RQSHFANAQP | Luna-Vital et al. (2015) | |
| Chickpea | – | • VW GQ | ||
| Amaranth | – | • GLTSK | ||
| – | • LSGNK | |||
| Phaseolus vulgaris |
• GEGSGA • MPACGSSMTEEY |
|||
| Animal-based bioactive peptides | ||||
| Milk | – | • PGPIPN |
Zhou et al. (2017) |
|
| Casein | Fermentation | • GFHI, DFHING | ||
| • FHG | ||||
| • GLSDGEWQ | ||||
| Whey | Fermentation | • GFHI | ||
| Marine-based bioactive peptide | ||||
| Callyspongia species | – | – |
Shaala et al. (2016) Huang et al.(2017) |
|
| Crassostrea gigas | Enzymatic digestion | – | ||
| Smenospongia aurea | – | – | ||
| Crassostrea gigas | Enzyme treatment | • HFNIGNRCLC | ||
| Tuna dark muscle | – | – | ||
| Red Sea Moses sole | – | – | ||
Different repositories of bioactive peptides
Plant- and animal-based sources
Most of the BPs have been extracted and purified both from animal and plant origins. Pulses, defatted cereals, pseudocereals, oilseeds, nuts, fruits, and vegetable seeds have been encouraging sources of proteins (Gorguç et al. 2020). Similarly, bioactivity is reported in the bulk of peptides produced from different animal sources such as milk, meat, eggs and fishes. Consequently, a bioactive balanced diet might help to provide a quantifiable physiological effect and its bioactivity should be studied at a physiologically sensible level. Ensuring such effect, milk, meat, egg and fish-derived BPs are able to affect some physiological functions, ultimately on the health conditions (Ayyash et al. 2018). There is also rising demand in developing BPs from the plant-based sources. Walnuts and pine nuts, flaxseed (Linum usitatissimum) protein and its derived peptides, wild hazelnut (Corylus heterophylla), source of six peptides (ADGF, AWDPE, AGGF, ETTL, DWDPK, and SGAF), peptide-SMRKPPG from peony (Paeonia suffruticosa) seed protein hydrolysate, Chia seeds (Salvia hispanica) with high antioxidant, anti-cancerous, antidiabetic, and antihypertensive activity are reported so far (Acevedo‐Juárez et al. 2022). Similarly, a peptide—AYLQYTDFETR, extracted from pecan meal is found to exhibit significant scavenging activities against the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical, ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) and hydroxyl radicals (Hu et al. 2018; Tadesse and Emire 2020). A protein hydrolysate extracted from the amaranth seeds was shown to have anti-cancer potentials against cancer cell lines (Taniya et al. 2020). Plant proteins possess less fat and have the least health complications compared to animal-derived proteins, so it is critical to have insights into the plant-derived BPs (Kumar et al. 2022). Mellander in 1950 was the first to state the BPs by proposing that casein-phosphopeptide (casein-derived phosphorylated peptides) strengthens bone calcification in infants, suffering from rickets (Mellander 1950). Ibrahim et al. (2018) separated camel milk proteins and hydrolyzed them, using the enzyme-Pepsin. In another study, Gong et al. (2020) obtained BPs–αS2-casein (NPWDQVKR), αS1-casein (SDIPNPIGSE), and β-casein (QEPVLGPVRGPFP) (SLSSSEESITH) and these peptides were involved in decreasing the blood glucose levels and were isolated from goat milk hydrolysates. Atanasova et al. (2021) identified active peptides in goat and sheep milk-based brined cheese. The identified peptides were having ACE inhibition, casein-phosphopeptide, αs1-casokinins, and immune peptide activity, and decrease the risks of obesity and type-II diabetes (Zhang et al. 2022). Because of their physiological and physico-chemical characteristics, milk, egg, and meat derived BPs are considered as tremendously eminent components by encouraging as functional foods or pharmaceuticals (Redha et al. 2022). Goat milk fermented with various Lactobacillus kefir strains showed antimicrobial effect against microbes- E. coli, Proteus mirabilis, Micrococcus luteus, and Salmonella (Biadała et al. 2020). Industrial scale generation of these peptides are recently established on the international market levels.
Marine sources of bioactive peptides
A diverse range of BPs isolated form marine sources possess biological properties against various health diseases such as anti-hypertensive activities, anti-oxidant, anti-cancer, and immune-modulatory (Zhong et al. 2019). BPs derived from marine origin showed diversity in their sources such as, from crustaceans, fishes, molluscs, algae etc. (Lafarga et al. 2020). Similar to animal and plant peptides, the structure and composition of various marine BPs is highly dependent on the sources from which they are derived (Blunt et al. 2018). Furthermore, BPs with more hydrophobic amino acid residues such as, Gly, Val, Ala, Ile, Leu, Phe and Pro, enhance the antioxidant activity of BPs. Marine peptides enriched with phenolic compounds with improved emulsifying and foaming properties results its application in the food industry for functional food development (Halim et al. 2016). Attempts are made to use peptides extracted from seaweeds in the prebiotics and nutraceuticals (Charoensiddhi et al. 2017). Therefore, marine sources have been determined as excellent reservoirs for extracting potential biologically active compounds.
Algae- and fungi-based sources of bioactive peptides
Algae are nutrient dense with beneficial micro- and macro-nutrients, encompassing carbohydrates, proteins, phenols, minerals and vitamins (Skjånes et al. 2021). These nutrients are primarily utilised by the animal feed and food industries as these are rich sources of essential amino acids and the utilisation of non-digestible carbohydrates from seaweeds, act as a source of fibers. These ample and varying constitutions of algae are being investigated for its possibilities to acquire BPs and carbohydrates and reported antihypertensive, antioxidant, and immune modulatory properties (Bleakley et al. 2017). A protein hydrolysate isolated from Dunaliella salina was able to inhibit cancer of the colon and also possesses anti-microbial effects. However, the peptides were not tested on non-cancerous cells and thus, further observations, like in-vivo experiment is required (Darvish et al. 2018). The synthesis of health-promoting compounds from microalgae is a promising field of research (Hamidi et al. 2019). Functionally active foods comprising seaweeds procured peptides are recently capitalised in Japan. Seaweed-acquired peptide-comprising products with FOSHU accepted anti-hypertensive assertion involve Wakame peptide jelly and Nori peptides (Cian et al. 2022). Similarly, the secondary metabolites acquired from fungal strains disclosed their biological and pharmacological properties (Abdel-Wahab et al. 2019). Large metabolites such as, peptides, alkaloids, terpenoids, additionally polyketides, steroids, and lactones have been identified with beneficial health effects (Zhang et al. 2019). Marine microorganisms characterised by fungi and bacteria, but also marine invertebrates, are considered as a treasured source of bioactive compounds. On the reports of the Food and Drug Administration (FDA), plentiful accepted BPs are expanding (Lee et al. 2019). Mushroom BPs possess a high ACE inhibitory activity for example peptide derived from the marine edible animal Styela clava (Kang et al. 2020). The fruiting body and even the by-products of the mushroom Agaricus bisporus are found rich in biologically active compounds (Ramos et al. 2019). About eight novel ACE inhibitory peptides were isolated from the fruiting body of Agaricus bisporus and the most active ones were RIGLF, AHEPVK, and PSSNK. The BPs derived from the extracts of tiger milk mushroom (Lignosus rhinocerus), showed a potential HIV-RT inhibitory activity (Sillapachaiyaporn and Chuchawankul 2019). Thus, mushroom-based HIV-RT inhibitory peptides may be a potential supplement as an anti-HIV drug. Various other bioactive functions, such as decreasing cholesterol activity, were identified in other foods (Karami and Akbari-Adergani 2019). Thus, mushrooms are potential and enormous rich sources of active peptides, and need to be explored more for the generation of BPs.
Extraction of bioactive peptides
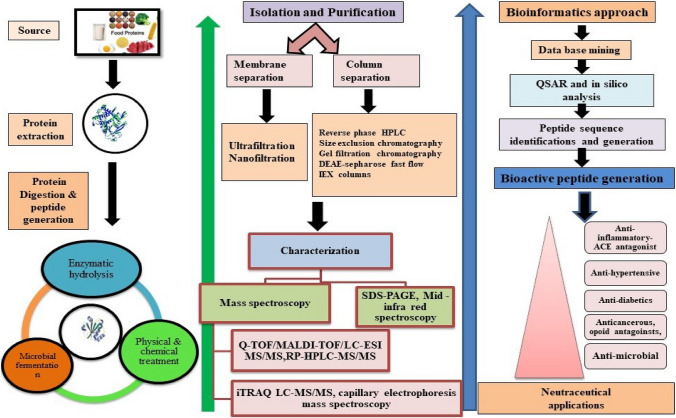
Enzyme hydrolysis and microbial fermentation are two of the most frequent techniques for producing BPs (Chalamaiah et al. 2018). These approaches result in the release of a wide range of BPs which are digested and absorbed easily by humans with no side effects. Implementation of certain novel approaches, such as ultrasound- or microwave-assisted extraction methods also increase the production of BPs. Some of the major extraction technologies frequently employed for BP isolation are discussed below (Fig. 2).
Fig. 2.
Extraction, isolation and purification procedures of BPs from diverse sources of living organisms. In extraction different methods used are enzymatic hydrolysis, which aids in maintaining the higher antioxidant activity and also enzyme to substrate ratio, hydrolysis period, type and ratio of the enzyme utilized, all affects the peptide sequences and their biological functions of BPs. microbial fermentation, physical and chemical treatment based extraction also helps in specific physico-chemical features. The figure also demonstrates use of bioinformatics tools to retain the data pertaining to BPs and in-silico evaluation of biological properties
Enzymatic hydrolysis and microbial fermentation
Based on the ideal temperature and pH of the enzymes, a set of crude or purified enzymes are added either simultaneously or sequentially to the target proteins which leads to the hydrolysis of peptide bonds. This approach has the advantage of being easy to scale up and having a quicker reaction time (Abd El-Salam and El-Shibiny 2017). The enzymatic hydrolysis is believed to be more applicable since amino acid residues remain intact during hydrolysis (Akaberi et al. 2019). Moaveni et al. (2022) reported production of BPs obtained by enzymatic hydrolysis of microalgae proteins possessing higher antioxidant activity. Enzyme to substrate ratio, hydrolysis period, type and ratio of the enzyme utilized, all affects the peptide sequences and their biological functions. Some non-protein bioactive molecules may be separated during the BPs generation such as phenolics that interfere with biological functions and must be removed. Before hydrolysis, these components are frequently extracted using acetone, or ethanol (Patil et al. 2020). Proteolysis produces protons, which alters the medium pH and interferes with the hydrolytic process, as a result, use of a buffer is recommended. The mixture is then centrifuged to separate active peptides after the enzymatic digestion. The peptides are recovered from the supernatant. Other methods such as desalting, freeze-drying, cross flow membrane filtrations, and column chromatography are used to recover peptides. Among these, gel filtration is an excellent method for separating peptides based upon their size (Patil et al. 2020). Enzyme extraction is one of the most important methods for rupturing macro-algal cell wall (Pradhan et al. 2022). The use of polysaccharide-digesting enzymes such as cellulases, glucanases, hemi-cellulases, and xylanases are regarded as a food-grade technique. As a result, commercial enzyme combinations have been shown to be effective in this application (Mendez and Kwon 2021). Because red and green seaweeds have a lighter component than brown seaweeds, enzyme-assisted extraction is mostly studied on them (Vásquez et al. 2019). Similarly, a large protein yield was obtained when Solieria chordalis, Sargassum muticum and Ulva seaweed species were treated with enzymes in which cellulase typically alone was more effective and further enhanced BP output. Other studies discovered that Palmaria palmata when treated with a combination of commercial glucanase-Celluclast and Shearzyme, increased protein synthesis (Vásquez et al. 2019).
