Abstract
Background and Aims
Unlike classically described polycythemia, anemia is found to be more prevalent in patients with chronic obstructive pulmonary disease (COPD). Anemia increases the cost of hospital stay and causes an increased risk of adverse outcomes including death in COPD patients. This study was done to find the prevalence of anemia in COPD patients, the factors associated, and the outcomes of anemic COPD.
Methods
It was a quantitative, descriptive‐analytical, and cross‐sectional study conducted in Tribhuvan University Teaching Hospital's medical wards and the Emergency Room from September 2019 to September 2020. A simple random sampling method was used. Clinical information was obtained, and patients were followed up 3 months after discharge to document the number of exacerbations and deaths if present.
Results
The patients in our study had a mean age of 70.80 ± 11.16 years. Most were female. Most (85.5%) had a history of exposure to firewood smoke. Twenty‐three percent of the patients had anemia and these patients had significantly greater mortality 3 months postdischarge. Middle‐old and old were more likely to have anemia with odds ratio (OR) of 2.55 (confidence interval [CI]: 0.48–13.5) and 13.6 (CI: 1.12–24.2), respectively. Current smokers had less likelihood of having anemia (OR: 0.05, CI: 0.006–0.49). Multivariate analysis showed that age, sex, and smoking status were significant determinants of anemia in COPD. There was no association between anemia and duration of hospital stay. However, mortality was higher at 3 months in COPD patients with anemia (p < 0.001).
Conclusion
In COPD patients, anemia is prevalent comorbidity that is significantly linked to higher mortality but not to exacerbations. It is unknown, though, if treating anemia in COPD patients will affect the patient's outcome. Additional research in this area may be possible.
Keywords: anemia, chronic obstructive pulmonary disease, COPD, impact, prevalence
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a common preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases. The most encountered risk factor for COPD is smoking, with exposure to noxious stimuli also contributing to nonsmokers. 1 The prevalence, morbidity, and mortality rates of COPD differ by nation and among various populations within nations. According to estimates, it is more than 210 million. 2 There were 960,737 persons in Nepal who had COPD in 2016, and it was estimated that 16,302 of them passed away that year. 3
Comorbidities are believed to result from a spillover of systemic inflammation, despite the presence of shared risk factors. Anemia is one of the comorbidities, even though polycythemia has traditionally been emphasized in COPD. According to various retrospective and prospective studies by Parveen et al., Shorr et al., and Halpern et al., anemia is a prevalent comorbidity in COPD and may be a risk factor for unfavorable outcomes, including death, in several cases. 4 Patients with COPD who have anemia have decreased exercise capacity, worsening hypoxemia, declining life quality, and worsening exacerbations. 5
Numerous studies that studied the prevalence of anemia in COPD patients have been reported. Most studies are retrospective in nature, making it challenging to evaluate because they are prone to many types of biases. To ascertain the true prevalence of anemia in COPD, the effects of anemia in COPD patients, and the effect of treating anemia on clinical outcomes, we needed a sizable prospective study. The primary goal of this investigation was to determine the prevalence and clinical effects of anemia in COPD patients. The clinical effects evaluated included the rate of exacerbations, hospital stay, rate of corpulmonale, and mortality rate among anemic patients and they were compared with the cohort of patients without anemia.
2. METHODS
It was a quantitative, descriptive‐analytical, and cross‐sectional study followed by a cohort study done in medical wards and the Emergency Room of Tribhuvan University Teaching Hospital, Kathmandu, Nepal. The Institutional Review Committee of the Institute of Medicine granted ethical clearance for this investigation, which was carried out from September 2019 to September 2020. The research included 200 eligible COPD patients in total. The study comprised COPD patients who were at least 40 years old and had been diagnosed with the disease. Diagnosis of the disease was established at some point between the past and the present (if first‐time presentation to the hospital) by spirometry. All the patients labeled as COPD had nonreversible airflow limitation after bronchodilator administration. Patients with common confounders such as malignancy, chronic liver disease, active gastrointestinal bleeding, and chronic liver disease were eliminated. The sample size was calculated using the following formula:
Using this formula, based on the prevalence study done in New Delhi, India, the prevalence of anemia in COPD was found to be 14% of COPD patients. 6
With z = 1.96 and a level of significance of 5%, the sample size was 185, and 199 with 10% nonresponders. So, a total of 200 samples were taken.
