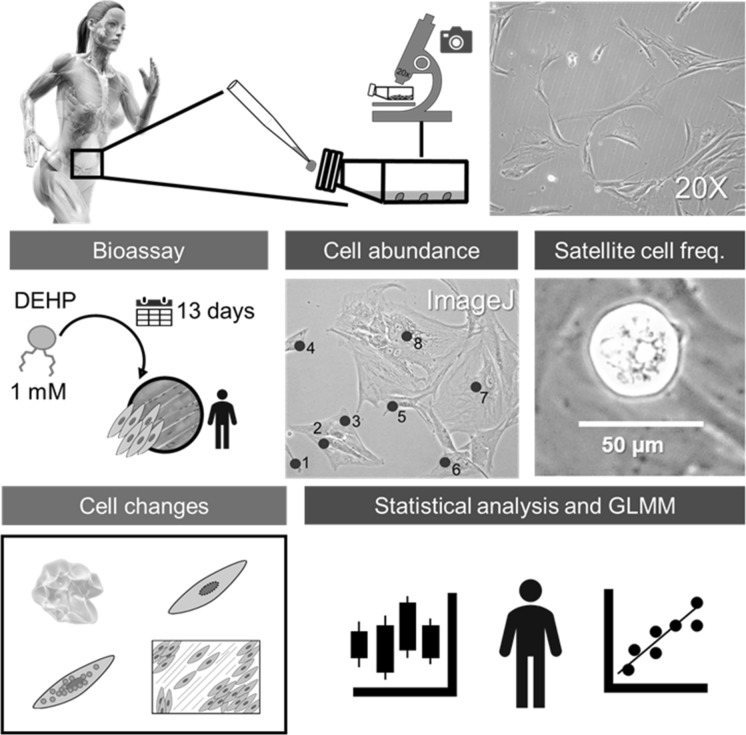
Abstract
The plasticizer di (2-ethylhexyl) phthalate (DEHP) inhibits differentiation, impairs glucose metabolism, and decreases mitochondrial function in murine muscle satellite cells; however, if these effects are translated to human cells is unknown. The goal of this study was to evaluate changes in morphology and proliferation of primary human skeletal muscle cells exposed to DEHP. Rectus abdominis muscle samples were obtained from healthy women undergoing programed cesarean surgery. Skeletal muscle cells were isolated and grown under standard primary culture conditions, generating two independent sample groups of 25 subcultures each. Cells from the first group were exposed to 1 mM DEHP for 13 days and monitored for changes in cell morphology, satellite cell frequency and total cell abundance, while the second group remained untreated (control). Differences between treated and untreated groups were compared using generalized linear mixed models (GLMM). Cell membrane and nuclear envelope boundary alterations, loss of cell volume and presence of stress bodies were observed in DEHP-treated cultures. DEHP-treated cultures also showed a significant reduction in satellite cell frequency compared to controls. Exposure to DEHP reduced human skeletal muscle cell abundance. Statistical differences were found between the GLMM slopes, suggesting that exposure to DEHP reduced growth rate. These results suggest that exposure to DEHP inhibits human skeletal muscle cell proliferation, as evidenced by reduced cell abundance, potentially compromising long-term culture viability. Therefore, DEHP induces human skeletal muscle cell deterioration potentially inducing an inhibitory effect of myogenesis by depleting satellite cells.
Graphical abstract
Keywords: Cytotoxicity, Emerging pollutants, Phthalates, Primary culture, Striated muscle, Women
Introduction
Di (2-ethylhexyl) phthalates (DEHP) are widely used as additives in plastic products to increase flexibility, endurance and transparency (ATSDR 2019). However, these chemical compounds are toxic and teratogenic (Park et al. 2020). According to the U.S. Environmental Protection Agency (EPA), DEHP is the most widespread plasticizer and one of the six phthalates considered priority pollutants worldwide (EPA 2012). Because DEHP is not covalently bound to the plastic polymer, this phthalate can be released and transported by air, water and soil (Halden 2010). DEHP concentrations in nature depend on the environment where DEHP is deposited. Net et al. (2015) reported that DEHP dust concentration varies between 600 µg g−1 d−1 in the U.S. and 3000 µg g−1 d−1 in Denmark, estimating a general absorption per person of 70 µg kg−1 d−1. Despite a maximum exposure concentration of DEHP per day established at 25 µg kg− 1 for a human adult (60 kg) (World Health Organization 2017), concentrations as low as 100 µM could be harmful for different tissues and systems (Brassea-Pérez et al. 2022).
Human exposure to DEHP mostly occurs by consuming food and beverages packaged in plastic (Kim et al. 2014; Gurdemir et al. 2019), but also through inhalation (Franken et al. 2017), across the skin (Wu et al. 2015) and via parenteral administration (Fromme 2011; Kelley et al. 2012; Steiner et al. 1998) found that DEHP concentration in human saliva after sucking on a PVC film, common in dental vacuum forming sheet, vinyl toys, and snorkel mouthpieces, is 1017 µg g−1 equivalent to 2.6 mM (2604 µM). DEHP is a lipophilic compound that can cross biological membranes; it is absorbed and metabolized in the intestine, and distributed through the vascular system reaching the liver, before excretion takes place (Rael et al. 2009; Choi et al. 2013). Within cells, esterase and lipase-mediated metabolism hydrolyze DEHP into its primary metabolite mono-(2-ethylhexyl) phthalate (MEHP) (Koch and Calafat 2009; Choi et al. 2013). This monoester is lighter (lower molar mass) than DEHP and, therefore, preferentially transported into the vascular system. Thus, MEHP is a more reactive and potentially hazardous compound to human health than DEHP (Choi et al. 2018). Chronic exposure to phthalates in humans and other animals is associated with endocrine dysfunction (Cho et al. 2015), developmental alterations (Agarwal et al. 1986; Zuo et al. 2014), cancer (Wang et al. 2012; Yavasoglu et al. 2014; Crobeddu et al. 2019), and loss of cell proliferation and viability in different tissues (Ma et al. 2018; Molino et al. 2019; Chen et al. 2020). Despite the available information on the hazardous effects of DEHP in mammals, the potential impact of this compound in human skeletal muscle is still unclear (ATSDR 2019).
Skeletal muscle is composed of cells with multiple nuclei that form long fibers; these cells are involved in voluntary movements and represent 30% and 38% of body mass in adult women and men, respectively (Janssen et al. 2000; Hill and Olson 2012). As a contractile apparatus, skeletal muscle fibers need to be continuously repaired. The proliferation or population growth capacity of skeletal muscle cells and fibers depends on self-renewal of myogenic satellite cells (Snijders et al. 2015). Skeletal muscle satellite cells maintain cell populations which would spread or proliferate, differentiate into myoblasts, fuse forming multinuclear myotubes, and lead to myofiber formation (Etienne et al. 2020). This regeneration process compensates for tissue loss due to attrition, exposure to xenobiotics or injury (Snijders et al. 2015; Chen et al. 2020; Etienne et al. 2020).