Microbes (bacteria or yeast) are cultured on media enriched with protein substrates. Proteolytic enzymes secreted by bacteria cause protein break down and release BPs (Patil et al. 2020). Some bacterial species have a different proteolytic process, resulting in peptides with different bioactivities (Patil et al. 2020). Yeast and filamentous fungi have also been reported to be employed in the production of BPs (Chourasia et al. 2021). Even a combination of yeast and bacteria can be used to boost proteolysis. After centrifugation, the recovered supernatant typically contains peptides, which can be further hydrolyzed to obtain short peptide sequences. As a result, the peptides were isolated, and mass spectroscopy has been used to sequence their amino acids (Daliri et al. 2017).
Chemical and physical synthesis
Chemical synthesis uses chemical reagents to mediate the generation of peptides. It is an important method for creating BPs with specific physico-chemical features (Akbarian et al. 2022). The therapeutic properties of BPs focus researchers' interest nowadays in chemically synthesising the peptides to treat a variety of oxidation-related diseases (Patil et al. 2020). For instance, the tripeptides tyrosine-Histidine-tyrosine (YHY) & Proline-Histidine-Histidine (PHH) were found primarily active in stabilizing oxygen species and other radicals, as well as lipid peroxide and peroxynitrite, among the antioxidant tripeptide library. In-vitro designing and production of innovative peptides that resemble protein secondary structure conformation to generate potential peptide analogues as well as peptidomimetics with distinct medicinal properties has become increasingly popular in recent years. Chemically synthesised BPs are a new breed of physiologically active regulators that can help to treat diseases by preventing the oxidation and microbial degradation of meals (Patil et al. 2020).
Solid–liquid extraction (SLE)
SLE is one of the important methods for extracting BPs which uses a variety of solvents, including buffered solutions, distilled water, and lytic solutions (García-Vaquero et al. 2017). Factors like solute/solvent ratio (w/v), duration, or temperature should be tweaked to improve the efficacy of the method (Wijesekara et al. 2017). SLE has traditionally chosen hot water to extract algal polysaccharides, allowing for the coextraction of proteins. As a result, for BPs solvent deletion to retain protein quality and low temperatures (about 4 °C) are necessary, whereas, thermal stresses might also be employed for the production of protein hydrolysates, necessitating heat-assisted extractions (HAE) or a mixture of enzyme catalyzing methods (Veide Vilg et al. 2017). The technology is called food-grade since it is utilized to extract proteins from a range of food matrices (Naseri et al. 2020). The change in pH has shown good consequences in sample preparation for instance the use of acidic SLE succeeded by alkaline separation from the Ascophyllum nodosum, resulting in a high yield. Porphyra dioica and Ulva sp. protein content was extracted utilizing a reaction mixture comprising urea, solvent, and other reactants.
Pulse electric field assisted-extractions (PEFs)
The use of electrochemical reactions for the lysis of plasma membranes and cell walls as a method of collecting molecules is becoming extremely pervasive (Galanakis 2021). By producing significant voltages (kV), and electric pulse of varied durations (from micro to milli-seconds), PEFs assist in protein extractions from the macro-algae by electroporations of the membranes and cell walls. PEF is established as an effective and rapid green approach that solves its own limitations, such as conductance and electrode gaps (Silva et al. 2020). PEF has been frequently utilized to boost extraction yield from eco-friendly seaweeds. Common protein yields are obtained in similar species when PEF was assisted with water pressures. PEF and mechanical pressing were used to extract proteins from Ulva sp. An improved approach showed a seven-fold enhancement in total protein yields (protein percentages in the extracts) (Robin et al. 2018). PEF was also considered a suitable method for BP extraction since PEF-extracted proteins possess high anti-oxidant potentials than non-PFE assisted protein extractions.
High hydrostatic pressure extraction (HHPE)
Hydrostatic pressure extraction (HHPE) enhances protein extractions using pressure up to 1000 bars to stimulate cellular disintegration and ease in release of proteins from cell structures. HHPE efficiency is affected by the solvent utilized, as well as the operating parameters, temperature, and time (Silva et al. 2020). HHPE is regarded an effective green extraction method because of low operating temperatures, quick processing time and higher recovery rates. As a result, this method is suitable for thermal compounds, while pressure-induced protein molecular change must be taken into account. Protein recovery from two brown seaweeds; Alaria esculenta and Fucus vesiculosus, and two red seaweeds; Palmaria palmata and Chondrus crispus, were aided by HHPE. The application of HHPE assisted with other extraction techniques was also assayed, notably as HHP-assisted enzyme extraction (Suwal et al. 2019). It has been found that a lesser proportion of unwanted polyphenols also results. Optimising extraction parameters to limit phenolic extraction may benefit in the optimization and amplification of BP productions. While further investigation is required to establish HHPE as a feasible, specific BP extraction technology, controlling process conditions to reduce polyphenol extraction would help to optimise and improve BP production.
Ultrasound-assisted extraction (UAE)
Through the use of UAE, as a sonication process or as a core element of the extraction process, is recently attracting attention to increasing protein extraction from different sources (Silva et al. 2020). Cell wall breakdown is triggered by the cavitational process, in which the air bubbles generate a tremendous kinetic work that breaches the cell wall. The key benefits of this generation process method are its temperature independence and short processing time (Kazir et al. 2019), and the water as the solvent, is especially significant for algae samples. Applying high ultrasonic power over longer periods of time, on the other hand, leads to excess heat production and can drastically alter the protein configurations. UAE can be associated with other techniques such as EAE. As a consequence of combining conventional methods with these novel techniques enhance protein extraction yields. The red seaweed- Grateloupia turuturu generated considerably more phycobiliprotein when UAE and EAE were coupled than when EAE was used alone. Using NaOH as a solvent, UAE generated a higher percent yield of protein from the red seaweed Neoporphyra haitanensis. However, combining sonication with ammonium sulphate precipitations results in the high recovery of protein from seaweeds-Chondrus crispus and Fucus vesiculosus. On a broader scale, the UAE application as a food grade extraction technology has previously been established as total proteins recovered from Gracilaria sp., and Ulva sp. respectively (Kazir et al. 2019).
Microwave-assisted extraction (MAE)
Throughout the use of MAE, micro-wave waves were used for producing heat by ion conduction and dipole rotation (Silva et al. 2020). As a result, MAE may not be the most appropriate method for extracting heat sensitive biologically active peptides. MAE has been used to extract carbohydrate or flavonoid elements from seaweed studies rather than bioactive proteins/peptides, despite its reputation as an effective low-energy extraction technique (Magnusson et al. 2019). Due to the microwave’s damaging impact on the cell walls and membranes, microwave-induced ionization can stimulate the release of cellular contents from the matrix into the extraction solution. Because edible seaweeds have high ash content, which adds to the ionic conductions, MAE is an efficient technique used for extracting macro-algae proteins (Magnusson et al. 2019). While compared to standard extraction, MAE minimizes extraction yield and solvent type was completed in less than 5 min and exclusively with the aqueous BP fraction in this study. In conclusion, while MAE efficiency in terms of BP generation enhances their yield, lowering power usage and thereby, increasing the experiment’s productivity (Magnusson et al. 2019).
Protection and delivery of bioactive peptides
Protection
BPs that exist in a microenvironment inside the colloidal particles have a significant effect on their physical and chemical constancy. There are certain substances (buffers and antacids) that hinder local pH inside the colloidal particle, and stabilise protein from the alkaline- or acid-induced change in their configurations (Zhang et al. 2017a, b). Some constituents like polyols, salts, and surfactants can aid in the implementation of protein structure, thereby protecting them from loss of activity due to protein denaturation. To improve the stability, defense, and performance of a BP, it is better to co-encapsulate the BPs with stabilising molecules in the form of colloidal particles (Mc Clements 2018). Moreover, when the particle size is large, the capacity of the colloidal particle to protect a BP increases with time due to the accommodation of a number of proteins inside a particle. Furthermore, during production, storage, and utilisation, the colloidal particles experience numerous physiological changes like: variation in pH, ionic composition, enzyme activities, ingredient interactions, oxygen, light, and temperatures, as well as upon their passage through the gastro-intestinal tract. As a result, it is necessary to prepare a colloidal particle in such a manner that remains stable throughout different environmental conditions. It is significant to balance the colloidal particle for protecting BPs using different wall materials. Polysaccharide-based delivery systems have functional groups, interacts with a large range of biologically active chemicals, making these as multipurpose transporters for attaching and entrapping a number of hydrophobic and hydrophilic biologically active food ingredient. Alehosseini et al. (2018) investigated the solution properties and electrospraying of different polysaccharides including dextran, a resistant starch, maltodextrin, and fructo-oligosaccharides as well as two proteins (whey protein concentrated from milk and a soy-protein extract), as a matrix material, in order to characterize and compare the hydro-colloid aqueous solution made from the flexible polymer in water as polyethylene oxide (PE-O) and polyvinyl alcohol (PV-OH). Furthermore, Chitosan (co-polymer of d-glucosamine and N-acetyl-d-glucosamine that is made by de-acetylation of chitin) is soluble in an aqueous acidic solution and is used to make micro/nanoparticles (Motiei et al. 2021). Electrospraying was used to make poly-ethylene glycol-based hydrogel micro-spheres, according the reports of Qayyum et al. (2017). They enhanced the gelation duration of PEG hydro-gels while by using the Michael-addition reaction between thiol and acrylate to make microspheres more easily (Jain et al. 2017). Similarly, polylactide often known as poly-lactic acid (PLA), is a bio-degradable thermoplastic polyester made from tapioca roots, maize starch, or sugarcane, and is ubiquitous (Mustățea et al. 2019).
Delivery of bioactive peptides
To provide the anticipated health benefit, BPs must be passing through all the gatekeepers from the mouth to the target organ while retaining their structure and biological activity (Gianfranceschi et al. 2018). The selection of appropriate food matrices is critical in delivering BPs. Using chemically inert or least active vehicles to limit the interaction, enhance bioaccessibility and maintain the native structure and functionality of BPs may be entailed. Several nano delivery BPs systems have been reported for efficient targeting in humans as demonstrated in Fig. 3. Fiber-rich foods, for example, are found to be comparatively inert when used for delivering BPs, whereas lipid-based food matrix is found to form complexes that affect BP functionality, also bio-accessibility (Sun et al. 2020). Non-covalently bound BPs to divalent metals in the food matrix may be delivered depending upon the pH gradient across the GI tract. BPs can be prepared in covalently linked peptide-lipid conjugates in a way that the peptides are cleavable by endogenous enzymes in the GI tract. The second technique is to use food processing tools that do not cause harmful actions and effect the native form of BPs. For example, the use of processing tools that do not involve heating, such as pulsed electric field, ultrasound, and ultra-high hydrostatic pressure, is likely to have a lower impact on the sequence and conformation of BPs thus, increasing their bio accessibility. The third technique involves the use of inclusion complexes or guest host system (such as nanoencapsulation) to prevent and deliver BPs only at the target site. This involves the BP incorporation into a nano-sized colloidal delivery system to prevent from the unfavourable conditions of GI tract (Hosseini et al. 2021). Solid–lipid nanoparticles, emulsions, liposomes, and biopolymer micro-gels, have all been proposed as carriers for BP oral delivery. The natural lipid-based delivery systems like soy lecithin-derived nanoliposomes and chitosan-fabricated nanocarriers, as well as methacrylate-based microgels and alginates, have been proposed as effective inclusion complexes for increasing BP bio-accessibility (Mc Clements 2018).
Fig. 3.
Bioactive peptide delivery systems, such as nanoemulsions (can pass easily through the cell membrane, increasing BP biocompatibility and bioavailability, and the surface groups can be associated with target ligands), nano structured lipid carriers, nano-complexation, lipid-based nano-delivery, mesoporous silica nanoparticles, protein based nano-delivery, solid lipid nanoparticles, polymeric nanocarriers. The nanoparticle based delivery improves stability, easy validation, the elimination of the need for a solvent in the process, suitability for hydrophobic as well as hydrophilic nutraceutical, biodegradability, increased loading potentials, and bio-compatibility of the lipids
Nano-delivery of bioactive peptides
Protein-based delivery systems: Protein-based nano-carriers are derived from bacteria, plants, animals, and fungi and can be used to deliver hydrophobic as well as hydrophilic biologically active agents. Gel extrusion, inclusion complexes, emulsification, and spray drying techniques are effective methods for producing proteins from nano-delivery systems such as nano-assemblies, hydrocolloids, micelles, hydrogels, films, micro- as well as nanoparticle (Assadpour and Jafari 2019). As protein carriers, casein, gelatin, albumin, collagen, whey proteins, and elastin from the animal origins, and wheat gliadin, zein, and soy glycinin, from plant sources, are commonly used (Assadpour and Jafari 2019). Meat derived proteins such as collagen and gelatin, particularly gelatin extracted from collagen, are used for nutraceutical encapsulation due to their bio-compatibility, ease of transport, bio-degradability, whey proteins, high-temperature stability, and its safety (Hong et al. 2020).
