Abstract
The M2 macrophages are major components in the tumor microenvironment and are closely linked to immune suppression and tumor metastasis. This work focuses on how M2 macrophage-derived extracellular vesicles (EVs) affect colorectal cancer (CRC) progression. THP-1 monocytes were induced to differentiate to M0 or M2 macrophages, and the macrophage-derived EVs (M0-EVs and M2-EVs, respectively) were collected and identified. The M2-EVs stimulation augmented proliferation, mobility, and the in vivo tumorigenic activity of CRC cells. Circular RNA_CCDC66 (circ_CCDC66) was highly enriched in M2-EVs and could be delivered into CRC cells. The RNA pull-down and luciferase assays showed that circ_CCDC66 could competitively bind to microRNA (miR)-342-3p, therefore restoring the expression of metadherin (MTDH) mRNA, a target transcript of miR-342-3p. Suppression of circ_CCDC66 in the M2-EVs or specific knockdown of MTDH in CRC significantly blocked the growth and mobility of CRC cells. However, miR-342-3p inhibition restored the malignant phenotype of cancer cells. Moreover, the MTDH knockdown was found to increase the cytotoxicity of CD8+ T and reduce the protein level of the immune checkpoint PDL1 in CRC cells. In summary, this study reveals that the M2-EVs augment immune evasion and development of CRC by delivering circ_CCDC66 and restoring the MTDH level.
Keywords: M2 macrophages, Extracellular vesicles, Circ_CCDC66, MicroRNA-342-3p, MTDH, Colorectal cancer
Introduction
Colorectal cancer (CRC) ranks the third (10.0%) in terms of the incidence rate and the second (9.4%) in terms of cancer death in 2020 in the global scale (Sung et al. 2021). In general, tumor resection represents the most effective strategy for early stages of CRC, and combination of chemotherapeutic regimens is required for cancers at different stages, especially those at advanced stages (Wen et al. 2019). Unfortunately, approximately 35% of patients are diagnosed with a metastatic disease, and another 50% of patients with nonmetastatic cancer develop metastasis during the disease course (Piawah and Venook 2019). In the event of metastasis, the outcomes of treatment were disappointing with the 5-year survival rate reportedly slightly over 10% (Ganesh et al. 2019).
It has been increasingly acknowledged that the tumor microenvironment (TME) is critical to mediate the distant metastasis of CRC (Yoon et al. 2020). Tumor associated macrophages (TAMs) are major components in the TME and regulate a variety of factors to promote tumor progression (Bao et al. 2022). The macrophages also represent the major type of immune cells in CRC and usually present with the immunosuppressive type (M2) (Stadler et al. 2021). The M2 macrophages trigger growth, angiogenesis, metastasis and affect the anti-tumor functions of the immune cells, which leads to immunosuppression in the TME (Min et al. 2021). Moreover, M2 macrophages can release extracellular vesicles (EVs) to further trigger the dissemination of CRC cells (Lan et al. 2019). EVs are nanometer-sized vesicles released by all cells of different sizes containing a multitude of proteins, nucleic acids (RNA and DNA), and lipids (Ortiz 2021). The EVs transfer the containers to participate in the intercellular communication, and the “cargoes” included are protected from degradation during transportation by the lipid bilayer membrane (Kim et al. 2017). This work aims to validate the role of EVs from M2 macrophages (M2-EVs) in the development of CRC and the molecules implicated.
Circular RNAs (circRNAs) are a highly abundant and heterogeneous class of non-coding RNAs enriched in EVs (Kim et al. 2017). They are single-stranded, covalently closed, and highly stable molecules formed by pre-mRNAs via a process termed “back-splicing” and are frequently involved in tumorigenesis (Vo et al. 2019). Interestingly, circ_CCDC66 has been reported as one of the diagnostic markers in the plasma of patients with CRC (Lin et al. 2019). Upregulation of circ_CCDC66 was found to be responsible for the growth and metastasis of colon cancer (Hsiao et al. 2017). One of the most widely investigated mechanisms of circRNAs is that they may competitively bind to microRNAs (miRNAs) or RNA-binding proteins to manipulate gene expression (Abdelmohsen et al. 2017; Hansen et al. 2013; Smillie et al. 2018). We obtained a candidate circ_CCDC66/miR-342-3p/metadherin (MTDH) axis in CRC via bioinformatics predictions. Downregulation of miR-342-3p has reportedly been linked to tumorigenesis of CRC (Zhou et al. 2021). By contrast, the MTDH has been recently found as a positive regulator of programmed death ligand 1 (PDL1), a critical immune checkpoint protein responsible for immunosuppression and tumor development (Wan et al. 2022). Taken together, we postulated that the M2-EVs possibly deliver circ_CCDC66 to restore the MTDH expression by sponging miR-342-3p, which induces immunosuppression and progression of CRC.
Material and methods
Cell treatment
Human monocytes THP-1 and human embryonic kidney (HEK)-293 T cells were procured from Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cytotoxic CD8+ T cells, two CRC cell lines (SW480 and HCT116), and human normal colon cells (FHC) were procured from American Type Culture Collection (Manassas, VA, USA). The THP-1 and CD8+ T cells were cultured in RPMI-1640, HEK-293 T cells in DMEM, FHC cells in DMEM: F12K, SW480 cells in L-15 medium, and HCT116 cells were cultured in McCoy’s 5a medium. All media were supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and glutamine. SW480 cells were cultured at 37℃ without CO2, and the other cells were cultured at 37℃ with 5% CO2.
Short hairpin (sh) RNA vectors including sh-circ_CCDC66 and sh-MTDH corresponding negative control (NC) were provided from VectorBuilder Technologies (Guangdong, China). The miR-342-3p-inhibitor (chemically engineered antisense oligonucleotide suppressing miR-342-3p expression via highly complementary interaction) and miR-342-3p-mimic were procured from RiboBio Co., Ltd, (Guangdong, China). The sh-circ_CCDC66 (1, 2, 3#) and the sh-NC were transfected into M2-macrophages (see details below), the miR-342-3p inhibitor, sh-MTDH (1, 2, 3#), and the controls were transfected into CRC cells, and miR-342-3p-mimic or the NC-mimic were transfected into the HEK-293 T cells. All the transfections were performed following the instructions of the Lipofectamine 3000 kit (L3000150, Invitrogen, Thermo Fisher Scientific, Rockford, IL, USA) when the cell density reached ~ 70%. The stem-loop sequence for shRNA construction was CTCGAG, and the target sequences are listed in Table 1.
Table 1.