Data were collected in the self‐made pro‐forma sheet after getting informed expressed consent from the patient or patient party and were entered in MS Excel 2016.
2.1. Case definition
Anemia was defined with World Health Organization criteria of <13 g/dL for males and <12 g/dL for females.
- Corpulmonale was defined with echocardiography when available and by electrocardiography(ECG)/X‐ray when echocardiography was not available. ECG features of corpulmonale are:
-
○Peaked P wave with amplitude >2.5 mm in inferior leads and 1.5 mm in leads V1 and V2.
-
oRight ventricular hypertrophy is defined with a right axis deviation of 110° or more, dominant R wave in V1 (>7 mm tall or R/S ratio > 1). Dominant S wave in V5 or V6 (>7 mm deep or R/S ratio < 1), QRS duration < 120 ms (i.e., changes not due to right bundle branch block).
-
○
Smoker: A person who has smoked 100 cigarettes in a lifetime.
Never smoker: A person who has smoked less than 100 cigarettes in a lifetime.
Ex‐smoker: A person who has smoked more than 100 cigarettes in their lifetime and has left smoking for more than a year.
These definitions were obtained from https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm.
2.2. Statistical analysis
To learn more about the participant characteristics, descriptive statistics were used. The number of exacerbations was compared between the anemic and nonanemic patients. Bivariate odds ratios (ORs), 95% confidence intervals (CIs), and p value were used to analyze the relationship between the outcome variable (anemia) and probable variables. To determine the relationship between variables and the outcome variable binary logistic regression was applied. All the variables with a p value less than 0.05 and independent variables with biological plausibility for the occurrence of the outcomes with p < 0.2 were considered for the multivariate logistic regression model. Finally, model validation was done by checking the interaction between the variables by multicollinearity test where the variance inflation factor (VIF) test was run in the final model and VIF for all the variables was found to be less than 10. Moreover, for the goodness of fit analysis, the model was found to be significant with regression analysis of variance where the p < 0.05, the mean of residuals was zero (linearity fits) and the p value was less than 0.05 by Shapiro–Wilk's normality test (normality fits). In addition, residuals do not have an equal variance by the Reusch–Pagan test where the p < 0.05. The Hosme–Lemenshow χ 2 test shows the model is fitted with the data (p < 0.05). A sensitivity analysis with the E value to measure the robustness of the associations for unmeasured or unadjusted confounding was performed. Stata version 13 and the statistical software Easy R version 3.5 were utilized throughout the entire analysis.
3. RESULTS
The study involved 200 patients, with a mean age of 70.80 ± 11.16 years. The minimum and maximum diagnosis ages were 41 and 97 years, respectively. Most patients (74.5%) were former smokers, followed by 15% who were still smoking today, and 10.5% who had never smoked. Most (85.5%) of the patients had a history of being exposed to firewood smoke. Out of 200 individuals with COPD, 47 (23.5%) had anemia, while 153 (66.5%) did not (Table 1). Out of these, 1% had macrocytic anemia, 2% had microcytic anemia, 18.5% had normocytic normochromic anemia, and 1% had mixed normocytic, normochromic and micarocytic hypochromic anemia. Three of the four people with microcytic anemia had a Fecal Occult Blood Test positive.
Table 1.
