TO THE EDITOR:
Hairy cell leukemia (HCL) is a rare, indolent B-cell malignancy comprising 1.4% of all lymphoid neoplasms, with 1910 new cases in the United States in 2016.1 Patients generally present with cytopenias, splenomegaly, and an increased risk of infections. Newly diagnosed patients are treated with purine nucleoside analogs (PNAs), which have demonstrated efficacy in HCL—overall response rate (ORR) of 97% and median relapse-free survival of 16 years.2 For patients who do not respond to PNAs or who relapse quickly or after therapy for relapsed/refractory disease, the preferred treatment strategies include clinical trial participation, retreatment with a PNA plus rituximab, moxetumomab pasudotox, vemurafenib with or without rituximab, peginterferon-alfa 2a, or ibrutinib.3 Patients with 2 or more relapses will continue to relapse, with steadily decreasing durations of response.4 Thus, additional targeted therapies are needed.
Zanubrutinib (BGB-3111) is a second-generation Bruton tyrosine kinase (BTK) inhibitor designed to maximize BTK occupancy and minimize off-target inhibition of TEC- and EGFR-family kinases. It has demonstrated efficacy in a various B-cell malignancies and has a favorable safety profile.5,6 We report the outcomes of 12 patients with relapsed/refractory HCL treated with single-agent zanubrutinib in a phase 1/2 open-label study (NCT02343120).
Eligible patients had relapsed/refractory HCL per the World Health Organization classification.7 Eligible patients were ≥18 years old, had an Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate organ function and peripheral blood counts (absolute neutrophil count ≥1.0 × 109/L; platelet count ≥50 × 109/L; patients with marrow infiltration could receive growth factor/transfusion support to achieve eligibility). Key exclusion criteria were central nervous system disease, prior BTK inhibitor use, or requirement for treatment with concurrent strong cytochrome P450 3A inhibitors or inducers. Information on the mutational status of cancer was not collected. Patients received zanubrutinib until disease progression or intolerable toxicity, and were transferred to a long-term extension study after completion of the initial trial if they continued to derive benefits. Efficacy and safety were assessed by an investigator. Consensus guidelines for the diagnosis and management of patients with classic HCL were followed, evaluating the response to treatment by target normalization of peripheral blood counts, regression of splenomegaly, and reduction in morphologic evidence of HCL in peripheral blood and bone marrow.8 The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines and the Declaration of Helsinki. The protocol was approved by the institutional review boards and independent ethics committees at each study site, and all patients provided written informed consent before participation. Investigators and their research teams collected the data, and all authors had full access to and were responsible for analyzing and interpreting the data.
A total of 12 male patients with HCL were enrolled in the study. This study consisted of a dose-escalation phase followed by an expansion cohort at the recommended phase 2 dose (RP2D). Dose-limiting toxicity was not observed during the escalation phase. The RP2D was determined using pharmacokinetic and pharmacodynamic data, as either 320 mg once daily or 160 mg twice daily. One patient with HCL was enrolled in the dose-escalation phase, initially receiving a dose of 40 mg once daily and escalating to 320 mg once daily after the RP2D was established; 11 patients were enrolled in the HCL expansion cohort, with 9 patients starting zanubrutinib at 160 mg twice daily and 2 starting at 320 mg once daily. The median patient age was 55.5 years (range, 38-78). The baseline characteristics of the patients are summarized in Table 1. The median time from diagnosis to study entry was 5.1 years (range, 3-24), with patients receiving a median of 2 (range, 1-5) previous therapies. All patients had previously received cladribine (2 in combination with rituximab), and 1 had had a previous splenectomy. The median relative dose intensity (ratio of actual to planned dose intensity) was 98.6%. The median follow-up time was 33.2 months (range, 18.8-74.0). At study completion (March 2021), 9 patients remained on treatment, and 3 had discontinued treatment owing to disease progression (n = 1), an adverse event (grade 3 cerebral aspergillosis [n = 1]), or investigator decision (n = 1). Two patients died during the study owing to progressive disease and >30 days after discontinuation of zanubrutinib.
Table 1.