Chen et al. (2020) suggested that DEHP/MEHP induces mitochondrial dysfunction and inhibits myogenesis in murine skeletal muscle cells. Moreover, exposure to phthalates promotes fragmentation of the mitochondrial reticulum, compromising mitochondrial efficiency (Hoppins 2014; Lackner 2014). By decreasing mitochondrial energy production, DEHP/MEHP could compromise muscle satellite cell viability and myogenic regeneration. Furthermore, skeletal muscle dysfunction may lead to metabolic disorders such as insulin resistance, obesity (Rabinowitz and Zierler 1962) and even sarcopenia (Yang et al. 2022). As plasticizers are found in many daily products, exposure to DEHP has increased in the last decades (Ferguson et al. 2011; Kim et al. 2014; Gurdemir et al. 2019). The aim of this study was to analyze potential changes in skeletal muscle cell proliferation, abundance and morphology in primary human skeletal muscle cells exposed to DEHP.
Materials and methods
Sample collection
Rectus abdominis muscle biopsies (~ 3 g) were collected from five healthy adult (18–35 years old) females undergoing programed cesarean surgery and antenatal care at Instituto Mexicano del Seguro Social (IMSS). Prior to sampling, informed consent was obtained from all volunteers. The research protocol and informed consent forms were registered and approved by Comité de Ética en Investigación and Comité Hospitalario de Bioética (F-CNIC 2019-174 and R 2000-785-008), Comisión Nacional de Investigación Científica del Instituto Mexicano del Seguro Social (IMSS; 2018-785-010), as well as by Comisión Nacional de Bioética (CONBIOÉTICA-09-CEI-009-20160601). Sample collection was carried out in accordance with the guidelines of CONBIOÉTICA and the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Primary cell isolation and culture
Human skeletal muscle cells were grown from tissue explants in culture medium consisting of Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12, 1X; Corning®), calf serum (FBS, 12.4% v/v; Gibco™), penicillin/streptomycin (10 U mL−1; GibcoTM), l-alanine-l-glutamine (GlutaMAX, 100X; Gibco™), sodium pyruvate (100 mM; Gibco™) and sulfonic acid (HEPES, 1 M; Gibco™). Skeletal muscle cells were incubated at 35 ± 1 °C in a humidified 5% CO2 incubator. Medium was changed every 3 days. Cells were allowed to reach ~ 98–100% confluence before being sub-cultured until enough biomass for the bioassays was obtained.
Cell viability at different di (2-ethylhexyl) phthalate (DEHP) doses
DEHP toxicity in skeletal muscle cells was tested prior to bioassays to establish the in vitro DEHP theoretical concentration at which cell viability declines. The theoretical concentration was estimated according to the ratio (v/v) of dissolved DEHP in FBS and culture medium (Jones et al. 1975; Li et al. 2015). DEHP partially dissolves in cell culture medium; thus, initial concentration of DEHP in a stock solution is not maintained and the actual DEHP content (approximately 15% of the initial concentration) can be estimated as follows:
| 1 |
where represents the DEHP concentration in the stock solution.
The proportion of live skeletal muscle cells was determined by trypan blue exclusion using a hematocytometer (Ehrlich 1904; Louis and Siegel 2011). By estimating cell viability, it was found that, at a theoretical concentration of 925 µM DEHP, 40% of the cells were dead between 10 and 13 days of exposure (Fig. 1). Considering the apparent self-diffusion coefficient (Dapp) of DEHP in the medium to be 4.04 × 10−7 cm min−1 (Hara 1993; Bernard et al. 2021), the concentration of dissolved DEHP in culture medium was estimated to be approximately 1000 µM (1 mM). Where dissolved DEHP is the portion of the phthalate in contact with the cells in culture and can be absorbed by them. Therefore, further bioassays were carried out for 13 days using a 1 mM DEHP concentration. Prior to each assay, DEHP was diluted in FBS (12.4% v/v; Gibco™) for 24 h and added to the culture medium.
Fig. 1.
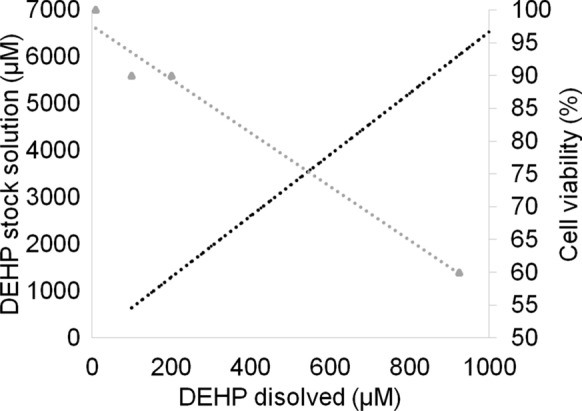
Relationship between the theorical concentration (Jones et al. 1975; Li et al. 2015) of dissolved di (2-ethylhexyl) phthalate (DEHP) and DEHP stock solution (black circles) and cell viability (gray triangles) in human skeletal muscle cells in primary culture
Di (2-ethylhexyl) phthalate (DEHP) bioassay
Skeletal muscle cells were trypsinized (Freshney 2016) and seeded in 25 cm2 T flasks. From each donor (biological replicate), 10 primary cell cultures, in independent T flasks, were obtained, for a total of 50 T flasks (subcultures or experimental units; Mead et al. 2012). Cells were then assigned to two groups, one group was treated with a theoretical concentration of DEHP (1 mM, n = 25 T flasks) (Jones et al. 1975) for 13 days; the second group was kept as an untreated control (n = 25 T flasks). At the beginning of each bioassay, each T flask started at ~ 40–50% confluence.
Cell morphology, proliferation, and regeneration capacity
Human skeletal muscle cell cultures were monitored using an inverted microscope and ZEN 2.0 software© (Carl Zeiss Microscopy GmbH 2011). Photographs were taken under a 20× objective from random quadrants of each flask and saved in JPEG format (5 mp). Photograph quality parameters, including brightness (normal 0%), contrast ratio (20%), resolution (96 ppp), and noise (0%) were considered to select representative images.