The target sequences of shRNAs
| shRNA ID | Target sequence |
|---|---|
| sh-circ_CCDC66 1# | CAATTAGAGCATCAGTGTCTT |
| sh-circ_CCDC66 2# | AATTAGAGCATCAGTGTCTTA |
| sh-circ_CCDC66 3# | AGAGCATCAGTGTCTTAAAGT |
| sh-MTDH 1# | GATGAATGGTCTGGGTTAAAT |
| sh-MTDH 2# | GTTGGTTCCAAGAAGAATAAA |
| sh-MTDH 3# | TACTACAAGAGACAGATAAAT |
shRNA, short hairpin RNA; MTDH, metadherin
Induction of macrophages and phenotype analysis
The THP-1 cells were incubated with 10 ng/mL phorbol-12-myristate-13-acetate (PMA, P8139, Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 24 h to differentiate to M0 macrophages. The M0 macrophages were further incubated with 20 ng/mL IFN-γ (300–02, PeproTech Inc., NJ, USA) and 10 pg/mL lipopolysaccharide (L5293, Sigma-Aldrich) to obtain an M1 phenotype, or incubated with 20 ng/mL interleukin (IL)-4 (AF-200–04, PeproTech Inc., NJ, USA) and IL-13 (AF-200–13, Preprotech) for 48 h to obtain an M2 phenotype.
The CD68 and CD163 expression in macrophages was analyzed by flow cytometry. In short, the cells were collected into tubes, washed with PBS and resuspended. Thereafter, the cells were incubated with anti-CD68 (Cat. No. 562117, BD Biosciences) or anti-CD163 (Cat. No. 568202, BD Biosciences, Franklin Lakes, NJ, USA) for 1 h and then analyzed by the flow cytometer (BD Biosciences). The M2 macrophages co-cultured with Alexa Fluor™ 647 Mouse IgG1 (Cat. No. 565571, BD Biosciences) and FITC Mouse IgG2b (Cat. No. 555067, BD Biosciences) were set to the isotype controls.
Extraction and identification of macrophages-derived EVs
The macrophages were cultured for 48 h, and then the culture solution was collected, in which the EVs were extracted by differential centrifugation. In short, the cell culture supernatant was centrifuged at 4℃ at 2000 × g for 15 min and at 10,000 × g for 30 min to discard the dead cells and cell debris. The supernatant was then collected and ultra-centrifuged at 110,000 × g for 1 h to collect the pellets, which were washed and resuspended in PBS, and centrifuged at 110,000 × g for 1 h to obtain the precipitated particles (EVs). The EVs were resuspended in PBS and stored at -80℃ for further use. The concentration and particle size were analyzed by nanoparticle tracking analysis (NTA). The EVs were dropped on copper mesh, fixed with 1% glutaraldehyde, counter-stained with 2% uranyl acetate solution, and then observed under the TEM. The EVs were further identified by detecting the positive marker proteins CD63, CD9 and CD81 and negative protein Calnexin. The EVs were separately packaged at 10 μg/tube for co-culture with the CRC cells.
RNA extraction and quantification
The TRIzol reagent (T9424, Sigma-Aldrich) was used to extract total RNA from cells, and an Exosome RNA Purification kit (EZB-exo-RN1, HifunBio, Shanghai, China) was used to extract total RNA from EVs. The cytoplasmic and nuclear RNA was separated by the PARIS™ kit (AM1921, Invitrogen). Reverse transcription of circRNA or mRNA was performed using the PrimeScript™ RT reagent Kit (RR037A, Takara Holdings Inc., Kyoto, Japan), and that of miRNA was performed using the miRNA First Strand cDNA Synthesis Kit (B532451, Sangon Biotech Co., Ltd., Shanghai, China). Later, the quantitative polymerase chain reaction (qPCR) analysis was performed in compliance with the instructions of the TB Green® Premix Ex Taq™ II (RR820A, Takara). The primer information is presented in Table 2, in which the U6 and β-actin were used as loading controls. The RNA expression was quantified by the 2−ΔΔCt method.
Table 2.
Primer sequences for qPCR analysis
| Gene symbol | Forward (5ʹ-3ʹ) | Reverse (5ʹ-3ʹ) |
|---|---|---|
| IL-10 | TCTCCGAGATGCCTTCAGCAGA | TCAGACAAGGCTTGGCAACCCA |
| Arg-1 | TCATCTGGGTGGATGCTCACAC | GAGAATCCTGGCACATCGGGAA |
| TGF-β | TACCTGAACCCGTGTTGCTCTC | GTTGCTGAGGTATCGCCAGGAA |
| circ_CCDC66 | CGAGACAGACGACGACAAAA | CCTGCAAGTAAAGCCATCAA |
| miR-342-3p | GGGTGCTATCTGTGATTG | GAACATGTCTGCGTATCTC |
| miR-129-5p | TTTTGCGGTCTGGGCTT | GAACATGTCTGCGTATCTC |
| MTDH | GGAGTCAAGACACTGGAGATGC | GGGTTGATTACGGCTAACATCCC |
| β-actin | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
| U6 | CTCGCTTCGGCAGCACAT | TTTGCGTGTCATCCTTGCG |
Note: IL-10, interleukin; Arg-1, arginase 1; transforming growth factor beta, TGF-β; miR, microRNA; MTDH, metadherin
Western blot (WB) analysis
Total protein in cells was extracted using the RIPA lysis buffer (Cat. No. 89901, Thermo Fisher Scientific), and that in EVs was extracted using the Exosome Protein Extraction Kit (EZN-exo-PRO1, HifunBio). The protein concentration was detected by the BCA kit (Cat. No. 23225, Thermo Fisher Scientific). After protein separation by SDS-PAGE, the protein sample was loaded onto polyvinylidene fluoride membranes, which were blocked with 5% non-fat milk for 1 h and reacted with the primary antibodies (Table 3) at 4℃ for 12 h, and then with goat anti-rabbit IgG (1:2,000, ab6721, Abcam Inc., Cambridge, MA, USA) at room temperature (~ 23℃) for 1 h. The immunoblots were developed by the ECL kit (ab65623, Abcam).
Table 3.
Primary antibodies for western blot analysis
| Antibodies | Dilution | Cat. No | Manufacture |
|---|---|---|---|
| CD63 | 1:1,000 | ab134045 | Abcam |
| CD9 | 1:1,000 | ab236630 | Abcam |
| CD81 | 1:1,000 | ab109201 | Abcam |
| Calnexin | 1:1,000 | ab22595 | Abcam |
| PD-L1 | 1:1,000 | #13684 | Cell signaling technology |
| β-actin | 1:1,000 | ab115777 | Abcam |
PD-L1, programmed death ligand 1; Abcam Inc., Cambridge, MA, USA; Cell Signaling Technology (CST), Beverly, MA, USA
Uptake of EVs by CRC cells
The EVs were labeled with a PKH67 kit (PKH67GL, Sigma-Aldrich). The PKH67-labeled EVs were added to the culture medium of CRC cells for 24 h of co-culture. Cells co-cultured with equal volume of PBS were set to controls. Thereafter, the CRC cells were fixed with 4% paraformaldehyde, penetrated with Triton X-100, blocked with bovine serum albumin, stained with DAPI, and then observed and captured under the fluorescence microscopy.