Lipid-based delivery systems: the lipid-based nano delivery systems allows hydrophobic compounds such as fatty acids (conjugated linoleic acid and omega-3), carotenoids (carotene, lycopene or lutein), fat-soluble vitamins (A, D, E, and K), antioxidants (polyphenols which includes oleuropein, tocopherols, gallic acid, caffeic acid, flavonoids), and phytosterols lipid nanospheres, particularly nanoliposomes, lipid nanoparticles, and archaeosomes work as lipid-based nanostructures for the delivery of biologically active compounds (Díaz-Gómez et al. 2020), oil-in-water (O/W) nano emulsions or microemulsions, emulsification, filled hydrogel particles, multilayer emulsions, liposomes, or solid-lipid nanoparticles are the some of the main techniques to create a lipid-based delivery system (Assadpour and Jafari 2019).
Nano emulsions
Nano-emulsions are heterogeneous systems which consist of nano-scale droplet dispersions and are generated by shear-induced rupture. They are composed of two immiscible liquids; one is a droplet and the second is immersed into a liquid phase and stabilised by a surfactant (Peng et al. 2018). It is observed that double-layer techniques use for formation of W/O/W multi-emulsions (oil droplet which is coated by double-layer interfacial membranes), efficiently coat oil particle during emulsification (Peng et al. 2018). Because the Brownian motion is highest generated by gravity, nano emulsions do not sediment. High-pressure homogenization and micro fluidization are the most common methods for preparing nano emulsions, but other techniques such as ultra-sonication and in situ emulsification are also used (Singh et al. 2017). The advantages of using nano emulsions include their high kinetic stabilities against creaming and flocculation, thermal stability, and their important application for health care and personal products. They can also pass easily through the cell membrane, increasing their biocompatibility and bioavailability, and the surface groups can be associated with target ligands.
Solid lipid-nanoparticles (SLNs) and nano-structured lipid carriers (NLCs)
The lipid phase of a nano-structured lipid carrier consists of the solid lipid–fat and liquid lipid at the normal temperature (Liu et al. 2021a, b). These are kind of lipid-based deliver systems which solubilise the lipophilic nutraceutical and contains inner matrix of acyl-glycerols, fatty acids & waxes, and are stabilised by surfactants such as bile salts, sphingomyelins, phospholipids, and sterols. In contrast with other nanocarriers such as nano emulsions, SLNs possess benefits like protection against degradation in GI tract, providing stability to the unstable hydrophobic compounds, and provides targeted deliver of a nutraceutical (Nandvikar Lala and Shinde 2019). At normal temperatures, the lipid phase of nano-structured carriers contains both solid-lipid (fat) and liquid lipid. Solid lipid-nanoparticles (SLNs) are important lipid-based delivery methods that solubilizes lipo-philic nutraceutical within inner matrix (fatty acids, waxes, and acyl-glycerols) and are stabilised by the surfactants (such as phospholipids, sphingomyelins, bile salts and sterols) (Shah et al. 2017). On the basis of the preparation procedures used for SLN, NLC is formed by combining different lipid molecules which includes solid and liquid lipids. The benefits of NLC includes the ability to achieve focussed and sustained nutraceutical deliver, improved stability, easy validation, the elimination of the need for a solvent in the process, suitability for hydrophobic as well as hydrophilic nutraceutical, biodegradability, increased loading potentials, and bio-compatibility of the lipids (Nandvikar et al. 2019).
Carbohydrate-based delivery systems
Carbohydrate-based systems provide natural and suitable shells under high temperature processes, cheap food ingredients, and degradable substances and interacts with various types of biologically active substances through their reactive groups. It encloses a broad variety of hydrophobic and hydrophilic biologically active compounds (Kim et al. 2021). As a result, many carbohydrates or their chemically conjugated forms such as cellulose, alginate, chitosan, gums, pectin, cyclodextrins, starch, and dextrin have been used to assemble nano delivery systems. Furthermore, these may be employed as a proper shell for the encapsulation of pharmaceuticals or nutraceuticals due to their great thermal stability in contrast to protein- or lipid-based delivery system, which are susceptible to denaturation or melting.
Nano-complexation
The nano-complex approach is another appropriate nano-system for the preservation of biologically active food ingredients or nutraceutical (Desai et al. 2020). In general, bio-polymer is organised through electrostatic absorptions to link proteins and polysaccharides for improved conservation. As a result, at pH lower than the isoelectric point, the net positive charge of the protein interacts with the anionic groups on the polysaccharides. The layer-by-layer approach of biopolymer complexation begins with the adsorption of ionic polysaccharide upon the protein nano-particles (Rajabi et al. 2020). The stability of nanocomplex carrier is determined by various parameters such as ionic strength, charge density, pH values, biopolymer ratios, conformation, and polysaccharide-protein content (Okagu et al. 2020). Furthermore, the complex of proteins or carbohydrates with phenolic compounds has received great attention to increase the stability and release of these compounds, mostly those having low solubility and bio availabilities. The biodegradability, decreased toxicity, and biocompatibility with cells are all advantages of employing these complexes. Numerous studies are carried out in recent years for the encapsulation of BPs, such as ferulic acid through complexes of malto-dextrin (FA-MD) and hydroxy-propyl methyl-cellulose (FA-HPMC) by using spray drying method, with higher encapsulation efficiency percentage (Yu et al. 2021); curcumin through a complex of an insect protein as mealworm chitosan and protein using homogenization followed by freeze dried methods; curcumin through an ovalbumin (OVA) and sodium alginate complex formation (300–330 nm particle size) using homogenization (Feng et al. 2019); folic acid was produced by a complex of folic acid (FA) and 11 S and 7 S globulins using this approach (Ochnio et al. 2018), Epigallocatechin gallate was produced through a complex of piperine into a zein nano-carrier using an anti-solvent precipitation technique (Dahiya et al. 2018).
Polymeric nanocarriers
Polymeric carriers such as polylactic acid (PLA), poly L-glycolide (PLG), and poly cyanoacrylate (PCA), are found to entrap both hydrophobic and hydrophilic nutraceuticals such as BPs having medicinal properties (Mahapatro and Singh 2011). They should have enough mechanical strength, biocompatibility and biodegradability. The most appealing is polylactic-co-glycolic acid (PLGA), a copolymer of poly lactic acid (PLA) and poly glycolic acid (PGA) (Zorkina et al. 2020). Low toxicity, more potent, small size, greater mechanical and chemical stability, biocompatibility, targeted release of nutraceuticals, ease of modifications and production, and increased reproducibility are all advantages of nanocarriers based on polymeric micelles. Furthermore, polymeric nano-particle as a drug or bioactive agent delivery system can be employed for localised or target drug or nutraceutical release systems to specific organ or tissues site with higher deliver rates (Zorkina et al. 2020).
Mesoporous silica nanoparticles (MSNs)
Surface dynamicity, high drug loading capacities, constant release, better biocompatibility, chemical resistance, stability and target release of a variety of drugs by the unique mesoporous silica nanoparticles (MSNs) are various features that favours them as nanocarriers for biologically active molecules (Hu et al. 2016). They possess a honeycomb-like conformation and an active preface that is applied to change surface features for association with pharmacological or biologically active compounds. MSNs can be manufactured using sol–gel or spray drying processes and used to transport hydrophobic, hydrophilic, and positively or negatively charged bioactive chemicals (Rashidi et al. 2017). For many decades, MSNs are being used in a varied range of sectors, including cosmetics, feed, and medicinal items. These materials have no environmental or health concerns, according to ecotoxicology, epidemiology data, and toxicological, safety.
Nutraceutical potential of BPs: a molecular perspective
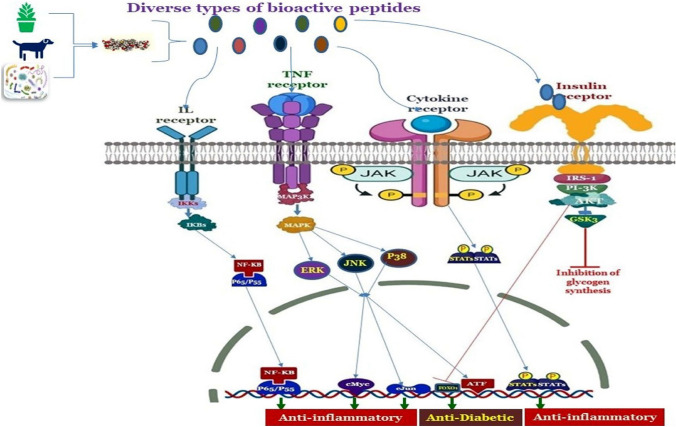
Bioactive peptides have been identified as an important functional food ingredient which imparts potential benefits on the human health apart from their nutritive values (Hernández-Ledesma et al. 2022). The potential health benefits of peptides against different metabolic disorders such as hypertension, diabetes, obesity, and cancer are due to their higher binding specificities and affinities with the enzymes, receptors, and particular biomolecules inside the cellular organisms (Chelliah et al. 2021). The bioactive based activation defensive mechanism against inflammatory response, obesity, and diabetes through activation of TNF receptor, cytokine receptor, insulin receptor and interleukin receptor is shown in Fig. 4. The different molecular mechanisms and therapeutic potentials behind these bioactive peptides over various metabolic disorders are discussed below:
Fig. 4.
The proposed signalling cascade showing the bioactive based activation defensive mechanism against inflammatory response, obesity, and diabetes through activation of TNF receptor, cytokine receptor, insulin receptor and IL receptor. Different types of membrane receptors interact with different BPs and initiate signalling cascades through activation of transcription factors. Figures also demonstrates that BPs suppress JNK/p38 and NF-κB MAPKs pathways in the liver, adipose, and muscle tissue as reported in diabetic and obese rats and decreases the resistance of insulin such as derived from bitter melon
Bioactive peptides against hypertension
There are millions of cases of hypertension globally and the number is estimated to reach in billions within a few years (Balwan et al. 2021). The angiotensin-converting enzyme is found to play a crucial role in the maintaining of blood pressure, and catalyses the inactive angiotensin I form (decapeptide) to active angiotensin II (octapeptide). Angiotensin II regulates the enzyme-cellular lipoxygenase which improves the low-density lipoprotein (LDL) oxidation and leads to atherogenesis. The hypertension drugs have many varied side effects such as coughing, pimples, taste changes, and oedema, therefore, signifies a great deal of potential in natural anti-hypertensive peptide use. The different sources of anti-hypertensive peptides are extensively investigated. These sources mostly include egg protein, milk proteins, meat protein, gelatin fish skin protein, beef haemoglobin, and various plant protein sources, such as soy (Wang et al. 2019), sesame (Aondona et al. 2021), broccoli (Dang et al. 2019), buckwheat, and transgenic rice proteins. In most cases, to use the anti-hypertensive peptides for human use, these compounds must be absorbed through the intestines and pass finally into the bloodstream. The mechanism of action of various BPs and their molecular targets regulating hypertension are recently observed and reviewed. Various studies reported BPs as significant inhibitors of Angiotensin converting enzyme (ACE) to regulate blood pressure, salt balance and water, and therefore, prevents hypertension (Karami et al. 2019). Clinical trials have reported that ACE inhibitor peptides potentially reduce the death rates in patients suffering with myocardial infarctions (Messerli et al. 2018). The BPs which possess ACE inhibition obtained from different sources like Ile-Gln-Trp, Leu-Lys-Pro and Ile-Arg-Trp (egg protein ovotransferrin-derived) Val-Tyr and Leu-Lys-Pro-Asn-Met (fish-derived) and Val-Pro-Pro and Ile-Pro-Pro (sour milk derived) (Li et al. 2018). In an observation lactoferrin derived RRWQWR, RPYL and LIWKL peptides were found to reduce hypertensive effects in rats and in rabbit’s carotid arterial segment (Fernández-Musoles et al. 2014) and among all these three BPs-RPYL has been reported to show highest hypertensive effect and inhibition of Angiotensinogen II binding to its AT1 receptor Therefore, studies have revealed that the BPs derived from different sources act upon the AT1 receptors as well as act on renin–angiotensinogen converting enzymes as inhibitors to impart there in vivo anti- hypertensive benefits (Majumder 2015). Thus, it must be concluded that BPs have great potential to lessen the impact of hypertension on the human body.