Demographic profile of patients included in the study.
| Frequency | Percentage | |
|---|---|---|
| Age, years | ||
| 40–59 | 38 | 19 |
| 60–69 | 43 | 21.5 |
| 70–79 | 66 | 33 |
| 80 and above | 53 | 26.5 |
| Sex | ||
| Male | 67 | 33.5 |
| Female | 133 | 66.5 |
| Occupation | ||
| Dependent | 115 | 57.5 |
| Housewife | 45 | 22.5 |
| Farmer | 36 | 18 |
| laborer | 2 | 1 |
| Business | 1 | 0.5 |
| Ex‐army | 1 | 0.5 |
Patients selected in the initial sample were followed up for 3 months. (Figure 1) Figure 2 illustrates the total number of exacerbations in COPD patients with and without anemia over the course of 3 months. Of these, 7.5% had no exacerbations in anemic COPD, 8.5% had just one episode, 6.5% had two episodes, and 1% had three episodes of acute exacerbations. In contrast, among patients without anemia, only 35% experienced no acute exacerbation, 24.5% did so just once, 14.5% did so twice, and 2.5% experienced three acute exacerbations. At 3 months after discharge, there was no significant difference in the number of exacerbations between the anemic and nonanemic COPD patients (p > 0.05).
Figure 1.
Patient selection and follow‐up flowchart. AECOPD, acute exacerbations of chronic obstructive pulmonary disease.
Figure 2.
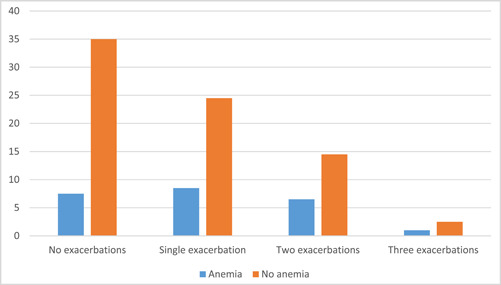
Exacerbations in patients with chronic obstructive pulmonary disease.
Nineteen of the 75 people with corpulmonale had anemia whereas 28 of 97 people who did not have corpulmonale had anemia. Corpulmonale did not differ significantly in the anemic and nonanemic COPD. Bivariate analysis showed that the odds of having anemia are increased in the elderly population of age 70–79 years and more than 80 years (Table 2). Compared to people aged 40–59, those aged 60–69 had a 2.55 times greater risk of anemia. In a similar vein, anemia was 13.6 and 5.2 times more common in persons aged 70–79 compared to people in late adulthood. Males were 2.11 times more likely than females to have anemia. In comparison to independent people, dependent people had 2.11 times the odds of getting anemia. Compared to never smokers, current and former smokers had a lower risk of anemia. Significantly, the odds of getting anemia were 0.05 for current smokers and 0.55 for ex‐smokers. In our study, the prevalence or absence of anemia was not substantially correlated with exposure to firewood. When adjusted with other factors and variables put in multivariate analysis, age, sex, and smoking status were significant determinants of anemia in COPD patients (Table 3).
Table 2.
Bivariate analysis of anemia in COPD patients by selected variables.
| Covariate | Anemia in COPD patients | ||
|---|---|---|---|
| Unadjusted OR | 95% CI | p Value | |
| Age group (years) | |||
| Late adulthood (40–59) | 1.00 | <0.001 | |
| Young old (60–69) | 2.55 | 0.48–13.50 | |
| Middle old (70–79) | 13.60 | 2.96–62.40 | |
| Old old (80+) | 5.20 | 1.12–24.20 | |
| Sex | |||
| Female | 1.00 | <0.01 | |
| Male | 2.11 | 1.08–4.13 | |
| Occupation enrollment | |||
| Independent | 1.00 | 0.02 | |
| Dependent | 2.30 | 1.14–4.74 | |
| Smoking status | |||
| Never | 1.00 | 0.03 | |
| Current | 0.05 | 0.006–0.49 | |
| Ex | 0.55 | 0.21–1.45 | |
| Firewood exposure | |||
| No | 1.00 | 0.93 | |
| Yes | 0.96 | 0.38–2.41 | |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Table 3.