Disease history and baseline characteristics of patients with HCL
| Characteristic | N = 12 |
|---|---|
| Median age (range), y | 55.5 (38-78) |
| Sex, n (%) | |
| Male | 12 (100.0) |
| Baseline ECOG performance status score, n (%) | |
| 0 | 7 (58.3) |
| 1 | 5 (41.7) |
| Median time from initial diagnosis to first dose (range), y | 5.1 (3.0-24.0) |
| Median time from end of last regimen to first dose (range), mo | 31.6 (3.0-53.8) |
| Median number of previous therapies (range) | 2 (1-5) |
| Cytopenia at study entry, n (%) | |
| Anemia (≤110 g/L) | 6 (50.0) |
| Neutropenia (≤1.5 × 109/L) | 6 (50.0) |
| Thrombocytopenia (≤100 × 109/L) | 7 (58.3) |
| Missing | 2 (16.7) |
| Median laboratory values (range)∗ | |
| Hemoglobin (g/dL) | 109.5 (91.0-161.0) |
| Platelet count (109/L) | 64.5 (32.0-182.0) |
| Absolute neutrophil count (109/L) | 1.55 (0.8-3.8) |
ECOG, Eastern Cooperative Oncology Group.
Ten patients had cytopenias as the reason for starting a new HCL therapy. Neutropenia was given as the reason for 3 patients, anemia for 3 patients, and thrombocytopenia for 4 patients. The reason for new therapy was missing in 2 patients.
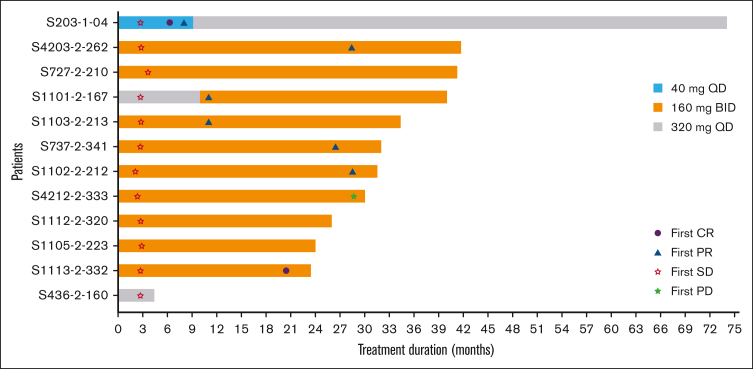
The ORR was 58.3% (n = 7), and 16.7% (n = 2) of the patients achieved a complete response. The median time to response was 20.5 months (range, 6.3-28.6). None of the 7 responding patients had disease progression on study, and the progression-free rate at 48 months was 100% for these patients. Five patients (41.7%) had the best overall response to stable disease (Figure 1). The median progression-free survival (PFS) and overall survival (OS) were not reached. At 36 months, PFS and OS rates were 80.0% and 81.5%, respectively.
Figure 1.
Swimmer plot of treatment duration and investigator-assessed response. BID, twice daily; CR, complete response; PD, progressive disease; PR, partial response; QD, once daily; SD, stable disease.
Eleven patients had baseline cytopenias. Of these, 5 of 6 patients (83.3%) had a hemoglobin response, 6 of 7 (85.7%) had a platelet response, and all had a neutrophil response. The median time to recovery to an absolute neutrophil count >1.5 × 109/L was 3.2 months, to a platelet count >100 × 109/L was 5.5 months, and to a hemoglobin value >11 g/dL was 6.4 months.
Treatment-emergent adverse events (TEAEs) are listed in supplemental Table 1. Eleven (91.7%) of the 12 patients experienced TEAEs, and 7 (58.3%) patients experienced grade ≥3 events. One (8.3%) patient experienced a TEAE leading to treatment discontinuation (grade 3 cerebral aspergillosis), and 4 (33.3%) patients experienced TEAEs leading to dose modification (neutropenia [n = 1], thrombocytopenia/anemia/neutropenia/pneumonia [n = 1], pyrexia/pulmonary aspergillosis [n = 1], vomiting [n = 1]).
TEAEs commonly observed with BTK inhibitor treatment were also observed. Eight (66.7%) patients experienced TEAEs of infections; all were grade 1 and 2 except for 1 event of grade 3 pneumonia and 1 event of grade 3 cerebral aspergillosis. One patient developed an infection defined as opportunistic (pulmonary and cerebral aspergillosis) on study day 122. Five patients experienced minor hemorrhage events (contusion [n = 2] and epistaxis [n = 2]; 1 event of petechiae, ecchymosis, conjunctival hemorrhage, and gingival bleeding). No major hemorrhage or atrial fibrillation events were observed. One TEAE of hypertension was noted on study day 278, and 2 patients experienced TEAEs of basal cell carcinoma during treatment.
New or worsening treatment-emergent neutropenia was observed in 5 (41.7%) patients; all were grade 3 or 4. However, no febrile neutropenia was observed. Three (25.0%) patients experienced TEAEs of thrombocytopenia and 1 patient (8.3%) experienced grade 3 anemia. Only grade 3 anemia led to a dose reduction.