Co-culture of M2 macrophages and CRC cells
To examine the transfer of circ_CCDC66, Cy3-labeled circ_CCDC66 (Cy3-circ_CCDC66) and the control Cy3-NC were acquired from RiboBio and transfected into M2 macrophages. CRC cells were seeded in 0.4-μm Transwell basolateral chambers (3412, Corning Glass Works, Corning, NY, USA) and co-cultured with the macrophages in apical chambers (Dong et al. 2021). Later, the CRC cells were collected, fixed, penetrated, stained with DAPI, and captured under the fluorescence microscopy. 5-ethynyl-2’-deoxyuridine (EdU) labeling assay.
Cell proliferation was analyzed using the EdU labeling kit (C0071S, Beyotime). In short, the cells were digested and seeded into 96-well plates. After incubation in a 37℃ incubator overnight, the cells were incubated with EdU reagent for 2 h. Later, the cells were fixed with formaldehyde for 15 min, treated with Triton X-100 for 15 min, incubated with the Click reaction solution in the dark for 30 min, and then treated with Hoechst 33342 for nucleus staining. The staining was captured under microscopy.
Transwell assays
Stably transfected CRC cells were resuspended in serum-free medium and then loaded into 0.8-μm Transwell chambers (~ 2 × 105 cells per chamber) without (for migration detection) or with (for invasion detection) Matrigel precoating. The basolateral plates were added with complete medium (containing 10% FBS) for inducer. The chambers were placed in a 37℃ incubator. After 48 h, the plates were taken out, and the migratory or invasive cells were fixed with formaldehyde, stained with crystal violet, and counted under the microscopy.
RNA pull-down assay
Biotin-labeled miR-342-3p and miR-129-5p and the NC were procured from VectorBuilder. The CRC cells were lysed in RIPA buffer, and then the lysates were incubated with the biotin-labeled RNA. The biotinylated RNA-conjugates complexes were separated by magnetic beads (Cat. No. 11641786001, Roche Ltd., Basel, Switzerland). The complexes were eluted, and the expression of enriched circ_CCDC66 and MTDH mRNA was analyzed by qPCR analysis.
Luciferase assay
The binding sites of circ_CCDC66 and MTDH mRNA with miR-342-3p were obtained from Starbase (http://starbase.sysu.edu.cn/). The wild-type (WT) circ_CCDC66 and MTDH mRNA sequences containing the binding sites with miR-342-3p and the designed mutant-type (MUT) sequences were inserted to the pGL4-promoter vector (Promega Corporation, Madison, WI, USA) to construct luciferase reporter vectors. These vectors were transfected into the HEK293T cells along with miR-342-3p mimic or NC-mimic using the Lipofectamine. After 48 h, the luciferase activity in cells was analyzed using the dual luciferase reporter gene system (E1910, Promega).
Lactate dehydrogenase (LDH) detection to analyze cytotoxicity of CD8+ T cells on CRC cells
Activated CD8+ cytotoxic T cells represent a major population of anti-tumor immune cells directly killing tumor cells. The procured CD8+ T cells were activated by CD3/CD28 beads (ACROBiosystems Group, Beijing, China). The cytotoxicity of CD8+ T cells on CRC cells was analyzed be detecting the LDH release in cells using the LDH kit (C0016, Beyotime). In short, the cytotoxic CD8+ T cells were co-cultured with CRC cells in 96-well plates at a ratio of 10:1. Wells added with CRC cells only were set to NC, and wells added with LDH release reagent only were set to positive control (max release wells). After 24 h, the LDH developer was configured following the kit’s instructions and added to the wells. The optical density (OD) at 490 nm was analyzed by the microplate reader. The cytotoxicity was calculated as follows: .
Xenograft tumorigenesis in nude mice
The animal experiment protocol was ratified by the Animal Ethics Committee of the First Affiliated Hospital of Gannan Medical College, and the procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Bethesda, MD, USA). Forty BALB/c nude mice (one month old) were procured from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and allocated into the following 8 groups: M0-EVs, M2-EVs, M2-EVssh−NC, M2-EVssh−circ_CCDC66, M2-EVssh−circ_CCDC66 + NC-inhibitor, M2-EVssh−circ_CCDC66 + miR-342-3p-inhibitor, M2-EVs + sh-NC, and M2-EVs + sh-MTDH, 5 in each. HCT116 cells with corresponding treatments were digested and loaded into sterile tubes, washed in PBS, and then injected into the right flank of mice subcutaneously (3 × 106 cells per mouse). Meanwhile, the mice were injected with EVs through the tail vein. The tumor volume was then evaluated every 5 d as follows: V = (long axis × short axis2)/2. The mice were sacrificed by injection of 150 mg/kg 1% nembutal after day 25 to collect and weigh the tumors.
Statistical analysis
GraphPad Prism 8.0 (GraphPad, La Jolla, CA, USA) was used for data analysis and plotting. Measurement data are expressed as the mean ± SD. Differences between groups were analyzed by the t test or the one/two-way ANOVA. Cut-off value for statistical significance was set at p < 0.05.
Results
Identification of the M2 macrophages and their derived EVs
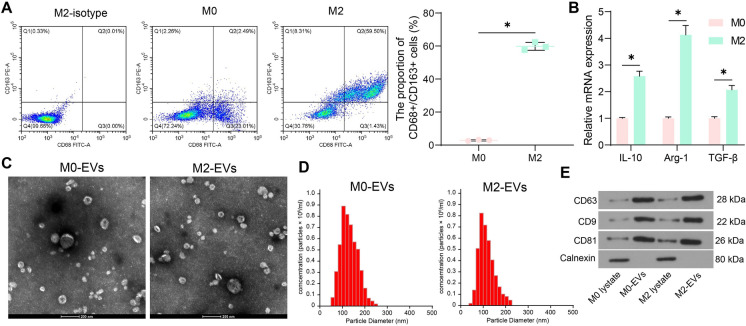
In colorectal tumorigenesis, monocytes mainly differentiate into M2-phenotype macrophages (CD68+/CD163+) (Stadler et al. 2021), which are critical components in the TME leading to cancer development. We treated THP-1 monocytes with PMA to induce M0 macrophages and then with IL-4 and IL-13 to obtain the M2 phenotype. The flow cytometry showed that the macrophages exhibited high expression of CD68 and CD163 (Fig. 1A). Compared to the M0 macrophages, the M2 macrophages showed elevated mRNA levels of IL-10, arginase 1 (Arg-1) and transforming growth factor beta (TGF-β) (Fig. 1B). The EVs from both M0 and M2 macrophages were extracted, designated as M0-EVs and M2-EVs, respectively. Under the TEM, the EVs showed typical cup shape (Fig. 1C). NTA results showed that the diameter of the nanoparticles was approximately 100 nm (Fig. 1D). The WB analysis showed that the EVs showed positive expression of CD63, CD9, and CD81 whereas negative expression of Clanexin (Fig. 1E).
Fig. 1.