Bioactive peptides against diabetes
Diabetes is a grave deep-rooted illness determined by persistent hyperglycemia, which evolves when the pancreas does not make sufficient insulin or when the body do not competently make use of the insulin generated. Lifelong untreated hyperglycemia can distress various body systems, mostly the nervous and cardiovascular systems (WHO 2018). Food-derived bioactive molecules from animal and plant sources, has been found to aid in modulating glycemic functions, such as increasing insulin production, insulin action, or inhibiting glucose absorption. (Domnguez-Pérez et al. 2020). A wide range of plant-derived peptides assist diabetic patients in a variety of biological pathways. The different pathways are affected by different targets such as inhibitory action on the glucose transporter system, dipeptidyl peptidase IV, alpha amylase, and mimics insulin action (Patil et al. 2020). The important enzymes- dipeptidyl peptidase IV (DPP-IV) and α-Glucosidase plays a significant role in the onset of diabetes (mostly Type 2 Diabetes—a type of diabetes) (Patil et al. 2015). Therefore, the increase or decrease in their activity is one of the significant mechanisms to monitor and control diabetes. The studies are recently carried out to determine the involvement of BPs derived from the dietary proteins as inhibitors for DPP-IV and α-glucosidase enzymes thus, acting as natural sources of inhibition (Acquah et al. 2022). Various studies carried out on derived BPs are found to play an important role in regulating multiple signalling pathways which prevents the glucose synthesis and increases insulin sensitivity inside the body (Chelliah et al. 2021). Such peptides suppress JNK/p38 and NF-κB MAPKs pathways in the liver, adipose, and muscle tissue as reported in diabetic and obese rats and decreases the resistance of insulin such as derived from bitter melon (Momordica charantia) (Li et al. 2018). The peptides increase GLUT 4 expression which enhances the uptake of the glucose as well as activation of MAPK pathway and regulates the insulin signal transduction (Wang et al. 2020a, b). In observation, a study was carried out on the diabetic mice in which bioactive peptides (Momordica charantia) bind insulin receptors (IR) and stimulated AKT phosphorylation. This increased GLUT4 expression to enhance glucose uptake in the tissues majorly adipose tissues, leading to glucose clearance in diabetic mice (Jahandideh et al. 2022). Similar effects were observed for soybean derived peptides which increased and improved glucose uptake and clearance through the enhanced insulin receptor-IR phosphorylation, IRS1, AKT and therefore, GLUT-4 expression on the cell membranes and thus, showing antidiabetic activities (Kim et al. 2021). The BPs or their hydrolysates have been also reported to enhance the sensitivity of insulin by activating AMPK or insulin signalling pathways. The AMPK activation is found recently proposed as a potential target for the management and diagnosis of diabetes (Jahandideh et al. 2022). The AMPK activation results in the phosphorylation of AKT substrate. This results in the decreased activity of GTPase- Rab guanosine triphosphatase but increases the GTP on the GLUT4 storage site and promotes the translocations of GLUT4 to the target cell membranes which triggers the transport of glucose in the adipose and skeletal muscle tissues (Lankatillake et al. 2019). For example, Ile-Pro-Pro-Lys-Lys-Asn-Gln-Asp-Lys-Thr-Glu peptide, isolated from casein is found to prevent higher glucose/insulin resistances in cells (hepG2) by the insulin activation as well as AMPK signalling transduction through AMPK and Akt phosphorylation (Li et al. 2018). The cross talk between insulin, mTOR signalling and AMPK pathway is found to be the important targets for diabetes mostly type 2 diabetes due to the involvement of antidiabetic BPs. Peptides derived from soybean-Trp-His dipeptide are found to increases uptake of glucose in muscle cells by activating AMPK in an insulin-independent pathway (Li et al. 2018) and increase GLUT4 translocation to the membrane majorly plasma membrane.
Bioactive peptides against cancer
Altered gene functions and genetic expression are important characteristics of cancers. Different BPs with anticancer properties were reported to disrupt the plasma membrane of cancerous cells specifically. These show therapeutic abilities for different cancers, which are not responsive to conventional pharmaceutical therapies. The anti-cancerous effect of BPs is found to be mediated though membrane as well as non-membrane mediated mechanisms. The synthetic anticancer drugs have neurotoxic, nephrotoxic, cardiotoxic, and gonadotoxic like disadvantageous effects. Subsequently, the search for anti-cancer BPs in food has enhanced a cell-specific peptide- HVLSRAPR, isolated from Spirulina platensis hydrolysates, had a significant inhibitory effect on cancer cell growth but had no consequence on normal liver cells (Gutierrez et al. 2016). Enhancing p21 and p27 expression levels and reducing cyclin A expressions, the peptides cause arrest in the S phase of the cell cycle. The peptides further cleaved caspase 3, which decreased PARP, aBcl-2, and caspase 9 expression while increasing p53 and Bax expression.
Membrane based: one of the key distinguishing features between a cancerous and non-cancerous cell is the negatively charged phosphatidyl serine exposure on the outer leaflet of the plasma membranes.It observed that BPs specifically bind the membrane components of cancer cells mainly phosphatidylserine (PS), heparan sulphate or sialic acid, which causes depolarization of cytoplasmic membranes. This results in the membrane swelling and blebbing as reported with fluorescently labelled peptides. Thus, target the cancerous cells by disrupting their cellular membranes and eventually cause cell death (Farsinejad et al. 2015). The cell cytotoxicity is because of pore formation in the membranes about 3.7 nm approximately that increases permeability of anionic molecules rather than cations. Various models are given to describe the plasma membrane lysis mediated by BPs. These models are barrel stave model, toroidal model and carpet models. The barrel stave model states that the peptides diffuse laterally through the lipid bilayer and arrange into helices and form barrel or stave like channel which spans the membranes. For example, BPs derived from animal sources are reported to follow this model and cause cell lysis (Pino-Angeles et al. 2016). Similarly, according to the toroidal model, the BPs show a parallel orientation with the plasma membrane and a hollow core is formed at the center of pore while as the lipid groups and BPs forms the pore wall.
Non-membrane/Mitochondria dependent apoptosis: in addition, to the membrane-based lysis, anticancer BPs are found to cause apoptosis using mitochondrial pathways. The mitochondrial apoptotic disruption is an important therapeutical management of cancer cells therefore, understanding these pathways are very significant in using BPs (Whelan et al. 2012). The various bioactive peptides derived from plant and animal sources have been observed to strongly inhibit the fate of cancer cells in a dose dependent and time-dependent manner thus, showing oncogenic activity (Orafaie et al. 2021). These peptides target the mitochondria and cause a loss of membrane potential by producing reactive oxygen species (ROS) and lead to inhibition of DNA replication enhance the pro-apoptotic protein levels such as, Bax and decrease the antiapoptotic protein levels such as, Bcl-XL, Bcl-Xs, Bcl-2, and XIAP. Thus, BPs induce apoptosis by caspase independent as well as caspase dependent pathways in the mitochondria (Wang et al. 2017a, b).
Effect of BPs on cell cycle regulation
Various food derived peptides have been reported to prevent various cancer stages which includes initiation, promotion and finally progression. These BPs induce proapoptotic factors and block the cell division by reducing the expression of genes of cyclin D, bcl-2, c-myc and the expression of PCNA protein. They are also reported to increase p21, p16, p27 and Bax expression. The peptides also induce apoptosis in-vitro as well as in vivo cancer cells by activating P53, PARP, and Mcl-1 which mediates apoptosis. The cation charge strongly binds to the negative charge on the cancer cells imparted due to the sphingocholine and results in the destabilisation of the membranes of cancer cells. The anticancer peptides induce apoptosis by activation of voltage gated calcium channels due to which influx of calcium ions occurs and causes depolarization of the cancerous cells (Perego et al. 2012), modulate expression of genes, cell cycle arrest and prevents angiogenesis as observed under in-vitro studies (De Mejia and Dia 2010). The different sources of peptides responsible for anti-cancerous activity are given in Table 1.
Bioactive peptides as antioxidants (redox balance)
An imbalance between antioxidants and reactive oxygen species (ROS) may bring about oxidative damage to proteins, lipids, and nucleic acids (Nwachukwu and Aluko 2018). Moreover, oxidative stress may lead to illnesses such as cancer, diabetes, cardiovascular disease, and inflammatory disorders. Various antioxidant peptides from diverse dietary proteins have been found and their antioxidant activity has been explored. Moreover, BPs extracted by proteinase K hydrolysates from peptic fractions of Spirulina platensis exhibited higher antioxidant activity (Yu et al. 2016). Similarly, BPs extracted from Chlorella ellipsoidea aid in scavenging DPPH and peroxyl radicals and help to scavenge free radicals in monkey kidney cells. Food-derived antioxidant peptides are healthy compounds and safe with high activity, low weight, low cost, and easy absorption. The antioxidant capacity has been examined with reference to peptides' potential to inactivate reactive oxygen species (ROS), scavenge free radicals, and safeguard cells from oxidative stress, chelate oxidative metals, and enhance the activation of intracellular antioxidant enzymes. The antioxidant peptides using a different molecular mechanism, induces synthesis of major antioxidant enzymes catalase (CAT), superoxide dismutase (SOD) and peroxidases (Px) and stimulates the nuclear factor erythroid-related factor-2 (Nrf2) anti-oxidant defense mechanism. The mechanism of antioxidants using dietary BPs depends upon the peptide length, hydrophobicity and peptide composition. The main anti-oxidant pathway which prevents oxidative stress and help in maintaining the redox balance inside the body involves Nrf2-antioxidant response element (ARE) and Kelch-like ECH-associated protein 1-(Keap1-) (Huang et al. 2015). It is a leucine zipper Transcription factor. The BPs are reported to prevent the degradation of Nrf2 and GSK-3β phosphorylation and protein kinase B (Akt) activation. Keap1 acts as suppressor protein to the Nrf2, under a normal ROS level, Keap1 is bound with Nrf2 and causes proteasomal degradation of the Keap1-Nrf2 complex. Under oxidative stress the Nrf2 gets separated from the Keap1 and enters the nucleus where it binds to the ARE to promote the expression of antioxidant enzymes. Peptide-EDYGA, derived from the soft-shelled turtle increased the Nrf2 levels by binding of glutamate residue of the derived peptide to the Arg 415 of the Kelch receptors (Wang et al. 2020a, b).
Bioactive peptide against inflammation (the NF-κB pathway)
Inflammation is one of the important body’s immune response against external or internal stimulus, such as pathogen invasion, tissue damages, injury, or any infection (Korniluk et al. 2017). The BPs mainly food-derived are found to have anti-inflammatory activity were analysed under both in-vitro as well as in vivo animal models (Guha and Majumder 2019). These are reported to show inhibitory role against MAPK-JNK pathway or inhibit the Renin–Angiotensin system (RAS) in endothelium and leukocytes and in macrophages and adipocytes respectively (Li et al. 2018). The severe inflammation involves nuclear factor-kappa B (NF-κB) pathway, janus kinase-signal inducer pathway (JAK-STAT) under the stimulus of tumor necrosis factor-α (TNFα), lipopolysaccharides (LPS) and interleukin-1 (IL-1), and the mitogen activated protein kinase-c-jun N-terminal kinase (MAPK-JNK) pathway (Soliman et al. 2022). The activated MAPK phosphorylates different transcriptional factors such as c-Myc, c-Jun, and ATF-2 that in turn activates numerous cellular functions such as cell proliferation, differentiation, survival for the ERK-1/2 signalling cascade, autophagy, inflammation and apoptotic stress (Kassouf et al. 2020). Different peptides are reported to have anti-inflammatory properties (Güneş et al. 2022). However, it is still yet to understand about peptide specific receptors and the signalling pathways associated with the anti-inflammatory activity and furthermore, the crosstalk in between RAS components and the inflammatory signalling pathways requires further investigation and elucidation.