Multivariate analysis of anemia in COPD patients by selected variables.
| Covariate | Anemia in COPD patients | ||
|---|---|---|---|
| Adjusted OR | 95% CI | p Value | |
| Age group (years) | |||
| Late adulthood (40–59) | 1.00 | 0.01 | |
| Young old (60–69) | 1.70 | 0.29–9.78 | |
| Middle old (70–79) | 9.31 | 1.64–52.80 | |
| Old (80+) | 4.47 | 0.85–23.30 | |
| Sex | |||
| Female | 1.00 | <0.01 | |
| Male | 3.45 | 1.56–7.59 | |
| Occupation enrollment | |||
| Independent | 1.00 | 0.95 | |
| Dependent | 1.03 | 0.42–2.48 | |
| Exposure hours per day in the past week | |||
| 0 (no exposure) | 1.00 | 0.26 | |
| 0–1 h | 0.84 | 0.54–1.34 | |
| 1–2 | 0.55 | 0.28–1.05 | |
| 2 and more (2+) | 0.59 | 0.24–1.45 | |
| Smoking status | |||
| Never | 1.00 | 0.04 | |
| Current | 0.05 | 0.004–0.53 | |
| Ex | 0.39 | 0.12–1.25 | |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Anemic COPD patients spent an average of 9.7 days in the hospital overall, compared to 8.9 days for nonanemic COPD patients. Patients with COPD who were anemic or not did not have a statistically different cumulative length of hospital stay. Out of 200 patients, anemia was present in 45.9% of those who died within 3 months, but not in the other patients. Additionally, 81.6% of those who continued to live after 3 months did not develop anemia. When compared to cohorts of nonanemic COPD patients, there was a substantial difference in mortality (Tables 4 and 5).
Table 4.
Association between anemia status and cumulative duration of hospital stay among COPD patients.
| Group by hemoglobin status | Cumulative duration of hospital stay | ||||
|---|---|---|---|---|---|
| Observation | Mean | Standard deviation | 95% Confidence Interval | ||
| Nonanemic | 153 | 8.85 | 5.24 | 8.02 | 9.69 |
| Anemic | 47 | 9.70 | 4.15 | 8.48 | 10.92 |
| Combined | 200 | 9.05 | 5.01 | 8.35 | 9.75 |
| p = 0.84 | |||||
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 5.
Comparison of mortality of anemic and nonanemic COPD patients at 3 months of discharge.
| Variable (n = 200) | Presence of anemia in COPD patients | |||
|---|---|---|---|---|
| Mortality status at 3 months | Yes | No | Total | p Value |
| Yes | 17 (45.90) | 20 (54.10) | 37 (100) | <0.0001 |
| No | 30 (18.40) | 133 (81.60) | 163(100) | |
Abbreviation: COPD, chronic obstructive pulmonary disease.
The calculated value of E with the point estimate of anemia in COPD (23.5%), and confidence interval of 17.92 and 29.08 at 95% is obtained as 9.17. Since the E value is obtained less, it shows that little unmeasured confounding on the anemic COPD with its independent variables, thus proving the robustness of the associations.
4. DISCUSSION
COPD is a disease of the elderly. The average age of the patients included in the study was 70.80 ± 11.16 years which is comparable to the Nepalese studies done. A study done by Khatri et al. in the Nepalese Army Institute of Health Science in 2018 showed a mean age of 69.85 ± 10.36 years, which is comparable to our study. 7 It was also comparable to the mean age seen in the study done by Shrestha et al. in NMCTH which showed a mean age of 66.1 ± 10.9 years. 8 The minimum age of diagnosis was 41 years and the maximum age was 97 years. COPD is said to be the disease of the elderly; however, the diagnosis of COPD at 40s and 50s in this study seems to be a true representation of Nepalese society in which people start smoking in late childhood or early adulthood. Nearly one‐third of people were in the age group 70–79 years. In the study done by Shrestha et al., the age group 60–75 had nearly half of the people. 8
In our study, it was seen that 66.5% were female and 33.5% were male. The published sex prevalence has been shown to vary appreciably among different regions in different studies. A study including the data from 2006 to 2009 in the midwestern region of Nepal showed 60% of the admitted patients of COPD to be female. 9 This is probably due to the increased duration of exposure to indoor household smoking in females. Traditional energy source (cow dung, firewood and agricultural residue) was the primary source of energy in Nepal comprising 85% of energy consumption in the residential sector. 10 , 11 The patriarchal society of Nepal leaves women for indoor cooking and hence is probably the reason why COPD is more prevalent in Nepal. However, in the community, the prevalence of COPD is found to be more in males.