Identification of the M2 macrophages and their derived EVs. A, expression of CD68 and CD163 in macrophages analyzed by flow cytometry; B, mRNA expression of the M2 macrophage markers IL-10, Arg-1 and TGF-β in the macrophages examined by qPCR analysis; C, shape of the extracted EVs under TEM; D, particle size of the extracted EVs analyzed by NTA; E, expression of the EV marker proteins in the extracted EVs analyzed by WB analysis. Repetitions = 3. Differences were analyzed by the two-way ANOVA. *p < 0.05
M2-EVs deliver circ_CCDC66 and trigger CRC cell development
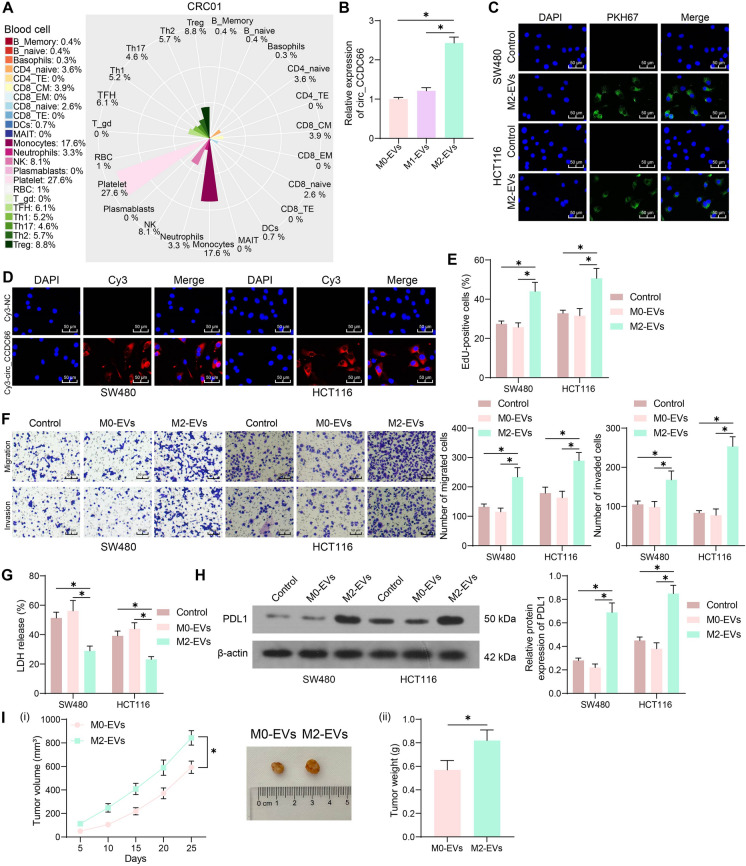
Circ_CCDC66 has been demonstrated as a potential new diagnostic biomarker in the plasma of patients with CRC (Lin et al. 2019). The biomarkers in plasma are usually released by blood cells. Therefore, we analyzed the enrichment of blood cells in CRC patients via exoRBase 2.0 (http://www.exorbase.org/exoRBaseV2/toIndex). The result showed that monocytes had the greatest enrichment level following the common platelet (Fig. 2A). As mentioned above, monocytes mainly differentiate into M2-phenotype macrophages in cancer progression. Interestingly, we identified increased circ_CCDC66 expression in M2-EVs relative to that in M0-EVs or M1-EVs (Fig. 2B). The CRC cells were co-cultured with M2-EVs or PBS, and it was observed that the M2-EVs could be directly absorbed by CRC cells (Fig. 2C). Moreover, we transfected Cys-circ_CCDC66 into M2 macrophages, which were then co-cultured with the CRC cells. Later, the red fluorescence was detected in the cytoplasm of CRC cells (Fig. 2D). Therefore, we believe that the M2 macrophages can deliver circ_CCDC66 to the CRC cells via the M2-EVs. Of note, the CRC cells showed increased proliferation, migration, and invasion potentials when co-cultured with the M2-EVs compared to those co-cultured with M0-EVs or those cultured in PBS (Fig. 2E–F). In addition, the effect of the EVs on immune evasion was determined. Compared to the M0-EVs or PBS treatment, the M2-EVs-stimulated CRC cells showed significantly reduced LDH release after CD8+ T cell attack (Fig. 2G). Western blot assay showed that the protein level of PDL1 in CRC cells was increased after M2-EVs stimulation (Fig. 2H). Moreover, we found that the M2-EVs treatment enhanced the tumorigenic activity of HCT116 cells in nude mice (Fig. 2I).
Fig. 2.
M2-EVs deliver circ_CCDC66 and trigger CRC cell development. A, enrichment of blood cells in the plasma of CRC patients; B, circ_CCDC66 expression in the collected EVs examined by qPCR analysis; C, uptake of PKH67-labeled M2-EVs by CRC cells; D, fluorescence images of in CRC cells after co-cultured with the Cy3-NC- or Cy3-circ_CCDC66-infected M2 macrophages; E, EdU assay for the proliferation of CRC cells co-cultured with EVs; F, Transwell assays for the migration and invasion of CRC cells co-cultured with EVs; G, LDH release in CRC cells upon CD8+ T cell attack; H, WB analysis for the PDL1 protein expression in CRC cells with or without EVs stimulation; I, tumorigenic activity of the HCT116 cells in nude mice with EVs treatment. For cellular experiments, repetitions = 3; for animal studies, n = 5 in each group. Differences were analyzed by the t test or two-way ANOVA. *p < 0.05
Suppression of circ_CCDC66 in M2-EVs blocks CRC cell development
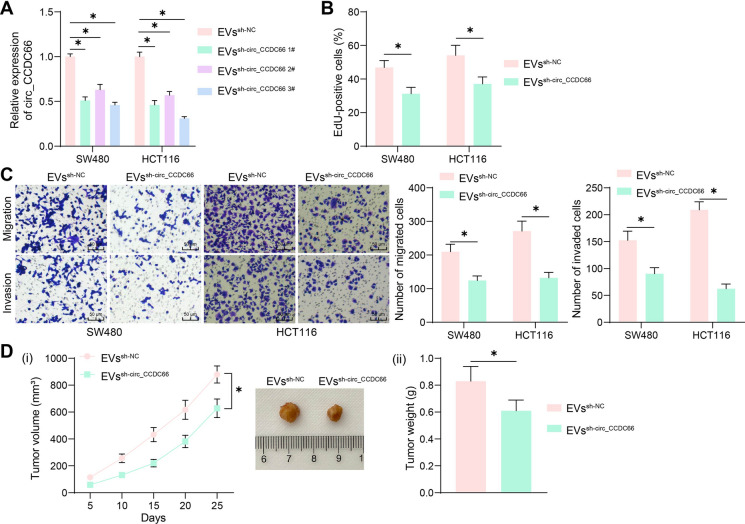
To confirm the participation of circ_CCDC66 in CRC tumorigenesis triggered by M2-EVs, we transfected three shRNAs of circ_CCDC66 (sh-circ_CCDC66 1, 2, 3#) and the sh-NC into M2 macrophages and then had the M2-EVs collected, termed EVssh−circ_CCDC66 and EVs−sh−NC, respectively. The EVs were co-cultured with the CRC cells. The EVssh−circ_CCDC66 3#, which led to the lowest circ_CCDC66 expression in CRC cells, were collected for subsequent use (Fig. 3A). Compared to those co-cultured with M2-EVssh−NC, the CRC cells co-cultured with M2-EVssh−circ_CCDC66 showed significantly reduced activity in proliferation, migration, and invasion (Fig. 3B–C). Likewise, the EVssh−circ_CCDC66 treatment markedly reduced the tumorigenic activity of HCT116 cells in nude mice (Fig. 3D).