The images that met the required quality parameters were used for further processing, including determination of cell morphology, cell abundance, and satellite cell frequency. Changes in physical cell characteristics, including cell volume loss (plasmolysis), presence of bodies in the cytosol (Ravel-Chapuis et al. 2016), and changes in the nuclear envelope (Ye et al. 2017), were registered. Cell confluence measured as the percentage of the surface culture area that is covered with cells was also considered. Cell proliferation was quantified based on changes in cell abundance and used as an indicator of cell population growth. Cell abundance was estimated using ImageJ (Schneider et al. 2012). To ensure consistency across images, a reference scale was set for each image using ImageJ software (Schneider et al. 2012), considering that 574 pixels in each 20× objective image is equivalent to 0.02 cm. Total cell abundance was then calculated by extrapolating the number of cells counted in each photograph (0.0061178 cm2) to the total surface of each flask (25 cm2). Total cell count was reported as the mean of total number of cells in 25 cm2 per day in each culture flask. This method provides accurate and consistent measurement of the cell population over the course of the experiment. The frequency of satellite cells, which are a major component of the regenerative capacity of muscles (Charifi et al. 2003), was used as a proxy of the regeneration capacity of skeletal muscle. Satellite cells were identified as those showing a characteristic round shape and approximate size of 25 ± 15 μm (Allbrook 1981; Gregory 2004) using a 2D landmark-based geometric morphometric analysis conducted in ImageJ software (Schneider et al. 2012), following the methodology described by Labno in 2014 for automated cell counting in mixed samples. The following parameters were used for particle analysis: (1) size exclusion, where cells larger than 50 μm were excluded by the software; (2) structure, by setting cell shape circularity between 0.8 and 1; (3) color, using the minimum method for thresholding in hue, saturation, and brightness (HSB). The data obtained following this automated process was visually confirmed. Total satellite cell numbers were estimated using the same extrapolation parameters used for total cell abundance. Satellite cell absolute frequency was divided into two sets to calculate the average daily frequency of satellite cells in the first (1–7 days) and second (8–13 days) weeks of the bioassays, respectively.
Statistical analyses
Shapiro–Wilks (W) and Levene’s tests were used to evaluate statistical assumptions of normality and homoscedasticity, respectively, before statistical analyses were performed (Hector 2015). Non-parametric Wilcoxon test was applied to estimate statistical differences in satellite cell frequency between control and treatment groups through the first (1–7 days) and second week (8–13 days) of bioassays.
To avoid observation bias during cell counts, the full data set was subjected to bootstrapping, using 1000 iterations; no statistical differences were observed between sample and resample distributions (X2 = 2400, p = 0.2405). Hence, raw data were used to compute further analyses. Cell abundance quantified in each flask was treated as an independent subsample of each human skeletal muscle cell culture (n = 5). For each culture condition (control, DEHP exposure), generalized linear mixed-effects models (GLMMs) (Bates et al. 2015; Handayani et al. 2017) were adjusted to analyze skeletal muscle cells response, in terms of cell proliferation (response variable). Random slopes, intercepts and non-random effect from independent cultures were considered for model building (Table 1).
Table 1.
Factors used to build generalized linear mixed-effects models (GLMMs) to analyze human skeletal muscle cell population growth in untreated (control) and di (2-ethylhexyl) phthalate (DEHP, 1 mM) treated cell cultures for 13 days
| Treatment | Variable | Type | Description | Formula |
|---|---|---|---|---|
| Control | Cell abundance | Continuous | Response variable | y ~ β0 + β1x + (x||human) |
| Culture time | Continuous | x: Explanatory variable | ||
| Human | Categorical | Random effect | ||
| DEHP | Cell abundance | Continuous | Response variable | y ~ β0 + β1x + (x||human) |
| Culture time | Continuous | x: Explanatory variable | ||
| Human |
Categorical random effect |
Random effect |
(||) Indicates non-random effect from independent samples
GLMM were designed following a gamma distribution with an identity link function (f(x) = x) as it yielded the best fit for model building and data distribution showed no statistical differences with respect to a reference gamma distribution (X2 = 32,680, p = 0.275). Goodness of fit and model selection for correlated and uncorrelated intercepts and slopes were based on Akaike’s information criterion (AIC). Nagelkerke R-squared, an alternative test for fitted models, was estimated as well as the significant relationship described by the model (p value), by using the likelihood ratio test (Chi-square test) (Kabacoff 2015). For this estimation, each GLMM is compared against a null model which is nested in the fitted models. GLMM data are presented as means and standard deviations. The slope coefficient and constants were statistically tested to evaluate differences in skeletal muscle cells between DEHP-treated and control cells using Student’s t-test (Ferson and Burgman 2000). All analyses were performed using RStudio 4.0.3® (RStudio Team 2020) and all reported p values lower than 0.05 (α = 0.05) were considered statistically significant.
Results
A DEHP theorical concentration of 1 mM (cell viability < 60%) was used for further analysis as other tested concentrations (10, 100 and 200 µM) did not affect skeletal muscle cell viability (≥ 90%).
Di (2-ethylhexyl) phthalate (DEHP) induces morphological changes in human skeletal muscle cells in primary culture
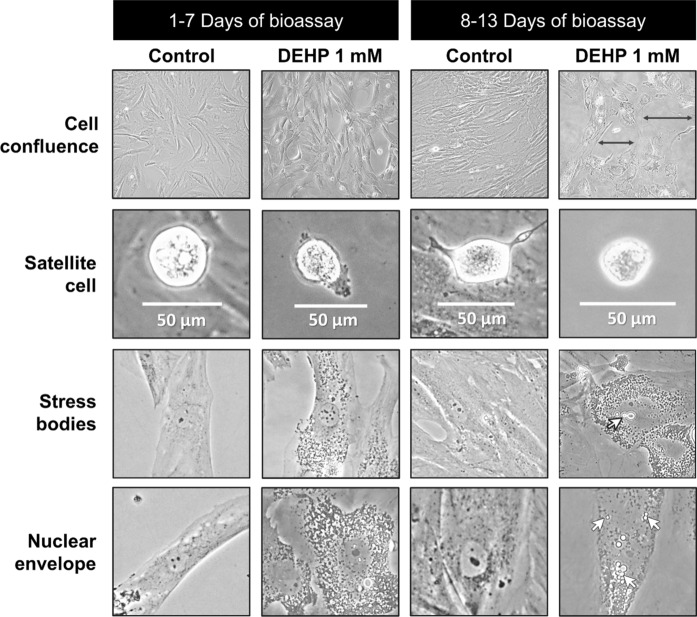
From the 526 total photographs obtained, 413 (78.5%) images met the required quality parameters; total cell abundance and satellite cell frequency were calculated throughout the bioassay. Figure 2 shows representative images of the morphological changes observed in primary human skeletal muscle cells exposed to DEHP (1 mM). From day 3 of the bioassay, phthalate micelles were observed in the cytosol. Satellite cell membranes shrunk and contracted, and some skeletal cells lost volume; these changes were observed especially in cells without adjacent or neighboring cells. On day 6, DEHP particles were observed inside the nucleus and the nuclear envelope appeared dissociated. At this time, some satellite and skeletal muscle cells were seen floating in the media. Plasmolysis and stress bodies formation in the cytosol increased with DEHP exposure time. On day 9, plasmolyzed cells were prevalent and the intercellular spaces became more evident with time in cells exposed to DEHP. During the second week of DEHP exposure, the cytoplasmic material was almost covered by stress bodies surrounding the nuclei. Control cultures started to show cells growing on top of other cells, forming layers, when 85–90% confluence was reached. By day 13, more DEHP micelles were observed in the cytosol and nuclei, leading to cell shape change, loss of cell adherence to the culture flask, loss of cell layering, and cell death (Fig. 2). In contrast, in the control samples skeletal muscle cell layers were piled one on top of the other (~ 3 layers) and large multinucleated myotubes were observed.