In a 2018 research, Adhikari et al. noted that while age‐standardized death rates and DALYs were higher among women in Nepal, age‐standardized prevalence and incidence of COPD were higher among men. 3 Our study done in a hospital setting includes exacerbated COPD which means that the women are likely to lose their life or live with a disability as exacerbations have been shown to be a risk factor for future exacerbations or death.
Anemia was prevalent in 23.5% of the total 200 samples taken. The prevalence in this study was comparable with the prevalence reported in a study done in Kashmir, India by Parveen et al., who found anemia to be prevalent in 18% of COPD cases. 12 A study done in the United States found anemia to be present in 21% of cases after excluding anemia due to nutritional factors. Our study had 18.5% of people anemic (excluding nutritional causes) which is comparable to the study in the United States. 13
A study by Chambellan et al. in 2012 revealed the probable modification of erythropoiesis in COPD and linked tobacco smoking, hypoxemia, and chronic inflammation as contributors to the development of anemia. 14 It has been also hypothesized that the polymorphism of the genes to control erythropoiesis cause the differences in the responses to the stimuli and manifestation as either polycythemia or anemia. 15 Polycythemia in COPD is due to increased production of erythropoietin. A prospective study published in the Egyptian Journal of Chest and Tuberculosis showed that erythropoietin increased up to GOLD stage III of COPD and then declined. 16 The same study concluded that the probability of blunting of erythropoietin response to hypoxia is present in advanced COPD which might be the cause of anemia in advanced COPD. The same study showed anemia to be present in half of the study population and polycythemia occurred in only the advanced stages. 16 As the primary mechanism underlying the development of anemia in patients with COPD, most of the literature has focused on chronic disease‐related anemia involving the hepcidin‐ferroportin axis and cytokine‐mediated development of erythropoietin resistance. 17
In 3 months of postdischarge, the rate of exacerbations was not significantly different among the anemic versus nonanemic COPD. In a study done by Husebø et al., in predictors of exacerbations of COPD‐Bergen COPD cohort study, inhaled steroids, chronic cough, COPD III and IV, and female sex were the predictors of exacerbations. They didn't analyze anemia in the study. 18 In susceptibility of exacerbation in COPD, an article by Hurst et al., besides previous exacerbations, heartburn, poor quality of life, and leukocytosis were the predictors of exacerbation in COPD. This study didn't analyze anemia as a predictor of exacerbation. 19 So, exacerbation is probably better predicted by the degree of airway obstruction and retention of secretion or chronic micro‐aspiration. However, the use of proton pump inhibitor was not associated with a decrease in exacerbations.
The cumulative duration of hospital stay didn't seem to differ in our study. The difference seen isn't statistically significant. However, previous studies have shown to increase the cost of treatment of anemic COPD. This is probably due to the increased number of investigations done during the hospital stay in COPD patients.
Corpulmonale wasn't associated with anemia in our study. Corpulmonale is traditionally said to be the result of hypoxic vasoconstriction, polycythemia, and destruction of lung parenchyma by emphysema. Since these aren't related to anemia, the finding might have been nonsignificant. However, if analyzed with the GOLD stage, the findings might be significant in the late stages where severe emphysema occurs leading to hypoxic vasoconstriction and anemia is prevalent in the late stages of COPD. Probably, when stratified according to the GOLD stages, anemia and corpulmonale may be related in GOLD III and IV COPD.