Fig. 3.
Suppression of circ_CCDC66 in M2-EVs blocks CRC cell development. A, qPCR analysis for circ_CCDC66 expression in CRC cells after EVssh−circ_CCDC66 or EVssh−NC stimulation; B, EdU assay for the proliferation of CRC cells co-cultured with EVssh−circ_CCDC66 or EVssh−NC; C, Transwell assays for the migration and invasion of CRC cells co-cultured with EVssh−circ_CCDC66 or EVssh−NC; D, tumorigenic activity of the HCT116 cells in nude mice with EVssh−circ_CCDC66 treatment. For cellular experiments, repetitions = 3; for animal studies, n = 5 in each group. Differences were analyzed by the t test or by two-way ANOVA. *p < 0.05
Circ_CCDC66 suppresses miR-342-3p expression
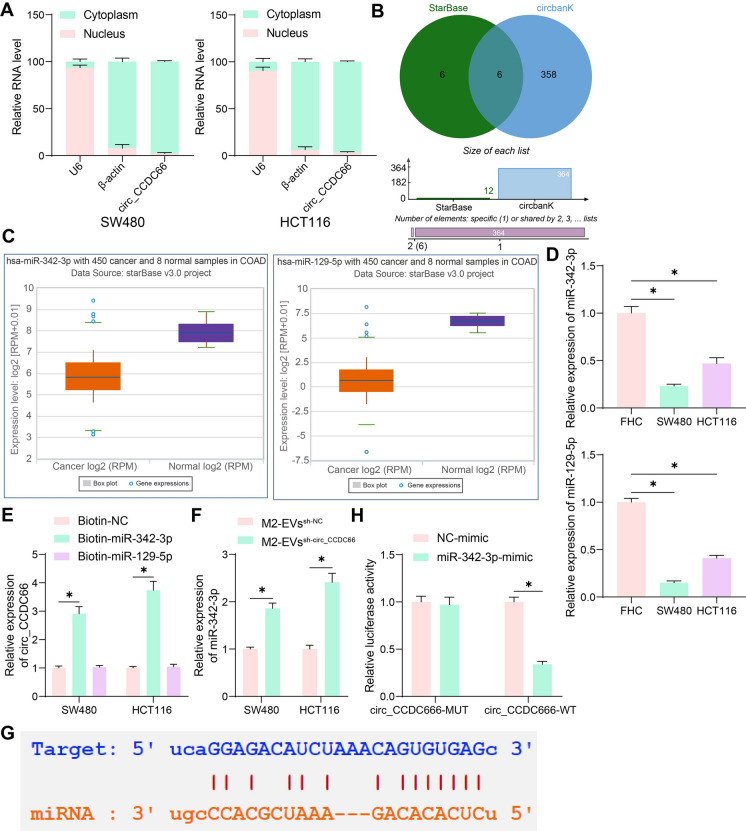
The qPCR analysis results showed that the circ_CCDC66 was mainly expressed in cytoplasmic RNA (Fig. 4A). We therefore explored its target miRNAs in the bioinformatics systems StarBase and circBank (http://www.circbank.cn/index.html), with six intersected outcomes predicted (Fig. 4B). Amongst the six candidate miRNAs, only miR-342-3p and miR-129-5p showed a significant low-expression pattern in CRC according to the data in StarBase (Fig. 4C). Thereafter, we identified significantly reduced expression of miR-342-3p and miR-129-5p in the CRC cell lines (Fig. 4D). However, the subsequent RNA pull-down assay revealed that circ_CCDC66 could only bind to miR-342-3p but not miR-129-5p (Fig. 4E). Moreover, significantly increased miR-342-3p expression was detected in CRC cells co-cultured with EVssh−circ_CCDC66 compared to those with EVssh−NC (Fig. 4F). The putative binding site of circ_CCDC66 to miR-342-3p was obtained from StarBase (Fig. 4G) to construct WT (containing the binding site) or MUT (with the binding site mutation) luciferase vectors. The luciferase assay showed that the miR-342-3p mimic in 293 T cells significantly suppressed the activity of circ_CCDC66-WT, but it did not significantly affect the activity of circ_CCDC66-MUT luciferase vector (Fig. 4H), which indicated the direct binding between circ_CCDC66 and miR-342-3p.
Fig. 4.
Interaction between circ_CCDC66 and miR-342-3p is identified in CRC. A, circ_CCDC66 expression in nuclear/cytoplasmic RNA in CRC cells examined by qPCR analysis; B, target miRNAs of circ_CCDC66 predicted in two bioinformatics systems; C, miR-342-3p and miR-129-5p with low-expression patterns in CRC predicted in StarBase; D, qPCR analysis for the expression of miR-342-3p and miR-129-5p in CRC cells and normal FHC cells; E, binding of circ_CCDC66 to miR-342-3p/miR-129-5p analyzed by RNA pull-down assay; F, qPCR analysis for miR-342-3p expression in CRC cells co-cultured with EVssh−circ_CCDC66 or EVssh−NC; G, putative binding sites between circ_CCDC66 and miR-342-3p obtained from StarBase; H, binding between circ_CCDC66 and miR-342-3p validated by the luciferase assay. Repetitions = 3. Differences were analyzed by the one-way or two-way ANOVA. *p < 0.05
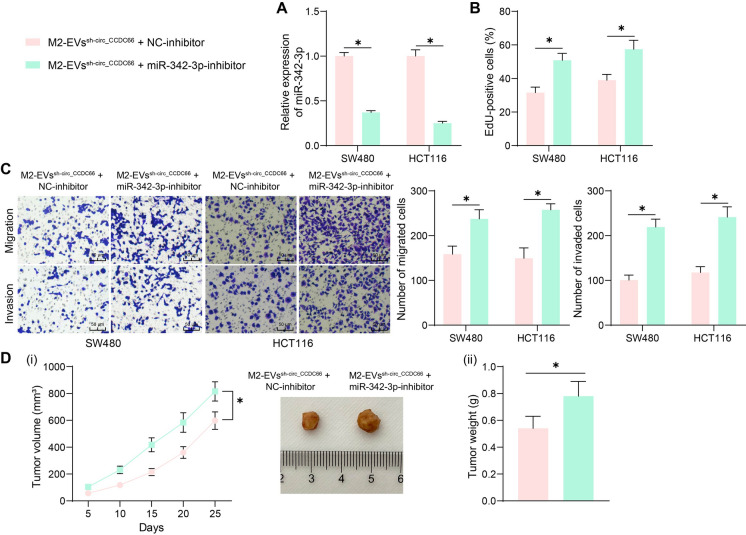
miR-342-3p inhibitor restores the malignant phenotype of CRC cells
To examine the function of miR-342-3p in CRC development, we transfected the miR-342-3p inhibitor or the NC-inhibitor into CRC cells after co-culture with the EVssh−circ_CCDC66 or EVssh−NC. The successful miR-342-3p inhibition in cells was detected by qPCR analysis (Fig. 5A). Importantly, it was found that the proliferation of the CRC cells suppressed by EVssh−circ_CCDC6 was rescued upon the miR-342-3p inhibition (Fig. 5B). Moreover, the counts of migratory and invasive CRC cells were significantly increased by miR-342-3p inhibitor (Fig. 5C). In vivo, the miR-342-3p inhibition also increased the growth rate and weight of xenograft tumors formed by HCT116 cells (Fig. 5D).