Antimicrobial activity of bioactive peptides
From last few years, different peptides with antibacterial, antifungal and antiviral activities have been isolated in vertebrates as well as invertebrates, which being a vital part of the innate immune system of the host as given in Table 1. In majority of the cases, the mechanism of action of antimicrobial peptides is different from the conventional antibiotics. This is the reason why these peptides are proving efficient as new drugs to fight infectious agents. The effectiveness of these biologically active peptides as antimicrobial agents depends on structural properties e.g. amino acid composition, peptide size, or charge (Akbarian et al. 2022). It has been concluded that in the case of some antimicrobial peptides, although the peptides reduce the harmful microbial growth, they do not directly interact with the target microbes or microorganisms, but do so with the stimulation of the host immune system. These activities include stimulating of the stimulating macrophage phagocytosis, natural killer cells proliferation, and encouraging the expression of many antibodies, cytokines, and chemokine. Antimicrobial peptides on the one hand, have a dual potential as they protect the host against harmful pathogens through antimicrobial activity, and on the other hand, these prevent the host from the adverse effects of excess of inflammatory responses (Patil et al. 2015).
Cholesterol lowering activity of bioactive peptides
Different BPs have been reported which are responsible for lowering the cholesterol, reduce cholesterol micelle production, inhibit lipase activity, and bind strongly to bile acids, suggesting that they may decrease cholesterol when ingested (Siow et al. 2016). For instance, the rats when fed with a high-cholesterol diet, sericin-derived oligopeptides, reduced total cholesterol levels in blood and non-high-density lipoprotein (HDL) cholesterol levels. The peptides decreased cholesterol absorption and reduce cholesterol solubility in lipid micelles. They also bind to taurocholate, deoxytaurocholate, and glycodeoxycholate, may be turning down cholesterol absorption in the intestine (Lapphanichayakool et al. 2017). LPYP, IAVPGEVA, and IAVPTGVA are soybean derived peptides that have been shown to inactivate the LDLR-SREBP 2 pathway (LDL receptor mediated sterol regulatory element binding protein-2 pathway) and decreases LDL uptake (Lammi et al. 2015). The peptides also decrease HMG-CoA reductase activity to prevent cholesterol biosynthesis.
Conclusion and future directions
Modern technological interventions and recent literature are strongly proven that our daily food stuff is enriched by bioactive peptides (BP) evolved by peptide linkage of amino acids or encrypted from the native protein structures having desirable bioactive potential. Biological activities-associated with BPs are antioxidants, antihypertensive, antimicrobials, anti-inflammatory and anti-cancerous for the prevention of various health related diseases. Moreover, BPs are nowadays evolved as biologically active molecules with the potential scope to enhance microbial degradation in foods, ward off oxidation of foods, amend a diverse range of human diseases to enhance the overall quality of human life. In conclusion, BP are naturally produced biologically active compounds which possibly act as valuable tool kits to restore human health. However, we are still facing issues pertaining to the in-depth information regarding the several aspects of bioactive peptides such as, pharmacokinetics, peptide bioavailability and metabolic role in humans. Further progress in BPs regarding their extraction, delivery and biological properties under both in-vitro and in vivo conditions requires complete discovery and technical intervention. Recently we observed that some of the underutilised crops like Buckwheat, which is considered as nutrient dense food sources with gluten-free protein has potential of being an excellent sources of BP (Sofi et al. 2022; Mir et al. 2022).
Acknowledgements
We acknowledge European Commission for award of Erasmus + Fellowship to MM and AH.
Abbreviations
- BP
Bioactive peptide
- DPPH
2,2-Diphenyl-1-picrylhydrazyl radical
- ABTS
2,2′-Azinobis-3-ethylbenzothiazoline-6-sulfonic acid and hydroxyl radicals
- ACE
Angiotensin-converting enzyme
- FOSHU
Foods for specified health uses
- SLE
Solid–liquid extraction
- HAE
Heat-assisted extraction
- PEF
Pulse electric field assisted-extractions
- SLN
Solid lipid-nanoparticles
- NLC
Nano-structured lipid carriers
Author contributions
MM, RAM, AF have equally contributed in compiling of different sections of manuscript. RAM majorly contributed in designing of figures. AH and MMP compiled the table. SAS, FAM, KH, MAB, BAB, NR have edited the manuscript. SMZ developed the concept, formed the outline, and guide in finalising the manuscript. The authors MM, RAM, AF, AH and MMP have equally contributed for the review article. The authors AP, MKK, MH have edited and reviewed to finalise the manuscript.
Declarations
Conflict of interest
The authors declare that there are no known conflicts of interest among authors associated with this publication and agreed to publish.
Research involving human and animal participants
This article does not contain any studies with human participants.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
We hereby give consent for publication.
Footnotes
Madhiya Manzoor, Rakeeb Ahmad Mir, Asmat Farooq, Ammarah Hami and Mohammad Maqbool Pakhtoon are contributed equally for the review article.
References
- Abd El-Salam MH, El-Shibiny S. Preparation, properties, and uses of enzymatic milk protein hydrolysates. Crit Rev Food Sci Nutr. 2017;57:1119–1132. doi: 10.1080/10408398.2014.899200. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab NM, Harwoko H, Müller WE, Hamacher A, Kassack MU, Fouad MA, et al. Cyclic heptapeptides from the soil-derived fungus Clonostachys rosea. Bioorg Med Chem. 2019;27(17):3954–3959. doi: 10.1016/j.bmc.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Acevedo-Juárez S, Guajardo-Flores D, Heredia-Olea E, Antunes-Ricardo M. Bioactive peptides from nuts: a review. Int J Food Sci. 2022;57(4):2226–2234. doi: 10.1111/ijfs.15543. [DOI] [Google Scholar]
- Acquah C, Dzuvor CK, Tosh S, Agyei D. Anti-diabetic effects of bioactive peptides: recent advances and clinical implications. Crit Rev Food Sci Nutr. 2022;62(8):2158–2171. doi: 10.1080/10408398.2020.1851168. [DOI] [PubMed] [Google Scholar]
- Akaberi S, Gusbeth C, Silve A, Senthilnathan DS, Navarro-Lopez E, MolinaGrima E, Frey W. Effect of pulsed electric field treatment on enzymatic hydrolysis of proteins of Scenedesmus almeriensis. Algal Res. 2019;43:101656. doi: 10.1016/j.algal.2019.101656. [DOI] [Google Scholar]
- Akbarian M, Khani A, Eghbalpour S, Uversky VN. Bioactive peptides: synthesis, sources, applications, and proposed mechanisms of action. Int J Mol Sci. 2022;23(3):1445. doi: 10.3390/ijms23031445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alehosseini A, Ghorani B, Sarabi-Jamab M, Tucker N. Principles of electrospraying: a new approach in protection of bioactive compounds in foods. Crit Rev Food Sci Nutr. 2018;58(14):2346–2363. doi: 10.1080/10408398.2017.1323723. [DOI] [PubMed] [Google Scholar]
- Atanasova J, Dalgalarrondo M, Iliev I, Moncheva P, Todorov SD, Ivanova IV. Formation of free amino acids and bioactive peptides during the ripening of Bulgarian white brined cheeses. Probiotics Antimicrob. 2021;13(1):261–272. doi: 10.1007/s12602-020-09669-0. [DOI] [PubMed] [Google Scholar]
- Ayala-Niño A, Contreras-López E, Castañeda-Ovando A, Sánchez-Franco JA, González-Olivares LG. Amaranth proteins as a source of bioactive peptides: a review. Int Food Res J. 2020;27(1):1–15. [Google Scholar]
- Ayyash M, Al-Dhaheri AS, Al Mahadin S, Kizhakkayil J, Abushelaibi A. In-vitro investigation of anticancer, antihypertensive, antidiabetic, and antioxidant activities of camel milk fermented with camel milk probiotic: a comparative study with fermented bovine milk. J Dairy Sci. 2018;101(2):900–911. doi: 10.3168/jds.2017-13400. [DOI] [PubMed] [Google Scholar]
- Balwan WK, Kour S. A systematic review of hypertension and stress-the silent killers. Sch Acad J Biosci. 2021;6:150–154. doi: 10.36347/sajb.2021.v09i06.002. [DOI] [Google Scholar]
- Biadała A, Szablewski T, Lasik-Kurdyś M, Cegielska-Radziejewska R. Antimicrobial activity of goat’s milk fermented by single strain of kefir grain microflora. Eur Food Res Technol. 2020;246(6):1231–1239. doi: 10.1007/s00217-020-03483-2. [DOI] [Google Scholar]
- Bleakley S, Hayes M, O’Shea N, Gallagher E, Lafarga T. Predicted release and analysis of novel ACE-I, renin, and DPP-IV inhibitory peptides from common oat (Avena sativa) protein hydrolysates using in silico analysis. Foods. 2017;6(12):108. doi: 10.3390/foods6120108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt JW, Carroll AR, Copp BR, Davis RA, Keyzers RA, Prinsep MR. Marine natural products. Nat Prod Rep. 2018;35(1):8–53. doi: 10.1039/C7NP00052A. [DOI] [PubMed] [Google Scholar]
- Borawska-Dziadkiewicz J, Darewicz M, Tarczyńska AS. Properties of peptides released from salmon and carp via simulated human-like gastrointestinal digestion described applying quantitative parameters. PLoS ONE. 2021;16(8):e0255969. doi: 10.1371/journal.pone.0255969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhwar S, Chakraborty M, Sethi K, Chatterjee A. Antidiabetic properties of rice and wheat bran—a review. J Food Biochem. 2020;44(10):e13424. doi: 10.1111/jfbc.13424. [DOI] [PubMed] [Google Scholar]
- Chalamaiah M, Yu W, Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: a review. Food Chem. 2018;245:205–222. doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- Charoensiddhi S, Conlon MA, Franco CM, Zhang W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci Technol. 2017;70:20–33. doi: 10.1016/j.tifs.2017.10.002. [DOI] [Google Scholar]
- Chelliah R, Wei S, Daliri EBM, Elahi F, Yeon SJ, Tyagi A, et al. The role of bioactive peptides in diabetes and obesity. Foods. 2021;10(9):2220. doi: 10.3390/foods10092220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Li W, Santhanam RK, Wang C, Gao X, Chen Y, et al. Bioactive peptide with antioxidant and anticancer activities from black soybean [Glycine max (L.) Merr.] byproduct: isolation, identification and molecular docking study. Eur Food Res Technol. 2019;245(3):677–689. doi: 10.1007/s00217-018-3190-5. [DOI] [Google Scholar]
- Chourasia R, Abedin MM, Chiring Phukon L, Sahoo D, Singh SP, Rai AK. Biotechnological approaches for the production of designer cheese with improved functionality. Compr Rev Food Sci Food Saf. 2021;20(1):960–979. doi: 10.1111/1541-4337.12680. [DOI] [PubMed] [Google Scholar]
- Cian RE, Garzón AG, Ancona DB, Guerrero LC, Drago SR. Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT - Food Sci Technol. 2015;64(2):881–888. doi: 10.1016/j.lwt.2015.06.043. [DOI] [Google Scholar]