Fig. 2.
Morphological changes in primary human skeletal muscle cells exposed to 1 mM of di (2-ethylhexyl) phthalate (DEHP) for 13 days. All the images were taken under 20× objective and zoomed in for appreciation. Black arrows ( ) point at intercellular spaces and white arrows (
) point at intercellular spaces and white arrows ( ) indicate phthalate micelles
) indicate phthalate micelles
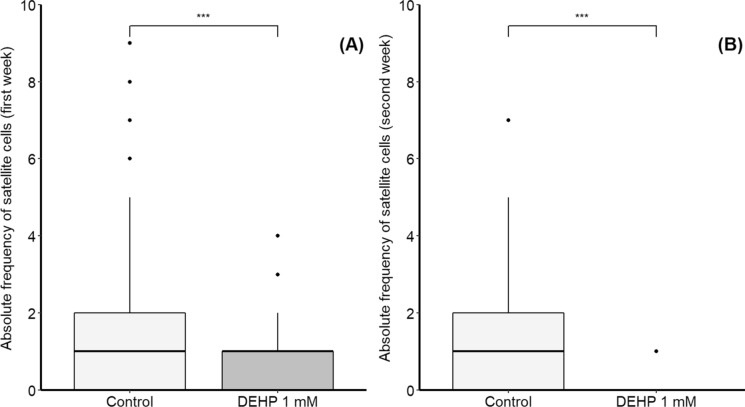
Table 2 summarizes the total number of human skeletal muscle cells and of satellite cells under control conditions and following exposure to di (2-ethylhexyl) phthalate (DEHP, 1 mM) for 13 days. Figure 3 shows the frequency of satellite cells within primary human skeletal muscle cells exposed to DEHP (1 mM) for 13 days. The absolute frequency of satellite cells was significantly lower in cells exposed to DEHP compared to controls (without DEHP); this was observed from day 1, and by day 7 (first week, Fig. 3A), as well as by day 8 to 13 (second week, Fig. 3B). DEHP-treated cells showed a decrease in satellite cell frequency despite available spaces in the culture flask. In contrast, skeletal muscle cells continued proliferating during the entire bioassay and satellite cells were frequently observed in the control group. Higher frequency of satellite cells in controls could be related to a higher potential of these cells to be resilient and cope with DEHP effects after 13 days of exposure.
Table 2.
Total number of human skeletal muscle cells and of satellite cells under control conditions and following exposure to di (2-ethylhexyl) phthalate (DEHP, 1 mM) for 13 days. Cell abundance was obtained by extrapolating the number of cells counted in each photograph to the total surface area (25 cm2) of each flask
| Treatment | Culture Day | Total number of skeletal muscle cells per 25 cm2 | Total number of satellite cells per 25 cm2 |
|---|---|---|---|
| Control | 1 | 275,046 ± 44,802 | 14,984 ± 0.82 |
| DEHP | 223,593 ± 32,389 | 16,346 ± 0.98 | |
| Control | 7 | 439,474 ± 133,585 | 6,837 ± 1.71 |
| DEHP | 319,332 ± 111,871 | 3,869 ± 1.09 | |
| Control | 13 | 577,979 ± 161,911 | 4,491 ± 1.26 |
| DEHP | 407,070 ± 144,338 | 774 ± 0.39 |
Data are shown as mean ± standard error
Fig. 3.
Absolute frequency of satellite cells within primary human skeletal muscle cells exposed to 1 mM of di (2-ethylhexyl) phthalate (DEHP) for 13 days. A Data from day 1 to 7 of bioassay; B data from day 8 to 13 of bioassay. Statistical differences were estimated using Wilcoxon tests. ***significant differences (p < 0.001) between treatments; black circles denote outliers
Di (2-ethylhexyl) phthalate (DEHP) reduces human skeletal muscle cell proliferation under primary culture conditions
Significant contribution of the explanatory variable culture time was found for both, control (t-test = 121.5, p < 0.001) and DEHP-treated GLMMs (t-test = 94.18, p < 0.001), meaning that in models, culture time is a predictor variable for cell proliferation, regardless of culture condition. The GLMMs suggest that the time of culture contributes to explain 91% and 90% of deviance of the total number of skeletal muscle cells in control and DEHP treatment conditions, respectively (Fig. 4). Residuals from GLMM for both control (W = 12, p = 0.055) and DEHP-treated (W = 15, p = 0.34) cells were normal, achieving linearity in both cases. The variable human, independent samples, was tested as random effects in the models, but its contribution does not improve model goodness of fit test, so no random effects from human independent samples were assumed for GLMM building.
Fig. 4.
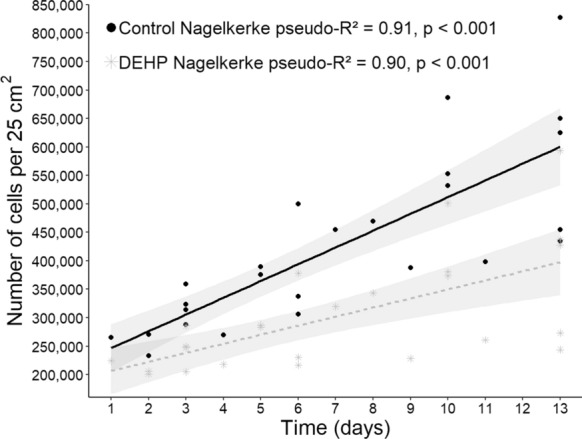
Generalized linear mixed models (GLMM) showing the total number of primary human skeletal muscle cells estimated in a 25 cm2 T flask throughout 13 days. Lines represent the calculated linear regression models describing the association between number of cells and time of culture for cells exposed to di (2-ethylhexyl) phthalate (DEHP, 1 mM) (---) and the corresponding controls ( ). Plotted values are those predicted by the GLMMs (n = 25, per treatment). The gray area represents the standard error for each GLMM.
). Plotted values are those predicted by the GLMMs (n = 25, per treatment). The gray area represents the standard error for each GLMM.
According to the GLMM for control cells, the average cell abundance after the average culture time (6.5 days) was 223,489 cells in 25 cm2 plus the starting cell quantity for the bioassay (~ 200,000 cells), considering a standard error of 231 cells. For DEHP-treated cells, the expected average cell abundance at 6.5 days was 203,660.3 cells in 25 cm2 plus the starting cell number (~ 200,000 cells), considering a standard error of 260 cells (Table 3).