Fig. 5.
Inhibition of miR-342-3p restores the malignant phenotype of CRC cells. A, qPCR analysis for miR-342-3p expression in CRC cells after further miR-342-3p inhibitor treatment; B, EdU assay for the proliferation of CRC cells after miR-342-3p inhibitor treatment; C, Transwell assays for the migration and invasion of CRC cells after miR-342-3p inhibitor treatment; D, tumorigenic activity of the HCT116 cells in nude mice after miR-342-3p inhibition. For cellular experiments, repetitions = 3; for animal studies, n = 5 in each group. Differences were analyzed by the t test or by two-way ANOVA. *p < 0.05
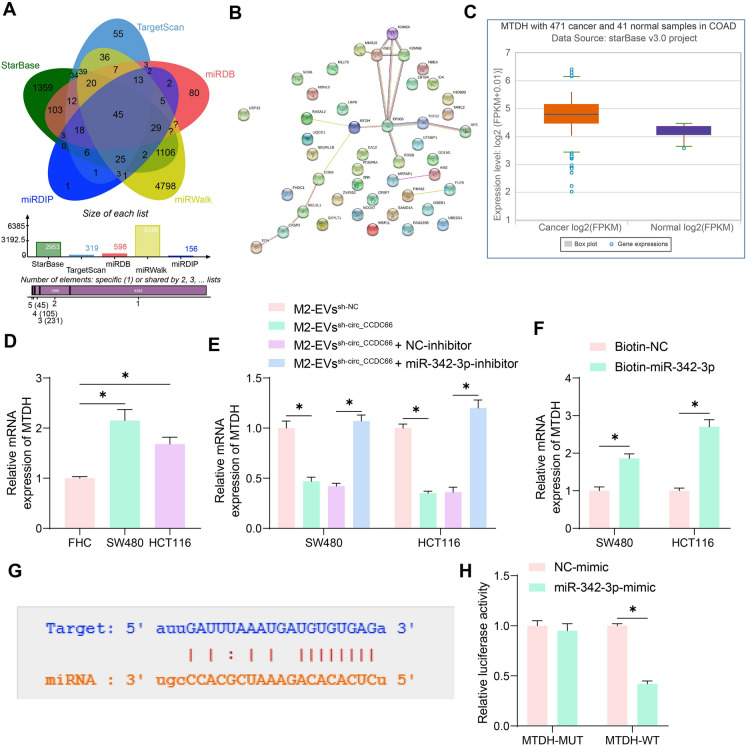
miR-342-3p targets MTDH mRNA
Next, we focused on the target genes of miR-342-3p by searching for several bioinformatics systems including StarBase, TargetScan (https://www.targetscan.org/vert_72/), miRDB (http://mirdb.org/), miRWalk (http://mirwalk.umm.uni-heidelberg.de/), and miRDIP (http://ophid.utoronto.ca/mirDIP/). A total of 45 common outcomes were obtained (Fig. 6A). These genes were collected for protein–protein-interaction (PPI) analysis using the String system (https://cn.string-db.org/cgi/input?sessionId=bktIYoIdkErF&input_page_show_search=on), with MTDH and EP300 identified as the hub genes (Fig. 6B). The MTDH was found to be aberrantly highly expressed in CRC in the StarBase system (Fig. 6C) and was selected for subsequent analysis. Elevated MTDH mRNA expression was detected in the CRC cell lines (Fig. 6D). It was noteworthy that the MTDH expression in CRC cells was suppressed upon EVssh−circ_CCDC66 stimulation but then restored after miR-342-3p inhibition (Fig. 6E). The RNA pull-down assay further confirmed that miR-342-3p was able to bind with MTDH mRNA (Fig. 6F). Likewise, the binding sequence of MTDH to miR-342-3p was downloaded from StarBase (Fig. 6G) for luciferase assay, and the results showed that the miR-342-3p mimic significantly suppressed the activity of the MTDH-WT luciferase vector in 293 T cells (Fig. 6H).
Fig. 6.
miR-342-3p targets MTDH mRNA. A, target mRNAs of miR-342-3p predicted in five bioinformatics systems; B, a PPI network of the predicted mRNAs; C, MTDH expression in CRC cells predicted in StarBase; D, qPCR analysis for the MTDH expression in CRC cells and normal FHC cells; E, qPCR analysis for MTDH expression in CRC cells upon EVssh−circ_CCDC66 stimulation or miR-342-3p inhibition F, RNA pull-down assay to detect the binding of miR-342-3p to MTDH mRNA; G, putative binding sites between miR-342-3p and MTDH obtained from StarBase; H, binding relationship of miR-342-3p to MTDH validated by luciferase assay. Repetitions = 3. Differences were analyzed by the one-way or two-way ANOVA. *p < 0.05
MTDH silencing suppresses immune evasion in CRC cells
Following the findings above, we postulated that M2-EVs possibly affect MTDH expression to mediate CRC tumorigenesis. To examine the role of MTDH, sh-MTDH was transfected into CRC cells after M2-EVs stimulation (Fig. 7A). Of note, it was observed that silencing of MTDH in CRC cells suppressed the activity in proliferation (Fig. 7B) as well as migration and invasion (Fig. 7C). Since MTDH has been recently identified involved in immune escape, we then focused on the role of MTDH in the immune response in CRC cells. The LDH release in CRC cells upon CD8+ T cell attacking was significantly increased after MTDH silencing (Fig. 7D). In addition, the WB analysis showed that the protein level of PDL1 in cells was decreased by sh-MTDH (Fig. 7E). Moreover, silencing of MTDH in HCT116 cells was found to suppress the growth rate and volume of xenograft tumors (Fig. 7F).
Fig. 7.
MTDH promotes immune escape in CRC cells. A, qPCR analysis for MTDH mRNA expression in CRC cells after further sh-MTDH transfection; B, EdU assay for the proliferation of CRC cells after MTDH knockdown; C, Transwell assays for the migration and invasion of CRC cells after MTDH knockdown; D, LDH release in CRC cells upon CD8+ T cell attacking; E, WB analysis for the PDL1 expression in CRC cells after MTDH knockdown; F, tumorigenic activity of the HCT116 cells in nude mice after MTDH knockdown. For cellular experiments, repetitions = 3; for animal studies, n = 5 in each group. Differences were analyzed by the t test or by two-way ANOVA. *p < 0.05
Discussion
The gastrointestinal tract holds the greatest enrichment of macrophages, and they recruit monocytes from the blood to the tumor site upon the external environmental stimulation, which then polarize to TAMs in the TME of CRC (Wang et al. 2021). This study focuses on the role of M2-EVs and their contained molecules in the progression of CRC and reports that the M2-EVs augment immune evasion and development of CRC via a circRNA_CCDC66/miR-342-3p/MTDB axis.