- Cian RE, Nardo AE, Garzón AG, Añon MC, Drago SR. Identification and in silico study of a novel dipeptidyl peptidase IV inhibitory peptide derived from green seaweed Ulva spp. hydrolysates. LWT. 2022;154:112738. doi: 10.1016/j.lwt.2021.112738. [DOI] [Google Scholar]
- Dahiya S, Rani R, Dhingra D, Kumar S, Dilbaghi N. Conjugation of epigallocatechin gallate and piperine into a zein nanocarrier: Implication on antioxidant and anticancer potential. Adv Nat Sci: Nanosci Nanotechnol. 2018;9(3):035011. [Google Scholar]
- Daliri EB, Oh DH, Lee BH. Bioactive Peptides. Foods. 2017;6(5):32. doi: 10.3390/foods6050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y, Zhou T, Hao L, Cao J, Sun Y, Pan D. In vitro and in vivo studies on the angiotensin-converting enzyme inhibitory activity peptides isolated from broccoli protein hydrolysate. JAgric Food Chem. 2019;67(24):6757–6764. doi: 10.1021/acs.jafc.9b01137. [DOI] [PubMed] [Google Scholar]
- Darvish M, Jalili H, Ranaei-Siadat SO, Sedighi M. Potential cytotoxic effects of peptide fractions from Dunaliella salina protein hydrolyzed by gastric proteases. J Aquat Food Prod Technol. 2018;27(2):165–175. doi: 10.1080/10498850.2017.1414095. [DOI] [Google Scholar]
- De Brucker K, Delattin N, Robijns S, Steenackers H, Verstraeten N, Landuyt B, Luyten W, Schoofs L, Dovgan B, Fröhlich M, et al. Derivatives of the mouse cathelicidin-related antimicrobial peptide (CRAMP) inhibit fungal and bacterial biofilm formation. Antimicrob Agents Chemother. 2014;58(9):5395–5404. doi: 10.1128/AAC.03045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Mane VK. Health perspective of nutraceutical fatty acids;(Omega-3 and Omega-6 Fatty Acids) Nutraceutical fatty acids from oleaginous microalgae: a human health perspective. 2020;4:227–248. doi: 10.1002/9781119631729.ch9. [DOI] [Google Scholar]
- Díaz-Gómez JL, Neundorf I, López-Castillo LM, Castorena-Torres F, Serna-Saldívar SO, García-Lara S. In silico analysis and in-vitro characterization of the bioactive profile of three novel peptides identified from 19 kDa α-Zein Sequences of Maize. Molecules. 2020;25(22):5405. doi: 10.3390/molecules25225405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Pérez LA, Beltrán-Barrientos LM, González-Córdova AF, Hernández-Mendoza A, Vallejo-Cordoba B. Artisanal cocoa bean fermentation: from cocoa bean proteins to bioactive peptides with potential health benefits. J Funct Foods. 2020;73:104134. doi: 10.1016/j.jff.2020.104134. [DOI] [Google Scholar]
- El-Sayed M, Awad S. Milk bioactive peptides: antioxidant, antimicrobial and anti-diabetic activities. Adv Biochem. 2019;7(1):22–33. doi: 10.11648/j.ab.20190701.15. [DOI] [Google Scholar]
- Faridy JCM, Stephanie CGM, Gabriela MMO, Cristian JM. Biological activities of chickpea in human health (Cicer arietinum L.). A review. Plant Foods Hum Nutr. 2020;75(2):142–153. doi: 10.1007/s11130-020-00814-2. [DOI] [PubMed] [Google Scholar]
- Farsinejad S, Gheisary Z, Ebrahimi Samani S, Alizadeh AM. Mitochondrial targeted peptides for cancer therapy. Tumor Biol. 2015;36(8):5715–5725. doi: 10.1007/s13277-015-3719-1. [DOI] [PubMed] [Google Scholar]
- Farzaneh P, Khanahamadi M, Ehsani MR, Sharifan A. Bioactive properties of Agaricus bisporus and Terfezia claveryi proteins hydrolyzed by gastrointestinal proteases. LWT. 2018;91:322–329. doi: 10.1016/j.lwt.2018.01.044. [DOI] [Google Scholar]
- Felix M, Cermeño M, Romero A, FitzGerald RJ. Characterisation of the bioactive properties and microstructure of chickpea protein-based oil in water emulsions. Food Res Int. 2019;121:577–585. doi: 10.1016/j.foodres.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Feng K, Ge Y, Chen Z, Li X, Liu Z, Li X, Li H, Tang T, Yang F, Wang X. Curcumin inhibits the perk-eif2α-chop pathway through promoting SIRT1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid Med Cell Longev. 2019;2019:8574386. doi: 10.1155/2019/8574386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Musoles R, Castello-Ruiz M, Arce C, Manzanares P, Ivorra MD, Salom JB. Antihypertensive mechanism of lactoferrin-derived peptides: angiotensin receptor blocking effect. J Agric Food Chem. 2014;62(1):173–181. doi: 10.1021/jf404616f. [DOI] [PubMed] [Google Scholar]
- Fu Y, Young JF, Løkke MM, Lametsch R, Aluko RE, Therkildsen M. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions. J Funct Foods. 2016;24:196–206. doi: 10.1016/j.jff.2016.03.026. [DOI] [Google Scholar]
- Furuta T, Miyabe Y, Yasui H, Kinoshita Y, Kishimura H. Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Mar Drugs. 2016;14(2):32. doi: 10.3390/md14020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanakis CM, editor. Nutraceutical and functional food components: effects of innovative processing techniques. Academic Press; 2021. [Google Scholar]
- García-Vaquero M, López-Alonso M, Hayes M. Assessment of the functional properties of protein extracted from the brown seaweed Himanthalia elongata (Linnaeus) SF Gray. Food Res Int. 2017;99:971–978. doi: 10.1016/j.foodres.2016.06.023. [DOI] [PubMed] [Google Scholar]
- Geng X, Tian G, Zhang W, Zhao Y, Zhao L, Wang H, Ng TB. A Tricholomamatsutake peptide with angiotensin converting enzyme inhibitory and antioxidative activities and antihypertensive effects in spontaneously hypertensive rats. Sci Rep. 2016;6(1):1–9. doi: 10.1038/srep24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfranceschi GL, Gianfranceschi G, Quassinti L, Bramucci M. Biochemical requirements of bioactive peptides for nutraceutical efficacy. J Funct Foods. 2018;47:252–263. doi: 10.1016/j.jff.2018.05.034. [DOI] [Google Scholar]
- Gizaw Z. Public health risks related to food safety issues in the food market: a systematic literature review. Environ Health Prev Med. 2019;24(1):1–21. doi: 10.1186/s12199-019-0825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorguç A, Özer P, Yilmaz FM. Simultaneous effect of vacuum and ultrasound assisted enzymatic extraction on the recovery of plant protein and bioactive compounds from sesame bran. J Food Composit Anal. 2020;87:103424. doi: 10.1016/j.jfca.2020.103424. [DOI] [Google Scholar]
- Gong H, Gao J, Wang Y, Luo QW, Guo KR, Ren FZ, Mao XY. Identification of novel peptides from goat milk casein that ameliorate high-glucose-induced insulin resistance in HepG2 cells. J Dairy Sci. 2020;103(6):4907–4918. doi: 10.3168/jds.2019-17513. [DOI] [PubMed] [Google Scholar]
- Guha S, Majumder K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: a brief review. J Food Biochem. 2019;43(1):e12531. doi: 10.1111/jfbc.12531. [DOI] [PubMed] [Google Scholar]
- Guo L, et al. In-vitro assessment of the multifunctional bioactive potential of Alaska pollock skin collagen following simulated gastrointestinal digestion. J Sci Food Agric. 2015;95(7):1514–1520. doi: 10.1002/jsfa.6854. [DOI] [PubMed] [Google Scholar]
- Güneş FE. Pro-/anti-inflammatory bioactive proteins and peptides. Res Anthol Recent Advanc Ethnopharmacol Nutrac. 2022 doi: 10.4018/978-1-6684-3546-5.ch030. [DOI] [Google Scholar]
- Gutierrez K, Glanzner WG, Chemeris RO, Rigo ML, Comim FV, Bordignon V, Gonçalves PB. Gonadotoxic effects of busulfan in two strains of mice. Reprod Toxicol. 2016;59:31–39. doi: 10.1016/j.reprotox.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Halim NRA, Yusof HM, Sarbon NM. Functional and bioactive properties of fish protein hydolysates and peptides: a comprehensive review. Trends Food Sci Technol. 2016;51:24–33. doi: 10.1016/j.tifs.2016.02.007. [DOI] [Google Scholar]
- Hamidi M, Kozani PS, Kozani PS, Pierre G, Michaud P, Delattre C. Marine bacteria versus microalgae: who is the best for biotechnological production of bioactive compounds with antioxidant properties and other biological applications? Mar Drugs. 2019;18(1):28. doi: 10.3390/md18010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnedy PA, O'Keeffe MB, FitzGerald RJ. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res Int. 2017;100:416–422. doi: 10.1016/j.foodres.2017.07.037. [DOI] [PubMed] [Google Scholar]
- Harnedy PA, Parthsarathy V, McLaughlin CM, O'Keeffe MB, Allsopp PJ, McSorley EM, et al. Blue whiting (Micromesistiuspoutassou) muscle protein hydrolysate with in-vitro and in vivo antidiabetic properties. J Funct Foods. 2018;40:137–145. doi: 10.1016/j.jff.2017.10.045. [DOI] [Google Scholar]
- Hernández-Ledesma B, Fernández-Tomé S, Amigo L. Bioactive peptides against inflammatory intestinal disorders and obesity. Bioactive food components activity in mechanistic approach. 2022;5:55–183. [Google Scholar]
- Hong H, Zheng Y, Song S, Zhang Y, Zhang C, Liu J, Luo Y. Identification and characterization of DPP-IV inhibitory peptides from silver carp swim bladder hydrolysates. Food Biosci. 2020;38:100748. doi: 10.1016/j.fbio.2020.100748. [DOI] [Google Scholar]
- Lankatillake C, Huynh T, Dias DA. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods. 2019;15(1):1–35. doi: 10.1186/s13007-019-0487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SF, Ramezanzade L, McClements DJ. Recent advances in nanoencapsulation of hydrophobic marine bioactives: bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends Food Sci Technol. 2021;109:322–339. doi: 10.1016/j.tifs.2021.01.045. [DOI] [Google Scholar]
- Hu JJ, Xiao D, Zhang XZ. Advances in peptide functionalization on mesoporous silica nanoparticles for controlled drug release. Small. 2016;12(25):3344–3359. doi: 10.1002/smll.201600325. [DOI] [PubMed] [Google Scholar]
- Hu F, Ci AT, Wang H, Zhang YY, Zhang JG, Thakur K, Wei ZJ. Identification and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chem. 2018;261:301–310. doi: 10.1016/j.foodchem.2018.04.025. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li W, Su ZY, Kong ANT. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. 2015;26(12):1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Ding G, Yang Z, Yu F. Two novel peptides derived from Sinonovacula constricta inhibit the proliferation and induce apoptosis of human prostate cancer cells. Mol Med Rep. 2017;16(5):6697–6707. doi: 10.3892/mmr.2017.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim HR, Isono H, Miyata T. Potential antioxidant bioactive peptides from camel milk proteins. Anim Nutr. 2018;4(3):273–280. doi: 10.1016/j.aninu.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilangala AB, Lechanteur A, Fillet M, Piel G. Therapeutic peptides for chemotherapy: trends and challenges for advanced delivery systems. Eur J Pharm Biopharm. 2021;167:140–158. doi: 10.1016/j.ejpb.2021.07.010. [DOI] [PubMed] [Google Scholar]
- Jahandideh F, Bourque SL, Wu J. A comprehensive review on the glucoregulatory properties of food-derived bioactive peptides. Food Chem. 2022 doi: 10.1016/j.fochx.2022.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain E, Sheth S, Polito K, Sell SA, Zustiak SP. Storage stability of biodegradable polyethylene glycol microspheres. Mater Res Express. 2017;4(10):105403. doi: 10.1088/2053-1591/aa8e37. [DOI] [Google Scholar]
- Kang N, Ko SC, Kim HS, Yang HW, Ahn G, Lee SC, Lee TG, Lee JS, Jeon YJ. Structural evidence for antihypertensive effect of an antioxidant peptide purified from the edible marine animal Styela clava. J Med Food. 2020;23(2):132–138. doi: 10.1089/jmf.2019.4415. [DOI] [PubMed] [Google Scholar]