Bivariate analysis showed that the odds of having anemia increased in the elderly population in the ages 70–79 years and more than 80 years. Also, the male population in the same group had higher anemia. This is similar to the studies done over a period of time along different populations of the globe. The prevalence of anemia in the elderly population has varied from 9.2% to 36.4% in males people in the elderly and 8.1% to 30.3% in females. 20 , 21 , 22 , 23 , 24 This might be the reason why males and the elderly in our study had a higher prevalence of anemia. Moreover, this is due to the fact that the cutoff level of Hemoglobin taken for males and females are different. Similarly, anemia was less likely to occur in the smokers and ex‐smokers which is probably due to the fact that smoking is a major cause of polycythemia. 25 Anemia didn't seem to differ in dependent or independent people, duration of noticed shortness of breath, and duration of exposure to firewood smoke. When adjusted with other factors, it didn't differ whether the person is dependent or not.
In our study, mortality at 3 months' postdischarge was significantly associated with anemia in COPD with p value of 0.000. In a study done to reveal the impact of anemia on hospitalization and death, anemia was associated with death and hospitalization even after adjusting for age, sex, diabetes, and hospitalization in the elderly. 26 Anemia also predicts death in patients admitted with decompensated heart failure. 27 Anemia has long been ignored to be present in COPD patients, so the study regarding the prognostic implication of anemia in COPD is lacking. Further studies are required to decipher other effects of anemia in COPD, including quality of life and whether correction improves survival.
This study has some limitations. The results of this study may not be generalized because it is based on a single institution, and because it was conducted in a hospital, it is impossible to determine the true prevalence of anemia among COPD patients. The connection between anemia and mortality in the population with COPD is likely to be complicated by a number of factors that were not examined in this study.
5. CONCLUSION
Our study has shown that anemia is a common comorbidity in COPD patients. Females formed the major bulk of hospitalized patients with COPD. Anemia was significantly associated with 3 months' mortality; however, was not associated with exacerbations. There was no difference in the presence or absence of anemia with corpulmonale. When covariate and multivariate analysis were done, the odds of having anemia is high in the elderly and females who never smoked.
AUTHOR CONTRIBUTIONS
Suman Rimal: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; validation; writing—original draft; writing—review and editing. Santa K. Das: Conceptualization; methodology; writing—review and editing. Anita Basnet: Data curation; formal analysis; investigation; resources; software; validation. Tej P. Rauniyar: Data curation; investigation; writing—original draft. Kundan Raj Pandey: Conceptualization; data curation; investigation; writing—original draft. Sandip Kuikel: Investigation; writing—original draft; writing—review and editing. All authors have read and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Suman Rimal affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Rimal S, Das SK, Basnet A, Rauniyar TP, Pandey KR, Kuikel S. Prevalence and clinical impact of anemia in patients diagnosed with chronic obstructive pulmonary disease: a cross‐sectional study. Health Sci Rep. 2023;6:e1371. 10.1002/hsr2.1371
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information: Materials. Additional information is available upon reasonable request to the corresponding author. The corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1. Global Initiative for Chronic Obstructive Lung Disease Inc . Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2018 report. Global Obstructive Lung Disease. 2018.