Table 3.
Summary of the effects of culture time in both control and di (2-ethylhexyl) phthalate (DEHP, 1 mM) treated cells on human skeletal muscle cell proliferation capacity during 13 days of bioassay
| Treatment | Variable | Estimate | Std. Error | t-test | p | AIC |
|---|---|---|---|---|---|---|
| Control | Intercept | 223,488.6 | 231.1 | 967.0 | < 0.001** | 615.5 |
| Time | 33,236.7 | 273.6 | 121.5 | < 0.001** | ||
| DEHP | Intercept | 203,660.3 | 259.8 | 783.91 | < 0.001** | 602.3 |
| Time | 17,866.9 | 519.6 | 34.39 | < 0.001** |
Std. Error, standard error; t-test, test for non-parametric Student’s t-distribution; p, significance level; AIC, Akaike information criterion
**Statistical differences
The prediction equations for the GLMMs are represented as follows:
| 2 |
| 3 |
where is indexed i for i-th human samples, x represents culture time.
For control cells, the expected increase in cell abundance per day was 33,237 ± 273.6 cells, which is higher than and statistically different from (t-test = − 56.18, p < 0.001) the expected value for DEHP-treated cells (17,867 ± 520 cells) (Fig. 4). These results suggest that human skeletal muscle cells proliferated at a lower rate when exposed to DEHP compared to control cells. Moreover, positive slopes were observed in both GLMMs, indicating that cell proliferation was maintained throughout the bioassay; however, cell abundance significantly decreased with time in DEHP-treated but not in control cells (Fig. 4).
Discussion
We found that exposure to DEHP concentration lower than 1 mM did not significantly reduce skeletal muscle cell viability, as reported in previous studies (Chen et al. 2020). High DEHP concentration, such as 1 mM, was used in prior studies with grass carp (Ctenopharyngodon idella) hepatocytes (cultured in 96-well plates) (Cui et al. 2020, 2021), but not in studies with other vertebrates’ skeletal muscle cells. Negative effects of DEHP exposure have been observed in other cells, such as human endometrial cells (Cho et al. 2015), erythrocytes (Melzak et al. 2018), placental cells (Tetz et al. 2013), and gametes (Al-Saleh et al. 2019). Some authors describe cell alterations, including decreased cell size or plasmolysis, nucleus fragmentation (Alberts 2013), DNA damage (Al-Saleh et al. 2019), vacuolization (Sung et al. 2003), and lower cell density (Patel et al. 2015). These changes have been related to programmed cell death and cellular senescence (Alberts 2013; Baar et al. 2018). In the freshwater prawn (Macrobrachium rosenbergii), exposure to phthalates (including DEHP) produced alterations in nuclear morphology of hemocytes and promoted cell vacuolization leading to cell death via apoptosis and necrosis (Sung et al. 2003). The authors observed that prawn hemocytes treated with 100 mg mL−1 of DEHP primarily die via necrosis on the first 10 min of exposure; then, at 40 min the main cell death pathway was apoptosis (Sung et al. 2003).
In the present study, the observed changes in skeletal muscle cell morphology, such as plasmolysis, could be associated to cell death, which could explain cell deterioration and population loss. The origin of cell stress bodies observed during the bioassays was not confirmed; but, according with the literature, they could be vacuoles (Sung et al. 2003) or apoptotic bodies produced as a consequence of cell disfunction (Alberts 2013). Alternatively, these bodies could be lipid droplets, which have cytoprotective functions against lipotoxic agents and lipid peroxidation promoters (Jarc and Petan 2019), or peroxisomes, which are produced massively as cytoprotective factors during stress processes (Elcombe and Mitchell 1986; Lapinskas et al. 2005). Stress granules, cytoplasmic aggregates of protein and RNA that contribute to cellular protection, have been observed in arsenite-treated (0.5 mM) C2C12 mouse myoblasts; these granules are more evident after 45 min of exposure (Ravel-Chapuis et al. 2016; Chen et al. 2020) observed stress granule formation in C2C12 mouse myoblasts at different stages of differentiation (proliferating, quiescent and differentiated) upon exposure to DEHP and its primary metabolite, MEHP.
Satellite cells, which are muscle stem cells, differentiate into myoblasts and have a crucial role in muscle maintenance and repair (Snijders et al. 2015). The presence of DEHP/MEHP in skeletal muscle cells promotes alterations in mitochondrial morphology, such as changes from its filamentary reticular network form into vesicles, which are less efficient at producing ATP (Chen et al. 2020). Likewise, phthalates block insulin-induced glucose cell uptake (Chen et al. 2020). Without glucose, and with less efficient mitochondria, satellite cells are not able to differentiate into myoblasts (Chen et al. 2020). This process leads to loss of cell abundance, decreased satellite cell recruitment and differentiation, and concomitant inhibition of muscle regeneration (Chen et al. 2020).
Human skeletal muscle cell proliferation was maintained, but satellite cell frequency decreased significantly after 13 days of DEHP exposure (1 mM = 390.564 µg mL−1). Gutiérrez-García et al. (2019) found that human hematopoietic stem cells from umbilical cord blood lost 82% of the cell population after 14 days of in vitro DEHP exposure (100 µg mL−1). Differentiated skeletal muscle cells were more resilient than satellite cells. This could be explained due to satellite cells being more sensitive to epigenetic alterations that impair cell function (Pérez et al. 2019), which reduce their capacity to deal with exogenous agents, including phthalates.
Differentiated skeletal muscle cells have high energy requirements due to their contractile function (Kanatous et al. 1999; Ravussin and Smith 2006). This activity is matched with high blood flow demand which makes skeletal muscle more vulnerable to circulating xenobiotics (Molina-Ortiz et al. 2013). It could be expected that metabolically active tissues that naturally deal with other kind of stressors, such as the contractile effort in muscle cells, could deal with xenobiotic effects (Rodrigues-Lima et al. 2003). Skeletal muscle, as a major organ in the human body, could have a role in degradation of xenobiotic compounds (Cooper and Plum 1987; Chen et al. 2020), including phthalates (ATSDR 2019). Further information is needed to assess the pathways in skeletal muscles that derive into DEHP/MEHP metabolization.
Based on the results from this study, it can be speculated that exposure to DEHP could aggravate muscular pathologies or syndromes such as sarcopenia, which is characterized by loss of muscle mass and function (Huang et al. 2021). Loss of satellite cells reduce myoblast recruitment, promoting cell culture deterioration and loss of cellular integrity. These processes are similar to those associated with cellular senescence (Serrano et al. 2008), which involves cellular aging and related diseases. In vivo skeletal muscle cell deterioration could involve other factors including xenobiotic exposure (Chen et al. 2020), chronic diseases (Morley 2001), malnutrition, vitamin D deficit (Malafarina et al. 2012), thermal, mechanical, oxidative, or pharmacological stresses (Mcardle et al. 2002), among others.