- Karami Z, Akbari-Adergani B. Bioactive food derived peptides: a review on correlation between structure of bioactive peptides and their functional properties. J Food Sci Technol. 2019;56(2):535–547. doi: 10.1007/s13197-018-3549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami Z, Peighambardoust SH, Hesari J, Akbari-Adergani B, Andreu D. Antioxidant, anticancer and ACE-inhibitory activities of bioactive peptides from wheat germ protein hydrolysates. Food Biosci. 2019;32:100450. doi: 10.1016/j.fbio.2019.100450. [DOI] [Google Scholar]
- Kassouf T, Sumara G. Impact of conventional and atypical MAPKs on the development of metabolic diseases. Biomolecules. 2020;10(9):1256. doi: 10.3390/biom10091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazir M, Abuhassira Y, Robin A, Nahor O, Luo J, Israel A, et al. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019;87:194–203. doi: 10.1016/j.foodhyd.2018.07.047. [DOI] [Google Scholar]
- Kęska P, Stadnik J, Bąk O, Borowski P. Meat proteins as dipeptidyl peptidase iv inhibitors and glucose uptake stimulating peptides for the management of a type 2 diabetes mellitus In Silico study. Nutrients. 2019;11(10):2537. doi: 10.3390/nu11102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Yang WS, Kim CH. Beneficial effects of soybean-derived bioactive peptides. Int J Mol Sci. 2021;22(16):8570. doi: 10.3390/ijms22168570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Ir J Med Sci (1971-) 2017;186(1):57–62. doi: 10.1007/s11845-016-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Tomar M, Punia S, Dhakane-Lad J, Dhumal S, Changan S, Kennedy JF. Plant-based proteins and their multifaceted industrial applications. LWT. 2022;154:112620. doi: 10.1016/j.lwt.2021.112620. [DOI] [Google Scholar]
- Lafarga T, Acién-Fernández FG, Garcia-Vaquero M. Bioactive peptides and carbohydrates from seaweed for food applications: natural occurrence, isolation, purification, and identification. Algal Res. 2020;48:101909. doi: 10.1016/j.algal.2020.101909. [DOI] [Google Scholar]
- Lammi C, Zanoni C, Arnoldi A. IAVPGEVA, IAVPTGVA, and LPYP, three peptides from soy glycinin, modulate cholesterol metabolism in HepG2 cells through the activation of the LDLR-SREBP2 pathway. J Funct Foods. 2015;14:469–478. doi: 10.1016/j.jff.2015.02.021. [DOI] [Google Scholar]
- Lammi C, Bollati C, Ferruzza S, Ranaldi G, Sambuy Y, Arnoldi A. Soybean-and lupin-derived peptides inhibit DPP-IV activity on in situ human intestinal Caco-2 cells and ex vivo human serum. Nutrients. 2018;10(8):1082. doi: 10.3390/nu10081082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapphanichayakool P, Sutheerawattananonda M, Limpeanchob N. Hypocholesterolemic effect of sericin-derived oligopeptides in high-cholesterol fed rats. J Nat Med. 2017;71(1):208–215. doi: 10.1007/s11418-016-1050-9. [DOI] [PubMed] [Google Scholar]
- Lee MR, Raman N, Ortiz-Bermudez P, Lynn DM, Palecek SP. 14-Helical beta-peptides elicit toxicity against C. albicans by forming pores in the cell membrane and subsequently disrupting intracellular organelles. Cell Chem Biol. 2019;26:289–299. doi: 10.1016/j.chembiol.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu L, He G, Wu J. Molecular targets and mechanisms of bioactive peptides against metabolic syndromes. Food Funct. 2018;9(1):42–52. doi: 10.1039/C7FO01323J. [DOI] [PubMed] [Google Scholar]
- Liao W, Jahandideh F, Fan H, Son M, Wu J. Egg protein-derived bioactive peptides: preparation, efficacy, and absorption. Adv Food Nutr Res. 2018;85:1–58. doi: 10.1016/bs.afnr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Liu M, Wang F, Pu C, Tang W, Sun Q. Nanoencapsulation of lutein within lipid-based delivery systems: characterization and comparison of zein peptide stabilized nano-emulsion, solid lipid nanoparticle, and nano-structured lipid carrier. Food Chem. 2021;358:129840. doi: 10.1016/j.foodchem.2021.129840. [DOI] [PubMed] [Google Scholar]
- Liu WY, Zhang JT, Miyakawa T, Li GM, Gu RZ, Tanokura M. Antioxidant properties and inhibition of angiotensin-converting enzyme by highly active peptides from wheat gluten. Sci Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-84820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Vital DA, Mojica L, de Mejía EG, Mendoza S, Loarca-Piña G. Biological potential of protein hydrolysates and peptides from common bean (Phaseolus vulgaris L.): a review. Food Res J. 2015;76:39–50. doi: 10.1016/j.foodres.2014.11.024. [DOI] [Google Scholar]
- Magnusson M, Glasson CR, Vucko MJ, Angell A, Neoh TL, de Nys R. Enrichment processes for the production of high-protein feed from the green seaweed Ulva ohnoi. Algal Res. 2019;41:101555. doi: 10.1016/j.algal.2019.101555. [DOI] [Google Scholar]
- Majumder K. Antihypertensive activity of egg white protein ovotransferrin-derived peptides. 2015 doi: 10.7939/R3GF0N486. [DOI] [Google Scholar]
- Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol. 2011;9(1):1–11. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor M, Singh J, Gani A. Exploration of bioactive peptides from various origin as promising nutraceutical treasures: in-vitro, in silico and in vivo studies. Food Chem. 2022;373:131395. doi: 10.1016/j.foodchem.2021.131395. [DOI] [PubMed] [Google Scholar]
- Mc Clements DJ. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: a review. Adv Colloid Interface Sci. 2018;253:1–22. doi: 10.1016/j.cis.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Mellander OLOF. The physiological importance of the casein phosphopeptide calcium salts. II. Peroral calcium dosage of infants. Some aspects of the pathogenesis of rickets. Acta Soc Bot Pol Pol. 1950;55:247–257. [PubMed] [Google Scholar]
- Mendez RL, Kwon JY. Effect of extraction condition on protein recovery and phenolic interference in Pacific dulse (Devaleraea mollis) J Appl Phycol. 2021;33:2497–2509. doi: 10.1007/s10811-021-02467-3. [DOI] [Google Scholar]
- Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol. 2018;71(13):1474–1482. doi: 10.1016/j.jacc.2018.01.058. [DOI] [PubMed] [Google Scholar]
- Minkiewicz P, Iwaniak A, Darewicz M. BIOPEP-UWM database of bioactive peptides: current opportunities. Int J Mol Sci. 2019;20(23):5978. doi: 10.3390/ijms20235978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir RA, Nazir M, Sabreena NS, Mukhtar S, Ganai BA, Zargar SM. Utilizing the underutilized plant resources for development of life style foods: putting nutrigenomics to use. Plant Physiol Biochem. 2022;171:128–138. doi: 10.1016/j.plaphy.2021.12.038. [DOI] [PubMed] [Google Scholar]
- Mishra J, Rajput R, Singh K, Bansal A, Misra K. Antioxidant-rich peptide fractions derived from high-altitude Chinese caterpillar medicinal mushroom Ophiocordyceps sinensis (Ascomycetes) inhibit bacterial pathogens. Int J Med Mushrooms. 2019 doi: 10.1615/IntJMedMushrooms.2019030013. [DOI] [PubMed] [Google Scholar]
- Moaveni S, Salami M, Khodadadi M, McDougall M, Emam-Djomeh Z. Investigation of S. limacinum microalgae digestibility and production of antioxidant bioactive peptides. LWT. 2022;154:112468. doi: 10.1016/j.lwt.2021.112468. [DOI] [Google Scholar]
- Motiei M, Mirahmadi-Zare SZ, Nasr-Esfahani MH. Chemical stabilization of γ-polyglutamate by chitosan and the effect of co-solvents on the stability. Biophys Chem. 2021;275:106605. doi: 10.1016/j.bpc.2021.106605. [DOI] [PubMed] [Google Scholar]
- Mustățea G, Ungureanu EL, Belc N. Polylactic acid (pla) for food packaging applications-a short overview. Ann Food Sci Technol (new York) 2019;20(1):9–14. [Google Scholar]
- Nandvikar NY, Lala RR, Shinde AS. Nanostructured lipid carrier: the advanced lipid carriers. Int J Pharm Sci Res. 2019;10(12):5252–5265. [Google Scholar]
- Naseri A, Marinho GS, Holdt SL, Bartela JM, Jacobsen C. Enzyme-assisted extraction and characterization of protein from red seaweed Palmaria palmata. Algal Res. 2020;47:101849. doi: 10.1016/j.algal.2020.101849. [DOI] [Google Scholar]
- Ochnio ME, Martínez JH, Allievi MC, Palavecino M, Martínez KD, Pérez OE. Proteins as nano-carriers for bioactive compounds. The case of 7S and 11S soy globulins and folic acid complexation. Polymers. 2018;10(2):149. doi: 10.3390/polym10020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagu OD, Verma O, McClements DJ, Udenigwe CC. Utilization of insect proteins to formulate nutraceutical delivery systems: Encapsulation and release of curcumin using mealworm protein-chitosan nano-complexes. Int J Biol Macromol. 2020;151:333–343. doi: 10.1016/j.ijbiomac.2020.02.198. [DOI] [PubMed] [Google Scholar]
- Orafaie A, Bahrami AR, Matin MM. Use of anticancer peptides as an alternative approach for targeted therapy in breast cancer: a review. Nanomed J. 2021;16(5):415–433. doi: 10.2217/nnm-2020-0352. [DOI] [PubMed] [Google Scholar]
- Patil P, Mandal S, Tomar SK, Anand S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur J Nutr. 2015;54(6):863–880. doi: 10.1007/s00394-015-0974-2. [DOI] [PubMed] [Google Scholar]
- Patil SM, Sujay S, Tejaswini M, Sushma PP, Prithvi S, Ramu R. Bioactive peptides: its production and potential role on health. Innov Food Sci Emerg Technol. 2020;7(1):167–182. [Google Scholar]
- Peng Y, Meng Q, Zhou J, Chen B, Xi J, Long P, et al. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2018;242:527–532. doi: 10.1016/j.foodchem.2017.09.094. [DOI] [PubMed] [Google Scholar]
- Perego S, Cosentino S, Fiorilli A, Tettamanti G, Ferraretto A. Casein phosphopeptides modulate proliferation and apoptosis in HT-29 cell line through their interaction with voltage-operated L-type calcium channels. J Nutr Biochem. 2012;23(7):808–816. doi: 10.1016/j.jnutbio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Pino-Angeles A, Leveritt JM, III, Lazaridis T. Pore structure and synergy in antimicrobial peptides of the magainin family. PLoS Comput Biol. 2016;12(1):e1004570. doi: 10.1371/journal.pcbi.1004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontonio E, Montemurro M, De Gennaro GV, Miceli V, Rizzello CG. Antihypertensive peptides from ultrafiltration and fermentation of the ricotta cheese exhausted whey: design and characterization of a functional Ricotta Cheese. Foods. 2021;10(11):2573. doi: 10.3390/foods10112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan B, Nayak R, Bhuyan PP, Patra S, Behera C, Sahoo S, et al. Algal phlorotannins as novel antibacterial agents with reference to the antioxidant modulation: current advances and future directions. Mar Drugs. 2022;20(6):403. doi: 10.3390/md20060403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qayyum AS, Jain E, Kolar G, Kim Y, Sell SA, Zustiak SP. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication. 2017;9(2):025019. doi: 10.1088/1758-5090/aa703c. [DOI] [PubMed] [Google Scholar]
- Rajabi H, Jafari SM, Feizy J, Ghorbani M, Mohajeri SA. Preparation and characterization of 3D graphene oxide nanostructures embedded with nanocomplexes of chitosan-gum Arabic biopolymers. Int J Biol Macromol. 2020;162:163–174. doi: 10.1016/j.ijbiomac.2020.06.076. [DOI] [PubMed] [Google Scholar]