The ORR with zanubrutinib in this study is similar to the ORR observed with the first-generation BTK inhibitor ibrutinib in a phase 2 study (58% vs 54%, respectively), and the 36-month PFS of with zanubrutinib appears similar to the estimated PFS at 36 months with ibrutinib9 (80.0% vs 73%, respectively), although there are no head-to-head trials of zanubrutinib and ibrutinib in this patient population.
Considering the similarity in response in patients with HCL, it should be noted that in head-to-head trials of zanubrutinib vs ibrutinib in Waldenström macroglobulinemia (ASPEN)10 and chronic lymphocytic leukemia (ALPINE)11 favorable differences in treatment tolerability were observed for zanubrutinib. Adverse events leading to treatment discontinuation and atrial fibrillation events of any grade were higher in patients treated with ibrutinib (ASPEN and ALPINE trials).10,11
Zanubrutinib demonstrated clinically significant and durable responses in relapsed/refractory HCL with a safety profile consistent with its known safety profile in other indications. In view of the PK data showing no differences in exposure-response or exposure-safety relationships with the twice daily and once daily doses,12 the zanubrutinib dosing schedule may represent a new treatment option for patients with resistant disease or those who are unable to tolerate PNAs. Future studies evaluating zanubrutinib monotherapy in patients with lower blood count thresholds and zanubrutinib in combination with anti-CD20 agents in high-risk patients (eg, variant HCL and early relapse after chemotherapy) would be of interest.
Conflict-of-interest disclosure: C.S.T. reports honoraria and institutional research funding from BeiGene, Janssen, and AbbVie. J.T. reports institutional research funding from BeiGene (for this study), Bristol Myers Squibb (BMS), Roche, PCYC, Takeda, Janssen, and Cellectar (outside of this work). S.O. has acted as a consultant/advisor for AbbVie, BeiGene, Janssen, Gilead, Roche, Mundipharma, Merck, and BMS; has received research funding from AbbVie, BeiGene, Janssen, Gilead, Roche, and Epizyme; and has received honoraria from AbbVie, BeiGene, Janssen, Gilead, Roche, Merck, and BMS. J.C.S., H.A., W.N., and J.H. are employees of and own stock in BeiGene. K.B. is an employee of BeiGene. A.T. reports serving on advisory boards and speaker bureaus for AbbVie, Janssen Spa, AstraZeneca, and BeiGene.
Acknowledgments
Acknowledgments: The authors thank the patients who participated in the study, their supporters, and the investigators and clinical research staff from the study centers. This study was supported by research funding from BeiGene Co, Ltd, Beijing, China. Third-party editorial assistance was provided by Holly Strausbaugh of Twist Medical and funded by BeiGene USA, Inc.
Contribution: J.C.S., H.A., W.N., and J.H. were responsible for study oversight, clinical data review, and data analyses; K.B. performed the statistical analyses; C.S.T., J.T., S.O., and A.T. recruited patients for the study; and all authors drafted and critically reviewed the manuscript and approved the final version of the manuscript.
Footnotes
Data are available on request, and subject to certain criteria, conditions, and exceptions. BeiGene will provide access to individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. Data requests may be submitted to DataDisclosure@beigene.com.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443–459. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 2.Else M, Dearden CE, Matutes E, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145(6):733–740. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. National Clinical Practice Guidelines in Oncology Hairy cell leukemia. Version 1.2022. 2022. https://www.nccn.org/professionals/physician_gls/pdf/hairy_cell.pdf [DOI] [PubMed]
- 4.Paillassa J, Troussard X. Patients with relapsed/refractory hairy-cell leukemia. Cancer Rep (Hoboken) 2022;5(3) doi: 10.1002/cnr2.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–859. doi: 10.1182/blood.2019001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tam CS, Dimopoulos M, Garcia-Sanz R, et al. Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv. 2022;6(4):1296–1308. doi: 10.1182/bloodadvances.2021005621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC; 2008. [Google Scholar]
- 8.Grever MR, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood. 2017;129(5):553–560. doi: 10.1182/blood-2016-01-689422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers KA, Andritsos LA, Wei L, et al. Phase 2 study of ibrutinib in classic and variant hairy cell leukemia. Blood. 2021;137(25):3473–3483. doi: 10.1182/blood.2020009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tam CS, Opat S, D'Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136(18):2038–2050. doi: 10.1182/blood.2020006844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillmen P, Eichhorst B, Brown JR, et al. 2021. First interim analysis of ALPINE study: Results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Presented at 2021 European Hematology Association Congress (Virtual) Abstract LB1900. [Google Scholar]
- 12.Tam CS, Ou YC, Trotman J, Opat S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev Clin Pharmacol. 2021;14(11):1329–1344. doi: 10.1080/17512433.2021.1978288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.