While the M2 macrophages have been broadly recognized as fundamental components in the TME leading to immunosuppression and tumor metastasis (Giannone et al. 2020; Wei et al. 2019), recent researches have paid increasing attentions to the roles of EVs derived from these macrophages in tumor progression. For instance, a recent publication by Cao et al. demonstrates that the M2-EVs augment metastasis of gastric cancer cells by delivering miR-15b-5p and suppressing the BRMS1 and DAPK1 expression (Cao et al. 2022). However, the authors did not provide the direct effect of the EVs on the malignant behavior of cancer cells. Another report by Chang et al. showed that the M2-EVs potentiated the differentiation and activities of the pancreatic cancer stem cells (Chang et al. 2021). More relevantly, a previous study by Lan et al. suggested that the culture in the conditioned medium of M2 macrophages augments the mobility of CRC cells, and they found that the M2-EVs treatment improved the tumorigenic activity of the CRC cells (Lan et al. 2019). In this work, we induced M0 and M2 macrophages and had their EVs collected. The M2-EVs significantly potentiated the proliferation as well as migration and invasion of CRC cells in vitro, and they promoted the tumorigenic activity of cancer cells in vivo. Moreover, the M2-EVs stimulation increased the immune tolerance of CRC cells. The results preliminarily validated the important oncogenic roles of the M2-EVs in CRC.
Of note, we identified increased abundance of circ_CCDC66 in the M2-EVs compared to the M0-EVs. This circRNA has been defined as a tumor driver triggering proliferation, invasiveness, epithelial-to-mesenchymal transition, and tumorigenic ability of cancer cells (Joseph et al. 2018; Xu et al. 2020). In CRC, likewise, circ_CCDC66 promoted the proliferation, migration, and dissemination of cancer cells by manipulating a subset of oncogenes (Mo et al. 2022). In addition, the circ_CCDC66 knockdown was found to suppress the chemo resistance in CRC cells (Lin et al. 2020). Importantly, we transfected shRNA of circ_CCDC66 in the M2 macrophages and had the M2-EVs collected. Upon circ_CCDC66 knockdown, the proliferation, migration, and invasiveness of the CRC cells potentiated by the M2-EVs were significantly blocked, indicating that circ_CCDC66 was, at least partly, accountable for the oncogenic functions of the M2-EVs.
The circ_CCDC66 has been frequently identified as a “sponge” for miRNAs to regulate downstream mRNAs implicated in tumor progression (Mo et al. 2022; Zhang et al. 2021). Here, we identified a cytoplasm-localization of circ_CCDC66 in two CRC cell lines and then confirmed miR-342-3p as its target miRNA. The ceRNA theory suggests that circRNAs or long non-coding RNAs bind to miRNAs to block their activity (Karreth and Pandolfi 2013). Interestingly, we found that the circ_CCDC66 knockdown led to an increase in miR-342-3p expression. Similar phenomena that the circRNA/long non-coding RNA alteration leading to expression change in miRNAs have been observed in several previous studies (Guo et al. 2021; Kong et al. 2019; Luo et al. 2021). However, the precise mechanism remains undefined yet. The miR-342-3p worked as a tumor suppressor in several human solid tumors (Komoll et al. 2021; Liu et al. 2021). Upregulation of miR-342-3p has been found to block the growth and dissemination of CRC cells triggered by exosomal LINC00659 (Zhou et al. 2021). Downregulation of miR-342-3p has also been found to be linked to increased chemo resistance in CRC (Zhang et al. 2019). Here, we found that the proliferation, migration, and invasiveness of the CRC cells suppressed upon circ_CCDC66 knockdown were blocked after further miR-342-3p inhibition. Later, we obtained via five bioinformatics systems that miR-342-3p can target the MTDH mRNA. MTDH has been revealed as a gene poorly expressed or absent in non-malignant tissues but markedly elevated in cancer cells, which participate in the tumorigenic and metastatic potential of transformed cells by regulating the cancer-related signaling such as PI3K/AKT, NF-κB, and Wnt/β-catenin (Dhiman et al. 2019). Therefore, it has been suggested as a therapeutic target for cancers including CRC (Shen et al. 2021). Not surprisingly, we performed loss-of-function assay and found that that the MTDH inhibition significantly blocked the malignant phenotype of CRC cells induced by the M2-EVs. Importantly, MTDH has been reported to increase the transcriptional activity of PDL1 via the β-catenin/LEF-1 signaling, and MTDH blockade was found to increase the anti-PD-1 response and cytotoxic T-cell infiltration in hepatocellular carcinoma cells (Wan et al. 2022). Considering that M2 macrophages can release PDL1 to trigger immunosuppression (Wei et al. 2019), we postulated that the M2-EVs possibly affects the MTDH expression to affect the immune response in CRC cells. Noteworthy, we found that the MTDH knockdown significantly enhanced the cytotoxicity of CD8+ T cells on CRC cells and reduced the expression of PDL1. Therefore, we believe that upregulation of MTDH may participate in the immunosuppression mediated by M2-EVs.
Taken together, this work demonstrates that M2-EVs restore the expression of MTDH by delivering circRNA_CCDC66 to augment the immune evasion and development of CRC cells. Specific inhibition of circRNA_CCDC66 or MTDH may help overcome the immunosuppression in CRC cells to suppress tumor metastasis and development. Direct extraction of macrophages from primary tumors might enhance the degree of translationally-relevant insight of the present work. However, we did not include primary tumor samples for analyses duo to the fund limits. We would like to focus on this issue in our future investigations. We also hope more researches will be conducted to verify the findings of the present work and develop more candidate targets for CRC management.
Author contributions
LFF contributed to the conception and design of the study, data acquisition, analysis and interpretation, and also drafted and critically revised the manuscript. GFX contributed to the analysis and interpretation of the data. XFZ contributed in the data collection and statistical analysis. All authors read and approved the final manuscript.
Funding
None.
Data availability
All the data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
No potential conflict of interest was reported by the author(s).
Ethical approval
The animal experiment proposal was ratified by the Animal Ethics Committee of the First Affiliated Hospital of Gannan Medical College, and the procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH, Bethesda, MD, USA).
Consent to participate
Not applicable.
Consent for publication
All authors read the journals guideline and agreed with consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Linfeng Fan, Email: Fanflf174@gmu.edu.cn.
Xiangfu Zeng, Email: zxf19681122@163.com.