Conclusion
Di (2-ethylhexyl) phthalate (DEHP) exposure for 13 days induces alterations in cell membrane and nuclear envelope boundaries, cell volume loss, presence of stress bodies, and reduced frequency of satellite cells in primary human skeletal muscle cell cultures. Based on the slope comparisons, we suggest that cells exposed to DEHP show lower proliferation rates than control cells. The results from this study suggest a potential link between DEHP exposure and functionality in human skeletal cells in primary culture. The lower frequency of satellite cells in DEHP-treated cells as compared to controls could overwhelm the repair function leading to cell biomass loss over time. Exposure to DEHP reduced human skeletal muscle cell proliferation capacity as evidenced by reduced cellular abundance, which is more evident at 13 days of xenobiotic exposure.
In summary, changes in both morphology and proliferation capacity were observed in human skeletal muscle cells under primary cell culture following exposure to DEHP (1 mM) for 13 days. These results contribute to understand the toxic effects of phthalates in human skeletal muscle and support previous research on the myogenic inhibitory effect of DEHP in skeletal muscle cells in other mammalian species and could suggest a potential link between DEHP exposure and muscle cell functionality.
Acknowledgements
Financial support for this study was provided by Consejo Nacional de Ciencia y Tecnología (CONACYT; Fronteras 2013-01-2305). EBP is a recipient of a graduate scholarship from CONACYT (# 613207). Authors are members of the CYTED network RIESCOS (ref. 419RT0578). All samples were collected in accordance with the guidelines of Mexico’s Comisión Nacional de Bioética (CONBIOÉTICA) and the Code of Ethics of the World Medical Association (Declaration of Helsinki). The research protocol and informed consent forms were registered and approved by Comité de Ética en Investigación and Comité Hospitalario de Bioética (F-CNIC 2019-174 and R 2000-785-008), Comisión Nacional de Investigación Científica del Instituto Mexicano del Seguro Social (IMSS; 2018-785-010), as well as by CONBIOÉTICA (09-CEI-009-20160601). Authors acknowledge personnel at IMSS for their help in sample collection, and O. Lugo-Lugo and P. Hernandez-Almaraz for their technical support and assistance during this study.
Author contributions
EB-P, sample collection and processing, bioassays, data collection and processing, manuscript production; VL-M, data processing, statistical analyses, manuscript edition; CH-C, sample collection, data analyses, manuscript edition; RG-R, sample collection, data analyses, manuscript edition; JPV-M, data analyses, manuscript edition; TZ-S, experimental design, sample collection and processing, bioassays, data processing, manuscript edition.
Funding
Financial support for this study was provided by Consejo Nacional de Ciencia y Tecnología (CONACYT; Fronteras 2013-01-2305).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elizabeth Brassea-Pérez, Email: ebrassea@pg.cibnor.mx.
Vanessa Labrada-Martagón, Email: vanessa.labrada@uaslp.mx.
Claudia J. Hernández-Camacho, Email: jcamacho@ipn.mx
Ramón Gaxiola-Robles, Email: ramon.gaxiola@imss.gob.mx.
José Pablo Vázquez-Medina, Email: jpv-m@berkeley.edu.
Tania Zenteno-Savín, Email: tzenteno04@cibnor.mx.
References
- Agarwal DK, Eustis S, Lamb JC, et al. Influence of dietary zinc on di(2-ethylhexyl)phthalate-induced testicular atrophy and zinc depletion in adult rats. Toxicol Appl Pharmacol. 1986;84:12–24. doi: 10.1016/0041-008x(86)90412-6. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) (2019) Toxicological Profile for Di (2-ethylhexyl) phthalate (DEHP). Life Systems, Atlanta, Georgia, U.S. [PubMed]
- Al-Saleh I, Coskun S, Al-Doush I, et al. Exposure to phthalates in couples undergoing in vitro fertilization treatment and its association with oxidative stress and DNA damage. Environ Res. 2019;169:396–408. doi: 10.1016/j.envres.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Alberts B. Essential cell biology. 4. New York: Garland Science; 2013. [Google Scholar]
- Allbrook D. Skeletal muscle regeneration. Muscle Nerve. 1981;4:234–245. doi: 10.1002/mus.880040311. [DOI] [PubMed] [Google Scholar]
- Baar MP, Perdiguero E, Muñoz-Cánoves P, de Keizer PL. Musculoskeletal senescence: a moving target ready to be eliminated. Curr Opin Pharmacol. 2018;40:147–155. doi: 10.1016/j.coph.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using. J Stat Soft. 2015 doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Bernard L, Bouattour Y, Masse M, et al. Association between urinary metabolites and the exposure of intensive care newborns to plasticizers of medical devices used for their care management. Metabolites. 2021;11:252. doi: 10.3390/metabo11040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassea-Pérez E, Hernández-Camacho CJ, Labrada-Martagón V, et al. Oxidative stress induced by phthalates in mammals: state of the art and potential biomarkers. Environ Res. 2022;206:112636. doi: 10.1016/j.envres.2021.112636. [DOI] [PubMed] [Google Scholar]
- Carl Zeiss Microscopy GmbH (2011) Software Guide ZEN 2. https://www.unige.ch/medecine/bioimaging/files/3914/3765/2064/ZEN_2_blue_edition_-_Software_Guide.pdf. Accessed 3 Mar 2020
- Charifi N, Kadi F, Féasson L, Denis C. Effects of endurance training on satellite cell frequency in skeletal muscle of old men: satellite cells, training, aging. Muscle Nerve. 2003;28:87–92. doi: 10.1002/mus.10394. [DOI] [PubMed] [Google Scholar]
- Chen Y-H, Wu Y-J, Chen W-C, et al. MEHP interferes with mitochondrial functions and homeostasis in skeletal muscle cells. Biosci Rep. 2020;40:BSR20194404. doi: 10.1042/BSR20194404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YJ, Park SB, Han M. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol. 2015;407:9–17. doi: 10.1016/j.mce.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Choi K, Joo H, Campbell JL, et al. In vitro intestinal and hepatic metabolism of Di(2-ethylhexyl) phthalate (DEHP) in human and rat. Toxicol In Vitro. 2013;27:1451–1457. doi: 10.1016/j.tiv.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Choi SM, Lim DS, Kim MK, et al. Inhibition of di(2-ethylhexyl) phthalate (DEHP)-induced endocrine disruption by co-treatment of vitamins C and E and their mechanism of action. J Toxicol Environ Health-A. 2018;81:748–760. doi: 10.1080/15287394.2018.1473262. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev. 1987;67:440–519. doi: 10.1152/physrev.1987.67.2.440. [DOI] [PubMed] [Google Scholar]