- Ramos M, Burgos N, Barnard A, Evans G, Preece J, Graz M, et al. Agaricus bisporus and its by-products as a source of valuable extracts and bioactive compounds. Food Chem. 2019;292:176–187. doi: 10.1016/j.foodchem.2019.04.035. [DOI] [PubMed] [Google Scholar]
- Rashidi L, Ganji F, Vasheghani-Farahani E. Fluorescein isothiocyanate-dyed mesoporous silica nanoparticles for tracking antioxidant delivery. IET nanobiotechnology. 2017;11(4):454–462. doi: 10.1049/iet-nbt.2016.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redha AA, Valizadenia H, Siddiqui SA, Maqsood S. A state-of-art review on camel milk proteins as an emerging source of bioactive peptides with diverse nutraceutical properties. Food Chem. 2022;373:131444. doi: 10.1016/j.foodchem.2021.131444. [DOI] [PubMed] [Google Scholar]
- Robin A, Kazir M, Sack M, Israel A, Frey W, Mueller G, et al. Functional protein concentrates extracted from the green marine macroalga Ulva sp., by high voltage pulsed electric fields and mechanical press. ACS Sustain Chem Eng. 2018;6(11):13696–13705. doi: 10.1021/acssuschemeng.8b01089. [DOI] [Google Scholar]
- Shaala LA, Youssef DT, Ibrahim SR, Mohamed GA. Callyptide A, a new cytotoxic peptide from the Red Sea marine sponge Callyspongia species. Nat Prod Res. 2016;30(24):2783–2790. doi: 10.1080/14786419.2016.1155577. [DOI] [PubMed] [Google Scholar]
- Shah MR, Imran M, Ullah S. Lipid-based nanocarriers for drug delivery and diagnosis. William Andrew; 2017. [Google Scholar]
- Sillapachaiyaporn C, Chuchawankul S. HIV-1 protease and reverse transcriptase inhibition by tiger milk mushroom (Lignosus rhinocerus) sclerotium extracts: in-vitro and in silico studies. J Tradit Complement Med. 2019 doi: 10.1016/j.jtcme.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A, Silva SA, Lourenço-Lopes C, Jimenez-Lopez C, Carpena M, Gullón P, et al. Antibacterial use of macroalgae compounds against foodborne pathogens. Antibiotics. 2020;9(10):712. doi: 10.3390/antibiotics9100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, Chourasia MK. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Siow HL, Choi SB, Gan CY. Structure–activity studies of protease activating, lipase inhibiting, bile acid binding and cholesterol-lowering effects of pre-screened cumin seed bioactive peptides. J Funct Foods. 2016;27:600–611. doi: 10.1016/j.jff.2016.10.013. [DOI] [Google Scholar]
- Skjånes K, Aesoy R, Herfindal L, Skomedal H. Bioactive peptides from microalgae: focus on anti-cancer and immunomodulating activity. Physiol Plant. 2021;173(2):612–623. doi: 10.1111/ppl.13472. [DOI] [PubMed] [Google Scholar]
- Sofi SA, Ahmed N, Farooq A, Rafiq S, Zargar SM, Kamran F, Dar TA, Mir SA, Dar BN, Khaneghah AM. Nutritional and bioactive characteristics of buckwheat, and its potential for developing gluten-free products: an updated overview. Food Sci Nutr. 2022 doi: 10.1002/fsn3.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AM, Sim RH, Das S, Mahakkanukrauh P. Therapeutic targeting of inflammatory pathways with emphasis on NLRP3 inflammasomes by natural products: a novel approach for the treatment of inflammatory eye diseases. Curr Med Chem. 2022;29(16):2891–2912. doi: 10.2174/0929867328666210910154330. [DOI] [PubMed] [Google Scholar]
- Sun X, Udenigwe CC. Chemistry and bio functional significance of bioactive peptide interactions with food and gut components. J Agric Food Chem. 2020;68(46):12972–12977. doi: 10.1021/acs.jafc.9b07559. [DOI] [PubMed] [Google Scholar]
- Suwal S, Perreault V, Marciniak A, Tamigneaux É, Deslandes É, Bazinet L, et al. Effects of high hydrostatic pressure and polysaccharidases on the extraction of antioxidant compounds from red macroalgae, Palmaria palmata and Solieria chordalis. J Food Eng. 2019;252:53–59. doi: 10.1016/j.jfoodeng.2019.02.014. [DOI] [Google Scholar]
- Tadesse SA, Emire SA. Production and processing of antioxidant bioactive peptides: a driving force for the functional food market. Heliyon. 2020;6(8):e04765. doi: 10.1016/j.heliyon.2020.e04765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamam B, Syah D, Suhartono MT, Kusuma WA, Tachibana S, Lioe HN. Proteomic study of bioactive peptides from tempe. J Biosci Bioeng. 2019;128(2):241–248. doi: 10.1016/j.jbiosc.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Taniya MS, Reshma MV, Shanimol PS, Krishnan G, Priya S. Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory effects in breast cancer cells. Food Biosci. 2020;35:100588. doi: 10.1016/j.fbio.2020.100588. [DOI] [Google Scholar]
- Thomas D, Latha MS. Bioactive proteins and peptides as functional foods. In Advances in nutraceuticals and functional foods. Apple Academic Press; 2022. pp. 173–198. [Google Scholar]
- Vásquez V, Martínez R, Bernal C. Enzyme-assisted extraction of proteins from the seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: characterization of the extracts and their bioactive potential. J Appl Phycol. 2019;31(3):1999–2010. doi: 10.1007/s10811-018-1712-y. [DOI] [Google Scholar]
- Verma ML, Chandel AK, editors. Biotechnological production of bioactive compounds. Elsevier; 2019. [Google Scholar]
- Vilg JV, Undeland I. pH-driven solubilization and isoelectric precipitation of proteins from the brown seaweed Saccharina latissima—effects of osmotic shock, water volume and temperature. J Appl Phycol. 2017;29(1):585–593. doi: 10.1007/s10811-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen H, Fu X, Li S, Wei J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: biochemical characterization and molecular docking study. Lwt. 2017;75:93–99. doi: 10.1016/j.lwt.2016.08.047. [DOI] [Google Scholar]
- Wang X, Yu H, Xing R, Li P. Characterization, preparation, and purification of marine bioactive peptides. BioMed Res Int. 2017 doi: 10.1155/2017/9746720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yu H, Zhao Z, Sheng X, Shen Y, Gu H. Natural variation of glucosinolates and their breakdown products in broccoli (Brassica oleracea var. italica) seeds. J Agric Food Chem. 2019;67(45):12528–12537. doi: 10.1021/acs.jafc.9b06533. [DOI] [PubMed] [Google Scholar]
- Wang L, Ding L, Xue C, Ma S, Du Z, Zhang T, Liu J. Corn gluten hydrolysate regulates the expressions of antioxidant defense and ROS metabolism relevant genes in H2O2-induced HepG2 cells. J Funct Foods. 2018;42:362–370. doi: 10.1016/j.jff.2017.12.056. [DOI] [Google Scholar]
- Wang J, Wu T, Fang L, Liu C, Liu X, Li H, et al. Peptides from walnut (Juglans mandshurica Maxim.) protect hepatic HepG2 cells from high glucose-induced insulin resistance and oxidative stress. Food Funct. 2020;11(9):8112–8121. doi: 10.1039/D0FO01753A. [DOI] [PubMed] [Google Scholar]
- Wang N, Wang W, Sadiq FA, Wang S, Caiqin L, Jianchang J. Involvement of Nrf2 and Keap1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent peptides from soft-shelled turtle. Process Biochem. 2020;92:174–181. doi: 10.1016/j.procbio.2019.12.022. [DOI] [Google Scholar]
- Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, et al. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci. 2012;109(17):6566–6571. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekara I, Lang M, Marty C, Gemin MP, Boulho R, Douzenel P, et al. Different extraction procedures and analysis of protein from Ulva sp. in Brittany, France. J Appl Phycol. 2017;29(5):2503–2511. doi: 10.1007/s10811-017-1239-7. [DOI] [Google Scholar]
- World Health Organization (2018) Noncommunicable diseases country profiles
- Wu S, Wang X, Qi W, Guo Q. Bioactive protein/peptides of flaxseed: a review. Trends Food Sci Technol. 2019;92:184–193. doi: 10.1016/j.tifs.2019.08.017. [DOI] [Google Scholar]
- Xu F, Zhang J, Wang Z, Yao Y, Atungulu GG, Ju X, Wang L. Absorption and metabolism of peptide WDHHAPQLR derived from rapeseed protein and inhibition of HUVEC apoptosis under oxidative stress. J Agric Food Chem. 2018;66(20):5178–5189. doi: 10.1021/acs.jafc.8b01620. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu Y, Xue M, Dun Y, Li S, Peng N, et al. Purification and identification of antioxidant peptides from enzymatic hydrolysate of Spirulina platensis. J Microbiol Biotechnol. 2016;26(7):1216–1223. doi: 10.4014/jmb.1601.01033. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Zhang YF, Yan J, Li SH, Chen Y. A novel glycoprotein emulsion using high-denatured peanut protein and sesbania gum via cold plasma for encapsulation of β-carotene. J Agric Food Chem. 2021;74:102840. doi: 10.1016/j.ifset.2021.102840. [DOI] [Google Scholar]
- Zaky AA, Abd El-Aty AM, Ma A, Jia Y. An overview on antioxidant peptides from rice bran proteins: extraction, identification, and applications. Crit Rev Food Sci Nutr. 2020 doi: 10.1080/10408398.2020.1842324. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang R, McClements DJ. Lactase (β-galactosidase) encapsulation in hydrogel beads with controlled internal pH microenvironments: impact of bead characteristics on enzyme activity. Food Hydrocoll. 2017;67:85–93. doi: 10.1016/j.foodhyd.2017.01.005. [DOI] [Google Scholar]
- Zhang Z, Zhang R, Sun Q, Park Y, McClements DJ. Confocal fluorescence mapping of pH profile inside hydrogel beads (microgels) with controllable internal pH values. Food Hydrocoll. 2017;65:198–205. doi: 10.1016/j.foodhyd.2016.11.018. [DOI] [Google Scholar]
- Zhang Q, Wu C, Fan G, Li T, Sun Y. Improvement of antioxidant activity of Morchellaesculenta protein hydrolysate by optimized glycosylation reaction. CYTA J Food. 2018;16(1):238–246. doi: 10.1080/19476337.2017.1389989. [DOI] [Google Scholar]
- Zhang F, Qu J, Thakur K, Zhang JG, Mocan A, Wei ZJ. Purification and identification of an antioxidative peptide from peony (Paeonia suffruticosa Andr.) seed dreg. Food Chem. 2019;285:266–274. doi: 10.1016/j.foodchem.2019.01.168. [DOI] [PubMed] [Google Scholar]
- Zhang L, Han B, Luo B, Ni Y, Bansal N, Zhou P. Characterization of endogenous peptides from Dromedary and Bactrian camel milk. Eur Food Res Technol. 2022 doi: 10.1007/s00217-021-03952-2. [DOI] [Google Scholar]
- Zhong Q, Wei B, Wang S, Ke S, Chen J, Zhang H, Wang H. The antioxidant activity of polysaccharides derived from marine organisms: an overview. Mar Drugs. 2019;17(12):674. doi: 10.3390/md17120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Yang X, Zhang W, Wang J, Wei C, Gu F, et al. Construction of an Anticancer Fusion Peptide (ACFP) derived from milk proteins and an assay of anti-ovarian cancer cells in-vitro. Curr Med Chem Anticancer. 2017;17(4):635–643. doi: 10.2174/1871520616666160627091131. [DOI] [PubMed] [Google Scholar]
- Zorkina Y, Abramova O, Ushakova V, Morozova A, Zubkov E, Valikhov M, Melnikov P, Majouga A, Chekhonin V. Nano carrier drug delivery systems for the treatment of neuropsychiatric disorders: Advantages and limitations. Molecules. 2020;25(22):5294. doi: 10.3390/molecules25225294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Wang M, Wang Z, Aluko RE, He R. Antihypertensive and antioxidant activities of enzymatic wheat bran protein hydrolysates. J Food Biochem. 2020;44(1):e13090. doi: 10.1111/jfbc.13090. [DOI] [PubMed] [Google Scholar]