References
- Abdelmohsen K, et al. (2017) Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017;10(1080/15476286):1279788. doi: 10.1080/15476286.2017.1279788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Z, et al. SNAIL induces EMT and lung metastasis of tumours secreting CXCL2 to promote the invasion of M2-type immunosuppressed macrophages in colorectal cancer. Int J Biol Sci. 2022;18:2867–2881. doi: 10.7150/ijbs.66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, et al. microRNA-15b-5p encapsulated by M2 macrophage-derived extracellular vesicles promotes gastric cancer metastasis by targeting BRMS1 and suppressing DAPK1 transcription. J Exp Clin Cancer Res. 2022;41:152. doi: 10.1186/s13046-022-02356-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chang J, Li H, Zhu Z, Mei P, Hu W, Xiong X, Tao J. microRNA-21–5p from M2 macrophage-derived extracellular vesicles promotes the differentiation and activity of pancreatic cancer stem cells by mediating KLF3. Cell Biol Toxicol. 2021 doi: 10.1007/s10565-021-09597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman G, et al. Metadherin: a therapeutic target in multiple cancers. Front Oncol. 2019;9:349. doi: 10.3389/fonc.2019.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, et al. Mechanical stretch induces osteogenesis through the alternative activation of macrophages. J Cell Physiol. 2021;236:6376–6390. doi: 10.1002/jcp.30312. [DOI] [PubMed] [Google Scholar]
- Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA., Jr Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G, Ghisoni E, Genta S, Scotto G, Tuninetti V, Turinetto M, Valabrega G. Immuno-metabolism and microenvironment in cancer: key players for immunotherapy. Int J Mol Sci. 2020 doi: 10.3390/ijms21124414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K, Qian K, Shi Y, Sun T, Wang Z. LncRNA-MIAT promotes thyroid cancer progression and function as ceRNA to target EZH2 by sponging miR-150–5p. Cell Death Dis. 2021;12:1097. doi: 10.1038/s41419-021-04386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, Tsai SJ. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77:2339–2350. doi: 10.1158/0008-5472.CAN-16-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph NA, et al. The role of HGF-MET pathway and CCDC66 cirRNA expression in EGFR resistance and epithelial-to-mesenchymal transition of lung adenocarcinoma cells. J Hematol Oncol. 2018;11:74. doi: 10.1186/s13045-018-0557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3:1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA. 2017 doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoll RM, et al. MicroRNA-342–3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol. 2021;74:122–134. doi: 10.1016/j.jhep.2020.07.039. [DOI] [PubMed] [Google Scholar]
- Kong X, et al. LncRNA-CDC6 promotes breast cancer progression and function as ceRNA to target CDC6 by sponging microRNA-215. J Cell Physiol. 2019;234:9105–9117. doi: 10.1002/jcp.27587. [DOI] [PubMed] [Google Scholar]
- Lan J, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon. Cancer Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- Lin J, Cai D, Li W, Yu T, Mao H, Jiang S, Xiao B. Plasma circular RNA panel acts as a novel diagnostic biomarker for colorectal cancer. Clin Biochem. 2019;74:60–68. doi: 10.1016/j.clinbiochem.2019.10.012. [DOI] [PubMed] [Google Scholar]
- Lin YC, Yu YS, Lin HH, Hsiao KY. Oxaliplatin-induced DHX9 phosphorylation promotes oncogenic circular RNA CCDC66 expression and development of chemoresistance. Cancers (basel) 2020 doi: 10.3390/cancers12030697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. Circular RNA hsa_circ_001783 regulates breast cancer progression via sponging miR-200c-3p. Cell Death Dis. 2019;10:55. doi: 10.1038/s41419-018-1287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cheng Y, Wang Y, Zhang Y. Circular RNA circVAPA contributes to non-small-cell lung cancer progression via miR-342–3p-dependent regulation of ZEB2. World J Surg Oncol. 2021;19:335. doi: 10.1186/s12957-021-02447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Liu S, Li X, Hu Y. Zhang K (2021) Circular RNA circHIPK3 promotes breast cancer progression via sponging MiR-326. Cell Cycle. 2021;10(1080/15384101):1939476. doi: 10.1080/15384101.2021.1939476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min AKT, et al. Therapeutic potential of anti-VEGF receptor 2 therapy targeting for M2-tumor-associated macrophages in colorectal cancer. Cancer Immunol Immunother. 2021 doi: 10.1007/s00262-020-02676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y, et al. Circular RNA CCDC66 improves murine double minute 4 (MDM4) expression through targeting miR-370 in colorectal cancer. Comput Math Methods Med. 2022 doi: 10.1155/2022/7723995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A. Extracellular vesicles in cancer progression. Semin Cancer Biol. 2021;76:139–142. doi: 10.1016/j.semcancer.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: a review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019;125:4139–4147. doi: 10.1002/cncr.32163. [DOI] [PubMed] [Google Scholar]
- Shen M, et al. Therapeutic targeting of metadherin suppresses colorectal and lung cancer progression and metastasis. Cancer Res. 2021;81:1014–1025. doi: 10.1158/0008-5472.CAN-20-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie CL, Sirey T, Ponting CP. Complexities of post-transcriptional regulation and the modeling of ceRNA crosstalk. Crit Rev Biochem Mol Biol. 2018;53:231–245. doi: 10.1080/10409238.2018.1447542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler M, et al. Stromal fibroblasts shape the myeloid phenotype in normal colon and colorectal cancer and induce CD163 and CCL2 expression in macrophages. Cancer Lett. 2021 doi: 10.1016/j.canlet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Vo JN, et al. The landscape of circular RNA. Cancer Cell. 2019 doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan JL, et al. MTDH antisense oligonucleotides reshape the immunosuppressive tumor microenvironment to sensitize Hepatocellular Carcinoma to immune checkpoint blockade therapy. Cancer Lett. 2022 doi: 10.1016/j.canlet.2022.215750. [DOI] [PubMed] [Google Scholar]
- Wang H, Tian T, Zhang J. Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): from mechanism to therapy and prognosis. Int J Mol Sci. 2021 doi: 10.3390/ijms22168470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64. doi: 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, et al. ACLY facilitates colon cancer cell metastasis by CTNNB1. J Exp Clin Cancer Res. 2019 doi: 10.1186/s13046-019-1391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, et al. Circular RNA CCDC66 promotes gastric cancer progression by regulating c-Myc and TGF-beta signaling pathways. J Cancer. 2020 doi: 10.7150/jca.37718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PS, et al. Advances in modeling the immune microenvironment of colorectal cancer. Front Immunol. 2020 doi: 10.3389/fimmu.2020.614300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PF, et al. The lncRNA SCARNA2 mediates colorectal cancer chemoresistance through a conserved microRNA-342–3p target sequence. J Cell Physiol. 2019 doi: 10.1002/jcp.27684. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li X, Zhang J, Mao L. Circ-CCDC66 upregulates REXO1 expression to aggravate cervical cancer progression via restraining miR-452–5p. Cancer Cell Int. 2021 doi: 10.1186/s12935-020-01732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li J, Tang Y, Yang M. Exosomal LncRNA LINC00659 transferred from cancer-associated fibroblasts promotes colorectal cancer cell progression via miR-342–3p/ANXA2 axis. J Transl Med. 2021 doi: 10.1186/s12967-020-02648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.