- 2. Bousquet J, Kiley J, Bateman ED, et al. Prioritised research agenda for prevention and control of chronic respiratory diseases. Eur Respir J. 2010;36(5):995‐1001. [DOI] [PubMed] [Google Scholar]
- 3. Adhikari TB, Neupane D, Kallestrup P. Burden of COPD in Nepal. Int J Chronic Obstruct Pulm Dis. 2018;13:583‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez‐Rivera C, Portillo K, Muñoz‐Ferrer A, et al. Anemia is a mortality predictor in hospitalized patients for COPD exacerbation. J Chronic Obstr Pulm Dis. 2012;9:243‐250. [DOI] [PubMed] [Google Scholar]
- 5. Xiong W, Xu M, Pudasaini B, Guo X, Liu J. The influence of anemia on one‐year exacerbation rate of patients with COPD‐PH. BMC Pulm Med. 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma RK, Chakrabarti S. Anaemia secondary to erythropoietin resistance: important predictor of adverse outcomes in chronic obstructive pulmonary disease. Postgrad Med J. 2016;92:636‐639. [DOI] [PubMed] [Google Scholar]
- 7. Khatri D, Karki P, Shrestha DB, et al. Echocardiographic findings in chronic obstructive pulmonary disease patients. Birat J Health Sci. 2018;3(1):342‐345. [Google Scholar]
- 8. Shrestha B, Dhungel S, Chokhani R. Echocardiography based cardiac evaluation in the patients suffering from chronic obstructive pulmonary disease. Nepal Med Coll J. 2009;11:14‐18. [PubMed] [Google Scholar]
- 9. Bhandari R, Sharma R. Epidemiology of chronic obstructive pulmonary disease: a descriptive study in the mid‐western region of Nepal. Int J Chronic Obstruct Pulm Dis. 2012;7:253‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lohani SP. Biomass as a source of household energy and indoor air pollution in Nepal. Iran J Energy Environ. 2011;2:74. [Google Scholar]
- 11. energypedia . Nepal energy situation. energypedia.info [Internet]. Accessed November 11, 2021. https://energypedia.info/wiki/Nepal_Energy_Situation
- 12. Parveen S, Rangreze I, Ahmad SN, Mufti SA, Khan SS. Prevalence of anemia in patients with COPD and its potential impact on morbidity of COPD patients. Int J Clin Med. 2014;5:452‐458. [Google Scholar]
- 13. Halpern MT, Zilberberg MD, Schmier JK, Lau EC, Shorr AF. Anemia, costs and mortality in chronic obstructive pulmonary disease. Cost Eff Resour Alloc. 2006;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chambellan A, Chailleux E, Similowski T, ANTADIR Observatory Group . Prognostic value of the hematocrit in patients with severe COPD receiving long‐term oxygen therapy. Chest. 2005;128(3):1201‐1208. [DOI] [PubMed] [Google Scholar]
- 15. Chambellan A, Coulon S, Cavailles A, Hermine O, Similowski T. COPD and erythropoiesis: interactions and consequences. Rev Mal Respir. 2012;29(2):213‐231. [DOI] [PubMed] [Google Scholar]
- 16. El‐Korashy RI, Amin YM, Moussa HA, Badawy I, Bakr SM. Study the relationship of erythropoietin and chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc. 2012;61(3):53‐57. [Google Scholar]
- 17. Sarkar M, Rajta P, Khatana J. Anemia in chronic obstructive pulmonary disease: prevalence, pathogenesis, and potential impact. Lung India. 2015;32(2):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Husebø GR, Bakke PS, Aanerud M, et al. Predictors of exacerbations in chronic obstructive pulmonary disease—results from the Bergen COPD Cohort Study. PLoS One. 2014;9(10):e109721. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4184893/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128‐1138. [DOI] [PubMed] [Google Scholar]
- 20. Salive ME, Cornoni‐Huntley J, Guralnik JM, et al. Anemia and hemoglobin levels in older persons: relationship with age, gender, and health status. J Am Geriatr Soc. 1992;40(5):489‐496. [DOI] [PubMed] [Google Scholar]
- 21. Inelmen EM, D'Alessio M, Gatto MRA, et al. Descriptive analysis of the prevalence of anemia in a randomly selected sample of elderly people living at home: some results of an Italian multicentric study. Aging Clin Exp Res. 1994;6(2):81‐89. [DOI] [PubMed] [Google Scholar]
- 22. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263‐2268. [DOI] [PubMed] [Google Scholar]
- 23. Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165(19):2214‐2220. [DOI] [PubMed] [Google Scholar]
- 24. Patel KV, Harris TB, Faulhaber M, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109(11):4663‐4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aitchison R, Russell N. Smoking—a major cause of polycythaemia. J R Soc Med. 1988;81(2):89‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107(10):3841‐3846. [DOI] [PubMed] [Google Scholar]
- 27. Felker GM, Gattis WA, Leimberger JD, et al. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol. 2003;92(5):625‐628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information: Materials. Additional information is available upon reasonable request to the corresponding author. The corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.