- Crobeddu B, Ferraris E, Kolasa E, Plante I. Di(2-ethylhexyl) phthalate (DEHP) increases proliferation of epithelial breast cancer cells through progesterone receptor dysregulation. Environ Res. 2019;173:165–173. doi: 10.1016/j.envres.2019.03.037. [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang Q, Yin K, et al. DEHP-induce damage in grass carp hepatocytes and the remedy of Eucalyptol. Ecotoxicol Environ Saf. 2020;206:111151. doi: 10.1016/j.ecoenv.2020.111151. [DOI] [PubMed] [Google Scholar]
- Cui Y, Yin K, Zheng Y, et al. Mixed plasticizers aggravated apoptosis by NOD2-RIP2-NF-κB pathway in grass carp hepatocytes. J Hazard Mater. 2021;402:123527. doi: 10.1016/j.jhazmat.2020.123527. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. Farben-therapeutische Versuche bei Trypanosomen Erkrankung. Berliner Klin Wochenschr. 1904;14:362–365. [Google Scholar]
- Elcombe CR, Mitchell AM. Peroxisome proliferation due to di(2-ethylhexyl) phthalate (DEHP): species differences and possible mechanisms. Environ Health Perspect. 1986;70:211–219. doi: 10.1289/ehp.8670211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne J, Liu C, Skinner CM, et al. Skeletal muscle as an experimental model of choice to study tissue aging and rejuvenation. Skelet Muscle. 2020;10:4. doi: 10.1186/s13395-020-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Loch-Caruso R, Meeker JD. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999–2006. Environ Res. 2011;111:718–726. doi: 10.1016/j.envres.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferson S, Burgman MA, editors. Quantitative methods for conservation biology. New York: Springer; 2000. [Google Scholar]
- Franken C, Lambrechts N, Govarts E, et al. Phthalate-induced oxidative stress and association with asthma-related airway inflammation in adolescents. Int J Hyg Environ Health. 2017;220:468–477. doi: 10.1016/j.ijheh.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Freshney RI. Culture of animal cells: a manual of basic technique and specialized applications. 7. New Jersey: John Wiley; 2016. [Google Scholar]
- Fromme H. Human exposure. Encyclopedia of environmental health. Amsterdam: Elsevier; 2011. pp. 498–510. [Google Scholar]
- Gregory M. Mobilisation of satellite cells following ischaemia and reperfusion in primate skeletal muscle. SA J Sports Med. 2004;16:17–24. doi: 10.17159/2078-516X/2004/v16i1a189. [DOI] [Google Scholar]
- Gurdemir G, Erkekoglu P, Balci A, et al. Oxidative stress parameters, selenium levels, DNA damage, and phthalate levels in Plastic Workers. J Environ Pathol Toxicol Oncol. 2019;38:253–270. doi: 10.1615/JEnvironPatholToxicolOncol.2019026470. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-García AK, Flores-Kelly JM, Ortiz-Rodríguez T, et al. Phthalates affect the in vitro expansion of human hematopoietic stem cell. Cytotechnology. 2019;71:553–561. doi: 10.1007/s10616-019-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halden RU. Plastics and health risks. Annu Rev Public Health. 2010;31:179–194. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- Handayani D, Notodiputro KA, Sadik K, Kurnia A. A comparative study of approximation methods for maximum likelihood estimation in generalized linear mixed models (GLMM) Indonesia: Jawa Barat; 2017. p. 020033. [Google Scholar]
- Hara M, editor. Polyelectrolytes: science and technology. New York: Marcel Dekker; 1993. [Google Scholar]
- Hector A. New statistics with R: an introduction for biologists. Oxford; New York, NY: Oxford University Press; 2015. [Google Scholar]
- Hill JA, Olson EN, editors. Muscle: fundamental biology and mechanisms of disease. 1. London; Waltham, MA: Academic Press; 2012. [Google Scholar]
- Hoppins S. The regulation of mitochondrial dynamics. Curr Opin Cell Biol. 2014;29:46–52. doi: 10.1016/j.ceb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wu B, Shen D, et al. Ferroptosis in a sarcopenia model of senescence accelerated mouse prone 8 (SAMP8) Int J Biol Sci. 2021;17:151–162. doi: 10.7150/ijbs.53126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 year. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Jarc E, Petan T. Lipid droplets and the management of cellular stress. Yale J Biol Med. 2019;92:435–452. [PMC free article] [PubMed] [Google Scholar]
- Jones AE, Kahn RH, Groves JT, Napier EA. Phthalate ester toxicity in human cell cultures. Toxicol Appl Pharmacol. 1975;31:283–289. doi: 10.1016/0041-008X(75)90163-5. [DOI] [PubMed] [Google Scholar]
- Kabacoff R. R in action: data analysis and graphics with R. Second. Shelter Island: Manning; 2015. [Google Scholar]
- Kanatous SB, DiMichele LV, Cowan DF, Davis RW. High aerobic capacities in the skeletal muscles of pinnipeds: adaptations to diving hypoxia. J Appl Physiol. 1999;86:1247–1256. doi: 10.1152/jappl.1999.86.4.1247. [DOI] [PubMed] [Google Scholar]
- Kelley KE, Hernández-Díaz S, Chaplin EL, et al. Identification of phthalates in medications and Dietary supplement formulations in the United States and Canada. Environ Health Perspect. 2012;120:379–384. doi: 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kang S, Lee G, et al. Urinary phthalate metabolites among elementary school children of Korea: sources, risks, and their association with oxidative stress marker. Sci Total Environ. 2014;472:49–55. doi: 10.1016/j.scitotenv.2013.10.118. [DOI] [PubMed] [Google Scholar]
- Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Phil Trans R Soc B. 2009;364:2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner LL. Shaping the dynamic mitochondrial network. BMC Biol. 2014;12:35. doi: 10.1186/1741-7007-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labno C (2014) Two ways to count cells with ImageJ. Integrated light microscopy core, University of Chicago, pp. 1–5.
- Lapinskas PJ, Brown S, Leesnitzer LM, et al. Role of PPARα in mediating the effects of phthalates and metabolites in the liver. Toxicology. 2005;207:149–163. doi: 10.1016/j.tox.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Li X, Fang EF, Scheibye-Knudsen M, et al. Di-(2-ethylhexyl) phthalate inhibits DNA replication leading to hyperPARylation, SIRT1 attenuation and mitochondrial dysfunction in the testis. Sci Rep. 2015;4:6434. doi: 10.1038/srep06434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis KS, Siegel AC. Cell viability analysis using trypan blue: manual and automated methods. Methods Mol Biol. 2011;740:7–12. doi: 10.1007/978-1-61779-108-6_2. [DOI] [PubMed] [Google Scholar]
- Ma Y, Guo Y, Wu S, et al. Analysis of toxicity effects of Di-(2-ethylhexyl) phthalate exposure on human bronchial epithelial 16HBE cells. Cytotechnology. 2018;70:119–128. doi: 10.1007/s10616-017-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafarina V, Úriz-Otano F, Iniesta R, Gil-Guerrero L. Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas. 2012;71:109–114. doi: 10.1016/j.maturitas.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Mcardle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev. 2002;1:79–93. doi: 10.1016/S0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- Mead R, Gilmour SG, Mead A. Statistical principles for the design of experiments. Cambridge: Cambridge University Press; 2012. [Google Scholar]
- Melzak KA, Uhlig S, Kirschhöfer F, et al. The blood bag plasticizer Di-2-ethylhexylphthalate causes red blood cells to form stomatocytes, possibly by inducing lipid flip-flop. Transfus Med Hemother. 2018;45:413–422. doi: 10.1159/000490502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Ortiz D, González-Zamora JF, Camacho-Carranza R, et al. Xenobiotic-metabolizing enzymes in skeletal muscle of children and adolescents. J Pharm Pharmacol. 2013;04:231–239. doi: 10.4236/pp.2013.42032. [DOI] [Google Scholar]
- Molino C, Filippi S, Stoppiello GA, et al. In vitro evaluation of cytotoxic and genotoxic effects of Di(2-ethylhexyl)-phthalate (DEHP) on european sea bass (Dicentrarchus labrax) embryonic cell line. Toxicol In Vitro. 2019;56:118–125. doi: 10.1016/j.tiv.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Morley JE. Anorexia, sarcopenia, and aging. Nutr J. 2001;17:660–663. doi: 10.1016/S0899-9007(01)00574-3. [DOI] [PubMed] [Google Scholar]
- Net S, Sempéré R, Delmont A, et al. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ Sci Technol. 2015;49:4019–4035. doi: 10.1021/es505233b. [DOI] [PubMed] [Google Scholar]
- Park CG, Sung B, Ryu CS, Kim YJ. Mono-(2-ethylhexyl) phthalate induces oxidative stress and lipid accumulation in zebrafish liver cells. Comp Biochem Physiol C Toxicol Pharmacol. 2020;230:108704. doi: 10.1016/j.cbpc.2020.108704. [DOI] [PubMed] [Google Scholar]
- Patel HP, White MC, Westbury L, et al. Skeletal muscle morphology in sarcopenia defined using the EWGSOP criteria: findings from the hertfordshire sarcopenia study (HSS) BMC Geriatr. 2015;15:171. doi: 10.1186/s12877-015-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez RF, Santamarina P, Fernández AF, Fraga MF. Epigenetics and Lifestyle: The Impact of Stress, Diet, and Social Habits on Tissue Homeostasis. Epigenetics and Regeneration. Amsterdam: Elsevier; 2019. pp. 461–489. [Google Scholar]
- Rabinowitz D, Zierler KL. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism*. J Clin Invest. 1962;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rael LT, Bar-Or R, Ambruso DR, et al. Phthalate esters used as plasticizers in packed red blood cell storage bags may lead to progressive toxin exposure and the release of pro-inflammatory cytokines. Oxid Med Cell Longev. 2009;2:166–171. doi: 10.4161/oxim.2.3.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel-Chapuis A, Klein Gunnewiek A, Bélanger G, et al. Staufen1 impairs stress granule formation in skeletal muscle cells from myotonic dystrophy type 1 patients. Mol Biol Cell. 2016;27:1728–1739. doi: 10.1091/mbc.e15-06-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Smith SR. Increased Fat Intake, impaired Fat Oxidation, and failure of Fat cell proliferation result in ectopic Fat Storage, insulin resistance, and type 2 diabetes Mellitus. Ann N Y Acad Sci. 2006;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Lima F, Cooper RN, Goudeau B, et al. Skeletal muscles Express the Xenobiotic-metabolizing enzyme arylamine N -acetyltransferase. J Histochem Cytochem. 2003;51:789–796. doi: 10.1177/002215540305100610. [DOI] [PubMed] [Google Scholar]
- RStudio T RStudio: Integrated Development for R., RStudio MA (2020) In: RStudio. http://www.rstudio.com/. Accessed 10 Oct 2020
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano AL, Baeza-Raja B, Perdiguero E, et al. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Snijders T, Nederveen JP, McKay BR, et al. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015 doi: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I, Scharf L, Fiala F, Washüttl J. Migration of di-(2‐ethylhexyl) phthalate from PVC child articles into saliva and saliva simulant. Food Addit Contam. 1998;15:812–817. doi: 10.1080/02652039809374715. [DOI] [PubMed] [Google Scholar]
- Sung H-H, Kao W-Y, Su Y-J. Effects and toxicity of phthalate esters to hemocytes of giant freshwater prawn, Macrobrachium rosenbergii. Aquat Toxicol. 2003;64:25–37. doi: 10.1016/S0166-445X(03)00011-0. [DOI] [PubMed] [Google Scholar]
- Tetz LM, Cheng AA, Korte CS, et al. Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicol Appl Pharmacol. 2013;268:47–54. doi: 10.1016/j.taap.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (US EPA) (2012) Phthalates:Action plan
- Wang Y-C, Chen H-S, Long C-Y, et al. Possible mechanism of phthalates-induced tumorigenesis. Kaohsiung J Med Sci. 2012;28:S22–S27. doi: 10.1016/j.kjms.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2017) Guidelines for drinking-water quality
- Wu Z, Li J, Ma P, et al. Long-term dermal exposure to diisononyl phthalate exacerbates atopic dermatitis through oxidative stress in an FITC-induced mouse model. Front Biol. 2015;10:537–545. doi: 10.1007/s11515-015-1382-y. [DOI] [Google Scholar]
- Yang Y, Ju L, Fan J, et al. Association of urinary phthalate metabolites with sarcopenia in US adults: NHANES 1999–2006. Environ Sci Pollut Res. 2022;29:7573–7582. doi: 10.1007/s11356-021-16202-5. [DOI] [PubMed] [Google Scholar]
- Yavasoglu NUK, Koksal C, Dagdeviren M, et al. Induction of oxidative stress and histological changes in liver by subacute doses of butyl cyclohexyl phthalate. Environ Toxicol. 2014;29:345–353. doi: 10.1002/tox.21813. [DOI] [PubMed] [Google Scholar]
- Ye H, Ha M, Yang M, et al. Di2-ethylhexyl phthalate disrupts thyroid hormone homeostasis through activating the Ras/Akt/TRHr pathway and inducing hepatic enzymes. Sci Rep. 2017;7:40153. doi: 10.1038/srep40153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo HX, Li JQ, Han B, et al. Di-(n-butyl)-phthalate-induced oxidative stress and depression-like behavior in mice with or without ovalbumin immunization. Biomed Environ Sci. 2014;27:268–280. doi: 10.3967/bes2014.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.